Abstract
Background
Radiomics is a promising tool for identifying imaging-based biomarkers. Radiomics-based models are often trained on single-institution datasets; however, multi-centre imaging datasets are preferred for external generalizability owing to the influence of inter-institutional scanning differences and acquisition settings. The study aim was to determine the value of preselection of robust radiomic features in routine clinical positron emission tomography (PET) images to predict clinical outcomes in locally advanced non-small cell lung cancer (NSCLC).
Methods
A total of 1404 primary tumour radiomic features were extracted from pre-treatment [18F]fluorodeoxyglucose (FDG)-PET scans of stage IIIA/N2 or IIIB NSCLC patients using a training cohort (n = 79; prospective Swiss multi-centre randomized phase III trial SAKK 16/00; 16 centres) and an internal validation cohort (n = 31; single centre). Robustness studies investigating delineation variation, attenuation correction and motion were performed (intraclass correlation coefficient threshold > 0.9). Two 12-/24-month event-free survival (EFS) and overall survival (OS) logistic regression models were trained using standardized imaging: (1) with robust features alone and (2) with all available features. Models were then validated using fivefold cross-validation, and validation on a separate single-centre dataset. Model performance was assessed using area under the receiver operating characteristic curve (AUC).
Results
Robustness studies identified 179 stable features (13%), with 25% stable features for 3D versus 4D acquisition, 31% for attenuation correction and 78% for delineation. Univariable analysis found no significant robust features predicting 12-/24-month EFS and 12-month OS (p value > 0.076). Prognostic models without robust preselection performed well for 12-month EFS in training (AUC = 0.73) and validation (AUC = 0.74). Patient stratification into two risk groups based on 12-month EFS was significant for training (p value = 0.02) and validation cohorts (p value = 0.03).
Conclusions
A PET-based radiomics model using a standardized, multi-centre dataset to predict EFS in locally advanced NSCLC was successfully established and validated with good performance. Prediction models with robust feature preselection were unsuccessful, indicating the need for a standardized imaging protocol.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13550-021-00809-3.
Keywords: Multi-centre, Radiomics, Lung cancer, PET, Robust
Background
Imaging is a fundamental tool in medicine and especially in personalized medicine [1]. Medical imaging in oncology is important for diagnosis, staging, treatment and response assessment. However, data extracted from radiological imaging have traditionally largely been qualitative, limiting the role of imaging in precision medicine. Only recently, imaging has been recognized as non-invasive biomarkers by extracting a large amount of data using mathematical image-analysis methodologies [2, 3]. Imaging-based biomarkers have therefore found their way into prognostic models to predict clinical outcome in investigative settings [4].
Radiomics refers to the extraction of a large number of quantitative features from medical images [5]. In addition to using standard imaging tumour characteristics, such as tumour volume, contrast enhancement, or maximum standardized uptake value (SUVmax), numerous other parameters, which may not be visible to the naked eye, may be extracted with radiomics [6, 7]. Radiomic features quantitatively describe different tissue characteristics, such as grey-value distribution or inter-pixel relationships. They can be categorized into shape, intensity, texture and filter-based (wavelet) features [5]. Radiomics has been applied to a variety of imaging, including computed tomography (CT), magnetic resonance imaging (MRI) and positron emission tomography (PET), with good prognostic power for different entities in a research setting [8–13].
Biomarkers are objective, quantifiable characteristics of a biological process [14]. Biomarkers are commonly seen as molecular markers, measured in biological samples, such as blood or tissue. However, medical parameters and indexes, such as heart rate or blood oxygen saturation, and imaging-based biomarkers can function as biomarkers following the same principles. Radiomics, as image-based biomarkers, has emerged as a novel approach in precision medicine, as it allows for thorough multi-modality image assessment accounting for intratumoural heterogeneity and change over time in a non-invasive, fast and affordable way by extracting a large number of phenotypic tumour characteristics from routine imaging [3, 15–18]. Radiomic features are currently being used in research studies, but before becoming part of clinical decision making, further validation and qualification are needed [19]. Addressing variability of PET imaging and radiomics methodology has been called for by several studies [20, 21].
One of the major strengths of radiomic biomarkers is that they can be extracted non-invasively, from routinely acquired imaging, which makes it cost-effective [5]. However, the protocols in these routinely acquired images have been mostly optimized for qualitative assessment. Therefore, image quality often varies among centres or among scanners, as well as over time. In PET imaging, several studies showed high instability rates of radiomic features depending for example on tumour motion, delineation, image reconstruction or image resampling [4, 22–27]. These robustness studies were based on phantom investigations or repeated imaging of the same patients. They are however often limited to investigation of one single cause of feature instability. Moreover, the robustness studies mostly focused on the stability of the features without its implication on the prognostic power of PET radiomics.
This study aims to investigate the value of preselection of robust radiomic features in clinical routine PET images, which are subject to the aforementioned variations, to predict clinical outcomes in locally advanced non-small cell lung cancer (NSCLC). If indeed prognostic models based on robust PET-based radiomic features alone are feasible, image acquisition standardization may not be necessary, allowing for wider implementation of the methodology and higher generalizability of the results. On the other hand, it might be that robust preselection removes features with high prognostic values and image standardization is therefore the preferred option. Patient-based datasets were used to evaluate three sources for radiomic feature instability: tumour delineation, attenuation correction and tumour motion. Consecutively, throughout stable features for tumour delineation, attenuation correction and tumour motion were tested for prediction of event-free survival (EFS) and overall survival (OS) using standardized image datasets. For comparability, two sets of models using standardized datasets were built, with the first set using all available features, independent of their robustness, and the second one using robust features only, to test the hypothesis whether EFS and OS prediction using robust features in locally advanced NSCLC using a real-life highly heterogenous dataset is feasible, or if image standardization is required.
Methods
Workflow
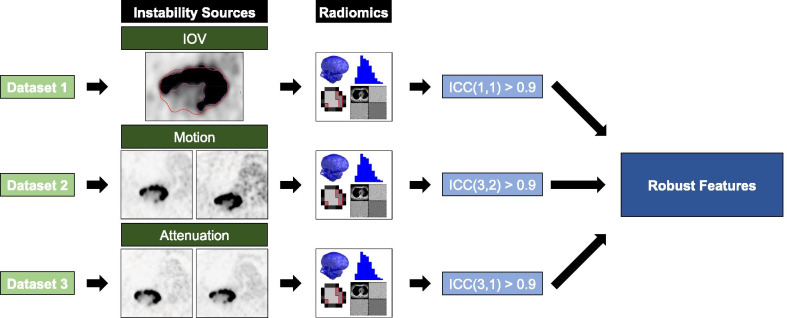
Robust radiomic features (delineation, attenuation correction, motion) were identified in different subsets of clinical [18F]fluorodeoxyglucose (FDG)-PET images (Figs. 1 and 2). Two predictive models, first using robust features only and second using all available features, were used for prediction of EFS and OS at 12, 18 and 24 months. For redundancy reasons, results were reported for 12- and 24-month outcomes only (18-month results are listed in Additional file 1: Supplement A1). Models were validated using an independent single-centre validation cohort. Finally, performance of all models was assessed.
