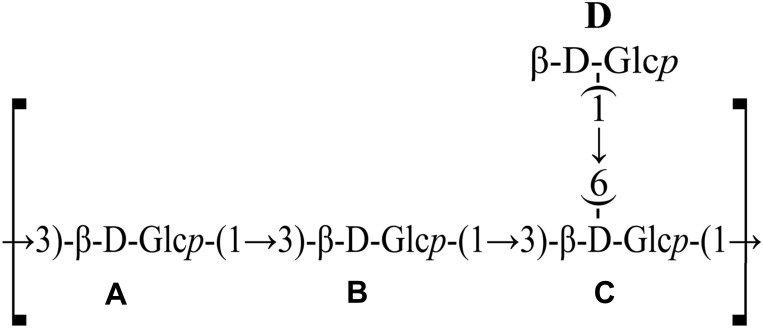Abstract
G. lucidum has a long history of thousands of years in China and is closely related with the lives of the Chinese people. It is reported to cure various diseases due to its high nutritional value and wide range of uses. The fascinating effects of G. lucidum have tethered a multitude of efforts to explore its effective ingredients and supplement functions. At present, many cancer research studies have reported the G. lucidum polysaccharides (GLPs) and G. lucidum triterpenes (GLTs) as the main active ingredients in G. lucidum, which have shown positive effects on radiotherapy and chemotherapy. GLPs or GLTs treatment synergizes with radiotherapy and chemotherapy through multiple pathways, including oxidative stress, apoptosis, immune microenvironment, etc. Therefore, this review aims to analyze and summarize these complex molecules from G. lucidum in order to create more treatment options for cancer patients in the future.
Keywords: GLP, GLTs, pharmacology, general structure, radiotherapy and chemotherapy
Introduction
G. lucidum is the dried fruit body of the polypore fungus G. lucidum Karst or Ganoderma sinense. G. lucidum has existed in China for thousands of years and is closely involved in the lives of the Chinese people, often being described as a kind of fairy grass that can cure various diseases.1 In a previous study, the medicinal history, cultivation methods, and species distribution of G. lucidum in China have been described in detail, filling the gaps for future research.2
Modern pharmacological studies have shown that G. lucidum contains a variety of bioactive components, including polysaccharides, nucleosides, furans, sterols, alkaloids, triterpenes, oils, various amino acids and proteins, enzymes, organic germanium, and many trace elements.3 Among them, G. lucidum polysaccharides (GLPs) and G. lucidum triterpenoids (GLTs) are the most important pharmacologically active substances. Recent studies have also highlighted the roles of GLPs in immune regulation and their hypoglycemic, hypolipidemic, anti-oxidant, anti-aging and anti-tumor effects.4 Moreover, GLTs have been found to purify blood and preserve liver function. Various Ganoderma preparations have sedative, anticonvulsant, anti-arrhythmic, anti-hypertensive and anti-tussive effects. In addition, G. lucidum displays anti-coagulant, anti-allergic, and platelet aggregation-inhibiting effects.5 The NIH3T3 cell line was employed to evaluate the cytotoxicity of G. lucidum using the XTT method, and no cytotoxicity related to the administration of G. lucidum was observed.6
G. lucidum is a safe, non-toxic, and widely used traditional Chinese medicine that is highly acknowledged by the world. GLPs and GLTs are the elite ingredients and are favored by researchers because of their high activity in G. lucidum. GLPs and GLTs have also showed remarkable biological effects.7 For example, they have been proven to play an effective role in cancer, neurodegenerative diseases, and diabetes, all of which are currently incurable diseases in humans.8,9 G. lucidum is extremely attractive to researchers, and it provides an in-depth explanation of its therapeutic effects on various cancers. Similarly, Rupeshkumar et al affirmed the magical power of G. lucidum from different angles.10
Currently, radiotherapy and chemotherapy represent the main outlets for cancer treatment in patients, but the side effects are also daunting.11 Maybe, the value of natural products shines exceptionally well. The flock of researchers focused their attention on whether they had the potential to change when combined with radiotherapy and chemotherapy. In a multitude of verification experiments, GLPs and GLTs have been shown to play versatile roles in responding to radiotherapy and chemotherapy, and they can be described as having full allure in future cancer research.12 Therefore, further research on polysaccharides and triterpenes, which are the elite components of G. lucidum, is urgent and valuable.
General Structure
GLPs are composed of three monosaccharide chains, possessing a helical stereo configuration similar to DNA and RNA. Among the more than 200 types of GLPs that have been isolated, in addition to glucose, most of them contain less monosaccharides such as arabinose, xylose, galactose, fucose, mannose, and rhamnose. Liu et al separated and purified GLPs from the fruiting body of G. lucidum, determining that the structure of the repeating unit is β-(1→3)-D-glucan as the main chain and β-(1→6)-D-glucopyranose as the side chain (Figure 1).
Figure 1.
Structure of GLP isolated from G. lucidum.
Note: Reprinted from Carbohydr Polym, 101, Liu Y, Zhang J, Tang Q, et al. Physicochemical characterization of a high molecular weight bioactive β-D-glucan from the fruiting bodies of Ganoderma lucidum. 968–974, Copyright (2014), with permission from Elsevier.45Abbreviation: Glcp, glucopyrano.
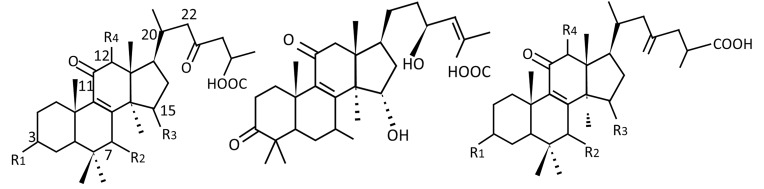
GLTs have 14 types of side chains and 7 types of original nuclei and have many different substituents, such as carboxyl, hydroxyl, ketone, methyl, methoxy, and acetyl groups. GLTs can be divided into C30, C27, and C24, depending on the number of carbon atoms present in the structure, and they can be further divided into classes based on the different functional groups and side chains, including acids, alcohols, aldehydes, and lactones (Figure 2 and Table 1).
