Abstract
The SARS-CoV-2 virus has caused a worldwide COVID-19 pandemic. In less than a year and a half, more than 200 million people have been infected and more than four million have died. Despite some improvement in the treatment strategies, no definitive treatment protocol has been developed. The pathogenesis of the disease has not been clearly elucidated yet. A clear understanding of its pathogenesis will help develop effective vaccines and drugs. The immunopathogenesis of COVID-19 is characteristic with acute respiratory distress syndrome and multiorgan involvement with impaired Type I interferon response and hyperinflammation. The destructive systemic effects of COVID-19 cannot be explained simply by the viral tropism through the ACE2 and TMPRSS2 receptors. In addition, the recently identified mutations cannot fully explain the defect in all cases of Type I interferon synthesis. We hypothesize that retinol depletion and resulting impaired retinoid signaling play a central role in the COVID-19 pathogenesis that is characteristic for dysregulated immune system, defect in Type I interferon synthesis, severe inflammatory process, and destructive systemic multiorgan involvement. Viral RNA recognition mechanism through RIG-I receptors can quickly consume a large amount of the body's retinoid reserve, which causes the retinol levels to fall below the normal serum levels. This causes retinoid insufficiency and impaired retinoid signaling, which leads to interruption in Type I interferon synthesis and an excessive inflammation. Therefore, reconstitution of the retinoid signaling may prove to be a valid strategy for management of COVID-19 as well for some other chronic, degenerative, inflammatory, and autoimmune diseases.
Keywords: Vitamin A, Vitamin D, Retinol, Retinoic acid, Retinoid signaling, COVID-19, Type-I IFN, RIG-I, CYP450, NF-κB, RORγt, FoxP3, Th17, Treg, Inflammation, Autoimmunity
Abbreviations: Akt, serine/threonine specific protein kinase B; ATRA, All-trans retinoic acid; BALF, bronchoalveolar lavage fluid; cisRA, cis isomers of retinoic acid; c-Myc, cellular Myc proto-oncogene; CDC, Centers for Disease Control and Prevention; COVID-19, coronavirus disease 2019; CRBP-I, cytoplasmic retinol binding protein 1; CRABP-1, cytoplasmic retinoic acid binding protein 1; CXCL9, C-X-C motif chemokine ligand 9; CYP26, cytochrome P450 family 26; CYP27, cytochrome P450 family 27; CYP450, cytochrome P450 superfamily of enzymes; Erk, extracellular-signal-regulated kinase; FoxP3, Forkhead box P3 transcription factor; GluR1, glutamate receptor 1; IFN, interferon; IL, interleukin cytokines; IL2RA, interleukin 2 receptor-alpha subunit; IRF, interferon-regulatory factors; Jak, Janus kinases; Lyn, Lyn protein kinase, a member of Src family tyrosine kinases; MAPK, mitogen-activated protein kinase; MDA5, melanoma differentiation-associated protein 5; NCoA, nuclear receptor co-activators; NCoR, nuclear receptor co-repressors; NF-κB, nuclear factor kappa light chain enhancer of activated B cells; PI3K, Phosphatidylinositol-3-Kinase; PPAR, peroxisome proliferator-activated receptor; PRR, pattern recognition receptors; RA, retinoic acid; RAR, retinoic acid receptors; RARE, retinoic acid response elements (regulatory DNA sequences bound by retinoic acid receptors); RBP, retinol binding protein; RALDH, retinaldehyde dehydrogenase enzyme; RDH, retinol dehydrogenase enzymes; RIG-I, retinoic acid-inducible gene-I; RLR, RIG-I-like receptor; RORγt, Retinoic acid-related orphan receptor gT transcription factor; RXR, retinoid X receptors; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; sST2, soluble suppression of tumorigenicity 2; STAT, signal transducer and activator of transcription; sTNFRSF1A, soluble TNF receptor superfamily member 1A; STRA6, signaling receptor and transporter of retinol; TGF-β, transforming growth factor-beta; Th17, T helper 17 cells; TLR, Toll-like receptors; TNF, Tumor necrosis factor; TRAF, TNF receptor (TNF-R)-associated factor; Treg, regulatory T cells; Type-I IFN, Type I interferon (IFN-α and IFN-β); VAD, vitamin A deficiency; VitA, vitamin A; VitD, vitamin D; WHO, World Health Organization
1. Introduction
Coronavirus disease 2019 (COVID-19) was initially detected as a pneumonia-like disease outbreak with unknown cause in Wuhan, China, in December 2019, and rapidly spread throughout the world [1], [2]. The World Health Organization (WHO) reported on January 9, 2020 that it was caused by a novel coronavirus, severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2), named the disease COVID-19 on February 11, and declared it as a pandemic on March 11, 2020 [1], [2]. The US Centers for Disease Control and Prevention (CDC) declared it as a public health emergency in the US at the end of January. Europe was declared the epicenter of the pandemic on March 13, and in a couple of weeks, US became the country with the highest number of infections and deaths ahead of any country. Later, it spread very fast in other countries such as Brazil and India. In a year and a half, by the beginning of May 2021, more than 200 million people have been infected and more than four million have died worldwide [1]. In addition, it has caused severe socioeconomic problems and continues to exist as an emergent public health problem all over the world [1]. Even though there have been some improved treatment strategies, no definitive COVID-19 treatment has been found so far [3], [4].
From the start of the pandemic, there have been very intense vaccine development approaches in the US, Europe, Russia, and China. As a result, vaccines against SARS-CoV2 had been developed with an unprecedented historical speed of less than a year. Soon, these vaccines began to be used after approval by regulatory agencies for emergency use. Preliminary results show high efficacy of at least some of the vaccines [5]. However, the long-term effectiveness and side effects of these COVID-19 vaccines are not yet known. Preliminary observations indicate some serious side effects such as allergy, anaphylaxis, and blood clotting [5], [6], [7], [8]. Furthermore, because of the frequent mutations of SARS-CoV-2 and the emergence of new variants of the virus, there have been concerns that the evolved novel mutants might evade the effectiveness of these vaccines [9], [10]. The delta variant has already become the dominant mutant spreading very fast in many countries infecting many including vaccinated people. The vaccine developers had already started incorporating the mutant variants in their vaccines. In addition, the production capacity of the vaccines and vaccine-related supply and logistic support pose important problems [11]. Considering that billions of people will be vaccinated, it seems impossible to meet this huge worldwide demand in a timely manner. Despite the rapid use of COVID-19 vaccines in some countries, it will not be easy to vaccinate billions of people around the world due to socioeconomical reasons. Therefore, detailed understanding of the COVID-19 pathogenesis will enable the development of more effective vaccines and effective treatment methods [12]. Hence, research aiming to further elucidate the COVID-19 pathogenesis will bear high importance in developing prevention and management strategies.
Although one and a half year has passed since the COVID-19 pandemic started, the pathogenesis of COVID-19 is still not well understood. Under the light from the current literature and research results, we aim to suggest that a defective retinoic acids (RA) metabolism and retinoid signaling disorder might be an important underlying factor in the COVID-19's destructive pathogenesis. Because the destructive systemic COVID-19 pathophysiology is much larger and severe compared to viral tissue tropism and mimics the systemic effect of retinoid signaling disorders, we hypothesize that the RA signaling disorder plays an essential role in the immunopathogenesis of COVID-19. In this study, we provide a systematic review of RA metabolism and a possible central role of retinoid signaling in COVID-19 pathogenesis. The likely mechanisms with which the retinoid signaling disorders may involve in the COVID-19 pathogenesis have been evaluated in the light of recent research data. More specifically, the immune system dysregulation observed in the COVID-19 patients and the literature data that have led to a likely misunderstanding of the regulation mechanisms of the immune system are discussed. This review aims to provide a new approach to the pathogenesis of COVID-19, to provide a critical analysis of existing knowledge in the field, to identify and discuss the main problems of misinterpretations, and to offer a new perspective for the research on the pathogenesis and treatment of COVID-19.
2. Retinol depletion and retinoid signaling disorder hypothesis in COVID-19 pathogenesis
This systemic involvement in COVID-19, which is different from many other viral infections and cannot be explained accurately by viral tropism, has made it difficult to clearly understand the COVID-19 pathogenesis. During the pandemic, most researchers focused their efforts on the tropism mechanism to delineate the pathogenesis of COVID-19. We believe that the retinol depletion and consequently impaired retinoid signaling play a central role in the COVID-19 pathogenesis and associated widespread systemic effects as seen in some other viral infections [13], [14]. Essentially, a main prominent difference that distinguishes COVID-19 pathogenesis from other viral infections is the excessive size of the SARS-CoV-2 genome which will result in an excessive immune response against viral RNA that will most likely end up with the depletion of body's retinol depot and subsequent defect in retinoid signaling. While the genome size of many RNA viruses is generally less than 10 kB, that of SARS-CoV-2 is 30 kB [15]. This makes SARS-CoV-2 the virus with the largest genome among RNA viruses [16], [17]. Because of such a large genome size, large amounts of RNA particles are released from viruses that are broken down by host immune response. These broken scattered large amount of viral RNA particles are recognized and processed by innate immune system, which leads to Type I interferon (IFN) synthesis, a process that also uses the RA signaling [18], [19]. The large amount of viral RNA and consequent overwhelming immune stimulation will cause increased RA consumption, and with the time, depletion of retinol reserves in the body [20], [21]. Liver damage incurred during COVID-19 infection may speed up the depletion of vitamin A (VitA) stores in the liver [22]. Depletion of retinol then leads to retinoid signaling disorders simultaneously in many organ systems. This wide-spread retinoid signaling disorder and interrupted type I IFN synthesis in turn lead to inflammatory cytokine discharge and systemic inflammation seen in the COVID-19.
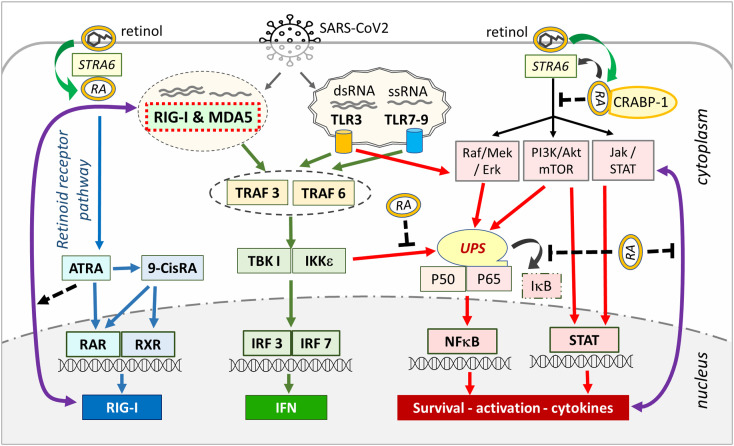
Pattern recognition receptors (PRR), such as Toll-like receptors (TLR) of the innate immune system are responsible for recognizing pathogens and viruses and inducing IFN production (Fig. 1 ). Viral RNA in the endosomes is mostly recognized byTLR3 and TLR7 while cytosolic viral RNA is recognized by RIG-I like receptor family proteins, such as retinoic acid-inducible gene-I (RIG-I) and melanoma differentiation-associated protein 5 (MDA5) [18], [19], [20], [23]. RIG-I is the main cytosolic receptor that recognizes cytosolic viral RNA molecules and is responsible for type I interferon synthesis [20], [24]. Expression of RIG-I is regulated through RA signaling and with the prolonged stimulation, the cellular retinol resources are used up causing depletion of retinol depot in the body [20], [25], [26], [27]. With the depletion of retinol and impaired retinoid signaling, the RIG-I pathway of the innate immune system collapses, and Type I interferon synthesis is interrupted [25], [28], [29]. This leads to dysregulation in the immune system with disrupted delicate balance between immune-suppressive regulatory T cells (Treg) and proinflammatory T helper 17 (Th17) cells [30], [31]. In this way, the coordination between the innate and adaptive components of the immune system is disrupted, causing a collapse of the innate immune system and the hyperactivation of the Th17 arm of the adaptive immune system, resulting in cytokine storm and systemic organ damage [32], [33]. However, when there is a normal serum level and adequate RA signaling, the balance favors the dominance of Treg cells and suppression of Th17 cells that prevent inflammatory cytokine release and establishment of self-tolerance (Fig. 2 ) [34], [35].
Fig. 1.
Retinoic acid signaling orchestrates innate and adaptive immune responses to viral infections. Extracellular and intra-vesicular viruses are recognized through TLR receptors activating the TRAF/IRF signaling cascade. After the viral genome is taken into the cell, the immune response switches to the RIG-I and MDA5 further activating TRAF/IRF pathway. Activated TRAF/IRF leads to Type I IFN synthesis (green arrows, center). RIG-I is synthesized through RAR/RXR (blue arrows, left side) as long as there is sufficient retinoid signaling in the cell, and type I interferon synthesis continues through RIG-I and TLR system (green arrows, center). TRAF can also activate IKK leading to degradation of I-κB and activation of NF-κB. In addition, the MAPK, PI3K/Akt and Jak/STAT pathways are also under the control of non-genomic signaling of retinoic acids. Here, non-genomic signaling cascades are modulated by STRA6 and membrane-associated RARs via RA recruitment, leading to activation or inactivation of NF-B (red arrows, right side). Here, the association of RA with CRABP-1 inhibits tonic MAPK, PI3K/Akt and Jak/STAT signaling. RA activates RIG-I expression (black dotted arrows) and inhibits cytokine feed-forward loop and UPS system (blunt-ended dotted black lines) preventing NF-кB activation through UPS. With retinol depletion, the RIG-I arm of the innate immune system that produces Type I interferon collapses (green arrows, center), but the UPS-NF-κB pathway continues operating through TLR receptors releasing cytokines and other inflammatory mediators (red arrows, right side). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
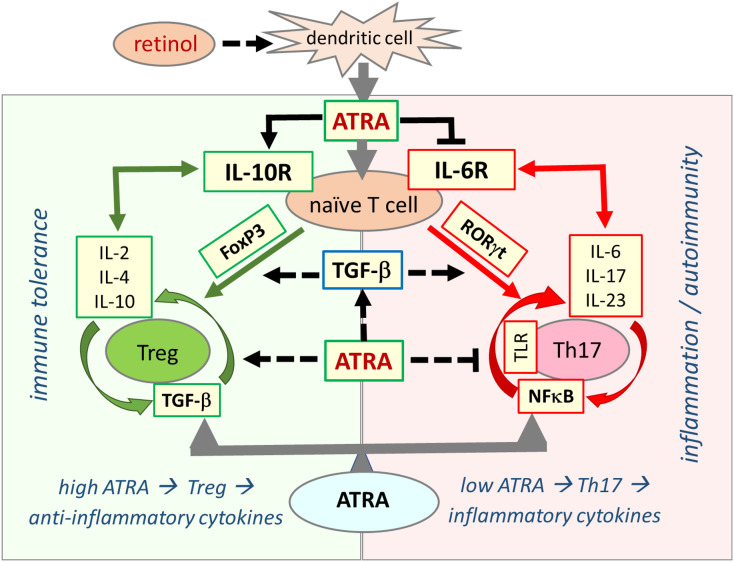
Fig. 2.
Retinoic acid signaling is essential in establishing immune tolerance through promoting differentiation of Tregs and inhibiting development of proinflammatory Th17 cells. The balance between Treg and Th17 cells defines immune tolerance or inflammation. Both Treg and Th17 cells differentiate from naive T cells under different cellular and tissue milieu requiring TGF-β. In the presence of sufficient ATRA levels, the interaction between signaling pathways from RA, TGF-β, and cytokines blocks IL-6 signaling and induce FoxP3 transcription factor that ultimately leads to differentiation of naïve T cells into Tregs, which induce expression of anti-inflammatory cytokines, such as IL-2, IL-4, and IL-10, and suppress the inflammatory response that culminate in establishment of self-tolerance (immune tolerance circle, green circled arrows, left side panel). In the presence of low or no ATRA, retinoid signaling is weak or absent and fails to block IL-6 signaling, leading to the expression and activation of RORγt that reprogram differentiation of naïve T cells into proinflammatory Th17 cells. Effector Th17 cells have high activity of NF-κB signaling that leads to expression and release of various proinflammatory cytokines such as IL-6, IL-17, and IL-23, generating an inflammatory response that may lead to development of autoimmune diseases (inflammation circle, red circled arrows, right side panel). ATRA exerts its effect by inducing expression of TGF-β and FoxP3 (dotted black arrows) and inhibiting activation of NF-κB (blunt-ended dotted black line). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
With the depletion of retinol and disrupted RA signaling, which may be the case in severe COVID-19, the transformation of naïve T cells to regular T cells is blocked, while transformation into effector Th17 cells is promoted [34], [35], [36]. As a result, excessive cytokine release and systemic organ damage may occur. Meanwhile, the cellular and mitochondrial DNA fragments emanating from dying host cells and RNA fragments emanating from fragmented viral particles will continue to activate the inflammatory NF-κB (nuclear factor kappa light chain enhancer of activated B cells) signaling cascade through TLR [37]. The continuous and intense stimulation of TLR causes hyperactivation of NF-κB pathway and excessive stimulation of the immune cells resulting in excessive cytokine discharge and reprogramming of B and T cell development that ultimately leads to devastating systemic effects seen in COVD-19 [38], [39], [40], [41].
Due to the widespread distribution and intensive activity of retinoid signaling in various organ systems, impairment in retinoid signaling affects almost all organ systems [42], [43], [44], [45]. Likewise, COVID-19 presents very common and similar various symptoms, clinical findings, and multi-organ involvement [36], [46], [47]. Damaged liver may lose retinol reserves faster [22] causing retinoid signaling impairment that may lead to the emergence of severe clinical pictures, multiple organ injuries, and chronic post-COVID syndromes [48], [49], [50].
Various studies have shown a link between increased inflammatory mediators and excessive cytokine production in COVID-19 [47], [51], [52], [53]. In almost all of these studies, treatments based on the blockade of the cytokines have been proposed for the treatment of COVID-19 due to its severe inflammation and cytokine storm phenotype [47], [54], [55], [56], [57]. However, we believe that the retinol depletion and the resulting low serum RA levels play an initial central role triggering the immune response towards increased cytokine expression and inflammation observed in COVID-19 and therefore, it needs further detailed studies.
The reason why the regulation mechanism of endogenous RAs in COVID-19 has not been noticed until now may be the assumption that retinol and RAs, which have large reserves, can always be present in the body's stores. However, the stored amount of RA in the human body is limited and is sufficient for about three months [41], [58], [59], [60]. The serum retinol level is kept constant as it is supplemented per need from body’s retinol depots, mainly liver. A drop in the serum retinol level occurs only after the deficiency progresses to severe levels when the liver retinol reserve is depleted. When serum retinol levels are low, the liver retinol stores are already depleted, and the disease is advanced [61], [62]. Retinol and RAs can be depleted rapidly, especially due to persistent and prolonged viral load, high fever, and catabolic destruction [60].
3. Vitamin A metabolism and retinoid signaling mechanism
Vitamin A (VitA) is one of the most important vitamins in the body. Its signaling is necessary for the regulation and maintenance of a wide variety of biological functions, such as growth, development, vision, differentiation, proliferation, and apoptosis, both during the intrauterine development and in the adulthood [44], [63], [64]. It is essential for organogenesis during embryonic development as well as for metabolic homeostasis. VitA functions as hormones in regulating body's metabolism and promoting growth [65], [66]. When first discovered, it was named as hormone A and growth factor due to its vital role in cell survival and development [67]. Later, it was found to have an anti-infection affect and therefore was referred to as an anti-infectious vitamin [27], [68]. The WHO added VitA to its infection prevention programs during measles epidemics in the 1950s, which proved to achieve successful results, as it reduced mortality from measles pneumonia by 50% during the epidemic [69], [70]. Likewise, similar successful results had been obtained from supportive therapies with VitA in AIDS patients [71]. Furthermore, VitA and its derivative retinoids are currently being used as vaccine adjuvants [69], [72]. VitA deficiency (VAD) causes various pathologies such as impaired vision, reduced growth, increased morbidity and mortality of certain infections due to an impairment of innate and adaptive immune responses and increased inflammation [29], [73]. VAD is also associated with increased mortality in children [74], [75], [76].
The biologically active form of VitA, retinol, is taken up by gastrointestinal epithelial cells and transferred into blood where it binds to serum retinol binding protein (RBP). In tissues, the RBP-retinol complex is taken up into the cells through Signaling Receptor and Transporter of Retinol (STRA6) and retinol is then transferred to the Cytoplasmic Retinol Binding Protein 1 (CRBP-I) [77]. Once in the storage tissue cells, the retinol and other chemically related retinoids and carotenoids are stored as retinal esters in the VitA-storing cells, also known as stellate cells, lipocytes, or Ito cells, mostly in liver but also in other organs such as kidney and lung [78], [77]. Per need, it is released into the circulation to be taken up into the target cells. Retinoid target cells are present in many tissues and organs of the body including hormonal glands, reproductive tissues, neural tissues, epithelial tissues, and immune cells [45], [79]. In the target cells, it generates retinoid signaling leading to various physiological outcomes. Retinoid signaling is seminal in various developmental, physiological, and biochemical processes including, vision, spermatogenesis, embryogenesis, and development and function of nervous and immune systems [63], [64], [80]. During mammalian embryogenesis, it is important for the initiation of development of body axis and many organs including the brain, spinal cord, forelimb buds, skeleton, heart, eye, pancreas, lung, and genitourinary tract [45], [81].
