Abstract
Notwithstanding the “one-module-one-elongation-cycle” paradigm of assembly line polyketide synthases (PKSs), some PKSs harbor modules that iteratively elongate their substrates through a defined number of cycles. While some insights into module iteration, also referred to as “stuttering”, have been derived through in vivo and in vitro analysis of a few PKS modules, a general understanding of the mechanistic principles underlying module iteration remains elusive. This report serves as the first interrogation of a stuttering module from a trans-AT subfamily PKS that is also naturally split across two polypeptides. Previous work has shown that Module 5 of the NOCAP (nocardiosis associated polyketide) synthase iterates precisely three times in the biosynthesis of its polyketide product, resulting in an all trans-configured triene moiety in the polyketide product. Here we describe the intrinsic catalytic properties of this NOCAP synthase module. Through complementary experiments in vitro and in E. coli, the “split-and-stuttering” module was shown to catalyze up to five elongation cycles, although its dehydratase domain ceased to function after three cycles. Unexpectedly, the central olefinic group of this truncated product had a cis configuration. Our findings set the stage for further in-depth analysis of a structurally and functionally unusual PKS module with contextual biosynthetic plasticity.
Graphical Abstract

Introduction
Polyketide synthases (PKSs) are multifunctional enzymes responsible for the biosynthesis of structurally diverse polyketide products, many of which are clinically used as medicines1. Therefore, understanding their catalytic diversity and mechanisms has been of high interest to researchers in the hope of engineering novel pharmacologically useful molecules. One class of PKSs, called assembly line PKSs, consists of multiple catalytic modules each of which is responsible for a well-defined set of chemical transformations on the growing polyketide chain. Minimally a PKS module harbors a ketosynthase (KS), acyltransferase (AT), and acyl carrier protein (ACP) domain2. Some PKS modules (hereafter designated cis-AT PKSs) include the AT as an integral domain of a multifunctional polypeptide, whereas other modules (designated trans-AT PKSs) engage stand-alone AT proteins3.
Canonically, each module of an assembly line PKS catalyzes a single elongation and modification cycle, where after the growing polyketide chain is either translocated onto the KS domain of the next module or it is off-loaded from the assembly line4,5. Such a collinear architecture has facilitated prediction of structural features of the resulting polyketide products6. However, not all PKSs operate according to a collinear one-module-one-elongation-cycle model3. For example, the NOCAP synthase7,8 harbors a trans-AT module (Module 5) that catalyzes three successive elongation and modification cycles on the growing polyketide chain yielding products 1 and 2 (Figure 1)9,10.
Figure 1.

Biosynthetic pathway of 1 via Modules L-8-TR + tAT-TEII of NOCAP synthase. 2 is an alternative product generated when Module 3 is skipped. Key: KS, ketosynthase; AT, acyltransferase; DH, dehydratase; KR, ketoreductase; ER, enoylreductase; cMT, C-methyltransferase; ACP, acyl carrier protein; TR, thioester reductase; and TEII, thioesterase II. 0; subscript implies inactive domain.
After each cycle, the elongated and modified polyketide product is passed from the ACP domain of Module 5 back to its own KS domain11. This type of programmed module iteration, also known as “stuttering”12,13, has been identified in a few other assembly line PKSs14 such as the stigmatellin15, lankacidin16, borrelidin17, neoaureothin and aureothin18 PKS pathways.
While the governing principles that control programmed iteration are not well understood, some insights have been gained through in vitro and in vivo analysis of cis-AT PKSs. Module 1 of the aureothin synthase (cis-AT PKS), which catalyzes two elongation and modification cycles in the context of the complete assembly line PKS, was expressed in Streptomyces lividans19. The resulting strain produced a spectrum of compounds that had undergone up to 4 rounds of elongation, although products resulting from 3 and 4 rounds of iteration were orders of magnitude less abundant. Additionally, an in vitro analysis of Module 5 of the borrelidin PKS revealed that the KS domain of this stuttering module accepted non-native substrates corresponding to the chain lengths of its natural intermediates20. Collectively, these and other findings have led to a mechanistic model in which the KS specificity at least partly dictates the number of iterations observed. However, the downstream module also has been suggested to exhibit a gatekeeping role21. Given the lack of prior studies on stuttering modules from trans-AT PKSs, we interrogated Module 5 of the NOCAP synthase. Of particular interest to us was its additionally unusual “split-and-stuttering” feature, with its KS and ACP domains distributed across separate proteins (Figure 1). Whereas there are examples of split trans-AT assembly line PKS modules (e.g., KirAIV)22, Module 5 of the NOCAP synthase is a trans-AT that has both “split-and-stuttering” properties. We therefore sought to study this module further.
Results and Discussion
In order to investigate the potential gatekeeping role of downstream modules in the NOCAP synthase we sought to develop a truncated assembly line excluding Modules 6–8-TR. Fortunately, a system for functionally expressing the entire NOCAP synthase in E. coli BAP123 was recently developed which was adapted for the present study10. Three plasmids with unique antibiotic resistance markers were introduced into E. coli BAP1 (Figure 2, Figure S1).
Figure 2.

