Abstract
A 39-year-old woman with progressive loss of vision left eye was referred for evaluation. Notably, she had been diagnosed with COVID-19 two weeks beforehand. Examination and ancillary testing confirmed atypical multifocal evanescent white dot syndrome. Possible other masquerades were excluded. A few weeks later, visual acuity improved in the left eye and symptoms resolved together with normalization of ancillary testing, including visual fields.
Keywords: Multiple evanescent white dot syndrome (MEWDS), COVID 19, Indocyanine Angiography, Retinal lesions, Non infectious uveitis
1. Case report
An otherwise healthy 39-year-old woman with COVID-19 viral infection confirmed by SARS-CoV-2 throat and nasal swab PCR developed floaters 2 weeks following the diagnosis. She then experienced progressive loss of vision in the left eye and attended the ophthalmology emergency department 3 weeks from the onset of symptoms and 5 days following a negative repeated PCR. Her medical history included postpartum depression, acephalgic migraine, and asthma. Family history was positive for age-related macular degeneration and primary open-angle glaucoma. She was a nonsmoker and was not taking any medications.
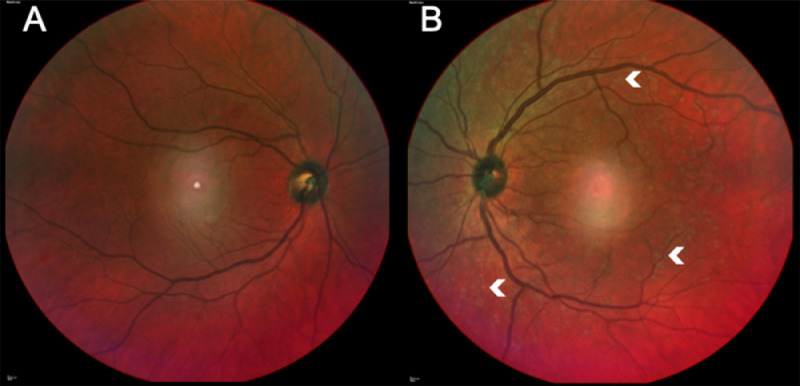
Best-corrected visual acuity was 20/40 in the right eye (OD) and 20/200 in the left eye (OS). There was no relative afferent pupillary defect (RAPD). Intraocular pressures and extraocular motility were normal. Her Ishihara color test was full in OD, while she recognized only the test plate on OS. Ocular adnexa and anterior segments were unremarkable. Dilated fundus examination OD was unremarkable (Fig. 1 A); OS presented tortuous dilated veins and multiple yellowish lesions spread across the retina (Fig. 1B).
Fig. 1.
(A) Multicolor image of the right eye shows a central artifact, otherwise it is unremarkable. (B) The left eye shows dilated veins and multiple scattered yellowish retinal lesions (arrowheads). A central artifact is also seen in this image.
In the current scenario what should one consider as differential diagnoses?
What initial systemic and ophthalmic workup should be done?
Comments by Doran Spencer, MD, PhD
In this case, the most salient features guiding a differential are the degree of vision loss, presence of floaters, findings of tortuous vessels and retinal lesions, and medical history of recent COVID-19 infection in an otherwise healthy patient. The floaters are suggestive of vitritis in the absence of a posterior vitreous detachment or vitreous hemorrhage. Vitritis may be seen in the setting of uveitis syndromes such as pars planitis, posterior segment infections, or malignant masquerade syndromes. The retinal lesions seen here are small, discrete, multifocal and subretinal, consistent with an autoimmune etiology. Work-up should exclude common infectious etiologies and systemic uveitis syndromes that may present with vitritis and retinal lesions, such as sarcoidosis and lymphoma/leukemia.
