Abstract
Preclinical and early phase clinical studies suggest that an appropriately dosed umbilical cord blood (CB) infusion has the potential to help improve motor function in young children with cerebral palsy (CP). As many children with CP do not have their own CB available, use of allogeneic cells would extend access to this potentially beneficial therapy to more children. In this phase I, open‐label study, 15 children, aged 1 to 6 years, with moderate to severe spastic CP were treated with a single intravenous infusion of allogeneic human leukocyte antigen (HLA) matched or partially matched sibling CB with a cell dose of ≥2.5 × 107 cells/kg based on the pre‐cryopreservation count (median infused cell dose, 3.3 × 107; range, 1.8‐5.2 × 107). There were a total of 49 adverse events (AEs) over a 2‐year time period, but there were no AEs related to the CB infusions. Specifically, there were no acute infusion reactions and no antibody formation against platelets, red blood cells, or donor‐specific HLA antigens. Donor cells were not detected in peripheral blood 6 months later. Six months after infusion, participants were assessed for response and experienced a mean ± SD increase of 4.7 ± 2.5 points on the Gross Motor Function Measure‐66 and 1 ± 2.9 points on the Peabody Gross Motor Quotient. Appropriately dosed, allogeneic partially or fully HLA‐matched sibling CB infusion is well tolerated and potentially beneficial in young children with CP.
Keywords: cellular therapy, clinical trials, cord blood, human cord blood, nervous system, umbilical cord blood
Lessons learned
Sibling umbilical cord blood (CB) infusions are well tolerated in young children with cerebral palsy (CP).
Although children treated with sibling CB demonstrated gains in motor function, this cannot be definitively attributed to CB treatment in this phase I study.
Randomized clinical trials are needed to determine efficacy of CB therapy and should evaluate the use of donor products.
Significance statement
In this study, 15 children with cerebral palsy (CP) were treated with sibling cord blood (CB) infusions. The infusions were well tolerated, and children demonstrated motor improvements, laying the groundwork for efficacy trials using umbilical CB from unrelated donors.
1. INTRODUCTION
Cerebral palsy (CP) is a form of acquired brain injury caused by nonprogressive insults to the fetal or neonatal brain.1 In the USA alone, approximately 10 000 babies and infants are diagnosed with CP each year, making it the most common motor disorder of childhood.2 For many affected children, the cause of CP is often an injury sustained before or shortly after birth, resulting in a wide range of symptoms including abnormal muscle tone and motor function. Children with CP embark on a lifelong journey of physical and occupational therapies to try to maximize function and quality of life. Although some pharmacologic, surgical, and other supportive care options are available to help reduce spasticity in selected children, no curative therapies are available.
Preclinical and early phase clinical studies suggest that umbilical cord blood (CB) infusion has potential to help improve motor function in CP.3, 4, 5, 6, 7, 8 We recently reported results of a phase II randomized, placebo‐controlled trial of autologous CB infusion, which demonstrated that children who received higher doses of CB cells (>2.5 × 107 cells/kg cryopreserved and >2.0 × 107 cells/kg infused) exhibited a greater degree of improvement in gross motor function than children who received lower cell doses or placebo.9 As many children with CP do not have their own CB available, we hypothesized that the use of related allogeneic cells would be a way to extend this therapy to more children who may benefit. Since allogeneic cells theoretically posed risks not seen with autologous cells, such as graft‐vs‐host disease or alloimmunization, we conducted a phase I, open‐label study of a higher dosed, single human leukocyte antigen (HLA)‐matched or partially matched sibling CB infusion in children with CP to describe the safety and efficacy of this approach.
2. MATERIALS AND METHODS
2.1. Study design
This study was designed as a phase I, open‐label trial of a single intravenous infusion of HLA‐matched or partially HLA‐matched allogeneic sibling CB in young children with CP (Figure 1). Eligible patients whose parent(s) provided informed consent were treated with a single CB infusion at baseline and assessed for safety and motor function outcomes at 6 months after infusion. No immunosuppression or conditioning was given prior to CB infusion. Additional safety endpoints were assessed remotely at 12 and 24 months. The study was approved by the Duke University Institutional Review Board (Pro00065043), conducted under IND 16615 from the U.S. Food and Drug Administration, and registered at ClinicalTrials.gov (NCT02599207).
