Abstract
Purpose
Neutralizing antibodies can reduce SARS-CoV-2 cellular entry, viral titers, and pathologic damage. CT-P59 (regdanvimab), a SARS-CoV-2 neutralizing monoclonal antibody, was examined in 2 randomized, double-blind, placebo-controlled, single ascending dose, Phase I studies.
Methods
In study 1.1, healthy adults were sequentially enrolled to receive CT-P59 10, 20, 40, or 80 mg/kg or placebo. In study 1.2, adult patients with mild SARS-CoV-2 infection were enrolled to receive CT-P59 20, 40, or 80 mg/kg or placebo. Primary objectives of both studies were safety and tolerability up to day 14 after infusion. Secondary end points included pharmacokinetic properties. Study 1.2 also measured virology and clinical efficacy.
Findings
Thirty-two individuals were randomized to study 1.1 (6 per CT-P59 dose cohort and 8 in the placebo cohort). By day 14 after infusion, adverse events (AEs) were reported in 2 individuals receiving CT-P59 20 mg/kg (headache and elevated C-reactive protein levels) and 1 receiving CT-P59 40 mg/kg (pyrexia) (all Common Terminology Criteria for Adverse Events grade 1). In study 1.2, 18 patients were randomized (5 per dose cohort and 3 in the placebo cohort). Sixteen AEs were reported in 10 patients receiving CT-P59. No AEs in either study led to study discontinuation. Greater reductions in viral titers were reported with CT-P59 than placebo in those with maximum titers >105 copies/mL. Mean time to recovery was 3.39 versus 5.25 days.
Implications
CT-P59 exhibited a promising safety profile in healthy individuals and patients with mild SARS-CoV-2 infection, with potential antiviral and clinical efficacy in patients with mild SARS-CoV-2 infection. ClinicalTrials.gov identifier: NCT04525079 (study 1.1) and NCT04593641 (study 1.2).
Keywords: COVID-19, CT-P59, neutralizing monoclonal antibody, regdanvimab, SARS-CoV-2
Introduction
The SARS-CoV-2 pandemic is a global public health crisis.1 , 2 Although public health containment measures represent the main mitigation strategy to restrict spread of the virus, effective treatments are also urgently required. Accelerated clinical research programs are evaluating a myriad of potential treatment approaches, including antiviral drugs, anti-inflammatory agents, and targeted immunomodulatory therapies, as well as vaccines.3
Entry of the coronavirus into host cells is an important determinant of viral infectivity and pathogenesis. This process is mediated through binding of the SARS-CoV-2 spike (S) protein to the angiotensin-converting enzyme 2 (ACE2) receptor located on epithelial and endothelial cells.4 Patients with SARS-CoV-2 infection exhibit a neutralizing antibody response against the S protein, which reduces cellular entry of SARS-CoV-2, decreases viral titers, reduces pathologic damage arising from infection, and alleviates the symptoms of infection in rodent and primate models of SARS-CoV-2 infection.5, 6, 7, 8 There is also evidence that neutralizing S-reactive antibodies may confer immunity to SARS-CoV-2 infection in humans.9 Collectively, these studies provide proof of principle for the use of antibody therapy against the SARS-CoV-2 S protein in the treatment of SARS-CoV-2 infection.
CT-P59, a potent neutralizing monoclonal antibody against various SARS-CoV-2 isolates, was identified through screening human monoclonal antibodies from the peripheral blood mononuclear cells of a SARS-CoV-2 convalescent patient.10 Complex crystal structure studies of the CT-P59 antigen-binding fragment/SARS-CoV-2 receptor-binding domain (RBD) suggest that CT-P59 blocks the interaction regions between SARS-CoV-2 S protein RBD and ACE2. CT-P59 inhibits SARS-CoV-2 replication in vitro with an MIC50 of 8 ng/mL, and animal models of SARS-CoV-2 infection indicate that CT-P59 is associated with a substantial reduction in viral load and alleviation of clinical symptoms.10 We report the outcomes from the first-in-human clinical evaluations of the SARS-CoV-2 antibody CT-P59. We conducted 2 Phase I, single ascending dose studies primarily to assess the safety and pharmacokinetic properties of CT-P59 in healthy volunteers and the safety, virologic efficacy, and pharmacokinetic properties in patients with mild SARS-CoV-2 infection.
Participants and Methods
Study Design
Study 1.1 and study 1.2 were randomized, double-blind, placebo-controlled, parallel-group, single ascending dose, Phase I studies. In both studies, all participants received a single dose of CT-P59 or placebo via an intravenous infusion. The final protocols for each study are included in the Appendix.
Study 1.1 was conducted at Chungnam National University Hospital, Daejeon, Republic of Korea. This study consisted of 4 sequential dose cohorts, with each including 8 healthy individuals (CT-P59, n = 6; placebo, n = 2). Cohorts 1 (CT-P59 10 mg/kg or placebo) and 2 (CT-P59 20 mg/kg or placebo) included a sentinel group of 2 individuals who were initially randomized 1:1 to receive CT-P59 or placebo. If no tolerability concerns were reported within 48 hours of dosing the sentinel participants, the remaining 6 participants from the same cohort were randomized 5:1 to receive CT-P59 or placebo. In cohorts 3 (CT-P59 40 mg/kg or placebo) and 4 (CT-P59 80 mg/kg or placebo), 8 participants were randomized in a 3:1 ratio to receive CT-P59 or placebo without a sentinel group. All participants were followed up for a total of 90 days after infusion.
Study 1.2 was conducted at 3 centers in the Republic of Korea and Romania. This study comprised 3 sequential dose cohorts (CT-P59 20, 40, or 80 mg/kg or placebo), each including 6 patients who were randomized in a 5:1 ratio to receive CT-P59 or placebo via intravenous infusion. All patients will be followed up for a total of 180 days after infusion. Dose escalation within and among studies is described in the Appendix.
Healthy male or female participants 19 to 55 years of age without SARS-CoV-2 infection were eligible for enrollment in study 1.1. All participants were required to weigh ≥50 kg, have a body mass index (BMI) of 18.0 to 29.9 kg/m2, and be able to comply with all requirements of the protocol.