Fig. 1.
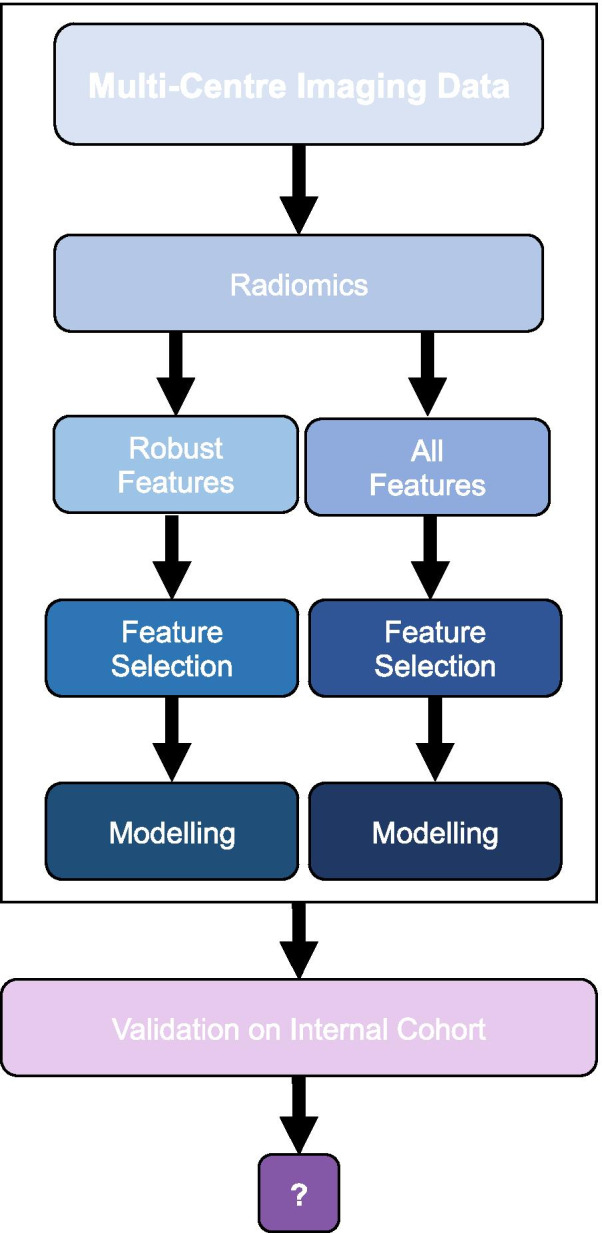
Workflow. Robust radiomic features (delineation, attenuation correction, 3D vs. 4D acquisition) were identified. Two prognostic models, using robust features only and using all available features, respectively, were constructed for 12- and 24-month event-free survival (EFS) and overall survival (OS) based on a standardized multi-centre imaging dataset. Model performance was validated using a separate internal validation cohort
Fig. 2.
Robustness studies. The influence of delineation variability or image was investigated using either a pair of scans for each patient as it is the case for the PET/CT/PET/MR and motion comparison, or different methods of defining the regions of interest (ROI), as applied for the delineation study. Radiomics calculation was performed on each individual ROI. Robust features were determined using the intraclass correlation coefficient (ICC)
Prognostic modelling
Studied cohorts
The training cohort (TC) was derived from a prospective Swiss multi-centre randomized phase III trial (SAKK 16/00) on IIIA/N2 NSCLC patients [28]. In this multi-modality treatment comparison trial, patients underwent neoadjuvant chemotherapy or chemoradiotherapy prior to surgery (43 vs. 36). Radiomic features of pre-treatment PET scans of primary tumours of ≥ 72 voxels (after resizing to 5.5 mm cubic voxels) were included in the TC (n = 79). Small tumours (< 72 voxels) were excluded to ensure meaningful wavelet feature calculations. In the validation cohort (VC), separate pre-treatment PET scans of 31 stage IIIA/N2 or stage IIIB NSCLC patients were included. Patients were treated with induction chemotherapy or chemoradiotherapy in curative intent (30 vs. 1) prior to surgery at the University Hospital Zurich (USZ). Initial datasets consisted of 103 and 38 patients, of which 24 and 7 cases were excluded due to small tumour size in the TC and VC, respectively. Median EFS was 13.3 and 16.3 months, and median OS was 55.6 and 53.6 months, for the TC and VC, respectively.
Ethics amendment approvals were received from all involved Swiss canton ethics committees and informed consent was obtained from all individual participants. Ethics amendments requesting inclusion of the current study were documented in Additional file 1: Supplement A2. Ethics board approval and written consent were obtained for the VC as well (KEK ZH 2018-02405).
Patients were staged according to the 6th edition of the TNM classification as defined in the SAKK 16/00 trial. The two outcomes of interest were EFS and OS. For the TC, clinical outcomes were defined according to the SAKK 16/00 trial, with EFS being time from randomization to relapse, progression, second tumour, or death, whichever occurred first, and OS being defined by death [28]. The same definitions were applied for the VC with the date of diagnosis being used instead of the date of randomization.
Imaging, delineation and radiomics
The analysis was performed based on pre-treatment [18F]FDG-PET/CT imaging. The TC imaging dataset consisted of imaging from different centres (n = 16). The VC dataset included 31 pre-treatment PET scans. The imaging datasets were standardized for tumour delineation, attenuation correction and tumour motion. Additional file 1: Supplement A3 lists technical details on the used PET scanners.
Primary tumours were contoured based on CT and PET images manually by a medical student and radiation oncology trainee using MIM VISTA (Version 6.7.9., MIM Software Inc., Cleveland, USA). The contours were checked for consistency by a senior radiation oncologist. Initial registration of the PET and CT scans was optimized manually. Contouring was based on the PET signal and CT findings. Images were resized to cubic voxels (5.5 mm) with linear interpolation. A fixed bin size of 0.25 SUV was used for texture calculation. A Hounsfield unit (HU) range of − 300 to 200 was used to exclude bone and lung tissue from the analysis based on CT intensities and PET/CT registration.
Robustness studies
Three potential sources of radiomic feature instability (tumour motion, attenuation correction and tumour delineation) were studied individually while two factors were kept constant in each dataset used for robustness studies. Each dataset consisted of 9–10 separate, single-centre IIIA/N2 or IIIB NSCLC patients. First, three delineation methods were investigated: manual delineation and semi-automated delineation (threshold based and gradient based). Contours were created in the MIM VISTA software (Version 6.7.9., MIM Software Inc., Cleveland, USA). Primary lesions were manually delineated using fused PET/CT images. For semi-automated segmentation, threshold and gradient tools in MIM VISTA were used. The threshold was adapted for individual patients and ranged from 27 to 41% of SUVmax [29]. Second, motion effects were evaluated using free-breathing 3D data acquisition and gated 4D phase acquisition. In the gated acquisition, the quiescent phase of the respiratory cycle was chosen for comparison as it was considered the most stable phase [4]. Third, attenuation correction was evaluated by comparing PET radiomic features from PET/CT and PET/MR scans of the same patient according to Vuong et al. [29]. The summary of imaging, reconstruction and delineation protocols for the three datasets is presented in Table 1.
