This work highlights the importance of CNS transduction for treatment of neurological diseases, a finding with significant clinical implications considering the long-lasting effects of gene therapy.
Abstract
Niemann–Pick C1 disease (NPC1) is a rare, fatal neurodegenerative disease caused by mutations in NPC1, which encodes the lysosomal cholesterol transport protein NPC1. Disease pathology involves lysosomal accumulation of cholesterol and lipids, leading to neurological and visceral complications. Targeting the central nervous system (CNS) from systemic circulation complicates treatment of neurological diseases with gene transfer techniques. Selected and engineered capsids, for example, adeno-associated virus (AAV)-PHP.B facilitate peripheral-to-CNS transfer and hence greater CNS transduction than parental predecessors. We report that systemic delivery to Npc1m1N/m1N mice using an AAV-PHP.B vector ubiquitously expressing NPC1 led to greater disease amelioration than an otherwise identical AAV9 vector. In addition, viral copy number and biodistribution of GFP-expressing reporters showed that AAV-PHP.B achieved more efficient, albeit variable, CNS transduction than AAV9 in Npc1m1N/m1N mice. This variability was associated with segregation of two alleles of the putative AAV-PHP.B receptor Ly6a in Npc1m1N/m1N mice. Our data suggest that robust improvements in NPC1 disease phenotypes occur even with modest CNS transduction and that improved neurotrophic capsids have the potential for superior NPC1 AAV gene therapy vectors.
Introduction
Niemann–Pick disease, type C (NPC) is a fatal, autosomal recessive lysosomal storage disorder with an estimated incidence of 1 in ∼100,000 live births (Vanier, 2013). Unesterified cholesterol and sphingolipid accumulation in the lysosome is a primary hallmark of NPC. In 95% of NPC patients, mutations in NPC1 (NPC1 disease, OMIM #257220), which encodes the NPC1 transmembrane protein found in the limiting lysosomal membrane (Carstea et al, 1997), is causative. The remaining 5% of patients have mutations in the soluble lysosomal protein NPC2 (NPC2 disease, OMIM #607625), which binds cholesterol and physically interacts with NPC1 (Naureckiene et al, 2000). The two forms of the disease are clinically indistinguishable, consistent with the many studies demonstrating that NPC1 and NPC2 work together to regulate cholesterol efflux from the lysosome (Infante et al, 2008; Cologna & Rosenhouse-Dantsker, 2019; Pfeffer, 2019). NPC patients exhibit a wide array of neurological symptoms, including motor impairment and learning deficits, as well as visceral complications such as hepatosplenomegaly, with a highly heterogeneous disease severity and age of onset (Garver et al, 2007; Vanier, 2010; Patterson et al, 2013; Geberhiwot et al, 2018). Currently there are no FDA-approved therapies for NPC in the United States, thus there is an urgent need for discovery of effective treatments for this debilitating, fatal disease.
Gene therapy represents a promising treatment for monogenic diseases such as NPC1 and related lysosomal diseases. Recent technological advances such as improved vector design, RNA-based therapies, and CRISPR/Cas9 technology have brought gene therapy for these disorders closer to reality (Ma et al, 2019; Shahryari et al, 2019). In particular, engineered capsids derived from various serotypes of adeno-associated virus (AAV) have been found to be highly efficient for gene delivery and exhibit a wide range of tissue specificity, thus allowing their use in numerous in vivo gene therapy techniques (Hudry & Vandenberghe, 2019; Li & Samulski, 2020). Many treatments targeting neurodegenerative diseases have focused on AAV9 because preclinical trials in various animal models have shown that it crosses the blood–brain barrier (BBB), efficiently transduces cells in the central nervous system (CNS), and can be safely administered in nonhuman primates (Bevan et al, 2011; Samaranch et al, 2012; Saraiva et al, 2016; Lykken et al, 2018; Hudry & Vandenberghe, 2019). Furthermore, AAV9-derived vectors have already achieved clinical translation, with the successful FDA approval of Zolgensma representing the first use of an AAV vector for the treatment of spinal muscular atrophy type 1, a fatal neurodegenerative disorder of infancy and childhood (Saraiva et al, 2016; Lykken et al, 2018; Al-Zaidy et al, 2019; Lowes et al, 2019). Use of AAV9-based vectors has been extended to clinical trials for other disorders, including giant axonal neuropathy, Pompe disease, GM1 gangliosidosis, Duchenne muscular dystrophy, Batten disease, Danon disease, Gaucher disease, and Mucopolysaccharidosis type IIIA.
Despite theoretical limitations to the application of gene therapy to treat lysosomal storage diseases which feature cell autonomous pathology, AAV9 vectors can be effective in alleviating phenotypes of NPC1 in mouse models. We previously reported the systemic delivery of an AAV9-EF1a(s)-hNPC1 vector significantly increased survival and delayed disease progression in the Npc1m1N/m1N null mouse model (Chandler et al, 2017), even after treatment of the mice as juveniles. Subsequent studies similarly noted improvement in Npc1m1N/m1N mice after intra-cardiac delivery of an AAV9-CMV-NPC1 vector (Xie et al, 2017) or intracerebroventricular delivery of an AAV9-hSynapsin-NPC1 vector delivered in the neonatal period (Hughes et al, 2018). Although encouraging, these results also indicate that careful optimization can significantly improve the therapeutic efficacy of gene therapy in NPC1.
The promising results of the aggregate AAV9 studies have led us to explore AAV9 capsid variants with improved CNS penetration from the systemic circulation as a means to increase the potency of AAV gene therapy for NPC disease. This is particularly important for the more complex NPC disease type 1 as opposed to type 2, where the secretion of soluble NPC2 provides the added benefit of cross-correction (Markmann et al, 2018). Moreover, neuronal deficiency of NPC1 has proven sufficient to mediate CNS disease (Yu et al, 2011), further highlighting the need for vectors with enhanced neuronal tropism. The prototypical capsid, AAV-PHP.B, emerged from an in vivo screen and displayed ∼40-fold greater gene transfer efficiency to the central nervous system (CNS) with transduction of a variety of cell types including astrocytes, oligodendrocytes, and neurons after peripheral injection (Deverman et al, 2016). Studies with AAV-PHP.B vectors have demonstrated widespread and efficient gene delivery to the rodent CNS and alleviation of neurological phenotypes (Jackson et al, 2016; Gao et al, 2017; Morabito et al, 2017; Zelikowsky et al, 2018; Lim et al, 2019; Luoni et al, 2020; Yang et al, 2020). However, the ability of systemically administered AAV-PHP.B to transduce the CNS in mice is dependent on the presence of a strain-specific haplotype that includes the gene encoding the GPI-linked protein Ly6a; without this permissive Ly6a allele, AAV-PHP.B CNS transduction is severely limited (Hordeaux et al, 2019; Huang et al, 2019; Batista et al, 2020). Importantly, studies with AAV-PHP.B and other engineered capsids (Deverman et al, 2016; Tordo et al, 2018; Wang et al, 2018; Hanlon et al, 2019) can provide preclinical paradigms for future applications of AAV serotypes and/or engineered capsids that display enhanced CNS transduction in other species, including non-human primates (Ivanchenko et al, 2020).
In this study, we compared AAV-PHP.B and AAV9 vectors in the well-established NPC1 null mouse model, Npc1m1N. Transgene expression of GFP reporter constructs showed higher transduction throughout the brain for the AAV-PHP.B vector in comparison to AAV9, particularly notable in the hippocampus and midbrain regions. Comparative analyses of Npc1m1N/m1N mice that received comparable doses in otherwise identical vectors showed that mice treated with the AAV-PHP.B-NPC1 construct lived longer and showed greater reduction of disease symptoms. Variability in disease phenotypes and CNS copy numbers of AAV-PHP.B–treated mice correlated with the segregation of the permissive and restrictive alleles of Ly6a. Interestingly, despite the superior performance of the AAV-PHP.B-NPC1 vector in improving survival, weight, and behavior, clear reduction in pathology within the CNS in comparison to the AAV9-NPC1 vector was difficult to detect. Both vectors exhibited only moderate correction of brain disease pathology (cholesterol accumulation, inflammation, loss of cerebellar Purkinje cells) compared to untreated Npc1m1N/m1N mice. Overall, results from this proof of concept study suggest that relatively small numbers of CNS cells were effectively transduced by AAV-PHP.B, and this moderate cell correction was enough to markedly improve disease phenotypes, including survival, in Npc1m1N/m1N mice.
Results
AAV-PHP.B-GFP reporter construct showed biodistribution throughout the brain in Npc1m1N/m1N mice
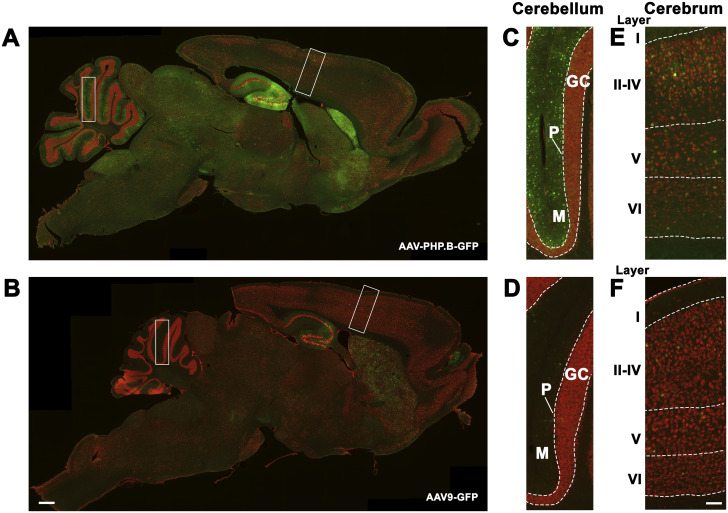
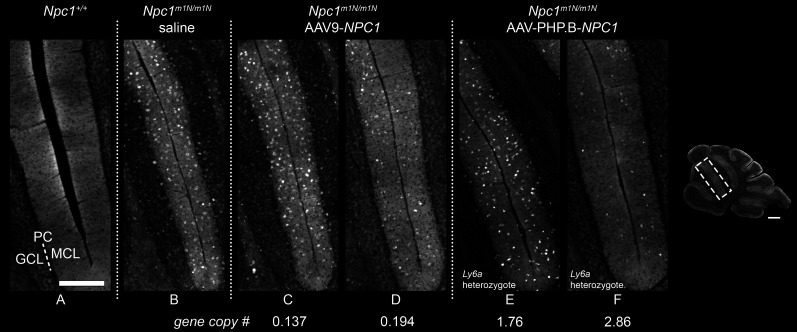
Transduction efficacy of AAV-PHP.B versus AAV9 vectors was compared in Npc1m1N/m1N and Npc1+/+ mice treated with retro-orbital injections of either an AAV-PHP.B-GFP or AAV9-GFP vector. Each vector contained a GFP reporter construct under control of the ubiquitous elongation factor 1a (shortened) promoter (AAV-PHP.B-EF1a(s)-GFP and AAV9-EF1a(s)-GFP, respectively). Mice were given 1.21 × 1012 genome copies (GC) (∼8.55 × 1013 GC/kg) of each vector construct between postnatal (P) days 24–27, and GFP expression was analyzed 5.5 wk later (∼P63/9 wk). Mice receiving AAV-PHP.B-GFP showed GFP expression throughout the brain (Fig 1A, C, and E), and this expression was greater than that seen in mice given AAV9-GFP (Fig 1B, D, and F). GFP expression was particularly high in the hippocampus, striatum, molecular cell layer of the cerebellum, and vestibular nucleus of Npc1m1N/m1N mice that received the AAV-PHP.B-GFP vector relative to mice that received AAV9-GFP. Similar results were seen in Npc1+/+ mice that received AAV-PHP.B-GFP or AAV9-GFP (data not shown). These findings are consistent with previous studies that suggest greater CNS transduction by AAV-PHP.B vectors in comparison to AAV9 vectors, and these results also demonstrate that the underlying disease state does not interfere with AAV-PHP.B CNS transduction.