Adult male or female patients 18 to 60 years of age (or 18 to 70 years of age if enrolled in the Republic of Korea) with mild symptoms of SARS-CoV-2 infection, confirmed by reverse transcription–polymerase chain reaction (RT-PCR), were eligible for enrollment in study 1.2. Patients were required to exhibit mild symptoms of SARS-CoV-2 infection, defined as oxygen saturation ≥94% on room air. Evidence of prespecified symptoms was required ≤7 days before study drug administration. Comprehensive eligibility criteria are provided in the supplementary material.
Randomization and Masking
In study 1.1, a randomization code was generated by an unblinded statistician before the study and was provided to an unblinded pharmacist in the study center. The pharmacist dispensed study drug based on the provided randomization lists. In study 1.2, randomization was performed using an interactive web response system by biostatisticians who were unblinded to treatment allocation. The study drug was dispensed by unblinded study center personnel who were not permitted to conduct any participant assessments. CT-P59 and placebo were prepared in identical 20-mL single-use vials that contained identical excipients. The only difference between the CT-P59 and placebo formulations was the exclusion of the SARS-CoV-2 neutralizing monoclonal antibody from the placebo formulation. All other study center personnel, all participants, and investigators remained blinded to treatment allocation.
Study Oversight
Both studies were conducted in compliance with the International Council for Harmonisation Good Clinical Practice and applicable regulatory requirements in accordance with the Declaration of Helsinki. The protocols and all applicable amendments were reviewed and approved by all relevant local independent ethics committees at each participating institution, and national approval was granted, where required, before commencing the study. All participants provided written informed consent before any study-related procedures.
Procedures
In study 1.1, participants were admitted to the study center on day −1 before administration of study drug on day 1. Participants were then discharged on day 4 after completion of a posttreatment 72-hour assessment. Confinement at the study site could be extended up to day 14 after drug administration according to the availability of the participants and study center and at the discretion of the investigator. In study 1.2, eligible patients were admitted to the study site and underwent screening. If participants still satisfied the eligibility criteria before dosing, they received the study drug on day 1 of the study. Confinement at the study site was required for a minimum of 72 hours.
In both studies, CT-P59 and placebo were administered via a 250-mL infusion solution of 0.9% weight/volume sodium chloride for 90 minutes. Monitoring for hypersensitivity reactions was conducted before dosing, at 15, 30, and 60 minutes from the start of the infusion, at completion of the infusion, and at 2, 3, 6, 12, and 24 hours after the start of infusion. Blood samples for pharmacokinetic assessment were taken before administration of the study drug, at the end of the infusion, at 1 hour after completion of the infusion on day 1 (also at 4, 8, and 12 hours after infusion in study 1.1 only), and on days 2, 3, 5, 7, 10, 14, 28, 56, and 90. In study 1.2, nasopharyngeal swabs for assessment of viral shedding were taken before study drug administration, daily on days 2 to 7, and on days 10, 14, 21, and 28.
Patients in study 1.2 completed a SARS-CoV-2 Infection Symptom Checklist twice daily at approximately 12-hour intervals in the morning and evening until clinical recovery or day 28, whichever came first. The symptom checklist provides a patient rating of the intensity of 7 symptoms (feeling feverish, cough, shortness of breath or difficulty breathing, sore throat, body pain or muscle pain, fatigue, and headache) using a 4-point scoring scale of absent (0), mild (1), moderate (2), and severe (3).
Outcomes
The final report describes study outcomes up to completion of the 90-day follow-up period in study 1.1, and the interim report describes study outcomes up to day 14 of follow-up in study 1.2. The primary objective of both studies was to evaluate the safety and tolerability of CT-P59 up to day 14 after study drug administration. Safety assessments included monitoring of adverse events (AEs), serious adverse events (SAEs), AEs of special interest (infusion-related reactions [IRRs], including hypersensitivity and anaphylactic reactions), vital signs, hypersensitivity monitoring, 12-lead ECG, physical examination findings, and clinical laboratory tests. The severity of AEs was graded based on the Common Toxicity Criteria for Adverse Events (CTCAE), version 5.0. In study 1.2, additional safety evaluations included the incidence of antibody-dependent enhancement, defined as excessive progression of symptoms or other signs or symptoms of SARS-CoV-2 infection considered to be manifestations of antibody-dependent enhancement, SARS-CoV-2 infection–related signs and symptoms, and radiography.
Secondary end points in both studies included pharmacokinetic analyses of CT-P59, additional safety monitoring, and the incidence of antidrug antibodies (ADAs) and neutralizing antibodies during the entire study period. This report presents pharmacokinetic analyses conducted up to follow-up day 90 in study 1.1 and follow-up day 14 in study 1.2. Pharmacokinetic end points include CT-P59 Cmax, Tmax, and dose normalized Cmax. Additional pharmacokinetic end points assessed in study 1.1 AUC0–∞, dose normalized AUC0–∞, t½, CL, and Vd during the terminal phase. Secondary end points in study 1.2 included virology, assessed using quantitative RT-PCR and next-generation sequencing, to evaluate SARS-CoV-2 genetic variants at baseline and after treatment (Viroclinics Biosciences BV, Rotterdam, the Netherlands). Efficacy evaluations included time to clinical recovery (defined as all symptoms on the SARS-CoV-2 Infection Symptom Checklist recorded as absent or mild for at least 24 hours) and the number and proportion of patients with clinical recovery up to day 14.
Statistical Analysis
Sample sizes in both studies were set empirically based on first-in-human clinical trial guidelines from the US Food and Drug Administration.11 Planned enrollment was for 32 patients in study 1.1 and 18 patients in study 1.2.
Safety analyses are based on the safety set that included all participants who had received a full or partial dose of CT-P59 or placebo. Pharmacokinetic analyses are based on the pharmacokinetic set, which includes all participants who had received a full dose of CT-P59 and had ≥1 evaluable posttreatment pharmacokinetic concentration result. Virologic and clinical efficacy analyses are based on the intent-to-treat set, defined as all participants randomly assigned to receive study drug, regardless of whether dosing was completed.
Continuous variables are reported using descriptive statistics, including the number of observations, means (SDs), and medians (interquartile ranges [IQRs]), unless otherwise specified. Categorical variables are summarized using frequency tables giving the number and percentage of participants within each category.
