Abstract
Introduction
Psoriatic arthritis (PsA) is considered a multifaceted disease, with patients reporting low health-related quality of life (HRQoL). Data on disease burden are substantial and there exists a need for properly designed studies to learn more about the evolution of HRQoL in this condition. This study aims to identify factors associated to HRQoL evolution in PsA patients followed-up in a real-world setting in Spain.
Methods
We conducted a retrospective longitudinal observational study including incident patients from the rheumatology outpatient clinic of Hospital Clínico San Carlos (Madrid, Spain), diagnosed for the first time of PsA, defined as having received any ICD9/ICD10 diagnosis code of PsA, from 2007 to 2016, and followed-up until loss of follow-up, death, or November 2017. The influence of demographic and clinical variables in baseline HRQoL [assessed with the Rosser Classification Index (RCI)] was analyzed using bivariable and multivariable generalized linear models. The influence of those variables and of treatment-related factors in repeated measures of HRQoL was analyzed using bivariable and multivariable generalized estimating equations (GEE) models nested by patient.
Results
Two hundred and thirty patients were included in the analysis, with 3384 registered visits. At baseline, older age, a previous diagnosis of obesity, and the presence of enthesitis were significantly associated with worse HRQoL. During follow-up, using an exchangeable working correlation structure, the presence of enthesitis was also associated with worse HRQoL, coefficient (95% CI) − 0.006 (− 0.01 to − 0.002), p = 1.00E−03; conversely, treatment with methotrexate or antimalarials was associated with better HRQoL with 0.007 (0.001–0.014), p = 0.020 and 0.003 (0.001–0.005), p = 3.00E−03, respectively.
Conclusions
Musculoskeletal manifestations and comorbidities exert a deleterious effect in HRQoL of PsA patients. Therefore, the optimal management of this condition needs to also address these manifestations in order to try to restore the QoL of these patients.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40744-021-00349-1.
Keywords: Arthritis, Psoriatic, Health-related quality of life, Comorbidities, Generalized estimating equations, Outpatient care
Key Summary Points
| PsA patients report low health-related quality of life (HRQoL) influenced by different disease related factors such as disability, disease activity, pain, particular comorbidities, and musculoskeletal manifestations. |
| Most available studies on the role of the different factors associated to HRQoL in PsA patients are based on cross-sectional methodologies not considering its evolution over a follow-up period or how it is influenced by different disease variables. |
| The objective of this study is to analyze the influence of several clinical aspects, comorbidities, and medications in the evolution of HRQoL in PsA patients followed-up in a rheumatology clinic in Spain. |
| Our study revealed that older age, a previous diagnosis of obesity, and the presence of enthesitis were significantly associated with worse HRQoL at baseline. Presence of enthesitis also resulted in worse HRQoL during the follow-up, while methotrexate or antimalarial treatments were significantly associated with an improvement of this condition. |
| Study findings point to musculoskeletal manifestations and comorbidities as the main factors associated with a worse HRQoL evolution, thus suggesting that an optimal management of the disease needs to properly address these points. |
Introduction
Psoriatic arthritis (PsA) is a chronic and inflammatory disease characterized by a heterogeneous involvement of peripheral and spinal/sacroiliac joints and the presence of psoriasis (both of the skin and nails), enthesitis, and dactylitis [1, 2]. Joint disease includes asymmetric sacroiliitis, new bone formation, ankyloses, and the presence of erosions (sometimes causing destructive arthritis). Moreover, PsA is frequently linked to comorbidities (obesity, hypertension, dyslipidemia, type II diabetes mellitus, cardiovascular disease, depression and sleep disturbances [3–5]), and increased mortality [6, 7].
The burden of PsA is comparable to that of RA [8], and impacts both psychological and physical well-being. PsA patients report lower health-related quality of life (HRQoL) than the general population and psoriasis patients without articular involvement [8–11]. Moreover, the presence of both cutaneous and articular disease was associated with a significant burden across multiple outcomes, such as work productivity, even in patients with mild symptoms [12].
Several studies have analyzed in PsA the role of several factors in HRQoL, including disability, disease activity, pain, particular comorbidities, and musculoskeletal manifestations (presence of peripheral arthritis, axial involvement, and enthesitis [13–18]). However, most studies were cross-sectional, including patients with longstanding disease, and therefore there is a need for properly designed studies to learn more about the evolution of the HRQoL throughout the follow-up in this heterogeneous condition, analyzing the influences of different clinical aspects, comorbidities, and disease treatments.
In this sense, the purpose of this study was to analyze the impact of several clinical aspects, comorbidities, and medications in the evolution of the HRQoL in PsA patients during their follow-up in an outpatient rheumatology clinic using real-world data extracted from a departmental electronic health record (EHR).
Methods
Patients
This is a retrospective longitudinal observational study, including patients diagnosed with PsA belonging to the Hospital Clínico San Carlos musculoskeletal cohort (HCSC-MSKC). This routine clinical practice cohort includes subjects seen at the rheumatology outpatient clinic of the Hospital Clínico San Carlos (Madrid, Spain) whose clinical information and management was carried out using a departmental EHR. Further details have been previously reported [19].
In the present study, we selected patients with the following criteria: (a) first visit at our outpatient clinic between January 2007 and December 2016; (b) diagnosed for the first time of PsA in our outpatient clinic, defined as having received any ICD9/ICD10 diagnosis code of PsA by their attending rheumatologist (Supplementary Table S1), and no record of a previous PsA diagnosis before attending our clinic; (c) at least two registered visits with their respective HRQoL measures, and; (d) symptoms onset after 16 years of age. Patients were followed-up until loss of follow-up, death, or November 2017 (whichever occurred first). Only patients with impossibility to access their clinical information were excluded.
HCSC Ethics Review Board approval was obtained as a retrospective study (Reference 19/342-E) and waiver of informed consent was granted for use of de-identified clinical records. Furthermore, the study was conducted in accordance with the Declaration of Helsinki.
Data Source
Demographic and clinical information was obtained from our departmental EHR, which is used in all outpatient interactions between health professionals and patients. In each patient visit, information is collected both as free text (including clinical notes, and comorbidity or medications prescribed by other physicians) and codified [including rheumatology diagnoses (using the ICD9/ICD10), prescribed drugs (using the Spanish Drug and Medical Device Agency codification system), HRQoL (using the Rosser Classification Index: RCI [20]), and the patient’s follow-up plan.
Variables
Dependent Variable
The outcome of interest in this study was the patient’s HRQoL, measured using the RCI [20–22]. This measure of overall health status is a standardized instrument applicable to a wide range of health conditions. It evaluates two dimensions: disability (in a seven-level scale) and distress/pain (in a four-level scale). The combination from both subscales provides a single index value for HRQoL, ranging from 1 (perfect health) to 0 (value assigned to a state of death), with negative values representing states worse than death.
