Abstract
Introduction
The aim of this work was to evaluate the prevalence of malignancies in a multicenter cohort of Chinese patients with immunoglobulin G4-related disease (IgG4-RD) and to identify the related risk factors of malignancy in IgG4-RD patients.
Methods
We retrospectively analyzed 602 IgG4-RD patients who were recruited in five medical centers from 2009 to 2020. Standardized prevalence ratios (SPRs) against the general Chinese population were calculated along with 95% confidence intervals (CIs). We identified the risk factors of malignancy in IgG4-RD and calculated the odds ratios (ORs) of different factors. We then developed and validated a prediction model for malignancy risk of IgG4-RD based on our cohort.
Results
We observed a significantly increased prevalence of total malignancies in this cohort compared to the general Chinese population (SPR 8.66 [95% CI 5.84, 12.31]). Logistic regression analysis indicated that eosinophil percentage (OR 1.096 [95% CI 1.019–1.179], P = 0.016), serum albumin-to-globulin ratio (AGR) (OR 0.185 [95% CI 0.061–0.567], P = 0.002) and autoimmune pancreatitis (OR 2.400 [95% CI 1.038–5.549], P = 0.041) were three potential risk factors of malignancy in IgG4-RD patients. Four predictors were included in our final prediction model: age at IgG4-RD diagnosis, eosinophil percentage, AGR and autoimmune pancreatitis. The nomogram performed well in the internal validation cohort, with a concordance index (C-index) of 0.738.
Conclusions
A significantly increased prevalence of total malignancies was observed in our multicenter cohort. Eosinophil percentage and autoimmune pancreatitis are risk factors, whereas AGR is negatively associated with malignancy in IgG4-RD. A prediction model for malignancy risk of IgG4-RD was first developed and validated in our study.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40744-021-00326-8.
Keywords: IgG4-related disease, Malignancy, Risk factors, Prediction model
Key Summary Points
| Why carry out this study? |
| The association between IgG4-related disease (IgG4-RD) and malignancy remains controversial. |
| This study aims to evaluate the prevalence of malignancies in a multicenter cohort of Chinese IgG4-RD patients and to identify the potential related factors of malignancy. |
| What was learned from the study? |
| We reported a significantly increased prevalence of malignancies in IgG4-RD patients. |
| Eosinophil percentage and autoimmune pancreatitis are potential risk factors, whereas serum albumin-to-globulin ratio (AGR) is negatively associated with malignancy in IgG4-RD. |
| A nomogram for assessment of malignancy in IgG4-RD patients was first developed and validated. |
Digital Features
This article is published with digital features, including a summary slide, to facilitate understanding of the article. To view digital features for this article go to 10.6084/m9.figshare.14617950.
Introduction
IgG4-related disease (IgG4-RD) is a systemic autoimmune disease characterized by clinicopathological evidence of single or multiple tumefactive lesions, frequent elevation of serum IgG4 concentration and pathological findings such as fibrosis arranged in a storiform pattern, obliterative phlebitis, and IgG4-positive plasma cell infiltration in tissue [1–3]. As a recently recognized fibroinflammatory condition, IgG4-RD is manifested as sialadenitis, dacryoadenitis, autoimmune pancreatitis (AIP), sclerosing cholangitis (SC) [4], tubulointerstitial nephritis (TIN), membranous glomerulonephropathy (MGN) [5] and retroperitoneal fibrosis, etc.
As an important cause of polyclonal hypergammaglobulinemia (PHGG), IgG4-RD is usually considered as mimickers of some neoplastic disorders, such as B cell lymphoma, angioimmunoblastic T cell lymphoma, non-hematological malignancy associated with inflammation, histiocyte disorders (especially Rosai–Dorfman disease), as well as multicentric Castleman disease (MCD) [6, 7]. Definitive diagnosis of IgG4-RD requires close clinical-pathological correlation and long-term follow-up [8].
Whether patients with IgG4-RD have a higher risk of malignancy than general population is still controversial. Several studies reported an increased risk for malignancies among patients with IgG4-RD [9–13]. However, other studies observed no significant increase in the incidence of malignancies compared with the general population [14–16]. It is still too early to draw a definitive conclusion based on limited samples in a single center cohort.
In this study, we evaluated the prevalence of malignancy in a multicenter large cohort of Chinese IgG4-RD patients and compared clinical characteristics between patients with and without malignancies. Risk factors of malignancy in IgG4-RD patients were identified. A model capable of assessing malignancy risk of patients with IgG4-RD was developed and validated.
Methods
Patients
For this multicenter study, we included 602 patients who were referred to as IgG4-RD by the 2019 ACR/EULAR IgG4-RD classification criteria from five medical centers in China between April 2009 and January 2020 [17]. Malignant tumors were diagnosed according to available medical records and reliable pathological evidence, fulfilling International Classification of Diseases (ICD-11) criteria. Malignancy diagnosed within 1 year before or after the diagnosis of IgG4-RD was defined as on the diagnosis of IgG4-RD. Patients with malignancy diagnosed more than 1 year before or after the diagnosis of IgG4-RD were defined as before or after the diagnosis of IgG4-RD, respectively. The study has been approved by the Medical Ethics Committee of Peking University People's Hospital (Beijing, China).
Variables of Interest
During the follow-up period, we retrospectively collected baseline data pertaining to demographic characteristics, personal history, past medical history, laboratory results and organ involvement. As possible risk factors of malignancy in IgG4-RD patients, we included the following variables for univariate and multivariate analysis: personal history such as smoking and alcohol, past medical history including allergic diseases, laboratory predictors such as complement, ESR, CRP, serum globulin level, serum albumin to globulin ratio (AGR), serum immunoglobulin level (IgA, IgM, IgE, and IgG), serum IgG4 level, eosinophil, ANA and RF, as well as organ involvement. We selected predictors from variables above for our final model.
Statistical Analysis
Baseline Characteristics
Categorical variables were presented as the ratio or percentage of subjects, and continuous variables as mean ± standard deviation (SD) (for normally distributed data) or median (interquartile range, IQR) (for non-normally distributed data). The statistical significance of differences in frequencies between groups was determined using the Chi-square test or the Fisher’s exact test as appropriate, while continuous variables were compared by the Mann–Whitney U test.
