Abstract
Human bone marrow‐derived mesenchymal stem/stromal cells (BM‐MSCs) represent promising stem cell therapy for the treatment of type 2 diabetes mellitus (T2DM), but the results of autologous BM‐MSC administration in T2DM patients are contradictory. The purpose of this study was to test the hypothesis that autologous BM‐MSC administration in T2DM patient is safe and that the efficacy of the treatment is dependant on the quality of the autologous BM‐MSC population and administration routes. T2DM patients were enrolled, randomly assigned (1:1) by a computer‐based system into the intravenous and dorsal pancreatic arterial groups. The safety was assessed in all the treated patients, and the efficacy was evaluated based on the absolute changes in the hemoglobin A1c, fasting blood glucose, and C‐peptide levels throughout the 12‐month follow‐up. Our data indicated that autologous BM‐MSC administration was well tolerated in 30 T2DM patients. Short‐term therapeutic effects were observed in patients with T2DM duration of <10 years and a body mass index <23, which is in line with the phenotypic analysis of the autologous BM‐MSC population. T2DM duration directly altered the proliferation rate of BM‐MSCs, abrogated the glycolysis and mitochondria respiration of BM‐MSCs, and induced the accumulation of mitochondria DNA mutation. Our data suggest that autologous administration of BM‐MSCs in the treatment of T2DM should be performed in patients with T2DM duration <10 years and no obesity. Prior to further confirming the effects of T2DM on BM‐MSC biology, future work with a larger cohort focusing on patients with different T2DM history is needed to understand the mechanism underlying our observation.
Keywords: autologous, autologous stem cell transplantation, bone marrow, diabetes
Human bone marrow‐derived mesenchymal stem/stromal cells (BM‐MSCs) represent promising stem cell therapy for the treatment of type 2 diabetes, but the results of autologous BM‐MSC admniistration in T2DM patients are contradicted. Our data indicated that autologous BM‐MSC administration was well tolerated in 30 T2DM patients. The potential therapeutic effects of the treatments were observed in patients with less than 10 years of T2DM and a BMI<23, and this finding could be explained by reductions in autologous stem cell phenotypes, including prolonged cell proliferation, reduced metabolic functions, and alterations in mtDNA.
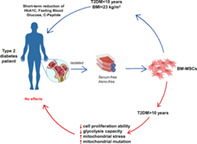
Lessons learned.
Autologous bone marrow‐derived mesenchymal stem cell administration exhibited a reduction in HbA1c and fasting blood glucose level in patients with a type 2 diabetes mellitus (T2DM) duration of <10 years and a body mass index of <23, and the effectiveness appeared to diminish after 6 months.
The duration of T2DM directly altered the phenotype of bone marrow‐derived mesenchymal stem cells by reducing the proliferation rate, compromising the glycolysis process, reducing mitochondrial functions, and changing mitochondrial variants.
These results support the need for stem cell intervention earlier after diagnosis, rather than later, for patients diagnosed with T2DM.
Significance statement.
This study revealed that autologous administration of bone marrow‐derived mesenchymal stem cells (BM‐MSCs) is safe for patients with type 2 diabetes mellitus (T2DM) and that there is no significant difference in efficacy between intravenous and dorsal pancreatic artery infusion. Administration leads to short‐term reduction in hemoglobin A1c and fasting blood glucose levels in patients with T2DM history <10 years and body mass index <23. Phenotypic analysis provisionally links treatment effectiveness to duration of T2DM. This study suggests that autologous administration of BM‐MSCs in treatment of T2DM should be performed in patients with T2DM duration <10 years and no obesity.
1. INTRODUCTION
Diabetes mellitus (DM) is one of the leading causes of death and disability worldwide. This chronic metabolic disease occurs when the pancreas does not produce sufficient insulin or when insulin resistance results in hyperglycemia.1 Type 2 diabetes mellitus (T2DM), one of the three types of DM, accounts for 85% to 95% of all cases. Patients with T2DM often suffer from insulin resistance in insulin‐regulated cell types and/or impaired insulin secretion.2 In 2017, approximately 425 million people were diagnosed with DM worldwide, and this number is expected to increase by 50% in 2035.3 Vietnam experienced a twofold increase in the incidence of DM from 2.7% in 2002 to 5.4% in 2010.4 An impaired quality of life, comorbidities, and financial burdens are major problems for patients with T2DM.
Stem cell administration has recently emerged as a potential candidate treatment for T2DM because of its immunomodulatory properties and regenerative ability to stimulate the recovery of the injured pancreas.5 Based on a large number of preclinical studies using animal models, several human clinical trials were conducted to evaluate the safety and efficacy of stem cell therapy in the treatment of T2DM.6 Among the different types of stem cells, bone marrow‐derived mononuclear cells (BM‐MNCs) and BM‐derived mesenchymal stem/stromal cells (BM‐MSCs) are the most tested cell types and have been suggested to be safe in the treatment of T2DM.7, 8
BM‐MSCs are multipotent progenitor cells that differentiate into mesodermal lineages, including adipocytes, chondrocytes, and osteoblasts, according to the International Society for Cell and Gene Therapy (ISCT).9 They were first identified by Friedenstein in the 1960s and have been proven to play a significant role in bone homeostasis.10 BM‐MSCs have since become a prominent candidate in regenerative medicine because of their special biological characteristics, including ease of isolation, proliferation capacity, and immunomodulatory effects via paracrine mechanisms.11 Moreover, these cells can also activate other tissue‐specific stem cells and stimulate neoangiogenesis processes.12 Because of their special therapeutic applications, BM‐MSCs have been widely used to treat various disorders, including cardiovascular disease, liver regeneration, bone and cartilage healing, neurodegenerative conditions, and pulmonary distress syndrome.11
In patients with T2DM, autologous BM‐MSC infusion reportedly reduces insulin requirement and hemoglobin A1c (HbA1c) levels, although the duration of the effectiveness of the treatment is relatively short.7 Two clinical trials have shown that a loss of stem cell effectiveness was observed 9 months and 2 years after administration.13, 14 The efficacy of BM‐MSC administration in patients with T2DM is also contradictory, as a systematic review conducted in 2016 reported that stem cell therapy improves C‐peptide levels, whereas a second review in 2018 reported an opposite conclusion.15, 16 To date, only one study has attempted to evaluate the effects of autologous BM‐MNCs administered via three routes, including the superior pancreaticoduodenal artery, splenic artery, and peripheral intravenous route.8 Therefore, an evaluation of the safety and effectiveness of autologous BM‐MSC administration in patients with T2DM based on the quality of the stem cells themselves and the administration routes is important.
The relationship between cellular metabolism and function has been reported since the early 1960s.17 BM‐MSCs maintain their quiescence in the BM niche for a long period or initiate a sequential differentiation process to regenerate functional cells in response to exogenous signals, such as inflammatory signals. In their quiescent state, BM‐MSCs depend on glycolysis to maintain their undifferentiated state, cease their proliferation and minimize the effects of reactive oxygen species.18 However, the effects of diseases, such as diabetes and other metabolic diseases, on mesenchymal stem cell (MSC) behavior and metabolic function remain poorly understood.19
At the molecular level, alterations in mitochondrial DNA (mtDNA) termed homoplasmic and heteroplasmic mutations might also lead to mitochondrial dysfunction and metabolic deregulation, and these effects contribute to the development of many complex diseases, including diabetes.20 The polyploid nature of the mitochondrial genome gives rise to an important feature of mitochondrial genetics, which is known as “homoplasmy” and “heteroplasmy.” The value of these terms is apparent when considering mtDNA mutations in a disease context. Some mutations affect all copies of the mitochondrial genome and are termed “homoplasmic mutations,” whereas others are only present in some mtDNA copies and are thus known as “heteroplasmic mutations”.20 The accumulation of mtDNA variants was shown to be involved in the differentiation of stem cells, ATP production,21 and T2DM pathogenesis in different ethnic populations.5 However, to the best of our knowledge, the genetic alterations in mtDNA in patients with T2DM have not been previously investigated in the context of patient‐derived BM‐MSCs, particularly in patients with different disease states.
