Abstract
Purpose
To compare the imaging quality, T stage and extramural venous invasion (EMVI) evaluation between the conventional and synthetic T2-weighted imaging (T2WI), and to investigate the role of quantitative values obtained from synthetic magnetic resonance imaging (MRI) for assessing nodal staging in rectal cancer (RC).
Methods
Ninety-four patients with pathologically proven RC who underwent rectal MRI examinations including synthetic MRI were retrospectively recruited. The image quality of conventional and synthetic T2WI was compared regarding signal-to-noise ratio (SNR), contrast-to-noise (CNR), sharpness of the lesion edge, lesion conspicuity, absence of motion artifacts, and overall image quality. The accuracy of T stage and EMVI evaluation on conventional and synthetic T2WI were compared using the Mc-Nemar test. The quantitative T1, T2, and PD values were used to predict the nodal staging of MRI-evaluated node-negative RC.
Results
There were no statistically significant differences between conventional and synthetic T2WI in SNR, CNR, overall image quality, lesion conspicuity, and absence of motion artifacts (p = 0.058–0.978). There were no significant differences in the diagnostic accuracy of T stage and EMVI between conventional and synthetic T2WI from two observers (p = 0.375 and 0.625 for T stage; p = 0.625 and 0.219 for EMVI). The T2 value showed good diagnostic performance for predicting the nodal staging of RC with the area under the receiver operating characteristic, sensitivity, specificity, and accuracy of 0.854, 90.0%, 71.4%, and 80.3%, respectively.
Conclusions
Synthetic MRI may facilitate preoperative staging and EMVI evaluation of RC by providing synthetic T2WI and quantitative maps in one acquisition.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13244-021-01063-w.
Keywords: Rectal cancer, Magnetic resonance imaging, Synthetic imaging, Evaluation study
Key Points
Synthetic T2WI provides comparable image quality with the conventional T2WI.
Synthetic T2WI enables similar diagnostic accuracy in local staging of rectal cancer.
Synthetic MRI facilitate preoperative evaluation of rectal cancer by providing multiple images.
Introduction
Colorectal cancer is the third most common malignancy worldwide, ranking third in mortality among female and male, respectively [1]. Rectal cancer (RC) accounts for approximately 30%–35% of colorectal cancer cases, which are mostly adenocarcinoma [2]. Magnetic resonance imaging (MRI), especially high-resolution T2-weighted imaging (T2WI), is recommended to be routinely used for assessing RC local stage, which is critical for treatment decisions and the prognosis prediction [3, 4].
Besides the conventional contrast-weighted imaging, quantitative relaxation mappings have been shown to play a certain role in identifying the tumor grade, metastatic lymph nodes, lymphovascular invasion, and therapeutic response of several tumors [5–9]. However, the separate acquisitions of quantitative mappings and contrast-weighted images can be time-consuming. Synthetic MRI, in which a multi-echo and multi-delay acquisition scheme is adopted, can be advantageous of shorting scanning time by simultaneously quantifying T1, T2 and proton density (PD) relaxometry and generating synthetic contrast-weighted images in a single scan [10, 11].
Synthetic MRI has been demonstrated to have excellent correlation with conventional mapping methods and comparable image quality to that of conventional contrast-weighted images in brain and knee [12–14]. Additionally, there have been some promising findings of synthetic MRI in various tumors, such as prostate cancer, breast cancer, bone metastasis [6, 15, 16]. Our previous study has proven that quantitative T1 and T2 values generated from synthetic MRI were useful for predicting prognostic factors of RC [17]. Another study showed that radiomics model based on synthetic MRI could improve the diagnostic performance of extramural venous invasion (EMVI) in rectal cancer [18]. To the best of our knowledge, the feasibility of the contrast-weighted imaging generated from synthetic MRI has not yet been reported.
Recently, the diagnostic accuracy and interobserver agreement of conventional nodal staging in RC remain unsatisfactory [19]. A previous study suggested that T2 value may be useful in differentiating metastatic lymph nodes in RC, but is limited by the node-by-node approach to match the lymph node on MRI with the pathological specimen [9]. Although previous studies have confirmed the associations between the characteristics of the primary tumor and the nodal staging, few studies have focused on the MRI-evaluated node-negative RC.
The aim of this study was to compare the image quality, T stage, and EMVI assessment between the conventional and synthetic T2WI, and to investigate the quantitative values for a more accurate nodal staging of MRI-evaluated node-negative RC.
Materials and methods
Participants
Our Institutional Review Board approved this retrospective single-center study and the informed consent was waived. In total, 143 patients with pathologically confirmed rectal adenocarcinoma who underwent rectal MRI examinations including the synthetic MRI sequence between November 2018 and February 2020 were enrolled. Exclusion criteria were as follows: (1) those who received neoadjuvant treatment before the surgery; (2) time interval between the MRI examination and surgery greater than 4 weeks. Finally, 94 patients were included in the study. Of these, 18 participants with suspicious metastatic lymph node on preoperative MRI were excluded from quantitative evaluation of nodal staging. The flowchart of the study cohort is shown in Fig. 1.