Table 1.
Overview of image acquisition characteristics
| Robustness study | Delineation (3 methods) | Attenuation correction | Motion | ||
|---|---|---|---|---|---|
| CT based | MR based | Average | Gating | ||
| Number of patients | 9 | 9 | 10 | ||
| Scanner manufacturer | GE Healthcare, Waukesha | GE Healthcare, Waukesha | GE Healthcare, Waukesha | GE Healthcare, Waukesha | |
| Scanner model | Discovery 690 | Discovery 690 | SIGNA PET/MR | SIGNA PET/MR | SIGNA PET/MR |
| Reconstruction method | VPFXS | VPFXS | VPFXS | VPFXS | VPFXS |
| Attenuation correction | MR based: LAVA-flex pulse sequence | CT based | MR based: LAVA-Flex pulse sequence | MR based: LAVA-Flex pulse sequence | |
| Time delay between FDG injection and PET scan start (min) | 71.5–92.5 | 71.5–92.5 | 40.3–117.6 | 57.1–75.9 | 36.8–82.0 |
| Injected activity (MBq) | 181.2–252.3 | 181.2–252.3 | 136.2–259.3 | ||
| Acquisition type | 3D | 3D | 3D | 4D (phase-gated) | |
| Time per bed position (min) | 2 | 2 | 2 | 2 | 2 |
| Resolution (mm) | 2.73 × 2.73 × 3.27 | 2.73 × 2.73 × 3.27 | 2.34 × 2.34 × 2.78 | 2.34 × 2.34 × 2.78 | 2.34 × 2.34 × 2.78 |
| Delineation | Gradient-based threshold-based manual | Gradient based | Gradient based | Gradient based | Gradient based |
| Intraclass correlation coefficient (ICC) | ICC(1,1) | ICC(3,1) | ICC(3,2) | ||
PET image dataset acquisition characteristics including reconstruction and delineation protocols for the three datasets are listed
Radiomics calculation
Radiomics calculation was performed with an in-house developed radiomics software Z-rad implemented in Python programming language (Version 2.7.10). Resampling of the images to 5.5 mm cubic voxels was performed using linear interpolation. A fixed bin size of 0.25 standardized uptake value (SUV) was chosen. In total, 1404 radiomic features were calculated, i.e. shape (n = 18), intensity (n = 17), texture (n = 137) and wavelets (n = 1232) (for further details see https://medical-physics-usz.github.io/). Shape, intensity and texture feature definition were standardized to the image biomarker standardization initiative (IBSI) [18]. To compare the radiomic features within a certain robustness dataset, the intraclass correlation coefficient (ICC) was calculated [30]. Type of ICC used for each individual robustness study is listed in Table 1. An ICC larger than 0.9 was considered stable.
Statistical analysis
PET radiomics prognostic models were trained to predict EFS and OS at 12, 18 and 24 months as defined by the SAKK 16/00 trial protocol [28]. Models were trained separately using all 1404 features and robust features alone, referred to as standard and robust models, respectively. Principal component analysis (PCA) was performed to group correlated features. The Horn method [31] was used to select the number of retained components. Features were grouped based on their correlations to the principal component group. As a group surrogate, the feature with the largest area under receiver operating characteristics curve (AUC) in the univariable analysis was selected. Only features with a p value < 0.05 were considered. Final feature selection was performed in the multivariable logistic regression with backward selection of variables based on Akaike information criterion (AIC). Performance of the models was tested in fivefold cross-validation using the TC.
Per clinical endpoint (EFS, OS) and feature set (standard, robust), models performing best in the TC, defined as best trade-off between the largest average AUC and the smallest range of AUCs in the cross-validation folds, were validated in the independent VC. To further investigate effects of different robustness factors on the prognostic value PET radiomics, a set of features with AUC > 0.6 in both training and validation cohorts was selected (12-month EFS and 12-month OS). Within this set, the percentage of features robust against each of the 3 factors (tumour motion, attenuation and delineation) was reported separately. Model building, validation and comparison were performed using R (Version 3.5.3), with packages base, survival [32], survcomp [33], boot [34], pROC [35] and glmnet [36].
Results
Robustness studies
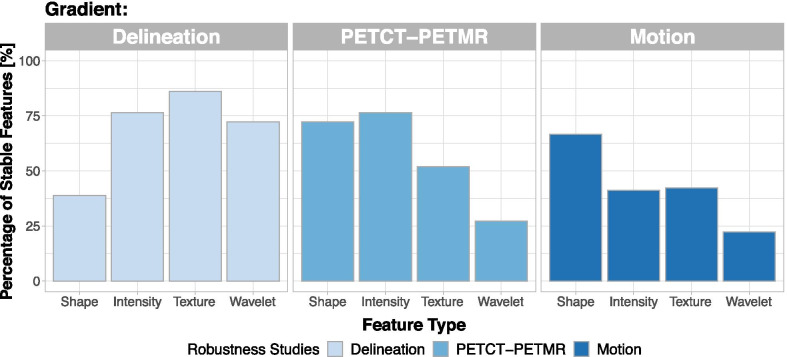
Robustness results are shown in Fig. 3. The majority of features (78%) were stable with regard to delineation differences. Attenuation correction method and motion had a stronger influence on feature stability. Thirty-one per cent of the features were not affected by attenuation correction, and 25% were stable regardless of motion. Altogether, only 13% of the features were robust in respect to all three studied factors. Shape features showed poor reproducibility in the delineation dataset, but they were robust against motion and attenuation correction. On the other hand, wavelet features were highly dependent on motion and attenuation correction, but they showed high stability in the delineation dataset. The overlap of the three studies is visualized with Venn diagrams (Additional file 1: Supplement A4). Overall, the overlap of robust features was small among all feature types, with shape and intensity features showing the highest overlap (> 35% of robust features stable), followed by texture (23.4%) and wavelet features (10.3%).
Fig. 3.
Robust radiomic features. Percentage of stable features for each of the robustness studies, i.e. the interobserver delineation variability, PET/CT/PET/MR, and respiratory motion are depicted. For each robustness study, the percentage of stable features is shown for the four feature types: shape, intensity, texture and wavelet. The feature type with the highest stability differed between the studies, with the lowest stability in the delineation study being shape, and wavelet in the PET/CT / PET/MR and motion studies
Prognostic modelling
Robust features
Univariable analysis identified no significant robust features for EFS at 12 and 24 months, and OS at 24 months. Only one significant robust feature was identified for OS at 12 months: HHL NGLDM dependence count entropy, a wavelet feature (Table 2). This 12-month OS model using robust features only was tested in the validation cohort, but did not perform well (AUC = 0.53, 95% confidence interval (95% CI) 0.26–0.81).
Table 2.