Results
Study 1.1: CT-P59 in Healthy Volunteers
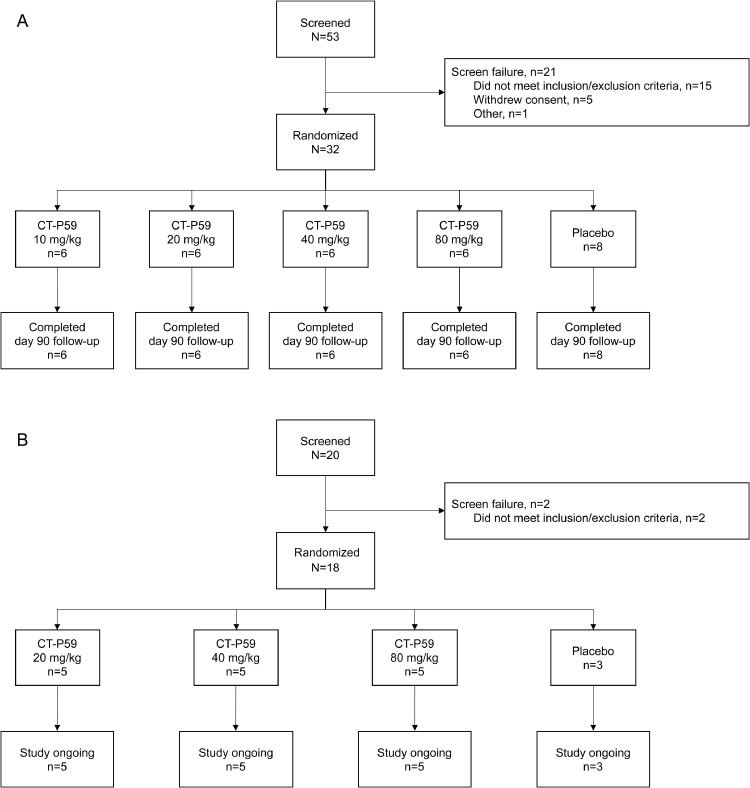
Study 1.1 was initiated on July 21, 2020, with the last visit on November 5, 2020, wherein a total of 53 individuals were screened and 32 were randomized (Figure 1 A). All randomized individuals were male and Asian, with a median age across cohorts of 24.0 years (IQR, 22–30 years) (Table I ). No difference was noted between dose cohorts in terms of age or BMI. In all 32 participants, the actual dose administered was equivalent to the prescribed dose.
Figure 1.
Participant flow diagrams for study 1.1 (A) and study 1.2 (B).
Table I.
Baseline demographic and clinical characteristics of healthy volunteers (study 1.1).
| Characteristic | CT-P59 10 mg/kg (n = 6) |
CT-P59 20 mg/kg (n = 6) |
CT-P59 40 mg/kg (n = 6) |
CT-P59 80 mg/kg (n = 6) |
Placebo (n = 8) |
Total (N = 32) |
|---|---|---|---|---|---|---|
| Age, median (IQR), y | 33.5 (25–43) | 23.5 (22–24) | 21.5 (20–23) | 28.5 (24–30) | 25.0 (21–34) | 24.0 (22–30) |
| Male sex, No. (%) | 6 (100) | 6 (100) | 6 (100) | 6 (100) | 8 (100) | 32 (100) |
| Asian race, No. (%) | 6 (100) | 6 (100) | 6 (100) | 6 (100) | 8 (100) | 32 (100) |
| BMI, median (IQR), kg/m2 | 26.140 (24.72–27.47) |
20.960 (20.54–23.03) |
23.175 (22.94–25.74) |
23.345 (22.00–24.57) |
24.665 (22.64–26.46) |
23.400 (21.88–25.87) |
BMI = body mass index; IQR = interquartile range.
Safety Profile
Predefined stopping criteria were not met; dosing of study drug in each cohort was completed per protocol. By day 14 after infusion, AEs were reported in 2 participants receiving CT-P59 20 mg/kg: 1 receiving CT-P59 40 mg/kg and 1 receiving placebo (all mild severity; CTCAE grade 1). No AEs were reported in the CT-P59 10 mg/kg or 80 mg/kg cohorts. One participant receiving CT-P59 20 mg/kg developed a headache on day 3 after infusion, which was considered as probably related to the study drug and resolved on the same day with oral paracetamol. Another participant receiving CT-P59 20 mg/kg had increased C-reactive protein levels on day 13 after infusion. C-reactive protein levels increased from 0.3 mg/dL on the day before study drug administration to 3.0 mg/dL on day 14 after infusion. This event was considered as possibly related to the study drug and resolved 2 weeks later without further intervention. One participant who received CT-P59 40 mg/kg developed pyrexia 1 day after study drug administration, with a body temperature increase from 37.6°C to 38.2°C. The investigator considered the event as probably related to the study drug; the fever resolved 1 day later without further intervention. One participant receiving placebo developed an IRR that initially manifested as dyspnea that developed 15 minutes after the start of infusion but resolved with ongoing infusion. Two hours after starting study drug administration, the participant had a mild fever (temperature of 38°C) that resolved the following day without further intervention. There were no IRRs and no evidence of a hypersensitivity reaction in any participant receiving CT-P59.
No SAEs were reported when all participants had completed 14 days of follow-up. There were no clinically meaningful changes in vital signs, electrocardiogram, or physical examination, and only 1 participant had a clinically meaningful change in laboratory values (increased C-reactive protein level, as previously described). Neither ADAs nor neutralizing antibodies were detected in any participant receiving CT-P59 up to day 7 of follow-up.
Between follow-up day 14 and follow-up day 90, an additional 5 AEs and 1 SAE were reported (Table II and eAppendix). The SAE was the result of an unintentional event and was considered unrelated to CT-P59. ADAs were not detected in any participant up to follow-up day 90.
Table II.