Reliability and validity of the RCI for musculoskeletal conditions has been previously assessed [20, 23]. This variable is codified and collected in every patient’s visit of our rheumatology clinic.
Independent Variables
We collected data at every outpatient visit of variables potentially relevant regarding their influence in the evolution of the HRQoL, including demographic; disease-related; musculoskeletal manifestations; extra-articular manifestations recorded by the rheumatologist; comorbidities; and treatment-related. A detailed list of the analyzed variables can be found at Supplementary Data S1.
Statistical Analysis
Continuous variables were expressed as median values (and first and third quartiles). Categorical variables were expressed as frequencies and percentages.
For the analyses of categorical risk factors for RCI, only independent variables with prevalence ≥ 5% (baseline analysis) or ≥ 1% (longitudinal analysis) were studied. In case of variables with more than two categories, those categories with prevalence below the thresholds were grouped until the new categories surpassed those thresholds.
Factors influencing the RCI at first visit in our outpatient clinic were analyzed using bivariable generalized linear regression (GLM) models. Those variables with a p value < 0.15 (plus age, gender, calendar year of diagnosis, and those variables which could be of clinical importance, as defined by clinicians) were introduced in a multivariable GLM model. Different multivariable models were compared using the Akaike information criteria (AIC).
To compare the evolution of the RCI at pre-specified time points (first visit, 1, 2, 5, and 10 years of follow-up), those visits closest to the time points (± 3 months) were selected, and median and first and third quartiles for the RCI were calculated for each time point. A comparison with the baseline visit (meaning, the first visit in our outpatient clinic) was analyzed out using generalized estimating equations (GEE) models nested by patient [24, 25], analyzing the variable time (considered as the “elapse time from the first visit to the pre-specified time point”) as a categorical variable. No other covariates were included in this model. We used GEE, as these models allow us to take into account both the inter-subject and intra-subjects variability, and they are robust when the main outcome variable (RCI) does not have a Gaussian distribution [26]. In all the generalized estimating equations models a Gaussian family, and an identity link function were used. Different covariable structures were tested (independent and exchangeable) and compared using the quasi-Akaike information criterion (QIC [27]).
Factors influencing repeated measures of the RCI throughout the follow-up were also analyzed using GEE models nested by patient. Bivariable and multivariable analyses were carried out as with the GLM models. In these GEE models, the dependent variable was the RCI score at each visit, with each independent variable observed at each visit as predictors. We want to point out that unlike in the previous GEE model, in this analysis the variable time (defined as the elapsed time, in years, from the first visit to each successive visit) was analyzed as a continuous variable. In the multivariable GEE model, those variables with a p value < 0.15 in the bivariable analysis were included (plus age, gender, follow-up time, calendar year of diagnosis, and those variables which could be of clinical importance, as defined by clinicians). Different multivariable models were compared using the QIC.
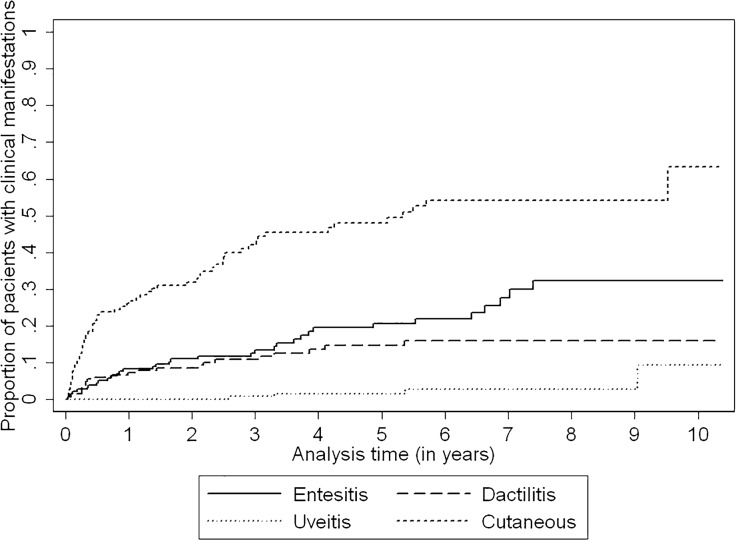
The incidence of clinical manifestations during follow-up was measured using the incidence rate of enthesitis, dactylitis, skin involvement, and uveitis, by dividing the number of events occurring during the time of observation by the number of person-years of exposure and given per 100 person-years with 95% confidence intervals (CI). The time of observation was the time from the first visit to our outpatient clinic until the occurrence of any of the following cut-off points: (a) loss of follow-up; (b) end of the study (November 30th, 2017), or (c) development of enthesitis, dactylitis, skin involvement, or uveitis. A different analysis was carried out for each manifestation. Only the patients not presenting a particular manifestation at the first visit were included in the analysis of that outcome. All statistical analyses were performed using STATA software version 13 (Stata Corp, College Station, TX, USA).
Results
Patient Characteristics
After reviewing the patients whose information was stored in the departmental EHR, 726 patients had received at least one diagnostic ICD9/10 code of PsA. Of those patients, 474 were excluded due to a first visit at our outpatient clinic before January 2007 or after December 2016; ten were excluded due to a diagnosis of PsA before attending our clinic; 11 were excluded due to having only one register visit with HRQoL data; and one patient was excluded due to symptoms onset before 16 years of age. Consequently, 230 patients were included in the analysis, with 3384 registered visits.
Table 1 outlines the baseline demographic and clinical characteristics of the included patients. The most frequent musculoskeletal manifestation at baseline was peripheral arthritis, followed by inflammatory low back pain, enthesitis, and dactylitis. Several patients had more than one PsA feature present (52 patients, 22.6%), being the presence of peripheral arthritis and dactylitis the most frequent presentation (18 patients, 7.8%), followed by peripheral arthritis and enthesitis (six, 2.6%). Several patients have had active psoriasis (76, 33.0%), the majority being plaque psoriasis and a small percentage presenting nail psoriasis manifestation (five, 2.2%). Only one patient presented at baseline an extra-cutaneous and extra-articular manifestation (uveitis).
Table 1.
