Abstract
(a). Purpose.
A central neuropeptide mediator of ocular immune privilege is α-MSH, which can be used to therapeutically suppress experimental autoimmune uveitis (EAU). A part of α-MSH-regulation of immune activity is through its melanocortin 5 receptor (MC5r). One of the mechanisms of ocular immune privilege mediated by α-MSH is RPE suppression of phagolysosome activation associated with antigen presenting cell (APC) processing of antigen. Therefore, we examined the possible role of MC5r-expression in the recovery of RPE suppression of macrophage phagolysosome activation following α-MSH-treatment of EAU.
(b). Methods.
The conditioned media of cultured in situ RPE-eyecup from α-MSH-treated EAU wild-type and MC5r(−/−) mice were used to treat macrophages phagocytizing opsonized-pHrodoRed-bacterial bioparticles to assay for phagolysosome activation. In addition, the phagocytic activity of macrophages from MC5r(−/−) mice was assayed.
(c). Results.
The RPE from MC5r(−/−) mice that have recovered from EAU after α-MSH-therapy do suppress phagosome maturation in wildtype macrophages; but do not suppress phagosome maturation in MC5r(−/−) macrophages. In addition, α-MSH does not suppress phagolysosome activation in MC5r(−/−) macrophages, and the macrophages are highly enhanced in their phagocytic activity. Along with the enhanced macrophage activity was observed an increase in damage of the EAU MC5r(−/−) retinas.
(d). Conclusion.
The results demonstrated that treatment of EAU with α-MSH mediated recovery of RPE suppression of phagolysosome activation in macrophages and protected the retina from inflammatory damage. This was dependent on the expression of MC5r. Moreover, through MC5r the neuropeptide α-MSH potentially acts as a homeostatic moderator of phagosome-maturation within macrophages.
Keywords: Immune Privilege, Retinal Pigment Epithelial Cells, Macrophages, Phagocytosis, Experimental Autoimmune Uveitis, Melanocortin 5 Receptor
INTRODUCTION
The eye has adapted within its tissue microenvironment several mechanisms, which work to prevent and suppress the activation of inflammation that establishes immune privilege.1 The mechanisms of immune privilege are mediated in part by neuropeptides produced within the eye. These molecules mediate localized suppression of inflammation, prevent activation of effector T cells, and promote immune tolerance to antigen expressed within the eye. An essential mediator of these immunomodulating mechanisms is the neuropeptide alpha-melanocyte stimulating hormone (α-MSH).1–3 The neuropeptide α-MSH is constitutively expressed within the eye, and retinal pigment epithelial cells (RPE) are a source of α-MSH.4, 5 In the aqueous humor, α-MSH mediates aqueous humor suppression of effector T cell activation and induces T cell production of TGF-β that further suppresses immune cell activity.6 In the conditioned media of RPE-eyecups, α-MSH suppresses pro-inflammatory activity, promotes anti-inflammatory cytokine production, alters the maturation of phagosomes, and induces suppressor cell activity in macrophages.5, 7–12
The neuropeptide α-MSH is part of the highly conserved melanocortin family of molecules that includes Adrenocorticotropic hormone (ACTH), and the five G-protein-coupled melanocortin receptors.13–15 While ACTH binds all five melanocortin receptors (MCr), α-MSH binds all but MC2r.15, 16 The MC2r is exclusively expressed on the adrenal glands through which ACTH induces corticosteroid production. The melanocortin receptors MC1r, MC3r, and MC5r are expressed on immune cells, and cells of the retina.8, 17–23 The literature demonstrates that there is differential regulation of immune cell activity through the different melanocortin receptors. The neuropeptide α-MSH through MC1r and MC3r suppresses pro-inflammatory activity in activated macrophages.21, 24–27 Through MC5r, α-MSH promotes the induction of Treg cells, and induces suppressor APC with the capacity in an antigen-specific manner to mediate counter-conversion of effector CD4+ T cells into Treg cells.8, 18, 22, 28–31
Immune privilege is an evolutionary adaptation that protects the eye from the collateral damage of inflammation to its delicate and non-replicating light-gathering tissues and reduces susceptibility to autoimmune disease. Mice with experimental autoimmune uveitis (EAU), a well-studied rodent model of human endogenous uveitis, enter a stage of chronic retinitis that resolves without therapeutic interventions.32, 33 As the untreated EAU mice begin to resolve the retinitis there is an expansion of the suppressor APC in their spleens.8, 31 These suppressor APC counter-convert retinal-autoantigen effector T cells into inducible Treg cells, which provide long-term protection from reactivation of autoimmune uveitis.22, 30, 31 The expansion of the suppressor APC is dependent on the expression of MC5r, and without MC5r expression there are no detectible retinal-autoantigen specific Treg cells in the spleen post-EAU. When EAU mice are therapeutically treated with α-MSH there is an accelerated resolution of retinitis, and expansion of the suppressor antigen presenting cells (APC) in the spleen.8, 31 Moreover, α-MSH-treated EAU mice have well preserved retinal structures, and are resistant to re-inducing EAU.18, 34
When treated with α-MSH, EAU mice with MC5r knocked-out (MC5r(−/−)) have an accelerated resolution of EAU, but without the post-EAU suppressor APC, and without the induction of protective inducible-Treg cells in the spleen.22, 30, 31, 33 This suppression of uveitis by α-MSH-treatment is presumably the anti-inflammatory activity mediated by α-MSH through MC1r and MC3r. However, α-MSH-therapy in EAU MC5r(−/−) mice had less preservation of retinal structures.34 Recent reports have demonstrated that α-MSH promotes retinal cell survival through MC1r and MC5r.19, 20, 35 Also, the MC5r(−/−) mice are highly susceptible to an immediate and severe reactivation of EAU.18
Our previous findings showed that RPE through its production of α-MSH regulates phagocytic maturation within APC that can change with disease.11 This regulation is important since phagocytic activity is linked to both the induction of inflammation and the processing and presentation of antigen, of the innate and adaptive immune interface.36 Phagocytes (macrophages, dendritic cells, and microglial cells) phagocytize proteins, dead cell material, and toxic molecules to prevent them from adversely affecting tissue structure and cell survival. As the phagosome matures it fuses with the lysosome. Within the acidic environment of the activated phagolysosome there is an efficient degradation of proteins into peptides that can be shuttled into the MHC class II compartment. If the peptides have high affinity to the MHC class II molecule, they are loaded onto MHC class II and presented on the surface of the phagocyte that is now an antigen presenting cell. Altering this pathway would prevent the activation of autoreactive effector T cells and be beneficial to maintaining a healthy ocular microenvironment. To suppress the phagosome maturation pathway in autoimmune disease would help to rob the autoreactive effector T cells their central driving mechanism of presented autoantigen. Since suppression of EAU by α-MSH-therapy may require MC5r-expression, we assayed whether MC5r expression is needed for α-MSH-therapy to restore RPE-regulation of phagosome maturation in EAU.
