Abstract
Background:
Falls are a devastating complication of cirrhosis. The risk of falls in contemporary patients without HE is unclear. Further, bedside tools for predicting falls are lacking
Methods:
We prospectively enrolled 299 subjects with currently compensated Child A-B (70% Child A) cirrhosis and portal hypertension without prior HE from 7/2016–8/2018. We followed patients for a median of 1003 days (IQR640-1102) for incident falls accounting for the competing risk of death or transplantation. Candidate baseline fall predictors included patient reported outcomes (e.g. SF-8), physical function (e.g. chair-stands), blood tests (e.g. MELD-Na and its components), and cognitive function (using Inhibitory Control Testing). We internally validated a predictive model for falls and evaluated the association between incident falls and mortality.
Results:
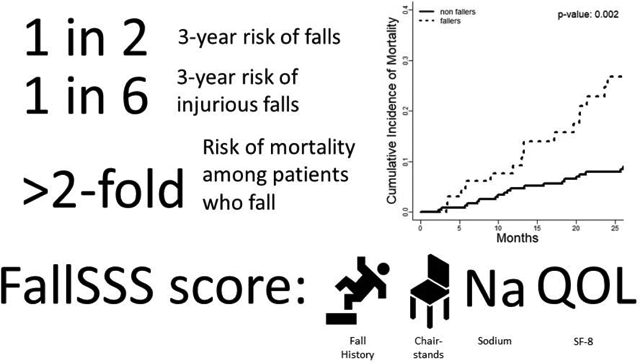
During follow-up, 141(47%) patients experienced falls, 38(13%) with injuries, 49(16%) died, and 13(4%) underwent transplants. Median time to a fall was 279(98–595) days. The overall probability of falls was 28.8% and 50.2% at years 1 and 3; the probability of injurious falls was 9.1% and 16.5%. We derived a predictive model for falls. The FallSSS score (prior falls, chair-stands, sodium, and SF-8) had an AUROC for injurious falls at 6- and 12-months of 0.79 and 0.81 while MELD-Na’s AUROC was 0.57 for both. Adjusting for baseline Child class, MELD-Na, albumin level, disability status, and comorbidities, both incident falls – sHR 2.76 95%CI(1.46–5.24) – and HE – sHR 4.25 95%CH(2.15–8.41) – were strongly and independently associated with mortality.
Conclusion:
Our prospective study of patients with cirrhosis without a baseline history of HE demonstrates that falls are common, morbid, and predictable. These data highlight both the value of expanding screening to patients with cirrhosis and the potential for benefit in studies of interventions to address fall-risk in this vulnerable population.
Keywords: Frailty, patient reported outcomes, MELD, ascites
Graphical Abstract
Lay summary
Falls are a devastating complication of cirrhosis. Bedside tools for predicting falls are lacking. We found that falls were very common and often associated with serious injuries. Falls were also associated with an increased risk of death. Falls could be predicted with an algorithm called FallSSS - based on prior history of falls, blood sodium level, number of chair-stands performed in 30 seconds, and quality of life.
Background
Falls are associated with poor health-related quality of life (HRQOL), death, and disability in patients with cirrhosis.[1, 2] Understanding fall-risk may allow for closer monitoring, lifestyle modification, and therapeutic interventions to improve functional status, preserve HRQOL, and reduce morbidity. Although the vast majority of the more than 1 million Americans living with cirrhosis are compensated, data on the risk of falls is lacking for this important population.
Prior estimates of fall risk include an annual rate of 1 in 8 (or 2 in 5 among those with cognitive dysfunction) and 1.7/1000 patient-days among hospitalized patients with cirrhosis.[3, 4] We previously evaluated the National Emergency Department Sample and found that over a 4-year study period, patients with cirrhosis incurred 116,526 falls, with 6,313 (5.4%) of patients presenting with severe injuries such as intracranial hemorrhage and major fractures.[5] However, these data all highlight hepatic encephalopathy (HE) as a primary risk factor. In the only prospective study of fall risk, Soriano followed 122 patients (87% decompensated) for a median of 10 months and found that the 40 patients with cognitive dysfunction (40% of whom had prior HE) were most likely to fall. Unfortunately, evaluations for cognitive dysfunction are rarely performed in clinical practice and, as such, simpler risk assessments are needed. Furthermore, it is unclear how such data would generalize to patients who have not decompensated or developed HE.
Herein, we prospectively 1) determine the incidence of falls in patients with cirrhosis, portal hypertension and no prior overt HE, 2) compare strategies utilizing psychometrics, liver function, HRQOL, and frailty to predict the development of falls, and 3) determine the impact of falls on the risk of mortality.
Methods
Study Population
We prospectively enrolled 299 patients from the Hepatology clinic at the University of Michigan from 7/2016–4/2018 and followed them through 2/2021. We included adult patients with cirrhosis from all etiologies and portal hypertension. Diagnosis of cirrhosis was based on histology, radiology, and/or elastography. The presence of portal hypertension was defined by at least 1 of the following: ascites or hydrothorax (current or controlled, within the prior year), varices or history of variceal hemorrhage, platelet count ≤80/nL (in the absence of hematological causes of thrombocytopenia). We excluded all patients with Child C cirrhosis, a current or prior of overt HE or treatment for HE (history of hospitalization for HE, lactulose or rifaximin/neomycin prescription), non-English speaking, estimated life expectancy <12 months, pregnancy, severe mobility/cognitive impairment, prior liver transplantation, or history of transjugular intrahepatic portosystemic shunt (TIPS). Additional details are available in the Supplementary Methods. This study was approved by the University of Michigan Health System Institutional Review Board (HUM00132678) and all patients provided written informed consent.