Figure 1. Biodistribution of GFP expression in Npc1m1N/m1N mice that received either adeno-associated virus (AAV)-PHP.B-GFP or AAV9-GFP vectors.
(A, B) Sagittal brain sections with immunohistochemical staining of GFP (green) and neuronal nuclei (red). Npc1m1N/m1N mice treated with AAV-PHP.B-GFP showed increased GFP expression compared to Npc1m1N/m1N mice treated with AAV9-GFP. (C, D, E, F) Higher magnification views of lobule IV/V in the cerebellum and the dorsal neocortex. Vector was administered to mice between 24 and 27 d old and tissues were analyzed at 9 wk. Scale bars = 500 (A, B) and 100 μm (C, D, E, F).
Npc1m1N/m1N mice treated with an AAV-PHP.B-NPC1 vector showed increased survival and delayed disease phenotype progression
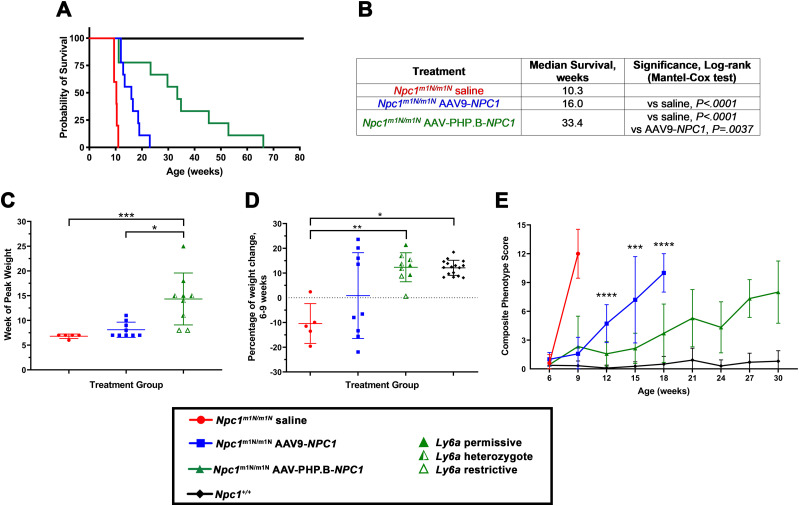
To compare the efficacy of AAV-PHP.B to AAV9, Npc1m1N/m1N mice were treated with identical transgenes in the different capsids. The vectors express NPC1 under control of the EF1a (shortened) promoter (AAV-PHP.B-EF1a(s)-hNPC1 or AAV9-EF1a(s)-hNPC1, hereafter referred to as AAV-PHP.B-NPC1 or AAV9-NPC1, respectively). Three cohorts of Npc1m1N/m1N mice were injected, as follows: nine mice received AAV-PHP.B-NPC1 vector at 1.43 × 1012 GC (∼1.24 × 1014 GC/kg), nine mice received AAV9-NPC1 vector at 1.84 × 1012 GC (∼1.42 × 1014 GC/kg), and five mice received saline-only injections. Technical variability with vector titer assays led to these moderately different doses for AAV-PHP.B-NPC1 versus AAV9-NPC1. Comparison of survival among these three groups (Fig 2A and B) showed that Npc1m1N/m1N mice treated with AAV-PHP.B-NPC1 exhibited a significantly higher median survival of 33.4 wk in comparison to mice treated with AAV9-NPC1, for which median survival was only 16 wk (P < 0.005, Mantel–Cox log rank test). Both vectors showed significantly higher median survival than saline-injected Npc1m1N/m1N mice (P < 0.0001), which had all reached terminal end point by 11 wk.
Figure 2. Npc1m1N/m1N mice treated with an NPC1 adeno-associated virus (AAV)-PHP.B vector showed increased survival and delayed disease phenotype progression.
(A) Kaplan–Meier curve depicts survival of the following: Npc1m1N/m1N mice treated with an AAV-PHP.B vector containing human NPC1 (1.43 × 1012 genome copy), Npc1m1N/m1N mice treated with an AAV9 vector containing human NPC1 (1.84 × 1012 genome copy), saline-injected Npc1m1N/m1N mice, and untreated Npc1+/+ controls. All mice were administered retro-orbital injections between P24 and P27. (A, B) Table of treatment group, median survival, and significance (Mantel–Cox log rank test) of data shown in (A). (C) AAV-PHP.B-NPC1–treated Npc1m1N/m1N mice reached peak weight at a significantly older age than saline-injected Npc1m1N/m1N mice and Npc1m1N/m1N mice receiving the AAV9-NPC1 vector (Kruskal–Wallis test with Dunn’s multiple comparisons test). AAV9-treated Npc1m1N/m1N mice showed no significant difference from saline-injected mice. (D) Graphical depiction of the percentage of weight change between 6 and 9 wk of age. AAV-PHP.B-NPC1–treated Npc1m1N/m1N mice and normal Npc1+/+ mice gained weight at a similar rate during this time period, both of which were significantly different from the marked weight loss exhibited by saline-injected Npc1m1N/m1N mice (Welch’s ANOVA test with Dunnett’s multiple comparisons test). AAV9-NPC1–treated mice showed wide variability spanning across the other three groups. (E) Composite phenotype scores for each treatment group, measured at 3-wk intervals starting at 6 wk of age. Npc1m1N/m1N mice treated with AAV-PHP.B-NPC1 maintained significantly lower composite scores compared to Npc1m1N/m1N mice treated with AAV9-NPC1 from weeks 12–18 (two-way ANOVA with Tukey’s multiple comparisons test). A full table of 2-way ANOVA results is presented in Table S1. Composite phenotype scores include disease-relevant measures of gait, kyphosis, ledge test, hind limb clasp, grooming, and tremor, with a higher score indicating a more severe disease phenotype. (A, B, C, D) For panels (A, B, C, D), n’s are as follows: Npc1m1N/m1N saline = 5, Npc1m1N/m1NAAV9-NPC1 = 9, Npc1m1N/m1N AAV-PHP.B-NPC1 = 9, Npc1+/+ = 14. (E) Of note for panel (E), the n of mice at each time point in all three Npc1m1N/m1N groups became smaller at later time periods because of animals reaching end stage (see Table S1). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
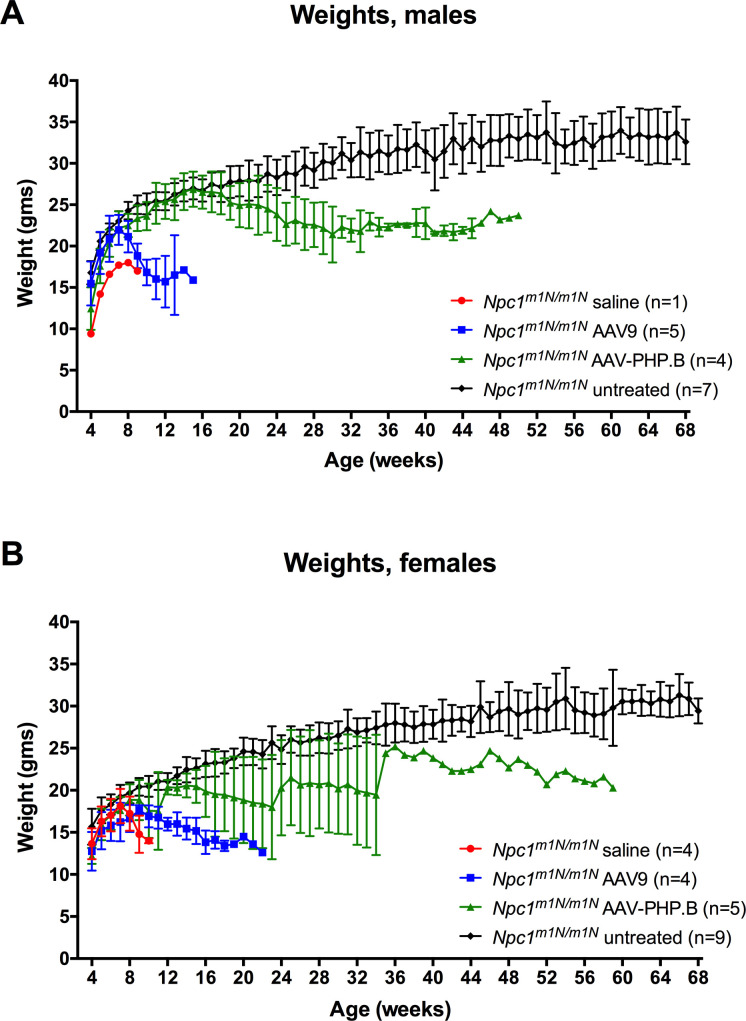
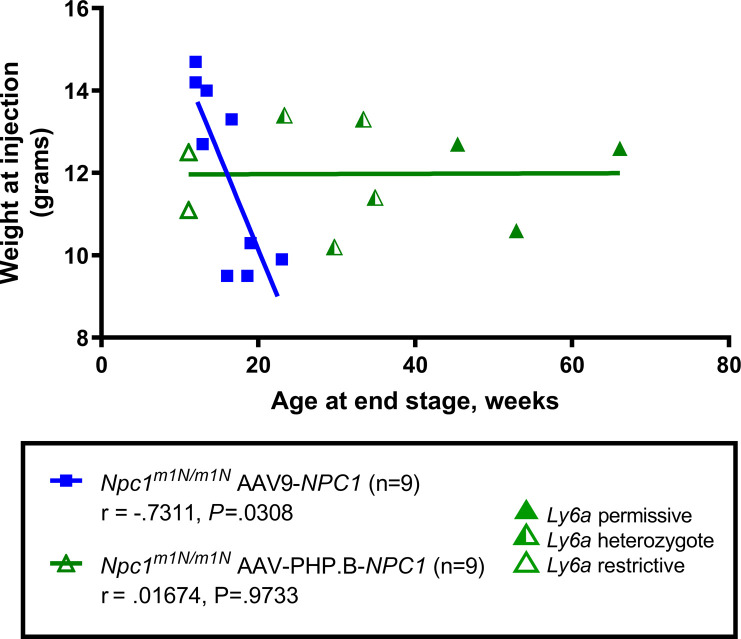
Normal disease progression in Npc1m1N/m1N mice includes a marked decline in weight starting at about 6 wk of age; therefore, the week at which mice in both vector-treated cohorts reached peak weight was monitored. Npc1m1N/m1N mice receiving AAV-PHP.B-NPC1 reached peak weight significantly later than Npc1m1N/m1N mice receiving AAV9-NPC1 (mean of 14.3 ± 5.2 versuss 8.1 ± 1.6 wk, respectively, Fig 2C). In addition, the age of peak weight of AAV-PHP.B-NPC1–treated Npc1m1N/m1N mice was significantly different from that of saline-injected Npc1m1N/m1N mice, which showed a peak weight at 6.8 ± 0.45 wk (Fig 2C). Longitudinal weight data are shown in Fig S1A and B (males and females, respectively), illustrating the longer maintenance of weight and longer lifespan of AAV-PHP.B-NPC1–treated mice. The lifespan of AAV-PHP.B-NPC1–treated mice did not correlate with their weight at the time of injection, suggesting no dosage effect for these mice, although a correlation was seen in AAV9-NPC1–treated mice (r = −.7311, P = 0.0308, Fig S2). Interestingly, the percent of weight change between 6 and 9 wk of age (Fig 2D) showed that whereas saline-injected Npc1m1N/m1N mice lost weight, AAV-PHP.B-NPC1–treated Npc1m1N/m1N mice and normal Npc1+/+ mice gained weight at a similar rate during this time period (AAV-PHP.B–treated Npc1m1N/m1N mean = 12.3% ± 5.6%; Npc1+/+ mean = 12.1% ± 3.0%). In contrast, the cohort of AAV9-NPC1–treated mice showed wide variability (mean = 0.88% ± 17.3%, Fig 2D).