Results of the multivariable analysis
| Outcome | Radiomic features (feature type) | Robustness delineation (ICC) | Robustness attenuation correction (ICC) | Robustness motion (ICC) | AUC training (range) | AUC validation (95% CI) |
|---|---|---|---|---|---|---|
| 12-month EFS | LHL coefficient of variation (wavelet) | 0.79 | 0.81 | 0.00 | 0.73 (0.65–0.81) | 0.74 (0.55–0.93) |
| LHH neighbouring grey-level dependence matrix high dependence high grey-level emphasis (wavelet) | 0.91 | 0.35 | 0.92 | |||
| HLH skewness (wavelet) | 0.58 | 0.37 | 0.43 | |||
| 24-month EFS | HLH mean (wavelet) | 0.49 | 0.17 | 0 | 0.74 (0.58–0.94) | |
| LHH neighbouring grey-level dependence matrix high dependence high grey-level emphasis (wavelet) | 0.91 | 0.35 | 0.92 | |||
| 12-month OS | LLH skewness (wavelet) | 0.74 | 0.06 | 0.47 | 0.85 (0.6–1) | 0.67 (0.43–0.91) |
| HLL kurtosis (wavelet) | 0.93 | 0.93 | 0.75 | |||
| HHL skewness (wavelet) | 0.85 | 0.00 | 0.49 | |||
| LLH grey-level run length matrix short run high grey-level emphasis (wavelet) | 1.00 | 0.83 | 0.93 | |||
| 24-month OS | HLL skewness (wavelet) | 0.82 | 0.91 | 0.39 | 0.69 (0.57–0.8) | |
| 12-month OS robust preselection | HHL NGLDM dependence count entropy (wavelet) | 0.95 | 0.98 | 0.96 | 0.67 (0.46–0.85) | 0.53 (0.26–0.81) |
Results multivariable analysis for EFS and OS. Radiomic features were selected using backward selection. Good classification performances of models without robust preselection were observed for the training cohort (AUC = 0.69–0.85) and the validation cohort (AUC = 0.67–0.74). Performance of the robust model was moderate in training (AUC = 0.67) and weak in validation (AUC = 0.53)
The different impact of the robustness factors on the prognostic value of PET radiomics was observed. For 12-month EFS, 9 features showed AUC > 0.6 in both training and validation, but only 22% were stable against motion and delineation, and 0% were stable against attenuation. Similarly, for 12-month OS, 116 prognostic features were identified, from which 68% were stable against delineation and 19% were stable against attenuation and motion.
All features
Prognostic models using features irrespective of their robustness were identified for EFS and OS at all timepoints (Table 2). The final multivariable models consisted of 3 and 2 radiomic features for 12- and 24-month EFS, respectively. For 12- and 24-month OS, 4 and 1 significant radiomic features were identified, respectively.
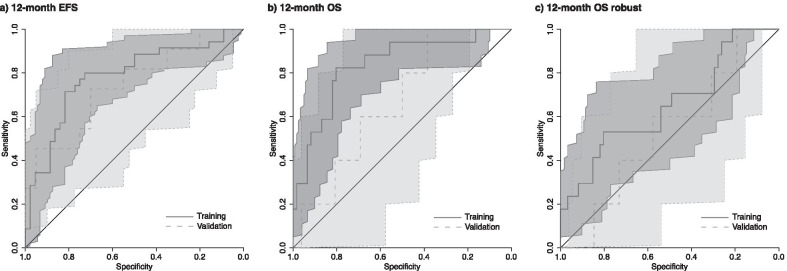
The best trade-off between the largest average AUC and smallest AUC range in cross-validation was observed for 12-month EFS (average AUC = 0.73) and 12-month OS models (average AUC = 0.85). Only the 12-month EFS model was successfully validated, resulting in AUC = 0.74 with a 95% CI of 0.55–0.93 (Table 2 and Fig. 4).
Fig. 4.
ROC curves. Receiver operating characteristic (ROC) curves with 95%-CI for a 12-month EFS, b 12-month OS and c robust 12-month OS for training (dark grey, solid lines) and validation cohorts (light grey, interrupted lines) are shown. Only the 12-month EFS model trained on the entire feature set without robust preselection was successful in the validation cohort
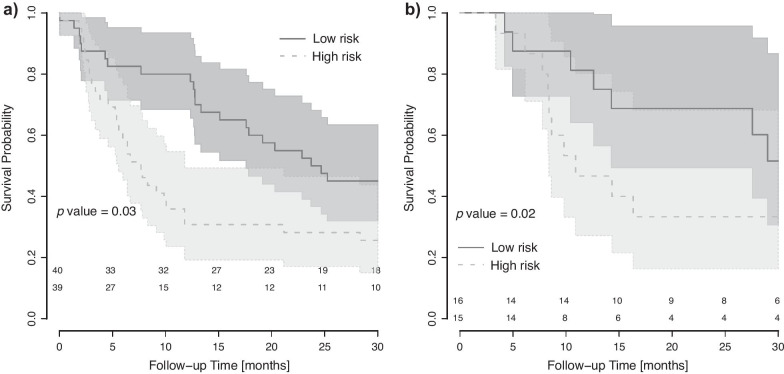
The probabilities from the 12-month EFS model were used to create Kaplan–Meier curves, based on the median in the TC (Fig. 5). The split was significant, both in the TC (g-rho test, p value = 0.03) and VC (p value = 0.02). The low-risk group had significantly longer median EFS, 8 versus 25 months in the TC and 11 versus 29 months in the VC.
Fig. 5.
Patient stratification based on 12-month EFS. Patient stratification into high risk (light grey, interrupted lines, 95%-CI in light grey) and low risk (dark grey, solid lines, 95%-CI in dark grey) was significant for 12-month EFS: a training cohort, p value = 0.03 and b validation cohort, p value = 0.02
Discussion
Event-free survival prediction of locally advanced NSCLC based on radiomics models was successfully performed using multi-centre clinical routine FDG-PET datasets and validated using an independent internal VC. The 12-month EFS model using LHL coefficient of variation (wavelet feature), LHH neighbouring grey-level dependence matrix high dependence high grey-level emphasis (wavelet feature) and HLH skewness (wavelet feature) resulted in the highest AUC in validation (AUC = 0.74). Increased LHL coefficient of variation as well as decreased LHH neighbouring grey-level dependence matrix high dependence high grey-level emphasis and HLH skewness were associated with no events at 12 months, suggesting that more heterogeneous FDG uptake pattern is associated with worse prognosis, which has been observed in other studies [37, 38]. Except for 12-month OS, models using preselected robust features only, could not be built using our feature selection scheme. The 12-month OS model with robust feature preselection yielded poor performance (AUC = 0.53). This indicates that robust feature preselection excluded a prohibitive large number of important radiomic features from the prognostic models. It can therefore be extrapolated that a clean, standardized dataset with similar imaging quality, especially in terms of attenuation correction and motion compensation, is required for generalizable and transferable EFS and OS prediction allowing for all available features to be potentially included.