Calculation of SPRs
Standardized prevalence ratios (SPRs) against the general Chinese population were calculated along with 95% confidence intervals (CIs). The SPR was calculated by comparing the observed to the expected number of cases, and the 95% CI was calculated based on the Poisson's distribution model.
Logistic Regression Analysis
Univariate and multivariate analyses were performed to identify the risk factors of malignancy in IgG4-RD and calculate the odds ratios (ORs) of different factors based on a logistic regression model. In the multivariate analysis, we applied those variables with P < 0.1 in univariate analysis and possible risk factors of malignancy in previous studies [1, 3, 7] and used the backward elimination method.
Development and Validation of an Assessment Model
We developed a nomogram for assessing malignancy risk of IgG4-RD based on backward stepwise logistic regression and used the bootstrap method with 1000 repetitions for internal validation, Harrell’s C statistic was calculated as well.
The final model was developed and validated using R software. All other statistical analyses were performed by SPSS version 25.0.
Results
Baseline Characteristics of IgG4-RD Patients in the Cohort
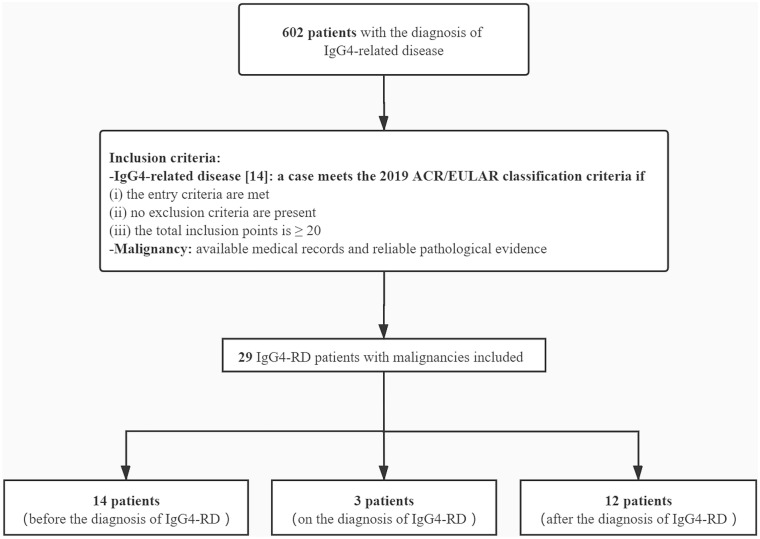
A total of 602 patients with IgG4-RD meeting the inclusion criteria in Fig. 1 were enrolled in this study. As presented in Table 1, the baseline characteristics were collected at the time of diagnosis of IgG4-RD before clinical interventions. According to the 2019 ACR/EULAR IgG4-RD classification criteria [14], 59.63% patients in our cohort were male. The mean age at IgG4-RD diagnosis was 54.6 ± 13.4 years. The median follow-up time of the patients was 47.0 (27.0–65.0) months. The most frequently involved organs were the submandibular glands (56.81%), followed by the lymph nodes (48.01%) and the lacrimal glands (41.36%). Twenty-nine patients (18 male and 11 female) were identified as IgG4-RD accompanied by malignancy. Of all cases with malignancy, the mean age was 58.9 ± 12.1 years at IgG4-RD diagnosis and 56.8 ± 13.7 years at malignancy diagnosis, respectively.
Fig. 1.
Flowchart of the inclusion of patients with immunoglobulin G4-related disease (IgG4-RD) and malignancy in this study
Table 1.
Characteristics of IgG4-RD patients with and without malignancies (n = 602)
| Variable | Total (n = 602) | IgG4-RD with malignancies (n = 29) | IgG4-RD without malignancies (n = 573) | P value |
|---|---|---|---|---|
| Demographic data | ||||
| Agea, years | 54.6 ± 13.4 | 58.9 ± 12.1 | 54.4 ± 13.4 | 0.081 |
| Male:Female | 359:243 (1.48: 1) | 18:11 (1.64: 1) | 341:232 (1.47: 1) | 0.784 |
| Follow-up time, months | 47.0 (27.0–65.0) | 54.0 (23.0–73.0) | 46.0 (27.0–65.0) | 0.515 |
| Time from onset to diagnosis, months | 12.0 (6.0–36.0) | 24.0 (7.0–60.0) | 12.0 (6.0–36.0) | 0.052 |
| Personal history | ||||
| Smoke, n (%) | 92 (15.28%) | 6 (20.69%) | 86 (15.01%) | 0.407 |
| Alcohol, n (%) | 47 (7.81%) | 2 (6.90%) | 45 (7.85%) | 1.000 |
| Past medical history | ||||
| Allergic diseasesb | 223 (37.04%) | 14 (48.28%) | 209 (36.47%) | 0.199 |
| Cardiovascular disease, n (%) | 64 (10.63%) | 4 (13.79%) | 60 (10.47%) | 0.797 |
| Hypertension, n (%) | 150 (24.92%) | 11 (37.93%) | 139 (24.26%) | 0.097 |
| Diabetes, n (%) | 99 (16.45%) | 8 (27.59%) | 91 (15.88%) | 0.097 |
| Hyperlipidemia, n (%) | 59 (9.80%) | 3 (10.34%) | 56 (9.77%) | 1.000 |
| Laboratory results | ||||
| Complement 3 (C3), g/l | 0.897 (0.736–1.090) | 0.873 (0.762–1.100) | 0.898 (0.731–1.090) | 0.965 |
| Complement 4 (C4), g/l | 0.210 (0.155–0.273) | 0.180 (0.137–0.233) | 0.210 (0.159–0.275) | 0.135 |
| ESR, mm/h | 13.0 (7.0–34.0) | 15.0 (6.5–46.0) | 13.0 (7.0–34.0) | 0.792 |
| CRP, mg/l | 2.21 (0.91–7.28) | 2.79 (1.05–5.32) | 2.20 (0.91–7.51) | 0.789 |
| Serum globulin level, g/l | 31.60 (27.50–37.48) | 33.30 (30.80–35.33) | 31.50 (27.50–37.60) | 0.399 |
