Abstract
Background:
Current treatment options for atrial fibrillation (AF), the most common arrhythmia in clinical practice, have limited success. Previous attempts at treating AF using implantable devices have been limited by the painful nature of high-voltage shocks.
Objectives:
This first-in-human feasibility study was undertaken to translate the novel low-voltage MultiPulse Therapy (MPT), which was previously been shown to be effective in preclinical studies in terminating AF, into clinical use.
Methods:
42 patients undergoing AF ablation were recruited at 6 investigational centers worldwide. Prior to ablation, electrode catheters were placed in the coronary sinus, right and/or left atrium for recording and stimulation. After the induction of AF, MPT, which consists of up to a 3-stage sequence of far- and near-field stimulation pulses of varied amplitude, duration and inter-pulse timing, was delivered via temporary intracardiac leads. MPT parameters and delivery methods were iteratively optimized.
Results:
In the 14 patients from the Efficacy Phase, MPT terminated 37/52 (71%) of AF episodes with the lowest median energy of 0.36J (IQR 0.14–1.21) and voltage of 42.5V (25–75). 38% of AF terminations occurred within 2 seconds of MPT delivery (p<0.0001). Shorter time between AF induction and MPT predicted success of MPT in terminating AF (p<0.001).
Conclusions:
MultiPulse Therapy effectively terminated AF at voltages and energies known to be well-tolerated or painless in some patients. Our results support further studies of the concept of implanted devices for early AF conversion to reduce AF burden, symptoms and progression.
Keywords: Atrial fibrillation, Multipulse therapy, cardioversion, defibrillation
CONDENSED ABSTRACT
Current treatments options for atrial fibrillation (AF), such as catheter ablation or anti-arrhythmic medication, have limited success rates. Previous attempts at using implantable devices to cardiovert AF soon after its onset were abandoned as the shocks were deemed too painful for patients to tolerate. This first-in-human feasibility study was undertaken to translate the novel low-voltage MultiPulse Therapy (MPT), into clinical use. MPT, which consists of up to a 3-stage sequence of far- and near-field stimulation pulses of varied amplitude, duration and inter-pulse timing, effectively terminated AF at voltages and energies known to be well-tolerated or painless in some patients.
INTRODUCTION
Atrial fibrillation (AF) is a global epidemic with approximately 33.5 million individuals affected worldwide and close to 5 million new cases occurring each year (1), costing the US healthcare system between $6 and $26 billion annually (2). Atrial fibrillation is a progressive disease, with many patients first developing paroxysmal AF, and if left untreated, can progress from paroxysmal to persistent or permanent AF, due to electrical and structural remodeling (3), changes in the autonomic nervous system and Ca2+ handling abnormalities (4), all of which create a more proarrhythmic atria that further exacerbates AF (5). Because of these rapid changes in the atria after AF onset, a therapy to treat AF soon after onset may slow the progressive nature of AF.
In the 1990s, there was significant interest in the atrial defibrillator, an implantable device designed with the intent of cardioverting AF soon after its onset to restore sinus rhythm, reduce symptoms, prevent atrial remodeling and potentially negate the need for long-term anticoagulation (6,7). The atrial defibrillator was successful in acutely cardioverting AF though it was ultimately abandoned as the shocks were deemed too painful for patients to tolerate for a non-life threatening arrhythmia such as AF, when repeated shocks were required following early AF recurrences (8–11).
In several preclinical studies, we reported that multiple low-energy, low-voltage pulses can terminate both AF and ventricular tachycardia as successfully as a conventional high-energy biphasic shock (12–14). As the energy and voltage required to cardiovert the arrhythmias varied according to the timing of the pulse in relation to the phase of the tachyarrhythmia cycle (15,16), a series of low-energy far-field pulses increased the probability of a pulse being applied within the critical time window for successful termination of AF. Critically-timed low-energy far-field and near-field electrical pulses can induce multiple virtual electrodes at areas of structural heterogeneity, which generate new wavefronts that interact with fibrillatory drivers, leading to the destabilization and unpinning of these drivers (16–19). Here, we present the results of our first-in-human translational study investigating the safety and feasibility of low-energy MultiPulse Therapy (MPT) in terminating AF in patients undergoing catheter ablation.
METHODS
Study design
This was an open-label, multicenter study involving the first-in-human acute testing of MPT algorithms in patients undergoing catheter ablation of AF. The study consisted of 42 patients undergoing transvenous catheter ablation of AF for documented paroxysmal or persistent AF at 6 centers worldwide (Imperial College London, United Kingdom; University Hospital Brno, Brno, Czech Republic; Hôpital Cardiologique du Haut-Lévêque, Bordeaux-Pessac, France; Na Homolce, Prague, Czech Republic; Institut klinické a experimentální medicíny, Prague, Czech Republic; Sequoia Hospital, Redwood City, California). Exclusion criteria included NYHA Class III-IV heart failure at the time of enrollment, left ventricular ejection fraction < 40%, history of thromboembolic events, or the presence of a mechanical tricuspid heart valve or an active implantable device. The study was performed in compliance with the Declaration of Helsinki and the institutional review board or research ethics committee of each participating center approved the study protocol. All patients gave written informed consent prior to the start of the study after a complete discussion of benefits and risks. This was a single arm study with no pre-defined statistical endpoints, and without blinding. The subject demographics are reported in Table 1.
Table 1.
Patients Demographics and Clinical Characteristics
| Parameters | Overall Population (N = 42) | Efficacy Phase (N = 16) |
|---|---|---|
| Age (years) | 60.5 (51.7–64.9) (41/42) | 60.5 (52.7, 64.5) (15/16) |
|
| ||
| Sex (male) (%) | 79 (33/42) | 69 (11/16) |
|
| ||
| LA Size (mm) | 42.0 (38.5–46.0) (32/42) | 40.0 (38.0–43.0) (12/16) |
|
| ||
| LVEF (%) | 60.0 (58.5,−65.5) (36/42) | 62.5 (60.0–68.0) (14/16) |
|
| ||
| PAF Durations (months) | 40.2 (19.8–73.7) (32/42) | 57.0 (14.9–100.6) (12/16) |
|
| ||
| Beta Blocker Use | 57% (24/42)* | 69% (11/16) |
|
| ||
| AAD Use | 62% (26/42)* | 69% (11/16) |
| Class Ic | 38% (16/42)** | 44% (7/16) |
| Class III | 21% (9/42)** | 25% (4/16) |
Results are median (Q1-Q3) (n/N)
Abbreviations: LA: left atrial roof; LVEF: Left ventricular ejection fraction; PAF: Paroxysmal atrial fibrillation : AAD: Antiarrhythmic drug
1 patient had no Beta Blocker or AAD information entered
1 patient who reported AAD use did not have a class listed
Study Protocol
All procedures were performed according to each center’s standard of care for AF ablation. The study protocol was performed prior to the clinically indicated AF catheter ablation procedure and is summarized in Figure 1A. Following the introduction of femoral venous sheaths and catheters, defibrillation leads were placed in the heart (Figure 1B & 1C). Multipolar catheters were positioned in the right and left atrium (RA and LA respectively) and, in some cases, also at the LA roof for electrogram recordings. Multiple electrode configurations were used in this study. In the majority of cases, a single-coil cardioversion/defibrillation lead (Model 6937/A, Medtronic, Dublin, Ireland) was placed in the distal coronary sinus (CS) and a second defibrillation lead (Model 6944), utilizing the distal coil, was positioned to maintain tissue contact along the septal wall or crista terminalis of the RA (Figure 1B). A CS to LA vector was also tested in some patients with a multipolar catheter placed in the LA (Figure 1C), via a transseptal puncture. Following the placement of leads and electrodes, MPT was delivered by the Cardiac External Stimulation System research stimulator (Cardialen Inc., Minneapolis, MN) to terminate AF.