Compounds 3 and 4 identified in (a) an E. coli BAP1 strain housing NOCAP synthase Modules L-5 [pCK-KPY178/pCK-KPY222/pKMG14]. (b) HPLC UV chromatogram (at 280 ± 10 nm) comparing crude extracts from E. coli BAP1 supernatants. E. coli BAP1 strains: [pCK-KPY178/pCK-KPY222] (black, omit Module 4-KS5 and DH5-ACP5-KR5); [pCK-KPY178/pCK-KPY222/pKMG15] (grey, omit KR5); [pCK-KPY178/pCK-KPY102/pKMG14] (blue, omit Module 3); and [pCK-KPY178/pCK-KPY222/pKMG14] (red, Modules L- 5). (c) Enlarged region of HPLC UV trace for clarity. Data acquired on an Agilent 6545 Q-TOF LC-MS system.
Plasmid pCK-KPY178 encodes the malonyl-CoA synthetase MatB from Streptomyces coelicolor24, the Rhizobium leguminosarum malonate carrier protein MatC25, the loading module (Module L) and trans-AT-TEII (tAT-TEII) of the NOCAP synthase. Plasmid pCK-KPY222 encodes Modules 1–3 with engineered docking domains to facilitate protein-protein interactions26. Finally, pKMG14 or alternatively pKMG15 encodes Module 4 and the DH-ACP-KR or the DH-ACP domains of Module 5, respectively. In the absence of a specific product release mechanism in these truncated assembly lines, we relied on spontaneous hydrolysis of polyketide products from ACP5.
One to three liters of E. coli BAP1 [pCK-KPY178/pCK-KPY222/pKMG14] supernatant was subjected to solid phase extraction (SPE) followed by high performance liquid chromatography (HPLC) (Figure S2). Encouragingly, two compounds 3 and 4 with distinct UV signatures were identified (Figure 2, Figures S3–S4). Control strains harboring pKMG15 in lieu of pKMG14 or lacking either plasmid failed to produce either 3 or 4 (Figure S5). High-resolution mass spectrometry (HRMS) analysis revealed that 3 had a molecular formula of C20H28O5 (HRMS (ESI) m/z: [M − H]− calcd for C20H27O5 347.1864; found 347.1845), while 4 had a molecular formula of C18H26O5 (HRMS (ESI) m/z: [M − H]− calcd for C18H25O5 321.1707; found 321.1704) (Figure S6). Similar to the relationship between 1 and 2, these predicted formulas differed by a mass shift consistent with an unsaturated two-carbon (C2H2) moiety. To verify the anticipated biosynthetic relationship between 3 and 4, an E. coli BAP1 strain lacking Module 3 [pCK-KPY178/pCK-KPY102/pKMG14] was engineered (Supporting Information). The resulting strain yielded 4 but not 3 (Figure S5), confirming a requirement of Module 3 for the biosynthesis of the latter compound. However, because these HRMS values were inconsistent with our expectation that Module 5 catalyzed three elongation and modification cycles, we turned to NMR spectroscopy to elucidate the structures of 3 and 4.
The structures of 3 and 4 were uncovered by extensive 1-D and 2-D NMR analysis, including 1H 1D, 13C 1D, COSY, HSQC, HMBC, and ROESY experiments (Figure 3, Figures S7–S31).
Figure 3.
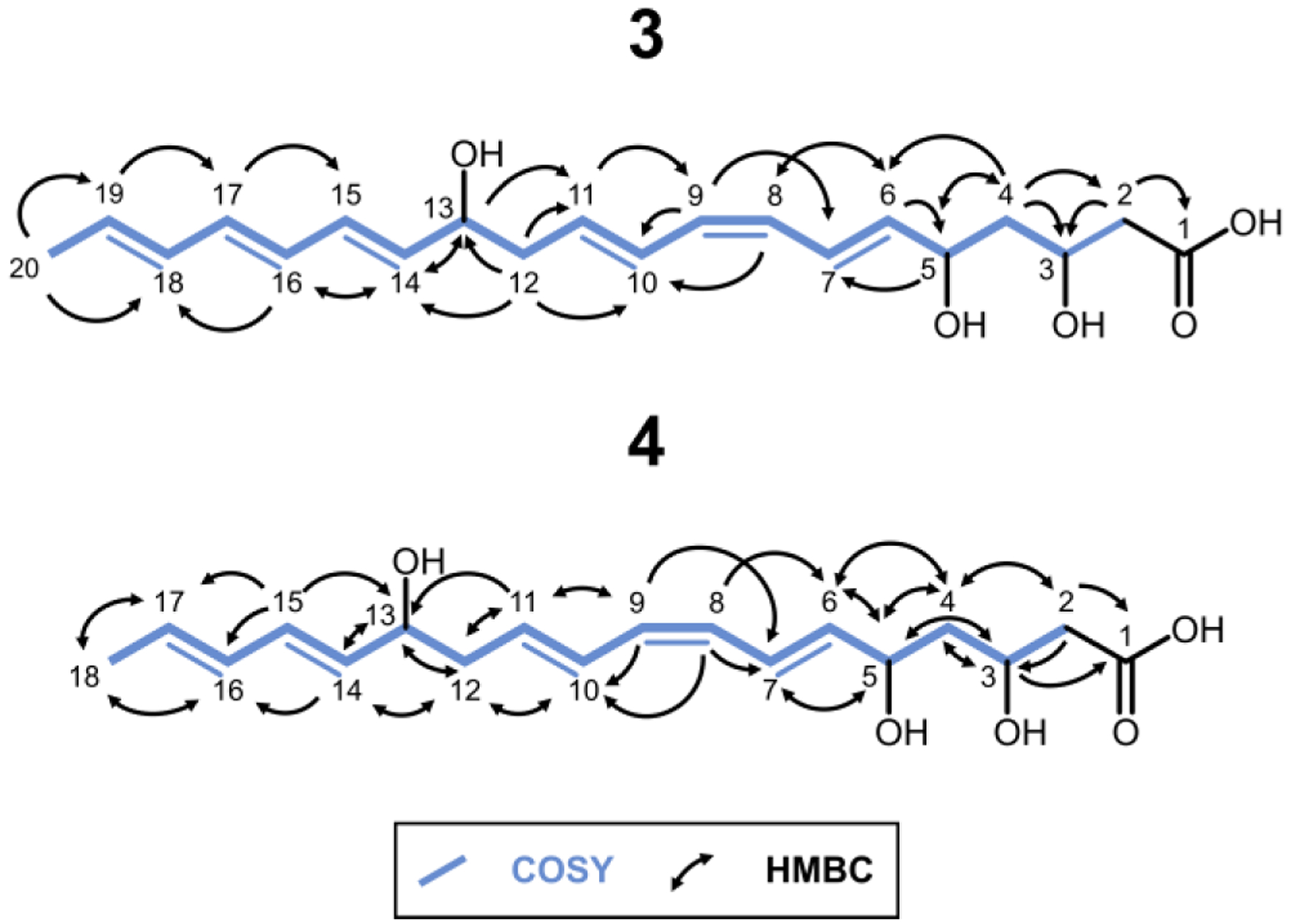
Structures of 3 and 4 assembled from 2D NMR data.
