Abstract
Introduction
The characteristics of leukopenia in patients with systemic lupus erythematosus (SLE) in different studies are different, which may be related to region, race, and sample size. Moreover, the extent of leukocyte count decline remains to be studied. This study aimed to analyze the clinical characteristics of leukopenia in patients with SLE of Han ethnicity in China.
Methods
A single-center, retrospective, cross-sectional study was conducted in Chinese Han patients with SLE from June 2013 to August 2020.
Results
A total of 125 patients with SLE were included in the study, and 104 age- and sex-matched healthy controls were recruited. The prevalence of leukopenia, neutropenia, and lymphopenia was 40.0, 20.8, and 55.2%, respectively. The median leukocyte count in the leukopenia group was 2.80 × 109/l, the median neutrophil count in the neutropenia group was 1.40 × 109/l, and the median lymphocyte count in the lymphopenia group was 0.60 × 109/l, which was 47.06, 40.58, and 30.00% of the median of the healthy control group, respectively. The lymphocyte count of SLE patients without lymphopenia was also lower than that of healthy controls, and the lymphocyte count was negatively correlated with the SLE disease activity index 2000 score in all patients with SLE. Independent risk factors for neutropenia include decreased platelet count and lymphocyte count, as well as the presentation of cylindruria. For lymphopenia, the independent risk factors were positivity for anti-dsDNA antibody and Coombs’ test, decreased platelet count, and cylindruria.
Conclusions
In Han Chinese patients with SLE, leukopenia, neutropenia, and lymphopenia are common clinical manifestations, and the degree of reduction in blood cell count was also remarkable. Lymphopenia is associated with disease severity in patients with SLE. The correlation between Coombs’ test results and lymphopenia deserves further study.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40744-021-00336-6.
Keywords: Leukopenia, Lymphopenia, Systemic lupus erythematosus, Coombs’ test, SLEDAI
Key Summary Points
| The characteristics of leukopenia in patients with systemic lupus erythematosus (SLE) in different studies are different, and the extent of leukocyte count decline remains to be studied. |
| In Han Chinese patients with SLE, leukopenia, neutropenia, and lymphopenia are common clinical manifestations, and the degree of reduction in blood cell count was also remarkable. |
| The lymphocyte count of SLE patients without lymphopenia was lower than that of healthy controls, and the lymphocyte count was negatively correlated with the SLEDAI 2000 score in patients with SLE. |
Digital Features
This article is published with digital features, including a summary slide, to facilitate understanding of the article. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.14763141.
Introduction
Systemic lupus erythematosus (SLE) is an autoimmune disease that mainly affects women of childbearing age, which has serious adverse effects on the physiology, psychology, and fertility of patients. Leukopenia is a common clinical manifestation in patients with SLE and is usually secondary to lymphopenia, neutropenia, or a combination of both. A study from the Netherlands found that neutropenia occurred in 47% and lymphopenia in 20% of patients with SLE, and life-table analysis showed no adverse influence on survival with neutropenia or lymphopenia [1]. In Turkey, the rates of leukopenia, lymphopenia, and neutropenia in patients with SLE were 57, 82, and 20%, respectively, and leukopenia was commonly observed in patients with skin/mucosal involvement [2]. In a multiethnic, longitudinal outcome study, cumulative lymphopenia was observed in 64.6% and 81.9% at the enrollment and last visit of patients, respectively, and was positively associated with renal involvement, leukopenia, and the presence of anti-double stranded DNA (dsDNA) antibody and anti-Ro antibody, but was negatively associated with photosensitivity [3]. Another study from Japan found that the level of anti-ribosomal P antibody was inversely correlated with the peripheral lymphocyte count [4]. Therefore, the characteristics of leukopenia in different studies are different, which may be related to region, race, and sample size.
Moreover, many studies have not provided a detailed description of the exclusion criteria of patients, such as whether they are accompanied by infection, liver disease, and other diseases that may affect the white blood cell count. Therefore, it is not clear whether the occurrence of leukopenia, lymphopenia, and neutropenia in patients with SLE is solely caused by the disease itself. In addition, current studies only focused on the prevalence of leukopenia, neutropenia, and lymphopenia; however, the degree of decrease in these blood cells relative to healthy controls remains to be studied.
Finally, the clinical characteristics of leukopenia in Chinese patients with SLE have not been reported. Therefore, we analyzed the clinical characteristics of leukopenia in patients with SLE of Han ethnicity in Suzhou, China, and excluded patients with diseases that may have potential effects on leukocytes and those with drug-induced myelosuppression.
Methods
Study Design and Period
A single-center, retrospective, cross-sectional study design was used to assess the clinical characteristics of leukopenia in Han patients with SLE. A retrospective study based on inpatients’ medical records from June 2013 to August 2020 was conducted in the Department of Rheumatology and Immunology, the Second Affiliated Hospital of Soochow University. Healthy subjects of Han ethnicity from the physical examination center were also included in the control group. This study was conducted in accordance with the World Medical Association Declaration of Helsinki and was approved by the Human Ethics Review Committee of the Second Affiliated Hospital of Soochow University. Informed consent was not required from patients and healthy subjects, which was approved by the Human Ethics Review Committee, because the study was based on the participants’ previous medical records.