Figure 1:
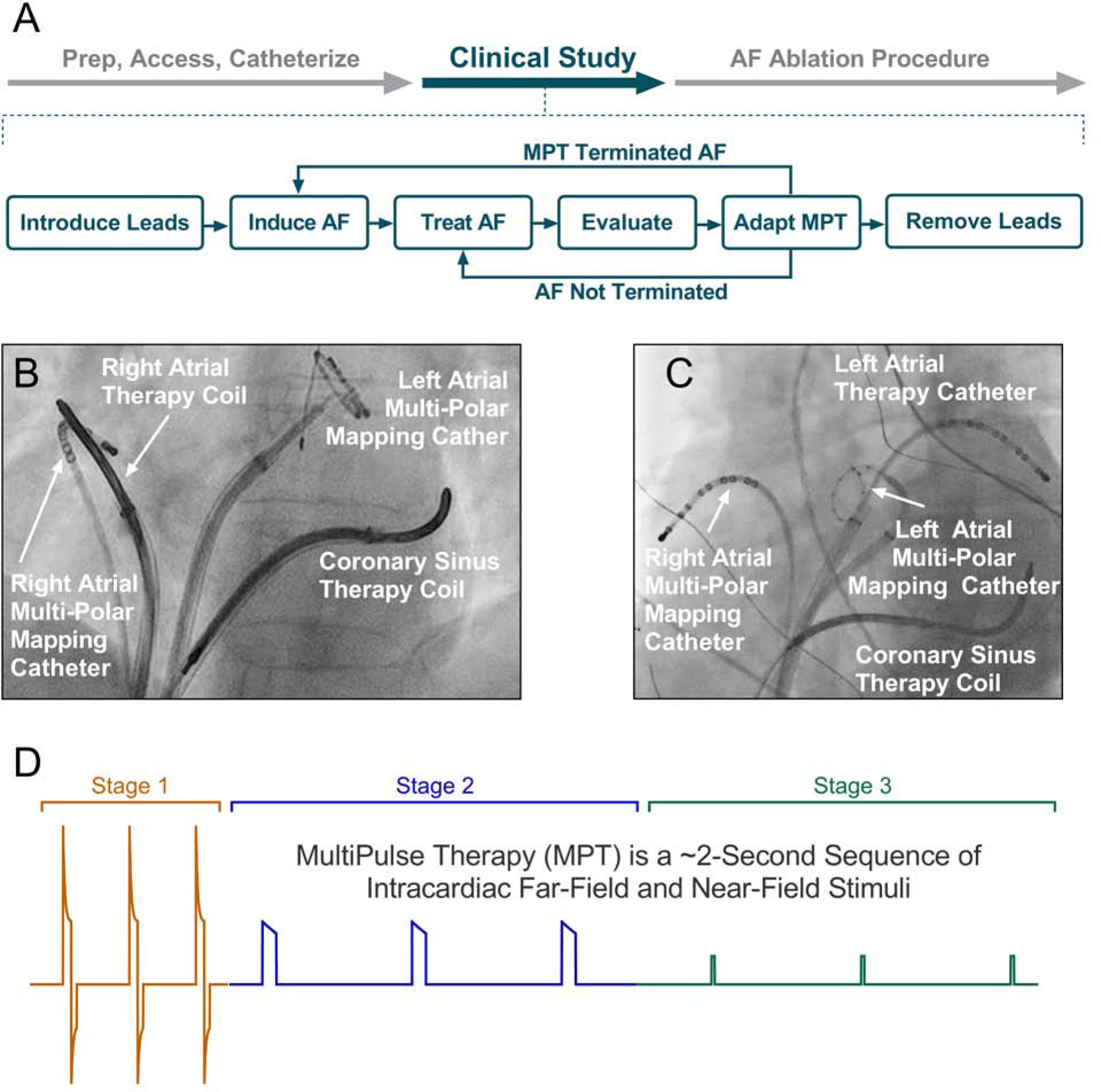
MultiPulse Therapy: (A) Study protocol at the start of the atrial fibrillation ablation procedure; (B and C, RA and LA, respectively) Lead positioning for MPT studies. Defibrillation leads were placed in the right atrium and in the coronary sinus and catheters were also placed in the left atria, to allow different vectors for MPT delivery. (D) Schematic of the 3 MultiPulse Therapy stages.
MultiPulse Therapy
MultiPulse Therapy (Cardialen, Inc., Minneapolis, MN) is a novel low-energy therapy designed to treat tachycardia and fibrillation. The MPT algorithm consists of up to a 3-stage sequence of far- and near-field stimulation pulses of varied amplitude, duration, and inter-pulse timing (Figure 1D). In this study, varying numbers of MPT stages were delivered per episode, with all MPT deliveries consisting of at least of Stage 1 therapy. Stage 1 involves far-field biphasic shocks triggered by an R-wave and delivered between the defibrillation coils during the ventricular effective refractory period. Stages 2 and 3 are far-field and near-field monophasic pulses and delivered with a programmable interval.
In the course of translating this therapy from experimental models to human subjects, changes were made iteratively to the methods. The MPT therapy parameters were changed, the therapy vectors were varied (CS-RA; CS-LA roof), the time from induction of AF to therapy onset varied (120 sec, 15 seconds), and the locations of the leads and electrode catheters were varied.
The study objectives were to determine the safety and efficacy of MPT therapy in humans, and to describe the atrial response to MPT therapy at a variety of programmed settings in patients with AF. The specific details on therapy (outcome, time in AF, time to Normal Sinus Rhythm (NSR) if applicable and voltage) for all 296 MPT deliveries in the Efficacy Phase with detailed data analysis are provided in Online Table 1. The electrogram traces for 276 of these 296 MPT deliveries are presented in Online Figure 1. Twenty therapies were not recorded on the EP recording system. These electrogram traces include when a low-voltage 5V MPT was delivered to assess impedance and is denoted in the traces.
Statistical Analysis
Continuous variables are presented as median (IQR, Q1-Q3) and categorical variables are represented as frequencies and percentages. Post-hoc comparison was performed by non-parametric tests as noted in the text and online supplement. A p value < 0.05 was considered statistically significant. All analyses were performed with SAS software version 9.1 (SAS Institute Inc, USA), R v 3.5.1 and Prism 8.02 (GraphPad, USA). The statistical methods used to measure the efficacy of MPT, the causal relationship between MPT delivery and AF termination, and the determinants of MPT efficacy are presented in detail in the Online Supplement.
RESULTS
A total of 42 patients were enrolled in the study. Because of the nature of this translational study, whereby the MPT parameters and vectors were iteratively optimized, the patients were retrospectively classified into two sequential groups. The first group (Group 1 – Translational Phase) consisted of the initial 26 patients. Of those 26 patients 13 were excluded due to incomplete waveform data, and 1 because AF was not reliably induced and the lead position was unstable thus MPT was not delivered.. The remaining 12 patients in this group had MPT delivered ~120 seconds after AF induction, and there was variation of MPT parameters and vectors as the therapy was being optimised. The latter 16 consecutive patients (Group 2 – Efficacy Phase) is the focus of this manuscript and all efficacy analysis. Group 2 had MPT therapy delivered ~15 seconds post-AF induction, and had more uniform MPT parameters. Of these 16 consecutive patients in Group 2, 1 was excluded from the analysis due to AF refractory to transthoracic cardioversion and 1 due to lack of waveform data resulting in 14 analyzed patients. Patient demographics and clinical characteristics are reported in Table 1 and the flow of patients is depicted in Figure 2. The specific details on MPT therapy including outcome, time in AF, time to Normal Sinus Rhythm (NSR) when applicable, and voltage for all 296 MPT deliveries and the electrograms of the Efficacy Phase are provided in Online Table 1 and Online Figure 1, respectively.
