Abstract
We report a case of myocarditis in an adult patient with recent coronavirus disease 2019 (COVID-19) infection presenting as recurrent ST-segment elevation, mimicking coronary vasospasm. This case highlights the wide range of presentations of COVID-19–related myocarditis. The novel teaching point is that COVID-19 myocarditis can present with acute manifestations such as chest pain and transient ST-segment elevation even several weeks after complete recovery from the initial infection. Cardiac magnetic resonance imaging should be considered in patients with chest pain syndromes and angiographically normal coronary arteries, as the presence of late gadolinium enhancement and a high T2 signal can be diagnostic. Follow-up cardiac magnetic resonance imaging may be used to assess resolution.
Résumé
Nous présentons un cas de myocardite chez un patient adulte infecté par le nouveau coronavirus (COVID-19) qui s’est traduit par une élévation récurrente du segment ST évoquant un vasospasme coronarien. Ce cas illustre le large éventail de tableaux cliniques de la myocardite associée à la COVID-19. Le nouveau point à retenir est que la myocardite associée à la COVID-19 peut se traduire par des manifestations aiguës telles que la douleur thoracique l’élévation transitoire du segment ST, même plusieurs semaines après le rétablissement complet de l’infection initiale. L’imagerie cardiaque par résonance magnétique devrait être envisagée chez les patients qui ont des syndromes de douleur thoracique et des artères coronariennes normales à l’angiographie, puisque la présence d’un rehaussement tardif après injection de gadolinium et d’un signal élevé en T2 peut servir à poser le diagnostic. Le suivi en imagerie cardiaque par résonance magnétique peut être utilisé pour évaluer la résolution.
Case
A 25-year-old man with a history of mild coronavirus disease-2019 (COVID-19) infection, characterized by a low-grade fever and malaise for several days, with complete recovery 6 weeks prior, presented to the emergency room with intermittent episodes of substernal chest pain, with radiation to both arms. He denied diaphoresis and shortness of breath. On initial evaluation, the patient was awake and conversant, with a heart rate of 82 beats per minute, blood pressure of 131/73 mm Hg, a temperature of 36.4°C, and an oxygen saturation of 100% on room air. While in the emergency room, he experienced another episode of similar chest pain. An electrocardiogram (ECG) performed during the episode demonstrated normal sinus rhythm with 1-mm ST-segment elevations in leads II, III, and aVF, without reciprocal changes (Fig. 1A). Initial laboratory workup was significant for a positive COVID-19 polymerase chain reaction test, elevated high-sensitivity troponin (hs-troponin) level of 10,739 ng/L (normal: < 34 ng/L), and an elevated high-sensitivity C-reactive protein (CRP) level of 27.1 mg/L (normal: < 3 mg/L). A repeat ECG a few minutes after the initial ECG showed normal sinus rhythm with resolution of the ST-segment changes (Fig. 1B).
Figure 1.
(A) Electrocardiogram (ECG) results demonstrate ST-segment elevation in the II, III, and aVF leads on presentation. (B) Repeat ECG demonstrates resolution of ST-segment elevation. (C) ECG at time of recurrence of chest pain shows ST-segment elevation in the II, III, and aVF leads. (D) Combined near-infrared spectroscopy and intravascular ultrasound demonstrates minimal lipid burden. (E) Optical coherence tomography shows normal right coronary artery.
Etiologies of chest pain with ST-segment elevation and elevated troponin level include ST elevation myocardial infarction (STEMI), myocarditis, perimyocarditis, coronary vasospasm (such as that secondary to illicit drug use), and stress cardiomyopathy. The patient was treated with aspirin at 325 mg, ticagrelor at 180 mg, and intravenous heparin, and he underwent emergent left heart catheterization (LHC), which demonstrated angiographically normal coronary arteries (Video 1, A and B
 , view video online), with subsequent transfer to the intensive care unit for observation. Dihydropyridine calcium-channel blocker was started for suspicion of coronary vasospasm.
, view video online), with subsequent transfer to the intensive care unit for observation. Dihydropyridine calcium-channel blocker was started for suspicion of coronary vasospasm.