While a majority of radiomics studies in locally advanced NSCLC assessed the prognostic value of CT-based features, our study is adding to the emerging body of the literature on PET-based models [4, 16, 22, 23, 37–44]. Although it is challenging to systematically compare NSCLC PET-based radiomics studies at this time due to different cohorts in terms of stage and treatment, as well a different radiomic, biological and clinical features tested, varied statistical methodology employed and common scarcity of model validation, some studies indicate a link between higher heterogeneity and worse prognosis [37, 38]. Interpretation of radiomic features as biomarkers may be challenging for clinicians, given their large number and not yet well-understood association with tumour characteristics and biological processes. Imaging variations not only affect the robustness of features but may also influence the association between imaging features and the underlying tumour activity distribution. In a phantom study, the association between PET radiomic features in the lung and underlying intratumoural heterogeneity was shown to be strongly influenced by image acquisition and PET imaging reconstruction [45]. While these results need additional validation for wavelet features, they point to an inherent problem in radiomics. In addition to modelling strategies, where our results indicate improved modelling performance with standardized imaging, feature association with tumour activity distribution can be maintained across all patients when using standardized imaging settings. On the other hand, simpler imaging-based biomarkers have already found their way into clinical practice, such as the SUVmax [4]. However, conventional PET-parameters, such as SUVmax, SUVpeak or SUVmean were not selected into our final models, as they showed lower prognostic value than more complex radiomic features. While this is in agreement with previous publications, other studies have shown some prognostic value of SUV descriptors [37, 38, 42, 43, 46]. In our study, a comprehensive number of radiomic features (n = 1404) were tested for their predictive value, including shape, texture, intensity and wavelet features. This is in contrast to other studies, where a more selective number of features was assessed [16, 38–40, 42–44]. This comprehensive approach allowed to identify the prognostic value of wavelet features, which is different from other studies, where mostly textural features were included in predictive models. The majority of PET-based outcome prediction studies in NSCLC used single-institution imaging, thereby minimizing heterogeneity within datasets. Ohri et al. tested 43 textural features, metabolic tumour volume (MTV) and SUVmax, using a multi-centre trial dataset, and identified one texture feature, SumMean, an indicator of homogeneity, as prognostic for OS among patients with large primary tumours [40]. The authors hypothesize that the strong association may indicate feature robustness in their multi-institutional dataset, thereby increasing generalizability [40]. A second multi-centre study by Arshad et al. claimed that slice thickness and matrix size did not significantly affect the predictive feature vector discovered (FVX), concluding that robustness of FVX permits this variable to be applied in multi-institutional studies [42]. In our study, standardized multi-centre PET imaging was used to allow for generalizable comparison of prediction models, using robust feature preselection and all available features.
After the initial success of radiomic models for prediction of different outcomes, robustness of the features started to be frequently discussed in the context of multi-centre validation [3, 4]. By its design, PET imaging characterizes tumour biology by the use of different radiopharmaceuticals, which makes it a perfect modality for prognosis assessment. However, an inherent complex nature of image acquisition (processes linked to radiotracer uptake, signal acquisition, image reconstruction and postprocessing) makes it a challenging modality to analyse with quantitative methods. Several initiatives exist worldwide to improve the comparability between images acquired in different institutions [47–51]. Rapid development of detector technology and reconstruction methods makes collection of large and homogenous datasets difficult. Recently, in the context of quantitative texture analysis, specialized PET radiomics phantoms have been investigated to depict heterogeneity of PET tracer uptake [4]. While phantoms may facilitate the analysis of a larger number of confounding factors in a single study, to date, most radiomics robustness studies have focused on a single factor only.
The secondary investigation focus of our study was the robustness of radiomic features in presence of multiple confounding factors (delineation variability, attenuation correction and tumour motion). Only 13% of the features were robust against all three studied factors. In the individual studies, a higher percentage of stable features was observed for delineation variability (78%) than for attenuation correction (31%) and motion difference (25%). This translated into a larger number of stable and prognostic features (AUC > 0.6) in the presence of interobserver delineation variability than different attenuation correction and motion compensation. However, the types of stable features differed considerably between robustness studies. For delineation variability, shape features were found to be the least stable ones, which is expected since shape is directly affected by different delineations. On the other hand, wavelet and texture features, which are less dependent on boundary definition, displayed a higher number of robust features. In the case of attenuation correction and motion studies, findings were the opposite. Shape features were less affected by these factors, but the number of stable texture and wavelet features was lower. Texture and wavelet features constitute a majority of the studied features, and thus, the overall percentage of features stable against attenuation correction and motion was low. Impact of delineation variability and motion on robustness of PET radiomic features was also studied by other groups and results were comparable to ours. For delineation variability, a study by Leijenar et al. reported robustness of 91% of the features for NSCLC patients [23]. The value is 13% higher than in our study; however, they used a less strict criterium of ICC > 0.8 [23]. A study conducted by Takeda et al. found 86% (ICC > 0.8) robust features for interobserver variability, but only seven radiomic features were investigated [39]. The impact of motion on feature robustness was studied by Oliver et al. finding that the percentage of stable features between respiration-gated images and averaged images over all phases was 26.2% [22]. This value is very close to the one obtained in our study; however, a detailed comparison is not possible as stability was not defined using ICC.
Strengths of our study include a prospective multi-centre training dataset, a large number of tested radiomic features, radiomic feature robustness assessment, model validation on a separate dataset and stratification by disease stage and treatment. This study adds to our recent publication on CT-based radiomics to predict OS of locally advanced NSCLC and shows that in contrast to CT-based radiomics, prognostic PET-based radiomics models require harmonized PET imaging, as robust feature preselection excluded a prohibitive large number of important radiomic features from the prognostic models [52]. Generalizability of our models is therefore restricted to patients who underwent similar PET imaging. The importance of using a clean imaging dataset was further illustrated by a recent phantom study by Ger et al. which found that most radiomic feature values showed good reliability when PET imaging protocol parameters were within clinically used ranges, but that interscanner variability was similar to interpatient variability, leading the authors to caution radiomics analyses on patients scanned on different PET scanners [53]. While our results support the need for harmonized PET imaging and we advocate for standardization of protocols, the impact assessment of different PET scanners was not the thrust of our study. Often heterogeneity by different PET scanners is unavoidable in a clinical setting. Another limitation of our study may be the restricted reproducibility of our results, as they depend on an in-house software. However, our software was benchmarked within the Image Biomarker Standardization Initiative (IBSI) [18]. While the TC consisted of prospectively acquired data following a strict trial protocol, the VC consisted of a retrospective dataset potentially allowing for introduction of patient selection bias. Further, while our study stratified for stage and surgical treatment, it did not include other clinical or molecular outcome predictors, such a smoking habits or epidermal growth factor receptor (EGFR) status, which may influence EFS and OS and could hypothetically improve the models’ predictive power. However, inclusion of clinical parameters was out of scope of this study, as we aimed to investigate the optimal modelling strategy for robust multi-centre PET radiomics models. Similarly, only the primary tumour was taken into account in our study, as it is commonly the case in radiomics studies and in accordance with the study objectives. While we were able to categorize the patient cohort into low-risk and high-risk groups, biological correlation of these groups and individual radiomic factors used in the prognostic models remain unknown. In addition, sample size is another limitation of our study, which is related to availability of data. However, methodological steps were taken to address potential related statistical issues such as using PCA to reduce dimensionality, excluding correlated features and evaluating model performance in cross-validation using the TC as well as testing the models in a completely separate VC as recommended by the Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis (TRIPOD) statement [54]. Additional data is needed to further validate the study findings. Further, although this study investigated more than one robustness factor, the set of factors was still limited. However, two factors (motion and attenuation correction) are linked to new developments in the field, motion correction (with the recent advent of data-driven deviceless gating techniques) and PET/MR hybrid devices, and thus make them relevant factors to be studied. A recent study showed that randomization of voxel intensities had an impact on model prognostication [55]. Since the goal of this study was to simulate a real-world environment, this aspect remained outside the scope of this work. Another limitation is the fact that all the robustness studies were conducted on different datasets with a small number of patients. This may be solved in the future with introduction of highly specialized heterogenous PET phantoms. Only one setting of radiomics calculation parameters was investigated (bin size, voxel size, HU range). The results of robustness studies might be influenced by this choice but considering the low number of patients in the robustness studies and moderate size of datasets in the prognostic modelling step, it was deemed important to limit the number of extracted features.
