Abstract
Introduction
Systemic lupus erythematosus (SLE) can affect bone metabolism and homeostasis of serum electrolytes that are associated with abnormal levels of vitamin D. Mycophenolate mofetil (MMF) is a commonly used immunosuppressant with the active metabolite mycophenolic acid (MPA). The area under the plasma concentration–time curve (AUC) of MPA is often monitored during the treatment to assess the exposure levels. This study aims to explore the association between exposure levels of MPA and 25-hydroxyvitamin D [25(OH)D] levels in children with SLE.
Methods
Repeated measured data of children with SLE who were treated with MMF and under therapeutic drug monitoring (TDM) were retrospectively collected from the electronic medical records. MPA exposure levels were reflected by the area under the concentration–time curve over 24 h (AUC0–24h). Univariate and multivariate linear regression models were employed to analyze factors associated with 25(OH)D levels. Hierarchical linear models were developed to analyze the intra- and inter-individual effects of AUC0–24h on the variance of 25(OH)D levels.
Results
Data from 184 children with SLE (142 female and 42 male) with 518 follow-ups were collected. The median age was 14 years (range 3–18 years) at TDM. Children with normal 25(OH)D levels had significantly higher AUC0–24h than children with low 25(OH)D levels (98.71 vs. 84.05 mg·h/L, P = 0.004). Intra- and inter-individual effects of AUC0–24h on 25(OH)D levels were similar ( = 0.034 vs. = 0.037) but only the intra-individual effect was significant (P = 0.001) in hierarchical models. Other associated factors include age, sex, season at measurement, glucocorticoid daily dose, and external vitamin D3 supplements.
Conclusion
25(OH)D levels are associated with MPA exposure levels, and may serve as a potential indicator to optimize the exposure level of MPA during treatment. AUC0–24h of 98.71 mg·h/L or AUC0–12h of 49.36 mg·h/L could be the targeted exposure level for children with SLE.
Keywords: Children, Exposure level, Mycophenolate mofetil, Mycophenolic acid, Systemic lupus erythematosus, Vitamin D
Key Summary Points
| Why carry out this study? |
| Systemic lupus erythematosus (SLE) can affect bone metabolism and homeostasis of serum electrolytes that are associated with abnormal levels of vitamin D. |
| Mycophenolate mofetil (MMF) is a commonly used immunosuppressant with the active metabolite mycophenolic acid (MPA). The optimal exposure level of MPA has not yet been settled. |
| This study aims to explore the association between the exposure levels of MPA, as indicated by the area under the plasma concentration–time curve (AUC), and the 25-hydroxyvitamin D [25(OH)D] levels in children with SLE, and to optimize MPA exposure levels. |
| What was learned from the study? |
| 25(OH)D levels are associated with MPA exposure levels in children with SLE. |
| 25(OH)D may serve as a potential indicator to optimize the exposure level of MPA during treatment. |
| AUC0–24h of 98.71 mg·h/L could be the targeted exposure level for children with SLE. |
Digital Features
This article is published with digital features, including a summary slide to facilitate understanding of the article. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.14597163.
Introduction
Systemic lupus erythematosus (SLE) is a chronic autoimmune disease with systemic manifestations and multi-organ involvement, which has a more severe phenotype in children than in adults [1–3]. SLE can affect bone metabolism and serum electrolyte homeostasis through renal function impairment and endocrinological disorder [4–6]. Vitamin D is an important modulator of bone metabolism and calcium and phosphorus balance [5]. 25-Hydroxyvitamin D [25(OH)D] is recognized as the main indicator of vitamin D levels of the body [7, 8]. External vitamin D supplements (i.e., vitamin D2 or vitamin D3) or vitamin D receptor activators (VDRAs, i.e., calcitriol or alfacalcidol) are used for either prevention or treatment purpose in patients with SLE given the concerns for abnormal renal function and chronic administration of glucocorticoids [5, 9, 10]. Other bone turnover markers include β-CrossLaps (β-CTx) (a marker of bone resorption) and osteocalcin (a predictor of osteoporosis), which are also closely monitored in patients at a risk of osteoporosis or decreased bone density [11–13].
Mycophenolate mofetil (MMF) is an immunosuppressant that is metabolized into the active form called mycophenolic acid (MPA) after oral administration [14]. The mechanism of immunosuppressive action of MPA is inhibiting inosine monophosphate dehydrogenase (IMPDH), which is the rate-limiting enzyme in the de novo synthesis pathway of guanine in T and B lymphocytes [14]. MMF is part of the treatment strategy of SLE in helping with disease control and glucocorticoid tapering [1, 15]. Exposure levels of MPA indicated by area under the concentration–time curve (AUC) were reported to be associated with SLE disease activity, where higher AUC is associated with lower SLE disease activity index (SLEDAI) score [16, 17]. Therapeutic drug monitoring (TDM) is often conducted to help achieve exposure target by measuring the plasma concentration of MPA and estimating the AUC with pharmacokinetic models [18]. There are still controversies about the optimal exposure level of MPA in the treatment of SLE. Some studies recommended AUC0–12h > 30 mg·h/L [19–21], while others reported AUC0–12h > 45 mg·h/L was associated with better clinical outcome [22, 23]. Therefore, it is of clinical importance to find more evidence to help decide the treatment target.
Vitamin D status of the body could be a potential indicator to help guide the treatment with MMF, which is based on the rationale that bone metabolism is one of the main concerns during the treatment of SLE and the use of immunosuppressants should be appropriate to avoid the negative effect on homeostasis of other systems [24–27]. However, limited research has looked into the relationship between MMF treatment effect and vitamin D levels in patients with SLE. Therefore, this study is the first one that aims to explore the association between exposure levels of MPA and 25(OH)D levels in children with SLE and recommend the optimal exposure levels of MPA based on the normal range of serum 25(OH)D.