Case report continued:
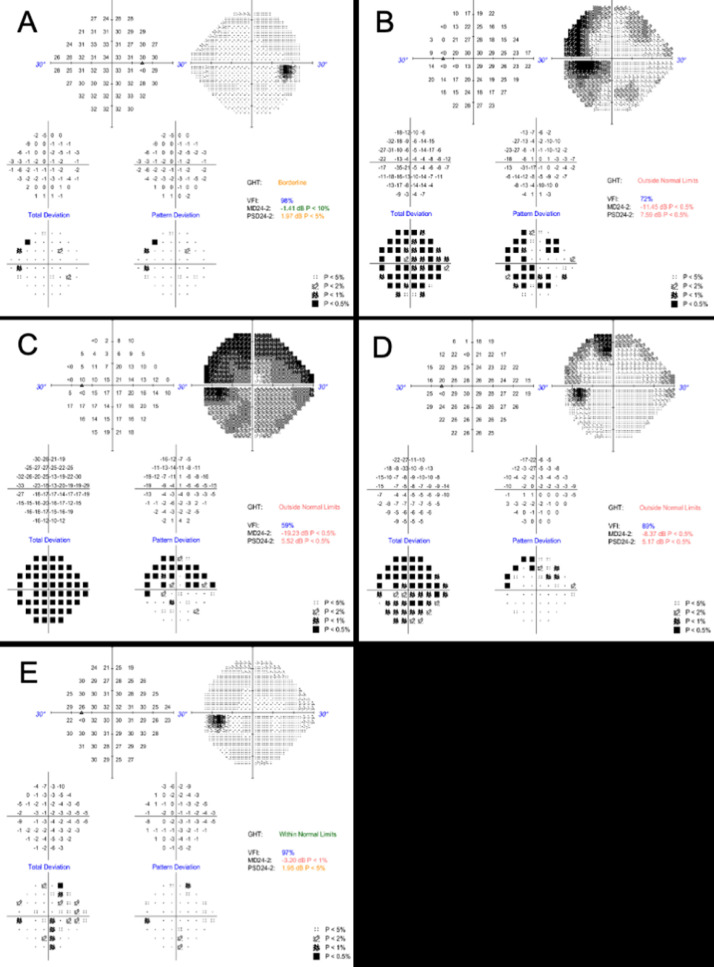
Possible masquerades such as HIV, syphilis, tuberculosis, and sarcoidosis were excluded. Complete blood count, C-reactive protein, and erythrocyte sedimentation rate values were all within normal range. A chest X-ray was normal. Positive anti-SARS-CoV-2 IgG confirmed the recent infection. Humphrey visual fields using a 24-2 protocol (VF), fundus autofluorescence (FAF), and spectral domain optical coherence tomography (SD-OCT) were unremarkable OD (Figs. 3A, 2 A and 2D). In OS, VF highlighted reduced sensitivity, mainly temporally (Fig. 3 B). FAF showed multiple, small, widespread hyperautofluorescent lesions in the outer retina; SD-OCT revealed ellipsoid zone (EZ) disruption and extension of hyper-reflective material through the EZ into the outer nuclear layer (Figs. 4 A and B). These findings were compatible with a diagnosis of multiple evanescent white dot syndrome (MEWDS). Five days later she presented to the emergency ophthalmology department complaining of worsening of vision OS. VA OS was 20/400, the Ishihara control plate was unrecognizable, and there was pain on eye movements. No RAPD was elicited. Cells were seen in the anterior chamber, the yellowish retinal lesions were more diffuse, and the VF had worsened (Fig. 3C).
Fig. 3.
A) Baseline automated visual field 24-2 (HVF) of the right eye is essentially normal. (B) Baseline HFV 24-2 of the left eye (OS) demonstrating a dense scotoma mainly involving the temporal field. (C) HVF 24-2 OS repeated five days later showing worsening of the scotoma. (D) HFV 24-2 after three weeks reveals improvement of the scotoma. (E) HVF 24-2 OS repeated two months from presentation showing complete resolution of the scotoma.
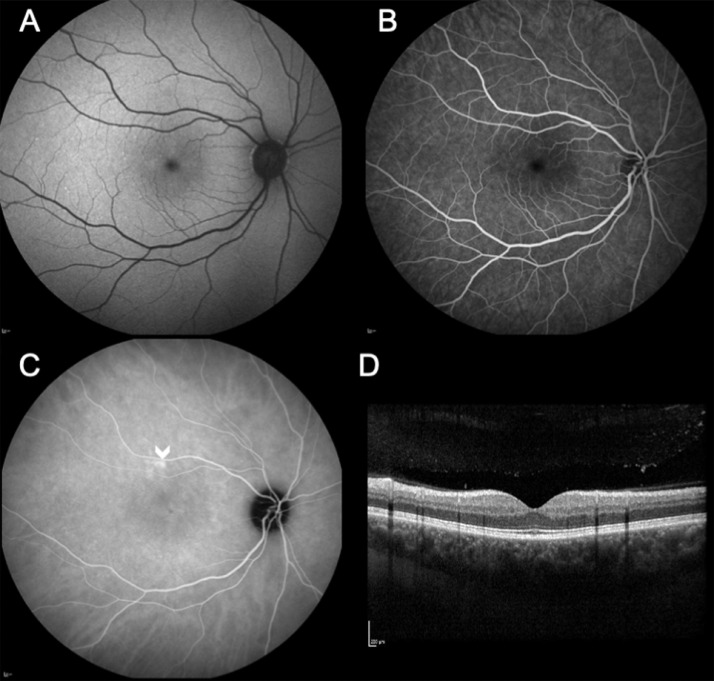
Fig. 2.