MATERIALS AND METHODS
Animals
The mice used in these experiments were 6–8 week old C57BL/6J and MC5r(−/−) (C57BL/6J background) mice. The C57BL/6J mice were purchased from Jackson Laboratories (Bar Harbor, ME), and housed in the Boston University Animal Science Center (ASC). The MC5r(−/−) mice were rederived mice supplied to us from Dr. Robert Cone, Oregon Health Science Center, and bred in the Boston University ASC. All experimental use of animals was in accordance with procedures approved by the Boston University Institutional Animal Care and Use Committee and ARVO statement for the use and care of animals in vision research.
Induction of experimental autoimmune uveitis, and α-MSH peptide therapy
The EAU was induced in the mice as previously described.8, 11, 30, 34 Human interphotoreceptor retinoid binding protein peptide (IRBP) spanning amino acid residues 1–20 emulsified in complete Freund’s adjuvant was injected subcutaneously. This was followed by intraperitoneal injections of 10 ng of pertussis toxin in 100μl of PBS two days apart. Every three to four days eye fundus was examined and scored for severity of retinal inflammation on a score of 0–5.29, 37, 38 Retinas with no inflammation were scored as 0, with only white focal lesions of vessels were scored as 1, with linear lesions of the vessels within half of retina were scored as 2, with linear lesions of vessels over more than half of the retina were scored as 3, with severe chorioretinal exudates or hemorrhages in addition to the vasculitis were scored as 4, and retinas with subretinal hemorrhage or retinal detachments were scored as 5. No mouse under our housing and care reached a clinical score of 4. Data presented are from 3–5 experiments, and in each experiment 10 mice per group were used. The α-MSH peptide was purchased from Bachem (Torrance, CA). The α-MSH was reconstituted to a concentration of 500μg/mL in sterile PBS carrier. When the uveitis reached the start of its chronic inflammatory plateau, a sustained EAU score of 3, 50μg of α-MSH was intraperitoneally injected, and two days later a second injection was given.
Preparation of Mouse RPE Eyecups
After treatment, EAU was scored every 3 to 4 days per week until the mice treated with α-MSH had resolved the inflammation. The eyes were enucleated for preparation of RPE eyecups. The RPE eyecups were made based on our previously published method.5, 7, 11, 39 Eyes were placed in ice-cold 0.01M PBS (Lonza) and the connective tissue and optic nerve were removed from the eyeball. A circumferential cut was made around the limbus to remove the entire anterior segment including the cornea, iris, ciliary body and lens. The neural retina was gently removed from the RPE monolayer and discarded. The remaining posterior of the eye (RPE eyecups) contain only the RPE monolayer, choroid and the sclera. The RPE eyecup was placed into the well of a 96-well round bottom tissue culture plate (Corning, Corning, NY, USA) containing 200uL Serum-free Media (SFM): RPMI 1640 supplemented with a 1/500 dilution of ITS+ (Insulin/Transferrin/Selenium + linoleic acid) (Sigma-Aldrich, St. Louis, MO), and 0.1% sterile BSA solution (Sigma-Aldrich). The cultures were incubated for 24 hours at 37C in 5% CO2, and the conditioned media (CM) was collected, centrifuged at 2100g for 5 minutes, and the supernatant was used immediately in the assays as RPE eyecup CM.
Collection of primary resting macrophages
Resting peritoneal macrophages were used to assay for phagolysosome activation. Macrophages were obtained from a peritoneal lavage of naive WT or MC5r(−/−) mice. Five mL 0.01M PBS (Lonza) was injected into the peritoneal cavity and was recovered after 5 minutes. The lavage was centrifuged at 400g for 5 minutes, and then mixed with 1 mL red blood cell lysing buffer (Sigma-Aldrich) for 5 minutes on ice, centrifuged at 400g for 5 minutes. The cells were resuspended in RPMI 1640 with 10% fetal bovine serum (Hyclone, Logan, UT, USA).
Phagolysosome activation
To assay the effects of RPE CM on phagolysosome activation in macrophages, the peritoneal macrophages were seeded at 1 × 105 cells per well in a Nunc Lab-Tek 8-chamber slide (ThermoFisher, Waltham, MA) and incubated at 37°C for 2 hours. Each well was washed once with 200 μL SFM, and the macrophages were treated with a 1:2 dilution of RPE CM for 30 minutes at 37°C in 5% CO2. Opsonized pHrodo-Red Staphylococcus aureus bioparticles (0.25 ug) from Invitrogen (ThermoFisher) were added to each well, and cultures were incubated at 37°C in 5% CO2 for 24 hours. Each culture well was assayed by fluorescence microscopy using a FSX100 inverted fluorescence imaging-microscope (Olympus, Center Valley, PA, USA). Ten images were captured from each well at constant exposure and time for all the wells in an experiment, and at least 100 cells were measured per culture.
Phagocytosis activities
To investigate the difference between the phagocytosis and phagolysosome activation of WT and MC5r(−/−) mice, the peritoneal macrophages were cultured the same as above, and a solution of equal weight of opsonized AF488-conjugates of Escherichia coli bioparticles (0.125 μg), and opsonized pHrodo-Red S. aureus bioparticles (0.125 μg) were added to the cultures. The cells were incubated for 2 hours at 37°C in 5% CO2 and washed with warm serum free media to remove bioparticles that were not phagocytized. Fresh serum-free media was added, and the cells incubated for a total of 48 hours. The cultures were imaged using the FSX100 inverted fluorescence imaging-microscope at 2, 24, and 48 hours of incubation, and the fluorescent intensity of at least 100 cells was measured per culture.
Effect of α-MSH and Neuropeptide Y (NPY) on phagolysosome activation
To find out whether WT and MC5r(−/−) macrophages responded differently to the neuropeptides of α-MSH and NPY, macrophages were cultured the same as above, and treated with either 1ng/ml of α-MSH, 1 ng/ml of NPY (1 ng/ml), or a mixture of α-MSH and NPY at 1 ng/ml each.9 The cultures were incubated for 30 minutes at 37°C in 5% CO2, and 2 μl of opsonized pHrodo-Red S. aureus bioparticles (0.25 μg) were added. The cultures were incubated for 30 minutes at 37°C in 5% CO2. The cells were examined by fluorescence microscopy. Ten images were captured from each well at a constant exposure and time for all wells in an experiment, and at least 100 cells were measured per culture.
Image Analysis
Each fluorescent image was analyzed using a MATLAB program (R2017b) to calculate an objective measure of fluorescence. analyzed using a MATLAB program (R2017b) to calculate an objective measure of fluorescence. For experiments analyzing activation of phagolysosome, relative intensity was calculated as a ratio of the sum of intensities above the “positive” threshold to the number of pixels occupied by cells This was done by creating a combination of binary masking techniques and filters using MATLAB’s built-in functions (more information in supplementary). To do the analysis, the image was processed to isolate relevant artifacts in the image such as the cells from noise (i.e. random artifacts). The fluorescence was calculated for each image from this analysis (Sub 1).
Histology
Enucleated eyes from EAU mice with or without treatment were fixed in Davidson’s fixative for 24 hours, and then transferred to 10% buffered formalin for an additional 24 hours. The eyes were subsequently embedded in paraffin, after which 5 μm sections were cut and stained with H&E. The images were taken using a CX33 microscope (Olympus) and QColor 5 camera system and software (Olympus). Presented are the retinal sections at two optic disk diameters from the center of the optic nerve. Thickness of the retina and the outer nuclear layer (ONL) were measured using ImageJ software of full-length retinal sections centered on the optic nerve (representative images in Supplemental Fig. 2). Measurements were made at 0.5, 1.0, 1.5, 2 mm to the right and left (−0.5, −1.0, −1.5, −2 mm) of the optic nerve head.
