Abstract
Type 2 diabetes is associated with several potential comorbidities, among them impaired wound healing, chronic ulcerations, and the requirement for lower extremity amputation. Disease-associated abnormal cellular responses, infection, immunological and microvascular dysfunction, and peripheral neuropathy are implicated in the pathogenesis of the wound healing impairment and the diabetic foot ulcer. The skin houses a dense network of sensory nerve afferents and nerve-derived modulators, which communicate with epidermal keratinocytes and dermal fibroblasts bidirectionally to effect normal wound healing after trauma. However, the mechanisms through which cutaneous innervation modulates wound healing are poorly understood, especially in humans. Better understanding of these mechanisms may provide the basis for targeted treatments for chronic diabetic wounds. This review provides an overview of wound healing pathophysiology with a focus on neural involvement in normal and diabetic wound healing, as well as future therapeutic perspectives to address the unmet needs of diabetic patients with chronic wounds.
INTRODUCTION
In the United States, more than 30 million individuals above the age of 18 years have type 2 diabetes (T2D)1 and more than 34% of the US adult population are prediabetic (an estimated 88 million adults).2 From 2012 to 2017, the costs of diabetes increased by 26% to $327 billion USD,1 and the global economic burden is projected to exceed $627 billion USD by 2035.3 Smoking,4 a sedentary lifestyle,5 and obesity6 are recognized risk factors for developing diabetes-related complications, including life-threatening heart attacks and strokes.
Chronic, nonhealing wounds, particularly ulcerations of the foot, are the leading cause of nontraumatic lower extremity amputations in the United States at a frequency of approximately 200,000 annually.7,8 Disease-associated abnormal cellular responses, infection, immunological and microvascular dysfunction, and peripheral neuropathy are implicated in the pathogenesis of the wound healing impairment and diabetic foot ulcer.9,10 Current treatment options are limited, particularly pathogenesis-based therapeutic approaches for preventing or healing diabetic ulcers.
MECHANISM OF NORMAL WOUND HEALING
The skin acts as a protective barrier, preventing desiccation and representing the body’s key site of protection against the environment. Receptors on keratinocytes and epidermal sensory nerve afferents detect extrinsic stimuli and respond to prevent and react to an injury. When a wound occurs in a healthy individual, communication among keratinocytes, nerves, and other cells leads to a series of distinct, yet overlapping phases as part of the “cascade of healing” (Fig 1). Hemostasis, an immediate response, involves the constriction of injured blood vessels and activation of platelets to form a fibrin clot,11 which serves as a scaffold for incoming inflammatory cells.12 Proinflammatory cytokines, among them interleukin (IL)-1β, IL-6, tumor necrosis factor (TNF)-α,13 and interferon-gamma (IFN-γ),14 recruit neutrophils into the wound bed, followed by monocytes, which become tissue-activated macrophages 48–96 hours postinjury.15 These cytokines and growth factors stimulate epithelial cells, endothelial cells, and fibroblasts to proliferate and migrate into the wound area. Fibroblasts fill the injured area and differentiate into myofibroblasts, first synthesizing collagen III, but also collagen I, proteoglycans, and matrix metalloproteinases (MMPs),16 to form and remodel the extracellular matrix. Angiogenesis occurs in this developing granulation tissue matrix due to the presence of low oxygen,17 low pH, and high lactate levels.18 Keratinocytes begin the re-epithelization process through successful migration across the matrix, with proliferation and then differentiation into a functional epidermis. Migration ends when keratinocytes from opposing edges meet. A thin epithelial layer is established as keratinocytes form new adhesions to the underlying matrix.19 Through keratinocyte proliferation and differentiation, the multilayer epidermis is ultimately formed. Growth factors, such as epidermal growth factor (EGF),20 keratinocyte growth factor (KGF),21 insulin-like growth factor (IGF)-1,22 and transforming growth factor (TGF)-α,23 regulate keratinocyte activity.24 Although re-epithelization is a clinical indicator of wound healing, granulation tissue reinforcement completes the reparative process. The remodeling phase of wound healing, also known as the maturation phase, involves strengthening the scarred area by crosslinking and improving collagen fiber alignment, with apoptosis of cells from the healing process that are no longer needed.
Fig 1.
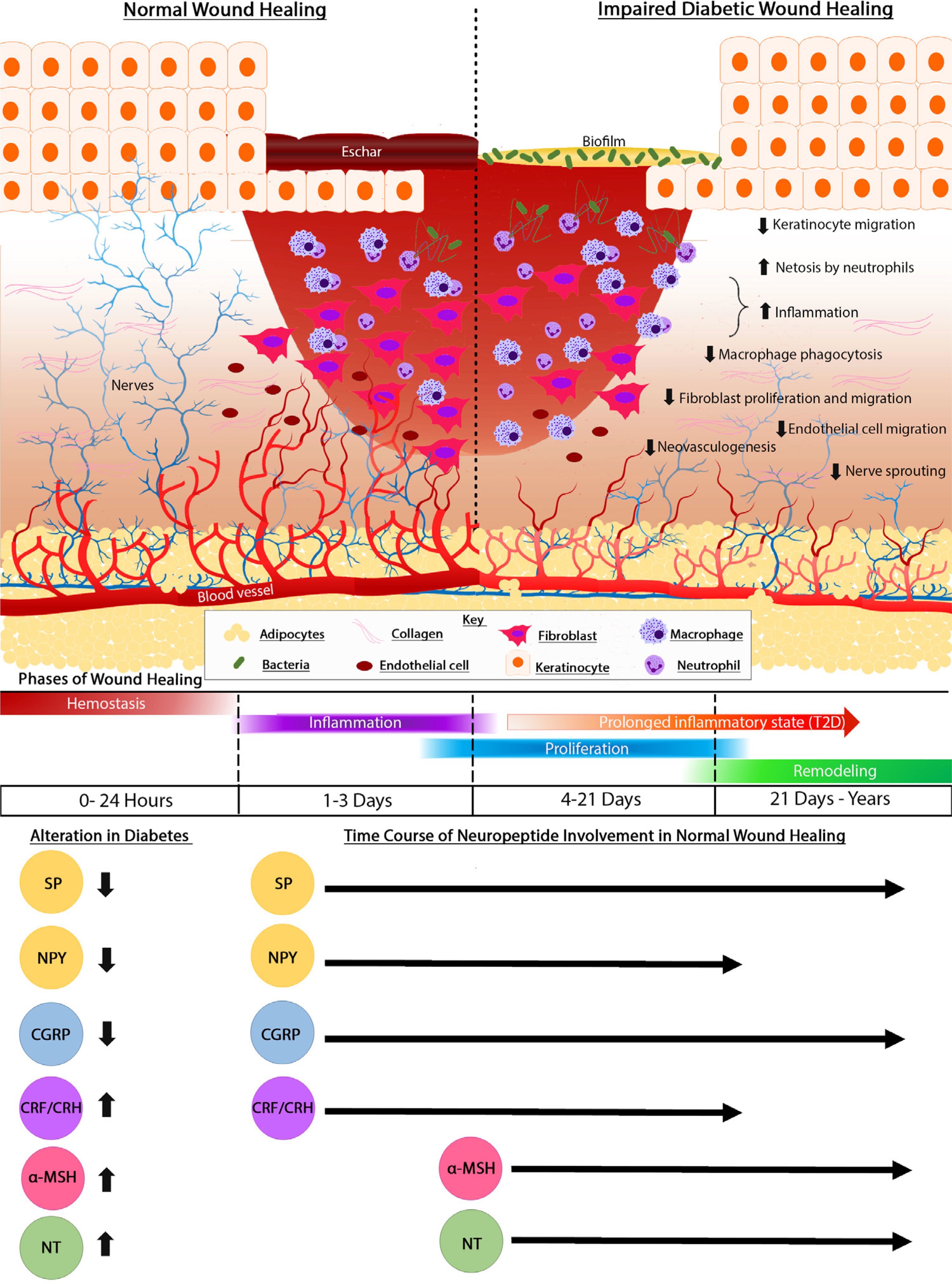
Cellular mechanisms involved in normal wound healing and their impairment in chronic diabetic wounds. Top: In contrast to healing in normal skin (left), healing in diabetic skin (right) is impaired. Chronic diabetic wounds have an epidermis that migrates poorly, an influx of dysfunctional inflammatory cells, and surface biofilm. In addition to impaired proliferation and migration of fibroblasts and endothelial cells in diabetic wounds, sensory innervation is deficient, with a reduction in intraepidermal nerve fiber density. Bottom: Normal wound repair involves a temporal sequence of overlapping phases: hemostasis, inflammation, cell proliferation and migration, and remodeling. Unlike normal wounds, chronic wounds are stalled in the inflammatory phase. Neuropeptides have crucial roles at each stage of wound repair and are dysregulated in diabetes. While tachykinins substance P (SP) and neuropeptide Y (NPY), as well as calcitonin gene-related peptide (CGRP) are downregulated during diabetic wound healing, corticotropin releasing factor (CRF), α-melanocorticotropin releasing hormone (α-MSH), and neurotensin (NT) are upregulated, contributing to delayed healing.
WOUND HEALING ABNORMALITIES IN DIABETES: TISSUE AND CELLULAR FACTORS
Diabetes affects inflammation,25 matrix deposition,26 and angiogenesis27 of the wound healing cascade as a result of numerous factors (Fig 1; Table I). Many studies of diabetic wounds have investigated the impaired blood flow leading to poor oxygenation and nutrient delivery, chronic exposure to hyperglycemia, immune cell dysregulation, and propensity for bacterial colonization and infection. Importantly, chronic induction of proinflammatory cytokines, such as TNF-α28 and IL-1β,29,30 stalls the inflammatory phase and disrupts wound healing. Defects have been described in neutrophil function, leukocyte chemotaxis, macrophage phagocytosis, and bactericidal capacity in diabetic wounds, leading to inadequate bacterial clearance.
Table I.
Changes in diabetes that affect stages of healing in animal models and patients with diabetes26,27,34,36,231–247
| Alterations in inflammatory phase | Alterations in proliferative phase | Alterations in remodeling phase |
|---|---|---|
| ↑ Levels of proinflammatory cytokines (IL-6, MCP1, TNF-α) | ↓ Fibroblast proliferation and migration | ↑ MMPs |
| ↓ Phagocytosis | ↓ Keratinocyte differentiation and migration | ↓ Collagen and elastin content |
| ↑ NETosis | ↓ Functional levels of growth factors (PDGF, IGF-1, VEGF) | ECM glycation and ↑ crosslinking |
| ↓ Numbers of CD4+ T cells | Impaired angiogenesis | ↓ Levels of TIMPs (MMP inhibitors) |
| ↑ Oxidative stress | Endothelial cell dysfunction | ↑ Presence of nonsolubilized and fragmented ECM fibrils |
| Impaired macrophage polarization (proinflammatory to proreparative phenotype switch) | Pericyte dysfunction | ↓ Wound contraction and wound strengthening |
| Impaired neutrophil function | Mast cell dysfunction | Fibroblast senescence |
Abbreviations: ECM, extracellular matrix; IGF-1, insulin-like growth factor-1; IL-6, interleukin-6; MCP1, monocyte chemoattractant protein-1; MMPs, matrix metalloproteinases; NET, neutrophil extracellular traps; PDGF, platelet-derived growth factor; TIMPs, tissue inhibitors of metalloproteinases; TNF-α, tumor necrosis factor alpha; VEGF, vascular endothelial growth factor.
Normal healing is characterized by a transition in the ratio of macrophage phenotypes M1 and M2, reflecting the shift from inflammatory to proliferative functions. Classically activated M1 macrophages secrete high amounts of proinflammatory cytokines (eg, IL-1, IL-6, IL-12, TNF-α)31 and oxidative metabolites (eg, nitric oxide and superoxide) to facilitate pathogen killing activity and wound debridement early after wounding. Varied stimuli, including IL-4 and IL-13 signaling,32 evoke M2 macrophage activation, and aid in the resolution of inflammation. The phenotypic switch to an M2 macrophage is marked by upregulation of classical M2 cytokines and growth factors, such as IL-10, vascular endothelial growth factor (VEGF), transforming growth factor (TGF)-β1, and platelet-derived growth factor (PDGF), to encourage granulation tissue formation, angiogenesis, and cellular proliferation.32,33
In diabetes, the ratio of M1 (proinflammatory) to M2 (anti-inflammatory) macrophages is increased, impeding the proliferative phase in wound healing.34 Diabetes also increases neutrophil extracellular traps (NETs), aggregates of de-condensed chromatin formed by neutrophils to neutralize organisms, which suggests another deleterious effect of the enhanced inflammatory response.35,36 Hyperglycemia accelerates the formation of advanced glycation end product (AGE),37 which are thought to contribute to impaired healing by increasing oxidative stress,38 changing the expression and function of proteins that are critical to wound repair,39,40 enhancing the inflammatory response by activation of transcription factors,41,42 and potentially leading to exaggerated cellular apoptosis.43
In addition to increased levels of glycosylated proteins, a measure of poor diabetic control,44,45 glycosphingolipids, and particularly ganglioside GM3, are increased in diabetic tissues, including skin,46–48 and have been implicated in impaired healing. Increases in GM3 suppress growth factor-induced responses, including insulin, IGF-1,49 and EGF receptor signaling, leading to delayed skin cell migration and inhibition of cell proliferation. Suppression of expression of GM3 synthase (GM3S), the enzyme required for synthesis of GM3, using genetic (knockout or topically applied siRNA nanoconstructs) or biochemical (glucosylceramide synthesis small molecule inhibition) approaches accelerates healing in wounded mouse models of diet-induced type 2 diabetes.46,48 Inhibition of GM3S in cultured keratinocytes reverses the glucose- and TNF-α-induced slowing of cell migration through increasing insulin- and IGF-1-induced IGF-1 receptor phosphorylation and activating Rac1.46–48 These data suggest GM3 depletion as a pathogenesis-based direction in therapy.
Diabetic cells retain “metabolic memory,” including at the level of histone modification,50,51 genome-wide DNA methylation,51 and microRNA expression patterns.52,53 Hyperglycemia leads to an altered miRNA signature in wound healing, which may be linked to the dysregulated inflammation in diabetes54 and has been positively correlated to the severity of diabetic foot ulcers.55 Dysregulated expression of miRNAs influences the cellular transcriptome and may impair wound healing in diabetes. For example, miR-27–3p overexpression in diabetic foot ulcer-derived fibroblasts (DFUFs) and diabetic mice,56 as well as downregulation of miR-129 and −355 in human diabetic wounds,57 hinders healing by impairing fibroblast function and inhibiting MMP-9 expression, respectively. In a comparative analysis of miRNA expression profiles in human DFUFs vs normal foot-derived fibroblasts (NFF), aberrant expression of miR–21–5p, miR–34a–5p, and miR–145–5p was linked to DFUF dysfunction in proliferation, migration, and differentiation.58
CONTRIBUTIONS OF PERIPHERAL NERVE DYSFUNCTION TO POOR DIABETIC WOUND HEALING
Both sensory and autonomic nerves populate skin.59 Autonomic nerves are restricted to the dermis and regulate lymphatic function, blood circulation, and appendageal function.60 In contrast, cutaneous sensory nerves predominate and are widely distributed in skin, including extending into the upper epidermis to interface with the environment. The trigeminal ganglia comprise many of the sensory neurons innervating the head. Conversely, the dorsal root ganglia largely innervate the rest of the body. Neuronal afferents that traverse the dermis to the epidermis originate in dorsal root ganglia in the spinal cord and only their dendritic extensions populate skin, with sensations transmitted from the peripheral nerve terminals to the body in the spinal cord. Distal foot skin represents the longest extension of a dorsal root ganglion in the body.