Methods
Patients and Data Collection
From November 2015 to March 2021, data were retrospectively collected from pediatric patients who were diagnosed with SLE and treated with MMF (dispersible tablets, Saikeping, Huangzhou Zhongmei Huadong Pharmaceutical Co. Ltd.; OR capsules, Cellcept, Shanghai Roche Pharmaceuticals Co. Ltd.) in the Children’s Hospital of Fudan University, National Children’s Medical Center in Shanghai. This study only included patients with follow-up data when TDM of MPA was conducted. The daily dosing amount of methylprednisolone was converted to the equivalent amount of prednisolone by multiplying it by 1.25 [28].
This study was conducted according to the ethical guidelines of the Declaration of Helsinki, the protocol of which was approved by the Ethics Committee of the Children’s Hospital of Fudan University [No. (2020) 490]. Written informed consent was waived because of the retrospective nature of this study.
Mycophenolic Acid Concentration Measurement and Area Under the Curve Calculation
The routine TDM protocol of MPA includes collecting 2 mL of blood samples at 30 min prior to the administration of MMF, and 20 min, 60 min, and 180 min post dose. Blood samples were centrifuged at 3000×g for 5–10 min and plasma was isolated for MPA concentration measurement. The enzyme-multiplied immunoassay technique (EMIT) was used to measure the MPA plasma concentration on the Viva System (Siemens Healthcare Diagnostics, Eschborn, Germany) at room temperature. The calibration range was 0.10–15.00 mg/L with the lower limit of quantification of 0.10 mg/L. The AUC over 24 h (AUC0–24h) was estimated with the four-point MPA concentrations using Bayesian methods [18] in the MwPharm++ software (Version 1.6.1.128, Mediware, Prague, Czech Republic).
Statistical Analysis
The age at SLE diagnosis was obtained by calculating the time difference between the date of SLE diagnosis and the birth date of each patient. Likewise, the age at TDM was obtained by calculating the time difference between the date of TDM and the birth date. The SLE duration was from the time difference between the date of TDM and the date of SLE diagnosis. Values of both age and SLE duration were rounded to years. Descriptive analyses included summarizing the frequency of each category for nominal or ordinal variables, and the center and variability for variables measured on a ratio scale. β-CTx, osteocalcin, 25(OH)D, calcium, and phosphorus were treated both as continuous variables and categorical variables. These originally continuous variables were categorized into three levels according to the normal reference range on the biochemical report. The normal reference ranges for the biochemical indices are β-CTx 0.30–0.60 ng/mL, osteocalcin 24.00–70.00 ng/mL, 25(OH)D 15.00–35.00 ng/mL, calcium 2.20–2.65 mmol/L, phosphorus 1.29–2.26 mmol/L (age ≤ 14 years), 0.81–1.45 (age > 14 years). Bartlett test was used to test the hypothesis of homoscedasticity of AUC0–24h across different levels of categorical variables. For variables that conformed to the assumption of homoscedasticity, analysis of variance (ANOVA) was conducted to test the statistical difference across different groups. Tukey’s honestly significant difference (HSD) test was employed as post hoc statistical test for two of the three groups.
Linear regression models with 25(OH)D as the dependent variable were used to identify factors associated with the 25(OH)D level. Repeated measures hierarchical linear models (RM-HLM) were developed to analyze the intra- and inter-individual effect of AUC0–24h on the variance of 25(OH)D levels. The AUC0–24h was separated into two variables, i.e., subject-centered AUC0–24h (AUC_SC) and subject mean of AUC0–24h (AUC_SM) by the following equations:
In the above equations, stands for the subject mean of AUC0–24h of the jth individual. means the jth individual having data from follow-ups. is the AUC0–24h for the jth individual at the ith follow-up. stands for the subject-centered AUC0–24h for the jth individual at the ith follow-up.
The unadjusted RM-HLM was as follows:
Level-1 model:
Level-2 model:
Overall model:
where:
= intercept for the jth individual;
= regression coefficient associated with for the jth individual;
= random error for the level-1 model;
= overall mean intercept adjusted for for the level-1 slope;
= overall mean intercept for the level-1 slope;
= regression coefficient associated with relative to level-1 intercept;
= random effects of the level-2 model on the intercept;
= variance of the random error for the level-1 model;
= variance of the random error for the level-2 model.
Further, the relation of AUC0–24h and 25(OH)D with SLEDAI score was analyzed with a mediation model in subjects with complete observations of the three variables. SLEDAI score was tested as the mediator with 25(OH)D as the dependent variable.
All the statistical analyses were conducted in R (version 4.0.4, the R foundation for Statistical Computing). RM-HLM was developed with the “nlme” package. Mediation effect was analyzed with the “mediation” package. Two-sided P < 0.05 was considered as statistically significant.
Results
Patients
Data were available from 184 pediatric patients (142 female, 42 male) with a total of 518 MPA TDM follow-ups. The median age at MPA TDM was 14 years with a range of 3–18 years. The median AUC0–24h was 88.88 mg·h/L, ranging from 6.00 to 294.98 mg·h/L. The descriptive statistics of patients’ demographic and biochemical data are presented in Table 1. The use of medications of the patients is shown in Table 2. Most of the patients were using glucocorticoids at follow-up (98.84%), with a median daily dose of 17.50 mg. Patients were also using oral vitamin D3 supplements, VDRAs, and hydroxychloroquine (HCQ) as prescribed.
Table 1.