Statistical analysis.
Statistical significance was calculated using an ordinary one-way ANOVA with a post-analysis Dunnett’s multiple comparisons test to compare means between test groups and controls. For analysis of EAU scores a non-parametric Mann-Whitney T-test was used to measure significance. Statistical differences for retinal layer thickness were determined using a two-way ANOVA with a post-analysis Tukey multiple comparison of the whole curve. In all cases significance was detected when P ≤ 0.05. The data is presented as the mean ± SEM for each experimental group. Presented micrograph images represent the group mean of each experiment.
RESULTS
The effects of α-MSH-therapy on EAU MC5r(−/−) mice.
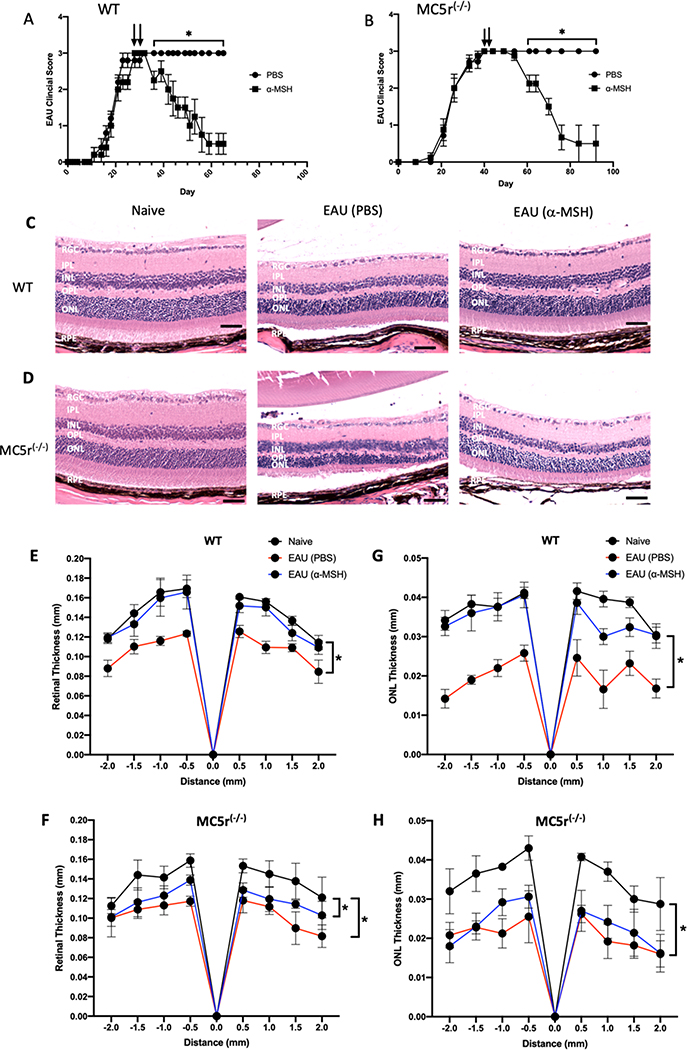
We have previously demonstrated that the resolution of EAU in mice is in part associated with the α-MSH receptor MC5r. To demonstrate the importance of MC5r in resolving EAU following α-MSH-treatment, we assayed the clinical EAU score, and by histology examined the structure of the retina in EAU MC5r(−/−) mice treated with α-MSH. In both the wild type (WT) and MC5r(−/−) mice, the course of EAU was similar (Figs 1A and 1B) with both reaching a level of chronic inflammation at a clinical score of 3. The MC5r(−/−) mice took an extra week to reach the chronic stage of EAU. As we have shown before in WT EAU mice following α-MSH therapy, the inflammation begins to resolve within a week and reaches clearance in 30 days (Fig. 1A). In contrast, the α-MSH-treated MC5r(−/−) EAU-mice took almost 2 weeks for resolution to start, but still reached clearance in 30 days after treatment (Fig 1B). This suggests that in MCr5(−/−) mice, EAU is a prolonged process of inflammation both in development and in α-MSH-induced resolution.
Figure 1. The effects of α-MSH-treatment on EAU in MC5r(−/−) mice.
EAU was induced in Wildtype and MC5r(−/−) mice and treated with α-MSH when clinical scores reached the chronic phase of inflammation (clinical score = 3) on Day 30 for WT mice and Day 40 for the MC5r(−/−) with a second injection of α-MSH 2 days later. The mean EAU scores ± SEM of each treated group (N = 10) of A) WT and B) MC5r(−/−) mice are presented over time. Representative retinal sections on Day 65 of WT mice and Day 90 of MC5r(−/−) mice were stained and presented. C) The retinas of α-MSH treated EAU mice (EAU (α-MSH)) appeared the same as retinas of naive mouse eyes. The vehicle injected (PBS) EAU retina was the thinnest with cell loss in both inter-nuclear layer and outer-nuclear layer. D) Both α-MSH and PBS injected MC5r(−/−) EAU mice had retinas that were thinner than the naive mouse retina with about half the photoreceptor outer-segment layer lost in the PBS-injected MC5r(−/−) EAU mouse retinas. The size bar equals 50μm. RGC – Retinal Ganglion Cells Layer; IPL – Inner Plexiform Layer; INL – Inner Nuclear Layer; OPL – Outer Plexiform Layer; ONL – Outer Nuclear Layer; RPE – Retinal Pigment Epithelium. The thickness of retina (E, F) and ONL (G, H) (N=5 each) were measured by ImageJ and presented as mean (mm) of thickness ± SEM at 0.5 mm intervals from the optic nerve head. There is no statistical difference between WT and α-MSH-treated EAU-WT retinal and ONL thickness (E, G); whereas there were (* P ≤ 0.001) significant loss of retinal and ONL thickness in the untereated and α-MSH-treated EAU MC5r(−/−) mice.
To see if there is a difference in the retinal structures of the EAU WT and MC5r(−/−) mice, the eyes were collected on Day 65 for the WT and Day 90 for the MC5r(−/−) α-MSH treated. Naive mouse eyes were also sectioned and stained for histological analysis (Figs. 1C and 1D). The retinal structures of Naive WT and MC5r(−/−) mice were similar (Figs. 1C and 1D Naive), but the retinal structure in untreated-EAU MC5r(−/−) mice had severe retinal degeneration with loss of photoreceptor outer-segments and nuclear layer thinning compared to the untreated WT EAU mouse retinas (Figs. 1C and 1D EAU(PBS)). The retinas from α-MSH-treated EAU WT-mice retained almost normal retinal layer thickness and structure, and retention of ganglion cells (Fig. 1C EAU(α-MSH)). In contrast, the retinas of α-MSH-treated EAU-MCr5(−/−) mice had detectible thinner nuclear and photoreceptor layers with ganglion cell dropout (Fig 1D EAU(α-MSH)). In eyes with EAU there is a significant thinning of the retina and the outer nuclear layer (ONL) In both WT and MC5r(−/−) mice (Fig 1 E,F,G,H). Treatment with α-MSH preserved the thickness of the retina and ONL in EAU wild type mice (Fig 1E and Fig 1G); however, α-MSH-therapy had no effect on preventing retinal and ONL thinning in EAU MC5r(−/−) mice (Fig. 1F and Fig 1H). These results demonstrate that the resolution of clinical inflammation in EAU following α-MSH-treatment is not fully dependent on MC5r expression; however, MC5r expression is needed for α-MSH-treatment to protect the retinal structure from the damage of inflammation within the retina.