Safety and tolerability profiles of CT-P59 up to day 90 of follow-up in healthy volunteers (study 1.1).
| System organ class preferred term* | No. (%) of healthy volunteers |
|||||
|---|---|---|---|---|---|---|
| CT-P59 10 mg/kg (n = 6) |
CT-P59 20 mg/kg (n = 6) |
CT-P59 40 mg/kg (n = 6) |
CT-P59 80 mg/kg (n = 6) |
Placebo (n = 8) |
Total (N = 32) |
|
| Total AEs | 0 | 5 | 4 | 0 | 1 | 10 |
| Participants with ≥1 AE | 0 | 4 (66.7) | 3 (50.0) | 0 | 1 (12.5) | 8 (25.0) |
| AEs selated to study treatment | 0 | 2 (33.3) | 1 (16.7) | 0 | 1 (12.5) | 4 (12.5) |
| Grade 1 | 0 | 2 (33.3) | 1 (16.7) | 0 | 1 (12.5) | 4 (12.5) |
| AEs unrelated to study treatment | 0 | 2 (33.3) | 2 (33.3) | 0 | 0 | 4 (12.5) |
| Grade 2 | 0 | 1 (16.7) | 2 (33.3) | 0 | 0 | 3 (9.4) |
| Grade 3 | 0 | 1 (16.7) | 0 | 0 | 0 | 1 (3.1) |
| General disorders and administration-site conditions | ||||||
| Pyrexia (grade 1, related) | 0 | 0 | 1 (16.7) | 0 | 0 | 1 (3.1) |
| Injury, poisoning, and procedural complications | ||||||
| Infusion-related reaction† (grade 1, related) | 0 | 0 | 0 | 0 | 1 (12.5) | 1 (3.1) |
| Limb injury‡ (grade 3, unrelated) | 0 | 1 (16.7) | 0 | 0 | 0 | 1 (3.1) |
| Investigations | ||||||
| Blood creatine phosphokinase increased (grade 2, unrelated) | 0 | 1 (16.7) | 1 (16.7) | 0 | 0 | 2 (6.3) |
| C-reactive protein increased (grade 1, related) | 0 | 1 (16.7) | 0 | 0 | 0 | 1 (3.1) |
| γ-Glutamyl transferase increased (grade 2, unrelated) | 0 | 0 | 1 (16.7) | 0 | 0 | 1 (3.1) |
| White blood cells urine positive (grade 1, unrelated) | 0 | 0 | 1 (16.7) | 0 | 0 | 1 (3.1) |
| Nervous system disorders | ||||||
| Headache (grade 1, related) | 0 | 1 (16.7) | 0 | 0 | 0 | 1 (3.1) |
| Skin and subcutaneous tissue disorders | ||||||
| Urticaria‡ (grade 3, unrelated) | 0 | 1 (16.7) | 0 | 0 | 0 | 1 (3.1) |
| Any SAE | 0 | 1 (16.7)c | 0 | 0 | 0 | 1 (3.1) |
| AEs leading to interruption or discontinuation of infusion | 0 | 0 | 0 | 0 | 0 | 0 |
| Hypersensitivity or anaphylactic reaction | 0 | 0 | 0 | 0 | 1 (12.5)b | 1 (3.1) |
| Death | 0 | 0 | 0 | 0 | 0 | 0 |
AE = adverse event; SAE = serious adverse event.
Coded from Medical Dictionary for Regulatory Activities (MedDRA), version 23.1.
Grade 1 infusion-related reaction also listed as an AE manifested as onset of dyspnea 15 minutes after commencement of infusion that resolved with continued infusion and pyrexia (38°C) approximately 2 hours after completion of infusion that resolved within 24 hours.
Grade 3 limb injury was reported as an SAE because of hospitalization for the event and was determined by the investigator as unrelated to the treatment; grade 3 urticaria occurred shortly after receiving a transfusion for the limb injury. The other AE reported in this participant was grade 3 urticaria, which occurred on the same day as the grade 3 limb injury and resolved on the same day.
Pharmacokinetic Properties
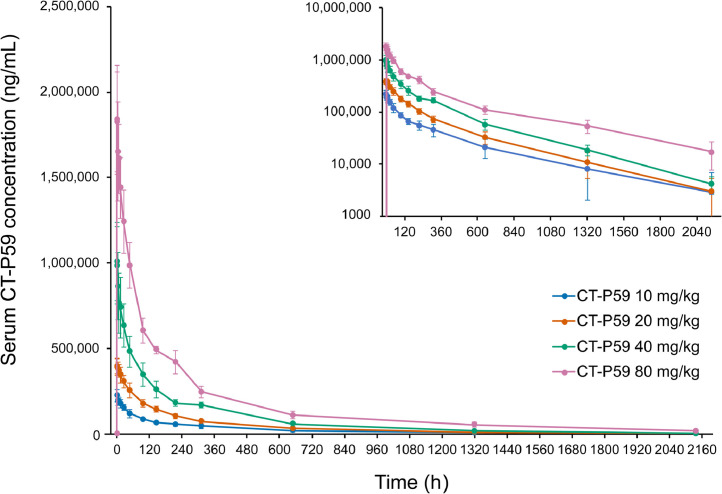
CT-P59 Cmax, Tmax, dose normalized Cmax, AUC0–∞, dose normalized AUC0–∞, t½, CL, and Vd during the terminal phase are presented in the eAppendix. The increase in the CT-P59 Cmax was proportional to dose over the dose range of 10 to 80 mg/kg, reaching Tmax at approximately 2 hours after administration in all dose cohorts (Figure 2 and Supplemental Table I). The 90% CI for the slope among CT-P59 dose, Cmax, and AUC0–∞ (log-transformed) included 1, with an 8.3-fold increase of Cmax and 6.6-fold increase in geometric mean AUC0–∞ (Supplemental Table II). Mean CT-P59 t½ was between 16.6 and 22.0 days and appeared to be unrelated to dose.
Figure 2.
Mean serum concentrations of CT-P59 in healthy volunteers (study 1.1). Data are presented on a semilogarithmic scale shown as an insert. Error bars indicate SDs.