Outcomes
The primary outcome was a fall. Patients were surveyed in-person or by telephone quarterly to ask “have you fallen in the past 12 months and landed on the floor or hit an object?” and “Did (that fall/any of those falls) cause a broken bone, a serious injury like a head injury, or cause you to seek medical care?” Medical records of all visits/hospitalizations (including to outside facilities) were recorded. Incident overt HE was also recorded as previously described.[6] The risk of falls was evaluated in the context of competing risks of death (confirmed by family report, review of medical records, and the social security death index) or liver transplantation.
Assessment
Comorbidities were defined by Charlson Comorbidity Index, modified to exclude liver disease.[7] All medications were recorded. Alcohol use during the prior 12 months was recorded using a validated questionnaire.[8] Alcohol abuse was defined by binge drinking (>4 drinks/2 hours for women and >5 for men) or chronic use >7 or >14 drinks/week for women and men, respectively. Lab values at the time of enrollment were recorded. Severity of liver disease was assessed using the Child classification and MELD-Na.[9]
Assessment of frailty and function
Patients were also asked about falls in the prior 6 months. Frailty was assessed in 3 ways. Hand-grip strength in the patient’s dominant hand was evaluated using a dynamometer. We counted the number of chair stands (repeatedly rising from a seated position to standing and sitting again) performed within 30 seconds. Disability was assessed by Katz Activity of Daily Living (ADL) scale of independence in 6 ADLs which was scored as completely-independent or not.(Supplementary Table 1) We assessed psychometric performance using the computerized Inhibitory Control Test (ICT),[10] defining covert HE according to Amodio criteria (weighted-lures>24).[11] Additional details regarding the ICT and its scoring are available in the Supplementary Methods.
Assessment of HRQOL
First, we used the Short-Form 8 (SF-8) which has been validated in patients with liver disease, that has a range from 0–100 and can be dichotomized as good (>50) or poor (≤50). Second, we determined each patient’s Work-Productivity-Activity Impairment (WPAI). Because many (63%) patients were not working at enrollment, we focused on the final one-question scale “During the past seven days, how much did your cirrhosis affect your ability to do your regular daily activities, other than work at a job,” which ranged from 0 (no effect on daily activities) to 10 (completely prevented me from doing my daily activities).
Analysis
All analyses were performed using R and SAS (version 9.4). Cumulative incidence curves were drawn to show the risk of falls over time in the presence of the competing risk of death or transplantation.[12] Next, we selected a set of predictors that best predict the risk of falls. We first used univariate Cox proportional hazards regression to select predictors with p-value <0.10 as candidates, accounting for competing risks.[13, 14] We also used random survival forest (with default hyperparameters) to further expand the candidate list based on variable importance scores.[15, 16] We determined a list of candidate predictors based on results from univariate Cox models, random survival forest, and clinical knowledge (for example, bilirubin and MELD-Na could not both meet inclusion). Finally, we applied the penalized Cox regression with Lasso penalty to select a small set of predictors from the candidate predictors, and the penalty parameter was determined by cross-validation method. Penalized Cox regression can handle a large number of predictors. The consequence of imposing the lasso penalty is to reduce (i.e., shrink) the regression coefficient values towards zero. This allows the less contributive predictors to have a coefficient equal to zero. The final predictors included falls prior to the baseline assessment, serum sodium, SF-8, and chair-stands (the FallSSS score). A cause-specific competing risk model was used to assess the effect of each predictor.
Model Performance, Validation and Accuracy
Model performance was evaluated using the C-statistic (a measure of discrimination) and time-dependent receiver operating characteristic (ROC) curve.[17] In predictive models, a c-statistic describes how well the model can rank order cases and non-cases. We contrasted the performance of our best model, FallSSS, with ICT weighted-lures, chair-stands and hand-grip, SF-8 and serum sodium, and MELD-Na. An internal validation was performed using a 5-fold cross-validation. This method divides the dataset into 5 sets of 80/20 splits (folds) where each 80%-fold is used as the training set for the remaining 20%. Summary statistics for this procedure include the c-statistic, AUROC at 6-month intervals, and the integrated Brier score (a measure of model accuracy).[18] We conducted two sensitivity analyses – 1) evaluating the SSS model in the sample of patients without prior falls and 2) re-deriving the individual predictors in the sample without prior falls.
Mortality
We sought to determine the association of incident falls with the risk of mortality, independent of the impact of incident HE, using a cause-specific competing risk framework. To do so, we treated falls and HE as time-varying covariates. We selected 5 baseline factors for risk-adjustment on the basis of biological plausibility: Child class (A versus B), MELD-Na, Albumin, ADL independence, covert HE (based on Amodio’s ICT criteria, weighted lures >24), and Charlson comorbidity index. Albumin and MELD-Na were log-transformed.
Results
Cohort characteristics are delineated in Table 1. At enrollment, median age was 60 years, 56% were male, 70% were Child Class A and median MELD-Na was 9. Overall, 22% reported a prior history of falls, none of which were injurious.