FIGURE 1.
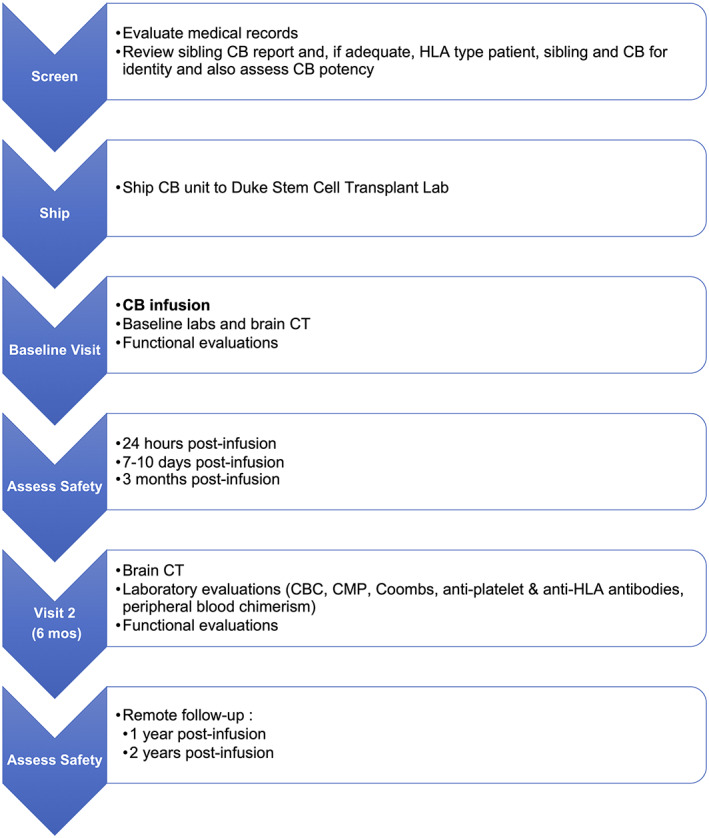
Study schema. CB, cord blood; CBC, complete blood count; CMP, complete metabolic panel; CT, computed tomography; HLA, human leukocyte antigen
2.2. Patient population
Fifteen participants aged 1 to 6 years with isolated CP and an available HLA‐matched or haploidentical, qualified, CB unit from a sibling with the same biological parents were enrolled. The Gross Motor Function Classification System (GMFCS) was assessed by a pediatric neurologist and used to classify participants' functional level. Children with CP and a GMFCS level of 2 to 4 were eligible to participate. Children who had GMFCS level 1 were eligible if they had bilateral CP and were ≥2 years of age or had hemiplegia and used their affected hand as an assist only. Children had to have normal blood counts and lymphocyte counts. Participants' prior brain imaging was reviewed, and children with possible brain malformations or known genetic conditions were excluded. In addition, children with autism, hypsarrhythmia, intractable seizures, need for respiratory support, or evidence of a genetic or progressive neurologic disease, active systemic infection, prior chemotherapy, or immunosuppressive therapy were ineligible. Children who had received prior cell therapy or who had an available qualified autologous CB unit were also excluded.
2.3. Sibling CB units
Study participation required the availability of a banked unit of allogeneic sibling CB that had a pre‐cryopreservation total nucleated cell count (TNCC) ≥2.5 × 107 cells/kg, pre‐cryopreservation cell viability ≥80%, negative sterility cultures, negative maternal infectious disease screening (minimally including hepatitis B, hepatitis C, HIV, human T‐cell lymphotropic virus I/II, and syphilis) and was at least a 4/8 HLA molecular match with the participant. HLA matching and CB unit identity confirmation was determined via testing of the participant, sibling donor, and sibling CB sample via low‐resolution typing of HLA‐A, ‐B, and ‐C and high‐resolution typing of HLA‐DRB1. Prior to study enrollment, a sample of the sibling CB unit was shipped to Duke for potency testing on a segment attached to the CB unit and had to have a post‐thaw CD45 viability of ≥40% to be eligible. Post‐thaw, a sample from the infused CB unit was tested for TNCC, viability, viable CD34 cell content, myeloerythroid progenitor cell content (colony forming units [CFU]), and sterility.