Study 1.2: CT-P59 in Patients With Mild SARS-CoV-2 Infection
Study 1.2 was initiated on September 4, 2020. A total of 20 patients with mild SARS-CoV-2 infection were screened and 18 were randomized (Figure 1B). Patients in the 20 mg/kg dose cohort were enrolled at 2 study sites in the Republic of Korea; those in the 40 mg/kg and 80 mg/kg dose cohorts were enrolled at 1 study site in Romania. All patients in the CT-P59 20 mg/kg dose cohort were Asian; all those in the CT-P59 40 mg/kg and 80 mg/kg cohorts were White (Table III ). Median age across all cohorts was 52.0 years (IQR, 49–57 years). Among the risk factors for developing severe illness, no patients were >65 years of age, and 5 patients had hypertension, obesity, or diabetes mellitus. All the higher-risk patients were included in the CT-P59 treatment groups. All 18 patients received a dose of study drug equivalent to the prescribed dose. Median time from symptom onset to administration of study drug was 4 to 6 days across the CT-P59 treatment groups and 4 days in the placebo group.
Table III.
Demographic and disease characteristics of patients with mild SARS-CoV-2 infection (study 1.2).
| Characteristic | CT-P59 20 mg/kg (n = 5) |
CT-P59 40 mg/kg (n = 5) |
CT-P59 80 mg/kg (n = 5) |
Placebo (n = 3) |
Total (N = 18) |
|---|---|---|---|---|---|
| Age, median (IQR), y | 59.0 (56–59) |
51.0 (48–52) |
52.0 (43–57) |
50.0 (49–57) |
52.0 (49–57) |
| Male sex, No. (%) | 2 (40) | 2 (40) | 5 (100) | 2 (66.7) | 11 (61.1) |
| Race, No. (%) | |||||
| White | 0 | 5 (100) | 5 (100) | 2 (66.7) | 12 (66.7) |
| Asian | 5 (100%) | 0 | 0 | 1 (33.3) | 6 (33.3) |
| BMI, median (IQR), kg/m2, | 24.610 (24.34–25.96) | 26.890 (23.01–30.86) | 28.630 (27.46–29.40) |
27.770 (24.45–27.80) | 27.175 (24.45–28.63) |
| Time since symptom onset, median (IQR), d* | 4.0 (3–5) |
6.0 (5–6) |
4.0 (4–4) |
4.0 (4–6) |
4.0 (4–5) |
| Quantitative RT-PCR Ct value, mean (SD) | 24.406 (5.0934) | 28.176 (6.6642) | 24.651 (5.5092) | 24.827 (7.7960) | 25.516 (6.2297) |
BMI = body mass index; Ct = cycle threshold; IQR = interquartile range; RT-PCR = reverse transcription–polymerase chain reaction.
Number of days is calculated as [date of study drug administration − first start date of SARS-CoV-2 infection-associated symptoms + 1].
Safety Profile
Predefined stopping criteria were not met; dosing of study drug in each cohort was completed per protocol. A total of 16 AEs were reported in 10 patients receiving CT-P59, and 3 AEs were reported in 1 patient receiving placebo (Table IV ). AEs of diarrhea and increased alanine aminotransferase levels were each reported by 2 patients receiving CT-P59; all other AEs were reported by a single patient. All AEs were considered unrelated to the study drug. Grade 3 AEs were reported by 2 patients receiving CT-P59 40 mg/kg (hepatocellular injury and increased alanine aminotransferase concentration) and 1 patient receiving placebo (COVID-19 pneumonia). One patient with a medical history of obesity and hypertriglyceridemia who received CT-P59 40 mg/kg experienced grade 4 hypertriglyceridemia that was considered a worsening of the patient's preexisting condition. There were no SAEs, AEs that led to study discontinuation, or AEs of special interest (IRRs). There were no clinically meaningful changes in vital signs, hypersensitivity monitoring, ECG, radiography, physical examination, or SARS-CoV-2 infection–related signs and symptoms. There was also no evidence leading to suspicion of ADE or ADA-positive results at posttreatment visit up to day 14.
Table IV.
Safety and tolerability profiles of CT-P59 up to day 14 of follow-up in patients with mild SARS-CoV-2 infection (study 1.2).
| System organ class preferred term* |
No. (%) of patients with mild SARS-CoV-2 |
||||
|---|---|---|---|---|---|
| CT-P59 20 mg/kg (n = 5) |
CT-P59 40 mg/kg (n = 5) |
CT-P59 80 mg/kg (n = 5) |
Placebo (n = 3) |
Total (N = 18) |
|
| Total AEs† | 5 | 8 | 3 | 3 | 19 |
| Patients with ≥1 AE‡ | 3 (60) | 4 (80) | 3 (60) | 1 (33.3) | 11 (61.1) |
| Grade 1 | 3 (60) | 0 | 1 (20) | 0 | 4 (22.2) |
| Grade 2 | 0 | 1 (20) | 2 (40) | 0 | 3 (16.7) |
| Grade 3 | 0 | 2 (40) | 0 | 1 (33.3) | 3 (16.7) |
| Grade 4 | 0 | 1 (20) | 0 | 0 | 1 (5.6) |
| Cardiac disorders | |||||
| Palpitations, grade 2 | 0 | 1 (20) | 0 | 0 | 1 (5.6) |
| Gastrointestinal disorders | |||||
| Diarrhea, grade 1 | 2 (40) | 0 | 0 | 0 | 2 (11.1) |
| Dysphagia, grade 1 | 0 | 1 (20) | 0 | 0 | 1 (5.6) |
| Hepatocellular disorders | |||||
| Hepatocellular injury, grade 3 | 0 | 1 (20) | 0 | 0 | 1 (5.6) |
| Infections and infestations | |||||
| COVID-19 pneumonia, grade 3 | 0 | 0 | 0 | 1 (33.3) | 1 (5.6) |
| Candida infection, grade 2 | 0 | 0 | 1 (20) | 0 | 1 (5.6) |
| Investigations | |||||
| ALT increased, grade 1 | 0 | 0 | 1 (20) | 0 | 1 (5.6) |
| ALT increased, grade 3 | 0 | 1 (20) | 0 | 0 | 1 (5.6) |
| Blood CPK increased, grade 2 | 0 | 0 | 1 (20) | 0 | 1 (5.6) |
| Metabolism and nutrition disorders | |||||
| Hypertriglyceridemia, grade 4 | 0 | 1 (20) | 0 | 0 | 1 (5.6) |
| Musculoskeletal and connective tissue disorders | |||||
| Flank pain, grade 1 | 1 (20) | 0 | 0 | 0 | 1 (5.6) |
| Psychiatric disorders | |||||
| Insomnia, grade 1 | 1 (20) | 0 | 0 | 0 | 1 (5.6) |
| Respiratory, thoracic, and mediastinal disorders | |||||
| Productive cough, grade 1 | 1 (20) | 0 | 0 | 0 | 1 (5.6) |
| Vascular disorders | |||||
| Hypertension, grade 2 | 0 | 0 | 0 | 1 (33.3) | 1 (5.6) |
AE = adverse event; ALT = alanine aminotransferase; CPK = creatine phosphokinase.