The structures deduced from NMR analysis suggested that Module 5 catalyzed five elongation cycles in the biosynthesis of these compounds, but that the final two cycles were not accompanied by dehydration of the β-hydroxyacyl intermediate (Figure 4, Figure S32).
Figure 4.

Proposed biosynthesis of 3 from NOCAP synthase Modules L-5 + tAT-TEII.
Furthermore, the presence of a cross-peak in the ROESY spectrum implied the existence of a cis-olefinic bond between C8-C9 in contrast to the all-trans configuration of 1 and 2 (Figures S28 and S31). To our knowledge this is the first reported instance of “imperfect stuttering”, where the programmed sequence of chain modification reactions is not faithfully catalyzed in every iteration (compared to the full assembly line). The unexpected chain length and configuration of 3 (and 4) raised the possibility that endogenous E. coli enzymes played a role in its biosynthesis. For instance, FabA from the fatty acid synthase of E. coli iteratively catalyzes the dehydration of ACP-bound β-hydroxyacyl intermediates generated during fatty acid biosynthesis. FabA can also reversibly isomerize trans-2-decenoyl-ACP to cis-3-decenoyl-ACP27 Therefore, to rule out the possibility of artifactual involvement of host enzymes, we sought to functionally reconstitute the truncated hexamodular derivative of the NOCAP synthase in vitro using purified proteins.
The following proteins were expressed and purified via immobilized metal ion chromatography followed by anion exchange chromatography: DH5-ACP5-KR5, DH5-ACP5 and tAT-TEII from the NOCAP synthase, and S. coelicolor MatB (Figure S33, Experimental Section). Additionally, Module L, Modules 1–2, Module 3 and Module 4-KS5 were purified on a StrepII-Tag affinity column. Modules L, 1–2, 3, 4-KS5 and DH5-ACP5-KR5 were incubated in the presence of tAT-TEII, MatB, NADPH, S-adenosyl methionine (SAM), malonate, ATP, and CoASH. Parallel reactions were performed without malonate or with [2-13C]-, [1,3-13C2]-, or [13C3]-malonate to ensure the products detected were of polyketide origin. Product formation relied on spontaneous hydrolysis from ACP5. The predominant product of the truncated hexamodular PKS in vitro was identical to 3, as judged by HRMS (Figure 5).
Figure 5.

(a) EICs of 3 synthesized in E. coli BAP1 and (b) in vitro (upper red traces). Negative controls (lower grey traces) omitted Module 4-KS5 and DH5-ACP5-KR5.
The observation of +10, +10, and +20 mass shifts in reactions containing [2-13C]-, [1,3-13C2]-, and [13C3]-malonate, respectively, supported our hypothesis that in vitro-derived 3 a) originates from the truncated assembly line and b) had undergone five rounds of elongation at Module 5 (Figure S34). 4 was not synthesized in detectable amounts in vitro. These results largely support our proposed biosynthetic pathway. However, despite considerable effort, these in vitro reactions did not yield sufficient material for ROESY verification of 3’s geometry.