Figure 2.
Common nuclei structures of GLTs.
Table 1.
Species of GLTs
| Number | Name | Molecular Formula | Source | Reference |
|---|---|---|---|---|
| 1 | Ganosporeric acid A | C30H44O7 | Spores | [41] |
| 2 | Ganosporeric acid B | C30H44O7 | Fruiting body | [41] |
| 3 | Ganosporeric acid C | C30H46O7 | Fruiting body | [41] |
| 4 | Ganosporeric acid D | C30H42O7 | Fruiting body | [41] |
| 5 | Ganosporeric acid E | C30H40O7 | Fruiting body | [41] |
| 6 | Ganosporeric acid F | C32H42O9 | Fruiting body | [41] |
| 7 | Ganosporeric acid G | C30H44O8 | Fruiting body | [41] |
| 8 | Ganosporeric acid H | C32H44O9 | Gill surfaces | [41] |
| 9 | Ganosporeric acid J | C30H42O7 | Fruiting body | [41] |
| 10 | Ganosporeric acid K | C30H46O9 | Fruiting body | [41] |
| 11 | Ganosporeric acid α | C32H46O9 | Fruiting body | [3] |
| 12 | Ganosporeric acid β | C30H44O6 | Spores | [3] |
| 13 | Lucidumol A | C30H48O4 | Spores | [3] |
| 14 | Ganoderic acid DM | C30H44O4 | Fruiting body | [3] |
| 15 | Ganoderic acid γ | C30H44O7 | Spores | [42] |
| 16 | Ganoderic acid δ | C30H44O7 | Spores | [42] |
| 17 | Ganoderic acid ε | C30H44O7 | Spores | [42] |
| 18 | Ganoderic acid ζ | C30H42O7 | Spores | [42] |
| 19 | Ganoderic acid η | C30H44O8 | Spores | [42] |
| 20 | Ganoderic acid θ | C30H42O8 | Spores | [42] |
| 21 | Lucidenic acid O | C27H38O7 | Fruiting body | [3] |
| 22 | Ganoderic acid Ma | C34H52O7 | Fruiting body | [41] |
| 23 | Ganoderic acid Mb | C36H54O9 | Fruiting body | [41] |
| 24 | Ganoderic acid Mc | C36H54O9 | Fruiting body | [41] |
| 25 | Ganoderic acid Md | C35H54O7 | Mycelial mats | [41] |
| 26 | Ganoderic acid Mg | C35H54O8 | Mycelial mats | [41] |
| 27 | Ganoderic acid Mh | C34H52O8 | Mycelial mats | [41] |
| 28 | Ganoderic acid Mj | C33H52O6 | Mycelial mats | [41] |
| 29 | Lucidenic acid lactone | C27H40O7 | Fruiting body | [3] |
| 30 | Lucidenic acid P | C29H42O8 | Fruiting body | [43] |
| 31 | Lucidenic acid N | C27H40O5 | Fruiting body | [43] |
| 32 | Methyl lucidenates D | C30H40O8 | Fruiting body | [43] |
| 33 | Methyl lucidenates E | C30H42O8 | Fruiting body | [43] |
| 34 | Methyl lucidenates F | C28H38O6 | Fruiting body | [43] |
| 35 | Methyl lucidenates P | C30H44O8 | Fruiting body | [43] |
| 36 | Methyl lucidenates Q | C28H44O6 | Fruiting body | [43] |
| 37 | Lucidenic acid LM1 | C27H40O6 | Fruiting body | [44] |
| 38 | Lucidenic acid LM2 | C30H42O7 | Fruiting body | [44] |
| 39 | Ganosporelactone A | C30H40O7 | Spores | [44] |
| 40 | Ganosporelactone B | C30H42O7 | Spores | [44] |
| 41 | Ganolactone | C27H36O6 | Fruiting body | [44] |
| 42 | Tsugaric acid A | C32H50O4 | Fruiting body | [44] |
| 43 | Tsugaric acid B | C33H52O5 | Fruiting body | [44] |
| 44 | Compound VI | C28H8O8 | Fruiting body | [44] |
| 45 | Compound VII | C31H44O9 | Fruiting body | [44] |
| 46 | Lucialdehyde A | C30H46O2 | Fruiting body | [44] |
| 47 | Lucialdehyde B | C30H44O3 | Fruiting body | [44] |
| 48 | Lucialdehyde C | C30H46O3 | Fruiting body | [44] |
| 49 | 8β,9α-dihydroga noderic acid J | C30H44O7 | Fruiting body | [3] |
| 50 | Methyl 8β,9α-dihydroga noderic acid J | C31H46O7 | Fruiting body | [3] |
| 51 | 20-hydroxylga noderic acid G | C30H44O9 | Fruiting body | [3] |
| 52 | Ganoderic acid Sz | C30H44O3 | Fruiting body | [41] |
| 53 | Ganoderic acid S1 | C30H44O3 | Fruiting body | [41] |
Protective Effects of GLPs and GLTs on Radiotherapy and Chemotherapy
Radiotherapy and chemotherapy directly kill tumor cells and suppress tumor growth, representing the most important treatment options for malignant tumors. However, their side effects are unavoidable and harmful to human health and can lead to decreased gastrointestinal immune function and bone marrow suppression. In recent years, the emergence of natural products derived from traditional Chinese medicine has greatly enriched methods of tumor treatment and has quickly become a research hotspot, gradually evolving into a potential adjuvant therapy for tumor treatment. G. lucidum, which has existed in China for thousands of years, has proved to demonstrate resistance to radiotherapy and chemotherapy side effects. GLPs and GLTs are believed to play a crucial role in resisting radiotherapy and chemotherapy side effects.