The aims of this study were as follows:
To investigate the safety and correlated factors of BM‐MSC administration via the intravenous route vs through the pancreatic artery route in the management of T2DM.
To investigate the effect of T2DM on the molecular and cellular features of BM‐MSCs with a focus on metabolic activities and mtDNA alterations.
2. MATERIALS AND METHODS
2.1. Study design and participants
This study is an open‐label, uncontrolled, randomized clinical trial. Patients were enrolled at the Internal Medicine Department at Vinmec Times City International Hospital, Hanoi, Vietnam, between December 2017 and February 2019. A patient (older than 18 years) was selected if she or he was diagnosed with T2DM as defined by a high HbA1c level (between 7.5% and 9%) and a fasting blood glucose (FBG) level less than 10 mmol/L. The details of the inclusion and exclusion criteria are shown in Panel 1. The clinical trial was approved by the Ethics Committee of Vinmec International Hospital (number 130817/2017/QĐ‐VMEC) and Vietnam Ministry of Health (number 5393/QĐ‐BYT). This study was registered at ClinicalTrials.gov (number NCT03343782). BM‐MSCs from a control group were obtained with informed consent from healthy donors approved by the Ethics Committee of Vinmec International Hospital (number 122/2019/QĐ‐VMEC). Thirty patients who met the inclusion criteria listed in Panel 1 were enrolled in this study and randomly assigned into two groups: one received intravenous infusion, and the other received dorsal pancreatic artery delivery (Figure 1).
FIGURE 1.
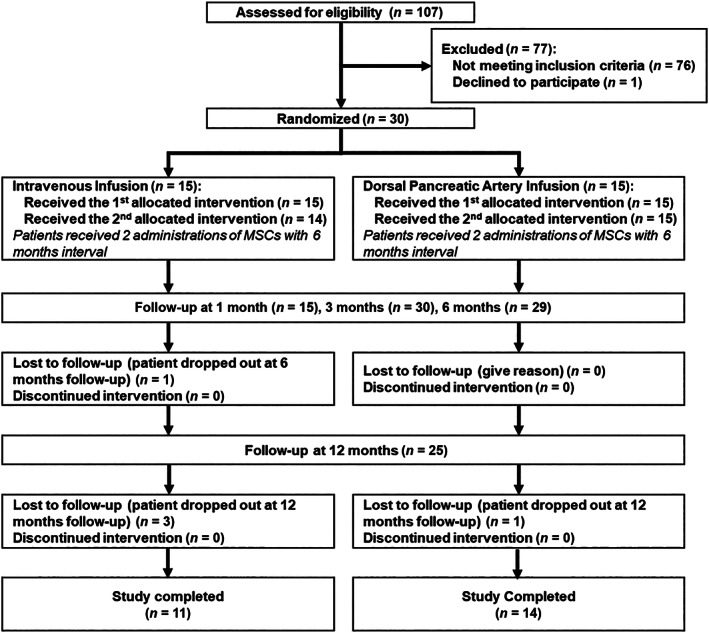
Treatment procedure for this trial. One hundred seven patients were screened to select 30 patients who met all the inclusion criteria of the study. The patients were randomized into two groups divided based on the transplantation route: one group received stem cells via intravenous infusion, and the other group received stem cells through the dorsal pancreatic artery. Abbreviation: MSC, mesenchymal stem cell
Panel 1. Inclusion and exclusion criteria.
Inclusion criteria
Aged 18 years and older.
Patients from both sexes diagnosed with T2DM.
7.5% < HbA1c < 9.0% and FBG <10 mmol/L.
Exclusion criteria
Patients diagnosed with type 1 diabetes mellitus.
Patients diagnosed with cancer, chronic diseases, or internal organ failure.
Pregnant or breastfeeding women.
Inability to obtain consent.
Patients with clinical coagulation disorders.
Patients currently undergoing any treatments for a severe acute illness.
For the phenotypic and molecular analysis of BM‐MSCs, the samples collected from both nondiabetic donors (age‐matched) and patients with T2DM were classified into four groups: group 1 (healthy donors without T2DM, age‐matched), group 2 (average T2DM duration of 5 years), group 3 (average T2DM duration of 10 years), and group 4 (T2DM duration of more than 15 years).
2.2. Randomization
After a 10‐week preliminary run before commencing the administration procedure to stabilize the HbA1c and FBG levels, all patients were randomized (1:1) into either the intravenous infusion (IV) group (n = 15) or the dorsal pancreatic arterial infusion (DPA) group (n = 15). Computer software was used to randomize the allocations into the IV and DPA groups.
2.3. Procurement and expansion of autologous BM‐MSCs
BM was aspirated from the anterior iliac crest of patients under general anesthesia in the operating theater. BM‐MNCs were separated from the aspirate by density gradient centrifugation (Ficoll method).22 Freshly harvested BM‐MNCs were collected and cultured under xeno‐free and serum‐free conditions to passage 3 to reach the administration dose and enrich and expand the BM‐MSC population. If no bacterial, fungal, mycoplasma, and endotoxin contamination was detected, the cells were suspended in 20 mL (IV group) and 10 mL (DPA group) of 0.9% NaCl (B. Braun Medical Inc., Bethlehem, Pennsylvania) to a final dose of 1 × 106 cells/km of the patient's body weight for use in the transplantation ward. The release criteria and stem cell quality are shown in Table S1.
2.4. Mode of administration
In the group IV, stem cells were delivered via the intravenous route for 30 minutes at a delivery rate of 40 mL/hour. In the DPA group, a catheter was introduced into the femoral artery under local anesthesia and then directed to the dorsal pancreatic artery. Stem cells were infused for 30 minutes at a rate of 20 mL/hour.
2.5. Outcomes
2.5.1. Safety evaluation (primary outcomes)
Administration‐associated risks, including hyperglycemia, anaphylaxis, urticaria, dyspnea, hypoxia, vomiting, fever, pain, local infection, local hematoma, hypertension, hypotension, bradycardia, tachycardia, rigors, and hemoglobinuria, were monitored for 48 hours after administration and assessed at 1, 3, 6, and 12 months after the first administration.
2.5.2. Evaluation of therapeutic effectiveness (secondary outcomes)
All patients were requested to participate in an assessment at 1, 3, 6, and 12 months after the first administration. The assessment at each visit included the following parameters: general condition, hematological analysis, blood glucose level, FBG level, HbA1c level, C‐peptide level, and documented antidiabetic medications (including insulin doses and medication usage). An evaluation of the patient's quality of life was conducted using the Treatment‐Related Impact Measurement‐Diabetes (TRIM‐D) tool at baseline and 6 and 12 months after administration.
2.6. Statistical analysis
Skyler (2015) revealed that the mean HbA1c level was 8.2% ± 0.8%,23 and we hypothesized that this value would decrease by 4.9% after 12 months of intervention; thus, based on an alpha of .05 and a power of 80%, we determined that 30 patients should be included in the trial. Descriptive statistics were used to illustrate the demographics of the patients with T2DM. Categorical variables are presented as proportions, whereas quantitative variables are described as the mean values ± SD or as the medians and their interquartile ranges. A t test or Wilcoxon rank‐sum test was used to assess the relationship between the outcomes after the two infusions, whereas one‐way analysis of variance (ANOVA) or the Kruskal‐Wallis test was used to assess the changes in the HbA1c, FBG, and C‐peptide levels over time. A multiple linear regression model was used to measure the correlation between the HbA1c level and the duration of the illness, body mass index (BMI), growth curve, and demographic information (age, gender, smoking, and alcohol consumption). Values of P < .05 were considered statistically significant. The analyses were performed using Stata version 14 (StataCorp, College Station, Texas).