Early sensory nerve classification was strictly based on the size, speed of impulse conduction, and function (including neuropeptide secretion and type of sensation recognized). The thinly myelinated Aδ low-threshold mechanoreceptors are nerve fibers that carry thermal, mechanoreceptive (pressure), and acute nociceptive (pain) signals. The small, unmyelinated C fibers (~70%) transmit information related to pain, temperature, and itch,61 sending slower and more sustained impulses than Aδ fibers.62,63 C fibers are classically divided into 2 subsets, peptidergic (PEP) and nonpeptidergic (NP). Peptidergic C fibers produce neuropeptides such as substance P (SP) and calcitonin gene–related peptide (CGRP), and express the tropomyosin receptor kinase A (TrkA). In contrast, nonpeptidergic C fibers bind to isolectin B4 (IB4) and express the ATP-binding purinergic receptor P2 × 3. However, markers for the PEP and NP C fibers are not absolute, for example, 1 subset of NP C fibers expresses Calca, the gene that encodes for neuropeptide CGRP (Table II). Indeed, use of single cell transcriptomics has demonstrated a previously unappreciated level of heterogeneity among DRG neurons, indicating that their functions may be very precisely tuned according to phenotype. Moreover, this type of analysis has highlighted important differences in the properties of DRG neurons based on sex and species, illustrating the translational challenges that face novel therapies for pain and itch based on regulation of sensory neuron function. Aδ and C fibers are considered polymodal due to their ability to sense various different stimuli.65 TH-expressing C fiber low-threshold mechanoreceptors (C-LTMRs), which are unmyelinated and express tyrosine hydroxylase and dopamine/L-DOPA, are also likely involved in pain sensation but not well understood.66
Table II.
Classification of sensory neurons from adult mouse DRGs and markers128
| Neuron size | Small neurons |
Large neurons |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Principal neuron types Classical markers | PEP Tac1, Calca |
NP IB4, P2 × 3, Plxnc1 |
TH Th |
NF Nefh, Ldhd |
|||||||
| Subpopulations | PEP1 | PEP2 | NP1 | NP2 | NP3 | - | NF1 | NF2 | NF3 | NF4 | NF5 |
| Myelinated/unmyelinated | Unmyelinated | Myelinated | Unmyelinated | Unmyelinated | Myelinated | ||||||
| Gene markers/products | TRPV1 | Nav1.8/9 | TRPA1 | TRPV1 | TRPV1 | TRPA1 | TrkBhigh | TrkBlow | TrkChigh | TrkClow | TrkClow |
| Nav1.8/9 | TrkA | TRPC3 | TRPA1 | TRPA1 | NAV1.8/9 | NEFH | RET | RET | ASIC1 | ASIC1 | |
| TrkA | CGRP | MRGPRD | TRPC3 | TRPC3 | VGLUT3 | NEFH | NEFH | CNTNAP2 | CNTNAP2 | ||
| CGRP | Fam19A1 | P2 × 3 | Nav1.8/9 | Nav1.8/9 | Piezo2 | NEFH | NEFH | ||||
| PLXNC1low | NEFH | MRGPRA3 | P2 × 3 | ||||||||
| Gene expression/subtype | Plxnc1 | Fam19a1+ | P2 × 3hgh | Ntrk1+ | Sst+ | Th+ | Ntrk2high Necab2 | Ntrk2low | Ntrk3hgh | Ntrk3low | |
| Tac1+ | Calca+ | Calca+ | P2 × 3low | Plxnc1− | Calb1 | Pv− | Pv+ | ||||
| Calca+ | Nefh+ | Ntrk1− | Fam19a1+ | Fam19a1− | |||||||
| Nefh− | Cntnap2+ | ||||||||||
| Spp1+ | |||||||||||
| Modality-specific function | Peptidergic | Nonpeptidergic | C-LTMRs | AS-LTMRs | Aβ-LTMRs (RA, SA) | Proprioceptors | |||||
Abbreviations: ASIC1, acid sensing ion channel subunit 3; Aβ-LTMRs (RA, SA), alpha beta low threshold mechanoreceptor (rapidly adapting or slowly adapting); Aδ-LTMRs, alpha-delta low-threshold mechanoreceptor; Calb1, calbindin 1; Calca, calcitonin-related polypeptide-α; CGRP, calcitonin gene-related peptide; C-LTMRs, C-low threshold mechanoreceptors; Cntnap2+, contactin associated protein 2-positive; Fam19A1, family with sequence similarity 19 member A1; IB4, isolectin-B4; Ldhd, lactate dehydrogenase B; MRGPR, Mas-related G-protein coupled receptor member; Nav1.8/9, voltage-gated sodium (NaV) channels 1.8/1.9; Necab2, N-terminal EF-hand calcium binding protein 2; Nefh, neurofilament heavy; NF, neurofilament-positive; NP, nonpeptidergic; PEP, peptidergic; Piezo2, piezo type mechanosensitive ion channel component 2; Plxnc1, plexin C1; Pv, parvalbumin; P2 × 3, P2X purinoceptor 3; RET, RET proto-oncogene; Spp1+, secreted phosphoprotein 1; Sst+, somatostatin-positive; Tac1, tachykinin precursor 1; TH/Th, tyrosine hydroxylase; Trpa1, transient receptor potential ankyrin 1; Trpc3, transient receptor potential cation channel subfamily C member 3; Trpv, transient receptor potential cation channel; Trk, tyrosine receptor kinase; VGLUT3, vesicular glutamate transporter 3.
Diabetic neuropathy occurs in almost 90% of diabetic foot ulcers.67 Neuropathies selectively target C and Aδ fibers, with degeneration linked to impaired healing in both type 1 and type 2 diabetes.68 Sensory neuropathy can lead to neuropathic pain and/or loss of sensation, increasing the risk of injury and foot ulceration by 8- to 18-fold and lower extremity amputation by 2- to 15-fold.69 Length-dependent “dying back” of axons, primarily involving the distal portions of the longest myelinated and unmyelinated sensory axons, results in nerve dysfunction.70 Given the predominant degeneration of the small C and Aδ fibers, a common initial presentation is symmetric loss of temperature, light touch sensation, or painful prickling sensations in a “stocking and glove” distribution.
Diagnosis of small fiber neuropathy is based on the presence of specific sensory deficits, discovered through examination and validated by structural and functional assessments. Structural assessment relies on skin biopsy71–75 for nerve fiber assessment that combines quantification of intraepidermal nerve fiber density (IENFD) and dermal nerve bundles. Functional assessment involves quantitative sensory testing, pain-related and laser induced potential recording, and single axon recording using microneurography.76 Noninvasive measures, such as quantifying axon-reflex mediated vasodilation (LDIflare technique)77–79 and measuring nerve conduction velocity,80,81 have also been investigated to evaluate abnormal sensory nerves. These measures have shown the reduction in cutaneous innervation in biopsies of diabetic human subjects based on reduced immunoreactivity to PGP9.5 (detecting sensory neurons) and a variety of neuropeptides (particularly calcitonin gene-related peptide (CGRP), substance P (SP), and neuropeptide Y) (Table III).71,72 Characteristic findings in human diabetic skin include fewer and more fragmented nerves throughout the dermis82,83 and reduction in nerve afferents in the epidermis84–88 and papillary dermis,89 even in the absence of clinically-detectable sensory neuropathy.90 Diabetic subjects can show markedly reduced amplitudes and neural conduction velocities associated with nerve fiber loss.91 Similar changes in the anatomy of cutaneous sensory afferents have also been observed in rodent models of diabetes, in which different subpopulations of DRG neurons, such as the sodium channels NaV1.8 or the G-protein coupled receptor MrgD (member D of the Mas-related G-protein coupled receptors or Mrgprs), can be precisely identified by making use of the genetic expression of fluorescent markers.
Table III.
| Neuropeptide/dysregulation in diabetes | Neuropeptide receptor(s) | Target cell-types in skin | Cytokines in wound healing | Biological function in skin | Involvement in wound healing phase |
|---|---|---|---|---|---|
| Substance P (SP) ↓ | NK-1R | Endothelial cell, Keratinocyte, Fibroblast, Mast cell, Monocyte & Macrophages, Granulocytes, Lymphocytes | TNF-α, IL-1β, IL-2, IL-6, IL-8, TGF-β | Vasodilation; Vascular permeability; Cell proliferation and migration; Leukocyte attraction and adhesion; Polymorphonuclear cell infiltration; Plasma extravasation; Angiogenesis; Neurite Outgrowth; Immune cell proliferation and ↑ chemotaxis; ↑ NGF; Collagen remodeling | Inflammatory Proliferative Remodeling |
| Calcitonin gene-related peptide (CGRP) ↓ | CL-R/RAMP1 | Endothelial cell, Keratinocyte, Mast cell, Monocyte & Macrophage, Melanocyte, Granulocyte, Lymphocyte | TNF-α, IL-1β, IL-1α, IL-8, IL-2, IL-6, VEGF | Vasodilation; Vascular permeability; Cell proliferation; Immunomodulation; Angiogenesis; Acts in combination with SP; ↑ NGF; Collagen remodeling | Inflammatory Proliferative Remodeling |
| Neuropeptide Y (NPY) ↓ | Y1–Y6 | Endothelial cell, Mast cell, Monocyte & Macrophage, Granulocyte, Lymphocyte | TNF-α and IL-2 | Migration of macrophages; Cell proliferation; vasoconstriction; angiogenesis | Inflammatory Proliferative |
| Neurotensin (NT) ↑ | NTR1–R3 | Keratinocyte, Fibroblast, Mast cell, Dendritic cell | IL-6, TNF-α, IL-8 | ↑ EGF; Angiogenesis; Anti-inflammatory; Mast cell degranulation; Cell migration | Proliferative Remodeling |
| α-Melanocyte stimulating hormone (α-MSH) ↑ | MC1R–5R | Keratinocyte, Mast cell, Fibroblast, Endothelial cell, Monocyte & Macrophage, Melanocytes | TNF-α, IL-β, IL-8, IL-10, IFN-γ | Anti-inflammatory; Angiogenesis; Immunomodulation | Proliferative Remodeling |
| Corticotropin releasing factor (CRF) ↑ | CRFR1–R2 | Keratinocyte, Fibroblast, Monocyte | TNF-α, IL-1β, IL-1α, IL-2, IL-6, IL-4, IL-10, IL-8, IFN-α, IFN-γ, MIP-1α, KGF-1 | Proinflammatory cytokine release; Angiogenesis; Mast cell activation and degranulation; Cell migration and proliferation; Immunomodulation | Inflammatory Proliferative |
Abbreviations: CL-R/RAMP1, calcitonin receptor-like receptor, receptor activity-modifying protein 1; CRFR, corticotropin releasing factor receptor; EGF, epidermal growth factor; IFN-α, interferon-α; IFN-γ, interferon-γ; IL, interleukin; KGF-1, keratinocyte growth factor-1; MIP-1α, macrophage inflammatory protein-1α; NGF, nerve growth factor; NK-1R, neurokinin 1 receptor; NTR, neurotensin receptor; MCR, melanocortin receptor; SP, substance P; TGF-β, transforming growth factor-β; TNF-α, tumor necrosis factor-α; VEGF, vascular endothelial growth factor.
Reduction in VEGFR-expressing dermal blood vessels82,92 and presence of a low-grade inflammatory cell infiltration93 have been associated with the innervation abnormalities in diabetes.90 Despite these known abnormalities, the role of cutaneous innervation and specific neuronal subsets in normal healing is more poorly understood than the role of other tissue types, such as the vasculature, keratinocytes, fibroblasts, and immune cells.94–106
POOR WOUND HEALING AND REDUCED INNERVATION: RESULTS FROM EXPERIMENTAL DENERVATION
Skin nociceptive effectors modulate gene expression of extracellular matrix (ECM), transcription factors, cytoskeleton, proteases, receptors, intracellular transducers, and adhesion molecules,107 suggesting a role in wound healing. Studies in the chick embryo suggest a positive reciprocal association between nerves and wound repair.108 Chemical and surgical denervation studies support a role for cutaneous innervation in wound healing, with reduction in small nerve fibers by at least 70% leading to features of poor wound repair.109 Reduction of sensory nerves by subcutaneous capsaicin treatment in nondiabetic mice and rats delayed re-epithelialization, reduced epidermal stem cell migration, and suppressed angiogenesis and VEGF expression.110–112 In both nondiabetic and diabetic mouse models, chemical ablation of sympathetic nerves using intraperitoneal injection of 6-hydroxydopamine (6-OHDA) delayed wound re-epithelization and reduced inflammation,113 but in leptin-deficient diabetic mice also increased wound contraction.114
Surgical denervation in nondiabetic mice, rats, and rabbits led to markedly delayed closure of wounds on the ear pinnae and dorsum and have shown delayed wound contraction,115 altered keratinocyte proliferation,116 delayed re-epithelialization,117 and reduced granulation tissue.118 Transplantation into denervated wounds of skin-derived precursors (SKPs),119 a population of neural crest-related stem cells within the dermis that participate in cutaneous nerve regeneration,120 leads to wound cell proliferation, increased nerve fiber density, and higher neuropeptide levels (nerve growth factor [NGF], SP, and CGRP) in mice.
Experimental intracutaneous excision axotomy in human subjects with diabetes and neuropathy leads to slower healing than in healthy controls.121 Punch biopsies of the distal thigh skin were performed, followed by concentric overlapping biopsies at various time points. Compared to healthy controls, the diabetic wounds had reduced re-epithelialization and granulation tissue, poor vascularization, and diminished dermal innervation and Schwann cells in the axotomy site.121 Blood vessel growth into the excision site preceded dermal nerve fiber regeneration in both diabetics and nondiabetics, suggesting that blood vessels act as a framework for later axon and Schwann cell growth.121 Diabetic subjects with epidermal denervation through capsaicin treatment also showed delayed rates of reinnervation when compared to healthy controls.121
Prevention of nerve degeneration improves healing.
Neuropathy has been noted in many of the rodent models of diabetes and poor wound healing (Table IV). The degree of obesity and severity of diabetes vary in these models.89,109,122 In general, obese mice with more severe diabetes show a more severe wound healing impairment. Regardless of the extent of diabetes and obesity, however, the reversal of neuropathy has been shown to be convergent with improvement in wound healing. Knockout of GM3 synthase (with resultant ganglioside GM3 reduction) reversed both the neuropathy (characterized by loss of sensory neurons and increased sensitivity to pain with von Frey testing) and the wound healing defect in mice fed a high-fat diet, regardless of the extent of obesity or diabetes.123 Diet-induced obese diabetic mice with selective chemokine receptor CXCR4 deletion from Nav1.8-positive dorsal root ganglia (DRG) neurons failed to develop of mechanical allodynia and small fiber degeneration, despite diet-induced obesity and diabetes.124 Antagonism of CXCR4 by AMD 3100, a small molecule inhibitor, improved wound healing in db/db mice. These observations suggest some potential therapeutic directions for diabetic neuropathy and improved healing.
Table IV.
Type 2 diabetes models with delayed healing and known evidence of neuropathy
| Model | Comments | References |
|---|---|---|
| Mouse models | ||
| B6.Cg-Lepob/J (ob/ob) | Very obese, high glucose and insulin levels | 282–285 |
| Monogenic JAX stock #000632 | ||
| BTBR-ob/ob | Very obese, high glucose and insulin levels | 286–289 |
| Monogenic | Retinopathy | |
| JAX stock #004824 | Nephropathy | |
| BKS.Cg-Dock7m +/+ Leprdb/J (db/db) | Very obese, high glucose and insulin levels | 290–293 |
| Monogenic | Myocardial disease | |
| JAX stock #000642 | ||
| C57BL/6J diet-induced obese | Less obesity than genetic models and moderate increase in glucose and insulin | 294–299 |
| JAX stock # 380050 | Hypertension Endothelial dysfunction |
|
| Rat models | ||
| Zucker diabetic fatty (ZDF) | Obese, high glucose and insulin levels | 300–305 |
| Monogenic | Hydronephrosis and hypertension | |
| Otsuka Long-Evans Tokushima fatty | Late onset obesity and increases in glucose and insulin levels (moderate range) | 303,304 |
| Polygenic | ||
| Zucker diabetic Sprague-Dawley (ZDSD/Pco) | Obesity and early onset high glucose and insulin | 305,306 |
| Polygenic | Osteoporosis and nephropathy | 307–309 |
| Goto-Kakizaki | Not obese | |
| Polygenic | High glucose and insulin | |
| High-fat diet-low dose streptozotocin (STZ)-treated | Obesity, high glucose and insulin Retinopathy Renal dysfunction Cardiomyopathy |
310–312 |
Cutaneous afferents are not static, but undergo remodeling based on environmental cues. Deep wounding has been linked to active retraction of preexisting axons from the wound region in streptozotocin-induced (type 1) diabetic mice, as evidenced by reduced expression of the growth-related axon plasticity marker, GAP43.125 In contrast, superficial perturbation may increase remodeling. Hair clipping in transgenic mice with fluorescent axons led to epidermal proliferation, increases in the expression of follicular stem cell markers, and axon remodeling.126 Schwann cells in peripheral nerves also possess exceptional plasticity. Injury to peripheral nerves has been shown to activate peripheral glia by reprogramming them into “repair cells”, which prompts glial cell dedifferentiation, proliferation, and dissemination into the wound bed to promote healing.127 Better understanding of these responses is important for uncovering the role of nerve plasticity in normal and diabetic wound repair.