Fig. 1.
Flow chart of the study cohort
Pathological characteristics of surgical specimens were assessed according to the eighth edition of the American Joint Committee on Cancer (AJCC) TNM staging system [20].
MRI acquisition
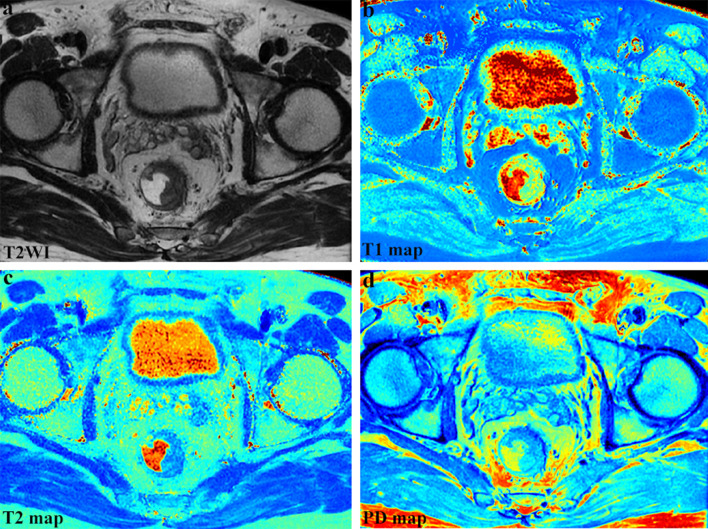
All MRI examinations were performed on a 3.0 T scanner (SIGNA Pioneer, GE Healthcare, Milwaukee, WI) equipped with a 32-channel phased-array body coil. For bowel preparation, glycerin enema was used to empty the feces before the examination. Anisodamine hydrochloride (10 mg, except those with contraindications) was intramuscularly injected 15 min before the examination to reduce peristaltic artifacts. Axial T1-weighted imaging (T1WI), diffusion-weighted imaging (DWI) (b values of 0 and 1000 s/mm2), oblique axial/coronal (perpendicular/parallel to the maximum tumor length), sagittal T2WI, synthetic MRI, and dynamic contrast-enhanced sequence were obtained. Synthetic MRI was performed before the injection of contrast agent in the oblique axial plane, using a QRAPMASTER (quantification of relaxation times and proton density by multiecho acquisition of a saturation-recovery using turbo spin-echo readout) sequence with two echo times (19.5/97.3 ms) and four saturation delay times (210/610/1810/3810 ms). The detailed acquisition parameters are listed in Table 1. Raw data of synthetic MRI were loaded into SyMRI 8.0 (SyntheticMR, Linköping, Sweden) for postprocessing, and quantitative T1, T2, PD maps and synthetic contrast-weighted images were automatically generated within 10 s (Fig. 2).
Table 1.
MRI parameters
| T1WI | T2WI | Synthetic MRI | DWI | DCE | |
|---|---|---|---|---|---|
| Sequence | FSE | FSE | QRAPMASTER | SS-EPI | DISCO |
| Imaging plane | Axial | Oblique axial, oblique coronal, sagittal | Oblique axial | Axial | Axial |
| Repetition time (ms) | 588 | 5115, 5912, 5062 | 4000 | 4574 | 4.8 |
| Echo time (ms) | Min full | 85.0, 98.8, 90.0 | 19.5/97.3 | Minimum | 2.0 |
| Slice thickness/gap (mm) | 5/1 | 3/0.3 | 3/0.3 | 5/1 | 2/0 |
| Field of view (cm) | 40 × 40 | 18 × 18, 24 × 24, 24 × 24 | 24 × 24 | 40 × 40 | 36 × 36 |
| Matrix | 320 × 256 | 320 × 224, 320 × 256, 320 × 224 | 320 × 256 | 128 × 128 | 288 × 192 |
| Echo train length | 3 | 24, 32, 24 | 16 | – | – |
| Bandwidth (kHz) | 50.00 | 50.00, 63.50, 50.00 | 27.78 | 250 | 142.86 |
| Acquisition time (min: s) | 0:57 | 02:39, 02:40, 2:37 | 04:32 | 01:36 | Per phase 0:09 |
MRI, magnetic resonance imaging; T1WI, T1-weighted imaging; T2WI, T2-weighted imaging; DWI, diffusion-weighted imaging; DCE, dynamic contrast enhanced; FSE, fast spin-echo; QRAPMASTER, quantification of relaxation times and proton density by multiecho acquisition of a saturation recovery using turbo spin-echo readout; SS-EPI, single-shot echo-planar imaging; DISCO, differential sub-sampling with cartesian ordering
Fig. 2.