Demographic and biochemical data of children with SLE
| Variables | No. of observations | Values |
|---|---|---|
| No. of patients | – | 184 |
| Sex (F/M) | 184 | 142/42 |
| Age at SLE diagnosis (years) | 184 | 10.82 (2.79), 11 [12–17] |
| MPA TDM times | 518 | 2 [1–18] |
| SLE duration (years) | 518 | 2.87 (2.64), 2 [0–12] |
| Age at MPA TDM (years) | 518 | 13.45 (2.79), 14 [3–18] |
| Season at MPA TDM | 518 | |
| Spring (March to May) | 110 (21.24%) | |
| Summer (June to August) | 175 (33.78%) | |
| Autumn (September to November) | 107 (20.66%) | |
| Winter (December to February) | 126 (24.32%) | |
| MPA concentrations (mg/L) | ||
| 30 min pre-dose/trough | 509 | 2.31 (1.75), 1.94 [0.12–15.45] |
| 20 min post-dose | 515 | 8.43 (9.46), 4.91 [0.13–71.40] |
| 60 min post-dose | 514 | 10.65 (9.14), 8.39 [0.34–79.80] |
| 180 min post-dose | 514 | 4.48 (3.12), 3.66 [0.30–25.10] |
| MPA AUC over 24 h (mg·h/L) | 515 | 95.05 (45.40), 88.88 [6.00–294.98] |
| SLEDAI | 380 | 5.96 (6.73), 4 [0–35] |
| β-CTx (ng/mL) | 376 | 0.88 (0.82), 0.66 [0.03–5.85] |
| < 0.30 ng/mL (low) | 61 (16.22%) | |
| 0.30–0.60 ng/mL (normal) | 110 (29.26%) | |
| > 0.60 ng/mL (high) | 205 (54.52%) | |
| Osteocalcin (ng/mL) | 378 | 37.21 (42.55), 24.63 [0.50–256.00] |
| < 24.00 ng/mL (low) | 184 (48.68%) | |
| 24.00–70.00 ng/mL (normal) | 149 (39.42%) | |
| > 70.00 ng/mL (high) | 45 (11.90%) | |
| 25(OH) vitamin D (ng/mL) | 378 | 19.81 (8.80), 18.43 [3.00–67.64] |
| < 15.00 ng/mL (low) | 109 (28.84%) | |
| 15.00–35.00 ng/mL (normal) | 248 (65.61%) | |
| > 35.00 ng/mL (high) | 21 (5.56%) | |
| Serum calcium (mmol/L) | 482 | 2.31 (0.17), 2.33 [1.68–2.89] |
| < 2.20 mmol/L (low) | 96 (19.92%) | |
| 2.20–2.65 mmol/L (normal) | 380 (78.84%) | |
| > 2.65 mmol/L (high) | 6 (1.24%) | |
| Serum phosphorus (mmol/L) | 480 | 1.33 (0.31), 1.33 [0.31–3.08] |
| Low | 113 (23.54%) | |
| Normal* | 321 (66.88%) | |
| High | 46 (9.58%) | |
Data are presented as mean (standard deviation), median [range], or frequency (percentage)
SLE systemic lupus erythematosus, MPA mycophenolic acid, TDM therapeutic drug monitoring, AUC area under the MPA concentration–time curve, SLEDAI SLE disease activity index, β-CTx beta-CrossLaps, No. Number
*Normal serum phosphorus range 1.29–2.26 mmol/L for children ≤ 14 years old; 0.81–1.45 for children > 14 years old
Table 2.
Use of medications in children with SLE
| Medication | Frequency | Daily dosing amount (mg) |
|---|---|---|
| MMF | 518 (100%) | 958 (338), 1000 [125–2000] |
| Glucocorticoids | 512 (98.84%) | 22.19 (26.04), 17.50 [0–468.75] |
| Oral vitamin D3 supplements | 457 (88.22%) | – |
|
Vitamin D receptor activators (calcitriol or alfacalcidol) |
296 (57.14%) | – |
| HCQ | 428 (82.63%) | – |
MMF mycophenolate mofetil, HCQ hydroxychloroquine
Data are presented as mean (standard deviation), median [range], or frequency (percentage)
Comparisons Across Different Levels of Bone Metabolism Markers and Electrolytes
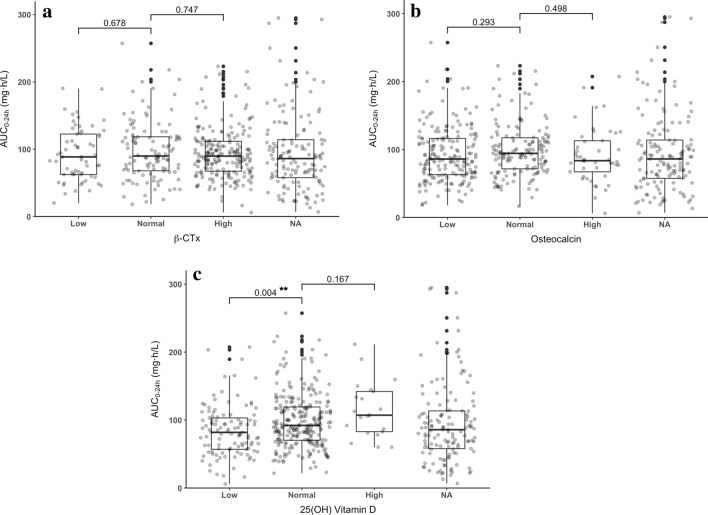
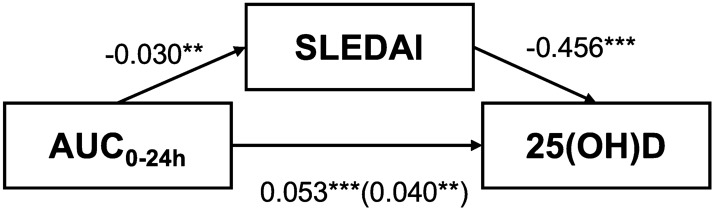
The comparisons of AUC0–24h across different levels of β-CTx, osteocalcin, 25(OH)D, calcium, and phosphorus are shown in Figs. 1 and 2. There was no significant difference in AUC0–24h between patients with normal and abnormal levels of β-CTx, osteocalcin, or serum phosphorus. However, patients with normal 25(OH)D levels had significantly higher AUC0–24h than patients with low levels of 25(OH)D (mean AUC0–24h, 98.71 vs. 84.05 mg·h/L, P = 0.004). There was also significant difference in AUC0–24h across patients with different levels of serum calcium. Patients with normal levels of calcium had significantly higher AUC0–24h than patients in the low-level group.
Fig. 1.
AUC of MPA by levels of bone turnover markers. a β-CTx. b Osteocalcin. c 25(OH) vitamin D. MPA mycophenolic acid, AUC0–24h area under the MPA concentration–time curve over 24 h, β-CTx beta-CrossLaps, NA missing data. ***P < 0.001, **P < 0.01, *P < 0.05
Fig. 2.