A COMPLEX NETWORK OF SENSORY NERVES IN MOUSE SKIN
As indicated above, our understanding of the subtypes of sensory nerves in skin is rapidly evolving through transcriptomic128–130 and proteomic big data analysis of murine (and more recently monkey and human) DRGs. More than a dozen morphologically, physiologically, and genetically distinct primary somatosensory neuron subtypes have been described based on studies that utilize single cell RNA-sequencing techniques and microarrays.128,131–134 For example, studies of the molecular properties and receptor and ion channel expression of DRG neurons have led to identification of the neuropeptide Y (NPY) receptor,135 MRGPRs,136 voltage gated Na+ (Nav) channels,137,138 transient receptor potential (TRP) channels,138–140, ATP receptors (such as purinoceptor P2 × 3 and P2 × 4),141 and tyrosine kinase receptors (TRKs)142 (Table II). The TRP family of receptor ion channels are major signal detectors and transducers in nociceptive neurons and, with Nav1.8 143 and MRGPRD,144 are thought to play a major role in transmitting the sensation of chronic skin pain.
More recently, the number of DRG neuron subtypes in mice and human has expanded into at least 14 subtypes, although the ultimate degree of heterogeneity may well be greater. These subtypes are based on coupling single cell RNA sequencing (scRNA-seq) and single-cell polymerase chain reaction (PCR) confirmation128,145 with in vivo whole-cell patch-clamp recording of randomly selected DRG neurons. Moreover, another interesting benefit of a single cell transcriptional approach is the possibility of discovering transcriptional plasticity associated with pathological states, which may increasingly guide our choice of therapeutic targets. Determining the functional consequences of different types of transcriptomal patterning is of great interest (Table V).146 The rapid progress in determining sensory neuronal subsets involved in pain promises to open the door to delineating their roles in diabetic neuropathy and wound healing. Transcriptome profiling of DRG neurons has now been performed in rodents with and without pathological conditions, such as chronic pain induced by inflammation or injury133,147,148 and diabetes.149 In rats, 66 RNA transcripts related to inflammation, hyperalgesia/analgesia, cell growth, and cell survival were differentially expressed between diabetics and controls. Diabetics showed not only an increase in pain-related genes, but in regenerative-related genes, suggesting an attempt to switch to a regenerative program.149
Table V.
Classification of sensory neurons from adult mouse DRGs based on scRNA-seq clusters129 and compared with previous marker-based classification
| Neuron cluster based on transcriptional analysis and predicted function | Markers | Subtypes | Subtype markers | Classification of DRGs based on size, myelination, and markers——-Small neurons——Large neurons |
Highly expressed TR channel types per cluster subtype | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PEPPeptidergic (myelinated+unmyelinated) | NPNonpeptidergic (unmyelinated) | TH (unmyelin-ated)C-LTMRsTh | NF(myelinated) | ||||||||
| Tac1 | Calca | IB4 | P2 × 3r | Plxnc1 | Nefh | Ldhb | |||||
| Small fiberneurons | |||||||||||
| C1 | Gal | C1–1 | Asic3high | Trpv1high | |||||||
| MHN | Adcyapl | Cldn9 | Trpm8hgh | ||||||||
| C1–2 |
Zcchcl2
Sstr2 |
Trpm3high | |||||||||
| C2 | Nppb | 02–1 | Pvalb+ | Trpv2high | |||||||
| MHN (MI, IS) | Il31ra | S100b− | |||||||||
| C2–2 |
Pva1b−
S100b+ |
Trpm3high | |||||||||
| C3 | Th | – | Trpv2high | ||||||||
| C-LTMR | Zfp521 | Trpv4high | |||||||||
| C4 | Mrgpra3 | C4–1 |
Mrgprb4−
Ptpn6− |
||||||||
| MHN (IS) | Rspo1 | C4–2 |
Mrgprb4+
Ptpn6+ |
||||||||
| C5 | Mrgprd (highly expressed) | C5–1 |
Gfra3+
Sstr2+ |
Trpm3high | |||||||
| MHN (IS) | Prkcq, Lpar3 | C5–2 |
Gfra3+
Sstr2+ |
Trpv2high | |||||||
| C6 | Mrgprd (moderately expressed) S100b | C6–1 |
Ntrk1+
Prkcqlow |
||||||||
| MHN | C6–2 |
Ntrk1
Prkcqhigh |
Trpv2high | ||||||||
| Large fiber neurons | |||||||||||
| C7 | Nxph1, Wnt7a, S100b | – | – | ||||||||
| MHN (MS) | |||||||||||
| C8 | Trappc31, Cgn11, S100b | C8–1 | Ntrk3high | Trpm8high | |||||||
| MR | Htr1dhigh | ||||||||||
| C8–2 |
Ntrk1high
Htr3ahigh |
Trpm8high | |||||||||
| C9 | Baiap2l1, Cadps2, S100b | C9–1 |
Asic3−
Cgnl1low |
||||||||
| MN | C9–2 |
Asic3+
Cgnl1high |
|||||||||
| C10 | Gal, | – | – | Trpv2high | |||||||
| MR or P | Rspo1 | – | – |
Trpv1high
Trpa1high Trpm3high |
|||||||
Gray-filled squares indicate highly expressed markers per cluster.
Abbreviations: Adcyap1, pituitary adenylate cyclase-activating polypeptide 1; Asic3, acid sensing ion channel subunit 3; Baiap2l1, brain-specific angiogenesis inhibitor 1-associated protein 2-like protein 1; Cadps2, calcium-dependent secretion activator 2; Calca, calcitonin-related polypeptide-α; Cgnl1, cingulin-like 1; C-LTMR, C fiber low-threshold mechanoreceptor; Cldn9, claudin 9; Gal, galanin; Gfra3, GDNF family receptor-α3; Htr1d, 5-hydroxytryptamine receptor 1D; Il31ra, interleukin-31 receptor A; IS, itch-sensitive; Ldhb, lactate dehydrogenase B; Lpar3, lysophosphatidic acid receptor 3; MHN, mechanoheat nociceptor; MI, mechanically insensitive; MN, mechanical nociceptor; MR, mechanoreceptor; Mrgpra3, Mas-related G-protein coupled receptor member A3; Mrgprb4, Mas-related G-protein coupled receptor member B4; Mrgprd, Mas-related G-protein coupled receptor member D; MS, mechanically sensitive; Nefh, neurofilament heavy; NF, neurofilament-positive; NP, nonpeptidergic; Nppb, natriuretic peptide B; Ntrk1, neurotrophic receptor tyrosine kinase 1; Nxph1, neurexophilin 1; P, proprioceptor; Plxnc1, plexin C1; Ptpn6, protein tyrosine phosphatase non receptor type 6; Prkcq, protein kinase c theta; Pvalb, parvalbumin; P2 × 3r, purinergic receptor P2 × 3; PEP, peptidergic; Rspo1, R-spondin 1; Sstr2, somatostatin receptor 2; S100b, S100 calcium binding protein B; Tac1, tachykinin precursor 1; TH/Th, tyrosine hydroxylase; Trappc3l, trafficking protein particle complex subunit 3-like protein; Trpm, transient receptor potential melastatin; Trpv, transient receptor potential cation channel; Wnt7a, Wnt family member 7A; Zcchc12, zinc finger CCHC domain-containing protein 12; Zfp521, zinc finger protein 521.
Early studies suggest differences between human and mouse sensory neuronal subsets.
Importantly, early studies of scRNA-seq of human DRGs have shown differences in the subsets of mouse (see Table VI) vs human peripheral afferents.150 An integrative analysis with RNA-seq data of human and mouse DRGs revealed broad conservation of nociceptor-enriched genes (eg, P2XR3 [P2 × 3 receptor], SCN10A [Nav1.8], SCN11A [Nav1.9], NTRK1 [TrkA], and MRGPRD [MRGPRD]) across mouse and human DRGs,151 although the relative expression of different subsets and co-expression of markers on various subsets differed between mice and humans.150 For example, in situ hybridization by multiplex RNAscopy showed overlap of CGRP and P2 × 3R neuronal subpopulations present in human lumbar DRGs, but not in mouse DRGs.152 Differences in the mRNA expression patterns of human vs mouse DRGs for transient receptor potential channels, cholinergic receptors, potassium channels, and sodium channels was also found.152 Overall, there is only partial concordance of preclinical results related to wound healing in vitro (57%) and in small laboratory mammals (53%) with clinical results in humans.153 These differences between human and mouse DRG subsets and their function may explain the poor correlation in results of preclinical rodent vs human clinical trials in response to therapeutics for diabetic healing.
Table VI.
Spatial transcriptomics to define human DRG neuronal subtype clusters313
| Neuron dass | Aβ and Aδ fibers |
C fibers |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Subtype | C1 | C2 | C3 | C4 | C5 | C6 | C7 | C8 | C9 | C10 |
| Markers | NTRK3 HI | NTRK3 | NTRK2 HI | NTRK3 | TRPM8 | LPAR3 | PENK | TRPA1 | CHRNA3 | SCN11A |
| NTRK2 LOW | NTRK2 LOW | NTRK3 LOW | SCN10A |
TRPV1
SCN10A |
SCN11A
NPPB GFRA2 IL31RA |
|||||
| Putative Functlonal Classification | Aβ-SA LTIMR | Aβ-RA LTMR | Aδ LTMR | Putative Aβ nociceptor | Cold nociceptor | LPAR3+ nociceptor | PENK+ nociceptor | TRPA1+ nociceptor | Putative silent nociceptor | Putative itch nociceptor |
| Preferentlal upregulatlon of genes by sex | Females | Females | Females | None | Males | Females | Females | Females | Males | Males |
Abbreviations: Aβ-RA, alpha-beta rapidly adapting fibers; Aβ-SA, alpha beta sLOWly adapting fibers; Aδ LTMR, alpha delta LOW threshold mechanoreceptor; CHRNA3, cholinergic receptor nicotinic alpha 3; GFRA2, GDNF family receptor alpha 3; IL31RA, interleukin-31 receptor A; LPAR3, lysophosphatidic acid receptor 3; NPPB, natriuretic peptide B; NTRK, neurotrophic receptor tyrosine kinase; PENK, proenkephalin; SCN10A, sodium voltage-gated channel alpha subunit 10/ also NaV 1.8; SCN11A, sodium voltage-gated channel alpha subunit 11/ also NaV 1.9; TRPA1, transient receptor potential ankyrin 1; TRPM8, transient receptor potential cation channel subfamily melastatin member 8.
Given these molecular and electrophysiological differences between rodent and human DRG sensory neuron and cutaneous afferent subtypes, it will be crucial to validate the molecular mechanisms underlying impaired wound healing in diabetes and the potential therapeutic targets using human samples (eg, human 3D skin equivalent models of diabetes154 and diabetic wound biopsies). Nevertheless, transcriptional heterogeneity among neuronal clusters contributes to the functional specificity and responses to cutaneous stimuli of specific neuronal subtypes in both mice and humans. Given the diversity of neurons, elucidating key neuronal subpopulations in the context of diabetic neuropathy would advance our understanding of impaired diabetic wound healing.
BIDIRECTIONAL COMMUNICATION BETWEEN NEURONS AND OTHER CELLS IN HEALTHY AND DIABETIC SKIN
More recently, attention has focused on neuropeptides, such as CGRP and SP (one set of neurotransmitters typically released by many DRG neurons), and neuromodulators (acting on neurons) as messengers for bidirectional communication between skin cells and nerve afferents, including in studying wound repair. In skin, neuropeptides released by peripheral sensory nerves bind to receptors on a variety of skin cells, among them keratinocytes,155–157 dermal vascular endothelial cells,158,159 dermal dendritic cells,160,161 Langerhans cells,162–165 mast cells,166,167 and fibroblasts168 (Table III). Neuropeptide-specific receptor expression in both neuronal and skin cells suggest a close functional interaction between neurons and skin.169 In addition, epidermal cells (keratinocytes, Langerhans cells, and Merkel cells) can also express neuroactive molecules and participate in neurogenic inflammation.170 For example, ATP, neurotrophins, and cytokines171,172 are secreted by keratinocytes and are capable of modulating sensory neurons. Keratinocytes have been shown to communicate with nonpeptidergic (ie, MrgD+/IB4-binding C fibers) and deeper projecting peptidergic C fibers, as well as Aδ fiber nociceptors.131,132,173–175 For example, ATP release by keratinocytes activates P2 × 4 receptors on sensory neurons to relay touch perception from skin, but P2 × 4 knockdown in mice also dampens the firing rate of deeper afferents.173 Furthermore, skin cells are capable of releasing axon guidance cues through netrins and semaphorins, which may direct nerve fiber growth.176,177
NEUROPEPTIDES AS MEDIATORS IN CUTANEOUS WOUND HEALING
Although barely detectable in unstimulated skin, neuron-derived neuropeptides are significantly increased by wounding98 and direct chemical and electrical stimulation.178–182 In fact, increases in specific neuropeptides are linked temporally to the inflammation, proliferation, and migration phases of wound healing (Fig 1). Several neuropeptides, among them CGRP, SP, corticotropin releasing factor (CRF), α-melanocorticotropin releasing hormone (α-MSH), neurotensin (NT), neurokinin A (NKA), and neuropeptide Y (NPY), mediate important wound healing functions (Table III). These neuropeptides have also been shown to influence vasodilation183,184 and inflammatory responses,185 which are critical to normal healing in animal models.
Chronic nonhealing wounds and skin from subjects with diabetes and disease-associated peripheral neuropathy have increased expression and activation of neutral endopeptidase (NEP),186 a cell surface metalloprotease that degrades SP. Consistent with the observation in humans, over-expressed NEP in mutant diabetic mice diminishes the proinflammatory effects of SP that promote healing.187 Similarly, genetically modified nondiabetic mice without neuropeptide Y receptor (NPY-2Ra)188 or CGRP112 have a significant delay in cutaneous wound healing and decreased neovascularization. In diabetic mouse models, neuropeptide application to wounds, including neurotensin (NT)189 and SP,190 improves healing, variably reducing the inflammatory cell infiltrate,191 increasing angiogenesis,192 and increased fibroblast proliferation and collagen deposition.193 In nondiabetic mice, intraperitoneal injection of α-MSH194 before skin wounding antagonizes inflammation, accelerates wound healing, and improves collagen fiber organization. A more holistic investigation into which neuropeptides are predominantly impacted by diabetes (especially human) is needed.