Figure S1. Longitudinal weight data for mice in different treatment groups.
Both male (A) and female (B) Npc1m1N/m1N mice administered adeno-associated virus-PHP.B-NPC1 vector maintain weight longer than Npc1m1N/m1N mice treated with adeno-associated virus 9-NPC1.
Figure S2. The lifespan of adeno-associated virus-PHP.B-NPC1–treated Npc1m1N/m1N mice did not correlate with their weight at the time of injection.
A modest correlation was seen between weight at injection and lifespan in adeno-associated virus 9-NPC1–treated Npc1m1N/m1N mice (Spearman’s correlation coefficient).
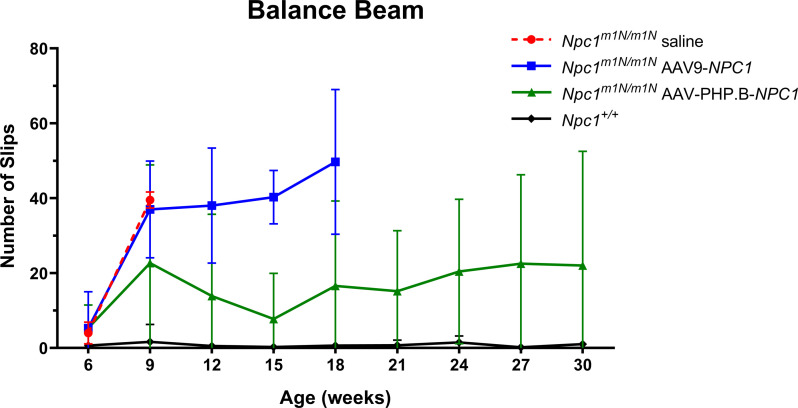
To compare the effects of AAV-PHP.B-NPC1 and AAV9-NPC1 on Npc1m1N/m1N disease-associated traits, two behavioral assays were used: phenotype score and balance beam. The phenotype score assesses six measures of the NPC disease phenotype (gait, kyphosis, ledge test, hind limb clasp, grooming, and tremor) while the balance beam is an indicator of motor coordination. A higher composite score for the phenotype assay or greater number of slips for the balance beam correlates with a worsened disease state. From weeks 12–18, Npc1m1N/m1N mice treated with AAV-PHP.B-NPC1 maintained significantly lower composite phenotype scores than Npc1m1N/m1N mice treated with AAV9-NPC1 (P < 0.001, two-way ANOVA with Tukey’s multiple comparisons test, Fig 2E). Two additional comparisons showed treatment with AAV-PHP.B-NPC1 delayed disease progression: AAV-PHP.B-NPC1–treated mice had a composite score that did not differ from Npc1+/+ control mice at weeks 12 and 15, and from weeks 18–24, AAV-PHP.B-NPC1–treated mice maintained scores that were better than those of end-stage AAV9-NPC1–treated mice (Table S1). Similarly, AAV-PHP.B-NPC1–treated mice showed delayed loss of motor coordination on the balance beam assay (Fig S3). From weeks 12–18, Npc1m1N/m1N mice treated with AAV-PHP.B-NPC1 had fewer slips on the balance beam than Npc1m1N/m1N mice treated with AAV9-NPC1 (P < 0.01, two-way ANOVA with Tukey’s multiple comparisons test; Table S1). In addition, enhanced therapeutic benefit of AAV-PHP.B-NPC1 on ambulation and ataxia relative to AAV9-NPC1 can be appreciated, as demonstrated in Videos 1 and 2.
Figure S3. Adeno-associated virus-PHP.B-NPC1–treated Npc1m1N/m1N mice showed a delay in loss of motor coordination on the balance beam assay when compared with adeno-associated virus 9-NPC1–treated Npc1m1N/m1N mice.
Table S1 Phenotype scores and balance beam: two-way ANOVA. (90.6KB, docx)
Table S2 Genotype, sample size, and gender of mice used in study. (14.6KB, docx)
This video demonstrates the enhanced therapeutic benefit of adeno-associated virus-PHP.B-NPC1 (left cage, mouse with red tail) versus adeno-associated virus 9-NPC1 (right cage, mouse with no tail color). An Npc1+/+ littermate is also present for comparison (left cage, mouse with no tail color) and all mice are age matched (15-wk-old). Download video (7.2MB, mp4)
This video shows the longest surviving adeno-associated virus-PHP.B-NPC1–treated Npc1m1N/m1N mouse (faint green tail; survived to 66.1 wk) with an age-matched Npc1+/+ littermate at 37 wk of age. Video 2 This adeno-associated virus-PHP.B-NPC1–treated Npc1m1N/m1N mouse exhibits relatively good overall body condition and modest motor impairment, although moderate kyphosis and ruffled fur persist. Download video (11.7MB, mp4)
Differential impact of AAV-PHP.B and AAV9 vectors on NPC1 transduction efficiency in brain and liver
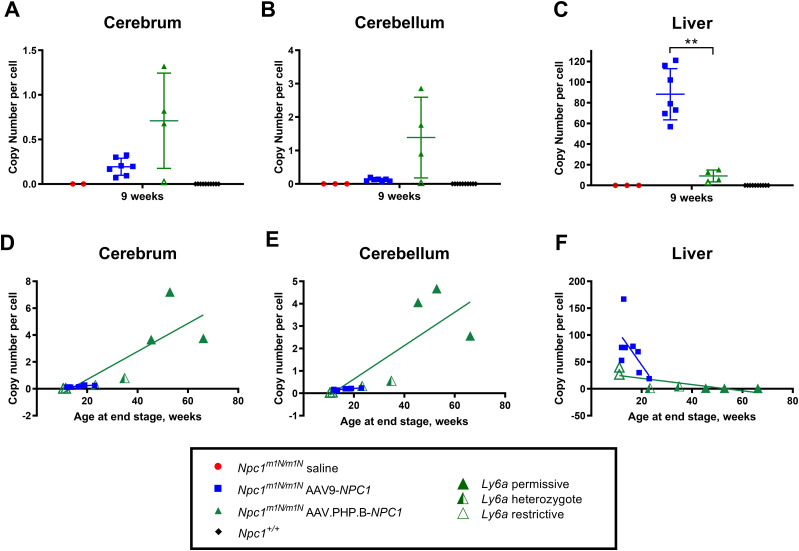
Further analyses were performed on Npc1m1N/m1N mice to examine the transduction efficiency of AAV-PHP.B-NPC1 and AAV9-NPC1 vectors. Transduction efficiency of each vector was evaluated by measuring NPC1 copy number in cerebrum, cerebellum, and liver by droplet digital PCR (ddPCR) at 9 wk of age and at end stage. At 9 wk, AAV-PHP.B-NPC1–treated mice showed higher NPC1 copy numbers than AAV9-NPC1-transduced mice in both brain regions; however, these differences were not statistically significant likely because of small sample size and variability (Fig 3A and B). In contrast, liver showed significantly higher NPC1 copy number in Npc1m1N/m1N mice that received AAV9-NPC1 in comparison to AAV-PHP.B-NPC1 (Fig 3C and P < 0.001, unpaired t test). These patterns of relatively higher AAV-PHP.B-NPC1 brain transduction and relatively higher AAV9-NPC1 liver transduction persisted in end stage Npc1m1N/m1N mice (Fig S4). Interestingly, positive correlations were seen between age at end stage disease and NPC1 copy numbers in cerebrum and cerebellum and in both the AAV-PHP.B-NPC1– and AAV9-NPC1–transduced mice (Fig 3D and E), suggesting the levels of NPC1 transduction in the brain were important for extending lifespan. In contrast, the liver NPC1 copy number did not positively correspond to lifespan in either group of mice (Fig 3F).
Figure 3. Differential transduction efficiency of adeno-associated virus (AAV)-PHP.B-NPC1 and AAV9-NPC1 vectors in Npc1m1N/m1N mice in brain and liver.