Table 1:
Characteristics of the 299-person Cohort at Enrollment
| Age, years | 60 (52–66) | |
| Education, years | 14 (12 – 16) | |
| Sex, male | 169 (56.3%) | |
| Body Mass Index, kg/m2 | 29 (26 – 34) | |
| Etiology* | ||
| Other | 25% | |
| Hepatocellular Carcinoma | 22 (7.3%) | |
| Child class A | 209 (70%) | |
| Varices | 229 (76%) | |
| Ascites | 122 (41%) | |
| Ascites requiring paracentesis (at any point) | 30 (10%) | |
| Platelet count < 80,000 | 111 (37%) | |
| Any current alcohol use | 92 (31%) | |
| Current alcohol abuse | 22 (7.3%) | |
| Charlson Comorbidity Index | 4 (1 – 4) | |
| Laboratory Values | ||
| MELD-Na | 9 (7 – 13) | |
| Bilirubin (mg/dL) | 1 (0.7 – 1.6) | |
| Creatinine (mg/dL) | 0.9 (0.7 – 1.04) | |
| INR | 1.1 (1 – 1.2) | |
| Sodium (meq/L) | 140 (138 – 141) | |
| Albumin (mg/dL) | 4 (3.6–4.3) | |
| Markers of Frailty | ||
| Incompletely independent in ADLs | 27 (9%) | |
| Chair stands | 10 (7– 13) | |
| Hand grip, kilograms | 31 (22 – 39) | |
| Self-reported falls in past 6 months | 65 (22%) | |
| Medication Reconciliation (chronic current use) | ||
| Diuretics | 120 (40%) | |
| Nonselective Beta-blockers | 180 (60%) | |
| Benzodiazepine | 55 (18%) | |
| Opioid | 66 (22%) | |
| Antidepressant | 54 (18%) | |
| Inhibitory Control Test (ICT) Performance | ||
| Lures | 12 (7 –22) | |
| Targets | 94.3 (84 – 98) | |
| Weighted lures | 14.5 (8.2 – 37.8) | |
| Cannot complete ICT | 38 (13%) | |
ADL = activities of daily living, INR = international normalized ratio, MELD-Na = model for endstage liver disease – sodium, NAFLD = nonalcoholic fatty liver disease
Outcomes
During a median follow-up of 1003 days (IQR 640–1102), 141 (47%) patients experienced falls, 38 (13%) with injuries. In Figure 1, we detail the cumulative incidence of falls stratified by Child class. There were 68 (23%) cases of incident HE, 49 (16%) deaths, and 13 (4%) transplants. Median time to a fall was 279 (98–595) days. The injuries included 17 hospitalizations for pain/soft-tissue injury, 8 arm/wrist fractures, 5 hip/femur fractures, 4 rib fractures, 4 lower-leg fractures, 2 skull fractures/intracranial hemorrhage, and 1 cervical spine fracture.
Figure 1: Incidence and Risk of Falls.
The cumulative incidence of falls (accounting for the competing risk of death or transplantation) is displayed stratified by baseline Child class (Gray’s test, p=0.22).
In Table 2, we detail the probability of falls and injurious falls at 1 and 3 years accounting for the competing risk of death or liver transplantation across multiple subgroups. The overall probability of falls was 28.8% and 50.2% at years 1 and 3; the probability of injurious falls was 9.1% and 16.5%. The subgroups with the highest risk of falls and injurious falls were those with prior falls and any disability. The groups with the lowest risk of falls were those aged <60 years and those without prior falls, however in both cases the 3-year probability of falls rose to >40%.
Table 2:
Probability of Falls
| Number of at-risk patients | Cases of Incident Falls | Cases of injurious falls | Probability of Falls at 1 year* (Standard Deviation) | Probability of Injurious falls at 1 year* (Standard Deviation) | Probability of Falls at 3 year3 * (Standard Deviation) | Probability of Injurious falls at 3 years* (Standard Deviation) | |
|---|---|---|---|---|---|---|---|
| Overall | 299 | 141 | 38 | 28.8 ± 2.6% | 9.1 ± 1.7% | 50.2 ± 3.1% | 16.5 ± 2.7% |
| Child A | 209 | 93 | 27 | 28.4 ± 3.1% | 8.9 ± 3.2% | 46.8 ± 3.7% | 16.2 ± 3.2% |
| Child B | 90 | 48 | 11 | 30.0 ± 4.9% | 9.2 ± 2.1% | 57.8 ± 5.7% | 17.2 ± 5.3% |
| Ascites | 122 | 63 | 18 | 31.3 ± 4.2% | 12.8 ± 3.2% | 55.7 ± 4.9% | 18.7 ± 4.3% |
| Paracentesis | 30 | 15 | 4 | 36.7 ± 9.0% | 11.4 ± 6.3% | 50.8 ± 9.6% | 18.9 ± 9.6% |
| No Ascites | 177 | 78 | 20 | 27.2 ± 3.4% | 6.6 ± 1.9% | 46.3 ± 4.0% | 14.9 ± 3.5% |
| Age ≥ 60 years | 158 | 81 | 25 | 30.5 ± 3.7% | 11.8 ± 2.7% | 55.4 ± 4.4% | 19.5 ± 3.9% |
| Age <60 years | 141 | 60 | 13 | 27.0 ± 3.8% | 6.0 ± 3.6% | 44.7 ± 4.4% | 12.8 ± 3.6% |
| Weighted Lures >24 | 91 | 45 | 9 | 29.8 ± 4.8% | 7.3 ± 2.9% | 53.0 ± 5.7% | 14.0 ± 4.8% |
| Weighted Lures ≤ 24 | 170 | 73 | 18 | 26.5 ± 4.2% | 7.1 ± 3.5% | 46.1 ± 4.2% | 14.1 ± 3.5% |
| Alcohol-related liver disease | 65 | 34 | 15 | 30.8 ± 5.8% | 14.6 ± 4.6% | 56.4 ± 6.7% | 28.7 ± 4.6% |
| Alcohol abuse | 21 | 10 | 5 | 14.3 ± 7.8% | 5.0 ± 5.0% | 47.6 ± 11.4% | 26.6 ± 10.7% |