AUC of MPA by levels of serum calcium (a) and phosphorus (b). MPA mycophenolic acid, AUC0–24h area under the MPA concentration–time curve over 24 h, NA missing data. ***P < 0.001, **P < 0.01, *P < 0.05
Linear Association Between Area Under the Curve and 25-Hydroxyvitamin D
The results of linear regression analyses are presented in Table 3. The concentration of 25(OH)D is significantly associated with AUC0–24h in both univariate (B = 0.040, P < 0.001) and multivariate regression models (B = 0.034, P < 0.001). Other factors including age, sex, season, glucocorticoid daily dose, vitamin D3 supplements, and VDRAs were also associated with 25(OH)D levels.
Table 3.
Univariate and multivariate linear regression models with 25(OH) vitamin D as the dependent variable
| Predictors | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| Coefficient | P value | Coefficient | P value | |
| Age at TDM | − 0.959 | < 0.001*** | − 0.934 | < 0.001*** |
| Sex | ||||
| Female | Reference | Reference | ||
| Male | 5.958 | < 0.001*** | 4.370 | < 0.001*** |
| Season | ||||
| Spring | 0.379 | 0.785 | − 0.219 | 0.859 |
| Summer | 4.213 | < 0.001*** | 3.162 | 0.002** |
| Autumn | 3.497 | 0.010* | 2.632 | 0.030* |
| Winter | Reference | Reference | ||
| MPA AUC0–24h | 0.040 | < 0.001*** | 0.034 | < 0.001*** |
| Glucocorticoid daily dose | − 0.094 | < 0.001*** | − 0.101 | < 0.001*** |
|
Vitamin D3 supplements |
4.834 | 0.002** | 4.946 | < 0.001*** |
| Vitamin D receptor activators | − 2.768 | 0.003** | − 1.089 | 0.206 |
TDM therapeutic drug monitoring, MPA mycophenolic acid, AUC0–24h area under the MPA concentration–time curve over 24 h
***P < 0.001, **P < 0.01, *P < 0.05
In RM-HLMs, intra- and inter-individual effects of AUC0–24h were analyzed. The intra-individual effect of AUC0–24h had statistical significance in both unadjusted models ( = 0.034, P = 0.001) and adjusted models ( = 0.028, P < 0.001), while the inter-individual effect was not significant. The coefficient estimates and P values of the RM-HLMs are displayed in Table 4.
Table 4.
Repeated measures hierarchical linear models with 25(OH) vitamin D as the dependent variable
| Fixed effects | Unadjusted models | Adjusted models | ||
|---|---|---|---|---|
| Coefficient | P value | Coefficient | P value | |
|
MPA AUC0–24h (subject-centered) |
0.034 | 0.001** | 0.028 | < 0.001*** |
|
MPA AUC0–24h (subject mean) |
0.037 | 0.074 | 0.028 | 0.139 |
| Age at TDM | – | – | − 0.793 | < 0.001*** |
| Sex | ||||
| Female | – | – | Reference | |
| Male | – | – | 3.199 | 0.018* |
| Season | ||||
| Spring | – | – | 0.546 | 0.560 |
| Summer | – | – | 2.970 | < 0.001*** |
| Autumn | – | – | 1.818 | 0.054 |
| Winter | – | – | Reference | |
| Glucocorticoid daily dose | – | – | − 0.108 | < 0.001*** |
| Vitamin D3 supplements | – | – | 2.190 | 0.051 |
| Vitamin D receptor activators | – | – | − 0.171 | 0.822 |
| Random effects | ||||
| συ02 | 46.559 | 35.203 | ||
| σϵ2 | 25.150 | 23.062 | ||
MPA mycophenolic acid, AUC0–24h area under the MPA concentration–time curve over 24 h, TDM therapeutic drug monitoring
συ02 variance of the random intercept, σϵ2 variance of the residual
***P < 0.001, **P < 0.01, *P < 0.05
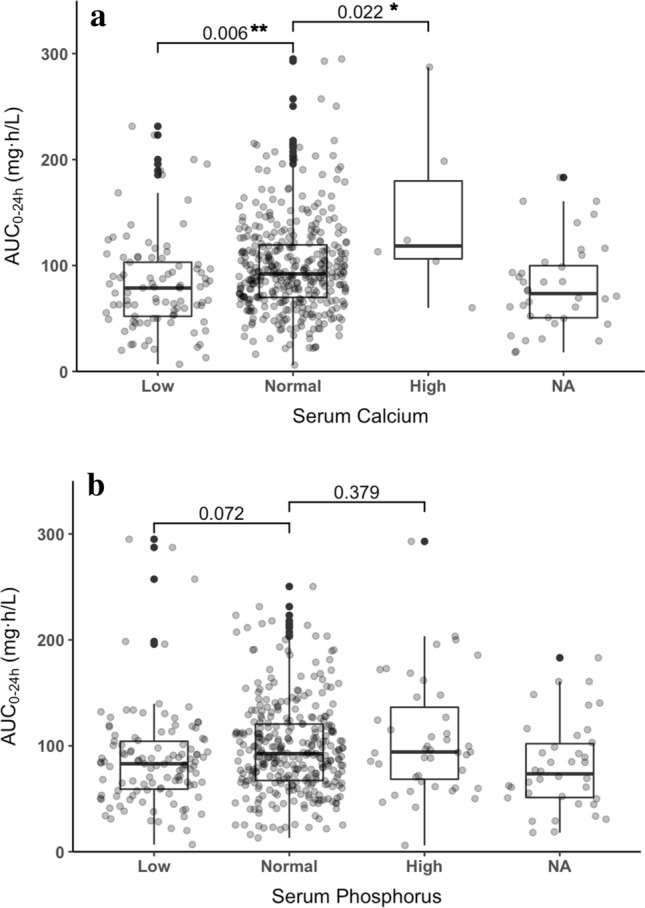
25-Hydroxyvitamin D Levels Were Associated Calcium and Phosphorus Homeostasis
As is shown in Fig. 3, serum calcium concentrations were positively correlated with 25(OH)D levels (y = 0.006x + 2.197, P < 0.001). For children over 14 years old, serum phosphorus concentrations were negatively correlated with 25(OH)D levels (y = − 0.012x + 1.517, P < 0.001), while for children 14 years old or younger, the correlation was not significant (P = 0.912).