Figure 2:
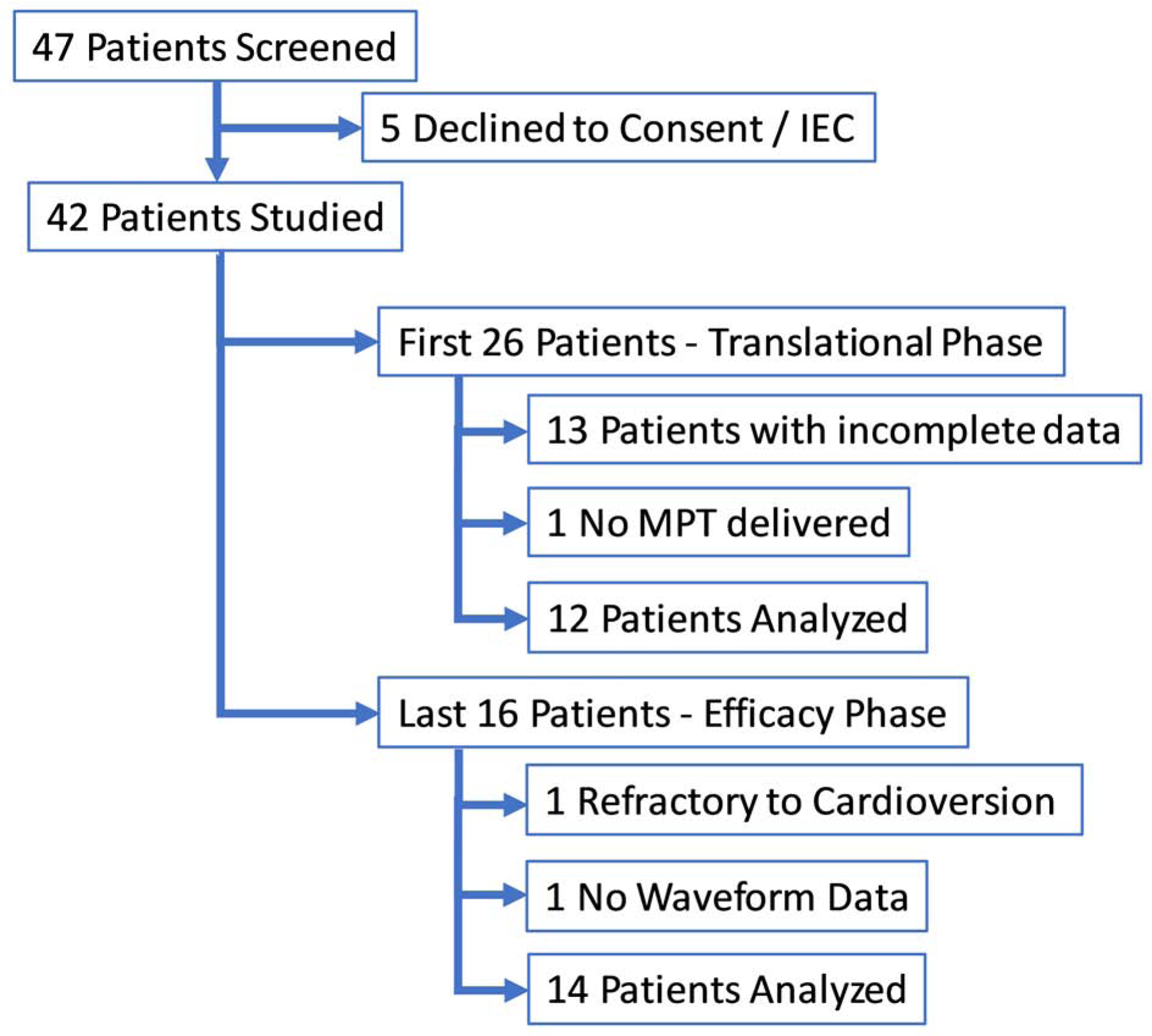
Patient Disposition Flow Chart: A total of 42 patients were enrolled in the study. and retrospectively divided into two groups. In the first group (n=26), Translational Phase, MPT therapy was not delivered until at least 120 seconds post AF induction. Of the 26 patients 13 patients were excluded due to incomplete waveform data and 1 because AF was not able to be induced and MPT was not delivered resulting in 12 patients in this group. In the second group (n=16), Efficacy Phase, MPT therapy was not delivered until at least 15 seconds post AF induction. Of the 16 consecutive patients in the Efficacy Phase, 2 were excluded from the analysis due to AF refractory to transthoracic cardioversion and because of the lack waveform data.
Group 1 – “Translational Phase”
In this first group of patients, a safe and reproducible method of placing transvenous leads via the femoral venous approach was developed, and we confirmed that MPT can be safely and reliably delivered in humans. The preclinical MPT parameters, lead placements and electrotherapy vectors were systematically modified and optimized for use in humans. For these first 12 patients analysed in this group, 241 MPTs were delivered with 3 terminations (1.2% success), with a median voltage and energy of 25V (25–37.5) and 0.16J (0.14–0.29) respectively.
Efficacy of MPT (Group 2 – “Efficacy Phase”)
Of the 14 patients analysed in this group, MPT converted AF in 10/14 (71%) patients, using an arbitrary definition of success of AF termination within 20 seconds of MPT delivery. Representative intracardiac electrograms showing termination of AF with MPT are shown in Figure 3A. MPT terminated AF at a median lowest energy of 0.36J (0.14 – 1.21) and a median lowest leading-edge voltage of 42.5V (25–75) (Figure 3B and 3C). On a per episode termination basis, 37 of the 52 AF episodes (71%) terminated by MPT, the median energy leading-edge voltage were 0.58J (0.15–1.15) and 50V (25 – 75), respectively (Figure 3D). We also report other stricter definitions of success, such as termination of AF within 5, 10 and 15 seconds of MPT, which resulted in lower success rates (33%, 44% and 58%, respectively), but without affecting the median energy and voltage (Figure 3E, 3F, 3G).