| Non-alcoholic fatty liver | 99 | 57 | 11 | 40.4 ± 5.0% | 11.0 ± 3.5% | 59.0 ± 5.3% | 12.8 ± 3.9% |
| Prior falls | 65 | 52 | 16 | 63.1 ± 6.1% | 25.7 ± 6.1% | 83.7 ± 5.2% | 39.5 ± 6.1% |
| No prior falls | 234 | 89 | 22 | 19.3 ± 2.6% | 5.1 ± 1.5% | 41.0 ± 3.5% | 11.9 ± 2.6% |
| Any disability | 26 | 18 | 4 | 53.8 ± 10.1% | 20.2 ± 9.6 | 75.9 ± 11.3% | 20.2 ± 9.6% |
| No disability | 273 | 123 | 34 | 26.5 ± 2.7% | 8.2 ± 1.7% | 48.0 ± 3.2% | 16.0 ± 2.8% |
| Benzodiazepine user | 55 | 36 | 8 | 41.9 ± 6.7% | 14.5 ± 5.2% | 70.7 ± 7.1% | 17.4 ± 5.9% |
| Benzodiazepine nonuser | 244 | 105 | 30 | 25.9 ± 2.8% | 8.0 ± 1.8% | 45.7 ± 3.4% | 15.8 ± 2.9% |
| Opioid user | 66 | 41 | 14 | 41.9 ± 6.7% | 18.0 ± 5.0% | 64.0 ± 6.3% | 18.0 ± 7.2% |
| Opioid nonuser | 233 | 100 | 24 | 25.9 ± 2.8% | 6.6 ± 1.7% | 46.2 ± 3.5% | 13.4 ± 2.8% |
| Beta-blocker user | 120 | 58 | 15 | 27.7 ± 4.1% | 7.2 ± 2.5% | 52.3 ± 5.1% | 17.8 ± 4.9% |
| Beta-blocker non-user | 179 | 83 | 23 | 29.6 ± 3.4% | 10.4 ± 2.4% | 48.9 ± 4.0% | 15.9 ± 3.2% |
competing risk analysis.
Risk factors for Falls
Using competing-risks regression, we determined univariable associations with falls, accounting for the competing risk of death or transplantation.(Supplementary Table 2) The strongest predictors (sHR) were baseline reports of prior falls (4.08), sodium (0.92 per meq/L), chair-stands (0.92 per stand), SF-8 score (0.97 per point), albumin (0.66 per mg/dL), ADL-independence (0.44), anti-depressant use (2.07), gabapentin use (2.05), benzodiazepine use (1.90) and opioid use (1.82). Several factors that were associated with falls in univariable analysis were not included in the final model after multivariable adjustment and statistical variable selection. These include MELD-Na, benzodiazepines, opioids, antidepressants, and cognitive dysfunction (>24 weighted-lures). The final multivariable model for fall risk included prior falls, sodium, SF-8, and chair-stands – the FallSSS score.
Validation Performance (Discrimination and Calibration) and Accuracy
The results of the 5-fold cross-validation are summarized in Table 3. We compare the FallSSS score to MELD-Na, cognitive function (ICT weighted-lures), physical function (chair-stands and hand-grip), and FallSSS components. For the prediction of injurious falls, whereas MELD-Na was poorly discriminative (AUROC ≤0.57 at multiple time-points), inaccurate (c-index 0.54), FallSSS was much stronger. The AUROC for FallSSS at 6-months was 0.79, 0.81 at 12-months, with the highest c-index of the models (0.74) and the best (lowest) Brier score (0.11) indicating better calibration. In Supplementary Table 3, these relationships hold for predictions of any fall. The AUROC for any fall at 6- and 12-months is 0.75 and 0.73 while MELD-Na performs no better than for injurious falls.
Table 3:
Discrimination, Accuracy and Validation of Models in Predicting Injurious Falls
| Results of 5-Fold Cross-Validation | ||||||
|---|---|---|---|---|---|---|
| AUROC | C-Index | Brier Score | ||||
| Model |
6
months |
12
months |
18
months |
24
months |
Overall | Overall |
| MELD-Na | 0.51 | 0.56 | 0.57 | 0.56 | 0.54 | 0.12 |
| ICT weighted lures | 0.53 | 0.54 | 0.53 | 0.53 | 0.53 | 0.12 |
| Chair-stands and hand-grip | 0.72 | 0.75 | 0.74 | 0.71 | 0.69 | 0.11 |
| SF-8 and Sodium | 0.71 | 0.76 | 0.75 | 0.70 | 0.69 | 0.11 |
| FallSSS Score | 0.79 | 0.81 | 0.80 | 0.76 | 0.74 | 0.11 |
AUROC (area under the receiver operating characteristic curve), ICT (inhibitory control test), MELD-Na (Model for Endstage Liver Disease-Sodium), SF-8 (Short-form 8), and FallSSS score (Prior Falls, serum Sodium, SF-8, and number of chair-Stands performed within 30 seconds). AUROC and c-indices lack confidence intervals as they the product of cross-validations.
We conducted two sensitivity analyses of the cross-validated models. First, we assessed the performance of the SSS score in patients without a prior history of falls. In this case, the c-index was 0.60 and the AUROC peaked at 18 months with 0.64. MELD-Na had a c-index of 0.53 and an AUROC at 18-months of 0.54 while ICT weighted-lures had a c-index of 0.51 and an AUROC of 0.51 at 18-months. Second, we repeated the variable selection procedures excluding patients with prior falls. In this case, the most predictive variables were INR, NAFLD, sodium, age, and chair-stand performance. However, the cross-validated AUROC also peaked at 18-months with 0.68.