Post-EAU recovery of RPE suppression of phagolysosome activation in macrophages after α-MSH-treatment.
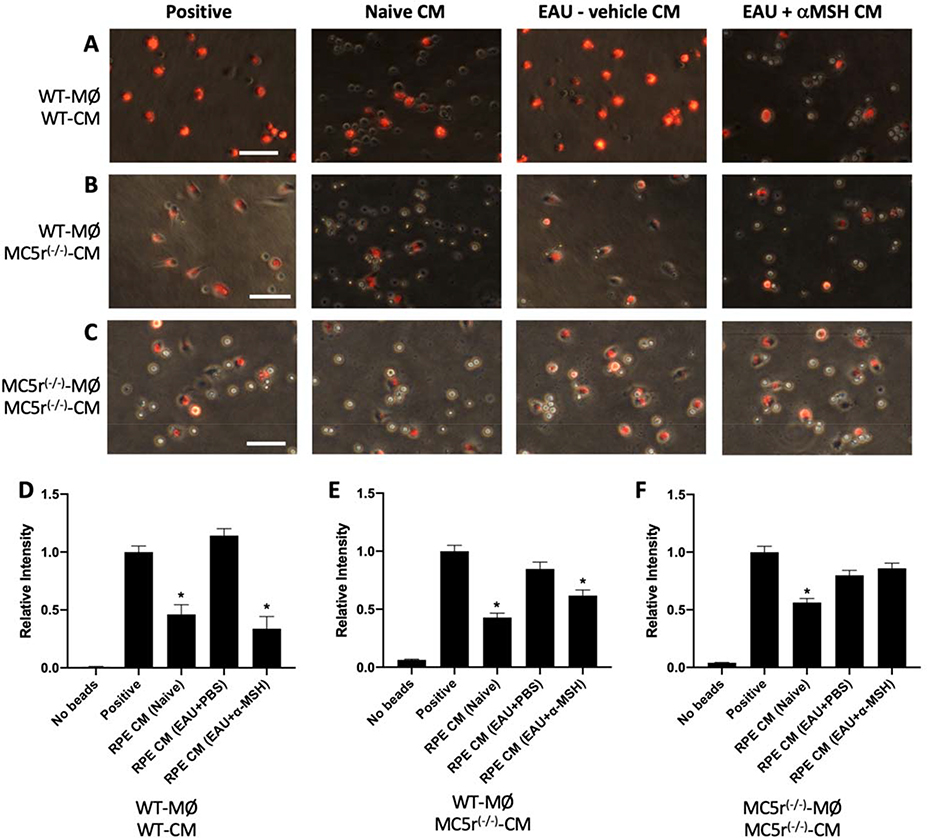
Since it is considered that tissue damage is collateral to an inflammatory response, we examined the possibility that without MC5r expression there is missing tissue sparing RPE suppression of immune cell activity. Our previous studies demonstrated that RPE suppress phagosome maturation at an early stage, down-regulate lysosomal-associated membrane protein 1 (LAMP-1) expression needed for phagolysosome formation and activation in macrophages.11, 12 Therefore, we examined the possibility that α-MSH-therapy promoted RPE recovery of its ability to govern innate immune cell activity by suppressing phagolysosome activation in macrophages. Cultures of RPE eyecups from EAU WT and MC5r(−/−) mouse eyes were collected on EAU Day 65 for the WT and Day 90 for the MC5r(−/−) mice. These mice were either untreated or treated with α-MSH. Age matched naive mouse RPE eyecups were also collected. The conditioned media of cultured RPE-eyecups were used to treat macrophages given opsonized pHrodo-Red bioparticles to phagocytize. The phagocytized pHrodo-Red-bioparticles are weakly fluorescent before they are in an activated phagolysosome where the acidic conditions make the bioparticles fluoresce at their maximum intensity. This allows for quantifying phagolysosome activation.
The RPE from both naive WT and MC5r(−/−) mice significantly suppressed phagolysosome-activation in WT macrophages (Fig. 2A, B, and D, E (Naive)), and in contrast the RPE from untreated EAU WT and MC5r(−/−) mice did not suppress phagolysosome-activation in the WT macrophages (Fig. 2A, B, and D, E (EAU+PBS)). Treatment of both EAU WT and MC5r(−/−) mice with α-MSH significantly recovered RPE suppression of phagolysosome-activation in the WT macrophages (Fig. 2A, B, and D, E (EAU+α-MSH)). Since in MC5r(−/−) mice both the RPE and the macrophages are MC5r(−/−), we assayed the effects of conditioned media of RPE-eyecups from MC5r(−/−) mice on phagolysosome activation in macrophages from MC5r(−/−) mice (Fig. 2C and F). While the RPE from Naive MC5r(−/−) mice suppressed phagolysosome-activation in the MC5r(−/−) macrophages, the RPE from α-MSH-treated EAU MC5r(−/−) mice did not suppress phagolysosome-activation in the MC5r(−/−) macrophages as it did in WT macrophages. These results demonstrated that α-MSH-treatment of EAU promotes recovery of RPE suppression of phagolysosome activation in macrophages whether or not the RPE are in eyes expressing MC5r; however the benefit is dependent on MC5r expression by the macrophages.
Figure 2. The effects of α-MSH-treatment of EAU on recovery of RPE suppression of phagolysosome activity.
RPE-conditioned media (CM) were collected from 24-hr cultures of RPE eyecups from WT (A and B) and MC5r(−/−) (C) eyes of naive mice, EAU mice injected with PBS-vehicle (EAU+PBS), or EAU mice treated with α-MSH (EAU+α-MSH). Primary resting macrophages from A) WT mice or B and C) MC5r(−/−) mice were treated with the CM and given opsonized pHrodoRed-bioparticles to phagocytize. The cells were incubated for 24-hours, imaged and fluorescent intensity was measured. Presented are representative images of the pHrodoRed-bioparticle fluorescence in the macrophages indicating activated phagolysosomes. The size bar equals 50μm. D) The relative fluorescent intensity of phagolysosome activation in WT macrophages treated with WT-CM was calculated vs the positive control macrophage cultures and presented are the mean ± SEM of 3 independent experiments (N = 5). Both naive CM and EAU+α-MSH CM (*) significantly (P ≤ 0.001) suppressed phagolysosome activation while vehicle (PBS)-treated EAU-CM showed no suppression. E) The relative fluorescent intensity of phagolysosome activation in WT macrophages treated with MC5r(−/−)-CM was calculated vs the positive control macrophage cultures and presented are the mean ± SEM of 3 independent experiments (N = 5). The naive MC5r(−/−)-CM and the α-MSH-treated EAU MC5r(−/−)-CM (*) significantly (P ≤ 0.001) suppressed phagolysosome activation. F) Using primary resting MC5r(−/−) peritoneal macrophages instead of wildtype-macrophages showed that only the naive MC5r(−/−) RPE-CM (*) significantly (P ≤ 0.001) suppressed phagolysosome activation. Presented are the mean ± SEM of 3 independent experiments (N = 5).