Description of baseline demographic and clinical characteristics of a cohort of psoriatic arthritis patients
| Variable | n = 230 |
|---|---|
| Women, n (%) | 96 (41.74) |
| Age at symptoms onset, median (Q1–Q3) | 48.3 (39.6–59.6) |
| Age at first visit, median (Q1–Q3) | 49.1 (40–60.2) |
| Age at psoriatic arthritis diagnosis, median (Q1–Q3) | 49.2 (40.4–60.6) |
| Follow-up duration, in years, median (Q1–Q3) | 4.1 (1.4–7.6) |
| Year of visit, n (%) | |
| 2007 | 11 (4.78) |
| 2008 | 29 (12.61) |
| 2009 | 22 (9.57) |
| 2010 | 36 (15.65) |
| 2011 | 25 (10.87) |
| 2012 | 22 (9.57) |
| 2013 | 19 (8.26) |
| 2014 | 30 (13.04) |
| 2015 | 16 (6.96) |
| 2016 | 20 (8.7) |
| Rosser Classification Index, median (Q1–Q3) | 0.986 (0.973–0.995) |
| Comorbidities at baseline | |
| History of cutaneous psoriasis, n (%) | 172 (74.78) |
| Arterial hypertension, n (%) | 48 (20.87) |
| Dyslipidemia, n (%) | 57 (24.78) |
| Diabetes mellitus, n (%) | 15 (6.52) |
| Obesity, n (%) | 31 (13.48) |
| Ischemic heart disease, n (%) | 4 (1.74) |
| Cerebrovascular disease, n (%) | 8 (3.48) |
| Depression, n (%) | 14 (6.09) |
| Cognitive impairment, n (%) | 2 (0.87) |
| Sleep disorders, n (%) | 15 (6.52) |
| Cancer, n (%) | 11 (4.78) |
| Fibromyalgia, n (%) | 0 (0) |
| Osteoporosis, n (%) | 6 (2.61) |
| Osteoporotic fracture, n (%) | 0 (0) |
| Hyperuricemia, n (%) | 12 (5.22) |
| Interstitial lung disease, n (%) | 1 (0.43) |
| Chronic obstructive pulmonary disease, n (%) | 9 (3.91) |
| Restrictive pulmonary disease, n (%) | 0 (0) |
| Renal disease, n (%) | 2 (0.87) |
| Liver disease, n (%) | 6 (2.61) |
| Peptic ulcer, n (%) | 13 (5.65) |
| Thyroid disease, n (%) | 20 (8.7) |
| Clinical manifestations at baseline | |
| Inflammatory low back pain, n (%) | 42 (18.26) |
| Peripheral arthritis, n (%) | 165 (71.74) |
| Enthesitis, n (%) | 34 (14.78) |
| Dactylitis, n (%) | 27 (11.74) |
| Uveitis, n (%) | 1 (0.43) |
| Inflammatory bowel disease, n (%) | 0 (0) |
| Chronic kidney disease, n (%) | 0 (0) |
| Cutaneous manifestations, n (%) | |
| Cutaneous psoriasis | 76 (33.04) |
| Other | 5 (2.17) |
| Cardiac manifestations | 0 (0) |
| Pulmonary manifestations | 0 (0) |
| Treatment prescribed at baseline | |
| NSAIDs, n (%) | |
| Non-selective | 98 (42.61) |
| COX2 inhibitors | 3 (1.3) |
| Oral corticosteroids, n (%) | 58 (25.22) |
| Oral corticosteroids dosage, mg/day, median (Q1–Q3) | 10 (5–10) |
| Calcium and vitamin D, n (%) | 32 (13.91) |
| Proton inhibitors, n (%) | 55 (23.91) |
| Anti-osteoporotic, n (%) | |
| Bisphosphonates | 2 (0.87) |
| Other | 1 (0.43) |
| Any DMARD, n (%) | 69 (30.0) |
| Conventional synthetic DMARDs, n (%) | 61 (26.52) |
| Biologic DMARDs, n (%) | 15 (6.52) |
| TNFi, n (%) | 12 (5.22) |
| Type of DMARD, n (%) | |
| Conventional synthetic DMARDs | 54 (23.48) |
| TNFi | 12 (5.22) |
| Other biologic DMARDs | 3 (1.3) |
| Number of any DMARDs, n (%) | |
| 1 | 59 (25.65) |
| 2 | 10 (4.35) |
| Methotrexate (oral), n (%) | 45 (19.57) |
| Methotrexate (subcutaneous), n (%) | 5 (2.17) |
| Sulfasalazine, n (%) | 6 (2.61) |
| Antimalarials, n (%) | 2 (0.87) |
| Leflunomide, n (%) | 3 (1.3) |
| Cyclosporine, n (%) | 2 (0.87) |
| Gold salts, n (%) | 1 (0.43) |
| Azathiorpine, n (%) | 0 (0) |
| Adalimumab, n (%) | 7 (3.04) |
| Infliximab, n (%) | 4 (1.74) |
| Etanercept, n (%) | 0 (0) |
| Certolizumab, n (%) | 0 (0) |
| Golimumab, n (%) | 1 (0.43) |
| Secukinumab, n (%) | 0 (0) |
| Ustekinumab, n (%) | 3 (1.3) |
DMARDs disease-modifying anti-rheumatic drugs, ILD interstitial lung disease, Q1–Q3 first and third quartiles, NSAIDs non-steroidal anti-inflammatory drugs, TNFi TNF-alpha inhibitors
Regarding baseline comorbidities, besides a medical history of psoriasis in the majority of patients (74, 8%), the most frequent were traditional cardiovascular risk factors (dyslipidemia, arterial hypertension, diabetes mellitus, and obesity). Five percent of the patients presented ischemic heart or cerebrovascular disease, and 6% depression.
Regarding treatments, most patients were prescribed with non-selective NSAIDs, and 25% were prescribed with oral corticosteroids, receiving up to 15 mg/day of prednisone. Thirty percent were prescribed with DMARDs, being the most frequent methotrexate MTX and TNF inhibitors.
Influence of Demographic and Clinical-Related Variables in Baseline HRQoL
Several variables showed an association with baseline HRQoL in the bivariable GLM analysis (see Supplementary Table S2 for further details). Women, older age, a previous diagnosis of hypertension, obesity or depression, the presence of enthesitis and three or more concomitant musculoskeletal manifestations were associated with a lower baseline RCI value, with a p value < 0.15. In the multivariable analysis (Table 2), only older age, a previous diagnosis of obesity, and the presence of enthesitis remained significantly and independently associated with worse HRQoL.
Table 2.
Multivariable generalized linear model analysis to assess the association between demographic and clinical variables with baseline health related quality of life in a cohort of psoriatic arthritis patients
| Variable | Coefficient (95% CI) | Std. error | p |
|---|---|---|---|
| Women | − 0.003 (− 0.008 to 0.001) | 0.002 | 0.13 |
| Age at symptoms onset | − 0.003 (− 0.004 to − 0.001) | 7.8E−04 | 5.10E−04 |
| Year of visit | − 0.0003 (− 0.001 to 0.001) | 4.2E−04 | 0.45 |
| Obesity | − 0.008 (− 0.014 to − 0.001) | 0.003 | 0.017 |
| Enthesitis | − 0.007 (− 0.013 to − 0.001) | 0.003 | 0.030 |
CI confidence interval
Evolution of the HRQoL During Follow-Up
We analyzed the HRQoL at baseline, 1, 2, 5, and 10 years of follow-up using a GEE model with time fitted as a categorical variable. RCI’s median and first and third quartiles values were 0.986 (0.973–0.995; 230 patients), 0.986 (0.986–0.995; 96 patients), 0.986 (0.986–0.995; 123 patients), 0.990 (0.986–1.000; 85 patients), and 0.990 (0.986–0.998; 16 patients), respectively. Comparing with the baseline visit, we did not identify significant differences between the HRQoL at 1, 2, 5, or 10 years of follow-up (p = 0.38, p = 0.25, p = 0.11, and p = 0.65, respectively; Fig. 1).
