Abstract
Background
Despite the success of combined antiretroviral therapy (cART) in reducing viral load, a substantial portion of Human Immunodeficiency Virus (HIV)+ patients report chronic pain. The exact mechanism underlying this co-morbidity even with undetectable viral load remains unknown, but the transactivator of transcription (HIV-Tat) protein is of particular interest. Functional HIV-Tat protein is observed even in cerebrospinal fluid of patients who have an undetectable viral load. It is hypothesized that Tat protein exposure is sufficient to induce neuropathic pain-like manifestations via both activation of microglia and generation of oxidative stress.
Method
iTat mice conditionally expressed Tat(1–86) protein in the central nervous system upon daily administration of doxycycline (100 mg/kg/d, i.p., up to 14 days). The effect of HIV-Tat protein exposure on the well-being of the animal was assessed using sucrose-evoked grooming and acute nesting behavior for pain-depressed behaviors, and the development of hyperalgesia assessed with warm-water tail withdrawal and von Frey assays for thermal hyperalgesia and mechanical allodynia, respectively. Tissue harvested at select time points was used to assess ex vivo alterations in oxidative stress, astrocytosis and microgliosis, and blood-brain-barrier integrity with assays utilizing fluorescent-based indicators.
Results
Tat protein induced mild thermal hyperalgesia but robust mechanical allodynia starting after 4 days of exposure, reaching a nadir after 7 days. Changes in nociceptive processing were associated with reduced sucrose-evoked grooming behavior without altering acute nesting behavior; and in spinal cord dysregulated free radical generation as measured by DCF fluorescent intensity, altered immunohistochemical expression of the gliotic markers, Iba-1 and GFAP, and increased permeability of the blood-brain-barrier to the small molecule fluorescent tracer, sodium fluorescein in a time-dependent manner. Pretreatment with the anti-inflammatory, indomethacin (1 mg/kg/d, i.p), the antioxidant, methylsulfonylmethane (100 mg/kg/d i.p) or the immunomodulatory agent, dimethylfumarate (100 mg/kg/d p.o) thirty minutes prior to daily injections of doxycycline (100 mg/kg/d i.p) over 7 days significantly attenuated the development of Tat-induced mechanical allodynia.
Conclusions:
Collectively, the data suggests that even acute exposure to HIV-1 Tat protein at pathologically relevant levels is sufficient to produce select neurophysiological and behavioral manifestations of chronic pain consistent with that reported by HIV-positive patients.
Keywords: HIV, Tat, Pain, Allodynia, Microglia, Oxidative Stress
Graphical Abstract
Introduction
While neuropathic pain is attributed to lesions or diseases of the somatosensory system [1], precise causes vary widely, and the underlying pathophysiology is complex [2]. Despite the success of combined antiretroviral therapy (cART) in sustaining patients infected with Human Immunodeficiency Virus (HIV), 30% of HIV patients manifest detectable peripheral neuropathy and pain [3] such as tactile allodynia in the extremities that progresses over time, and nearly 60% of HIV-positive patients report chronic pain of some type [4], significantly decreasing the quality of life [5]. Reports suggest conventional analgesics may have reduced effect against HIV-related pain, complicating treatment [6,7]. Postmortem analysis of HIV-positive patients who suffered from chronic pain demonstrated no significant increase in viral load in the brain, cerebrospinal fluid, or blood compared to HIV positive patients who did not report pain prior to death [8]. Accordingly, the etiology of HIV-associated pain remains unknown, although the neurotoxic effects of HIV proteins, cytokines, and mitochondrial damage have each been implicated [6]. For instance, elevated levels of the HIV viral protein gp120 were found in HIV+ patients who reported pain prior to death [8], and earlier animal work demonstrated that intrathecal administration of gp120 induces mechanical allodynia and thermal hyperalgesia [9]. These manifestations of neuropathic pain are associated with increases in free radical generation, inflammation [10] and immune activation [11] that are exacerbated with opioid co-treatment [12]. Likewise, expression of HIV viral protein R (vpr) in immunodeficient mice also increased intracellular calcium, extracellular cytokine release and mechanical allodynia associated with spinal neuronal damage [13].
Another HIV regulatory protein, the transactivator of transcription (Tat) has garnered significant attention in its potential role in HIV-associated sensory neuropathy. Tat is profoundly neurotoxic and contributes to neuronal injury after release from intact HIV-infected glial cells [14]. Tat continues to be detected in cerebrospinal fluid samples from virally suppressed patients on cART [15,16], indicating the availability of this viral protein to mediate HIV-associated sensory neuropathy. Acute exposure to the Tat protein produces axonal injury, apoptosis, and hyperexcitability of dorsal root ganglion neurons in vitro [17,18], and exposure in vivo to Tat protein produces microgliosis in the spinal cord and reduced epidermal nerve fiber density in the periphery [17–19], all of which might contribute mechanistically to increased pain-like symptomology [19,20]. However, functional tests of mechanisms by which Tat protein increases pain responding remain understudied.
In this study, we evaluated Tat-induced pain-like behaviors, pathological markers associated with HIV-sensory neuropathy (oxidative stress and gliosis)[21], and examined possible functional mechanisms that might contribute to a potential pain state after relevant central exposure to HIV Tat protein [15,22] in male Tat-inducible (iTat) mice [23–25]. We hypothesized that exposure to Tat protein would increase oxidative stress and microglial activation in the spinal cord and reduce the spinal-cord-blood barrier integrity similar to reports observed at the supraspinal level [26], thereby promoting nociceptive behavior in models of evoked, spinally mediated reflexive measures of pain (thermal and mechanical nociception) [27] and supraspinally modulated, spontaneous pain-related depression of naturalistic behavior (nesting and grooming) indicative of general well-being [28–30].
Additional studies were conducted to evaluate potential underlying mechanisms of Tat-induced neuropathy. Specifically, daily prophylactic pretreatment during induction of Tat protein with methylsulfonylmethane (MSM), an antioxidant that was found to successfully attenuate Tat-induced increases in oxidative stress and depression-like behavior [24]; the anti-inflammatory, indomethacin, used previously to block impairment of Tat-induced sensorimotor gating [25]; or the immunomodulatory agent used to treat multiple sclerosis [31], dimethyl fumarate were administered to determine if these respective mechanisms mediated Tat-induced hyperalgesic responses.
Materials and Methods
Animals, Environmental and Drug Conditions
C57BL/6J mice weighing 22–26 g were obtained from Jackson Labs (Bar Harbor, ME, USA). The iTat bigenic mice, which contain a tetracycline sensitive GFAP promoter next to the Tat1–86 (Tat) gene [23], and the G-tg transgenic mouse lacking the Tat gene [24] were obtained from breeding colonies established at the University of Florida (Gainesville, Florida, USA). All mice were housed in a temperature and humidity-controlled room at the University of Florida vivarium. Animals remained on a regular light/dark (12:12) cycle with the light phase starting at 7 a.m. and ending at 7 p.m., and given food and water ad libitum except during experimental sessions. All testing was done during the light phase of the animals’ cycle but was limited mainly from 10:00 a.m. to 3:00 p.m. All studies were conducted in 8–12-week-old male mice.
Drug Treatment
All drugs were dissolved prior to injection, and administered within 0.25 mL per 25 grams of body weight. Doxycycline (10 mg/mL), methylsulfonylmethane (MSM, 10 mg/mL), and indomethacin (0.1 mg/mL) were dissolved in saline and administered through the intraperitoneal (i.p) route, while dimethyl fumarate (DMF, 10 mg/mL) was dissolved in 10% dimethyl sulfoxide (DMSO) and administered via oral gavage (p.o.). MSM, indomethacin, and DMF were administered 30 minutes prior to doxycycline treatment each day. Doxycycline was administered once daily at a dose (100 mg/kg/d, i.p.) established earlier to induce Tat protein [23,25].