Methods for the phenotypic analysis and mtDNA sequencing are provided in Data S1.
3. RESULTS
3.1. Autologous BM‐MSC administration is safe for patients with T2DM
One hundred seven patients were screened to select 30 patients who met all the inclusion criteria of the study (Figure 1). The detailed characteristics of enrolled patients are described in Table 1. No severe adverse events (SAEs) occurred during the study period. The patients' general health conditions after each administration were stable, and all vital criteria were in a normal and acceptable range. At the end of the trial, four infusion‐associated AEs were confirmed to be related to incidences of hyperglycemia (three events) and hypoglycemia (one case). AEs that were more likely related to administration, such as feeling pain, were observed in three patients. Single incidences of AEs that were potentially related to therapy, such as splenomegaly, insomnia, vomiting, headache, and hypertension, occurred in individual patients within 12 months after stem cell administration. Other AEs were deemed not related to the intervention. The number of occurrences of AEs was comparable in both groups (IV vs DPA; P > .05). The details of the AEs and their relevance to stem cell intervention are shown in Table S2.
TABLE 1.
Characteristics of the patients with T2DM before stem cell transplantation
| Characteristic | IV group (n = 15) | DPA group (n = 15) | All |
|---|---|---|---|
| Age, median (interpercentile range), years | 62.0 (57.0‐66.0) | 57.0 (55.0‐61.0) | 59.5 (34‐68) |
| Gender, M:F | 10:05 | 11:04 | 21:09 |
| Duration of T2DM, n (%) | |||
| ≤10 years | 6 (40) | 8 (53.3) | 14 (46.7) |
| >10 years | 9 (60) | 7 (46.7) | 16 (53.3) |
| BMI, n (%) | |||
| <23 kg/m2 | 5 (33.3) | 7 (46.7) | 12 (40) |
| >23 kg/m2 | 10 (66.7) | 8 (53.3) | 18 (60) |
| HbA1c, median (interpercentile range), % | 8.3 (8.0‐9.1) | 7.9 (7.4‐8.6) | 8.2 (7.8‐8.9) |
| FBG, mean ± SD, mmol/L | 9.0 ± 2.4 | 8.2 ± 2.2 | 8.6 ± 2.3 |
| C‐peptide, median (interpercentile range), ng/mL | 1.9 (1.0‐3.0) | 1.4 (0.7‐2.0) | 1.6 (0.97‐2.21) |
Abbreviations: BMI, body mass index; DPA, dorsal pancreatic artery infusion; F, female; FBG, fasting blood glucose; HbA1c, hemoglobin A1c; IV, intravenous infusion; M, male; T2DM, type 2 diabetes mellitus.
3.2. Outcomes of autologous BM‐MSC administration
A slight reduction in HbA1c levels was observed in both groups in the first 3 months after administration, but the level returned to normal after 6 months and even increased in the group IV. The FBG concentration in both groups was reduced after the first 3 months and maintained at the baseline level after 12 months. Notably, the C‐peptide level was reduced at 12 months after administration in the IV group (1.86 [0.97‐3.03] vs 1.43 [0.53‐2.39]) but increased in the DPA group (1.42 [0.72‐2.01] vs 1.59 [1.08‐1.95]). No statistically significant differences in the changes in HbA1c, FBG, and C‐peptide levels were observed in groups with different administration routes (P > .05).
Further analysis of the treatment outcomes based on the T2DM duration revealed a significant reduction in the HbA1c levels in the patients who had suffered from T2DM for less than 10 years and had a BMI less than 23 kg/m2 (P = .045; Table 2). Patients with T2DM for less than 10 years showed a reduction in the FBG level at 12 months after administration; in particular, patients with a BMI less than 23 kg/m2 showed a decrease in the FBG level from 8.5 ± 3.5 mmol/L at baseline to 6.8 ± 2.4 mmol/L at 12 months after administration. All patients with T2DM for more than 10 years did not exhibit any significant changes in their HbA1c, FBG, and C‐peptide levels, with the exception of patients with a BMI higher than 23 kg/m2, who showed a slight increase in their HbA1c and FBG levels after the intervention. The baseline C‐peptide levels were relatively low compared with the normal range and regardless of the duration of T2DM and the patients' BMI, and a decreasing trend in the levels of C‐peptide was observed after administration, particularly in patients with a T2DM history of 10 years (P < .07; Table 2). Taken together, the results suggested the short‐term efficacy of the therapy and a strong correlation to the T2DM duration and patient's BMI.
TABLE 2.
Analysis of the changes in the HbA1c, fasting blood glucose, and C‐peptide levels over time in different groups (BMI <23 or >23 kg/m2 and T2DM duration ≤10 years or >10 years) using one‐way analysis of variance
| Clinical Assessments | T2DM duration ≤10 years | T2DM duration >10 years | ||
|---|---|---|---|---|
| BMI <23 (n = 6) | BMI >23 (n = 8) | BMI <23 (n = 6) | BMI >23 (n = 10) | |
| HbA1c | ||||
| Baseline | 8.9 ± 1.6 | 8.4 ± 0.9 | 8.4 ± 0.8 | 8.0 ± 0.6 |
| 1 month | 8.1 ± 0.7 | 8.6 ± 1.1 | 8.6 ± 1.1 | 7.9 ± 0.3 |
| 3 months | 7.6 ± 0.6 | 8.4 ± 0.9 | 8.4 ± 1.1 | 8.2 ± 0.6 |
| 6 months | 7.9 ± 0.5 | 8.3 ± 1.0 | 9.6 ± 0.8 (5) | 8.6 ± 0.9 |
| 12 months | 8.2 ± 0.5 (5) | 8.7 ± 0.9 (6) | 8.6 ± 1.3 (5) | 8.3 ± 1.1 (9) |
| P value | .044 | .995 | .846 | .003 |
| Fasting blood glucose | ||||
| Baseline | 8.5 ± 3.5 | 9.1 ± 1.5 | 9.5 ± 2.4 | 7.8 ± 1.9 |
| 1 month | 7.0 ± 2.2 | 11.9 ± 6.4 | 11.1 ± 5.5 | 7.7 ± 1.1 |
| 3 months | 7.4 ± 1.6 | 9.3 ± 2.5 | 9.8 ± 2.9 | 8.3 ± 1.2 |
| 6 months | 7.9 ± 4.9 | 8.8 ± 1.7 | 12.7 ± 3.0 (5) | 9.7 ± 3.3 |
| 12 months | 6.8 ± 2.4 (5) | 8.7 ± 2.8 (6) | 9.7 ± 3.3 (5) | 8.4 ± 2.4 (9) |
| P value | .133 | .001 | .423 | .007 |
| C‐peptide | ||||
| Baseline | 1.5 ± 0.9 | 2.4 ± 1.8 | 1.9 ± 0.6 | 1.7 ± 1.9 |
| 1 month | 1.6 ± 1.3 | 2.1 ± 0.8 | 2.0 ± 1.5 | 1.2 ± 0.7 |
| 3 months | 1.4 ± 1.1 | 1.9 ± 1.3 | 1.7 ± 0.4 | 1.3 ± 0.8 |
| 6 months | 1.3 ± 1.0 | 1.9 ± 0.9 | 1.7 ± 0.9 (5) | 1.4 ± 0.9 |
| 12 months | 1.3 ± 1.3 (5) | 2.0 ± 1.5 (6) | 1.6 ± 0.3 (5) | 1.4 ± 1.0 (9) |
| P value | .935 | .201 | .007 | .007 |
Note: All results are presented as means ± SD. The number in parentheses is the number of patients involved in the follow‐up.
Abbreviations: BMI, body mass index; HbA1c, hemoglobin A1c; T2DM, type 2 diabetes mellitus.