2.4. CB infusion
On the day of infusion, the allogeneic sibling CB unit was thawed and washed in the standard fashion10 in dextran 40 + 5% human serum albumin (DA) and placed in 1.25 mL/kg DA for administration with a goal dose of 2.5 to 5 × 107 total nucleated cells/kg. Participants were premedicated with single intravenous doses of 0.5 mg/kg diphenhydramine and 0.5 mg/kg methylprednisolone and, if compliant, oral 10 to 15 mg/kg acetaminophen 15 to 60 minutes prior to the infusion. The allogeneic sibling CB was administered intravenously through a peripheral intravenous catheter over 15 minutes in the outpatient setting under direct physician supervision. Participants received maintenance intravenous fluids and were monitored for 1 to 2 hours after infusion.
2.5. Safety and motor evaluations
Participants were evaluated at baseline and 6 months with a complete neurologic examination, brain computed tomography, laboratory studies (blood counts, chemistries, Coombs, anti‐platelet and anti‐HLA antibodies, peripheral blood chimerism via whole blood restriction fragment length polymorphism), and motor evaluations. The Gross Motor Function Measure‐66 (GMFM‐66), Peabody Developmental Motor Scales, and Assisting Hand Assessment were performed by pediatric physical and occupational therapists trained in these measures who underwent reliability training. Additional safety assessments were conducted in person 1 day after infusion and remotely via parental questionnaires at 2 weeks and 3, 12, and 24 months after infusion, including specific questions regarding symptoms of graft‐vs‐host disease (ie, diarrhea, rash, transfusion, immune conditions). Adverse events (AEs) were captured at these scheduled time points and reported by parents between time points. When available, supporting documentation regarding reported AEs was obtained and reviewed. AEs were adjudicated by the study team and classified based on Common Terminology Criteria for AEs version 5.0.
2.6. Statistical analysis
The frequency of AEs was analyzed descriptively by participant, severity, and relatedness to study product as determined by the investigator. Motor function outcomes were analyzed descriptively, and 95% confidence intervals (CIs) were reported based on the t distribution. With 15 enrolled participants, the study had a high probability (~80%) of identifying at least one common product‐related AE that occurs in 10% of infusions, based on binomial probabilities.
3. RESULTS
3.1. Participant characteristics
Fifteen children (5 boys, 10 girls), median age 3.7 years (range, 1‐6 years), with spastic CP were enrolled between November 2015 and April 2016 (Table 1). The majority of patients (n = 13) had bilateral CP, with 10 of 15 having all 4 limbs affected. Baseline GMFCS levels ranged from 2 to 4, with a median of 3. Etiologies of CP included periventricular leukomalacia (n = 6), hypoxic ischemic encephalopathy (n = 3), in utero stroke or bleed (n = 3), kernicterus (n = 1), meningitis (n = 1), and postnatal ischemia secondary to cardiac disease (n = 1).
TABLE 1.
Participant and sibling umbilical cord blood characteristics (n = 15)
| Characteristics | Median (range) or n (%) |
|---|---|
| Patient characteristics | |
| Age, years | 3.7 (1.4‐6.0) |
| Sex | |
| Male | 5 (33.3) |
| Female | 10 (66.7) |
| Race | |
| White | 14 (93.3) |
| Non‐White | 1 (6.7) |
| Type of cerebral palsy | |
| Spastic quadriplegia | 10 (66.7) |
| Spastic tetraplegia | 1 (6.7) |
| Spastic diplegia | 2 (13.3) |
| Spastic hemiplegia | 2 (13.3) |
| GMFCS level | |
| II | 3 (20) |
| III | 5 (33.3) |
| IV | 7 (46.7) |
| Baseline GMFM‐66 score | 37 (23‐58) |
| Cord blood characteristics | |
| Precryopreservation | |
| TNCC, ×108 | 7.2 (3.3‐14.9) |
| Cell dose used, ×107 cells/kg | 4.6 (2.7‐7.7) |
| Total CD34+, ×106 | 2.8 (0.7‐8.5) |
| Viability, % | 88 (80‐98) |
| Post‐thaw | |
| TNCC, ×108 | 4.3 (1.8‐6.6) |
| Cell dose infused, ×107 cells/kg | 3.3 (1.8‐5.2) |
| TNCC recovery, % | 67 (45‐84) |
| Viable CD34+ dose infused, ×105 cells/kg | 0.6 (0.1‐1.8) |
| Viability, % | 97 (93‐99) |
| CFU per 105 cells | 62.5 (0‐133) |
| HLA match | |
| 8/8 | 4 (26.7) |
| 5/8 | 1 (6.7) |
| 4/8 | 10 (66.7) |
Abbreviations: CFU, colony forming units; GMFCS, Gross Motor Function Classification System; GMFM‐66, Gross Motor Function Measure‐66; HLA, human leukocyte antigen; TNCC, total nucleated cell count.