In the target cells, retinol and other retinoid molecules exert their functional effect through activating cellular signaling pathways. Cellular retinol is first reversibly metabolized to retinal, which is then irreversibly metabolized to retinoic acid (RA) [45]. RA has a few isomers including all-trans RA (ATRA) and a few Cis isomers (CisRA), including 9-CisRA, 11-CisRA, and 13-CisRA. ATRA is the major RA isomer mediating cellular signaling leading to the metabolic outcome of the retinoid signaling in the cells [82]. The level of RA in the cells is tightly regulated by two sets of enzymatic reactions: those that synthesize RA and those that degrade or metabolize RA. Synthesis of RA starts with retinol, which is first reversibly metabolized to retinal by retinol dehydrogenase (RDH) enzymes, such as RDH10 [45]. Retinal is then irreversibly metabolized to RA by retinaldehyde dehydrogenase enzymes (RALDH/ALDH1A) [45]. The half-life of free RA and other related retinoids in cells is very short as they are quickly being degraded by retinoic acid hydroxylase CYP26 enzymes such as CYP26A1 (cytochrome P450 family 26 subfamily A member 1), CYP26B1, CYP26C1, and their isomers, as well as members of other subfamilies of cytochrome P450 (CYP450) enzymes, such as CYP2, CYP3, and CYP4, providing a short time window for RA to initiate signaling [83], [84]. CYP26 and other CYP's are members of the larger CYP superfamily monooxygenase enzymes that are important regulators of various cellular metabolic pathways, including steroids, lipids, xenobiotics, and cellular respiration, and therefore has been implicated in various clinical pathologies and explored for therapeutic approaches [84], [85], [86].
RA signaling pathway involves transcriptional (genomic) and non-transcriptional (non-genomic) mechanisms [79], [87], [88]. At the transcriptional level, the canonical RA signaling leads to the transcription of various target genes. RAs act as ligands for members of RA receptors (RARs) and related Retinoid X Receptors (RXRs) as well as peroxisome proliferator-activated receptor (PPAR) and other related receptors to activate transcription of target genes [42], [44], [89]. RAR and RXR can constitutively form homodimers or heterodimers bound to RA Response Elements (RARE) on the regulatory sequences of the target genes. In the absence of cognate retinoic acid ligands, RAR/RXR heterodimers are bound to nuclear receptor corepressors (NCoR) that keep the transcription of target genes off. Binding of ligands displaces the corepressors, allowing the recruitment of nuclear receptor co-activators (NCoA) to bind, resulting in the transcriptional activation of target genes [90], [91], [92], [93], [94]. In this way, retinoid-activated RAR/RXR signaling pathways directly or indirectly regulate hundreds of RARE-containing genes including Hox family genes, which are important in embryonic development, and genes important in the development and function of the immune system such as NF-κB, c-Myc, Lyn, IL2RA (interleukin 2 receptor-alpha subunit), and TGF-β (transforming growth factor-beta) [42], [45], [87].
RA signaling at the non-transcriptional level, the non-canonical RA signaling, involves in direct modulation of intracellular signaling cascades through membrane associated STRA6 and RARs and RA-binding cytoplasmic proteins such as cellular RA-binding protein 1 (CRABP-1). During retinol transport into the cells, the association of STRA6 and retinol can rapidly initiate tonic cellular signaling through phosphorylation of cellular kinases. Retinol-bound STRA6 can activate cytokine signaling such as Jak/STAT pathway [95]. Janus kinases (Jak) and transcription factors of signal transducer and activator of transcription (STAT) are family of cellular signaling molecules that generate immediate signaling mechanisms from membrane to transcription that affects many aspects of the immune system including cytokine and interferon signaling and expression [96]. In addition, a subpopulation of RAR, especially RAR-alpha (RARα), has been localized in the membrane lipid rafts and upon binding to RA, it can similarly activate cytoplasmic kinases leading to the activation of various intracellular signaling pathways, including PI3K/Akt, MAPK/Erk, Jak/STAT, RIG-I, non-genomic GluR1, and calcium flux pathways, many of which are highly active signaling cascades in many cells especially in nervous and immune cells [42], [87], [95], [96], [97], [98], [99], [100], [101], [102]. Furthermore, association of RA with CRABP-1 form a nucleus for a larger signaling complex that leads to inhibition of cellular signaling such as Raf/Mek/Erk, mTOR, Jak/STAT pathways [103], [104], [105], [106], [107], [108] (Fig 1).
The retinoid signaling mechanisms, both at transcriptional and non-transcriptional levels, are responsible for RA's global effect on the formation, development, and function of organs and tissues from the embryonic development to the adulthood. Disruption of this signaling causes serious congenital malformations during the embryonic period and causes serious developmental disorders affecting many organ systems in adulthood including immune system [45], [64]. Even though the lack of VitA has serious consequences, overdose of retinol and related retinoids and RA in the body may pose serious consequences such as developmental and teratogenic side effects [109], [110], [111]. In the case of VAD, retinol and related carotenoid supplementation may be recommended. However, the use of retinoids and ATRA for treatment or prophylactic purposes must be under medical supervision.
4. Viral tropism, retinoid signaling disorder, and COVID-19 pathogenesis
Pathological damages caused by many viral infections usually occur in certain tissues and organs associated with the cell and tissue tropism of the virus [112]. Many viruses are further classified according to their tissue and cell tropism such as neurotrophic, hepatotropic, enterotropic viruses, etc. The prominent factor defining viral tropism is the specific interaction of viral receptors to their cognate host cell receptor needed for the viral entry into the host cells. The tropism of SARS-CoV-2, the causative agent of COVID-19, is provided through host cell receptor Angiotensin-converting enzyme 2 (ACE2), a critical carboxypeptidase in the renin-angiotensin hormone system that regulates blood volume and cardiovascular homeostasis [113], [114]. ACE2 is expressed mainly on endothelial and epithelial cells of a few organs, such as lung, gastrointestinal tract, hearth, kidney, and testes. Cellular entry of SARS-CoV-2 into the target cells, in addition to ACE2, requires protease activity of surface receptors Transmembrane Protease Serine 2 (TMPRSS2), TMPRSS13, or endosomal protease cathepsin L [115], [116].
Although the main target of SARS-CoV2 infection is the respiratory and gastrointestinal tract, its infection causes involvement of many organ systems, including nervous, auditory, olfactory, circulation, and inflammation [36], [117], [118], [119]. The most prominent pathogenesis of COVID-19 is lung infection. SARS-CoV-2 invades the lungs through the respiratory tract and causes severe pneumonia [36], [118], [120]. The main symptoms of patients with COVID-19 infection are interstitial changes in the lung which may become severe and lead to hypoxemia and type I respiratory distress. While it may be asymptomatic or mild in many infected individuals, about 15% of infected individuals may develop severe symptoms, and about 6% may progress to acute respiratory distress syndrome (ARDS) and multiorgan failure leading to death [121], [122].
The clinical presentation and immunological profile of COVID-19 is unique with end-organ damage mediated through host immune response [17], [122]. The immunopathophysiology of COVID-19 manifests itself as a biphasic illness at least in severe cases [17], [121], [123], [124]. The initial viral phase lasts about a week followed by a second “hyperinflammatory” phase leading to host immune response-mediated organ damage. In severe cases, the hyperinflammatory phase is characteristic with hemophagocytosis, neutrophilia, lymphocytopenia, thrombocytopenia, and increased proinflammatory mediators and cytokines [118], [123], [125], [126], [127], [128], [129]. The respiratory manifestation of this systemic inflammatory response is acute respiratory distress syndrome (ARDS), which leads to a need for mechanical ventilation in severe cases [130]. The resulting process is the severe COVID-19 picture characterized by systemic inflammatory response syndrome (SIRS), ARDS, hypoxia, and systemic organ damage leading to death [17], [121], [131].
The COVID-19 has been a unique and very challenging pandemic. How COVID-19 affects all organ systems beyond tissue tropism is less understood. The destructive systemic effects caused by SARS-CoV-2 cannot be explained merely by the interaction between viral spike glycoprotein and the host cell receptors ACE2 and TMPRSS2 [17], [132]. The severity of COVID-19 infection is due to impaired immune response causing tissue and organ destruction [118], [120]. It will be of paramount importance to identify the immune system components that drive the pathophysiology of the COVID-19.
Many COVID-19 symptoms related to the immune and central nervous systems as well as the cardiovascular, respiratory, digestive systems, kidney, suprarenal gland, the smell, taste, hearing, vision, and balance disorders are also related to retinoid signaling disorders [89], [133], [134]. The enzymes and molecules important in the RA metabolism, such as RALDH enzyme activity, ATRA synthesis, RA receptors, and CYP450 enzymes, are also highly expressed in these organ systems that are significantly affected and damaged in severe COVID-19 patients [64], [83], [135]. Therefore, the RA and retinoid signaling mechanism has a widespread distribution and activity in almost all organ systems [63], [136], [137]. RA signaling disorders can cause common and severe symptoms and signs similar to those seen in COVID-19 [132], [134], [138]. Interestingly, the way the COVID-19 affects organs parallels the widespread positioning and activity of retinoid signaling [133], [134], [139]. Furthermore, RA signaling has been implicated in the pathogenesis of the COVID-19 [140] and high retinol level is inversely corelated with COVID-19 infection [141].
COVID-19 patients display multiple signs and symptoms that typically resemble those of VAD that develop due to retinol depletion and impaired retinoid signaling. For example, some laboratory and radiological imaging findings, clinical observations, and symptoms of COVID-19 resemble those of VAD [132], [133], [134], [142], [143]. Many of these signs and symptoms, such as changes in the immune system, central nervous system, and eyes, and especially the smell and taste disturbance, are also very common in VAD [142], [143], [144]. Likewise, the changes observed in immune system in COVID-19 infections are drastically similar to those of immune system suppression seen in VAD [123], [126], [145], [146].
5. CNS involvement and retinoid signaling disorder inCOVID-19
VitA and its derivative retinoids and their signaling are necessary for normal embryonic development, neural tube patterning, neural differentiation, and gene expression in the brain. RA metabolism and the retinoid signaling through RARs are constantly active at a basal level in the nervous system [64], [139], [147]. RA is synthesized in various cells of nervous system including oligodendrocytes, astrocytes, and Schwann cells, the myelin sheaths of the axons in the brain [45], [64], [79], [139]. RA signaling is necessary not only for development of organs and tissues but also for continuation of physiological activities and homeostatic regulation [87], [139]. However, deviations from the basal levels of retinoid activity may cause disruption in the synthesis and functions of downstream structural and functional proteins, enzymes, and hormones manifesting as organ damage and loss of function phenotypes [64], [148]. Most of the COVID-19 symptoms resemble those caused by organ damage and loss of functions seen in the retinoid acid signaling disorders [64], [134]. Therefore, most of the systemic signs and symptoms of COVID-19, especially those related to the central nervous system, sensory organs, and immune system may well be due to the VAD and ensuing impaired retinoid signaling [63], [73], [149], [150].
Ionotropic glutamate receptor subunit 1 (GluR-1/GRIA1/GluR-A) has a vital function in neurons [151], [152], [153]. It is the predominant excitatory neurotransmitter receptor in the brain and is mainly responsible for providing neuronal plasticity and synaptic transmission in the nervous system [154], [155], [156]. There are alternatively spliced four isomers (GluR1-4), which are differentially activated by L-glutamate in a variety of normal neurophysiologic processes. These receptor isomers are heteromeric transmembrane protein complexes with multiple subunits that form a ligand-gated ion channel [152], [153]. They belong to a family of alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionate (AMPA) receptors family. Proper activation of GluR1 is very important in the functions of the limbic system, also known as paleomammalian cortex, which contains a set of brain structures including the amygdala, thalamus, hypothalamus, and hippocampus [151], [154], [155], [156]. It coordinates vital functions such as nutrition, smell, taste, vision, hearing, balance, fear, anger, sleep, and memory. The signaling disorders within the limbic system are responsible for etiopathogenesis of many acute and chronic, degenerative, and neuropsychiatric diseases, especially Alzheimer's, dementia, schizophrenia, depression, and insomnia [154], [155], [157], [158], [159]. In the limbic system, memory and alertness are regulated by neuronal plasticity provided by retinoid signaling [154], [155], [156], [160].
The expression and activity of GluR1 is regulated through RA signaling including genomic and non-genomic cytoplasmic action of RAR signaling [87], [161]. The defect in the non-genomic function of retinoid signaling is especially seen in various pathologies, such as smell, taste, memory, sleep, and mood disorders [144], [162], [163]. Synaptic transmission in such cases is conducted mainly through activation ofthe GluR1 by glutamate and non-transcriptional regulation through RAR signaling [161], [162], [163]. The non-transcriptional regulation of GluR1 through the RAR signaling involves phosphorylation and activation of downstream signaling molecules [161]. The expression of RARα, which regulates the synthesis of GluR1, is being regulated by retinoid signaling making a self-perpetuating regulatory loop for neurotransmission and plasticity [87], [164]. With retinol depletion in COVID-19, the defects in the synthesis and function of the RAR causes defects in the GluR1 synthesis and a consequent disruption of GluR1-mediated synaptic transmission in various neural tissues including sensory path of smell and taste, leading to the loss of these senses [4], [144], [165]. Furthermore, such a loss of proper GluR1-mediated synaptic transmission may play a central role in various neuropsychiatric symptoms [162], [163], [165]. Retinoid signaling defect may also cause odor loss and neurological symptoms similar to those of COVID-19 [149], [166]. RALDH3 enzyme activity, which synthesizes ATRA from retinal, is detected in the olfactory pit during the embryological period [167]. The therapeutic efficacy of VitA and RAs in restoring the sense of smell has been demonstrated in some cases [168], [169].
In the COVID-19, it is highly likely that impaired retinoid signaling has an important role in the development of in the nervous system-related symptoms [170], [171]. In the course of COVID-19, the involvement of these areas of the nervous system where retinoid signaling is normally highly intense, may likely suggest a cause-and-effect association that needs further investigation. In addition to the mild symptoms and severe clinical picture of acute illness, neuropsychiatric manifestations such as autism, dementia, schizophrenia, depression, and insomnia may occur as post-COVID syndromes that can well be, at least partially, due to impaired retinoid signaling [172], [173]. We believe that the interrupted RA signaling mechanism might underlie the neuropsychiatric complaints observed in the pathogenesis of COVID-19 and deserves further research.
In addition to the nervous and sensory systems, some other organ systems are also affected by COVID-19 infection in proportion to the intensities of their retinoid activities. For example, the adrenal glands and testicles are the organs where the synthesis and signaling of steroid hormones and RA are highly active [174], [175]. These are also implicated in the severe COVID-19 infection [122], [176], [177]. The synthesis of steroid hormones provided by aromatase enzymes in these organs involves CYP450 enzymes, which are also important in the regulation of RA metabolism [178]. In addition, a number of chronic inflammatory disorders, degenerative, and autoimmune diseases are variably associated with retinoid signaling disorder in the late phase of COVID-19 infection [173]. The brain regions, sensory system, and neuroendocrine organs where RA metabolism and retinoid activity are very active are also affected by COVID-19 [173], [175], [179], [180], [181], [182] suggesting that involvement in all these organ systems is a strong indication that the pathogenesis in COVID-19 might well be caused by defects in the retinoid signaling.
6. Immune system involvement and retinoid signaling disorder in COVID-19
VitA has an important immunomodulatory effect on the components of innate and adaptive immunity [100]. It regulates the activities of innate and adaptive immune cells such as macrophages, neutrophils, and lymphocytes during inflammatory responses [183], [184]. VitA supplementation induces NK and NKT cell activities and oxidative killing and downregulates inflammatory IL-6, IL-10, and IL-17 [61]. Retinol induces the T cell activation through CD3 likely through non-genomic activity of membrane bound RAR [185]. VitA also has an anti-inflammatory effect that is required for maintaining intestinal immune tolerance [68], [186], [187]. VitA deficiency (VAD) is associated with inflammation and dietary intake of VitA is required for the maintenance of the anti-inflammatory state of tissue-resident intestinal macrophages [68], [188]. VAD has been shown to increase infectious disease burden and decrease host resistance against viral infections [27], [61], [75], [142], [189] and impairs B and T cell responses to viral vaccines [28]. In addition, VitA levels decrease during viral infections indicating the importance of active usage of VitA in the anti-viral immunity [142], [190]. The immune system's response to viral and parasitic infections is compromised in VAD, and if not restored, the duration and severity of this deficiency-related conditions worsen [191], [192].
While the major signaling molecule inside the cell is RA, lymphocytes may also respond to other forms of VitA metabolic products such as retinal and hydroxy retinol [193]. RA metabolism is highly dynamic in the immune cells. ATRA is synthesized in various circulatory and tissue resident immune cells, primarily monocyte, macrophage, dendritic cells, gut-associated lymphoid tissue cells (GALT) in the intestine, plasmacytoid dendritic cells in the lung, alveolar macrophages, and type II pneumocytes [43], [45], [64], [79]. RA production is increased in dendritic cells and macrophages stimulated through TLR2 as well as through cytokines Granulocyte and Monocyte Colony Stimulating Factor (GM-CSF) and Interleukin-4 (IL-4, 194, 195]. RA is also important in inducing homing after immunization through upregulation of homing receptors in the T and B cells [193], [196], [197], [198].
NF-κB is a family of transcriptional factors important in the development and regulation of innate and adaptive immune cells as well as in neural plasticity, learning, and memory [199], [200], [201], [202], [203], [204]. In immune cells, NF-κB proteins regulate immune cell development, activation, survival, and cytokine production [200], [201]. NF-κB proteins are activated through stimulants such as cytokines, stress, free radicals, ultraviolet radiation, and infections [200]. They play a central role in regulating immune response to infections. They are highly active in inflammatory diseases and control transcription of many genes involved in immune response and cytokine production [200], [201].
The levels and activities of NF-кB proteins are highly regulated. Inappropriate regulation of NF-κB has been linked to various immune disorders, including inflammation, septic shock, autoimmune diseases, infections, and cancers [200], [201]. NF-κB proteins are heterodimer of two proteins, such as Rel and p50 proteins, and are bound to I-κB, which sequester the complex in the cytoplasm in an inactive state [200], [201]. When activated through various signals, the enzyme I-κB kinase (IKK) phosphorylates the I-κB protein, resulting in its ubiquitination and dissociation form NF-κB complex leading to the degradation of I-κB by the proteasomes in the ubiquitin-proteasome system (UPS) [200], [205]. The freed NF-κB complex is then translocated into the nucleus where it binds to specific NF-κB response elements on target genes activating their transcription through recruiting RNA polymerase and other transcription regulatory proteins, such as coactivators [200], [201] (Fig. 1).
RA signaling converge with NF-κB signaling in immune cells (Fig. 1), 199, 206, 207]. PRR of innate immune system, such as TLR and RIG-I are activated after pathogen recognition and this activation leads to the activation of cellular signaling pathways, such as RA, NF-κB, and Jak/STAT, and production of IFN and cytokines [19], [40], [208], [209] (Fig. 1). I-κB, the inhibitory component of the NF-κB, can directly associate with RXR and modulates retinoid signaling [210]. In addition, activation of UPS that leads to the degradation of I-κB is under constant inhibitory pressure of RA signaling [201], [211] (Fig. 1). VAD increases activation of NF-κB and exacerbates NF-κB-mediated inflammation and supplementation of RA suppresses NF-κB activation [143], [188], [212]. However, in COVID19, the inhibition pressure of RAs on the UPS system is eliminated due to the defect in the retinoid signaling after retinol depletion [41], [46]. As a result, UPS degradation valves open leading to the ubiquitination and degradation of I-κB and subsequent activation of NF-κB, leading to the synthesis and release of inflammatory cytokines [213].
COVID-19 immunopathogenesis displays a unique and inappropriate inflammatory response with three prominent features: leukocytosis with lymphocytopenia, inefficient production of type I IFNs, and increased expression of pro-inflammatory cytokines and chemokines [126], [214], [215], [216], [217], [218]. Cytopenia with impaired type I IFN production leads to a viral-induced immune suppression while excessive cytokine production leads to uncontrolled non-specific immune response that causes damage in organ systems [215], [219]. Leukocytosis involves increased number of innate immune cells especially dendritic cells, macrophages, and neutrophils while lymphocytopenia is characteristic with low number of NK, B, and T cells [218], [220], [221]. Furthermore, monocyte/macrophage associated markers are also upregulated in the blood of COVID-19 patients [222]. In addition, like some viruses, SARS-CoV2 can evade innate immune response mediated through PRR [18]. Upregulation of NKG2A and decreased NK, cytotoxic T, memory CD4, and T regulatory cells are associated with the severity of the COVID-19 disease [127], [218], [223], [224]. Taken together, the delicate and dynamic balance between inflammation and anti-inflammatory and antiviral defenses is impaired in COIVD-19.
6.1. The role of retinol depletion and retinoid signaling disorder on the Treg /Th17 imbalance in COVID-19: implication of inflammation and autoimmunity
The balance between regulatory T (Treg) and T helper 17 (Th17) cells is critical for a proper immune response against infections. Increased Treg is associated with decreased Th17 and absence of inflammation [217], [225]. Increased Th17 level, on the other hand, is associated with the increased inflammation and autoimmunity [226]. RA signaling in T cell as well as in dendritic cells is important in orchestrating the balance between Treg and Th17 cells. Skin-originated CD103-negative dendritic cells synthesize ATRA at basal level and this synthesis is further induced through stimulation, which is required for induction of Treg development [227]. CD103-positive dendritic cells in the gut produce high levels of ATRA that corresponds to the induced immune tolerance in mesenteric lymph nodes [228], [229]. In the presence of ATRA, dendritic cells produce high levels of anti-inflammatory cytokine IL-10 and induces T regulatory cell development and maintenance [230], [231], [232] .
