Abstract
A diagnosis of paroxysmal nocturnal hemoglobinuria (PNH) was elicited during acute COVID‐19 infection. COVID‐19 spike proteins trigger the alternative pathway of complement. Acute SARS‐CoV‐2 infection possibly expanded an existing PIG‐A mutation.
Keywords: COVID‐19, PNH, SARS‐CoV2
A diagnosis of paroxysmal nocturnal hemoglobinuria (PNH) was elicited during acute COVID‐19 infection. COVID‐19 spike proteins trigger the alternative pathway of complement. Acute SARS‐CoV‐2 infection possibly expanded an existing PIG‐A mutation.

1. CASE
We present the case of a 35‐year‐old male with no past medical history who presented with pancytopenia and melena and was diagnosed with paroxysmal nocturnal hemoglobinuria (PNH) in the setting of acute COVID‐19 infection. To our knowledge, this is the first case of PNH presenting during an acute COVID‐19 infection.
A 35‐year‐old African‐American man with prior history of migraines presented to the emergency room with headaches and melena associated with fatigue and inappetence for two weeks. Due to severe pancytopenia, he was initially admitted to the intensive care unit for gastrointestinal bleed concerns. He denied any fevers, chills, cough, myalgias, dyspnea, difficulty breathing, diarrhea, anosmia, or ageusia. Initial laboratory tests showed white blood cell count (1.70 K/ul), absolute neutrophil count (0.82 K/ul), absolute lymphocyte count (0.65 K/ul), hemoglobin (5.0 g/dl), platelet count (12 K/ul), with normal prothrombin time, partial thromboplastin time, international normalized ratio, D‐dimer, fibrinogen, and normal renal and hepatic function. He was transfused two units of packed red blood cells and 1 unit of single donor platelet then transferred to general medical floor. Prior to admission, he reported taking ibuprofen 800 mg daily for one week. No further episodes of melena occurred once NSAIDs were stopped, thus endoscopy was deferred.
COVID‐19 by nasopharyngeal polymerase chain reaction (PCR) was positive on Day 1. Computed tomography of chest/abdomen/pelvis revealed bilateral lower lobe opacities. Other viral studies, including parvovirus, human immunodeficiency virus, cytomegalovirus, Epstein‐Barr, varicella zoster, hepatitis B and C, were negative. Iron studies, vitamin B12, folate, Coombs, and urinalysis were normal. Exhaustive Rheumatological testing was unremarkable including complement 3/4/5 levels, Aldolase, cyclic citrullinated peptide antibody, anti‐nuclear factor antibody, anti‐smith antibody, anti‐RNP antibody, anti‐double stranded DNA antibody, anti‐SSA and anti‐SSB antibody, scleroderma and centromere antibodies. Abnormal laboratory studies included undetectable haptoglobin (<20 mg/dl), with elevated lactate dehydrogenase (794 U/L), ferritin (503 ng/dl), and erythrocyte sedimentation rate (38 mm/hr). Poikilocytosis and large granular lymphocytes (LGL) were noted [Figure 1]. Peripheral T‐cell receptor (TCR) gene rearrangement showed clonality in TCRβ and TCRγ [Figure 2]. Peripheral flow cytometry revealed normal myeloid and lymphocyte immunophenotype with increased natural killer‐like T cells (20%). Serum protein electrophoresis was normal with increased serum kappa/lambda free light chain ratio (2.09; normal 0.26–1.65), which normalized by day 17. Serum and urine immunofixation showed no monoclonality. Bone marrow biopsy showed trilineage hematopoiesis with maturation. Glycosylphosphatidylinositol‐linked antigen testing revealed “classical Paroxysmal Nocturnal Hemoglobinuria (PNH)” showing RBC‐Complete Antigen (Ag) Loss 4.34% (Reference Range (RR) 0.00–0.01), Granulocytes 42.55% (RR 0.00–0.01), Monocytes 45.84% (RR 0.00–0.05) [Figure 3]. Due to his persistent headaches a magnetic resonance imaging (MRI) of the brain was performed which showed nonspecific patchy elliptically shaped areas of restricted diffusion and low marrow signal within the left parietal calvarium, cervical vertebral bodies, posterior elements, and clivus with minimal if any associated enhancement. There was no evidence of intracranial bleeding or thrombosis on imaging. Given thrombocytopenia, the patient refused lumbar puncture. Empiric treatment with high‐dose pulse steroid and intravenous immunoglobulin showed no improvement. He was discharged home to start eculizumab as outpatient. Over time, he has responded minimally to eculizumab requiring transfusional support over the last 8 months.
FIGURE 1.
