Abstract
Objectives
Scarce data are currently available on the kinetics of antibodies after vaccination with mRNA vaccines as a whole and, with mRNA-1273, in particular. We report here an ad-interim analysis of data obtained after a 6-month follow-up in a cohort of healthcare workers (HCWs) who received the mRNA-1273 vaccine. These new data provide more insight into whether and in whom a 3rd dose could be necessary.
Methods
Our study compared the anti-S antibody kinetics at 2 weeks (T1), 3 months (T3) and 6 months (T4) after the first injection, and 2 weeks after the second injection (T2). The 201 participating HCWs were stratified according to their initial serological status. The vaccine effectiveness was also assessed through a medical questionnaire.
Results
We report here a marked and statistically significant antibody decrease (P < 0.05) between T3 and T4, especially in naïve vaccinees. The analysis of potential confounding factors or known risk factors for severe COVID-19 disease did not reveal any influence on the drop observed. Six-month after vaccination, only one, symptomatic, infection was reported in our cohort.
Conclusions
In a supply-limited environment, our results plead for reserving the 3rd dose scheme, in the upcoming months, to seronegative individuals prior to vaccination, especially when the serological status is easily accessible.
Keywords: SARS-CoV-2, COVID-19, Immunogenicity, Efficacy, mRNA-1273 vaccine
Introduction
Vaccines are currently one of the most effective weapons to fight the novel coronavirus severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and eradicate the enduring pandemic.1 The health situation remains worrying. On August 7, the Johns Hopkins’ University assessment reported that the number of confirmed cases exceeds 201.783.223, the number of deaths worldwide stands at 4.278.676 and the vaccine doses administered reached 4.370.877.650.2
At the eighth meeting of its emergency committee, WHO launched a global call to step up immunization and apply social and public health measures in a rational manner. The committee also examined critical issues such as inequalities in access to vaccines against the 2019 coronavirus disease (COVID-19) globally, further exacerbated by the use of available vaccines for groups larger than priority populations recommended by the Strategic Advisory Group of Experts on Immunization (SAGE),3 and by the administration of booster doses, when many countries do not have access to sufficient initial doses.4 The question of the need for booster doses remains open. Although some countries like France, Belgium and Israel have already decided and introduced a 3rd dose for severely immunocompromised people, it is still unclear to what extent adding a 3rd dose in the general population offers additional protection not only against disease, but also against infection and transmission.
Among the different vaccine platforms developed against SARS-CoV-2, mRNA vaccines (BNT162b2 and mRNA-1273) were the firsts to obtain marketing authorization from both the European Medicines Agency (EMA) and the Food and Drug Administration (FDA).5 Moderna vaccine (mRNA-1273) is a lipid nanoparticle vaccine containing mRNA that codes for the Spike (S) protein. This protein is located on the surface of the SARS-CoV-2 envelope and allows it to bind to a cellular receptor (ACE-2) and then enter cells: its role in infection is therefore central.6 The efficacy of this vaccine against COVID-19 was initially evaluated at 94.5% in the large-scale phase 3 study conducted by the manufacturer.7 More recently, on August 5, during the second quarter financial results’ presentation, Stéphane Bancel, the CEO of the company, unveiled the final blinded analysis of Phase 3 COVE study showing 93% of efficacy of their vaccine and affirming that it lasts through six months after the second dose.8 Scientific data results are awaiting publication.
However, despite this reassuring announcement, the data currently available on the durability of the antibody response after mRNA-1273 vaccination is very limited 9, 10, 11, 12, 13, 14, 15 and only the study conducted by the firm Moderna goes up to 6 months after vaccination. However, other limitations appear: this study was carried out on a limited number of participants (33) only stratified according to age, without categorizing them according to their initial vaccination status and without analyzing whether other confounding factors could influence the humoral response.11
To provide new data on the kinetics of the evolution of antibodies, which would allow part of the response to this important public health problem, namely whether a 3rd dose is necessary, we report here an ad-interim analysis of data obtained after a 6-month follow-up in a cohort of healthcare workers (HCWs) who received the mRNA-1273 vaccine. If a significant drop in antibodies is observed, this study also aims to identify the factors influencing the decrease in antibodies to target the group (s) of individuals who should receive a 3rd dose as a priority.