Both Th17 and Tregs develop from naïve T cells through combination of different stimuli [233]. TGF-β signaling is needed for the development of both Tregs and Th17 cells from naïve T cells but, IL-6 signaling favors Th17 development [233], [234] (Fig. 2). RA switches this balance towards Treg by blocking the IL-6 receptor-induced signaling needed for Th17 development [35], [234]. When ATRA is absent or its level is low, IL-6 receptor signaling is not blocked and elevated IL-6 signaling transforms naive T cells into effector proinflammatory Th17 cells [225], [235], [236] (Fig. 2).
ATRA also suppresses NF-κB activity and associated cytokine discharge through its inhibitory activity on the ubiquitin proteasome system (UPS) [212], [237], [238]. In the inflammatory tissue environment, with reduced amount or lack of ATRA, the UPS activation through TRAF and cytokine signaling is not properly inhibited and as a result, UPS-mediated NF-κB activity increases in the adaptive immune cells leading to the development of naive T cells into Th17 cells [41], [212], [234], [237], [239], [240], [241]. Low ATRA levels are initially responsible for diverting the immune response towards inflammation [234]. Once inflammation has started, Th17 cells produce proinflammatory cytokines that further aggravate the inflammation and tissue damage [54], [226], [233], [242] (Fig. 2).
One of the most important differentiating transcription factors for Treg is forkhead box protein 3 (FoxP3) while RA Related Orphan Receptor gamma (RORγt) is important for the development of Th17 cells [233] (Fig 2). FoxP3 is crucial for development of Treg, and its expression and stability need RA signaling in cooperation with T cell receptor (TCR) and TGF-β signaling [243], [244], [245]. ATRA regulates differentiation of naive T cells to Treg cells through induction of FoxP3 transcription factor [229], [246], [247] and TGF-β potentiates this effect of ATRA [244]. Hence, RA blocks the differentiation of Th17 cells and induces Tregs in the presence of TGF-β by reciprocally down-regulating RORγt and activating FoxP3 expression in T cells [233], [248], [249]. Like a regulatory switch, ATRA largely inhibits Th17-mediated inflammation and autoimmunity [234], [241].
The immune dysregulation pathogenesis observed in COVID-19 is likely due to an imbalance between Treg and pathological Th17 cells (Fig 2). In COVID-19, there is a decrease in Treg and an increase in the Th17 cells [119], [250], [251], [252]. The increase in the number and activity of these effector Th17 cells has been blamed for excessive inflammatory cytokine release and systemic effects in COVID-19 [119], [250], [252]. However, the role of inflammatory cytokines is secondary to the initial role of retinoid signaling, and cytokines have a synergistic effect on further synthesis and release of more cytokines due to their bidirectional interactions in activating NF-κB [200], [253]. Because of this synergistic and bilateral interaction, a vicious cycle occurs in the synthesis and secretion of proinflammatory cytokines [128], [179], [217], [254], [255]. This mechanism causes an uncontrolled increase in cytokine synthesis and activity [255], [256]. Cytokines are therefore responsible for aggravating the secretion of secondary inflammatory mediators leading to systemic inflammation and multi-organ damage [46], [119].
In the studies conducted within the scope of the COVID Human Genetic Effort Project, in addition to the defects in the Type I interferon synthesis, autoantibodies against Type I interferons in severe COVID-19 patients were detected [257]. This reaction was more common in elderly males. However, this autoimmune response can be seen in many COVID-19 patients, including children [257], [258], [259]. Likewise, the development of autoantibodies against many host antigens other than Type I interferon has been shown in severe COVID-19 patients and dozens of other diseases associated with such autoimmune responses [260], [261], [262], [263].
By favoring an increase in the number and activity of Tregs, RA signaling provides self-tolerance to host antigens [99], [226], [264], [265]. In autoimmune and inflammatory pathogenesis, self-tolerance is impaired due to an imbalance between Treg and Th17 cells in favor of Th17 cells [234], [244]. Loss of control in self-tolerance ultimately results in autoimmune diseases [247], [265], [266], [267], [268], [269]. Proper RAR signaling prevents inflammatory and autoimmune diseases through changing this balance towards Tregs [187], [241], [270], [271]. Administration of RA and TGF-β together prevents experimental autoimmune diabetes with indication of involvement of regulatory B lymphocytes [272].
PRRs, such as TLR and RIG-I-like receptors, are highly expressed on neutrophil, monocyte, macrophage, and dendritic cells [246], [273], [274]. These receptors are innate immune receptors that recognize pathogen associated molecular patterns such as bacterial and viral RNA and DNA [19], [273], [275]. Host immune cells recognize self-DNA fragments in autoimmune diseases such as lupus, psoriasis, arthritis, and multiple sclerosis [38], [228], [267], [276], [277], [278], [279], [280]. In COVID-19, autoimmune pathogenesis proceeds with the severe inflammatory process that develops due to dysregulation between Treg and Th17 cells [126], [258], [261], [262]. This autoimmune response in COVID-19 may well be caused, at least partially, by the dysregulation and loss of self-tolerance in the immune system that develops most likely as a result of retinol depletion and retinoid signaling disorders. The decrease in Treg cell numbers due to the low levels of retinol and ATRA in COVID-19 can lead to a breakdown of immune tolerance and development of autoimmune pathogenesis [233], [236], [281].
6.2. The role of retinol depletion and retinoid signaling disorder on cytokine storm in COVID-19
The excessive cytokines released by the overactive monocytes, macrophages, and especially dendritic and Th17 cells leads to systemic inflammatory response and serious damage to the organ systems [46], [119], [215], [256], [282]. In severe COVID-19 cases, macrophage activation syndrome, strong IL-6 and IL-10 production, and low Type I IFN expression are observed and adversely related to the disease course and prognosis [121], [129], [177], [218], [221], [283]. The severity and mortality of COVID-19 have strongly been associated with increased expression of various cytokines and immune-related molecules including IL-17, IL-15, IL-10, IL-6, sTNFRSF1A (soluble tumor necrosis factor (TNF) receptor (TNFR superfamily member 1A), CXCL9 (C-X-C motif chemokine ligand 9), and lactoferrin [222], [282]. In addition, among the hospitalized COVID-19 patients, the amount of IL-10, IL-15, sST2 (soluble suppression of tumorigenicity 2), and sTNFRSF1A were consistently higher in died patients versus recovered ones [222].
A recent large scale, multicenter, multi-tissue genetically regulated gene expression profiling study have identified high IL-10 receptor beta (IL10RB) expression as the key regulator of COVID susceptibility and severity [141]. This study showed that IL10RB is significantly upregulated in individuals susceptible to COVID-19 hospitalization and downregulated in non-hospitalized infected individuals. Therefore, IL10RB expression level was found to be the best prediction of the worst COVID-19 outcome. The same study also demonstrated 10–100 times increased expression of some pathway markers including dendritic cell migration, chemokine and cytokine receptor signaling, and inflammatory response, in severe COVID-19 cases and found that retinol and immunosuppressant drug Azothioprine are significantly associated with strong decrease of COVID-19 infection [141]. Furthermore, bioinformatics-based simulation analysis has identified MAPK1, IL10, EGFR, ICAM1, MAPK14, CAT, and PRKCB, which are signaling molecules important in the immune cell function, as the possible targets of VitA for the treatment of COVID-19 [284]. In addition to the excessive cytokine expression, complement activation has also been indicated in the COVID-19 pathogenesis and may adversely affect the outcome of infection [285], [286]. These and other similar studies indicate that the COVID-19 pathogenesis is prominent with systemic highly dysregulated cytokines, chemokines, complement, and other related inflammatory mediators.
6.3. The role of retinol depletion and retinoid signaling disorder on interferon production in COVID-19
In severe COVID-19 infections, there is a profound defect in the Type I IFN synthesis [214], [219]. The defect in the Type I IFN synthesis was initially attributed to some gene mutations seen in COVID-19 patients [220], [259], [287]. However, it is unlikely that the same type of mutations seen in this study conducted in diverse patient groups with various genetic backgrounds in wide geographical areas, was the main cause of this defect in Type I IFN production. In addition, a mutation affecting the synthesis of such vital protective molecule might have very likely been fatal early in life as a consequence of getting exposed to various viral infections. However, other studies showed that these mutations could only be seen in 3.5% of severe COVID-19 patients and that these mutations could not fully explain the defect in the Type I interferon synthesis [216].
Type I IFNs are the strongest endogenous antiviral mediators in host defense to clear the virus from the body [288], [289]. They orchestrate antiviral immune response to prevent viral replication through induction of gene expression involving antigen presentation leading to activation of virus specific T cells, including cytotoxic and helper T cells, and T cell-dependent B cell activation and antibody production [290], [291], [292]. For example, IFN-α potentiates the cytotoxic effects of tumor necrosis factor alpha (TNF-α) by suppressing TNF-induced activation of NF-кB and AP-1 [293]. In this way, Type I IFN provides the development of a strong and effective antiviral immune response [288], [291], [292]. For this reason, the lack of Type I interferon synthesis has an important causality in the impairment of antibody response, persistent infection, and reinfection in COVID-19 and therefore play an important role in the immunopathogenesis of COVID-19 infection [294].
Viral RNA is recognized by PRRs, particularly by TLR3, TLR7, and RIG-I-like receptor (RLR) in immune and infected cells [19], [20], [195], [295], [296]. This recognition of viral RNA leads to synthesis of Type I IFNs through RA signaling [288], [296], [297], [298], [299]. Type I interferon synthesis is particularly prominent in plasmacytoid dendritic cells in the lungs where ATRA is also highly synthesized [18], [19], [196], [266], [300], [301]. Some viruses including SARS-CoV2 have developed mechanisms that evade and antagonize the RIG-I-mediated IFN production pathway [18], [55], [302].
VitA and its derivatives provide their main protective effects against viral infections through upregulating Type I IFN synthesis [51]. They also strengthen the mucosal immunity by increasing secretory IgA production and mucosal barrier formation [288], [299]. In the case of COVID-19, Riva et al., have reported that the expression of genes within RAR signaling pathway were significantly decreased in Vero E6 culture cells infected with SARS-CoV-2 [140]. They further found that RA receptor agonists inhibited SARS-CoV-2 replication, and that this inhibition was reversed with RA receptor antagonist molecules indicating the importance of RA receptor signaling in COVID-19 pathogenesis [140]. These data demonstrate that retinol metabolism and retinoid signaling can function as a critical host-pathogen-interaction circuit in controlling SARS-CoV-2 infection, which indicates that VitA supplementation may help fight COVID-19 [41], [59], [303].
6.4. Retinoid signaling disorders and dysbiosis in COVID-19
Dysbiosis is an imbalance of intestinal microbiota and has been the subject of numerous studies in recent years with increasing popularity in inflammatory pathogenesis research [304]. Many of such studies show that dysbiosis is associated with inflammatory pathogenesis [304], [305]. So far, no concrete evidence has been found to be the main cause of dysbiosis-related inflammation. As a result, no compound or drug has been found for effective treatment of inflammation through the dysbiosis mechanism other fecal microbiota transplantation for antibiotic resistant Clostridioides difficile infections [306], [307]. Due to this association between inflammatory events and dysbiosis, some researchers have suggested that the excessive inflammatory mechanism in COVID-19 may have been caused by the disorder of the intestinal microbiota in patients [308]. While some researchers have even recommended prebiotics and probiotics for the treatment of the inflammatory process in COVID-19, others have recommended fecal transplantation [309], [310], [311].
In COVID-19, the inflammatory knot is dissolved in the intestines and surrounding lymphoid tissues. We believe that rather than the change in the amount and scope of microbiota, it is the impaired RAR signaling that triggers inflammatory mechanisms leading to immune dysregulation and the resulting increased activity of Th17 cells in the intestine and mesenteric lymphoid tissues, and the consequent secretion of excessive cytokines and other inflammatory mediators. Previous studies have shown that RA plays an important role in modulating the function of dendritic cells to induce immune tolerance in the GI tract [30], [280], [300], [312]. We believe that this might also be true in the case of COVID19 infection and that the primary triggering role in inflammatory pathogenesis is the impaired RAR signaling while cytokines and dysbiosis are responsible for the aggravation of the inflammatory process. Therefore, VitA supplementation may help turn off the inflammatory response and help decrease the severe immunopathogenesis at initial stage of COVID-19 infection [41], [59], [141].
6.5. Biphasic immunopathogenesis in COVID-19 and bimodal effect of VitA
In a balanced functional immune response, the production of Type I IFNs in the early stage of the infection is essential to arrest the viral replication until the delayed adaptive immune response develops and produces B and T cell-mediated specific immunity [288], [290], [292]. Maintaining a balanced transition between these two systems is associated with a good prognosis and is essential for the host survival [288], [291]. However, in severe COVID-19 cases, there is a characteristic dysregulated biphasic immune response. The first phase is the normal immune response at the beginning of the infection when there is enough retinoic signaling, which activates RIG-I signaling that leads to the high level of Type I IFN synthesis. The second phase is the disruption of Type I IFN synthesis and overactivation of the Th17 arm of the adaptive immune system leading to the release of excessive inflammatory cytokines that causes systemic organ damage [41], [59], [124], [131], [216], [313]. The resulting process is the severe COVID-19 picture characterized by SIRS, ARDS, hypoxia, and systemic organ damage leading to mortality [118], [131], [225].
The balance between Type I interferon synthesis and inflammatory cytokine discharge is a main factor determining the prognosis in COVID-19 [124], [216], [314]. The biphasic immune pathogenesis of COVID-19 is correlated well with the clinical manifestation of the VitA's mode of action [143]. Even though retinoid signaling has not well been studied in the COVID-19 pathogenesis, the current data and understanding indicate that retinoid signaling [315] might be an important driving force in this dysregulated biphasic immune response observed in the COVID-19. The depletion of VitA store in the body during COVID-19 infection results in a deregulated balance between Treg and Th17 cells, where the number and activity of Treg cells decrease, while those of effector Th17 cells increase, leading to increased production of inflammatory mediators and cytokines resulting in systemic organ damage [217], [256], [316], [317], [318]. The suppressed innate immune response and hyperactivated adaptive immune response observed in COVID-19 correlate well with the metabolic phenotype of low levels of VitA and ATRA [31], [246]. Therefore, there is a need to further study the importance of this correlation and if proven true, it will have a huge application potential not only in COVID-19 but also in other diseases such as autoimmune and infectious diseases, sepsis, cancer, and psychiatric disorders.
VitA shows a bimodal activity in the inflammatory mechanisms. While ATRA, the VitA's active signaling molecule, at high doses, promotes production of Type I IFNs and suppresses inflammatory processes, at low doses, this suppression mechanism is disabled leading to an increased inflammation [241], [312] (Fig. 2). This change seen in VAD is fully compatible with immunological dysregulation and biphasic immune pathogenesis seen in COVID-19 patients [251], [312]. In the body, it is not necessary to deplete VitA completely, rather, a low VitA level that falls below a threshold level in the serum is sufficient to trigger inflammatory pathway [27], [319]. The changes in the immune response and clinical findings seen in COVID-19 are very similar to those seen in VAD [246], [247]. Therefore, the inflammatory process in COVID-19 may well be prevented or managed through reconstituting the RA signaling since the cytokines and other inflammatory mediators can be secondary to the consequence of impaired retinoid signaling.
The sudden and unexpected improper transition between these two components of the immune system in COVID-19 has been in the core of scientific and medical interest. Although the dose-dependent bimodal activity of ATRA in the immune system, especially on inflammatory processes, has been previously studied [192], [320], it has not been investigated in the case of COVID-19. We believe that this sudden and unexpected transition between the innate and the adaptive components of the immune system in COVID-19 is ignited by the retinol depletion and consequently impaired retinoid signaling disorder [59]. Depletion of retinol causes a transition from a proper innate immune response to the exacerbated cytokine storm [41]. In this way, RA signaling acts as a switch in balancing the innate and adaptive immune response [186] and the signaling for this switch is provided by ATRA [280]. Therefore, we believe that the immune system dysregulation and the biphasic immunological pathogenesis of COVID-19 are associated with retinoid signaling disorder.
7. Vitamin D effect and retinoid signaling mechanisms in COVID-19
Vitamin D (VitD), like VitA, is a steroid hormonal molecule important in many aspects of cellular physiology including bone metabolism, cell proliferation, regulation of innate and adaptive immunity, and preventive effect on cardiovascular and neurodegenerative diseases [321], [322]. It has been shown to modulate innate and adaptive immunity in a similar way as VitA does [323]. The role of VitD has been recognized in the prophylaxis and treatment of COVID-19 [324], [325]. At high doses, VitD has been shown to decrease the severity and provide therapeutic benefit in the treatment of COVID-19 [326]. However, VitD supplementation did not seem to be effective in hospitalized seriously ill COVID-19 patients [327], which we believe is due to the depleted retinol and subsequently dysregulated retinoid signaling mechanisms [315]. The lack of response to the supplementation of VitD in these severe COVID-19 patients may have well been due to the dysregulated retinoid signaling [327] because VitD exerts its effect through retinoid signaling mechanism [321]. In this sense, an optimum response would be accomplished when the proper signaling cascades through both of VitD and retinoids converge.
VitD exerts its biological action through binding to its receptor VitD receptor (VDR) and activating downstream transcriptional activation [321], [328]. The VitD signaling converges with the retinoid signaling. VDR can heterodimerize with RXR and bind to VDR elements (VDRE) on the target genes [321], [322]. Binding of VitD to VDR/RXR heterodimers on the DNA recruits other regulatory factors to activate the transcription of the target genes [93], [94], [324], [329]. In this way, the VitD signaling converges with that of VitA through the use of common RXR in modulating the expression of downstream genes [94].
VDR is expressed in various immune cells including monocytes, dendritic cells, T and B cells [321], [323]. VitD exerts similar effect in immune cells as VitA does. In innate immune cells, it upregulates the expression of the PRR that leads to an increased innate immune response against infections [323], [330]. It also induces anti-inflammatory response through development of Tregs and suppresses development of Th17 cells, proinflammatory cytokines such as IL-17 and IL-23, and autoimmunity [330], [331].
A recent transcriptomic analysis shows that the metabolism and signaling of both VitD and RARs have been dysregulated in COVID-19 patients [315]. This study analyzed the transcriptomic data from bronchoalveolar lavage fluid (BALF) of COVID-19 patients and controls and showed that, in addition to the VitD signaling components, the retinoid signaling components were also downregulated in the lungs of COVID-19 patients [315]. Among the downregulated genes, were the expression of a large set of genes, including Vitamin D receptor (VDR), RXR, and CYP27A1. The RXR is an essential component of the VitD and retinoid signaling pathways [91], [92] while CYP27A1, a member of CYP450 protein family, is essential in metabolism of VitD and is strongly upregulated through activated retinoid signaling [315], [332]. This downregulation of CYP27A1 in these patients is a strong indication of dysregulated retinoid signaling. The majority of the downregulated genes were enriched in the immune cells. Among these genes, the top predictors belonged to NF-кB/cytokine signaling and cell cycle regulators [315]. Most of these genes are also regulated through retinoid signaling pathways indicating interdependence of the two signaling pathways.
The therapeutic and prophylactic effect of VitD on COVID-19 appears to be through the retinoid signaling mechanism. Despite the large number of studies on VitD in the management and treatment of COVID-19, unfortunately, adequate clinical studies have not been conducted on VitA, which forms the basis of the retinoid signaling mechanism. The mechanism of retinoid signaling provided by VitA and VitD is intriguing, and more research is needed to identify their role in the pathogenesis of COVID-19. Therefore, a detailed investigation of the roles of VitA and VitD signaling mechanisms will be rewarding in understanding the pathogenesis, management, and treatment of COVID-19.
8. Conclusion
VAD is a nutritional immunodeficiency. Its relationship with immunosuppression has been clearly demonstrated and its role in regulating the primary immune defense and in keeping the inflammatory process under control during infections has been clearly demonstrated [29], [209], [300]. The prophylactic and therapeutic values of VitA and its derivatives in infectious and inflammatory processes should carefully be reviewed, including monitoring serum retinol levels during infections, especially in COVID-19 infections.
While COVID-19 infection causes very mild or no symptoms in some individuals, it results in very severe clinical pictures and death in other individuals [121], [179], [333]. Although some comorbidity conditions and diseases that predispose individuals to severe clinical pictures have been recognized, the main reasons for predisposition have not been clearly identified [118], [119], [334]. We hypothesize that the state of retinol storage, the changes in the activities of the enzymes in RA metabolic pathway, and the retinoid signaling have a central role in severe COVID-19 immunopathogenesis [46], [59]. Diseases and conditions that cause comorbidity in COVID-19, such as malnutrition, chronic liver and lung diseases, chronic kidney disease, obesity, hepatosteatosis, chronic inflammatory autoimmune and rheumatic diseases, malignancies, organ transplants, and senility, also drain body's retinol reserves [22], [41], [335]. Since retinol stores are weakened in these diseases, the immune defense against SARS-CoV-2 is also impaired.
The Type I interferon production and immune response against SARS-CoV-2 in the early stage of COVID-19 infection is closely related to the retinol depot and RA levels [58]. Good liver retinol reserve and especially serum levels of RAs at the therapeutic range may be an effective strategy against SARS-CoV-2. Early depletion of retinol depot in COVID-19 may cause an aggravated clinical picture as previously observed for rotaviruses [28]. Therefore, VitA and carotenoids can work for COVID-19 prophylaxis and similar other infections [51]. However, well-planned controlled clinical studies are needed to clarify the role of VitA or RA derivatives in the pathogenesis and treatment of COVID-19.