Figure 3:
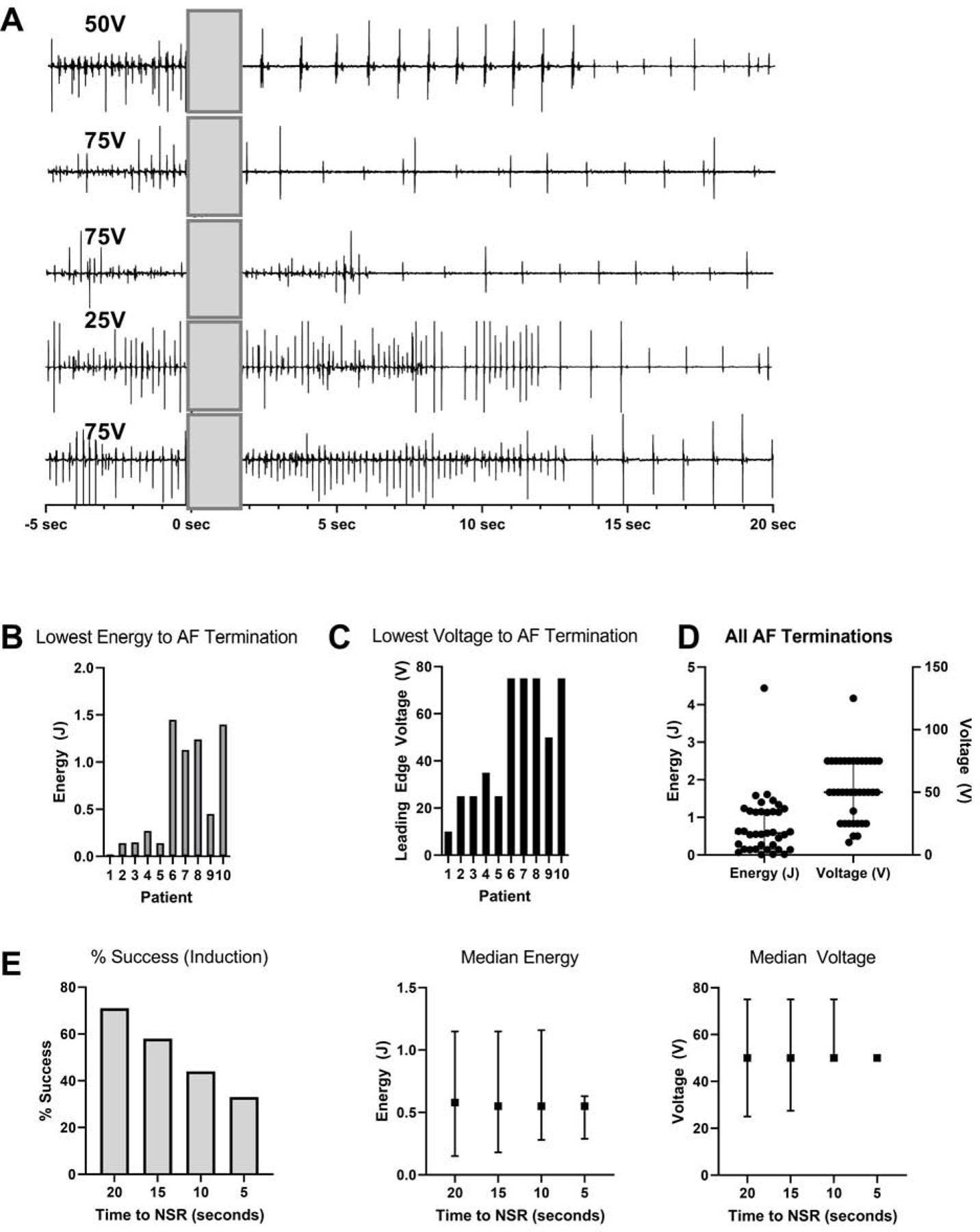
Lowest energy and voltage for AF termination: (A) Representative intracardiac electrograms showing termination of AF with MPT (delivered at t=0s). (B & C) The lowest energy and voltage required for AF termination per patient. (D) The median energy and voltage required for successful AF termination. Error bars represent Q1-Q3 (IQR).
Data for B, C & D are based on the definition of success as AF termination within 20 seconds of MPT. (E) The % success and median energy and voltage required for cardioversion based on different definitions of success, i.e. termination within 20, 15, 10 and 5 seconds of MPT. Error bars represent Q1-Q3 (IQR).
Causal relationship between MPT and AF termination
Because most patients had paroxysmal AF and MPT was delivered into induced AF, which can terminate spontaneously, analyses were performed to support a causal relationship between MPT delivery and AF termination. Figure 4A shows the AF durations for each MPT delivery and Figure 4B shows that the temporal relationship between MPT delivery and AF termination follows a skewed distribution, with a median time to conversion of 6.1± 6.8s. (Kolmogorov-Smirnov test, p < 0.0001).
Figure 4:
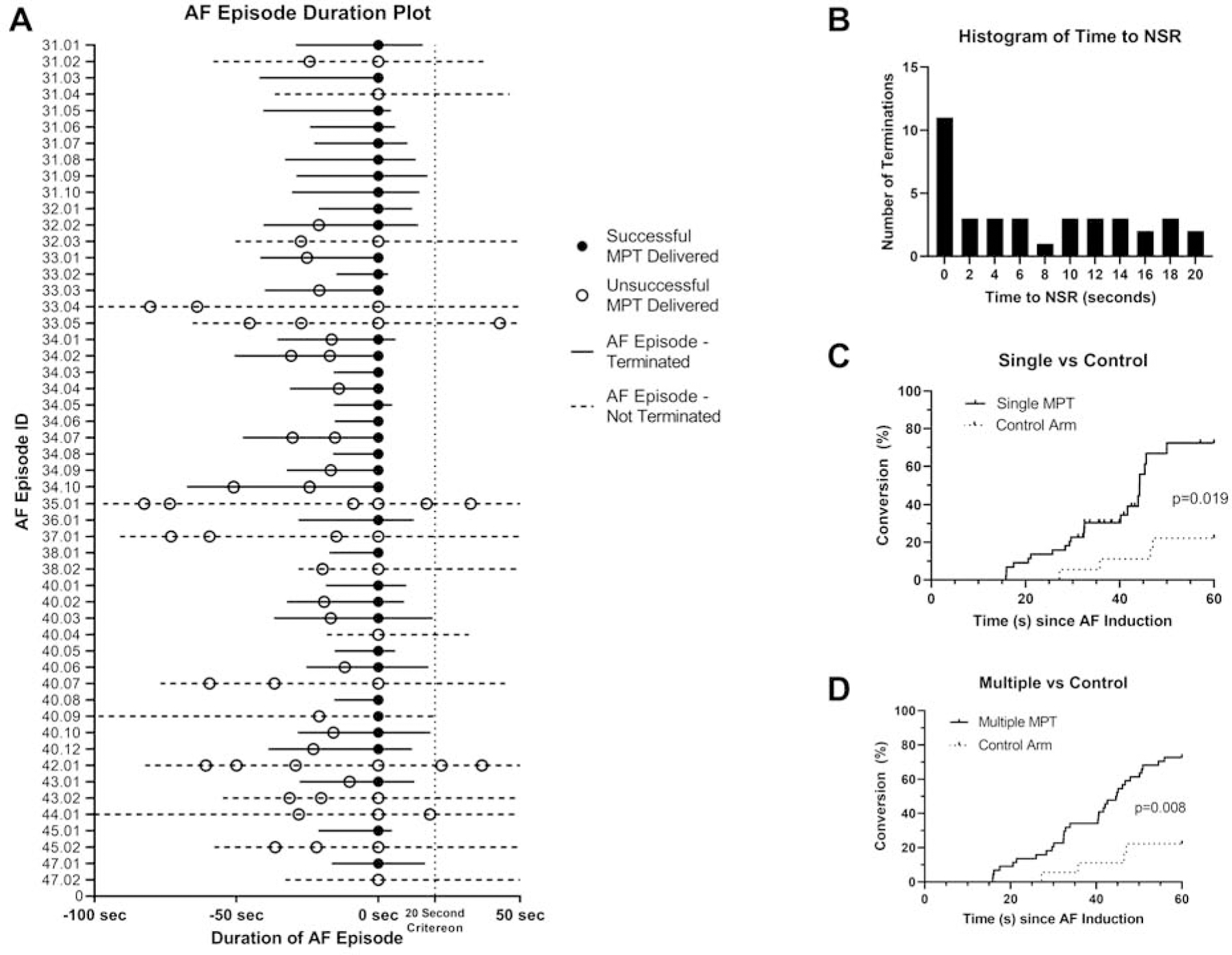
Causality analysis for AF terminations after MPT: (A) AF duration plot for all 52 episodes of MPT deliveries (aligned at time of MPT delivery, t=0 seconds), showing the temporal relationship between MPT and AF termination. (B) Distribution of time to AF termination after MPT (time=0 seconds) for all 37 successful terminations. The distribution was skewed towards therapy time (p<0.0001) (Kolmogorov-Smirnov test), with many AF terminations occurring within 2 seconds of MPT, consistent with a causal relationship between MPT and AF termination. (C & D) Kaplan Meier curves comparing AF terminations when assigned to MPT (n=12 patients with 44 episodes) versus a retrospective control untreated arm (n=7 patients with 18 episodes) to an MPT therapy arm. The effect of both a single MPT or multiple MPTs on AF termination are shown.