Materials and methods
Study design and clinical procedures
This large prospective study started in January 2021, at the Iris Sud Hospitals (HIS-IZZ, Brussels, Belgium) a 550-bed public hospital group distributed on four sites, in the specific context of the vaccination of healthcare personnel. This hospital is one of the first chosen in Belgium by the federal and regional authorities to participate in the vaccination pilot project for the Brussels region, which began on Monday, January 18. Thanks to this initiative, mature and new data for the antibody response at 6 months following vaccination were collected.
201 participants have been enrolled in this study to evaluate the kinetics of anti-SARS-CoV-2 antibodies after COVID-19 vaccination over a 2-year period. The main inclusion criteria for participation in this study were: eligibility for vaccination according to the Belgian immunization program, be a member of the HIS-IZZ healthcare personnel, have given their written consent after being informed about the purpose of the research and have carried out a first SARS-CoV-2 serology maximum 7 days before the first vaccination or on the day of the vaccination accompanied by a negative PCR test. The study exclusion criteria are as follows: refusal to give consent to the study and contraindications to close blood samples (anemia, etc.).
This study is in accordance with the Declaration of Helsinki and International Conference for Harmonization for good practice and has been approved by the Ethical Committee of the HIS-IZZ (ethical agreement number: CEHIS/2021-007). Subject data were kept confidential in accordance with the rules of the General Data Protection Regulation and all HCWs gave informed consent before participating in the study.
Clinical data
For each recruited HCW, demographic information (sex, age, ABO and RH1 red blood cell antigens) as well as data on the presence of risk factors for developing severe COVID-19 disease (Body Mass Index (BMI), immunodeficiency, chronic lung disease, cardiovascular disease, diabetes, liver disease)16 were collected on the informed consent form and entered into a standardized database by trained clinicians.
When taking each new sample, the participants were asked to declare any results of RT-qPCR tests regardless of the reason behind, even in asymptomatic situations, and any eventual COVID-19 infection after vaccination (including severity of symptoms if any).
Measurement of antibodies
The evaluation of the antibody response of HCWS having received two doses of the mRNA-1273 vaccine was carried out according to the following sequence: at T0 (the day of vaccination or maximum 7 days before), 2 weeks after the first injection (T1), 2 weeks after the second injection (T2), 3 months (T3) and 6 months (T4) after the first injection. The second dose was administered 28 days after the first one. After vein puncture, blood samples were collected in serum collection tubes (BD Vacutainer SST II advance, BD, Plymouth, UK) according to standardized operating procedure. Samples were centrifuged at 3500 rpm (2451 g) for 10 min. Serum was then collected, and samples were analyzed as soon as possible. In case the analyzes were delayed, samples were aliquoted and stored between 2 and 8°C for a maximum of 3 days.
Only participants who took at least 4 samples out of 5 (T0 mandatory), were included in this intermediate analysis 6 months post-vaccination. The quantification of the anti-SARS-CoV-2 IgG antibodies directed against the subunits (S1) and (S2) of the virus spike protein was carried out using the LIAISON®SARS-CoV-2 IgG kit on a LIAISON®XL analyzer in accordance with the manufacturer's instructions.17
Statistics
In each cohort of participants (naïve and previously infected vaccines), the influence of different confounding factors (sex, age, BMI, immunodeficiency, chronic lung disease, cardiovascular disease, diabetes, liver disease, ABO and RH1 group) on the antibody fall observed between T3 and T4 was assessed using univariable logistic regression. Results are reported as odds ratio with 95% confidence interval.
Paired and independent sample values were compared using a Wilcoxon test and a Mann-Whitney test respectively. A P-value < 0.05 was considered significant. A Wilcoxon test was used to assess the changes in IgG levels between T0–4 times within seronegative and seropositive subjects. A Mann-Whitney test was used to compare the antibody decrease at T4 vs. T3 between the 2 groups. Data analysis was performed using Graphpad Prism software (version 9.1.0, San Diego, CA, USA) and MedCalc version 10.4.0.0 (MedCalc Software, Ostend, Belgium).