Representative synthetic magnetic resonance images from a 57-year-old male patient with rectal cancer. (a–d) T2-weighted imaging (T2WI), T1 map, T2 map, and PD map
Subjective image quality analysis
The subjective image quality of conventional and synthetic T2WI was evaluated by two radiologists with 5 and 8 years of experience in rectal imaging, who were blinded to pathological information and acquisition methods. The observers scored the image quality based on following 4 factors on a 5-point-Likert scale: (1) Sharpness of the lesion edge (1 = not sharp; 2 = a little sharp; 3 = moderately sharp; 4 = well sharp; 5 = very sharp); (2) Lesion conspicuity (1 = difficult to find; 2 = minimally perceivable; 3 = recognizable; 4 = easy to detect, good contrast of lesion; 5 = excellent contrast of lesion); (3) Motion artifacts (1 = severe, difficult to diagnose; 2 = a little severe, accessible to diagnose; 3 = moderate; 4 = mild; 5 = absence of artifacts); (4) Overall image quality (the three factors above added together, 1 = unacceptable; 2 = poor; 3 = moderate; 4 = good; 5 = excellent). The patient order was randomized, as was the review order of the conventional or synthetic T2WI.
Objective image quality analysis
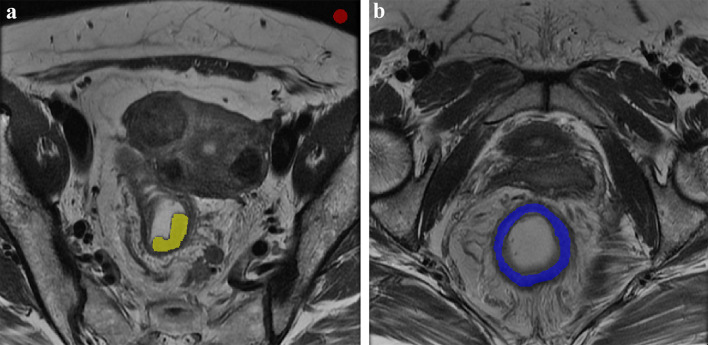
Regions of interest (ROI) were manually drawn on conventional oblique axial T2WI and synthetic T2WI using ITK-SNAP software (version 2.2.0, www.itksnap.org) by the same two radiologists. The ROI for the tumor was delineated on each slice along the margin of the tumor, resulting in a 3D whole tumor ROI. The normal tissue ROI was drawn in homogeneous normal rectum tissue distant from the tumor area, including the entire rectal wall at a single slice. The background ROI was a circular area with a diameter of 1 cm, placed within the field of view but outside the body surface. The mean and standard deviation of the signal intensity were obtained from each ROI. Signal-to-noise ratio (SNR) and contrast-to-noise (CNR) were calculated based on following formulas [21]:
where Stumor is the mean signal intensity within the tumor, SDbackground represents the standard deviation of the background noise, and Stissue denotes the mean signal intensity of the normal tissue (Fig. 3).
Fig. 3.
Signal-to-noise ratio (SNR) and contrast-to-noise (CNR) were calculated based on three regions of interest (ROIs). The yellow ROI represents the tumor, which was delineated slice by slice along the tumor margin. The red ROI on background was placed within the field of view but outside the body surface. The blue ROI represents normal tissue, which was drawn in normal rectum tissue at a single slice. The mean and standard deviation of the signal intensity were obtained from each ROI to calculate SNR and CNR
Preoperative evaluation of RC
The same two radiologists independently assessed the T stage and EMVI status on conventional and synthetic T2WI. The T stage was evaluated based on the European Society for Medical Oncology guidelines [3]. In addition, EMVI status was assessed based on a 5-point scoring system proposed by Smith et al. [22]: (0 = definitely absent; 1 = probably absent; 2 = indeterminate; 3 = probably present; 4 = definitely present). Scores of 3 or 4 were regarded as positive EMVI. Criteria for malignant node was according to the European Society of Gastrointestinal and Abdominal Radiology recommendations [23]: (1) short axis diameter ≥ 9 mm; (2) short axis diameter 5 − 8 mm and ≥ 2 morphologically suspicious characteristics; (3) short axis diameter < 5 mm and 3 morphologically suspicious characteristics; (4) all mucinous lymph nodes (any size). Morphologically suspicious criteria included round shape, irregular border, and heterogeneous signal. If there was any obvious discordance of nodal staging between two radiologists, a senior radiologist with 21 years of experience in rectal imaging made the final decision.
Quantitative assessment
The whole tumor ROIs drawn on synthetic T2WI by two radiologists above were transferred to the quantitative maps using ITK-SNAP software. The mean T1, T2, and PD values were automatically obtained. Quantitative values acquired by the senior radiologist were used to assess the nodal staging of MRI-evaluated node-negative RC.