ANIMAL MODELS OF NEUROPATHY AND DIABETIC WOUND HEALING
Rodents are commonly used models for wound healing studies, but have been criticized because of the propensity of rodent skin to heal by contraction, rather than primarily by re-epithelialization, and the marked difference in epidermal thickness (ie, mice have 2–3 layers vs the 7–10 layers of human epidermis).195,196 Nevertheless, several murine models of diabetes and associated neuropathy (Table IV) have been splinted to prevent wound contracture and encourage healing by re-epithelialization.197,198 Rodent models traditionally utilize wounds on the back rather than the typical human location on the foot, although an open full-thickness excision wound on the footpad of T2D rodents has recently been used.199 Despite their limitations, rodent models are the most feasible and cost-effective systems for studying genetic or biochemical alterations in sensory nerves or nerve subsets and their impact on wound healing in diabetes.110,200
Larger animals, however, can also serve as models of diabetes for studies with neuropeptide supplementation or tracking changes in cutaneous nerves. Rabbit ears as the site for wound experiments have the advantage being cartilaginous (naturally splinted) to limit contraction.201,202 The alloxan-induced type 1 diabetic rabbit model was used to show dysregulation in cytokine and neuropeptide gene expression in diabetic wounds.95,97 Similarly, a rabbit model of diabetic neuroischemic wound healing illustrated how a combination of a high M1/M2 ratio, failure to mount postinjury cytokine response, and diminished neuropeptide expression, contribute to wound-healing impairment in diabetes.95
Porcine skin is thought to be most similar to human skin with respect to anatomy (including neuroanatomy), immune cells, and collagen biochemistry, although the dermal microvascular density is less than in human skin.203,204 In contrast to the relatively poor concordance of rodent with human wound healing study results, pig models were 78% concordant with human studies regarding wound healing therapies.203 In the porcine neuropathic pain model, as in human neuropathic pain,205 cutaneous (especially epidermal) small caliber C and Aδ afferents are decreased, keratinocyte expression of Nav1.7, the endothelin A receptor, and CGRP are increased (all expected to lead to nociceptor excitatory algesia), and expression of the keratinocyte endothelin B receptor, which mediates inhibitory analgesic mechanisms, is decreased.205
The Ossabaw pig is a relatively new model of T2D206 and has been used to study wound healing impairment. Ossabaw swine are obesity-prone. When fed a high-fat diet, they develop at least 5 of the 6 criteria of the metabolic syndrome, including primary insulin resistance, obesity with significant visceral adipose expansion, hypertriglyceridemia and increased LDL: HDL cholesterol, mild hypertension, and coronary artery disease.206,207 Wounds in high fat diet Ossabaw pigs have exaggerated and persistent inflammation, lower abundance of endothelial cells in the granulation tissue (impaired vascularization), reduced fibroblast markers, and disorganized granulation tissue.208 Ocular neuronal and vascular alterations in the early time course of diabetic retinopathy pathogenesis were observed by electron microscopy in young Ossabaw pigs,209 suggesting that the diabetic Ossabaw pig model may be used for examining neurologic influences and treatment responses in diabetic wound healing that could more easily translate to humans with diabetes and chronic wounds.
IN VITRO MODELS OF WOUND HEALING
As an additional means to consider the impact of diabetic conditions in human models, researchers have studied cocultures of human skin cells and/or neurons210 to mimic in vivo conditions.211–214 Primary human keratinocytes cocultured with rat cutaneous primary afferent DRGs have up-regulated NGF production, and show both directed neurite outgrowth and enhanced keratinocyte proliferation, further emphasizing the dynamic interaction of sensory neurons and keratinocytes.215 A 3D coculture system of injured human skin explants with either rat sensory neurons or neuropeptides enabled the study of sensory neuron and neuropeptide influences on wound healing processes. The cocultures with rat sensory neurons promoted keratinocyte and fibroblast proliferation, stimulated collagen expression, and increased the enzymatic activity of matrix metalloproteins; addition of the neuropeptides led to human dermal fibroblasts proliferation, adherence, differentiation into myofibroblast, and increased matrix metalloprotein enzymatic activities in the early phases of wound healing.99 The quality of most 3D models is compromised, however, by having nonhuman components, with all-human cell models preferred.
Primary human dorsal root ganglia (DRG) from cadavers216 or sensory neurons from induced pluripotent stem cells217 have also been cultured with cutaneous immune cells, keratinocytes, or fibroblasts.218–220 A coculture model of human keratinocytes and neural crest cell-derived sensory neurons demonstrated functional cross-talk between cell types through Ca2+ imaging experiments.221,222 A tissue-engineered skin model of peripheral nerve regeneration by incorporating collagen sponge populated with human endothelial cells and/or human fibroblasts was used to assess the influence of endothelial and epidermal cells on neurite growth. Spontaneous formation of numerous thick myelin sheaths surrounding motor fibers after long-–term culture was observed.223
In vitro human 3-dimensional (3D) tissue models (human skin equivalents) have also been engineered to resemble normal human skin in their morphology, proliferation, differentiation, and transcriptional patterns and responses.224 These models have been adapted to study the keratinocyte-fibroblast interactions in diabetes during wounding using 3D models with human diabetic foot ulcer fibroblasts embedded into the bed underlying normal keratinocytes.154 Healing is delayed, with reduced keratinocyte migration to re-epithelialize the wound and impaired extracellular matrix deposition compared to 3D cultures with foot fibroblasts from healthy controls.154,225 Incorporating neurons (and vasculature) into this model could be useful in understanding the influence of nerves in diabetic healing. Indeed, a tissue-engineered wound healing model made of: i) a perforated epidermal compartment with green fluorescent human keratinocytes; ii) a dermal compartment; and iii) sensory neurons demonstrated the impact of sensory neurons on wound closure via secretion of neuropeptide SP.96 Microfluidic cell culture systems226,227 also provide a platform for probing functional properties of neurons and investigating neuronal-non-neuronal cell crosstalk.
FUTURE THERAPEUTIC PERSPECTIVES
Beyond cell cultures and 3D skin equivalents, the development of 3D printed skin equivalents offers the ability to incorporate skin features that traditional cell cultures lack, such as blood vessels and glands. A recently developed vascularized 3D printed skin model composed of epidermis, dermis, and hypodermis reflected the complexity of the human skin, including epidermal stemness and stratification.228 Similarly, fabrication of synthetic biocompatible vascular networks in combination with electrospinning and 3D printing techniques enabled the study of cutaneous angiogenesis in a more physiologically relevant environment229 with endothelial cell migration and tube formation in vitro. The development of more dynamic in vitro approaches through tissue engineering allows closer modeling of native human cell behaviors and may be a potential avenue for human neuronal investigations.
CONCLUSIONS
Current treatment options for individuals with diabetic foot ulcerations are limited, resulting in amputations and a large unmet need for improved management, ideally based on understanding disease pathogenesis. Much of the basic research that addresses nerves in wound healing is associative, but nevertheless supports the notion that cutaneous sensory innervation, local neuropeptide release, and other mediating factors affect healing, including in diabetic models.
At this time, therapies that can reduce the pain of diabetic neuropathy, such as gabapentin, pregabalin, duloxetine, and amitriptyline, do not reverse the neuropathy itself and have not been noted to ameliorate the wound healing issues.230 Better understanding of the specific roles of nerve subtypes within DRGs in wound healing will be critical and may well suggest novel therapeutic targets. While not without their limitations, emerging in vivo and in vitro large animal and human models provide an opportunity to further investigate the molecular and cellular features of wound repair and advance our understanding of neural involvement in wound healing pathology. Translation of these observations related to reversal of the neuropathy and better healing in animal models could lead to a disease-modifying approach.
ACKNOWLEDGMENTS
Conflicts of Interest: The authors have read the journal’s authorship agreement and policy on disclosure of potential conflicts of interest. The authors declare no conflict of interest.
Writing was not supported by a funding source.
The manuscript has been reviewed by and approved by all named authors.
Drs Paller, Menichella, and Miller acknowledge salary support from NIAMS/NIH grant R01AR077691 for research related to sensory innervation in diabetic wounds.
Abbreviations:
- α-MSH
alpha-melanocorticotropin releasing hormone
- CGRP
calcitonin gene–related peptide
- C-LTMR
C fiber low-threshold mechanoreceptor
- CRF
corticotropin releasing factor
- DRG
dorsal root ganglia
- EGF
epidermal growth factor
- GM3
monosialo-dihexosylganglioside
- IFN-γ
interferon-gamma
- IGF-1
insulin-like growth factor-1
- IL
interleukin
- MMPs
matrix metalloproteinases
- MRGPRs
Mas-related G-protein coupled receptors
- NGF
nerve growth factor
- NPY
neuropeptide Y
- NT
neurotensin
- SP
substance P
- TNF-α
tumor necrosis factor-α
- TRKs
tyrosine kinase receptors
- TRP
transient receptor potential
- T2D
type 2 diabetes
- VEGF
vascular endothelial growth factor
REFERENCES
- 1.American Diabetes Association. Economic costs of diabetes in the U.S. in 2017. Diabetes Care 2018;41:917–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.CDC. Estimates of diabetes and its burden in the United States. National diabetes statistics report, 2020. Atlanta, GA: Centers for Disease Control and Prevention, U.S. Dept of Health and Human Services; 2020. [Google Scholar]
- 3.Ogurtsova K, da Rocha Fernandes JD, Huang Y, et al. IDF Diabetes Atlas: global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract 2017;128:40–50. [DOI] [PubMed] [Google Scholar]
- 4.Manson JE, Ajani UA, Liu S, Nathan DM, Hennekens CH. A prospective study of cigarette smoking and the incidence of diabetes mellitus among US male physicians. Am J Med 2000;109:538–42. [DOI] [PubMed] [Google Scholar]
- 5.Zimmet P, Alberti KG, Shaw J. Global and societal implications of the diabetes epidemic. Nature 2001;414:782–7. [DOI] [PubMed] [Google Scholar]
- 6.Danaei G, Finucane MM, Lu Y, et al. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2.7 million participants. Lancet 2011;378:31–40. [DOI] [PubMed] [Google Scholar]
- 7.Armstrong DG, Boulton AJM, Bus SA. Diabetic foot ulcers and their recurrence. N Engl J Med 2017;376:2367–75. [DOI] [PubMed] [Google Scholar]
- 8.Geiss LS, Li Y, Hora I, Albright A, Rolka D, Gregg EW. Resurgence of diabetes-related nontraumatic lower-extremity amputation in the young and middle-aged adult U.S. population. DiabCare 2019;42:50–4. [DOI] [PubMed] [Google Scholar]
- 9.Menke A, Casagrande S, Geiss L, Cowie CC. Prevalence of and trends in diabetes among adults in the United States, 19882012. JAMA 2015;314:1021–9. [DOI] [PubMed] [Google Scholar]
- 10.Feldman EL, Nave KA, Jensen TS, Bennett DLH. New horizons in diabetic neuropathy: mechanisms, bioenergetics, and pain. Neuron 2017;93:1296–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weisel JW, Litvinov RI. Fibrin formation, structure and properties. Subcell Biochem 2017;82:405–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Broszczak DA, Sydes ER, Wallace D, Parker TJ. Molecular aspects of wound healing and the rise of venous leg ulceration: Omics approaches to enhance knowledge and aid diagnostic discovery. Clin Biochem Rev 2017;38:35–55. [PMC free article] [PubMed] [Google Scholar]
- 13.Serra MB, Barroso WA, da Silva NN, et al. From inflammation to current and alternative therapies involved in wound healing. Int J Inflam 2017;2017:3406215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ishida Y, Kondo T, Takayasu T, Iwakura Y, Mukaida N. The essential involvement of cross-talk between IFN-γ and TGF-β in the skin wound-healing process. J Immunol 2004;172:1848–55. [DOI] [PubMed] [Google Scholar]
- 15.Park JE, Barbul A. Understanding the role of immune regulation in wound healing. Am J Surg 2004;187:11S–6S. [DOI] [PubMed] [Google Scholar]
- 16.Lindner D, Zietsch C, Becher PM, et al. Differential expression of matrix metalloproteases in human fibroblasts with different origins. Biochem Res Int 2012;2012:875742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Woodley DT, Wysong A, DeClerck B, Chen M, Li W. Keratinocyte migration and a hypothetical new role for extracellular heat shock protein 90 alpha in orchestrating skin wound healing. Adv Wound Care (New Rochelle) 2015;4:203–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bhushan M, Young HS, Brenchley PE, Griffiths CE. Recent advances in cutaneous angiogenesis. Br J Dermatol 2002;147:418–25. [DOI] [PubMed] [Google Scholar]
- 19.Hopkinson SB, Hamill KJ, Wu Y, Eisenberg JL, Hiroyasu S, Jones JC. Focal contact and hemidesmosomal proteins in keratinocyte migration and wound repair. Adv Wound Care (New Rochelle) 2014;3:247–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim CS, Mitchell IP, Desotell AW, Kreeger PK, Masters KS. Immobilized epidermal growth factor stimulates persistent, directed keratinocyte migration via activation of PLCgamma1. FASEB J 2016;30:2580–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Denzinger M, Link A, Kurz J, et al. Keratinocyte growth factor modified messenger RNA accelerating cell proliferation and migration of keratinocytes. Nucleic Acid Ther 2018;28:335–47. [DOI] [PubMed] [Google Scholar]
- 22.Achar RA, Silva TC, Achar E, Martines RB, Machado JL. Use of insulin-like growth factor in the healing of open wounds in diabetic and non-diabetic rats. Acta Cir Bras 2014;29:125–31. [DOI] [PubMed] [Google Scholar]
- 23.Li Y, Fan J, Chen M, Li W, Woodley DT. Transforming growth factor-alpha: a major human serum factor that promotes human keratinocyte migration. J Invest Dermatol 2006;126:2096–105. [DOI] [PubMed] [Google Scholar]
- 24.Seeger MA, Paller AS. The roles of growth factors in keratinocyte migration. Adv Wound Care (New Rochelle) 2015;4:213–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boniakowski AE, Kimball AS, Jacobs BN, Kunkel SL, Gallagher KA. Macrophage-mediated inflammation in normal and diabetic wound healing. J Immunol 2017;199:17–24. [DOI] [PubMed] [Google Scholar]
- 26.Loots MA, Lamme EN, Zeegelaar J, Mekkes JR, Bos JD, Middelkoop E. Differences in cellular infiltrate and extracellular matrix of chronic diabetic and venous ulcers versus acute wounds. J Invest Dermatol 1998;111:850–7. [DOI] [PubMed] [Google Scholar]
- 27.Fadini GP, Albiero M, Bonora BM, Avogaro A. Angiogenic abnormalities in diabetes mellitus: mechanistic and clinical aspects. J Clin Endocrinol Metab 2019;104:5431–44. [DOI] [PubMed] [Google Scholar]