Results
Characteristics of participants
This study included 201 HCWs, who received two doses of mRNA-1273 vaccine according to the Moderna initial notice (28-day delays between injections). The demographic characteristics of the HCWs are shown in Table 1 .
Table 1.
Demographic characteristics of the HCWs.
| Overall | Seronegative | Seropositive | ||
|---|---|---|---|---|
| Demography | N | 201 | 158 (78,6%) | 43 (21,4%) |
| Age (median - 95%CI) | 50,1 (46,9-52,4) | 49,4 (46,6-52,7) | 51,2 (45,5-54,1) | |
| Age < = 50 | 100 (49,8%) | 81 | 19 | |
| Age > 50 | 101 (50,2%) | 77 | 24 | |
| Female | 150 (74,6%) | 118 | 32 | |
| Male | 51 (25,4%) | 40 | 11 | |
| Blood Groups | A | 71 (39,7%) | 56 | 15 |
| B | 17 (9,5%) | 13 | 4 | |
| AB | 3 (1,7%) | 3 | 0 | |
| O | 88 (49,2%) | 68 | 20 | |
| RH1 | 146 (81,6%) | 117 | 29 | |
| Not documented | 22 | |||
| Risk Factors | BMI (N = 167) (median - 95%CI) | 24,6 (24,1-25,6) | 24,4 (23,6-25,1) | 26,9 (24,4-28,9) |
| Pulmonary diseases | 13 (9,2%) | 11 | 2 | |
| Cardiovascular diseases | 6 (4,3%) | 5 | 1 | |
| Diabetes | 4 (2,8%) | 3 | 1 | |
| Immunodeficiency | 4 (2,8%) | 3 | 1 |
Kinetics of SARS-CoV-2 IgG antibodies
To assess the serological status of the participants (n = 201), a first dosage was carried out with a median time (± 95% confidence interval [CI]) of 2 [1,2] days before the initial injection (T0). Among those, 158 participants were found to be seronegative and 43 seropositive. Only participants with at least 4 over the following 5 sampling timepoints were included in our analysis; 2 weeks after the first injection (T1) (median time [± 95% CI]: 16 [16,17] days), and the second injection (T2) (median time [± 95% CI]: 42 [42-42] days),); 3 months (T3) (median time [± 95% CI]: 86 [85,86] days) and 6 months (T4) (median time [± 95% CI]: 174 [174-174] days) after the first injection.
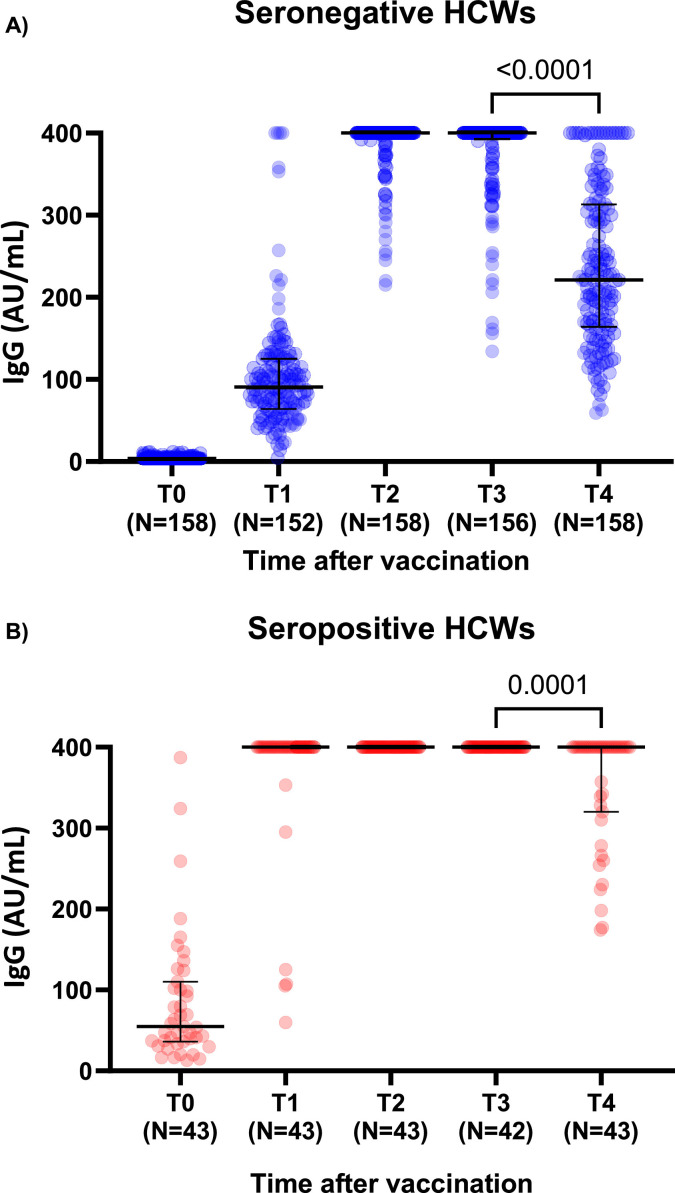