Behavioral Testing
Thermal Hyperalgesia
Thermal hyperalgesia was assessed using the warm-water tail-withdrawal assay and acetone tests. Briefly, each mouse was tested for baseline tail-withdrawal latency prior to drug administration, and again 1, 4, 7 and 14 days later after doxycycline treatment. The 52°C warm-water tail-withdrawal assay was conducted as a measure of acute thermal nociception [27,32–34]. A maximum response time of 15 seconds for 52°C was utilized to prevent tissue damage. The 48°C warm-water tail-withdrawal assay was conducted as a measure of acute thermal hyperalgesia with a maximum response time of 30 seconds [35]. The acetone test was used to assess cold allodynia by applying acetone (50 μL) to the hind paw of the animal, recording the duration of licking [36]. Increased licking in response to acetone is a measure of cold allodynia. All data is reported in seconds ± SEM.
Mechanical Allodynia
Mechanical hyperalgesia was assessed in a separate cohort of animals using calibrated von Frey filaments (Stoelting, Wood Dale, IL) ranging from 0.4 to 6.0 grams of pressure. Mice stood on a metal mesh covered with a plastic dome during each testing session. Mice were first habituated for at least one hour prior to each testing session. During testing, the plantar surface of the hind paws was touched with different von Frey filaments with a bending force from 0.4 to 6.0 g until the threshold that induces paw withdrawal was found [37]. Unresponsive mice received a maximal score of 6.0 grams. Paw withdrawal threshold was determined as the average of 6 measurements per animal, with three measurements per paw. An experimentally induced decrease in paw withdrawal threshold is considered mechanical allodynia [37–40].
Nestling Displacement Test
In a separate cohort of animals, spontaneous nesting behavior was evaluated as a non-reflexive model of pain-depressed behavior in a separate cohort of animals [29]. A 5 cm X 5 cm Nestlet™ was divided into 6 pieces, and evenly distributed across a cage resembling the animal’s home cage. Group-housed mice were separated and placed into a new cage, and the number of nestlet pieces displaced was scored (0–6) after two hours. Depression of nest building is indicative of nociception and is sensitive to common analgesics such as morphine and ketoprofen [29].
Sucrose-Evoked Grooming Test
After nesting behavioral analysis, induction of self-grooming was initiated using 1 mL of 10 percent (v/v) sucrose placed on the dorsal lumbar region of the animal. Latency to initiate grooming on the animal’s flank, along with total grooming time was recorded in seconds for 10 minutes. Increased latency to groom and decreased total time spent grooming are indicative of pro-depressant behaviors that have been associated with chronic pain, and were assessed here to evaluate the affective component of pain [41].
Ex Vivo Analysis
Immunohistochemistry
In a separate cohort of animals (G-tg or iTat), 7- or 14-day doxycycline-treated mice were anesthetized with isoflurane, and perfused with 12 mL of PBS followed by 12 mL of 10% formalin (Fisher Scientific). Spinal cords were harvested and placed in 10% formalin at 4°C overnight. Afterwards, tissue was transferred and maintained in 30% sucrose at 4°C for at least 1 day until sectioned. The spinal cord was removed from sucrose, and the lumbar region was isolated using a razor blade and transfixed onto a specimen disk using O.C.T embedding media (Tissue-Tek VWR). A cryostat (Leica CM3050 S) was used to section tissue coronally at 40 μM thickness. Slices were collected and stored in cryoprotectant (30% Sucrose and 30% Ethylene Glycol in 0.1M Phosphate Buffer) at −20°C for future analysis. Slices were mounted on positively charged slides (SuperFrost Plus, Fisher Scientific), washed in potassium-PBS (KPBS) five times and then incubated in sodium citrate buffer (10 mM Tri-sodium citrate, pH 8.5) for an hour at 80°C. Afterwards, tissue was incubated in blocking buffer (2% NDS, 0.5% Triton, 0.3M Glycine, and 10% BSA in KPBS) for 1 hour at room temperature.
Subsequently, primary antibody (either Iba-1 (Abcam ab178846) at 1:1000 or GFAP (Abcam ab68428) at 1:500 diluted in blocking buffer) was applied to the tissue on the slide and left at 4°C overnight. After primary antibody incubation, slides were rinsed in KPBS five times and incubated in secondary antibody (Donkey Anti-Rabbit IgG H/L AlexaFluor 488, Abcam ab150073) for 2 hours at room temperature. Following secondary antibody incubation, slides were washed in KPBS five times, with a final rinse in KPB. Slides were dehydrated using serial applications of increasing concentrations of ethanol (60–80-100%) on the slide. Excess ethanol was removed by inverting the slide, and slides were immediately cover-slipped using DAPI mounting medium (SouthernBiotech). Images were obtained at 20X using an epifluorescent microscope and processed using ImageJ. To control for changes in baseline intensity changes across slides, tissue were processed in batches containing both control and Tat exposed tissue, with the fluorescent intensity reported as a percentage of the control (% control = [fluorescent intensity of doxycycline treated iTat mouse / fluorescent intensity of doxycycline treated G-tg mouse] * 100).
Oxidative Stress Measurement
Whole brain or spinal cord ROS/RNS levels were quantified ex vivo [24]. Briefly, mice were anesthetized with isoflurane, and brains and spinal cords from the same cohort of iTat or G-tg animals were harvested after 7 or 14 days of saline or doxycycline treatment (100 mg/kg/d, i.p), flash frozen in liquid nitrogen, and stored at −80°C for future analysis. Tissue was homogenized at 25 mg of tissue per mL of phosphate buffered saline (PBS, pH 7.2) with a QSonica sonicator. Homogenates (1 mL) were centrifuged for 5 minutes at 6000 rpm using a Corning® LSE™ Mini-Microcentrifuge, and supernatant was immediately assayed in the OxiSelect fluorescent kit (STA-347, Cell Biolabs, Inc.), with fluorescence detected using a Synergy H1 Multi-mode reader (BioTek Instruments, Inc.). Increased DCF fluorescence indicates an increase in free radical generation, a marker of oxidative stress. Arbitrary fluorescent units were recorded from brain and spinal cord in triplicate and were used for statistical comparisons.
Blood-Brain-Barrier Integrity
In a separate cohort of animals, untreated C57BL/6J mice, 7- or 14-day doxycycline treated iTat mice, and 14-day doxycycline treated G-tg mice were anesthetized with isoflurane. Animals were transcardially perfused with 50 μL of sodium fluorescein (2% w/v), a small fluorescent tracer (0.376 kDa) with low central nervous system permeability, 10 minutes prior to 12 mL of PBS perfusion [26]. Whole brains, spinal cords and hearts were harvested from each animal, flash frozen, and stored at −80°C for future analysis. Tissue was homogenized at 200 mg per 1 mL using the QSonica sonicator. Ten μL of homogenate was diluted into 190 μL of PBS, and directly measured for fluorescence (440/525 nm) using the Synergy H1 reader. Arbitrary fluorescent units were recorded from brain, spinal cord and heart of each animal in quadruplicate. The fluorescent units in the brain or spinal cord were normalized as a percent fluorescence to the fluorescent units measured from the heart where injection occurred. This normalization was conducted for each replicate, across all mice, and was used for statistical comparisons.
Statistical Analysis
All data are presented as mean ± SEM, with significance set at p < 0.05, denoted by the asterisk (*). All data were statistically evaluated with Prism 8.0 software (GraphPad Software, La Jolla, California, USA). Significant differences in behavioral data were assessed using either Student’s t-test, a Two-Way Repeated Measures ANOVA or a One-Way ANOVA as appropriate; ANOVA testing was followed by Bonferroni post-hoc analysis for significant pairwise comparisons. Significant differences in oxidative stress, blood brain-barrier integrity or glial fluorescent staining was measured using either a One or Two-Way ANOVA with Bonferroni’s post-hoc analysis when appropriate.