(A) Fundus autofluorescence (FAF) of the right eye and (B) Fundus fluorescein angiography (FFA) shows no abnormalities. (C) Early phase indocyanine green angiography showing an area of choroidal hyperpermeability above the macula (arrowhead). (D) Spectral domain optical coherence tomography (SD-OCT) is also structurally normal.
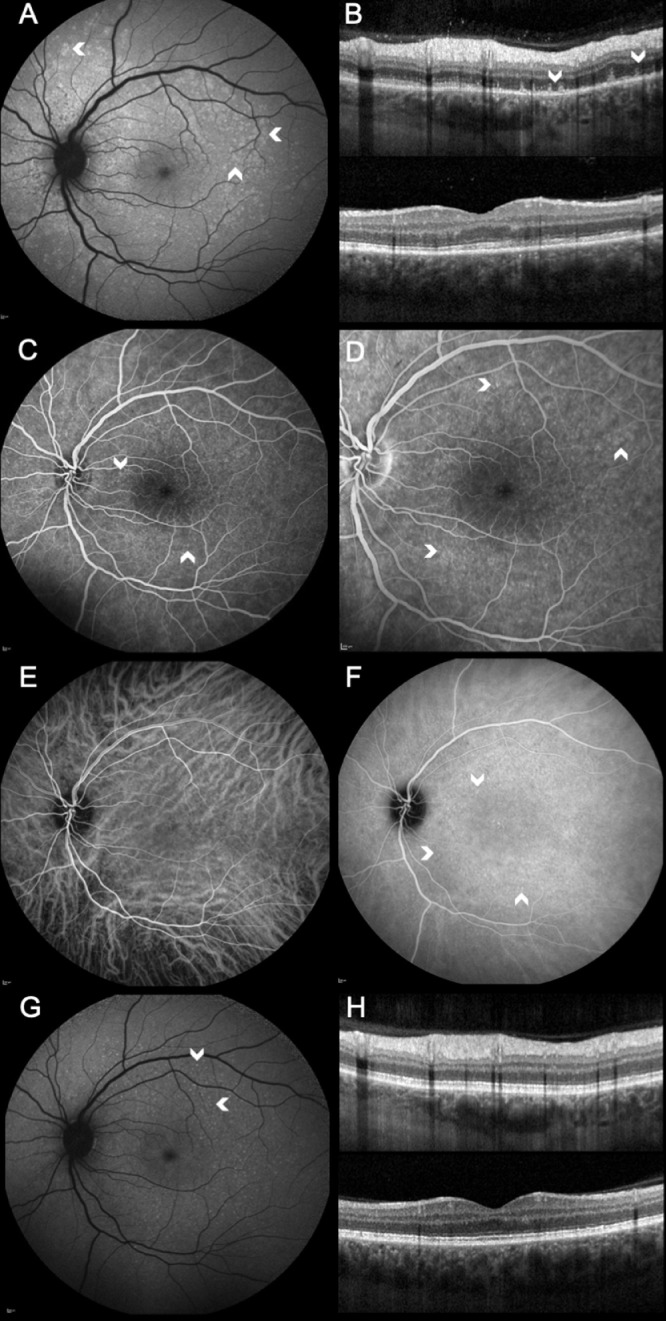
Fig. 4.
(A) Fundus autofluorescence (FAF) of the left eye showing multiple, small, widespread hyperautofluorescent lesions in the outer retina (arrowheads). (B) Same day SD-OCT revealed ellipsoid zone (EZ) disruption and extension of hyper-reflective material through the EZ into the outer nuclear layer (arrowheads) in the nasal (top) and central macula (bottom). (C) Fundus fluorescein angiography showed dilated veins, hyperfluorescent spots (arrowheads) spread throughout the retina and late hot disc (D) due to mild anterior chamber inflammation. (E) Indocyanine green angiography lacked hypofluorescent lesions, showing instead multiple hyperfluorescent lesions (arrowheads) in the intermediate phase with late-phase resolution (F). (G) Follow-up FAF reveals improved appearance of the hyperautofluorescent spots as smaller and more defined (arrowheads). (H) EZ at matching SD-OCT again clearly visible.