Coded from Medical Dictionary for Regulatory Activities (MedDRA), version 23.1.
There were no SAEs or AEs leading to study discontinuation, AEs of special interest (infusion-related reaction, including hypersensitivity and anaphylactic reaction), or deaths.
All AEs were considered by the investigator to be unrelated to the study drug.
Viral Shedding
All patients had positive results for viral shedding based on quantitative RT-PCR at baseline. Because of the variability in viral shedding at baseline, mean viral titers based on quantitative RT-PCR were analyzed according to maximum viral titers. Overall, viral shedding in both the CT-P59 treatment groups and placebo group decreased over time. In patients with low maximum viral titers (<105 copies/mL), titers decreased regardless of study drug administration (Figure 3 A). In patients with maximum viral titers of >105 copies/mL, decrease in viral load was more rapid among patients receiving CT-P59 (20, 40, or 80 mg/kg) than those receiving placebo (Figure 3B–D). Of the 3 patients who received placebo, 2 had maximum viral titers of <105 copies/mL, and 1 had maximum titer of >105 copies/mL. Overall, mean change from baseline for viral titers in nasopharyngeal swab specimens based on quantitative RT-PCR was generally greater in the CT-P59 treatment groups than placebo up to day 14 (Supplemental Table III).
Figure 3.
Change in viral titer according to maximum viral load (A) <105 copies (cp)/mL, (B) >105 cp/mL, (C) >106 cp/mL, and (D) >107 cp/mL in patients with mild SARS-CoV-2 infection (study 1.2). Dotted horizontal lines represent lower limit of detection.
Next-Generation Sequencing Analysis
None of the enrolled patients had an allele frequency of ≥15% for Q493K or S494P variants. Such substitutions in the S protein RBD have been identified in nonclinical neutralization assays as reducing susceptibility to CT-P59. Variants with N501Y, E484K, and K417N mutations, associated with the Alpha (United Kingdom) and Beta (South African) variants, were also not observed at an allele frequency of ≥15% at baseline or after study drug administration.
Clinical Efficacy
Except for 1 placebo recipient, all patients recovered by day 14 (Supplemental Table IV). Mean time to recovery was 3.39 days across all CT-P59 treatment groups compared with 5.25 days among patients who had received placebo (the patient who did not recover was censored at day 14 and included in this analysis at the point of censor). The mean time to clinical recovery was 4.43 days among those who had received CT-P59 20 mg/kg, 3.21 days among those who had received CT-P59 40 mg/kg, and 2.52 days among those who had received CT-P59 80 mg/kg. No patient in CT-P59 treatment groups received other antiviral therapy or had decreased oxygen saturation <94% as a result of worsening symptoms of SARS-CoV-2 infection. The patient in the placebo group who had not recovered by follow-up day 14 received dexamethasone orally for 11 days, remdesivir intravenously for 5 days, and tocilizumab intravenously (3 doses for 2 days); however, symptoms of SARS-CoV-2 infection persisted up to and beyond day 14.
Pharmacokinetic Properties
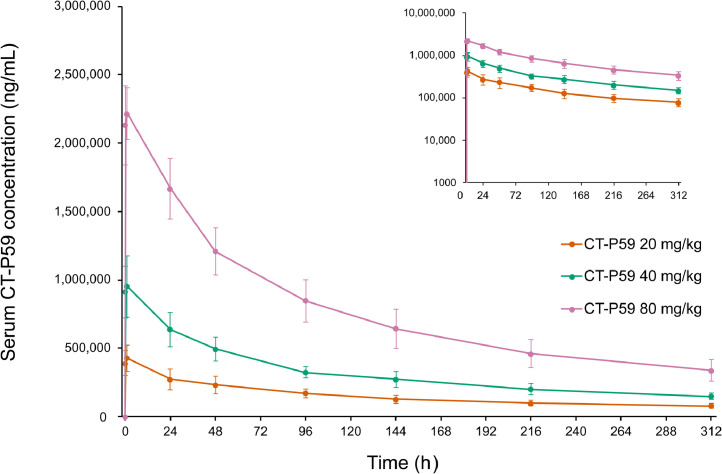
Overall, pharmacokinetic results confirmed that exposure to CT-P59 increased with dose during the studied range of 20 to 80 mg/kg (Figure 4 and Supplemental Table V). CT-P59 Cmax increased in a greater than dose-proportional manner. The 90% CI for the slope between CT-P59 dose and Cmax (both log-transformed) was 1.21, with 90% CIs that excluded 1 (90% CI, 1.074–1.355) (Supplemental Table II). In all cohorts, median Tmax was reached 2.5 hours after administration (Supplemental Table V), and CT-P59 levels remained detectable up to 14 days after administration (Figure 4).
Figure 4.
Serum concentrations of CT-P59 in patients with mild SARS-CoV-2 infection (study 1.2). Data are presented on a semilogarithmic scale shown as an insert. Error bars indicate SDs.