The GLP Effects of Radiotherapy and Chemotherapy
Experiments with GLPs in Swiss albino mice showed that β-glucan (BG), a polysaccharide derived from G. lucidum, possessed a certain degree of protection against radiation and could effectively resist radiation-induced damage. In the control group receiving only radiotherapy, 80% of the animals died after receiving radiation for 20 days, while no mice in this group had survived by day 30. Before radiation, the mice were given BG at a dose of 500 μg/kg body weight (bw)/day, and the survival rates on days 20 and 20 were 66% and 33%, respectively. After radiotherapy, mice were given the same dose, but the results showed that the survival rates on days 25 and 30 were 83% and 66%, respectively. These results showed that the survival rate of mice treated with the same dose of BG was significantly higher than that before radiotherapy (P < 0.001), and they confirmed that administering BG at a dose of 500 mg/kg body weight (bw)/day was nontoxic.13
Radiotherapy and chemotherapy are effective methods for treating malignant tumors, but cancer is an expendable disease. As a possible consequence of radiotherapy and chemotherapy, chemotherapy-related fatigue is also an urgent problem to address. In a study on chemotherapy-related fatigue, a weight-loaded swimming test was used to assess the degree of fatigue in rats with A549 lung cancer cells, which showed that, compared to the control group, the duration of weight-loaded swimming in rats with GLPs was longer than that of the control group.14
In an interesting set of binding experiments, the combination of synthetic bismuth sulfide nanoparticles (BiNP) and GLPs presented new prospects in the development of radiotherapies. When combined with radiation, GLP-BiNP achieved a significant inhibitory effect on tumor growth through radio sensitization and immune activity, and mitigated the risk of bismuth nephrotoxicity. For future treatment prospects, this strategy shows huge potential.15 Similarly, the combination of gold nanocomposites and GLPs (GLP-Au) also has broad prospects.16
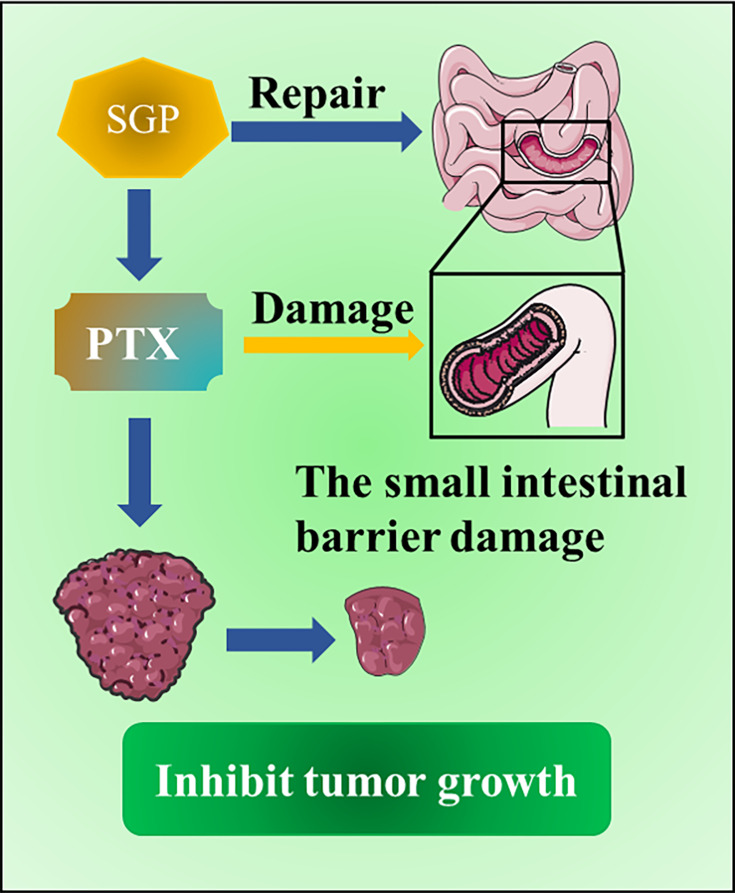
Paclitaxel (PTX) has become a broad-spectrum first-line chemotherapy drug due to its complex and novel chemical structure, unique biological mechanism of action, and reliable anti-cancer activity. According to research led by Su et al in 2018,17 the combination of G. lucidum spore polysaccharide (SGP) and PTX had incredible effects on tumor treatment. In preliminary studies of PTX in vitro, SGP did not increase the cytotoxicity of PTX. During the 21-day observation, the use of PTX alone in inhibiting tumor growth was effective from day 15, resulting in a reduction in tumor weight (p < 0.05). On the contrary, the inhibitory effect of PTX and SGP combination therapy on tumor growth might have occurred earlier. Additionally, experiments proved that the combined use of PTX and SGP could restore intestinal biological diseases caused by PTX monotherapy, and it helps to inhibit tumor metabolism, thereby inhibiting tumor growth. Another combination therapy of SGP and PTX was proven to ameliorate the intestinal barrier damage caused by PTX. The integrity of the small intestinal barrier of mice induced by PTX can be exceedingly adjusted by SGP. Intestinal injury caused by the use of PTX is closely correlated to the increase in epithelial permeability and the destruction of tight junctions. In view of the side effects of PTX, the combination of PTX and SGP for the protection of the small intestinal barrier damage caused by PTX may be accomplished by promoting the renewal of the intestinal epithelium to enhance the permeability and integrity of the epithelium. The mechanism involved may be related to the suppression of microtubule aggregates to inhibit cell proliferation and apoptosis18 (Figure 3).
Figure 3.
