Abstract
Acacia catechu (L.f.) Willd is a profoundly used traditional medicinal plant in Asia. Previous studies conducted in this plant are more confined to extract level. Even though bioassay-based studies indicated the true therapeutic potential of this plant, compound annotation was not performed extensively. This research is aimed at assessing the bioactivity of different solvent extracts of the plant followed by annotation of its phytoconstituents. Liquid chromatography equipped with high resolution mass spectrometry (LC-HRMS) is deployed for the identification of secondary metabolites in various crude extracts. On activity level, its ethanolic extract showed the highest inhibition towards α-amylase and α-glucosidase with an IC50 of 67.8 ± 1 μg/mL and 10.3 ± 0.1 μg/mL respectively, inspected through the substrate-based method. On the other hand, the plant extract showed an antioxidant activity of 23.76 ± 1.57 μg/mL, measured through radical scavenging activity. Similarly, ethyl acetate and aqueous extracts of A. catechu showed significant inhibition against Staphylococcus aureus with a zone of inhibition (ZoI) of 13 and 14 mm, respectively. With the LC-HRMS-based dereplication strategy, we have identified 28 secondary metabolites belonging to flavonoid and phenolic categories. Identification of these metabolites from A. catechu and its biological implication also support the community-based usage of this plant and its medicinal value.
1. Introduction
Acacia catechu (L.f.) Willd is a deciduous and gregarious tree with a light feathery crown native to Nepal, India, and Myanmar and one of the most promising medicinal plants of the family Fabaceae [1]. A. catechu has received attention as a potential source of bioactive secondary metabolites to be used for the formulation of pharmaceutical products [2, 3]. Various parts of plant extracts were known to have strong antioxidant [2], antimicrobial [4], anti-inflammatory [5], antihyperglycemic [6], and immunomodulatory [7] activities. Although several secondary metabolites have been identified from A. catechu, the molecules, catechin, epicatechin, and quercetin, are the principal contributor to therapeutical properties [8]. Nowadays, plant-based secondary metabolites are extensively used in the management of various infectious diseases and achieved clinical benefits in the health care system.
Diabetes mellitus (DM) is a leading cause of hyperglycemia, and carbohydrate-hydrolase inhibitors, such as inhibitors for α-amylase and α-glucosidase, offer an effective strategy to lower the level of postprandial hyperglycemia via control of dietary carbohydrates and glycogen breakdown [9]. Moreover, microbial infections have undermined the existing antibiotic-based treatment era and raised the mortality rate in patients with higher medical expenses and extended hospital stays [10]. Around the world, about 28,000 plant taxa have been known for their medicinal values and about 3000 plant species possess ethnopharmacological uses for the management of DM and others [11]. Different explorers had effectively shown the inhibitory abilities of natural products towards digestive enzymes, hence reducing hyperglycemia [12–14].
Hyperglycemia stimulates the autooxidation of glucose to generate free radicals [15]. Free radicals are created constantly in the body during metabolism as they are required to serve various essential functions essential for survival [16]. A body with a weak defense system is unable to counteract these increased radicals, which ultimately leads to imbalance, and this condition of more free radicals than antioxidants is known as oxidative stress. Excess free radicals and reactive oxygen species, beyond the scavenging capacities of the cellular antioxidant system, are involved in several human diseases and complications like arteriosclerosis, cancer, the aging process, diabetes, cardiovascular disease, nerve damage, blindness, and nephropathy [15, 17–20]. Antioxidants from natural products especially fruits, vegetables, herbs, and spices are effective in reducing diabetic vascular complications [15] and have diverse pharmaceutical properties [21, 22].
Plant products have played the important role in the development of new therapeutics with milder adverse side effects than commercial drugs [23]. Consequently, it is important to recognize and measure all the secondary metabolites to ensure the biological research reliability and reproducibility over the pharmacological benefits and/or hazards. Currently, liquid chromatography with high resolution mass spectrometry (LC-HRMS) has emerged as a leading tool for detecting and identifying pharmacologically active secondary metabolites [24, 25]. Nuclear magnetic resonance spectroscopy and mass spectrometry followed by chemometric tools are the most used analytical methods of annotation [26]. Additionally, LC-HRMS is useful for exact mass measurement as well as for molecular formula generation of any unknown molecules, parent ions, and fragment ions in the plant extracts [27]. LC-HRMS not only allows for the determination of chemical structure but also offers excellent sensitivity to the low amount of sample in a short time and also plays an important role in the screening of flavonoids and other phenolic contents [28, 29]. Thus, this study was carried out for method validation for some biochemical assays and molecular profiling in the ethanolic extract of A. catechu.
2. Materials and Methods
2.1. Chemicals
The α-glucosidase from Saccharomyces cerevisiae (CAS No. 9001-42-7), porcine pancreas α-amylase (CAS No. 9000-90-2), 4-nitrophenyl-α-D-glucopyranoside (pNPG) (CAS No. 3767-28-0), 2-chloro-4-nitrophenyl-α-D-maltotrioside (CNPG3) (CAS No.118291-90-0), acarbose (CAS No. 56180-94-0), and quercetin (CAS No. 117-39-5) were purchased from Sigma-Aldrich, Germany. Gallic acid, 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical, Folin-Coicalteu reagent, resazurin sodium, and other chemicals and solvents were purchased from Molychem, Himedia, and Fisher Scientific, India.
2.2. Collection and Identification of the Plant
A. catechu was collected from Syangja District of Gandaki Province with coordinates 27° 58′ N, 83° 46′ E Nepal, in January 2020. Its taxonomy was verified in voucher specimen TUCH-201011 by the Central Department of Botany, Tribhuvan University, Kirtipur, Nepal. Its major phytoconstituents and ethnopharmacological application are mentioned in Table S1.
2.3. Preparation of Crude Extracts and Fractionation
The barks of A. catechu were shade dried at room temperature and pulverized. The powder was soaked in ethanol for 24 hours and filtered. The same process was repeated for three successive days, and collected filtrate was concentrated in a vacuum in a rotary evaporator below 45°C. Fractionation was carried out by dissolving 50 g of crude ethanolic extract into distilled water and then partitioned three times with hexane, dichloromethane (DCM), and ethyl acetate successively to obtain respective solvent-solvent fractions.
2.4. Total Phenolic Content
The Folin-Coicalteu method was employed for the estimation of total phenolic content (TPC) as described by Lu et al. [30] with slight modifications. 20 μL of sample (0.5 mg/mL) plant extracts/standard followed by 100 μL of Folin-Coicalteu reagent (1 : 10; v/v) was added in a 96-well microtiter plate, and initial absorbance was taken. Then, 80 μL of 1 M Na2CO3 solution was added to the above mixture making a final volume of 200 μL and incubated for 25 minutes. Absorbance was measured at 765 nm by using Synergy LX Multi-Mode Reader with Gen5 3.08.01 software. The TPC was determined using a calibration curve with gallic acid, and results were expressed as milligrams of gallic acid equivalent per gram of dry weight of the extract (mg GAE/g). Triplicates of each measurement were carried out for validation.
2.5. Total Flavonoid Content
Total flavonoid content (TFC) were estimated by the AlCl3 method, based on the formation of a complex between AlCl3 and flavonoid with a maximum absorbance at 415 nm [31]. Briefly, 20 μL of each extract (0.5 mg/mL) was separately mixed with 60 μL ethanol and 5 μL 10% AlCl3. Subsequently, 5 μL of 1 M potassium acetate and 110 μL distilled water were supplemented into each well, and the reaction mixture was allowed to stand for 25 minutes. Triplicates of each measurement were carried out to verify experimental reproducibility. The TFC was determined using a calibration curve with quercetin, and results were expressed as milligrams of quercetin equivalent per gram dry weight of extract (mg QE/g).
2.6. Free Radical Scavenging Activity
The radical scavenging activity was observed to demonstrate the antioxidant ability of plant extracts as described previously with slight modifications [32]. The reaction was done in 200 μL volume by mixing 100 μL DPPH (0.1 mM) and 100 μL plant extract. Then, it was incubated for 25 minutes in the dark and absorbance was taken at 517 nm. The percent scavenging was calculated by the following formula:
| (1) |
where Ao is absorbance of DPPH radical with 50% DMSO and At is absorbance of DPPH radical with test or reference sample.
2.7. In Vitro α-Glucosidase Inhibitory Assay
The competitive inhibition-based assay with α-glucosidase was carried out using a method adopted by Fouotsa et al. [33] and Aryal et al. [34] with slight modifications. Briefly, 20 μL of plant extract prepared on 30% DMSO and 80 μL of the α-glucosidase enzyme (0.5 U/mL) prepared in 50 mM phosphate saline buffer (pH 6.8) were added, and initial absorbance was taken at 410 nm in a microplate reader. Then, the microtiter plate was preincubated at 37°C for 15 minutes. 100 μL of 1.4 mM substrate (pNPG) was added and incubated at 37°C for 25 minutes, and absorbance was measured. Acarbose was used as the reference compound, and each assay was performed in triplicate for validation. The percentage inhibition of α-glucosidase by plant extract was calculated by using the following formula:
| (2) |
where Ac is the absorbance of enzyme-substrate reaction with 30% DMSO and At is the absorbance of enzyme-substrate with plant extract.
2.8. In Vitro α-Amylase Inhibition Assay
The competitive inhibition-based assay with α-amylase was carried out by following the method adopted by Khadayat et al. [32] with slight modifications. 20 μL of plant extracts in 50% DMSO and 80 μL of porcine pancreas α-amylase (1.5 U/mL) in phosphate buffer of pH 7.0 were loaded, and initial absorbance was taken at 405 nm. The microtiter plate was incubated at 37°C for 15 minutes. Then, 100 μL of 1 mM CNPG3, the substrate was added to initiate the reaction with incubation for 25 minutes, and the change in absorbance was monitored at 405 nm [32]. Acarbose was used as a reference compound. The percentage inhibition of α-amylase enzyme was estimated using equation (2) mentioned earlier.
2.9. Antibacterial Assay
The antibacterial activity of A. catechu extract against Gram-positive (Staphylococcus aureus ATCC 25923) and Gram-negative (Escherichia coli ATCC 25922, Klebsiella pneumonia ATCC 13883, Salmonella typhi ATCC 14028, and Shigella sonnei ATCC 25931) was assayed through Agar well diffusion method in a Mueller Hinton Agar (MHA) plate [35]. The inoculum of microorganisms in Mueller Hinton broth (MHB) adjusted to 0.5 McFarland equivalents was spread on the surface of the MHA plate using a sterile cotton swab. Then, wells were punched aseptically into the agar surface by using a sterile cork borer of 6 mm diameter and filled with 50 μL (50 mg/mL) of plant extract prepared in 50% DMSO. The plates were allowed to diffuse at room temperature for 2 hours. 50 μL of neomycin (1 mg/mL) and DMSO was used as positive and negative control, respectively. The zone of inhibition (ZoI) was determined by measuring the diameters of bacterial growth on plates after incubation at 37°C for 24 hours.
The extracts with maximum ZoI were further evaluated for minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) via the resazurin-based turbidimetric broth microdilution method [36]. The minimum concentration before the color change (from blue to pink) was taken as MIC value, and higher concentrations were streaked onto the MHA plates at different compartments and incubated overnight at 37°C to observe concentration corresponding to no growth of bacteria, i.e., MBC.