Fig. 1.
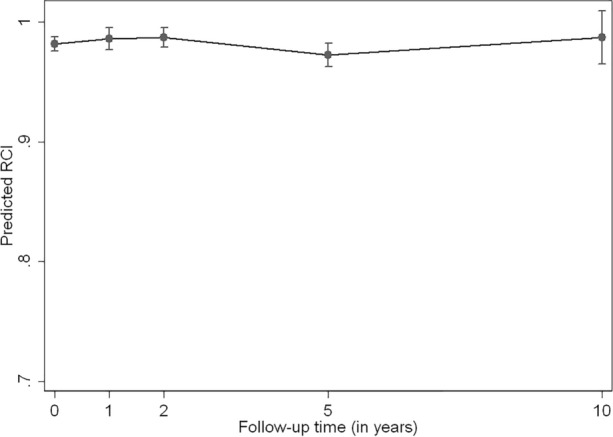
Mean estimated Rosser classification index at baseline, 1, 2, 5, and 10 years of follow-up in a cohort of patients diagnosed with psoriatic arthritis
Influence of Demographic and Clinical-Related Variables in the Evolution of the HRQoL During Follow-Up
Supplementary Table S3 shows the prevalence of the demographic and clinical-related variables in all the visits included in the analyses. Bivariable analysis for both the exchangeable and independent working correlation structures are presented in Supplementary Table S4. Overall, the most significant variables for one correlation structured also showed a significant association with the other correlation structure. In the multivariable models (Table 3), the one using the exchangeable working correlation structure resulted in the lowest QIC.
Table 3.
Multivariable generalized estimating equation analysis to assess the association between demographic and clinical variables with repeated measures of health-related quality of life in a cohort of psoriatic arthritis patients using an exchangeable and independent working correlation structures
| Exchangeable | Independent | |||||
|---|---|---|---|---|---|---|
| Coefficient (95% CI) | p | Std. error | Coefficient (95% CI) | Std. error | p | |
| Women | − 0.001 (− 0.007 to 0.004) | 0.65 | 0.003 | 0.002 (− 0.005 to 0.008) | 0.003 | 0.60 |
| Age at psoriatic arthritis diagnosis | − 0.005 (− 0.01 to 0.0002) | 0.058 | 2.47E−04 | − 0.004 (− 0.009 to 0.001) | 2.44E−04 | 0.10 |
| Follow-up time | − 0.0002 (− 0.002 to 0.001) | 0.73 | 7.17E−04 | 0.0001 (− 0.001 to 0.001) | 5.49E−04 | 0.84 |
| Year of visit | − 0.0003 (− 0.001 to 0.0003) | 0.39 | 3.08E−04 | − 0.001 (− 0.001 to 0.00003) | 3.44E−04 | 0.063 |
| Dyslipidemia | 0.008 (− 0.000003 to 0.015) | 0.050 | 0.004 | 0.01 (− 0.001 to 0.021) | 0.005 | 0.073 |
| Obesity | − 0.009 (− 0.019 to 0.001) | 0.074 | 0.005 | − 0.012 (− 0.025 to 0.002) | 0.007 | 0.091 |
| Hyperuricemia | 0.003 (− 0.001 to 0.007) | 0.097 | 0.002 | – | – | – |
| Enthesitis | − 0.006 (− 0.01 to -0.002) | 1.00E−03 | 0.002 | – | – | – |
| Calcium and vitamin D | – | – | – | − 0.009 (− 0.016 to − 0.001) | 0.004 | 0.023 |
| Methotrexate (oral) | 0.007 (0.001 to 0.014) | 0.020 | 0.003 | 0.012 (0.004 to 0.019) | 0.004 | 3.00E−03 |
| Antimalarials | 0.003 (0.001 to 0.005) | 3.00E−03 | 0.001 | 0.008 (− 0.002 to 0.018) | 0.005 | 0.097 |
| Leflunomide | – | – | – | 0.007 (0.0003 to 0.013) | 0.003 | 0.040 |
| QIC | 103.303 | 132.004 | ||||
CI confidence interval
In this model, the presence of enthesitis was associated with worse HRQoL. Conversely, treatment with MTX or antimalarials (AM) was associated with better HRQoL. To further explore the association between enthesitis and lower HRQoL, we analyzed if those patients developing this manifestation were associated with an overall worse HRQoL (meaning that those patients presenting anytime that manifestation had a worse HRQoL in most visits, even in those without enthesitis) compared with those that do not present it during follow-up. With this aim, we tested if the presence of this manifestation anytime during follow-up, or its presence at baseline, or the number or proportion of visits when it was present was associated with an overall worse HRQoL during the patient’s follow-up, using GEE. None of those situations were associated with worse HRQoL (p = 0.28, p = 0.26, p = 0.65, p = 0.56, respectively). Therefore, although enthesitis is associated with a worse HRQoL in those visits when it is present, those patients developing this manifestation are not associated with an overall worse HRQoL compared with those that do not present it during follow-up.
To further characterize the association between csDMARDs and HRQoL during follow-up, we compared the baseline characteristics of those patients who were treated with either AM or MTX during follow-up, and those who did not (Supplementary Table S5 and S6 for AM, and MTX, respectively). Twenty-six patients (11.3%) received AM during follow-up. Patients ever treated with these drugs had a lower prevalence of history of cutaneous psoriasis (57.7 vs. 77.0%; p = 0.033); a higher prevalence of cognitive impairment (7.7 vs. 0%, p = 0.012); and a higher prevalence of being prescribed with AM at baseline (7.7 vs. 0%, p = 0.012). Overall, although it did not reach statistical significance, patients treated with AM exhibited a higher prevalence of peripheral arthritis (84.6 vs. 70.1%; p = 0.17), and a lower prevalence of inflammatory low back pain (3.9 vs. 20.1%; p = 0.056), enthesitis (3.9 vs. 16.2%; p = 0.14) and dactylitis (7.7 vs. 12.3%; p = 0.75).
Regarding oral MTX, 153 (66.5%) patients were prescribed with this drug during follow-up. These patients had a statistically significant longer follow-up (median time in years: 4.7 vs. 3.2; p = 1.70E−03); had a lower proportion of inflammatory low back pain (14.4 vs. 26.0%; p = 0.032) and higher proportion of peripheral arthritis (77.8 vs. 59.7%; p = 4.00E−03) and dactylitis (15.0 vs. 5.2%; p = 0.029); and higher proportion of patients prescribed with oral corticosteroids (29.4 vs. 16.9%; p = 0.039), and csDMARDs (33.3 vs. 13.0%; p = 1.00E−03), and lower proportion of patients prescribed with bDMARDs (3.9 vs. 11.7%; p = 0.024).