Association of Falls with Mortality
Overall, patients incurring falls in follow-up were at higher cumulative risk of mortality.(Figure 2) We evaluated the association of incident falls and HE on the risk of mortality using a time-varying multivariable cox model accounting for the competing risk of transplantation.(Table 4) We selected a model for mortality based on conventional, baseline risk factors – Child class, MELD-Na, Albumin, Charlson comorbidity index, and ADL-based disability – and evaluated falls and HE as two time-varying factors to determine their independent association with morality. In this model, both falls – sHR 2.91 95%CI(1.55–5.44) – and HE – sHR 4.36 95%CI(2.33–8.53) – were strongly and independently associated with mortality. Falls and incident HE were not associated, with most cases of HE happening long after the index fall.(Supplementary Figure 1A–B)
Figure 2: Falls and Mortality.
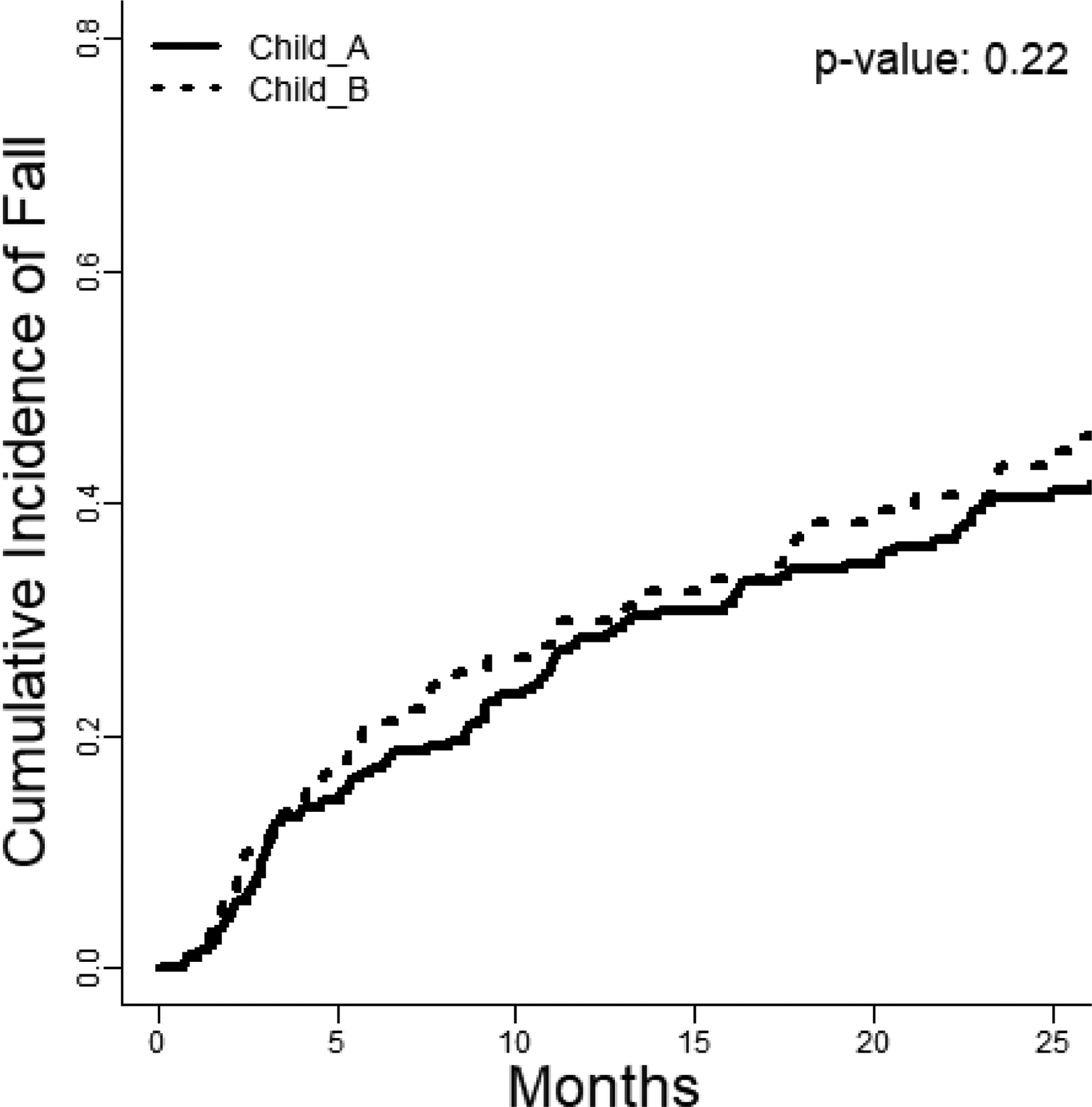
The cumulative incidence of mortality (accounting for the competing risk of transplantation) is displayed stratified according to whether the patients incurred a fall or not (Gray’s test, p=0.002).
Table 4:
A multivariable model for mortality accounting for the competing risk of liver transplantation
| Multivariable sHR (95% Confidence Interval) |
p-value | |
|---|---|---|
| Baseline factors | ||
| Child A (versus Child B) |
0.36 (0.16 – 0.83) | 0.02 |
| MELD-Na (log-transformed) |
0.94 (0.37 – 2.36) | 0.89 |
| Albumin (log-transformed) |
0.004 (0.0004–0.03) | <0.0001 |
| Independence in activities of daily living | 0.58 (0.25 –1.32) | 0.19 |
| Weighted Lures > 24 | 2.08 (1.00–4.35) | 0.05 |
| Charlson score | 1.05 (0.97–1.14) | 0.19 |
| Time-varying factors | ||
| Incident Falls | 2.76 (1.46–5.24) | 0.002 |
| Incident Hepatic Encephalopathy | 4.25 (2.15–8.41) | <0.0001 |
MELD-Na (Model for Endstage Liver Disease-Sodium), Weighted-lures >24 is a measure obtained from Inhibitor Control Testing that is associated with covert hepatic encephalopathy.