Inclusion Criteria
Han patients diagnosed with SLE were included in this study. The establishment of SLE diagnosis needed to meet one of the following three criteria: (a) the American College of Rheumatology (ACR) 1997 revised criteria [5, 6]; (b) 2012 Systemic Lupus International Collaborating Clinics classification criteria [7]; or (c) 2019 European League Against Rheumatism/ACR classification criteria [8].
Exclusion Criteria
Patients with SLE were excluded if they had at least one of the following conditions: (a) other autoimmune diseases, such as rheumatoid arthritis, ankylosing spondylitis, systemic sclerosis, antiphospholipid antibody syndrome, or thrombotic microangiopathy; (b) myelosuppression induced by drugs, (c) infectious diseases, (d) pregnancy, (e) malignant disease, (f) hematological disease, (g) blood transfusion, (h) hepatosplenic disease, (i) coronary artery disease, heart failure, or acute coronary syndrome; and (J) diabetes.
Data Collection
The following information was extracted from each SLE patient’s electronic medical records during the same hospitalization: demographic data, clinical findings, organ involvement, serological profile (antinuclear antibody [ANA], anti-dsDNA, anti-Sm, anti-U1RNP, anti-Ro52, anti-Ro60, anti-La, anti-Scl70, anti-Jo, anti-centromere protein B, anti-ribosomal P protein), anti-phospholipid antibody (IgG and IgM anti-cardiolipin, IgG anti-β2-glicoprotein 1), complement 3 (C3), C4, immunoglobulin G (IgG), IgA, IgM, hemocytes, urine routine, direct Coombs’ test, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), and immunosuppressive agents, including corticosteroids. In addition, the SLE disease activity index (SLEDAI) 2000 score was calculated. In healthy controls, only demographic characteristics and blood cell count were collected.
Operational Definitions
Hemocytes were tested using an automatic blood analyzer within 2 h of blood sample collection in EDTA tubes. Leukopenia was defined as a repeated leukocyte count of < 3.5 × 109/l, neutropenia as a repeated neutrophil count of < 1.8 × 109/l, lymphopenia as repeated lymphocyte counts of < 1.1 × 109/l, thrombocytopenia as a repeated platelet count of < 125 × 109/l, and anemia as repeated a hemoglobin level of < 115 g/l.
Untreated patients with SLE were those who had never received immunosuppressive agents (including glucocorticoids, mycophenolate mofetil, methotrexate, leflunomide, cyclophosphamide, etc.), hydroxychloroquine, and immunoglobulin, or have completely interrupted the above treatment for at least 6 months. Otherwise, Otherwise, they were classified as treated patients.
Hypocompleminemia means that the C3 and/or C4 levels of a patient were lower than the lower limit of the normal reference value.
Data Analysis
All variables were analyzed using the IBM Corp. Released 2012. IBM SPSS Statistics for Windows, version 21.0. Armonk, NY: IBM Corp. Continuous variables were presented as mean and standard deviation (for those that have normal distribution) or median and range (for those that have non-normal distribution). Categorical variables were reported as absolute frequencies and percentages from each group or subgroup. Normality was tested using the Shapiro–Wilk test. The Kruskal–Wallis test was used to compare the blood cell count among SLE patients with hemocytopenia, SLE patients without hemocytopenia, and healthy controls. For the comparison of clinical characteristics between the leukopenia group, neutropenia group, lymphopenia group, and the corresponding SLE group without hemocytopenia, normally distributed data were analyzed using Student’s t test, skewed data were analyzed using the Mann–Whitney U test, and categorical variables were analyzed using the Chi-square test or Fisher’s exact test. The correlation between lymphocyte count and SLEDAI 2000 was analyzed using Spearman rank correlation analysis. The binary logistic regression model was applied to identify multiple independent risk factors for the dependent variables neutropenia and lymphopenia. The significance level was set at p < 0.05.
Results
Baseline Characteristics
A total of 172 patients with SLE were reviewed. Among them, 46 cases met the exclusion criteria, including 22 cases of infection, seven cases of rheumatoid arthritis, five cases of liver cirrhosis, three cases of systemic sclerosis, two cases of drug-induced myelosuppression, two cases of pregnancy, and five cases of other diseases (Supplementary Material).
The remaining 125 patients were included in this study. Of these, 60.8% (76 out of 125) patients were treated, 92.8% (116 out of 125) patients were female, their median age was 36.0 years (range, 13.0–81.0 years), and their median symptom duration was 24.0 months (range, 0.1–360.0 months) (Table 1).
Table 1.