Six months after vaccination, we observed a statistically significant drop in antibody levels in both seronegative and seropositive participants (Wilcoxon; P < 0.05). Antibody values went from 400 [400-400] AU/mL at T3 to 221.0 [202.3-241.2] AU/mL at T4, and from 400 [400-400] AU/mL at T3 to 400 [365.0-400] AU/mL at T4, in the seronegative and seropositive groups, respectively (Fig. 1 A and B). At T4, the levels seen in the seronegative group (221.0 [203.0–241.4] AU/mL) are closer to the ones observed at T1 (90.8 [82.8–100.0] AU/mL) (P > 0.05) than the ones obtained at T3 (400 [400-400] AU/mL). All participants still had detectable SARS-CoV-2 IgG antibodies up to 6 months after a complete vaccination scheme.
Fig. 1.
Immunogenicity of mRNA-1273 COVID vaccine after 6 months surveillance in health care workers; a third dose is necessary
It shows the titers of SARS-CoV-2 IgG antibodies directed against the subunits (S1) and (S2) of the virus spike protein before (T0), 2 weeks after the first injection (T1), 2 weeks after the second injection (T2), 3 months (T3) and 6 months (T4) after the first infection according to the participant serological status (n = 201). Data are presented with median and interquartile range. A P-value < 0.05 was considered significant.
A total of 152/201 (137 seronegative and 15 seropositive) participants had their antibody levels drop 6 months after the first injection, more specifically, between T3 and T4. A sharper decrease is seen in the naïve vaccinees compared to the previously exposed participants (Mann-Whitney; P < 0.0001).
At T4, among the seropositive group, only 15/43 had their antibody values fell. Of note, 5 out of 6 needed a second injection in order to reach the 400 AU/mL threshold . Moreover, all were older than 40 years and RH:1. The subgroup was gender-balanced, 4 had a BMI>26 kg/m2 and 3 had risk factors.