3.2. CB characteristics
Allogeneic sibling CB units were obtained from eight different family CB banks. The first four participant/sibling pairs were 8/8 HLA matches at HLA‐A, ‐B, ‐C, and ‐DRB1. The remaining participant/sibling pairs were 5/8 (n = 1) or 4/8 (n = 10) HLA matches. The median pre‐cryopreservation cell dose of the entire sibling CB unit was 5.5 × 107 total nucleated cells/kg (range, 2.8‐13.3 × 107 cells/kg). To achieve the targeted cell dose, the entire sibling CB unit was used for infusion in eight participants. In the other seven participants, a portion of the sibling CB unit was used for infusion, and the remainder was stored for potential future use. Allogeneic sibling CB infusions delivered a median TNCC of 3.3 × 107 cells/kg (range, 1.8‐5.2 × 107 cells/kg) and median viable CD34 dose of 0.6 × 105 cells/kg (range, 0.1‐1.8 × 105 cells/kg). All CB products had negative post‐thaw sterility cultures. Fourteen of fifteen CB units exhibited CFU growth post‐thaw.
3.3. Safety of sibling CB infusions
All participants received their allogeneic sibling CB infusion as intended. There were no acute infusion reactions. At 6 months after infusion, there were no unexpected findings on brain computed tomography and no donor cells detected in peripheral blood. Prior to infusion, 5 of 15 participants had detectable HLA antibodies. Six months after infusion, two participants with undetectable HLA antibodies at baseline demonstrated new low‐titer HLA antibodies that were not were donor specific. No patients developed cytopenias, required transfusions, had any evidence of graft‐vs‐host disease, or developed an autoimmune or alloimmune condition during the study. Thus, the HLA antibodies were not considered to be clinically significant. No participants developed new antibodies to platelets or red blood cells.
With 2 years of follow‐up, there were a total of 49 AEs in 14 participants (Figure 2). None of these events were related to the CB infusion, and most (28/49) were attributed to common childhood ailments. Four participants reported skin changes during the study period. One had an isolated rash on their cheek a week after infusion, one had a transient rash associated with a febrile illness 2 months after infusion, one was diagnosed with eczema 3 months after infusion, and one had some skin breakdown on their finger 5 months after infusion. One participant experienced diarrhea associated with fever and vomiting approximately 11 weeks after CB infusion. This illness was self‐limited and consistent with viral gastroenteritis. None of these instances were consistent with graft‐vs‐host disease.
FIGURE 2.

Adverse events observed up to 24 months after sibling umbilical cord blood infusion
Ten of the 49 AEs were serious and resulted in hospitalization in 9 participants. Of these, four admissions were for elective surgical procedures after the 6‐month evaluation, two were for increased or prolonged seizures in patients with a history of seizures, two were for dehydration in the setting of suspected viral illnesses, one was for constipation, and one was for cellulitis requiring intravenous antibiotics.