Does this change the diagnosis and are other tests needed?
Comments by Dr. Spencer continued:
Reassuringly, common infectious etiologies and uveitis syndromes were excluded by serologic testing and imaging. These results and progression of the unilateral subretinal lesions narrowed the diagnosis to an idiopathic autoimmune process, although the pain with eye movement and worse vision (despite the absence of a RAPD) warrants exclusion of pars planitis associated with optic neuritis and multiple sclerosis. Further characterization of the fundus lesions via FAF and angiography will aid in delineating the exact layer of ocular tissue involvement.
Case report continued
She underwent an ultrasound to exclude posterior scleritis; no T-sign was seen. Fundus fluorescein angiography (FFA) and indocyanine green angiography (ICGA) were performed. FFA was unremarkable OD (Fig. 2B); OS showed hyperfluorescence from the diffuse retinal lesions and an enhanced optic disc, presumably caused by the intraocular inflammation (Figs. 4C and 4D). ICGA OD was mainly unremarkable, but with a small area of choroidal hyperpermeability in the early phase (Fig. 2C). OS lacked the classic MEWDS hypocyanescent lesions and showed instead multiple hypercyanescent lesions in the intermediate phase with late-phase resolution (Figs. 4E and F).
She was referred to neurology for further evaluation, and perimetry was repeated (Fig. 3D). Neurologic examination was normal. Further blood tests (anti-MOG, anti-Aquaporin 4 antibodies, immunoglobulins and electrophoresis, connective tissue ANA screening, ANCA) and a brain and orbit MRI with gadolinium excluded possible central nervous system (CNS) involvement. The patient did not receive any medication and was followed up 8 weeks later by when OS FAF and SD-OCT had improved in appearance (Figs. 4G and 4H),along with the VA (20/50) and VF (Fig. 3E).
How could the lack of hypocyanescent lesions seen on ICGA be explained in this MEWDS-like case?
Would you consider any treatment?
Comments by Dr. Spencer concluded
MEWDS is an idiopathic inflammatory syndrome affecting the outer retina that is typically attributed to an aberrant immune response to an antecedent viral respiratory infection. In this case, the temporal association with a documented and symptomatic COVID-19 infection is strongly suggestive of causality, along with the low likelihood of a concomitant second viral infection. Given the novelty of SARS-CoV-2 infections in the human population, it is not altogether unexpected that a resultant immune response may lead to previously uncharacterized clinical features; rather, this appears to be a hallmark of systemic, symptomatic COVID-19 infections (e.g., MIS-C and “long COVID”). The exact nature of the subretinal and choroidal infiltrates in MEWDS is unknown, but likely highly variable immune molecules such as immunoglobulins that differ in their anatomical localization depending on the nature of the inciting agent. For this reason, it is unsurprising that novel angiographic features may be noted following a novel infection. As for treatment, the uveitis symptoms of floaters, decreased vision from subretinal and choroidal infiltrates and optic nerve inflammation often benefit from local (topical) or preferably systemic (oral) corticosteroids, although the natural history of MEWDS is often excellent without intervention.
2. Discussion
Only a few reports describing retinal features related to the coronavirus pandemic have been published to date, and some remain debated.2 Our patient, an otherwise healthy 39-year-old woman with COVID-19 viral infection confirmed by SARS-CoV-2 throat and nasal swab PCR developed ocular symptoms 2 weeks following the diagnosis and experienced progressive and profound loss of vision in OS. A multimodal imaging approach confirmed a clinical picture compatible with MEWDS; however, the ICGA lacked the classic hypocyanescent lesions usually seen in these cases and showed multiple hypercyanescent lesions instead. This particular finding has not been previously reported in MEWDS. Masquerades such as HIV, syphilis, tuberculosis, sarcoidosis, posterior scleritis and possible involvement of the CNS have therefore been excluded. She was followed for 8 weeks without receiving any local or systemic treatment. Repeated multimodal images showed improvement of the clinical picture along with the visual acuity.