- 28.Xu F, Zhang C, Graves DT. Abnormal cell responses and role of TNF-alpha in impaired diabetic wound healing. Biomed Res Int 2013;2013:754802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kheiralla ZM, Maklad SS, Ashour SM, El-Sayed Moustafa E. Association of complement C3 and interleukin-1 with foot infections in diabetic patients. Eur J Microbiol Immunol (Bp) 2012;2:220–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mirza RE, Fang MM, Ennis WJ, Koh TJ. Blocking interleukin-1beta induces a healing-associated wound macrophage phenotype and improves healing in type 2 diabetes. Diabetes 2013;62:2579–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krzyszczyk P, Schloss R, Palmer A, Berthiaume F. The role of macrophages in acute and chronic wound healing and interventions to promote pro-wound healing phenotypes. Front Physiol 2018;9:4–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vannella KM, Wynn TA. Mechanisms of organ injury and repair by macrophages. Annu Rev Physiol 2017;79:593–617. [DOI] [PubMed] [Google Scholar]
- 33.Ferrante CJ, Leibovich SJ. Regulation of macrophage polarization and wound healing. Adv Wound Care (New Rochelle) 2012;1:10–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fujisaka S, Usui I, Bukhari A, et al. Regulatory mechanisms for adipose tissue M1 and M2 macrophages in diet-induced obese mice. Diabetes 2009;58:2574–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Menegazzo L, Ciciliot S, Poncina N, et al. NETosis is induced by high glucose and associated with type 2 diabetes. Acta Diabetol 2015;52:497–503. [DOI] [PubMed] [Google Scholar]
- 36.Wong SL, Demers M, Martinod K, et al. Diabetes primes neutrophils to undergo NETosis, which impairs wound healing. NatMed 2015;21:815–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.El-Mesallamy HO, Hamdy NM, Ezzat OA, Reda AM. Levels of soluble advanced glycation end product-receptors and other soluble serum markers as indicators of diabetic neuropathy in the foot. J Investig Med 2011;59:1233–8. [DOI] [PubMed] [Google Scholar]
- 38.Tian M, Qing C, Niu Y, et al. The relationship between inflammation and impaired wound healing in a diabetic rat burn model. J Burn Care Res 2016;37:e115–24. [DOI] [PubMed] [Google Scholar]
- 39.Sekido H, Suzuki T, Jomori T, Takeuchi M, Yabe-Nishimura C, Yagihashi S. Reduced cell replication and induction of apoptosis by advanced glycation end products in rat Schwann cells. Biochem Biophys Res Commun 2004;320:241–8. [DOI] [PubMed] [Google Scholar]
- 40.Williams SK, Howarth NL, Devenny JJ, Bitensky MW. Structural and functional consequences of increased tubulin glycosylation in diabetes mellitus. Proc Natl Acad Sci U S A 1982;79:6546–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schmidt AM, Yan SD, Yan SF, Stern DM. The multiligand receptor RAGE as a progression factor amplifying immune and inflammatory responses. J Clin Invest 2001;108:949–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Byun K, Yoo Y, Son M, et al. Advanced glycation end-products produced systemically and by macrophages: a common contributor to inflammation and degenerative diseases. Pharmacol Ther 2017;177:44–55. [DOI] [PubMed] [Google Scholar]
- 43.Alikhani M, Maclellan CM, Raptis M, Vora S, Trackman PC, Graves DT. Advanced glycation end products induce apoptosis in fibroblasts through activation of ROS, MAP kinases, and the FOXO1 transcription factor. Am J Physiol Cell Physiol 2007;292:C850–6. [DOI] [PubMed] [Google Scholar]
- 44.Markuson M, Hanson D, Anderson J, et al. The relationship between hemoglobin A(1c) values and healing time for lower extremity ulcers in individuals with diabetes. Adv Skin Wound Care 2009;22:365–72. [DOI] [PubMed] [Google Scholar]
- 45.Christman AL, Selvin E, Margolis DJ, Lazarus GS, Garza LA. Hemoglobin A1c predicts healing rate in diabetic wounds. J Invest Dermatol 2011;131:2121–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dam DHM, Wang XQ, Sheu S, et al. Ganglioside GM3 mediates glucose-induced suppression of IGF-1 receptor-Rac1 activation to inhibit keratinocyte motility. J Invest Dermatol 2017;137:440–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Randeria PS, Seeger MA, Wang XQ, et al. siRNA-based spherical nucleic acids reverse impaired wound healing in diabetic mice by ganglioside GM3 synthase knockdown. Proc Natl Acad SciUSA 2015;112:5573–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang XQ, Lee S, Wilson H, et al. Ganglioside GM3 depletion reverses impaired wound healing in diabetic mice by activating IGF-1 and insulin receptors. J Invest Dermatol 2014;134:1446–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sadagurski M, Yakar S, Weingarten G, et al. Insulin-like growth factor 1 receptor signaling regulates skin development and inhibits skin keratinocyte differentiation. Mol Cell Biol 2006;26:2675–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reddy MA, Zhang E, Natarajan R. Epigenetic mechanisms in diabetic complications and metabolic memory. Diabetologia 2015;58:443–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Park LK, Maione AG, Smith A, et al. Genome-wide DNA methylation analysis identifies a metabolic memory profile in patient-derived diabetic foot ulcer fibroblasts. Epigenetics 2014;9:1339–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu YF, Ding M, Liu DW, Liu Y, Mao YG, Peng Y. MicroRNA profiling in cutaneous wounds of diabetic rats. Genet Mol Res 2015;14:9614–25. [DOI] [PubMed] [Google Scholar]
- 53.Madhyastha R, Madhyastha H, Nakajima Y, Omura S, Maruyama M. MicroRNA signature in diabetic wound healing: promotive role of miR-21 in fibroblast migration. Int Wound J 2012;9:355–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kantharidis P, Wang B, Carew RM, Lan HY. Diabetes complications: the microRNA perspective. Diabetes 2011;60:1832–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu J, Xu Y, Shu B, et al. Quantification of the differential expression levels of microRNA-203 in different degrees of diabetic foot. Int J Clin Exp Pathol 2015;8:13416–20. [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang P, Song X, Dong Q, Zhou L, Wang L. miR-27–3p inhibition restore fibroblasts viability in diabetic wound by targeting NOVA1. Aging (Albany NY) 2020;12:12841–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang W, Yang C, Wang XY, et al. MicroRNA-129 and −335 promote diabetic wound healing by inhibiting Sp1-mediated MMP-9 expression. Diabetes 2018;67:1627–38. [DOI] [PubMed] [Google Scholar]
- 58.Liang L, Stone RC, Stojadinovic O, et al. Integrative analysis of miRNA and mRNA paired expression profiling of primary fibroblast derived from diabetic foot ulcers reveals multiple impaired cellular functions. Wound Repair Regen 2016;24:943–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bove D, Lupoli A, Caccavale S, Piccolo V, Ruocco E. Dermatological and immunological conditions due to nerve lesions. Funct Neurol 2013;28:83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Roosterman D, Goerge T, Schneider SW, Bunnett NW, Steinhoff M. Neuronal control of skin function: the skin as a neuroimmunoendocrine organ. Physiol Rev 2006;86:1309–79. [DOI] [PubMed] [Google Scholar]
- 61.Ruscheweyh R, Forsthuber L, Schoffnegger D, Sandkuhler J. Modification of classical neurochemical markers in identified primary afferent neurons with Abeta-, Adelta-, and C-fibers after chronic constriction injury in mice. J Comp Neurol 2007;502:325–36. [DOI] [PubMed] [Google Scholar]
- 62.Fang X, McMullan S, Lawson SN, Djouhri L. Electrophysiological differences between nociceptive and non-nociceptive dorsal root ganglion neurones in the rat in vivo. J Physiol 2005;565:927–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Willis WD, Coggeshall RE. Sensory receptors and peripheral nerves. Sensory mechanisms of the spinal cord: volume 1 primary afferent neurons and the spinal dorsal horn. Boston, MA: Springer US;2004:19–90. [Google Scholar]
- 64.Pinto LG, Souza GR, Kusuda R, et al. Non-peptidergic nociceptive neurons are essential for mechanical inflammatory hypersensitivity in mice. Mol Neurobiol 2019;56:5715–28. [DOI] [PubMed] [Google Scholar]
- 65.Glatte P, Buchmann SJ, Hijazi MM, Illigens BM-W, Siepmann T. Architecture of the cutaneous autonomic nervous system. Front Neurol 2019;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brumovsky PR. Dorsal root ganglion neurons and tyrosine hydroxylase—an intriguing association with implications for sensation and pain. PAIN 2016;157:314–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kumar S, Ashe HA, Parnell LN, et al. The prevalence of foot ulceration and its correlates in type 2 diabetic patients: a population-based study. Diabet Med 1994;11:480–4. [DOI] [PubMed] [Google Scholar]
- 68.Barker AR, Rosson GD, Dellon AL. Wound healing in denervated tissue. Ann Plast Surg 2006;57:339–42. [DOI] [PubMed] [Google Scholar]
- 69.Tellechea A. Inflammatory and angiogenic abnormalities in diabetic wound healing: role of neuropeptides and therapeutic perspectives. Open CircVascJ 2012;3:43–55. [Google Scholar]
- 70.Kazamel M, Dyck PJ. Sensory manifestations of diabetic neuropathies: anatomical and clinical correlations. Prosthet Orthot Int 2015;39:7–16. [DOI] [PubMed] [Google Scholar]
- 71.Levy DM, Karanth SS, Springall DR, Polak JM. Depletion of cutaneous nerves and neuropeptides in diabetes mellitus: an immunocytochemical study. Diabetologia 1989;32:427–33. [DOI] [PubMed] [Google Scholar]
- 72.Lindberger M, Schröder HD, Schultzberg M, et al. Nerve fibre studies in skin biopsies in peripheral neuropathies. I. Immunohistochemical analysis of neuropeptides in diabetes mellitus. J Neurol Sci 1989;93:289–96. [DOI] [PubMed] [Google Scholar]
- 73.Polydefkis M, Hauer P, Griffin JW, McArthur JC. Skin biopsy as a tool to assess distal small fiber innervation in diabetic neuropathy. Diabetes Technol Ther 2001;3:23–8. [DOI] [PubMed] [Google Scholar]
- 74.Griffin JW, McArthur JC, Polydefkis M. Assessment of cutaneous innervation by skin biopsies. Curr Opin Neurol 2001;14:655–9. [DOI] [PubMed] [Google Scholar]
- 75.Lauria G, Lombardi R. Skin biopsy: a new tool for diagnosing peripheral neuropathy. BMJ 2007;334:1159–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Terkelsen AJ, Karlsson P, Lauria G, Freeman R, Finnerup NB, Jensen TS. The diagnostic challenge of small fibre neuropathy: clinical presentations, evaluations, and causes. Lancet Neurol 2017;16:934–44. [DOI] [PubMed] [Google Scholar]
- 77.Krishnan ST, Rayman G. The LDIflare: a novel test of C-fiber function demonstrates early neuropathy in type 2 diabetes. Diabetes Care 2004;27:2930–5. [DOI] [PubMed] [Google Scholar]
- 78.Sharma S, Vas PR, Rayman G. Assessment of diabetic neuropathy using a point-of-care nerve conduction device shows significant associations with the LDIFLARE method and clinical neuropathy scoring. J Diabetes Sci Technol 2015;9:123–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Green AQ, Krishnan S, Finucane FM, Rayman G. Altered C-fiber function as an indicator of early peripheral neuropathy in individuals with impaired glucose tolerance. Diabetes Care 2010;33:174–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dupuis JE, Li J, Callaghan BC, Reynolds EL, London ZN. Bilateral nerve conduction studies in the evaluation of distal symmetric polyneuropathy. Muscle Nerve 2019;60:305–7. [DOI] [PubMed] [Google Scholar]
- 81.Cabre JJ, Mur T, Costa B, et al. Feasibility and effectiveness of electrochemical dermal conductance measurement for the screening of diabetic neuropathy in primary care. DECODING Study (Dermal Electrochemical Conductance In Diabetic Neuropathy). J Clin Med 2019;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Krishnan ST, Quattrini C, Jeziorska M, Malik RA, Rayman G. Neurovascular factors in wound healing in the foot skin of type 2 diabetic subjects. Diabetes Care 2007;30:3058–62. [DOI] [PubMed] [Google Scholar]
- 83.Hsieh ST, Chiang HY, Lin WM. Pathology of nerve terminal degeneration in the skin. J Neuropathol Exp Neurol 2000;59:297–307. [DOI] [PubMed] [Google Scholar]
- 84.Bonhof GJ, Strom A, Puttgen S, et al. Patterns of cutaneous nerve fibre loss and regeneration in type 2 diabetes with painful and painless polyneuropathy. Diabetologia 2017;60:2495–503. [DOI] [PubMed] [Google Scholar]
- 85.Luo KR, Chao CC, Chen YT, et al. Quantitation of sudomotor innervation in skin biopsies of patients with diabetic neuropathy. J Neuropathol Exp Neurol 2011;70:930–8. [DOI] [PubMed] [Google Scholar]
- 86.Luo KR, Chao CC, Hsieh PC, Lue JH, Hsieh ST. Effect of glycemic control on sudomotor denervation in type 2 diabetes. Diabetes Care 2012;35:612–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ziegler D, Papanas N, Zhivov A, et al. Early detection of nerve fiber loss by corneal confocal microscopy and skin biopsy in recently diagnosed type 2 diabetes. Diabetes 2014;63:2454–63. [DOI] [PubMed] [Google Scholar]
- 88.Pittenger GL, Ray M, Burcus NI, McNulty P, Basta B, Vinik AI. Intraepidermal nerve fibers are indicators of small-fiber neuropathy in both diabetic and nondiabetic patients. Diabetes Care 2004;27:197–9. [DOI] [PubMed] [Google Scholar]
- 89.Gibran NS, Jang YC, Isik FF, et al. Diminished neuropeptide levels contribute to the impaired cutaneous healing response associated with diabetes mellitus. J Surg Res 2002;108:122–8. [DOI] [PubMed] [Google Scholar]
- 90.Galkowska H, Olszewski WL, Wojewodzka U, Rosinski G, Karnafel W. Neurogenic factors in the impaired healing of diabetic foot ulcers. J Surg Res 2006;134:252–8. [DOI] [PubMed] [Google Scholar]
- 91.Shun CT, Chang YC, Wu HP, et al. Skin denervation in type 2 diabetes: correlations with diabetic duration and functional impairments. Brain 2004;127:1593–605. [DOI] [PubMed] [Google Scholar]
- 92.Quattrini C, Jeziorska M, Boulton AJ, Malik RA. Reduced vascular endothelial growth factor expression and intra-epidermal nerve fiber loss in human diabetic neuropathy. Diabetes Care 2008;31:140–5. [DOI] [PubMed] [Google Scholar]
- 93.Galkowska H, Olszewski WL, Wojewodzka U. Expression of natural antimicrobial peptide beta-defensin-2 and Langerhans cell accumulation in epidermis from human non-healing leg ulcers. Folia Histochem Cytobiol 2005;43:133–6. [PubMed] [Google Scholar]
- 94.Jain M, LoGerfo FW, Guthrie P, Pradhan L. Effect of hyperglycemia and neuropeptides on interleukin-8 expression and angiogenesis in dermal microvascular endothelial cells. J Vasc Surg 2011;53:1654–60, e1652. [DOI] [PubMed] [Google Scholar]
- 95.Pradhan Nabzdyk L, Kuchibhotla S, Guthrie P, et al. Expression of neuropeptides and cytokines in a rabbit model of diabetic neuroischemic wound healing. 2013;58:766–775. e712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Blais M, Mottier L, Germain MA, Bellenfant S, Cadau S, Berthod F. Sensory neurons accelerate skin reepithelialization via substance P in an innervated tissue-engineered wound healing model. Tissue Eng Part A 2014;20:2180–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pradhan L, Cai X, Wu S, et al. Gene expression of pro-inflammatory cytokines and neuropeptides in diabetic wound healing. J Surg Res 2011;167:336–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Da Silva L, Carvalho E, Cruz MT. Role of neuropeptides in skin inflammation and its involvement in diabetic wound healing. Exp Opin Biol Ther 2010;10:1427–39. [DOI] [PubMed] [Google Scholar]
- 99.Chéret J, Lebonvallet N, Buhé V, Carre JL, Misery L, Le Gall-Ianotto C. Influence of sensory neuropeptides on human cutaneous wound healing process. J Dermatol Sci 2014;74:193–203. [DOI] [PubMed] [Google Scholar]