With large-scale epidemiological studies, determining low VitA levels on a social scale and feeding the society with foods rich in retinoids and carotenoids, giving prophylactic retinol supplements, when necessary, as in previous measles epidemics [336] will also ensure the protection of the society from many viral infections, especially from COVID-19. Another important issue is related to vaccination programs. Vaccines aim to induce development of pathogen-specific adaptive immunity by directly or indirectly introducing pathogen-specific antigens, which are processed and presented to T cells, which then induce T-cell dependent antibody production and immune memory [337]. The whole procedure ends up generating antigen-specific long-lived protective memory B and T lymphocytes, which also depends on RA signaling [28], [72], [190], [301]. In addition, RA can be used as adjuvant to increase the efficacy of vaccines [72]. Therefore, it is important to access the level of retinoid and RA signaling to design better strategies for vaccine efficacy [72]. Hence, by checking VitA levels before vaccination, the effectiveness of vaccines can be increased by supplementing VitA to those with VAD [338]. During the clinical course of COVID-19, the decrease in retinol levels and the susceptibility of VAD to the disease make prophylactic and adjuvant applications with VitA even more important. We believe that retinol and retinoic acid signaling in the context of COVID-19 infection and increasing effectiveness of vaccines merit further study.
VitA and VitD both exert their physiologic effects through retinoid receptor signaling, which is wide spread in various tissue and organ systems. Both have similar effect especially on CNS and immune system [26], [76], [321], [330]. Their immunomodulatory effect is noteworthy as it may have implications in many human illnesses including autoimmune and COVID-19. The therapeutic effect of VitD in COVID-19 has been recognized but that of VitA has been overlooked [323], [339]. Even though there has not yet been any experimental study on the dynamics of the retinoic signaling in COVID-19, a recent study by George et al., [315] supports our hypothesis. We believe that VitA and VitD signaling have similar but central role in the pathogenesis of COVID-19, and therefore needs further investigation, the results of which might provide a useful knowledge platform for development of vaccines and treatment strategies not only for COVID-19 but also for various other illnesses.
Detailed clinical studies of retinol and RA metabolism and retinoid signaling in COVID-19 may also prove useful in the development of effective vaccines and effective adjuvant applications against COVID-19. Likewise, the basic condition for developing effective vaccines and drugs depends on a clear understanding of how the virus affects the immune system. Based on the available data, we can predict that enriching the plasma retinol store can both strengthen the innate immune system and inhibit the severe inflammatory process of the adaptive immune system [14], [141], [185], [301]. Because of their potent immunomodulatory activities, retinol, RAs, some specific CYP450 inhibitors (RAMBAs), and RAR/RXR receptor agonists (rexinoids) can act as prophylactic and therapeutic agents against COVID-19 [187], [242], [336]. However, although we strongly believe that our hypothesis provided here is correct, it should not be considered as a recipe for treatment of COVID-19 without experimental data support and that the use of vitA and related supplements should be under the supervision of medical professionals.
Disclosure statement
All authors declare no conflict of interest.
Credit author statement
Aziz Rodan Sarohan wrote initial draft, discussed, reviewed, and revised throughout the process.
Murat Kızıl discussed, reviewed, and provided feedback for revised draft.
Ahmet Çağkan İnkaya discussed, reviewed, and provided feedback for revised draft.
Shokhan Mahmud discussed, reviewed, and provided feedback for revised draft.
Muhammad Akram discussed, reviewed, and provided feedback for revised draft.
Osman Cen discussed, reviewed, reorganized, and wrote the revised draft.
References
- 1.Lai C.C., Wang C.Y., Wang Y.H., Hsueh S.C., Ko W.C., Hsueh P.R. Global epidemiology of coronavirus disease 2019 (COVID-19): disease incidence, daily cumulative index, mortality, and their association with country healthcare resources and economic status. Int. J. Antimicrob. Agents. 2020;55(4) doi: 10.1016/j.ijantimicag.2020.105946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jin Y., Yang H., Ji W., Wu W., Chen S., Zhang W., Duan G. Virology, epidemiology, pathogenesis, and control of COVID-19. Viruses. 2020;12(4) doi: 10.3390/v12040372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gavriatopoulou M., Ntanasis-Stathopoulos I., Korompoki E., Fotiou D., Migkou M., Tzanninis I.G., Psaltopoulou T., Kastritis E., Terpos E., Dimopoulos M.A. Emerging treatment strategies for COVID-19 infection. Clin. Exp. Med. 2021;21(2):167–179. doi: 10.1007/s10238-020-00671-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trivedi N., Verma A., Kumar D. Possible treatment and strategies for COVID-19: review and assessment. Eur. Rev. Med. Pharmacol. Sci. 2020;24(23):12593–12608. doi: 10.26355/eurrev_202012_24057. [DOI] [PubMed] [Google Scholar]
- 5.O'Leary K. COVID-19 vaccine and blood clotting. Nat. Med. 2021 doi: 10.1038/d41591-021-00025-5. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 6.D'Agostino V., Caranci F., Negro A., Piscitelli V., Tuccillo B., Fasano F., Sirabella G., Marano I., Granata V., Grassi R., Pupo D., Grassi R. A rare case of cerebral venous thrombosis and disseminated intravascular coagulation temporally associated to the COVID-19 vaccine administration. J. Personal. Med. 2021;11(4) doi: 10.3390/jpm11040285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Long B., Bridwell R., Gottlieb M. Thrombosis with thrombocytopenia syndrome associated with COVID-19 vaccines. Am. J. Emerg. Med. 2021;49:58–61. doi: 10.1016/j.ajem.2021.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seneff S., Nigh G. Worse than the Disease? Reviewing some possible unintended consequences of the mRNA vaccines against COVID-19. Int. J. Vaccine Theory Practice Res. 2021;2(1):402–443. [Google Scholar]
- 9.Cascella M., Rajnik M., Aleem A., Dulebohn S.C., Napoli R.Di. StatPearls Publishing. Copyright © 2021, StatPearls Publishing LLC.; Treasure Island (FL): 2021. Features, Evaluation, and Treatment of Coronavirus (COVID-19), StatPearls. [PubMed] [Google Scholar]
- 10.Hagens A., Inkaya A., Yildirak K., Sancar M., van der Schans J., Acar Sancar A., Ünal S., Postma M., Yegenoglu S. COVID-19 vaccination scenarios: a cost-effectiveness analysis for Turkey. Vaccines. 2021;9(4) doi: 10.3390/vaccines9040399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sinha P., Kumar S., Chandra C. Strategies for ensuring required service level for COVID-19 herd immunity in Indian vaccine supply chain. Eur. J. Oper. Res. 2021 doi: 10.1016/j.ejor.2021.03.030. PubMed is: https://pubmed.ncbi.nlm.nih.gov/33776195/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou B., Kojima S., Kawamoto A., Fukushima M. COVID-19 pathogenesis, prognostic factors, and treatment strategy: urgent recommendations. J. Med. Virol. 2021;93(5):2694–2704. doi: 10.1002/jmv.26754. [DOI] [PubMed] [Google Scholar]
- 13.Brown C.C., Esterhazy D., Sarde A., London M., Pullabhatla V., Osma-Garcia I., Al-Bader R., Ortiz C., Elgueta R., Arno M., de Rinaldis E., Mucida D., Lord G.M., Noelle R.J. Retinoic acid is essential for Th1 cell lineage stability and prevents transition to a Th17 cell program. Immunity. 2015;42(3):499–511. doi: 10.1016/j.immuni.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liang Y., Yi P., Wang X., Zhang B., Jie Z., Soong L., Sun J. Retinoic acid modulates hyperactive T cell responses and protects vitamin A-deficient mice against persistent lymphocytic choriomeningitis virus infection. J. Immunol. (Baltimore, Md. : 1950) 2020;204(11):2984–2994. doi: 10.4049/jimmunol.1901091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Masters P.S. The molecular biology of coronaviruses. Adv. Virus Res. 2006;66:193–292. doi: 10.1016/S0065-3527(06)66005-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Naqvi A.A.T., Fatima K., Mohammad T., Fatima U., Singh I.K., Singh A., Atif S.M., Hariprasad G., Hasan G.M., Hassan M.I. Insights into SARS-CoV-2 genome, structure, evolution, pathogenesis and therapies: structural genomics approach, biochimica et biophysica acta. Mol. Basis Dis. 2020;1866(10):165878. doi: 10.1016/j.bbadis.2020.165878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harrison A.G., Lin T., Wang P. Mechanisms of SARS-CoV-2 transmission and pathogenesis. Trends Immunol. 2020;41(12):1100–1115. doi: 10.1016/j.it.2020.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dixon D.C.K., Ratan C., Nair B., Mangalath S., Abraham R., Nath L.R. RNA sensors as a mechanism of innate immune evasion among SARS-CoV2, HIV and Nipah viruses. Curr. Protein Peptide Sci. 2021 doi: 10.2174/1389203722666210322142725. https://pubmed.ncbi.nlm.nih.gov/33749559/. PMID: 33749559. [DOI] [PubMed] [Google Scholar]
- 19.Yamada S., Shimojima M., Narita R., Tsukamoto Y., Kato H., Saijo M., Fujita T. RIG-I-like receptor and toll-like receptor signaling pathways cause aberrant production of inflammatory Cytokines/Chemokines in a severe fever with thrombocytopenia syndrome virus infection mouse model. J. Virol. 2018;92(13) doi: 10.1128/JVI.02246-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kawai T., Akira S. Toll-like receptor and RIG-I-like receptor signaling. Ann. N. Y. Acad. Sci. 2008;1143:1–20. doi: 10.1196/annals.1443.020. [DOI] [PubMed] [Google Scholar]
- 21.Liu Y., Olagnier D., Lin R. Host and viral modulation of RIG-I-mediated antiviral immunity. Front. Immunol. 2016;7:662. doi: 10.3389/fimmu.2016.00662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Motomura K., Ohata M., Satre M., Tsukamoto H. Destabilization of TNF-alpha mRNA by retinoic acid in hepatic macrophages: implications for alcoholic liver disease. Am. J. Physiol. Endocrinol. Metab. 2001;281(3) doi: 10.1152/ajpendo.2001.281.3.E420. E420-9. [DOI] [PubMed] [Google Scholar]
- 23.Okamoto M., Tsukamoto H., Kouwaki T., Seya T., Oshiumi H. Recognition of viral RNA by pattern recognition receptors in the induction of innate immunity and excessive inflammation during respiratory viral infections. Viral Immunol. 2017;30(6):408–420. doi: 10.1089/vim.2016.0178. [DOI] [PubMed] [Google Scholar]
- 24.Onomoto K., Onoguchi K., Yoneyama M. Regulation of RIG-I-like receptor-mediated signaling: interaction between host and viral factors. Cell. Mol. Immunol. 2021;18(3):539–555. doi: 10.1038/s41423-020-00602-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chow K.T., Gale M., Jr., Loo Y.M. RIG-I and other RNA sensors in antiviral immunity. Annu. Rev. Immunol. 2018;36:667–694. doi: 10.1146/annurev-immunol-042617-053309. [DOI] [PubMed] [Google Scholar]
- 26.Ross A.C., Stephensen C.B. Vitamin a and retinoids in antiviral responses. FASEB J. 1996;10(9):979–985. [PubMed] [Google Scholar]
- 27.Stephensen C.B. Vitamin a, infection, and immune Function*. Annu. Rev. Nutr. 2001;21(1):167–192. doi: 10.1146/annurev.nutr.21.1.167. [DOI] [PubMed] [Google Scholar]
- 28.Chattha K.S., Kandasamy S., Vlasova A.N., Saif L.J. Vitamin a deficiency impairs adaptive B and T cell responses to a prototype monovalent attenuated human rotavirus vaccine and virulent human rotavirus challenge in a gnotobiotic piglet model. PloS one. 2013;8(12) doi: 10.1371/journal.pone.0082966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wiedermann U., Hanson L.A., Kahu H., Dahlgren U.I. Aberrant T-cell function in vitro and impaired T-cell dependent antibody response in vivo in vitamin A-deficient rats. Immunology. 1993;80(4):581–586. [PMC free article] [PubMed] [Google Scholar]
- 30.Kim C.H. Regulation of FoxP3 regulatory T cells and Th17 cells by retinoids. 2008;2008 doi: 10.1155/2008/416910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Raverdeau M., Mills K.H. Modulation of T cell and innate immune responses by retinoic acid. J. Immunol. (Baltimore, Md. : 1950) 2014;192(7):2953–2958. doi: 10.4049/jimmunol.1303245. [DOI] [PubMed] [Google Scholar]
- 32.Casadevall A., Pirofski L.A. In fatal COVID-19, the immune response can control the virus but kill the patient. Proc. Natl Acad. Sci. USA. 2020;117(48):30009–30011. doi: 10.1073/pnas.2021128117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dorward D.A., Russell C.D., Um I.H., Elshani M., Armstrong S.D., Penrice-Randal R., Millar T., Lerpiniere C.E.B., Tagliavini G., Hartley C.S., Randle N.P., Gachanja N.N., Potey P.M.D., Dong X., Anderson A.M., Campbell V.L., Duguid A.J., Al Qsous W., BouHaidar R., Baillie J.K., Dhaliwal K., Wallace W.A., Bellamy C.O.C., Prost S., Smith C., Hiscox J.A., Harrison D.J., Lucas C.D. Tissue-specific immunopathology in fatal COVID-19. Am. J. Respir. Crit. Care Med. 2021;203(2):192–201. doi: 10.1164/rccm.202008-3265OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Elias K.M., Laurence A., Davidson T.S., Stephens G., Kanno Y., Shevach E.M., O’Shea J.J. Retinoic acid inhibits Th17 polarization and enhances FoxP3 expression through a Stat-3/Stat-5 independent signaling pathway. Blood. 2008;111(3):1013–1020. doi: 10.1182/blood-2007-06-096438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lu L., Lan Q., Li Z., Zhou X., Gu J., Li Q., Wang J., Chen M., Liu Y., Shen Y., Brand D.D., Ryffel B., Horwitz D.A., Quismorio F.P., Liu Z., Li B., Olsen N.J., Zheng S.G. Critical role of all-trans retinoic acid in stabilizing human natural regulatory T cells under inflammatory conditions. Proc. Natl Acad. Sci. USA. 2014;111(33):E3432–E3440. doi: 10.1073/pnas.1408780111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chatterjee S.K., Saha S., Munoz M.N.M. Molecular pathogenesis, immunopathogenesis and novel therapeutic strategy against COVID-19. Front. Mol. Biosci. 2020;7:196. doi: 10.3389/fmolb.2020.00196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gebhardt A., Laudenbach B.T., Pichlmair A. Discrimination of self and non-self ribonucleic acids. J. Interf. Cytokine ReS. 2017;37(5):184–197. doi: 10.1089/jir.2016.0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hua Z., Hou B. TLR signaling in B-cell development and activation. Cell. Mol. Immunol. 2013;10(2):103–106. doi: 10.1038/cmi.2012.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jin B., Sun T., Yu X.H., Yang Y.X., Yeo A.E. The effects of TLR activation on T-cell development and differentiation. Clin. Dev. Immunol. 2012;2012 doi: 10.1155/2012/836485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kong L., Sun L., Zhang H., Liu Q., Liu Y., Qin L., Shi G., Hu J.H., Xu A., Sun Y.P., Li D., Shi Y.F., Zang J.W., Zhu J., Chen Z., Wang Z.G., Ge B.X. An essential role for RIG-I in toll-like receptor-stimulated phagocytosis. Cell Host Microbe. 2009;6(2):150–161. doi: 10.1016/j.chom.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 41.Sarohan A.R. COVID-19: endogenous retinoic acid theory and retinoic acid depletion syndrome. Med. Hypotheses. 2020;144 doi: 10.1016/j.mehy.2020.110250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Allenby G., Bocquel M.T., Saunders M., Kazmer S., Speck J., Rosenberger M., Lovey A., Kastner P., Grippo J.F., Chambon P., et al. Retinoic acid receptors and retinoid X receptors: interactions with endogenous retinoic acids. Proc. Natl. Acad. Sci. U. S. A. 1993;90(1):30–34. doi: 10.1073/pnas.90.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Balmer J.E., Blomhoff R. Gene expression regulation by retinoic acid. J. Lipid Res. 2002;43(11):1773–1808. doi: 10.1194/jlr.r100015-jlr200. [DOI] [PubMed] [Google Scholar]
- 44.Cunningham T.J., Duester G. Mechanisms of retinoic acid signalling and its roles in organ and limb development. Nat. Rev. Mol. Cell Biol. 2015;16(2):110–123. doi: 10.1038/nrm3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ghyselinck N.B., Duester G. Retinoic acid signaling pathways. Development (Cambridge, England) 2019;146(13) doi: 10.1242/dev.167502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sarohan A.R. Systemic organ involvement and retinoid signaling disorder in COVID-19. Immunogenetics. 2021;6(3):1–6. [Google Scholar]
- 47.Yang L., Liu S., Liu J., Zhang Z., Wan X., Huang B., Chen Y., Zhang Y. COVID-19: immunopathogenesis and immunotherapeutics. Sign. Transduct. Targeted Therapy. 2020;5(1):128. doi: 10.1038/s41392-020-00243-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Halpin S., O'Connor R., Sivan M. Long COVID and chronic COVID syndromes. J. Med. Virol. 2021;93(3):1242–1243. doi: 10.1002/jmv.26587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lim M.A., Pranata R., Huang I., Yonas E., Soeroto A.Y., Supriyadi R. Multiorgan failure with emphasis on acute kidney injury and severity of COVID-19: systematic review and meta-analysis. Can. J. Kidney Health Dis. 2020;7 doi: 10.1177/2054358120938573. 2054358120938573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang F., Kream R.M., Stefano G.B. Long-term respiratory and neurological sequelae of COVID-19. Med. Sci. Monitor. 2020;26 doi: 10.12659/MSM.928996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Trasino S.E. A role for retinoids in the treatment of COVID-19? Clin. Exp. Pharmacol. Physiol. 2020;47(10):1765–1767. doi: 10.1111/1440-1681.13354. [DOI] [PubMed] [Google Scholar]
- 52.Iddir M., Brito A., Dingeo G., Fernandez Del Campo S.S., Samouda H., La Frano M.R., Bohn T. Strengthening the immune system and reducing inflammation and oxidative stress through diet and nutrition: considerations during the COVID-19 crisis. Nutrients. 2020;12(6) doi: 10.3390/nu12061562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hojyo S., Uchida M., Tanaka K., Hasebe R., Tanaka Y., Murakami M., Hirano T. How COVID-19 induces cytokine storm with high mortality. Inflammat. Regenerat. 2020;40:37. doi: 10.1186/s41232-020-00146-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu D., Yang X.O. TH17 responses in cytokine storm of COVID-19: an emerging target of JAK2 inhibitor fedratinib. J. Microbiol. Immunol. Infect. = Wei mian yu gan ran za zhi. 2020;53(3):368–370. doi: 10.1016/j.jmii.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xia H., Cao Z., Xie X., Zhang X., Chen J.Y., Wang H., Menachery V.D., Rajsbaum R., Shi P.Y. Evasion of type I interferon by SARS-CoV-2. Cell Rep. 2020;33(1) doi: 10.1016/j.celrep.2020.108234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shi Y., Wang Y., Shao C., Huang J., Gan J., Huang X., Bucci E., Piacentini M., Ippolito G., Melino G. COVID-19 infection: the perspectives on immune responses. Cell Death Different. 2020;27(5):1451–1454. doi: 10.1038/s41418-020-0530-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang C., Wu Z., Li J.W., Zhao H., Wang G.Q. Cytokine release syndrome in severe COVID-19: interleukin-6 receptor antagonist tocilizumab may be the key to reduce mortality. Int. J. Antimicrob. Agents. 2020;55(5) doi: 10.1016/j.ijantimicag.2020.105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.di Bari I., Franzin R., Picerno A., Stasi A., Cimmarusti M.T., Di Chiano M., Curci C., Pontrelli P., Chironna M., Castellano G., Gallone A., Sabbà C., Gesualdo L., Sallustio F. Severe acute respiratory syndrome coronavirus 2 may exploit human transcription factors involved in retinoic acid and interferon-mediated response: a hypothesis supported by an in silico analysis. New Microb. New Infect. 2021;41 doi: 10.1016/j.nmni.2021.100853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sarohan A.R., Akelma H., Araç E., Aslan Ö. Retinol depletion in severe COVID-19. medRxiv. 2021 doi: 10.1016/j.nutos.2022.05.007. https://www.medrxiv.org/content/10.1101/2021.01.30.21250844v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wolf G. Retinoic acid homeostasis: retinoic acid regulates liver retinol esterification as well as its own catabolic oxidation in liver. Nut. Rev. 2001;59(12):391–394. doi: 10.1111/j.1753-4887.2001.tb06968.x. [DOI] [PubMed] [Google Scholar]