To exclude the possibility that the AF terminations were spontaneous and unrelated to MPT, the effect of single and multiple MPTs on AF termination rates (n = 12, 44 AF episodes) were compared to the rates in patients where no MPT was delivered for > 60 seconds (n = 7, 18 AF episodes) (only episodes where AF was sustained for > 15 seconds were included, Figure 4C and 4D). Overall, the MPT arms had greater AF termination rates (single MPT 73%, multiple MPT 72% vs controls 22% at 60 seconds following AF induction, p = 0.019 and p = 0.008, respectively), suggesting MPT causally contributed to AF terminations.
Determinants of MPT efficacy
Univariate and multivariate analysis demonstrated that MPT success was significantly related to the time between AF induction and MPT delivery (Figure 5). A trend between AF cycle length and MPT terminations was observed in some statistical models (p = 0.06). Unlike single biphasic shocks, there was no relationship between MPT strength (voltage and energy) and the conversion rate, suggesting of a different mechanism of action for MPT. Beta-blocker (p=0.16) and anti-arrhythmic drugs (III vs none, p=0.53, IC vs none, p=0.50) also did not affect MPT efficacy. Although the therapy vectors were not associated with MPT success in the multivariate analysis, there was a trend towards statistical significance for the Stage 1 vector being a determinant of the energy required to terminate AF. In the Efficacy Phase (Group 2), 81% of the 296 therapies delivered were via the LA to CS vector with the remaining 20% delivered via the RA to CS vector. The energies required to terminate AF with the CS-LA vector was 0.45J (0.14–0.61) (n = 27) compared with 1.16J (1.14–1.54), for the CS-RA vector (n=10) (p = 0.089, permutation test).
Figure 5:
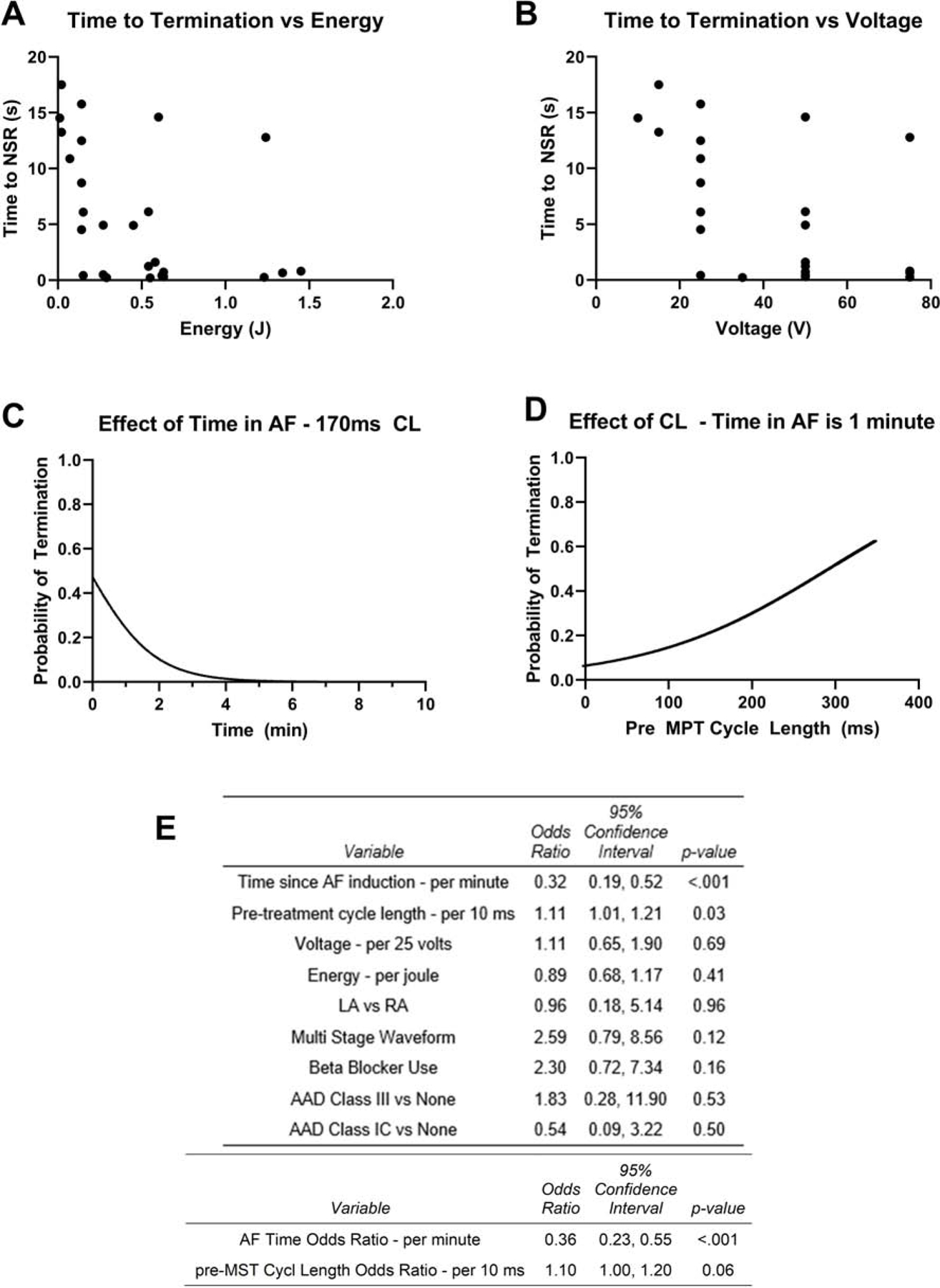
Determinants of MPT success: (A & B) The energy and voltage of MPT were not related to time to termination of AF. (C & D) The effects of Time in AF and Cycle Length on probability of AF Termination via multivariate analysis are shown (E) Univariate and multivariate analysis of the predictors of MPT success. For the multivariate analysis, the duration of AF before MPT delivery predicted MPT success. There was a trend towards significance for AF cycle length predicting MPT success.
Clinical safety of MPT
MPT therapy was delivered in total 666 times in 42 subjects during this study. There were 11 adverse events (AE) in the study. Of those 11 AEs, one event was unrelated to the study and 10 were related to MPT delivery. Eight AEs in 5 subjects occurred when MPT was delivered into sinus rhythm due to AF termination prior to MPT therapy, resulting in reinitiation of AF. The remaining 2 adverse events were observed early in the study in one subject, when MPT extended into the T-wave and induced VF. In one AE, VF was non-sustained having a duration of < 5 seconds. In the second AE, the subject was treated with one successful transthoracic defibrillation shock and no untoward effects were reported after the procedure.
In these two instances of VF being induced by MPT, the prior ventricular rate was >120bpm and the R-T interval was approximately 110ms, measured from the peak of the R-wave to the onset of the T-wave. Unlike standard high-energy therapy, where a short 10ms (6/4ms) biphasic shock is delivered, MPT consists of multiple pulses with an S1-S1 interval, thus creating a longer duration during which therapy is delivered. There is therefore a higher probability of extending therapy delivery into the T-wave, which is exacerbated by a short R-T interval. An implantable device would have a minimum R-R interval criterion, which must be met before therapy is delivered, to avoid this occurrence.