The effects of α-MSH and NPY on phagolysosome activation in MC5r(−/−) macrophages
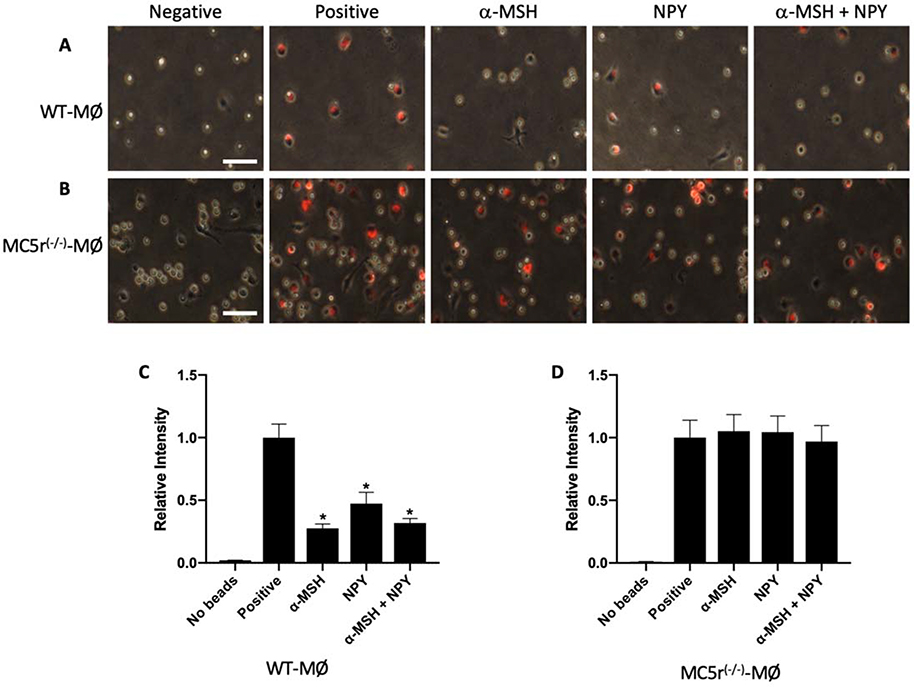
The results suggest that the macrophages from MC5r(−/−) mice were not affected by the RPE mediated suppression of phagolysosome activation. The neuropeptides α-MSH and NPY are two soluble factors produced by the RPE that suppress phagolysosome activation.9, 11 Resting macrophages obtained from WT and MC5r(−/−) mice were treated with α-MSH, NPY, or both, and then assayed for phagolysosome activation with opsonized-pHrodo-Red bioparticles (Fig 3). The phagolysosome activation in WT macrophages was significantly suppressed by α-MSH, NPY, and co-treatment of α-MSH and NPY (Figs 3A, C). In contrast, the two neuropeptides and in combination did not have an effect on phagolysosome activation in MC5r(−/−) macrophages (Figs 3B, D). Therefore, without expression of MC5r α-MSH does not suppress phagolysosome activation in macrophages. Interestingly, the MC5r(−/−) macrophages are not suppressed by NPY which does not work through MC5r. This suggests the possibility that resting MC5r(−/−) macrophages are differently regulated in their phagocytic activity than WT macrophages.
Figure 3. The effects of neuropeptides on phagolysosome activation in MC5r(−/−) macrophages.
Wildtype and MC5r(−/−) peritoneal macrophages were collected and treated with α-MSH, NPY or both for 30 minutes, and then given opsonized-pHrodoRed-bioparticles. The cells were incubated for 24 hours and imaged by microscopy. Presented are representative images of red-fluorescence expressed in A) wildtype and B) MC5r(−/−) macrophages indicating activated phagolysosomes. The size bar equals 50μm. C) The relative fluorescent intensity of the bioparticles in the untreated (Positive) and treated wildtype-macrophages were measured. The relative fluorescent intensity was calculated to the positive control and presented as the mean ± SEM of 3 independent experiments. WT macrophages treated with α-MSH, NPY or α-MSH+NPY were (*) significantly suppressed (P ≤ 0.001) in phagolysosome activation. D) The relative fluorescent intensity of the bioparticles in the untreated (Positive) and treated MC5r(−/−) macrophages. The relative fluorescent intensity was calculated to the positive control and presented as the mean ± SEM of 3 independent experiments. No treatment suppressed phagolysosome activation in the MC5r(−/−) macrophages. The negative controls are macrophages cultured with no bioparticles.
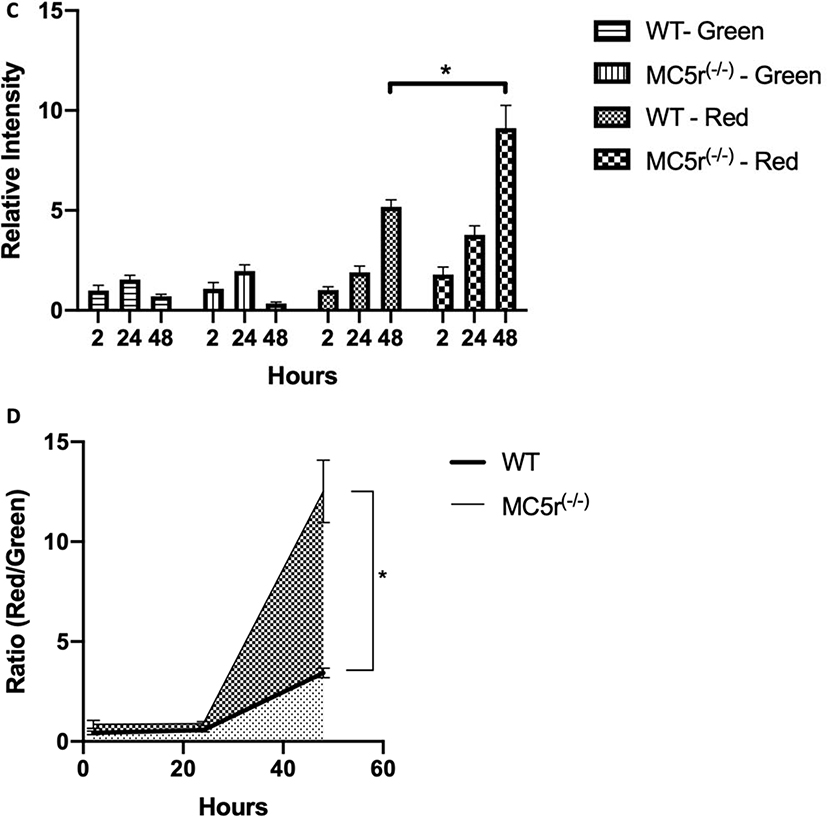
The phagocytic activity of MC5r(−/−) macrophages
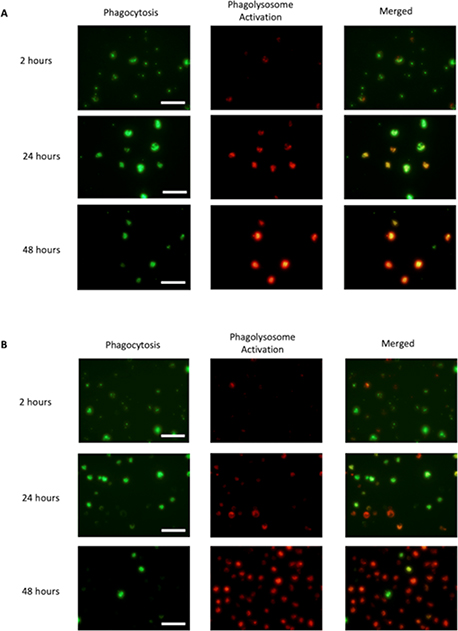
To find out whether the phagocytic activity of MC5r(−/−) macrophages was different from WT macrophages, we performed a timed assay that traced the maturation of phagocytized bioparticles. This was done by culturing the macrophages with a mixture of opsonized-Alexa488-conjugated-biopaticles and pHrodo-Red-conjugated-bioparticles for 2 hours then washed the cultures of any unphagocytized bioparticles from the cultures. As the phagocytized Alexa488-bioparticles move through the maturing phagosomes fluoresce until they are degraded in an active phagolysosome. In contrast, the phagocytized pHrodo-Red bioparticles we used above are weakly fluorescent until they reach the activated-phagolysosome. We took images of the macrophage cultures at 2, 24 and 48 hours after adding the opsonized-bioparticle mixture (Figs 4A, and 4B). The phagocytic activity (green fluorescence intensity per pixel) in both WT macrophages and MC5r(−/−) macrophages were the same showing increased intensity at 24-hours, due to the coalescing of the maturing phagosomes within the macrophages (Fig. 4C). This was followed by the expected decrease at 48 hours as the phagosomes further matured into phagolysosomes and degraded the bioparticles. The rate of phagolysosome activation was significantly different between the WT and MC5r(−/−) macrophages, with the MC5r(−/−) macrophages having nearly 2-fold greater intensity than the WT macrophages (Fig. 4C). When the ratio of Red to Green intensity was calculated, indicating the relative progression of phagosome maturation into a phagolysosome, a 3-fold increase in phagolysosome maturation in the MC5r(−/−) macrophages was seen at 48 hours (Fig. 4D). These results demonstrated a higher level of phagosome maturation in MC5r(−/−) macrophages, suggesting that the macrophages are more active when stimulated for phagocytosis than WT macrophages.
Figure 4. Comparison of phagocytic activity in Wildtype and MC5r(−/−) macrophages.