Further tests, including a urine drug screen, were negative. His hs-troponin levels were serially followed and peaked at 14,122 ng/L without chest pain recurrence. An echocardiogram demonstrated a normal left ventricular ejection fraction of 55%-60%, without wall-motion abnormalities. Within 30 hours, the patient developed another episode of chest pain, with ECG demonstrating recurrent ST-segment elevations in leads II, III, and avF (Fig. 1C). The hs-troponin level rose to 18,235 ng/L. A repeat LHC (Video 1C
 , view video online) was performed along with optical coherence tomography (OCT; Abbott, Abbott Park, IL) and combined near-infrared spectroscopy and intravascular ultrasound (NIRS-IVUS; Abbott, Abbott Park, IL). Both NIRS-IVUS (Fig. 1D; Video 2
, view video online) was performed along with optical coherence tomography (OCT; Abbott, Abbott Park, IL) and combined near-infrared spectroscopy and intravascular ultrasound (NIRS-IVUS; Abbott, Abbott Park, IL). Both NIRS-IVUS (Fig. 1D; Video 2
 , view video online) and OCT (Fig. 1E; Video 3
, view video online) and OCT (Fig. 1E; Video 3
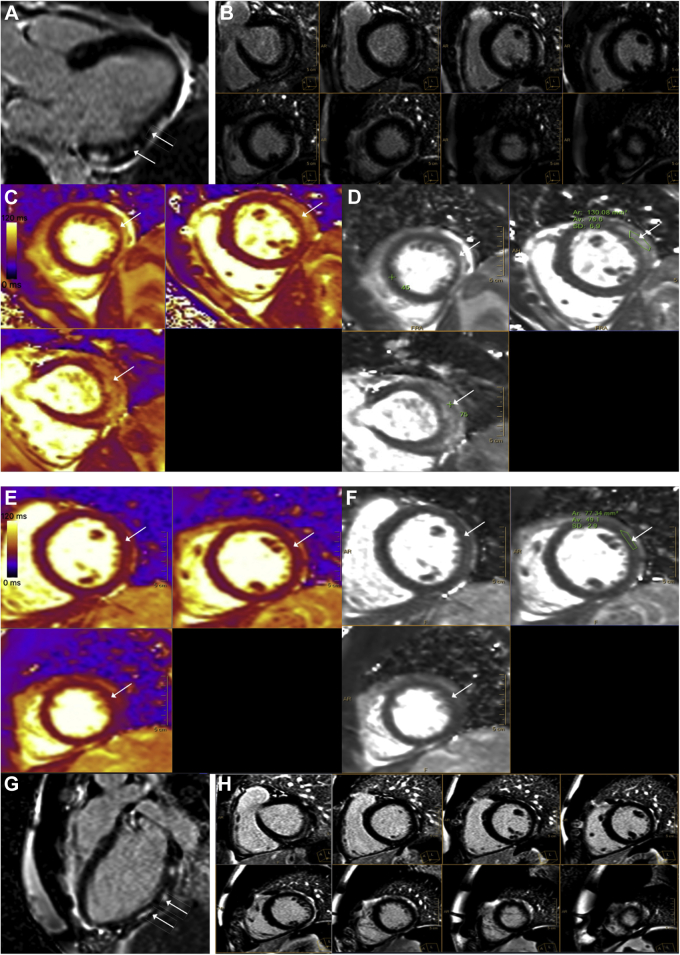
 , view video online) of right coronary artery showed normal intravascular morphology with minimal lipid burden. Cardiac magnetic resonance (CMR) imaging demonstrated predominantly subepicardial enhancement of the basal to mid inferolateral wall and the apical lateral wall (Fig. 2, A and B), suggestive of myocarditis. The enhancement also included pericardium adjacent to the affected myocardium, suggestive of concomitant pericardial involvement and myopericarditis. The T2 signal (Fig. 2, C and D) was increased to 77 ms (for our clinical reference range, the value of 45 ± 6 ms is used for abnormal values, consistent with the Society of Cardiovascular Magnetic Resonance recommendation1) in the lateral wall, indicating presence of edema and acute inflammation. There was mild left ventricular systolic dysfunction (left ventricular ejection fraction: 52%) with mild hypokinesis of the lateral wall of the left ventricle (Video 4
, view video online) of right coronary artery showed normal intravascular morphology with minimal lipid burden. Cardiac magnetic resonance (CMR) imaging demonstrated predominantly subepicardial enhancement of the basal to mid inferolateral wall and the apical lateral wall (Fig. 2, A and B), suggestive of myocarditis. The enhancement also included pericardium adjacent to the affected myocardium, suggestive of concomitant pericardial involvement and myopericarditis. The T2 signal (Fig. 2, C and D) was increased to 77 ms (for our clinical reference range, the value of 45 ± 6 ms is used for abnormal values, consistent with the Society of Cardiovascular Magnetic Resonance recommendation1) in the lateral wall, indicating presence of edema and acute inflammation. There was mild left ventricular systolic dysfunction (left ventricular ejection fraction: 52%) with mild hypokinesis of the lateral wall of the left ventricle (Video 4
 , view video online), corresponding to the late gadolinium enhancement (LGE) and increased T2 signal. Serial hs-troponin levels continued to downtrend, and the patient remained free of chest pain. The patient was discharged with a recommendation to refrain from high-level physical activity and to follow-up in an outpatient clinic for a repeat CMR. At a 6-week follow-up clinic visit, the patient denied any recurrence of symptoms, with laboratory work showing an hs-troponin level of 10 ng/L. A repeat CMR performed at that time showed interval resolution of the T2 signal (Fig. 2, E and F) and a minimal qualitative decrease in the extent of LGE (Fig. 2, G and H) in the involved segments, suggesting resolution of the acute/subacute phase of myocarditis, with residual fibrosis.