Incidence Rate of Clinical Manifestations
Table 4 shows the incidence rate (IR) of onset of clinical manifestation during follow-up.
Table 4.
Incidence rate of onset of clinical manifestation during follow-up in a cohort of psoriatic arthritis patients
| Subjects (events) | Time-at-risk (in person-years) | Incidence rate per 100 person-years (95% CI) | |
|---|---|---|---|
| Enthesitis | 196 (34) | 712.7 | 4.77 (3.41–6.68) |
| Dactylitis | 203 (23) | 764.4 | 3.00 (2.00–4.53) |
| Uveitis | 229 (4) | 934.9 | 0.43 (0.16–1.14) |
| Cutaneous manifestations | 149 (63) | 428.6 | 14.70 (11.48–18.81) |
CI confidence interval
Cutaneous manifestations showed the higher IR, followed by enthesitis, dactylitis, and uveitis. Kaplan–Meier cumulative incidence curves for the onset of these manifestations are shown in Fig. 2.
Fig. 2.
Kaplan–Meier cumulative incidence curves representing the onset of cutaneous manifestations, enthesitis, dactylitis, and uveitis in a cohort of psoriatic arthritis patients
Discussion
In our study, we assessed the added burden of different factors in the evolution of HRQoL in PsA patients in daily clinical practice. After adjustment for different demographic and clinical-related variables, older age at symptoms onset, and presence of obesity and enthesitis were significantly and independently associated with worse HRQoL. Conversely, prescription of MTX and AM were associated with better HRQoL.
Although most studies analyzing the impact of PsA in HRQoL have been focused on the burden of cutaneous and joint manifestations [28–30], the study of the effect of other musculoskeletal manifestations has recently gained attention [31, 32]. In the case of enthesitis, it is now considered a hallmark of this condition, with auto inflammatory phenomena taking place at the entheses and triggering the disease, reinforcing the idea of the relationship between the enthesitis physiopathology and PsA [33–35]. Moreover, as suggested by the CASPAR criteria and GRAPPA recommendations [36], enthesitis is an important domain for the identification, diagnosis, and treatment of PsA [37, 38]. In addition, it is becoming an important outcome in many clinical trials [39–42], as several recent studies have shown a deleterious impact of this manifestation. In a study conducted in a Dutch early PsA cohort, enthesitis showed a negative effect in the HRQoL [43], measured with the SF-36 questionnaire. Moreover, Gezer et al. [44], previously demonstrated that enthesitis was significantly correlated with the PsA Quality of Life Index and sleep disturbances in patients with PsA and recently Sunar et al. [45] found higher worse HRQoL determined with SF-36 and PsAQoL scores in patients with enthesitis. In a multi-national, real-world PsA population, enthesitis was significantly associated with higher disability levels, greater patient-reported pain and fatigue, and patients suffering from this manifestation were significantly more likely to experience overall impairment while working [15]. Finally, Polachek et al. [46] showed an association between higher echographic enthesitis scores and greater radiographic joint damage, axial damage, and a greater chance of patients developing joint ankyloses and arthritis mutilans. Our results contribute evidence to the relevance of enthesitis, showing its detrimental effect in HRQoL both considered at baseline and during follow-up.
Comorbidities have also been studied as risk factors of poor HRQoL in PsA. A higher prevalence of different comorbidities has been shown in these patients compared with the general population [3–5], specially traditional cardiovascular risk factors. However, not all seem to affect the HRQoL, with several studies concluding that only particular comorbidities have a greater effect than the overall number of comorbidities [18, 47]. Therefore, to correctly assess the burden of PsA, it would be necessary to identify which precise comorbidities are present in the patients. In our study, obesity showed an independent association with HRQoL. Similar to our results, Husted et al. [18] observed a strong association between obesity and a decreased SF-36 physical health subscale, even after adjustment for inflammatory disease-related and demographic characteristics.
Contrary to previous reports, we did not observe a negative effect of dyslipidemia in the HRQoL [48]. This is probably due to study design differences, where dyslipidemia is usually evaluated along with cholesterol-lowering treatment, so the results may indicate that asymptomatic people diagnosed and treated for dyslipidemia may not perceive themselves as being as healthy as those not reporting dyslipidemia treatment. In our study, we have not collected treatment for this condition.
MTX plays a key role in the treatment of PsA, being the most commonly used drug in this condition [49], mostly due to its safety and efficacy profile both in articular and cutaneous manifestations [50]. Despite the scarcity of randomized clinical trials, observational studies support the role of MTX in this pathology and current treatment recommendations approve its use as the first-line DMARD therapy for the management of PsA with predominant peripheral arthritis [36, 51]. Regarding the effect of MTX in HRQoL, although a randomized controlled trial showed a greater improvement of HRQoL (measured using the SF‐36) compared with cyclosporine [52], in a recent Cochrane database systematic review, benefits of MTX on HRQoL beyond 6 months have not been measured or reported in randomized placebo-control trials [53]. In our study, we showed an overall benefit of this drug, regardless of the duration of follow-up. Our results are in accordance with the benefit MTX has in patients with peripheral arthritis, particularly in those with many swollen joints, structural damage in the presence of inflammation, as is recommended on the available literature as the first-choice csDMARD (51).
Regarding the benefit of AM on HRQoL in the PsA patients, it may reflect the unselected patients treated in everyday practice probably aimed at treating joint symptoms and supported in the evidence that the drug can be given safely in PsA [49, 54], further in our study, patients ever treated with antimalarials had a lower prevalence of past history of psoriasis.
Our study is one of the few assessing HRQoL over time using real-world data. There seems to be an increasing interest in this topic, with the recent publication of a similar study evaluating HRQoL with the EQ-5D [55] in a 2-year, prospective, multicenter, non-interventional study. In our study, we did not identify changes in the evolution of the HR-QoL of these patients, reflected in the lack of statistically significant differences between RCI values at baseline, 1, 2, 5, and 10 years of follow-up. It is important to take into account that the RCI lacks the granularity of other HRQoL measurements, such as the EuroQoL 5D [20]. Therefore, it is possible that the patients experience changes in HRQoL during follow-up that are not reflected in the RCI.
Regarding the study limitations, the main ones are those that affect any observational retrospective study. In our study, the clinical measurement of enthesitis consisted in a clinical evaluation of entheses, detecting clinical signs of this manifestation, such as tenderness and general soft-tissue swelling. We did not apply any standardized index developed to aid clinical recognition and quantification of enthesitis, such as those frequently used in clinical trials. In addition, we did not report direct parameters of disease activity in PsA. Finally, we used a HRQoL measurement (the RCI), instead of a disease specific QoL questionnaire, and therefore, we possibly missed disease-specific effects in the QoL of our patients.