3.4. Motor evaluations
Six months after CB infusion, children improved by a mean ± SD of 4.7 ± 2.5 points on the GMFM‐66 (95% CI, 3.4‐6.2) and 1 ± 2.9 points on the Peabody Gross Motor Quotient (95% CI, −0.6, 2.6). Regarding fine motor assessments, participants improved a mean ± SD of 0.1 ± 7.2 points on the Peabody Fine Motor Quotient (95% CI, −3.9, 4.1) and 5.3 ± 3.2 points on the Assisting Hand Assessment Interval Score (95% CI, 3.5‐7.1). Motor evaluation results are shown in aggregate in Table 2 and by patient in Table 3.
TABLE 2.
Motor evaluations
| Measure | Baseline, mean ± SD | 6 months, mean ± SD | Change score, mean ± SD (95% CI) |
|---|---|---|---|
| GMFM‐66 | 37.0 ± 9.1 | 41.7 ± 9.1 | 4.7 ± 2.5a (3.4‐6.2) |
| PDMS‐Gross Motor Quotient | 47.7 ± 7.7 | 48.7 ± 8.4 | 1.0 ± 2.9 (−0.6, 2.6) |
| PDMS‐Fine Motor Quotient | 63.3 ± 15.9 | 63.4 ± 12.9 | 0.1 ± 7.2 (−3.9, 4.1) |
| AHA interval score | 44.6 ± 20.4 | 49.9 ± 19.6 | 5.3 ± 3.2 (3.5‐7.1) |
Exceeds minimum clinically important difference of large effect size.
Abbreviations: AHA, Assisting Hand Assessment; CI, confidence interval; GMFM‐66, Gross Motor Function Measure‐66; PDMS, Peabody Developmental Motor Scales.
TABLE 3.
Patient and donor characteristics and GMFM‐66 scores by participant
| Participant | Cerebral palsy | CB unit | GMFM‐66 scores | Elective surgerya | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age, years | Sex | Typography | Etiology | GMFCS level | HLA match | Infused TNCC, ×107 cells/kg | Baseline | 6 months | ||
| 1 | 3.7 | F | Quadriplegia | HIE | 4 | 8/8 | 3.2 | 50 | 52 | Hip reconstruction |
| 2 | 3.8 | F | Hemiplegia | in utero stroke | 3 | 8/8 | 4.1 | 40 | 42 | — |
| 3 | 2.8 | M | Quadriplegia | PVL | 4 | 8/8 | 2.7 | 32 | 38 | SDR |
| 4 | 3.7 | F | Quadriplegia | PVL | 3 | 8/8 | 1.9 | 33 | 36 | — |
| 5 | 2.4 | F | Quadriplegia | HIE | 3 | 4/8 | 5.0 | 43 | 49 | — |
| 6 | 5.9 | M | Quadriplegia | kernicterus | 2 | 4/8 | 3.3 | 50 | 53 | — |
| 7 | 1.8 | M | Hemiplegia | in utero stroke | 2 | 4/8 | 1.8 | 37 | 48 | — |
| 8 | 5.6 | F | Quadriplegia | meningitis | 4 | 4/8 | 4.0 | 25 | 31 | — |
| 9 | 3.7 | F | Quadriplegia | HIE | 4 | 5/8 | 2.3 | 23 | 30 | — |
| 10 | 1.7 | F | Diplegia | PVL | 3 | 4/8 | 5.2 | 32 | 36 | — |
| 11 | 4.7 | M | Quadriplegia | PVL | 4 | 4/8 | 1.8 | 47 | 52 | — |
| 12 | 1.0 | M | Triplegia | in utero bleed | 2 | 4/8 | 4.7 | 37 | 42 | SDR |
| 13 | 4.8 | F | Quadriplegia | stroke | 4 | 4/8 | 3.9 | 37 | 39 | SDR |
| 14 | 1.4 | M | Diplegia | PVL | 4 | 4/8 | 3.6 | 23 | 25 | — |
| 15 | 6.6 | F | Quadriplegia | PVL | 3 | 4/8 | 2.3 | 46 | 52 | — |
All elective surgeries were performed after the 6‐month motor assessment.
Abbreviations: CB, umbilical cord blood; F, female; GMFCS, Gross Motor Function Classification System; GMFM‐66, Gross Motor Function Measure‐66; HIE, hypoxic ischemic encephalopathy; HLA, human leukocyte antigen; M, male; PVL, periventricular leukomalacia; SDR, selective dorsal rhizotomy; TNCC, total nucleated cell count.
