Abstract
Background
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the cause of ongoing global pandemic of coronavirus disease 2019 (COVID-19), has infected millions of people around the world, especially the elderly and immunocompromised individuals. The infection transmission rate is considered more rapid than other deadly pandemics and severe epidemics encountered earlier, such as Ebola, Zika, Influenza, Marburg, SARS, and MERS. The public health situation therefore is really at a challenging crossroads.
Main body
The internal and external and resident microbiota community is crucial in human health and is essential for immune responses. This community tends to be altered due to pathogenic infections which would lead to severity of the disease as it progresses. Few of these resident microflora become negatively active during infectious diseases leading to coinfection, especially the opportunistic pathogens. Once such a condition sets in, it is difficult to diagnose, treat, and manage COVID-19 in a patient.
Conclusion
This review highlights the various reported possible coinfections that arise in COVID-19 patients vis-à-vis other serious pathological conditions. The local immunity in lungs, nasal passages, oral cavity, and salivary glands are involved with different aspects of COVID-19 transmission and pathology. Also, the role of adaptive immune system is discussed at the site of infection to control the infection along with the proinflammatory cytokine therapy.
Keywords: COVID-19, SARS-CoV-2, Microbiota, Resident microflora, Black fungus, Coinfection
Background
The ongoing human-to-human transmitted coronavirus disease 2019 (COVID-19), caused by the latest coronavirus strain (severe acute respiratory syndrome coronavirus 2; SARS-CoV-2), is debatably believed to have originated from pangolins and/or bats that has spread rapidly worldwide [1, 2]. SARS-CoV-2 infection as the ongoing pandemic, resulting in increased number of COVID cases with the current second wave, is a serious global health concern, especially for the immunocompromised and the elderly. Viral infections like Ebola, Zika, Influenza, SARS-CoV, MERS-CoV-2, and Marburg have been infecting millions of humans, animals, and birds equally either as a seasonal epidemic or as a pandemic and a global health disaster [3]. These spread through person-to-person contact with body fluids, and there is no effective therapeutics to treat them being viral entities. Like other CoVs, SARS-CoV-2 possesses membrane glycoprotein, spike protein, nucleocapsid protein, small membrane protein, and hemagglutinin esterase [4, 5]. The glycoprotein spikes present on the outer surface of the virus are mostly responsible for its attachment and entry to the host cell [6]. SARS-CoV and MERS-CoV recognize exopeptidases as the key receptor in case of humans [7], while aminopeptidases or carbohydrates in others. MERS-CoV binds to DPP4 while SARS-CoV and SARS-CoV-2 bind to angiotensin-converting enzyme 2 (ACE2) as a key receptor [7, 8]. The virus spike (S)-protein may bind to ACE2 receptors present on various human cells to initiate its entry into the human host cells [6, 9]. ACE2 is found in human cells like in lung alveolar epithelial cells [10]. However, understanding the dynamics of SARS-CoV-2 in humans and its impact is at its infancy [11]. This viral infection reportedly has caused pulmonary, cardiac, renal, circulatory, gastrointestinal, and neurological fatal tissue damage in patients.
The most common symptoms for COVID-19 are cold, fever, and cough, followed by pneumonia. Apart from these respiratory affections, the virus may further affect the heart, kidneys, and the nervous system. It may cause severe complications among the immunocompromised, including those having diabetes and cardiovascular disorders [12, 13]. SARS-CoV-2 is mainly transmitted through the respiratory droplets from the infected, and also through direct/indirect contacts (i.e., contaminated object/surface/fomite) and fecal-oral route [14]. The WHO till date reported millions of deaths due to this novel virus. Respiratory viral infections lead to secondary coinfections and increase the disease severity and mortality outcomes [15]. Microbial coinfection also increases the risk of disease severity in humans [16]. The mechanism of virus interactions with other microbes is still unclear. It is very essential to study the source and the mechanism infection of the coinfecting pathogens. In 1918 influenza outbreak, Morens et al. [17] suggested that most fatalities occurred due to a subsequent coinfection by Streptococcus pneumonia. Bacterial coinfection was also associated with the 2009 H1N1 influenza pandemic [18, 19]. There are reports on the bacterial and fungal coinfections (Fig. 1) in COVID-19 pandemic, and the related fatalities [20, 21]. The state-of-art mNGS technique helps to investigate and identify the novel pathogen directly from clinical samples [22] which has confirmed the presence of an elevated level of oral and upper respiratory commensal bacteria [23]. An oral-lung aspiration axis may be a key factor for many infectious diseases [24].
Fig. 1.
Coinfections observed in COVID-19 cases
Main text
Viral coinfection
Coinfection is commonly encountered in respiratory diseases [25] which influences disease prognosis and treatment (Table 1). Viral coinfections in COVID-19 patients have been reported globally, and are critical during early misdiagnosis [50]. Possibly due to their immunity status, the middle-aged and the elderly are more prone to viral coinfection [26]. However, it may not be true, healthy people may also be coinfected [27]. Majority of COVID-19 patients coinfected with other viruses have been reported to be around 30–60 years old [51]. An in vitro study by Lin and coworkers at Shenzhen Third People’s Hospital confirmed 3.2% viral coinfection, and at least two viruses were detected in 2.2% of those patients [52]. A study in Wuhan confirmed 5.8% coinfections with other coronavirus, hRV, and influenza (H3N2) [27]. Additional pathogens in 20.7% COVID-19-positive specimens were reported from Northern California, predominantly by RSV, entero-/rhinovirus, and non-SARS-CoV-2 CoV [28]. Due to the infectivity nature of SARS-CoV-2, respiratory viruses like hepatitis virus [29] and HIV [30] coinfections were noticed along with simultaneous detection of common respiratory viruses like RSV, hMPV, hRV, PIV2, and HKU1. Also, C. pneumoniae, parainfluenza 3, influenza A, M. pneumoniae, rhinovirus, and non-SARS-CoV-2 CoV are common coinfections [26].
Table 1.
Microbial groups reportedly active in coinfection of COVID-19 patients
| Microbial group | Microbe(s) | Origin | Endogenous/exogenous | Found in | References |
|---|---|---|---|---|---|
| Virus | C. pneumoniae, parainfluenza 3, influenza A, M. pneumoniae, rhinovirus, non-SARS-CoV-2 | Environment/birds/animals | Exogenous | Upper/lower respiratory tract | [26] |
| Other coronavirus, hRV, influenza (H3N2) | Environment/birds/animals | Exogenous | Upper/lower respiratory tract, intestine | [27] | |
| RSV, entero/rhinovirus, non-SARS-CoV-2 | Environment/birds/animals | Exogenous | Upper/lower respiratory tract, intestine | [28] | |
| Hepatitis virus | Human | Endogenous/exogenous | Blood, tissues, body secretions | [29] | |
| HIV | Human | Endogenous/exogenous | Blood, tissues, body secretions | [30] | |
| hMPV, hRV, PIV2, HKU1 | Human, animals | Exogenous | Upper/lower respiratory tract | [29, 30] | |
| Bacteria | Veillonella, Capnocytophaga, | Human | Endogenous | Oral cavity | [23, 31–33] |
| Neisseria, Streptococcus pneumoniae, Corynebacterium, Leptotrichia, Prevotella, Fusobacterium periodonticum | Human | Endogenous | Oral cavity | [34, 35] | |
| Pseudomonas aeruginosa, Streptococcus pneumoniae, Fusobacterium periodonticum, Veillonella, Prevotella, Capnocytophaga | Human | Endogenous/exogenous | Upper/lower respiratory tract | [23, 31–33] | |
| Staphylococcus aureus, Haemophilus influenza, Escherichia coli | Human/environment | Endogenous/exogenous | Respiratory tract, skin, and intestines | [36] | |
| Coprobacillus, Clostridium hathewayi, C. ramosum | Human, environment | Endogenous/exogenous | Gut | [37] | |
| Clostridium, Veillonella, Actinomyces, Streptococcus pneumoniae, Rothia | Human, environment | Endogenous/exogenous | Gut | [25, 37–40] | |
| Mycobacterium tuberculosis | Human, environment | Endogenous/exogenous | Lower respiratory tract, other organs of the body | [41–43] | |
| Fungi | Candida tropicalis, C. albicans, C. glabrata, C. dubliniensis, C. krusei | Human | Endogenous | Upper/lower respiratory tract, intestine | [44] |
| Aspergillus fumigatus | Human, environment | Endogenous/exogenous | Upper/lower respiratory tract | [45] | |
| Aspergillus fumigatus, Rhizopus oryzae, Absidia mucor | Human, environment | Endogenous/exogenous | Respiratory tract, other organs of the body | [46] | |
| Black fungus (Rhizomucor species, Syncephalastrum species, Cunninghamella bertholletiae, Apophysomyces, Lichtheimia, Saksenaea, Rhizomucor) | Environment | Majorly exogenous | Respiratory tract, eye, broken skin and its appendages, sinuses, and brain | [47–49] |
Viral coinfection and immune response
The respiratory viral infections normally affect the airways and lungs. Among all, the Influenza virus is responsible for causing frequent seasonal viral infections [3]. Other viruses responsible for respiratory infections include coronavirus, human adenovirus, rhinovirus, enterovirus, parainfluenza virus, and human metapneumovirus. Viral coinfection influences the prognosis and treatment of COVID-19, and such patients need higher level of care [53]. Development of such coinfections affects the host immune response, especially in the immunocompromised and elderly people [54]. Reportedly, the patients having hepatitis C virus and HIV infections more likely lead to drug-induced liver injury (DILI) [55]. COVID-19 infection may cause liver damage [56]. As coinfection causes serious damage to immunity [57], so patient’s condition may be more serious, the treatment could be more complicated, and the treatment cycle may be longer [58]. Patients that are coinfected with SARS-CoV-2 and HIV had a longer disease progression attributed to the slower specific antibody generation [59]. Genome sequencing confirms that SARS-CoV-2 is 79.5% identical with SARS-CoV [5].