Regarding the study strengths, the main strength is the use of real-world data from a large number of visits in patients with PsA with a prolonged follow-up. Notably, all patients included had more than two HRQoL assessments throughout the follow-up, including different moments in the course of the disease and not only longstanding disease. Besides, we were able to analyze patients with different musculoskeletal manifestation. Our PsA cohort therefore reflects the population as seen in daily clinical practice, not influenced by selection criteria from clinical trials.
Conclusions
In summary, when analyzing the evolution of the HRQoL in PsA patients over time, we found that age at symptoms onset, obesity, and enthesitis was associated with a worse HRQoL and conversely, the use of MTX and antimalarials were associated with better HRQoL. Our results suggest that the optimal management of PsA needs to extend beyond the treatment of the inflammatory joint and skin disease and also include other musculoskeletal manifestation and comorbidities.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Funding
This work and the journal’s Rapid Service Fee were supported by a grant from Eli Lilly and Company.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author’s Contributions
LRR and LA, study’s conception and design; DDFN, LL, and GC: acquisition of the data; LRR, LA, and AMG: analysis of the data; LRR, LA, MN, NB, SD, and BFG: interpretation of data. DDFN and LRR: drafting the manuscript; AMG, LL, GC, MN, NB, SD, BFG, and LA: revising the manuscript critically for important intellectual content; all authors gave the final approval of the version to be published and have participated sufficiently in the work to take public responsibility for appropriate portions of the content.
Disclosures
Mercedes Núñez, Natalia Bello, and Silvia Díaz are employees of Eli Lilly and Co. and own company stock. Dalifer Freites, Alfredo Madrid-García, Leticia Leon, Gloria Candelas, Benjamín Fernández-Gutiérrez, Lydia Abasolo, and Luis Rodriguez-Rodriguez have nothing to disclose.
Compliance with Ethics Guidelines
HCSC Ethics Review Board approval was obtained as a retrospective study (Reference 19/342-E) and waiver of informed consent was granted for use of de-identified clinical records. Furthermore, the study was conducted in accordance with the Declaration of Helsinki.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Footnotes
Lydia Abasolo and Luis Rodriguez-Rodriguez share senior authorship of this paper.
References
- 1.Gossec L, Smolen JS, Ramiro S, de Wit M, Cutolo M, Dougados M, et al. European League against Rheumatism (EULAR) recommendations for the management of psoriatic arthritis with pharmacological therapies: 2015 update. Ann Rheum Dis. 2016;75:499–510. doi: 10.1136/annrheumdis-2015-208337. [DOI] [PubMed] [Google Scholar]
- 2.Gladman DD. Psoriatic arthritis. N Engl J Med. 2017;376:957–970. doi: 10.1056/NEJMra1505557. [DOI] [PubMed] [Google Scholar]
- 3.Polachek ARI, Touma Z, Anderson M, Eder L. Risk of cardiovascular morbidity in patients with psoriatic arthritis: a meta-analysis of observational studies. Arthritis Care Res. 2017;69(1):67–74. doi: 10.1002/acr.22926. [DOI] [PubMed] [Google Scholar]
- 4.Kotsis K, Voulgari PV, Tsifetaki N, Machado MO, Carvalho AF, Creed F, Drosos AA, Hyphantis T. Anxiety and depressive symptoms and illness perceptions in psoriatic arthritis and associations with physical health-related quality of life. Arthritis Care Res. 2012;64(10):1593–1601. doi: 10.1002/acr.21725. [DOI] [PubMed] [Google Scholar]
- 5.Ba PK, Gordon KB, Mph JIS. Association of psoriasis and psoriatic arthritis with osteoporosis and pathological fractures. J Am Dermatol. 2019;76(6):1045–1053.e3. doi: 10.1016/j.jaad.2016.11.046. [DOI] [PubMed] [Google Scholar]
- 6.Gladman DD. Natural history of psoriatic arthritis. Bailliere’s Clin Rheumatol. 1994;8(2):379–394. doi: 10.1016/S0950-3579(94)80024-3. [DOI] [PubMed] [Google Scholar]
- 7.Brockbank J, Gladman D. Diagnosis and management of psoriatic arthritis. Drugs 2002;62(17):2447–57. [DOI] [PubMed]
- 8.Husted JA, Gladman DD, Farewell VT, Cook RJ. Health-related quality of life of patients with psoriatic arthritis: a comparison with patients with rheumatoid arthritis. Arthritis Rheum. 2001;45(2):151–158. doi: 10.1002/1529-0131(200104)45:2<151::AID-ANR168>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 9.Rosen CF, Mussani F, Chandran V, Eder L, Thavaneswaran A, Gladman DD. Patients with psoriatic arthritis have worse quality of life than those with psoriasis alone. Rheumatology. 2011;2012:571–576. doi: 10.1093/rheumatology/ker365. [DOI] [PubMed] [Google Scholar]
- 10.Zachariae H, Zachariae R, Blomqvist K, Davidsson S, Molin L, Mørk C, et al. Quality of life and prevalence of arthritis reported by 5795 data from the Nordic Quality of Life Study. Acta Dermato-Venereol. 2002;82:108–113. doi: 10.1080/00015550252948130. [DOI] [PubMed] [Google Scholar]
- 11.Leung YY, Ho KW, Zhu TY, Tam LS, Kun EWL, Li EKM. Testing scaling assumptions, reliability and validity of medical outcomes study short-form 36 health survey in psoriatic arthritis. Rheumatology. 2010;49(8):1495–1501. doi: 10.1093/rheumatology/keq112. [DOI] [PubMed] [Google Scholar]
- 12.Tezel N, Yilmaz Tasdelen O, Bodur H, Gul U, Kulcu Cakmak S, Oguz ID, et al. Is the health-related quality of life and functional status of patients with psoriatic arthritis worse than that of patients with psoriasis alone? Int J Rheum Dis. 2015;18(1):63–69. doi: 10.1111/1756-185X.12283. [DOI] [PubMed] [Google Scholar]
- 13.Edson-Heredia E, Zhu B, Guo J, Maeda-Chubachi T, Lebwohl M. Disease burden and quality of life in psoriasis patients with and without comorbid psoriatic arthritis: results from National Psoriasis Foundation panel surveys. Cutis. 2015;95(3):173–8. [PubMed]