Demographic features and organ involvements of SLE patients
| Variables | All patients (n = 125) |
|---|---|
| Age (years), median (range) | 36.0 (13.0–81.0) |
| Female | 116 (92.8) |
| Disease duration (months), median (range) | 24.0 (0.1–360.0) |
| Treated patients | 76 (60.8) |
| ESR (mm/H), median (range) | 28.0 (2.0–142.0) |
| CRP (mg/l), median (range) | 5.4 (0.2–139.8) |
| C3 (g/l), median (range) | 0.520 (0.060–1.120) |
| C4 (g/l),median (range) | 0.100 (0.017–0.389) |
| Hypocompleminemia | 103 (82.4) |
| Fever | 17 (8.0) |
| Rash | 43 (34.4) |
| Alopecia | 18 (14.4) |
| Oral ulcers | 7 (5.6) |
| Pleurisy | 14 (11.2) |
| Pericarditis | 12 (9.6) |
| Joint involvement | 34 (27.2) |
| Myositis | 5 (4.0) |
| Vasculitis | 10 (8.0) |
| Renal involvement | 68 (54.4) |
| Albuminuria | 37 (29.6) |
| Hematuria | 40 (32.0) |
| Leukocyturia | 45 (36.0) |
| Cylindruria | 12 (9.6) |
| Neurologic involvement | 6 (4.8) |
| Leukopenia | 50 (40.0) |
| Leukocyte count (109/l), median (range) | 4.0 (1.3–12.5) |
| Neutropenia | 26 (20.8) |
| Neutrophil count (109/l), median (range) | 2.5 (0.5–9.5) |
| Lymphopenia | 69 (55.2) |
| Lymphocyte count (109/l), median (range) | 0.9 (0.2–3.6) |
| Anemia | 73 (58.4) |
| Hemoglobin (g/l), mean ± SD | 109.1 ± 22.6 |
| Thrombocytopenia | 41 (32.8) |
| Platelet count (109/l), mean ± SD | 167.6 ± 91.2 |
| Anti-dsDNA | 89 (71.2) |
| Anti-Smith | 34 (27.2) |
| Anti-U1RNP | 65 (52.0) |
| Anti-ribosomal P protein | 31 (24.8) |
| Anti-Ro60 | 74 (59.2) |
| Anti-Ro52 | 64 (51.2) |
| Anti-SSB | 16 (12.8) |
| Anti-centromere protein B | 9 (7.2) |
| Anti-phospholipid | 30 (24.0) |
| Direct Coombs’ test | 16 (12.8) |
| SLEDAI 2000 score | 11.0 (0–39.0) |
Except where indicated otherwise, values are n (%)
anti-dsDNA anti-double stranded DNA, C3 complement 3, C4 complement 4, CRP C-reactive protein, ESR erythrocyte sedimentation rate, SLE systemic lupus erythematosus, SLEDAI 2000 systemic lupus erythematosus disease activity index 2000
The prevalence of leucopenia, neutropenia, and lymphopenia were 40.0, 20.8, and 55.2% in total patients, respectively, and were 26.3, 13.2, and 51.3% in treated patients, and were 61.2, 32.7, and 61.2% in untreated patients. There were significant differences in the prevalence of leucopenia (p < 0.001) and neutropenia (p = 0.009) between treated and untreated patients, but there was no difference in the prevalence of lymphopenia (p = 0.277).
The Comparison of Hematocyte Count between Patients with SLE and Healthy Controls
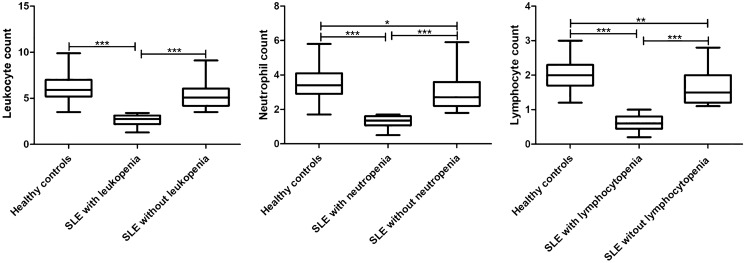
To further clarify the degree of reduction in leukocyte, neutrophil, and lymphocyte counts in patients with SLE, 104 healthy subjects were included as a control group. A total of 91.4% (95 out of 104) healthy controls were female, with a median age of 33.0 years (range, 11.0–81.0 years), and there were no significant differences in these two parameters between the patients with SLE and healthy controls. The median leukocyte, neutrophil, and lymphocyte counts in healthy controls were 5.95 × 109/l, 3.45 × 109/l, and 2.00 × 109/l, respectively. The median leukocyte count in SLE patients with leukopenia was 2.80 × 109/l, the median neutrophil count in SLE patients with neutropenia was 1.40 × 109/l, and the median lymphocyte count in SLE patients with lymphopenia was 0.60 × 109/l, which was 47.06, 40.58, and 30.00% of the healthy control group, respectively. There was no difference in the leukocyte levels between SLE patients without leukopenia and healthy controls, while the neutrophil count of SLE patients without neutropenia was slightly higher than that of healthy controls. However, the lymphocyte levels of SLE patients without lymphopenia were still lower than those of healthy controls (Fig. 1).