- 61.Ahmad S.M., Haskell M.J., Raqib R., Stephensen C.B. Markers of innate immune function are associated with vitamin a stores in men. J. Nutr. 2009;139(2):377–385. doi: 10.3945/jn.108.100198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gatica L., Alvarez S., Gomez N., Zago M.P., Oteiza P., Oliveros L., Gimenez M.S. Vitamin a deficiency induces prooxidant environment and inflammation in rat aorta. Free Radic. Res. 2005;39(6):621–628. doi: 10.1080/10715760500072214. [DOI] [PubMed] [Google Scholar]
- 63.Cañete A., Cano E., Muñoz-Chápuli R., Carmona R. Role of vitamin A/Retinoic acid in regulation of embryonic and adult hematopoiesis. Nutrients. 2017;9(2) doi: 10.3390/nu9020159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Das B.C., Thapa P., Karki R., Das S., Mahapatra S., Liu T.C., Torregroza I., Wallace D.P., Kambhampati S., Van Veldhuizen P., Verma A., Ray S.K., Evans T. Retinoic acid signaling pathways in development and diseases. Bioorg. Med. Chem. 2014;22(2):673–683. doi: 10.1016/j.bmc.2013.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hogarth C.A., Griswold M.D. The key role of vitamin a in spermatogenesis. J. Clin. Invest. 2010;120(4):956–962. doi: 10.1172/JCI41303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zadik Z., Sinai T., Zung A., Reifen R. Vitamin a and iron supplementation is as efficient as hormonal therapy in constitutionally delayed children. Clin. Endocrinol. 2004;60(6):682–687. doi: 10.1111/j.1365-2265.2004.02034.x. [DOI] [PubMed] [Google Scholar]
- 67.Pinnock C., Vitamin A. Nurs. J. India. 1991;82(11):307–308. [PubMed] [Google Scholar]
- 68.Reifen R. Vitamin a as an anti-inflammatory agent. Proc. Nutr. Soc. 2002;61(3):397–400. doi: 10.1079/PNS2002172. [DOI] [PubMed] [Google Scholar]
- 69.Benn C.S. Combining vitamin a and vaccines: convenience or conflict? Danish Med. J. 2012;59(1):B4378. [PubMed] [Google Scholar]
- 70.Huiming Y., Chaomin W., Meng M. Vitamin A for treating measles in children. Cochr. Database Syst. Rev. 2005;2005(4):Cd001479. doi: 10.1002/14651858.CD001479.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mehta S., Fawzi W. Effects of vitamins, including vitamin a, on HIV/AIDS patients. Vitam. Horm. 2007;75:355–383. doi: 10.1016/S0083-6729(06)75013-0. [DOI] [PubMed] [Google Scholar]
- 72.Tan X., Sande J.L., Pufnock J.S., Blattman J.N., Greenberg P.D. Retinoic acid as a vaccine adjuvant enhances CD8 T cell response and mucosal protection from viral challenge. J. Virol. 2011;85(16):8316–8327. doi: 10.1128/JVI.00781-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wiedermann U., Chen X.J., Enerbäck L., Hanson L.A., Kahu H., Dahlgren U.I. Vitamin a deficiency increases inflammatory responses. Scand. J. Immunol. 1996;44(6):578–584. doi: 10.1046/j.1365-3083.1996.d01-351.x. [DOI] [PubMed] [Google Scholar]
- 74.Imdad A., Mayo-Wilson E., Herzer K., Bhutta Z.A. Vitamin A supplementation for preventing morbidity and mortality in children from six months to five years of age. Cochr. Database Syst. Rev. 2017;3(3):Cd008524. doi: 10.1002/14651858.CD008524.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sommer A. Large dose vitamin a to control vitamin a deficiency. Int. J. Vitamin Nutr. Res. Suppl. = Internationale Zeitschrift fur Vitamin- und Ernahrungsforschung. 1989;(Suppl. 30):37–41. [PubMed] [Google Scholar]
- 76.Sommer A., Vyas K.S. A global clinical view on vitamin a and carotenoids. Am. J. Clin. Nutr. 2012;96(5):1204s-6s. doi: 10.3945/ajcn.112.034868. [DOI] [PubMed] [Google Scholar]
- 77.Kawaguchi R., Zhong M., Kassai M., Ter-Stepanian M., Sun H. Vitamin a transport mechanism of the multitransmembrane cell-surface receptor STRA6. Membranes. 2015;5(3):425–453. doi: 10.3390/membranes5030425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Senoo H., Kojima N., Sato M. Vitamin A-storing cells (stellate cells) Vitamins Hormon. 2007;75:131–159. doi: 10.1016/S0083-6729(06)75006-3. [DOI] [PubMed] [Google Scholar]
- 79.Blomhoff R., Blomhoff H.K. Overview of retinoid metabolism and function. J. Neurobiol. 2006;66(7):606–630. doi: 10.1002/neu.20242. [DOI] [PubMed] [Google Scholar]
- 80.Duester G. Retinoic acid synthesis and signaling during early organogenesis. Cell. 2008;134(6):921–931. doi: 10.1016/j.cell.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Clagett-Dame M., Plum L.A. Retinoid-regulated gene expression in neural development. Crit. Rev. Eukaryot. Gene Expr. 1997;7(4):299–342. doi: 10.1615/critreveukargeneexpr.v7.i4.20. [DOI] [PubMed] [Google Scholar]
- 82.Alatshan A., Kovács G.E., Aladdin A., Czimmerer Z., Tar K., Benko S. All-trans retinoic acid enhances both the signaling for priming and the glycolysis for activation of NLRP3 inflammasome in human macrophage. Cells. 2020;9(7) doi: 10.3390/cells9071591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ross A.C., Zolfaghari R. Cytochrome P450s in the regulation of cellular retinoic acid metabolism. Annu. Rev. Nutr. 2011;31:65–87. doi: 10.1146/annurev-nutr-072610-145127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Thatcher J.E., Isoherranen N. The role of CYP26 enzymes in retinoic acid clearance. Expert Opin. Drug Metab. Toxicol. 2009;5(8):875–886. doi: 10.1517/17425250903032681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Manikandan P., Nagini S. Cytochrome P450 structure, function and clinical significance: a review. Curr. Drug Targets. 2018;19(1):38–54. doi: 10.2174/1389450118666170125144557. [DOI] [PubMed] [Google Scholar]
- 86.Mukhopadhyay R. Human cytochrome P450s: the work of Frederick Peter guengerich. J. Biol. Chem. 2012;287(19):15798–15800. doi: 10.1074/jbc.O112.000003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Al Tanoury Z., Piskunov A., Rochette-Egly C. Vitamin a and retinoid signaling: genomic and nongenomic effects. J. Lipid Res. 2013;54(7):1761–1775. doi: 10.1194/jlr.R030833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rochette-Egly C. Retinoic acid signaling and mouse embryonic stem cell differentiation: cross talk between genomic and non-genomic effects of RA. Biochim. Biophys. Acta. 2015;1851(1):66–75. doi: 10.1016/j.bbalip.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 89.Mora J.R., Iwata M., von Andrian U.H. Vitamin effects on the immune system: vitamins a and D take centre stage. Nat. Rev. Immunol. 2008;8(9):685–698. doi: 10.1038/nri2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chen Z.P., Iyer J., Bourguet W., Held P., Mioskowski C., Lebeau L., Noy N., Chambon P., Gronemeyer H. Ligand- and DNA-induced dissociation of RXR tetramers. J. Mol. Biol. 1998;275(1):55–65. doi: 10.1006/jmbi.1997.1413. [DOI] [PubMed] [Google Scholar]
- 91.Egea P.F., Rochel N., Birck C., Vachette P., Timmins P.A., Moras D. Effects of ligand binding on the association properties and conformation in solution of retinoic acid receptors RXR and RAR. J. Mol. Biol. 2001;307(2):557–576. doi: 10.1006/jmbi.2000.4409. [DOI] [PubMed] [Google Scholar]
- 92.Westin S., Kurokawa R., Nolte R.T., Wisely G.B., McInerney E.M., Rose D.W., Milburn M.V., Rosenfeld M.G., Glass C.K. Interactions controlling the assembly of nuclear-receptor heterodimers and co-activators. Nature. 1998;395(6698):199–202. doi: 10.1038/26040. [DOI] [PubMed] [Google Scholar]
- 93.Dong D., Noy N. Heterodimer formation by retinoid X receptor: regulation by ligands and by the receptor’s self-association properties. Biochemistry. 1998;37(30):10691–10700. doi: 10.1021/bi980561r. [DOI] [PubMed] [Google Scholar]
- 94.Fadel L., Rehó B., Volkó J., Bojcsuk D., Kolostyák Z., Nagy G., Müller G., Simandi Z., Hegedüs É., Szabó G., Tóth K., Nagy L., Vámosi G. Agonist binding directs dynamic competition among nuclear receptors for heterodimerization with retinoid X receptor. J. Biol. Chem. 2020;295(29):10045–10061. doi: 10.1074/jbc.RA119.011614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Berry D.C., O’Byrne S.M., Vreeland A.C., Blaner W.S., Noy N. Cross talk between signaling and vitamin A transport by the retinol-binding protein receptor STRA6. Mol. Cell. Biol. 2012;32(15):3164–3175. doi: 10.1128/MCB.00505-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Villarino A.V., Kanno Y., O'Shea J.J. Mechanisms and consequences of Jak-STAT signaling in the immune system. Nat. Immunol. 2017;18(4):374–384. doi: 10.1038/ni.3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yen A., Roberson M.S., Varvayanis S., Lee A.T. Retinoic acid induced mitogen-activated protein (MAP)/extracellular signal-regulated kinase (ERK) kinase-dependent MAP kinase activation needed to elicit HL-60 cell differentiation and growth arrest. Cancer Res. 1998;58(14):3163–3172. [PubMed] [Google Scholar]
- 98.Ben-Sasson H., Ben-Meir A., Shushan A., Karra L., Rojansky N., Klein B.Y., Levitzki R., Ben-Bassat H. All-trans-retinoic acid mediates changes in PI3K and retinoic acid signaling proteins of leiomyomas. Fertil. Steril. 2011;95(6):2080–2086. doi: 10.1016/j.fertnstert.2011.01.155. [DOI] [PubMed] [Google Scholar]
- 99.Koprivica I., Gajic D., Saksida T., Cavalli E., Auci D., Despotovic S., Pejnovic N., Stosic-Grujicic S., Nicoletti F., Stojanovic I. Orally delivered all-trans-retinoic acid- and transforming growth factor-ß-loaded microparticles ameliorate type 1 diabetes in mice. Eur. J. Pharmacol. 2019;864 doi: 10.1016/j.ejphar.2019.172721. [DOI] [PubMed] [Google Scholar]
- 100.Larange A., Cheroutre H. Retinoic acid and retinoic acid receptors as pleiotropic modulators of the immune system. Annu. Rev. Immunol. 2016;34:369–394. doi: 10.1146/annurev-immunol-041015-055427. [DOI] [PubMed] [Google Scholar]
- 101.Mucida D., Cheroutre H. TGFbeta and retinoic acid intersect in immune-regulation. Cell Adhes. Migr. 2007;1(3):142–144. doi: 10.4161/cam.1.3.5062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ganor Y., Levite M. The neurotransmitter glutamate and human T cells: glutamate receptors and glutamate-induced direct and potent effects on normal human T cells, cancerous human leukemia and lymphoma T cells, and autoimmune human T cells. J. Neural Transmiss. (Vienna, Austria : 1996) 2014;121(8):983–1006. doi: 10.1007/s00702-014-1167-5. [DOI] [PubMed] [Google Scholar]
- 103.Guo Y., Zhang H., Chen X., Liu Y. All-trans retinoic acid reduces mammalian target of rapamycin via a Sirtuin1-dependent mechanism in neurons. Neuroreport. 2021;32(12):975–982. doi: 10.1097/WNR.0000000000001672. [DOI] [PubMed] [Google Scholar]
- 104.Nagpal I., Wei L.N. All-trans retinoic acid as a versatile cytosolic signal modulator mediated by CRABP1. Int. J. Mol. Sci. 2019;20(15) doi: 10.3390/ijms20153610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Park S.W., Nhieu J., Persaud S.D., Miller M.C., Xia Y., Lin Y.W., Lin Y.L., Kagechika H., Mayo K.H., Wei L.N. A new regulatory mechanism for raf kinase activation, retinoic acid-bound Crabp1. Sci. Rep. 2019;9(1):10929. doi: 10.1038/s41598-019-47354-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Persaud S.D., Park S.W., Ishigami-Yuasa M., Koyano-Nakagawa N., Kagechika H., Wei L.N. All trans-retinoic acid analogs promote cancer cell apoptosis through non-genomic Crabp1 mediating ERK1/2 phosphorylation. Sci. Rep. 2016;6:22396. doi: 10.1038/srep22396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ramsey H.E., Stengel K., Pino J.C., Johnston G., Childress M., Gorska A.E., Arrate P.M., Fuller L., Villaume M., Fischer M.A., Ferrell P.B., Jr., Roe C.E., Zou J., Lubbock A.L.R., Stubbs M., Zinkel S., Irish J.M., Lopez C.F., Hiebert S., Savona M.R. Selective inhibition of JAK1 primes STAT5-driven human leukemia cells for ATRA-induced differentiation. Target. Oncol. 2021 doi: 10.1007/s11523-021-00830-5. https://pubmed.ncbi.nlm.nih.gov/34324169/ PMID: 34324169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Syed Z., Cheepala S.B., Gill J.N., Stein J., Nathan C., Digiovanni J., Batra V., Adegboyega P., Kleiner H.E., Clifford J.L. All-trans retinoic acid suppresses Stat3 signaling during skin carcinogenesis. Cancer Prevent Res. (Philadelphia, Pa.) 2009;2(10):903–911. doi: 10.1158/1940-6207.CAPR-09-0041. [DOI] [PubMed] [Google Scholar]
- 109.Broulík P.D., Raška I., Brouliková K. Prolonged overdose of all-trans retinoic acid enhances bone sensitivity in castrated mice. Nutrition (Burbank Los Angeles County, Calif.) 2013;29(9):1166–1169. doi: 10.1016/j.nut.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 110.Piersma A.H., Hessel E.V., Staal Y.C. Retinoic acid in developmental toxicology: teratogen, morphogen and biomarker. Reprod. Toxicol. (Elmsford, N.Y.) 2017;72:53–61. doi: 10.1016/j.reprotox.2017.05.014. [DOI] [PubMed] [Google Scholar]
- 111.Russell R.M. The vitamin A spectrum: from deficiency to toxicity. Am. J. Clin. Nutr. 2000;71(4):878–884. doi: 10.1093/ajcn/71.4.878. [DOI] [PubMed] [Google Scholar]
- 112.Maginnis M.S. Virus-receptor interactions: the key to cellular invasion. J. Mol. Biol. 2018;430(17):2590–2611. doi: 10.1016/j.jmb.2018.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Marian A.J. The discovery of the ACE2 gene. Circ. Res. 2013;112(10):1307–1309. doi: 10.1161/CIRCRESAHA.113.301271. [DOI] [PubMed] [Google Scholar]
- 114.Donoghue M., Hsieh F., Baronas E., Godbout K., Gosselin M., Stagliano N., Donovan M., Woolf B., Robison K., Jeyaseelan R., Breitbart R.E., Acton S. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1–9. Circ. Res. 2000;87(5):E1–E9. doi: 10.1161/01.res.87.5.e1. [DOI] [PubMed] [Google Scholar]
- 115.Laporte M., Raeymaekers V., Van Berwaer R., Vandeput J., Marchand-Casas I., Thibaut H.J., Van Looveren D., Martens K., Hoffmann M., Maes P., Pöhlmann S., Naesens L., Stevaert A. The SARS-CoV-2 and other human coronavirus spike proteins are fine-tuned towards temperature and proteases of the human airways. PLoS Pathog. 2021;17(4) doi: 10.1371/journal.ppat.1009500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Murgolo N., Therien A.G., Howell B., Klein D., Koeplinger K., Lieberman L.A., Adam G.C., Flynn J., McKenna P., Swaminathan G., Hazuda D.J., Olsen D.B. SARS-CoV-2 tropism, entry, replication, and propagation: considerations for drug discovery and development. PLoS Pathog. 2021;17(2) doi: 10.1371/journal.ppat.1009225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A., Müller M.A., Drosten C., Pöhlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Olwenyi O.A., Dyavar S.R., Acharya A., Podany A.T., Fletcher C.V., Ng C.L., Reid S.P., Byrareddy S.N. Immuno-epidemiology and pathophysiology of coronavirus disease 2019 (COVID-19) J. Mol. Med. 2020;98(10):1369–1383. doi: 10.1007/s00109-020-01961-4. (Berlin, Germany) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ye Q., Wang B., Mao J. The pathogenesis and treatment of the `Cytokine Storm' in COVID-19. J. Infect. 2020;80(6):607–613. doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mortaz E., Tabarsi P., Varahram M., Folkerts G., Adcock I.M. The immune response and immunopathology of COVID-19. Front. Immunol. 2020;11:2037. doi: 10.3389/fimmu.2020.02037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Cao X. COVID-19: immunopathology and its implications for therapy. Nat. Rev. Immunol. 2020;20(5):269–270. doi: 10.1038/s41577-020-0308-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C., Liu S., Zhao P., Liu H., Zhu L., Tai Y., Bai C., Gao T., Song J., Xia P., Dong J., Zhao J., Wang F.S. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chalmers J.D., Chotirmall S.H. Rewiring the immune response in COVID-19. Am. J. Respir. Crit. Care Med. 2020;202(6):784–786. doi: 10.1164/rccm.202007-2934ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Jamal N., Whittier S., Carter R.C., Zachariah P. Biphasic variation over time in presenting features of patients with COVID-19. Pediatrics. 2020;146(5) doi: 10.1542/peds.2020-014902. [DOI] [PubMed] [Google Scholar]
- 125.McElvaney O.J., McEvoy N.L., McElvaney O.F., Carroll T.P., Murphy M.P., Dunlea D.M., N.C. O J.Clarke., E. O'Connor Characterization of the inflammatory response to severe COVID-19 illness. Am. J. Respirat. Crit. Care Med. 2020;202(6):812–821. doi: 10.1164/rccm.202005-1583OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Qin C., Zhou L., Hu Z., Zhang S., Yang S., Tao Y., Xie C., Ma K., Shang K., Wang W., Tian D.S. Dysregulation of immune response in patients with coronavirus2019 (COVID-19) in Wuhan, China. Clin. Infect. Dis. 2020;71(15):762–768. doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Liu X., Zhang R., He G. Hematological findings in coronavirus disease 2019: indications of progression of disease. Ann. Hematol. 2020;99(7):1421–1428. doi: 10.1007/s00277-020-04103-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Rahi M.S., Jindal V., Reyes S.P., Gunasekaran K., Gupta R., Jaiyesimi I. Hematologic disorders associated with COVID-19: a review. Ann. Hematol. 2021;100(2):309–320. doi: 10.1007/s00277-020-04366-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Rutkowska E., Kwiecien I., Zabicka M., Maliborski A., Raniszewska A., Klos K., Urbanska W., Klajnowicz I., Rzepecki P., Chcialowski A. Cytokines and leukocytes subpopulations profile in SARS-CoV-2 patients depending on the CT score severity. Viruses. 2021;13(5) doi: 10.3390/v13050880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Agbuduwe C., Basu S. Haematological manifestations of COVID-19: from cytopenia to coagulopathy. Eur. J. Haematol. 2020;105(5):540–546. doi: 10.1111/ejh.13491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Cevik M., Kuppalli K., Kindrachuk J., Peiris M. Virology, transmission, and pathogenesis of SARS-CoV-2. BMJ (Clinical research ed.) 2020;371 doi: 10.1136/bmj.m3862. [DOI] [PubMed] [Google Scholar]
- 132.Park S.E. Epidemiology, virology, and clinical features of severe acute respiratory syndrome -coronavirus-2 (SARS-CoV-2; coronavirus Disease-19) Clin. Exp. Pediatrics. 2020;63(4):119–124. doi: 10.3345/cep.2020.00493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gavriatopoulou M., Korompoki E., Fotiou D., Ntanasis-Stathopoulos I., Psaltopoulou T., Kastritis E., Terpos E., Dimopoulos M.A. Organ-specific manifestations of COVID-19 infection. Clin. Exp. Med. 2020;20(4):493–506. doi: 10.1007/s10238-020-00648-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Munjal M., Das S., Chatterjee N., Setra A.E., Govil D. Systemic involvement of novel coronavirus (COVID-19): a review of literature. 2020;24(7):565–569. doi: 10.5005/jp-journals-10071-23498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lidén M., Eriksson U. Understanding retinol metabolism: structure and function of retinol dehydrogenases. J. Biol. Chem. 2006;281(19):13001–13004. doi: 10.1074/jbc.R500027200. [DOI] [PubMed] [Google Scholar]