3.3. Changes in the diabetic medication dose after administration
The 30 patients enrolled in our study were divided into 3 subgroups based on the medication they took prior to administration: the patients who used only insulin (n = 4), those who used only drugs (n = 14), and those who used a combination of both insulin and multiple drugs (n = 12) to maintain their blood glucose level. Because 5 patients were excluded at the end of the study, 25 patients were included in the evaluation of the results. Based on our results, two of the four patients who used only insulin reported a decrease in their insulin dosage at the 12‐month follow‐up visit, and the average reduction was 6 IU/day. Among the 10 patients who used multiple drugs, 5 patients reduced their use of oral drugs at 12 months after the intervention, and the average reduction was 868 mg. One of the three patients who used insulin in combination with a single drug had decreased their drug dose but increased their insulin usage at the 12‐month follow‐up. A similar observation was reported for 8 of the 11 patients who used insulin in combination with multiple drugs; 5 of these 8 patients reduced their use of oral drugs, and 2 other patients decreased their use of insulin at 12 months after administration (Table S3). The results of the excluded patients are shown in Table S4, and one patient who used insulin in combination with a single drug reduced his or her use of the oral drug at 6 months after administration.
Multiple linear regression analysis was used to predict the changes in the HbA1c, FBG, and C‐peptide levels at 1, 3, 6, and 12 months compared with the baseline levels based on age, gender, type of infusion, BMI, and duration of T2DM. A significant regression equation was found for HbA1c levels at 1, 3, and 6 months (P < .05). The efficiency in reducing the HbA1c levels based on various variables (age, gender, type of infusion, duration of illness, and BMI) decreased over time (R 2 = 48.6% > 47.1% > 37.9% > 13%; Table S5). The total TRIM‐D scores indicated improvements in the patients' quality of life (significantly reduced from 68.4 ± 6.0 at baseline to 63.2 ± 7.9 at 6 months and 57 ± 4.6 at 12 months after administration; P = .026), particularly values related to their daily life and psychological status (P < .05; Table S6).
3.4. The T2DM duration alters the proliferation rate of autologous BM‐MSCs but not their stem cell phenotypes
The mean ± SD volume of BM aspirated for the enrichment of BM‐MSCs was 30.6 ± 4.8 mL (n = 30), and the median (interquartile range) number of MSCs obtained after gradient centrifugation was 88.8 (60‐132) × 106 MNCs (n = 30). When cultured under xeno‐free and serum‐free conditions, these cells exhibited the typical MSC morphology, including plastic‐adherent features, a spindle shape, and a fibroblast‐like phenotype (Figure 2A). No significant difference in the morphology of these cells was observed between groups. The results of the calculated population doubling times revealed that BM‐MSCs isolated from patients with T2DM had a slower proliferation rate than those isolated from individuals without diabetes (control group, group 1; Figure 2B). The population doubling times (mean ± SEM) of groups 1 (n = 6), 2 (n = 8), 3 (n = 17), and 4 (n = 5) were 42.54 ± 6.13, 52.08 ± 9.22, 85.94 ± 27.14, and 60.97 ± 13.97 hours, respectively. BM‐MSCs isolated from patients with T2DM for more than 5 years (groups 3 and 4) displayed a significantly slower proliferation rate compared with those isolated from group 1 (control group) and group 2 (one‐way ANOVA; P < .05). An analysis of MSC markers revealed that after two expansions for administration, all these cells expressed more than 98% of positive markers of MSCs (including CD73, CD90, and CD105) and less than 2% of negative markers (CD11b, CD19, CD34, CD45, and HLR‐DR) (Figure 2C). These cells maintained a normal karyotype at the administration stage (passage 3) (Figure 2D), and the mean ± SD cell viability at the time of the first and second administrations was 97.8% ± 1.3% (n = 30) and 96.2% ± 2.5% (n = 29), respectively. No difference was observed in terms of adipogenic, chondrogenic, and osteogenic differentiation between the control and T2DM groups (Figure 2E). These data indicate that although autologous BM‐MSCs isolated from patients with T2DM exhibited normal morphology, accepted MSC marker expression according to ISCT guidelines, and the ability to differentiate into the mesodermal cell lineage, their proliferation rate decreased relative to the duration of T2DM.
FIGURE 2.
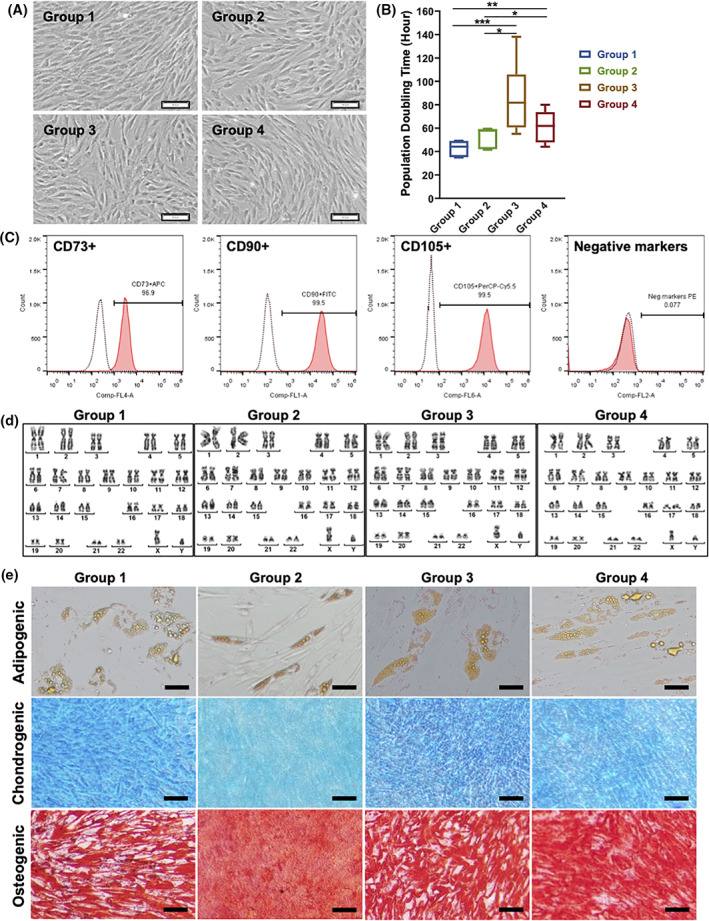
Characterization of autologous bone marrow‐derived mesenchymal stem cells (BM‐MSCs) isolated from the control group (group 1) and from patients with type 2 diabetes mellitus (T2DM) (duration of T2DM: group 2: <5 years, group 3:5‐10 years, and group 4: >10 years). A, Typical morphology of BM‐MSCs cultured under xeno‐ and serum‐free culture conditions. These cells were plastic‐adherent, spindle‐shaped, and fibroblast‐like. B, The population doubling time (hours) of BM‐MSCs indicated a significant difference in the growth rates of groups 3 and 4 compared with those of group 1 (control group) and group 2. C, The flow cytometry analysis of mesenchymal stem cell surface markers, including the positive markers CD73, CD90, and CD105, and the absence of hematopoietic markers (CD34, CD45, CD14 or CD11b, CD79α or CD19, and HLA‐DR) confirmed that all BM‐MSCs expressed more than 95% of positive markers and less than 2% of negative markers. D, A normal karyotype was maintained in all cells cultured under xeno‐ and serum‐free conditions. E, All BM‐MSCs from the four groups were able to differentiate into adipocytes, chondrocytes, and osteoblasts. Scale bar: 100 μm. APC, allophycocyanin; Cy5.5, cyanine dyes 5.5; FITC, fluorescein isothiocyanate; HLA‐DR, human leukocyte antigen ‐ DR isotype; PE, phycoerythrin; PerCP, peridinin chlorophyll protein complex; *P<.05; **P<0.01; ***P<0.001
3.5. T2DM alters the metabolic profile of BM‐MSCs
The “Cell Energy Phenotype Test” results revealed decreases in the baseline extracellular acidification rate (ECAR) and oxygen consumption rate (OCR) in both the control and T2DM groups, but these differences did not reach statistical significance (Figure 3A). In addition, no differences in the ECAR and OCR were observed under baseline and control conditions among the groups. Based on these results, BM‐MSCs isolated from either nondiabetic donors or patients with T2DM maintained their energy generation at similar levels under normal conditions. The assessment of the metabolic potential showed that BM‐MSCs derived from either the control group or T2DM groups had higher ECAR than OCR values, suggesting that these cells exhibited the glycolytic phenotype (Figure 3B). Mitochondrial respiration was measured in BM‐MSCs using the “Seahorse XF Cell Mito Stress Test” to obtain further insights into the metabolic activities of these cells under T2DM conditions. The OCR profile of the age‐matched control group was higher than that of the diabetic groups (Figure 3C). The data calculated from the Seahorse Generator revealed that the BM‐MSCs isolated from age‐matched nondiabetic individuals exhibited significantly higher values for maximal respiration, ATP production, and spare respiratory capacity than cells derived from patients with T2DM, and no significant differences were found among the T2DM groups (Figure 3D). In the assessment of glycolysis, the Seahorse XF Glycolysis Stress Test indicated higher ECAR values for the control group than the T2DM groups (Figure 3E). Moreover, no significant difference in glycolysis was observed among the T2DM groups, but BM‐MSCs from nondiabetic donors exhibited a significantly higher glycolytic capacity and significantly higher glycolysis reserves than those from patients with T2DM (Figure 3F). Taken together, T2DM introduced mitochondrial respiratory stress in BM‐MSCs and reduced the glycolysis activities of these cells.