In conclusion, a PET-based radiomics model using multi-centre datasets to predict EFS in locally advanced NSCLC was successfully established. However, PET acquisition standardization is necessary, as prediction models using robust features alone could not be built or showed poor performance. Therefore, a standardized dataset with similar image acquisition and reconstruction is required for EFS prediction based on PET-based radiomics models.
Supplementary Information
Additional file 1.Supplement A1: 18-Month Results. Supplement A2: Training Cohort Ethics Board Amendment Documents. Supplement A3: PET-Specific Information from the Training Cohort and Validation Cohort. Supplement A4: Venn Diagrams of the Robustness Studies.
Acknowledgements
This work was supported by the Swiss National Science Foundation (310030_173303).
Abbreviations
- AIC
Akaike information criterion
- AUC
Area under the receiver operating characteristic curve
- CT
Computed tomography
- EFS
Event-free survival
- EGFR
Epidermal growth factor receptor
- FDG
Fluorodeoxyglucose
- FVX
Predictive feature vector discovered
- HU
Hounsfield unit
- IBSI
Image biomarker standardization initiative
- ICC
Intraclass correlation coefficient
- MRI
Magnetic resonance imaging
- MTV
Metabolic tumour volume
- NSCLC
Non-small cell lung cancer
- OS
Overall survival
- PCA
Principal component analysis
- PET
Positron emission tomography
- ROC
Receiver operating characteristic
- ROI
Regions of interest
- SUV
Standardized uptake value
- TC
Training cohort
- VC
Validation cohort
Authors' contributions
CO/FA contributed to data collection, analysis and interpretation, manuscript drafting, critical review and revision; DV/MB contributed to study design, data analysis and interpretation, manuscript drafting, critical review and revision; MH contributed to data interpretation and manuscript critical review; RF contributed to study design, data collection and manuscript critical review; LB/CS/EIE/MP/ST/SP/SH contributed to data collection and manuscript critical review; STL and MG contributed to study design, data interpretation and manuscript critical review; all authors read and approved the final manuscript.
Funding
This study was funded by Swiss National Science Foundation (310030_173303).
Availability of data and material
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request (data sharing agreements needed). In-house developed radiomics software Z-rad implemented in Python programming language (Version 2.7.10) was used. For further details, see https://medical-physics-usz.github.io/. Software is available upon request to the authors.
Declarations
Ethics approval and consent to participate
Ethics amendment approvals to include the current study were received from all involved Swiss canton ethics committees as documented in Supplement A2. Informed consent was obtained from all individual participants. Ethics board approval and written consent were also obtained for the validation cohort (KEK ZH 2018-02405).
Consent for publication
All listed authors consent to publication of this manuscript. No consent for publication of images needed as no details on individuals reported within the manuscript.
Competing interests
M.H. is a recipient of grants from GE Healthcare, grants for translational and clinical cardiac and oncological research from the Alfred and Annemarie von Sick Grant legacy and grants from the Artificial Intelligence in oncological Imaging Network by the University of Zurich.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Carol Oliveira and Florian Amstutz shared first co-authorship
References
- 1.Radiology ESo Medical imaging in personalised medicine: a white paper of the research committee of the European Society of Radiology (ESR) Insights Imaging. 2015;6(2):141–155. doi: 10.1007/s13244-015-0394-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gillies RJ, Kinahan PE, Hricak H. Radiomics: images are more than pictures, they are data. Radiology. 2016;278(2):563–577. doi: 10.1148/radiol.2015151169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lambin P, Leijenaar RTH, Deist TM, Peerlings J, de Jong EEC, van Timmeren J, et al. Radiomics: the bridge between medical imaging and personalized medicine. Nat Rev Clin Oncol. 2017;14(12):749–762. doi: 10.1038/nrclinonc.2017.141. [DOI] [PubMed] [Google Scholar]
- 4.Bogowicz M, Vuong D, Huellner MW, Pavic M, Andratschke N, Gabrys HS, et al. CT radiomics and PET radiomics: ready for clinical implementation. Q J Nucl Med Mol Imaging. 2019 doi: 10.23736/S1824-4785.19.03192-3. [DOI] [PubMed] [Google Scholar]
- 5.Aerts HJ, Velazquez ER, Leijenaar RT, Parmar C, Grossmann P, Carvalho S, et al. Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nat Commun. 2014;5:4006. doi: 10.1038/ncomms5006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yip SSF, Liu Y, Parmar C, Li Q, Liu S, Qu F, et al. Associations between radiologist-defined semantic and automatically computed radiomic features in non-small cell lung cancer. Sci Rep. 2017;7(1):3519. doi: 10.1038/s41598-017-02425-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cook GJR, Azad G, Owczarczyk K, Siddique M, Goh V. Challenges and promises of PET radiomics. Int J Radiat Oncol Biol Phys. 2018;102(4):1083–1089. doi: 10.1016/j.ijrobp.2017.12.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang Y, Liu Z, He L, Chen X, Pan D, Ma Z, et al. Radiomics signature: a potential biomarker for the prediction of disease-free survival in early-stage (I or II) non-small cell lung cancer. Radiology. 2016;281(3):947–957. doi: 10.1148/radiol.2016152234. [DOI] [PubMed] [Google Scholar]
- 9.Bogowicz M, Riesterer O, Ikenberg K, Stieb S, Moch H, Studer G, et al. Computed tomography radiomics predicts HPV status and local tumor control after definitive radiochemotherapy in head and neck squamous cell carcinoma. Int J Radiat Oncol Biol Phys. 2017;99(4):921–928. doi: 10.1016/j.ijrobp.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 10.Bogowicz M, Riesterer O, Stark LS, Studer G, Unkelbach J, Guckenberger M, et al. Comparison of PET and CT radiomics for prediction of local tumor control in head and neck squamous cell carcinoma. Acta Oncol. 2017;56(11):1531–1536. doi: 10.1080/0284186X.2017.1346382. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Y, Oikonomou A, Wong A, Haider MA, Khalvati F. Radiomics-based prognosis analysis for non-small cell lung cancer. Sci Rep. 2017;7:46349. doi: 10.1038/srep46349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tanadini-Lang S, Bogowicz M, Veit-Haibach P, Huellner M, Pauli C, Shukla V, et al. Exploratory radiomics in computed tomography perfusion of prostate cancer. Anticancer Res. 2018;38(2):685–690. doi: 10.21873/anticanres.12273. [DOI] [PubMed] [Google Scholar]