A combined therapeutic effect of SGP and PTX. The combination of PTX and SGP can restore the small intestinal barrier damage caused by PTX treatment alone, which helps to inhibit tumor metabolism and ultimately inhibit tumor growth.
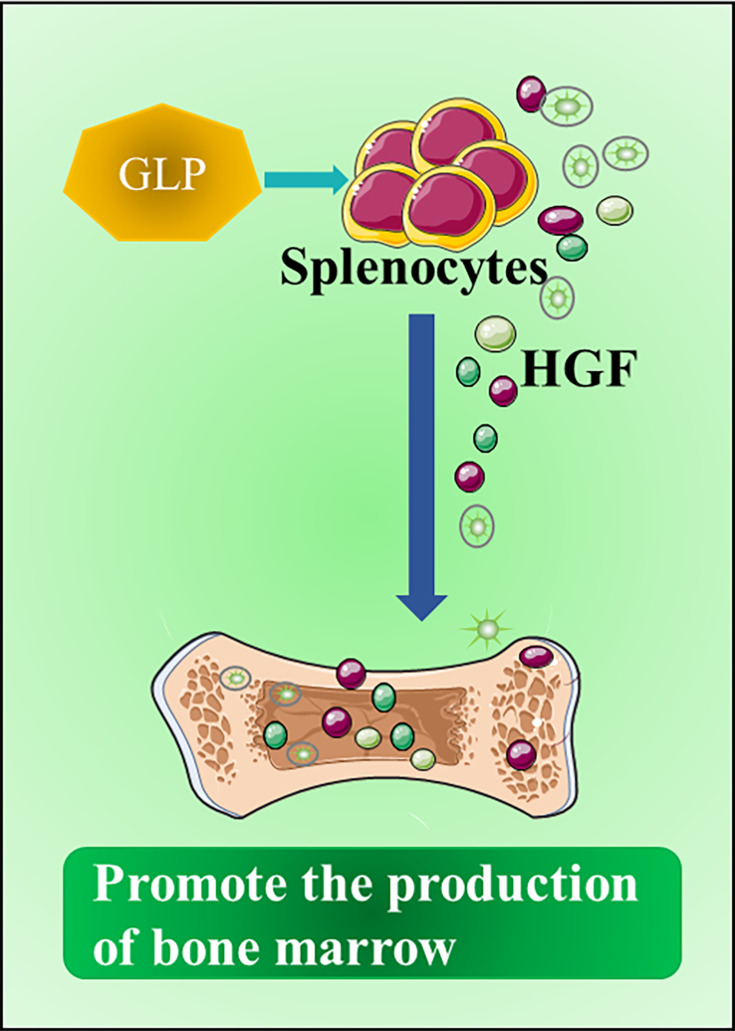
Another well-known side effect of chemotherapy is myelosuppression. A related study found that GLP could be used as a promoter for myelopoiesis to reduce the effects of myelosuppression induced by chemotherapy, thereby achieving protective effects on chemotherapy. It was recognized that GLPs do not directly stimulate the proliferation of hematopoietic progenitor cells to promote myelopoiesis, rather they indirectly stimulate splenocytes to produce hematopoietic growth factors (HGF), which mainly include granulocyte colony stimulating factor, interleukin-1, and interleukin-6, and stem cell factor19 (Figure 4).
Figure 4.
GLP is a promoter of myelopoiesis. GLPs stimulate splenocytes to produce hematopoietic growth factors (HGF), which can act as a promoter of bone marrow production, reduce the bone marrow suppression induced by chemotherapy, and maximize the anti-tumor effect of chemotherapy.
The GLTs Effects of Radiotherapy and Chemotherapy
Smina et al reported that GLTs could strongly mitigate oxidation and scavenge free radicals, and they demonstrated the protective effects of GLTs on DNA and cell membranes after radiation damage using Thiobarbituric acid reactive substances (TBARS), comet assay, and micronucleus assay.20 Another study published by Smina et al revealed the potential therapeutic use of GLTs as an adjuvant in radiation therapy. GLTs were orally administered continuously for 14 days at doses of 50 and 100 mg/kg body weight (bw)/day before exposure of the whole body of Swiss albino mice to radiation. GLTs were shown to reduce the levels of lipid peroxidation and protein oxidation, effectively restore the activities of antioxidant enzymes and glutathione in the liver and brain of irradiated mice, and significantly reduce DNA strand breaks.21 It has also been reported that GLTs could be used as an alternative dietary supplement to prevent cancer-associated colitis.22
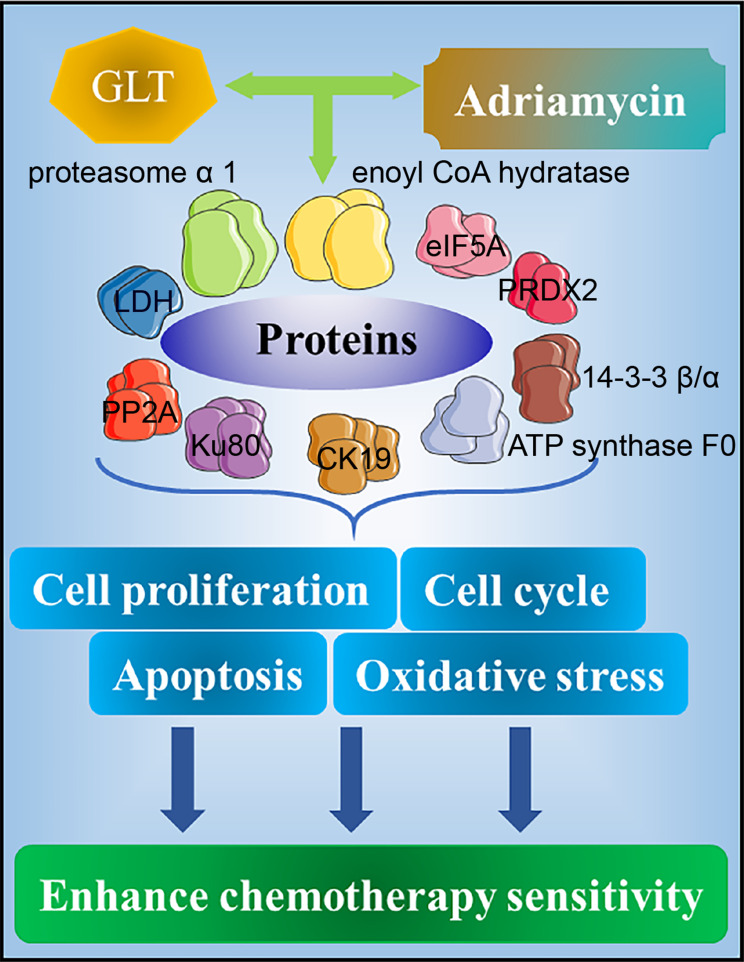
In a study using HeLa cells, GLTs and adriamycin displayed a synergistic effect that caused the regulated expression of 14 proteins that play significant roles in cell proliferation, the cell cycle, apoptosis, and oxidative stress. Furthermore, GLTs enhanced the production of reactive oxygen species (ROS) by adriamycin. It was suggested that the synergistic effect between GLTs and adriamycin may be based on the fact that GLTs enhance their sensitivity to chemotherapy by enhancing oxidative stress, DNA damage, and apoptosis23 (Figure 5).