(A, B, C) Droplet digital PCR was used to measure NPC1 copy number at 9 wk of age. There was a trend for higher copy number in cerebrum and cerebellum of AAV-PHP.B-NPC1- compared with AAV9-NPC1–treated Npc1m1N/m1N mice. In contrast, liver tissue showed the opposite result, with significantly lower copy numbers in Npc1m1N/m1N mice treated with AAV.PHP.B-NPC1 (unpaired t test). (D, E, F) NPC1 copy number of AAV9-NPC1– and AAV-PHP.B-NPC1–treated Npc1m1N/m1N mice graphed as a function of age at the end stage. The AAV-PHP.B-NPC1–treated mice with the highest NPC1 copy number in cerebrum and cerebellum also survived the longest. Ly6a allelic determination is shown for AAV-PHP.B-NPC1–treated Npc1m1N/m1N mice as well. Both groups showed a positive correlation between age at end stage and NPC1 copy numbers in cerebrum (AAV-PHP.B-NPC1, r = 0.929, P = 0.007; AAV9-NPC1, r = 0.886, P = 0.006) and cerebellum (AAV-PHP.B-NPC1, r = 0.893, P = 0.012; AAV9-NPC1, r = 0.778, P = 0.03; Spearman’s correlation coefficient test). AAV-PHP.B-NPC1 copy number in liver did not positively correlate with age at the end stage.
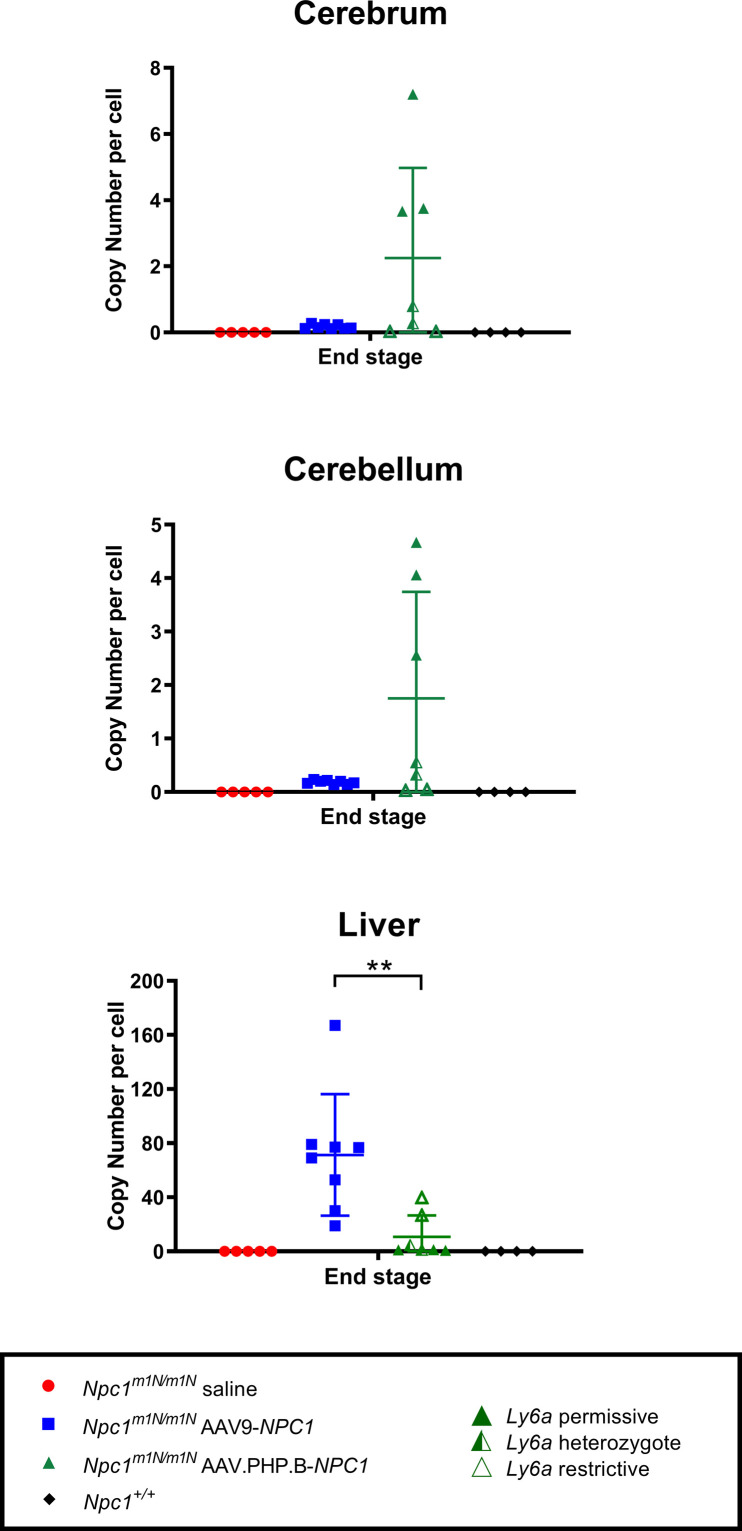
Figure S4. Copy number per cell at end stage for cerebrum, cerebellum, and liver of all treatment groups.
Similar to what was observed in the 9-wk-old age-group, Npc1m1N/m1N mice administered adeno-associated virus-PHP.B-NPC1 trended towards higher copy numbers in brain, whereas Npc1m1N/m1N mice given adeno-associated virus 9-NPC1 had significantly higher copy numbers in the liver (unpaired t test).
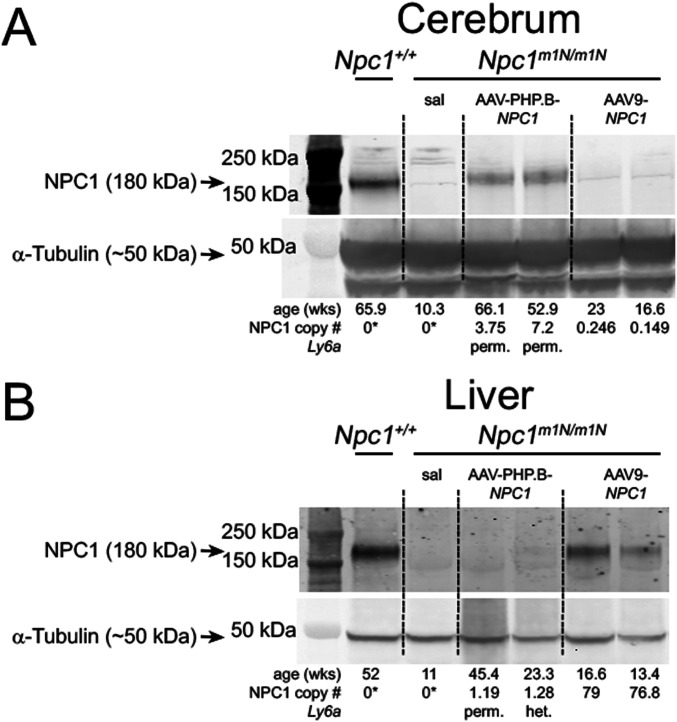
The higher transduction of AAV-PHP.B-NPC1 in brain was corroborated by assessment of NPC1 protein levels. Western blot analysis of cerebrum tissue collected from AAV-PHP.B-NPC1–transduced Npc1m1N/m1N mice showed modest levels of NPC1 protein with the highest NPC1 copy number and longest survival (Fig 4A), whereas immunoreactive NPC1 was undetectable in mice with the lowest vector copy number and shortest lifespan (data not shown). None of the AAV9-NPC1–transduced Npc1m1N/m1N mice showed detectable NPC1 by Western blot (Fig 4A). In contrast, Western blot analyses of liver revealed that AAV9-NPC1–transduced Npc1m1N/m1N mice showed consistently higher protein levels of NPC1 than AAV-PHP.B-NPC1–transduced mice (Fig 4B). Similar to cerebrum, liver showed a correspondence between the NPC1 copy numbers found in ddPCR analysis and the presence or absence of NPC1 protein on Western blots.
Figure 4. NPC1 protein levels correspond with differential transduction efficiency of adeno-associated virus (AAV)-PHP.B-NPC1 and AAV9-NPC1 vectors.
(A, B) Western blots were used to confirm the presence or absence of NPC1 protein in cerebrum and liver tissue. The Npc1m1N/m1N model is a null, thus the only NPC1 protein present would arise from the transduced vectors. Age in weeks and NPC1 copy number for each mouse is under α-tubulin loading control. (A) In cerebrum, NPC1 protein was detectable only in the longest surviving AAV-PHP.B-NPC1–treated Npc1m1N/m1N and Npc1+/+ mice, and was not detected in AAV9-NPC1–treated Npc1m1N/m1N mice. (B) Conversely, analysis of liver showed that NPC1 protein was present in most AAV9-NPC1–treated Npc1m1N/m1N mice, but only rarely in Npc1m1N/m1N mice receiving AAV-PHP.B-NPC1. *Droplet digital PCR assayed only human NPC1, not murine NPC1, hence zero values for non-gene therapy treated mice. Full unedited gels for Fig 4: Please see green outlines below for cerebrum and blue outlines below for liver to denote the portion of the gel used in Fig 4. Each gel was co-labeled with NPC1 and α-tubulin and different secondaries were used for each primary antibody.
Source data are available for this figure.
Allelic differences at the Ly6a locus are associated with the variable phenotypes of AAV-PHP.B-NPC1–treated mice
Notable variability was present in lifespan, phenotype score, and copy number in brain tissues of AAV-PHP.B-NPC1–treated Npc1m1N/m1N mice (Figs 2 and 3). After the completion of the AAV-PHP.B-NPC1 experiments, other groups published work identifying strain-specific effects on the CNS transduction efficiency of AAV-PHP.B that are associated with different haplotypes at Ly6a, a gene encoding a GPI-anchored protein expressed at the BBB (Hordeaux et al, 2019; Huang et al, 2019; Batista et al, 2020). These Ly6a studies showed that BALB/cJ mice had lower AAV-PHP.B transduction efficiency, of relevance because the spontaneous Npc1m1N allele arose on a BALB/c-derived strain (Morris et al., 1977, 1982). Therefore, we sought to examine the Ly6a genotype in the Npc1m1N/m1N mice used in our experiments.