In summary, this is the first description of a trans-AT “split-and-stuttering” module that preferentially iterates for additional cycles beyond those observed in the context of the full PKS assembly line. Although our lack of a releasing enzyme may have resulted in additional iterations28, our aim was focused on the intrinsic capabilities of module 5 lacking downstream modules. We therefore speculate that Module 6 of the NOCAP synthase regulates the overall polyketide chain length by preferentially recognizing the ACP5-bound intermediate after it has undergone three elongation and modification cycles on Module 5. The inability of DH5 to catalyze dehydration after three elongation cycles revealed an unprecedented feature of this assembly line PKS domain and is reminiscent of iterative PKSs (iPKSs) such as the lovastatin synthase29. Future efforts will aim to elucidate the underlying mechanisms that govern module 5’s particularly unusual iterative behavior, including the origin of the unexpected cis-olefinic bond. Overall, our findings demonstrate the remarkable contextual biosynthetic plasticity of Module 5 of the NOCAP synthase while highlighting the opportunity for future polyketide engineering through a deeper understanding of iterative PKS modules.
Experimental Section
General HPLC Solvents
HPLC A: 99.9% (v/v) water, 0.1% (v/v) formic acid HPLC Grade; HPLC B: 99.9% (v/v) acetonitrile, 0.1% (v/v) formic acid HPLC Grade
Biosynthesis (and Extraction) of 3 and 4 in (from) E. coli10
Growth medias used in this protocol had the following compositions: a) LB Broth: 25.0 g/L LB Broth, Miller granulated powder; supplemented with 100 mg/L carbenicillin disodium, 50 mg/L kanamycin monosulfate and 50 mg/L streptomycin sulfate; b) Terrific Broth: 47.6 g/L Terrific Broth (modified) powder, 10 g/L glycerol; supplemented with 100 mg/L carbenicillin disodium, 50 mg/L kanamycin monosulfate and 50 mg/L streptomycin sulfate. LB Broth was sterilized in an autoclave, while Terrific Broth was sterilized by flow through a 0.2 μm PES bottle filter attachment. A single colony of E. coli BAP1 [pCK-KPY178/pCK-KPY222/pKMG14] was used to inoculate an overnight seed culture of LB Broth (15 mL) grown at 30°C in a 50 mL Falcon tube. The overnight seed culture was pelleted by centrifugation at 4000 × g for 15 min at room temperature and re-suspended in LB Broth (15 mL). 12 mL of starter culture were used to inoculate 1 L of Terrific Broth in a 2.5 L Tunair shake flask and agitated at 30°C (200 rpm) until the OD600 reached ~0.2. After the addition of 10 mL/L culture of 500 mM sodium malonate, pH 7.4, 1 mL/L culture of 50 mM calcium D-pantothenate and 0.5 mL/L culture of 200 mM isopropyl β-D-1-thiogalactopyranoside (IPTG) the cultures were agitated (200 rpm) at 16°C for an additional 72 hrs. 1–3 L of culture were emptied into centrifuge bottles. The Tunair shake flask was then rinsed with ~200 mL of distilled H2O to ensure maximum yield and collection of foam that arose during culturing. Bottles were then centrifuged at 5000 × g for 15 min at 16°C. Supernatant and pellets were separated and frozen at −20°C. Pellet and supernatant were processed separately. Compounds 3 and 4 were identified in the supernatant, judged by crude LC-MS (Waters SQ Detector 2 LC-MS system¶) extract comparative analysis. With these results the following protocol was adopted for the extraction of 3 and 4. A 60-mL Solid-Phase Extraction column (SPE) was retrofitted onto either a 50 mL Falcon tube with a vacuum syringe line or a 2 L Erlenmeyer flask with an in-house vacuum line depending on the extraction step. The following were gently passed through the SPE column (flow rate ~1 drop/s): 25 mL of HPLC grade Methanol – Wash; 25 mL of HPLC A – Equilibration; 500 mL of E. coli supernatant – Load; 5 × 5 mL HPLC B – Elutions. Note: The E. coli supernatant was filtered using a 0.2 μm PES membrane filtering unit prior to loading on the SPE column. 1 SPE column was used/500 mL of E. coli supernatant processed. Samples were protected from light at all times. Elution fractions 1–5 identified by LC-MS¶ to contain 3 and 4 were pooled and dried under a gentle N2 stream. Once a final volume of 10 mL was achieved, 10 mL of HPLC A was added, mixed and then dried in vacuo. The sample was removed from vacuum and dissolved in 2.4 mL 75:25 (% v/v) HPLC A:HPLC B and filtered through a 0.45 μm PTFE membrane. This mixture was separated with a gradient elution method (70:30 % HPLC A:HPLC B to 45:55 % HPLC A:HPLC B over 1 hr at 1 mL/min) on an Agilent 1260 Infinity LC system equipped with an Agilent Eclipse XDB-C8 column (5 μm, 250 mm × 9.4 mm). Fractions identified by LC-MS (Figure S2–4) to contain 3 and 4 were diluted with D2O, applied to a 200 mg C18 solid-phase extraction column, washed with D2O to remove HPLC A and HPLC B solvents30, and eluted with 0.25–0.75 mL CD3OD for NMR experiments. Unfortunately, since the compounds were unable to be lyophilized without compromising their quality, the exact quantity (mass) obtained is unknown. Compounds were analyzed on an Agilent Infinity 1290 II HPLC/Agilent Q-TOF 6545 LC-MS system‖: 3: HRMS (ESI) m/z: [M − H]− calcd for C20H27O5 347.1864; found 347.1845. 4: HRMS (ESI) m/z: [M − H]− calcd for C18H25O5 321.1707; found 321.1704.