Both wildtype (A) and MC5r(−/−) (B) macrophages were cultured with a mixture of opsonized AF488-bioparticles and pHrodoRed-bioparticles. Two hours later the cultures were washed to remove unphagocytized bioparticles. At 2, 24, and 48 hours fluorescent images were taken and the expression of green-fluorescent (indication of phagocytic uptake), and red-fluorescent (indication of phagolysosome activation) were imaged. The green and red-fluorescent images of (A) WT and (B) MC5r(−/−) macrophages are presented. The size bar equals 50μm. C) From the images of 4A and B the relative green and red-fluorescent intensity, mean ± SEM of 6 cultures, were calculated relative to 2-hour WT macrophages. A significant difference (P ≤ 0.001) was seen between relative red-fluorescence intensity between WT and MC5r(−/−) macrophages at 48 hours of incubation. D) The ratio of red to green fluorescent-intensity was calculated from the images of 4A and 4B and presented as mean ± SEM to measure the progression of the bioparticles from phagocytosis to phagolysosome activation. There is a significantly (P ≤ 0.006) greater progression of phagosome maturation seen in the MC5r(−/−) macrophages compared to WT macrophages.
DISCUSSION
The role of melanocortins in regulating immune cell activity has been well documented, including in our own studies that show the importance of the melanocortins in the mechanisms of ocular immune privilege.4–6 Our current results demonstrated that the highly conserved neuropeptide α-MSH, and its equally conserved melanocortin receptors, in a therapeutic manner have the potential to reestablish ocular immune privilege within eyes with autoimmune uveitis. In addition, as we have seen before, there is a differential effect of α-MSH through its melanocortin receptors on immune cell activity with a dependence of macrophage expression of MC5r to have their phagocytic activity regulated by RPE after recovery from uveitis. Our results also demonstrated that there may be a different mechanism of suppressing phagocytic activity of macrophages by RPE in MC5r(−/−) mice, and that without MC5r macrophages had an enhanced level of phagosome maturation. Therefore, α-MSH through MC5r mediates RPE suppression of antigen processing in potential APC and may be a homeostatic moderator of phagosome-maturation within macrophages.
Rodent EAU has been the best model to identify important conserved pathways in immune activity associated with autoimmune disease of the retina. This includes recent findings of the potential for the gut microbiome to induced autoreactive effector T cells to antigens within the eye.40 Also, it has revealed the role of Th1 and Th17 cells in severity and duration of uveitis, and that targeting their cytokines with biologics is effective in suppressing uveitis.41 A unique feature of EAU in C57BL/6 mice is that these mice resolve autoimmune uveitis without intervention, and are resistant to reactivation of ocular-autoimmune disease.18 It has been seen that there is at the resolution of EAU a significant increase in Treg cells both within the eye and in the spleen.33 In the spleen of EAU-recovered mice there is present a suppressor APC that is dependent on MC5r-expression for its activation, and its presence in the spleen.8 These suppressor APC counter-convert effector T cells into Treg cells.31 These Treg cells are retinal-antigen specific, and express markers of inducible Treg (iTreg) cells.30 The suppressor APC are induced when α-MSH-therapy is applied to mice with EAU. And similar to the results in this manuscript, α-MSH-therapy suppresses EAU in MC5r(−/−) mice; however, it does not induce the suppressor APC nor the subsequent retinal-antigen specific iTreg cells. These findings, including the results in this report, demonstrate an importance in MC5r-expression for α-MSH to mediate regulatory immunity.
The ability of RPE to suppress phagolysosome activation provides the immune privileged ocular-microenvironment with a mechanism to block locally the engine of antigen presentation that drives activation of CD4+ effector T cell that mediate autoimmune disease. Through the melanocortin pathway, RPE alter the maturation of the phagosome that can result in the presentation of a set of antigens that are not recognized by effector T cells. Since α-MSH treated APC promote activation of Treg cells, it suggests that any APC still presenting antigen within the retina after α-MSH therapy may well be presenting antigens recognized by Treg cells within the eye. 8, 22, 30, 31 In addition, α-MSH induces production of anti-inflammatory cytokines that further suppress the activity of effector T cells. Therefore, α-MSH-treatment of uveitis not only suppresses the retinitis, but also has the potential through MC5r to reestablish immune tolerance to retinal antigens.