Discussion
Data from these phase 1, single ascending dose studies suggest that the SARS-CoV-2 S protein antibody CT-P59 has an acceptable safety profile in healthy volunteers, up to 90 days after intravenous administration, and in patients with mild SARS-CoV-2 infection, up to 14 days after intravenous administration. In healthy volunteers, most AEs were mild or moderate in severity and did not require intervention. Similarly, in patients with mild SARS-CoV-2 infection, most AEs were mild or moderate in severity, and all AEs, including those of CTCAE grade 3 or 4, were considered unrelated to study drug. There were no IRRs or anaphylactic events among individuals who had received CT-P59 in either study. Protocol-specified dose escalation rules were satisfied before each dose escalation in both studies, and there was no indication of a dose-related increase in toxicity. In healthy participants and patients with mild SARS-CoV-2 infection, pharmacokinetic analyses indicated that plasma levels of CT-P59 increased with increasing dose; maximum concentrations were reached approximately 2.5 hours after infusion. The mean t½ of CT-P59 at doses of 10 to 80 mg/kg, observed by day 90 in healthy individuals, was approximately 19 days. Quantitative RT-PCR analysis of nasopharyngeal swab specimens indicated that decrease in viral load was similar with CT-P59 and placebo in patients with low maximum viral load (<105 copies/mL) but that greater reductions in viral titers were achieved with CT-P59 than with placebo in patients with maximum titers >105 copies/mL. Furthermore, the mean time to clinical recovery of SARS-CoV-2 symptoms (assessed by SARS-CoV-2 Infection Symptom Checklist) was shorter in patients who had received CT-P59 compared with placebo.
Several lines of evidence support evaluation of S protein antibodies in the treatment of SARS-CoV-2 infection. Preclinical data indicate that S protein antibodies from patients with SARS-CoV-2 infection may offer protection in models of infection. SARS-CoV-2 neutralizing antibodies exhibit potent inhibition of viral replication in vitro and reduce viral load, improve pathologic mechanisms, and alleviate symptoms of infection in rodent and primate models of SARS-CoV-2 infection.5 , 7 Convalescent plasma therapy, in which blood plasma from a recovered patient is transfused to a symptomatic patient, is also undergoing evaluation.12, 13, 14 Finally, the RBD-specific antibodies REGN-COV-2 and LY-CoV555 are both being studied for the treatment of SARS-CoV-2 infection.15, 16, 17 REGN-COV-2 is a cocktail of 2 antibodies that target nonoverlapping epitopes on the S protein, developed to mitigate the risk of escape mutations emerging under therapeutic pressure.15 , 17 LY-CoV555 is also an RBD-specific antibody that protects the upper and lower airways from SARS-CoV-2 infection in a primate model of infection.16 Early clinical data indicate a reduction in viral load with LY-CoV555 (2800 mg) relative to placebo in patients with mild or moderate SARS-CoV-2 infection; however, these findings were not replicated in patients receiving the higher 7000 mg dose.18
There are limitations to drawing statistically robust conclusions about the differences between the groups in these Phase I studies, given the small sample size. However, the safety, virology, efficacy, and pharmacokinetic properties of CT-P59 will be further evaluated in a large number of patients in 2 Phase II studies of CT-P59, which will offer greater statistical power to determine differences between CT-P59 and placebo. Because studies 1.1 and 1.2 were the first to evaluate CT-P59 in healthy volunteers and patients with mild SARS-CoV-2 infection, patients with comorbidities considered clinically significant by the investigator were not enrolled. Furthermore, evaluation of efficacy in study 1.2 was limited by the inclusion of only 3 patients in the placebo group, 2 of whom had low maximum viral titers (<105 copies/mL). Efficacy comparisons between CT-P59 and placebo in patients with high maximum viral titers (>105 copies/mL), therefore, include only 1 patient in the placebo group. Moreover, because this was an interim analysis of study 1.2, additional data from patients with SARS-CoV-2 infection who complete 180 days of follow-up, including further clinical outcomes, will form the basis of a later report.
Since initiating this study, a number of key variants of SARS-CoV-2 have been identified and become prevalent. Because study 1.2 was initiated on September 4, 2020, patients enrolled in this study are unlikely to have been exposed to the emerging variants in the countries where the study was performed. Further studies are ongoing to determine the effectiveness of regdanvimab against SARS-CoV-2 variants. Recently, in vivo ferret challenge studies found that a therapeutic dosage of CT-P59 decreased upper and lower respiratory tract viral load of the Beta (South African) variant, termed B.1.351 (K417N/E484K/N501Y), to a comparable extent as that observed with the wild-type virus.19 The finding of sufficient antiviral effect in B.1.351-infected animals treated with a clinical dosage of CT-P59 suggests that CT-P59 has therapeutic potential for patients infected with the Beta variant of SARS-CoV-2.19
Notably, the limited study locations resulted in a relatively homogenous study population and, in the case of study 1.1, a coincidental imbalance of male and female participants. Therefore, we are cautious about drawing conclusions about more diverse populations until further studies have been completed.
Conclusion
CT-P59, an experimental antibody therapy for patients with SARS-CoV-2 infection, exhibited a promising safety profile in healthy volunteers and patients with mild SARS-CoV-2 infection and potential antiviral efficacy in patients with mild symptoms of SARS-CoV-2 infection. On the basis of the promising results from these studies, CT-P59 was granted Investigational New Drug applications from several countries, including the Republic of Korea and the United States, and additional Phase II and III studies are ongoing in outpatients with SARS-CoV-2 infection. With 2 Phase I studies and a Phase II study, CT-P59 was granted marketing authorization in the Republic of Korea in February 2021. In March 2021, the European Medicines Agency Committee for Medicinal Products for Human Use gave a positive scientific opinion for the use of CT-P59 for the treatment of confirmed COVID-19 in adult patients at high risk of progressing to severe disease (not requiring supplemental oxygen therapy). A separate rolling review of CT-P59, which began in February 2021, is ongoing and will provide the basis for a European Union marketing authorization application once completed.
Acknowledgments
Acknowledgment
We thank all participants and investigators involved in the studies. Medical writing support (including development of a draft outline and subsequent drafts in consultation with the authors, assembling tables and figures, collating author comments, copyediting, fact checking, and referencing) was provided by Duncan Campbell, PhD, CMPP, at Aspire Scientific (Bollington, United Kingdom) and funded by Celltrion Inc. (Incheon, Republic of Korea). Jin Yong Kim, Young Rock Jang, Jang Hee Hong, Jin Gyu Jung, Jae-Hyeong Park, Adrian Streinu-Cercel, Anca Streinu-Cercel, Oana Săndulescu, and Yeon-Sook Kim contributed to patient enrollment and monitoring and data collection and analysis. Sang Joon Lee, Sung Hyun Kim, Na Hyun Jung, Seul Gi Lee, Jeong Eun Park, Min Kyung Kim, Da Bee Jeon, Yeo Jin Lee, Bum Soo Kim, and Yeon Mi Lee contributed to trial design and data analysis. All authors drafted and revised the manuscript and approved the final version for submission.