Results
HIV-1 Tat Expression Induces Select Alterations in Thermal Hyperalgesia and Mechanical Allodynia in Male Mice
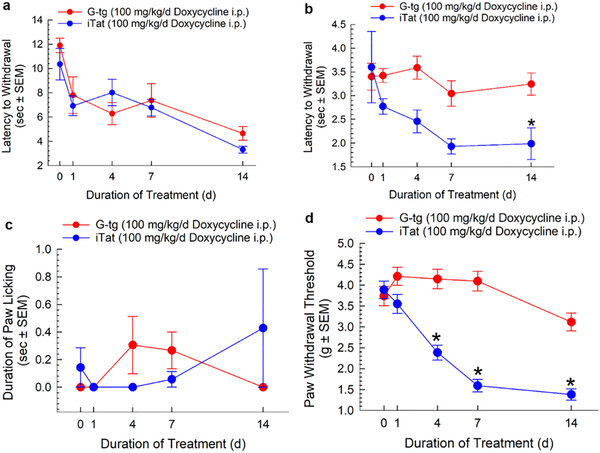
Male iTat or G-tg mice (n = 8 per group) displayed no significant differences between strains in baseline tail-withdrawal latencies at either 48°C (11.9 ± 0.60 vs. 10.4 ± 1.30, p > 0.82) or 52°C (3.40 ± 0.30 vs. 3.60 ± 0.80, p > 0.99), nor duration of paw licking after acetone application (0.00 ± 0.00 vs. 0.10 ± 0.10, p > 0.97) (Figure 1, day 0). Mice were then treated daily for 14 days with doxycycline (100 mg/kg/d, i.p) with measures of potential hyperalgesia monitored repeatedly across treatments. Two-way repeated measures ANOVA with Tat as a between subject factor and duration of treatment as a within subject factor indicated that Tat protein expression did not produce a significant main effect, as no differences in algesic response were observed between doxycycline treated iTat or G-tg mice using the 48°C tail-withdrawal test (Figure 1A; F1, 14 = 0.40, p = 0.53), nor in the acetone test (Figure 1C; F1, 14 = 0.01, p = 0.93). However, a significant Tat main effect in thermal hyperalgesia over the duration of treatment was observed using the 52°C water bath (Figure 1B; F1, 14 = 16.9, p < 0.01).
Figure 1.
Evaluation of HIV-Tat induced hyperalgesia and mechanical allodynia. G-tg and iTat mice were tested before, during and after 14 days of doxycycline treatment (n = 8 per group). Latency of tail-withdrawal after various durations of exposure to the Tat protein was assessed using a 48°C (A) and a 52°C (B) water bath. The duration of paw licking after acetone application (C), as well as mechanical allodynia (D) were assessed as well. Triangles represent mean and SEM. Two-Way Repeated Measures ANOVA with Bonferroni’s Post Hoc analysis was conducted. * = p < 0.05 vs. matching time point of control G-tg mice.
Post-hoc analysis using Tukey’s multiple comparisons test showed trending decreases in tail withdrawal latency for 52°C water in Tat-exposed mice after 4 and 7 days of doxycycline treatment (Figure 1B; p < 0.07), with a statistically significant reduction occurring at 14 days (p = 0.03). In contrast, mechanical allodynia developed quickly and robustly after exposure to Tat protein (Figure 1D; Tat main effect, F1, 81 = 99.7, p < 1.00*10−4), with statistically significant decreases in paw withdrawal threshold observed starting after 4 days of exposure (p < 1.00*10−4), and reaching a nadir after 7 days.
HIV-Tat Protein Exposure Reduces Evoked Grooming Behavior without Altering Nesting Behavior
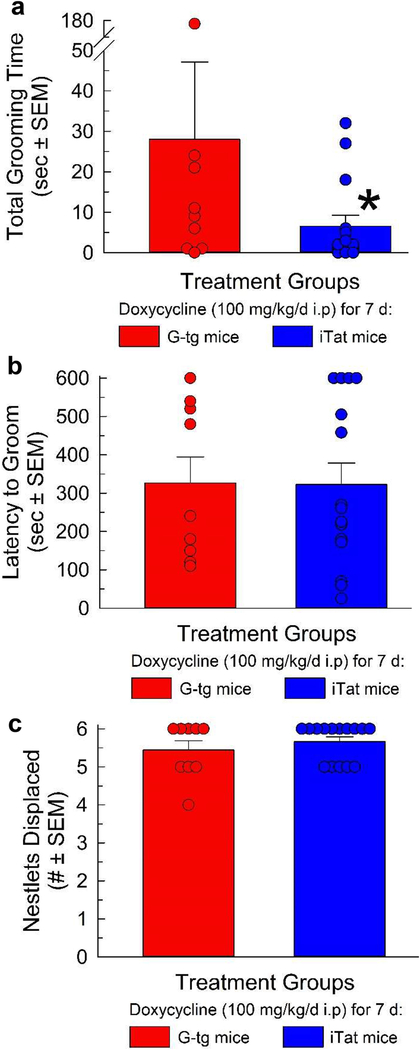
Following the demonstration of algesia and allodynia after 7 days’ Tat exposure, a separate cohort of G-tg (n = 9) and iTat mice (n = 15) treated for 7 days with doxycycline were exposed to 6 equally sized pieces of nestlet for 2 hours, with the number of nestlets displaced from their original position being measured. Immediately afterwards, 30% sucrose was applied to the dorsal rump of the animal and directed grooming towards this area was measured. A Student’s t-test indicated that 7 days of Tat protein exposure in vivo significantly reduced the total grooming time evoked by application of 30% sucrose on the dorsal coat as compared to control mice (Figure 2A, t (18) = 2.72; p = 0.01). No changes in latency to groom (Figure 2B, t (18) = 0.42; p = 0.97), or nestlet displacement (Figure 2C, t (18) = 0.89; p = 0.38) were otherwise detected during this period in this cohort of animals.
Figure 2.
HIV-Tat protein exposure reduces evoked grooming but not nesting behavior. Total grooming time (A), latency to first groom (B), and nesting behavior (C) were evaluated after 7 days of Tat protein exposure (n = 9 for G-tg and 15 for iTat group). Bars represent mean with SEM, with data points (circles) plotted representing the response of each individual animal. Student’s t-test was used to compare between these two groups for each behavioral outcome. * = p <0.05 vs. response of G-tg mice.
HIV-1 Tat Expression Modifies Markers of Gliosis, Oxidative Stress, and Blood-Brain-Barrier Integrity
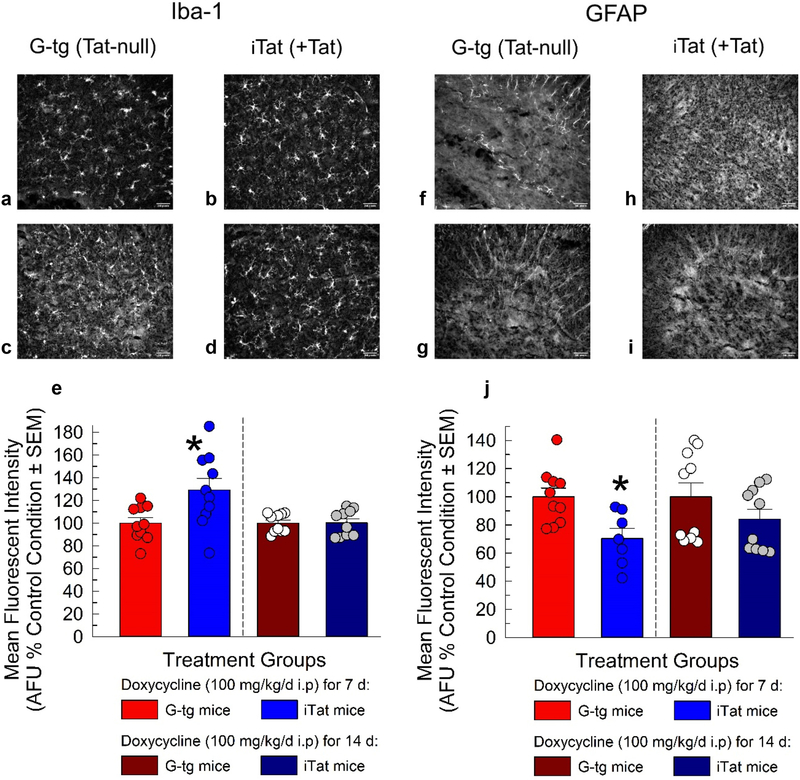
To determine if previously reported alterations in gliosis with exposure to the Tat protein at the supraspinal level [25] correlated to changes in gliosis at the spinal level, we examined the macrophage/microglia marker, ionized calcium-binding adapter molecule 1(Iba-1), and the astrocyte marker, glial acidic fibrillary protein (GFAP), in the lumbar spinal cord (Figure 3). Two-way ANOVA was conducted with Tat exposure and duration of exposure as between subject factors for both Iba-1 and GFAP marker intensity analysis. Tat displayed a significant main (F1, 36 = 5.86, p = 0.02) and interactive effect with duration of treatment (F1, 36 = 5.67, p = 0.02) on Iba-1 expression (Figure 3A–E). Specifically, only 7 days of Tat exposure produced significant increases in Iba-1 staining intensity (p = 3.00*10−3), with no significant effects observed after 14 days of protein exposure compared to controls (p = 0.99). GFAP intensity was significantly decreased with Tat protein expression (Figure 3G–J; main effect, F1, 33 = 8.08, p = 0.01), predominately after 7 days of exposure (p = 0.04), and with no statistically significant effects observed after 14 days (p = 0.28).