2.10. LC-HRMS Analysis
The LC-HRMS analysis of ethyl acetate and the aqueous fraction was performed using an Agilent 6520, Accurate-Mass Q-TOF Mass Spectrometer equipped with a G1311A quaternary pump, G1329A autosampler, and G1315D diode array detector at Sophisticated Analytical Instrument Facility (SAIF), CSIR-Central Drug Research Institute, Lucknow. Source and scan parameter settings include gas temp: 30°C, gas flow: 11.01/min, nebulizer: 40 psi, VCap: 3500, fragmentor: 175, skimmer1: 65.0, and octopoleRF Peak: 750. The solvent elution consists of acetonitrile, 5 mM acetate buffer, and water at the flow rate of 1.5 mL/min. The elution gradient was started from 5% acetonitrile for 0.1 min to 30% acetonitrile for 10 min, 80% acetonitrile for 32 minutes, and back to its initial conditions. During the whole process, column temperature was maintained at 30°C. After passing through the flow cell of the diode array detector, the column elute was directed to Q-TOF HRMS fitted with an electrospray interface. The mass spectrum analysis was carried out using positive electron spray ionization (ESI-positive mode) within the mass range of 100-2000 daltons at a scan rate of 1.03.
2.11. Data Analysis
The results were processed by using Gen5 Microplate Data Collection and Analysis Software and then by MS Excel. Inhibition of enzymatic hydrolysis of the substrates (pNPG and CNPG3) by 50% (IC50) was calculated using the GraphPad Prism Software version 8. All the experiments were carried out in triplicate, and data were presented in mean ± standard deviation. Raw data files acquired from the LC-HRMS were processed using MZmine 2 and then Mestre Nova 12.0 for compound annotation using PubChem, Dictionary of Natural Products 2, ChemSpider, and METLIN database.
3. Results
3.1. Total Phenolic and Flavonoid Content
The TPC and TFC were expressed as the GAE/g and QE/g of extract using calibration curves of gallic acid and quercetin, respectively. The TPC of A. catechu was found to be 175.48 ± 4.67 mg GAE/g which was greater than its TFC examined, i.e., 7.66 ± 1.0 mg QE/g.
3.2. Free Radical Scavenging Activity
The antioxidant of ethanolic extracts of A. catechu was evaluated by using a quick, reliable, and reproducible method through the measurement of 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging. DPPH radical scavenging results were reported as IC50 and compared with the IC50 value of quercetin (6.3 ± 1.0 μg/mL) as a standard. The radical scavenging ability of plant extract is 23.76 ± 1.57 μg/mL.
3.3. α-Glucosidase and α-Amylase Inhibitory Activities
The results of α-glucosidase and α-amylase inhibitory activity of the extracts are expressed in Table 1. Among the tested fraction, aqueous and ethyl acetate fraction showed the most activity with an IC50 value of 82.6 ± 0.3 and 130.2 ± 0.6 μg/mL against the α-glucosidase as compared to acarbose (IC50 = 344.23 ± 1.03 μg/mL). In the α-amylase assays, the crude ethanol extract showed antidiabetic activity with an IC50 of 67.8 ± 1.8 μg/mL, compared to acarbose (IC50 = 6.1 ± 0.1 μg/mL).
Table 1.
α-Glucosidase and α-amylase inhibitory activity of different fractions of A. catechu.
| Extracts | IC50 (μg/mL) | |
|---|---|---|
| α-Glucosidase | α-Amylase | |
| Crude ethanol extract | 10.3 ± 0.1 | 67.8 ± 1.8 |
| Hexane fraction | <50 % | <50 % |
| Dichloromethane fraction | 174.7 ± 0.6 | <50 % |
| Ethyl acetate fraction | 130.2 ± 0.6 | <50 % |
| Aqueous fraction | 82.6 ± 0.3 | <50% |
| Standard (acarbose) | 344.2 ± 1.03 | 6.02 ± 0.1 |
3.4. Antibacterial Activity
The antibacterial activity of plant extracts against S. aureus ATCC 25923, E. coli ATCC 25922, K. pneumoniae ATCC 13883, S. typhi ATCC 14028, and S. sonnei ATCC 25931 was performed. The antibacterial activity was measured in terms of ZoI diameter in millimeters (mm) as shown in Table 2. Based on the ZoI, MIC and MBC were evaluated against S. aureus ATCC 25923. The MIC and MBC of the aqueous fraction of A. catechu bark extract were 6.25 and 12.5 mg/mL while those of neomycin are 0.0625 and 0.25 mg/mL respectively.
Table 2.
Zone of inhibition of each fraction of plants.
| Microorganisms | Zone of inhibition (ZoI) in millimeters (mm) | ||||
|---|---|---|---|---|---|
| Hexane Fraction | Dichloromethane fraction | Ethyl acetate fraction | Aqueous fraction | Positive control | |
| Staphylococcus aureus | 11 mm | 9 mm | 13 mm | 14 mm | 10 mm |
| Escherichia coli | — | — | — | — | 16 mm |
| Klebsiella pneumonia | — | — | 8 mm | 10 mm | 19 mm |
| Salmonella typhi | — | — | — | — | 18 mm |
| Shigella sonnei | 6 mm | 7 mm | 12 mm | 10 mm | 25 mm |
Note: positive control (50 mg/mL neomycin).
3.5. LC-HRMS-Driven Molecular Annotation
The raw data of LC-HRMS were processed using MZmine, and the fraction with the best total ion chromatogram (TIC) was proceeding for further analysis using MestreNova 12.0 software. The TIC obtained using MZmine of ethyl acetate and aqueous fraction is shown in Figure S1. Details of identified compounds with their theoretical and observed mass to charge ratio, double bond equivalence (DBE), molecular formula, absolute errors in parts per million (ppm), and retention time (Rt) in positive ion mode in ESI are presented in Table 3. The compounds were identified based on the observed mass spectra and also compared with the literature data (Figure 1). We observed the presence of flavonoids and phenolic compounds in the extract of A. catechu such as catechin/epicatechin (m/z = 291.08) (Figure S2), gallocatechin/epigallocatechin (m/z = 307.08) (Figure S3), procyanidin B1/procyanidin B3 (m/z = 579.15) (Figure S4), emodin (m/z = 271.06) (Figure S5), epiafzelechin/afzelechin (m/z = 275.08) (Figure S6), maclurin (m/z = 263.05) (Figure S7), irisflorentin (m/z = 387.10) (Figure S8), naringenin (m/z = 273.07) (Figure S9), isoquercetin (m/z = 465.10) (Figure S10), diosmetin (m/z = 301.07) (Figure S11), chrysin (m/z = 255.06) (Figure S12), myricetin (m/z = 319.04) (Figure S13), kaempferol (m/z = 287.05) (Figure S14), avicularin (m/z = 435.09) (Figure S15), prodelphinidin B3 (m/z = 595.14) (Figure S16), prodelphinidin B (m/z = 611.14) (Figure S17), quercetin (m/z = 303.05) (Figure S18), taxifolin (m/z = 305.06) (Figure S19), acacetin (m/z = 285.07) (Figure S20), aciculatinone (m/z = 413.12) (Figure S21), gossypin (m/z = 481.09) (Figure S22), pterocarpin (m/z = 299.09) (Figure S23), isorhamnetin (m/z = 317.06) (Figure S24), and trihydroxy dimethoxyflavone (m/z = 331.08) (Figure S25).
Table 3.
Identified compounds from A. catechu with names, base peak, double bond equivalence (DBE), retention time (Rt), and m/z values.
| Annotated compounds | Systematic name | Calculated mass | Observed mass | Formula | DBE | Absolute error (ppm) | Rt (minute) | Fragment peak | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Catechin (1) | [(2R,3S)-2-(3,4-Dihydroxyphenyl)-3,4-dihydro-2H-chromene-3,5,7-triol] | 290.07 | 291.08 | C15H14O6 | 9 | 0.16 | 10.4 | 313.07 [M + Na]+ and 139 | [8, 37, 38] LC/MS and LC/MS/MS, NMR, UV |
| Epicatechin (2) | [(2R,3R)-2-(3,4-Dihydroxyphenyl)-3,4-dihydro-2H-chromene-3,5,7-triol] | 290.07 | 291.08 | C15H14O6 | 9 | 0.16 | 10.4 | 313.07 [M + Na]+ and 139.03 | [8, 37, 38] LC/MS and LC/MS/MS, UV, NMR |
| Gallocatechin (3) | [(2R,3S)-2-(3,4,5-Trihydroxyphenyl)-3,4-dihydro-2H-chromene-3,5,7-triol] | 306.07 | 307.08 | C15H14O7 | 9 | 2.26 | 7.15 | 313.07 [M + Na]+, 289.07, and 139.03 | [8, 38, 39] LC/MS and LC/MS/MS, UV, NMR |
| Epigallocatechin (4) | [(2R,3R)-2-(3,4,5-Trihydroxyphenyl)-3,4-dihydro-2H-chromene-3,5,7-triol] | 306.07 | 307.08 | C15H14O7 | 9 | 2.26 | 7.15 | 313.07 [M + Na]+, 289.07, and 139.03 | [8, 38, 39] LC/MS and LC/MS/MS, UV, NMR |