Rationale of viral coinfection
Viral coinfection increases the CRP and PCT levels, damaging the immunity and the airway [60, 61]. Viral coinfections arise as the airway epithelium is destroyed by SARS-CoV-2 virus. COVID-19 could cause immune system disorders leading to a possibility of coinfection by other viruses [62]. Coinfection mechanism is unclear in COVID-19 patients due to very little available information about the virus kinetics. Coinfection rate in COVID-19 with other viruses is reportedly not very high [63]. Prevention and control of infection is suggested in COVID-19 patients to avoid coinfection [64]. Social distancing is arguably the best prevention in the spread of infection [65–67]. Isolating the patients during treatment in a clinical setting is suggested to understand the transmission risk of the infection [67]. Patients with HIV infection history are more likely to encounter COVID-19 coinfection due to their reduced specific antibody responses [68].
Bacterial and fungal coinfection
Bacterial coinfection is a worrying problem in the COVID-19 management and also is the major cause of morbidity and mortality in other respiratory infections [69]. However, the rate of coinfection in COVID-19 patients is relatively low possibly due to limited available studies. Contou et al. [70] reported 28% bacterial coinfection in French ICU patients with SARS-CoV-2, mostly related to Haemophilus influenzae, Staphylococcus aureus, Streptococcus pneumonia, and bacteria of Enterobacteriaceae family. A recent meta-analysis also confirmed bacterial and viral coinfections in COVID-19 patients [71]. Bacterial coinfection is reportedly more (14%) in COVID-19 patients in the ICU [72]. Calcagno and coworkers reported coinfections with other respiratory pathogens such as Staphylococcus aureus, Moraxella catarrhalis, Haemophilus influenzae, Streptococcus agalactiae, Enterobacter cloacae, Klebsiella pneumoniae, and Escherichia coli in COVID-19 patients [73]. A study on 989 COVID-19 patients showed nosocomial superinfections [74]. A total of 51 hospital-acquired bacterial superinfections by Escherichia coli and Pseudomonas aeruginosa along with S. pneumoniae, S. aureus and Klebsiella pneumoniae were diagnosed. Also, mycobacterium tuberculosis coinfection was observed in COVID-19 patients [41–43], although such coinfections reportedly do not frequently occur. Mohamed and coworkers reported multi-triazole resistant Aspergillus fumigates coinfection in respiratory samples and suggested that early diagnosis would help to understand the antifungal therapy to improve the diseases condition [45]. In a case report, Pal and coworkers found Streptococcus pneumoniae coinfection in SARS-CoV-2-infected patients [75]. S. pneumoniae, M. pneumoniae, L. pneumoniae, and C. pneumoniae coinfections are also observed in COVID-19 patients and suggested for combination therapy with non-anti-SARS-CoV-2 agents [76]. In a multicentre cohort study, Russell and his group reported 70.6% secondary nosocomial infections in COVID-19 cases during the first wave [36]. Staphylococcus aureus, Haemophilus influenzae, and Escherichia coli (Enterobacteriaceae) were the most commonly encountered pathogens as diagnosed within two days post hospitalization.
Human saliva and COVID-19
Human saliva constituting 94–99% water content, produced by the salivary gland, is important in food digestion, oral mucosa lubrication, cleaning, and preservation of oral cavity. It also contains food particles, oral microbes and their metabolites, serum elements, white blood cells, and exfoliated epithelial cells. Although more than 700 microbial species are detected in it, saliva prevents overgrowth of specific pathogens and serves as a gatekeeper (the first level of defense), and prevents them from spreading to the respiratory and gastrointestinal tracts [65]. Also, it is crucial in preventing viral infection [77]. SARS-CoV-2 may enter human saliva through the lower and upper respiratory tract droplet nuclei. It may enter the mouth through the blood from gingival crevicular fluid, and through salivary ducts from infected salivary gland [78].
A previous study on SARS-CoV confirmed infection of epithelial cells of salivary gland having elevated angiotensin-converting enzyme 2 (ACE2) expressions [79]. Moreover, ACE-2 expression in minor salivary glands was found to be more than in lungs. Before the onset of lung lesions, SARS-CoV RNA may be found in saliva samples. Live virus may be cultured in saliva samples. Thus, salivary gland is a significant virus reservoir. It suggests that SARS-CoV-2 spreads through contaminated saliva for asymptomatic infections [80].
Oral bacterial microbiota
Significant number of viral, bacterial, and fungal coinfections in COVID-19 originating from the oral cavity has been observed, similar to other pandemics. Oral pathogens like Veillonella and Capnocytophaga were confirmed by mNGS in bronchoalveolar lavage fluid (BALF) of COVID-19 cases [31]. A higher nasal virus load in the throat has been reported [81]. Oral cavity houses the second largest microbiota containing bacteria, viruses, fungi, and archaea in human body [82]. Major bacterial genera in human oral cavity are Neisseria, Prevotella, Streptococcus, Corynebacterium, Fusobacterium, Leptotrichia, Veillonella, and Capnocytophaga [34]. Many such pathogens may colonize the respiratory tract of healthy individuals asymptomatically [83]. Thus, oral microbiome regulates mucosal immunity and affects pathogenicity [84].
Lung microbiota
In COVID-19, the virus infects epithelial cells of the upper respiratory tract (URT) like the nasal passages and throat, and lungs (bronchi and lung alveoli). The local immunity in lungs, nasal passages, oral cavity, and salivary glands are involved with different aspects of SARS-CoV-2 transmission and pathology. The lung microbiota community is another complex variety and found in lower respiratory track (LRT) like the epithelial and mucous layers. There is a relationship between the microbial community in lungs and the oral cavity [85]. Under normal conditions, the microbiota from oral cavity migrates as an important source of lungs microbiota [86]. Human lungs contain Pseudomonas, Streptococcus, Prevotella, Fusobacterium, Veillonella, and Capnocytophaga that is found in oral cavity as well [23, 32, 33]. Sometimes, potentially harmful bacteria responsible for respiratory disorders like S. pneumonia, H. influenza, and M. catarrhalis are also found in respiratory specimens. Further, the fungal genera include Candida, Aspergillus, Saccharomyces, and Malassezia. Studies confirm that lung microbiota is quite similar to those in the oropharynx and nasopharynx [44].
Reports mention that 72% COVID-19 patients received antimicrobial therapy to treat fungal and bacterial coinfections [87, 88], although the pathogenesis was unclear. As active microbiota of the oral cavity is found in the BALF of COVID-19 patients, it could be a natural reservoir of opportunistic pathogens in COVID-19 patients. Metagenomic sequencing confirms that the nasopharyngeal Fusobacterium periodonticum population in SARS-CoV-2 patients varied with the duration of the infection and decreased significantly beyond 3 days [35].
Intestinal microbiota
Ingestion is a frequent mode of pathogen transmission; gastrointestinal infection is common among the pediatric age group attributable to their playing habits. Environmental microbes are accidentally ingested by both humans and animals, although most of them do not necessarily result in infection. This could be attributed to the unfriendly acidic environment in the stomach and the various proteolytic enzymes in the alimentary system. The mucus lining, the peristaltic movements of the intestinal villi, the secretory immunoglobulins, the local immune defence mechanisms like mucosa-associated lymphoid tissue (MALT) and gut-associated lymphoid tissue (GALT) also aid in the first line of defense. However, microbes like bacteria and viruses occasionally succeed in causing gastrointestinal disorders. SARS-CoV-2 is transmitted through the respiratory route and not much is known about the presence/survival of it in the intestine and transmission through the fecal-oral route. The consequence of SARS-CoV-2 infection in the gastrointestinal tracts is unclear [89, 90]. Studies report the potential of SARS-CoV-2 in the faeces of infected persons and its possible faecal-oral transmission. This is supported by several reports hinting at diarrhoea as a clinical presentation among a quarter of patients infected by the pandemic. Common gastrointestinal symptoms include nausea, diarrhea, vomiting, and abdominal pain [91–96], that persist in throat swabs in SARS-CoV-2 convalescence with diminished respiratory symptoms.