Figure 3.
Evaluation of HIV-Tat induced gliosis in the lumbar spinal cord. G-tg and iTat mice (n = 3 mice per group) were administered doxycycline (100 mg/kg/d i.p) for either 7 or 14 days and their spinal cords were harvested. Sections of the lumbar spinal cord were stained for either Iba-1 (A-D) or GFAP (F-I). Images were collected for 3 sections of the lumbar spinal cord per mouse and analyzed for mean fluorescent intensity (arbitrary fluorescent units) (E, H). Bars represent mean and SEM, with superimposed circles representing data collected from a unique tissue section. Two-way ANOVA with Bonferroni’s Post Hoc analysis was conducted. * = p < 0.05 vs. control G-tg time-point.
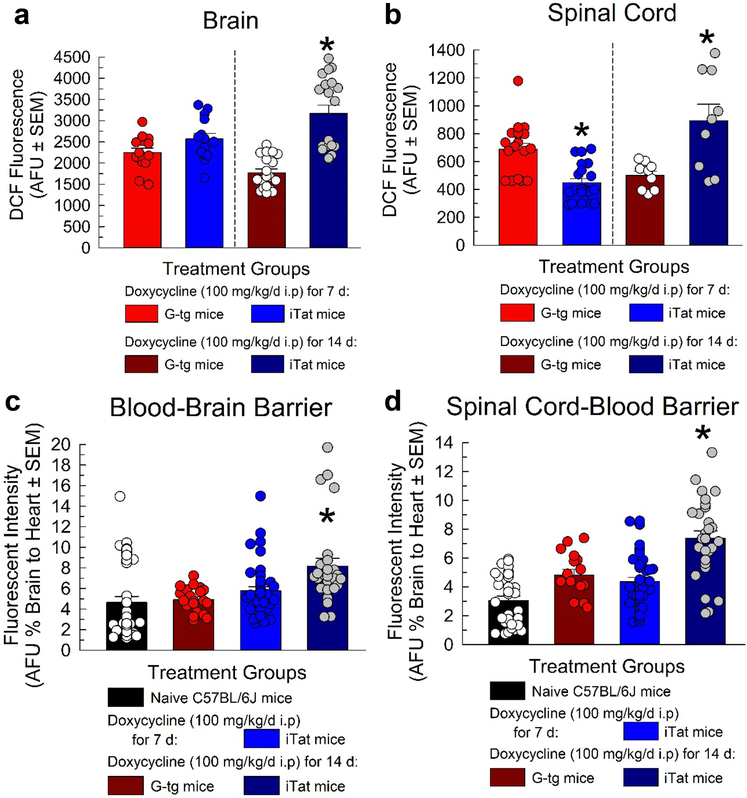
Oxidative stress was assessed from supernatant of both brain and spinal cord of iTat and G-tg mice pretreated 7 or 14 days with doxycycline (100 mg/kg/d, i.p) (n = 4 mice per group, with brain and spinal cord run in triplicate) utilizing the OxiSelect In Vitro ROS/RNS assay. Supernatant from each sample was exposed to a reduced probe, DCFH, where ROS/RNS in the sample oxidized DCFH into highly fluorescent DCF. Fluorescence intensity is directly proportional to ROS/RNS levels in the sample, with average fluorescence intensities for each group reported in Figure 4A and B. Two-way ANOVA with Tat and duration of treatment as between subject factors found that exposure to HIV-Tat protein produced a significant Tat main effect (F1, 62 = 34.4, p < 1.00*10−4) and interaction (Tat X duration of treatment; F1, 62 = 13.5, p = 5.00*10−4) on fluorescent intensities observed from brain supernatant.
Figure 4.
Evaluation of HIV-Tat induced oxidative stress and blood-brain-barrier dysregulation. DCF fluorescence (arbitrary fluorescent units) was used as a direct measure of ROS/RNS levels in supernatant from brain (A) and spinal cord (B) of G-tg (red) and iTat (blue) mice treated with 7 or 14 days of doxycycline (100 mg/kg/d i.p). In a separate cohort of animals treated in the same conditions, the penetration of the small fluorescent tracer, sodium fluorescein, a marker of blood-brain-barrier integrity was measured in the brain (C) and spinal cord (D) of each animal. Bars represent mean and SEM for each treatment group, with superimposed circles representing individual responses (including replicates) from subjects. One-Way ANOVA was utilized with post-hoc comparisons utilizing Bonferroni correction. * = p < 0.05 to respective control condition (doxycycline treated G-tg group).
In spinal cord supernatant, expression of the HIV Tat protein did not yield a significant main Tat effect (F1, 54 = 1.88, p = 0.18), however a significant [Tat X duration of treatment] interaction was observed (F1, 54 = 32.9, p = 1.00*10−4). Tukey’s post-hoc analysis revealed significant increases in ROS/RNS levels in the brain (Figure 4A) occurred only after 14 days of doxycycline treatment (p < 1.00*10−4). Interestingly, in the spinal cord (Figure 4B), 7 days of Tat protein exposure significantly decreased ROS/RNS levels (p = 3.00*10−4), while 14 days of Tat exposure significantly increased those levels compared to controls (p = 2.00*10−4).
To assess blood-brain-barrier integrity, brains and spinal cords were extracted from doxycycline treated G-tg and iTat mice or naïve C57BL/6J mice (n = 5–7 mice per group run in quadruplicate) that were first perfused with the small molecule fluorescent tracer, sodium fluorescein, homogenized, and then assayed for fluorescence. HIV Tat protein exposure displayed a significant main effect on brain (Figure 4C, F3, 124 = 7.40, p = 4.00*10−4) and spinal cord (Figure 4D, F3, 32 = 22.6, p < 1.00*10−4) fluorescence intensity, using One-way ANOVA. Post hoc analysis with Tukey’s Honest Significant Difference (HSD) demonstrated that when compared to naïve C57BL/6J mice, doxycycline administration in control G-tg mice did not display any significant differences in the % fluorescence detected in the brain or spinal cord normalized to the heart (p > 0.05). However, although 7 days of exposure to Tat protein did not increase the % fluorescence detected in brain (p = 0.77) or spinal cord (p = 0.87), 14 days of exposure increased it significantly (p < 0.01).