- 14.Gratacós J, Daudén E, Gómez-reino J, Carlos J, Ángel M. Health-related quality of life in psoriatic arthritis patients in Spain. Reumatología Clínica. 2014;10(1):25–31. doi: 10.1016/j.reuma.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 15.Mease PJ, Karki C, Palmer JB, Etzel CJ, Kavanaugh A, Ritchlin CT, et al. Clinical characteristics, disease activity, and patient-reported outcomes in psoriatic arthritis patients with dactylitis or enthesitis: results from the Corrona Psoriatic Arthritis/Spondyloarthritis Registry. Arthritis Care Res. 2017;69(11):1692–1699. doi: 10.1002/acr.23249. [DOI] [PubMed] [Google Scholar]
- 16.Mease PJ, Palmer JB, Liu M, Kavanaugh A, Ritchlin CT, Karki C, et al. Influence of axial involvement on clinical characteristics of psoriatic arthritis: analysis from the Corrona Psoriatic Arthritis/Spondyloarthritis Registry. J Rheumatol. 2018;45(10):1389–1396. doi: 10.3899/jrheum.171094. [DOI] [PubMed] [Google Scholar]
- 17.Bavière W, Deprez X, Houvenagel E, Philippe P, Deken V, Flipo RM, Paccou J. Association Between Comorbidities and Quality of Life in Psoriatic Arthritis: Results from a Multicentric Cross-sectional Study. J Rheumatol. 2020;47(3):369–76. [DOI] [PubMed]
- 18.Husted JA, Thavaneswaran A, Chandran V, Gladman DD. Incremental effects of comorbidity on quality of life in patients. J Rheumatol. 2013;40(8):1349–1356. doi: 10.3899/jrheum.121500. [DOI] [PubMed] [Google Scholar]
- 19.Madrid-García A, Font-Urgelles J, Vega-Barbas M, León-Mateos L, Freites DD, Lajas CJ, et al. Outpatient readmission in rheumatology: a machine learning predictive model of patient’s return to the clinic. J Clin Med. 2019;8(8):1156. doi: 10.3390/jcm8081156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leon L, Rodriguez-Rodriguez L, Aguilar MD, Jover JÁ, Vadillo C, Redondo M, Abasolo L. Validation of a Quality of Life Instrument in Spanish Patients With Rheumatic Diseases: The Rosser Classification System. J Clin Rheumatol. 2019;25(2):78–84. [DOI] [PubMed]
- 21.Rosser R, Kind P. A scale of valuations of states of illness: is there a social consensus? Int J Epidemiol. 1978;7(4):347–358. doi: 10.1093/ije/7.4.347. [DOI] [PubMed] [Google Scholar]
- 22.Rosser RM, Watts VC. The measurement of hospital output. Int J Epidemiol. 1972;1:361–368. doi: 10.1093/ije/1.4.361. [DOI] [PubMed] [Google Scholar]
- 23.Leon L, Angel J, Estibaliz J, Maria L, Zunzunegui V. Health-related quality of life as a main determinant of access to rheumatologic care. Rheumatol Int. 2013;33:1797–1804. doi: 10.1007/s00296-012-2599-6. [DOI] [PubMed] [Google Scholar]
- 24.Martínez C, Ortiz AM, Juarranz Y, Lamana A, Seoane IV, Leceta J, et al. Serum levels of vasoactive intestinal peptide as a prognostic marker in early arthritis. PLoS ONE. 2014;9(1):e85248. doi: 10.1371/journal.pone.0085248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abasolo L, Ivorra-Cortes J, Leon L, Jover JA, Fernández-Gutiérrez B, Rodriguez-Rodriguez L. Contribution of the bone and cartilage/soft tissue components of the joint damage to the level of disability in rheumatoid arthritis patients: a longitudinal study. Clin Rheumatol. 2019;38(3):691–700. [DOI] [PubMed]
- 26.Ballinger GA. Using generalized estimating equations for longitudinal data analysis. Organ Res Methods. 2004;7(2):127–150. doi: 10.1177/1094428104263672. [DOI] [Google Scholar]
- 27.Pan W. Akaike’s information criterion in generalized estimating equations. Biometrics. 2001;57(1):120–125. doi: 10.1111/j.0006-341X.2001.00120.x. [DOI] [PubMed] [Google Scholar]
- 28.Borman P, Toy GG, Babaoǧlu S, Bodur H, Ciliz D, Alli N. A comparative evaluation of quality of life and life satisfaction in patients with psoriatic and rheumatoid arthritis. Clin Rheumatol. 2007;26(3):330–334. doi: 10.1007/s10067-006-0298-y. [DOI] [PubMed] [Google Scholar]
- 29.de Vlam K, Merola JF, Birt JA, Sandoval DM, Lobosco S, Moon R, et al. Skin involvement in psoriatic arthritis worsens overall disease activity, patient-reported outcomes, and increases healthcare resource utilization: an observational cross-sectional study. Rheumatol Ther. 2018;5(2):423–436. doi: 10.1007/s40744-018-0120-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ogdie A, Zhu B, Spraberry A, Malatestinic W, Dong Y, Shrom DSP. Relative contributions of improvements in the psoriasis area and severity index (PASI) and disease activity index for psoriatic arthritis (DAPSA) to improvements in quality of life and function in patients with psoriatic arthritis. Arthritis Rheumatol. 2018;70:729. [Google Scholar]
- 31.Walsh JA, Ogdie A, Michaud K, Peterson S, Holdsworth E, Karyekar C, et al. Fri0463 enthesitis, dactylitis, and axial disease in psoriatic arthritis (PSA): impact on patient quality of life and work productivity. Ann Rheum Dis. 2019;78:925–926. [Google Scholar]
- 32.Wervers K, Luime JJ, Tchetverikov I, Gerards AH, Kok MR, Appels CWY, et al. Influence of disease manifestations on health-related quality of life in early psoriatic arthritis influence of disease manifestations on health-related quality of life in early psoriatic arthritis. J Rheumatol. 2018;45(11):1526–1531. doi: 10.3899/jrheum.171406. [DOI] [PubMed] [Google Scholar]
- 33.McGonagle D. Enthesitis: an autoinflammatory lesion linking nail and joint involvement in psoriatic disease. J Eur Acad Dermatol Venereol. 2009;23(SUPPL. 1):9–13. doi: 10.1111/j.1468-3083.2009.03363.x. [DOI] [PubMed] [Google Scholar]
- 34.Veale DJ, Fearon U. The pathogenesis of psoriatic arthritis. The Lancet. 2018;391(10136):2273–2284. doi: 10.1016/S0140-6736(18)30830-4. [DOI] [PubMed] [Google Scholar]
- 35.Kaeley GS, Eder L, Aydin SZ, Gutierrez M, Bakewell C. Enthesitis: A hallmark of psoriatic arthritis. Semin Arthritis Rheum. 2018;48(1):35–43. [DOI] [PubMed]