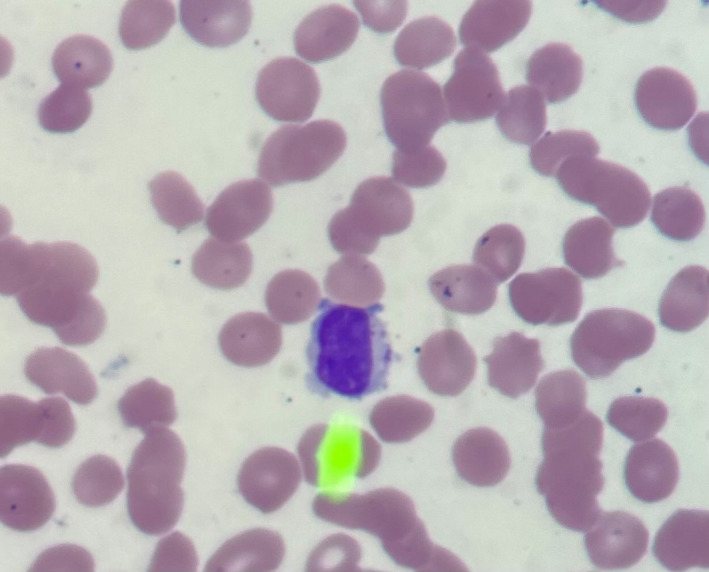
Peripheral blood smear with arrow pointing to large granular lymphocyte
FIGURE 2.
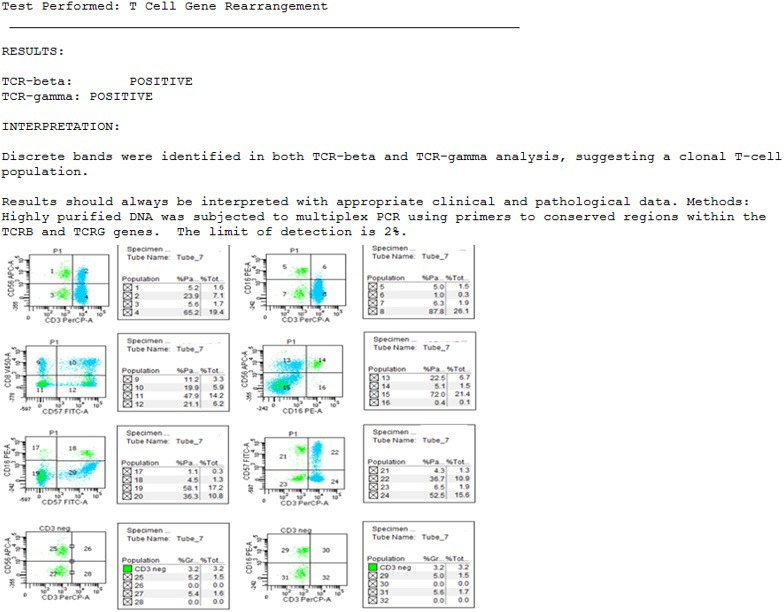
TCR gene rearrangement shows positive TCRβ and TCRγ. C. Peripheral blood flow cytometry histogram depicting an increase in NK‐like T cells CD57+/CD3+ comprising 10.3% of the total population with different subtypes like CD57+/CD8+ comprising 5.9% of the total population
FIGURE 3.
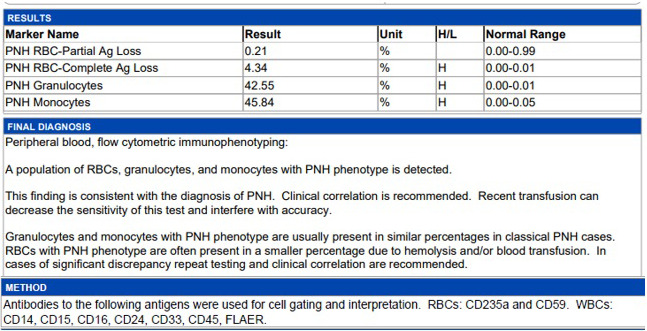
Glycosylphosphatidylinositol‐linked antigen results reveal PNH clones in Type III red blood cells (4.34%), granulocytes (42.55%), and monocytes (45.84%)
Here, we present the case of a patient with new‐onset PNH in the setting of acute COVID‐19 infection without any of the typical symptoms traditionally associated with a COVID infection. PCR testing for COVID was positive on days 1 and 7 of hospitalization, testing negative by day 13 (confirmed on day 14). COVID IgG antibodies were initially negative on days 2 and day 4, becoming positive on day 5 of admission. To our knowledge, this is the first report of a case of COVID‐19 infection presenting with isolated pancytopenia from PNH without any of the commonly associated symptoms from the acute infection. Review of the literature shows the report of a single case of a patient with a known history of PNH developing acute flare possibly precipitated by COVID‐19 infection.1There is a known association with LGL clonal expansion in PNH, though unlikely relevant unless persistently cytopenic post PNH therapy.2 There was an increase in NK‐like T cells, CD57+/CD3+ comprising 10.3% of the total population with different subtypes such as CD57+/CD8+ comprising 5.9% of the total population. An increase in these populations can be seen in conditions such inflammatory disease, viral infections, following treatment with chemotherapy or transplant patients and in large granular lymphocyte leukemia. T‐cell rearrangement studies showed discrete bands in both TCR‐beta and TCR‐gamma analysis, suggesting a clonal T‐cell population. However, the clinical significance of all these findings is unclear in the setting of pancytopenia and the acute COVID‐19 infection.