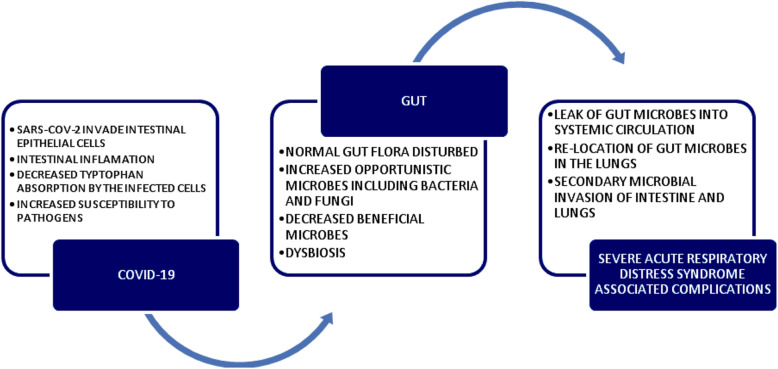
Intestinal microbiota influence pulmonary diseases [97]. Studies demonstrate that respiratory viral infection may disturb intestinal microbiota [94, 98–101]. Gut microbiota may downregulate the ACE2 expression with virus load in COVID-19 cases [37]. Results demonstrate that SARS-CoV-2, which attaches to the ACE2 receptors and transmembrane serine protease 2, could infect intestinal epithelial cells. These cells exhibited receptors to bind to the virus as do the respiratory epithelial cells and other cells. Thus, SARS-CoV-2 efficiently adheres to intestinal epithelial cells, causes inflammation, and could initiate infection via the gastrointestinal tract [102]. SARS-CoV-2 may cause local inflammation in the gut and could lead to coinfection taking advantage of suppressed immune system resulting in severe infection, especially among the elderly. The novel virus caused dysbiosis of the gut microbiome potentially facilitating its invasion and survival. Disturbed normal gut microbiome predisposes the patients to secondary microbial infections and dissemination of virus to other body parts [103, 104]. The susceptibility to SARS-CoV-2 in patients with irritable bowel disease (IBD) and other luminal diseases has been reported. This could be supporting evidence that a healthy gut with normal microbiome prevents potential SARS-CoV-2 spread by faecal-oral route.
Indians have a comparatively healthy gut attributable to their eating habits, ensuring the existence of health-benefiting microbes [105]. There is an increased belief that probiotics help in managing and prognosis of COVID-19. Probiotics could prevent excessive immune response (cytokine storm), reduce inflammation and prevent virus multiplication and invasion [106, 107]. COVID-19 remains mild and becomes self-limiting in healthy individuals with a robust immunity. SARS-CoV-2 infection increases in severity causing complications and death with a compromised immunity and other debilitating conditions like diabetes and increased age. This supports the argument that the immunity status of individuals plays a key role in COVID-19 disease prognosis. As gut microflora influences the immune system, disturbances of the gut microbiome may predispose people to COVID-19 via intestinal invasion. Also, there could be an increased likelihood of secondary microbial coinfections as observed in HIV infection and acquired immunodeficiency syndrome (AIDS) wherein the patients suffer from serious intestinal parasitic infections involving opportunistic microbes [108].
A comparison between the gut microbiome of COVID-19, H1N1 influenza patients, and the healthy controls indicated that opportunistic bacterial (Clostridium, Veillonella, Actinomyces, Streptococcus and Rothia) and fungal (Candida and Aspergillus) pathogens replaced beneficial microbes/commensals like Proteobacteria, Bacteroides, Actinobacteria, Blautia, Romboutsia, Collinsella, and Bifidobacterium in COVID-19 patients. Also, unique bacterial species were noticed in COVID-19 patients that could be infection indicators in SARS-CoV-2 [25, 37–40]. Coprobacillus, Clostridium hathewayi, and C. ramosum have been reported to be associated in severe COVID-19. Bacteroides sp. downregulated ACE2 expression in the murine gut and were correlated inversely with the SARS-CoV-2 load [37, 109].
An assessment of the pulmonary and intestinal microflora that influences the prognostic to determine the clinical outcome of COVID-19 patients and therapeutics has been reported. The ACE-2 on intestinal epithelial cells facilitates absorption of tryptophan, an antimicrobial peptide. As SARS-CoV-2 attaches to the ACE-2 receptors on the epithelial cells causing reduced absorption of tryptophan and increased survival of microbes, it predisposes COVID-19 patients to severe complications and secondary microbial coinfections [110]. Fecal shedding of SARS-CoV-2 in convalescing patients and dysbiosis of gut microbiome even after a month has been reported. Study proposed the screening of fecal specimen for SARS-CoV-2 before fecal microbiota transplantation procedures [111]. It is important that the normal pulmonary and intestinal microflora is maintained in equilibrium as there is a harmonious relationship between the gut microbiome and the respiratory health [112]. Because COVID-19 disturbs the gut and the airway microbiome, it predisposes the patients to gastrointestinal and respiratory complications (Fig. 2).
Fig. 2.
Gut microbiota and COVID-19
Proinflammatory cytokine therapy
The fundamental components (B cells, CD4+ T cells, and CD8+ T cells) of the adaptive immune system are important to control the viral infections. These play different roles in different viral infections and it is very essential to understand the COVID-19-associated mechanism. Adaptive immune system should act at the site of infection to control an infection [113]. Although the mechanism of viral entry is still ambiguous [114, 115], severe COVID-19 patients demonstrated an over-reactive immune response leading to cytokine storm and developing acute respiratory distress syndrome (ARDS) [114]. ARDS leads to other complications like secondary bacterial infections and lung fibrosis. In severe cases, host-directed immunotherapy is an adjunct therapy that could reduce inflammation and related lung damage and prevent ICU hospitalization. Cytokine storm syndrome is a major cause of mortality associated with hospitalized COVID-19 patients [116]. Cytokine storm from several viral infections is well-known to be involved in enhancing immunopathology of the disease [117]. A high level of inflammatory cytokine (IL-6) was reported during early pandemic days in COVID-19 patients, with more than 80 pg/mL IL-6 levels, a good indicator of respiratory failure and death [118]. Targeting IL-1 (another pro-inflammatory cytokine) could be a successful strategy to improve survival in COVID-19 patients [119, 120]. Cavalli and coworkers compared the effectiveness of IL-1 and IL-6 inhibition in treating COVID-19 cytokine storm syndrome [121]. In a significant number of mortality cases, SARS-CoV-2 was associated with extensive multiorgan inflammation suggesting a maladaptive immune response, resulting in continuous neutrophil activation and organ damage [117].
Anti-inflammatory therapy is being explored in morbidity and mortality reduction. Immunosuppressive therapies like cytokine blockade and JAK inhibition is also suggested [122]. The first therapy that reduced mortality was dexamethasone. Recent studies have shown the benefit of tocilizumab in critically ill patients, and baricitinib in hospitalized patients providing substantial evidence that COVID-19 patients benefit from immunosuppressive therapies [123]. Glucocorticoid therapy may also be beneficial for COVID-19 treatment [124]. Cytokine-targeted treatment by anakinra was promising in saving lives in COVID-19 cases, although randomized controlled trails results are awaited [124, 125].
Animal models in coinfection study
Various microbial coinfections are a common occurrence in several epidemics and pandemics including three lethal CoVs witnessed in last two decades. It is very essential to understand the pathogenesis and nosocomial management of SARS-CoV-2 and related coinfections. Mouse model is widely used for different viral pathogenesis investigations due to its small size and easy low cost of operation. Studies to determine the role of immune effectors in the CoV infection report the use of immunocompromized mice [126].
The current SARS-CoV-2 mouse model may be critical in line with considering the use of MHV to study its biological mechanisms [127], using gene editing technology to understand mouse genes (ACE2 and TMPRSS2) related to viral binding and entry [128, 129], or transfer of human ACE2 for direct infection [130], and using wild SARS-CoV-2 virus to establish a mouse model for significant clinical phenotype [131]. To understand the coinfection mechanism by inoculating other pathogens, a coinfection mouse model may be recommended [132]. Furthermore, small animals like human ACE2 transgenic mice, wild-type mice, Syrian hamsters, and large animals such as ferrets, cats, Rhesus macaques, and Cynomolgus macaques may contribute significantly as animal models to evaluate vaccines and drugs against SARS-CoV-2 [133, 134].