- 100.Pradhan L, Nabzdyk C, Andersen ND, Logerfo FW, Veves A. Inflammation and neuropeptides: the connection in diabetic wound healing. Exp Rev Molec Med 2009;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Scholzen T, Armstrong CA, Bunnett NW, Luger TA, Olerud JE, Ansel JC. Neuropeptides in the skin: interactions between the neuroendocrine and the skin immune systems. Exp Dermatol 1998;7:81–96. [DOI] [PubMed] [Google Scholar]
- 102.Delgado AV, McManus AT, Chambers JP. Production of tumor necrosis factor-alpha, interleukin 1-beta, interleukin 2, and interleukin 6 by rat leukocyte subpopulations after exposure to substance P. Neuropeptides 2003;37:355–61. [DOI] [PubMed] [Google Scholar]
- 103.Shi X, Wang L, Clark JD, Kingery WS. Keratinocytes express cytokines and nerve growth factor in response to neuropeptide activation of the ERK1/2 and JNK MAPK transcription pathways. Regul Pept 2013;186:92–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mehta D, Granstein RD. Immunoregulatory effects of neuropeptides on endothelial cells: relevance to dermatological disorders. Dermatology 2019;235:175–86. [DOI] [PubMed] [Google Scholar]
- 105.Bohm M, Luger T. Are melanocortin peptides future therapeutics for cutaneous wound healing? Exp Dermatol 2019;28:219–24. [DOI] [PubMed] [Google Scholar]
- 106.Ma Q, Cai JL, Pan XJ, et al. Effects of neuro-immuno-modulation on healing of wound combined with local radiation injury in rats. Chin J Traumatol 2017;20:270–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Fundin BT, Rice FL, Ernfors P. Patterned gene programs and target remodeling following axotomy at a major site for sensory innervation. J Neurobiol 2002;53:370–80. [DOI] [PubMed] [Google Scholar]
- 108.Harsum S, Clarke JD, Martin P. A reciprocal relationship between cutaneous nerves and repairing skin wounds in the developing chick embryo. Dev Biol 2001;238:27–39. [DOI] [PubMed] [Google Scholar]
- 109.Wallengren J, Chen D, Sundler F. Neuropeptide-containing C-fibres and wound healing in rat skin. Neither capsaicin nor peripheral neurotomy affect the rate of healing. Br J Dermatol 1999;140:400–8. [DOI] [PubMed] [Google Scholar]
- 110.Smith PG, Liu M. Impaired cutaneous wound healing after sensory denervation in developing rats: effects on cell proliferation and apoptosis. Cell Tissue Res 2002;307:281–91. [DOI] [PubMed] [Google Scholar]
- 111.Martínez-Martínez E, Galván-Hernández CI, Toscano-Márquez B, Gutiarréz-Ospina G. Modulatory role of sensory innervation on hair follicle stem cell progeny during wound healing of the rat skin. PLoS One 2012;7:e36421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Toda M, Suzuki T, Hosono K, et al. Roles of calcitonin gene-related peptide in facilitation of wound healing and angiogenesis. Biomed Pharmacother 2008;62:352–9. [DOI] [PubMed] [Google Scholar]
- 113.Souza BR, Cardoso JF, Amadeu TP, Desmouliere A, Costa AM. Sympathetic denervation accelerates wound contraction but delays reepithelialization in rats. Wound Repair Regen 2005;13:498–505. [DOI] [PubMed] [Google Scholar]
- 114.Zheng Z, Wan Y, Liu Y, et al. Sympathetic denervation accelerates wound contraction but inhibits reepithelialization and pericyte proliferation in diabetic mice. J Diab Res 2017;2017:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Engin C, Demirkan F, Ayhan S, Atabay K, Baran NK. Delayed effect of denervation on wound contraction in rat skin. Plast Reconstr Surg 1996;98:1063–7. [DOI] [PubMed] [Google Scholar]
- 116.Hsieh ST, Choi S, Lin WM, Chang YC, McArthur JC, Griffin JW. Epidermal denervation and its effects on keratinocytes and Langerhans cells. JNeurocytol 1996;25:513–24. [DOI] [PubMed] [Google Scholar]
- 117.Buckley G, Wong J, Metcalfe AD, Ferguson MW. Denervation affects regenerative responses in MRL/MpJ and repair in C57BL/6 ear wounds. J Anat 2012;220:3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Alapure BV, Lu Y, Peng H, Hong S. Surgical denervation of specific cutaneous nerves impedes excisional wound healing of small animal ear pinnae. Mol Neurobiol 2018;55:1236–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Shu B, Xie JL, Xu YB, et al. Effects of skin-derived precursors on wound healing of denervated skin in a nude mouse model. Int J Clin Exp Pathol 2015;8:2660–9. [PMC free article] [PubMed] [Google Scholar]
- 120.Khuong HT, Kumar R, Senjaya F, et al. Skin derived precursor Schwann cells improve behavioral recovery for acute and delayed nerve repair. Exp Neurol 2014;254:168–79. [DOI] [PubMed] [Google Scholar]
- 121.Ebenezer GJ, O’Donnell R, Hauer P, Cimino NP, McArthur JC, Polydefkis M. Impaired neurovascular repair in subjects with diabetes following experimental intracutaneous axotomy. Brain 2011;134:1853–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Schwarz D, Hidmark AS, Sturm V, et al. Characterization of experimental diabetic neuropathy using multicontrast magnetic resonance neurography at ultra high field strength. Sci Rep-Uk 2020;10:7593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Menichella DM, Jayaraj ND, Wilson HM, et al. Ganglioside GM3 synthase depletion reverses neuropathic pain and small fiber neuropathy in diet-induced diabetic mice. Mol Pain 2016;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Jayaraj ND, Bhattacharyya BJ, Belmadani AA, et al. Reducing CXCR4-mediated nociceptor hyperexcitability reverses painful diabetic neuropathy. J Clin Invest 2018;128:2205–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Cheng C, Singh V, Krishnan A, Kan M, Martinez JA, Zochodne DW. Loss of innervation and axon plasticity accompanies impaired diabetic wound healing. PLoS One 2013;8: e75877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Cheng C, Guo GF, Martinez JA, Singh V, Zochodne DW. Dynamic plasticity of axons within a cutaneous milieu. J Neurosci 2010;30:14735–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Parfejevs V, Debbache J, Shakhova O, et al. Injury-activated glial cells promote wound healing of the adult skin in mice. Nat Commun 2018;9:236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Usoskin D, Furlan A, Islam S, et al. Unbiased classification of sensory neuron types by large-scale single-cell RNA sequencing. Nat Neurosci 2015;18:145–53. [DOI] [PubMed] [Google Scholar]
- 129.Li CL, Li KC, Wu D, et al. Somatosensory neuron types identified by high-coverage single-cell RNA-sequencing and functional heterogeneity. Cell Res 2016;26:967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gatto G, Smith KM, Ross SE, Goulding M. Neuronal diversity in the somatosensory system: bridging the gap between cell type and function. Curr Opin Neurobiol 2019;56:167–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Abraira VE, Ginty DD. The sensory neurons of touch. Neuron 2013;79:618–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell 2009;139:267–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Xiao HS, Huang QH, Zhang FX, et al. Identification of gene expression profile of dorsal root ganglion in the rat peripheral axotomy model of neuropathic pain. Proc Natl Acad Sci U S A 2002;99:8360–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Manteniotis S, Lehmann R, Flegel C, et al. Comprehensive RNA-Seq expression analysis of sensory ganglia with a focus on ion channels and GPCRs in Trigeminal ganglia. PLoS One 2013;8:e79523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhang X, Shi T, Holmberg K, et al. Expression and regulation of the neuropeptide Y Y2 receptor in sensory and autonomic ganglia. Proc Natl Acad Sci U S A 1997;94:729–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Liu Y, Yang FC, Okuda T, et al. Mechanisms of compartmentalized expression of Mrg class G-protein-coupled sensory receptors. J Neurosci 2008;28:125–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Baker MD, Wood JN. Involvement of Na+ channels in pain pathways. Trends Pharmacol Sci 2001;22:27–31. [DOI] [PubMed] [Google Scholar]
- 138.Thakur M, Crow M, Richards N, et al. Defining the nociceptor transcriptome. Front Mol Neurosci 2014;7:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 1997;389:816–24. [DOI] [PubMed] [Google Scholar]
- 140.Dhaka A, Viswanath V, Patapoutian A. Trp ion channels and temperature sensation. Annu Rev Neurosci 2006;29:135–61. [DOI] [PubMed] [Google Scholar]
- 141.Kobayashi K, Yamanaka H, Noguchi K. Expression of ATP receptors in the rat dorsal root ganglion and spinal cord. Anat Sci Int 2013;88:10–6. [DOI] [PubMed] [Google Scholar]
- 142.Kashiba H, Uchida Y, Senba E. Distribution and colocalization of NGF and GDNF family ligand receptor mRNAs in dorsal root and nodose ganglion neurons of adult rats. Brain Res Mol Brain Res 2003;110:52–62. [DOI] [PubMed] [Google Scholar]
- 143.Ramachandra R, McGrew SY, Baxter JC, Howard JR, Elmslie KS. NaV1.8 channels are expressed in large, as well as small, diameter sensory afferent neurons. Channels (Austin) 2013;7:34–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wang H, Zylka MJ. Mrgprd-expressing polymodal nociceptive neurons innervate most known classes of substantia gelatinosa neurons. J Neurosci 2009;29:13202–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Chiu IM, Barrett LB, Williams EK, et al. Correction: transcriptional profiling at whole population and single cell levels reveals somatosensory neuron molecular diversity. Elife 2015;4:e06720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Li CL, Li KC, Wu D, et al. Somatosensory neuron types identified by high-coverage single-cell RNA-sequencing and functional heterogeneity. Cell Res 2016;26:83–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Renthal W, Tochitsky I, Yang L, et al. Transcriptional reprogramming of distinct peripheral sensory neuron subtypes after axonal injury. Neuron 2020;108:128–44, e129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Chandran V, Coppola G, Nawabi H, et al. A systems-level analysis of the peripheral nerve intrinsic axonal growth program. Neuron 2016;89:956–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Athie MCP, Vieira AS, Teixeira JM, et al. Transcriptome analysis of dorsal root ganglia’s diabetic neuropathy reveals mechanisms involved in pain and regeneration. Life Sci 2018;205:54–62. [DOI] [PubMed] [Google Scholar]
- 150.Ray P, Torck A, Quigley L, et al. Comparative transcriptome profiling of the human and mouse dorsal root ganglia: an RNA-seq-based resource for pain and sensory neuroscience research. Pain 2018;159:1325–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Price T, Torck A, Ray P, et al. RNA-seq based transcriptome profiling of human and mouse dorsal root ganglion reveals a potential role for Protease Activated Receptor 3 (PAR3) in pain processing. FASEB J 2016;30:710.716–710.716. [Google Scholar]
- 152.Shiers S, Klein RM, Price TJ. Quantitative differences in neuronal subpopulations between mouse and human dorsal root ganglia demonstrated with RNAscope in situ hybridization. Pain 2020;161:2410–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Parnell LKS, Volk SW. The evolution of animal models in wound healing research: 1993–2017. Adv Wound Care (New Rochelle) 2019;8:692–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Maione AG, Brudno Y, Stojadinovic O, et al. Three-dimensional human tissue models that incorporate diabetic foot ulcer-derived fibroblasts mimic in vivo features of chronic wounds. Tissue Eng Part C Methods 2015;21:499–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Hou Q, Barr T, Gee L, et al. Keratinocyte expression of calcitonin gene-related peptide beta: implications for neuropathic and inflammatory pain mechanisms. Pain 2011;152:2036–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Stander S, Moormann C, Schumacher M, et al. Expression of vanilloid receptor subtype 1 in cutaneous sensory nerve fibers, mast cells, and epithelial cells of appendage structures. Exp Dermatol 2004;13:129–39. [DOI] [PubMed] [Google Scholar]
- 157.Takahashi K, Nakanishi S, Imamura S. Direct effects of cutaneous neuropeptides on adenylyl cyclase activity and proliferation in a keratinocyte cell line: stimulation of cyclic AMP formation by CGRP and VIP/PHM, and inhibition by NPY through G protein-coupled receptors. J Invest Dermatol 1993;101:646–51. [DOI] [PubMed] [Google Scholar]
- 158.Hartmeyer M, Scholzen T, Becher E, Bhardwaj RS, Schwarz T, Luger TA. Human dermal microvascular endothelial cells express the melanocortin receptor type 1 and produce increased levels of IL-8 upon stimulation with alpha-melanocyte-stimulatinghormone. J Immunol 1997;159:1930–7. [PubMed] [Google Scholar]
- 159.Scholzen TE, Brzoska T, Kalden DH, et al. Expression of functional melanocortin receptors and proopiomelanocortin peptides by human dermal microvascular endothelial cells. Ann N Y Acad Sci 1999;885:239–53. [DOI] [PubMed] [Google Scholar]
- 160.Kashem SW, Riedl MS, Yao C, Honda CN, Vulchanova L, Kaplan DH. Nociceptive sensory fibers drive interleukin-23 production from CD301b+ dermal dendritic cells and drive protective cutaneous immunity. Immunity 2015;43:515–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Perner C, Flayer CH, Zhu X, et al. Substance P release by sensory neurons triggers dendritic cell migration and initiates the type-2 immune response to allergens. Immunity 2020;53:1063–77, e1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Torii H, Yan Z, Hosoi J, Granstein RD. Expression of neurotrophic factors and neuropeptide receptors by Langerhans cells and the Langerhans cell-like cell line XS52: further support for a functional relationship between Langerhans cells and epidermal nerves. J Invest Dermatol 1997;109:586–91. [DOI] [PubMed] [Google Scholar]
- 163.He Y, Ding G, Wang X, Zhu T, Fan S. Calcitonin gene-related peptide in Langerhans cells in psoriatic plaque lesions. Chin Med J (Engl) 2000;113:747–51. [PubMed] [Google Scholar]
- 164.Kodali S, Ding W, Huang J, Seiffert K, Wagner JA, Granstein RD. Vasoactive intestinal peptide modulates Langerhans cell immune function. J Immunol 2004;173:6082–8. [DOI] [PubMed] [Google Scholar]
- 165.Staniek V, Misery L, Peguet-Navarro J, et al. Binding and in vitro modulation of human epidermal Langerhans cell functions by substance P. Arch Dermatol Res 1997;289:285–91. [DOI] [PubMed] [Google Scholar]
- 166.Arifuzzaman M, Mobley YR, Choi HW, et al. MRGPR-mediated activation of local mast cells clears cutaneous bacterial infection and protects against reinfection. Sci Adv 2019;5: eaav0216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Green DP, Limjunyawong N, Gour N, Pundir P, Dong X. A mast-cell-specific receptor mediates neurogenic inflammation and pain. Neuron 2019;101:412–20, e413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Hochman B, Tucci-Viegas VM, Monteiro PKP, Franc a JP, Gaiba S, Ferreira LM. The action of CGRP and SP on cultured skin fibroblasts. Central Eur J Biol 2014;9:717–26. [Google Scholar]
- 169.Choi Y-H, Moh SH, Kim KW. The role of neuropeptides in skin wound healing. In: Farage MA, Miller KW, Maibach HI, eds. Textbook of aging skin, Berlin, Heidelberg: Springer, 2017:1937–48. [Google Scholar]
- 170.Shipton EA. Skin matters: identifying pain mechanisms and predicting treatment outcomes. Neurol Res Int 2013;2013:329364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Olah A, Szollosi AG, Biro T. The channel physiology of the skin. Rev Physiol Biochem Pharmacol 2012;163:65–131. [DOI] [PubMed] [Google Scholar]