- 136.Marill J., Idres N., Capron C.C., Nguyen E., Chabot G.G. Retinoic acid metabolism and mechanism of action: a review. Curr. Drug Metab. 2003;4(1):1–10. doi: 10.2174/1389200033336900. [DOI] [PubMed] [Google Scholar]
- 137.Rhinn M., Dollé P. Retinoic acid signalling during development. Development (Cambridge, England) 2012;139(5):843–858. doi: 10.1242/dev.065938. [DOI] [PubMed] [Google Scholar]
- 138.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., Liu L., Shan H., Lei C.L., Hui D.S.C., Du B., Li L.J., Zeng G., Yuen K.Y., Chen R.C., Tang C.L., Wang T., Chen P.Y., Xiang J., Li S.Y., Wang J.L., Liang Z.J., Peng Y.X., Wei L., Liu Y., Hu Y.H., Peng P., Wang J.M., Liu J.Y., Chen Z., Li G., Zheng Z.J., Qiu S.Q., Luo J., Ye C.J., Zhu S.Y., Zhong N.S. Clinical characteristics of coronavirus disease 2019 in China. New Engl. J. Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Tafti M., Ghyselinck N.B. Functional implication of the vitamin a signaling pathway in the brain. Arch. Neurol. 2007;64(12):1706–1711. doi: 10.1001/archneur.64.12.1706. [DOI] [PubMed] [Google Scholar]
- 140.Riva L., Yuan S., Yin X., Martin-Sancho L., Matsunaga N., Pache L., Burgstaller-Muehlbacher S., De Jesus P.D., Teriete P., Hull M.V., Chang M.W., Chan J.F., Cao J., Poon V.K., Herbert K.M., Cheng K., Nguyen T.H., Rubanov A., Pu Y., Nguyen C., Choi A., Rathnasinghe R., Schotsaert M., Miorin L., Dejosez M., Zwaka T.P., Sit K.Y., Martinez-Sobrido L., Liu W.C., White K.M., Chapman M.E., Lendy E.K., Glynne R.J., Albrecht R., Ruppin E., Mesecar A.D., Johnson J.R., Benner C., Sun R., Schultz P.G., Su A.I., García-Sastre A., Chatterjee A.K., Yuen K.Y., Chanda S.K. Discovery of SARS-CoV-2 antiviral drugs through large-scale compound repurposing. Nature. 2020;586(7827):113–119. doi: 10.1038/s41586-020-2577-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Voloudakis G., Hoffman G., Venkatesh S., Lee K.M., Dobrindt K., Vicari J.M., Zhang W., Beckmann N.D., Jiang S., Hoagland D., Bian J., Gao L., Corvelo A., Cho K., Lee J.S., Iyengar S.K., Luoh S.W., Akbarian S., Striker R., Assimes T.L., Schadt E.E., Merad M., Charney A.W., Brennand K.J., Lynch J.A., Fullard J.F., Roussos P., tenOever B.R. IL10RB as a key regulator of COVID-19 host susceptibility and severity. medRxiv. 2021 doi: 10.1101/2021.05.31.21254851. https://pubmed.ncbi.nlm.nih.gov/34100031/ PMID: 34100031. [DOI] [Google Scholar]
- 142.Stephensen C.B., Lietz G. Vitamin a in resistance to and recovery from infection: relevance to SARS-CoV2. Br. J. Nutr. 2021:1–10. doi: 10.1017/S0007114521000246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wiseman E.M., Bar-El Dadon S., Reifen R. The vicious cycle of vitamin a deficiency: a review. Crit. Rev. Food Sci. Nutr. 2017;57(17):3703–3714. doi: 10.1080/10408398.2016.1160362. [DOI] [PubMed] [Google Scholar]
- 144.Wong D.K.C., Gendeh H.S., Thong H.K., Lum S.G., Gendeh B.S., Saim A., Salina H. A review of smell and taste dysfunction in COVID-19 patients. Med. J. Malaysia. 2020;75(5):574–581. [PubMed] [Google Scholar]
- 145.Chowdhury M.A., Hossain N., Kashem M.A., Shahid M.A., Alam A. Immune response in COVID-19: A review. J. Infect. Public Health. 2020;13(11):1619–1629. doi: 10.1016/j.jiph.2020.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Paces J., Strizova Z., Smrz D., Cerny J. COVID-19 and the immune system. Physiol. Res. 2020;69(3):379–388. doi: 10.33549/physiolres.934492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Olson C.R., Mello C.V. Significance of vitamin a to brain function, behavior and learning. Mol. Nutr. Food Res. 2010;54(4):489–495. doi: 10.1002/mnfr.200900246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Marquez H.A., Chen F. Retinoic acid signaling and development of the respiratory system. Subcell. Biochem. 2020;95:151–174. doi: 10.1007/978-3-030-42282-0_6. [DOI] [PubMed] [Google Scholar]
- 149.Biesalski H.K., Wellner U., Stofft E., Bässler K.H. Vitamin a deficiency and sensory function. Acta vitaminologica et enzymologica. 1985;7(Suppl):45–54. [PubMed] [Google Scholar]
- 150.Timoneda J., Rodríguez-Fernández L., Zaragozá R., Marín M.P., Cabezuelo M.T., Torres L., Viña J.R., Barber T. Vitamin a deficiency and the lung. Nutrients. 2018;10(9) doi: 10.3390/nu10091132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Chourbaji S., Vogt M.A., Fumagalli F., Sohr R., Frasca A., Brandwein C., Hörtnagl H., Riva M.A., Sprengel R., Gass P. AMPA receptor subunit 1 (GluR-A) knockout mice model the glutamate hypothesis of depression. FASEB J. 2008;22(9):3129–3134. doi: 10.1096/fj.08-106450. [DOI] [PubMed] [Google Scholar]
- 152.Bai X., Kirchhoff F., Scheller A. Oligodendroglial GABAergic signaling: more than Inhibition! Neurosci. Bull. 2021;37(7):1039–1050. doi: 10.1007/s12264-021-00693-w. https://pubmed.ncbi.nlm.nih.gov/33928492/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Lee H.K. In: The Dynamic Synapse: Molecular Methods in Ionotropic Receptor Biology. Kittler J.T., Moss S.J., editors. CRC Press/Taylor & Francis; 2006. Frontiers in neuroscience - AMPA receptor phosphorylation in synaptic plasticity: insights from knockin mice. Copyright © 2006, Taylor & Francis Group, LLC., Boca Raton (FL) [PubMed] [Google Scholar]
- 154.Nakanishi S., Nakajima Y., Masu M., Ueda Y., Nakahara K., Watanabe D., Yamaguchi S., Kawabata S., Okada M. Glutamate receptors: brain function and signal transduction, brain research. Brain Res. Rev. 1998;26(2–3):230–235. doi: 10.1016/s0165-0173(97)00033-7. [DOI] [PubMed] [Google Scholar]
- 155.Petroff O.A. GABA and glutamate in the human brain. Neuroscientist. 2002;8(6):562–573. doi: 10.1177/1073858402238515. [DOI] [PubMed] [Google Scholar]
- 156.Traynelis S.F., Wollmuth L.P., McBain C.J., Menniti F.S., Vance K.M., Ogden K.K., Hansen K.B., Yuan H., Myers S.J., Dingledine R. Glutamate receptor ion channels: structure, regulation, and function. Pharmacol. Rev. 2010;62(3):405–496. doi: 10.1124/pr.109.002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Maden M. Retinoic acid in the development, regeneration and maintenance of the nervous system. Nat. Rev. Neurosci. 2007;8(10):755–765. doi: 10.1038/nrn2212. [DOI] [PubMed] [Google Scholar]
- 158.Aoto J., Nam C.I., Poon M.M., Ting P., Chen L. Synaptic signaling by all-trans retinoic acid in homeostatic synaptic plasticity. Neuron. 2008;60(2):308–320. doi: 10.1016/j.neuron.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Perfilova V.N., Tyurenkov I.N. Glutamate lonotropic receptors: structure, localisation, function. Usp. Fiziol. Nauk. 2016;47(1):80–96. [PubMed] [Google Scholar]
- 160.Lane M.A., Bailey S.J. Role of retinoid signalling in the adult brain. Prog. Neurobiol. 2005;75(4):275–293. doi: 10.1016/j.pneurobio.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 161.Piskunov A., Al Tanoury Z., Rochette-Egly C. Nuclear and extra-nuclear effects of retinoid acid receptors: how they are interconnected. Subcell. Biochem. 2014;70:103–127. doi: 10.1007/978-94-017-9050-5_6. [DOI] [PubMed] [Google Scholar]
- 162.Hamilton K.A., Parrish-Aungst S., Margolis F.L., Erdélyi F., Szabó G., Puche A.C. Sensory deafferentation transsynaptically alters neuronal GluR1 expression in the external plexiform layer of the adult mouse main olfactory bulb. Chem. Senses. 2008;33(2):201–210. doi: 10.1093/chemse/bjm079. [DOI] [PubMed] [Google Scholar]
- 163.Rawson N.E., LaMantia A.S. A speculative essay on retinoic acid regulation of neural stem cells in the developing and aging olfactory system. Exp. Gerontol. 2007;42(1–2):46–53. doi: 10.1016/j.exger.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 164.Khatib T., Whiting A., Chisholm D.R., Redfern C., Müller B., McCaffery P. A bioluminescence reporter assay for retinoic acid control of translation of the GluR1 subunit of the AMPA glutamate receptor. Mol. Neurobiol. 2019;56(10):7074–7084. doi: 10.1007/s12035-019-1571-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kumar S., Veldhuis A., Malhotra T. Neuropsychiatric and cognitive sequelae of COVID-19. Front. Psychol. 2021;12(553) doi: 10.3389/fpsyg.2021.577529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Marshall M. How COVID-19 can damage the brain. Nature. 2020;585(7825):342–343. doi: 10.1038/d41586-020-02599-5. [DOI] [PubMed] [Google Scholar]
- 167.Asson-Batres M.A., Ahmad O., Smith W.B. Expression of the cellular retinoic acid binding proteins, type II and type I, in mature rat olfactory epithelium. Cell Tissue Res. 2003;312(1):9–19. doi: 10.1007/s00441-003-0709-1. [DOI] [PubMed] [Google Scholar]
- 168.Reden J., Lill K., Zahnert T., Haehner A., Hummel T. Olfactory function in patients with postinfectious and posttraumatic smell disorders before and after treatment with vitamin a: a double-blind, placebo-controlled, randomized clinical trial. Laryngoscope. 2012;122(9):1906–1909. doi: 10.1002/lary.23405. [DOI] [PubMed] [Google Scholar]
- 169.Hummel T., Whitcroft K.L., Rueter G., Haehner A. Intranasal vitamin a is beneficial in post-infectious olfactory loss. Eur. Arch. Oto-rhino-Laryngol. 2017;274(7):2819–2825. doi: 10.1007/s00405-017-4576-x. [DOI] [PubMed] [Google Scholar]
- 170.Iadecola C., Anrather J., Kamel H. Effects of COVID-19 on the nervous system. Cell. 2020;183(1):16–27.e1. doi: 10.1016/j.cell.2020.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Zhou Y., Xu J., Hou Y., Leverenz J.B., Kallianpur A., Mehra R., Liu Y., Yu H., Pieper A.A., Jehi L., Cheng F. Network medicine links SARS-CoV-2/COVID-19 infection to brain microvascular injury and neuroinflammation in dementia-like cognitive impairment. Alzheimers Res. Ther. 2021;13(1):110. doi: 10.1186/s13195-021-00850-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Garg P., Arora U., Kumar A., Wig N. The "post-COVID" syndrome: how deep is the damage? J. Med. Virol. 2021;93(2):673–674. doi: 10.1002/jmv.26465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Yong S.J. Persistent brainstem dysfunction in long-COVID: a hypothesis. ACS Chem. Neurosci. 2021;12(4):573–580. doi: 10.1021/acschemneuro.0c00793. [DOI] [PubMed] [Google Scholar]
- 174.Balaphas A., Gkoufa K., Meyer J., Peloso A., Bornand A., McKee T.A., Toso C., Popeskou S.G. COVID-19 can mimic acute cholecystitis and is associated with the presence of viral RNA in the gallbladder wall. J. Hepatol. 2020;73(6):1566–1568. doi: 10.1016/j.jhep.2020.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Pal R., Banerjee M. COVID-19 and the endocrine system: exploring the unexplored. J. Endocrinol. Investig. 2020;43(7):1027–1031. doi: 10.1007/s40618-020-01276-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Nalbandian A., Sehgal K., Gupta A., Madhavan M.V., McGroder C., Stevens J.S., Cook J.R., Nordvig A.S., Shalev D., Sehrawat T.S., Ahluwalia N., Bikdeli B., Dietz D., Der-Nigoghossian C., Liyanage-Don N., Rosner G.F., Bernstein E.J., Mohan S., Beckley A.A., Seres D.S., Choueiri T.K., Uriel N., Ausiello J.C., Accili D., Freedberg D.E., Baldwin M., Schwartz A., Brodie D., Garcia C.K., Elkind M.S.V., Connors J.M., Bilezikian J.P., Landry D.W., Wan E.Y. Post-acute COVID-19 syndrome. Nat. Med. 2021;27(4):601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Chen G., Wu D., Guo W., Cao Y., Huang D., Wang H., Wang T., Zhang X., Chen H., Yu H., Zhang X., Zhang M., Wu S., Song J., Chen T., Han M., Li S., Luo X., Zhao J., Ning Q. Clinical and immunological features of severe and moderate coronavirus disease 2019. J. Clin. Invest. 2020;130(5):2620–2629. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Usmani K.A., Tang J. 2004. Human Cytochrome P450: Metabolism of Testosterone by CYP3A4 and Inhibition by Ketoconazole, Current Protocols in Toxicology Chapter 4. Unit4.13. [DOI] [PubMed] [Google Scholar]
- 179.Kirtipal N., Bharadwaj S., Kang S.G. From SARS to SARS-CoV-2, insights on structure, pathogenicity and immunity aspects of pandemic human coronaviruses. Infect. Genet. Evolut. 2020;85 doi: 10.1016/j.meegid.2020.104502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Ritchie K., Chan D., Watermeyer T. The cognitive consequences of the COVID-19 epidemic: collateral damage? Brain Commun. 2020;2(2):fcaa069. doi: 10.1093/braincomms/fcaa069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Wu Y., Xu X., Chen Z., Duan J., Hashimoto K., Yang L., Liu C., Yang C. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav. Immun. 2020;87:18–22. doi: 10.1016/j.bbi.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Yachou Y., El Idrissi A., Belapasov V., Ait Benali S. Neuroinvasion, neurotropic, and neuroinflammatory events of SARS-CoV-2: understanding the neurological manifestations in COVID-19 patients. Neurol. Sci. 2020;41(10):2657–2669. doi: 10.1007/s10072-020-04575-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Brown C.C., Noelle R.J. Seeing through the dark: new insights into the immune regulatory functions of vitamin A. Eur. J. Immunol. 2015;45(5):1287–1295. doi: 10.1002/eji.201344398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Bono M.R., Tejon G., Flores-Santibañez F., Fernandez D., Rosemblatt M., Sauma D. Retinoic acid as a modulator of T cell immunity. Nutrients. 2016;8(6) doi: 10.3390/nu8060349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Allende L.M., Corell A., Madroño A., Góngora R., Rodríguez-Gallego C., López-Goyanes A., Rosal M., Arnaiz-Villena A. Retinol (vitamin A) is a cofactor in CD3-induced human T-lymphocyte activation. Immunology. 1997;90(3):388–396. doi: 10.1111/j.1365-2567.1997.00388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Erkelens M.N., Goverse G., Konijn T., Molenaar R., Beijer M.R., Van den Bossche J., de Goede K.E., Verberk S.G.S., de Jonge W.J., den Haan J.M.M., Mebius R.E. Intestinal macrophages balance inflammatory expression profiles via vitamin a and Dectin-1-mediated signaling. Front. Immunol. 2020;11:551. doi: 10.3389/fimmu.2020.00551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Erkelens M.N., Mebius R.E. Retinoic acid and immune homeostasis: a Balancing Act. Trends Immunol. 2017;38(3):168–180. doi: 10.1016/j.it.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 188.Reifen R., Nur T., Ghebermeskel K., Zaiger G., Urizky R., Pines M. Vitamin a deficiency exacerbates inflammation in a rat model of colitis through activation of nuclear factor-kappaB and collagen formation. J. Nutr. 2002;132(9):2743–2747. doi: 10.1093/jn/132.9.2743. [DOI] [PubMed] [Google Scholar]
- 189.Sommer A. Vitamin a, infectious disease, and childhood mortality: a 2 solution? J. Infect. Dis. 1993;167(5):1003–1007. doi: 10.1093/infdis/167.5.1003. [DOI] [PubMed] [Google Scholar]
- 190.McGill J.L., Kelly S.M., Guerra-Maupome M., Winkley E., Henningson J., Narasimhan B., Sacco R.E. Vitamin A deficiency impairs the immune response to intranasal vaccination and RSV infection in neonatal calves. Sci. Rep. 2019;9(1):15157. doi: 10.1038/s41598-019-51684-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet (London, England) 2020:497–506<span style="color: rgb(0, 0, 0); font-family: "Times New Roman"; font-size: 18px; font-style: normal; font-variant-ligatures: normal; font-variant-caps: normal; font-weight: 400; letter-spacing: normal; orphans: 2; text-align: left; text-indent: 0px; text-transform: none; white-space: normal; widows: 2; word-spacing: 0px; -webkit-text-stroke-width: 0px; background-color: rgb(250, 250, 250); text-decoration-thickness: initial; text-decoration-style: initial; text-decoration-color: initial; display: inline !important; float: none;"><year>2020</year><span/></span>. [Google Scholar]
- 192.Huang Z., Liu Y., Qi G., Brand D., Zheng S.G. Role of vitamin a in the immune system. J. Clin. Med. 2018;7(9) doi: 10.3390/jcm7090258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Garbe A., Buck J., Hämmerling U. Retinoids are important cofactors in T cell activation. J. Exp. Med. 1992;176(1):109–117. doi: 10.1084/jem.176.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Manicassamy S., Pulendran B. Retinoic acid-dependent regulation of immune responses by dendritic cells and macrophages. Semin. Immunol. 2009;21(1):22–27. doi: 10.1016/j.smim.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Yokota A., Takeuchi H., Maeda N., Ohoka Y., Kato C., Song S.Y., Iwata M. GM-CSF and IL-4 synergistically trigger dendritic cells to acquire retinoic acid-producing capacity. Int. Immunol. 2009;21(4):361–377. doi: 10.1093/intimm/dxp003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Iwata M. Retinoic acid production by intestinal dendritic cells and its role in T-cell trafficking. Semin. Immunol. 2009;21(1):8–13. doi: 10.1016/j.smim.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 197.Iwata M., Hirakiyama A., Eshima Y., Kagechika H., Kato C., Song S.Y. Retinoic acid imprints gut-homing specificity on T cells. Immunity. 2004;21(4):527–538. doi: 10.1016/j.immuni.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 198.Hammerschmidt S.I., Friedrichsen M., Boelter J., Lyszkiewicz M., Kremmer E., Pabst O., Förster R. Retinoic acid induces homing of protective T and B cells to the gut after subcutaneous immunization in mice. J. Clin. Invest. 2011;121(8):3051–3061. doi: 10.1172/JCI44262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Ahn H.J., Hernandez C.M., Levenson J.M., Lubin F.D., Liou H.C., Sweatt J.D. c-Rel, an NF-kappaB family transcription factor, is required for hippocampal long-term synaptic plasticity and memory formation. Learn. Memory (Cold Spring Harbor, N.Y.) 2008;15(7):539–549. doi: 10.1101/lm.866408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Brasier A.R. The NF-kappaB regulatory network. Cardiovasc. Toxicol. 2006;6(2):111–130. doi: 10.1385/ct:6:2:111. [DOI] [PubMed] [Google Scholar]
- 201.Gilmore T.D. Introduction to NF-kappaB: players, pathways, perspectives. Oncogene. 2006;25(51):6680–6684. doi: 10.1038/sj.onc.1209954. [DOI] [PubMed] [Google Scholar]
- 202.Mattson M.P., Meffert M.K. Roles for NF-kappaB in nerve cell survival, plasticity, and disease. Cell Death Differ. 2006;13(5):852–860. doi: 10.1038/sj.cdd.4401837. [DOI] [PubMed] [Google Scholar]
- 203.Smith E.M., Gregg M., Hashemi F., Schott L., Hughes T.K. Corticotropin releasing factor (CRF) activation of NF-kappaB-directed transcription in leukocytes. Cell. Mol. Neurobiol. 2006;26(4–6):1021–1036. doi: 10.1007/s10571-006-9040-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Levenson J.M., Choi S., Lee S.Y., Cao Y.A., Ahn H.J., Worley K.C., Pizzi M., Liou H.C., Sweatt J.D. A bioinformatics analysis of memory consolidation reveals involvement of the transcription factor c-rel. J. Neurosci. 2004;24(16):3933–3943. doi: 10.1523/JNEUROSCI.5646-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Chen Z.J. Ubiquitin signalling in the NF-kappaB pathway. Nat. Cell Biol. 2005;7(8):758–765. doi: 10.1038/ncb0805-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Albensi B.C., Mattson M.P. Evidence for the involvement of TNF and NF-kappaB in hippocampal synaptic plasticity. Synapse (New York, N.Y.) 2000;35(2):151–159. doi: 10.1002/(SICI)1098-2396(200002)35:2<151::AID-SYN8>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 207.Lee H.C., Headley M.B., Iseki M., Ikuta K., Ziegler S.F. Cutting edge: inhibition of NF-kappaB-mediated TSLP expression by retinoid X receptor. J. Immunol. (Baltimore, Md. : 1950) 2008;181(8):5189–5193. doi: 10.4049/jimmunol.181.8.5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Rafa H., Benkhelifa S., AitYounes S., Saoula H., Belhadef S., Belkhelfa M., Boukercha A., Toumi R., Soufli I., Moralès O., de Launoit Y., Mahfouf H., Nakmouche M., Delhem N., Touil-Boukoffa C. All-trans retinoic acid modulates TLR4/NF-?B signaling pathway targeting TNF-a and nitric oxide synthase 2 expression in colonic mucosa during ulcerative colitis and colitis associated cancer. Mediat. Inflamm. 2017;2017:7353252. doi: 10.1155/2017/7353252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Sirisinha S. The pleiotropic role of vitamin a in regulating mucosal immunity. Asian Pac. J. Allergy Immunol. 2015;33(2):71–89. [PubMed] [Google Scholar]