| Procyanidin B1 (5) | (2R,2′R,3R,3′S,4R)-2,2′-Bis(3,4-dihydroxyphenyl)-3,3′,4,4′-tetrahydro-2H,2'H-4,8′-bichromene-3,3′,5,5′,7,7′-hexol | 578.15 | 579.15 | C30H26O12 | 18 | 2.21 | 9.87 | 427.10 [M + H − 152]+, 289.07 (kampferol) | [8, 40] HPLC, LC/MS, and LC/MS/MS |
| Procyanidin B3 (6) | (2R,2′R,3S,3′S,4S)-2,2′-Bis(3,4-dihydroxyphenyl)-3,3′,4,4′-tetrahydro-2H,2′H-4,8′-bichromene-3,3′,5,5′,7,7′-hexol | 578.15 | 579.15 | C30H26O12 | 18 | 2.21 | 9.87 | 427.10 [M + H − 152]+, 289.07 (kampferol) | [8, 41] NMR, (Q-Trap), LC/MS and LC/MS/MS |
| Emodin (7) | 1,3,8-Trihydroxy-6-methyl-9,10-anthraquinone | 270.05 | 271.06 | C15H10O5 | 11 | 4.18 | 18.60 | 253.16, 243.17, 229.14, 225.13, and 197.08 | [42] UPLC-DAD-MS/MS |
| Afzelechin (8) | (2R,3S)-2-(4-Hydroxyphenyl)-3,4-dihydro-2H-chromene-3,5,7-triol | 274.08 | 275.08 | C15H14O5 | 9 | 3.51 | 12.77 | 257.17, 233.08 | [43–45] UHPLC-PDA-HRMS, HPLC/ESI-MS and NMR, HPLC/MS/MS |
| Epiafzelechin (9) | (2R,3R)-2-(4-Hydroxyphenyl)-3,4-dihydro-2H-chromene-3,5,7-triol | 274.08 | 275.08 | C15H14O5 | 9 | 3.51 | 12.77 | 257.17, 233.08 | [43–45] UHPLC-PDA-HRMS, HPLC/ESI-MS and NMR, HPLC/MS/MS |
| Maclurin (10) | (3,4-Dihydroxyphenyl)-(2,4,6-trihydroxyphenyl)methanone | 262.04 | 263.05 | C13H10O6 | 9 | 4.24 | 2.96 | 153.08 | [46] HPLC/ESI-MS |
| Irisflorentin (11) | 9-Methoxy-7-(3,4,5-trimethoxyphenyl)-[1,3]dioxolo[4,5-g]chromen-8-one | 386.09 | 387.1 | C20H18O8 | 12 | 2.45 | 5.07 | 357.09 [M + H − CH3 × 2]+, 372.07 [M + H − CH3]+ | [47–49] HPLC–DAD–ESI-MS, NMR, LC-MS |
| Naringenin (12) | 5,7-Dihydroxy-2-(4-hydroxyphenyl)chroman-4-one | 272.06 | 273.07 | C15H12O5 | 10 | 2.07 | 12.56 | 153.03 | [50] (LC–MS/MS) |
| Isoquercetin (13) | 2-(3,4-Dihydroxyphenyl)-5,7-dihydroxy-3-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxychromen-4-one | 464.09 | 465.1 | C21H20O12 | 12 | 3.02 | 12.95 | 303.05 (quercetin), 289.07 (kampferol) | [51] |
| Diosmetin (14) | 5,7-Dihydroxy-2-(3-hydroxy-4-methoxyphenyl)-4H-chromen-4-one | 300.06 | 301.07 | C16H12O6 | 11 | 4.94 | 13.72 | 289.09, 149.09 | [52, 53] UHPLC-LTQOrbitrap MS, NMR |
| Chrysin (15) | 5,7-Dihydroxy-2-phenylchromen-4-one | 254.05 | 255.06 | C15H10O4 | 11 | 2.26 | 16.18 | 153.12 | [54] UPLC-MS/MS |
| Myricetin (16) | 3,5,7-Trihydroxy-2-(3,4,5-trihydroxyphenyl)chromen-4-one | 318.03 | 319.04 | C15H10O8 | 11 | 3.54 | 12.64 | [55] HPLC-PAD, UV/MS + NMR |
|
| Kaempferol (17) | 3,5,7-Trihydroxy-2-(4-hydroxyphenyl)chromen-4-one | 286.04 | 287.05 | C15H10O6 | 11 | 1.16 | 17.17 | 259.13, 165.09, 153.12 | [56] Q-TOF-HRMS |
| Avicularin (18) | 3-[(2S,3R,4R,5S)-3,4-Dihydroxy-5-(hydroxymethyl)oxolan-2-yl]oxy-2-(3,4-dihydroxyphenyl)-5,7-dihydroxychromen-4-one | 434.08 | 435.09 | C21H20O13 | 12 | 3.39 | 13.38 | 303.05, 287.06, and 183.10 | [57] UHPLC-DAD-ESI-HRMS/MS, NMR |
| Prodelphinidin B3 (19) | (2R,2′R,3S,3′S,4S)-2,2′-Bis(3,4,5-trihydroxyphenyl)-3,3′,4,4′-tetrahydro-2H,2′H-4,8′-bichromene-3,3′,5,5′,7,7′-hexol | 594.13 | 595.14 | C30H26O13 | 18 | 3.03 | 5.78 | 427.08, 169.07, 291.09, 305.07 | [40, 58] HPLC, ESI-Q-TOF |
| Prodelphinidin B (20) | 2-(3,4,5-Trihydroxyphenyl)-8-[3,5,7-trihydroxy-2-(3,4,5-trihydroxyphenyl)-3,4-dihydro-2H-chromen-4-yl]-3,4-dihydro-2H-chromene-3,5,7-triol | 610.13 | 611.14 | C30H25O14 | 18 | 2.52 | 8.05 | 307.08 | [59] UPLC-ESI-MS |
| Quercetin (21) | 2-(3,4-Dihydroxyphenyl)-3,5,7-trihydroxychromen-4-one | 302.04 | 303.05 | C15H10O7 | 11 | 1.6 | 13.96 | 285.15, 257.13, 247.15, 229.13, 201.12, 183.10, 165.08, 153.11, 137.08, 121.03, 111.06 | [60] FTMS, HPLC, LC MS/MS, and LCMS |
| Taxifolin (22) | (2R,3R)-2-(3,4-Dihydroxyphenyl)-3,5,7-trihydroxy-2,3-dihydrochromen-4-one | 304.05 | 305.06 | C15H12O7 | 10 | 1.78 | 13.51 | 287.05, 179.09 | [61, 62] 1UHPLC-DAD-FLD, UHPLC-HRMS/MS, and HPLC-ESI-IT-TOF-MS |
| Acacetin (23) | 5,7-Dihydroxy-2-(4-methoxyphenyl)chromen-4-one | 284.06 | 285.07 | C16H12O5 | 11 | 2.64 | 16.38 | 245.15 (M + H − CO2)+, 213.13(M − CO2 − C0 + H)+ | [63] UHPLC-Q-TOF-MS/MS |
| Aciculatinone (24) | 5-Hydroxy-8-[(2R,5R,6R)-5-hydroxy-6-methyl-4-oxooxan-2-yl]-2-(4-hydroxyphenyl)-7-methoxychromen-4-one | 412.11 | 413.12 | C22H20O8 | 13 | 3.80 | 8.18 | 325.10,296.10, 295.09 | [64] NMR, HREIMS |
| Gossypin (25) | 2-(3,4-Dihydroxyphenyl)-3,5,7-trihydroxy-8-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxychromen-4-one | 480.09 | 481.09 | C21H20O13 | 12 | 2.32 | 11.79 | 319.04, | [65, 66] HRMS, LCMS/MS |
| Pterocarpin (26) | (1R,12R)-16-Methoxy-5,7,11,19-tetraoxapentacyclo[10.8.0.02,10.04,8.013,18]icosa-2,4(8),9,13(18),14,16-hexaene | 298.08 | 299.09 | C17H14O5 | 11 | 3.59 | 14.59 | [67] DART-TOF-MS | |
| Isorhamnetin (27) | 3,5,7-Trihydroxy-2-(4-hydroxy-3-methoxyphenyl)chromen-4-one | 316.05 | 317.06 | C16H12O7 | 11 | 1.03 | 17.70 | 303.21, 274.20, 153.12 | [68] LC-MS/MS |
| Trihydroxy dimethoxyflavone (28) | 5,6-Dihydroxy-2-(4-hydroxy-3-methoxyphenyl)-7-methoxychromen-4-one | 330.07 | 331.08 | C17H1407 | 11 | 2.36 | 14.25 | 301.08 and 315.09 | [69] UHPLC/Q-TOF MS |
Figure 1.
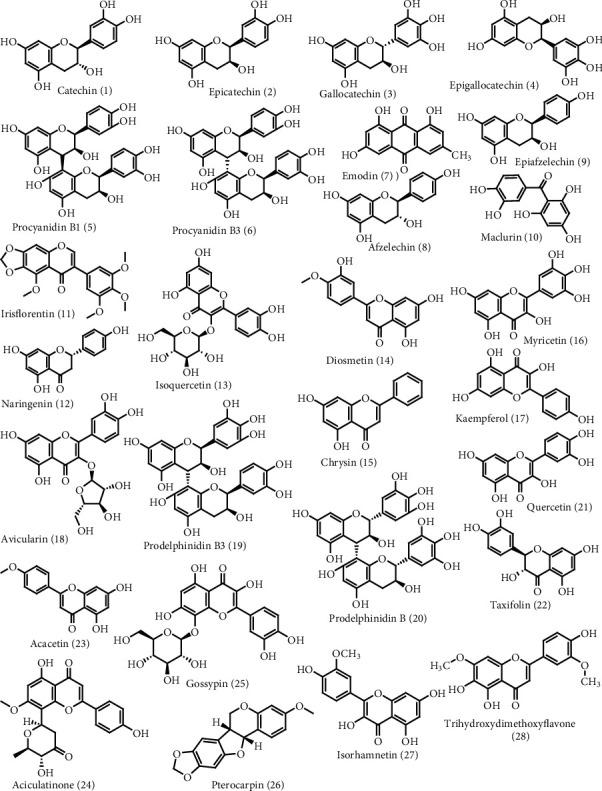
Annotated secondary metabolites in the ethanolic A. catechu bark extracts
4. Discussion
The study was aimed at making a comparative study of ethanolic and methanolic extract of A. catechu in terms of antidiabetic, antioxidant, antibacterial, and secondary metabolite profiling. Figure 2 shows the schematic workflow of the study. The TPC, TFC, and antioxidant activity of the crude ethanol extract of A. catechu were determined and compared with our previous work [34]. Studies have shown the valuable uses of plant-based bioactive secondary metabolites to mankind [8]. Plant extract contains many phenolic compounds with inhibitory activity against α-glucosidase supported by TPC and TFC of crude extract 175.48 ± 4.67 mg GAE/g and TFC 7.66 ± 1.0 mg QE/g respectively. The methanolic extract of A. catechu exhibited TPC and TFC of 186.675 ± 2.021 mg GAE/g and 10.24 ± 0.69 mg QE/g respectively [34]. Previous studies suggest that variation in TPC and TFC is due to differences in extracting solvents [70]. Likewise, the difference between antioxidant activity among the extracts might be due to the presence of metabolites at a different level which stabilizes the free radicals by donating hydrogen atoms [71, 72].
Figure 2.
Schematic workflow of the study
Compounds such as caprylic acid, methyl ester, lauric acid methyl ester, 2-ethyl-3-methyl-1-butene, myristic acid methyl ester [73], catechin, acacatechin, catechutannic acid, 4-hydroxybenzoic acid, afzelechin, epiafzelechin, mesquitol, ophioglonin, aromadendrin, kaempferol, baicalin, baicalein, and quercetin [74–77] and 5-hydroxy-2-[2-(4-hydroxyphenyl)acetyl]-3-methoxylbenzoic acid, (2S,3S)-3,7,8,3′,4′-pentahydroxyflavane, rhamnetin, 4-hydroxyphenyl ethanol, 3,3′,5,5′,7-pentahydroxyflavane, and fisetinidol [78] were isolated from A. catechu. Phytochemicals such as phenolic and flavonoid isolated from various parts of the plants are used as antioxidant, antimicrobial, antiulcer, antidiabetic, anticancer, antihyperlipidemic, antidiabetic, and hepatoprotective candidates [79, 80]. Previously, the IC50 value for inhibition of α-amylase activity by A. catechu ethanol extract was reported as 341.20 ± 15.30 μg/mL by Lakshmi et al. [2], while ethanol extract showed an inhibition of 67.8 ± 1.8 μg/mL in this study. Likewise, the methanolic extract of the A. catechu bark showed an IC50 of 115 ± 4.0 mg/mL and 23.7 ± 0.7 mg/mL against α-glucosidase and α-amylase, respectively [34], whereas leaf extracts showed an IC50 of 0.4977 mg/mL against α-glucosidase [81]. The difference in activities might be due to expression levels of secondary metabolites, which are greatly influenced by the regulation of biosynthetic gene clusters in climatic variation. Here, in our study, the crude extracts showed a potent inhibitory activity against carbohydrate hydrolases than that of the solvent fractions, which might be due to the synergistic effect of metabolites [82].