Statistical analysis
The inter-observer variability for image quality score and the tumor evaluation was assessed using kappa statistics (0.21–0.40, fair; 0.41–0.60, moderate; 0.61–0.80, good; 0.81–1.00, excellent). The intraclass correlation coefficient (ICC) was used to investigate the inter-observer agreement of the SNR, CNR and quantitative values (0.21–0.40, fair; 0.41–0.60, moderate; 0.61–0.80, good; 0.81–1.00, excellent) [5]. Continuous variables were compared using the independent samples t test or the Mann–Whitney U test according to the normality of data distribution. The Wilcoxon signed-rank test was adopted to compare the image quality scores, SNR and CNR between conventional and synthetic T2WI. With pathological results as the reference standard, the differences between conventional and synthetic T2WI in evaluating the T stage and EMVI were determined using the Mc-Nemar test. Receiver operating characteristic (ROC) curves were used to assess diagnostic performance of quantitative values in nodal staging. A two-sided p value < 0.05 was considered statistically significant. All statistical analyses were performed using SPSS 20.0 (IBM, Armonk, NY) and MedCalc 11.4 (MedCalc, Mariakerke, Belgium).
Results
Clinical characteristics
All 94 patients received radical surgical resection with pathological negative circumferential resection margin. The clinical characteristics of the patients are summarized in Table 2.
Table 2.
Clinical characteristics
| Characteristics | Number of patients |
|---|---|
| Mean age, years (range) | 57 (36–76) |
| Gender | |
| Male | 66 |
| Female | 28 |
| Surgical resection type | |
| Low anterior or anterior resection | 56 |
| Abdominoperineal resection | 33 |
| Hartman’s resection | 5 |
| Differentiation | |
| Well/Moderate | 59 |
| Poor | 35 |
| pT stage | |
| T1 | 6 |
| T2 | 33 |
| T3 | 44 |
| T4 | 11 |
| pN stage | |
| N0 | 59 |
| N1 | 20 |
| N2 | 15 |
| pEMVI | |
| Absent | 71 |
| Present | 23 |
| Tumor location | |
| Upper | 14 |
| Middle | 44 |
| Lower | 36 |
pEMVI, pathological extramural venous invasion
Image quality analysis
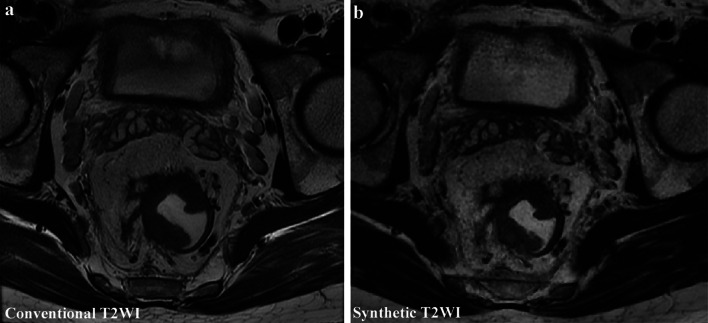
The image quality scores of conventional and synthetic T2WI are shown in Table 3. There were no statistically significant differences between conventional and synthetic T2WI in SNR, CNR, overall image quality, lesion conspicuity, and absence of motion artifacts (p = 0.058–0.978 for all comparison pairs, Table 3). Regarding the sharpness of the lesion edge, one observer’s evaluation showed that there was no significant difference between the conventional and synthetic T2WI (p = 0.127), while the other observer’s evaluation had a significant difference (p = 0.018). Representative images from one patient are shown in Fig. 4.
Table 3.
Comparison of image quality between conventional and synthetic T2WI
| Conventional T2WI | Synthetic T2WI | P value | |
|---|---|---|---|
| SNR | |||
| Observer 1 | 29.70 ± 12.17 | 26.85 (18.33, 41.24) | 0.978 |
| Observer 2 | 29.14 ± 11.94 | 25.85 (19.11, 41.06) | 0.717 |
| CNR | |||
| Observer 1 | 5.41 (2.00, 9.67) | 5.66 (2.89, 11.71) | 0.145 |
| Observer 2 | 4.99 (2.39, 9.37) | 5.27 (3.10, 11.84) | 0.058 |
| Overall image quality | |||
| Observer 1 | 4.63 ± 0.53 | 4.60 ± 0.54 | 0.592 |
| Observer 2 | 4.64 ± 0.51 | 4.57 ± 0.54 | 0.239 |
| Lesion conspicuity | |||
| Observer 1 | 4.69 ± 0.53 | 4.71 ± 0.52 | 0.617 |
| Observer 2 | 4.65 ± 0.56 | 4.66 ± 0.54 | 0.808 |
| Sharpness of the lesion edge | |||
| Observer 1 | 4.59 ± 0.50 | 4.69 ± 0.46 | 0.018* |
| Observer 2 | 4.64 ± 0.51 | 4.71 ± 0.46 | 0.127 |
| Absence of motion artifacts | |||
| Observer 1 | 4.68 ± 0.53 | 4.56 ± 0.58 | 0.069 |
| Observer 2 | 4.64 ± 0.55 | 4.53 ± 0.58 | 0.112 |
SNR and CNR following the normal distribution are expressed as mean ± standard deviation; otherwise, expressed as median (first quartile, third quartile). The subject image scores are expressed as means ± standard deviations
SNR, signal-to-noise ratio; CNR, contrast-to-noise ratio
*p < 0.05
Fig. 4.