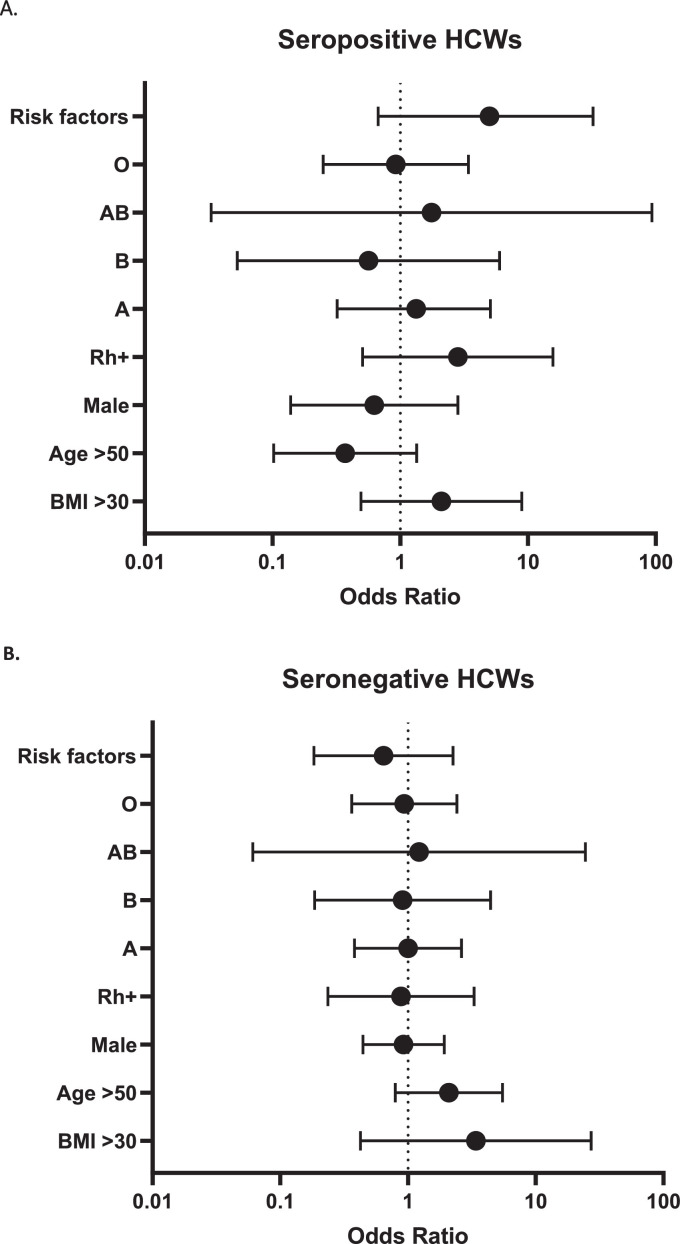
Influence of potential confounding factors
No influence of the factors studied (sex, age, ABO and RH1 group as well as risk factors for developing severe COVID-19 disease (BMI> 30 kg/m2, immunodeficiency, chronic lung disease, cardiovascular disease, diabetes, liver disease) was observed either among naïve participants or initially seropositive HCWs (Fig. 2 ).
Fig. 2.
Odds ratio with 95% confidence interval for univariate associationwith decline in IgG between T3 and T4 in seropositive (A) and seronegative (B) HCWs.
Clinical follow-up
During each sampling, a clinical follow-up questionnaire was sent to participants. 161 were returned at the time of redaction (T4). Analysis of the clinical follow-up questionnaires revealed that only one participant has been infected (on 11 August: result of RT-qPCR was positive). She presented with a flu syndrome and had 202 UA/mL antibodies against SARS-CoV-2 on July the 23rd (T4). A SARS-CoV-2 mutation identification method using RT-qPCR (AllplexTM SARS-CoV-2 Variants I and II, Seegene Technologies, Seoul, South Korea) revealed the presence of the E484K mutation, pointing to the delta variant.
The other respondents reported thinking they had not been infected (n = 160) since vaccination (6 months). 46 of them underwent RT-qPCR which all tested negative.
Discussion
Kinetics of antibody development 6 months after vaccination
Very little data has been published on the evolution of antibodies after vaccination with mRNA vaccines as a whole (BNT162b2 and mRNA-1273) 9, 10, 11, 12, 13, 14, 15 , 18, 19, 20, studies on mRNA-1273 being even rarer. The results of our study extend the evidence provided by Doria-Rose et al.11 that anti-SARS-Cov-2 antibodies persist 6 months after vaccination with mRNA-1273. Although no seronegativation was observed in our participants, we report a marked and statistically significant decrease (P < 0.05) in antibodies observed between T3 and T4, especially in naïve vaccinees. These results are not seen in the Doria-Rose et al. study probably because of the smaller cohort (33 participants) and the absence of stratification following prior exposure to the virus.
The analysis of potential confounding factors on the drop in antibodies observed between T3 and T4 did not reveal any influence of all the parameters studied: sex, age, BMI, ABO and RH1 group as well as risk factors to develop severe COVID-19 disease (immunodeficiency, chronic lung disease, cardiovascular disease, diabetes, liver disease). This applies to both naïve participants and previously exposed HCWs. Worth mentioning, 3-month studies conducted with BNT162b2 showed that younger age was associated with superior antibody responses and older age with lower ones.20,21 Further studies at 6 months are expected to confirm our results.