4. DISCUSSION
We report results of an open‐label pilot study testing the safety of intravenous infusions of allogeneic fully or partially matched sibling umbilical CB in young children with CP. Infusions were administered without prior conditioning or immunosuppression and were well tolerated without any evidence of AEs related to the CB therapy immediately or within the first 2 years following treatment. CB was dosed to deliver a minimum of 2 × 107 cells/kg based on observations in prior studies suggesting a potential dose threshold for efficacy.9 In this study, all participants received a dose that was significantly greater than this threshold, and all patients demonstrated improvement in gross motor function 6 months after the CB infusion.
The use of allogeneic sibling CB units extended CB therapy beyond autologous use. This is particularly relevant in treating children with CP as many affected children are born prematurely or under traumatic circumstances, often making CB collection challenging or impossible at the time of their birth. This was a limitation in terms of both enrollment and accessibility to treatment in our prior study of autologous CB infusion in children with CP.9 The use of allogeneic sibling CB in the current study enabled the treatment of children whose own CB was not collected at the time of their birth. Notably, a higher median infused dose was achieved with the sibling CB used in this study compared with autologous CB used in our prior study (3.3 × 107 vs 2.0 × 107 cells/kg). As all of the CB units were banked at family CB banks, collection and banking practices were similar between the two studies. The pre‐cryopreservation TNCC of allogeneic sibling units also exceeded that of the autologous units (7.2 × 108 vs 4.9 × 108), suggesting that the siblings of children with CP may have had less complicated births and also may have been healthier at birth, resulting in larger CB collections.
In using CB cell therapies for neurologic conditions, donor cell engraftment is not necessary for therapeutic benefit, as the cells act via paracrine effects.8, 11 This has been demonstrated in vitro and in vivo in multiple preclinical models.12, 13 Although the mechanism of action by which CB may improve motor function in children with CP is not completely understood, improved connectivity in the motor tracks has been demonstrated in treated children.9 We have also explored potential ways that this could be occurring and have demonstrated that CB CD14+ cells protect brain tissue from hypoxic injury in organotypic models.7 In a mouse brain organotypic slice culture model of hypoxic injury via oxygen and glucose deprivation, CB reduced microglial and astrocyte activation and prevented neuronal death with or without cell‐to‐cell contact. We hypothesized that this was through signaling to endogenous macrophages and oligodendrocytes that in turn remyelinate damaged neuronal tracks. The CD14+ monocyte population was identified as the potential active cell type, as treatment with CD14+ cell‐depleted CB was not neuroprotective.7 A similar finding was reported in a rat model of brain injury following middle cerebral artery occlusion (MCAO), in which administration of human CB improved motor function following MCAO, but depletion of the CD14+ cell fraction did not result in motor function improvement.14 Almost all such models are xenogeneic, demonstrating that CB cells do not need to be autologous to have potential therapeutic effects.
Nevertheless, the use of donor cells carries theoretical risks beyond those of autologous cells, including the potential for graft‐vs‐host disease and immune sensitization. Without employing a preconditioning regimen, donor cell engraftment after allogeneic sibling CB infusion is not anticipated in immunocompetent patients, and this was evidenced by the fact that we did not detect any donor cells in the peripheral blood of CB recipients 6 months after infusion. In the absence of donor cell engraftment, there was no reason to anticipate the development graft‐vs‐host disease, which was not seen in any of the patients treated on this study. Also importantly, there was no evidence of immune activation as assessed by the lack of donor‐specific antibodies against HLA, red blood cells, or platelets following sibling CB infusion in this study.