Conventional and synthetic T2-weighted imaging (T2WI) from a 46-year-old male patient with rectal cancer. Synthetic T2WI provided similar image quality with the conventional T2WI. The patient was evaluated as T3 stage with extramural venous invasion using both conventional T2WI and synthetic T2WI
Evaluation of T stage and EMVI
Comparisons of the diagnostic accuracy of T stage and EMVI using conventional and synthetic T2WI are presented in Table 4. With pathological T stage as the reference standard, there were no significant differences in the diagnostic accuracy of T stage between conventional and synthetic T2WI from both observers (p = 0.375 and 0.625). Similarly, the differences in the accuracy for evaluating EMVI between conventional and synthetic T2WI were not statistically significant from both observers (p = 0.625 and 0.219).
Table 4.
Comparison of diagnostic accuracy of T stage and EMVI using conventional and synthetic T2WI
| Conventional T2WI | Synthetic T2WI | P value | |
|---|---|---|---|
| mrT stage | |||
| Observer 1 | 90.4% (85/94) | 85.1% (80/94) | 0.375 |
| Observer 2 | 91.5% (86/94) | 87.2% (82/94) | 0.625 |
| mrEMVI | |||
| Observer 1 | 81.9% (77/94) | 79.8% (75/94) | 0.625 |
| Observer 2 | 85.1% (80/94) | 78.7% (74/94) | 0.219 |
Numbers used to calculate percentages are in parentheses. mrT stage, T stage on T2-weighted imaging; mrEMVI, extramural venous invasion on T2-weighted imaging
Quantitative assessment
Among the 76 patients without suspicious metastatic lymph node on preoperative MRI, 56 were pathologically confirmed pN0 stage, and 20 were pN1-2. The differences in the T1, T2 and PD values between the pN0 and pN1-2 groups are listed in Table 5. The T2 values of the pN1-2 group were significantly lower than those of the pN0 group (p < 0.001). The T2 value demonstrated good diagnostic performance for predicting nodal staging of preoperatively node-negative RC with the area under the ROC (AUC), sensitivity, specificity, and accuracy of 0.854 (95% confidence interval, CI, 0.755 − 0.925), 90.0%, 71.4%, and 80.3%, respectively. The ROC curve is shown in Fig. 5.
Table 5.
Differences of quantitative parameters between the pN0 and pN1-2 groups
| Parameters | pN0 (n = 56) | pN1-2 (n = 20) | P value |
|---|---|---|---|
| T1 (ms) | 1492.47 (1437.33, 1619.47) | 1492.62 ± 173.93 | 0.273 |
| T2 (ms) | 95.15 ± 4.60 | 87.44 ± 5.66 | < 0.001* |
| PD (pu) | 61.45 (54.65, 65.91) | 60.44 ± 9.017 | 0.860 |
Data following the normal distribution are expressed as mean ± standard deviation. Otherwise, data are expressed as median (first quartile, third quartile)
PD, proton density
*p < 0.05
Fig. 5.
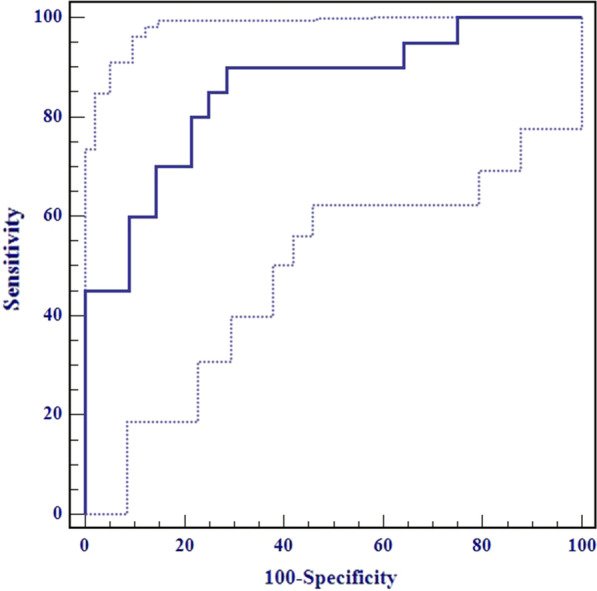
Receiver operating characteristics (ROC) curve of the T2 value for predicting nodal staging of preoperatively node-negative rectal cancer with the area under the ROC, sensitivity, specificity, and accuracy of 0.854, 90.0%, 71.4%, and 80.3%, respectively. The solid line represents the area under the ROC curve. The dotted line represents the 95% confidence interval
Interobserver reliability
The results of interobserver agreements are summarized in Additional file 1: Table S1. The SNR and CNR of conventional and synthetic T2WI all demonstrated excellent interobserver agreements (ICC > 0.9). The interobserver agreements of subjective image quality scores for conventional and synthetic T2WI were good or excellent (kappa values = 0.800–0.957). The interobserver agreements of mrT stage and mrEMVI were all excellent for conventional and synthetic T2WI (kappa values = 0.892 and 0.810 for mrT stage; 0.854 and 0.865 for mrEMVI). The T1, T2, and PD value demonstrated excellent interobserver agreement (ICC = 0.916, 0.953, and 0.973, respectively).