NMR of 3 and 4
Samples were prepared in 0.25–0.75 mL of CD3OD in standard borosilicate glass NMR tubes (5 mm × 8 inch) or in matched symmetrical Shigemi tubes. NMR spectra of 3 were acquired on a 900 MHz Bruker AVANCE II spectrometer (Central California 900 MHz NMR Facility, California Institute for Quantitative Biosciences, University of California, Berkeley) with a 5 mm TCI (H{CN}, Z-gradient) cryoprobe, running TopSpin v3.2. Sample temperature was regulated at 25°C. Experiments acquired include: 1H 1D, 13C 1D, COSY, HSQC, HMBC, and ROESY. This instrument was also used to obtain the 13C 1D spectra of 4. The remaining NMR spectra of 4 were acquired on a 600 MHz Varian Inova spectrometer (NMR facility, Department of Chemistry, Stanford University) with a 5 mm (H{CN}, Z-gradient) probe. Sample temperature was regulated at 25°C. Experiments acquired include: 1H 1D, COSY, HSQC, HMBC, and ROESY. Data files were analyzed using Mnova software. Automatic baseline corrections, phasing, and referencing (when applicable; CD3OD; H 3.31 ppm, C 49.0 ppm) were used to correct offsets in the acquired data. Additionally, t1 noise was reduced using processing in Mnova. Samples were protected from light at all times. NMR Experimental Parameters*: Compound 3: nt; (np,ni); sw; d1; at; 1H 1D: 16; -; 12626.3; 2.00; 5.1905; 13C 1D: 9900; -; 59523.8; 2.80; 0.3588; COSY: 4; (4096, 400); (12626.3, 12594.5); 1.50; 0.1622; HSQC: 32; (2048, 128); (12626.3, 37313.4); 1.50; 0.0811; HMBC: 32; (2048, 128); (12626.3, 37313.4); 1.50; 0.0811; ROESY (300 ms mixing): 8; (4096, 400); (12626.3, 12594.5); 2.00; 0.1622. NMR Experimental Parameters*: Compound 4: nt; (np,ni); sw; d1; at; 1H 1D: 1; -; 5950.6; 1.00; 5.5067; 13C 1D**: 1218; -; 59523.8; 2.80; 0.3599; COSY: 4; (2048, 400); (5950.6, 5950.6); 1.50; 0.1721; HSQC: 16; (2048, 200); (5950.6, 25641.0); 1.50; 0.1721; HMBC: 80; (1786, 300); (5950.6, 36199.1); 1.50; 0.1501; ROESY (200 ms mixing): 8; (1786, 200); (5950.6, 5950.6); 3.00; 0.1501. Compound 3***: 1H NMR (CD3OD, 900 MHz): δ 6.73 (dd, 1H, J = 15.1, 11.0 Hz), 6.61 (dd, 1H, J = 15.0, 11.0 Hz), 6.21–6.19 (ddd, 2H, J = 22.1, 15.0, 10.6 Hz), 6.09–6.08 (m, 2H), 5.96 (t, 1H, J = 11.0 Hz), 5.91 (t, 1H, J = 11.0 Hz), 5.74–5.72 (m, 3H), 5.66 (dd, 1H, J = 15.2, 6.8 Hz), 4.40 (q, 1H, J = 6.6 Hz), 4.26 (m, 1H), 4.16 (q, 1H, J = 6.4 Hz), 2.48 (m, 2H), 2.37 (m, 2H), 1.75 (d, 3H, J = 6.7 Hz), 1.65 (t, 2H, J = 6.4 Hz). 13C{1H} NMR (CD3OD, 226 MHz): δ 175.9, 137.7, 135.4, 134.3, 132.7, 132.3, 131.9, 131.1, 130.6, 130.6, 129.1, 128.3, 126.1, 72.9, 69.4, 66.0, 44.9, 43.4, 41.8, 18.4. Compound 4***: 1H NMR (CD3OD, 600 MHz): δ 6.73 (dd, 1H, J = 15.0, 10.8 Hz), 6.60 (dd, 1H, J = 15.2, 10.7 Hz), 6.16 (dd, 1H, J = 15.2, 10.4 Hz), 6.04 (m, 1H), 5.95–5.90 (m, 2H), 5.73–5.70 (m, 3H), 5.53 (dd, 1H, J = 15.2, 6.8 Hz), 4.40 (q, 1H, J = 6.4 Hz), 4.26 (m, 1H), 4.12 (q, 1H, J = 6.6 Hz), 2.46 (m, 2H), 2.36 (m, 2H), 1.73 (d, 3H, J = 6.8 Hz), 1.65 (t, 2H, J = 6.4 Hz). 13C{1H} NMR (CD3OD, 226 MHz): δ 176.4, 137.7, 133.4, 132.2, 131.9, 131.7, 130.4, 130.4, 128.9, 128.2, 125.9, 72.7, 69.4, 65.9, 44.7, 43.2, 41.7, 17.9.