Discussion
The epidemiology of cirrhosis is shifting. Patients are presenting with cirrhosis at older ages with metabolic or alcohol-related etiologies.[19] The comorbidities associated with these conditions – sarcopenia, frailty, neuropathy, retinopathy, polypharmacy – are each associated with increased fall-risk in contemporary patients with cirrhosis.[20, 21] Our prospective study highlights the value of expanding fall-risk screening to patients with cirrhosis, even those who are compensated without prior HE. We also demonstrate the substantial harms associated with falls and therefore the potential for benefit in interventions to address fall-risk.
Falls are common and serious in people with cirrhosis
Our study shows that the risk of falls is high in persons with cirrhosis, irrespective of baseline Child classification. These data confirm and extend a prior short-term (<1 year) study in decompensated patients with highly prevalent HE.[22] Falls impact patients with compensated cirrhosis at a high-rate that is not simply commensurate with MELD-Na score or prior history of cirrhosis complications. Even among patients without prior falls who have a lower 1-year probability of falls, that probability still rises to 41% by 3-years. We also show that fall-related injuries are common. For patients with alcohol-related liver disease, any degree of disability, or prior falls at baseline the probability of an injurious fall at 3-years ranges from 1-to-2 in 5. Whereas we previously showed that patients with cirrhosis seeking emergent care for falls were more likely to incur serious injuries compared to others including those with congestive heart failure,[23] these data are derived from a well-phenotyped prospective cohort providing incidence rates and granular risk-adjustments. Our study shows that, adjusting for baseline indices of disease severity as well as impact of incident HE, incident falls are associated with an increased risk of mortality, sHR 2.76 95%CI(1.46–5.24).
Falls are predictable
We have identified a predictive model for falls – FallSSS (Fall history, sodium, SF-8, and chair-stands). The most potent predictor of falls is a past history of falls. As above, the USPSTF already recommends screening for fall history among community-dwelling adults. Our modeling shows that in addition to fall history, there is incremental risk discrimination in the patient’s sodium level, frailty (measured best in this instance with timed chair-stands), and HRQOL. One prior study by Soriano et al evaluated the ability of psychometric testing to predict falls and demonstrated utility. Unfortunately, psychometric testing is rarely performed in clinical practice,[24] particularly among persons with prior HE where the clinical utility of testing is unclear.[25] In our study of people without prior HE, 70% of whom were Child A, the ICT, a point-of-care psychometric test, performed poorly in predicting falls. Additional data are needed, however, to evaluate promising, universally applicable tests of cognitive function such as the Animal Naming Test.[26]
Our study adds 3 novel independent predictors. First, chair-stands predicted falls. They are a validated measure of frailty in cirrhosis that correlates with sarcopenia reflects lower body strength and co-ordination.[21, 27, 28] Second, serum sodium predicts falls. It has been associated with disordered neurotransmission, cognitive function, and poor HRQOL.[29] Third, just as we found that HRQOL was strongly associated with incident HE, so too is it linked with incident falls.[6] Poor HRQOL is a pragmatic biomarker that efficiently reflects causal factors that vary from patient-to-patient, many of which are associated with fall-risk including cognitive dysfunction, psychoactive medications, poor sleep, and frailty.[6, 30, 31] In patients without prior falls, the SSS model, performs best at 18-months. When we repeated the modeling procedures, we find that age, INR, and NAFLD are also predictors of falls in models including sodium and chair-stands, however the incremental predictive value is limited.
It is also notable that sedative medications, which are associated with falls in older adults with mild-moderate Alzheimer’s,[32]were not associated in our multivariable modeling. Our model includes HRQOL and frailty which are better functional expressions of the indications for (or effects of) sedative load. We previously found that a model including MELD-Na, HRQOL and frailty (timed chair-stands) predicted the development of incident HE.[6] We found, similarly, that psychoactive medications were not associated with HE when accounting for these other factors.
Building interventions
The United States Preventive Services Task Force (USPSTF) recommends assessing community-dwelling persons over the age of 65 for fall-risk as simply as inquiring about a history of falls in the prior year in order to suggest exercise interventions.[33] Most falls occur due to slipping, stumbling, or tripping during walking and the subsequent failure to swiftly correct the perturbation.[21] As we have found, each individual patient with cirrhosis may have various deficits in the neuromuscular capacities underlying fall risk relating to cognitive function, muscle strength, tactile sensation, visual contrast, and/or proprioception.[20] Interventions should be tailored to the specific deficits. Patients can be referred to physical or occupational therapists, undergo balance perturbation training, and consideration of HE-directed therapy for those with cognitive dysfunction could be considered.