Mucormycosis
Mucormycosis, also known as black fungus or zygomycosis, is found in the environment and is caused by a group of molds called mucormycetes that mainly affect the sinuses or the lungs of people with reduced immunity [135]. It is a rare albeit deadly fungal infection and is now detected in COVID-19 patients in India too. Many Indian states have reported such infections among the COVID-19 patients. Once a person is infected, this opportunistic pathogenic fungus manifests in the skin or could affect the brain or lungs. As per the Centre for Disease Control and Prevention (CDC) of the USA, it may be rhinocerebral mucormycosis (sinus and brain), pulmonary mucormycosis (lung), gastrointestinal mucormycosis (gut and intestines), cutaneous mucormycosis (skin), and disseminated mucormycosis (in people having other medical conditions). Usually developing in 10–14 days post-hospitalization, the infection spreads through the bloodstream to other body parts. The patients may be treated with Amphotericin B (an antifungal), and surgery may be required in some cases.
The symptoms are pain and redness around the eyes or nose, blurred or double vision, loosening of teeth, toothache, blackish/bloody discharge from nose, bloody vomits, swelling in cheekbones, skin lesion, chest pain, fever, headache, dyspnea, coughing, and may also alter mental status [135, 136]. This infection is observed in the convalescing COVID-19 patients having issues related to diabetes, prolonged ICU stay, prolonged medical oxygen use, high blood sugars, chronic kidney disease, HIV/AIDS, hematological malignancies, solid organ transplant, etc. [137] Such infections spread due to the rampant misuse or overuse of steroids, monoclonal antibodies, and broad-spectrum antibiotics during COVID-19 treatment [47]. As India has second largest diabetic population with around 70% are uncontrolled cases, such coinfection has become more common here [135]. Hence, higher mortality rate (~ 87%) is observed these days as compared to earlier reports (~ 50%) during non-COVID times [48, 138].
Garg and coworkers [48] reported a COVID-19-associated pulmonary mucormycosis in a 55-year-old COVID-19 patient with diabetes, end-stage kidney disease. With 5 g of liposomal amphotericin B treatment, the patient was discharged from the hospital after 54 days. They also analyzed seven other cases of COVID-19-associated mucormycosis. According to them, diabetes mellitus was the most common risk factor. The incidence of acute invasive fungal rhinosinusitis is prominent in post-COVID-19 patients especially in the immunocompromised [46], the most common infecting organisms being Aspergillus fumigatus, Rhizopus oryzae, and Absidia mucor.
In India, along with black fungus, white and yellow fungus infections detected during endoscopy proved fatal in COVID-19 patients [139]. While mucormycosis relates to black fungus, however the latter are referred to as aspergillosis, candidiasis, and cryptococcosis. All such fungal infections were observed in immunocompromised COVID-19 patients by invading the immune system leading to dysregulation and reduced numbers of T lymphocytes, CD4+T, and CD8+T cells [46]. Physicians need to be careful about the possibility of such secondary invasive fungal infections in COVID-19 patients during and after the onset of the disease [49].
Conclusions
The internal and external resident microbiota is crucial in human health and is essential for immune responses. The microbial coinfection increases the risk of disease severity in humans. However, their mechanism of interaction with the infecting virus with other pathogens is still unclear. It is very essential to study the source and the mechanism of the coinfecting pathogens. This will help in early diagnosis and to understand the antimicrobial and antifungal therapy to effectively treat the disease. The use of health microbiobata, probiotics, and other health promoting regimens need to be explored to counter coinfections during COVID-19 pandemic. Experimental therapy to support the treatment outcomes and prevention of the consequences of respiratory coinfection is imminent. This review has attempted to summarize previous studies describing the viral, bacterial, and fungal pathogens involved in COVID-19 coinfections, and it also discusses the role of adaptive immune system at the site of infection to control the infection along with the proinflammatory cytokine therapy.
Acknowledgements
All authors acknowledge their respective institute and university.
Abbreviations
- CoV
Coronavirus
- COVID-19
Coronavirus disease 2019
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2
- RSV
Respiratory syncytial virus
- mNGS
Metagenomic next-generation sequencing
- BALF
Bronchoalveolar lavage fluid
- PIV2
Parainfluenza virus type 2
- M. pneumoniae
Mycoplasma pneumoniae
- C. pneumoniae
Chlamydia pneumoniae
- DILI
Drug-induced liver injury
- CRP
C-reactive protein
- PCT
Procalcitonin
- MALT
Mucosa associated lymphoid tissue
- GALT
Gut-associated lymphoid tissue
- MHV
Mouse hepatitis virus
- ARDS
Acute respiratory distress syndrome
Authors’ contributions
Conceptualization and idea for the article: RKM. Literature search and design of the work: RKM and AKS. Data analysis and interpretation: VK, SM, and LP. Writing—original draft preparation: RKM, SM, VK, and RT. Writing—review and editing: KD and LP. All authors have read and approved the final manuscript.
Funding
No funding received for the work.
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Mohapatra RK, Perekhoda L, Azam M, Suleiman M, Sarangi AK, Semenets A, Pintilie L, Al-Resayes SI. Computational investigations of three main drugs and their comparison with synthesized compounds as potent inhibitors of SARS-CoV-2 main protease (Mpro): DFT, QSAR, molecular docking, and in silico toxicity analysis. J King Saud Univ Sci. 2021;33:101315. doi: 10.1016/j.jksus.2020.101315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mohapatra RK, Das PK, Kandi V. Challenges in controlling COVID-19 in migrants in Odisha, India. Diabetes Metab Syndr Clin Res Rev. 2020;14:1593–1594. doi: 10.1016/j.dsx.2020.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mahal A, Duan M, Zinad DS, Mohapatra RK, Obaidullah AJ, Wei X, Pradhan MK, Das D, Kandi V, Zinad HS, Zhu Q. Recent progress in chemical approaches for the development of novel neuraminidase inhibitors. RSC Adv. 2021;11:1804–1840. doi: 10.1039/D0RA07283D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu P, Hao X, Lau EHY, Wong JY, Leung KSM, Wu JT, et al. Real-time tentative assessment of the epidemiological characteristics of novel coronavirus infections in Wuhan, China, as at 22 January 2020. Euro Surveill. 2020;25(3):2000044. doi: 10.2807/1560-7917.ES.2020.25.3.2000044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou P, Yang X-L, Wang X-G, Hu B, Zhang L, Zhang W, Si HR, Zhu Y, Li B, Huang CL, Chen HD, Chen J, Luo Y, Guo H, Jiang RD, Liu MQ, Chen Y, Shen XR, Wang X, Zheng XS, Zhao K, Chen QJ, Deng F, Liu LL, Yan B, Zhan FX, Wang YY, Xiao GF, Shi Z-L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mohapatra RK, Pintilie L, Kandi V, Sarangi AK, Das D, Sahu R, Perekhoda L. The recent challenges of highly contagious COVID-19; causing respiratory infections: symptoms, diagnosis, transmission, possible vaccines, animal models and immunotherapy. Chem Biol Drug Des. 2020;96(5):1187–1208. doi: 10.1111/cbdd.13761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang N, Shi X, Jiang L, Zhang S, Wang D, Tong P, et al. Structure of MERS-CoV spike receptor-binding domain complexed with human receptor DPP4. Cell Res. 2013;23(8):986–993. doi: 10.1038/cr.2013.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raj VS, Mou H, Smits SL, Dekkers DH, Muller MA, Dijkman R, et al. Dipeptidyl peptidase 4 is a functional receptor for the emerging human coronavirus-EMC. Nature. 2013;495(7440):251–254. doi: 10.1038/nature12005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mohapatra RK, Rahman M (2021) Is it possible to control the outbreak of COVID-19 in Dharavi, Asia’s largest slum situated in Mumbai? Anti-Infect Agents. 10.2174/2211352518999200831142851