Protein Expression and Purification of Modules L-5, tAT-TEII10
LB Broth was supplemented with either 100 mg/L carbenicillin disodium or 50 mg/L kanamycin monosulfate – see “Expression vectors for In Vitro Assays” list. The following buffers were used for protein purification: Ni-NTA Lysis/Wash Buffer: 50 mM sodium phosphate, 500 mM sodium chloride, 20 mM imidazole, 1 mg/mL chicken egg white lysozyme, 10% (v/v) glycerol, pH 7.4. Ni-NTA Elution Buffer: 50 mM sodium phosphate, 20 mM sodium chloride, 500 mM imidazole, 10% (v/v) glycerol, pH 7.4. Strep-Tactin Lysis Buffer: 50 mM sodium phosphate, 500 mM sodium chloride, 10% (v/v) glycerol, 1 mg/mL chicken egg white lysozyme, 4 mg/L chicken egg white avidin, pH 7.4. Strep-Tactin Wash Buffer: 50 mM sodium phosphate, 500 mM sodium chloride, 10% (v/v) glycerol, pH 7.8. Strep-Tactin Elution Buffer: 50 mM sodium phosphate, 500 mM sodium chloride, 10% (v/v) glycerol, 5 mM d-desthiobiotin, pH 7.8. FPLC A: 50 mM sodium phosphate, 10% (v/v) glycerol, pH 7.4. FPLC B: 50 mM sodium phosphate, 1 M sodium chloride, 10% (v/v) glycerol, pH 7.4. A single colony of E. coli BAP1 housing the appropriate plasmid was used to inoculate an overnight seed culture of LB Broth (10–80 mL) and grown at 37°C. The overnight seed culture was pelleted by centrifugation at 4000 × g for 15 min at room temperature and re-suspended in fresh LB Broth (10–80 mL). 4–5 mL of seed culture was added into each 2.5 L Tunair shake flask (2–8 flasks with 1 L fresh LB Broth in each flask) and agitated (200 rpm) at 37°C until the OD600 reached 0.2–0.3. At this point, the incubator temperature was set to 18°C, and the flasks were allowed to shake for 1 hr before the addition of 0.5–1.0 mL/L culture of 200 mM IPTG at an OD600 of 0.5–0.7. The cultures were further agitated (180 rpm) at 18°C for an additional 15–17 hrs. Cells were harvested by centrifugation at 5000 × g for 15 min at 4°C, frozen in liquid nitrogen and stored at −80°C. Thawed cells were re-suspended either in Ni-NTA Lysis Buffer or Strep-Tactin Lysis Buffer, incubated at 4°C for 1 hr and sonicated. Lysates were clarified twice by centrifugation at 25,000 × g for 1 hr at 4°C. Strep-II tagged protein lysates were incubated overnight while rotating end-over-end with 0.5 mL/L culture Strep-Tactin Sepharose resin. Whereas, 6×His tagged protein lysates were incubated for 1–2 hrs while rotating end-over-end with 2 mL/L culture HisPur Ni-NTA resin. The protein-bound resin was washed (25 mL×3) with either Ni-NTA Wash Buffer or Strep-Tactin Wash Buffer. Protein was eluted off the resin with either Ni-NTA Elution Buffer (5–10 mL × 2) or Strep-Tactin Elution Buffer (2.5 mL × 6) and further purified with a gradient elution method (FPLC A to FPLC B over 20 column volumes at 4 mL/min, 4 column volume wash with FPLC A prior to gradient) on an ÄKTA pure chromatography system (GE Healthcare Life Sciences) equipped with a HiTrap Q HP anion exchange chromatography column (5 mL). Fractions identified by SDS-PAGE to contain proteins of interest were pooled, concentrated using Amicon Ultra Centrifugal Filters, frozen in liquid nitrogen and stored at −80°C. All steps were carried out at 4°C or on ice. Approxiamate protein yields were 15 mg/L for JK52, 10 mg/L for CK-KPY059, 3 mg/L for CK-KPY102, 1 mg/L for CK-KPY293, 5 mg/L for CK-KPY294, 5 mg/L for JK65, and 10 mg/L for JK66.
In Vitro Reconstitution of Modules L-5, tAT-TEII10
Reaction Components: 100 mM HEPES, pH 7.5, 2.5 mM coenzyme A, 10 mM adenosine 5′-triphosphate, 5 mM NADPH, 10 mM magnesium chloride, 10 mM tris(2-carboxyethyl)phosphine, 5 μM MatB, and 4–8 μM of all remaining proteins depending on scale (tAT-TEII, MBP-module L, module 1–2-DEBS(4), DEBS(5)-module 3-DEBS(2), DEBS(3)-module 4-KS5, DH5-ACP5-KR5). [12C3]-, [13C1,3]-, [13C2]-, or [13C3]-malonate was added to start the reaction (5 mM final concentration), which was incubated overnight (24 hrs) at room temperature in the dark. Reactions were quenched by the addition of LC-MS grade methanol (1:1 reaction volume/methanol). The mixture was vortexed followed by centrifugation at 16,000 × g for 10 min at room temperature. The supernatant was transferred to a 0.2 μm PTFE spin filter and centrifuged at 16,000 × g for 1 min before transferring to a LC-MS vial. For LC-MS analysis, compounds were separated with a gradient elution method an Agilent Infinity 1290 II HPLC/6545 Q-TOF MS systemǁ. In this and all assays utilizing both malonate and coenzyme A, the Streptomyces coelicolor malonyl-CoA synthetase MatB generated malonyl-CoA in situ. Agilent Q-TOF HRMS 3: HRMS (ESI) m/z: [M − H]− calcd for C20H27O5 347.1864; found 347.1868.