In addition, NPY did not suppress phagolysosome activation in the MC5r(−/−) macrophages. We have seen NPY and α-MSH work together to suppress phagocytic activity and promote differentiation of macrophages into suppressor cells expressing both M1 and M2 characteristics.5, 9, 11, 12 It is not known if there is a dependency of NPY receptor signaling on MC5r expression. While there are only a few reports on NPY suppression of phagocytic activity, it appears that the effects of NPY on macrophage activity, pro or anti-inflammatory, is influenced by the microenvironment, the stimulating agents, and aging.42–44 It is possible that since the MC5r(−/−) macrophages have enhanced phagosome activity, there also is a change in NPY receptors or signaling. Without MC5r the macrophages act like conventional APC and stimulate effector T cells8, 31; therefore, it is to be seen if NPY may on the same macrophages induce cytokine production that is proinflammatory.
We demonstrate that α-MSH can suppress EAU without MC5r expression. 8, 30, 31 This corresponds with observed differential regulation of immunity by α-MSH with anti-inflammatory activity mediated through MC1r and MC3r.21, 25 With MC5r-expression there is a recovery in RPE suppression of phagolysosome activation within macrophages. However, the recovery of RPE regulatory activity may be either a direct effect of α-MSH on the RPE cells, or indirectly from the immunosuppressive actions of α-MSH on the macrophages/microglial cells in the retina. The finding that macrophages in MC5r(−/−) mice have enhanced levels of phagosome maturation could mean that within retinas of MC5r(−/−) mice the expected regulatory activity of the RPE is different in response to potentially heightened myeloid cell-activity. Also, it suggests that in MC5r(−/−) mice their macrophages when stimulated may mediate or prolong inflammation that is more damaging to the retina than in WT mice. Recently, there are reports that retinal cell survival is enhanced by α-MSH through MC1r and MC5r.19, 35 Severe damage is seen in the retinas of EAU MC5r(−/−) mice in contrast to the relatively protected retinal structures of α-MSH-treated EAU WT-mice. This demonstrated that MC5r-expression is needed to fully maintain ocular immune privilege, to provide survival of retinal cells, and to recover immune privilege after uveitis.
Supplementary Material
Acknowledgments:
We thank David Yee for his technical assistance, and support from the Boston University Undergraduate Research Program (UROP) and the Massachusetts Lions Eye Research Foundation with funding by the NIH/NEI grant EY025961.
Footnotes
Disclosure statement: In accordance with Taylor & Francis policy and my ethical obligation as a researcher, I (AWT) am reporting that I am a consultant to a company that may be affected by the research reported in the enclosed paper. I have disclosed those interests fully to Taylor & Francis, and I have in place an approved plan for managing any potential conflicts.
REFERENCES:
- 1.Taylor AW, Ng TF. Negative regulators that mediate ocular immune privilege. J Leukoc Biol 2018;103:1179–1187. doi: 10.1002/JLB.3MIR0817-337R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clemson CM, Yost J, Taylor AW. The Role of Alpha-MSH as a Modulator of Ocular Immunobiology Exemplifies Mechanistic Differences between Melanocortins and Steroids. Ocul Immunol Inflamm 2017;25:179–189. doi: 10.3109/09273948.2015.1092560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taylor AW. Ocular immunosuppressive microenvironment. Chemical immunology and allergy 2007;92:71–85. doi: 10.1159/000099255. [DOI] [PubMed] [Google Scholar]
- 4.Taylor AW, Streilein JW, Cousins SW. Identification of Alpha-Melanocyte Stimulating Hormone as a Potential Immunosuppressive Factor in Aqueous-Humor. Curr Eye Res 1992;11:1199–1206. doi: 10.3109/02713689208999545. [DOI] [PubMed] [Google Scholar]
- 5.Kawanaka N, Taylor AW. Localized retinal neuropeptide regulation of macrophage and microglial cell functionality. J Neuroimmunol 2011;232:17–25. doi: 10.1016/j.jneuroim.2010.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taylor AW, Alard P, Yee DG, Streilein JW. Aqueous humor induces transforming growth factor-beta (TGF-beta)-producing regulatory T-cells. Curr Eye Res 1997;16:900–908. doi: 10.1076/ceyr.16.9.900.5043. [DOI] [PubMed] [Google Scholar]
- 7.Lau CH, Taylor AW. The immune privileged retina mediates an alternative activation of J774A.1 cells. Ocul Immunol Inflamm 2009;17:380–389. doi: 10.3109/09273940903118642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee DJ, Taylor AW. Following EAU recovery there is an associated MC5r-dependent APC induction of regulatory immunity in the spleen. Invest Ophthalmol Vis Sci 2011;52:8862–8867. doi: 10.1167/iovs.11-8153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Phan TA, Taylor AW. The neuropeptides alpha-MSH and NPY modulate phagocytosis and phagolysosome activation in RAW 264.7 cells. J Neuroimmunol 2013;260:9–16. doi: 10.1016/j.jneuroim.2013.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taylor AW, Dixit S, Yu J. Retinal Pigment Epithelial Cell Line Suppression of Phagolysosome Activation. Int J Ophthalmol Eye Sci 2015;Suppl 2:1–6. doi. [PMC free article] [PubMed] [Google Scholar]
- 11.Wang E, Choe Y, Ng TF, Taylor AW. Retinal Pigment Epithelial Cells Suppress Phagolysosome Activation in Macrophages. Invest Ophthalmol Vis Sci 2017;58:1266–1273. doi: 10.1167/iovs.16-21082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benque IJ, Xia P, Shannon R, Ng TF, Taylor AW. The Neuropeptides of Ocular Immune Privilege, alpha-MSH and NPY, Suppress Phagosome Maturation in Macrophages. Immunohorizons 2018;2:314–323. doi: 10.4049/immunohorizons.1800049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schioth HB, Haitina T, Ling MK, et al. Evolutionary conservation of the structural, pharmacological, and genomic characteristics of the melanocortin receptor subtypes. Peptides 2005;26:1886–1900. doi: 10.1016/j.peptides.2004.11.034. [DOI] [PubMed] [Google Scholar]
- 14.Dores RM, Baron AJ. Evolution of POMC: origin, phylogeny, posttranslational processing, and the melanocortins. Ann N Y Acad Sci 2011;1220:34–48. doi: 10.1111/j.1749-6632.2010.05928.x. [DOI] [PubMed] [Google Scholar]
- 15.Voisey J, Carroll L, van Daal A. Melanocortins and their receptors and antagonists. Curr Drug Targets 2003;4:586–597. doi. [DOI] [PubMed] [Google Scholar]
- 16.Dores RM, Londraville RL, Prokop J, Davis P, Dewey N, Lesinski N. MOLECULAR EVOLUTION OF GPCRS: Melanocortin/melanocortin receptors. J Mol Endocrinol: (c) 2014 Society for Endocrinology; 2014:T29–t42. [DOI] [PubMed] [Google Scholar]