, view video online), corresponding to the late gadolinium enhancement (LGE) and increased T2 signal. Serial hs-troponin levels continued to downtrend, and the patient remained free of chest pain. The patient was discharged with a recommendation to refrain from high-level physical activity and to follow-up in an outpatient clinic for a repeat CMR. At a 6-week follow-up clinic visit, the patient denied any recurrence of symptoms, with laboratory work showing an hs-troponin level of 10 ng/L. A repeat CMR performed at that time showed interval resolution of the T2 signal (Fig. 2, E and F) and a minimal qualitative decrease in the extent of LGE (Fig. 2, G and H) in the involved segments, suggesting resolution of the acute/subacute phase of myocarditis, with residual fibrosis.
Figure 2.
Cardiac magnetic resonance (CMR) imaging on 1.5T. (A, B) Late gadolinium enhancement imaging in (A) 3-chamber view and (B) short-axis view shows areas of patchy, mid to epicardial enhancement in the basal, mid, and apical lateral and inferolateral wall. (C, D) T2 maps in the short-axis projection show prolonged T2 relaxation times in the anterolateral and lateral wall, consistent with edema. For our clinical reference range, the value of 45 ± 6 ms is used for abnormal values consistent with the Society for Cardiovascular Magnetic Resonance recommendation.1 Follow-up cardiac magnetic resonance on 1.5T. (E, F) Late gadolinium enhancement imaging in 3-chamber- and short-axis view shows small interval decrease in late gadolinium enhancement. (G, H) T2 maps in the short-axis projection show resolution of the previously increased T2 signal. For our clinical reference range, the value of 45 ± 6 ms is used for abnormal values, consistent with the Society for Cardiovascular Magnetic Resonance recommendation.1
Discussion
Cardiac catheterization of COVID-19 patients presenting with ST-segment elevation has shown a variety of findings, ranging from obstructive coronary artery disease to angiographically normal coronary arteries.2
This is, to our knowledge, the first reported case in the literature of COVID-19 myocarditis presenting late after initial COVID-19 infection with transient and recurrent ST-segment elevation. Our patient was young, without cardiovascular risk factors, and had a history of complete recovery from mild COVID-19 infection 6 weeks prior to presentation. LHC demonstrated normal coronary arteries; OCT and NIRS-IVUS excluded occult plaque/coronary artery dissection and confirmed normal coronary arteries, including the right coronary artery. In this setting, he was referred for CMR imaging for further evaluation of the myocardial injury. Of note, the diagnostic algorithm used in this patient has been used in a published trial that demonstrated the utility of sequential OCT and CMR in patients with myocardial infarction with normal coronary arteries (MINOCA).3 Parametric imaging (myocardial mapping) is of particular interest in the diagnosis of myocarditis, and elevated T2 values, in addition to T1 parameters, are part of the CMR consensus criteria for nonischemic myocardial inflammation.4 In our patient, the abnormal T2 signal indicating myocardial edema of the inferolateral wall, and LGE with subendocardial sparing, pointed to acute myocarditis as the underlying mechanism. In absence of other known causes of myocarditis, including vaccination, COVID-19 infection remained the most likely etiology.
Most reported cases of COVID-19 myocarditis describe myocarditis at the time of active infection5; however, in our case, the patient presented 6 weeks after recovering from mild COVID-19 infection. The treatment for COVID-19 myocarditis remains uncertain, with options including corticosteroids, interleukin-6 inhibitors, and antivirals. Given his clinical improvement and the lack of data on the use of immunosuppressive therapy, our patient was treated supportively with plans for follow-up CMR imaging as an outpatient. He was additionally advised to restrict physical activity for 3 to 6 months, a recommendation consistent with the American Heart Association/American College of Cardiology scientific statement on myocarditis.6
Our findings of residual LGE on follow-up CMR imaging, several months after the acute myocarditis have been previously described in non-COVID-19 myocarditis.7 Although baseline LGE represents a known predictor of cardiac mortality, the long-term impact of persistent LGE on cardiac events during follow-up remains uncertain.8 Further study is needed to determine whether COVID-19 myocarditis has similar outcomes, compared with myocarditis from other causes.