| AGR | 1.46 (1.28–1.65) | 1.15 (0.93–1.43) | 1.49 (1.29–1.65) | < 0.001** |
| Serum IgA level, g/l | 1.99 (1.35–2.79) | 1.56 (1.14–2.88) | 2.01 (1.36–2.78) | 0.235 |
| Serum IgM level, g/l | 0.84 (0.62–1.18) | 0.77 (0.62–1.34) | 0.84 (0.61–1.17) | 0.833 |
| Serum IgE level, IU/ml | 236.80 (77.89–633.25) | 320.50 (169.00–701.00) | 234.00 (73.92–625.60) | 0.172 |
| Serum IgG level, g/l | 17.42 (13.70–25.50) | 21.94 (16.30–29.80) | 17.36 (13.65–24.74) | 0.015* |
| Serum IgG4 level, mg/dl | 584.0 (273.0–1400.0) | 610.0 (444.0–2330.0) | 565.9 (269.0–1382.5) | 0.065 |
| Eosinophil count, 109/l | 0.16 (0.08–0.30) | 0.26 (0.10–0.47) | 0.16 (0.08–0.30) | 0.102 |
| Eosinophil percentage, % | 2.40 (1.00–4.50) | 6.10 (2.65–7.53) | 2.30 (1.00–4.30) | < 0.001** |
| ANA (+), n (%) | 96 (15.95%) | 4 (13.79%) | 92 (16.06%) | 0.948 |
| Elevated RF, n (%) | 80 (13.29%) | 4 (13.79%) | 76 (13.26%) | 1.000 |
| Number of involved organs | 3.0 (2.0–5.0) | 4.0 (2.0–5.0) | 3.0 (2.0–4.3) | 0.252 |
| Organ involvement | ||||
| Head and neckc | 409 (67.94%) | 22 (75.86%) | 387 (67.54%) | 0.349 |
| Aorta | 29 (4.82%) | 1 (3.45%) | 28 (4.89%) | 1.000 |
| Bile duct system | 102 (16.94%) | 6 (20.69%) | 96 (16.75%) | 0.581 |
| Endocranium | 5 (0.83%) | 0 (0) | 5 (0.87%) | 1.000 |
| Gall bladder | 76 (12.62%) | 3 (10.34%) | 73 (12.74%) | 0.926 |
| Heart and pericardium | 6 (1.00%) | 0 (0) | 6 (1.05%) | 1.000 |
| Kidney | 115 (19.10%) | 4 (13.79%) | 111 (19.37%) | 0.615 |
| Lacrimal gland | 249 (41.36%) | 15 (51.72%) | 234 (40.84%) | 0.245 |
| Liver | 28 (4.65%) | 3 (10.34%) | 25 (4.36%) | 0.298 |
| Lung | 138 (22.92%) | 8 (27.59%) | 130 (22.69%) | 0.540 |
| Lymph node | 289 (48.01%) | 18 (62.07%) | 271 (47.29%) | 0.120 |
| Mediastinum | 2 (0.33%) | 0 (0) | 2 (0.35%) | 1.000 |
| Mesentery | 11 (1.83%) | 1 (3.45%) | 10 (1.75%) | 1.000 |
| Middle ear and mastoid | 3 (0.50%) | 0 (0) | 3 (0.52%) | 1.000 |
| Orbit and peri-orbit | 58 (9.63%) | 4 (13.79%) | 54 (9.42%) | 0.649 |
| Pancreas | 167 (27.74%) | 12 (41.38%) | 155 (27.05%) | 0.093 |
| Parotid gland | 193 (32.06%) | 7 (24.14%) | 186 (32.46%) | 0.349 |
| Pituitary gland | 3 (0.50%) | 0 (0) | 3 (0.52%) | 1.000 |
| Prostate | 32 (5.32%) | 1 (3.45%) | 31 (5.41%) | 0.972 |
| Retroperitoneum | 87 (14.45%) | 4 (13.79%) | 83 (14.49%) | 1.000 |
| Sublingual gland | 19 (3.16%) | 2 (6.90%) | 17 (2.97%) | 0.524 |
| Submandibular gland | 342 (56.81%) | 20 (68.97%) | 322 (56.20%) | 0.176 |
| Thyroid | 54 (8.97%) | 0 (0) | 54 (9.42%) | 0.098 |
Values are expressed as mean ± standard deviation (SD), median (interquartile range, IQR), ratio or number (percentage)
ESR erythrocyte sedimentation rate, CRP C-reactive protein, AGR albumin-to-globulin ratio, RF rheumatoid factor, ANA anti-nuclear antibodies
aAge, age at IgG4-related disease diagnosis
bAllergic diseases include asthma, urticaria, eczema, and allergic rhinitis
cHead and neck involvement includes orbit and peri-orbit, lacrimal gland, submandibular gland, parotid gland, sublingual gland and thyroid
*P < 0.05
**P < 0.01
Clinical Characteristics of IgG4-RD Patients with Malignancies
Baseline clinical characteristics in patients with and without malignancies were compared as shown in Table 1. There were no significant differences in demographic data, personal history, and past medical history between IgG4-RD patients with and without malignancies. However, we observed statistical differences in laboratory results. The laboratory results revealed that IgG4-RD patients with malignancies had lower serum AGR (1.15 vs. 1.49, P < 0.001), higher serum IgG level (21.94 vs. 17.36 g/l, P = 0.015) and higher eosinophil percentage (6.10 vs. 2.30%, P < 0.001) than those without malignancies. In terms of the distribution of organ involvement, submandibular glands, lymph nodes, and lacrimal glands were the most frequently affected organs whether the patients developed malignancies or not.