- 172.Hanel KH, Cornelissen C, Luscher B, Baron JM. Cytokines and the skin barrier. IntJMol Sci 2013;14:6720–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Moehring F, Cowie AM, Menzel AD, et al. Keratinocytes mediate innocuous and noxious touch via ATP-P2X4 signaling. Elife 2018;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Zylka MJ, Rice FL, Anderson DJ. Topographically distinct epidermal nociceptive circuits revealed by axonal tracers targeted to Mrgprd. Neuron 2005;45:17–25. [DOI] [PubMed] [Google Scholar]
- 175.Le Pichon CE, Chesler AT. The functional and anatomical dissection of somatosensory subpopulations using mouse genetics. Front Neuroanat 2014;8:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Tominaga M, Ogawa H, Takamori K. Decreased production of semaphorin 3A in the lesional skin of atopic dermatitis. Br J Dermatol 2008;158:842–4. [DOI] [PubMed] [Google Scholar]
- 177.Botchkarev VA, Yaar M, Peters EM, et al. Neurotrophins in skin biology and pathology. J Invest Dermatol 2006;126:1719–27. [DOI] [PubMed] [Google Scholar]
- 178.Croom JE, Foreman RD, Chandler MJ, Barron KW. Cutaneous vasodilation during dorsal column stimulation is mediated by dorsal roots and CGRP. Am J Physiol 1997;272:H950–7. [DOI] [PubMed] [Google Scholar]
- 179.Wu M, Komori N, Qin C, Farber JP, Linderoth B, Foreman RD. Roles of peripheral terminals of transient receptor potential vanilloid-1 containing sensory fibers in spinal cord stimulation-induced peripheral vasodilation. Brain Res 2007;1156:80–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Sauerstein K, Klede M, Hilliges M, Schmelz M. Electrically evoked neuropeptide release and neurogenic inflammation differ between rat and human skin. J Physiol 2000;529(Pt 3):803–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.De Col R, Messlinger K, Hoffmann T. Differential conduction and CGRP release in visceral versus cutaneous peripheral nerves in the mouse. J Neurosci Res 2018;96:1398–405. [DOI] [PubMed] [Google Scholar]
- 182.Emmerson E Efficient healing takes some nerve: electrical stimulation enhances innervation in cutaneous human wounds. J Invest Dermatol 2017;137:543–5. [DOI] [PubMed] [Google Scholar]
- 183.Delay-Goyet P, Satoh H, Lundberg JM. Relative involvement of substance P and CGRP mechanisms in antidromic vasodilation in the rat skin. Acta Physiol Scand 1992;146:537–8. [DOI] [PubMed] [Google Scholar]
- 184.Hu CL, Xiang JZ, Hu FF. Vanilloid receptor TRPV1, sensory C-fibers, and activation of adventitial mast cells. A novel mechanism involved in adventitial inflammation. Med Hypotheses 2008;71:102–3. [DOI] [PubMed] [Google Scholar]
- 185.Carr RW, Delaney CA, Westerman RA, Roberts RG. Denervation impairs cutaneous microvascular function and blister healing in the rat hindlimb. Neuroreport 1993;4:467–70. [DOI] [PubMed] [Google Scholar]
- 186.Antezana M, Sullivan SR, Usui M, et al. Neutral endopeptidase activity is increased in the skin of subjects with diabetic ulcers. J Invest Dermatol 2002;119:1400–4. [DOI] [PubMed] [Google Scholar]
- 187.Spenny ML, Muangman P, Sullivan SR, et al. Neutral endopeptidase inhibition in diabetic wound repair. Wound Repair Regen 2002;10:295–301. [DOI] [PubMed] [Google Scholar]
- 188.Ekstrand AJ, Cao R, Bjorndahl M, et al. Deletion of neuropeptide Y (NPY) 2 receptor in mice results in blockage of NPY-induced angiogenesis and delayed wound healing. Proc Natl Acad Sci USA 2003;100:6033–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Moura LI, Dias AM, Suesca E, et al. Neurotensin-loaded collagen dressings reduce inflammation and improve wound healing in diabetic mice. Biochim Biophys Acta 2014;1842:32–43. [DOI] [PubMed] [Google Scholar]
- 190.Um J,Yu J, Park KS. Substance P accelerates wound healing in type 2 diabetic mice through endothelial progenitor cell mobilization and Yes-associated protein activation. Mol Med Rep 2017;15:3035–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Park JH, Kim S, Hong HS, Son Y. Substance P promotes diabetic wound healing by modulating inflammation and restoring cellular activity of mesenchymal stem cells. Wound Repair Regen 2016;24:337–48. [DOI] [PubMed] [Google Scholar]
- 192.Kohara H, Tajima S, Yamamoto M, Tabata Y. Angiogenesis induced by controlled release of neuropeptide substance P. Biomaterials 2010;31:8617–25. [DOI] [PubMed] [Google Scholar]
- 193.Kant V, Kumar D, Kumar D, et al. Topical application of substance P promotes wound healing in streptozotocin-induced diabetic rats. Cytokine 2015;73:144–55. [DOI] [PubMed] [Google Scholar]
- 194.de Souza KS, Cantaruti TA, Azevedo GM Jr, et al. Improved cutaneous wound healing after intraperitoneal injection of alpha-melanocyte-stimulating hormone. Exp Dermatol 2015;24:198–203. [DOI] [PubMed] [Google Scholar]
- 195.Phillips KG, Samatham R, Choudhury N, Gladish JC, Thuillier P, Jacques SL. In vivo measurement of epidermal thickness changes associated with tumor promotion in murine models. J Biomed Opt 2010;15:041514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Gudjonsson JE, Johnston A, Dyson M, Valdimarsson H, Elder JT. Mouse models of psoriasis. J Invest Dermatol 2007;127:1292–308. [DOI] [PubMed] [Google Scholar]
- 197.Buck DW 2nd, Jin DP, Geringer M, Hong SJ, Galiano RD, Mustoe TA. The TallyHo polygenic mouse model of diabetes: implications in wound healing. Plast Reconstr Surg 2011;128:427e–37e. [DOI] [PubMed] [Google Scholar]
- 198.Michaels Jt, Churgin SS Blechman KM, et al. db/db mice exhibit severe wound-healing impairments compared with other murine diabetic strains in a silicone-splinted excisional wound model. Wound Repair Regen 2007;15:665–70. [DOI] [PubMed] [Google Scholar]
- 199.Yu CO, Leung KS, Fung KP, et al. The characterization of a full-thickness excision open foot wound model in n5-streptozotocin (STZ)-induced type 2 diabetic rats that mimics diabetic foot ulcer in terms of reduced blood circulation, higher C-reactive protein, elevated inflammation, and reduced cell proliferation. Exp Anim 2017;66:259–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Younan G, Ogawa R, Ramirez M, Helm D, Dastouri P, Orgill DP. Analysis of nerve and neuropeptide patterns in vacuum-assisted closure-treated diabetic murine wounds. Plast Reconstr Surg 2010;126:87–96. [DOI] [PubMed] [Google Scholar]
- 201.Breen A, Mc Redmond G, Dockery P, O’Brien T, Pandit A. Assessment of wound healing in the alloxan-induced diabetic rabbit ear model. J Invest Surg 2008;21:261–9. [DOI] [PubMed] [Google Scholar]
- 202.O’Loughlin A, Kulkarni M, Vaughan EE, et al. Autologous circulating angiogenic cells treated with osteopontin and delivered via a collagen scaffold enhance wound healing in the alloxan-induced diabetic rabbit ear ulcer model. Stem Cell Res Ther 2013;4:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Sullivan TP, Eaglstein WH, Davis SC, Mertz P. The pig as a model for human wound healing. Wound Repair Regen 2001;9:66–76. [DOI] [PubMed] [Google Scholar]
- 204.Zilic L, Garner PE, Yu T, Roman S, Haycock JW, Wilshaw SP. An anatomical study of porcine peripheral nerve and its potential use in nerve tissue engineering. J Anat 2015;227:302–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Rice FL, Castel D, Ruggiero E, et al. Human-like cutaneous neuropathologies associated with a porcine model of peripheral neuritis: a translational platform for neuropathic pain. Neurobiol Pain 2019;5:100021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Brown C, Byrd JP, Eggenberger C, et al. Wound healing in diabetic Ossabaw pigs. 2018. [Google Scholar]
- 207.Spurlock ME, Gabler NK. The development of porcine models of obesity and the metabolic syndrome. J Nutr 2008;138:397–402. [DOI] [PubMed] [Google Scholar]
- 208.Sinha M, Ghosh N, Steiner S, et al. Ossabaw pigs as a natural preclinical model of metabolic syndrome mediated impaired wound healing. Wound Rep Regen 2019:A8., [abstr]. [Google Scholar]
- 209.Lim RR, Grant DG, Olver TD, et al. Young Ossabaw pigs fed a western diet exhibit early signs of diabetic retinopathy. Invest Ophthalmol Vis Sci 2018;59:2325–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Le Gall-Ianotto C, Andres E, Hurtado SP, Pereira U, Misery L. Characterization of the first coculture between human primary keratinocytes and the dorsal root ganglion-derived neuronal cell line F-11. Neuroscience 2012;210:47–57. [DOI] [PubMed] [Google Scholar]
- 211.Biedermann T, Bottcher-Haberzeth S, Klar AS, et al. Rebuild, restore, reinnervate: do human tissue engineered dermo-epidermal skin analogs attract host nerve fibers for innervation? Pediatr Surg Int 2013;29:71–8. [DOI] [PubMed] [Google Scholar]
- 212.Lebonvallet N, Boulais N, Le Gall C, et al. Effects of the reinnervation of organotypic skin explants on the epidermis. Exp Dermatol 2012;21:156–8. [DOI] [PubMed] [Google Scholar]
- 213.Sevrain D, Le Grand Y, Buhe V, et al. Two-photon microscopy of dermal innervation in a human re-innervated model of skin. Exp Dermatol 2013;22:290–1. [DOI] [PubMed] [Google Scholar]
- 214.Lebonvallet N, Pennec JP, Le Gall-Ianotto C, et al. Activation of primary sensory neurons by the topical application of capsaicin on the epidermis of a re-innervated organotypic human skin model. Exp Dermatol 2014;23:73–5. [DOI] [PubMed] [Google Scholar]
- 215.Radtke C, Rennekampff HO, Reimers K, Vogt PM, Kocsis JD. Paracrine loop of keratinocyte proliferation and directed neuritic outgrowth in a neuroepithelial coculture. Ann Plast Surg 2013;70:162–7. [DOI] [PubMed] [Google Scholar]
- 216.Valtcheva MV, Copits BA, Davidson S, et al. Surgical extraction of human dorsal root ganglia from organ donors and preparation of primary sensory neuron cultures. Nat Protoc 2016;11:1877–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Gu Y, Xue C, Zhu J, et al. Basic fibroblast growth factor (bFGF) facilitates differentiation of adult dorsal root ganglia-derived neural stem cells toward Schwann cells by binding to FGFR-1 through MAPK/ERK activation. J Mol Neurosci 2014;52:538–51. [DOI] [PubMed] [Google Scholar]
- 218.Chateau Y, Dorange G, Clement JF, et al. In vitro reconstruction of neuro-epidermal connections. J Invest Dermatol 2007;127:979–81. [DOI] [PubMed] [Google Scholar]
- 219.Pereira U, Boulais N, Lebonvallet N, Lefeuvre L, Gougerot A, Misery L. Development of an in vitro coculture of primary sensitive pig neurons and keratinocytes for the study of cutaneous neurogenic inflammation. Exp Dermatol 2010;19:931–5. [DOI] [PubMed] [Google Scholar]
- 220.Roggenkamp D, Kopnick S, Stab F, Wenck H, Schmelz M, Neufang G. Epidermal nerve fibers modulate keratinocyte growth via neuropeptide signaling in an innervated skin model. J Invest Dermatol 2013;133:1620–8. [DOI] [PubMed] [Google Scholar]
- 221.Krishnan-Kutty V, Bigliardi PL, Dykas MM, et al. Peripheral nerve fibres form multifacet interactions with keratinocytes in a novel complete human 2D culture model. Exp Dermatol 2017;26:281–4. [DOI] [PubMed] [Google Scholar]
- 222.Sondersorg AC, Busse D, Kyereme J, et al. Chemosensory information processing between keratinocytes and trigeminal neurons. J Biol Chem 2014;289:17529–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Gingras M, Beaulieu MM, Gagnon V, Durham HD, Berthod F. In vitro study of axonal migration and myelination of motor neurons in a three-dimensional tissue-engineered model. Glia 2008;56:354–64. [DOI] [PubMed] [Google Scholar]
- 224.Carlson MW, Alt-Holland A, Egles C, Garlick JA. Three-dimensional tissue models of normal and diseased skin. Curr Protoc Cell Biol 2008:Chapter 19:Unit19 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Egles C, Garlick JA, Shamis Y. Three-dimensional human tissue models of wounded skin. Methods Mol Biol 2010;585:345–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Karimi M, Bahrami S, Mirshekari H, et al. Microfluidic systems for stem cell-based neural tissue engineering. Lab Chip 2016;16:2551–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Tsantoulas C, Farmer C, Machado P, Baba K, McMahon SB, Raouf R. Probing functional properties of nociceptive axons using a microfluidic culture system. PLoS One 2013;8:e80722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Kim BS, Gao G, Kim JY, Cho DW. 3D cell printing of perfusable vascularized human skin equivalent composed of epidermis, dermis, and hypodermis for better structural recapitulation of native skin. Adv Healthc Mater 2019;8:e1801019. [DOI] [PubMed] [Google Scholar]
- 229.Dikici S, Claeyssens F, MacNeil S. Bioengineering vascular networks to study angiogenesis and vascularization of physiologically relevant tissue models in vitro. ACS Biomater Sci Eng 2020;6:3513–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Finnerup NB, Attal N, Haroutounian S, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol 2015;14:162–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Khanna S, Biswas S, Shang Y, et al. Macrophage dysfunction impairs resolution of inflammation in the wounds of diabetic mice. PLoS One 2010;5:e9539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Lerman OZ, Galiano RD, Armour M, Levine JP, Gurtner GC. Cellular dysfunction in the diabetic fibroblast: impairment in migration, vascular endothelial growth factor production, and response to hypoxia. Am J Pathol 2003;162:303–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Liu Y, Min D, Bolton T, et al. Increased matrix metalloproteinase-9 predicts poor wound healing in diabetic foot ulcers. Diabetes care 2009;32:117–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Rodgers KE, Ellefson DD, Espinoza T, Hsu YH, diZerega GS, Mehrian-Shai R. Expression of intracellular filament, collagen, and collagenase genes in diabetic and normal skin after injury. Wound Repair Regen 2006;14:298–305. [DOI] [PubMed] [Google Scholar]
- 235.Zgheib C, Hodges M, Hu J, et al. Mechanisms of mesenchymal stem cell correction of the impaired biomechanical properties of diabetic skin: the role of miR-29a. Wound Repair Regen 2016;24:237–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Dolivo D, Xie P, Hou C, et al. Application of decellularized human reticular allograft dermal matrix promotes rapid re-epithelialization in a diabetic murine excisional wound model. Cytotherapy 2021. 10.10167/j.jcyt.2020.11.009 S1465-3249(20)30961-0Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 237.Strieder-Barboza C, Baker NA, Flesher CG, et al. Advanced glycation end-products regulate extracellular matrix-adipocyte metabolic crosstalk in diabetes. Sci Rep 2019;9:19748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Beer HD, Longaker MT, Werner S. Reduced expression of PDGF and PDGF receptors during impaired wound healing. J Invest Dermatol 1997;109:132–8. [DOI] [PubMed] [Google Scholar]
- 239.Muller M, Trocme C, Lardy B, Morel F, Halimi S, Benhamou PY. Matrix metalloproteinases and diabetic foot ulcers: the ratio of MMP-1 to TIMP-1 is a predictor of wound healing. Diabet Med 2008;25:419–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Mudge BP, Harris C, Gilmont RR, Adamson BS, Rees RS. Role of glutathione redox dysfunction in diabetic wounds. Wound Repair Regen 2002;10:52–8. [DOI] [PubMed] [Google Scholar]
- 241.Argyropoulos AJ, Robichaud P, Balimunkwe RM, et al. Alterations of dermal connective tissue collagen in diabetes: molecular basis of aged-appearing skin. PLoS One 2016;11:e0153806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Kolluru GK, Bir SC, Kevil CG. Endothelial dysfunction and diabetes: effects on angiogenesis, vascular remodeling, and wound healing. Int J Vasc Med 2012;2012:918267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Braverman IM, Sibley J, Keh A. Ultrastructural analysis of the endothelial-pericyte relationship in diabetic cutaneous vessels. J Invest Dermatol 1990;95:147–53. [DOI] [PubMed] [Google Scholar]