Funding Sources
This work was supported by Celltrion Inc. The sponsor funded both studies and contributed to the study design and conduct, data collection, and statistical analyses; reviewed the manuscript throughout development for scientific accuracy; and approved the final version. Both studies were also supported by the Korea Drug Development Fund, funded by the Korean Ministry of Science and ICT; Ministry of Trade, Industry, and Energy; and Ministry of Health and Welfare (HQ20C0045, Republic of Korea). All coauthors had access to the study data, approved the final draft of the manuscript, and assume full responsibility for the accuracy of the data.
Disclosure
Jin Yong Kim and Young Rock Jang have been investigators in COVID-19 clinical trials by Daewoong Pharmaceuticals, Enzychem Lifesciences, and GC Pharma, outside the scope of the submitted work, and by Celltrion Inc, within the scope of the submitted work. Adrian Streinu-Cercel, Anca Streinu-Cercel, and Oana Săndulescu, have been investigators in COVID-19 clinical trials by Algernon Pharmaceuticals, Atea Pharmaceuticals, Diffusion Pharmaceuticals, and Regeneron Pharmaceuticals, outside the scope of the submitted work, and by Celltrion Inc, within the scope of the submitted work. Sang Joon Lee and Sung Hyun Kim are employees of and hold shares in Celltrion Inc. Na Hyun Jung, Seul Gi Lee, Jeong Eun Park, Min Kyung Kim, Da Bee Jeon, Yeo Jin Lee, Bum Soo Kim, and Yeon Mi Lee are employees of Celltrion Inc. The authors have indicated that they have no other conflicts of interest regarding the content of this article.
References
- 1.Johns Hopkins Coronavirus Research Center . JHU; 2021. COVID-19 dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University.https://coronavirus.jhu.edu/map.html accessed 1 Jul 2021. [Google Scholar]
- 2.World Health Organization . 2021. WHO Coronavirus Disease (COVID-19) Dashboard.https://covid19.who.int/ accessed 1 Jul 2021. [PubMed] [Google Scholar]
- 3.Wiersinga WJ, Rhodes A, Cheng AC, et al. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;324:782–793. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- 4.Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu L, Wang P, Nair MS, et al. Potent neutralizing antibodies against multiple epitopes on SARS-CoV-2 spike. Nature. 2020;584:450–456. doi: 10.1038/s41586-020-2571-7. [DOI] [PubMed] [Google Scholar]
- 6.Shi R, Shan C, Duan X, et al. A human neutralizing antibody targets the receptor-binding site of SARS-CoV-2. Nature. 2020;584:120–124. doi: 10.1038/s41586-020-2381-y. [DOI] [PubMed] [Google Scholar]
- 7.Zost SJ, Gilchuk P, Case JB, et al. Potently neutralizing and protective human antibodies against SARS-CoV-2. Nature. 2020;584:443–449. doi: 10.1038/s41586-020-2548-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu Y, Wang F, Shen C, et al. A noncompeting pair of human neutralizing antibodies block COVID-19 virus binding to its receptor ACE2. Science. 2020;368:1274–1278. doi: 10.1126/science.abc2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Addetia A, Crawford KHD, Dingens A, et al. Neutralizing antibodies correlate with protection from SARS-CoV-2 in humans during a fishery vessel outbreak with high attack rate. J Clin Microbiol. 2020;58:e02107–e02120. doi: 10.1128/JCM.02107-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim C, Ryu DK, Lee J, et al. A therapeutic neutralizing antibody targeting receptor binding domain of SARS-CoV-2 spike protein. Nat Commun. 2021;12:288. doi: 10.1038/s41467-020-20602-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.US Food and Drug Administration. Expansion cohorts: use in first-in-human clinical trials to expedite development of oncology drugs and biologics guidance for industry. August 2018, https://www.fda.gov/regulatory-information/search-fda-guidance-documents/expansion-cohorts-use-first-human-clinical-trials-expedite-development-oncology-drugs-and-biologics; 2018 (accessed 1 Jul 2021).
- 12.Salazar E, Perez KK, Ashraf M, et al. Treatment of coronavirus disease 2019 (COVID-19) patients with convalescent plasma. Am J Pathol. 2020;190:1680–1690. doi: 10.1016/j.ajpath.2020.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hartman WR, Hess AS, Connor JP. Hospitalized COVID-19 patients treated with convalescent plasma in a mid-size city in the Midwest. Transl Med Commun. 2020;5:17. doi: 10.1186/s41231-020-00068-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li L, Zhang W, Hu Y, et al. Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: a randomized clinical trial. JAMA. 2020;324:460–470. doi: 10.1001/jama.2020.10044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baum A, Fulton BO, Wloga E, et al. Antibody cocktail to SARS-CoV-2 spike protein prevents rapid mutational escape seen with individual antibodies. Science. 2020;369:1014–1018. doi: 10.1126/science.abd0831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones BE, Brown-Augsburger PL, Corbett KS, et al. The neutralizing antibody, LY-CoV555, protects against SARS-CoV-2 infection in nonhuman primates. Sci Transl Med. 2021;13:eabf1906. doi: 10.1126/scitranslmed.abf1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baum A, Ajithdoss D, Copin R, et al. REGN-COV2 antibodies prevent and treat SARS-CoV-2 infection in rhesus macaques and hamsters. Science. 2020;370:1110–1115. doi: 10.1126/science.abe2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen P, Nirula A, Heller B, et al. SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with Covid-19. N Engl J Med. 2021;384:229–237. doi: 10.1056/NEJMoa2029849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ryu DK, Song R, Kim M, et al. Therapeutic effect of CT-P59 against SARS-CoV-2 South African variant. Biochem Biophys Res Commun. 2021;566:135–140. doi: 10.1016/j.bbrc.2021.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]