Fig. 1.
Comparison of the blood cell count among SLE patients with hemocytopenia, SLE patients without hemocytopenia, and healthy controls. The results are presented as the median (range). (*p < 0.05, **p < 0.01, ***p < 0.001)
Leukopenia
A comparison of the clinical and laboratory features of all patients with SLE, with and without leukopenia, showed that leukopenia was positively associated with neutropenia, lymphopenia, thrombocytopenia, and anti-dsDNA antibody and anti-ribosomal P protein antibody. Neutrophil count, lymphocyte count, platelet count, and C3 levels were lower in patients with leukopenia. In addition, the number of treated patients in the leukopenia group was lower than that in the group without leukopenia (Table 2).
Table 2.
Comparison of clinical and laboratory features between SLE patients with and without leukopenia
| Variables | With leukopenia (n = 50) | Without leukopenia (n = 75) | p |
|---|---|---|---|
| Age (years), median (range) | 35.5 (13.0–74.0) | 37.0 (15.0–81.0) | 0.747 |
| Female | 47 (94.0) | 69 (92.0) | 0.740 |
| Disease duration (months), median (range) | 18.0 (0.2–324.0) | 24.0 (0.1–360.0) | 0.197 |
| Treated patients | 20 (40.0) | 56 (74.7) | < 0.001 |
| C3 (g/l), median (range) | 0.430 (0.060–0.960) | 0.600 (0.200–1.120) | 0.004 |
| Neutropenia | 26 (52.0) | 0 (0) | < 0.001 |
| Neutrophil count (109/l), median (range) | 1.7 (0.5–2.8) | 3.5 (1.9–9.5) | < 0.001 |
| Lymphopenia | 43 (86.0) | 26 (34.7) | < 0.001 |
| Lymphocyte count (109/l), median (range) | 0.7 (0.2–1.4) | 1.3 (0.2–3.6) | < 0.001 |
| Thrombocytopenia | 25 (50.0) | 16 (21.3) | 0.001 |
| Platelet count (109/l), mean ± SD | 131.2 ± 73.0 | 191.9 ± 94.5 | < 0.001 |
| Anti-dsDNA | 41 (82.0) | 48 (64.0) | 0.029 |
| Anti-ribosomal P protein | 18 (36.0) | 13 (17.3) | 0.018 |
Except where indicated otherwise, values are n (%)
anti-dsDNA anti-double stranded DNA, C3 complement 3, SLE systemic lupus erythematosus
Neutropenia
Upon the comparison of patients with and without neutropenia, a significantly higher incidence of leukopenia, lymphopenia, thrombocytopenia, and cylindruria was observed in patients with neutropenia. Along with neutropenia, patients had lower leukocyte counts, lymphocyte counts, platelet counts, and C3 levels. Moreover, fewer patients received treatment in the neutropenia group than in the control group. However, there was no significant difference in the antibody spectra between the two groups (Table 3).
Table 3.
Comparison of clinical and laboratory features between SLE patients with and without neutropenia
| Variables | With neutropenia (n = 26) | Without neutropenia (n = 99) | p |
|---|---|---|---|
| Age (years), median (range) | 42.4 ± 17.3 | 39.0 ± 14.9 | 0.368 |
| Female | 25 (96.2) | 91 (91.9) | 0.684 |
| Disease duration (months), median (range) | 18.0 (0.2–324.0) | 24.0 (0.1–360.0) | 0.434 |
| Treated patients | 10 (38.5) | 66 (66.7) | 0.009 |
| C3 (g/l), mean ± SD | 0.390 (0.060–0.960) | 0.530 (0.200–1.120) | 0.004 |
| Leukopenia | 26 (100.0) | 24 (24.2) | < 0.001 |
| Leukocyte count (109/l), median (range) | 2.3 (1.3–3.4) | 4.6 (2.4–12.5) | < 0.001 |
| Lymphopenia | 20 (76.9) | 49 (49.5) | 0.012 |
| Lymphocyte count (109/l), median (range) | 0.7 (0.3–1.4) | 1.1 (0.2–3.6) | 0.001 |
| Thrombocytopenia | 18 (69.2) | 23 (23.2) | < 0.001 |
| Platelet count (109/l), mean ± SD | 116.3 ± 73.3 | 181.1 ± 91.0 | 0.001 |
| Cylindruria | 6 (23.1) | 6 (6.1) | 0.009 |
Except where indicated otherwise, values are n (%)
C3 complement 3, SLE systemic lupus erythematosus
Lymphopenia
Lymphopenia was positively associated with a number of clinical and laboratory parameters, including leukopenia, neutropenia, thrombocytopenia, anti-dsDNA antibody, anti-ribosomal P protein antibody, Coombs’ test, hypocompleminemia, and cylindruria. Patients with lymphopenia developed decreased leukocyte count, neutrophil count, platelet count, and C3 and C4 levels. However, there was no difference in the treatment factors between the two groups (Table 4).