- 10.Xu X, Chen P, Wang J, Feng J, Zhou H, Li X, et al. Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission. Sci China Life Sci. 2020;63:457–460. doi: 10.1007/s11427-020-1637-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tetro JA. Is COVID-19 receiving ADE from other coronaviruses? Microbes Infect. 2020;22(2):72–73. doi: 10.1016/j.micinf.2020.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arumugam VA, Thangavelu S, Fathah Z, et al. COVID-19 and the World with Co-Morbidities of Heart Disease, Hypertension and Diabetes. J Pure Appl Microbiol. 2020;14(3):1623–1638. doi: 10.22207/JPAM.14.3.01. [DOI] [Google Scholar]
- 13.Mohapatra RK, Mishra S, Azam M, Dhama K. COVID-19, WHO guidelines, pedagogy, and respite. Open Med. 2021;16:491–493. doi: 10.1515/med-2021-0266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mohapatra RK, Das PK, Pintilie L, Dhama K. Infection capability of SARS-CoV-2 on different surfaces. Egypt J Basic Appl Sci. 2021;8(1):75–80. [Google Scholar]
- 15.Cox MJ, Loman N, Bogaert D, O’Grady J (2020) Co-infections: potentially lethal and unexplored in COVID-19. Lancet. 10.1016/S2666-5247(20)30009-4 [DOI] [PMC free article] [PubMed]
- 16.Bao L, Zhang C, Dong J, Zhao L, Li Y, Sun J (2020) Oral Microbiome and SARS-CoV-2: Beware of Lung Co-infection. Front Microbiol. 10.3389/fmicb.2020.01840 [DOI] [PMC free article] [PubMed]
- 17.Morens DM, Taubenberger JK, Fauci AS. Predominant role of bacterial pneumonia as a cause of death in pandemic influenza: implications for pandemic influenza preparedness. J Infect Dis. 2008;198(7):962–970. doi: 10.1086/591708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.MacIntyre CR, Chughtai AA, Barnes M, et al. The role of pneumonia and secondary bacterial infection in fatal and serious outcomes of pandemic influenza a (H1N1) pdm09. BMC Infect Dis. 2018;18:637. doi: 10.1186/s12879-018-3548-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rawson TM, Moore LSP, Zhu N, Ranganathan N, Skolimowska K, Gilchrist M, Satta G, Cooke G, Holmes A. Bacterial and Fungal Coinfection in Individuals With Coronavirus: A Rapid Review To Support COVID-19 Antimicrobial Prescribing. Clin Infect Dis. 2020;71(9):2459–2468. doi: 10.1093/cid/ciaa530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shi M, Zhang YZ, Holmes EC. Meta-transcriptomics and the evolutionary biology of RNA viruses. Virus Res. 2018;243:83–90. doi: 10.1016/j.virusres.2017.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shen Z, Xiao Y, Kang L, Ma W, Shi L, Zhang L et al (2020) Genomic diversity of SARS-CoV-2 in coronavirus disease 2019 patients. Clin Infect. 10.1093/cid/ciaa203
- 24.Mammen MJ, Scannapieco FA, Sethi S. Oral-lung microbiome interactions in lung diseases. Periodontology. 2000;2020(83):234–241. doi: 10.1111/prd.12301. [DOI] [PubMed] [Google Scholar]
- 25.Chen X, Liao B, Cheng L, Peng X, Xu X, Li Y, Hu T, Li J, Zhou X, Ren B. The microbial coinfection in COVID-19. Appl Microbiol Biotechnol. 2020;104:7777–7785. doi: 10.1007/s00253-020-10814-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, Barnaby DP, Becker LB, Chelico JD, Cohen SL, Cookingham J, Coppa K, Diefenbach MA, Dominello AJ, Duer-Hefele J, Falzon L, Gitlin J, Hajizadeh N, Harvin TG, Hirschwerk DA, Kim EJ, Kozel ZM, Marrast LM, Mogavero JN, Osorio GA, Qiu M, Zanos TP. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City Area. Jama. 2020;323:2052. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lim YK, Kweon OJ, Kim HR, Kim TH, Lee MK. Impact of bacterial and viral coinfection in community-acquired pneumonia in adults. Diagn Microbiol Infect Dis. 2019;94(1):50–54. doi: 10.1016/j.diagmicrobio.2018.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim D, Quinn J, Pinsky B, Shah NH, Brown I. Rates of coinfection between SARS-CoV-2 and other respiratory pathogens. Jama. 2020;323:2085. doi: 10.1001/jama.2020.6266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kiley JL, Chung KK, Blyth DM (2020) Viral infections in burns. Surg Infect. 10.1089/sur.2020.130 [DOI] [PubMed]
- 30.Blanco JL, Ambrosioni J, Garcia F, Martínez E, Soriano A, Mallolas J, Miro JM. COVID-19 in patients with HIV: clinical case series. Lancet HIV. 2020;7(5):e314–e316. doi: 10.1016/s2352-3018(20)30111-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu F, Zhao S, Yu B, Chen YM, Wang W, Song ZG, Hu Y, Tao ZW, Tian JH, Pei YY, Yuan ML, Zhang YL, Dai FH, Liu Y, Wang QM, Zheng JJ, Xu L, Holmes EC, Zhang YZ. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579(7798):265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beck JM, Schloss PD, Venkataraman A, Twigg H, Jablonski KA, Bushman FD, et al. Multicenter comparison of lung and oral microbiomes of HIV-infected and HIV-uninfected individuals. Am J Respir Crit Care Med. 2015;192(11):1335–1344. doi: 10.1164/rccm.201501-0128OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wypych TP, Wickramasinghe LC, Marsland BJ. The influence of the microbiome on respiratory health. Nat Immunol. 2019;20(10):1279–1290. doi: 10.1038/s41590-019-0451-9. [DOI] [PubMed] [Google Scholar]
- 34.Li Y, He J, He Z, Zhou Y, Yuan M, Xu X, Sun F, Liu C, Li J, Xie W, Deng Y, Qin Y, VanNostrand JD, Xiao L, Wu L, Zhou J, Shi W, Zhou X. Phylogenetic and functional gene structure shifts of the oral microbiomes in periodontitis patients. ISME J. 2014;8(9):1879–1891. doi: 10.1038/ismej.2014.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moore SC, Penrice-Randal R, Alruwaili M, Dong X, Pullan ST, Carter D et al (2020) Amplicon based MinION sequencing of SARS-CoV-2 and metagenomic characterisation of nasopharyngeal swabs from patients with COVID-19. medRxiv[Preprint. 10.1101/2020.03.05.20032011
- 36.Russell CD, Fairfield CJ, Drake TM, Turtle L, Seaton RA, Wootton DG, Sigfrid L et al on behalf of the ISARIC4C investigators, (2021) Co-infections, secondary infections, and antimicrobial use in patients hospitalised with COVID-19 during the first pandemic wave from the ISARIC WHO CCP-UK study: a multicentre, prospective cohort study. Lancet Microbe. 10.1016/S2666-5247(21)00090-2 [DOI] [PMC free article] [PubMed]
- 37.Zuo T, Zhang F, Lui GCY, Yeoh YK, Li AYL, Zhan H, Wan Y, Chung ACK, Cheung CP, Chen N, Lai CKC, Chen Z, Tso EYK, Fung KSC, Chan V, Ling L, Joynt G, Hui DSC, Chan FKL, Chan PKS, Ng SC. Alterations in gut microbiota of patients with COVID-19 during time of hospitalization. Gastroenterology. 2020;159(3):944–955.e8. doi: 10.1053/j.gastro.2020.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ferreira C, Viana SD, Reis F. Gut Microbiota Dysbiosis-Immune Hyperresponse-Inflammation Triad in Coronavirus Disease 2019 (COVID-19): Impact of Pharmacological and Nutraceutical Approaches. Microorganisms. 2020;8(10):1514. doi: 10.3390/microorganisms8101514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gu S, Chen Y, Wu Z, Chen Y, Gao H, Lv L, Guo F, Zhang X, Luo R, Huang C, Lu H, Zheng B, Zhang J, Yan R, Zhang H, Jiang H, Xu Q, Guo J, Gong Y, Tang L, Li L. Alterations of the Gut Microbiota in Patients with COVID-19 or H1N1 Influenza. Clin Infect Dis. 2020;4:ciaa709. doi: 10.1093/cid/ciaa709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zuo T, Zhan H, Zhang F, et al. Alterations in fecal fungal microbiome of patients with COVID-19 during time of hospitalization until discharge. Gastroenterology. 2020;159(4):1302–1310.e5. doi: 10.1053/j.gastro.2020.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Singh A, Gupta A, Das K (2020) Severe acute respiratory syndrome coronavirus-2 and pulmonary tuberculosis coinfection: double trouble. Res Square Preprint. 10.21203/rs.3.rs-22464/v4 [DOI] [PubMed]