Genotype analysis was performed for two exonic SNPs in Ly6a that exhibit nonsynonymous variants (relative to C57Bl6/J reference sequence) in BALB/cJ and other inbred strains, as follows: rs32279213, p.D63G, and rs213983347, p.V106A. Two haplotypes that include these SNPs associate with brain transduction levels of AAV-PHP.B that are either higher (permissive genotype, p.D63 and p.V106) or lower (restrictive genotype, p.G63 and p.A106) (Hordeaux et al, 2019; Huang et al, 2019; Batista et al, 2020). Interestingly, genotyping results revealed that both Ly6a genotypes were segregating in AAV-PHP.B–treated Npc1m1N/m1N mice (Table 1), and the Ly6a genotypes correlated with the biodistribution of GFP, lifespan and NPC1 copy number in the brain. Enhanced biodistribution of the GFP vector (data not shown), as well as increased lifespan and greater NPC1 copy number occurred in the AAV-PHP.B-NPC1–treated Npc1m1N/m1N mice that were homozygous for the permissive Ly6a genotype (Table 1 and Fig 3D–F). In contrast, AAV-PHP.B-NPC1–treated Npc1m1N/m1N mice homozygous for the restrictive Ly6a genotype showed the shortest lifespan and lowest NPC1 copy number (Table 1). These findings suggest the variability of phenotype improvement in AAV-PHP.B–treated mice was associated with their Ly6a genotype. A genome-wide scan of two randomly selected Npc1m1N/m1N mice in this study also showed the presence of BALB/cJ and non-BALB/cJ genetic markers (homozygous BALB/cJ markers were present for only 76%–81% of the informative markers analyzed). This genetic variability could be specific to our colony, as genotyping from the Jackson Laboratory colony (Stock #003092, BALB/cNctr-Npc1<m1N>/J) showed these mice were homozygous for the restrictive Ly6a genotype. Moreover, two distinct cohorts from collaborative studies done at the Jackson Laboratory in the Npc1m1N/m1N mice also revealed only the restrictive Ly6a genotype (Pavan, unpublished observation).
Table 1.
Npc1m1N/m1N mice treated with adeno-associated virus-PHP.B show allelic variance at the Ly6a locus that correlates with lifespan and copy number per cell.
| SNP genotyping at Ly6a locus | Copy number per cell | ||||
|---|---|---|---|---|---|
| Age, wka | rs32279213b | rs213983347c | Cerebrum | Cerebellum | Liver |
| 9 | G/G | C/C | 0.0229 | 0.032 | 3.1 |
| 9 | A/G | T/C | 0.678 | 0.899 | 12.7 |
| 9 | A/G | T/C | 1.32 | 1.76 | 5.68 |
| 9 | A/G | T/C | 0.819 | 2.86 | 15.2 |
| 11.1 | G/G | C/C | 0.027 | 0.047 | 39.8 |
| 11.1 | G/G | C/C | 0.0286 | 0.03 | 26.8 |
| 34.9 | A/G | T/C | 0.799 | 0.558 | 4.55 |
| 23.3 | A/G | T/C | 0.278 | 0.329 | 1.28 |
| 33.4 | A/G | T/C | ND | ND | ND |
| 29.7 | A/G | T/C | ND | ND | ND |
| 45.4 | A/A | T/T | 3.66 | 4.06 | 1.19 |
| 66.1 | A/A | T/T | 3.75 | 2.56 | 0.82 |
| 52.9 | A/A | T/T | 7.2 | 4.67 | 1 |
9-wk-old mice were deliberately euthanized at this age; all other ages reflect end stage of disease.
G is the restrictive allele at rs32279213 (encoding p.G63) and A is the permissive allele (encoding p.D63).
C is the restrictive allele at rs213983347 (encoding p.A106) and T is the permissive allele (encoding p.V106).
ND, not determined.
Differential impact of NPC1 AAV-PHP.B-NPC1 and AAV9-NPC1 vectors and Ly6a genotype on Npc1m1N/m1N metabolomics
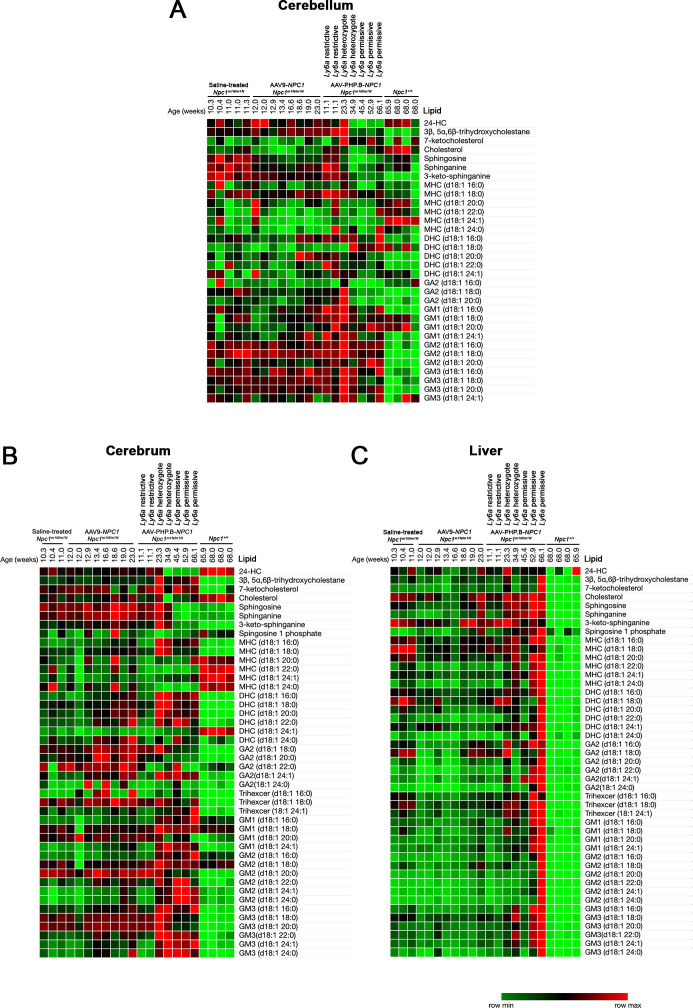
A hallmark of disease progression in NPC1 model mice is the accumulation of complex sphingolipid species and cholesterol oxidation products in various tissues as disease severity progresses (Pentchev et al, 1980; Goldin et al, 1992; Fan et al, 2013; Praggastis et al, 2015). Therefore, a broad panel of metabolites derived from cholesterol and sphingolipids was examined in cerebrum, cerebellum, and liver tissue from end stage AAV9-NPC1– and AAV-PHP.B-NPC1–treated Npc1m1N/m1N mice and compared with Npc1+/+ and Npc1m1N/m1N saline-injected mice (Fig S5). No differences were apparent between the saline-injected and AAV9-NPC1–treated Npc1m1N/m1N mice. The shortest-lived AAV-PHP.B-NPC1–treated Npc1m1N/m1N mice also did not show differences from untreated Npc1m1N/m1N mice. No consistent changes in cholesterol levels were apparent in the brain or liver tissues of AAV9-NPC1– and AAV-PHP.B-NPC1–treated Npc1m1N/m1N mice. Interestingly, the three longest lived AAV-PHP.B-NPC1–treated Npc1m1N/m1N mice with a homozygous permissive Ly6a genotype showed lower levels of sphingosine, sphinganine, and 3-keto-sphinganine in both cerebellum and cerebrum, suggesting modest correction in the brain (Fig S5A and B). In addition, these three mice showed lower levels than untreated Npc1m1N/m1N mice of GA2 species and 3β, 5α, 6β-trihydroxycholestane in the cerebellum. Both markers have shown improvement in mice, cats, and patients treated with 2-hydroxypropyl-β-cyclodextrin (HPβCD), a compound evaluated in clinical trial for NPC disease (Tortelli et al, 2014; Ory et al, 2017). In contrast, liver did not show notable correction of any individual lipid species in either AAV9-NPC1– or AAV-PHP.B-NPC1–treated Npc1m1N/m1N mice, and the longest lived AAV-PHP.B-NPC1–treated Npc1m1N/m1N mice showed higher levels of most lipids analyzed (Fig S5C). Of note, hierarchical clustering of the metabolomics data showed that AAV-PHP.B-NPC1–treated mice homozygous for the permissive Ly6a allele clustered together in cerebrum, cerebellum, and liver (data not shown), further suggesting that there are Ly6a-correlated differences in the lipidomic profiles of AAV-PHP.B-NPC1–treated Npc1m1N/m1N mice at end stage.
Figure S5. Metabolomics at the end stage reveal only modest changes resulting from treatment with either adeno-associated virus (AAV)9-NPC1 or AAV-PHP.B-NPC1.
(A, B) Metabolomics analysis of cerebellum and cerebrum tissues overall demonstrated little correction of the lipid pathology by either vector. The colorimetric scale (lower right) reflects the minimum and maximum levels of individual lipids in each row, with green indicating lowest levels, red indicating highest levels, and black indicating intermediate levels. Of note, several lipid species showed lower levels in brain in the three longest-lived AAV-PHP.B-NPC1–treated Npc1m1N/m1N mice that showed permissive Ly6a genotypes. These mice showed reduced levels of sphingosine, sphinganine, and 3-keto-sphinganine in both cerebellum and cerebrum (indicated by bright green), thus bringing the levels closer to those of Npc1+/+ mice. In addition, these three mice showed lower levels of GA2 species and 3β, 5α, 6β-trihydroxycholestane in the cerebellum in comparison with untreated Npc1m1N/m1N mice. (C) Metabolomics analysis of liver showed no notable correction of any individual lipid species in AAV-PHP.B-NPC1–treated Npc1m1N/m1N mice in comparison to saline-treated Npc1m1N/m1N mice, and the oldest AAV-PHP.B-NPC1–treated Npc1m1N/m1N mice showed broadly elevated lipid levels (indicated by red). However, AAV-PHP.B-NPC1–treated Npc1m1N/m1N mice did have an overall metabolomics signature more similar to Npc1+/+ mice, suggesting modest correction of liver disease in alignment with pathology findings. Heat maps were generated using Morpheus (https://software.broadinstitute.org/morpheus).
Differential impact of NPC1 AAV-PHP.B and AAV9 vectors on pathology
The impact of gene therapy on accumulation of unesterified cholesterol in the CNS and liver, a pathological hallmark of NPC disease, was evaluated with filipin complex (a macrolide that labels unesterified cholesterol in immunofluorescent staining) (Vanier & Latour, 2015). Additional known pathological changes, such as neuroinflammation and GM2 ganglioside accumulation were also investigated. Hematoxylin & eosin (H&E) staining provided further information with respect to vacuolization of neurons in the hippocampus and Kupffer cells (KCs) in the liver.