The phytochemicals exhibit a wide range of antioxidant, antidiabetic, and antimicrobial activity [83, 84]. The antimicrobial activity of extracts from different parts of A. catechu [85–87] has been shown previously. A. catechu resin exhibits an inhibitory effect against B. subtilis (MIC: 20 μg/mL), S. aureus (MIC: 40 μg/mL), P. aeruginosa (MIC: 220 μg/mL), and E. coli (MIC: 330 μg/mL) [87]. In our study, the fractions of A. catechu from ethanol extract showed comparable antibacterial activities to methanol extract fraction as reported by Aryal et al. [34]. It might be due to the same experimental conditions, the only difference in the extracting solvent.
The antioxidant mechanisms of flavonoid and phenolic compounds play an important role in protecting humans against infections and degenerative diseases [88]. Polyphenols with the number of hydroxyl groups can act as a source of hydrogen and electron donor to radicals to stabilize them and hence reduce the oxidative stress which plays an important role in the management of diabetes [89, 90]. Hydroxyl groups at 3′, 4′, and 5′of ring B (a pyrogallol group) and the double bond between carbon-2 and carbon-3 conjugated with the 4-oxo (=O) and 3-hydroxyl (-OH) group in ring C enhance the radical scavenging activity of flavonoids [91]. From the study of a structure-activity relationship, hydroxyl groups and their configuration, a ketonic functional group at carbon-4, and a double bond at carbons (C2–C3) are quite important structural features on flavonoids to show their antioxidant and antidiabetic ability [92, 93].
Isolation of plant metabolites is considerably challenging due to the lack of standardized instruments, income sources, and the availability of the laboratory in our context. To identify the metabolites within a short instance of time with a small number of samples, LC-HRMS of ethyl acetate and aqueous fractions of A. catechu bark extract were carried out. To further evaluate the metabolites, MZmine 2 was used to study the peak features of the raw MS files. The TIC obtained by overlaying through MZmine 2 is shown in Figure S1. Then, Mesternova 12.0 software is utilized to annotate the metabolites based on m/z, retention time, and molecular formula, and other databases are used to search and assign formulas and compound structures. Twenty-eight phenolic and flavonoids were annotated during our study by comparing our spectral data with the literature. The base peak atm/z 291.08, molecular formula C15H14O6, and DBE 9 and fragment peaks at 313.07 [M + Na]+ and 139.03 are speculated as catechin (1) or epicatechin (2). This data is consistent with Shen et al. [8] and Ibrahim et al. [37]. The fragmentation pattern of catechin and epicatechin is shown in Figure S26. Catechin/epicatechin was already reported with antidiabetic [94] (IC50 of 160 ± 67 μg/mL against α-amylase; 31 μg/mL and >290 μg/mL against α-glucosidase), antimicrobial [95], anti-inflammatory [96], and antioxidant [97] abilities and also reduces the risk of ischemic heart disease [98]. Likewise, [M + H]+ at m/z 307.08, molecular formula C15H14O7, DBE 9, and along with fragment peak at 289.07 and 139.03 is considered as gallocatechin (3) or epigallocatechin (4) based on result analysis from Shen et al. [8]. The fragmentation pattern of gallocatechin/epigallocatechin is shown in Figure S27. It has shown different pharmacological activities such as antiviral [99, 100], antioxidant [101], and antibacterial [102] activities. Compounds with characteristic fragment ions 427.10 [M + H − 152]+ and 289.07 (kaempferol) and base peak [M + H]+ at m/z 579.15, molecular formula C30H26O12, and DBE 18 are annotated as procyanidin B1 (5) or procyanidin B (6). The spectral data coincides with data from Shen et al. [8]. Likewise, it is reported to be used as antioxidant, antibacterial, antiviral, anticarcinogenic, anti-inflammatory, antiallergic, and vasodilatory candidate. They can inhibit lipid peroxidation, platelet aggregation, and capillary hyperpermeability [62, 103]. The base peak [M + H]+ at m/z 271.06, molecular formula C30H26O12, and DBE 11 with characteristic fragment ions 253.16, 243.17, 229.14, 225.13, and 197.08 is explicated as emodin (7). The data is in agreement with Zhan et al. [42]. The fragmentation pattern of emodin is shown in Figure S28. Emodin also exhibits a wide spectrum of pharmacological properties including antiallergic, antiosteoporotic, antidiabetic, anti-inflammatory, anticancer, antiviral, antimicrobial, antioxidant, hepatoprotective, and immunosuppressive activities [104]. The compound with base peak 275.08, molecular formula C15H14O5, and DBE 9 and fragment peaks at 257.17, 233.08, 191.07, and 169.11 is expected to be afzelechin (8) or epiafzelechin (9). The data is consistent with Mittal et al. [43]. It has been reported to have antidiabetic [105], antimicrobial [106], and antioxidant [107] abilities.
The base peak at m/z 263.05 with molecular formula C13H10O6 and DBE 9 and fragment peaks at 153.08 are assigned as maclurin (10), consistent with the result of Berardini et al. [46]. Maclurin (10) has been reported to have antioxidant [108] and anticancer activities [109]. Similarly, the compound at base peak m/z 387.1 and fragment peaks at 357.09 (M + H − CH3 × 2)+, 372 [M + H − CH3]+ is assigned as irisflorentin (11) based on result analysis by Zhang et al. [49]. It is used as anti-inflammatory [110], antiallergic [111], anticholinesterase [112], and antimicrobial agent [113]. Likewise, mass spectrum with a base peak at m/z 273.07, molecular formula C15H12O, and DBE 10 and fragment peak at 153.10 is predicted to be naringenin (12) corresponding to fragment pattern analyzed by Tong et al. [50]. The fragmentation pattern of naringenin is shown in Figure S29. It shows antibacterial [114], anticancer [115], antioxidant, anti-inflammatory, hepatoprotective, nephroprotective, immunomodulatory, and antidiabetic properties [116]. Similarly, a base peak with m/z 465.1 and molecular formula C21H20O12 and the fragment ion at 303.05 and 289.07 are interpreted as isoquercetin (13) comparing our spectra with Liu et al. [51]. The fragmentation pattern of isoquercetin is shown in Figure S30. It acts as antioxidation, anticancer, anticardiovascular, antidiabetes, and antiallergic candidate [117]. Likewise, the compound with fragment peaks at 289.09 and 149.09 is annotated as diosmetin (14) having a base peak at m/z 301.07. The spectral data matches with data from Chen et al. [52] and Park et al. [53]. The fragmentation pattern of diosmetin is shown in Figure S31. It is used as an anticancer, anti-inflammatory, antioxidant, and antimicrobial agent [118]. The base peak at m/z 255.06, molecular formula C15H10O4, and DBE 11 and characteristic fragment ion at 153.12 are expected to be chrysin (15) with reference from the data collected from our spectra and Zhao et al. [54]. The fragmentation pattern of diosmetin is shown in Figure S32. It shows anticancer, antidiabetic, neuroprotective, antiallergic, antihyperlipidemic, antimicrobial, antiobesity, anti-inflammatory, hepatoprotective, cardiovascular, reproductive, and antioxidant activities [119]. The other flavonoid is expected to be myricetin (16) with base peak m/z 319.04, molecular formula C15H10O8, and DBE 11 with reference from the data collected from our spectra and Saldanha et al. [55]. It is used as antimicrobial, antioxidant, and anti-inflammatory agent [120, 121]. Likewise, a base peak with m/z 287.05, molecular formula C15H10O6, and DBE 11 and fragment peak at m/z 259.13, 165.09, and 153.12 were predicted to be kaempferol (17) as spectral figures match with the literature of March and Miao [56]. The fragmentation pattern of kaempferol is shown in Figure S33. It has neuroprotective, antimicrobial, antioxidant, anti-inflammatory, and anticancer effects [122]. Moreover, in our spectra, a base peak with m/z 435.09, molecular formula C21H20O13, and DBE 12 and fragment peak at m/z 303.05, 287.06, and 183.10 were annotated as avicularin (18) taking reference of Santos et al. [57]. The fragmentation pattern of avicularin is shown in Figure S34. It is used as an anticancer, anti-inflammatory, and anti-infectious candidate [123].
The molecular ion peak at m/z 595.14, molecular formula C30H26O13, and DBE 18 and fragment ions at m/z 427.08, 169.07, 291.09, and 305.06 were annotated as prodelphinidin B3 (19). These spectral data are consistent with Friedrich et al. [40] and Pinto et al. [58]. The fragmentation pattern of prodelphinidin B3 is shown in Figure S35. It is used as antidiabetic, antiviral, and anti-inflammatory activities [124]. Additionally, [M + H]+ at m/z 611.14, molecular formula C30H26O14, and DBE 18 and fragment ions at m/z 307.08 are considered as prodelphinidin B (20) relying on result analysis from Navarro et al. [59]. The fragmentation pattern of prodelphinidin B is shown in Figure S36. It is used as antidiabetic, antiviral, and anti-inflammatory candidate [124]. Base peak m/z 303.05, molecular formula C15H10O7, DBE 11, and fragments ions at m/z 285.15, 257.13, 247.15, 229.13, 201.12, 183.10, 165.08, 153.11, 137.08, 121.03, and 111.06 were annotated as quercetin (21) whose spectra exactly match with the spectra data of Scigelova et al. [60]. It is used as an anti-inflammatory, antioxidant, antiviral, antimicrobial, and anticancer agent [125]. A. catechu may contain taxifolin (22) with the base peak at m/z 305.06, molecular formula C15H12O7, and DBE 10, and characteristic fragment ions are 287.05 (M + H − H2O)+ and 177.0253 (M + H − H2O − C3O2 − C2H2O]+ comparing our result with Michel et al. [61] and Yang et al. [62]. It is known to have potential antibacterial, antifungal, anti-inflammatory, analgesic, antioxidant, antipyretic, platelet inhibitory, and even anticancer actions [126, 127]. Likewise, a base peak at m/z 284.06, molecular formula C16H12O5, and DBE 11 and characteristic fragment ions at m/z 245.15 (M + H − CO2)+, 213.13(M − CO2 − CO + H)+ are annotated as acacetin (23). The data is the same as the spectral data of Yin et al. [63]. The molecular ion at m/z 413.12, molecular formula C22H20O8, and DBE 13 and fragments ions at m/z 325.10, 296.10, and 295.09 are characterized as aciculatinone (24). The spectral data exactly match with Shen et al.[64]. The base peak at m/z 481.09 and fragment peaks 319.04 which are in agreement with previous studies done by Giorio et al. [65] and Petsalo et al. [66] are speculated as gossypin (25). The base peak at m/z 299.09, molecular formula C17H14O5, and DBE 11 manifested that it could be pterocarpin (26) comparing spectral data with Geiger et al. [67]. Moreover, another annotated compound is isorhamnetin (27) with the base peak at m/z 317.06, molecular formula C16H12O7, and DBE 11. The data coincides with Chen et al. [68]. The fragmentation pattern of isorhamnetin is shown in Figure S37. Likewise, [M + H]+ at 331.08 along with fragment peak at 315.09, and 301.08 is considered as trihydroxy dimethoxyflavone (28) relying on result analysis from Zhang et al. [69]. The fragmentation pattern of trihydroxy dimethoxyflavone is shown in Figure S38.
In our study, we have annotated 28 secondary metabolites from the ethanol extract of A. catechu where seven of the secondary metabolites, namely, catechin, epicatechin, gallocatechin, epigallocatechin, procyanidin, emodin, and quercetin, were already annotated by Aryal et al. [34] in methanol extract. To support our annotation, more spectroscopic data are required. Hence, further investigation is required for the separation of potential enzyme inhibitors, antioxidant, and antibacterial compounds and hence the inhibitory activities and enzymatic kinetics of such compounds to develop them as future drug candidates or food supplements.