Fig. 3.
Relationship between serum electrolyte levels and 25(OH) vitamin D. a Calcium. b Phosphorus
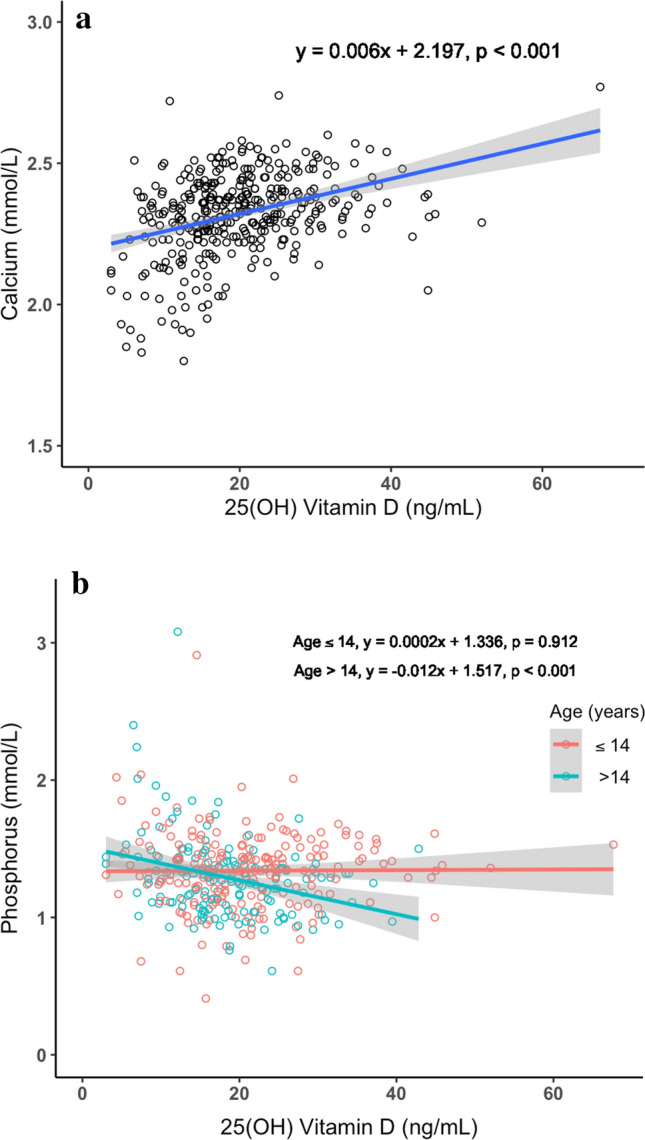
Mediation Effect of Disease Activity Score in Relationship Between 25-Hydroxyvitamin D Levels and Area Under the Curve
In the mediation model, data from 303 follow-ups with complete observations of the three variables were included for the analysis. The mediation relation of SLEDAI with AUC0–24h and 25(OH)D is illustrated in Fig. 4. Both the direct (B = 0.040, P = 0.004) and indirect effects (B = 0.014, P < 0.001) of AUC0–24h were significant. Of the total effect, 25.4% could be explained by mediation effect of SLEDAI.
Fig. 4.
Mediation effect of SLEDAI score in the relationship between AUC and 25(OH)D. AUC0–24h area under the MPA concentration–time curve over 24 h, SLEDAI systemic lupus erythematosus disease activity index, 25(OH)D 25-hydroxyvitamin D. The number outside the parentheses is the total effect. The number inside the parentheses is the direct effect. ***P < 0.001, **P < 0.01, *P < 0.05
Discussion
This is the first study that has explored the association between vitamin D levels and MPA exposure levels in children with SLE. A significantly positive association was found between the two variables. As the main indicators of vitamin D status in the human body, 25(OH)D may also serve as a potential indicator to optimize the exposure level of MPA during treatment. The optimal exposure level of MPA in children with SLE could be 98.71 mg·h/L in AUC0–24h or 49.36 mg·h/L in AUC0–12h, which is consistent with a previous study using SLEDAI score to evaluate the ideal exposure levels of MPA, with AUC0–12h over 50 mg·h/L being recommended as the treatment target [16].
Vitamin D status is important for monitoring patients with SLE and it is closely related to the bone metabolism [4, 29]. Chronic glucocorticoid use in children with SLE could lead to abnormal bone metabolism and growth [6, 9, 30]. Studies have shown that serum 25(OH)D levels of 15 ng/mL or less are independently associated with a lower bone formation rate and an increased fracture risk [31] in adults as well as in children [32]. In our study, higher 25(OH)D levels were found to be positively associated with higher levels of serum calcium and lower levels of phosphorus, which is related to the role of parathyroid hormone in regulating the levels of calcium under the circumstances of vitamin D deficiency and hypocalcemia [5].
The relationship between blood drug concentrations and vitamin D has also been investigated by other researchers. Lindh et al. [33] observed seasonal variation in plasma concentrations of cytochrome P450 3A4 (CYP3A4) like tacrolimus and sirolimus, which was highly consistent with seasonal changes in vitamin D. A possible explanation could be the stimulatory effect of vitamin D on drug metabolism by inducing the expression of CYP3A4. In their study, MPA was included as a control drug that is independent of CYP3A4 metabolism. Another study by Wang et al. [34] found that the co-administration of 1-alpha,25-dihydroxyvitamin D3 [1α,25(OH)2D3] could alter the pharmacokinetics of MPA by regulating the extrahepatic enzymes UGT1A8 and UGT1A10 in renal transplant recipients. However, Wang’s study used within-individual repeated-measure comparison to demonstrate the significant change in AUC0–12h of MPA from the pre-administration of 1α,25(OH)2D3 (on day 8) to the post-administration (on day 16). One potential problem they might not have considered was that the post-transplantation days could affect the pharmacokinetics of MPA, thus confounding the effect of 1α,25(OH)2D3 [35].