Types of Malignancy in Chinese IgG4-RD Patients and SPRs
Clinical characteristics of the IgG4-RD patients with malignancies are shown in Table S1 (see Supplementary Material). Fourteen (48.3%), 3 (10.3%) and 12 (41.4%) patients developed malignancies before, on, and after the diagnosis of IgG4-RD, respectively. Twenty-five out of 29 (86.2%) IgG4-RD patients were diagnosed with solid tumors, which consisted of lung cancer, stomach cancer, cervical cancer, thyroid cancer, bladder cancer, testicular cancer, kidney cancer, intra-abdominal soft tissue sarcoma, colon cancer, prostate cancer, pancreatic cancer, and esophageal squamous cell carcinoma (ESCC). Another four (13.8%) patients developed hematological malignancy, including two non-Hodgkin’s lymphoma (NHL) cases, one Hodgkin’s lymphoma (HL) case, and one multiple myeloma (MM) case. Among IgG4-RD patients with malignancy in this study, lung cancer (eight cases) was the most common malignancy. Patients were treated for malignancies with a variety of approaches (Table S1), including surgery, chemotherapy, radiation, traditional Chinese medicine (TCM), and supporting treatment.
As shown in Table 2, the expected total malignancies in a cohort of 602 IgG4-RD patients would be 3.347 based on general Chinese population estimates. In our study, 29 (4.82%) patients were identified as IgG4-RD accompanied malignancy. The SPR for total malignancy compared to the general Chinese population was 8.66 (95% CI 5.84, 12.31). Among male and female IgG4-RD patients, the expected total malignancies according to the general Chinese population would be 1.672 and 1.333, respectively. However, we observed 18 males and 11 females in our cohort, corresponding to SPRs of 10.77 (95% CI 6.41, 16.86) and 8.25 (95% CI 4.14, 14.66). Also, we calculated the SPRs for different malignancies. There was a significantly increased SPR for lymphoma (42.86 [95% CI 8.79, 123.88]) as listed in Table 2.
Table 2.
Standardized prevalence ratios for malignancy in the IgG4-RD cohort compared to the general Chinese population
| Stratification variables | Observed malignancies, n | Chinese prevalence, % | Expected malignancies, n | SPR (95% CI) |
|---|---|---|---|---|
| Total | 29 | 0.556 | 3.347 | 8.66 (5.84, 12.31) |
| Sex | ||||
| Male | 18 | 0.2778 | 1.672 | 10.77 (6.41, 16.86) |
| Female | 11 | 0.2214 | 1.333 | 8.25 (4.14, 14.66) |
| Malignancy | ||||
| Lung | 8 | 0.0656 | 0.395 | 20.25 (8.77, 39.66) |
| Lymphoma | 3 | 0.0117 | 0.070 | 42.86 (8.79, 123.88) |
| Thyroid | 3 | 0.0299 | 0.180 | 16.67 (3.44, 48.47) |
| Cervix | 3 | 0.0233 | 0.140 | 21.43 (4.42, 62.21) |
| Bladder | 2 | 0.0173 | 0.104 | 19.23 (2.33, 69.07) |
| Stomach | 2 | 0.0648 | 0.390 | 5.13 (0.62, 18.44) |
IgG4-RD IgG4-related disease, SPR standardized prevalence ratio, 95% CI 95% confidence interval
Predictive Factors for Malignancy in IgG4-RD Patients
As shown in Table 3, odds ratios (ORs) were calculated by univariate analysis and we identified the following four variables as potential risk factors (P < 0.1): age at IgG4-RD diagnosis, eosinophil percentage, AGR, and autoimmune pancreatitis. Among the above variables, age at IgG4-RD diagnosis (OR 1.028 [95% CI 0.996–1.062], P = 0.082), eosinophil percentage (OR 1.101 [95% CI 1.042–1.164], P = 0.001) and autoimmune pancreatitis (OR 1.904 [95% CI 0.889–4.077], P = 0.098) were positively correlated with malignancies in IgG4-RD patients, while AGR (OR 0.112 [95% CI 0.040–0.308], P < 0.001) was negatively correlated with malignancies. Based on univariate analysis and previous studies, we entered the following seven variables into a multivariate logistic regression model: age at IgG4-RD diagnosis, sex, serum IgG level, AGR, eosinophil percentage, serum IgG4 level [7], and autoimmune pancreatitis. Multivariate analysis confirmed that eosinophil percentage (OR 1.096 [95% CI 1.019–1.179], P = 0.016), AGR (OR 0.185 [95% CI 0.061–0.567], P = 0.002) and autoimmune pancreatitis (OR 2.400 [95% CI 1.038–5.549], P = 0.041) were three possible risk factors of malignancy in IgG4-RD patients. Moreover, eosinophil percentage and autoimmune pancreatitis were positive correlation factors, whereas AGR was negatively associated with malignancy risk in IgG4-RD patients.
Table 3.
ORs of related factors for malignancy in IgG4-RD patients: logistic regression analysis
| Variables | Univariate analysis | Multivariate analysis | ||||||
|---|---|---|---|---|---|---|---|---|
| Odds ratio (OR) | 95% CI | Wald Z | P value | Odds ratio (OR) | 95% CI | Wald Z | P value | |
| Age at IgG4-RD diagnosis | 1.028 | (0.996–1.062) | 3.028 | 0.082 | ||||
| Eosinophil percentage | 1.101 | (1.042–1.164) | 11.627 | 0.001 | 1.096 | (1.019–1.179) | 6.057 | 0.016* |
| AGR | 0.112 | (0.040–0.308) | 17.946 | < 0.001 | 0.185 | (0.061–0.567) | 8.744 | 0.002** |
| AIP | 1.904 | (0.889–4.077) | 2.744 | 0.098 | 2.400 | (1.038–5.549) | 4.187 | 0.041* |
AGR albumin-to-globulin ratio, AIP autoimmune pancreatitis
*P < 0.05
**P < 0.01
Development of an Assessing Model for Malignancy Risk of IgG4-RD
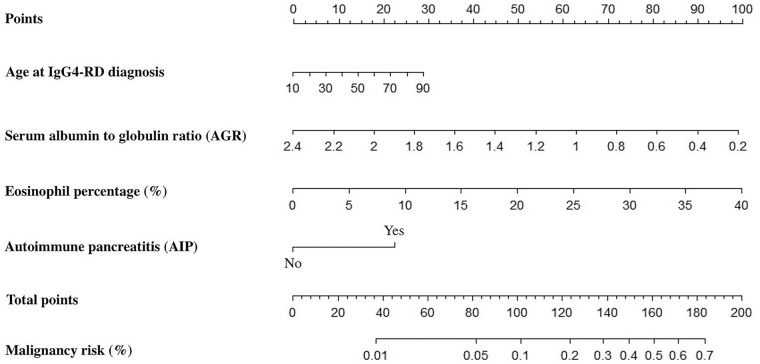
Based on the analyses above, four predictors were included in our final prediction model: age at IgG4-RD diagnosis, eosinophil percentage, AGR, and autoimmune pancreatitis. To visualize the logistic regression model, a nomogram incorporating each of these variables was configured as shown in Fig. 2. Malignancy risk assessment of a patient with IgG4-RD contains three main steps. First, determine and locate the patient’s position on each predictor axis. Second, draw perpendiculars from the corresponding axis of each predictor until the lines intersect with the top line labeled ‘Points’. Third, sum up the points for all predictors and draw a line descending from the axis labeled ‘Total points’ until it reaches the bottom line labeled ‘Malignancy risk’ to determine the probability of malignancy.