DISCUSSION
This first-in-human study demonstrates the efficacy of low-energy, low-voltage MPT for the treatment of AF. The median lowest energy and leading-edge voltage required for AF termination in the Efficacy Phase patients were 0.36J (0.14–1.21) and 42.5V (25–75) respectively. Kaplan-Meier analysis comparing of MPT-treated patients to non-treated patients showed that MPT significantly increased AF conversion to sinus rhythm compared to no treatment.
Low-energy low-voltage cardioversion with MPT
In this study, the energies and voltages required to successfully cardiovert AF in humans were comparable to those reported in our in vivo preclinical studies. In our previous studies in acute and persistent AF in canine models, the mean defibrillation thresholds were 0.19±0.12 J and 0.16±0.16 J respectively (12,14). For comparison, the median lowest energy in the Efficacy Phase in this study was higher at 0.36J (0.14–1.21), but was still lower than the energy previously reported to be required for a single biphasic shock (mean±SD 1.48±0.91 J) (13). This higher atrial cardioversion threshold with MPT in man might be due to the larger size of the human atrium versus the canine model, and in addition the human subjects all had an intrinsic atrial pathologic process leading to spontaneous development clinically of atrial fibrillation while the canine model had no spontaneous atrial fibrillation. Variations in the minimum energy required for AF cardioversion between humans and canines also may in part be due to anatomical differences and the resultant differences in positioning of the intracardiac catheters and defibrillation coils. In our study, Stages I and Stage II of MPT were delivered across either a CS to RA vector or CS to LA roof vector, whereas the canine studies used either a left atrial disc electrode (12) or a defibrillator lead in the left pulmonary artery (14), in addition to defibrillator leads in the coronary sinus and right atrium. In both of the canine studies, the vectors for Stages I & II of MPT were more extensive, across both atria.
Pain perception with MPT was not formally assessed in this study as patients were either under general anesthesia or deep sedation for the catheter ablation procedure. However, the energies employed in our study were possibly below the pain threshold for humans, reported to range from <1 to 2 J (20–23). Even if the MPT therapy is perceptible, the energies and voltages are below the reported tolerability thresholds. In previous pain threshold studies with the atrial defibrillator, patients were able to tolerate a mean of 3.4±2 shocks, with a tolerability threshold of 255±60 V, 2.5±1.3 J (24), whilst patients tolerated shocks without sedation up to 116±51 V in a separate study (11).
Mechanism of AF termination with MPT
Our data provide indirect support for the potential mechanisms for MPT, as previously examined in prior preclinical studies (12,14,15,19). Unlike with conventional high-energy biphasic shocks, AF did not terminate immediately following MPT, but can often continue for several seconds before termination, suggesting destabilization of AF prior to AF termination.
These observations are consistent with previous preclinical and computational studies (12,14,15,19,25–27), which suggested that MPT terminated AF by causing virtual electrode polarization at sites of structural heterogeneities, which then serve as initiation sites of new electrical wavefronts that interact with reentrant AF drivers, destabilize them and cause termination of the arrhythmia (16–19). A proposed mechanism for MPT is that Stage 1 shocks disrupt AF drivers, Stage 2 entrainment shocks continue to silence the AF drivers, whilst MPT Stage 3 pacing stimuli provide consistent atrial activation until sinus rhythm is restored (Figure 1D)(14). In support of MPT having a different mode of action than single biphasic shock, is that unlike single biphasic shocks, which are more successful with increasing voltage, we did not see a relationship between increasing voltage and energy with MPT termination of AF.
The mechanism of delayed AF termination with MPT created two significant challenges for the data analysis. One challenge was selecting an appropriate definition of success. Our analyses were based on an arbitrary definition of success of AF termination within 20 seconds of MPT delivery. Using this criterion, MPT converted AF in 71% of patients at the lowest median energy of 0.36 J and lowest median leading-edge voltage of 42.5 V. Similar results were also observed on a per episode termination basis. However, when stricter definitions of success were used (termination of AF within 5, 10 and 15 seconds of MPT), MPT was associated with lower success rates but without affecting the median energy and voltage. The other challenge was determining if a specific AF termination was attributable to the MPT, as our study involved mainly patients with paroxysmal AF, where MPT was delivered into induced AF, which can sometimes spontaneously terminate. Additional statistical analyses confirmed a temporal relationship between MPT and AF terminations, and that the termination rate with MPT was greater than the expected spontaneous termination rate, both supporting that MPT causally contributed to AF terminations.
Determinants of MPT efficacy
Based on univariate and multivariate analyses, MPT success was significantly related to the duration of time between AF induction and MPT delivery suggesting that AF may be more organized early after induction and more amenable to termination with MPT. Our results are in agreement with a previous study in a goat model showing that the AF cycle length shortens very rapidly thereby suggesting a rapid functional remodeling within the first few hours after AF onset (5). This observation supports our own that MPT is most successful if delivery early during an AF episode. We also observed a trend for AF cycle length to be associated with MPT termination of AF which further supports the hypothesis that MPT is more effective when AF is more organized (28,29). Unlike single biphasic shocks, we did not find a relationship between MPT strength (voltage and energy) and conversion rate, suggesting a different mechanism of action for MPT.
Potential Clinical Application of MPT
The first target patient population for clinical implementation of MPT would be patients with an existing indication for an ICD or CRT-D device. In these patients, and additional or a modified coronary sinus lead with a defibrillation coil will allow delivery of MPT therapy. There is a 29% prevalence of paroxysmal AF in patients receiving ICD devices, (30) who will benefit from this therapy. While the results for the patient population enrolled in this study (NYHA Class I-II and average LVEF of 61%) are promising, further study is needed to determine if MPT will perform in a similar manner for heart failure patients with reduced LVEF who are likely to have indications for an ICD or CRTD device. This therapy may not only reduce symptoms, but if paroxysmal AF can be treated early in the AF episode, the progression of AF from paroxysmal to persistent may be slowed. (3,5)
Due to a high number of delivered therapies, previous atrial cardioverters suffered from rapid battery depletion. The Jewel AF study reported 7 treated episodes of AF per patient-month, of which ~60% were treated by high-energy shocks.(31) The average MPT threshold of 0.8J in our study is approximately 5% of the energy delivered in Jewel AF. Accordingly, MPT would not be expected to have the same effect of rapid battery depletion as that associated with higher-energy atrial cardioversion therapies of prior devices.
Limitations
In the Efficacy Phase, all patients had paroxysmal AF and AF had to be induced prior to delivery of MPT. Acute electrically-induced AF may be qualitatively different to clinical AF, with longer AFCLs (32), and may require different energies and voltages to terminate. A further limitation is that the patients were either sedated or anesthetized for the catheter ablation procedure, which prevented a formal assessment of pain perception of MPT. While no data was obtained regarding pain or patient tolerability of MPT, the voltage and energy of MPT are within the range of tolerability in previous studies.(11) Further studies will be required to specifically address the question of patient discomfort and tolerability.
Conclusions
Low-energy MPT is a promising therapy for the treatment of AF. The voltages and energies required for successful termination of AF with MPT are lower than those using conventional biphasic shocks, and at levels that are previously known to be painless or well-tolerated. The results of this first-in-man study justify further systematic investigation of MPT. Delivery of MPT via chronically implanted devices may represent a novel therapeutic approach that allows for early cardioversion of AF to reduce symptom burden and delay AF progression. Further investigation into MPT to treat both paroxysmal and persistent AF are currently underway.