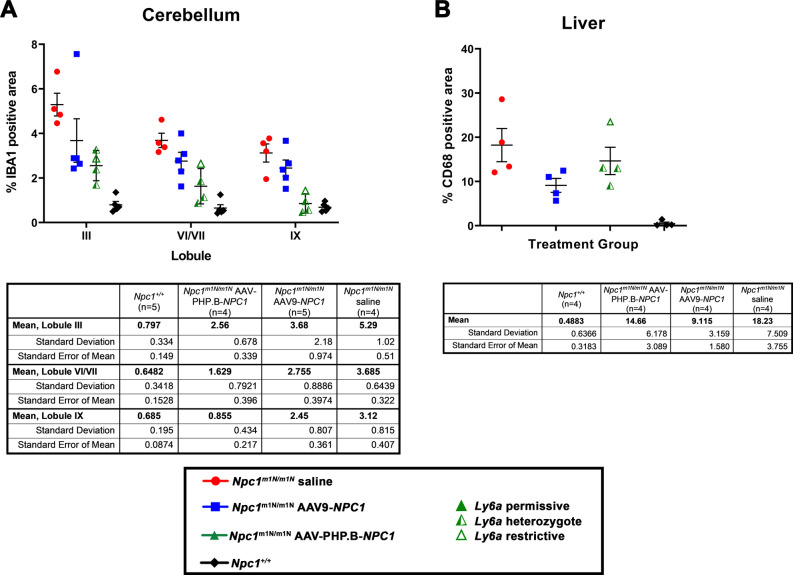
Filipin staining revealed prominent accumulation of unesterified cholesterol in the cerebellum in saline-injected Npc1m1N/m1N mice when compared to Npc1+/+ mice, as highlighted in Lobules III/IV (Fig 5A and B, Npc+/+ versus Npc1m1N/m1N, respectively). After treatment with either AAV9-NPC1 or AAV-PHP.B-NPC1, the anticipated reduction in cholesterol accumulation was modest (Fig 5C–F), a somewhat unexpected result given the significant impact on disease course. Even in regions with high expression observed in GFP studies, such as the hippocampus, reduction of pathology was minimal (Fig S6A–H). Only modest improvement of gliosis in gene therapy treated mice compared with the saline-injected Npc1m1N/m1N cohort was observed, as evidenced by microglial and astrocytic staining (anti-IBA1 and anti-GFAP, respectively). Quantification of microgliosis, or the percentage of IBA1+ area, was determined in lobules III, VI/VII, and IX of cerebellar tissue sections. Saline-injected Npc1m1N/m1N mice displayed a significantly higher percentage IBA1+ area than Npc1+/+ mice (Fig S7A; Kruskal–Wallis test with Dunn’s multiple comparison post-test, P = 0.0029 for lobule III, P = 0.0034 for lobules VI/VII, P = 0.0264 for lobule IX). IBA1+ area in specified lobules of cerebella from Npc1m1N/m1N mice treated with either gene therapy vector were not significantly different from saline-injected Npc1m1N/m1N or Npc1+/+ mice, although AAV9-PHP.B-NPC1–treated Npc1m1N/m1N mice did trend toward greater reduction in pathology. This finding correlates with the higher AAV9-PHP.B-NPC1 vector copy number and NPC1 protein levels in brain. Overall, when considering the numerous different cell types in the brain, NPC1-associated pathology minimally improved after gene therapy treatment.
Figure 5. Impact of adeno-associated virus (AAV)9-NPC1 and AAV-PHP.B-NPC1 vectors on cerebellar pathology.
(A, B, C, D, E, F) Cholesterol accumulation (visualized by Filipin labeling) is seen as white punctae, predominantly in cells of the molecular layer, of lobules III/IV of the cerebellum in saline and gene therapy–treated Npc1m1N/m1N mice (B, C, D, E, F). Note heterogeneity of modest correction observed in both AAV9-NPC1– and AAV-PHP.B-NPC1–transduced mice (C, D, E, F). Abbreviations in panel (A): GCL, granule cell layer; PC, Purkinje cell layer (dotted line); MCL, molecular cell layer. Scale bars = 500 μm (inset of cerebellum) and 250 μm (A, B, C, D, E, F).
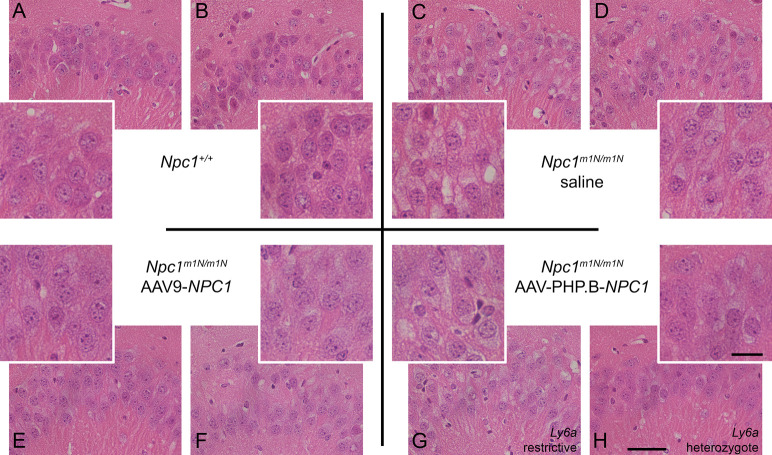
Figure S6. Region of interest from the hippocampus of hematoxylin & eosin–stained brain sections of 9-wk-old mice show little to no noticeable improvement of vacuolization in Npc1m1N/m1N mice, regardless of treatment.
(A, B) Quadrants depict two mice from each treatment group. Npc1+/+ mice (A, B) display normal cellular morphology absent of vacuolization. (C, D) Npc1m1N/m1N receiving saline injections (C, D) show prominent lighter areas throughout the cytoplasm which corresponds to lipid-laden storage bodies. (E, F, G, H) Npc1m1N/m1N mice given either adeno-associated virus (AAV)9-NPC1 (E and F) or AAV-PHP.B-NPC1 (G, H) display only modest improvement, with cytoplasmic vacuolization still visible in many cells and most noticeable in the AAV-PHP.B-NPC1–treated Npc1m1N/m1N mouse with restrictive Ly6a alleles (G). (A, C, D, E, H) Scale bars = 50 μm (A, C, D, E, H) and 20 μm (insets).
Figure S7. Quantification of percent positive area of macrophages versus total area assessed in cerebellum and liver reveals no significant differences in pathology, other than the expected increase in Npc1m1N/m1N saline-injected mice versus Npc1+/+ mice.
(A) Quantification of anti-IBA1 staining (microglial marker) in cerebellar lobules III, VI/VII, and IX suggests modestly reduced pathology in the Npc1m1N/m1N mice treated with either adeno-associated virus (AAV)9-NPC1 or AAV-PHP.B-NPC1 compared with saline-injected Npc1m1N/m1N mice, with AAV-PHP.B-NPC1 trending towards greater pathology reduction. (B) Quantification of anti-CD68 staining (macrophage marker) suggests only modest reduction in liver pathology of Npc1m1N/m1N mice after treatment with either AAV9-NPC1 or AAV-PHP.B-NPC1 compared with saline-injected Npc1m1N/m1N mice, with a trend towards greater pathology reduction by AAV9-NPC1.
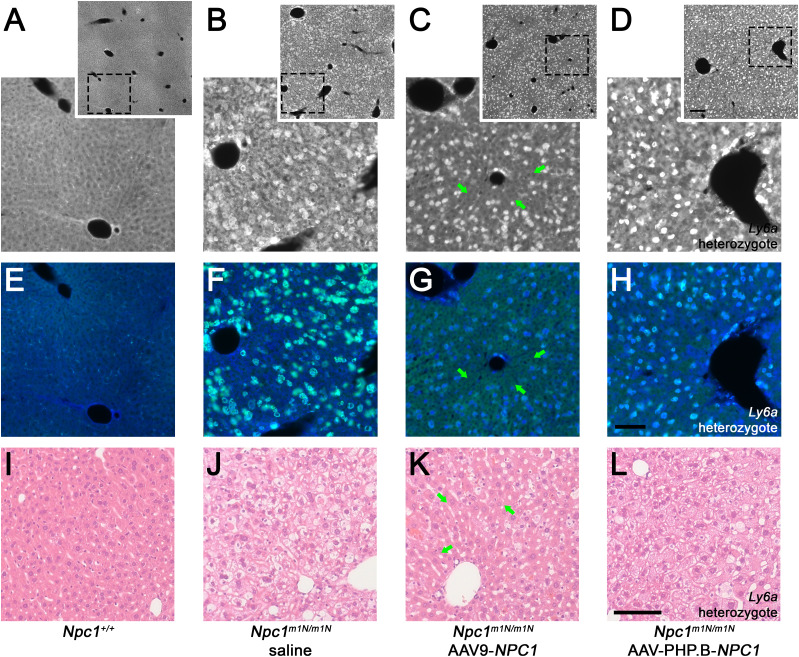
Filipin and immunofluorescent staining with anti-CD68 was performed on 9-wk-old liver tissue to visualize unesterified cholesterol accumulation and KCs, respectively (Fig 6). Untreated Npc1+/+ mice showed no cholesterol accumulation and normal cellular architecture of hepatocytes and KCs (Fig 6A, E, and I), whereas age-matched, saline-injected Npc1m1N/m1N mice showed extensive cholesterol accumulation and vacuolization in both hepatocytes and KCs (Fig 6B, F, and J). 9-wk-old Npc1m1N/m1N mice treated with AAV9-NPC1 and AAV-PHP.B-NPC1 showed similar relative abundance of lipid-laden KCs (green, Fig 6G and H) that corresponded to the very bright, rounded filipin positive cells (Fig 6C and D, depicting filipin channel alone). The only noticeable, although modest, pathological improvement in liver was found in AAV9-NPC1–treated Npc1m1N/m1N mice. Livers from these mice presented clusters of hepatocytes, frequently found near portal veins, that were free from cholesterol accumulation and vacuolization (Fig 6C, arrows and Fig 6K). On the other hand, livers from AAV-PHP.B-NPC1–treated Npc1m1N/m1N mice (Fig 6D, H, and L) showed very little correction of the storage phenotype, with only the occasional filipin-negative hepatocyte, consistent with the much lower copy number seen with ddPCR. Comparison of H&E staining for several mice from each treatment and age-group revealed heterogeneous pathological changes in AAV-PHP.B-NPC1–treated Npc1m1N/m1N mice, attributable to the Ly6a genotype, and extensive vacuolization in both gene therapy treatment groups at end stage disease (Fig S8; A-P 9-wk-old time point, Q-X end stage time point; column 1–4: Npc1+/+ mice, Npc1m1N/m1N saline-injected mice, Npc1m1N/m1N AAV9-NPC1-injected mice, and Npc1m1N/m1N AAV-PHP.B-NPC1-injected mice, respectively). Finally, the percentage of CD68+ area in sectioned liver tissue was compared between the different treatment groups (Fig S7B). Livers from saline-injected Npc1m1Nm1N mice displayed significantly larger CD68+ areas than did livers from Npc1+/+ mice (Kruskal–Wallis test with Dunn’s multiple comparison post-test; P = 0.0109). Although neither AAV9-NPC1- nor AAV-PHP.B-NPC1–treated Npc1m1N/m1N mice showed significant differences from values for Npc1m1N/m1N mice administered saline, there was a trend toward reduced CD68+ area in the AAV9-NPC1–treated mice which correlates with the assessments of vector copy number and NPC1 protein levels in liver.