Fig. 2.
The nomogram for assessing malignancy risk of IgG4-RD
Validation of the Nomogram
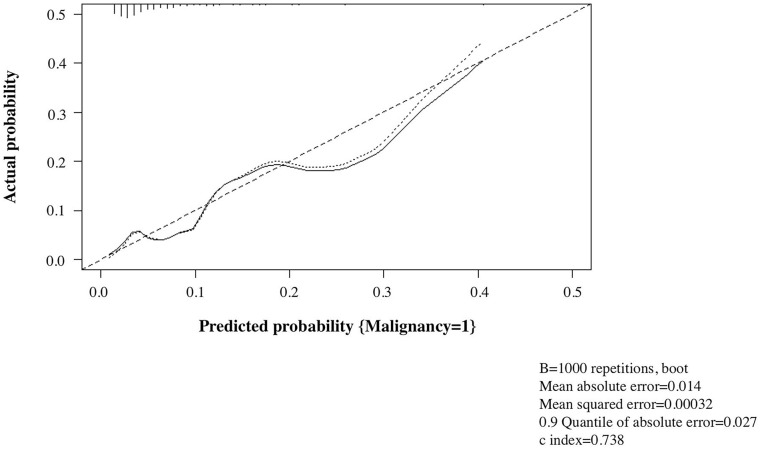
An internal validation was performed to test the performance of our nomogram using the bootstrap method with 1000 repetitions. Harrell’s C statistic was 0.738 (95% CI 0.635–0.842). Additionally, the calibration curve showed good agreement between the actual probability and the predicted probability by our nomogram (Fig. 3).
Fig. 3.
Calibration curve of the prediction model for malignancy risk. The calibration curve showed good agreement between the actual probability and the predicted probability by our nomogram. Harrell’s C statistic was 0.738 (95% CI 0.635–0.842)
Discussion
In the present study, we first observed a significantly increased prevalence of malignancies based on the largest multicenter cohort of Chinese IgG4-RD patients. We reported higher eosinophil percentage and more frequent AIP presented in IgG4-RD patients with malignancies. However, elevated serum AGR was a potential negative correlation factor for malignancy in IgG4-RD patients.
In this study, we observed a significantly increased prevalence of total malignancies in our cohort compared to the general Chinese population (SPR 8.66 [95% CI 5.84, 12.31]). We summarized the types of malignancies in our cohort and compared it between IgG4-RD patients and the general Chinese population. Similar to epidemiological studies of general population, lung cancer was the most common malignancy in IgG4-RD patients. However, lymphoma accounted for 10.3% (three cases) of the malignancies in our cohort, given a standard prevalence ratio of 42.86 (95% CI 8.79, 123.88). Several previous studies suggested discrepancies in the types of malignancies between patients with IgG4-RD and the general population [1, 11–13]. Besides, the distribution of malignant tumors in IgG4-RD observed in previous studies is varied. Wallace et al. [10] reported that prostate cancer and lymphoma were the most common malignancies based on a United States cohort, whereas only one prostate cancer case was observed in our study. Additionally, our cohort reported that lymphoma, thyroid, and stomach cancer were frequent malignancies associated with IgG4-RD, consistent with study by Ahn et al. [1]. The disparities may be explained by differences of race, environment, and sample capacity.
To date, the pathogenesis on the association between IgG4-RD and malignancy remains obscure. The chronic inflammatory state of IgG4-RD may play an important role in the development of malignancies. According to previous studies [19–23], mediators produced by activated inflammatory cells promote a variety of damages, including genetic mutations, post-translational modification of proteins involved in apoptosis, DNA repair, cell cycle control and signal transduction, as well as DNA and histone methylation, generating a pathologically conducive microenvironment, which may induce the growth and progression of malignancies. Several previous studies suggested that chronic antigenic stimulation, together with oncogenic events such as p53 inactivation and K-ras mutation in IgG4-RD, led to an increased risk of malignant transformation compared with the general population [9, 24]. Almost half of our patients developed malignancies after IgG4-RD diagnosis. Inflammation-associated oncogenesis may provide explanations for these cases.
In our study, three IgG4-RD patients developed lymphoma, including two B cell-derived non-Hodgkin’s lymphoma (NHL) cases and one Hodgkin’s lymphoma (HL) case. To our knowledge, few studies revealed close relationship between IgG4-RD and development of B cell lymphoma [25, 26]. Interestingly, as indicated by previous studies, an increased risk of lymphoma is also observed in patients with other autoimmune and inflammatory diseases [27, 28]. Goldin et al. emphasized the effects of secondary inflammation due to autoimmune stimulation on the processes, such as cytokine and chemokine release and viral reactivation (Epstein–Barr virus, for example). In addition, germline and somatic mutations are likely to induce autoimmunity and lymphomagenesis. Now that activation of B cells by increased Th2 cytokines including interleukin-4, 5, 10, and 13 contributes to the pathogenesis of IgG4-RD [29, 30], the superiority of B cell lymphoma as a secondary condition to IgG4-RD seems explicable. A study by Conde et al. identified the common genetic variants between NHL subtypes and autoimmune diseases, demonstrating a potential shared genetic mechanism [31]. A study reported the reciprocal chromosomal translocation t (14;19) (q32;q13.1) for rearrangements of IgH and BCL3 genes in the DLBCL cells, generating the dysfunction of the protein involved in nuclear factor (NF)-κB family regulation and complex cytogenetic abnormalities [26]. Accumulation of similar events may promote the process of lymphoma development from IgG4-RD. Moreover, the functional disorders of immune system in IgG4-RD may influence the interactions between components in the microenvironment and promote lymphomagenesis [32, 33]. The association between IgG4-RD and specific lymphoid malignancies and the potential etiologic mechanisms deserve further discussion.