Supplementary Material
Central Illustration:
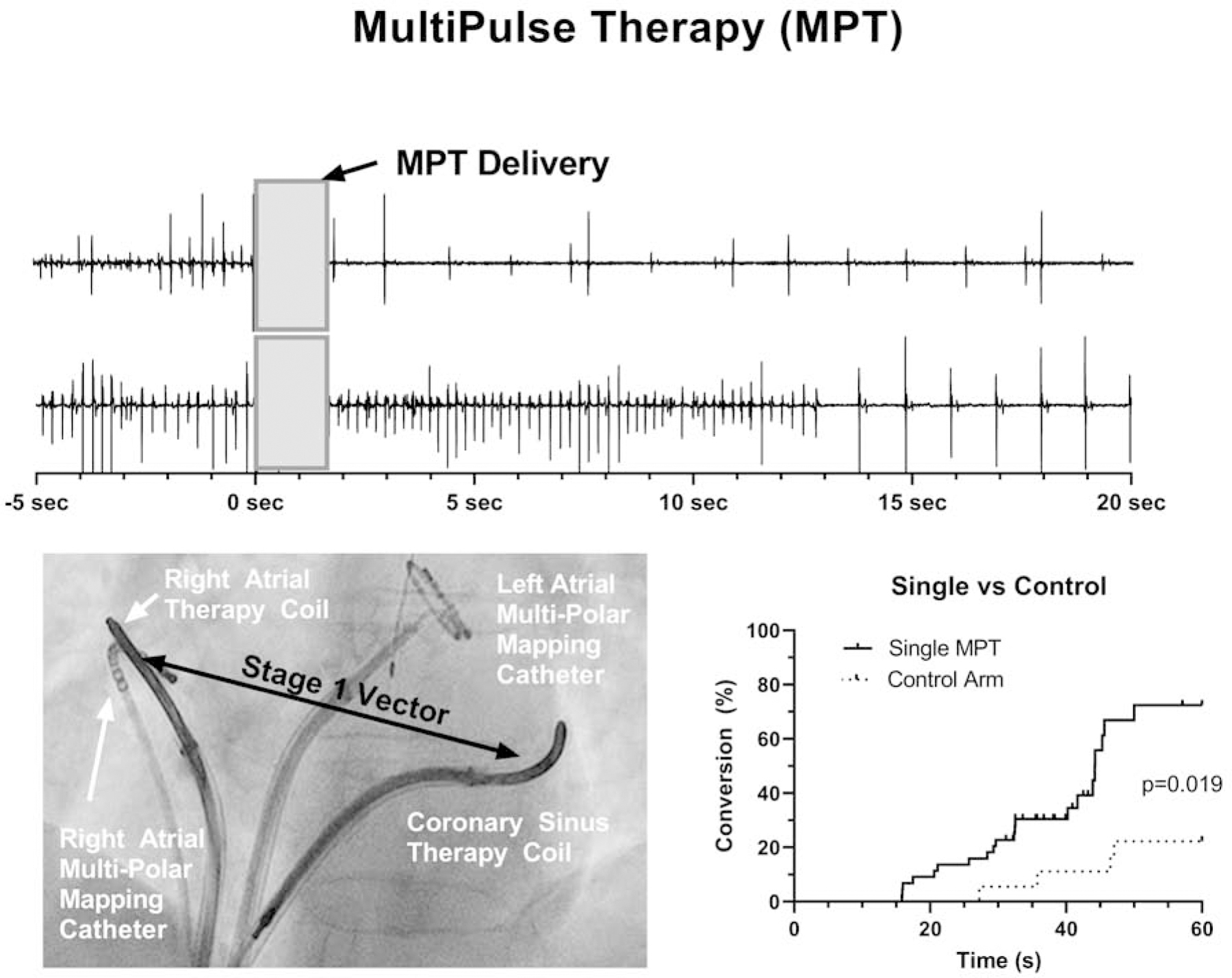
MultiPulse Therapy (MPT) is a sequence of far- and near-field stimulation that terminated AF with average voltage and energy of 50V (25–75) and 0.58J (0.15–1.15) respectively.
CLINICAL PERSPECTIVES.
Competency in Medical Knowledge:
MultiPulse Therapy, which consists of up to a 3-stage sequence of far- and near-field stimulation pulses of varied amplitude, duration and inter-pulse timing, can terminate atrial fibrillation at voltages and energies known to be well-tolerated or painless in some patients.
Translational Outlook:
Delivery of MultiPulse Therapy via chronically implanted devices may represent a novel therapeutic approach that allows for early cardioversion of AF to reduce symptom burden and delay AF progression. This therapy can be easily and readily implemented in patients with existing indications for an implantable cardioverter defibrillator (ICD) or a cardiac resynchronization device (CRT-D), with an additional or modified coronary sinus lead with a defibrillation coil.
Funding:
The study was supported by Cardialen Inc., Minneapolis, MN, Medtronic Plc., Dublin, Ireland and the National Heart, Lung, And Blood Institute of the National Institutes of Health under Award Number 1 R43 HL107055–01. FSN was supported by the National Institute of Health Research (NIHR) (Clinical Lectureship 1716) and British Heart Foundation (RG/16/3/32175). FSN, NAQ and NSP were supported the Imperial College Centre for Cardiac Engineering and the NIHR Imperial Biomedical Research Centre.
ABBREVIATIONS LIST
- AF
atrial fibrillation
- CS
coronary sinus
- J
Joules
- LA
left atrial appendage
- LVEF
Left ventricular ejection fraction
- MPT
MultiPulse therapy
- PAF
Paroxysmal Atrial Fibrillation
- RA
right atrium
- V
Volts
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: FSN, HRM, NSP and IRE have or had consulting agreements with Cardialen; DWB, MBS and ADS are current or former employees of Cardialen. IRE is a shareholder of Cardialen. The remaining authors have nothing to disclose.