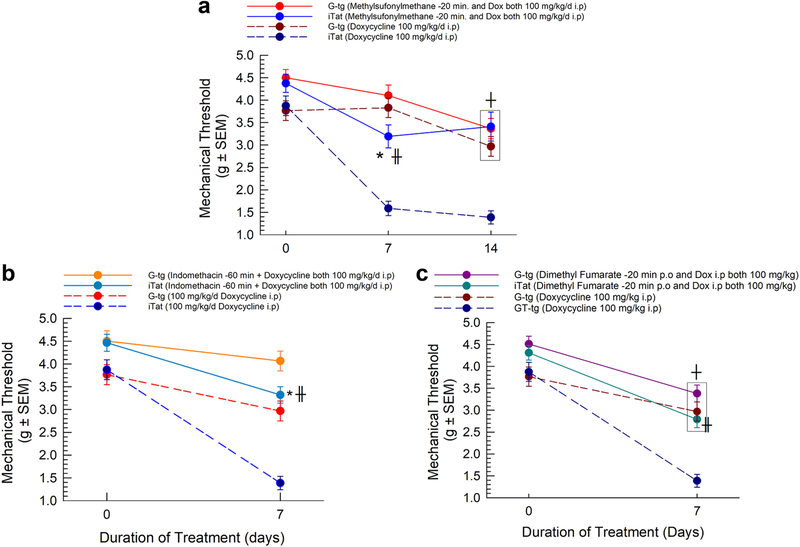
Pharmacological Reduction of Oxidative Stress and Inflammation Attenuates Tat-Induced Mechanical Allodynia
To determine whether the promotion of oxidative stress in the Tat-expressing animals functionally contributed to the robust development of Tat-induced mechanical allodynia, a separate cohort of animals (n = 8 per group) were treated with the antioxidant, methylsulfonylmethane (MSM, 100 mg/kg/d, i.p), prior to doxycycline each day for 14 days, and their mechanical threshold was assessed throughout (Figure 5A). Repeated measures Two-way ANOVA with treatment as a between-subject factor and duration of treatment as a within-subject factor was conducted on the results. Significant main effects of treatment (F3, 200 = 39.7, p < 1.00*10−4) and duration of treatment (F2, 400 = 53.8, p < 1.00*10−4) were observed, with a significant interaction [treatment X duration of treatment] (F6, 400 = 5.47, p < 1.00*10−4). Tukey post-hoc comparisons revealed that pretreatment with MSM significantly attenuated the development of mechanical allodynia after 7 (p < 1.00*10−4) and 14 (p < 1.00*10−4) days of Tat protein expression compared to doxycycline-treated iTat mice (Figure 5A). However, there was still a mild but statistically insignificant decrease in the mechanical allodynia threshold in the MSM-pretreated, Tat-expressing mice, compared to MSM-doxycycline treated G-tg mice (p = 0.09). Within-subject analysis of control G-tg mice indicated that doxycycline in and of itself began to have negative effects on paw withdrawal threshold after 14 days of administration (p < 2.00*10−3).
Figure 5.
Pharmacological interventions targeting oxidative stress/inflammation attenuate Tat-Induced mechanical allodynia. Pretreatment with methylsulfonylmethane (A), indomethacin (B) or dimethyl fumarate (C) were all able to significantly attenuate the development of mechanical allodynia after Tat protein exposure (n = 8 mice per group). Circles indicate mean and SEM. Two-Way Repeated Measures ANOVA was used. Post-hoc comparisons utilized Bonferroni correction. * = p < 0.05 to respective control condition, ╫ = p < 0.05 to respective iTat condition, and ┼ = p < 0.05 to respective baseline on day 0.
Similar to methylsulfonylmethane, separate cohorts of animals (n = 8 per group) were assessed for mechanical threshold before and after daily pretreatment with either the non-selective cyclo-oxygenase (COX) inhibitor, indomethacin (1 mg/kg/d, i.p), or the immunomodulatory nuclear factor erythroid 2-related factor 2 (Nrf2) inducer, dimethyl fumarate (100 mg/kg/d, p.o.), prior to doxycycline treatment (100 mg/kg/d i.p) for 7 days. Indomethacin (Figure 5B) or dimethyl fumarate (Figure 5C) pretreatment both produced a significant treatment effect on mechanical allodynia development (F2, 318 = 20.1 for indomethacin and F2, 171 = 29.7 for dimethyl fumarate, with p < 1.00*10−4 for both). Tukey’s post-hoc analysis demonstrated that pretreatment with either drug significantly reduced the development of mechanical allodynia after 7 days of Tat expression compared to iTat animals treated with doxycycline alone (p < 0.05).
Discussion
This study tested the hypothesis that HIV-1 Tat protein expression produces oxidative stress that contributes to the development of mechanical allodynia. Consistent with the findings of Wodarski and colleagues [19], where prolonged exposure to low levels of Tat protein produced mechanical but not thermal hyperalgesia in female mice, male animals presently expressing Tat protein displayed robust mechanical allodynia but not thermal hyperalgesia at 48°C. However, contrary to the findings of Wodarski [19], mice expressing Tat presently display a heightened sensitivity to thermal nociception at 52°C, and dynamic changes in microglia and astrocyte staining. Intriguingly, robust mechanical allodynia appears to coincide with only mild increases in sensitivity to thermal nociception at the 52°C temperature threshold, and modest changes in nesting behavior indicative of a pain-like state. Taken together, the data suggests Tat protein exposure induces both thermal hyperalgesia as well as mechanical allodynia in a time-dependent manner present after 7 days’ exposure. It is unlikely these responses are due to a loss of locomotor activity, as these mice have been characterized previously to possess normal baseline locomotion when expressing Tat protein [24,25]. Moreover, Wodarski and colleagues [19] demonstrated increased microglia profiles (number of microglia) only after 6 weeks of Tat expression, compared to 8 days herein, and without significant alteration in GFAP staining. In contrast, although utilizing slightly different methodology to measure staining intensity, we presently report increased Iba-1 and decreased GFAP fluorescent staining after 7 days of Tat protein exposure, which both normalize back to levels observed in doxycycline-treated control conditions after 14 days of exposure.
The decreases in GFAP staining in the spinal cord after 7 days of Tat exposure were not expected, although Wodarski and colleagues [19] likewise reported a statistically insignificant but appreciable decrease in GFAP staining after 8 days of exposure to low levels of Tat protein in female mice, suggesting a decrease in GFAP expression in astrocytes replicated across models and sexes. Since the induced Tat protein is generated in astrocytes in both mouse models, it is possible that early Tat protein exposure within astrocytes may promote apoptosis of said astrocytes that plateaus over exposure. Supporting this observation, the intensity of GFAP expression in the spinal cord has been demonstrated elsewhere to be inversely related to the display of me chanical allodynia after peripheral nerve injury; where lower levels of GFAP correlated with higher mechanical allodynia after chronic injury [43].
Similar to earlier cell culture studies showing Tat-induced activation of the microglial inflammasome and expression of inflammatory products such as IL-1β [44,45], present exposure to Tat protein induces dynamic changes in oxidative stress, another sequelae related to the inflammatory response. In contrast to the brain, where Tat protein produced initially negligible (at 7 days) but then substantial (at 14 days) enhancement of ROS/RNS levels, 7 days of exposure to Tat protein in the spinal cord was found presently to modestly lower ROS/RNS levels, and significantly increase oxidative stress only after 14 days. Lower levels of oxidative stress, although counter to the hypothesis, might be driven by the observed decrease in GFAP staining, and an increase in glutathione, a endogenous redox buffer that is upregulated in the prefrontal cortex of mice expressing the Tat protein for 7 days [24]. At 14 days of exposure, these compensatory mechanisms appear to be overcome, reducing the body’s ability to combat rampant ROS/RNS generation. Although not tested here, it could be speculated that Tat-induced mechanical allodynia is driven primarily by gliosis early on (e.g., 7 days of exposure), and then maintained at later time points (e.g., 14 days of exposure) by oxidative stress. Overall, there appears to be a dynamic shift across time in oxidative stress and inflammation markers, in that markers of gliosis are increased predominately after 7 days of Tat protein exposure when markers of oxidative stress are decreased, whereas after 14 days, levels of oxidative species become significantly higher while markers of gliosis return to control levels.