Table 4.
Comparison of clinical and laboratory features between SLE patients with and without lymphopenia
| Variables | With lymphopenia (n = 69) | Without lymphopenia (n = 56) | p |
|---|---|---|---|
| Age (years), median (range) | 33.0 (19.0–74.0) | 37.5 (13.0–81.0) | 0.221 |
| Female | 65 (94.2) | 51 (91.1) | 0.513 |
| Disease duration (months), median (range) | 36.0 (0.2–360.0) | 15.0 (0.1–240.0) | 0.691 |
| Treated patients | 39 (56.5) | 37 (66.1) | 0.277 |
| C3 (g/l), median (range) | 0.459 (0.060–1.110) | 0.624 (0.070–1.120) | 0.005 |
| C4 (g/l), median (range) | 0.083 (0.020–0.328) | 0.129 (0.017–0.389) | 0.008 |
| Hypocompleminemia | 63 (91.3) | 40 (71.4) | 0.004 |
| Leukopenia | 43 (62.3) | 7 (12.5) | < 0.001 |
| Leukocyte count (109/l), median (range) | 3.2 (1.3–10.6) | 5.3 (2.4–12.5) | < 0.001 |
| Neutropenia | 20 (29.0) | 6 (10.7) | 0.012 |
| Neutrophil count (109/l), median (range) | 2.2 (0.5–9.0) | 2.9 (1.0–9.5) | 0.001 |
| Thrombocytopenia | 29 (42.0) | 12 (21.4) | 0.015 |
| Platelet count (109/l), mean ± SD | 145.5 ± 81.3 | 194.9 ± 96.0 | 0.002 |
| Cylindruria | 10 (14.5) | 2 (3.6) | 0.039 |
| Anti-dsDNA | 56 (81.2) | 33 (58.9) | 0.006 |
| Anti-ribosomal P protein | 22 (31.9) | 9 (16.1) | 0.042 |
| Direct Coombs’ test | 14 (20.3) | 2 (3.6) | 0.006 |
| SLEDAI 2000 score | 12.0 (1.0–34.0) | 9.5 (0–39.0) | 0.016 |
Except where indicated otherwise, values are n (%)
anti-dsDNA anti-double stranded DNA, C3 complement 3, C4 complement 4, CRP C-reactive protein, ESR erythrocyte sedimentation rate, SLE systemic lupus erythematosus, SLEDAI 2000 systemic lupus erythematosus disease activity index 2000
It is worth noting that the SLEDAI 2000 score differed between patients with and without lymphopenia. We further examined the correlation between lymphocyte count and disease activity, and the results showed that lymphocyte count was negatively correlated with SLEDAI 2000 score (p = 0.0016, Spearman correlation coefficient = − 0.2788).
Multivariate Model
To identify multiple independent risk factors for the dependent variables neutropenia and lymphopenia, a binary logistic regression model was applied. Independent risk factors for neutropenia include decreased platelet count (p = 0.024, OR = 0.992, 95% CI = 0.986–0.999) and lymphocyte count (p = 0.042, OR = 0.313, 95% CI = 0.102–0.959), as well as the presentation of cylindruria (p = 0.046, OR = 4.825, 95% CI = 1.030–22.609). For lymphopenia, the independent risk factors were positivity for anti-dsDNA antibody (p = 0.046, OR = 2.542, 95% CI = 1.018–6.349) and Coombs’ test (p = 0.031, OR = 6.116, 95% CI = 1.179–31.716), decreased platelet count (p = 0.018, OR = 0.994, 95% CI = 0.989–0.999), and cylindruria (p = 0.037, OR = 10.683, 95% CI = 1.148–99.458).
Discussion
In the present study, we retrospectively analyzed 125 patients with SLE and found that the prevalence of leukopenia, neutropenia, and lymphopenia was 40.0, 20.8, and 55.2%, respectively. Moreover, the median leukocyte count, neutrophil count, and lymphocyte count in the corresponding hemocytopenia group were only 47.06, 40.58, and 30.00% of the healthy control group, respectively. Therefore, leukopenia, neutropenia, and lymphopenia are not only common clinical manifestations of SLE but also the degree of reduction in the number of leukocytes, neutrophils, and lymphocytes is very remarkable.
The prevalence of leukopenia in patients with SLE reported in the literature varies greatly from 22 to 60% [9, 10]. Leukopenia is usually caused by either or both lymphopenia and neutropenia. Low lymphocyte count commonly occurs in SLE, with a prevalence ranging from 15 to 93%, while neutropenia was described in 4.5% to 47% of patients [1, 9–12]. The significant differences in the prevalence of leukopenia, neutropenia, and lymphopenia among these studies may be related to the number of cases, race, region, treatment, and research method used. First, the prevalence of lymphopenia varies among ethnic populations [3, 13, 14]. This study focused on the characteristics of leukopenia in Han patients with SLE, so only the Han population was included. Second, the prevalence of leucopenia and neutropenia in the treated group was significantly lower than that in the untreated group, suggesting that the treatment reduced the prevalence of leukopenia and neutropenia. Third, accompanying diseases may affect the leukocyte levels. In order to detect the prevalence of leukopenia more accurately, we excluded patients with infection, liver cirrhosis, malignant tumors, other connective tissue diseases, and so on. Finally, this study confirmed that lymphocyte count was negatively correlated with the SLEDAI 2000 score, so the disease status of the included patients may affect the prevalence of lymphopenia.