Paroxysmal nocturnal hemoglobinuria is an X‐linked acquired somatic mutation of the phosphatidylinositol glycan class A (PIG‐A) gene that can cause bone marrow failure, complement‐mediated hemolysis, and thrombophilia.3 This mutation causes impaired expression of CD55 and CD59 (complement regulators) which causes dysregulated complement activation.3
Yu et al.4 demonstrated that SARS‐CoV‐2 spike proteins activate complement by engaging the alternative complement pathway. This activation can cause complement‐mediated damage such as endothelial injury, hemolysis, and contribute to end‐organ damage.5 Prior to this admission, the patient had not sought medical care in years, thus prior laboratory data for the patient are not available. It is possible that he had subclinical PNH that was discovered only during the acute COVID‐19 infection when he became symptomatic. It is possible the presenting symptoms were related to PNH with COVID‐19 infection being an incidental diagnosis. Given the temporal relationship and the clinical findings, it is not clear whether the acute COVID‐19 infection led to an acquired PIG‐A mutation or expanded an existing subclinical PIG‐A mutation. We report this case to inform the medical community to have a low threshold for testing for COVID‐19 infection particularly in areas of high prevalence of the disease as this patient presented without any of the typical symptoms associated with acute COVID.
CONFLICT OF INTEREST
Research funding: AstraZeneca, Oncternal, TG therapeutics, Pharmacyclics/Abbvie. Ad Board: AstraZeneca, Pharmacyclics/Abbvie, Beigene, Genentech, Gilead, Innate.
AUTHOR CONTRIBUTION
A.H. and N.H. conceived of the presented idea. J.B. verified and approved of the idea. A.H., N.H., and J.B. all contributed to writing and editing of the final manuscript.
ETHICAL APPROVAL
Hereby, I Jacqueline Barrientos consciously assure that for this manuscript the following is fulfilled: This manuscript is original work by the authors listed and has not been previously published elsewhere. The paper is not currently being considered for publication elsewhere. The paper properly credits the contributions of the co‐authors. All sources used are properly disclosed in the citation. All authors have been actively involved in substantial work leading to the paper and take full public responsibility of the content.
PATIENT CONSENTED STATEMENT
The patient consented to the publication of images and/or information in regard to this case.
Hines A, Hakim N, Barrientos J. COVID‐19 infection presenting as paroxysmal nocturnal hemoglobinuria. Clin Case Rep. 2021;9:e4636. 10.1002/ccr3.4636
Funding information
There was no funding provided for this manuscript.
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
REFERENCES
- 1.Schüller H, Klein F, Lübbert M, Prager EP. Hemolytic crisis in a patient treated with eculizumab for paroxysmal nocturnal hemoglobinuria possibly triggered by SARS‐CoV‐2 (COVID‐19): a case report. Ann Hematol. 2021;100(3):841‐842. 10.1007/s00277-020-04318-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Risitano A, Maciejewski J, Muranski P, et al. Large granular lymphocyte (LGL)‐like clonal expansions in paroxysmal nocturnal hemoglobinuria (PNH) patients. Leukemia. 2005;19:217‐222. 10.1038/sj.leu.2403617 [DOI] [PubMed] [Google Scholar]
- 3.Risitano AM. Paroxysmal nocturnal hemoglobinuria and the complement system: recent insights and novel anticomplement strategies. Adv Exp Med Biol. 2013;735:155‐172. 10.1007/978-1-4614-4118-2_10. PMID: 23402025. [DOI] [PubMed] [Google Scholar]
- 4.Yu J, Yuan X, Chen H, Chaturvedi S, Braunstein EM, Brodsky RA. Direct activation of the alternative complement pathway by SARS‐CoV‐2 spike proteins is blocked by factor D inhibition. Blood. 2020;136(18):2080‐2089. 10.1182/blood.2020008248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kulkarni HS, Atkinson JP. Targeting complement activation in COVID‐19. Blood. 2020;136(18):2000‐2001. 10.1182/blood.2020008925 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.