- 210.Na S.Y., Kim H.J., Lee S.K., Choi H.S., Na D.S., Lee M.O., Chung M., Moore D.D., Lee J.W. IkappaBbeta interacts with the retinoid X receptor and inhibits retinoid-dependent transactivation in lipopolysaccharide-treated cells. J. Biol. Chem. 1998;273(6):3212–3215. doi: 10.1074/jbc.273.6.3212. [DOI] [PubMed] [Google Scholar]
- 211.Karin M., Ben-Neriah Y. Phosphorylation meets ubiquitination: the control of NF-[kappa]B activity. Annu. Rev. Immunol. 2000;18:621–663. doi: 10.1146/annurev.immunol.18.1.621. [DOI] [PubMed] [Google Scholar]
- 212.Austenaa L.M., Carlsen H., Ertesvag A., Alexander G., Blomhoff H.K., Blomhoff R. Vitamin a status significantly alters nuclear factor-kappaB activity assessed by in vivo imaging. FASEB J. 2004;18(11):1255–1257. doi: 10.1096/fj.03-1098fje. [DOI] [PubMed] [Google Scholar]
- 213.Tian B., Brasier A.R. Identification of a nuclear factor kappa B-dependent gene network. Recent Prog. Horm. Res. 2003;58:95–130. doi: 10.1210/rp.58.1.95. [DOI] [PubMed] [Google Scholar]
- 214.Blanco-Melo D., Nilsson-Payant B.E., Liu W.C., Uhl S., Hoagland D., Møller R., Jordan T.X., Oishi K., Panis M., Sachs D., Wang T.T., Schwartz R.E., Lim J.K., Albrecht R.A. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell. 2020;181(5) doi: 10.1016/j.cell.2020.04.026. 6-1045.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Remy K.E., Mazer M., Striker D.A., Ellebedy A.H., Walton A.H., Unsinger J., Blood T.M., Mudd P.A., Yi D.J., Mannion D.A., Osborne D.F., Martin R.S., Anand N.J., Bosanquet J.P., Blood J., Drewry A.M., Caldwell C.C., Turnbull I.R., Brakenridge S.C., Moldwawer L.L., Hotchkiss R.S. Severe immunosuppression and not a cytokine storm characterizes COVID-19 infections. JCI Insight. 2020;5(17) doi: 10.1172/jci.insight.140329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Hadjadj J., Yatim N., Barnabei L., Corneau A., Boussier J., Smith N., Péré H., Charbit B., Bondet V., Chenevier-Gobeaux C., Breillat P., Carlier N., Gauzit R., Morbieu C., Pène F., Marin N., Roche N., Szwebel T.A., Merkling S.H., Treluyer J.M., Veyer D., Mouthon L., Blanc C., Tharaux P.L., Rozenberg F., Fischer A., Duffy D., Rieux-Laucat F., Kernéis S., Terrier B. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science (New York, N.Y.) 2020;369(6504):718–724. doi: 10.1126/science.abc6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Shibabaw T. Inflammatory cytokine: IL-17A signaling pathway in patients present with COVID-19 and current treatment strategy. J. Inflamm. Res. 2020;13:673–680. doi: 10.2147/JIR.S278335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Tan M., Liu Y., Zhou R., Deng X., Li F., Liang K., Shi Y. Immunopathological characteristics of coronavirus disease 2019 cases in Guangzhou, China. Immunology. 2020;160(3):261–268. doi: 10.1111/imm.13223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Acharya D., Liu G., Gack M.U. Dysregulation of type I interferon responses in COVID-19. Nat. Rev. Immunol. 2020;20(7):397–398. doi: 10.1038/s41577-020-0346-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Lucas C., Wong P., Klein J., Castro T.B.R., Silva J., Sundaram M., Ellingson M.K., Mao T., Oh J.E., Israelow B., Takahashi T., Tokuyama M., Lu P., Venkataraman A., Park A., Mohanty S., Wang H., Wyllie A.L., Vogels C.B.F., Earnest R., Lapidus S., Ott I.M., Moore A.J., Muenker M.C., Fournier J.B., Campbell M., Odio C.D., Casanovas-Massana A., Herbst R., Shaw A.C., Medzhitov R., Schulz W.L., Grubaugh N.D., Dela Cruz C., Farhadian S., Ko A.I., Omer S.B., Iwasaki A. Longitudinal analyses reveal immunological misfiring in severe COVID-19. Nature. 2020;584(7821):463–469. doi: 10.1038/s41586-020-2588-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Zhang W., Zhao Y., Zhang F., Wang Q., Li T., Liu Z., Wang J., Qin Y., Zhang X., Yan X., Zeng X., Zhang S. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): the perspectives of clinical immunologists from China. Clin. Immunol. 2020;214 doi: 10.1016/j.clim.2020.108393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Abers M.S., Delmonte O.M., Ricotta E.E., Fintzi J., Fink D.L., de Jesus A.A.A., Zarember K.A., Alehashemi S., Oikonomou V., Desai J.V., Canna S.W., Shakoory B., Dobbs K., Imberti L., Sottini A., Quiros-Roldan E., Castelli F., Rossi C., Brugnoni D., Biondi A., Bettini L.R., D'Angio M., Bonfanti P., Castagnoli R., Montagna D., Licari A., Marseglia G.L., Gliniewicz E.F., Shaw E., Kahle D.E., Rastegar A.T., Stack M., Myint-Hpu K., Levinson S.L., DiNubile M.J., Chertow D.W., Burbelo P.D., Cohen J.I., Calvo K.R., Tsang J.S., Su H.C., Gallin J.I., Kuhns D.B., Goldbach-Mansky R., Lionakis M.S., Notarangelo L.D. An immune-based biomarker signature is associated with mortality in COVID-19 patients. JCI Insight. 2021;6(1) doi: 10.1172/jci.insight.144455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Breton G., Mendoza P., Hägglöf T., Oliveira T.Y., Schaefer-Babajew D., Gaebler C., Turroja M., Hurley A., Caskey M., Nussenzweig M.C. Persistent cellular immunity to SARS-CoV-2 infection. J. Exp. Med. 2021;218(4) doi: 10.1084/jem.20202515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Zheng M., Gao Y., Wang G., Song G., Liu S., Sun D., Xu Y., Tian Z. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell. Mol. Immunol. 2020;17(5):533–535. doi: 10.1038/s41423-020-0402-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Muyayalo K.P., Huang D.H., Zhao S.J., Xie T., Mor G., Liao A.H. COVID-19 and Treg/Th17 imbalance: potentialrelationship to pregnancy outcomes. Am. J. Reprod. Immunol. (New York, N.Y. : 1989) 2020;84(5) doi: 10.1111/aji.13304. e13304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Fasching P., Stradner M., Graninger W., Dejaco C., Fessler J. Therapeutic potential of targeting the Th17/Treg axis in autoimmune disorders. Molecules (Basel, Switzerland) 2017;22(1) doi: 10.3390/molecules22010134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Guilliams M., Crozat K., Henri S., Tamoutounour S., Grenot P., Devilard E., de Bovis B., Alexopoulou L., Dalod M., Malissen B. Skin-draining lymph nodes contain dermis-derived CD103(-) dendritic cells that constitutively produce retinoic acid and induce Foxp3( ) regulatory T cells. Blood. 2010;115(10):1958–1968. doi: 10.1182/blood-2009-09-245274. [DOI] [PubMed] [Google Scholar]
- 228.Fujimoto K., Karuppuchamy T., Takemura N., Shimohigoshi M., Machida T., Haseda Y., Aoshi T., Ishii K.J., Akira S., Uematsu S. A new subset of CD103 CD8alpha dendritic cells in the small intestine expresses TLR3, TLR7, and TLR9 and induces Th1 response and CTL activity. J. Immunol. (Baltimore, Md. : 1950) 2011;186:11–95. doi: 10.4049/jimmunol.1004036. [DOI] [PubMed] [Google Scholar]
- 229.Schambach F., Schupp M., Lazar M.A., Reiner S.L. Activation of retinoic acid receptor-alpha favours regulatory T cell induction at the expense of IL-17-secreting T helper cell differentiation. Eur. J. Immunol. 2007;37(9):2396–2399. doi: 10.1002/eji.200737621. [DOI] [PubMed] [Google Scholar]
- 230.Jin C.J., Hong C.Y., Takei M., Chung S.Y., Park J.S., Pham T.N., Choi S.J., Nam J.H., Chung I.J., Kim H.J., Lee J.J. All-trans retinoic acid inhibits the differentiation, maturation, and function of human monocyte-derived dendritic cells. Leuk. Res. 2010;34(4):513–520. doi: 10.1016/j.leukres.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 231.Murai M., Turovskaya O., Kim G., Madan R., Karp C.L., Cheroutre H., Kronenberg M. Interleukin 10 acts on regulatory T cells to maintain expression of the transcription factor Foxp3 and suppressive function in mice with colitis. Nat. Immunol. 2009;10(11):1178–1184. doi: 10.1038/ni.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Wang S., Gao X., Shen G., Wang W., Li J., Zhao J., Wei Y.-Q., Edwards C.K. Interleukin-10 deficiency impairs regulatory T cell-derived neuropilin-1 functions and promotes Th1 and Th17 immunity. Sci. Rep. 2016;6(1):24249. doi: 10.1038/srep24249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Chen Z., Lin F., Gao Y., Li Z., Zhang J., Xing Y., Deng Z., Yao Z., Tsun A., Li B. FOXP3 and ROR?t: transcriptional regulation of treg and Th17. Int. Immunopharmacol. 2011;11(5):536–542. doi: 10.1016/j.intimp.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 234.Mucida D., Park Y., Kim G., Turovskaya O., Scott I., Kronenberg M., Cheroutre H. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. 2007;317:256–260. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 235.Orlov M., Wander P.L., Morrell E.D., Mikacenic C., Wurfel M.M. A case for targeting Th17 cells and IL-17A in SARS-CoV-2 infections. J. Immunol. (Baltimore, Md. : 1950) 2020;205(4):892–898. doi: 10.4049/jimmunol.2000554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Wu S., Wang W., Le Q. All-trans retinoic acid regulates the balance of treg-Th17 cells through ERK and P38 signaling pathway. Iran. J. Immunol.: IJI. 2019;16(1):1–10. doi: 10.22034/IJI.2019.39402. [DOI] [PubMed] [Google Scholar]
- 237.Skaug B., Jiang X., Chen Z.J. The role of ubiquitin in NF-kappaB regulatory pathways. Annu. Rev. Biochem. 2009;78:769–796. doi: 10.1146/annurev.biochem.78.070907.102750. [DOI] [PubMed] [Google Scholar]
- 238.Song K., Li S. The role of ubiquitination in NF-?B signaling during virus infection. Viruses. 2021;13(2) doi: 10.3390/v13020145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Bayon Y., Ortiz M.A., Lopez-Hernandez F.J., Gao F., Karin M., Pfahl M., Piedrafita F.J. Inhibition of IkappaB kinase by a new class of retinoid-related anticancer agents that induce apoptosis. Mol. Cell. Biol. 2003;23(3):1061–1074. doi: 10.1128/MCB.23.3.1061-1074.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Fang H., Jin H., Wang H. Effect of all-trans retinoic acid on airway inflammation in asthmatic rats and its mechanism. J. Huazhong Univ. Sci. Technol. Med. Scie. = hua zhong ke ji da xue xue bao. yi xue ying De wen ban = huazhong keji daxue xuebao Yixue Yingdewen ban. 2004;24(3):229–232. doi: 10.1007/BF02831997. [DOI] [PubMed] [Google Scholar]
- 241.Xiao S., Jin H., Korn T., Liu S.M., Oukka M., Lim B., Kuchroo V.K. Retinoic acid increases Foxp3 regulatory T cells and inhibits development of Th17 cells by enhancing TGF-beta-driven Smad3 signaling and inhibiting IL-6 and IL-23 receptor expression. J. Immunol. (Baltimore, Md.: 1950) 2008;181(4):2277–2284. doi: 10.4049/jimmunol.181.4.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Jetten A.M., Cook D.N. (Inverse) agonists of retinoic acid-related orphan receptor ?: regulation of immune responses inflammation, and autoimmune disease. Ann. Rev. Pharmacol. Toxicol. 2020;60:371–390. doi: 10.1146/annurev-pharmtox-010919-023711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Xu L., Kitani A., Stuelten C., McGrady G., Fuss I., Strober W. Positive and negative transcriptional regulation of the Foxp3 gene is mediated by access and binding of the Smad3 protein to enhancer I. Immunity. 2010;33(3):313–325. doi: 10.1016/j.immuni.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Mucida D., Park Y., Cheroutre H. From the diet to the nucleus: vitamin a and TGF-beta join efforts at the mucosal interface of the intestine. Semin. Immunol. 2009;21(1):14–21. doi: 10.1016/j.smim.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Sierra-Mondragon E., Molina-Jijon E., Namorado-Tonix C., Rodríguez-Muñoz R., Pedraza-Chaverri J., Reyes J.L. All-trans retinoic acid ameliorates inflammatory response mediated by TLR4/NF-?B during initiation of diabetic nephropathy. J. Nutr. Biochem. 2018;60:47–60. doi: 10.1016/j.jnutbio.2018.06.002. [DOI] [PubMed] [Google Scholar]
- 246.Takeuchi H., Yokota-Nakatsuma A., Ohoka Y., Kagechika H., Kato C., Song S.Y., Iwata M. Retinoid X receptor agonists modulate Foxp3 regulatory T cell and Th17 cell differentiation with differential dependence on retinoic acid receptor activation. J. Immunol. (Baltimore, Md. : 1950) 2013;191(7):3725–3733. doi: 10.4049/jimmunol.1300032. [DOI] [PubMed] [Google Scholar]
- 247.Liu Z.M., Wang K.P., Ma J., Guo Zheng S. The role of all-trans retinoic acid in the biology of Foxp3 regulatory T cells. Cell Mol Immunol. 2015;12(5):553–557. doi: 10.1038/cmi.2014.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Jetten A.M., Takeda Y., Slominski A., Kang H.S. Retinoic acid-related orphan receptor ? (ROR?): connecting sterol metabolism to regulation of the immune system and autoimmune disease. Curr. Opin. Toxicol. 2018;8:66–80. doi: 10.1016/j.cotox.2018.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Lee I.K., Song H., Kim H., Kim I.S., Tran N.L., Kim S.H., Oh S.J., Lee J.M. RORa regulates cholesterol metabolism of CD8( ) T cells for anticancer immunity. Cancers. 2020;12(7) doi: 10.3390/cancers12071733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Parackova Z., Bloomfield M., Klocperk A., Sediva A. Neutrophils mediate Th17 promotion in COVID-19 patients. J. Leukoc. Biol. 2021;109(1):73–76. doi: 10.1002/JLB.4COVCRA0820-481RRR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Ragab D., Salah Eldin H., Taeimah M., Khattab R., Salem R. The COVID-19 cytokine storm; what we know so far. Front. Immunol. 2020;11:1446. doi: 10.3389/fimmu.2020.01446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Mendoza V.M.M. Interleukin-17: a potential therapeutic target in COVID-19. J. Infect. 2020;81(2):e136–e138. doi: 10.1016/j.jinf.2020.05.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Elshal M., Abu-Elsaad N., El-Karef A., Ibrahim T. Retinoic acid modulates IL-4, IL-10 and MCP-1 pathways in immune mediated hepatitis and interrupts CD4 T cells infiltration. Int. Immunopharmacol. 2019;75 doi: 10.1016/j.intimp.2019.105808. [DOI] [PubMed] [Google Scholar]
- 254.Marcinkiewicz J., Witkowski J.M., Olszanecki R. The dual role of the immune system in the course of COVID-19. The fatal impact of the aging immune system. Cent. Eur. J. Immunol. 2021;46(1):1–9. doi: 10.5114/ceji.2021.105240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Sun X., Wang T., Cai D., Hu Z., Chen J., Liao H., Zhi L., Wei H., Zhang Z., Qiu Y., Wang J., Wang A. Cytokine storm intervention in the early stages of COVID-19 pneumonia. Cytokine Growth Factor Rev. 2020;53:38–42. doi: 10.1016/j.cytogfr.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Wang J., Jiang M., Chen X., Montaner L.J. Cytokine storm and leukocyte changes in mild versus severe SARS-CoV-2 infection: review of 3939 COVID-19 patients in China and emerging pathogenesis and therapy concepts. J. Leukoc. Biol. 2020;108(1):17–41. doi: 10.1002/JLB.3COVR0520-272R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Bastard P., Rosen L.B., Zhang Q., Michailidis E., Hoffmann H.H., Zhang Y., Dorgham K., Philippot Q., Rosain J., Béziat V., Manry J., Shaw E., Haljasmägi L., Peterson P., Lorenzo L., Bizien L., Trouillet-Assant S., Dobbs K., de Jesus A.A., Belot A., Kallaste A., Catherinot E., Tandjaoui-Lambiotte Y., Le Pen J., Kerner G., Bigio B., Seeleuthner Y., Yang R., Bolze A., Spaan A.N., Delmonte O.M., Abers M.S., Aiuti A., Casari G., Lampasona V., Piemonti L., Ciceri F., Bilguvar K., Lifton R.P., Vasse M., Smadja D.M., Migaud M., Hadjadj J., Terrier B., Duffy D., Quintana-Murci L., van de Beek D., Roussel L., Vinh D.C., Tangye S.G., Haerynck F., Dalmau D., Martinez-Picado J., Brodin P., Nussenzweig M.C., Boisson-Dupuis S., Rodríguez-Gallego C., Vogt G., Mogensen T.H., Oler A.J., Gu J., Burbelo P.D., Cohen J.I., Biondi A., Bettini L.R., D'Angio M., Bonfanti P., Rossignol P., Mayaux J., Rieux-Laucat F., Husebye E.S., Fusco F., Ursini M.V., Imberti L., Sottini A., Paghera S., Quiros-Roldan E., Rossi C., Castagnoli R., Montagna D., Licari A., Marseglia G.L., Duval X., Ghosn J., Tsang J.S., Goldbach-Mansky R., Kisand K., Lionakis M.S., Puel A., Zhang S.Y., Holland S.M., Gorochov G., Jouanguy E., Rice C.M., Cobat A., Notarangelo L.D., Abel L., Su H.C., Casanova J.L. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science (New York, N.Y.) 2020;370(6515) doi: 10.1126/science.abd4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Ehrenfeld M., Tincani A., Andreoli L., Cattalini M., Greenbaum A., Kanduc D., Alijotas-Reig J., Zinserling V., Semenova N., Amital H., Shoenfeld Y. Covid-19 and autoimmunity. Autoimmun. Rev. 2020;19(8) doi: 10.1016/j.autrev.2020.102597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Zhang Q., Bastard P., Liu Z., Pen J.Le, Moncada-Velez M., Chen J., Ogishi M., Sabli I.K.D., Hodeib S., Korol C., Rosain J., Bilguvar K., Ye J., Bolze A., Bigio B., Yang R., Arias A.A., Zhou Q., Zhang Y., Onodi F., Korniotis S., Karpf L., Philippot Q., Chbihi M., Bonnet-Madin L., Dorgham K., Smith N., Schneider W.M., Razooky B.S., Hoffmann H.H., Michailidis E., Moens L., Han J.E., Lorenzo L., Bizien L., Meade P., Neehus A.L., Ugurbil A.C., Corneau A., Kerner G., Zhang P., Rapaport F., Seeleuthner Y., Manry J., Masson C., Schmitt Y., Schlüter A., Voyer T.Le, Khan T., Li J., Fellay J., Roussel L., Shahrooei M., Alosaimi M.F., Mansouri D., Al-Saud H., Al-Mulla F., Almourfi F., Al-Muhsen S.Z., Alsohime F., Turki S.Al, Hasanato R., van de Beek D., Biondi A., Bettini L.R., D'Angio M., Bonfanti P., Imberti L., Sottini A., Paghera S., Quiros-Roldan E., Rossi C., Oler A.J., Tompkins M.F., Alba C., Vandernoot I., Goffard J.C., Smits G., Migeotte I., Haerynck F., Soler-Palacin P., Martin-Nalda A., Colobran R., Morange P.E., Keles S., Çölkesen F., Ozcelik T., Yasar K.K., Senoglu S., Karabela N., Rodríguez-Gallego C., Novelli G., Hraiech S., Tandjaoui-Lambiotte Y., Duval X., Laouénan C., Snow A.L., Dalgard C.L., Milner J.D., Vinh D.C., Mogensen T.H., Marr N., Spaan A.N., Boisson B., Boisson-Dupuis S., Bustamante J., Puel A., Ciancanelli M.J., Meyts I., Maniatis T., Soumelis V., Amara A., Nussenzweig M., García-Sastre A., Krammer F., Pujol A., Duffy D., Lifton R.P., Zhang S.Y., Gorochov G., Béziat V., Jouanguy E., Sancho-Shimizu V., Rice C.M., Abel L., Notarangelo L.D., Cobat A., Su H.C., Casanova J.L. Inborn errors of type I IFN immunity in patients with life-threatening COVID-19. Science (New York, N.Y.) 2020;370(6515) doi: 10.1126/science.abd4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Bastard P., Michailidis E., Hoffmann H.H., Chbihi M., Voyer T.Le, Rosain J., Philippot Q., Seeleuthner Y., Gervais A., Materna M., de Oliveira P.M.N., Maia M.L.S., Bom A.P.Dinis Ano, Azamor T., da Conceição D.Araújo, Goudouris E., Homma A., Slesak G., Schäfer J., Pulendran B., Miller J.D., Huits R., Yang R., Rosen L.B., Bizien L., Lorenzo L., Chrabieh M., Erazo L.V., Rozenberg F., Jeljeli M.M., Béziat V., Holland S.M., Cobat A., Notarangelo L.D., Su H.C., Ahmed R., Puel A., Zhang S.Y., Abel L., Seligman S.J., Zhang Q., Donald M.R.Mac, Jouanguy E., Rice C.M., Casanova J.L. Auto-antibodies to type I IFNs can underlie adverse reactions to yellow fever live attenuated vaccine. J. Exp. Med. 2021;218(4) doi: 10.1084/jem.20202486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Gao Z.W., Zhang H.Z., Liu C., Dong K. Autoantibodies in COVID-19: frequency and function. Autoimmun. Rev. 2021;20(3) doi: 10.1016/j.autrev.2021.102754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Icenogle T. COVID-19: infection or autoimmunity. Front. Immunol. 2020;11:2055. doi: 10.3389/fimmu.2020.02055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Rodríguez Y., Novelli L., Rojas M., De Santis M., Acosta-Ampudia Y., Monsalve D.M., Ramírez-Santana C., Costanzo A., Ridgway W.M., Ansari A.A., Gershwin M.E., Selmi C., Anaya J.M. Autoinflammatory and autoimmune conditions at the crossroad of COVID-19. J. Autoimmun. 2020;114 doi: 10.1016/j.jaut.2020.102506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Liu Y., Sawalha A.H., Lu Q. COVID-19 and autoimmune diseases. Curr. Opin. Rheumatol. 2021;33(2):155–162. doi: 10.1097/BOR.0000000000000776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Yamazaki S., Steinman R.M. Dendritic cells as controllers of antigen-specific Foxp3 regulatory T cells. J. Dermatol. Sci. 2009;54(2):69–75. doi: 10.1016/j.jdermsci.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Bakdash G., Vogelpoel L.T., van Capel T.M., Kapsenberg M.L., de Jong E.C. Retinoic acid primes human dendritic cells to induce gut-homing, IL-10-producing regulatory T cells. Mucosal Immunol. 2015;8(2):265–278. doi: 10.1038/mi.2014.64. [DOI] [PubMed] [Google Scholar]