Figure 5.
Synergistic effect of GLTs and doxorubicin against chemotherapy sensitivity. The synergistic effect of GLT and adriamycin leads to the expression of multiple proteins, such as eIF5A, 14-3-3 β/α, and Ku80, which play important roles in cell proliferation, cell cycle, apoptosis, and oxidative stress, thereby enhancing the sensitivity to chemotherapy.
Experiments in HL-7702 cells found that GLTs were mainly concentrated in chloroform extracts, which exhibited significant inhibitory malignancy effects of cancer cells and on the repair or protection of normal cells damaged by radiotherapy and chemotherapy. These results showed that GLTs have protective effects against damaged normal cells induced by radiotherapy and chemotherapy.24
Research has shown that mycotherapy can improve the overall response rate during cancer treatment and reduce various chemotherapy-related adverse events. The GLT ganoderic acid A (GAA) was found to enforce QCT-induced apoptosis and Epstein-Barr virus (EBV) lytic reactivation at low concentrations, exhibiting similar biological effects to ganoderic lucidum extracts (GLE) in QCT-mediated antitumor activity. Thus, GAA can be used as a potential food adjunct for the prevention of EBV-associated gastric carcinoma (EBVaGC) development.25 Similarly, ganoderenic acid B (GAB) another type of GLTs could reverse the multidrug resistance of ABCB1-mediated liver cells to adriamycin, vincristine, and PTX, the mechanism of which may be due to the inhibition of ABCB1 transport and the increase in drug accumulation in MDR cells. GAB could also reverse the resistance of ABCB1-overexpressing MCF-7/ADR cells to doxorubicin.26
In addition, existing research shows that the Chinese medicinal herb complex (CCMH: a mixture of citronellol and extracts of G. lucidum, C. pilosula, and A. sinensis) increased the immune cell counts in cancer patients who received the treatment with chemotherapy and/or radiotherapy,27 which suggests that the Chinese medicinal herb has anti-radio-chemotherapy effects. Cao et al confirmed that G. lucidum and the immune system are inextricably linked in cancer treatment. It can activate immune cells, such as T or B lymphocytes, macrophages, and NK cells, and can also promote production of cytokines and antibodies, so as to achieve the purpose of inhibiting the growth of tumor cells.28
Ursolic acid is a naturally synthesized pentacyclic triterpenoid compound that has been widely found in various fruits and vegetables. It has not only demonstrated anti-cancer activities and anti-inflammatory effects but it has also been found to induce apoptosis in several human cancer cell lines. However, there is no clear evidence that there is an explicit limit to the use of ursolic acid in human studies.29 The compounds 2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oic acid (CDDO) and its C28-modified derivative, methyl-ester (CDDO-Me), are two synthetic derivatives of oleanolic acid that have been widely studied for their ability to induce apoptosis and aid in the differentiation of cancer cells. Although some progress has been made in clinical trials, some complications related to heart failure events have been observed.30
Another study demonstrated that Escin, a mixture of triterpenoid saponins extracted from the horse chestnut tree, had anti-cancer effects. However, clinical trial results showed that some patients exhibited diarrhea, dyspnea, dysphagia, and corestenoma, while healthy volunteers did not have similar symptoms.31 Compared to Escin, GLTs play a significant therapeutic role in the treatment of numerous diseases. Among these triterpenes, it has been discovered that GLTs display anti-cancer effects, and most importantly, natural nontoxic effects.
Discussion and Outlook
In the modern treatment of cancer, radiotherapy and chemotherapy are important methods for the treatment of malignant tumors, which have the advantages of killing tumor cells and inhibiting the growth of tumor cells. Specifically, the electron beams, X-rays and radioisotopes show efficacy in most cancers. As a common chemotherapeutic drug, PTX and cisplatin are widely used in the treatment of various cancers through inhibiting cancer cell division, arresting DNA replication process of cancer cells, destroying cancer cell membrane structure, etc. Both radiotherapy and chemotherapy can directly kill cancer cells while simultaneously triggering tumor microenvironment remodeling in which pro-inflammatory signaling pathways are activated and pro-inflammatory mediators are released, thereby recruiting tumor-infiltrating immune cells.