5. Conclusion
The bark of A. catechu has significant potential in inhibiting carbohydrate hydrolases due to the abundance of flavonoids and polyphenols. Our findings open the entryway for better utilization of phytoconstituents of A. catechu for the management of diabetes and pathogens. Further research on the isolation of potential inhibitors from solvent fraction, the study of pharmacokinetic parameters, kinetics, and in vivo experiments are required to develop an influential therapeutic approach against diabetes.
Acknowledgments
The authors are thankful to the Central Department of Botany, Tribhuvan University, Nepal, for plant identification. The authors are grateful to the Sophisticated Analytical Instrument Facility (SAIF), CSIR-Central Drug Research Institute, Lucknow, India, for LC-HRMS analysis. This research was funded by University Grants Commission- Nepal to B. Aryal (MRS-76/77-S&T-18) and B. Adhikari (MRS-76/77-S&T-l6). This project is also supported by the Higher Education Reform Project (HERP), Tribhuvan University, to N. Parajuli.
Abbreviations
- CNPG3:
2-Chloro-4-nitrophenyl-α-D-maltotrioside
- DBE:
Double bond equivalence
- DM:
Diabetes mellitus
- DPPH:
2,2-Diphenyl-1-picryl hydrazyl
- HRMS:
High-resolution mass spectrometry
- LC-HRMS:
Liquid chromatography-high resolution mass spectrometry
- MBC:
Minimum bactericidal concentration
- mg QE/g:
Milligrams of quercetin equivalent per gram dry weight of extract
- mg GAE/g:
Milligrams of gallic acid equivalent per gram dry weight of extract
- MHA:
Mueller Hinton Agar
- MIC:
Minimum inhibitory concentration
- MS:
Mass spectrometry
- pNPG:
4-Nitrophenyl-α-D-glucopyranoside
- Rt:
Retention time
- TFC:
Total flavonoid contents
- TIC:
Total ion chromatogram
- TPC:
Total phenolic contents
- ZoI:
Zone of inhibition.
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Conflicts of Interest
There is no conflict of interest among the authors.
Authors' Contributions
N.P. designed the research project; B. Aryal and B. Adhikari performed the experiments and analyzed the data; B. Aryal, B. Adhikari, B.R.B, N.A, K.K., and N.P prepared the manuscript. Babita Aryal and Bikash Adhikari contributed equally to this work.
Supplementary Materials
Table S1: Indigenous uses and chemical constituents' description of A. catechu used in the study. Figure S1: Total ion chromatogram (TIC) blue line for A. catechu ethyl acetate fraction and red line for A. catechu water fraction. Figure S2: Mass spectrum of catechin or epicatechin. Figure S3: Mass spectrum of gallocatechin/epigallocatechin. Figure S4: Mass spectrum of procyanidin B1/procyanidin B3. Figure S5: Mass spectrum of emodin. Figure S6: Mass spectrum of afzelechin/epiafzelechin. Figure S7: Mass spectrum of maclurin. Figure S8: Mass spectrum of irisflorentin. Figure S9: Mass spectrum of naringenin. Figure S10: Mass spectrum of isoquercetin. Figure S11: Mass spectrum of diosmetin. Figure S12: Mass spectrum of chrysin. Figure S13: Mass spectrum of myricetin. Figure S14: Mass spectrum of kaempferol. Figure S15: Mass spectrum of avicularin. Figure S16: Mass spectrum of prodelphinidin B3. Figure S17: Mass spectrum of prodelphinidin B. Figure S18: Mass spectrum of quercetin. Figure S19: Mass spectrum of taxifolin. Figure S20: Mass spectrum of acacetin. Figure S21: Mass spectrum of aciculatinone. Figure S22: Mass spectrum of gossypin. Figure S23: Mass spectrum of pterocarpan. Figure S24: Mass spectrum of isorhamnetin. Figure S25: Mass spectrum of trihydroxy dimethoxyflavone. Figure S26: Fragmentation pattern of catechin/epicatechin. Figure S27: Fragmentation pattern of gallocatechin/epigallocatechin. Figure S28: Fragmentation pattern of emodin. Figure S29: Fragmentation pattern of naringenin. Figure S30: Fragmentation pattern of isoquercetin. Figure S31: Fragmentation pattern of diosmetin. Figure S32: Fragmentation pattern of chrysin. Figure S33: Fragmentation pattern of kaempferol. Figure S34: Fragmentation pattern of avicularin. Figure S35: Fragmentation pattern of prodelphinidin B3. Figure S36: fragmentation pattern of prodelphinidin B. Figure S37: Fragmentation pattern of isorhamnetin. Figure S38: Fragmentation pattern of trihydroxy dimethoxyflavone.
References
- 1.Bhattarai R., Sharma P., Wagle B., Adhikari A., Acharya S. Revision and compilation of health management plan of Khair (Acacia catechu) Grassroots Journal of Natural Resources. 2020;3(1):15–28. doi: 10.33002/nr2581.6853.03012. [DOI] [Google Scholar]
- 2.Lakshmi T., Ramasamy R., Thirumalaikumaran R. Preliminary phytochemical analysis and in vitro antioxidant, FTIR spectroscopy, anti-diabetic activity of Acacia catechu ethanolic seed extract. Pharmacognosy Journal. 2015;7(6):356–362. doi: 10.5530/pj.2015.6.7. [DOI] [Google Scholar]
- 3.Patil A., Modak M. Comparative evaluation of oxidative stress modulating and DNA protective activities of aqueous and methanolic extracts of Acacia catechu. Medicines. 2017;4(3):p. 65. doi: 10.3390/medicines4030065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Negi B. S., Dave B. P. In vitro antimicrobial activity of Acacia catechu and its phytochemical analysis. Indian Journal of Microbiology. 2010;50(4):369–374. doi: 10.1007/s12088-011-0061-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yimam M., Brownell L., Hodges M., Jia Q. Analgesic effects of a standardized bioflavonoid composition from Scutellaria baicalensis and Acacia catechu. Journal of Dietary Supplements. 2012;9:155–165. doi: 10.3109/19390211.2012.708713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rahmatullah M., Hossain M., Mahmud A., et al. Antihyperglycemic and antinociceptive activity evaluation of ‘khoyer’ prepared from boiling the wood of Acacia catechu in water. African Journal of Traditional, Complementary and Alternative Medicines. 2013;10(4):1–5. doi: 10.4314/ajtcam.v10i4.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sunil M. A., Sunitha V. S., Radhakrishnan E. K., Jyothis M. Immunomodulatory activities of Acacia catechu, a traditional thirst quencher of South India. Journal of Ayurveda and Integrative Medicine. 2019;10(3):185–191. doi: 10.1016/j.jaim.2017.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shen D., Wu Q., Wang M., Yang Y., Lavoie E. J., Simon J. E. Determination of the predominant catechins in Acacia catechu by liquid chromatography/electrospray ionization−mass spectrometry. Journal of Agricultural and Food Chemistry. 2006;54:3219–3224. doi: 10.1021/jf0531499. [DOI] [PubMed] [Google Scholar]
- 9.Petersmann A., Nauck M., Müller-Wieland D., et al. Definition, classification and diagnosis of diabetes mellitus. Experimental and Clinical Endocrinology & Diabetes. 2019;127:S1–S7. doi: 10.1055/a-1018-9078. [DOI] [PubMed] [Google Scholar]
- 10.Lima R., Del Fiol F. S., Balcão V. M. Prospects for the use of new technologies to combat multidrug-resistant bacteria. Frontiers in Pharmacology. 2019;10 doi: 10.3389/fphar.2019.00692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kızıltaş H., Bingol Z., Gören A. C., et al. LC-HRMS profiling and antidiabetic, anticholinergic, and antioxidant activities of aerial parts of kınkor (Ferulago stellata) Molecules. 2021;26(9):p. 2469. doi: 10.3390/molecules26092469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Munhoz A. C. M., Frode T. S. Isolated compounds from natural products with potential antidiabetic activity - a systematic review. Current Diabetes Reports. 2017;14:36–106. doi: 10.2174/1573399813666170505120621. [DOI] [PubMed] [Google Scholar]
- 13.Bharti S. K., Krishnan S., Kumar A., Kumar A. Antidiabetic phytoconstituents and their mode of action on metabolic pathways. Therapeutic Advances in Endocrinology and Metabolism. 2018;9(3):81–100. doi: 10.1177/2042018818755019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harvey A. L. Plant natural products in anti-diabetic drug discovery. Current Organic Chemistry. 2010;14(16):1670–1677. doi: 10.2174/138527210792927681. [DOI] [Google Scholar]
- 15.Bajaj S., Khan A. Antioxidants and diabetes. Indian Journal of Endocrinology and Metabolism. 2012;16(Supplement 2):S267–S271. doi: 10.4103/2230-8210.104057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lobo V., Patil A., Phatak A., Chandra N. Free radicals, antioxidants and functional foods: impact on human health. Pharmacognosy Reviews. 2010;4:118–126. doi: 10.4103/0973-7847.70902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Noh S., Go A., Kim D. B., Park M., Jeon H. W., Kim B. Role of antioxidant natural products in management of infertility: a review of their medicinal potential. Antioxidants. 2020;9(10):p. 957. doi: 10.3390/antiox9100957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abdel-Hameed E.-S. S. Total phenolic contents and free radical scavenging activity of certain Egyptian Ficus species leaf samples. Food Chemistry. 2009;114(4):1271–1277. doi: 10.1016/j.foodchem.2008.11.005. [DOI] [Google Scholar]
- 19.Phaniendra A., Jestadi D. B., Periyasamy L. Free radicals: properties, sources, targets, and their implication in various diseases. Indian Journal of Clinical Biochemistry. 2015;30:11–26. doi: 10.1007/s12291-014-0446-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tiwari B. K., Pandey K. B., Abidi A. B., Rizvi S. I. Markers of oxidative stress during diabetes mellitus. Journal of Biomarkers. 2013;2013:8. doi: 10.1155/2013/378790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lourenço S. C., Moldão-Martins M., Alves V. D. Antioxidants of natural plant origins: from sources to food industry applications. Molecules. 2019;24(22):p. 4132. doi: 10.3390/molecules24224132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu D. P., Li Y., Meng X., et al. Natural antioxidants in foods and medicinal plants: extraction, Assessment and Resources. International Journal of Molecular Sciences. 2017;18(1):p. 96. doi: 10.3390/ijms18010096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pan S. Y., Zhou S. F., Gao S. H., et al. New Perspectives on How to Discover Drugs from Herbal Medicines: CAM's Outstanding Contribution to Modern Therapeutics. Evidence-Based Complementary and Alternative Medicine. 2013;2013:25. doi: 10.1155/2013/627375.627375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ladumor M. K., Tiwari S., Patil A., et al. Chapter 7 - High-resolution mass spectrometry in metabolite identification. In: Pérez S., Eichhorn P., Barceló D., editors. Comprehensive Analytical Chemistry. Elsevier; 2016. pp. 199–229. [DOI] [Google Scholar]