Leukopenia was positively associated with anti-dsDNA antibody, anti-ribosomal P protein antibody, neutropenia, lymphopenia, and thrombocytopenia in the present study, and patients with leukopenia developed decreased neutrophil counts, lymphocyte counts, platelet counts, and complement C3 levels. In another study, SLE patients with leukopenia also had an increased incidence of lymphopenia, thrombocytopenia, hemolytic anemia, anti-dsDNA antibody positivity, lupus anticoagulant and psychosis [15]. Recent research showed that leukopenia was associated with anti-dsDNA antibody positivity, low complement and ESR ≥ 25 mm/H [16]. We speculated that the correlation between leukopenia and other parameters may be due to the fact that neutropenia or lymphopenia was related to these parameters.
The present study showed that neutropenia was not associated with any of the antibodies studied, but was related to several clinical parameters, including lymphocytes, platelets, complement C3, and cylindruria. In a multivariate analysis, decreased platelet count and lymphocyte count, as well as the presence of cylindruria were identified as independent risk factors for neutropenia. Martinez-Banos et al. carried out a prospective study that included 33 SLE patients with moderate to severe neutropenia (< 1000/μl) [17]. Patients with neutropenia had a lower number of lymphocytes, hemoglobin, and platelets, but no differences in antinuclear antibodies were detected [17]. There was also no significant association between any autoantibody against nuclear antigens and neutropenia in a study including 82 lupus erythematosus patients [18]. A study including 208 SLE patients with neutropenia and 779 SLE patients without neutropenia showed that neutropenia was significantly associated with thrombocytopenia, lymphopenia, and low levels of complement C3 as determined in a multivariate analysis, which was similar to our results [19].
Anti-ribosomal P protein antibody has been reported to be associated with diffuse psychiatric/neuropsychological syndromes in SLE, and was also found to be associated with lymphopenia in several studies [20–24]. In a dataset (GIPT) originating from patients with SLE from six European tertiary centers, only anti-Sm antibody was associated with lymphopenia in a univariate model, while antibodies targeting other nuclear antigens were not associated with lymphopenia [25]. A cross-sectional, well-characterized SLE dataset from Sweden presented that anti-DNA antibody and anti-Sm antibody were associated with lymphopenia as determined using univariate and multivariable analyses [25]. In the present study, lymphopenia was significantly positively associated with anti-dsDNA antibody and anti-ribosomal P protein antibody, and multivariable analysis showed that anti-dsDNA antibody was an independent risk factor for lymphopenia.
Compared with neutropenia, lymphopenia showed a better correlation with the disease activity parameters of SLE, including the C3, C4, and SLEDAI 2000 scores, which was consistent with the results from the GIPT and Sweden SLE datasets. Moreover, in patients with lymphopenia, leukocyte count, neutrophil count, and platelet count decreased, and the presence of cylindruria increased. A multiethnic, longitudinal outcome study from the United States showed that lymphopenia was found to be positively associated with renal involvement, leukopenia and thrombocytopenia [3]. A GIPT and Sweden SLE dataset observed that SLE patients with lymphopenia were more likely to have leukopenia, thrombocytopenia, and neurological manifestations [25]. In addition, Spearman rank correlation analysis showed that the lymphocyte count was negatively correlated with the SLEDAI 2000 score in the present study. Therefore, the results of the above studies, including ours, are consistent despite some differences, suggesting that lymphopenia is associated with systemic damage and disease activity in SLE. In addition, the present study found that the lymphocyte levels of SLE patients without lymphopenia were still lower than those of healthy controls, so even if the lymphocytes of SLE patients are normal, the low level of normal lymphocytes may also indicate SLE disease activity.
Skare et al. found that Coombs’ test was positive in 12.8% of SLE samples studied, and was independently associated with hemolytic anemia, anti-RNP antibody and anti-SSB antibody [26]. In the present study, the positive rate of Coombs’ test was also 12.8%, and there was a positive correlation between Coombs’ test and lymphopenia, but we did not find any description of this relationship previously detected, so the relationship between Coombs’ test and lymphopenia is worthy of further study.
Certain limitations of this study must be acknowledged. First, this was a cross-sectional study, which may have led to the lower prevalence of leukopenia, neutropenia, and lymphopenia. Second, this was a single-center study of the Chinese Han population, so the results of this study may not be suitable for all patients with SLE. Finally, the size of this cohort was small, and the results may be biased; thus, a larger trial is needed to further confirm these results.