Several studies have found that AIP is associated with an increased risk of malignancies [3, 34]. In AIP patients, high levels of K-ras mutation have been detected in gastrointestinal tract, associated with persistent IgG4-related fibroinflammation and abundant infiltration of T lymphocytes and Foxp3+ cells, indicating that AIP may share a similar molecular pathogenesis with malignancies in IgG4-RD.
It is proved that eosinophils play a pivotal role in several immunological diseases and malignancies [35]. Several previous studies observed eosinophilia in patients with both solid tumors and lymphoma [36–38]. On the one hand, eosinophils exert great influence in tumoricidal response. On the other hand, eosinophils contribute to tumor angiogenesis through the release of proangiogenic molecules such as vascular endothelial growth factor (VEGF) and osteopontin (OPN), and promote endothelial cell proliferation [39, 40]. Accordingly, in this study, we found obviously elevated eosinophil percentage in IgG4-RD patients with malignancy. It is worth noting that eosinophilia may contribute to different clinical patterns in IgG4-RD patients as Zhang et al. reported [41]. As a possible marker of disease severity, the role of eosinophil in the development of malignancy relies on further pathological and molecular confirmation.
In this study, we observed higher serum globulin levels in IgG4-RD patients with malignancies. Besides, serum AGR was lower in patients with malignancies than those without, which is consistent with previous studies [42, 43]. As a pro-inflammatory factor, globulin consists of a variety of components, including acute-phase reactive proteins, immunoglobulins, interleukins, and tumor indicators, and participates in the regulation of osmotic pressure and the transportation of various compounds [42]. It is widely known that an increased level of globulin is related to chronic inflammation and various types of malignancies, especially hematological tumors. As serum globulin secreted by tumor-related cells is reported to promote the development, angiogenesis, immunosuppression, and metastasis of malignancies, high serum globulin may indicate the extent of severe chronic inflammation and poor prognosis [43]. As an important component, immunoglobulin G (IgG) expressed by cancer cells is reported to promote growth and survival of malignancies, by interacting with proteins involved in cell growth (RACK1, RAN and PRDX1, etc.) and activating the relevant signaling pathway. Down-regulation of IgG may arrest the S-phase of the cell cycle. In addition, several studies have reported IgG4 as a significant prognostic indicator in neoplastic disorders [44, 45]. Wu J et al. demonstrated that elevated serum IgG4:IgG ratio may predict poor clinical outcomes and recurrence of hepatocellular carcinoma (HCC) [46]. However, IgG and IgG subclass concentrations may differ according to sex and race. As reported, Asians with IgG4-RD have higher IgG and IgG4 production in general than white people, which may limit transferability of this finding to North American/European contexts [7, 47, 48].
This study has several limitations. First, as a retrospective study, it could not overcome the typical flaws inherent to the design itself. Second, examinations of the whole body, even the FDG-PET, may lead to the detection bias. Third, as an inevitable issue for a rare condition, low number of malignancies and wide 95% CI ranges were reported in our study. Moreover, the model was validated only internally. Collection of data based on an independent cohort for further verification is under way. In consideration of the low incidence of both IgG4-RD and malignancy, the nomogram for assessing malignancy risk in IgG4-RD deserves long-term verification and adjustment.
Conclusions
Our study first reported a comparatively large, multicenter cohort of Chinese patients with IgG4-RD and confirmed credibly the necessity of comprehensive examination during the follow-up of IgG4-RD patients. Eosinophil percentage and autoimmune pancreatitis were identified as potential risk factors, whereas AGR was negatively associated with malignancy risk in IgG4-RD. Furthermore, a nomogram for assessment of malignancy prevalence in IgG4-RD patients was first developed and validated in our study, leading to improved follow-up management and prognosis in IgG4-RD patients.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to thank all of the patients and staff who were involved in this study. We would like to thank Dr. HX. Liu from the Department of Clinical Epidemiology and Biostatistics at Peking University People’s Hospital for her assistance in statistical analyses.
Funding
This work and the Rapid Service Fee was supported by Peking University People’s Hospital Research and Development Funds [RDH 2020-03], Clinical Medicine Plus X - Young Scholars Project of Peking University (PKU2021LCXQ006), the Fundamental Research Funds for the Central Universities (PKU2021LCXQ006) and National Key R&D Program of China (2017YFA0105802).
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authors’ Contributions
Yanying Liu and Jiangnan Fu contributed equally to this paper. All named authors participated in the analyses and interpretation of data, wrote or critically reviewed the manuscript, and reviewed and approved the final version.
Disclosures
None of the authors (Yanying Liu, Jiangnan Fu, Xiaoran Ning, Huijuan Li, Xiangbo Ma, Kunkun Wang, Wenjie Bian, Yuxin Zhang, Guangyan Yu, Zhanguo Li) have conflicts of interest to disclose.
Compliance with Ethics Guidelines
This study was approved by the Medical Ethics Committee of Peking University People’s Hospital (Beijing, China).
Data Availability
Not applicable.
Footnotes
Yanying Liu and Jiangnan Fu contributed equally to this paper.
Contributor Information
Yanying Liu, Email: liuyanying20030801@msn.com.
Guangyan Yu, Email: gyyu@263.net.
Zhanguo Li, Email: zhanguolipph@163.com.