The contribution of oxidative stress to the development and maintenance of chronic pain may be germane to the treatment of HIV-associated sensory neuropathy. Substantial research has shown that various neuropathic pain models are sensitive to antioxidant treatment, that antioxidants do not develop tolerance or side effects characteristic of commonly prescribed drugs such as gabapentin or opiates, and antioxidants reduce central sensitization of nociception observed in the spinal cord after nerve injury [46–48]. Prophylactic intervention presently with the antioxidant methylsulfonylmethane (MSM) or other immunomodulatory agents was sufficient to attenuate Tat-induced mechanical allodynia.
Reactive oxygen species (ROS) generated from microglia activation after nerve injury in the spinal cord can produce mechanical allodynia, and is reportedly required for pro-inflammatory gene expression in primary microglia [49]. In addition to being regarded as a potent antioxidant, MSM also displays significant anti-inflammatory effects on both cytokine [50] and prostaglandin release [51], potentially contributing to the efficacy of the 7 day methylsulfonylmethane pretreatment. Consistent with the current findings, prior reports establish the potential cross-talk between inflammation and oxidative stress to promote neuropathic pain [46]. However, future studies are required to better understand the interaction of oxidative stress and inflammation in the development and maintenance of Tat-induced mechanical allodynia, as well as the possible contribution of mitochondrial dysfunction to these responses in mice exposed to Tat protein over time.
In a further contrast to present findings, a recent report found that Tat induction limited inflammatory paw swelling following administration of CFA to the paws of male, but not female mice [20], and reduced thermal and mechanical hypersensitivity following chronic nerve constriction injury in both sexes as compared to Tat(−) littermates [20]. These discrepancies in responses regarding the time-course of the development of Tat-induced mechanical allodynia and gliosis from previous reports [15,16,20] may be driven by the difference in the amount of Tat protein produced between the two mouse models and the sex of the subjects. Wodarski, Bagdas and colleagues utilized a model utilizing a single Tat transgene in Tat(+) mice [19, 20], while the iTat model in the current study utilizes multiple copies of the Tat transgene in the male iTat mouse [23], leading here to more protein being produced without the complications of an estrous cycle. These are important considerations given that the iTat mouse produces Tat protein levels in a similar manner to what is currently observed in the cerebrospinal fluid of virally suppressed patients [15,16], suggesting the alternative Tat(+) mouse model may perhaps experience lesser effects of the Tat protein on the nervous system.
Extending this discussion, it is clear that sex of the subject plays a critical role in nociceptive processing. Reduction of microglia through a variety of pharmacological and genetic manipulations rescued mechanical allodynia in spared nerve injured male, but not female, mice [42]. This raises the possibility that male mice experience hypersensitivity to pain from nerve injury in a microglia-dependent manner, while female mice may experience hypersensitivity in a microglia-independent manner. Although Tat-associated mechanical allodynia develops regardless of sex, further experiments are needed to determine if functional contributors to the mechanical allodynia observed by Wodarski, Bagdas and colleagues [19,20] are sex-dependent, as the literature cited above would suggest.
Another potential contributor to mechanical allodynia is the breakdown of the blood-brain-barrier, which could initially permit peripherally restricted immune cells and toxins into the central nervous system. In the present study, although mechanical allodynia developed rapidly, increased permeability at both the brain and spinal level were not observed until 14 days of Tat exposure, suggesting that the onset of mechanical allodynia is not derived from indirect effects such as the breakdown of the BBB. However, recent studies have indicated that BBB dysregulation occurs early on in HIV infection, and remains unresolved after a year of antiretroviral therapy [47].
Although exogenous [48,52–54] and inducible [26,55] HIV-Tat protein exposures have previously been reported to decrease BBB integrity, the current data suggests that duration of exposure may be critical. Acute and chronic pain states including inflammation, neuropathy, and migraine have been implicated with impaired blood-brain-barrier integrity and function [56,57]. Despite the presence of mechanical allodynia and dysregulated microglial and astrocytic marker expression in the brain and spinal cord after 7 days of Tat exposure, the absence of blood-brain-barrier dysfunction in the current study adds to the complexity between the intersection of pain, inflammation and blood-brain-barrier integrity.
In addition to the central nervous system, the peripheral nervous system is known to play a role in the development of HIV-sensory neuropathy. GFAP is expressed not just in brain and spinal cord astrocytes, but also in Schwann cells and satellite glia of the peripheral nervous system, where these cells have been implicated in various pain models [58–60]. Given that the iTat model used in the current study and the sister Tat(+) model used by Wodarski [19], Bagdas [20] and colleagues utilizes expression of Tat protein in GFAP-bearing cells, a possible alternative interpretation of the current findings is that the mechanical allodynia observed may arise, in part, from peripheral nociceptive mechanisms. Indeed, Tat mRNA has been detected in dorsal root ganglion and skin from female Tat(+) mice, suggesting the feasibility that Tat protein expressed there potentially modulates nociception at the peripheral level [19]. While this does not invalidate the current findings, future work is required to determine if Tat protein is expressed at various peripheral sites associated with nociception, and then to determine the mechanistic impact on nociceptive nerves by exposure to Tat protein, for instance with studies of nerve conduction and synaptic degeneration [11].
Conclusions
HIV Tat protein exposure produces hyperalgesia and depressed grooming that is associated with dynamic alterations in oxidative stress, gliotic marker expression and blood-brain-barrier integrity. Given their efficacy presently, antioxidants and anti-inflammatories like methylsulfonylmethane and indomethacin, respectively, and immunomodulatory agents like dimethyl fumarate may prove beneficial adjuvants for the treatment of HIV-associated sensory neuropathy.
Acknowledgements:
We thank Dr. Johnny J. He for the generous gift of the progenitor iTat mice.
Funding:
This research was supported by grants from the National Institute on Drug Abuse R01 DA039044 and R01 DA035714.
List of abbreviations
- cART
combinatorial anti-retroviral therapy
- HIV
human immunodeficiency virus
- Tat
transactivator of transcription
- iTat
Teton-GFAP inducible Tat mouse model
- DCF
oxidized dichlorofluorescein
- Iba-1
Ionized calcium binding adaptor molecule 1
- GFAP
glial fibrillary acidic protein
- MSM
methylsulfonylmethane
- NIH
National Institutes of Health
- DMF
dimethyl fumarate
- O.C.T
optimal cutting temperature
- KPBS
potassium phosphate buffer solution
- NDS
normal donkey serum
- BSA
bovine serum albumin
- PBS
phosphate buffer solution
- SEM
standard error of the mean
- ANOVA
analysis of variance
- i.p
intraperitoneal
- p.o
per os (by mouth)
- DCFH
reduced dichlorofluorescein
- ROS/RNS
reactive oxygen/nitrogen species
- HSD
honest significant difference
- COX
cyclo-oxygenase
- Nrf2
nuclear factor erythroid 2-related factor 2
- BBB
blood-brain-barrier
- gp120
glycoprotein 120
- VPR
viral protein R
- AFU
arbitrary fluorescent units
Footnotes
Competing interests:
The authors state they have no competing interests.
Declarations
Ethics Approval and Consent to Participate:
All experiments were conducted in accordance with the National Institutes of Health guide for the care and use of Laboratory animals (NIH Publications No. 8023, revised 1978). All procedures were preapproved and conducted in accordance with the Institutional Animal Care and Use Committee at the University of Florida as specified by the 2011 NIH Guide for the Care and Use of Laboratory Animals.
Consent for Publication:
Not applicable.