We identified several limitations of our study: Even though titers of antibodies against S1 spike protein seem to correlate with viral neutralization studies 1, 2, 3 , 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, other studies including measurement of viral neutralization are expected to confirm our observations.
In addition, the cohort studied is limited to HCWs with a median age of 50.1 (95% CI : 46,9-52,4) years. Our study only includes 4 participants over the age of 65. Finally, other parameters have been studied in a limited number, such as certain risk factors (pulmonary diseases, cardiovascular diseases, diabetes, immunodeficiency) and even certain blood groups (B, AB).
Vaccination strategy in the fight against SARS-CoV-2; national and global perspectives
In order to speed up vaccination campaigns, various strategies relating to mRNA vaccines have been studied. Based on modeling studies, a first approach focused on delaying the second dose administration but prioritizing it for people aged ≥ 65. This can result in lower mortality rates compared with the standard two dose strategy, even at vaccination rates up to 1% of the population per day.22 Other studies have shown that a single dose of mRNA vaccine may be sufficient in seropositive individuals prior to vaccination as the additional protective effect of the second dose has yet to be demonstrated in these individuals.1, 2, 3 , 5, 6, 7, 8 , 12, 13, 14, 15, 16 , 18 , 19 , 23 , 24
Thus, the vaccination schedule has already been reviewed by some countries: the French National Authority for Health (HAS) in France proposes to vaccinate patients who have had an infection (symptomatic or not) by SARS-CoV-2, confirmed by a PCR or antigenic test, to be vaccinated within a period of almost 6 months post-infection (and not before a period of 3 months post-infection) with a single administration of mRNA vaccine.25
Alongside this strategy, the question of the need for booster doses remains open. In a context where many countries do not have access to sufficient initial doses, sparing certain doses has a major impact in terms of public health. Our results argue for an additional dose of vaccine in the upcoming months primarily in the naïve population. The severely immunocompromised and seniors do, or soon will, benefit from a 3rd dose in various countries such as the United States, Belgium, France, the United Kingdom, Germany, or Israel.
Six-month post vaccination, only one infection was reported in our cohort. This not only confirms the high efficacy of the mRNA-1273 vaccine 7 but also aligns with the results of the study of Bergwerk et al. who reported very rare breakthrough infections among HCWs vaccines with BNT162b2.26 However, additional long-term studies will be necessary to confirm these observations.
Conclusion
The pandemic remains a challenge globally as countries face different health, economic and social imperatives. Introducing a booster dose, under certain conditions, could have a significant impact in terms of public health. We report here for the first time an ad-interim analysis of independent data obtained after a 6-month follow-up in a cohort of HCWs who received the mRNA-1273 vaccine. Our results plead, in a supply-limited environment, for reserving the third dose scheme, in the upcoming months, to seronegative individuals prior to vaccination.
Ethical approval
This study has been approved by the Ethical Committee of the HIS-IZZ (ethical agreement number: CEHIS/2021-007).
Funding
None declared.
CRediT authorship contribution statement
Marie Tré-Hardy: Visualization, Conceptualization, Investigation, Funding acquisition, Formal analysis, Data curation, Supervision, Writing – original draft, Writing – review & editing. Roberto Cupaiolo: Visualization, Conceptualization, Investigation, Funding acquisition, Formal analysis, Data curation, Writing – review & editing. Alain Wilmet: Visualization, Conceptualization, Investigation, Writing – review & editing. Thomas Antoine-Moussiaux: Visualization, Conceptualization, Writing – review & editing. Andrea Della Vecchia: Visualization, Conceptualization, Writing – review & editing. Alexandra Horeanga: Visualization, Conceptualization, Writing – review & editing. Emmanuelle Papleux: Visualization, Conceptualization, Writing – review & editing. Marc Vekemans: Visualization, Conceptualization, Writing – review & editing. Ingrid Beukinga: Visualization, Conceptualization, Investigation, Writing – review & editing. Laurent Blairon: Visualization, Conceptualization, Investigation, Funding acquisition, Formal analysis, Data curation, Supervision, Writing – review & editing.