Supplementary Material
Acknowledgements
We thank members of the Khosla Lab for thoughtful discussions. We also thank Theresa McLaughlin (Stanford University Mass Spectrometry facility) and Jeffrey G. Pelton (University of California, Berkeley) for technical assistance performing LC-MS and NMR experiments respectively. This work was supported by National Institutes of Health (NIH) Grant 5R01 GM087934 (to C.K.) (with extension -26S1 (to C.K and K.M.G.), NIH Grant F32 GM123637 (to K.P.Y.) and a Stanford Graduate Fellowship (SGF) Award (to K.M.G). This work utilized the Stanford Cancer Institute Proteomics/Mass Spectrometry Shared Resource, which is supported by NIH Grant P30 CA124435, and the 900 MHz Bruker NMR spectrometer (funded by NIH Grant P41 GM068933) at the Central California 900 MHz NMR Facility.
Footnotes
Compounds were separated with a gradient elution method (98/2 % HPLC A/HPLC B to 5/95 % HPLC A/HPLC B over 4 min at 0.3 mL/min) equipped with an Agilent InfinityLab Poroshell 120 SB-C18 column (2.7 μm, 2.1 × 50 mm) and operating in both positive and negative ion mode.
Compounds were separated with a gradient elution method (95/5 % HPLC A/HPLC B to 5/95 % HPLC A/HPLC B over 4 min at 0.6 mL/min) equipped with an Agilent ZORBAX RRHD Extend-C18 column (1.8 μm, 2.1 × 50 mm) and operating in negative ion mode.
Varian Inova/Agilent parameter terminology used;
Acquired on 900 MHz instrument;
Assignments based on HSQC shifts referenced to CD3OD (49, 3.31 ppm), except C1 based on 13C 1D data (226 MHz) referenced to CD3OD (49 ppm).
The authors declare no competing financial interest.
References
- [1].Walsh CT Polyketide and nonribosomal peptide antibiotics: modularity and versatility. Science 2004, 303, 1805–1810. [DOI] [PubMed] [Google Scholar]
- [2].Katz L The DEBS Paradigm for Type I Modular Polyketide Synthases and Beyond, Methods in Enzymol. 2009, 459, 113–142. [DOI] [PubMed] [Google Scholar]
- [3].Gay DC, Gay G, Axelrod AJ, Jenner M, Kohlhaas C, Kampa A, Oldham NJ, Piel J, and Keatinge-Clay AT A Close Look at a Ketosynthase from a Trans-Acyltransferase Modular Polyketide Synthase. Structure 2014, 22, 444–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Donadio S, Staver M, Mcalpine J, Swanson S, and Katz L Modular organization of genes required for complex polyketide biosynthesis. Science 1991, 252, 675–679. [DOI] [PubMed] [Google Scholar]
- [5].Khosla C, Herschlag D, Cane DE, and Walsh CT Assembly Line Polyketide Synthases: Mechanistic Insights and Unsolved Problems. Biochemistry 2014, 53, 2875–2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Helfrich EJN, Ueoka R, Dolev A, Rust M, Meoded RA, Bhushan A, Califano G, Costa R, Gugger M, Steinbeck C, Moreno P, and Piel J Automated structure prediction of trans-acyltransferase polyketide synthase products. Nat. Chem. Bio 2019, 15, 813–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Komaki H, Ichikawa N, Hosoyama A, Takahashi-Nakaguchi A, Matsuzawa T, Suzuki K, Fujita N, and Gonoi T Genome based analysis of type-I polyketide synthase and nonribosomal peptide synthetase gene clusters in seven strains of five representative Nocardia species. BMC Genomics 2014, 15, 323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kageyama A, Yazawa K, Mukai A, Kohara T, Nishimura K, Kroppenstedt RM, and Mikami Y Nocardia araoensis sp. nov. and Nocardia pneumoniae sp. nov., isolated from patients in Japan. Int. J. Syst. Evol. Microbiol 2004, 54, 2025–2029. [DOI] [PubMed] [Google Scholar]
- [9].Kuo J, Lynch SR, Liu CW, Xiao X, and Khosla C Partial In Vitro Reconstitution of an Orphan Polyketide Synthase Associated with Clinical Cases of Nocardiosis. ACS Chem. Bio 2016, 11, 2636–2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Yuet KP, Liu CW, Lynch SR, Kuo J, Michaels W, Lee RB, Mcshane AE, Zhong BL, Fischer CR, and Khosla C Complete reconstitution and deorphanization of the 3 MDa nocardiosis-associated polyketide synthase. J. Am. Chem. Soc 2020, 142 (13), 5952–5957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Busch B, Ueberschaar N, Sugimoto Y, and Hertweck C Interchenar Retrotransfer of Aureothin Intermediates in an Iterative Polyketide Synthase Module. J. Am. Chem. Soc 2012, 134, 12382–12385. [DOI] [PubMed] [Google Scholar]
- [12].He J, and Hertweck C Iteration as Programmed Event during Polyketide Assembly; Molecular Analysis of the Aureothin Biosynthesis Gene Cluster. Chemistry & Biology 2003, 10, 1225–1232. [DOI] [PubMed] [Google Scholar]