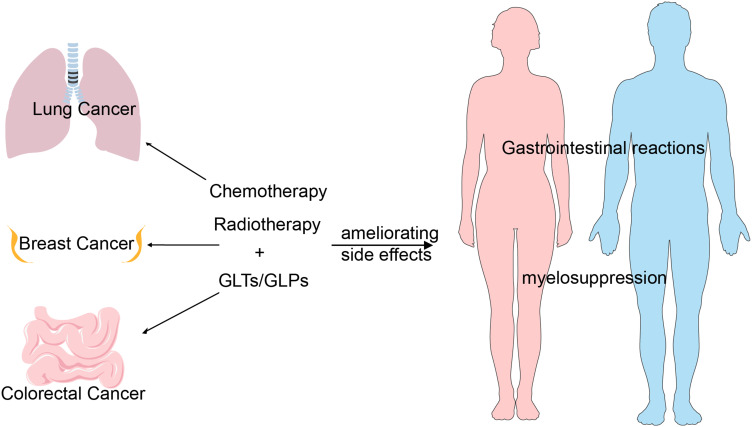
GLPs and GLTs are of the essence of G. lucidum, and have proved to play multi-faceted anti-cancer roles including direct cytotoxicity in tumor cells, antioxidant effect, inhibition of angiogenesis, induction of cell differentiation and immunomodulatory effect (activation of immune host response), etc. Currently, the clinical trials of GLPs and GLTs are under way. Polysaccharide extracts (Ganopoly) stimulate immune responses in advanced stage cancer patients. IL-2, IL-6, IFN-γ and NK cell activity in plasma were increased, while IL-1 and TNF-α were considerably reduced.32–34 Early research on G. lucidum against leukemia indicated that it is an ideal leukemia treatment drug, and its underlying mechanism may be that G. lucidum induces the differentiation of leukemia cells to the mature stage and inhibits their proliferation.35 In various nonrandomized clinical trials of different types of cancer, especially breast cancer, when combined with radiotherapy or chemotherapy, polysaccharide extracts of G. lucidum could be very efficient to reduce the metastasis potential and/or adverse effects, and enhance the effects of chemotherapy and radiotherapy.36–40 Therefore, combination therapy with GLPs/GLTs and radio-chemotherapy is becoming a general trend clinically, which can achieve the goal of increasing efficiency and reducing the side effects caused by drugs (Figure 6).
Figure 6.
The synergistic model of GLP and GLTs with chemotherapy and radiotherapy. Combination treatment of GLP/GLTs and chemo-radiotherapy showed synergistic effects in lung cancer, breast cancer and colorectal cancer treatments with ameliorating side effects such as gastrointestinal reactions and myelosuppression.
Future directions should focus on the molecular elaboration of GLPs and GLTs in cancer research. It is important to clarify the β-glucan contents in GLPs or SGP, also critical to decipher the structure and biological functions of these β-glucan in GLPs including the immunomodulatory mechanisms. The pharmacodynamics of GLPs in vivo is also important for future clinical translation. In addition, though the elite components of the G. lucidum are GLPs and GLTs, other components such as unsaturated long-chain fatty acids, appear to show the antitumoral activity. Elucidating the antitumor activity of G. lucidum has a great potential in improving human health and curing diseases.
Acknowledgments
All the staff have designed the study, data collected, prepared, written, reviewed and edited the entire manuscript. Hang Song and Teng Ma are co-correspondence authors for this study.
Funding Statement
This work was supported by Project of High-Level Talents in AHUTCM (Project code: 2019rcZD001).
Abbreviations
GLPs, G. lucidum polysaccharides; GLTs, G. lucidum triterpenoids; BG, β-glucan; BiNP, the combination of synthetic bismuth sulfide nanoparticles; GLP-Au, the combination of gold nanocomposites and GLPs; PTX, Paclitaxel; SGP, G. lucidum spore polysaccharide; HGF, hematopoietic growth factors; ROS, oxygen species; GAA, ganoderic acid A; EBV, Epstein-Barr virus; GLE, ganoderic lucidum extracts; EBVaGC, EBV-associated gastric carcinoma; GAB, ganoderenic acid B; CDDO and CDDO-Me, 2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oic acid and its C28-modified derivative, methyl-ester.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Ahmad MF. Ganoderma lucidum: a rational pharmacological approach to surmount cancer. J Ethnopharmacol. 2020;260:113047. doi: 10.1016/j.jep.2020.113047 [DOI] [PubMed] [Google Scholar]
- 2.Wang L, Li JQ, Zhang J, Li ZM, Liu HG, Wang YZ. Traditional uses, chemical components and pharmacological activities of the genus Ganoderma P. Karst.: a review. RSC Adv. 2020;10(69):42084–42097. doi: 10.1039/D0RA07219B [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gong T, Yan R, Kang J, Chen R. Chemical components of ganoderma. Adv Exp Med Biol. 2019;1181:59–106. [DOI] [PubMed] [Google Scholar]
- 4.Lu J, He R, Sun P, Zhang F, Linhardt RJ, Zhang A. Molecular mechanisms of bioactive polysaccharides from Ganoderma lucidum (Lingzhi), a review. Int J Biol Macromol. 2020;150:765–774. doi: 10.1016/j.ijbiomac.2020.02.035 [DOI] [PubMed] [Google Scholar]
- 5.Cör D, Knez Ž, Knez Hrnčič M. Antitumour, Antimicrobial, Antioxidant and Antiacetylcholinesterase Effect of Ganoderma Lucidum Terpenoids and Polysaccharides: a Review. Molecules. 2018;23(3):649. doi: 10.3390/molecules23030649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ergun B. Evaluation of antimicrobial, cytotoxic and genotoxic activities of Ganoderma lucidum (Reishi mushroom). Pak J Pharm Sci. 2017;30(5(Supplementary)):1991–1995. [PubMed] [Google Scholar]