REFERENCES
- 1.Chugh SS, Havmoeller R, Narayanan K, et al. Worldwide epidemiology of atrial fibrillation: A global burden of disease 2010 study. Circulation 2014;129(8):837–47. Doi: 10.1161/CIRCULATIONAHA.113.005119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim MH, Johnston SS, Chu BC, Dalal MR, Schulman KL Estimation of total incremental health care costs in patients with atrial fibrillation in the united states. Circ Cardiovasc Qual Outcomes 2011;4(3):313–20. Doi: 10.1161/CIRCOUTCOMES.110.958165. [DOI] [PubMed] [Google Scholar]
- 3.Ausma J, Wijffels M, Thoné F, Wouters L, Allessie M, Borgers M Structural changes of atrial myocardium due to sustained atrial fibrillation in the goat. Circulation 1997. Doi: 10.1161/01.CIR.96.9.3157. [DOI] [PubMed] [Google Scholar]
- 4.Greiser M Calcium signalling silencing in atrial fibrillation. J Physiol 2017. Doi: 10.1113/JP273045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wijffels MCEF, Kirchhof CJHJ, Dorland R, Allessie MA Atrial Fibrillation Begets Atrial Fibrillation. Circulation 1995;92(7):1954–68. Doi: 10.1161/01.CIR.92.7.1954. [DOI] [PubMed] [Google Scholar]
- 6.Wellens H, Lau C, Luderitz B, et al. Atrioverter: An implantable device for the treatment of atrial fibrillation. Circulation 1998;98(16):1651–6. [DOI] [PubMed] [Google Scholar]
- 7.Nattel S, Burstein B, Dobrev D Atrial remodeling and atrial fibrillation: mechanisms and implications. Circ Arrhythm Electrophysiol 2008;1(1):62–73. Doi: 10.1161/CIRCEP.107.754564. [DOI] [PubMed] [Google Scholar]
- 8.Daoud EG, Timmermans C, Fellows C, et al. Initial clinical experience with ambulatory use of an implantable atrial defibrillator for conversion of atrial fibrillation. Metrix Investigators. Circulation 2000;102(12):1407–13. Doi: 10.1161/01.CIR.102.12.1407. [DOI] [PubMed] [Google Scholar]
- 9.Geller JC, Reek S, Timmermans C, et al. Treatment of atrial fibrillation with an implantable atrial defibrillator — long term results. Eur Heart J 2003;24(23):2083–9. Doi: 10.1016/j.ehj.2003.09.033. [DOI] [PubMed] [Google Scholar]
- 10.Steinhaus DM, Cardinal DS, Mongeon L, Musley SK, Foley L, Corrigan S Internal defibrillation: pain perception of low energy shocks. Pacing Clin Electrophysiol 2002;25(7):1090–3. [DOI] [PubMed] [Google Scholar]
- 11.Murgatroyd FD, Slade a K., Sopher SM, Rowland E, Ward DE, Camm a J. Efficacy and tolerability of transvenous low energy cardioversion of paroxysmal atrial fibrillation in humans. J Am Coll Cardiol 1995;25(6):1347–53. [DOI] [PubMed] [Google Scholar]
- 12.Li W, Janardhan AH, Fedorov VV, Sha Q, Schuessler RB, Efimov IR Low-energy multistage atrial defibrillation therapy terminates atrial fibrillation with less energy than a single shock. Circ Arrhythm Electrophysiol 2011;4(6):917–25. Doi: 10.1161/CIRCEP.111.965830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Janardhan AH, Li W, Fedorov VV, et al. A novel low-energy electrotherapy that terminates ventricular tachycardia with lower energy than a biphasic shock when antitachycardia pacing fails. J Am Coll Cardiol 2012;60(23):2393–8. Doi : 10.1016/j.jacc.2012.08.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Janardhan AH, Gutbrod SR, Li W, Lang D, Schuessler RB, Efimov IR Multistage electrotherapy delivered through chronically-implanted leads terminates atrial fibrillation with lower energy than a single biphasic shock. J Am Coll Cardiol 2013;63(1):40–8. Doi: 10.1016/j.jacc.2013.07.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ambrosi CM, Ripplinger CM, Efimov IR, Fedorov VV Termination of sustained atrial flutter and fibrillation using low-voltage multiple-shock therapy. Hear Rhythm 2011;8(1):101–8. Doi: 10.1016/j.hrthm.2010.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ripplinger CM, Krinsky VI, Nikolski VP, Efimov IR Mechanisms of unpinning and termination of ventricular tachycardia. Am J Physiol Heart Circ Physiol 2006;291(1):H184–92. Doi: 10.1152/ajpheart.01300.2005. [DOI] [PubMed] [Google Scholar]
- 17.Ripplinger CM, Lou Q, Li W, Hadley J, Efimov IR Panoramic imaging reveals basic mechanisms of induction and termination of ventricular tachycardia in rabbit heart with chronic infarction: implications for low-voltage cardioversion. Heart Rhythm 2009;6(1):87–97. Doi: 10.1016/j.hrthm.2008.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li W, Ripplinger CM, Lou Q, Efimov IR Multiple monophasic shocks improve electrotherapy of ventricular tachycardia in a rabbit model of chronic infarction. Heart Rhythm 2009;6(7):1020–7. Doi: 10.1016/j.hrthm.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luther S, Fenton FH, Kornreich BG, et al. Low-energy control of electrical turbulence in the heart. Nature 2011;475(7355):235–9. Doi: 10.1038/nature10216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lévy S, Richard P Is There Any Indication for an Intracardiac Defibrillator for the Treatment of Atrial Fibrillation? J Cardiovasc Electrophysiol 1994;5(11):982–5. Doi: 10.1111/j.1540-8167.1994.tb01137.x. [DOI] [PubMed] [Google Scholar]
- 21.Kalman JM, Jones EF, Doolan L, Oliver LE, Power JM, Tonkin AM Low energy endocardial cardioversion of atrial arrhythmias in humans. Pacing Clin Electrophysiol 1995;18(10):1869–75. [DOI] [PubMed] [Google Scholar]
- 22.Saksena S, Prakash A, Mangeon L, et al. Clinical efficacy and safety of atrial defibrillation using biphasic shocks and current nonthoracotomy endocardial lead configurations. Am J Cardiol 1995;76(12):913–21. [DOI] [PubMed] [Google Scholar]
- 23.Ladwig K-H, Marten-Mittag B, Lehmann G, Gündel H, Simon H, Alt E Absence of an impact of emotional distress on the perception of intracardiac shock discharges. Int J Behav Med 2003;10(1):56–65. Doi: 10.1207/S15327558IJBM1001_05. [DOI] [PubMed] [Google Scholar]
- 24.Lok NS, Lau CP, Tse HF, Ayers GM Clinical shock tolerability and effect of different right atrial electrode locations on efficacy of low energy human transvenous atrial defibrillation using an implantable lead system. J Am Coll Cardiol 1997;30(5):1324–30. [DOI] [PubMed] [Google Scholar]
- 25.Cheng Y, Mowrey KA, Van Wagoner DR, Tchou PJ, Efimov IR Virtual electrode-induced reexcitation: A mechanism of defibrillation. Circ Res 1999;85(11):1056–66. Doi: 10.1161/01.RES.85.11.1056. [DOI] [PubMed] [Google Scholar]
- 26.Efimov I, Ripplinger CM Virtual electrode hypothesis of defibrillation. Hear Rhythm 2006;3(9):1100–2. Doi: 10.1016/j.hrthm.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 27.Nikolski VP, Sambelashvili AT, Efimov IR Mechanisms of make and break excitation revisited: paradoxical break excitation during diastolic stimulation. Am J Physiol Circ Physiol 2015;282(2):H565–75. Doi: 10.1152/ajpheart.00544.2001. [DOI] [PubMed] [Google Scholar]
- 28.Di Marco LY, Raine D, Bourke JP, Langley P Characteristics of atrial fibrillation cycle length predict restoration of sinus rhythm by catheter ablation. Hear Rhythm 2013;10(9):1303–10. Doi: 10.1016/j.hrthm.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 29.Haïssaguerre M, Sanders P, Hocini M, et al. Changes in atrial fibrillation cycle length and inducibility during catheter ablation and their relation to outcome. Circulation 2004;109(24):3007–13. Doi: 10.1161/01.CIR.0000130645.95357.97. [DOI] [PubMed] [Google Scholar]
- 30.Charitos EI, Pürerfellner H, Glotzer TV, Ziegler PD Clinical classifications of atria l fibrillation poorly reflect its temporal persistence: Insights from 1,195 patients continuously monitored with implantable devices. J Am Coll Cardiol 2014;63(25 PART A):2840–8. Doi: 10.1016/j.jacc.2014.04.019. [DOI] [PubMed] [Google Scholar]
- 31.Gold MR, Sulke N, Schwartzman DS, Mehra R, Euler DE Clinical experience with a dual-chamber implantable cardioverter defibrillator to treat atrial tachyarrhythmias. J Cardiovasc Electrophysiol 2001;12(11):1247–53. Doi: 10.1046/j.1540-8167.2001.01247.x. [DOI] [PubMed] [Google Scholar]
- 32.Sih HJ, Zipes DP, Berbari EJ, Adams DE, Olgin JE Differences in organization between acute and chronic atrial fibrillation in dogs. J Am Coll Cardiol 2000. Doi: 10.1016/S0735-1097(00)00788-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