FIGURE 3.
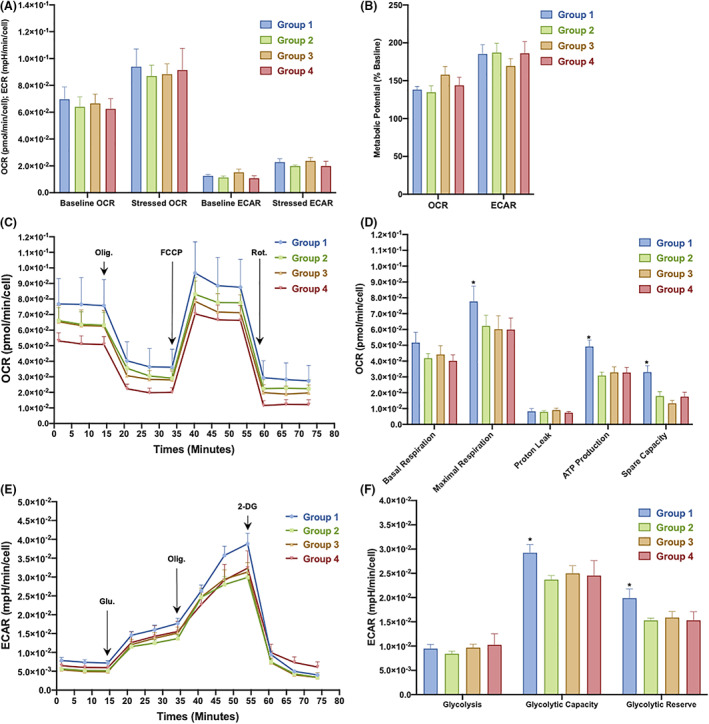
Metabolic profiles of bone marrow‐derived mesenchymal stem cells (BM‐MSCs) isolated from the control group and from patients with type 2 diabetes mellitus (T2DM). A, Phenotype analysis of the OCR (pmol/minute per cell) and ECAR (mpH/minute per cell) using the Seahorse XF Cell Energy Phenotype Test. B, Metabolic potential ([stressed OCR or ECAR ÷ baseline OCR or ECAR] × 100%) between samples from normal individuals and patients with T2DM. C, Profiles of mitochondrial respiration assessed using the Seahorse XF Cell Mito Stress Test. Arrows indicate injections of the specific stressors oligomycin, carbonyl cyanite‐4 phenylhydrazone, and rotenone/antimycin A into the assay medium. D, Calculated respiration values for the control and T2DM samples. E, Glycolysis profiles of BM‐MSCs obtained using the Seahorse XF Glycolysis Stress Test Kit; arrows indicate the injection of glucose, oligomycin, and 2‐deoxyglucose into the assay medium. F, Calculated glycolysis rate, glycolytic capacity, and glycolytic reserves of BM‐MSCs isolated from the control group and the T2DM groups. 2‐DG, 2‐deoxyglucose; ECAR, extracellular acidification rate; FCCP, carbonyl cyanite‐4 phenylhydrazone; Glu, glucose; OCR, oxygen consumption rate; Olig, oligomycin; Rot., rotenone/antimycin A. * P<.05
3.6. The genetic landscape of mtDNA in BM‐MSCs derived from patients with different T2DM durations
We sequenced the entire mtDNA genome from 24 samples derived from group 1 (n = 5), group 2 (n = 5), group 3 (n = 10), and group 4 (n = 4) with an average coverage of greater than 2000× (Figure S1). Coverage plots of two samples (one sample was a full‐length single mtDNA amplicon [black] and the other was two overlapping fragmented mtDNA amplicons [red]) are shown in Figure S2. The sequencing results from these samples showed a greater number of homoplasmic variants in the control group (141 variants) than in the T2DM groups, except for group 3; the numbers of homoplasmic variants in groups 2, 3, and 4 were 103, 206, and 73, respectively (Table S7; Figure 4A). However, in terms of heteroplasmic variants, group 1 had an average heteroplasmy of 2 ± 1 variants, which was relatively lower than that of groups 3 (12 ± 7 variants) and 4 (11 ± 8 variants) (all means ± SEM; Table S6; Figure 4B). Regarding homoplasmic changes, all four groups shared 10 variants (Table S8). These population‐specific variants are benign and unlikely to be involved in T2DM pathogenesis. Therefore, we excluded these variants from our further analyses.
FIGURE 4.
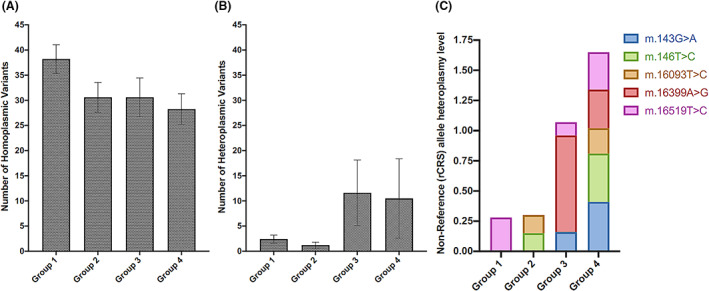
Characterization of mitochondrial DNA (mtDNA) isolated from bone marrow‐derived mesenchymal stem cells of the control group (group 1) and patients with type 2 diabetes mellitus (T2DM) (duration of T2DM: group 2: <5 years, group 3:5‐10 years, and group 4: >10 years). A,B, Number of mtDNA variants among the study groups, including homoplasmic variants (A) and heteroplasmic variants (B). C, Dynamics of the heteroplasmy level of several mtDNA variants across the study groups. The revised Cambridge Reference Sequence was applied as reference mitochondrial sequence. rCRS, revised Cambridge Reference Sequence
Further filtering of the homoplasmic and heteroplasmic variants revealed that T2DM‐specific variants frequently appeared in groups 2, 3, and 4. The results of 22 homoplasmic and 23 heteroplasmic variants are shown in Tables S9 and S10, respectively. Notably, m.A16182C and m.A16183C were the two homoplasmic variants that appeared with the most frequency in the T2DM groups (Table S9). All heteroplasmic variants appeared twice in the T2DM groups, with the exception of the m.C150T variant, which appeared three times (Table S10). Among the unique heteroplasmic variants specific to T2DM groups, the nonsynonymous single‐nucleotide polymorphism m.A10398G and four synonymous single nucleotide polymorphisms (m.10400T, m.T10873C, m.C12705T, and m.T14783C) were previously reported to be associated with T2DM.24, 25 Additionally, the heteroplasmy level of several specific variants, including m.143G>A, m.146T>C, m.16093T>C, m.16399A>G, and m.16519T>C, increased across the T2DM groups, especially in groups 3 and 4 (Figure 4C).