- 7.Li LF, Liu HB, Zhang QW, et al. Comprehensive comparison of polysaccharides from Ganoderma lucidum and G. sinense: chemical, antitumor, immunomodulating and gut-microbiota modulatory properties. Sci Rep. 2018;8(1):6172. doi: 10.1038/s41598-018-22885-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sohretoglu D, Zhang C, Luo J, Huang S. ReishiMax inhibits mTORC1/2 by activating AMPK and inhibiting IGFR/PI3K/Rheb in tumor cells. Signal Transduct Target Ther. 2019;4:21. doi: 10.1038/s41392-019-0056-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen SD, Yong TQ, Zhang YF, Hu HP, Xie YZ. Inhibitory effect of five Ganoderma species (Agaricomycetes) against key digestive enzymes related to type 2 diabetes mellitus. Int J Med Mushrooms. 2019;21(7):703–711. doi: 10.1615/IntJMedMushrooms.v21.i7.70 [DOI] [PubMed] [Google Scholar]
- 10.Rupeshkumar M, Chettri U, Paarakh PM. Ganoderma lucidum: a review with special emphasis on the treatment of various cancer. J App Pharm. 2016;8(4). doi: 10.21065/1920-4159.1000228 [DOI] [Google Scholar]
- 11.Cao W, Gu Y, Meineck M, Xu H. The combination of chemotherapy and radiotherapy towards more efficient drug delivery. Chem Asian J. 2014;9(1):48–57. doi: 10.1002/asia.201301294 [DOI] [PubMed] [Google Scholar]
- 12.Chen L, Abulizi A, Li M. Protective effect of Ganoderma (Lingzhi) on radiation and chemotherapy. Adv Exp Med Biol. 2019;1182:119–142. [DOI] [PubMed] [Google Scholar]
- 13.Pillai TG, Uma Devi P. Mushroom beta glucan: potential candidate for post irradiation protection. Mutat Res. 2013;751(2):109–115. doi: 10.1016/j.mrgentox.2012.12.005 [DOI] [PubMed] [Google Scholar]
- 14.Ouyang MZ, Lin LZ, Lv WJ, et al. Effects of the polysaccharides extracted from Ganoderma lucidum on chemotherapy-related fatigue in mice. Int J Biol Macromol. 2016;91:905–910. doi: 10.1016/j.ijbiomac.2016.04.084 [DOI] [PubMed] [Google Scholar]
- 15.Yu H, Yang Y, Jiang T, et al. Effective radiotherapy in tumor assisted by Ganoderma lucidum polysaccharide-conjugated bismuth sulfide nanoparticles through radiosensitization and dendritic cell activation. ACS Appl Mater Interfaces. 2019;11(31):27536–27547. doi: 10.1021/acsami.9b07804 [DOI] [PubMed] [Google Scholar]
- 16.Zhang S, Pang G, Chen C, et al. Effective cancer immunotherapy by Ganoderma lucidum polysaccharide-gold nanocomposites through dendritic cell activation and memory T cell response. Carbohydr Polym. 2019;205:192–202. doi: 10.1016/j.carbpol.2018.10.028 [DOI] [PubMed] [Google Scholar]
- 17.Su J, Li D, Chen Q, et al. Anti-breast cancer enhancement of a polysaccharide from spore of Ganoderma lucidum with paclitaxel: suppression on tumor metabolism with gut microbiota reshaping. Front Microbiol. 2018;9:3099. doi: 10.3389/fmicb.2018.03099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li D, Gao L, Li M, et al. Polysaccharide from spore of Ganoderma lucidum ameliorates paclitaxel-induced intestinal barrier injury: apoptosis inhibition by reversing microtubule polymerization. Biomed Pharmacother. 2020;130:110539. doi: 10.1016/j.biopha.2020.110539 [DOI] [PubMed] [Google Scholar]
- 19.Zhu XL, Liu JH, Li WD, Lin ZB. Promotion of myelopoiesis in myelosuppressed mice by Ganoderma lucidum polysaccharides. Front Pharmacol. 2012;3:20. doi: 10.3389/fphar.2012.00020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smina TP, Maurya DK, Devasagayam TP, Janardhanan KK. Protection of radiation induced DNA and membrane damages by total triterpenes isolated from Ganoderma lucidum (Fr.) P. Karst. Chem Biol Interact. 2015;233:1–7. doi: 10.1016/j.cbi.2015.03.019 [DOI] [PubMed] [Google Scholar]
- 21.Smina TP, Joseph J, Janardhanan KK. Ganoderma lucidum total triterpenes prevent γ-radiation induced oxidative stress in Swiss albino mice in vivo. Redox Rep. 2016;21(6):254–261. doi: 10.1080/13510002.2015.1126098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sliva D, Loganathan J, Jiang J, et al. Mushroom Ganoderma lucidum prevents colitis-associated carcinogenesis in mice. PLoS One. 2012;7(10):e47873. doi: 10.1371/journal.pone.0047873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yue QX, Xie FB, Guan SH, et al. Interaction of Ganoderma triterpenes with doxorubicin and proteomic characterization of the possible molecular targets of Ganoderma triterpenes. Cancer Sci. 2008;99(7):1461–1470. doi: 10.1111/j.1349-7006.2008.00824.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang DH, Weng XC. [Antitumor activity of extracts of Ganoderma lucidum and their protective effects on damaged HL-7702 cells induced by radiotherapy and chemotherapy]. Zhongguo Zhong Yao Za Zhi = Zhongguo Zhongyao Zazhi = China Journal of Chinese Materia Medica. 2006;31(19):1618–1622. Chinese. [PubMed] [Google Scholar]