- 42.Ata F, Yousaf Q, Parambil JV, Parengal J, Mohamedali MG, Yousaf Z. A 28-year-old man from India with SARS-Cov-2 and pulmonary tuberculosis co-infection with central nervous system involvement. Am J Case Rep. 2020;21:e926034. doi: 10.12659/AJCR.926034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.He G, Wu J, Shi J, Dai J, Gamber M, Jiang X, Sun W, Cai J. COVID-19 in tuberculosis patients: a report of three cases. J Med Virol. 2020;92:1802–1806. doi: 10.1002/jmv.25943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Segal LN, Alekseyenko AV, Clemente JC, Kulkarni R, Wu B, Gao Z, et al. Enrichment of lung microbiome with supraglottic taxa is associated with increased pulmonary inflammation. Microbiome. 2013;1:19. doi: 10.1186/2049-2618-1-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mohamed A, Hassan T, Trzos-Grzybowska M, Thomas J, Quinn A, O’Sullivan M, Griffin A, Rogers TR, Talento AF (2020) Multi-triazole-resistant Aspergillus fumigatus and SARS-CoV-2 co-infection: a lethal combination. Med Mycol Case Rep. 10.1016/j.mmcr.2020.06.005 [DOI] [PMC free article] [PubMed]
- 46.Ismaiel WF, Abdelazim MH, Eldsoky I, Ibrahim AA, Alsobky ME, Zafan E, Hasan A. The impact of COVID-19 outbreak on the incidence of acute invasive fungal rhinosinusitis. m J Otolaryngol Head Neck Med Surg. 2021;42:103080. doi: 10.1016/j.amjoto.2021.103080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mehta S, Pandey A. Rhino-Orbital Mucormycosis Associated With COVID-19. Cureus. 2020;12(9):e10726. doi: 10.7759/cureus.10726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Garg D, Muthu V, Sehgal IS, et al. Coronavirus disease (Covid-19) associated mucormycosis (CAM): case report and systematic review of literature. Mycopathologia. 2021;186(2):289–298. doi: 10.1007/s11046-021-00528-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Szarpak L, Chirico F, Pruc M, Szarpak L, Dzieciatkowski T, Rafique Z. Mucormycosis-a serious threat in the COVID-19 pandemic? J Infect. 2021;83(2):237–279. doi: 10.1016/j.jinf.2021.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lai CC, Wang CY, Hsueh PR (2020) Co-infections among patients with COVID-19: The need for combination therapy with non-anti-SARS-CoV-2 agents? J Microbiol Immunol Infect. 10.1016/j.jmii.2020.05.013 [DOI] [PMC free article] [PubMed]
- 51.Wang M, Wu Q, Xu W, Qiao B, Wang J, Zheng H, Jiang S, Mei J, Wu Z, Deng Y, Zhou F, Wu W, Zhang Y, Lv Z, Huang J, Guo X, Feng L, Xia Z, Li D, Xu Z, Liu T, Zhang P, Tong Y, Li Y (2020) Clinical diagnosis of 8274 samples with 2019-novel coronavirus in Wuhan. medRxiv:2020.02.12.20022327. 10.1101/2020.02.12.20022327
- 52.Lin D, Liu L, Zhang M, Hu Y, Yang Q, Guo J, Guo Y, Dai Y, Xu Y, Cai Y, Chen X, Zhang Z, Huang K. Co-infections of SARS-CoV-2 with multiple common respiratory pathogens in infected patients. Sci China Life Sci. 2020;63(4):606–609. doi: 10.1007/s11427-020-1668-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cawcutt K, Kalil AC. Pneumonia with bacterial and viral coinfection. Curr Opin Crit Care. 2017;23(5):385–390. doi: 10.1097/mcc.0000000000000435. [DOI] [PubMed] [Google Scholar]
- 54.Nikolich-Zugich J, Knox KS, Rios CT, Natt B, Bhattacharya D, Fain MJ (2020) SARS-CoV-2 and COVID-19 in older adults: what we may expect regarding pathogenesis, immune responses, and outcomes. 10.1007/s11357-020-00186-0 [DOI] [PMC free article] [PubMed]
- 55.Boeckmans J, Rodrigues RM, Demuyser T, Piérard D, Vanhaecke T, Rogiers V. COVID-19 and drug-induced liver injury: a problem of plenty or a petty point? Arch Toxicol. 2020;94(4):1367–1369. doi: 10.1007/s00204-020-02734-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li X, Wang L, Yan S, Yang F, Xiang L, Zhu J, Shen B, Gong Z. Clinical characteristics of 25 death cases with COVID-19: a retrospective review of medical records in a single medical center, Wuhan, China. Int J Infect Dis. 2020;94:128–132. doi: 10.1016/j.ijid.2020.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LFP. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20:363–374. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, Wu Y, Zhang L, Yu Z, Fang M, Yu T, Wang Y, Pan S, Zou X, Yuan S, Shang Y. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475–481. doi: 10.1016/s2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang M, Luo L, Bu H, Xia H. Case report: one case of coronavirus desease 2019(COVID-19) in patient co-nfected by HIV with a low CD4+ T cell count. Int J Infect Dis. 2020;96:148–150. doi: 10.1016/j.ijid.2020.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li Z, He L, Li S, He W, Zha C, Ou W, Hou Q, Wang W, Sun X, Liang H. Combination of procalcitonin and C-reactive protein levels in the early diagnosis of bacterial co-infections in children with H1N1 influenza. Influenza Other Respir Viruses. 2019;13(2):184–190. doi: 10.1111/irv.12621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vareille M, Kieninger E, Edwards MR, Regamey N. The airway epithelium: soldier in the fight against respiratory viruses. Clin Microbiol Rev. 2011;24(1):210–229. doi: 10.1128/cmr.00014-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rouse BT, Sehrawat S. Immunity and immunopathology to viruses: what decides the outcome? Nat Rev Immunol. 2010;10(7):514–526. doi: 10.1038/nri2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nowak MD, Sordillo EM, Gitman MR, Paniz Mondolfi AE (2020) Coinfection in SARS-CoV-2 infected patients: where are influenza virus and rhinovirus/enterovirus? J Med Virol. 10.1002/jmv.25953 [DOI] [PMC free article] [PubMed]
- 64.Yuen KS, Ye ZW, Fung SY, Chan CP, Jin DY. SARS-CoV-2 and COVID-19: the most important research questions. Cell Biosci. 2020;10:40. doi: 10.1186/s13578-020-00404-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li Y, Ren B, Peng X, Hu T, Li J, Gong T, Tang B, Xu X, Zhou X. Saliva is a non-negligible factor in the spread of COVID-19. Mol Oral Microbiol. 2020;35:141–145. doi: 10.1111/omi.12289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yetmar ZA, Issa M, Munawar S, Burton MC, Pureza V, Sohail MR, Mehmood T. Inpatient care of patients with COVID-19: a guide for hospitalists. Am J Med. 2020;S0002-9343(20):30349–30341. doi: 10.1016/j.amjmed.2020.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Alhazzani W, Møller MH, Arabi YM, Loeb M, Gong MN, Fan E, Oczkowski S, Levy MM, Derde L, Dzierba A, Du B, Aboodi M, Wunsch H, Cecconi M, Koh Y, Chertow DS, Maitland K, Alshamsi F, Belley-Cote E, Greco M, Laundy M, Morgan JS, Kesecioglu J, McGeer A, Mermel L, Mammen MJ, Alexander PE, Arrington A, Centofanti JE, Citerio G, Baw B, Memish ZA, Hammond N, Hayden FG, Evans L, Rhodes A. Surviving sepsis campaign: guidelines on the management of critically ill adults with Coronavirus Disease 2019 (COVID-19) Intensive Care Med. 2020;46(5):854–887. doi: 10.1007/s00134-020-06022-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ferretti L, Wymant C, Kendall M, Zhao L, Nurtay A, Abeler-Dörner L, Parker M, Bonsall D, Fraser C. Quantifying SARS-CoV-2 transmission suggests epidemic control with digital contact tracing. Science. 2020;368(6491):eabb6936. doi: 10.1126/science.abb6936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Prasetyoputri A (2021) Detection of bacterial coinfection in COVID-19 patients is a missing piece of the puzzle in the COVID-19 management in Indonesia. ACS Infect Dis [DOI] [PubMed]