References
- 1.Ahn SS, Song JJ, Park YB, et al. Malignancies in Korean patients with immunoglobulin G4-related disease. Int J Rheum Dis. 2017;20:1028–1035. doi: 10.1111/1756-185X.13093. [DOI] [PubMed] [Google Scholar]
- 2.Deshpande V, Zen Y, Chan JK, et al. Consensus statement on the pathology of IgG4-related disease. Mod Pathol. 2012;25:1181. doi: 10.1038/modpathol.2012.72. [DOI] [PubMed] [Google Scholar]
- 3.Tang H, Yang H, Zhang P, et al. Malignancy and IgG4-related disease: the incidence, related factors and prognosis from a prospective cohort study in China. Sci Rep. 2020;10:4910. doi: 10.1038/s41598-020-61585-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Culver EL, Chapman RW. IgG4-related hepatobiliary disease: an overview. Nat Rev Gastroenterol Hepatol. 2016;13:601–612. doi: 10.1038/nrgastro.2016.132. [DOI] [PubMed] [Google Scholar]
- 5.Cortazar FB, Stone JH. IgG4-related disease and the kidney. Nat Rev Nephrol. 2015;11:599–609. doi: 10.1038/nrneph.2015.95. [DOI] [PubMed] [Google Scholar]
- 6.Zhao EJ, Cheng CV, Mattman A, Chen LYC. Polyclonal hypergammaglobulinaemia: assessment, clinical interpretation, and management. Lancet Haematol. 2021;8:e365–e375. doi: 10.1016/S2352-3026(21)00056-9. [DOI] [PubMed] [Google Scholar]
- 7.Zhao EJ, Carruthers MN, Li CH, et al. Conditions associated with polyclonal hypergammaglobulinemia in the IgG4-related disease era: a retrospective study from a hematology tertiary care center. Haematologica. 2020;105:e121–e123. doi: 10.3324/haematol.2019.219725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen LYC, Mattman A, Seidman MA, Carruthers MN. IgG4-related disease: what a hematologist needs to know. Haematologica. 2019;104:444–455. doi: 10.3324/haematol.2018.205526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamamoto M, Takahashi H, Tabeya T, et al. Risk of malignancies in IgG4-related disease. Mod Rheumatol. 2012;22:414–418. doi: 10.3109/s10165-011-0520-x. [DOI] [PubMed] [Google Scholar]
- 10.Wallace ZS, Wallace CJ, Lu N, et al. Association of IgG4-related disease with history of malignancy. Arthritis Rheumatol. 2016;68:2283–2289. doi: 10.1002/art.39773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sekiguchi H, Horie R, Kanai M, et al. IgG4-related disease: retrospective analysis of one hundred sixty-six patients. Arthritis Rheumatol. 2016;68:2290–2299. doi: 10.1002/art.39686. [DOI] [PubMed] [Google Scholar]
- 12.Huggett MT, Culver EL, Kumar M, et al. Type 1 autoimmune pancreatitis and IgG4-related sclerosing cholangitis is associated with extrapancreatic organ failure, malignancy, and mortality in a prospective UK cohort. Am J Gastroenterol. 2014;109:1675–1683. doi: 10.1038/ajg.2014.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Asano J, Watanabe T, Oguchi T, et al. Association between immunoglobulin G4-related disease and malignancy within 12 years after diagnosis: an analysis after long-term follow-up. J Rheumatol. 2015;42:2135–2142. doi: 10.3899/jrheum.150436. [DOI] [PubMed] [Google Scholar]
- 14.Hart PA, Law RJ, Dierkhising RA, et al. Risk of cancer in autoimmune pancreatitis: a case-control study and review of the literature. Pancreas. 2014;43:417. doi: 10.1097/MPA.0000000000000053. [DOI] [PubMed] [Google Scholar]
- 15.Hirano K, et al. Incidence of malignancies in patients with IgG4-related disease. Intern Med. 2014;53:171–176. doi: 10.2169/internalmedicine.53.1342. [DOI] [PubMed] [Google Scholar]
- 16.Inoue D, Yoshida K, Yoneda N, et al. IgG4-related disease: dataset of 235 consecutive patients. Medicine. 2015;94:e680. doi: 10.1097/MD.0000000000000680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wallace ZS, Naden RP, Chari S, et al. The 2019 American College of Rheumatology/European League Against Rheumatism Classification Criteria for IgG4-related disease. Ann Rheum Dis. 2020;79:77–87. doi: 10.1136/annrheumdis-2019-216561. [DOI] [PubMed] [Google Scholar]
- 18.Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 19.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yajima R, Takano K, Konno T, et al. Mechanism of fibrogenesis in submandibular glands in patients with IgG4-RD. J Mol Histol. 2018;49:577–587. doi: 10.1007/s10735-018-9796-x. [DOI] [PubMed] [Google Scholar]
- 21.Pua KH, Chew CL, Lane DP, et al. Inflammation-associated genomic instability in cancer. Genome Instab Dis. 2020;1:1–9. doi: 10.1007/s42764-019-00006-6. [DOI] [Google Scholar]
- 22.Fest J, Ruiter R, Mulder M, et al. The systemic immune-inflammation index is associated with an increased risk of incident cancer—a population-based cohort study. Int J Cancer. 2020;146:692–698. doi: 10.1002/ijc.32303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gouravani M, Khalili N, Razi S, et al. The NLRP3 inflammasome: a therapeutic target for inflammation-associated cancers. Expert Rev Clin Immunol. 2020;16:175–187. doi: 10.1080/1744666X.2020.1713755. [DOI] [PubMed] [Google Scholar]
- 24.Gupta R, Khosroshahi A, Shinagare S, et al. Does autoimmune pancreatitis increase the risk of pancreatic carcinoma? A retrospective analysis of pancreatic resections. Pancreas. 2013;42:506–510. doi: 10.1097/MPA.0b013e31826bef91. [DOI] [PubMed] [Google Scholar]
- 25.Bledsoe JR, Wallace ZS, Stone JH, et al. Lymphomas in IgG4-related disease: clinicopathologic features in a Western population. Virchows Arch. 2018;472:839–852. doi: 10.1007/s00428-017-2286-9. [DOI] [PubMed] [Google Scholar]