- 25.Prasad B., Garg A., Takwani H., Singh S. Metabolite identification by liquid chromatography-mass spectrometry. Trends in Analytical Chemistry. 2011;2:360–387. [Google Scholar]
- 26.Salem M. A., Perez de Souza L., Serag A., et al. Metabolomics in the context of plant natural products research: from sample preparation to metabolite analysis. Metabolites. 2020;10(1):p. 37. doi: 10.3390/metabo10010037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Banerjee S., Mazumdar S. Electrospray ionization mass spectrometry: a technique to access the information beyond the molecular weight of the analyte. International Journal of Analytical Chemistry. 2012;2012:40. doi: 10.1155/2012/282574.e282574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tolonen A., Uusitalo J. Fast screening method for the analysis of total flavonoid content in plants and foodstuffs by high-performance liquid chromatography/electrospray ionization time-of-flight mass spectrometry with polarity switching. Rapid Communications in Mass Spectrometry. 2004;18(24):3113–3122. doi: 10.1002/rcm.1736. [DOI] [PubMed] [Google Scholar]
- 29.Huang X., Liu Y., Song F., Liu Z., Liu S. Studies on principal components and antioxidant activity of different radix astragali samples using high-performance liquid chromatography/electrospray ionization multiple-stage tandem mass spectrometry. Talanta. 2009;78(3):1090–1101. doi: 10.1016/j.talanta.2009.01.021. [DOI] [PubMed] [Google Scholar]
- 30.Lu X., Wang J., al-Qadiri H. M., et al. Determination of total phenolic content and antioxidant capacity of onion (Allium cepa) and shallot (Allium oschaninii) using infrared spectroscopy. Food Chemistry. 2011;129(2):637–644. doi: 10.1016/j.foodchem.2011.04.105. [DOI] [PubMed] [Google Scholar]
- 31.Ahmed I. A., Mikail M. A., bin Ibrahim M., et al. Antioxidant activity and phenolic profile of various morphological parts of underutilised Baccaurea angulata fruit. Food Chemistry. 2015;172:778–787. doi: 10.1016/j.foodchem.2014.09.122. [DOI] [PubMed] [Google Scholar]
- 32.Khadayat K., Marasini B. P., Gautam H., Ghaju S., Parajuli N. Evaluation of the alpha-amylase inhibitory activity of Nepalese medicinal plants used in the treatment of diabetes mellitus. Clinical Phytoscience. 2020;6(1):p. 34. doi: 10.1186/s40816-020-00179-8. [DOI] [Google Scholar]
- 33.Fouotsa H., Lannang A. M., Mbazoa C. D., et al. Xanthones inhibitors of α-glucosidase and glycation from Garcinia nobilis. Phytochemistry Letters. 2012;5(2):236–239. doi: 10.1016/j.phytol.2012.01.002. [DOI] [Google Scholar]
- 34.Aryal B., Niraula P., Khadayat K., et al. Antidiabetic, antimicrobial, and molecular profiling of selected medicinal plants. Evidence-Based Complementary and Alternative Medicine. 2021;2021:15. doi: 10.1155/2021/5510099.5510099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Balouiri M., Sadiki M., Ibnsouda S. K. Methods for in vitro evaluating antimicrobial activity: A review. Journal of Pharmaceutical Analysis. 2016;6(2):71–79. doi: 10.1016/j.jpha.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.CLSI. Performance Standards for Antimicrobial Susceptibility Testing: 25th Informational Supplement (M100-S23) Wayne, PA: Clinical and Laboratory Standards Institute; 2015. [Google Scholar]
- 37.Ibrahim T. A., Dib R. A. E., Al-Youssef H. M., Amina M. Chemical composition and antimicrobial and cytotoxic activities of Antidesm abunius L. Pakistan Journal of Pharmaceutical Sciences. 2019;32(1) [PubMed] [Google Scholar]
- 38.Shi M., Nie Y., Zheng X.-Q., Lu J.-L., Liang Y.-R., Ye J.-H. Ultraviolet B (UVB) photosensitivities of tea catechins and the relevant chemical conversions. Molecules. 2016;21(10):p. 1345. doi: 10.3390/molecules21101345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yuan Y., Song Y., Jing W., Wang Y., Yang X., Liu D. Simultaneous determination of caffeine, gallic acid, theanine, (−)-epigallocatechin and (−)-epigallocatechin-3-gallate in green tea using quantitative1H-NMR spectroscopy. Analytical Methods. 2014;6(3):907–914. doi: 10.1039/c3ay41369a. [DOI] [Google Scholar]
- 40.Friedrich W., Eberhardt A., Galensa R. Investigation of proanthocyanidins by HPLC with electrospray ionization mass spectrometry. European Food Research and Technology. 2000;211(1):56–64. doi: 10.1007/s002170050589. [DOI] [Google Scholar]
- 41.Klausen K., Mortensen A. G., Laursen B., Haselmann K. F., Jespersen B. M., Fomsgaard I. S. Phenolic compounds in different barley varieties: identification by tandem mass spectrometry (QStar) and NMR; quantification by liquid chromatography triple quadrupole-linear ion trap mass spectrometry (Q-Trap) Natural Product Communications. 2010;5(3, article 1934578X1000500) doi: 10.1177/1934578x1000500314. [DOI] [PubMed] [Google Scholar]
- 42.Zhan C., Xiong A., Shen D., Yang L., Wang Z. Characterization of the principal constituents of Danning Tablets, a Chinese formula consisting of seven herbs, by an UPLC-DAD-MS/MS approach. Molecules. 2016;21(5):p. 631. doi: 10.3390/molecules21050631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mittal A., Kadyan P., Gahlaut A., Dabur R. Nontargeted identification of the phenolic and other compounds of Saraca asoca by high performance liquid chromatography-positive electrospray ionization and quadrupole time-of-flight mass spectrometry. ISRN Pharmacology. 2013;2013:10. doi: 10.1155/2013/293935.293935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lin L.-Z., Sun J., Chen P., Monagas M. J., Harnly J. M. UHPLC-PDA-ESI/HRMSn profiling method to identify and quantify oligomeric proanthocyanidins in plant products. Journal of Agricultural and Food Chemistry. 2014;62(39):9387–9400. doi: 10.1021/jf501011y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.De Souza L. M., Cipriani T. R., Iacomini M., Gorin P. A. J., Sassaki G. L. HPLC/ESI-MS and NMR analysis of flavonoids and tannins in bioactive extract from leaves of Maytenus ilicifolia. Journal of Pharmaceutical and Biomedical Analysis. 2008;47(1):59–67. doi: 10.1016/j.jpba.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 46.Berardini N., Carle R., Schieber A. Characterization of gallotannins and benzophenone derivatives from mango (Mangifera indica L. cv. ‘Tommy Atkins’) peels, pulp and kernels by high-performance liquid chromatography/electrospray ionization mass spectrometry. Rapid Communications in Mass Spectrometry. 2004;18(19):2208–2216. doi: 10.1002/rcm.1611. [DOI] [PubMed] [Google Scholar]
- 47.Roger B., Jeannot V., Fernandez X., Cerantola S., Chahboun J. Characterisation and quantification of flavonoids in Iris germanica L. and Iris pallida Lam. resinoids from Morocco. Phytochemical Analysis. 2012;23(5):450–455. doi: 10.1002/pca.1379. [DOI] [PubMed] [Google Scholar]
- 48.Ślusarczyk S., Senol Deniz F. S., Woźniak D., et al. Selective in vitro and in silico cholinesterase inhibitory activity of isoflavones and stilbenes from Belamcandae chinensis rhizoma. Phytochemistry Letters. 2019;30:261–272. doi: 10.1016/j.phytol.2019.02.006. [DOI] [Google Scholar]
- 49.Zhang Y.-Y., Wang Q., Qi L.-W., Qin X.-Y., Qin M.-J. Characterization and determination of the major constituents in Belamcandae Rhizoma by HPLC-DAD-ESI-MS(n) Journal of Pharmaceutical and Biomedical Analysis. 2011;56:304–314. doi: 10.1016/j.jpba.2011.05.040. [DOI] [PubMed] [Google Scholar]
- 50.Tong L., Zhou D., Gao J., Zhu Y., Sun H., Bi K. Simultaneous determination of naringin, hesperidin, neohesperidin, naringenin and hesperetin of Fractus aurantii extract in rat plasma by liquid chromatography tandem mass spectrometry. Journal of Pharmaceutical and Biomedical Analysis. 2012;58:58–64. doi: 10.1016/j.jpba.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 51.Liu W., Huang J., Zhang F., et al. Comprehensive profiling and characterization of the absorbed components and metabolites in mice serum and tissues following oral administration of Qing- Fei-Pai-Du decoction by UHPLC-Q-Exactive-Orbitrap HRMS. Chinese Journal of Natural Medicines. 2021;19(4):305–320. doi: 10.1016/S1875-5364(21)60031-6. [DOI] [PubMed] [Google Scholar]
- 52.Chen X., Xu L., Guo S., et al. Profiling and comparison of the metabolites of diosmetin and diosmin in rat urine, plasma and feces using UHPLC-LTQ-Orbitrap MSn. Journal of Chromatography B: Analytical Technologies in the Biomedical and Life Sciences. 2019;1124:58–71. doi: 10.1016/j.jchromb.2019.05.030. [DOI] [PubMed] [Google Scholar]
- 53.Park Y., Moon B.-H., Yang H., Lee Y., Lee E., Lim Y. Complete assignments of NMR data of 13 hydroxymethoxyflavones. Magnetic Resonance in Chemistry. 2007;45(12):1072–1075. doi: 10.1002/mrc.2063. [DOI] [PubMed] [Google Scholar]
- 54.Zhao X., Su X., Liu C., Jia Y. Simultaneous determination of chrysin and tectochrysin from Alpinia oxyphylla fruits by UPLC-MS/MS and its application to a comparative pharmacokinetic study in normal and dementia rats. Molecules. 2018;23(7):p. 1702. doi: 10.3390/molecules23071702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Saldanha L., Vilegas W., Dokkedal A. Characterization of flavonoids and phenolic acids in Myrcia bella cambess. Using FIA-ESI-IT-MSn and HPLC-PAD-ESI-IT-MS combined with NMR. Molecules. 2013;18:8402–8416. doi: 10.3390/molecules18078402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.March R. E., Miao X.-S. A fragmentation study of kaempferol using electrospray quadrupole time-of-flight mass spectrometry at high mass resolution. International Journal of Mass Spectrometry. 2004;231(2-3):157–167. doi: 10.1016/j.ijms.2003.10.008. [DOI] [Google Scholar]
- 57.Santos A. L., Soares M. G., de Medeiros L. S., Ferreira M. J. P., Sartorelli P. Identification of flavonoid-3-O-glycosides from leaves of Casearia arborea (Salicaceae) by UHPLC-DAD-ESI-HRMS/MS combined with molecular networking and NMR. Phytochemical Analysis. 2021;2021 doi: 10.1002/pca.3032. [DOI] [PubMed] [Google Scholar]