- 244.Spampinato SF, Caruso GI, De Pasquale R, Sortino MA, Merlo S. The treatment of impaired wound healing in diabetes: looking among old drugs. Pharmaceuticals (Basel) 2020;13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Nishikori Y, Shiota N, Okunishi H. The role of mast cells in cutaneous wound healing in streptozotocin-induced diabetic mice. Arch Dermatol Res 2014;306:823–35. [DOI] [PubMed] [Google Scholar]
- 246.Wilkinson HN, Clowes C, Banyard KL, Matteuci P, Mace KA, Hardman MJ. Elevated local senescence in diabetic wound healing is linked to pathological repair via CXCR2. J Invest Dermatol 2019;139:1171–81, e1176. [DOI] [PubMed] [Google Scholar]
- 247.Alba-Loureiro TC, Hirabara SM, Mendonca JR, Curi R, Pithon-Curi TC. Diabetes causes marked changes in function and metabolism of rat neutrophils. J Endocrinol 2006;188:295–303. [DOI] [PubMed] [Google Scholar]
- 248.Avishai E, Yeghiazaryan K, Golubnitschaja O. Impaired wound healing: facts and hypotheses for multi-professional considerations in predictive, preventive and personalised medicine. EPMA J 2017;8:23–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Burbach GJ, Kim KH, Zivony AS, et al. The neurosensory tachykinins substance P and neurokinin A directly induce keratinocyte nerve growth factor. J Invest Dermatol 2001;117:1075–82. [DOI] [PubMed] [Google Scholar]
- 250.Amadesi S, Reni C, Katare R, et al. Role for substance p-based nociceptive signaling in progenitor cell activation and angiogenesis during ischemia in mice and in human subjects. Circulation 2012;125:1774–86, s1771–1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Mouritzen MV, Abourayale S, Ejaz R, et al. Neurotensin, substance P, and insulin enhance cell migration. J Pept Sci 2018;24:e3093. [DOI] [PubMed] [Google Scholar]
- 252.Bulut K, Felderbauer P, Deters S, et al. Sensory neuropeptides and epithelial cell restitution: the relevance of SP- and CGRP-stimulated mast cells. Int J Colorectal Dis 2008;23:535–41. [DOI] [PubMed] [Google Scholar]
- 253.Dallos A, Kiss M, Polyánka H, Dobozy A, Kemény L, Husz S. Effects of the neuropeptides substance P, calcitonin gene-related peptide, vasoactive intestinal polypeptide and galanin on the production of nerve growth factor and inflammatory cytokines in cultured human keratinocytes. Neuropeptides 2006;40:251–63. [DOI] [PubMed] [Google Scholar]
- 254.Paus R, Heinzelmann T, Robicsek S, Czarnetzki BM, Maurer M. Substance P stimulates murine epidermal keratinocyte proliferation and dermal mast cell degranulation in situ. Arch Dermatol Res 1995;287:500–2. [DOI] [PubMed] [Google Scholar]
- 255.Mathers AR, Tckacheva OA, Janelsins BM, Shufesky WJ, Morelli AE, Larregina AT. In vivo signaling through the neurokinin 1 receptor favors transgene expression by Langerhans cells and promotes the generation of Th1- and Tc1-biased immune responses. J Immunol 2007;178:7006–17. [DOI] [PubMed] [Google Scholar]
- 256.Suvas S Role of substance P neuropeptide in inflammation, wound healing, and tissue homeostasis. J Immunol (Baltimore, Md: 1950) 2017;199:1543–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Leal EC, Carvalho E, Tellechea A, et al. Substance P promotes wound healing in diabetes by modulating inflammation and macrophage phenotype. Am J Pathol 2015;185:1638–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Fong G, Backman LJ, Hart DA, Danielson P, McCormack B, Scott A. Substance P enhances collagen remodeling and MMP-3 expression by human tenocytes. J Orthopaed Res 2013;31:91–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Dianzani C, Collino M, Lombardi G, Garbarino G, Fantozzi R. Substance P increases neutrophil adhesion to human umbilical vein endothelial cells. Br J Pharmacol 2003;139:1103–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Kurashige C, Hosono K, Matsuda H, Tsujikawa K, Okamoto H, Majima M. Roles of receptor activity-modifying protein 1 in angiogenesis and lymphangiogenesis during skin wound healing in mice. FASEB J 2014;28:1237–47. [DOI] [PubMed] [Google Scholar]
- 261.Ghersi G, Chen W, Lee EW, Zukowska Z. Critical role of dipeptidyl peptidase IV in neuropeptide Y-mediated endothelial cell migration in response to wounding. Peptides 2001;22:453–8. [DOI] [PubMed] [Google Scholar]
- 262.Wallengren J, Badendick K, Sundler F, Hakanson R, Zander E. Innervation of the skin of the forearm in diabetic patients: relation to nerve function. Acta Derm Venereol 1995;75:37–42. [DOI] [PubMed] [Google Scholar]
- 263.Chen W-c, Liu Y-b, Liu W-f, Zhou Y-y, He H-f, Lin S. Neuropeptide Y is an immunomodulatory factor: direct and indirect. Front Immunol 2020;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.da Silva L, Neves BM, Moura L, Cruz MT, Carvalho E. Neurotensin downregulates the pro-inflammatory properties of skin dendritic cells and increases epidermal growth factor expression. Biochim Biophys Acta 2011;1813:1863–71. [DOI] [PubMed] [Google Scholar]
- 265.Moura LI, Dias AM, Leal EC, Carvalho L, de Sousa HC, Carvalho E. Chitosan-based dressings loaded with neurotensin–an efficient strategy to improve early diabetic wound healing. Acta Biomater 2014;10:843–57. [DOI] [PubMed] [Google Scholar]
- 266.Pereira da Silva L, Miguel Neves B, Moura L, Cruz MT, Carvalho E. Neurotensin decreases the proinflammatory status of human skin fibroblasts and increases epidermal growth factor expression. Int J Inflam 2014;2014:248240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Sheppard MC, Bailey CJ, Flatt PR, Swanston-Flatt SK, Shennan KI. Immunoreactive neurotensin in spontaneous syndromes of obesity and diabetes in mice. Acta Endocrinol (Copenh) 1985;108:532–6. [DOI] [PubMed] [Google Scholar]
- 268.Feldberg RS, Cochrane DE, Carraway RE, et al. Evidence for a neurotensin receptor in rat serosal mast cells. Inflamm Res 1998;47:245–50. [DOI] [PubMed] [Google Scholar]
- 269.Moura LI, Cruz MT, Carvalho E. The effect of neurotensin in human keratinocytes—implication on impaired wound healing in diabetes. Exp Biol Med (Maywood) 2014;239:6–12. [DOI] [PubMed] [Google Scholar]
- 270.Bohm M, Schulte U, Kalden H, Luger TA. Alpha-melanocyte-stimulating hormone modulates activation of NF-kappa B and AP-1 and secretion of interleukin-8 in human dermal fibroblasts. Ann N Y Acad Sci 1999;885:277–86. [DOI] [PubMed] [Google Scholar]
- 271.Redondo P, Garcia-Foncillas J, Okroujnov I, Bandres E. Alpha-MSH regulates interleukin-10 expression by human keratinocytes. Arch Dermatol Res 1998;290:425–8. [DOI] [PubMed] [Google Scholar]
- 272.Kiss M, Wlaschek M, Brenneisen P, et al. Alpha-melanocyte stimulating hormone induces collagenase/matrix metalloproteinase-1 in human dermal fibroblasts. Biol Chem Hoppe Seyler 1995;376:425–30. [DOI] [PubMed] [Google Scholar]
- 273.Catania A, Delgado R, Airaghi L, et al. Alpha-MSH in systemic inflammation. Central and peripheral actions. Ann N Y Acad Sci 1999;885:183–7. [DOI] [PubMed] [Google Scholar]
- 274.Muffley LA, Zhu KQ, Engrav LH, Gibran NS, Hocking AM. Spatial and temporal localization of the melanocortin 1 receptor and its ligand alpha-melanocyte-stimulating hormone during cutaneous wound repair. J Histochem Cytochem 2011;59:278–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Bohm M, Luger TA, Tobin DJ, Garcia-Borron JC. Melanocortin receptor ligands: new horizons for skin biology and clinical dermatology. J Invest Dermatol 2006;126:1966–75. [DOI] [PubMed] [Google Scholar]
- 276.Singh N, Bhattacharyya D. Biochemical and functional analysis of corticotropin releasing factor purified from an aqueous extract of human placenta used as wound healer. J Pharm Biomed Anal 2017;145:298–306. [DOI] [PubMed] [Google Scholar]
- 277.Zbytek B, Mysliwski A, Slominski A, Wortsman J, Wei ET, Mysliwska J. Corticotropin-releasing hormone affects cytokine production in human HaCaT keratinocytes. Life Sci 2002;70:1013–21. [DOI] [PubMed] [Google Scholar]
- 278.Rassouli O, Liapakis G, Lazaridis I, et al. A novel role of peripheral corticotropin-releasing hormone (CRH) on dermal fibroblasts. PLoS One 2011;6:e21654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Arbiser JL, Karalis K, Viswanathan A, et al. Corticotropin-releasing hormone stimulates angiogenesis and epithelial tumor growth in the skin. J Invest Dermatol 1999;113:838–42. [DOI] [PubMed] [Google Scholar]
- 280.Leu SJ, Singh VK. Stimulation of interleukin-6 production by corticotropin-releasing factor. Cell Immunol 1992;143:220–7. [DOI] [PubMed] [Google Scholar]
- 281.Slominski A, Zbytek B, Zmijewski M, et al. Corticotropin releasing hormone and the skin. Front Biosci 2006;11:2230–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Haluzik M, Colombo C, Gavrilova O, et al. Genetic background (C57BL/6J versus FVB/N) strongly influences the severity of diabetes and insulin resistance in ob/ob mice. Endocrinology 2004;145:3258–64. [DOI] [PubMed] [Google Scholar]
- 283.Kim AK, Hamadani C, Zeidel ML, Hill WG. Urological complications of obesity and diabetes in males and females of three mouse models: temporal manifestations. Am J Physiol Renal Physiol 2020;318:F160–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Leppin K, Behrendt AK, Reichard M, et al. Diabetes mellitus leads to accumulation of dendritic cells and nerve fiber damage of the subbasal nerve plexus in the cornea. Invest Ophthalmol Vis Sci 2014;55:3603–15. [DOI] [PubMed] [Google Scholar]
- 285.O’Brien PD, Sakowski SA, Feldman EL. Mouse models of diabetic neuropathy. ILAR J 2014;54:259–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.O’Brien PD, Hur J, Hayes JM, Backus C, Sakowski SA, Feldman EL. BTBR ob/ob mice as a novel diabetic neuropathy model: neurological characterization and gene expression analyses. Neurobiol Dis 2015;73:348–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Hudkins KL, Pichaiwong W, Wietecha T, et al. BTBR Ob/Ob mutant mice model progressive diabetic nephropathy. J Am Soc Nephrol 2010;21:1533–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Bjornson Granqvist A, Ericsson A, Sanchez J, et al. High-protein diet accelerates diabetes and kidney disease in the BTBRob/ob mouse. Am J Physiol Renal Physiol 2020;318: F763–71. [DOI] [PubMed] [Google Scholar]
- 289.Clee SM, Nadler ST, Attie AD. Genetic and genomic studies of the BTBR ob/ob mouse model of type 2 diabetes. Am J Ther 2005;12:491–8. [DOI] [PubMed] [Google Scholar]
- 290.Chua SC Jr, Chung WK, Wu-Peng XS, et al. Phenotypes of mouse diabetes and rat fatty due to mutations in the OB (leptin) receptor. Science 1996;271:994–6. [DOI] [PubMed] [Google Scholar]
- 291.Kampfer H, Paulukat J, Muhl H, Wetzler C, Pfeilschifter J, Frank S. Lack of interferon-gamma production despite the presence of interleukin-18 during cutaneous wound healing. Mol Med 2000;6:1016–27. [PMC free article] [PubMed] [Google Scholar]
- 292.Fan B, Li C, Szalad A, et al. Mesenchymal stromal cell-derived exosomes ameliorate peripheral neuropathy in a mouse model of diabetes. Diabetologia 2020;63:431–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Chen H, Charlat O, Tartaglia LA, et al. Evidence that the diabetes gene encodes the leptin receptor: identification of a mutation in the leptin receptor gene in db/db mice. Cell 1996;84:491–5. [DOI] [PubMed] [Google Scholar]
- 294.Seitz O, Schurmann C, Hermes N, et al. Wound healing in mice with high-fat diet- or ob gene-induced diabetes-obesity syndromes: a comparative study. Exp Diabetes Res 2010;2010:476969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Wang CY, Liao JK. A mouse model of diet-induced obesity and insulin resistance. Methods Mol Biol 2012;821:421–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Coppey L, Lu B, Gerard C, Yorek MA. Effect of inhibition of angiotensin-converting enzyme and/or neutral endopeptidase on neuropathy in high-fat-fed C57Bl/6J mice. J Obes 2012;2012:326806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Burke SJ, Batdorf HM, Burk DH, et al. db/db mice exhibit features of human type 2 diabetes that are not present in weight-matched C57BL/6J mice fed a western diet. J Diabetes Res 2017;2017:8503754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Molnar J, Yu S, Mzhavia N, Pau C, Chereshnev I, Dansky HM. Diabetes induces endothelial dysfunction but does not increase neointimal formation in high-fat diet fed C57BL/6J mice. Circ Res 2005;96:1178–84. [DOI] [PubMed] [Google Scholar]
- 299.Collins S, Martin TL, Surwit RS, Robidoux J. Genetic vulnerability to diet-induced obesity in the C57BL/6J mouse: physiological and molecular characteristics. Physiol Behav 2004;81:243–8. [DOI] [PubMed] [Google Scholar]
- 300.Schmidt RE, Dorsey DA, Beaudet LN, Peterson RG. Analysis of the Zucker diabetic fatty (ZDF) type 2 diabetic rat model suggests a neurotrophic role for insulin/IGF-I in diabetic autonomic neuropathy. Am J Pathol 2003;163:21–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Slavkovsky R, Kohlerova R, Tkacova V, et al. Zucker diabetic fatty rat: a new model of impaired cutaneous wound repair with type II diabetes mellitus and obesity. Wound Repair Regen 2011;19:515–25. [DOI] [PubMed] [Google Scholar]
- 302.Baynes J, Murray DB. Cardiac and renal function are progressively impaired with aging in Zucker diabetic fatty type II diabetic rats. Oxid Med Cell Longev 2009;2:328–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Choi R, Kim BH, Naowaboot J, et al. Effects of ferulic acid on diabetic nephropathy in a rat model of type 2 diabetes. Exp Mol Med 2011;43:676–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Kawano K, Mori S, Hirashima T, Man ZW, Natori T. Examination of the pathogenesis of diabetic nephropathy in OLETF rats. J Vet Med Sci 1999;61:1219–28. [DOI] [PubMed] [Google Scholar]
- 305.Peterson RG, Jackson CV, Zimmerman K, de Winter W, Huebert N, Hansen MK. Characterization of the ZDSD rat: a translational model for the study of metabolic syndrome and type 2 diabetes. J Diabetes Res 2015;2015:487816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Reinwald S, Peterson RG, Allen MR, Burr DB. Skeletal changes associated with the onset of type 2 diabetes in the ZDF and ZDSD rodent models. Am J Physiol Endocrinol Metab 2009;296:E765–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Tirabassi RS, Flanagan JF, Wu T, Kislauskis EH, Birckbichler PJ, Guberski DL. The BBZDR/Wor rat model for investigating the complications of type 2 diabetes mellitus. ILAR J 2004;45:292–302. [DOI] [PubMed] [Google Scholar]
- 308.Murakawa Y, Zhang W, Pierson CR, et al. Impaired glucose tolerance and insulinopenia in the GK-rat causes peripheral neuropathy. Diabetes Metab Res Rev 2002;18:473–83. [DOI] [PubMed] [Google Scholar]
- 309.Akash MS, Rehman K, Chen S. Goto-Kakizaki rats: its suitability as non-obese diabetic animal model for spontaneous type 2 diabetes mellitus. Curr Diabetes Rev 2013;9:387–96. [DOI] [PubMed] [Google Scholar]
- 310.Cheng KY, Lin ZH, Cheng YP, et al. Wound healing in streptozotocin-induced diabetic rats using atmospheric-pressure argon plasmajet. Sci Rep 2018;8:12214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Barriere DA, Noll C, Roussy G, et al. Combination of high-fat/high-fructose diet and low-dose streptozotocin to model long-term type-2 diabetes complications. Sci Rep 2018;8:424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Zhang M, Lv XY, Li J, Xu ZG, Chen L. The characterization of high-fat diet and multiple low-dose streptozotocin induced type 2 diabetes rat model. Exp Diabetes Res 2008;2008:704045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Tavares-Ferreira D, Shiers S, Ray PR, et al. Spatial transcriptomics reveals unique molecular fingerprints of human nociceptors. bioRxiv 2021;2021:2002.2006.430065. [Google Scholar]