- 70.Contou D, Claudinon A, Pajot O, Micaëlo M, Flandre PL, Dubert M, Cally R, Logre E, Fraissé M, Mentec H, Plantefève G. Bacterial and viral co-infections in patients with severe SARS-CoV-2 pneumonia admitted to a French ICU. Ann Intensive Care. 2020;10:119. doi: 10.1186/s13613-020-00736-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lansbury L, Lim B, Baskaran V, Lim WS. Co-infections in people with COVID- 19: a systematic review and meta-analysis. J Inf Secur. 2020;81(2):266–275. doi: 10.1016/j.jinf.2020.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jing R, Vunnam RR, Schnaubelt E, Vokoun C, Cushman-Vokoun A, Goldner D, Vunnam SR. Co-infection of COVID-19 and influenza A in a hemodialysis patient: a case report. BMC Infect Dis. 2021;21:68. doi: 10.1186/s12879-020-05723-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Calcagno A, Ghisetti V, Burdino E, Trunfio M, Allice T, Boglione L, Bonora S, Perri GD. Coinfection with other respiratory pathogens in COVID-19 patients. Clin Microbiol Infect. 2020;27(2):297–298. doi: 10.1016/j.cmi.2020.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Garcia-Vidal C, Sanjuan G, Moreno-García E, Puerta-Alcalde P, Garcia-Pouton N, Chumbita M, et al. Soriano, Incidence of co-infections and superinfections in hospitalized patients with COVID-19: a retrospective cohort study. Clin Microbiol Infect. 2021;27:83–88. doi: 10.1016/j.cmi.2020.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pal C, Przydzial P, Chika-Nwosuh O, Shah S, Patel P, Madan N (2020) Streptococcus pneumoniae coinfection in COVID-19: a series of three cases. Case Rep Pulmonol. 10.1155/2020/8849068 [DOI] [PMC free article] [PubMed]
- 76.Lai C-C, Wang C-Y, Hsueh P-R. Coinfections among patients with COVID-19: the need for combination therapy with non-anti-SARS-CoV-2 agents? Journal of Microbiology. Immunol Infect. 2020;53(4):505–512. doi: 10.1016/j.jmii.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Farshidfar N, Hamedani S (2020) Hyposalivation as a potential risk for SARS-CoV-2 infection: Inhibitory role of saliva. Oral Dis [DOI] [PMC free article] [PubMed]
- 78.Sabino-Silva R, Jardim AC, Siqueira WL (2020) Coronavirus COVID-19 impacts to dentistry and potential salivary diagnosis. Clin Oral Investig:1–3 [DOI] [PMC free article] [PubMed]
- 79.Liu L, Wei Q, Alvarez X, Wang H, Du Y, Zhu H, et al. Epithelial cells lining salivary gland ducts are early target cells of severe acute respiratory syndrome coronavirus infection in the upper respiratory tracts of rhesus macaques. J Virol. 2011;85(8):4025–4030. doi: 10.1128/JVI.02292-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Xu J, Li Y, Gan F, Du Y, Yao Y. Salivary glands: potential reservoirs for COVID-19 asymptomatic infection. J Dent Res. 2020;99(8):989. doi: 10.1177/0022034520918518. [DOI] [PubMed] [Google Scholar]
- 81.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dewhirst FE, Chen T, Izard J, Paster BJ, Tanner ACR, Yu WH, et al. The human oral microbiome. J Bacteriol. 2010;192:5002–5017. doi: 10.1128/jb.00542-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Arora SK, Dewan P, Gupta P. Microbiome: paediatricians’ perspective. Indian J Med Res. 2015;142:515–524. doi: 10.4103/0971-5916.171275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li Y, Wang K, Zhang B, Tu Q, Yao Y, Cui B, et al. Salivary mycobiome dysbiosis and its potential impact on bacteriome shifts and host immunity in oral lichen planus. Int J Oral Sci. 2019;11:1–10. doi: 10.1038/s41368-018-0031-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Segal LN, Clemente JC, Tsay JC, Koralov SB, Keller BC, Wu BG, et al. Enrichment of the lung microbiome with oral taxa is associated with lung inflammation of a Th17 phenotype. Nat Microbiol. 2016;1:16031. doi: 10.1038/nmicrobiol.2016.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bassis CM, Erb-Downward JR, Dickson RP, Freeman CM, Schmidt TM, Young VB, et al. Analysis of the upper respiratory tract microbiotas as the source of the lung and gastric microbiotas in healthy individuals. mBio. 2015;6:e00037–e00015. doi: 10.1128/mBio.00037-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rawson TM, Moore LSP, Zhu N, Ranganathan N, Skolimowska K, Gilchrist M et al (2020) Bacterial and fungal co-infection in individuals with coronavirus: a rapid review to support COVID-19 antimicrobial prescribing. Clin Infect Dis. 10.1093/cid/ciaa530 [DOI] [PMC free article] [PubMed]
- 88.Verweij PE, Gangneux J-P, Bassetti M, Brüggemann RJ, Cornely OA, Koehler P, et al. Diagnosing COVID-19-associated pulmonary aspergillosis. Lancet Microbe. 2020;1:e53–e55. doi: 10.1016/s2666-5247(20)30027-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cheng VC, Lau SK, Woo PC, Yuen KY. Severe acute respiratory syndrome coronavirus as an agent of emerging and reemerging infection. Clin Microbiol Rev. 2007;20:660–694. doi: 10.1128/CMR.00023-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ianiro G, Mullish BH, Kelly CR, Sokol H, Kassam Z, Ng SC, et al. Screening of faecal microbiota transplant donors during the COVID-19 outbreak: suggestions for urgent updates from an international expert panel. Lancet Gastroenterol Hepatol. 2020;5:430–432. doi: 10.1016/S2468-1253(20)30082-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hamid S, Mir MY, Rohela GK. Novel coronavirus disease (COVID-19): a pandemic (epidemiology, pathogenesis and potential therapeutics) New Microbes New Infect. 2020;35:100679. doi: 10.1016/j.nmni.2020.100679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Li G, Hu R, Gu X. A close-up on COVID-19 and cardiovascular diseases. Nutr Metab Cardiovasc Dis. 2020;30:1057–1060. doi: 10.1016/j.numecd.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cavaliere K, Levine C, Wander P, Sejpal DV, Trindade AJ. Management of upper GI bleeding in patients with COVID-19 pneumonia. Gastrointest Endosc. 2020;92:454–455. doi: 10.1016/j.gie.2020.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wan Y, Li J, Shen L, Zou Y, Hou L, Zhu L, Faden HS, Tang Z, Shi M, Jiao N, Li Y, Cheng S, Huang Y, Wu D, Xu Z, Pan L, Zhu J, Yan G, Zhu R, Lan P. Enteric involvement in hospitalised patients with COVID-19 outside Wuhan. Lancet Gastroenterol Hepatol. 2020;5(6):534–535. doi: 10.1016/S2468-1253(20)30118-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wong SH, Lui RN, Sung JJ. Covid-19 and the digestive system. J Gastroenterol Hepatol. 2020;35:744–748. doi: 10.1111/jgh.15047. [DOI] [PubMed] [Google Scholar]
- 96.Jin X, Lian J, Hu J, Gao J, Zheng L, Zhang YM, et al. Epidemiological, clinical and virological characteristics of 74 cases of coronavirus-infected disease 2019 (COVID-19) with gastrointestinal symptoms. Gut. 2020;69:1002–1009. doi: 10.1136/gutjnl-2020-320926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Vodnar D-C, Mitrea L, Teleky B-E, Szabo K, Călinoiu L-F, Nemes S-A, Martau G-A (2020) Coronavirus Disease (COVID-19) Caused by (SARS-CoV-2) Infections: a real challenge for human gut microbiota. Front Cell Infect Microbiol 10. 10.3389/fcimb.2020.575559 [DOI] [PMC free article] [PubMed]
- 98.Precup G, Vodnar DC. Gut Prevotella as a possible biomarker of diet and its eubiotic versus dysbiotic roles: a comprehensive literature review. Br J Nutr. 2019;122:131–140. doi: 10.1017/S0007114519000680. [DOI] [PubMed] [Google Scholar]
- 99.Pan L, Mu M, Yang P, Sun Y, Wang R, Yan J, et al. Clinical characteristics of COVID-19 patients with digestive symptoms in Hubei, China: a descriptive, cross-sectional, multicenter study. Am J Gastroenterol. 2020;115:766–773. doi: 10.14309/ajg.0000000000000620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Xiao F, Tang M, Zheng X, Liu Y, Li X, Shan H. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology. 2020;158:1831–1833. doi: 10.1053/j.gastro.2020.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhang W, Du RH, Li B, Zheng XS, Yang XL, Hu B, et al. Molecular and serological investigation of 2019-nCoV infected patients: implication of multiple shedding routes. Emerg Microbes Infect. 2020;9:386–389. doi: 10.1080/22221751.2020.1729071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Liang W, Feng Z, Rao S, Xiao C, Xue X, Lin Z, Zhang Q, Qi W. Diarrhoea may be underestimated: a missing link in 2019 novel coronavirus. Gut. 2020;69(6):1141–1143. doi: 10.1136/gutjnl-2020-320832. [DOI] [PubMed] [Google Scholar]