4. DISCUSSION
Our study confirmed that autologous BM‐MSC administration via the intravenous or dorsal pancreatic artery route was safe for the treatment of T2DM. No SAEs occurred during the study period from the initiation of BM stem cell collection and preparation to 12 months of follow‐up. Several AEs potentially related to the stem cell administration procedure, such as hyperglycemia, abdominal pain over 24 hours after administration, tiredness, vomiting, and headache, were well managed with conventional interventions. As illustrated in a meta‐analysis and review, stem cell administration for T2DM was conducted in 12 other studies with no administration‐related SAEs.16 Similar to our study, a previous study enrolled 30 patients in a randomized, placebo‐controlled comparative study for T2DM and showed that these patients tolerated the treatment well with no significant AEs during the course of the study, which supports the hypothesis that stem cell therapy is safe for T2DM treatment.7 Additionally, a phase III, placebo‐controlled, multicenter clinical trial was conducted with 61 patients with T2DM who received a single infusion of MSCs at different doses, including 0.3 × 106, 1 × 106, or 2 × 106 cells/km, or placebo.23 No toxicity or deaths occurred during the infusion, and no serious AEs that would cause patients to discontinue their participation were reported because the reported AEs were deemed related to MSC administration.
In terms of therapeutic potential, our results showed no difference between the IV and DPA groups in terms of the HbA1c, FBG, and C‐peptide levels, which did not change significantly during 12 months of follow‐up. Our data are similar to those obtained from a comparative study using BM‐MNCs showing that the C‐peptide levels and insulin sensitivity of the patients showed nonsignificant differences between baseline and 6 months after administration.8 Potential explanations for this finding are that both studies included a small cohort size, which did not provide sufficient statistical power, and the lack of a control group. For further analysis, we grouped the patients according to their T2DM duration (≤10 years vs >10 years) and BMI (<23 vs >23 kg/m2) to investigate the effectiveness of the therapy. A BMI value of 23 kg/m2 was selected for this analysis because the World Health Organization (WHO) reported that the BMI cutoff points for being overweight and obese in Asian countries (BMI = 23 kg/m2) are population‐specific and relatively lower than the existing WHO cutoff point for being overweight elsewhere (BMI >25 kg/m2).26 Our results revealed reductions in the HbA1c and FBG levels during the first 3 months after administration in patients with a T2DM duration of less than 10 years and a BMI <23 kg/m2, but the effectiveness appeared to diminish after 6 months. Moreover, the same group of patients presented a significant reduction in their HbA1c levels at 12 months after administration, and their FBG levels were reduced to normal levels (6.8 ± 2.4 mmol/L) at the end of the study. The reduction in FBG levels was not statistically significant but was deemed clinically significant because the reduction was maintained in a normal range at 12 months after administration.
Our trial provides the first evidence of the correlations between the outcomes of BM‐MSC administration and the duration of T2DM and the patients' BMI. The long‐term effects of autologous BM‐MSC transplantation were reported to be relatively narrow because the positive effects of MSCs were observed as early as 1 month after administration and started to diminish again at 3 to 6 months after administration.13, 27 Our data are consistent with the results from other studies that support the short‐term effects of stem cell therapy on T2DM.11, 13 Moreover, the disease duration and BMI are considered important clinical parameters that alter the outcomes of cell therapy by reducing the cell quality. Adipose‐derived MSCs (AD‐MSCs) isolated from patients with T2DM for more than 10 years and a BMI >25 kg/m2 were reported to exhibit reduced osteogenic differentiation and engraftment capabilities and increased the risks of T2DM‐related comorbidities.28, 29, 30, 31 Therefore, our results support two important points related to the effectiveness of the therapy: (a) the interval between the two infusions should be less than 3 months to maintain the positive effects of the therapy and (b) the T2DM duration and the patient's BMI potentially play a crucial role in the treatment efficacy of stem cell therapy in patients with T2DM. We suggest that future research using autologous BM‐MSCs should focus on patients with a T2DM history of less than 10 years and a BMI <23 kg/m2 to further enhance the therapeutic effectiveness.
A biological analysis performed according to ISCT guidelines of BM‐MSCs derived from patients with T2DM illustrated that these cells maintained similar characteristics compared with their age‐matched control counterpart, including MSC morphology, MSC markers, and differentiation ability. However, although the differentiation ability of T2DM‐derived BM‐MSCs was reported to be similar to the control group in our study, it is due to the limitation of our differentiation procedure, which is qualitative detection using staining chemicals and not a quantitative method. According to previous studies, T2DM compromised the osteogenic differentiation of the BM‐MSCs, resulting in the development of osteopathy, a recognized complication of T2DM.32, 33 An analysis of the growth rate of BM‐MSCs derived from patients with showed a slower proliferation rate during in vitro culture compared with BM‐MSCs from nondiabetic healthy donors, indicating that T2DM directly affects the expansion potential of MSCs. An increase in cell proliferation rate was detected when rodent BM‐MSCs were cultured under high‐glucose conditions.34 A similar observation was reported for healthy human BM‐MSCs expanded in the culture medium supplemented with serum from donors with diabetes.35 In contrast, comparable proliferation of BM‐MSCs isolated from donors with or without T2DM has also been reported.36 Although the surrounding environment, such as the glucose concentration, rather than permanent alterations induced by T2DM, has been suggested to influence the proliferation capacity of BM‐MSCs to the cells' capacity, our data provide a new set of information suggesting that prolonged exposure to T2DM might reduce the proliferation capacity of BM‐MSCs. The BM‐MSCs isolated from patients with T2DM were cultured under xeno‐ and serum‐free conditions with a standard glucose concentration in our study, suggesting that the proliferation capacity of T2DM‐derived BM‐MSCs is compromised by the T2DM condition itself but not the surrounding factors. Furthermore, a reduction in the proliferation capacity of BM‐MSCs was reported in studies assessing patients with T2DM and a disease duration of more than 10 years.37 MSCs derived from the adipose tissue of patient with a T2DM duration ranging from 5 to 15 years not only exhibited a reduced proliferation capacity but also decreased keratinocyte growth factor release, immunosuppressive potential, and survival rate.38, 39 These findings, together with our results, support a new hypothesis that the T2DM duration might decrease the self‐renewal capacity of MSCs, and thus therapeutic approaches using BM‐MSCs for T2DM treatment must consider the duration of T2DM as one of major factors that could alter the expansion capacity of these cells for autologous administration.
Stem cell proliferation and differentiation require energy and thus require an increase in mitochondrial activities to produce ATP through oxidative phosphorylation and a concurrent decrease in glycolysis.21 The results of our study revealed that T2DM compromises both glycolysis and mitochondrial respiration in BM‐MSCs, resulting in an increase in cell proliferation time and reduction in potential MSC functions. The increase in glycolysis and ATP generation was previously described in AD‐MSCs isolated from patients with T2DM and a BMI >35 kg/m2, and the authors concluded that these increases were associated with insulin resistance and predisposition to T2DM in obese patients.31, 40 In terms of mitochondrial respiration, our metabolic analysis supported a previous study showing that the T2DM duration altered respiratory capacity in the mitochondria of MSCs. Furthermore, T2DM might alter mitochondrial function and create defective oxidative phosphorylation, leading to the dysfunction of human skeletal muscle cells, cardiac cells, and MSCs.41, 42 Several studies have shown that T2DM directly alters mitochondrial functions by reducing the activity of rotenone‐sensitive NADH:O2 oxidoreductase, which leads to a decrease in ATP production via the electron transport chain of human skeletal muscle.43 Therefore, future research should investigate the direct link between T2DM‐induced mitochondrial dysfunction and BM‐MSC proliferation to provide a more comprehensive and in‐depth understanding of the field.