- 26.Kawaji Y, Nagata H, Muramatsu A, et al. Diffuse large B cell lymphoma with chromosomal translocation t(14;19)(q32;q13) occurring in IgG4-related disease. Ann Hematol. 2019;98:1785–1787. doi: 10.1007/s00277-019-03688-w. [DOI] [PubMed] [Google Scholar]
- 27.Gaetane N, Elena P, Michele B, et al. Lymphomas complicating primary Sjögren's syndrome: from autoimmunity to lymphoma. Rheumatology 2019; kez052. [DOI] [PMC free article] [PubMed]
- 28.Fallah M, Liu X, Ji J, et al. Autoimmune diseases associated with non-Hodgkin lymphoma: a nationwide cohort study. Ann Oncol. 2014;25:2025–2030. doi: 10.1093/annonc/mdu365. [DOI] [PubMed] [Google Scholar]
- 29.Tanaka A, Moriyama M, Nakashima H, et al. Th2 and regulatory immune reactions contribute to IgG4 production and the initiation of Mikulicz disease. Arthritis Rheumatol. 2012;64:254–263. doi: 10.1002/art.33320. [DOI] [PubMed] [Google Scholar]
- 30.Grados A, Ebbo M, Piperoglou C, et al. T cell polarization toward TH2/TFH2 and TH17/TFH17 in patients with IgG4-related disease. Front Immunol. 2017;8:235. doi: 10.3389/fimmu.2017.00235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Conde L, Bracci PM, Halperin E, Skibola CF. A search for overlapping genetic susceptibility loci between non-Hodgkin lymphoma and autoimmune diseases. Genomics. 2011;98:9–14. doi: 10.1016/j.ygeno.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vardhana S, Younes A. The immune microenvironment in Hodgkin lymphoma: T cells, B cells, and immune checkpoints. Haematologica. 2016;101:794–802. doi: 10.3324/haematol.2015.132761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khurana A, Ansell SM. Role of microenvironment in non-Hodgkin lymphoma: understanding the composition and biology. Cancer J. 2020;26:206–216. doi: 10.1097/PPO.0000000000000446. [DOI] [PubMed] [Google Scholar]
- 34.Schneider A, Hirth M, et al. Risk of cancer in patients with autoimmune pancreatitis: a single-center experience from Germany. Digestion. 2017;95:172–180. doi: 10.1159/000455963. [DOI] [PubMed] [Google Scholar]
- 35.Rosaria GM, Gilda V, Mansour S, et al. Bidirectional mast cell-eosinophil interactions in inflammatory disorders and cancer. Front Med. 2017;4:103. doi: 10.3389/fmed.2017.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leighton SEJ, Teo JGC, Leung SF, et al. Prevalence and prognostic significance of tumor-associated tissue eosinophilia in nasopharyngeal carcinoma. Cancer. 2015;77:436–440. doi: 10.1002/(SICI)1097-0142(19960201)77:3<436::AID-CNCR3>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 37.Kurose N, Mizuguchi S, Ohkanemasa Y, et al. Adenosquamous carcinoma of the uterine cervix displaying tumor-associated tissue eosinophilia. SAGE Open Med Case Rep. 2019;7:1–5. doi: 10.1177/2050313X19828235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hu G, Wang S, Zhong K, et al. Tumor-associated tissue eosinophilia predicts favorable clinical outcome in solid tumors: a meta-analysis. BMC Cancer. 2020;20:454. doi: 10.1186/s12885-020-06966-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kienzl M, Hasenoehrl C, Valadez-Cosmes P, Maitz K, Sarsembayeva A, Sturm E, Heinemann A, Kargl J, Schicho R. IL-33 reduces tumor growth in models of colorectal cancer with the help of eosinophils. Oncoimmunology. 2020;9:1776059. doi: 10.1080/2162402X.2020.1776059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao H, Chen Q, Alam A, et al. The role of osteopontin in the progression of solid organ tumour. Cell Death Dis. 2018;9:356. doi: 10.1038/s41419-018-0391-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang X, Zhang P, Li J, et al. Different clinical patterns of IgG4-RD patients with and without eosinophilia. Sci Rep. 2019;9:16483. doi: 10.1038/s41598-019-52847-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bozkaya Y, Erdem GU, Demirci NS, et al. Prognostic importance of albumin to globulin ratio in metastatic gastric cancer patients. Curr Med Res Opin. 2019;35:275–282. doi: 10.1080/03007995.2018.1479683. [DOI] [PubMed] [Google Scholar]
- 43.Yoshino Y, Taguchi A, Shimizuguchi T, et al. A low albumin to globulin ratio with a high serum globulin level is a prognostic marker for poor survival in cervical cancer patients treated with radiation-based therapy. Int J Gynecol Cancer. 2019;29:17–22. doi: 10.1136/ijgc-2018-000025. [DOI] [PubMed] [Google Scholar]
- 44.Harada K, Shimoda S, Kimura Y, et al. Significance of immunoglobulin G4 (IgG4)-positive cells in extrahepatic cholangiocarcinoma: molecular mechanism of IgG4 reaction in cancer tissue. Hepatology. 2012;56:157–164. doi: 10.1002/hep.25627. [DOI] [PubMed] [Google Scholar]
- 45.Karagiannis P, Gilbert AE, Josephs DH, et al. IgG4 subclass antibodies impair antitumor immunity in melanoma. J Clin Invest. 2013;123:1457–1474. doi: 10.1172/JCI65579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu J, Ma XL, Tian L, et al. Serum IgG4:IgG ratio predicts recurrence of patients with hepatocellular carcinoma after curative resection. J Cancer. 2017;8:1338–1346. doi: 10.7150/jca.18030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Qi R, Chen LYC, Park S, et al. Utility of serum IgG4 levels in a multiethnic population. Am J Med Sci. 2018;355:61–66. doi: 10.1016/j.amjms.2017.08.014. [DOI] [PubMed] [Google Scholar]
- 48.Harkness T, Fu X, Zhang Y, et al. Immunoglobulin G and immunoglobulin G subclass concentrations differ according to sex and race. Ann Allergy Asthma Immunol. 2020;125:190–5.e2. doi: 10.1016/j.anai.2020.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.