Discussion
We preliminarily investigated the feasibility of using synthetic MRI for the preoperative evaluation of RC. Synthetic T2WI provides similar image quality with the conventional T2WI, and achieves comparable diagnostic accuracy in assessing T stage and EMVI in RC patients. Additionally, T2 value demonstrated good diagnostic performance of nodal staging of MRI-evaluated node-negative patients.
The main advantage of synthetic MRI is the simultaneous generation of multiple images in a single scan, including quantitative relaxation mappings and synthetic morphological contrast-weighted images. The efficiency of synthetic MRI in the clinical application may depend on whether the total acquisition time is reduced and image quality is favorable. The acquisition time of synthetic MRI in our study was similar to that of T2 mapping in previous studies [9, 24], while synthetic MRI could simultaneously provide information of T1 mapping, PD mapping, T2WI, etc. Therefore, the scanning time of synthetic MRI is less than that with separately acquired contrast-weighted images and quantitative relaxation mappings.
Another primary issue is to ensure the image quality of synthetic MRI for further extensive clinical application. Our results demonstrated that the image quality scores of synthetic T2WI were comparable to that of conventional T2WI in terms of the SNR, CNR, overall image quality, lesion conspicuity, and the absence of motion artifacts. For the sharpness of the lesion edge, synthetic T2WI was rated superior to that of the conventional T2WI by one observer, which may be related to the observers’ interpreting experience. More experienced observers may be used to conventional images. Furthermore, there was overall good to excellent interobserver agreement among the image quality scores for both conventional and synthetic T2WI. Therefore, we preliminarily speculated that synthetic T2WI could achieve similar image quality to that of conventional T2WI.
To further confirm the clinical feasibility of synthetic MRI, we evaluated T stage and EMVI using synthetic T2WI and conventional T2WI. The diagnostic accuracy of EMVI by two observers was consistent with previous studies [25–27], as was the T stage [28–30]. Although the diagnostic accuracy with conventional T2WI was slightly higher than that with synthetic T2WI, the difference was not statistically significant. It may be due to the fact that the signal intensity of synthetic T2WI was generally lower than that of conventional T2WI [14, 31], and observers are more accustomed to the contrast and signal intensity of conventional T2WI. Therefore, we proposed that, with training and adaptation, synthetic T2WI might be as suitable for T stage and EMVI assessment of RC as conventional T2WI.
Our previous study confirmed the value of quantitative relaxation mapping in evaluating prognostic factors of RC, in which the potential of the T2 value for predicting RC nodal staging has been demonstrated [17]. Some RC patients with micro-nodal involvement may be underestimated as node-negative at initial MRI, so this study focused on these patients. A previous study established a nomogram based on clinical factors for predicting nodal staging in clinically node-negative RC patients with an AUC of 0.743 [32]. In our study, the T2 value demonstrated good diagnostic performance for predicting nodal staging of preoperatively node-negative RC with an AUC of 0.854 (95% CI 0.755–0.925), which was superior to the previous study. We speculated that the T2 value could provide additional information for improving nodal staging of MRI-evaluated node-negative patients, which was beneficial to correctly enroll RC patients for neoadjuvant treatment.
There were some limitations in this study. Firstly, this was a single-center study with a relatively small sample size. Further prospective multi-center studies with larger sample sizes are warranted to validate our preliminary foundings. Secondly, we only evaluated the image quality of T2WI generated by synthetic MRI and did not evaluate other images. At present, high resolution T2WI is mainly used in preoperative evaluation of RC, while the application value of other non-contrasted enhanced images such as T1WI is limited. Therefore, evaluating the image quality of other contrast-weighted images is of little clinical value. Finally, we did not evaluate all tumor characteristics including sphincter invasion, and quantitative analysis of lymph node was not performed in this preliminary study. These aspects will be included in further prospective studies.
Conclusions
Synthetic MRI may facilitate preoperative staging and EMVI evaluation of RC by providing synthetic T2WI and quantitative maps in one acquisition.
Supplementary Information
Additional file 1. Interobserver agreement of imaging quality score, mrT stage, and mrEMVI.