Conclusions
In conclusion, leukopenia, neutropenia, and lymphopenia are common clinical manifestations in Chinese Han patients with SLE, and the degree of reduction in the number of leukocytes, neutrophils, and lymphocytes is also very remarkable. Lymphopenia is associated with disease activity in SLE, and the independent risk factors for lymphopenia were positive anti-dsDNA antibody, positive Coombs’ test, decreased platelet count, and the presence of cylindruria. The correlation between Coombs’ test results and lymphopenia deserves further study.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank the participants of the study. We would also like to express our deepest heartfelt thanks to the Second Affiliated Hospital of Soochow University for allowing us to conduct this study.
Funding
This study was funded by the National Nature Science Foundation of China (81800622), the Natural Science Foundation of the Jiangsu Higher Education Institutions of China (18KJB320020), Suzhou Health and Key Talent Project (GSWS2019011), and Jiangsu Social Development Project (BE2019663). All authors had full access to all the data in this study and take full responsibility for the integrity and accuracy of the data analysis. The Rapid Service Fee was funded by the authors.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authorship Contributions
Study conception and design, and funding acquisition, Leixi Xue and Zhichun Liu; data collection and analysis, Wentian Lu, Ying Zhong, Yi Zhang and Leixi Xue; writing-original draft, Wentian Lu and Ying Zhong; writing-review and editing, Leixi Xue. All authors read and approved the final manuscript.
Disclosures
Wentian Lu, Ying Zhong, Yi Zhang, Zhichun Liu, and Leixi Xue have nothing to disclose.
Compliance with Ethics Guidelines
This study was conducted in accordance with the World Medical Association Declaration of Helsinki and was approved by the Human Ethics Review Committee of the Second Affiliated Hospital of Soochow University. Informed consent was not required from patients, which was approved by the Human Ethics Review Committee, because the study was based on the participants’ previous medical records.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Footnotes
Wentian Lu and Ying Zhong contributed equally to this work.
Contributor Information
Zhichun Liu, Email: liuzhichun5190@163.com.
Leixi Xue, Email: xueleixi2002@163.com.
References
- 1.Nossent JC, Swaak AJ. Prevalence and significance of haematological abnormalities in patients with systemic lupus erythematosus. Q J Med. 1991;80:605–612. [PubMed] [Google Scholar]
- 2.Beyan E, Beyan C, Turan M. Hematological presentation in systemic lupus erythematosus and its relationship with disease activity. Hematology. 2007;12:257–261. doi: 10.1080/10245330701214145. [DOI] [PubMed] [Google Scholar]
- 3.Vilá LM, Alarcón GS, McGwin G, Jr, Bastian HM, Fessler BJ, Reveille JD, et al. Systemic lupus erythematosus in a multiethnic US cohort, XXXVII: association of lymphopenia with clinical manifestations, serologic abnormalities, disease activity, and damage accrual. Arthritis Rheum. 2006;55:799–806. doi: 10.1002/art.22224. [DOI] [PubMed] [Google Scholar]
- 4.Takeda I, Iwadate H, Sugisaki K, Takahashi A, Nogae S, Kanno T, et al. Anti-ribosomal P antibodies are associated with nephritis, vascular thrombosis and lymphocytopenia in patients with systemic lupus erythematosus. Fukushima J Med Sci. 2005;51:11–18. doi: 10.5387/fms.51.11. [DOI] [PubMed] [Google Scholar]
- 5.Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271–1277. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 6.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 7.Petri M, Orbai AM, Alarcón GS, Gordon C, Merrill JT, Fortin PR, et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012;64:2677–2686. doi: 10.1002/art.34473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aringer M, Costenbader K, Daikh D, Brinks R, Mosca M, Ramsey-Goldman R, et al. 2019 European League Against Rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus. Ann Rheum Dis. 2019;78:1151–1159. doi: 10.1136/annrheumdis-2018-214819. [DOI] [PubMed] [Google Scholar]
- 9.Fayyaz A, Igoe A, Kurien BT, Danda D, James JA, Stafford HA, et al. Haematological manifestations of lupus. Lupus Sci Med. 2015;2:e000078. doi: 10.1136/lupus-2014-000078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carli L, Tani C, Vagnani S, Signorini V, Mosca M. Leukopenia, lymphopenia, and neutropenia in systemic lupus erythematosus: prevalence and clinical impact–A systematic literature review. Semin Arthritis Rheum. 2015;45:190–194. doi: 10.1016/j.semarthrit.2015.05.009. [DOI] [PubMed] [Google Scholar]