Although we cannot completely rule out the pharmacokinetic relationship between MPA exposure and vitamin D levels, the significant association found in our study was likely due to the indirect pharmacodynamic effect. A possible explanation for the observed association could be that full exposure to MPA is associated with disease control and reduced dose of prescribed glucocorticoids, thus leading to improved profiles of bone turnover markers like 25(OH)D. In our study, the AUC0–24h of MPA was found to have significantly negative association with SLEDAI score, with higher AUC0–24h predicting lower SLEDAI score, thus better disease control (B = − 0.030, P = 0.002). Meanwhile, higher SLEDAI score was significantly associated with lower 25(OH)D levels when AUC0–24h was controlled (B = − 0.456, P < 0.001), indicating the possible mediation effect by SLEDAI in the relationship between AUC0–24h and 25(OH)D. However, the effect of AUC0–24h on 25(OH)D levels remained significant after controlling for SLEDAI scores in the model (B = 0.040, P = 0.002). In the mediation analysis, both the direct and indirect effects of AUC0–24h were significant, with 25.4% of the total effect explained by the mediation effect of SLEDAI score.
In addition, the intra- and inter-individual effects of AUC0–24h were analyzed in hierarchical linear models. The effect sizes were similar while only the intra-individual effect was statistically significant, suggesting the importance of maintaining stable exposure levels during treatment.
However, we did not find significant differences in AUC0–24h across patient groups with different levels of β-CTx and osteocalcin. A study in female patients with SLE without the influence of glucocorticoids found lower osteocalcin and 25(OH)D levels and higher β-CTx levels compared to healthy controls, indicating that SLE itself is associated with changed bone metabolism [11]. Our study included both male and female patients, and most patients were put on glucocorticoids at TDM follow-up. Yet, the abnormal levels of β-CTx and osteocalcin were not associated with significant changes in AUC0–24h.
Besides the exposure levels of MPA, other factors were also associated with the variance of 25(OH)D, including age, sex, season at measurement, glucocorticoid daily dose, and use of vitamin D3 supplements and VDRAs. Age and glucocorticoid daily dose were negatively associated with 25(OH)D, while male sex, summer season, and external vitamin D3 supplements were positively associated with 25(OH)D, which are consistent with previous findings from other studies [9, 36–38]. One large population-based multicenter study in Jiangsu Province of China investigated 5289 children and revealed that children of older age and being a girl were at a higher risk of vitamin D deficiency [36]. In our study, boys with SLE had about fivefold higher 25(OH)D levels than girls with SLE (B = 5.958, P < 0.001) and increased age was associated with decreased 25(OH)D concentrations (B = − 0.959, P < 0.001).
Vitamin D3 supplements were significantly associated with increased levels of 25(OH)D in our study. However, the use of VDRAs (i.e., calcitriol or alfacalcidol) had a negative effect on 25(OH)D levels (B = − 2.768, P = 0.003), and this negative effect became nonsignificant after adjusting for other variables (B = − 1.089, P = 0.206). This is probably because VDRAs were used when vitamin D was insufficient and their effect on hyperparathyroidism bypassed 25(OH)D, thus their application would not increase the level of 25(OH)D.
There are some limitations in this study. First, this is a retrospective single-centered study. The data collection purely relied on the electronic medical records of the patients. This may lead to inaccuracy in recorded medications. Second, 25(OH)D levels of the patients had large variances, and factors considered in our study only explain a small portion of them. Third, as a result of the cross-sectional nature of this study, the causal relationship between exposure levels of MPA and 25(OH)D cannot be concluded. Therefore, further prospective and multicentered studies are needed to confirm these initial findings.
Conclusions
MPA exposure levels are positively associated with 25(OH)D independent of age, sex, season, glucocorticoids, and vitamin D3 supplements, but not significantly associated with β-CTx and osteocalcin levels. The intra-individual effect of MPA AUC0–24h is more significant than the inter-individual effect on 25(OH)D levels. On the basis of the normal reference range of 25(OH)D at 15.00–35.00 ng/mL, MPA AUC0–24h of 98.71 mg·h/L or AUC0–12h of 49.36 mg·h/L might be the targeted exposure levels for SLE treatment in children.
Acknowledgements
Funding
This study and the journal’s Rapid Service Fee were funded by the National Natural Science Foundation of China (No. 81874325), Scientific Research Project of Science and Technology Commission of Shanghai Municipality (No. 19DZ1910604/19XD1400900/18DZ1910604), and Key Innovative Team of Shanghai Top-Level University Capacity Building in Clinical Pharmacy and Regulatory Science at Shanghai Medical College, Fudan University (No. HJW-R-2019–66-19).
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICJME) criteria for authorship of this manuscript, take responsibility for the integrity of the whole work, and have given approval for the publication of this version of the manuscript.
Author Contributions
Conceptualization: Qiaofeng Ye, Guangfei Wang, and Zhiping Li; Methodology: Qiaofeng Ye, Guangfei Wang, Yidie Huang, Jinmiao Lu, and Xiaoxia Li; Formal analysis and investigation: Qiaofeng Ye, Guangfei Wang, Yidie Huang, Jinmiao Lu, Junqi Zhang, Lin Zhu, Yiqing Zhu, Xiaoxia Li, Jianger Lan, and Yubing Liu.; Writing – original draft preparation: Qiaofeng Ye; Writing – review and editing: Guangfei Wang, Yidie Huang, Jinmiao Lu, Yiqing Zhu, Lin Zhu, Xiaoxia Li, Ziwei Li, Jianger Lan, Yubing Liu, Hong Xu, and Zhiping Li; Funding acquisition: Zhiping Li; Resources: Zhiping Li, Hong Xu, and Yidie Huang; Supervision: Zhiping Li, Yidie Huang and Guangfei Wang.
Disclosures
Qiaofeng Ye, Guangfei Wang, Yidie Huang, Jinmiao Lu, Junqi Zhang, Lin Zhu, Yiqing Zhu, Xiaoxia Li, Jianger Lan, Ziwei Li, Yubing Liu, Hong Xu and Zhiping Li have nothing to disclose.
Compliance with Ethics Guidelines
This study was conducted according to the ethical guidelines of the Declaration of Helsinki, the protocol of which was approved by the Ethics Committee of the Children’s Hospital of Fudan University [No. (2020) 490]. Written informed consent was waived because of the retrospective nature of this study.