Abbreviations
- CI
Confidence interval
- CNR
Contrast-to-noise
- EMVI
Extramural venous invasion
- ICC
Intraclass correlation coefficient
- PD
Proton density
- RC
Rectal cancer
- ROC
Receiver operating characteristic
- ROI
Regions of interest
- SNR
Signal-to-noise ratio
- T2WI
T2-weighted imaging
Authors' contributions
LZ involved in methodology, data curation, and writing—original draft. ML involved in data curation and visualization. PW involved in software and formal analysis. YY involved in validation and investigation. HZ involved in supervision and writing—review and editing. XZ involved in conceptualization, supervision, and writing—review and editing. All authors read and approved the final manuscript.
Funding
There is no funding source.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This retrospective study was approved by our Institutional Review Board and the informed consent was waived.
Consent for publication
The authors of this manuscript consent for publication.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Hongmei Zhang, Email: 13581968865@163.com.
Xinming Zhao, Email: zhaoxinming@cicams.ac.cn.
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 3.Glynne-Jones R, Wyrwicz L, Tiret E, et al. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28:v22–v40. doi: 10.1093/annonc/mdx224. [DOI] [PubMed] [Google Scholar]
- 4.Benson AB, Venook AP, Al-Hawary MM, et al. Rectal cancer, version 2.2018, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2018;16:874–901. doi: 10.6004/jnccn.2018.0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li S, Liu J, Zhang F, et al. Novel T2 mapping for evaluating cervical cancer features by providing quantitative T2 maps and synthetic morphologic images: a preliminary study. J Magn Reson Imaging. 2020;52:1859–1869. doi: 10.1002/jmri.27297. [DOI] [PubMed] [Google Scholar]
- 6.Cui Y, Han S, Liu M, et al. Diagnosis and grading of prostate cancer by relaxation maps from synthetic MRI. J Magn Reson Imaging. 2020;52:552–564. doi: 10.1002/jmri.27075. [DOI] [PubMed] [Google Scholar]
- 7.Matsuda M, Kido T, Tsuda T, et al. Utility of synthetic MRI in predicting the Ki-67 status of oestrogen receptor-positive breast cancer: a feasibility study. Clin Radiol. 2020;75:391–398. doi: 10.1016/j.crad.2019.12.021. [DOI] [PubMed] [Google Scholar]
- 8.Adams LC, Bressem KK, Jurmeister P, et al. Use of quantitative T2 mapping for the assessment of renal cell carcinomas: first results. Cancer Imaging. 2019;19:35. doi: 10.1186/s40644-019-0222-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ge YX, Hu SD, Wang Z, et al. Feasibility and reproducibility of T2 mapping and DWI for identifying malignant lymph nodes in rectal cancer. Eur Radiol. 2020;31:3347–3354. doi: 10.1007/s00330-020-07359-7. [DOI] [PubMed] [Google Scholar]
- 10.Warntjes JB, Dahlqvist O, Lundberg P. Novel method for rapid, simultaneous T1, T2*, and proton density quantification. Magn Reson Med. 2007;57:528–537. doi: 10.1002/mrm.21165. [DOI] [PubMed] [Google Scholar]
- 11.Warntjes JB, Leinhard OD, West J, Lundberg P. Rapid magnetic resonance quantification on the brain: optimization for clinical usage. Magn Reson Med. 2008;60:320–329. doi: 10.1002/mrm.21635. [DOI] [PubMed] [Google Scholar]
- 12.Kumar NM, Fritz B, Stern SE, Warntjes J, Lisa CY, Fritz J. Synthetic MRI of the knee: phantom validation and comparison with conventional MRI. Radiology. 2018;289:465–477. doi: 10.1148/radiol.2018173007. [DOI] [PubMed] [Google Scholar]
- 13.Di Giuliano F, Minosse S, Picchi E, et al. Comparison between synthetic and conventional magnetic resonance imaging in patients with multiple sclerosis and controls. MAGMA. 2020;33:549–557. doi: 10.1007/s10334-019-00804-9. [DOI] [PubMed] [Google Scholar]
- 14.Tanenbaum LN, Tsiouris AJ, Johnson AN, et al. Synthetic MRI for clinical neuroimaging: results of the magnetic resonance image compilation (MAGiC) prospective, multicenter, multireader trial. AJNR Am J Neuroradiol. 2017;38:1103–1110. doi: 10.3174/ajnr.A5227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matsuda M, Tsuda T, Ebihara R, et al. Enhanced masses on contrast-enhanced breast: differentiation using a combination of dynamic contrast-enhanced MRI and quantitative evaluation with synthetic MRI. J Magn Reson Imaging. 2021;53:381–391. doi: 10.1002/jmri.27362. [DOI] [PubMed] [Google Scholar]
- 16.Arita Y, Takahara T, Yoshida S, et al. Quantitative assessment of bone metastasis in prostate cancer using synthetic magnetic resonance imaging. Invest Radiol. 2019;54:638–644. doi: 10.1097/RLI.0000000000000579. [DOI] [PubMed] [Google Scholar]