- 58.Pinto G., De Pascale S., Aponte M., Scaloni A., Addeo F., Caira S. Polyphenol profiling of chestnut pericarp, integument and curing water extracts to qualify these food by-products as a source of antioxidants. Molecules. 2021;26(8):p. 2335. doi: 10.3390/molecules26082335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Navarro M., Moreira I., Arnaez E., et al. Flavonoids and ellagitannins characterization, antioxidant and cytotoxic activities of Phyllanthus acuminatus Vahl. Plants. 2017;6(4):p. 62. doi: 10.3390/plants6040062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Scigelova M., Hornshaw M., Giannakopulos A., Makarov A. Fourier transform mass spectrometry. Molecular & Cellular Proteomics. 2011;10, article M111.009431 doi: 10.1074/mcp.M111.009431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Michel T., Khlif I., Kanakis P., et al. UHPLC-DAD-FLD and UHPLC-HRMS/MS based metabolic profiling and characterization of different Olea europaea organs of Koroneiki and Chetoui varieties. Phytochemistry Letters. 2015;11:424–439. doi: 10.1016/j.phytol.2014.12.020. [DOI] [Google Scholar]
- 62.Yang P., Xu F., Li H.-F., et al. Detection of 191 taxifolin metabolites and their distribution in rats using HPLC-ESI-IT-TOF-MSn. Molecules. 2016;21(9):p. 1209. doi: 10.3390/molecules21091209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yin J., Ma Y., Liang C., Gao J., Wang H., Zhang L. A systematic study of the metabolites of dietary acacetin in vivo and in vitro based on UHPLC-Q-TOF-MS/MS analysis. Journal of Agricultural and Food Chemistry. 2019;67(19):5530–5543. doi: 10.1021/acs.jafc.9b00330. [DOI] [PubMed] [Google Scholar]
- 64.Shen C.-C., Cheng J.-J., Lay H.-L., et al. Cytotoxic apigenin derivatives from Chrysopogon aciculatis. Journal of Natural Products. 2012;75(2):198–201. doi: 10.1021/np2007796. [DOI] [PubMed] [Google Scholar]
- 65.Giorio C., Moyroud E., Glover B. J., Skelton P. C., Kalberer M. Direct surface analysis coupled to high-resolution mass spectrometry reveals heterogeneous composition of the cuticle of Hibiscus trionum petals. Analytical Chemistry. 2015;87:9900–9907. doi: 10.1021/acs.analchem.5b02498. [DOI] [PubMed] [Google Scholar]
- 66.Petsalo A., Jalonen J., Tolonen A. Identification of flavonoids of Rhodiola rosea by liquid chromatography- tandem mass spectrometry. Journal of Chromatography A. 2006;1112(1-2):224–231. doi: 10.1016/j.chroma.2005.11.056. [DOI] [PubMed] [Google Scholar]
- 67.Geiger J., Armitage R. A., DeRoo C. S. Collaborative endeavors in the chemical analysis of art and cultural heritage materials. ACS Publications; 2012. Identification of organic dyes by direct analysis in real time-time of flight mass spectrometry; pp. 123–129. [Google Scholar]
- 68.Chen Y., Yu H., Wu H., et al. Characterization and quantification by LC-MS/MS of the chemical components of the heating products of the flavonoids extract in Pollen Typhae for transformation rule exploration. Molecules. 2015;20(10):18352–18366. doi: 10.3390/molecules201018352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang F., Li Z., Li M., et al. An integrated strategy for profiling the chemical components of Scutellariae Radix and their exogenous substances in rats by ultra-high-performance liquid chromatography/quadrupole time-of-flight mass spectrometry. Rapid Communications in Mass Spectrometry. 2020;34, article e8823 doi: 10.1002/rcm.8823. [DOI] [PubMed] [Google Scholar]
- 70.Van Ngo T., Scarlett C. J., Bowyer M. C., Ngo P. D., Van Vuong Q. Impact of different extraction solvents on bioactive compounds and antioxidant capacity from the root of Salacia chinensis L. Journal of Food Quality. 2017;2017 doi: 10.1155/2017/9305047.e9305047 [DOI] [Google Scholar]
- 71.Saeed N., Khan M. R., Shabbir M. Antioxidant activity, total phenolic and total flavonoid contents of whole plant extracts Torilis leptophylla L. BMC Complementary and Alternative Medicine. 2012;12:p. 221. doi: 10.1186/1472-6882-12-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dibacto R. E. K., Tchuente B. R. T., Nguedjo M. W., et al. Total polyphenol and flavonoid content and antioxidant capacity of some varieties of Persea americana peels consumed in Cameroon. The Scientific World Journal. 2021;2021:11. doi: 10.1155/2021/8882594.e8882594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Thakur A. V., Ambwani S., Kumar T. Preliminary phytochemical screening and GC-MS analysis of leaf extract of Acacia catechu (L.f.) Willd. International Journal of Herbal Medicine. 2018;6:81–85. doi: 10.20546/ijcmas.2017.601.011. [DOI] [Google Scholar]
- 74.Gunindro N., Devi K. P., Singh T. I. Effects of Acacia catechu on intestinal absorption of glucose in rats. Journal of Chemical and Pharmaceutical Research. 2013;5:78–81. [Google Scholar]
- 75.Chiaino E., Micucci M., Durante M., et al. Apoptotic-induced effects of Acacia catechu Willd Extract in Human Colon Cancer Cells. International Journal of Molecular Sciences. 2020;21(6):p. 2102. doi: 10.3390/ijms21062102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li X., Wang H., Liu C., Chen R. Chemical constituents of Acacia catechu. Zhongguo Zhong yao za zhi= Zhongguo zhongyao zazhi= China Journal of Chinese Materia Medica. 2010;35:1425–1427. [PubMed] [Google Scholar]
- 77.Wang L., Shen X., Le Mi J. J., et al. Simultaneous determinations of four major bioactive components in Acacia catechu (L.f.) Willd and Scutellaria baicalensis Georgi extracts by LC-MS/MS: application to its herb-herb interactions based on pharmacokinetic, tissue distribution and excretion studies in rats. Phytomedicine. 2019;56:64–73. doi: 10.1016/j.phymed.2018.09.239. [DOI] [PubMed] [Google Scholar]
- 78.Li X.-C., Liu C., Yang L.-X., Chen R.-Y. Phenolic compounds from the aqueous extract of Acacia catechu. Journal of Asian Natural Products Research. 2011;13:826–830. doi: 10.1080/10286020.2011.597384. [DOI] [PubMed] [Google Scholar]
- 79.Kis B., Avram S., Pavel I. Z., et al. Recent advances regarding the phytochemical and therapeutic uses of Populus nigra L Buds. Plants. 2020;9(11):p. 1464. doi: 10.3390/plants9111464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mujeeb F., Bajpai P., Pathak N. Phytochemical evaluation, antimicrobial activity, and determination of bioactive components from leaves of Aegle marmelos. BioMed Research International. 2014;2014 doi: 10.1155/2014/497606.497606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tunsaringkarn T., Rungsiyothin A., Ruangrungsi N. α-Glucosidase inhibitory activity of water soluble extract from Thai mimosaceous plants. 2009;4:54–63. August 2019, https://www.tci-thaijo.org/index.php/phjbuu/article/view/45607. [Google Scholar]
- 82.Komape N. P. M., Bagla V. P., Kabongo-Kayoka P., Masoko P. Anti-mycobacteria potential and synergistic effects of combined crude extracts of selected medicinal plants used by Bapedi traditional healers to treat tuberculosis related symptoms in Limpopo Province South Africa. BMC Complementary and Alternative Medicine. 2017;17:p. 128. doi: 10.1186/s12906-016-1521-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Noman O. M., Mothana R. A., Al-Rehaily A. J., et al. Phytochemical analysis and anti-diabetic, anti-inflammatory and antioxidant activities of Loranthus acaciae Zucc. grown in Saudi Arabia. Saudi Pharmaceutical Journal. 2019;27:724–730. doi: 10.1016/j.jsps.2019.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Savadi S., Vazifedoost M., Didar Z., Nematshahi M. M., Jahed E. Phytochemical analysis and antimicrobial/antioxidant activity of Cynodon dactylon (L.) Pers. rhizome methanolic extract. Journal of Food Quality. 2020;2020:10. doi: 10.1155/2020/5946541.e5946541 [DOI] [Google Scholar]
- 85.Ahamad S. T., Lakshmi T., Rajeshkumar S., Roy A., Gurunadhan D., Geetha R. V. Antibacterial activity of taxifolin isolated from Acacia catechu leaf extract-an invitro study. Indian Journal of Public Health Research & Development. 2019;10:p. 3540. doi: 10.5958/0976-5506.2019.04135.4. [DOI] [Google Scholar]
- 86.Dashtdar M., Dashtdar M. R., Dashtdar B., Shirazi M. K., Khan S. A. In-vitro, anti-bacterial activities of aqueous extracts of Acacia catechu (L.F.)Willd, Castanea sativa, Ephedra sinica stapf and Shilajita mumiyo against gram positive and gram negative bacteria. Journal of Pharmacopuncture. 2013;16:15–22. doi: 10.3831/kpi.2013.16.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Patel J. D., Kumar V., Bhatt S. A. Antimicrobial screening and phytochemical analysis of the resin part of Acacia catechu. Pharmaceutical Biology. 2009;47:34–37. doi: 10.1080/13880200802400527. [DOI] [Google Scholar]
- 88.Reznick A. Z., Shehadeh N., Shafir Y., Nagler R. M. Free radicals related effects and antioxidants in saliva and serum of adolescents with type 1 diabetes mellitus. Archives of Oral Biology. 2006;51:640–648. doi: 10.1016/j.archoralbio.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 89.Cai Y.-Z., Mei Sun, Jie Xing, Luo Q., Corke H. Structure-radical scavenging activity relationships of phenolic compounds from traditional Chinese medicinal plants. Life Sciences. 2006;78(25):2872–2888. doi: 10.1016/j.lfs.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 90.Huang D., Ou B., Prior R. L. The chemistry behind antioxidant capacity assays. Journal of Agricultural and Food Chemistry. 2005;53:1841–1856. doi: 10.1021/jf030723c. [DOI] [PubMed] [Google Scholar]
- 91.Balasundram N., Sundram K., Samman S. Phenolic compounds in plants and agri-industrial by-products: Antioxidant activity, occurrence, and potential use. Food Chemistry. 2006;99:191–203. doi: 10.1016/j.foodchem.2005.07.042. [DOI] [Google Scholar]
- 92.Sarian M. N., Ahmed Q. U., Mat So’ad S. Z., et al. Antioxidant and antidiabetic effects of flavonoids: a structure-activity relationship based study. BioMed Research International. 2017;2017:14. doi: 10.1155/2017/8386065.e8386065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Martinez-Gonzalez A. I., Díaz-Sánchez Á. G., Rosa L. A., Vargas-Requena C. L., Bustos-Jaimes I., Alvarez-Parrilla A. E. Polyphenolic compounds and digestive enzymes: in vitro non-covalent interactions. Molecules. 2017;22(4, article E669):p. 669. doi: 10.3390/molecules22040669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yilmazer-Musa M., Griffith A. M., Michels A. J., Schneider E., Frei B. Grape Seed and Tea Extracts and Catechin 3-Gallates Are Potent Inhibitors of α-amylase and α-glucosidase activity. Journal of Agricultural and Food Chemistry. 2012;60(36):8924–8929. doi: 10.1021/jf301147n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Veluri R., Weir T. L., Bais H. P., Stermitz F. R., Vivanco J. M. Phytotoxic and antimicrobial activities of catechin derivatives. Journal of Agricultural and Food Chemistry. 2004;52(5):1077–1082. doi: 10.1021/jf030653+. [DOI] [PubMed] [Google Scholar]