Conflicts of Competing Interest
The authors have no relevant competing interest to disclose in relation to this work.
Acknowledgments
The authors thank all the members of the clinical laboratory staff for technical assistance. We also thank the HCWs who participated in this study.
References
- 1.Harvey W T, Carabelli A M, Jackson B, Gupta R K, Thomson E C, Harrison E M, et al. SARS-CoV-2 variants, spike mutations and immune escape. Nat Rev Microbiol. 2021;19(7):409–424. doi: 10.1038/s41579-021-00573-0. Doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.John Hopkins University. Coronavirus Resource Center. Available at https://coronavirus.jhu.edu/map.html. Accessed August 7, 2021, 2021.
- 3.World Health Organisation. WHO SAGE Roadmap For Prioritizing Uses Of COVID-19 Vaccines In The Context Of Limited Supply. An approach to inform planning and subsequent recommendations based on epidemiological setting and vaccine supply scenarios. 2021.
- 4.World Health Organisation. Déclaration sur la huitième réunion du Comité d'urgence du Règlement sanitaire international (2005) concernant la pandémie de maladie à coronavirus 2019 (COVID-19) 2021.
- 5.Yan Y, Pang Y, Lyu Z, Wang R, Wu X, You C, et al. The COVID-19 Vaccines: Recent Development, Challenges and Prospects. Vaccines (Basel) 2021;9(4):349. doi: 10.3390/vaccines9040349. Doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walls A C, Park Y-J, Tortorici M A, Wall A, McGuire A T, Veesler D. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell. 2020;183(6):1735. doi: 10.1016/j.cell.2020.11.032. Doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baden L R, El Sahly H M, Essink B, Kotloff K, Frey S, Novak R, et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. Doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bancel S. Business Updates, Second Quarter 2021. Financial Results. 2021 [Google Scholar]
- 9.Widge A T, Rouphael N G, Jackson L A, Anderson E J, Roberts P C, Makhene M, et al. Durability of Responses after SARS-CoV-2 mRNA-1273 Vaccination. N Engl J Med. 2021;384(1):80–82. doi: 10.1056/NEJMc2032195. Doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anderson E J, Rouphael N G, Widge A T, Jackson L A, Roberts P C, Makhene M, et al. Safety and Immunogenicity of SARS-CoV-2 mRNA-1273 Vaccine in Older Adults. N Engl J Med. 2020;383(25):2427–2438. doi: 10.1056/NEJMoa2028436. Doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doria-Rose N, Suthar M S, Makowski M, O'Connell S, McDermott A B, Flach B, et al. Antibody Persistence through 6 Months after the Second Dose of mRNA-1273 Vaccine for Covid-19. N Engl J Med. 2021 doi: 10.1056/NEJMc2103916. Doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krammer F, Srivastava K, Alshammary H, Amoako A A, Awawda M H, Beach K F, et al. Antibody Responses in Seropositive Persons after a Single Dose of SARS-CoV-2 mRNA Vaccine. N Engl J Med. 2021 doi: 10.1056/NEJMc2101667. Doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tré-Hardy M, Cupaiolo R, Wilmet A, Beukinga I, Blairon L. Waning antibodies in SARS-CoV-2 naïve vaccinees: Results of a three-month interim analysis of ongoing immunogenicity and efficacy surveillance of the mRNA-1273 vaccine in healthcare workers. J Infect. 2021 doi: 10.1016/j.jinf.2021.06.017. S0163-4453(21)00314-5Doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tré-Hardy M, Cupaiolo R, Papleux E, Wilmet A, Horeanga A, Antoine-Moussiaux T, et al. Reactogenicity, safety and antibody response, after one and two doses of mRNA-1273 in seronegative and seropositive healthcare workers. J Infect. 