- 17.Teshigawara K, Takahashi S, Boswell T, Li Q, Tanaka S, Takeuchi S. Identification of avian alpha-melanocyte-stimulating hormone in the eye: temporal and spatial regulation of expression in the developing chicken. J Endocrinol 2001;168:527–537. doi: JOE04087 [pii]. [DOI] [PubMed] [Google Scholar]
- 18.Taylor AW, Kitaichi N, Biros D. Melanocortin 5 receptor and ocular immunity. Cellular and molecular biology (Noisy-le-Grand, France) 2006;52:53–59. doi. [PubMed] [Google Scholar]
- 19.Maisto R, Gesualdo C, Trotta MC, et al. Melanocortin receptor agonists MCR1–5 protect photoreceptors from high-glucose damage and restore antioxidant enzymes in primary retinal cell culture. J Cell Mol Med 2017;21:968–974. doi: 10.1111/jcmm.13036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rossi S, Maisto R, Gesualdo C, et al. Activation of Melanocortin Receptors MC 1 and MC 5 Attenuates Retinal Damage in Experimental Diabetic Retinopathy. Mediators Inflamm 2016;2016:7368389. doi: 10.1155/2016/7368389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li D, Taylor AW. Diminishment of alpha-MSH anti-inflammatory activity in MC1r siRNA-transfected RAW264.7 macrophages. J Leukoc Biol 2008;84:191–198. doi: 10.1189/jlb.0707463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee DJ, Preble J, Lee S, Foster CS, Taylor AW. MC5r and A2Ar Deficiencies During Experimental Autoimmune Uveitis Identifies Distinct T cell Polarization Programs and a Biphasic Regulatory Response. Sci Rep 2016;6:37790. doi: 10.1038/srep37790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spana C, Taylor AW, Yee DG, Makhlina M, Yang W, Dodd J. Probing the Role of Melanocortin Type 1 Receptor Agonists in Diverse Immunological Diseases. Frontiers in Pharmacology 2019;9: doi: 10.3389/fphar.2018.01535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lam CW, Getting SJ. Melanocortin receptor type 3 as a potential target for anti-inflammatory therapy. Curr Drug Targets Inflamm Allergy 2004;3:311–315. doi. [DOI] [PubMed] [Google Scholar]
- 25.Getting SJ, Lam CW, Chen AS, Grieco P, Perretti M. Melanocortin 3 receptors control crystal-induced inflammation. FASEB J 2006;20:2234–2241. doi: 10.1096/fj.06-6339com. [DOI] [PubMed] [Google Scholar]
- 26.Ignar DM, Andrews JL, Jansen M, et al. Regulation of TNF-alpha secretion by a specific melanocortin-1 receptor peptide agonist. Peptides 2003;24:709–716. doi. [DOI] [PubMed] [Google Scholar]
- 27.Taherzadeh S, Sharma S, Chhajlani V, et al. alpha-MSH and its receptors in regulation of tumor necrosis factor-alpha production by human monocyte/macrophages. Am J Physiol 1999;276:R1289–1294. doi. [DOI] [PubMed] [Google Scholar]
- 28.Taylor AW, Lee DJ. The alpha-melanocyte stimulating hormone induces conversion of effector T cells into treg cells. J Transplant 2011;2011:246856. doi: 10.1155/2011/246856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taylor A, Namba K. In vitro induction of CD25+ CD4+ regulatory T cells by the neuropeptide alpha-melanocyte stimulating hormone (alpha-MSH). Immunol Cell Biol 2001;79:358–367. doi: 10.1046/j.1440-1711.2001.01022.x. [DOI] [PubMed] [Google Scholar]
- 30.Lee DJ, Taylor AW. Recovery from experimental autoimmune uveitis promotes induction of antiuveitic inducible Tregs. J Leukoc Biol 2015;97:1101–1109. doi: 10.1189/jlb.3A1014-466RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee DJ, Taylor AW. Both MC5r and A2Ar are required for protective regulatory immunity in the spleen of post-experimental autoimmune uveitis in mice. Journal of immunology 2013;191:4103–4111. doi: 10.4049/jimmunol.1300182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ohta K, Wiggert B, Yamagami S, Taylor AW, Streilein JW. Analysis of immunomodulatory activities of aqueous humor from eyes of mice with experimental autoimmune uveitis. Journal of immunology 2000;164:1185–1192. doi. [DOI] [PubMed] [Google Scholar]
- 33.Kitaichi N, Namba K, Taylor AW. Inducible immune regulation following autoimmune disease in the immune-privileged eye. J Leukoc Biol 2005;77:496–502. doi: 10.1189/jlb.0204114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee DJ, Biros DJ, Taylor AW. Injection of an alpha-melanocyte stimulating hormone expression plasmid is effective in suppressing experimental autoimmune uveitis. Int Immunopharmacol 2009;9:1079–1086. doi: 10.1016/j.intimp.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Naveh N Melanocortins applied intravitreally delay retinal dystrophy in Royal College of Surgeons rats. Graefes Arch Clin Exp Ophthalmol 2003;241:1044–1050. doi: 10.1007/s00417-003-0781-y. [DOI] [PubMed] [Google Scholar]
- 36.Blum JS, Wearsch PA, Cresswell P. Pathways of antigen processing. Annu Rev Immunol 2013;31:443–473. doi: 10.1146/annurev-immunol-032712-095910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Namba K, Kitaichi N, Nishida T, Taylor AW. Induction of regulatory T cells by the immunomodulating cytokines alpha-melanocyte-stimulating hormone and transforming growth factor-beta 2. J Leukocyte Biol 2002;72:946–952. doi. [PubMed] [Google Scholar]
- 38.Taylor AW, Yee DG, Nishida T, Namba K. Neuropeptide regulation of immunity. The immunosuppressive activity of alpha-melanocyte-stimulating hormone (alpha-MSH). Ann N Y Acad Sci 2000;917:239–247. doi. [DOI] [PubMed] [Google Scholar]
- 39.Zamiri P, Masli S, Kitaichi N, Taylor AW, Streilein JW. Thrombospondin plays a vital role in the immune privilege of the eye. Invest Ophthalmol Vis Sci 2005;46:908–919. doi: 10.1167/iovs.04-0362. [DOI] [PubMed] [Google Scholar]
- 40.Zarate-Blades CR, Horai R, Mattapallil MJ, et al. Gut microbiota as a source of a surrogate antigen that triggers autoimmunity in an immune privileged site. Gut Microbes 2017;8:59–66. doi: 10.1080/19490976.2016.1273996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Luger D, Silver PB, Tang J, et al. Either a Th17 or a Th1 effector response can drive autoimmunity: conditions of disease induction affect dominant effector category. The Journal of experimental medicine 2008;205:799–810. doi: 10.1084/jem.20071258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dimitrijevic M, Stanojevic S. The intriguing mission of neuropeptide Y in the immune system. Amino Acids 2013;45:41–53. doi: 10.1007/s00726-011-1185-7. [DOI] [PubMed] [Google Scholar]
- 43.Bedoui S, von Horsten S, Gebhardt T. A role for neuropeptide Y (NPY) in phagocytosis: implications for innate and adaptive immunity. Peptides 2007;28:373–376. doi: 10.1016/j.peptides.2006.07.029. [DOI] [PubMed] [Google Scholar]
- 44.Dimitrijevic M, Stanojevic S, Vujic V, Beck-Sickinger A, von Horsten S. Neuropeptide Y and its receptor subtypes specifically modulate rat peritoneal macrophage functions in vitro: counter regulation through Y1 and Y2/5 receptors. Regul Pept 2005;124:163–172. doi: 10.1016/j.regpep.2004.07.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.