- 103.Dhar D, Mohanty A. Gut microbiota and Covid-19- possible link and implications. Virus Res. 2020;285:198018. doi: 10.1016/j.virusres.2020.198018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Aktas B, Aslim B. Gut-lung axis and dysbiosis in COVID-19. Turk J Biol. 2020;44(3):265–272. doi: 10.3906/biy-2005-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gupta R, Beg S, Jain A, Bhatnagar S. Paediatric COVID-19 and the GUT. Indian J Med Microbiol. 2020;38(3&4):261–264. doi: 10.4103/ijmm.IJMM_20_331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Akour A. Probiotics and COVID-19: is there any link? Lett Appl Microbiol. 2020;71(3):229–234. doi: 10.1111/lam.13334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bottari B, Castellone V, Neviani E. Probiotics and Covid-19. Int J Food Sci Nutr. 2020;12:1–7. doi: 10.1080/09637486.2020.1807475. [DOI] [PubMed] [Google Scholar]
- 108.Ramana KV, Mohanty SK. Opportunistic intestinal parasites and TCD4+ cell counts in human immunodeficiency virus seropositive patients. J Med Microbiol. 2009;58(Pt 12):1664–1666. doi: 10.1099/jmm.0.014043-0. [DOI] [PubMed] [Google Scholar]
- 109.Amirian ES. Potential fecal transmission of SARS-CoV-2: current evidence and implications for public health. Int J Infect Dis. 2020;95:363–370. doi: 10.1016/j.ijid.2020.04.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.He Y, Wang J, Li F, Shi Y. Main clinical features of COVID-19 and potential prognostic and therapeutic value of the microbiota in SARS-CoV-2 infections. Front Microbiol. 2020;11:1302. doi: 10.3389/fmicb.2020.01302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kaźmierczak-Siedlecka K, Vitale E, Makarewicz W. COVID-19 - gastrointestinal and gut microbiota-related aspects. Eur Rev Med Pharmacol Sci. 2020;24(20):10853–10859. doi: 10.26355/eurrev_202010_23448. [DOI] [PubMed] [Google Scholar]
- 112.Ahlawat S, Asha, Sharma KK. Immunological co-ordination between gut and lungs in SARS-CoV-2 infection. Virus Res. 2020;286:198103. doi: 10.1016/j.virusres.2020.198103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sette A, Crotty S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell. 2021;184:861–880. doi: 10.1016/j.cell.2021.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bonam SR, Kaveri SV, Sakuntabhai A, Gilardin L, Bayry J. Adjunct immunotherapies for the management of severely ill COVID-19 patients. Cell Rep Med. 2020;1(2):100016. doi: 10.1016/j.xcrm.2020.100016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Jafari AA, Ghasemi S. The possible of immunotherapy for COVID-19: a systematic review. Int Immunopharmacol. 2020;83:106455. doi: 10.1016/j.intimp.2020.106455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Henderson LA, Canna SW, Schulert GS, et al. On the alert for cytokine storm: immunopathology in COVID-19. Arthritis Rheum. 2020;72:1059–1063. doi: 10.1002/art.41285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chen LYC, Quach TTT (2021) COVID-19 cytokine storm syndrome: a threshold concept. Lancet:e49–e50 [DOI] [PMC free article] [PubMed]
- 118.Chen LYC, Hoiland RL, Stukas S, Wellington CL, Sekhon MS. Confronting the controversy: interleukin-6 and the COVID-19 cytokine storm syndrome. Eur Respir J. 2020;56:2003006. doi: 10.1183/13993003.03006-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Eloseily EM, Weiser P, Crayne CB, et al. Benefit of anakinra in treating pediatric secondary hemophagocytic lymphohistiocytosis. Arthritis Rheum. 2020;72:326–334. doi: 10.1002/art.41103. [DOI] [PubMed] [Google Scholar]
- 120.Huet T, Beaussier H, Voisin O, Jouveshomme S, Dauriat G, Lazareth I, Sacco E, Naccache JM, Bézie Y, Laplanche S, le Berre A, le Pavec J, Salmeron S, Emmerich J, Mourad JJ, Chatellier G, Hayem G. Anakinra for severe forms of COVID-19: a prospective cohort study. Lancet Rheumatol. 2020;2(7):e393–e400. doi: 10.1016/S2665-9913(20)30164-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Cavalli G, Larcher A, Tomelleri A et al (2021) Interleukin-1 and interleukin-6 inhibition compared with standard management in patients with COVID-19 and hyperinflammation: a cohort study. Lancet Rheumatol. 10.1016/S2665-9913(21)00012-6 [DOI] [PMC free article] [PubMed]
- 122.England JT, Abdulla A, Biggs CM et al (2020) Weathering the COVID-19 storm: lessons from hematologic cytokine syndromes. Blood Rev. 10.1016/j.blre.2020.100707 [DOI] [PMC free article] [PubMed]
- 123.Gordon AC (2021) Interleukin-6 receptor antagonists in critically ill patients with COVID-19—preliminary report. medRxiv (preprint). 10.1101/2021.01.07.21249390
- 124.The RECOVERY Collaborative Group (2020) Dexamethasone in hospitalized patients with Covid-19—preliminary report. N Engl J Med. 10.1056/NEJMoa2021436
- 125.Cron RQ (2021) COVID-19 cytokine storm: targeting the appropriate cytokine. Lancet Rheumatol 3. 10.1016/S2665-9913(21)00011-4 [DOI] [PMC free article] [PubMed]
- 126.Yuan L, Tang Q, Cheng T, Xia N. Animal models for emerging coronavirus: progress and new insights. Emerg Microbes Infect. 2020;9(1):949–961. doi: 10.1080/22221751.2020.1764871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Weiss SR. Forty years with coronaviruses. J Exp Med. 2020;217:e20200537. doi: 10.1084/jem.20200537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Iwata-Yoshikawa N, Okamura T, Shimizu Y, Hasegawa H, Takeda M, Nagata N. TMPRSS2 contributes to virus spread and immunopathology in the airways of murine models after coronavirus infection. J Virol. 2019;93:e01815–e01818. doi: 10.1128/JVI.01815-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280.e278. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Jiang RD, Liu MQ, Chen Y, Shan C, Zhou YW, Shen XR, et al. Pathogenesis of SARS-CoV-2 in transgenic mice expressing human angiotensin-converting enzyme 2. Cell. 2020;182:50–58.e8. doi: 10.1016/j.cell.2020.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Dinnon KH, Leist SR, Schäfer A, Edwards CE, Martinez DR, Montgomery SA et al (2020) A mouse-adapted SARS-CoV-2 model for the evaluation of COVID-19 medical countermeasures. bioRxiv[Preprint. 10.1101/2020.05.06.081497
- 132.Zhu X, Ge Y, Wu T, Zhao K, Chen Y, Wu B, et al. Co-infection with respiratory pathogens among COVID-2019 cases. Virus Res. 2020;285:198005. doi: 10.1016/j.virusres.2020.198005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Takayama K (2020) In vitro and animal models for SARS-CoV-2 research. Trends Pharmac Sci. 10.1016/j.tips.2020.05.005 [DOI] [PMC free article] [PubMed]
- 134.Tiwari R, Dhama K, Sharun K, Yatoo MI, Malik YS, Singh R, et al. COVID-19: animals, veterinary and zoonotic links. Vet Q. 2020;40(1):169–182. doi: 10.1080/01652176.2020.1766725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Suvvari TK, Arigapudi N, Kandi V, Kutikuppala LVS (2021) Mucormycosis: a killer in the shadow of COVID-19. J Med Mycol. 10.1016/j.mycmed.2021.101161 [DOI] [PMC free article] [PubMed]
- 136.Sharma S, Grover M, Bhargava S, Samdani S, Kataria T (2021) Post coronavirus disease mucormycosis: a deadly addition to the pandemic spectrum. J Laryngol Otol:1–6. 10.1017/S0022215121000992 [DOI] [PMC free article] [PubMed]
- 137.Claustre J, Larcher R, Jouve T, Truche A-S, Nseir S, Cadiet J, Zerbib Y, Lautrette A, Constantin J-M, Charles P-E, Daubin C, Coudroy R, Dellamonica J, Argaud L, Phelouzat P, Contou D, Pocquet J, Voiriot G, Navellou J-C, Lavagne P, Durand M, Cornet M, Schwebel C, Terzi N. Mucormycosis in intensive care unit: surgery is a major prognostic factor in patients with hematological malignancy. Ann Intensive Care. 2020;10:74. doi: 10.1186/s13613-020-00673-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Verma DK, Bali RK. COVID-19 and mucormycosis of the craniofacial skeleton: causal, contributory or coincidental? J Maxillofac Oral Surg. 2021;20(2):165–166. doi: 10.1007/s12663-021-01547-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gulf news, (2021) Why black fungus aggravates COVID-19 crisis in India, June 07, 2021. https://gulfnews.com/special-reports/why-black-fungus-aggravates-covid-19-crisis-in-india-1.1623068786523
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.