This trial was a phase I safety study and was not designed to confirm efficacy. However, treated patients demonstrated significant gains in both gross and fine motor function on the GMFM‐66 and the Assisting Hand Assessment within the 6 months after allogeneic sibling CB infusion. The GMFM‐66 is an accepted measure of motor function capability specific to CP and is highly recommended as a motor function outcome for CP research by the National Institute of Neurological Disorders and Stroke.15 Minimum clinically important differences (MCIDs) of large effect size have been reported as ranging from 1.2 to 2.7 points for the GMFM‐66 among ambulatory children with CP,16 and it has been suggested that MCIDs may be even lower in more severely affected children.17 The observed mean GMFM‐66 improvement of 4.7 points exceeds this threshold, indicating a clinically significant improvement. Limitations of the study included its small sample size, the lack of a control group, and the single point of follow‐up for efficacy (6 months) by the study team. Parents completed questionnaires at 2 weeks and 3, 12, and 24 months following treatment to report AEs or other events. These were reviewed by the study team and coded. All participants continued to receive medications and physical and occupational therapies as prescribed by their local physicians and therapists. The main objective of the study was to report the safety of allogeneic sibling CB infusions in this population, and we can conclude that they are well tolerated. Efficacy was described at a single point in time (6 months), and given that other concomitant therapies were not interrupted or stopped, the gains observed cannot be definitively attributed to the CB treatment. Nonetheless, these gains were also observed in our randomized, blinded, placebo‐controlled study of autologous blood and are interesting in that light.
5. CONCLUSION
Allogeneic sibling CB infusion was found to be well tolerated and feasible in young children with CP and extended the availability of CB treatment to children whose own CB was not available for use. To further expand access to this therapy and to further test the observed cell dose effect, the safety and efficacy of high‐dose allogeneic, unrelated‐donor, partially matched CB infusion is being studied in an ongoing clinical trial.
CONFLICT OF INTEREST
The authors report grant funding from the Julian Robertson Foundation (principal investigator [PI]: J.K.), the Marcus Foundation (PI: J.K.), and the Dana Foundation (PI: J.M.S.). J.M.S. declared intellectual property rights/patent ownership with Sinocell Technologies, Inc. (licensed data), advisory role with Medical Device Business Services, Biosense Webster (spouse), honoraria from Medtronic (spouse), and research funding from the Marcus Foundation. L.E.C. declared leadership position/employment at Duke University; advisory role with Genzyme Corporation of Sanofi; honoraria from Genzyme Corporation of Sanofi, Sarepta, Muscular Dystrophy Association; and research funding from the Robertson Foundation, the Marcus Foundation, Genzyme Corporation of Sanofi, Amicus, Biogen, Ultragenyx, NS Pharma, Reveragen, Pfizer, CINRG (Cooperative International Neuromuscular Research Group), TRiNDS (Therapeutic Research in Neuromuscular Disorders Solutions), AveXis, and AskBio. J.T. reported intellectual property rights/patent ownership from 62/470431, 16/493754, JP Appln No. 2019‐549 537, KR Appln No. 10‐2019‐7029 841, U.S. Appln No. 16/493754; advisory role AegisCN, LLC, Gamida Cell, Synthetic Biologics, The EMMES Corporation, and Cohortias International; honoraria from Gamida Cell, Synthetic Biologics, and The EMMES Corporation; and research funding from Seattle Genetics and Bristol Myers Squibb. J.K. declared intellectual property rights with pending licensing agreement with CryoCell and serves as the Director of the Carolinas Cord Blood Bank, a U.S. Food and Drug Administration‐licensed public cord blood bank located at Duke University and Medical Director of CryoCell, a hybrid cord blood bank. The other authors declared no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
J.M.S., J.K.: conception/design, collection and/or assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript; L.E.C., C.M., B.W.‐P.: collection and/or assembly of data, final approval of manuscript; M.A.M., J.J., G.W.: conception/design, collection and/or assembly of data, final approval of manuscript; J.T.: data analysis and interpretation, manuscript writing, final approval of manuscript.
ACKNOWLEDGMENTS
This study was supported by a grant from the Marcus Foundation. Many thanks to the participants and families for their dedication and commitment to participating in this trial. The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available because of privacy or ethical restrictions.