- 11.Rivero SJ, Díaz-Jouanen E, Alarcón-Segovia D. Lymphopenia in systemic lupus erythematosus. Clinical, diagnostic, and prognostic significance. Arthritis Rheum. 1978;21:295–305. doi: 10.1002/art.1780210302. [DOI] [PubMed] [Google Scholar]
- 12.Teke HÜ, Cansu DÜ, Korkmaz C. Detailed features of hematological involvement and medication-induced cytopenia in systemic lupus erythematosus patients: single center results of 221 patients. Eur J Rheumatol. 2017;4:87–92. doi: 10.5152/eurjrheum.2017.160086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pons-Estel BA, Catoggio LJ, Cardiel MH, Soriano ER, Gentiletti S, Villa AR, et al. The GLADEL multinational Latin American prospective inception cohort of 1,214 patients with systemic lupus erythematosus: ethnic and disease heterogeneity among “Hispanics”. Medicine (Baltimore) 2004;83:1–17. doi: 10.1097/01.md.0000104742.42401.e2. [DOI] [PubMed] [Google Scholar]
- 14.Mok CC, Lau CS. Lupus in Hong Kong Chinese. Lupus. 2003;12:717–722. doi: 10.1191/0961203303lu451xx. [DOI] [PubMed] [Google Scholar]
- 15.Skare T, Damin R, Hofius R. Prevalence of the American College of Rheumatology hematological classification criteria and associations with serological and clinical variables in 460 systemic lupus erythematosus patients. Rev Bras Hematol Hemoter. 2015;37:115–119. doi: 10.1016/j.bjhh.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kandane-Rathnayake R, Louthrenoo W, Golder V, Luo SF, Wu YJ, Lateef A, et al. Independent associations of lymphopenia and neutropenia in patients with systemic lupus erythematosus: a longitudinal, multinational study. Rheumatology (Oxford) 2021 doi: 10.1093/rheumatology/keab217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martínez-Baños D, Crispín JC, Lazo-Langner A, Sánchez-Guerrero J. Moderate and severe neutropenia in patients with systemic lupus erythematosus. Rheumatology (Oxford) 2006;45:994–998. doi: 10.1093/rheumatology/kel016. [DOI] [PubMed] [Google Scholar]
- 18.Wenzel J, Gerdsen R, Uerlich M, Bauer R, Tueting T, Bieber T. Lymphocytopenia in lupus erythematosus: close in vivo association to autoantibodies targeting nuclear antigens. Br J Dermatol. 2004;150:994–998. doi: 10.1111/j.1365-2133.2004.05848.x. [DOI] [PubMed] [Google Scholar]
- 19.Meyer A, Guffroy A, Blaison G, Dieudonne Y, Amoura Z, Bonnotte B, et al. Systemic lupus erythematosus and neutropaenia: a hallmark of haematological manifestations. Lupus Sci Med. 2020;7:e000399. doi: 10.1136/lupus-2020-000399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choi MY, FitzPatrick RD, Buhler K, Mahler M, Fritzler MJ. A review and meta-analysis of anti-ribosomal P autoantibodies in systemic lupus erythematosus. Autoimmun Rev. 2020;19:102463. doi: 10.1016/j.autrev.2020.102463. [DOI] [PubMed] [Google Scholar]
- 21.Arinuma Y, Kikuchi H, Hirohata S. Anti-ribosomal P protein antibodies influence mortality of patients with diffuse psychiatric/neuropsychological syndromes in systemic lupus erythematous involving a severe form of the disease. Mod Rheumatol. 2019;29:612–618. doi: 10.1080/14397595.2018.1508801. [DOI] [PubMed] [Google Scholar]
- 22.Shi ZR, Cao CX, Tan GZ, Wang L. The association of serum anti-ribosomal P antibody with clinical and serological disorders in systemic lupus erythematosus: a systematic review and meta-analysis. Lupus. 2015;24:588–596. doi: 10.1177/0961203314560003. [DOI] [PubMed] [Google Scholar]
- 23.Li J, Shen Y, He J, Jia R, Wang X, Chen X, et al. Significance of antibodies against the native ribosomal P protein complex and recombinant P0, P1, and P2 proteins in the diagnosis of Chinese patients with systemic lupus erythematosus. J Clin Lab Anal. 2013;27:87–95. doi: 10.1002/jcla.21543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barkhudarova F, Dähnrich C, Rosemann A, Schneider U, Stöcker W, Burmester GR, et al. Diagnostic value and clinical laboratory associations of antibodies against recombinant ribosomal P0, P1 and P2 proteins and their native heterocomplex in a Caucasian cohort with systemic lupus erythematosus. Arthritis Res Ther. 2011;13:R20. doi: 10.1186/ar3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yavuz S, Cansu DU, Nikolopoulos D, Crisafulli F, Antunes AM, Adamichou C, et al. Lymphopenia as a risk factor for neurologic involvement and organ damage accrual in patients with systemic lupus erythematosus: a multi-center observational study. Semin Arthritis Rheum. 2020;50:1387–1393. doi: 10.1016/j.semarthrit.2020.02.020. [DOI] [PubMed] [Google Scholar]
- 26.Skare T, Picelli L, Dos Santos T, Nisihara R. Direct antiglobulin (Coombs) test in systemic lupus erythematosus patients. Clin Rheumatol. 2017;36:2141–2144. doi: 10.1007/s10067-017-3778-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.