Availability of Data and Materials:
All data needed to evaluate the conclusions in the paper are present in the paper. Any raw data related to this manuscript may be requested from the authors.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- 1.Murnion BP: Neuropathic pain: current definition and review of drug treatment. Aust Prescr 2018, 41:60–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hughes RAC: Peripheral neuropathy. BMJ 2002, 324:466–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martin C, Solders G, Sonnerborg A, Hansson P: Painful and non-painful neuropathy in HIV-infected patients: an analysis of somatosensory nerve function. Eur J Pain 2003, 7:23–31. [DOI] [PubMed] [Google Scholar]
- 4.Keltner JR, Fennema-Notestine C, Vaida F, Wang D, Franklin DR, Dworkin RH, Sanders C, McCutchan JA, Archibald SL, Miller DJ, et al. : HIV-associated distal neuropathic pain is associated with smaller total cerebral cortical gray matter. J Neurovirol 2014, 20:209–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Phillips TJC, Brown M, Ramirez JD, Perkins J, Woldeamanuel YW, de C Williams AC, Orengo C, Bennett DLH, Bodi I, Cox S, Maier C, Krumova EK, Rice ASC: Sensory, psychological, and metabolic dysfunction in HIV-associated peripheral neuropathy: A cross-sectional deep profilng study. Pain, 2014, 155:1846–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gonzalez-Duarte A, Cikurel K, Simpson DM: Managing HIV peripheral neuropathy. Curr HIV/AIDS Rep 2007, 4:114–118. [DOI] [PubMed] [Google Scholar]
- 7.Phillips TJC, Cherry CL, Cox S, Marshall SJ, Rice ASC: Pharmacological treatment of painful HIV-associated sensory neuropathy: a systematic review and meta-analysis of randomized controlled trials. PLoS One 2010, 5:e14433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yuan SB, Shi Y, Chen J, Zhou X, Li G, Gelman BB, Lisinicchia JG, Carlton SM, Ferguson MR, Tan A, et al. : Gp120 in the pathogenesis of human immunodeficiency virus-associated pain. Ann Neurol 2014, 75:837–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Milligan ED, Mehmert KK, Hinde JL, Harvey LO, Martin D, Tracey KJ, Maier SF, Watkins LR: Thermal hyperalgesia and mechanical allodynia produced by intrathecal administration of the human immunodeficiency virus-1 (HIV-1) envelope glycoprotein, gp120. Brain Res 2000, 86:105–116. [DOI] [PubMed] [Google Scholar]
- 10.Milligan ED, O’Connor KA, Nguyen KT, Armstrong CB, Twining C, Gaykema RP, Holguin A, Martin D, Maier SF, Watkins LR: Intrathecal HIV-1 envelope glycoprotein gp120 induces enhanced pain states mediated by spinal cord proinflammatory cytokines. J Neurosci 2001, 21:2808–2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ru W, Liu X, Bae C, Shi Y, Walikonis R, Chung JM, Tan S-J: Microglia mediate HIV-1 gp120-induced synaptic degeneration in spinal pain neural circuits. J Neurosci 2019, 39:8408–8421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shi Y, Yuan S, Tang S-J: Reactive oxygen species (ROS) are critical for morphine exacerbation of HIV-1 gp120-induced pain. J Neuroimmune Pharmacol 2020. (online ahead of print; DOI: 10.1007/s11481-020-09951-6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Acharjee S, Noorbakhsh F, Stemkowski PL, Olechowski C, Cohen EA, Ballanyi K, Kerr B, Pardo C, Smith PA, Power C: HIV-1 viral protein R causes peripheral nervous system injury associated with in vivo neuropathic pain. FASEB J 2010, 24:4345–4353. [DOI] [PubMed] [Google Scholar]
- 14.Bagashev A, Sawaya BE: Roles and functions of HIV-1 Tat protein in the CNS: an overview. Virol J 2013, 10:358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Henderson LJ, Johnson TP, Smith BR, Reoma LB, Santamaria UA, Bachani M, Demarino C, Barclay RA, Snow J, Sacktor N, et al. : Presence of Tat and transactivation response element in spinal fluid despite antiretroviral therapy. AIDS 2019, 33 Suppl 2:S145–S157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson TP, Patel K, Johnson KR, Maric D, Calabresi PA, Hasbun R, Nath A: Induction of IL-17 and nonclassical T-cell activation by HIV-Tat protein. Proc Natl Acad Sci U S A 2013, 110:13588–13593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robinson B, Li Z, Nath A: Nucleoside reverse transcriptase inhibitors and human immunodeficiency virus proteins cause axonal injury in human dorsal root ganglia cultures. J Neurovirol 2007, 13:160–167. [DOI] [PubMed] [Google Scholar]
- 18.Chi X, Amet T, Byrd D, Chang KH, Shah K, Hu N, Grantham A, Hu S, Duan J, Tao F, et al. : Direct effects of HIV-1 Tat on excitability and survival of primary dorsal root ganglion neurons: possible contribution to HIV-1-associated pain. PLoS One 2011, 6:e24412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wodarski R, Bagdas D, Paris JJ, Pheby T, Toma W, Xu R, Damaj MI, Knapp PE, Rice ASC, Hauser KF: Reduced intraepidermal nerve fibre density, glial activation, and sensory changes in HIV type-1 Tat-expressing female mice: involvement of Tat during early stages of HIV-associated painful sensory neuropathy. Pain Rep 2018, 3:e654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bagdas D, Paris JJ, Carper M, Wodarski R, Rice ASC, Knapp PE, Hauser KF, Damaj MI: Conditional expression of HIV-1 tat in the mouse alters the onset and progression of tonic, inflammatory and neuropathic hypersensitivity in a sex-dependent manner. Eur J Pain 2020, 24:1609–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hao S: The Molecular and Pharmacological Mechanisms of HIV-Related Neuropathic Pain. Curr Neuropharmacol 2013, 11:499–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Langford D, Oh Kim B, Zou W, Fan Y, Rahimain P, Liu Y, He JJ: Doxycycline-inducible and astrocyte-specific HIV-1 Tat transgenic mice (iTat) as an HIV/neuroAIDS model. J Neurovirol 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim BO, Liu Y, Ruan Y, Xu ZC, Schantz L, He JJ: Neuropathologies in transgenic mice expressing human immunodeficiency virus type 1 Tat protein under the regulation of the astrocyte-specific glial fibriilary acidic protein promoter and doxycycline. Am J Pathol 2003, 162:1693–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McLaughlin JP, Paris JJ, Mintzopoulos D, Hymel KA, Kim JK, Cirino TJ, Gillis TE, Eans SO, Vitaliano GD, Medina JM, et al. : Conditional Human Immunodeficiency Virus Transactivator of Transcription Protein Expression Induces Depression-like Effects and Oxidative Stress. Biol Psychiatry Cogn Neurosci Neuroimaging 2017, 2:599–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paris JJ, Singh HD, Carey AN, McLaughlin JP: Exposure to HIV-1 Tat in brain impairs sensorimotor gating and activates microglia in limbic and extralimbic brain regions of male mice. Behav Brain Res 2015, 291:209218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leibrand CR, Paris JJ, Ghandour MS, Knapp PE, Kim WK, Hauser KF, McRae M: HIV-1 Tat disrupts blood-brain barrier integrity and increases phagocytic perivascular macrophages and microglia in the dorsal striatum of transgenic mice. Neurosci Lett 2017, 640:136–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deuis JR, Dvorakova LS, Vetter I: Methods Used to Evaluate Pain Behaviors in Rodents. Front Mol Neurosci 2017, 10:284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gaskill BN, Karas AZ, Garner JP, Pritchett-Corning KR: Nest building as an indicator of health and welfare in laboratory mice. J Vis Exp 2013:51012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Negus SS, Neddenriep B, Altarifi AA, Carroll FI, Leitl MD, Miller LL: Effects of ketoprofen, morphine, and kappa opioids on pain-related depression of nesting in mice. Pain 2015, 156:1153–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oliver VL, Thurston SE, Lofgren JL: Using Cageside Measures to Evaluate Analgesic Efficacy in Mice (J Am Assoc Lab Anim Sci 2018, 57:186–201. [PMC free article] [PubMed] [Google Scholar]