Our molecular‐level analysis characterized the mtDNA genetic profiles of patient‐derived BM‐MSCs. In our study, we identified 22 homoplasmic and 23 heteroplasmic variants that are unique to patients with T2DM compared with control individuals. The differences became more profound with an increase in the duration of T2DM. Moreover, the heteroplasmy level of five specific variants, including m.G143A, m.T146C, m.T16093C, m.A16399G, and m.T16519C, was increased relative to the duration of T2DM, although these variants have not been reported to be associated with T2DM. Based on our data, T2DM and its duration potentially affect the mtDNA profiles of patient‐derived BM‐MSCs. This result is consistent with many previous studies, although these studies were conducted in other ethnic populations using different tissues derived from patients with T2DM.25 Additionally, a previous study examining 6528 individuals showed that mitochondrial variants are associated with BMI in adults, supporting our observation of an increasing number of mtDNA variants in patients with T2DM and a BMI >23 kg/m2. 44 Hence, our study predicted that BM‐MSCs derived from patients with T2DM with increased mtDNA mutations might affect both the proliferation and metabolism of the cells and subsequently alter the outcomes of autologous cell administration.
4.1. Study limitations and suggestions
Although our study sought to assess the effect of the T2DM duration and patients' BMI on the biological and metabolic functions of autologous BM‐MSCs, the small cohort of patients and lack of a control group did not allow us to either accumulate experience from our trial or draw solid conclusions about the efficacy of autologous BM‐MSCs for T2DM. Additionally, the lack of an adequate control group reduces the confidence that the treatment effects are due to the BM‐MSC administration. Hence, based on our results and the short‐term efficacy of the autologous MSC therapy, we suggest that future trials should only use autologous MSC administration for patients with T2DM and a disease duration less than 10 years who are not obese. For patients who have suffered from T2DM more than 10 years and are obese, allogeneic administration of healthy MSC sources, such as umbilical cord‐derived MSCs, should be considered to achieve the therapeutic effect.
5. CONCLUSION
A double infusion of autologous BM‐MSCs through either the intravenous route or the dorsal pancreatic artery route was safe and well tolerated in patients with T2DM. The therapy exhibited short‐term efficacy in patients with T2DM for less than 10 years and a BMI <23 kg/m2. The duration of T2DM directly altered the phenotype of BM‐MSCs by reducing the proliferation rate, compromising the glycolysis process, reducing mitochondrial functions, and changing mitochondrial variants. Our data suggest that the autologous administration of BM‐MSCs to treat T2DM should be performed in patients with a T2DM duration <10 years and without obesity. Further studies are needed to confirm the effects of the T2DM duration on BM‐MSC functions and metabolic activities.
CONFLICT OF INTEREST
The authors indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
L.T.N.: conception/design, administrative support, provision of study materials or patients, manuscript writing, final approval of manuscript, other (performed the clinical assessments and follow‐ups), other (guarantee the study and accept full responsibility for the work and conduct of the study, had access to the data, and controlled the decision to publish); D.M.H.: conception/design, administrative support, provision of study materials or patients, manuscript writing, final approval of manuscript, other (performed the stem cell processing and characterization and prepared the stem cell products), other (performed the QC and the karyotyping of the stem cell products), other (performed the Seahorse metabolic experiments and analyzed the data), other (guarantee the study and accept full responsibility for the work and conduct of the study, had access to the data, and controlled the decision to publish); K.T.N.: conception/design, manuscript writing, final approval of manuscript; D.M.B.: final approval of manuscript, other (performed the clinical assessments and follow‐ups); H.T.N., H.M.L., N.Y.P., D.M.V.: final approval of manuscript, other (performed the mtDNA sequencing and data analyses); H.T.A.L., V.T.H., H.T.H.B., P.T.M.D.: final approval of manuscript, other (performed the stem cell processing and characterization and prepared the stem cell products); X.T.A.H., A.T.L.N.: final approval of manuscript, other (performed the Seahorse metabolic experiments and analyzed the data); T.T.D.: final approval of manuscript; T.D.N.: final approval of manuscript, other (performed the QC and the karyotyping of the stem cell products), other (performed the Seahorse metabolic experiments and analyzed the data); L.T.H., H.T.P.B.: final approval of manuscript, other (performed the QC and the karyotyping of the stem cell products); H.K.N.: data analysis and interpretation, final approval of manuscript; M.H.: final approval of manuscript, other (provided a scientific evaluation of the report and modified the report); A.V.B.: administrative support, provision of study materials or patients, final approval of manuscript.
Supporting information
Figure S1 Schematic visualization of the sampling design
Figure S2 Coverage plots of amplicons
Data S1 Materials and Methods for stem cell expansion, phenotypic analysis, and mtDNA sequencing
ACKNOWLEDGMENTS
This trial was supported by the Vingroup Scientific Research and Clinical Application Fund (grant ISC.17.07). The authors appreciate the support provided by leader boards at the Vinmec Health Care System and the Internal Medicine Department at Vinmec Times City International Hospital, Hanoi, Vietnam. The sponsor of the trial had no role in the study design, data collection, analysis, or interpretation, or the writing of this report. The first and corresponding authors (L.T.N. and D.M.H.) had full access to all the data and assume the final responsibility to submit the report for publication.
Nguyen LT, Hoang DM, Nguyen KT, et al. Type 2 diabetes mellitus duration and obesity alter the efficacy of autologously transplanted bone marrow‐derived mesenchymal stem/stromal cells. STEM CELLS Transl Med. 2021;10(9):1266–1278. 10.1002/sctm.20-0506
Liem Thanh Nguyen and Duc M. Hoang contributed equally to this study.
Contributor Information
Liem Thanh Nguyen, Email: v.liemnt@vinmec.com.
Duc M. Hoang, Email: v.duchm3@vinmec.com.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author.