- 13.Thawani R, McLane M, Beig N, Ghose S, Prasanna P, Velcheti V, et al. Radiomics and radiogenomics in lung cancer: a review for the clinician. Lung Cancer. 2018;115:34–41. doi: 10.1016/j.lungcan.2017.10.015. [DOI] [PubMed] [Google Scholar]
- 14.Strimbu K, Tavel JA. What are biomarkers? Curr Opin HIV AIDS. 2010;5(6):463–466. doi: 10.1097/COH.0b013e32833ed177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arimura H, Soufi M, Kamezawa H, Ninomiya K, Yamada M. Radiomics with artificial intelligence for precision medicine in radiation therapy. J Radiat Res. 2019;60(1):150–157. doi: 10.1093/jrr/rry077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fried DV, Mawlawi O, Zhang L, Fave X, Zhou S, Ibbott G, et al. Stage III non-small cell lung cancer: prognostic value of FDG PET quantitative imaging features combined with clinical prognostic factors. Radiology. 2016;278(1):214–222. doi: 10.1148/radiol.2015142920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morin O, Vallieres M, Jochems A, Woodruff HC, Valdes G, Braunstein SE, et al. A deep look into the future of quantitative imaging in oncology: a statement of working principles and proposal for change. Int J Radiat Oncol Biol Phys. 2018;102(4):1074–1082. doi: 10.1016/j.ijrobp.2018.08.032. [DOI] [PubMed] [Google Scholar]
- 18.Zwanenburg A, Vallieres M, Abdalah MA, Aerts H, Andrearczyk V, Apte A, et al. The image biomarker standardization initiative: standardized quantitative radiomics for high-throughput image-based phenotyping. Radiology. 2020;295(2):328–338. doi: 10.1148/radiol.2020191145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park JE, Kim HS. Radiomics as a quantitative imaging biomarker: practical considerations and the current standpoint in neuro-oncologic studies. Nucl Med Mol Imaging. 2018;52(2):99–108. doi: 10.1007/s13139-017-0512-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hatt M, Tixier F, Pierce L, Kinahan PE, Le Rest CC, Visvikis D. Characterization of PET/CT images using texture analysis: the past, the present... any future? Eur J Nucl Med Mol Imaging. 2017;44(1):151–165. doi: 10.1007/s00259-016-3427-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nyflot MJ, Yang F, Byrd D, Bowen SR, Sandison GA, Kinahan PE. Quantitative radiomics: impact of stochastic effects on textural feature analysis implies the need for standards. J Med Imaging (Bellingham) 2015;2(4):041002. doi: 10.1117/1.JMI.2.4.041002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oliver JA, Budzevich M, Zhang GG, Dilling TJ, Latifi K, Moros EG. Variability of image features computed from conventional and respiratory-gated PET/CT images of lung cancer. Transl Oncol. 2015;8(6):524–534. doi: 10.1016/j.tranon.2015.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leijenaar RT, Carvalho S, Velazquez ER, van Elmpt WJ, Parmar C, Hoekstra OS, et al. Stability of FDG-PET radiomics features: an integrated analysis of test-retest and inter-observer variability. Acta Oncol. 2013;52(7):1391–1397. doi: 10.3109/0284186X.2013.812798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gallivanone F, Interlenghi M, D'Ambrosio D, Trifiro G, Castiglioni I. Parameters influencing PET imaging features: a phantom study with irregular and heterogeneous synthetic lesions. Contrast Media Mol Imaging. 2018;2018:5324517. doi: 10.1155/2018/5324517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shiri I, Rahmim A, Ghaffarian P, Geramifar P, Abdollahi H, Bitarafan-Rajabi A. The impact of image reconstruction settings on 18F-FDG PET radiomic features: multi-scanner phantom and patient studies. Eur Radiol. 2017;27(11):4498–4509. doi: 10.1007/s00330-017-4859-z. [DOI] [PubMed] [Google Scholar]
- 26.Altazi BA, Zhang GG, Fernandez DC, Montejo ME, Hunt D, Werner J, et al. Reproducibility of F18-FDG PET radiomic features for different cervical tumor segmentation methods, gray-level discretization, and reconstruction algorithms. J Appl Clin Med Phys. 2017;18(6):32–48. doi: 10.1002/acm2.12170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reuze S, Orlhac F, Chargari C, Nioche C, Limkin E, Riet F, et al. Prediction of cervical cancer recurrence using textural features extracted from 18F-FDG PET images acquired with different scanners. Oncotarget. 2017;8(26):43169–43179. doi: 10.18632/oncotarget.17856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pless M, Stupp R, Ris HB, Stahel RA, Weder W, Thierstein S, et al. Induction chemoradiation in stage IIIA/N2 non-small-cell lung cancer: a phase 3 randomised trial. Lancet. 2015;386(9998):1049–1056. doi: 10.1016/S0140-6736(15)60294-X. [DOI] [PubMed] [Google Scholar]
- 29.Vuong D, Tanadini-Lang S, Huellner MW, Veit-Haibach P, Unkelbach J, Andratschke N, et al. Interchangeability of radiomic features between [18F]-FDG PET/CT and [18F]-FDG PET/MR. Med Phys. 2019;46(4):1677–1685. doi: 10.1002/mp.13422. [DOI] [PubMed] [Google Scholar]
- 30.Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull. 1979;86(2):420–428. doi: 10.1037/0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- 31.Hu P, Wang J, Zhong H, Zhou Z, Shen L, Hu W, et al. Reproducibility with repeat CT in radiomics study for rectal cancer. Oncotarget. 2016;7(44):71440–71446. doi: 10.18632/oncotarget.12199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Therneau TM, Grambsch PM. Modeling survival data: extending the Cox model. New York: Springer; 2013. [Google Scholar]
- 33.Schroder MS, Culhane AC, Quackenbush J, Haibe-Kains B. survcomp: an R/bioconductor package for performance assessment and comparison of survival models. Bioinformatics. 2011;27(22):3206–3208. doi: 10.1093/bioinformatics/btr511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Davison AC, Hinkley DV. Bootstrap methods and their application. Cambridge: Cambridge University Press; 1997. p. 582. [Google Scholar]