Conclusion
COVID-19 myocarditis is highly variable in presentation. We report a case of COVID-19 myocarditis developing 6 weeks after initial COVID-19 infection and presenting with transient and recurrent inferior ST-segment elevation, mimicking coronary vasospasm. This case demonstrates the clinical utility of CMR imaging in the diagnosis of myocarditis in the setting of an atypical presentation. The clinical course in this case was mild, and the patient improved with supportive management.
Novel Teaching Points.
-
•
COVID-19 myocarditis can present with acute manifestations, such as chest pain and transient ST-segment elevation, even several weeks after complete recovery from the initial infection.
-
•
CMR imaging should be considered in patients with chest pain syndromes and angiographically normal coronary arteries, as the presence of LGE and a high T2 signal can be diagnostic.
-
•
Follow-up CMR imaging may be used to assess resolution.
Funding Sources
The authors have no funding sources to declare.
Disclosures
The authors have no conflicts of interest to disclose.
Footnotes
Ethics Statement: The research reported has adhered to the relevant ethical guidelines.
See page 104 for disclosure information.
To access the supplementary material accompanying this article, visit CJC Open at https://www.cjcopen.ca/ and at https://doi.org/10.1016/j.cjco.2021.08.003.
Supplementary Material
LHC showing normal left and right coronary arteries.
Repeat LHC demonstrating normal RCA.
NIRS-IVUS showing absence of lipid rich plaque in RCA.
OCT showing no evidence of lipid rich plaque in RCA.
CMR with short axis demonstrating mild hypokinesis of the lateral segment of left ventricle.
References
- 1.Wiesmueller M., Wuest W., Heiss R., et al. Cardiac T2 mapping: robustness and homogeneity of standardized in-line analysis. J Cardiovasc Magn Reson. 2020;22:39. doi: 10.1186/s12968-020-00619-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bangalore S., Sharma A., Slotwiner A., et al. ST-segment elevation in patients with covid-19—a case series. N Engl J Med. 2020;382:2478–2480. doi: 10.1056/NEJMc2009020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reynolds H.R., Maehara A., Kwong R.Y., et al. Coronary optical coherence tomography and cardiac magnetic resonance imaging to determine underlying causes of myocardial infarction with nonobstructive coronary arteries in women. Circulation. 2021;143:624–640. doi: 10.1161/CIRCULATIONAHA.120.052008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferreira V.M., Schulz-Menger J., Holmvang G., et al. Cardiovascular magnetic resonance in nonischemic myocardial inflammation: expert recommendations. J Am Coll Cardiol. 2018;72:3158–3176. doi: 10.1016/j.jacc.2018.09.072. [DOI] [PubMed] [Google Scholar]
- 5.Siripanthong B., Nazarian S., Muser D., et al. Recognizing COVID-19-related myocarditis: the possible pathophysiology and proposed guideline for diagnosis and management. Heart Rhythm. 2020;17:1463–1471. doi: 10.1016/j.hrthm.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maron B.J., Udelson J.E., Bonow R.O., et al. Eligibility and disqualification recommendations for competitive athletes with cardiovascular abnormalities: task force 3: hypertrophic cardiomyopathy, arrhythmogenic right ventricular cardiomyopathy and other cardiomyopathies, and myocarditis: a scientific statement from the American Heart Association and American College of Cardiology. Circulation. 2015;132:e273–e280. doi: 10.1161/CIR.0000000000000239. [DOI] [PubMed] [Google Scholar]
- 7.Berg J., Kottwitz J., Baltensperger N., et al. Cardiac magnetic resonance imaging in myocarditis reveals persistent disease activity despite normalization of cardiac enzymes and inflammatory parameters at 3-month follow-up. Circ Heart Fail. 2017;10 doi: 10.1161/CIRCHEARTFAILURE.117.004262. [DOI] [PubMed] [Google Scholar]
- 8.Grün S., Schumm J., Greulich S., et al. Long-term follow-up of biopsy-proven viral myocarditis: predictors of mortality and incomplete recovery. J Am Coll Cardiol. 2012;59:1604–1615. doi: 10.1016/j.jacc.2012.01.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
LHC showing normal left and right coronary arteries.
Repeat LHC demonstrating normal RCA.
NIRS-IVUS showing absence of lipid rich plaque in RCA.
OCT showing no evidence of lipid rich plaque in RCA.
CMR with short axis demonstrating mild hypokinesis of the lateral segment of left ventricle.