- 17.Zhao L, Liang M, Xie L, Yang Y, Zhang H, Zhao X. Prediction of pathological prognostic factors of rectal cancer by relaxation maps from synthetic magnetic resonance imaging. Eur J Radiol. 2021;138:109658. doi: 10.1016/j.ejrad.2021.109658. [DOI] [PubMed] [Google Scholar]
- 18.Zhao L, Liang M, Wang S, Yang Y, Zhang H, Zhao X. Preoperative evaluation of extramural venous invasion in rectal cancer using radiomics analysis of relaxation maps from synthetic MRI. Abdom Radiol (NY) 2021 doi: 10.1007/s00261-021-03021-y. [DOI] [PubMed] [Google Scholar]
- 19.Li XT, Sun YS, Tang L, Cao K, Zhang XY. Evaluating local lymph node metastasis with magnetic resonance imaging, endoluminal ultrasound and computed tomography in rectal cancer: a meta-analysis. Colorectal Dis. 2015;17:O129–O135. doi: 10.1111/codi.12909. [DOI] [PubMed] [Google Scholar]
- 20.Weiser MR. AJCC 8th edition: colorectal cancer. Ann Surg Oncol. 2018;25:1454–1455. doi: 10.1245/s10434-018-6462-1. [DOI] [PubMed] [Google Scholar]
- 21.He M, Xu J, Sun Z, et al. Prospective comparison of reduced field-of-view (rFOV) and full FOV (fFOV) diffusion-weighted imaging (DWI) in the assessment of insulinoma: image quality and lesion detection. Acad Radiol. 2020;27:1572–1579. doi: 10.1016/j.acra.2019.11.019. [DOI] [PubMed] [Google Scholar]
- 22.Smith NJ, Barbachano Y, Norman AR, Swift RI, Abulafi AM, Brown G. Prognostic significance of magnetic resonance imaging-detected extramural vascular invasion in rectal cancer. Br J Surg. 2008;95:229–236. doi: 10.1002/bjs.5917. [DOI] [PubMed] [Google Scholar]
- 23.Beets-Tan R, Lambregts D, Maas M, et al. Magnetic resonance imaging for clinical management of rectal cancer: updated recommendations from the 2016 European Society of Gastrointestinal and Abdominal Radiology (ESGAR) consensus meeting. Eur Radiol. 2018;28:1465–1475. doi: 10.1007/s00330-017-5026-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jung Y, Gho SM, Back SN, Ha T, Kang DK, Kim TH. The feasibility of synthetic MRI in breast cancer patients: comparison of T(2) relaxation time with multiecho spin echo T(2) mapping method. Br J Radiol. 2018;92:20180479. doi: 10.1259/bjr.20180479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim TH, Woo S, Han S, Suh CH, Vargas HA. The Diagnostic performance of MRI for detection of extramural venous invasion in colorectal cancer: a systematic review and meta-analysis of the literature. AJR Am J Roentgenol. 2019;213:575–585. doi: 10.2214/AJR.19.21112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bae JS, Kim SH, Hur BY, et al. Prognostic value of MRI in assessing extramural venous invasion in rectal cancer: multi-readers' diagnostic performance. Eur Radiol. 2019;29:4379–4388. doi: 10.1007/s00330-018-5926-9. [DOI] [PubMed] [Google Scholar]
- 27.Ale AH, Kirsch R, Razaz S, et al. Extramural venous invasion in rectal cancer: overview of imaging, histopathology, and clinical implications. Abdom Radiol (NY) 2019;44:1–10. doi: 10.1007/s00261-018-1673-2. [DOI] [PubMed] [Google Scholar]
- 28.Xu L, Zhang Z, Qin Q, Zhang C, Sun X. Assessment of T and N staging with MRI(3)T in lower and middle rectal cancer and impact on clinical strategy. J Int Med Res. 2020;48:1220728237. doi: 10.1177/0300060520928685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu ZH, Hu CH, Qian WX, Cao WH. Preoperative diffusion-weighted imaging value of rectal cancer: preoperative T staging and correlations with histological T stage. Clin Imaging. 2016;40:563–568. doi: 10.1016/j.clinimag.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 30.Xu L, Zhang C, Zhang Z, Qin Q, Sun X. Value of 3Tesla MRI in the preoperative staging of mid-low rectal cancer and its impact on clinical strategies. Asia Pac J Clin Oncol. 2020;16:e216–e222. doi: 10.1111/ajco.13368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Drake-Pérez M, Delattre B, Boto J, et al. Normal values of magnetic relaxation parameters of spine components with the synthetic MRI sequence. AJNR Am J Neuroradiol. 2018;39:788–795. doi: 10.3174/ajnr.A5566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou C, Liu HS, Liu XH, et al. Preoperative assessment of lymph node metastasis in clinically node-negative rectal cancer patients based on a nomogram consisting of five clinical factors. Ann Transl Med. 2019;7:543. doi: 10.21037/atm.2019.09.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Interobserver agreement of imaging quality score, mrT stage, and mrEMVI.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.