- 96.Fan F.-Y., Sang L.-X., Jiang M. Catechins and their therapeutic benefits to inflammatory bowel disease. Molecules. 2017;22(3):p. 484. doi: 10.3390/molecules22030484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zanwar A. A., Badole S. L., Shende P. S., Hegde M. V., Bodhankar S. L. Chapter 21 - antioxidant role of catechin in health and disease. In: Watson R. R., Preedy V. R., Zibadi S., editors. Polyphenols in Human Health and Disease. San Diego: Academic Press; 2014. pp. 267–271. [DOI] [Google Scholar]
- 98.Arts I. C., Hollman P. C., Feskens E. J., Bueno de Mesquita H. B., Kromhout D. Catechin intake might explain the inverse relation between tea consumption and ischemic heart disease: the Zutphen Elderly Study. The American Journal of Clinical Nutrition. 2001;74:227–232. doi: 10.1093/ajcn/74.2.227. [DOI] [PubMed] [Google Scholar]
- 99.Ho H.-Y., Cheng M.-L., Weng S.-F., Leu Y.-L., Chiu D. T.-Y. Antiviral effect of epigallocatechin gallate on enterovirus 71. Journal of Agricultural and Food Chemistry. 2009;57:6140–6147. doi: 10.1021/jf901128u. [DOI] [PubMed] [Google Scholar]
- 100.Suedee A., Tewtrakul S., Panichayupakaranant P. Anti-HIV-1 integrase activity of Mimusops elengi leaf extracts. Pharmaceutical Biology. 2014;52:58–61. doi: 10.3109/13880209.2013.810649. [DOI] [PubMed] [Google Scholar]
- 101.Plumb G. W., Pascual-Teresa S., Santos-Buelga C., Rivas-Gonzalo J. C., Williamson G. Antioxidant properties of gallocatechin and prodelphinidins from pomegranate peel. Redox Report. 2002;7:41–46. doi: 10.1179/135100002125000172. [DOI] [PubMed] [Google Scholar]
- 102.Taylor P. W., Hamilton-Miller J. M. T., Stapleton P. D. Antimicrobial properties of green tea catechins. Food Science & Technology Bulletin. 2005;2:71–81. doi: 10.1616/1476-2137.14184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rauf A., Imran M., Abu-Izneid T., et al. Proanthocyanidins: a comprehensive review. Biomedicine & Pharmacotherapy. 2019;116, article 108999 doi: 10.1016/j.biopha.2019.108999. [DOI] [PubMed] [Google Scholar]
- 104.Dong X., Fu J., Yin X., et al. Emodin: a review of its pharmacology Toxicity and Pharmacokinetics. Phytotherapy Research. 2016;30:1207–1218. doi: 10.1002/ptr.5631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cao H., Chai T.-T., Wang X., et al. Phytochemicals from fern species: potential for medicine applications. Phytochemistry Reviews. 2017;16:379–440. doi: 10.1007/s11101-016-9488-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kuete V., Ngameni B., Simo C. C. F., et al. Antimicrobial activity of the crude extracts and compounds from Ficus chlamydocarpa and Ficus cordata (Moraceae) Journal of Ethnopharmacology. 2008;120:17–24. doi: 10.1016/j.jep.2008.07.026. [DOI] [PubMed] [Google Scholar]
- 107.Kpegba K., Agbonon A., Petrovic A. G., et al. Epiafzelechin from the root bark of Cassia sieberiana: detection by DART mass spectrometry, spectroscopic characterization, and antioxidant properties. Journal of Natural Products. 2011;74:455–459. doi: 10.1021/np100090e. [DOI] [PubMed] [Google Scholar]
- 108.Moon K. M., Kim C. Y., Ma J. Y., Lee B. Xanthone-related compounds as an anti-browning and antioxidant food additive. Food Chemistry. 2019;274:345–350. doi: 10.1016/j.foodchem.2018.08.144. [DOI] [PubMed] [Google Scholar]
- 109.Lee Y. J., Jung O., Lee J., et al. Maclurin exerts anti-cancer effects on PC3 human prostate cancer cells via activation of p38 and inhibitions of JNK, FAK, AKT, and c-Myc signaling pathways. Nutrition Research. 2018;58:62–71. doi: 10.1016/j.nutres.2018.07.003. [DOI] [PubMed] [Google Scholar]
- 110.Gao Y., Fang L., Liu F., et al. Suppressive effects of irisflorentin on LPS-induced inflammatory responses in RAW 264.7 macrophages. Experimental Biology and Medicine. 2014;239:1018–1024. doi: 10.1177/1535370214530081. [DOI] [PubMed] [Google Scholar]
- 111.Fu R.-H., Tsai C.-W., Tsai R.-T., et al. Irisflorentin modifies properties of mouse bone marrow-derived dendritic cells and reduces the allergic contact hypersensitivity responses. Cell Transplantation. 2015;24 doi: 10.3727/096368915x687002. [DOI] [PubMed] [Google Scholar]
- 112.Ullah F., Ayaz M., Sadiq A., et al. Phenolic, flavonoid contents, anticholinesterase and antioxidant evaluation of Iris germanica var; florentina. Natural Product Research. 2016;30:1440–1444. doi: 10.1080/14786419.2015.1057585. [DOI] [PubMed] [Google Scholar]
- 113.Sadgrove N. J., Oliveira T. B., Khumalo G. P., van Vuuren S. F., van Wyk B.-E. Antimicrobial isoflavones and derivatives from Erythrina (Fabaceae): structure activity perspective (Sar & Qsar) on experimental and mined values against Staphylococcus aureus. Antibiotics. 2020;9(5):p. 223. doi: 10.3390/antibiotics9050223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tiza N. u., Thato M., Raymond D., Jeremy K., Burtram C. F. Additive antibacterial activity of naringenin and antibiotic combinations against multidrug resistant Staphylococcus aureus. African Journal of Microbiology Research. 2015;9:1513–1518. [Google Scholar]
- 115.Choi J., Lee D.-H., Jang H., Park S.-Y., Seol J.-W. Naringenin exerts anticancer effects by inducing tumor cell death and inhibiting angiogenesis in malignant melanoma. International Journal of Medical Sciences. 2020;17:3049–3057. doi: 10.3390/biom9030099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Den Hartogh D. J., Tsiani E. Antidiabetic properties of naringenin: a citrus fruit polyphenol. Biomolecules. 2019;9 doi: 10.3390/biom9030099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Valentová K., Vrba J., Bancířová M., Ulrichová J., Křen V. Isoquercitrin: pharmacology, toxicology, and metabolism. Food and Chemical Toxicology. 2014;68:267–282. doi: 10.1016/j.fct.2014.03.018. [DOI] [PubMed] [Google Scholar]
- 118.Patel K., Gadewar M., Tahilyani V., Patel D. K. A review on pharmacological and analytical aspects of diosmetin: a concise report. Chinese Journal of Integrative Medicine. 2013;19:792–800. doi: 10.1007/s11655-013-1595-3. [DOI] [PubMed] [Google Scholar]
- 119.Wali A. F., Jabnoun S., Razmpoor M., et al. Chrysin, an important active ingredient of honey: beneficial pharmacological activities and molecular mechanism of action. In: Rehman M. U., Majid S., editors. Therapeutic Applications of Honey and its Phytochemicals : Volume II. Singapore: Springer; 2020. pp. 409–432. [DOI] [Google Scholar]
- 120.Taheri Y., Suleria H. A. R., Martins N., et al. Myricetin bioactive effects: moving from preclinical evidence to potential clinical applications. BMC Complementary Medicine and Therapies. 2020;20:p. 241. doi: 10.1186/s12906-020-03033-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sun F., Zheng Z., Lan J., et al. New micelle myricetin formulation for ocular delivery: improved stability, solubility, and ocular anti-inflammatory treatment. Drug Delivery. 2019;26:575–585. doi: 10.1080/10717544.2019.1622608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chen A. Y., Chen Y. C. A review of the dietary flavonoid, kaempferol on human health and cancer chemoprevention. Food Chemistry. 2013;138:2099–2107. doi: 10.1016/j.foodchem.2012.11.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Vo V. A., Lee J.-W., Chang J.-E., et al. Avicularin inhibits lipopolysaccharide-induced inflammatory response by suppressing ERK phosphorylation in RAW 264.7 Macrophages. Biomolecules and Therapeutics. 2012;20:532–537. doi: 10.4062/biomolther.2012.20.6.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Baskaran X., Geo Vigila A., Zhang S., Feng S., Liao W. A review of the use of pteridophytes for treating human ailments. Journal of Zhejiang University-Science B. 2018;19:85–119. doi: 10.1631/jzus.B1600344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Maalik A., Khan F. A., Mumtaz A., et al. Pharmacological applications of quercetin and its derivatives: a short review. Tropical Journal of Pharmaceutical Research. 2014;13:p. 1561. [Google Scholar]
- 126.Nawrot J., Budzianowski J., Nowak G., Micek I., Budzianowska A., Gornowicz-Porowska J. Biologically Active Compounds in Stizolophus balsamita Inflorescences: Isolation, Phytochemical Characterization and Effects on the Skin Biophysical Parameters. International Journal of Molecular Sciences. 2021;22:p. 4428. doi: 10.3390/ijms22094428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sunil C., Xu B. An insight into the health-promoting effects of taxifolin (dihydroquercetin) Phytochemistry. 2019;166, article 112066 doi: 10.1016/j.phytochem.2019.112066. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: Indigenous uses and chemical constituents' description of A. catechu used in the study. Figure S1: Total ion chromatogram (TIC) blue line for A. catechu ethyl acetate fraction and red line for A. catechu water fraction. Figure S2: Mass spectrum of catechin or epicatechin. Figure S3: Mass spectrum of gallocatechin/epigallocatechin. Figure S4: Mass spectrum of procyanidin B1/procyanidin B3. Figure S5: Mass spectrum of emodin. Figure S6: Mass spectrum of afzelechin/epiafzelechin. Figure S7: Mass spectrum of maclurin. Figure S8: Mass spectrum of irisflorentin. Figure S9: Mass spectrum of naringenin. Figure S10: Mass spectrum of isoquercetin. Figure S11: Mass spectrum of diosmetin. Figure S12: Mass spectrum of chrysin. Figure S13: Mass spectrum of myricetin. Figure S14: Mass spectrum of kaempferol. Figure S15: Mass spectrum of avicularin. Figure S16: Mass spectrum of prodelphinidin B3. Figure S17: Mass spectrum of prodelphinidin B. Figure S18: Mass spectrum of quercetin. Figure S19: Mass spectrum of taxifolin. Figure S20: Mass spectrum of acacetin. Figure S21: Mass spectrum of aciculatinone. Figure S22: Mass spectrum of gossypin. Figure S23: Mass spectrum of pterocarpan. Figure S24: Mass spectrum of isorhamnetin. Figure S25: Mass spectrum of trihydroxy dimethoxyflavone. Figure S26: Fragmentation pattern of catechin/epicatechin. Figure S27: Fragmentation pattern of gallocatechin/epigallocatechin. Figure S28: Fragmentation pattern of emodin. Figure S29: Fragmentation pattern of naringenin. Figure S30: Fragmentation pattern of isoquercetin. Figure S31: Fragmentation pattern of diosmetin. Figure S32: Fragmentation pattern of chrysin. Figure S33: Fragmentation pattern of kaempferol. Figure S34: Fragmentation pattern of avicularin. Figure S35: Fragmentation pattern of prodelphinidin B3. Figure S36: fragmentation pattern of prodelphinidin B. Figure S37: Fragmentation pattern of isorhamnetin. Figure S38: Fragmentation pattern of trihydroxy dimethoxyflavone.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.