Data Availability
The original data will be available upon reasonable request to the corresponding author.
Footnotes
Qiaofeng Ye, Guangfei Wang and Yidie Huang contributed equally to this paper.
Contributor Information
Hong Xu, Email: hxu@shmu.edu.cn.
Zhiping Li, Email: zpli@fudan.edu.cn.
References
- 1.Groot N, de Graeff N, Avcin T, et al. European evidence-based recommendations for diagnosis and treatment of childhood-onset systemic lupus erythematosus: the SHARE initiative. Ann Rheum Dis. 2017;76(11):1788–1796. doi: 10.1136/annrheumdis-2016-210960. [DOI] [PubMed] [Google Scholar]
- 2.Tang SP, Lim SC, Arkachaisri T. Childhood-onset systemic lupus erythematosus: Southeast Asian perspectives. J Clin Med 2021;10(4):559. [DOI] [PMC free article] [PubMed]
- 3.Liu Y, Yu C, Ji K, et al. Quercetin reduces TNF-alpha-induced mesangial cell proliferation and inhibits PTX3 production: involvement of NF-kappaB signaling pathway. Phytother Res. 2019;33(9):2401–2408. doi: 10.1002/ptr.6430. [DOI] [PubMed] [Google Scholar]
- 4.Bultink IE, Lems WF, Kostense PJ, Dijkmans BA, Voskuyl AE. Prevalence of and risk factors for low bone mineral density and vertebral fractures in patients with systemic lupus erythematosus. Arthritis Rheum. 2005;52(7):2044–2050. doi: 10.1002/art.21110. [DOI] [PubMed] [Google Scholar]
- 5.Goldsmith DJ, Massy ZA, Brandenburg V. The uses and abuses of vitamin D compounds in chronic kidney disease- mineral bone disease (CKD-MBD) Semin Nephrol. 2014;34(6):660–668. doi: 10.1016/j.semnephrol.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 6.Salman-Monte TC, Torrente-Segarra V, Vega-Vidal AL, et al. Bone mineral density and vitamin D status in systemic lupus erythematosus (SLE): a systematic review. Autoimmun Rev. 2017;16(11):1155–1159. doi: 10.1016/j.autrev.2017.09.011. [DOI] [PubMed] [Google Scholar]
- 7.Lertratanakul A, Wu P, Dyer A, et al. 25-Hydroxyvitamin D and cardiovascular disease in patients with systemic lupus erythematosus: data from a large international inception cohort. Arthritis Care Res (Hoboken) 2014;66(8):1167–1176. doi: 10.1002/acr.22291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chew C, Reynolds JA, Lertratanakul A, et al. Lower vitamin D is associated with metabolic syndrome and insulin resistance in systemic lupus: data from an international inception cohort. Rheumatology (Oxford) 2021. 10.1093/rheumatology/keab090. [DOI] [PMC free article] [PubMed]
- 9.Kipen Y, Buchbinder R, Forbes A, Strauss B, Littlejohn G, Morand E. Prevalence of reduced bone mineral density in systemic lupus erythematosus and the role of steroids. J Rheumatol. 1997;24(10):1922–1929. [PubMed] [Google Scholar]
- 10.Pazianas M, Miller PD. Osteoporosis and chronic kidney disease-mineral and bone disorder (CKD-MBD): back to basics. Am J Kidney Dis. 2021 doi: 10.1053/j.ajkd.2020.12.024. [DOI] [PubMed] [Google Scholar]
- 11.Wang T, Zhang Y, Hong B, Gu J, Wang H, Li Y. The changes in bone turnover markers of female systemic lupus erythematousus patients without glucocorticoid. Lupus. 2021 doi: 10.1177/09612033211000126. [DOI] [PubMed] [Google Scholar]
- 12.Zhou M, Ma X, Li H, et al. Serum osteocalcin concentrations in relation to glucose and lipid metabolism in Chinese individuals. Eur J Endocrinol. 2009;161(5):723–729. doi: 10.1530/EJE-09-0585. [DOI] [PubMed] [Google Scholar]
- 13.Thiering E, Bruske I, Kratzsch J, et al. Associations between serum 25-hydroxyvitamin D and bone turnover markers in a population based sample of German children. Sci Rep. 2015;5:18138. doi: 10.1038/srep18138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Allison AC, Eugui EM. Mycophenolate mofetil and its mechanisms of action. Immunopharmacology. 2000;47(2–3):85–118. doi: 10.1016/S0162-3109(00)00188-0. [DOI] [PubMed] [Google Scholar]
- 15.Groot N, de Graeff N, Marks SD, et al. European evidence-based recommendations for the diagnosis and treatment of childhood-onset lupus nephritis: the SHARE initiative. Ann Rheum Dis. 2017;76(12):1965–1973. doi: 10.1136/annrheumdis-2017-211898. [DOI] [PubMed] [Google Scholar]
- 16.Chen Y, Sun L, Xu H, et al. PK/PD study of mycophenolate mofetil in children with systemic lupus erythematosus to inform model-based precision dosing. Front Pharmacol. 2020;11:605060. doi: 10.3389/fphar.2020.605060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pourafshar N, Karimi A, Wen X, et al. The utility of trough mycophenolic acid levels for the management of lupus nephritis. Nephrol Dial Transplant. 2019;34(1):83–89. doi: 10.1093/ndt/gfy026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Woillard J-B, Bader-Meunier B, Salomon R, et al. Pharmacokinetics of mycophenolate mofetil in children with lupus and clinical findings in favour of therapeutic drug monitoring. Br J Clin Pharmacol. 2014;78(4):867–876. doi: 10.1111/bcp.12392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zahr N, Arnaud L, Marquet P, et al. Mycophenolic acid area under the curve correlates with disease activity in lupus patients treated with mycophenolate mofetil. Arthritis Rheum. 2010;62(7):2047–2054. doi: 10.1002/art.27495. [DOI] [PubMed] [Google Scholar]