- 267.Piccirillo C.A., d’Hennezel E., Sgouroudis E., Yurchenko E. CD4 Foxp3 regulatory T cells in the control of autoimmunity: in vivo veritas. Curr. Opin. Immunol. 2008;20(6):655–662. doi: 10.1016/j.coi.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 268.Sakaguchi S., Ono M., Setoguchi R., Yagi H., Hori S., Fehervari Z., Shimizu J., Takahashi T., Nomura T. Foxp3 CD25 CD4 natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol. Rev. 2006;212:8–27. doi: 10.1111/j.0105-2896.2006.00427.x. [DOI] [PubMed] [Google Scholar]
- 269.Sakaguchi S., Yamaguchi T., Nomura T., Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133(5):775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 270.Kiss M., Czimmerer Z., Nagy L. The role of lipid-activated nuclear receptors in shaping macrophage and dendritic cell function: from physiology to pathology. J. Allergy Clin. Immunol. 2013;132(2):264–286. doi: 10.1016/j.jaci.2013.05.044. [DOI] [PubMed] [Google Scholar]
- 271.Raverdeau M., Christofi M., Malara A., Wilk M.M., Misiak A., Kuffova L., Yu T., McGinley A.M., Quinn S.M., Massilamany C., Reddy J., Forrester J.V., Mills K.H. Retinoic acid-induced autoantigen-specific type 1 regulatory T cells suppress autoimmunity. EMBO Rep. 2019;20(5) doi: 10.15252/embr.201847121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Phillips B.E., Garciafigueroa Y., Engman C., Liu W., Wang Y., Lakomy R.J., Meng W.S., Trucco M., Giannoukakis N. Arrest in the progression of type 1 diabetes at the mid-stage of insulitic autoimmunity using an autoantigen-decorated all-trans retinoic acid and transforming growth factor Beta-1 single microparticle formulation. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.586220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Fischer S. Pattern recognition receptors and control of innate immunity: role of nucleic acids. Curr. Pharmaceut. Biotechnol. 2018;19(15):1203–1209. doi: 10.2174/138920112804583087. [DOI] [PubMed] [Google Scholar]
- 274.Takeuchi O., Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140(6):805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 275.Yamada T., Sato S., Sotoyama Y., Orba Y., Sawa H., Yamauchi H., Sasaki M., Takaoka A. RIG-I triggers a signaling-abortive anti-SARS-CoV-2 defense in human lung cells. Nat. Immunol. 2021 doi: 10.1128/JVI.02246-17. https://pubmed.ncbi.nlm.nih.gov/29643242/ PMID: 29643242 PMCID: PMC6002725. [DOI] [PubMed] [Google Scholar]
- 276.Iwata M., Eshima Y., Kagechika H. Retinoic acids exert direct effects on T cells to suppress Th1 development and enhance Th2 development via retinoic acid receptors. Int. Immunol. 2003;15(8):1017–1025. doi: 10.1093/intimm/dxg101. [DOI] [PubMed] [Google Scholar]
- 277.Kawai K., Uchiyama M., Hester J., Wood K., Issa F. Regulatory T cells for tolerance. Hum. Immunol. 2018;79(5):294–303. doi: 10.1016/j.humimm.2017.12.013. [DOI] [PubMed] [Google Scholar]
- 278.Oukka M. Th17 cells in immunity and autoimmunity. Ann. Rheumat. Dis. 2008;67, Suppl 3:iii26-9. doi: 10.1136/ard.2008.098004. [DOI] [PubMed] [Google Scholar]
- 279.Piccirillo C.A. Regulatory T cells in health and disease. Cytokine. 2008;43(3):395–401. doi: 10.1016/j.cyto.2008.07.469. [DOI] [PubMed] [Google Scholar]
- 280.Scott C.L., Aumeunier A.M., Mowat A.M. Intestinal CD103 dendritic cells: master regulators of tolerance? Trends Immunol. 2011;32(9):412–419. doi: 10.1016/j.it.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 281.Van Y.H., Lee W.H., Ortiz S., Lee M.H., Qin H.J., Liu C.P. All-trans retinoic acid inhibits type 1 diabetes by T regulatory (Treg)-dependent suppression of interferon-gamma-producing T-cells without affecting Th17 cells. Diabetes. 2009;58(1):146–155. doi: 10.2337/db08-1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Hu Z., Li S., Song X. Cytokine storm with rapidly elevated interleukin-6 indicates sudden death in patients with critical COVID-19. Cytokine Growth Factor Rev. 2021;58:30–31. doi: 10.1016/j.cytogfr.2020.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.McGonagle D., Sharif K., O'Regan A., Bridgewood C. The role of cytokines including Interleukin-6 in COVID-19 induced pneumonia and macrophage activation syndrome-like disease. Autoimmun. Rev. 2020;19(6) doi: 10.1016/j.autrev.2020.102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Li R., Wu K., Li Y., Liang X., Tse W.K.F., Yang L., Lai K.P. Revealing the targets and mechanisms of vitamin a in the treatment of COVID-19. Aging 2020;12(15):15784–15796. doi: 10.18632/aging.103888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Ramlall V., Thangaraj P.M., Meydan C., Foox J., Butler D., Kim J., May B., De Freitas J.K., Glicksberg B.S., Mason C.E., Tatonetti N.P., Shapira S.D. Immune complement and coagulation dysfunction in adverse outcomes of SARS-CoV-2 infection. Nat. Med. 2020;26(10):1609–1615. doi: 10.1038/s41591-020-1021-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Santiesteban-Lores L.E., Amamura T.A., da Silva T.F., Midon L.M., Carneiro M.C., Isaac L., Bavia L. A double edged-sword - the complement system during SARS-CoV-2 infection. Life Sci. 2021;272 doi: 10.1016/j.lfs.2021.119245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Casanova J.L., Su H.C. A global effort to define the human genetics of protective immunity to SARS-CoV-2 infection. Cell. 2020;181(6):1194–1199. doi: 10.1016/j.cell.2020.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Le Page C., Génin P., Baines M.G., Hiscott J. Interferon activation and innate immunity. Rev. Immunogenet. 2000;2(3):374–386. [PubMed] [Google Scholar]
- 289.Chen K., Liu J., Cao X. Regulation of type I interferon signaling in immunity and inflammation: a comprehensive review. J. Autoimmun. 2017;83:1–11. doi: 10.1016/j.jaut.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 290.Gessani S., Conti L., Del Cornò M., Belardelli F. Type I interferons as regulators of human antigen presenting cell functions. Toxins. 2014;6(6):1696–1723. doi: 10.3390/toxins6061696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Ivashkiv L.B., Donlin L.T. Regulation of type I interferon responses. Nat. Rev. Immunol. 2014;14(1):36–49. doi: 10.1038/nri3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Platanias L.C. Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat. Rev. Immunol. 2005;5(5):375–386. doi: 10.1038/nri1604. [DOI] [PubMed] [Google Scholar]
- 293.Manna S.K., Mukhopadhyay A., Aggarwal B.B. IFN-a suppresses activation of nuclear transcription factors NF-?B and activator protein 1 and potentiates TNF-induced apoptosis. J. Immunol. 2000;165(9):4927–4934. doi: 10.4049/jimmunol.165.9.4927. [DOI] [PubMed] [Google Scholar]
- 294.Sa Ribero M., Jouvenet N., Dreux M., Nisole S. Interplay between SARS-CoV-2 and the type I interferon response. PLoS Pathog. 2020;16(7) doi: 10.1371/journal.ppat.1008737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Yoneyama M., Fujita T. RIG-I family RNA helicases: cytoplasmic sensor for antiviral innate immunity. Cytokine Growth Factor Rev. 2007;18(5–6):545–551. doi: 10.1016/j.cytogfr.2007.06.023. [DOI] [PubMed] [Google Scholar]
- 296.Yoneyama M., Fujita T. RNA recognition and signal transduction by RIG-I-like receptors. Immunol. Rev. 2009;227(1):54–65. doi: 10.1111/j.1600-065X.2008.00727.x. [DOI] [PubMed] [Google Scholar]
- 297.Kell A.M., Gale M., Jr. RIG-I in RNA virus recognition. Virology. 2015;479–480:110–121. doi: 10.1016/j.virol.2015.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Matsumiya T., Stafforini D.M. Function and regulation of retinoic acid-inducible gene-I. Crit. Rev. Immunol. 2010;30(6):489–513. doi: 10.1615/critrevimmunol.v30.i6.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Onoguchi K., Yoneyama M., Fujita T. Retinoic acid-inducible gene-I-like receptors. J. Interf. Cytokine Res. 2011;31(1):27–31. doi: 10.1089/jir.2010.0057. [DOI] [PubMed] [Google Scholar]
- 300.Cassani B., Villablanca E.J., De Calisto J., Wang S., Mora J.R. Vitamin a and immune regulation: role of retinoic acid in gut-associated dendritic cell education, immune protection and tolerance. Mol. Asp. Med. 2012;33(1):63–76. doi: 10.1016/j.mam.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Hao X., Zhong X., Sun X. The effects of all-trans retinoic acid on immune cells and its formulation design for vaccines. AAPS J. 2021;23(2):32. doi: 10.1208/s12248-021-00565-1. [DOI] [PubMed] [Google Scholar]
- 302.Chen X., Saccon E., Appelberg K.S., Mikaeloff F., Rodriguez J.E., Vinhas B.S., Frisan T., Végvári Á., Mirazimi A., Neogi U., Gupta S. Type-I interferon signatures in SARS-CoV-2 infected huh 7 cells. Cell Death Discov. 2021;7(1):114. doi: 10.1038/s41420-021-00487-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Jovic T.H., Ali S.R., Ibrahim N., Jessop Z.M., Tarassoli S.P., Dobbs T.D., Holford P., Thornton C.A., Whitaker I.S. Could vitamins help in the fight against COVID-19? Nutrients. 2020;12(9) doi: 10.3390/nu12092550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Cani P.D. Human gut microbiome: hopes, threats and promises. Gut. 2018;67(9):1716–1725. doi: 10.1136/gutjnl-2018-316723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Xu W.T., Nie Y.Z., Yang Z., Lu N.H. The crosstalk between gut microbiota and obesity and related metabolic disorders. Future Microbiol. 2016;11:825–836. doi: 10.2217/fmb-2015-0024. [DOI] [PubMed] [Google Scholar]
- 306.Mounsey A., Lacy Smith K., Reddy V.C., Nickolich S. Clostridioides difficile infection: update on management. Am. Fam. Physician. 2020;101(3):168–175. [PubMed] [Google Scholar]
- 307.Ooijevaar R.E., van Beurden Y.H., Terveer E.M., Goorhuis A., Bauer M.P., Keller J.J., Mulder C.J.J., Kuijper E.J. Update of treatment algorithms for Clostridium difficile infection. Clin. Microbiol. Infect. 2018;24(5):452–462. doi: 10.1016/j.cmi.2017.12.022. [DOI] [PubMed] [Google Scholar]
- 308.Yeoh Y.K., Zuo T., Lui G.C., Zhang F., Liu Q., Li A.Y., Chung A.C., Cheung C.P., Tso E.Y., Fung K.S., Chan V., Ling L., Joynt G., Hui D.S., Chow K.M., Ng S.S.S., Li T.C., Ng R.W., Yip T.C., Wong G.L., Chan F.K., Wong C.K., Chan P.K., Ng S.C. Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19. Gut. 2021;70(4):698–706. doi: 10.1136/gutjnl-2020-323020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Yang M., Yang Y., He Q., Zhu P., Liu M., Xu J., Zhao M. Intestinal microbiota-a promising target for antiviral Therapy? Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.676232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Nejadghaderi S.A., Nazemalhosseini-Mojarad E., H. Asadzadeh Aghdaei, Fecal microbiota transplantation for COVID-19; a potential emerging treatment strategy. Med. Hypotheses. 2021;147 doi: 10.1016/j.mehy.2020.110476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Dhar D., Mohanty A. Gut microbiota and Covid-19- possible link and implications. Virus Res. 2020;285 doi: 10.1016/j.virusres.2020.198018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Oliveira L.M., Teixeira F.M.E., Sato M.N. Impact of retinoic acid on immune cells and inflammatory diseases. Mediat. Inflamm. 2018;2018:3067126. doi: 10.1155/2018/3067126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Lopez L., Sang P.C., Tian Y., Sang Y. Dysregulated interferon response underlying severe COVID-19. Viruses. 2020;(12) doi: 10.3390/v12121433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Trouillet-Assant S., Viel S., Gaymard A., Pons S., Richard J.C., Perret M., Villard M., Brengel-Pesce K., Lina B., Mezidi M., Bitker L., Belot A. Type I IFN immunoprofiling in COVID-19 patients. J. Allergy Clin. Immunol. 2020;146(1):206–208.e2. doi: 10.1016/j.jaci.2020.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.George B., Amjesh R., Paul A.M., Santhoshkumar T.R., Pillai M.R., Kumar R. Evidence of a dysregulated vitamin D endocrine system in SARS-CoV-2 infected patient's lung cells. Sci. Rep. 2021;11(1):8570. doi: 10.1038/s41598-021-87703-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Chiappelli F., Khakshooy A., Greenberg G. CoViD-19 immunopathology and immunotherapy. Bioinformation. 2020;16(3):219–222. doi: 10.6026/97320630016219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Sewell H.F., Agius R.M., Stewart M., Kendrick D. Cellular immune responses to covid-19. BMJ (Clin. Res. ed.) 2020;370 doi: 10.1136/bmj.m3018. [DOI] [PubMed] [Google Scholar]
- 318.Tian W., Zhang N., Jin R., Feng Y., Wang S., Gao S., Gao R., Wu G., Tian D., Tan W., Chen Y., Gao G.F., Wong C.C.L. Immune suppression in the early stage of COVID-19 disease. Nat. Commun. 2020;11(1):5859. doi: 10.1038/s41467-020-19706-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Ross A.C. Vitamin a and retinoic acid in T cell-related immunity. Am. J. Clin. Nutr. 2012;96(5) doi: 10.3945/ajcn.112.034637. 1166s-72s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Dierov J., Sawaya B.E., Prosniak M., Gartenhaus R.B. Retinoic acid modulates a bimodal effect on cell cycle progression in human adult T-cell leukemia cells. Clin. Cancer Res. 1999;5(9):2540–2547. [PubMed] [Google Scholar]
- 321.Gil Á., Plaza-Diaz J., Mesa M.D. Vitamin D: classic and novel actions. Ann. Nutr. Metabol. 2018;72(2):87–95. doi: 10.1159/000486536. [DOI] [PubMed] [Google Scholar]
- 322.Pike J.W., Christakos S. Biology and mechanisms of action of the vitamin D hormone. Endocrinol. Metab. Clin. N. Am. 2017;46(4):815–843. doi: 10.1016/j.ecl.2017.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Kumar R., Rathi H., Haq A., Wimalawansa S.J., Sharma A. Putative roles of vitamin D in modulating immune response and immunopathology associated with COVID-19. Virus Res. 2021;292 doi: 10.1016/j.virusres.2020.198235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Allegra A., Tonacci A., Pioggia G., Musolino C., Gangemi S. Vitamin deficiency as risk factor for SARS-CoV-2 infection: correlation with susceptibility and prognosis. Eur. Rev. Med. Pharmacol. Sci. 2020;24(18):9721–9738. doi: 10.26355/eurrev_202009_23064. [DOI] [PubMed] [Google Scholar]
- 325.Li Y., Tong C.H., Bare L.A., Devlin J.J. Assessment of the association of vitamin D Level with SARS-CoV-2 seropositivity among working-age adults. JAMA Netw. Open. 2021;4(5) doi: 10.1001/jamanetworkopen.2021.11634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Mansur J.L., Tajer C., Mariani J., Inserra F., Ferder L., Manucha W. Vitamin D high doses supplementation could represent a promising alternative to prevent or treat COVID-19 infection. Clin. e Invest. Arterioscl. 2020;32(6):267–277. doi: 10.1016/j.arteri.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Murai I.H., Fernandes A.L., Sales L.P., Pinto A.J., Goessler K.F., Duran C.S.C., Silva C.B.R., Franco A.S., Macedo M.B., Dalmolin H.H.H., Baggio J., Balbi G.G.M., Reis B.Z., Antonangelo L., Caparbo V.F., Gualano B., Pereira R.M.R. Effect of a single high dose of vitamin D3 on hospital length of stay in patients with moderate to severe COVID-19: a randomized clinical trial. Jama. 2021;325(11):1053–1060. doi: 10.1001/jama.2020.26848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Amrein K., Scherkl M., Hoffmann M., Neuwersch-Sommeregger S., Köstenberger M., Tmava Berisha A., Martucci G., Pilz S., Malle O. Vitamin D deficiency 2.0: an update on the current status worldwide. Eur. J. Clin. Nutr. 2020;74(11):1498–1513. doi: 10.1038/s41430-020-0558-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Meltzer D.O., Best T.J., Zhang H., Vokes T., Arora V., Solway J. Association of vitamin D status and other clinical characteristics with COVID-19 test results. JAMA Netw. Open. 2020;3(9) doi: 10.1001/jamanetworkopen.2020.19722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Bishop E., Ismailova A., Dimeloe S.K., Hewison M., White J.H. Vitamin D and immune regulation: antibacterial, antiviral, anti-inflammatory. JBMR Plus. 2020;5(1) doi: 10.1002/jbm4.10405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Arnson Y., Amital H., Shoenfeld Y. Vitamin D and autoimmunity: new aetiological and therapeutic considerations. Ann. Rheum. Dis. 2007;66(9):1137–1142. doi: 10.1136/ard.2007.069831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Langmann T., Liebisch G., Moehle C., Schifferer R., Dayoub R., Heiduczek S., Grandl M., Dada A., Schmitz G. Gene expression profiling identifies retinoids as potent inducers of macrophage lipid efflux. Biochim. Biophys. Acta. 2005;1740(2):155–161. doi: 10.1016/j.bbadis.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 333.Chung J.Y., Thone M.N., Kwon Y.J. COVID-19 vaccines: the status and perspectives in delivery points of view. Adv. Drug Deliv. Rev. 2021;170:1–25. doi: 10.1016/j.addr.2020.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Samudrala P.K., Kumar P., Choudhary K., Thakur N., Wadekar G.S., Dayaramani R., Agrawal M., Alexander A. Virology, pathogenesis, diagnosis and in-line treatment of COVID-19. Eur. J. Pharmacol. 2020;883 doi: 10.1016/j.ejphar.2020.173375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Paschaki M., Cammas L., Muta Y., Matsuoka Y., Mak S.S., Rataj-Baniowska M., Fraulob V., Dollé P., Ladher R.K. Retinoic acid regulates olfactory progenitor cell fate and differentiation. Neural Dev. 2013;8:13. doi: 10.1186/1749-8104-8-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Midha I.K., Kumar N., Kumar A., Madan T. Mega doses of retinol: a possible immunomodulation in Covid-19 illness in resource-limited settings. Rev. Med. Virol. 2020 doi: 10.1002/rmv.2204. e2204, https://pubmed.ncbi.nlm.nih.gov/33382930/ PMID: 33382930 PMCID: PMC7883262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Zepp F. Principles of vaccination. Methods Mol. Biology (Clifton, N.J.) 2016;1403:57–84. doi: 10.1007/978-1-4939-3387-7_3. [DOI] [PubMed] [Google Scholar]
- 338.Sarohan A.R. New strategies for COVID-19 vaccination and adjuvant prophylaxis. J. Vaccines Vaccin. 2021;12(2):1–8. [Google Scholar]
- 339.Vyas N., Kurian S.J., Bagchi D., Manu M.K., Saravu K., Unnikrishnan M.K., Mukhopadhyay C., Rao M., Miraj S.S. Vitamin D in prevention and treatment of COVID-19: current perspective and future prospects. J. Am. Coll. Nutr. 2020:1–14. doi: 10.1080/07315724.2020.1806758. [DOI] [PubMed] [Google Scholar]