REFERENCES
- 1.DeFronzo RA, Ferrannini E, Groop L, et al. Type 2 diabetes mellitus. Nat Rev Dis Primers. 2015;1:15019. [DOI] [PubMed] [Google Scholar]
- 2.Zaccardi F, Webb DR, Yates T, Davies MJ. Pathophysiology of type 1 and type 2 diabetes mellitus: a 90‐year perspective. Postgrad Med J. 2016;92:63‐69. [DOI] [PubMed] [Google Scholar]
- 3.Chatterjee S, Khunti K, Davies MJ. Type 2 diabetes. Lancet. 2017;389:2239‐2251. [DOI] [PubMed] [Google Scholar]
- 4.Nguyen CT, Pham NM, Lee AH, Binns CW. Prevalence of and risk factors for type 2 diabetes mellitus in Vietnam: a systematic review. Asia Pacific J Public Health. 2015;27:588‐600. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Y, Chen W, Feng B, Cao H. The clinical efficacy and safety of stem cell therapy for diabetes mellitus: a systematic review and meta‐analysis. Aging Dis. 2020;11:141‐153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berezin AE. Diabetes mellitus and cellular replacement therapy: expected clinical potential and perspectives. World J Diabetes. 2014;5:777‐786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhansali S, Dutta P, Kumar V, et al. Efficacy of autologous bone marrow‐derived mesenchymal stem cell and mononuclear cell transplantation in type 2 diabetes mellitus: a randomized, placebo‐controlled comparative study. Stem Cells Dev. 2017;26:471‐481. [DOI] [PubMed] [Google Scholar]
- 8.Sood V, Bhansali A, Mittal BR, et al. Autologous bone marrow derived stem cell therapy in patients with type 2 diabetes mellitus ‐ defining adequate administration methods. World J Diabetes. 2017;8:381‐389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315‐317. [DOI] [PubMed] [Google Scholar]
- 10.Friedenstein AJ, Piatetzky S II, Petrakova KV. Osteogenesis in transplants of bone marrow cells. J Embryol Exp Morphol. 1966;16:381‐390. [PubMed] [Google Scholar]
- 11.Squillaro T, Peluso G, Galderisi U. Clinical trials with mesenchymal stem cells: an update. Cell Transplant. 2016;25:829‐848. [DOI] [PubMed] [Google Scholar]
- 12.Saeedi P, Halabian R, Imani Fooladi AA. A revealing review of mesenchymal stem cells therapy, clinical perspectives and modification strategies. Stem Cell Investig. 2019;6:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu X, Zheng P, Wang X, et al. A preliminary evaluation of efficacy and safety of Wharton's jelly mesenchymal stem cell transplantation in patients with type 2 diabetes mellitus. Stem Cell Res Ther. 2014;5:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu J, Wang Y, Gong H, et al. Long term effect and safety of Wharton's jelly‐derived mesenchymal stem cells on type 2 diabetes. Exp Ther Med. 2016;12:1857‐1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.El‐Badawy A, El‐Badri N. Clinical efficacy of stem cell therapy for diabetes mellitus: a meta‐analysis. PLoS One. 2016;11:e0151938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rahim F, Arjmand B, Shirbandi K, et al. Stem cell therapy for patients with diabetes: a systematic review and meta‐analysis of metabolomics‐based risks and benefits. Stem Cell Investig. 2018;5:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oren R, Farnham AE, Saito K, Milofsky E, Karnovsky ML. Metabolic patterns in three types of phagocytizing cells. J Cell Biol. 1963;17:487‐501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu Y, Ma T. Metabolic regulation of mesenchymal stem cell in expansion and therapeutic application. Biotechnol Prog. 2015;31:468‐481. [DOI] [PubMed] [Google Scholar]
- 19.Fujimaki S, Wakabayashi T, Takemasa T, et al. Diabetes and stem cell function. Biomed Res Int. 2015;2015:592915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schon EA, DiMauro S, Hirano M. Human mitochondrial DNA: roles of inherited and somatic mutations. Nat Rev Genet. 2012;13:878‐890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang H, Menzies KJ, Auwerx J. The role of mitochondria in stem cell fate and aging. Development. 2018;145:dev143420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sharma S, Kumar L, Mohanty S, Kumar R, Datta Gupta S, Gupta DK. Bone marrow mononuclear stem cell infusion improves biochemical parameters and scintigraphy in infants with biliary atresia. Pediatr Surg Int. 2011;27:81‐89. [DOI] [PubMed] [Google Scholar]
- 23.Skyler JS, Fonseca VA, Segal KR, Rosenstock J, MSB‐DM003 Investigators . Allogeneic mesenchymal precursor cells in type 2 diabetes: a randomized, placebo‐controlled, dose‐escalation safety and tolerability pilot study. Diabetes Care. 2015;38:1742‐1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weissensteiner H, Forer L, Fuchsberger C, et al. mtDNA‐server: next‐generation sequencing data analysis of human mitochondrial DNA in the cloud. Nucleic Acids Res. 2016;44:W64‐W69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liou CW, Chen JB, Tiao MM, et al. Mitochondrial DNA coding and control region variants as genetic risk factors for type 2 diabetes. Diabetes. 2012;61:2642‐2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.WHO Expert Consultation . Appropriate body‐mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157‐163. [DOI] [PubMed] [Google Scholar]
- 27.Hu J, Li C, Wang L, et al. Long term effects of the implantation of autologous bone marrow mononuclear cells for type 2 diabetes mellitus. Endocr J. 2012;59:1031‐1039. [DOI] [PubMed] [Google Scholar]
- 28.Dentelli P, Barale C, Togliatto G, et al. A diabetic milieu promotes OCT4 and NANOG production in human visceral‐derived adipose stem cells. Diabetologia. 2013;56:173‐184. [DOI] [PubMed] [Google Scholar]
- 29.Koci Z, Turnovcova K, Dubsky M, et al. Characterization of human adipose tissue‐derived stromal cells isolated from diabetic patient's distal limbs with critical ischemia. Cell Biochem Funct. 2014;32:597‐604. [DOI] [PubMed] [Google Scholar]
- 30.Minteer DM, Young MT, Lin YC, et al. Analysis of type II diabetes mellitus adipose‐derived stem cells for tissue engineering applications. J Tissue Eng. 2015;6:2041731415579215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Serena C, Keiran N, Ceperuelo‐Mallafre V, et al. Obesity and type 2 diabetes alters the immune properties of human adipose derived stem cells. Stem Cells. 2016;34:2559‐2573. [DOI] [PubMed] [Google Scholar]
- 32.Deng X, Xu M, Shen M, et al. Effects of type 2 diabetic serum on proliferation and osteogenic differentiation of mesenchymal stem cells. J Diabetes Res. 2018;2018:5765478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mangialardi G, Madeddu P. Bone marrow‐derived stem cells: a mixed blessing in the multifaceted world of diabetic complications. Curr Diab Rep. 2016;16:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stolzing A, Coleman N, Scutt A. Glucose‐induced replicative senescence in mesenchymal stem cells. Rejuvenation Res. 2006;9:31‐35. [DOI] [PubMed] [Google Scholar]
- 35.Rezaie J, Nejati V, Khaksar M, et al. Diabetic sera disrupted the normal exosome signaling pathway in human mesenchymal stem cells in vitro. Cell Tissue Res. 2018;374:555‐565. [DOI] [PubMed] [Google Scholar]
- 36.Cassidy FC, Shortiss C, Murphy CG, et al. Impact of type 2 diabetes mellitus on human bone marrow stromal cell number and phenotypic characteristics. Int J Mol Sci. 2020;21:2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Phadnis SM, Ghaskadbi SM, Hardikar AA, Bhonde RR. Mesenchymal stem cells derived from bone marrow of diabetic patients portrait unique markers influenced by the diabetic microenvironment. Rev Diabet Stud. 2009;6:260‐270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gabr MM, Zakaria MM, Refaie AF, et al. Insulin‐producing cells from adult human bone marrow mesenchymal stem cells control streptozotocin‐induced diabetes in nude mice. Cell Transplant. 2013;22:133‐145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lafosse A, Dufeys C, Beauloye C, Horman S, Dufrane D. Impact of hyperglycemia and low oxygen tension on adipose‐derived stem cells compared with dermal fibroblasts and keratinocytes: importance for wound healing in type 2 diabetes. PLoS One. 2016;11:e0168058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Greenhill C. Mechanisms of insulin resistance. Nat Rev Endocrinol. 2018;14:565. [DOI] [PubMed] [Google Scholar]
- 41.Kornicka K, Houston J, Marycz K. Dysfunction of mesenchymal stem cells isolated from metabolic syndrome and type 2 diabetic patients as result of oxidative stress and autophagy may limit their potential therapeutic use. Stem Cell Rev Rep. 2018;14:337‐345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kelley DE, He J, Menshikova EV, Ritov VB. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes. 2002;51:2944‐2950. [DOI] [PubMed] [Google Scholar]
- 43.Ritov VB, Menshikova EV, Azuma K, et al. Deficiency of electron transport chain in human skeletal muscle mitochondria in type 2 diabetes mellitus and obesity. Am J Physiol Endocrinol Metab. 2010;298:E49‐E58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Flaquer A, Baumbach C, Kriebel J, et al. Mitochondrial genetic variants identified to be associated with BMI in adults. PLoS One. 2014;9:e105116. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Schematic visualization of the sampling design
Figure S2 Coverage plots of amplicons
Data S1 Materials and Methods for stem cell expansion, phenotypic analysis, and mtDNA sequencing
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author.