- 36.Coates LC, Gossec L, Ramiro S, Mease P, van der Heijde D, Smolen JS, et al. New GRAPPA and EULAR recommendations for the management of psoriatic arthritis. Rheumatology (Oxford) 2017;56(8):1251–1253. doi: 10.1093/rheumatology/kew390. [DOI] [PubMed] [Google Scholar]
- 37.Taylor W, Gladman D, Helliwell P, Marchesoni A, Mease P, Mielants H. Classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arthritis Rheum. 2006;54(8):2665–2673. doi: 10.1002/art.21972. [DOI] [PubMed] [Google Scholar]
- 38.Coates LC, Kavanaugh A, Mease PJ, Soriano ER, Laura Acosta-Felquer M, Armstrong AW, et al. Group for research and assessment of psoriasis and psoriatic arthritis 2015 treatment recommendations for psoriatic arthritis. Arthritis Rheumatol. 2016;68(5):1060–1071. doi: 10.1002/art.39573. [DOI] [PubMed] [Google Scholar]
- 39.McInnes IB, Mease PJ, Kirkham B, Kavanaugh A, Ritchlin CT, Rahman P, et al. Secukinumab, a human anti-interleukin-17A monoclonal antibody, in patients with psoriatic arthritis (FUTURE 2): a randomised, double-blind, placebo-controlled, phase 3 trial. The Lancet. 2015;386(9999):1137–1146. doi: 10.1016/S0140-6736(15)61134-5. [DOI] [PubMed] [Google Scholar]
- 40.Mease PJ, McInnes IB, Kirkham B, Kavanaugh A, Rahman P, Van Der Heijde D, et al. Secukinumab inhibition of interleukin-17A in patients with psoriatic arthritis. N Engl J Med. 2015;373(14):1329–1339. doi: 10.1056/NEJMoa1412679. [DOI] [PubMed] [Google Scholar]
- 41.Mease PJ, Van Der Heijde D, Ritchlin CT, Okada M, Cuchacovich RS, Shuler CL, et al. Ixekizumab, an interleukin-17A specific monoclonal antibody, for the treatment of biologic-naive patients with active psoriatic arthritis: results from the 24-week randomised, double-blind, placebo-controlled and active (adalimumab)-controlled period of the phase III trial SPIRIT-P1. Ann Rheum Dis. 2017;76(1):79–87. doi: 10.1136/annrheumdis-2016-209709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nash P, Kirkham B, Okada M, Rahman P, Combe B, Burmester GR, et al. Ixekizumab for the treatment of patients with active psoriatic arthritis and an inadequate response to tumour necrosis factor inhibitors: results from the 24-week randomised, double-blind, placebo-controlled period of the SPIRIT-P2 phase 3 trial. The Lancet. 2017;389(10086):2317–2327. doi: 10.1016/S0140-6736(17)31429-0. [DOI] [PubMed] [Google Scholar]
- 43.Wervers K, Luime JJ, Tchetverikov I, Gerards AH, Kok MR, Appels CWY, et al. Influence of disease manifestations on health-related quality of life in early psoriatic arthritis. J Rheumatol. 2018;45(11):1526–1531. doi: 10.3899/jrheum.171406. [DOI] [PubMed] [Google Scholar]
- 44.Gezer O, Batmaz İ, Sariyildiz MA, Sula B, Ucmak D, Bozkurt M, et al. Sleep quality in patients with psoriatic arthritis. Int J Rheum Dis. 2017;20(9):1212–1218. doi: 10.1111/1756-185X.12505. [DOI] [PubMed] [Google Scholar]
- 45.Sunar I, Ataman S, Nas K, Kilic E, Sargin B, Kasman SA, et al. Enthesitis and its relationship with disease activity, functional status, and quality of life in psoriatic arthritis: a multi-center study. Rheumatol Int. 2020;40(2):283–294. doi: 10.1007/s00296-019-04480-9. [DOI] [PubMed] [Google Scholar]
- 46.Polachek A, Cook R, Chandran V, Gladman DD, Eder L. The association between sonographic enthesitis and radiographic damage in psoriatic arthritis. Arthritis Res Ther. 2017;19(1):189. doi: 10.1186/s13075-017-1399-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bavière W, Deprez X, Houvenagel E, Philippe P, Deken V, Flipo RM, et al. Association between comorbidities and quality of life in psoriatic arthritis: results from a multicentric cross-sectional study. J Rheumatol. 2020;47(3):369–376. doi: 10.3899/jrheum.181471. [DOI] [PubMed] [Google Scholar]
- 48.Lalonde L, Clarke AE, Joseph L, Mackenzie T, Grover SA, Cassidy LE, et al. Health-related quality of life with coronary heart disease prevention and treatment. J Clin Epidemiol. 2001;54(10):1011–1018. doi: 10.1016/S0895-4356(01)00361-4. [DOI] [PubMed] [Google Scholar]
- 49.Helliwell PS, Taylor WJ. Treatment of psoriatic arthritis and rheumatoid arthritis with disease modifying drugs—comparison of drugs and adverse reactions. J Rheumatol. 2008;35(3):472–476. [PubMed] [Google Scholar]
- 50.Schmitt J, Rosumeck S, Thomaschewski G, Sporbeck B, Haufe E, Nast A. Efficacy and safety of systemic treatments for moderate-to-severe psoriasis: meta-analysis of randomized controlled trials. Br J Dermatol. 2014;170(2):274–303. doi: 10.1111/bjd.12663. [DOI] [PubMed] [Google Scholar]
- 51.Gossec L, Smolen JS, Ramiro S, de Wit M, Cutolo M, Dougados M, et al. European League Against Rheumatism (EULAR) recommendations for the management of psoriatic arthritis with pharmacological therapies: 2015 update. Ann Rheum Dis. 2016;75(3):499–510. doi: 10.1136/annrheumdis-2015-208337. [DOI] [PubMed] [Google Scholar]
- 52.Flytström I, Stenberg B, Svensson Å, Bergbrant IM. Methotrexate vs ciclosporin in psoriasis: effectiveness, quality of life and safety. A randomized controlled trial. Brit J Dermatol. 2008;158(1):116–121. doi: 10.1111/j.1365-2133.2007.08284.x. [DOI] [PubMed] [Google Scholar]
- 53.Wilsdon TD, Whittle SL, Thynne TR, Mangoni AA. Methotrexate for psoriatic arthritis. Cochrane Database Syst Rev. 2019;1(1):CD012722. [DOI] [PMC free article] [PubMed]
- 54.Gladman DD, Blake R, Brubacher B, Farewell VT. Chloroquine therapy in psoriatic arthritis. J Rheumatol. 1992;19(11):1724–1726. [PubMed] [Google Scholar]
- 55.Mlcoch T, Tuzil J, Sedova L, Stolfa J, Urbanova M, Suchy D, et al. Mapping quality of life (EQ-5D) from DAPsA, clinical DAPsA and HAQ in psoriatic arthritis. Patient. 2018;11(3):329–340. doi: 10.1007/s40271-017-0285-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.