Figure 6. Differential impact of adeno-associated virus (AAV)9-NPC1 and AAV-PHP.B-NPC1 vectors on liver pathology.
(A, B, C, D, E, F, G, H) Cholesterol accumulation (visualized by Filipin labeling: white in top row, blue in middle row) is pronounced in both hepatocytes and Kupffer cells (CD68+ green in middle row) of saline and AAV-PHP.B-NPC1–treated Npc1m1N/m1N mice (B, D, respectively). (A, B, C, D) Insets (A, B, C, D) provide an overview of pathology. (C, G, K) Groups of corrected hepatocytes, though not Kupffer cells, are visible in the AAV9-NPC1–treated Npc1m1N/m1N mice (arrows in C, G, K) consistent with moderate pathology reduction. (I. J, K, L) Hematoxylin and eosin staining support the mildly reduced pathology found in AAV9-NPC1–treated Npc1m1N/m1N mice (K) as compared to saline or AAV-PHP.B-NPC1 treatments (J, L). (A, E, I) Normal Npc1+/+ liver is shown in (A, E, I) for comparison. Scale bars = 200 μm (insets only, top row) or 100 μm (all other panels).
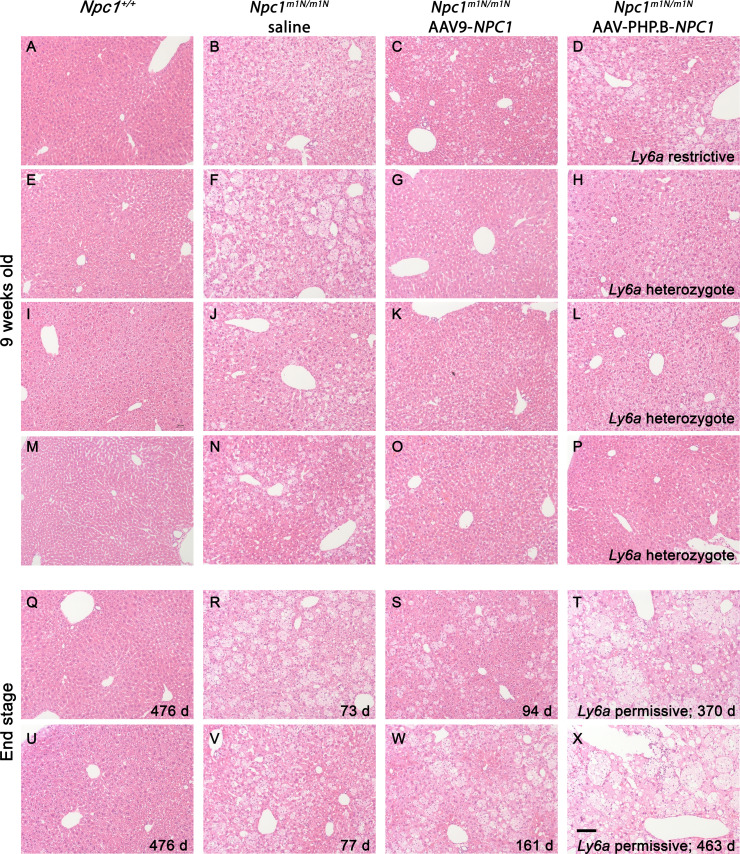
Figure S8. Liver pathology in 9-wk-old and end stage mice from different treatment groups.
(A, B, C, D, E, F, G, H, I, J, K, L, M, N, O, P, Q, R, S, T, U, V, W, X) Hematoxylin and eosin staining of liver from mice in the 9-wk (A, B, C, D, E, F, G, H, I, J, K, L, M, N, O, P) and end stage (Q, R, S, T, U, V, W, X) age-groups. (D, H, L, P) 9-wk-old mice demonstrate a correlation of pathology with Ly6a genotype in adeno-associated virus (AAV)-PHP.B-NPC1–treated group (D, H, L, P). (S, T, W, X) Representative mice from the end stage age group reveal significant liver pathology in the oldest surviving mice, whether treated with AAV9-NPC1 or AAV-PHP.B-NPC1 (S, W or T, X, respectively). Scale bar = 100 μm.
Whereas toxicity is possible with high-dosage AAV9 (Hinderer et al, 2018; Hordeaux et al, 2018), we found no histological indicators consistent with hepatic injury or genotoxicity. Upon examination of H&E staining for liver in Npc1m1N/m1N mice treated with AAV9-NPC1, the cytoarchitecture actually looked very similar to Npc1+/+ liver and showed no obvious signs of toxicity (Fig S8A, E, I, and M versus Fig S8C, G, K, and O; Npc1+/+ vs. Npc1m1N/m1N, respectively). The abnormal cells observed in the AAV9-treated livers are cholesterol-laden KCs as depicted in Fig 6, a known pathology of NPC1 disease. Furthermore, the mice had no adverse clinical symptoms as have been described in nonhuman primates or piglets treated with high doses of AAV9 early in life (Hinderer et al, 2018).
Discussion
This study demonstrates that treatment of Npc1m1N/m1N mice with an AAV-PHP.B vector containing human NPC1 significantly increased survival, delayed weight loss, and slowed disease progression compared to mice receiving an AAV9-pseudoserotyped NPC1 vector. Consistent with previous observations (Deverman et al, 2016), reporter studies revealed that AAV-PHP.B-EF1a(s)-GFP transgene expression was widespread throughout the brain after systemic delivery in both Npc1m1N/m1N mice and wild type littermates. The greater transduction efficiency observed with a neurotrophic NPC1 vector enabled increased CNS correction, and led to prolonged disease amelioration in the Npc1m1N/m1N mice. Although the importance of liver in NPC1 disease cannot be minimized, our data also suggest that significant improvement in disease course can be achieved in the absence of substantial liver correction. Finally, although preclinical data from animal models can provide essential proof-of-concept data, it is important to consider the limitations of these model systems, such as species effects of capsids and genetic admixture in mouse models, before translating therapies to the NPC1 patient population.
We noted a superior efficacy of an AAV-PHP.B vector compared with AAV9 in the Npc1m1N/m1N mice studied in our colony. This was evident by a significant increase in survival in the treated mutants, an important surrogate for NPC1 disease progression. The longest surviving AAV-PHP.B-NPC1–treated Npc1m1N/m1N mice were >1 yr old which, to our knowledge, represents the longest reported lifespan for mutant mice homozygous for this severe Npc1 allele. In the murine model, a single injection of AAV at weaning yielded survival benefits comparable to 2-hydroxypropyl-β-cyclodextrin, a promising NPC therapeutic which has advanced through a Phase 3 clinical trial (Ory et al, 2017). This cyclodextrin is a small cyclic sugar molecule that requires invasive delivery via the intrathecal route, lifelong dosing, and has significant ototoxicity as a frequent side effect (Ory et al, 2017). Therefore, a single administration of gene therapy, if it had long lasting effects, could represent an important new therapy that might be more effective, and perhaps synergize with other treatments. Combination AAV and cyclodextrin studies are underway, and might help define a new regimen to treat patients, one that hopefully would offer considerable improvement over current investigational or off-label treatments.
The gene therapy studies presented here are consistent with previous conditional and transgenic animal experiments that suggest greater CNS correction can lead to enhanced disease amelioration (Ko et al, 2005; Elrick et al, 2010; Yu et al, 2011). First, gene therapy–treated Npc1m1N/m1N mice with the longest survival had the highest NPC1 copy number in cerebrum and cerebellum, indicative of greater CNS transduction. Slower deterioration of motor coordination and disease phenotype, as demonstrated by the balance beam and phenotypic screening behavioral assays, was also noted in the mice with higher vector GC numbers. Importantly, AAV-PHP.B-NPC1–treated Npc1m1N/m1N mice displayed an even slower progression of disease than did AAV9-NPC1–treated Npc1m1N/m1N mice. Finally, maintenance of greater body weight was most apparent in the AAV-PHP.B-NPC1–treated Npc1m1N/m1N mice. All aforementioned improvements were exhibited in the treatment group displaying the highest NPC1 copy number in brain: AAV-PHP.B-NPC1. Although the results were variable, they consistently supported the observation that higher vector copy numbers in the brain correlated with greater improvement.
The extraneuronal disease of NPC1 is clinically significant, with hepatosplenomegaly and persistent liver disease, and even liver failure, documented in NPC1 patients (Kelly et al, 1993; Vanier, 2010; Patterson et al, 2013; Geberhiwot et al, 2018). However, whereas Npc1m1N/m1N mice treated with AAV9-NPC1 showed mild improvement in liver pathology, all other phenotypic measures were reduced compared with the more CNS-trophic AAV-PHP.B vector, highlighting the fact that correction of the CNS, broadly, at a very low level, drives phenotypic correction. Thus, our gene therapy experiments may serve to provide an alternative estimate to the mouse chimera mixing studies, which documented the need for ∼30% wild-type cells, to achieve phenotypic correction in Npc1 mice (Ko et al, 2005). Although we are uncertain as to the exact percent and cell type of CNS transduction achieved herein, the reported studies (Fig 1) and Western blotting (Fig 4) suggest it is <30%, and could perhaps inform the selection of a serotype, dose, and route of delivery for future human translation.
The influence of allelic variance at the Ly6a locus on treatment efficacy of the AAV-PHP.B vector is well exemplified in our study cohort. The protein encoded by Ly6a is expressed at the BBB, and transduction efficiency of AAV-PHP.B correlates with two different haplotypes across the Ly6a locus, which are present in various inbred strains of mice. These haplotypes include coding SNPs that may affect Ly6a function as well as upstream SNPs that may affect Ly6a protein expression at the BBB (Hordeaux et al, 2019; Huang et al, 2019; Batista et al, 2020). The Npc1m1N/m1N colony used in our studies harbors both permissive and restrictive genotypes that correlate with the effects seen in the outcome measures presented. In comparison, limited sampling from the Jackson Laboratory colony revealed only the restrictive genotype (Pavan, unpublished observation). Differences noted in published studies between Npc1m1N/m1N mice in distinct facilities, particularly in terms of average survival without therapeutic intervention, might be caused by genetic admixture and/or fixation of modifier alleles, and highlights the importance of exploring strain effects in murine models that are used to generate preclinical enabling data. Recent studies showed reduced efficacy of AAV-PHP.B in nonhuman primates that is likely attributable to the absence of LY6A in primates (Hordeaux et al, 2018; Matsuzaki et al, 2018; Liguore et al, 2019), suggesting that novel viral variants may not be readily transferred between species but instead would need to be generated in a species-specific manner. As such, translation from model organism to human must be considered and investigated. For many gene therapy studies, especially those using novel engineered capsids, nonhuman primate studies and relevant human culture models may be needed to validate and optimize a gene therapy vector for delivery to patients with NPC disease and related disorders. Advances in the identification of novel serotypes that cross the BBB in humans, and capsid engineering to derive CNS trophic variants (Castle et al, 2016; Hudry et al, 2018; Sullivan et al, 2018; Hanlon et al, 2019; Havlik et al, 2020) should help improve vectors, as recent studies highlight (Gray et al, 2013; Frederick et al, 2020; Yoon et al, 2020).