- 20.Sagcal-Gironella AC, Fukuda T, Wiers K, et al. Pharmacokinetics and pharmacodynamics of mycophenolic acid and their relation to response to therapy of childhood-onset systemic lupus erythematosus. Semin Arthritis Rheum. 2011;40(4):307–313. doi: 10.1016/j.semarthrit.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alexander S, Fleming DH, Mathew BS, et al. Pharmacokinetics of concentration-controlled mycophenolate mofetil in proliferative lupus nephritis: an observational cohort study. Ther Drug Monit. 2014;36(4):423–432. doi: 10.1097/FTD.0000000000000031. [DOI] [PubMed] [Google Scholar]
- 22.Lertdumrongluk P, Somparn P, Kittanamongkolchai W, Traitanon O, Vadcharavivad S, Avihingsanon Y. Pharmacokinetics of mycophenolic acid in severe lupus nephritis. Kidney Int. 2010;78(4):389–395. doi: 10.1038/ki.2010.170. [DOI] [PubMed] [Google Scholar]
- 23.Daleboudt GM, Reinders ME, den Hartigh J, et al. Concentration-controlled treatment of lupus nephritis with mycophenolate mofetil. Lupus. 2013;22(2):171–179. doi: 10.1177/0961203312469261. [DOI] [PubMed] [Google Scholar]
- 24.Fanouriakis A, Kostopoulou M, Cheema K, et al. 2019 update of the joint European League Against Rheumatism and European Renal Association-European Dialysis and Transplant Association (EULAR/ERA-EDTA) recommendations for the management of lupus nephritis. Ann Rheum Dis. 2020;79(6):713–723. doi: 10.1136/annrheumdis-2020-216924. [DOI] [PubMed] [Google Scholar]
- 25.Mendoza-Pinto C, Garcia-Carrasco M, Juarez-Melchor D, et al. A retrospective analysis of longitudinal changes in bone mineral density in women with systemic lupus erythematosus. Calcif Tissue Int. 2021. 10.1007/s00223-021-00845-0. [DOI] [PubMed]
- 26.Correa-Rodriguez M, Pocovi-Gerardino G, Callejas-Rubio JL, Rios-Fernandez R, Rueda-Medina B, Ortego-Centeno N. Disease damage accrual and low bone mineral density in female patients with systemic lupus erythematosus. Biol Res Nurs. 2021 doi: 10.1177/10998004211005550. [DOI] [PubMed] [Google Scholar]
- 27.Garelick D, Pinto SM, Farinha F, Pires T, Khan E, Isenberg D. Fracture risk in systemic lupus erythematosus patients over 28 years. Rheumatology (Oxford) 2020 doi: 10.1093/rheumatology/keaa705. [DOI] [PubMed] [Google Scholar]
- 28.Mager DE, Lin SX, Blum RA, Lates CD, Jusko WJ. Dose equivalency evaluation of major corticosteroids: pharmacokinetics and cell trafficking and cortisol dynamics. J Clin Pharmacol. 2003;43(11):1216–1227. doi: 10.1177/0091270003258651. [DOI] [PubMed] [Google Scholar]
- 29.Cutolo M. Vitamin D and autoimmune rheumatic diseases. Rheumatology (Oxford) 2009;48(3):210–212. doi: 10.1093/rheumatology/ken394. [DOI] [PubMed] [Google Scholar]
- 30.Blaslov K, Katalinic L, Kes P, Spasovski G, Smalcelj R, Basic-Jukic N. What is the impact of immunosuppressive treatment on the post-transplant renal osteopathy? Int Urol Nephrol. 2014;46(5):1019–1024. doi: 10.1007/s11255-013-0596-7. [DOI] [PubMed] [Google Scholar]
- 31.Garrett G, Sardiwal S, Lamb EJ, Goldsmith DJ. PTH–a particularly tricky hormone: why measure it at all in kidney patients? Clin J Am Soc Nephrol. 2013;8(2):299–312. doi: 10.2215/CJN.09580911. [DOI] [PubMed] [Google Scholar]
- 32.Varkal MA, Gulenc B, Yildiz I, et al. Vitamin D level, body mass index and fracture risk in children: vitamin D deficiency and fracture risk. J Pediatr Orthop B. 2021 doi: 10.1097/bpb.0000000000000867. [DOI] [PubMed] [Google Scholar]
- 33.Lindh JD, Andersson ML, Eliasson E, Bjorkhem-Bergman L. Seasonal variation in blood drug concentrations and a potential relationship to vitamin D. Drug Metab Dispos. 2011;39(5):933–937. doi: 10.1124/dmd.111.038125. [DOI] [PubMed] [Google Scholar]
- 34.Wang X, Wang H, Shen B, et al. 1-Alpha,25-dihydroxyvitamin D3 alters the pharmacokinetics of mycophenolic acid in renal transplant recipients by regulating two extrahepatic UDP-glucuronosyltransferases 1A8 and 1A10. Transl Res. 2016;178:54–62.e6. doi: 10.1016/j.trsl.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 35.Rong Y, Jun H, Kiang TKL. Population pharmacokinetics of mycophenolic acid in paediatric patients. Br J Clin Pharmacol. 2020 doi: 10.1111/bcp.14590. [DOI] [PubMed] [Google Scholar]
- 36.Zhao Y, Qin R, Hong H, et al. Vitamin D status and its dietary, lifestyle factors in children during the first five years of life: a cross-sectional multicenter Jiangsu bone study. J Hum Nutr Diet. 2021 doi: 10.1111/jhn.12883. [DOI] [PubMed] [Google Scholar]
- 37.Deng J, Silver Z, Huang E, et al. Pharmacological prevention of fractures in patients undergoing glucocorticoid therapies: a systematic review and network meta-analysis. Rheumatology (Oxford) 2021;60(2):649–657. doi: 10.1093/rheumatology/keaa228. [DOI] [PubMed] [Google Scholar]
- 38.Karnia MJ, Korewo D, Myslinska D, et al. The positive impact of vitamin D on glucocorticoid-dependent skeletal muscle atrophy. Nutrients. 2021;13(3):936. doi: 10.3390/nu13030936. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original data will be available upon reasonable request to the corresponding author.