- 31.Bomprezzi R: Dimethyl fumarate in the treatment of relapsing-remitting multiple sclerosis: an overview. Ther Adv Neurol Disord 2015, 8:20–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paul D, Mana MJ, Pfaus JG, Pinel JP: Attenuation of morphine analgesia by the S2 antagonists, pirenperone and ketanserin. Pharmacol Biochem Behav 1988, 31:641–647. [DOI] [PubMed] [Google Scholar]
- 33.Reilley KJ, Giulianotti M, Dooley CT, Nefzi A, McLaughlin JP, Houghten RA: Identification of two novel, potent, low-liability antinociceptive compounds from the direct in vivo screening of a large mixture-based combinatorial library. AAPSJ 2010, 12:318–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fitting S, Scoggins KL, Xu R, Dever SM, Knapp PE, Dewey WL, Hauser KF: Morphine efficacy is altered in conditional HIV-1 Tat transgenic mice. Eur J Pharmacol 2012, 689:96–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Journigan VB, Mesangeau C, Vyas N, Eans SO, Cutler SJ, McLaughlin JP, Mollereau C, McCurdy CR: Nonpeptide small molecule agonist and antagonist original leads for neuropeptide FF1 and FF2 receptors. J Med Chem 2014, 57:8903–8927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Colburn RW, Lubin ML, Stone DJ, Wang Y, Lawrence D, D’Andrea MR, Brandt MR, Liu Y, Flores CM, Qin N: Attenuated cold sensitivity in TRPM8 null mice. Neuron 2007, 54:379–386. [DOI] [PubMed] [Google Scholar]
- 37.Cirino TJ, Eans SO, Medina JM, Wilson LL, Mottinelli M, Intagliata S, McCurdy CR, McLaughlin JP: Characterization of Sigma 1 Receptor Antagonist CM-304 and Its Analog, AZ-66: Novel Therapeutics Against Allodynia and Induced Pain. Front Pharmacol 2019, 10:678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bennett GJ, Xie YK: A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain 1988, 33:87–107. [DOI] [PubMed] [Google Scholar]
- 39.Cheng H-YM, Pitcher GM, Laviolette SR, Whishaw IQ, Tong KI, Kockeritz LK, Wada T, Joza NA, Crackower M, Goncalves J, et al. : DREAM Is a Critical Transcriptional Repressor for Pain Modulation. Cell 2002, 108:31–43. [DOI] [PubMed] [Google Scholar]
- 40.Xu M, Petraschka M, McLaughlin JP, Westenbroek RE, Caron MG, Lefkowitz RJ, Czyzyk TA, Pintar JE, Terman GW, Chavkin C: Neuropathic pain activates the endogenous kappa opioid system in mouse spinal cord and induces opioid receptor tolerance. J Neurosci 2004, 24:4576–4584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sellmeijer J, Mathis V, Hugel S, Li XH, Song Q, Chen QY, Barthas F, Lutz PE, Karatas M, Luthi A, et al. : Hyperactivity of Anterior Cingulate Cortex Areas 24a/24b Drives Chronic Pain-Induced Anxiodepressive-like Consequences. J Neurosci 2018, 38:3102–3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sorge RE, Mapplebeck JC, Rosen S, Beggs S, Taves S, Alexander JK, Martin LJ, Austin JS, Sotocinal SG, Chen D, et al. : Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nat Neurosci 2015, 18:1081–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Deumens R, Jaken RJ, Knaepen L, van der Meulen I, Joosten EA: Inverse relation between intensity of GFAP expression in the substantia gelatinosa and degree of chronic mechanical allodynia. Neurosci Lett 2009, 452:101–105. [DOI] [PubMed] [Google Scholar]
- 44.Chivero ET, Guo ML, Periyasamy P, Liao K, Callen SE, Buch S: HIV-1 Tat Primes and Activates Microglial NLRP3 Inflammasome-Mediated Neuroinflammation. J Neurosci 2017, 37:3599–3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Periyasamy P, Thangaraj A, Bendi VS, Buch S: HIV-1 Tat-mediated microglial inflammation involves a novel miRNA-34a-NLRC5-NFκB signaling axis. Brain Behav Immun 2019, 80:227–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim M, Kwak C, Yu N-K, Kaang B-K: NADPH oxidase 2-derived reactive oxygen species in spinal cord microglia contribute to peripheral nerve injury-induced neuropathic pain. Proceedings of the National Academy of Sciences 2010, 107:14851–14856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rahimy E, Li FY, Hagberg L, Fuchs D, Robertson K, Meyerhoff DJ, Zetterberg H, Price RW, Gisslen M, Spudich S: Blood-Brain Barrier Disruption Is Initiated During Primary HIV Infection and Not Rapidly Altered by Antiretroviral Therapy. J Infect Dis 2017, 215:1132–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.András IE, Pu H, Deli MA, Nath A, Hennig B, Toborek M: HIV-1 Tat protein alters tight junction protein expression and distribution in cultured brain endothelial cells. J Neurosci Res 2003, 74:255–265. [DOI] [PubMed] [Google Scholar]
- 49.Kim D, You B, Jo EK, Han SK, Simon MI, Lee SJ: NADPH oxidase 2-derived reactive oxygen species in spinal cord microglia contribute to peripheral nerve injury-induced neuropathic pain. Proc Nad Acad Sc, U S A 2010, 107:14851–14856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ahn H, Kim J, Lee MJ, Kim YJ, Cho YW, Lee GS: Methylsulfonylmethane inhibits NLRP3 inflammasome activation. Cytokine 2015, 71:223–231. [DOI] [PubMed] [Google Scholar]
- 51.Kim YH, Kim DH, Lim H, Baek DY, Shin HK, Kim JK: The anti-inflammatory effects of methylsulfonylmethane on lipopolysaccharide-induced inflammatory responses in murine macrophages. Biol Pharm Bull 2009, 32:651–656. [DOI] [PubMed] [Google Scholar]
- 52.Pu H, Tian J, Flora G, Lee YW, Nath A, Hennig B, Toborek M: HIV-1 Tat protein upregulates inflammatory mediators and induces monocyte invasion into the brain. Mol Cell Neurosci 2003, 24:224–237. [DOI] [PubMed] [Google Scholar]
- 53.Pu H, Hayashi K, Andras IE, Eum SY, Hennig B, Toborek M: Limited role of COX-2 in HIV Tat-induced alterations of tight junction protein expression and disruption of the blood-brain barrier. Brain Res 2007, 1184:333–344. [DOI] [PubMed] [Google Scholar]
- 54.András IE, Pu H, Tian J, Deli MA, Nath A, Hennig B, Toborek M: Signaling mechanisms of HIV-1 Tat-induced alterations of claudin-5 expression in brain endothelial cells. J Cereb Blood Flow Metab 2005, 25:1159–1170. [DOI] [PubMed] [Google Scholar]
- 55.Leibrand CR, Paris JJ, Jones AM, Masuda QN, Halquist MS, Kim WK, Knapp PE, Kashuba ADM, Hauser KF, McRae M: HIV-1 Tat and opioids act independently to limit antiretroviral brain concentrations and reduce blood-brain barrier integrity. J Neurovirol 2019, 25:560–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.DosSantos MF, Holanda-Afonso RC, Lima RL, DaSilva AF, Moura-Neto V: The role of the blood-brain barrier in the development and treatment of migraine and other pain disorders. Front Cell Neurosci 2014, 8:302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rosenberg GA: Neurological diseases in relation to the blood-brain barrier. J Cereb Blood Flow Metab 2012, 32:1139–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Luo DS, Lin R, Luo LL, Li QH, Chen T, Qiu RH, Li YQ: Glial plasticity in the trigeminal root entry zone of a rat trigeminal neuralgia animal model. Neurochem Res 2019, 44:1893–1902. [DOI] [PubMed] [Google Scholar]
- 59.Xie AX, Madayag A, Minton SK, McCarthy KD, Malykhina AP: Sensory satellite glial Gq-GPCR activation alleviates inflammatory pain via peripheral adenosine 1 receptor activation. Sci Rep 2020, 10:14181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Trias E, Kovacs M, King PH, Si Y, Kwon Y, Varela V, Ibarburu S, Moura IC, Hermine O, Beckman JS, Barbeito L: Schwann cells orchestrate peripheral nerve inflammation through the expression of CSF1, IL-34, and SCF in amyotrophic lateral sclerosis. Glia 2020, 68:1165–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]