MEWDS, first described in 1984 by Jampol and coworkers,1 is an inflammatory disease of the photoreceptors that recovers in nearly all cases without leaving any visible abnormality of the outer retinal structure and/or the retinal pigment epithelium.4 A multimodal imaging approach may be necessary to confirm the diagnosis.3 In typical cases of MEWDS, the ICGA shows in the mid to late phases multiple hypocyanescent areas spread across the retina.5 During the recovery phase, those hypocyanescent spots disappear even though they may last longer on ICGA.7 It is possible that the coronavirus infection in this case may be causing the peculiar MEWDS-like appearance to the retina-choroid complex with lack of hypocyanescent lesions in the choroid. Another possibility to explain this unusual behavior of the choroid may be related to the descending slope in the curve of the natural history of the disease. The choroidal hypocyanescent spots seen on the ICGA usually last longer than those visible via funduscopy or FFA.7 In any case, the lack of typical hypocyanescence on ICGA in this case may confirm both sparing of the choriocapillaris and the theory that MEWDS is mainly a condition affecting the retinal photoreceptors recently described as a "photoreceptoritis".4 Furthermore, MEWDS has also been recently described as “the common cold of the retina” due to its nature: acute, benign, and self-limited.6 In our case, we refer to the condition as “the uncommon cold of the retina” because of its atypical ICGA appearance which we believe may be linked to the recent COVID-19 viral infection in our patient. This particular inflammatory process may be included among other ophthalmic manifestations of emerging viral diseases.8
Declarations
Funding
None.
Authors’ contributions
GDS collected the data and contributed to the conception and design of the work. GDS, AM and SVP contributed to data analysis and interpretation and drafted the manuscript. SVP and GDS elaborated the figures. All authors read and approved the final manuscript.
Availability of data and materials
The data is available from the corresponding author upon reasonable request.
Ethics approval and consent to participate
Informed consent was obtained from the patient.
Declaration of competing interest
GDS reports consultant fees from Allergan, Bayer, Heidelberg Engineering and Novartis, outside the submitted work.
AM declares no competing interests.
SVP reports consultant fees from Alimera Sciences, Allergan, Bayer, Novartis and Roche, outside the submitted work.
References
- 1.Jampol LM, Sieving PA, Pugh D, Fishman GA, Gilbert H. Multiple evanescent white dot syndrome. I. Clinical findings. Arch Ophthalmol. 1984;102(5):671–674. doi: 10.1001/archopht.1984.01040030527008. [DOI] [PubMed] [Google Scholar]
- 2.Marinho PM, Marcos AAA, Romano AC, Nascimento H, Belfort R., Jr. Retinal findings in patients with COVID-19. Lancet. 2020;395(10237):1610. doi: 10.1016/S0140-6736(20)31014-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marsiglia M, Gallego-Pinazo R, Cunha De Souza E, et al. Expanded clinical spectrum of multiple evanescent white dot syndrome with multimodal imaging. Retina. 2016;36(1):64–74. doi: 10.1097/IAE.0000000000000685. [DOI] [PubMed] [Google Scholar]
- 4.Pichi F, Srvivastava SK, Chexal S, et al. En face optical coherence tomography and optical coherence tomography angiography of multiple evanescent white dot syndrome: New Insights Into Pathogenesis. Retina. 2016;36(Suppl 1):S178–SS88. doi: 10.1097/IAE.0000000000001255. [DOI] [PubMed] [Google Scholar]
- 5.Slakter JS, Giovannini A, Yannuzzi LA, et al. Indocyanine green angiography of multifocal choroiditis. Ophthalmology. 1997;104(11):1813–1819. doi: 10.1016/s0161-6420(97)30022-0. [DOI] [PubMed] [Google Scholar]
- 6.Tavallali A, Yannuzzi LA. MEWDS, Common Cold of the Retina. J Ophthalmic Vis Res. 2017;12(2):132–134. doi: 10.4103/jovr.jovr_241_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsukamoto E, Yamada T, Kadoi C, et al. Hypofluorescent spots on indocyanine green angiography at the recovery stage in multiple evanescent white dot syndrome. Ophthalmologica. 1999;213(5):336–338. doi: 10.1159/000027449. [DOI] [PubMed] [Google Scholar]
- 8.Venkatesh A, Patel R, Goyal S, Rajaratnam T, Sharma A, Hossain P. Ocular manifestations of emerging viral diseases. Eye (Lond) 2021;35(4):1117–1139. doi: 10.1038/s41433-020-01376-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data is available from the corresponding author upon reasonable request.
Ethics approval and consent to participate
Informed consent was obtained from the patient.