Contextual Factors
Our data must be interpreted in the context of the study design. First, we included patients with portal hypertension who are at higher baseline risk of adverse events than those without.[34] Second, although Child A-B patients represent the vast majority of outpatients with cirrhosis, we excluded patients with prior HE and Child C cirrhosis, limiting generalizability to sicker patients. Nor did we compare outcomes to controls. However, national data from the US, England, and Denmark show that injurious falls and fractures are more common in patients with cirrhosis and that, based on English and Danish data, injury risk is not associated with disease severity.[22, 23, 35] Third, falls were ascertained by self-report, although injuries were confirmed with chart review. Fourth, although we assessed the robustness of our data using cross-validation, our model has not been validated in an external cohort. Fifth, we did not present data on the circumstances preceding falls and cannot exclude the possibility that our model predicts the risk of triggers (e.g. infection). However, as falls were associated with mortality independent of HE suggests that our model provides novel information. Finally, we evaluated chair-stands and hand-grip but not the liver frailty index (LFI) as our study was initiated prior to its validation.[36] Although we suspect the LFI and balance testing could discriminate risk further, fortunately hand-grip and chair-stands provide equal risk discrimination for mortality.[37]
Conclusion
Our study draws attention to falls, an overlooked cause of morbidity and mortality in patients with cirrhosis. Studies to define the specific neurophysiological perturbations underlying fall-risk will inform the design of interventions to reduce the harms of falls. Trials of tailored interventions are warranted. The population studied here, largely Child A, is at high risk but also likely more amenable to risk-reduction interventions owing to their lower medical complexity. These data provide both an estimate of fall incidence to power intervention studies and a method to stratify risk for trial inclusion.
Supplementary Material
Highlights.
The 1- and 3-year risk of falls is 29% and 50%; 9% and 16% for injurious falls
The top predictors of falls were prior falls, chair-stand performance, serum sodium, and quality of life (measured with SF-8), forming the FallSSS score
Incident falls were associated with an increased risk of mortality.
Financial support:
Dr. Tapper receives funding from the National Institutes of Health (K23DK117055, KL2TR002241). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health
Tapper is the guarantor of this article
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest. Tapper serves on advisory boards for Bausch Health, Rebiotix, and Mallinckrodt, consulted for Axcella, Kaleido, and Novo Nordisk. No other author has relevant conflicts of interest.
Data availability: De-identified data can be made available on request.
References
- [1].Román E, Córdoba J, Torrens M, Guarner C, Soriano G. Falls and cognitive dysfunction impair health-related quality of life in patients with cirrhosis. European journal of gastroenterology & hepatology 2013;25:77–84. [DOI] [PubMed] [Google Scholar]
- [2].Tapper E, Kanwal F, Asrani S, Ho C, Ovchinsky N, Poterucha J, et al. Patient Reported Outcomes in Cirrhosis: A Scoping Review of the Literature. Hepatology (Baltimore, Md) 2017. [DOI] [PubMed] [Google Scholar]
- [3].Román E, Córdoba J, Torrens M, Torras X, Villanueva C, Vargas V, et al. Minimal hepatic encephalopathy is associated with falls. The American journal of gastroenterology 2010. [DOI] [PubMed] [Google Scholar]
- [4].Tapper EB, Risech-Neyman Y, Sengupta N. Psychoactive medications increase the risk of falls and fall-related injuries in hospitalized patients with cirrhosis. Clinical Gastroenterology and Hepatology 2015;13:1670–1675. [DOI] [PubMed] [Google Scholar]
- [5].Gines P, Quintero E, Arroyo V, Teres J, Bruguera M, Rimola A, et al. Compensated cirrhosis: natural history and prognostic factors. Hepatology 1987;7:122–128. [DOI] [PubMed] [Google Scholar]
- [6].Tapper EB, Zhao L, Nikirk S, Baki J, Parikh ND, Lok AS, et al. Incidence and Bedside Predictors of the First Episode of Overt Hepatic Encephalopathy in Patients With Cirrhosis. Official journal of the American College of Gastroenterology| ACG 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. Journal of chronic diseases 1987;40:373–383. [DOI] [PubMed] [Google Scholar]
- [8].Force UPST. Screening and behavioral counseling interventions in primary care to reduce alcohol misuse: recommendation statement. Annals of Internal Medicine 2004;140:554. [DOI] [PubMed] [Google Scholar]
- [9].Kim WR, Biggins SW, Kremers WK, Wiesner RH, Kamath PS, Benson JT, et al. Hyponatremia and mortality among patients on the liver-transplant waiting list. New England Journal of Medicine 2008;359:1018–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Bajaj JS, Hafeezullah M, Franco J, Varma RR, Hoffmann RG, Knox JF, et al. Inhibitory control test for the diagnosis of minimal hepatic encephalopathy. Gastroenterology 2008;135:1591–1600 e1591. [DOI] [PubMed] [Google Scholar]
- [11].Amodio P, Ridola L, Schiff S, Montagnese S, Pasquale C, Nardelli S, et al. Improving the inhibitory control task to detect minimal hepatic encephalopathy. Gastroenterology 2010;139:510–518. e512. [DOI] [PubMed] [Google Scholar]
- [12].Gray RJ. A class of $ K $-sample tests for comparing the cumulative incidence of a competing risk. The Annals of statistics 1988;16:1141–1154. [Google Scholar]