- 35.Robin X, Turck N, Hainard A, Tiberti N, Lisacek F, Sanchez JC, et al. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinform. 2011;12:77. doi: 10.1186/1471-2105-12-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Friedman J, Hastie T, Tibshirani R. Regularization paths for generalized linear models via coordinate descent. J Stat Softw. 2010;33(1):1–22. doi: 10.18637/jss.v033.i01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kang SR, Song HC, Byun BH, Oh JR, Kim HS, Hong SP, et al. Intratumoral metabolic heterogeneity for prediction of disease progression after concurrent chemoradiotherapy in patients with inoperable stage III non-small-cell lung cancer. Nucl Med Mol Imaging. 2014;48(1):16–25. doi: 10.1007/s13139-013-0231-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cook GJ, Yip C, Siddique M, Goh V, Chicklore S, Roy A, et al. Are pretreatment 18F-FDG PET tumor textural features in non-small cell lung cancer associated with response and survival after chemoradiotherapy? J Nucl Med. 2013;54(1):19–26. doi: 10.2967/jnumed.112.107375. [DOI] [PubMed] [Google Scholar]
- 39.Takeda K, Takanami K, Shirata Y, Yamamoto T, Takahashi N, Ito K, et al. Clinical utility of texture analysis of 18F-FDG PET/CT in patients with Stage I lung cancer treated with stereotactic body radiotherapy. J Radiat Res. 2017;58(6):862–869. doi: 10.1093/jrr/rrx050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ohri N, Duan F, Snyder BS, Wei B, Machtay M, Alavi A, et al. Pretreatment 18F-FDG PET Textural features in locally advanced non-small cell lung cancer: secondary analysis of ACRIN 6668/RTOG 0235. J Nucl Med. 2016;57(6):842–848. doi: 10.2967/jnumed.115.166934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carvalho S, Leijenaar RT, Velazquez ER, Oberije C, Parmar C, van Elmpt W, et al. Prognostic value of metabolic metrics extracted from baseline positron emission tomography images in non-small cell lung cancer. Acta Oncol. 2013;52(7):1398–1404. doi: 10.3109/0284186X.2013.812795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Arshad MA, Thornton A, Lu H, Tam H, Wallitt K, Rodgers N, et al. Discovery of pre-therapy 2-deoxy-2-(18)F-fluoro-D-glucose positron emission tomography-based radiomics classifiers of survival outcome in non-small-cell lung cancer patients. Eur J Nucl Med Mol Imaging. 2019;46(2):455–466. doi: 10.1007/s00259-018-4139-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Krarup MMK, Nygard L, Vogelius IR, Andersen FL, Cook G, Goh V, et al. Heterogeneity in tumours: validating the use of radiomic features on (18)F-FDG PET/CT scans of lung cancer patients as a prognostic tool. Radiother Oncol. 2020;144:72–78. doi: 10.1016/j.radonc.2019.10.012. [DOI] [PubMed] [Google Scholar]
- 44.Ahn HK, Lee H, Kim SG, Hyun SH. Pre-treatment (18)F-FDG PET-based radiomics predict survival in resected non-small cell lung cancer. Clin Radiol. 2019;74(6):467–473. doi: 10.1016/j.crad.2019.02.008. [DOI] [PubMed] [Google Scholar]
- 45.Yang F, Young LA, Johnson PB. Quantitative radiomics: validating image textural features for oncological PET in lung cancer. Radiother Oncol. 2018;129(2):209–217. doi: 10.1016/j.radonc.2018.09.009. [DOI] [PubMed] [Google Scholar]
- 46.Carvalho S, Leijenaar RTH, Troost EGC, van Timmeren JE, Oberije C, van Elmpt W, et al. 18F-fluorodeoxyglucose positron-emission tomography (FDG-PET)-radiomics of metastatic lymph nodes and primary tumor in non-small cell lung cancer (NSCLC)—a prospective externally validated study. PLoS ONE. 2018;13(3):e0192859. doi: 10.1371/journal.pone.0192859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boellaard R, Oyen WJ, Hoekstra CJ, Hoekstra OS, Visser EP, Willemsen AT, et al. The Netherlands protocol for standardisation and quantification of FDG whole body PET studies in multi-centre trials. Eur J Nucl Med Mol Imaging. 2008;35(12):2320–2333. doi: 10.1007/s00259-008-0874-2. [DOI] [PubMed] [Google Scholar]
- 48.Bae H, Tsuchiya J, Okamoto T, Ito I, Sonehara Y, Nagahama F, et al. Standardization of [F-18]FDG PET/CT for response evaluation by the radiologic society of North America-quantitative imaging biomarker alliance (RSNA-QIBA) profile: preliminary results from the Japan-QIBA (J-QIBA) activities for Asian international multicenter phase II trial. Jpn J Radiol. 2018;36(11):686–690. doi: 10.1007/s11604-018-0780-x. [DOI] [PubMed] [Google Scholar]
- 49.Boellaard R. Need for standardization of 18F-FDG PET/CT for treatment response assessments. J Nucl Med. 2011;52(Suppl 2):93S–100S. doi: 10.2967/jnumed.110.085662. [DOI] [PubMed] [Google Scholar]
- 50.Kist JW, van der Vlies M, Hoekstra OS, Greuter HN, de Keizer B, Stokkel MP, et al. Calibration of PET/CT scanners for multicenter studies on differentiated thyroid cancer with (124)I. EJNMMI Res. 2016;6(1):39. doi: 10.1186/s13550-016-0191-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zukic D, Byrd DW, Kinahan PE, Enquobahrie A. Calibration software for quantitative PET/CT imaging using pocket phantoms. Tomography. 2018;4(3):148–158. doi: 10.18383/j.tom.2018.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vuong D, Bogowicz M, Denzler S, Oliveira C, Foerster R, Amstutz F, et al. Comparison of robust to standardized CT radiomics models to predict overall survival for non-small cell lung cancer patients. Med Phys. 2020;47(9):4045–4053. doi: 10.1002/mp.14224. [DOI] [PubMed] [Google Scholar]
- 53.Ger RB, Meier JG, Pahlka RB, Gay S, Mumme R, Fuller CD, et al. Effects of alterations in positron emission tomography imaging parameters on radiomics features. PLoS ONE. 2019;14(9):77. doi: 10.1371/journal.pone.0221877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Collins GS, Reitsma JB, Altman DG, Moons KG. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. BJOG. 2015;122(3):434–443. doi: 10.1111/1471-0528.13244. [DOI] [PubMed] [Google Scholar]
- 55.Welch ML, McIntosh C, Haibe-Kains B, Milosevic MF, Wee L, Dekker A, et al. Vulnerabilities of radiomic signature development: the need for safeguards. Radiother Oncol. 2019;130:2–9. doi: 10.1016/j.radonc.2018.10.027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1.Supplement A1: 18-Month Results. Supplement A2: Training Cohort Ethics Board Amendment Documents. Supplement A3: PET-Specific Information from the Training Cohort and Validation Cohort. Supplement A4: Venn Diagrams of the Robustness Studies.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request (data sharing agreements needed). In-house developed radiomics software Z-rad implemented in Python programming language (Version 2.7.10) was used. For further details, see https://medical-physics-usz.github.io/. Software is available upon request to the authors.