- [13].Moss SJ, Martin CJ, and Wilkinson B Loss of collinearity by modular polyketide synthases: a mechanism for the evolution of chemical diversity. Nat. Prod. Rep 2004, 21, 575–593. [DOI] [PubMed] [Google Scholar]
- [14].Nivina A, Yuet KP, Hsu J, and Khosla C Evolution and Diversity of Assembly-Line Polyketide Synthases. Chemical Reviews 2019, 119, 12524–12547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Gaitatzis N, Silakowski B, Kunze B, Nordsiek G, Blöcker H, Höfle G, and Müller R The biosynthesis of the aromatic myxobacterial electron transport inhibitor stigmatellin is directed by a novel type of modular polyketide synthase. J. Biol. Chem 2002, 277, 13082–13090. [DOI] [PubMed] [Google Scholar]
- [16].Mochizuki S, Hiratsu K, Suwa M, Ishii T, Sugino F, Yamada K, and Kinashi H The large linear plasmid pSLA2-L of Streptomyces rochei has an unusually condensed gene organization for secondary metabolism. Mol. Microbiol 2003, 48, 1501–1510. [DOI] [PubMed] [Google Scholar]
- [17].Olano C, Wilkinson B, Sánchez C, Moss SJ, Sheridan R, Math V, Weston AJ, Braña AF, Martin CJ, Oliynyk M, Méndez C, Leadlay PF, and Salas JA Biosynthesis of the angiogenesis inhibitor borrelidin by Streptomyces parvulus Tuü4055: cluster analysis and assignment of functions. Chem. Biol 2004, 11, 87–97. [DOI] [PubMed] [Google Scholar]
- [18].Traitcheva N, Jenke-Kodama H, He J, Dittmann E, and Hertweck C Non-colinear polyketide biosynthesis in the aureothin and neoaureothin pathways: an evolutionary perspective. ChemBioChem 2007, 8, 1841–1849. [DOI] [PubMed] [Google Scholar]
- [19].Busch B, Ueberschaar N, Behnken S, Sugimoto Y, Werneburg M, Traitcheva N, He J, and Hertweck C Multifactorial Control of Iteration Events in a Modular Polyketide Assembly Line. Angew. Chem. Int. Ed 2013, 52, 5285–5289. [DOI] [PubMed] [Google Scholar]
- [20].Curran SC, Hagen A, Poust S, Chan LJG, Garabedian BM, Rond TD, Baluyot M-J, Vu JT, Lau AK, Yuzawa S, Petzold CJ, Katz L, and Keasling JD Probing the Flexibility of an Iterative Modular Polyketide Synthase with Non-Native Substrates in Vitro. ACS Chem. Biol 2018, 13, 2261–2268. [DOI] [PubMed] [Google Scholar]
- [21].Sugimoto Y, Ishida K, Traitcheva N, Busch B, Dahse H-M, and Hertweck C Freedom and Constraint in Engineered Noncolinear Polyketide Assembly Lines. Chemistry & Biology 2015, 22, 229–240. [DOI] [PubMed] [Google Scholar]
- [22].Piel J Biosynthesis of polyketides by trans-AT polyketide synthases. Nat. Prod. Rep 2010, 27, 996–1047. [DOI] [PubMed] [Google Scholar]
- [23].Pfeifer BA; Admiraal SJ; Gramajo H; Cane DE; Khosla C Biosynthesis of complex polyketides in a metabolically engineered strain of E. coli. Science 2001, 291, 1790–1792 [DOI] [PubMed] [Google Scholar]
- [24].Hughes AJ; Keatinge-Clay A Enzymatic extender unit generation for in vitro polyketide synthase reactions: structural and functional showcasing of Streptomyces coelicolor MatB. Chem. Biol 2011, 18, 165–176. [DOI] [PubMed] [Google Scholar]
- [25].Lombó F; Pfeifer B; Leaf T; Ou S; Kim YS; Cane DE; Licari P; Khosla C Enhancing the atom economy of polyketide biosynthetic processes through metabolic engineering. Biotechnol. Prog 2001, 17, 612–617. [DOI] [PubMed] [Google Scholar]
- [26].Klaus M; Ostrowski MP; Austerjost J; Robbins T; Lowry B; Cane DE; Khosla C Protein-protein interactions, not substrate recognition, dominate the turnover of chimeric assembly line polyketide synthases. J. Biol. Chem 2016, 291, 16404–16415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Leesong M, Henderson BS, Gillig JR, Schwab JM, Smith JL. Structure of a dehydratase-isomerase from the bacterial pathway for biosynthesis of unsaturated fatty acids: two catalytic activities in one active site. Structure 1996, 4, 253–256. [DOI] [PubMed] [Google Scholar]
- [28].Zabala Angelica O., Chooi Yit-Heng, Choi Moon Seok, Lin Hsiao-Ching, and Tang Yi ACS Chemical Biology. 2014, 9, 1576–1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Wang J, Liang J, Chen L et al. Structural basis for the biosynthesis of lovastatin. Nat. Commun 2021, 12, 867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Wang B, Guo F, Huang C, Zhao H. Unraveling the iterative type I polyketide synthases hidden in Streptomyces. Proc Natl Acad Sci U S A. 2020, 117, 8449–8454. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