Sun JM, Case LE, Mikati MA, et al. Sibling umbilical cord blood infusion is safe in young children with cerebral palsy. STEM CELLS Transl Med. 2021;10(9):1258–1265. 10.1002/sctm.20-0470
Authored by a member of CBA.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1.Bax M, Goldstein M, Rosenbaum P, et al. Proposed definition and classification of cerebral palsy, April 2005. Dev Med Child Neurol. 2005;47(8):571‐576. [DOI] [PubMed] [Google Scholar]
- 2.Christensen D, Van Naarden BK, Doernberg NS, et al. Prevalence of cerebral palsy, co‐occurring autism spectrum disorders, and motor functioning—Autism and Developmental Disabilities Monitoring Network, USA, 2008. Dev Med Child Neurol. 2014;56:59‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kang M, Min K, Jang J, et al. Involvement of immune responses in the efficacy of cord blood cell therapy for cerebral palsy. Stem Cells Dev. 2015;24:2259‐2268. [DOI] [PubMed] [Google Scholar]
- 4.Min K, Song J, Kang JY, et al. Umbilical cord blood therapy potentiated with erythropoietin for children with cerebral palsy: a double‐blind, randomized, placebo‐controlled trial. Stem Cells. 2013;31:581‐591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee YH, Choi KV, Moon JH, et al. Safety and feasibility of countering neurological impairment by intravenous administration of autologous cord blood in cerebral palsy. J Transl Med. 2012;10:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vinukonda G, Liao Y, Hu F, et al. Human cord blood‐derived unrestricted somatic stem cell infusion improves neurobehavioral outcome in a rabbit model of intraventricular hemorrhage. Stem Cells Translational Medicine. 2019;8:1157‐1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saha A, Patel S, Xu L, et al. Human umbilical cord blood monocytes, but not adult blood monocytes, rescue brain cells from hypoxic‐ischemic injury: mechanistic and therapeutic implications. PLoS One. 2019;14:e0218906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drobyshevsky A, Cotten CM, Shi Z, et al. Human umbilical cord blood cells ameliorate motor deficits in rabbits in a cerebral palsy model. Dev Neurosci. 2015;37:349‐362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun JM, Song AW, Case LE, et al. Effect of autologous cord blood infusion on motor function and brain connectivity in young children with cerebral palsy: a randomized, placebo‐controlled trial. Stem Cells Translational Medicine. 2017;6:2071‐2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rubinstein P, Dobrila L, Rosenfield RE, et al. Processing and cryopreservation of placental/umbilical cord blood for unrelated bone marrow reconstitution. Proc Natl Acad Sci USA. 1995;92:10119‐10122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Surugiu R, Olaru A, Hermann DM, Glavan D, Catalin B, Popa‐Wagner A. Recent advances in mono‐ and combined stem cell therapies of stroke in animal models and humans. Int J Mol Sci. 2019;20:6029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bae SH, Kong TH, Lee HS, et al. Long‐lasting paracrine effects of human cord blood cells on damaged neocortex in an animal model of cerebral palsy. Cell Transplant. 2012;21:2497‐2515. [DOI] [PubMed] [Google Scholar]
- 13.Ahn SY, Chang YS, Sung DK, Sung SI, Ahn JY, Park WS. Pivotal role of brain‐derived neurotrophic factor secreted by mesenchymal stem cells in severe intraventricular hemorrhage in newborn rats. Cell Transplant. 2017;26:145‐156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Womble TA, Green S, Shahaduzzaman M, et al. Monocytes are essential for the neuroprotective effect of human cord blood cells following middle cerebral artery occlusion in rat. Mol Cell Neurosci. 2014;59:76‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schiariti V, Fowler E, Brandenburg JE, et al. A common data language for clinical research studies: the National Institute of Neurological Disorders and Stroke and American Academy for Cerebral Palsy and Developmental Medicine Cerebral Palsy Common Data Elements Version 1.0 recommendations. Dev Med Child Neurol. 2018;60:976‐986. [DOI] [PubMed] [Google Scholar]
- 16.Oeffinger D, Bagley A, Rogers S, et al. Outcome tools used for ambulatory children with cerebral palsy: responsiveness and minimum clinically important differences. Dev Med Child Neurol. 2008;50:918‐925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Storm FA, Petrarca M, Beretta E, et al. Minimum clinically important difference of gross motor function and gait endurance in children with motor impairment: a comparison of distribution‐based approaches. Biomed Res Int. 2020;2020:2794036. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