- [13].So Y, Lin G, Johnston G. Using the PHREG procedure to analyze competing-risks data. SAS Global Forum; 2014; 2014. p. 23–26. [Google Scholar]
- [14].Prentice RL, Kalbfleisch JD, Peterson AV Jr, Flournoy N, Farewell VT, Breslow NE. The analysis of failure times in the presence of competing risks. Biometrics 1978:541–554. [PubMed] [Google Scholar]
- [15].Friedman J, Hastie T, Tibshirani R. Regularization paths for generalized linear models via coordinate descent. Journal of statistical software 2010;33:1. [PMC free article] [PubMed] [Google Scholar]
- [16].Ishwaran H, Kogalur UB, Blackstone EH, Lauer MS. Random survival forests. The annals of applied statistics 2008;2:841–860. [Google Scholar]
- [17].Blanche P, Dartigues JF, Jacqmin-Gadda H. Estimating and comparing time-dependent areas under receiver operating characteristic curves for censored event times with competing risks. Statistics in medicine 2013;32:5381–5397. [DOI] [PubMed] [Google Scholar]
- [18].Hernández-Orallo J, Flach PA, Ramirez CF. Brier Curves: a New Cost-Based Visualisation of Classifier Performance. ICML; 2011; 2011. p. 585–592. [Google Scholar]
- [19].Moon AM, Singal AG, Tapper EB. Contemporary Epidemiology of Chronic Liver Disease and Cirrhosis. Clinical Gastroenterology and Hepatology 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Murphy SL, Richardson JK, Blackwood J, Martinez B, Tapper EB. Neurocognitive and Muscular Capacities Are Associated with Frailty in Adults with Cirrhosis. Digestive Diseases and Sciences 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Murphy SL, Tapper EB, Blackwood J, Richardson JK. Why Do Individuals with Cirrhosis Fall? A Mechanistic Model for Fall Assessment, Treatment, and Research. Digestive diseases and sciences 2018:1–8. [DOI] [PubMed] [Google Scholar]
- [22].Soriano G, Román E, Córdoba J, Torrens M, Poca M, Torras X, et al. Cognitive dysfunction in cirrhosis is associated with falls: a prospective study. Hepatology 2012;55:1922–1930. [DOI] [PubMed] [Google Scholar]
- [23].Ezaz G, Murphy SL, Mellinger J, Tapper EB. Increased Morbidity and Mortality Associated with Falls among Patients with Cirrhosis. The American journal of medicine 2018. [DOI] [PubMed] [Google Scholar]
- [24].Bajaj JS, Etemadian A, Hafeezullah M, Saeian K. Testing for minimal hepatic encephalopathy in the United States: an AASLD survey. Hepatology 2007;45:833–834. [DOI] [PubMed] [Google Scholar]
- [25].Tapper EB, Parikh ND, Waljee AK, Volk M, Carlozzi NE, Lok AS. Diagnosis of Minimal Hepatic Encephalopathy: A Systematic Review of Point-of-Care Diagnostic Tests. The American Journal of Gastroenterology 2018. [DOI] [PubMed] [Google Scholar]
- [26].Campagna F, Montagnese S, Ridola L, Senzolo M, Schiff S, De Rui M, et al. The animal naming test: an easy tool for the assessment of hepatic encephalopathy. Hepatology 2017;66:198–208. [DOI] [PubMed] [Google Scholar]
- [27].Lai JC, Volk ML, Strasburg D, Alexander N. Performance-Based Measures Associate With Frailty in Patients With End-Stage Liver Disease. Transplantation 2016;100:2656–2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Tapper EB, Derstine B, Baki J, Su GL. Bedside Measures of Frailty and Cognitive Function Correlate with Sarcopenia in Patients with Cirrhosis. Digestive diseases and sciences 2019:1–8. [DOI] [PubMed] [Google Scholar]
- [29].Ahluwalia V, Wade JB, Thacker L, Kraft KA, Sterling RK, Stravitz RT, et al. Differential impact of hyponatremia and hepatic encephalopathy on health-related quality of life and brain metabolite abnormalities in cirrhosis. J Hepatol 2013;59:467–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Tapper EB, Baki J, Parikh ND, Lok AS. Frailty, Psychoactive Medications, and Cognitive Dysfunction Are Associated With Poor Patient-Reported Outcomes in Cirrhosis. Hepatology 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Bajaj JS, Thacker LR, Heuman DM, Sterling RK, Stravitz RT, Sanyal AJ, et al. Cognitive performance as a predictor of hepatic encephalopathy in pretransplant patients with cirrhosis receiving psychoactive medications: a prospective study. Liver Transplantation 2012;18:1179–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Dyer AH, Murphy C, Lawlor B, Kennelly SP. Sedative Load in Community-Dwelling Older Adults with Mild-Moderate Alzheimer’s Disease: Longitudinal Relationships with Adverse Events, Delirium and Falls. Drugs & aging 2020;37:829–837. [DOI] [PubMed] [Google Scholar]
- [33].Grossmann M, Hoermann R, Gani L, Chan I, Cheung A, Gow PJ, et al. Low testosterone levels as an independent predictor of mortality in men with chronic liver disease. Clinical endocrinology 2012;77:323–328. [DOI] [PubMed] [Google Scholar]
- [34].D’Amico G, Pasta L, Morabito A, D’Amico M, Caltagirone M, Malizia G, et al. Competing risks and prognostic stages of cirrhosis: a 25-year inception cohort study of 494 patients. Aliment Pharmacol Ther 2014;39:1180–1193. [DOI] [PubMed] [Google Scholar]
- [35].Otete H, Deleuran T, Fleming KM, Card T, Aithal GP, Jepsen P, et al. Hip fracture risk in patients with alcoholic cirrhosis: A population-based study using English and Danish data. J Hepatol 2018;69:697–704. [DOI] [PubMed] [Google Scholar]
- [36].Lai JC, Covinsky KE, Dodge JL, Boscardin WJ, Segev DL, Roberts JP, et al. Development of a novel frailty index to predict mortality in patients with end-stage liver disease. Hepatology 2017;66:564–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Lai JC, Covinsky KE, McCulloch CE, Feng S. The Liver Frailty Index improves mortality prediction of the subjective clinician assessment in patients with cirrhosis. The American journal of gastroenterology 2018;113:235. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