2021;83(2):237–279. doi: 10.1016/j.jinf.2021.03.025. Doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iddins B O, Buck B, Cato T, Partin A, Attia K, Wesh C, et al. mRNA SARS-CoV-2 Immunization Confers Robust Antibody Response in Occupational Healthcare Workers and Fosters Workplace Safety. J Occup Environ Med. 2021;63(5):e314–e317. doi: 10.1097/JOM.0000000000002187. Doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gómez-Ochoa S A, Franco O H, Rojas L Z, Raguindin P F, Roa-Díaz Z M, Wyssmann B M, et al. COVID-19 in Health-Care Workers: A Living Systematic Review and Meta-Analysis of Prevalence, Risk Factors, Clinical Characteristics, and Outcomes. Am J Epidemiol. 2021;190(1):161–175. doi: 10.1093/aje/kwaa191. Doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tré-Hardy M, Wilmet A, Beukinga I, Dogné J-M, Douxfils J, Blairon L. Validation of a chemiluminescent assay for specific SARS-CoV-2 antibody. Clin Chem Lab Med. 2020;58(8):1357–1364. doi: 10.1515/cclm-2020-0594. Doi: [DOI] [PubMed] [Google Scholar]
- 18.Favresse J, Bayart J-L, Mullier F, Elsen M, Eucher C, Van Eeckhoudt S, et al. Antibody titres decline 3-month post-vaccination with BNT162b2. Emerg Microbes Infect. 2021;10(1):1495–1498. doi: 10.1080/22221751.2021.1953403. Doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Manisty C, Otter A D, Treibel T A, McKnight Á, Altmann D M, Brooks T, et al. Antibody response to first BNT162b2 dose in previously SARS-CoV-2-infected individuals. Lancet. 2021 doi: 10.1016/S0140-6736(21)00501-8. Doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Terpos E, Trougakos I P, Karalis V, Ntanasis-Stathopoulos I, Gumeni S, Apostolakou F, et al. Kinetics of Anti-SARS-CoV-2 Antibody Responses 3 Months Post Complete Vaccination with BNT162b2; A Prospective Study in 283 Health Workers. Cells. 2021;10(8):1942. doi: 10.3390/cells10081942. Doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salvagno G L, Henry B M, Pighi L, De Nitto S, Gianfilippi G L, Lippi G. Three-month analysis of total humoral response to Pfizer BNT162b2 mRNA COVID-19 vaccination in healthcare workers. J Infect. 2021;83(2):e4–e5. doi: 10.1016/j.jinf.2021.06.024. Doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Romero-Brufau S, Chopra A, Ryu A J, Gel E, Raskar R, Kremers W, et al. Public health impact of delaying second dose of BNT162b2 or mRNA-1273 covid-19 vaccine: simulation agent based modeling study. BMJ. 2021;373:n1087. doi: 10.1136/bmj.n1087. Doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wise J. Covid-19: People who have had infection might only need one dose of mRNA vaccine. BMJ. 2021;372:n308. doi: 10.1136/bmj.n308. Doi: [DOI] [PubMed] [Google Scholar]
- 24.Anichini G, Terrosi C, Gandolfo C, Gori Savellini G, Fabrizi S, Miceli G B, et al. SARS-CoV-2 Antibody Response in Persons with Past Natural Infection. N Engl J Med. 2021;385(1):90–92. doi: 10.1056/NEJMc2103825. Doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haute Autorité de Santé H.A.S. Saint-Denis La Plaine; 2021. Stratégie de vaccination contre le SARS-CoV-2 - Vaccination des personnes ayant un antécédent de Covid-19; p. 2021.https://www.has-sante.fr/upload/docs/application/pdf/2021-02/strategie_de_vaccination_contre_le_sars-cov-2___vaccination_des_personnes_ayant_un_antecedent_de_covid-19_-_synthese.pdf Available at. Accessed August 7. [Google Scholar]
- 26.Bergwerk M, Gonen T, Lustig Y, Amit S, Lipsitch M, Cohen C, et al. Covid-19 Breakthrough Infections in Vaccinated Health Care Workers. N Engl J Med. 2021 doi: 10.1056/NEJMoa2109072. Doi: [DOI] [PMC free article] [PubMed] [Google Scholar]