- 25.Huh S, Lee S, Choi SJ, et al. Quercetin synergistically inhibit EBV-associated gastric carcinoma with Ganoderma lucidum extracts. Molecules. 2019;24(21):3834. doi: 10.3390/molecules24213834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu DL, Li YJ, Yang DH, et al. Ganoderma lucidum derived ganoderenic acid B reverses ABCB1-mediated multidrug resistance in HepG2/ADM cells. Int J Oncol. 2015;46(5):2029–2038. doi: 10.3892/ijo.2015.2925 [DOI] [PubMed] [Google Scholar]
- 27.Zhuang SR, Chen SL, Tsai JH, et al. Effect of citronellol and the Chinese medical herb complex on cellular immunity of cancer patients receiving chemotherapy/radiotherapy. Phytother Res. 2009;23(6):785–790. doi: 10.1002/ptr.2623 [DOI] [PubMed] [Google Scholar]
- 28.Cao Y, Xu X, Liu S, Huang L, Gu J. Ganoderma: a cancer immunotherapy review. Front Pharmacol. 2018;9:1217. doi: 10.3389/fphar.2018.01217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yin R, Li T, Tian JX, Xi P, Liu RH. Ursolic acid, a potential anticancer compound for breast cancer therapy. Crit Rev Food Sci Nutr. 2018;58(4):568–574. doi: 10.1080/10408398.2016.1203755 [DOI] [PubMed] [Google Scholar]
- 30.Borella R, Forti L, Gibellini L, et al. Synthesis and anticancer activity of CDDO and CDDO-me, two derivatives of natural triterpenoids. Molecules. 2019;24(22):4097. doi: 10.3390/molecules24224097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheong DHJ, Arfuso F, Sethi G, et al. Molecular targets and anti-cancer potential of escin. Cancer Lett. 2018;422:1–8. doi: 10.1016/j.canlet.2018.02.027 [DOI] [PubMed] [Google Scholar]
- 32.Gao Y, Zhou S, Chen G, Dai X, Ye J. A phase I/II study of a (Curt.: fr.) P. Karst. Extract () in patients with advanced cancer. J Nucl Med. 2003;44(5):690.12732669 [Google Scholar]
- 33.Gao Y, Zhou S, Jiang W, Huang M, Dai X. Effects of ganopoly (a Ganoderma lucidum polysaccharide extract) on the immune functions in advanced-stage cancer patients. Immunol Invest. 2003;32(3):201–215. doi: 10.1081/IMM-120022979 [DOI] [PubMed] [Google Scholar]
- 34.Tang W, Gao Y, Chen G, et al. A randomized, double-blind and placebo-controlled study of a Ganoderma lucidum polysaccharide extract in neurasthenia. J Med Food. 2005;8(1):53–58. doi: 10.1089/jmf.2005.8.53 [DOI] [PubMed] [Google Scholar]
- 35.Zhong L, Jiang D, Wang Q. [Effects of Ganoderma lucidum (Leyss ex Fr) Karst compound on the proliferation and differentiation of K562 leukemic cells]. Hunan Yi Ke Da Xue Xue Bao = Hunan Yike Daxue Xuebao = Bulletin of Hunan Medical University. 1999;24(6):521–524. Chinese. [PubMed] [Google Scholar]
- 36.Jin X, Ruiz Beguerie J, Sze DM, Chan GC. Ganoderma lucidum (Reishi mushroom) for cancer treatment. Cochrane Database Syst Rev. 2012;6:CD007731. [DOI] [PubMed] [Google Scholar]
- 37.Unlu A, Nayir E, Kirca O, Ozdogan M. Ganoderma Lucidum (Reishi Mushroom) and cancer. J BUON. 2016;21(4):792–798. [PubMed] [Google Scholar]
- 38.Xie YZ, Yang F, Tan W, et al. The anti-cancer components of Ganoderma lucidum possesses cardiovascular protective effect by regulating circular RNA expression. Oncoscience. 2016;3(7–8):203–207. doi: 10.18632/oncoscience.316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu GQ, Zhang KC. Mechanisms of the anticancer action of Ganoderma lucidum (Leyss. ex Fr.) Karst.: a new understanding. 物学报(英文版). 2005;47(2):129–135. [Google Scholar]
- 40.Seleen J, Chen AW. Potential benefits of Ling Zhi or Reishi Mushroom Ganoderma lucidum (W. Curt.: fr.) P. Karst. (Aphyllophoromycetideae) to breast cancer patients. Int J Med Mushrooms. 2007;9(1):29–38. doi: 10.1615/IntJMedMushr.v9.i1.40 [DOI] [Google Scholar]
- 41.Liang C, Tian D, Liu Y, et al. Review of the molecular mechanisms of Ganoderma lucidum triterpenoids: ganoderic acids A, C2, D, F, DM, X and Y. Eur J Med Chem. 2019;174:130–141. doi: 10.1016/j.ejmech.2019.04.039 [DOI] [PubMed] [Google Scholar]
- 42.Ma B, Ren W, Zhou Y, Ma J, Ruan Y, Wen CN. Triterpenoids from the spores of Ganoderma lucidum. N Am J Med Sci. 2011;3(11):495–498. doi: 10.4297/najms.2011.3495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xia Q, Zhang H, Sun X, et al. A comprehensive review of the structure elucidation and biological activity of triterpenoids from Ganoderma spp. Molecules. 2014;19(11):17478–17535. doi: 10.3390/molecules191117478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baby S, Johnson AJ, Govindan B. Secondary metabolites from Ganoderma. Phytochemistry. 2015;114:66–101. doi: 10.1016/j.phytochem.2015.03.010 [DOI] [PubMed] [Google Scholar]
- 45.Liu Y, Zhang J, Tang Q, et al. Physicochemical characterization of a high molecular weight bioactive β-D-glucan from the fruiting bodies of Ganoderma lucidum. Carbohydr Polym. 2014;101:968–974. doi: 10.1016/j.carbpol.2013.10.024 [DOI] [PubMed] [Google Scholar]