In summary, our studies confirm that achieving even moderate transduction of the CNS using an AAV9-NPC1 vector can have profound effects on disease course, but that much greater correction can be demonstrated with a neurotrophic AAV-PHP.B vector, suggesting that eventual clinical translation may be best accomplished using a capsid that has similar properties in humans.
Materials and Methods
Vector construction and production
The transgene EF1a(s)-NPC1 was previously described (Chandler et al, 2017) and the analogous GFP reporter was prepared by replacing NPC1 with eGFP to make EF1a(s)-GFP. All therapeutic and control vectors were produced by the Beckman Institute CLOVER Center under direction of Dr V Gradinaru in the Division of Biology and Biological Engineering at the California Institutes of Technology as previously described (Deverman et al, 2016), and serotyped as AAV9 or AAV-PHP.B.
Animals
Animal work in these studies was performed according to the animal care and use protocols approved by the National Institutes of Health. Heterozygous mice from the BALB/cNctr-Npc1m1N/J strain were crossed to generate homozygous Npc1m1N/m1N mutants and Npc1+/+ control littermates. Mice were weighed once per week and then more frequently as disease progressed. Euthanasia was performed when end stage disease progression was reached, as determined by the presence of at least two of the following signs: 30% loss of maximum weight, reluctance to move about cage, repeated falling to side during forward ambulation, and palpebral closure/eyes appearing dull rather than bright.
Study design (based on guidelines in Percie du Sert et al [2020])
This study compared Npc1m1N/m1N mice administered either vehicle (saline) or gene therapy vector (AAV9 or AAV-PHP.B) with reporter construct (GFP) or NPC1. Npc1+/+ mice included as a control group for behavioral, survival, and pathology analyses did not receive saline, whereas a subset of Npc1+/+ mice received either the AAV9-GFP or AAV9-NPC1 for biodistrubution and gene copy number analyses. The Npc1m1N/m1N colony has been maintained in-house for 5+ yr but originated from the Jackson Laboratory (Stock #003092, BALB/cNctr-Npc1m1N/J). Each mouse was a single experimental unit and the sample size was based on previously published work from our group. A total of 82 mice were used in these studies (Table S2). No explicit criteria were set a priori for inclusion/exclusion criteria and no mice or data points were excluded from study or analyses. Group sample size for each analysis is stated in text, figure legend, or figure. Randomization was employed by using multiple cohorts and mice within each treatment group were included in every cohort to minimize confounders. In addition, order of mice in behavioral tests varied with each testing date and all animals were group housed. Researchers were not blinded to treatment at time of injections. However, behavioral analyses were carried out in a blinded fashion such that colored tails were used to denote individual mice within cages and only the cage number and tail color were available to the evaluator during testing. Main outcome measures, provided throughout text, included survival, behavioral phenotype, pathology, and gene copy number. Statistical test selection was based on particular data set and accounted for normalized (or not) data.
Table S3 Antibodies and dilutions for immunofluorescent staining. (13.8KB, docx)
Behavior testing
Two behavioral assays were used to determine the effect of gene therapy on motor performance: phenotype score and balance beam. Mice were tested beginning at 6 wk of age and then every 3 wk thereafter until euthanasia or unable to complete the task. The phenotype score evaluates six individual domains associated with the disease phenotype seen in Npc1m1N/m1N mice: gait, kyphosis, ledge test, and hind limb clasps from a cerebellar ataxia score (Guyenet et al, 2010) plus grooming and tremor (Alam et al, 2016). Each domain is given a score of 0–3 with a higher score indicating greater disease progression and the composite score of all domains is presented in results. The balance beam assay is a quantitative approach for assessing loss of motor function (Gulinello et al, 2010; Arteaga-Bracho et al, 2016). The number of hind limb foot slips is counted as mice traverse a four-foot long, round wooden beam (diameter = 18 or 24 mm). A more progressed disease state correlates to a higher number of slips.
Administration of vector
Npc1m1N/m1N mice received a 50 μl retro-orbital injection at weaning (24–27 d) of one of the following: 1.43 × 1012 GC of AAV-PHP.B-EF1a(s)-NPC1 (n = 13), 1.84 × 1012 GC of AAV9-EF1a(s)-NPC1 (n = 12), 1.21 × 1012 GC of AAV-PHP.B-EF1a(s)-GFP (n = 2), or 1.21 × 1012 GC of AAV9-EF1a(s)-GFP (n = 2). Control Npc1m1N/m1N mice received a 50-μl retro-orbital injection of 0.9% saline at weaning (24–27 d).
Tissue collection and homogenization
Euthanasia for tissue collection was initiated with an intraperitoneal injection of Avertin (lethal dose of 0.04 ml/gm). When mice were insensate, the chest cavity was opened to allow a transcardiac perfusion of 0.9% saline. One half of the brain and a piece of liver were then collected and flash frozen in liquid nitrogen, with long-term storage at −80°C. Subsequently, mice were re-perfused with 4% paraformaldehyde before collecting remaining organs for post-fixation overnight in 4% paraformaldehyde. Fixed tissues were rinsed and stored in PBS at 4°C.
Tissue homogenization was achieved using a Benchmark Scientific BeadBug homogenizer and UltraPure H2O (11005-060; IPM Scientific). Frozen tissues were placed in tubes prefilled with 3 mm glass beads and homogenized 3 × 30 s at a speed of 400. Homogenate was immediately aliquoted into three separate tubes for DNA (ddPCR), metabolomics, and protein (Western blot; WB). RIPA buffer with proteinase inhibitor cocktail (20-201; Millipore) was added to WB samples and then homogenates were spun down at 14,534g for 20 min at 4°C. Supernatant was collected and stored at −80°C.
Western blotting
Protein levels from cerebrum and liver WB homogenates were quantified using the BCA Protein Assay kit from Pierce (23227). Equal amounts of protein (80 μg for liver and 120 μg for cerebrum) were run on 4–12% Bis-Tris SDS-polyacrylamide gels (NW04120BOX; Thermo Fisher Scientific) to achieve separation of protein bands. After transferring to a nitrocellulose membrane (IB301002; Life Technologies) and blocking for 1 h in TBS-Tween + LI-COR Odyssey Blocking Buffer (927-40000), samples were incubated overnight with two antibodies: rabbit anti-NPC1 (ab 134113; 1:1,000; Abcam) and the loading control mouse anti-α-tubulin (T9026; 1:1,000; Millipore). The Odyssey donkey anti-rabbit 680 (926-68073; 1:5,000; LI-COR Biosciences) and the Odyssey donkey anti-mouse 800 (926-32212; 1:5,000; LI-COR Biosciences) were used as secondary antibodies. The LI-COR Odyssey Imaging System was used to capture results.
Immunohistochemistry
Brain and liver tissue from each treatment group at 9-wk-old and end stage were used for immunohistochemical staining. 24–48 h before sectioning of GFP biodistribution samples, tissues were transferred to a 30% sucrose solution where they remained until sinking. Using a cryostat, brain and liver were sectioned parasagittally at a 30 μm thickness and free-floating sections were collected and washed in PBS containing 0.25% Triton X-100 (PBSt). After a 1-h block at room temperature in PBSt/normal goat serum (NGS, S26-M; Sigma-Aldrich), primary antibodies were diluted in PBSt/NGS and sections incubated overnight. Subsequent to washing in PBSt, appropriate secondary antibodies were diluted in PBSt/NGS and incubated with sections for 30 min at room temperature. Refer to Table S2 for antibodies and dilutions. Filipin complex from Streptomyces filipinensis (F9765; 0.025 g/ml; Sigma-Aldrich) was diluted in PBSt to stain tissue for cholesterol accumulation. ProLong Gold mounting medium with or without DAPI (P36930 or P36935; Life Technologies) was used to coverslip slides after sections were mounted. H&E staining was performed by Histoserv, Inc..
Quantification of CD68+ and IBA1+ area
Percent CD68+ area relative to total area in liver tissue sections was performed according the methods previously described (Rodriguez-Gil et al, 2020). Percent IBA1+ area relative to total area in cerebellar tissue sections was performed in a similar manner with the use of a β version of Image-Pro v 10.5 software (Media Cybernetics, Inc.).
Copy number analysis by ddPCR
ddPCR was performed as previously described (Lissa et al, 2018) with the following modifications: NPC1 and GAPDH primers were obtained from Bio-Rad (unique Assay IDs dCNS361140976 with 6-FAM and dMmuCNS133125454 with HEX, respectively). From sample homogenates, 50 ng of DNA from cerebrum or cerebellum or 10 ng of DNA from liver was used to quantify gene copy number. PCR cycling conditions were identical with the exception of 60°C for annealing and extension. Droplet signal was read as being either positive or negative for NPC1 and/or GAPDH. Any samples with fewer than 10,000 positive droplets were excluded and the sample(s) re-run to obtain an accurate read.
Ly6a genotype analysis
Genomic DNA was amplified with primers and probes using a real-time SNP genotyping assay (Custom Taqman Assay Design Tool; Thermo Fisher Scientific) for SNPs rs32279213 and rs213983347, as follows: rs32279213 F: GCAGATGGGTAAGCAAAGATTGTTC, R: GTCCCTGCATAAGAAGTGAGTCA, FAM:TTCTTGCAGGTTCTCA,VIC: TTTCTTGCAGATTCTCA, rs213983347: F: AGGTGCTGCCTCCATTGG, R: CTAAGGTCAACGTGAAGACTTCCT, FAM: TCTGCAATGCAGCAGT, VIC: CCTCTGCAATGTAGCAGT, a universal 2x Taqman Master Mix (Thermo Fisher Scientific) and an ABI 7500 instrument for thermocycling and detection. For the genome-wide scan of two Npc1m1N/m1N mice, data were assessed at the DartMouseTM Speed Congenic Core Facility at the Geisel School of Medicine at Dartmouth, using a custom panel of 5,307 SNPs distributed throughout the mouse genome. Raw SNP data were analyzed using DartMouse’s SNaP-MapTM and Map-SynthTM software.
Lipidomics
Lipidomic analyses on cerebral, cerebellar, and liver homogenates were carried out as previously described (Praggastis et al, 2015; Davidson et al, 2019).
Image capture and analysis
Fluorescent images were captured on an inverted Zeiss Axio Observer.Z1 using an AxioCam MRm and ZEN 2.5 software. Brightfield images were captured on an inverted Zeiss Axio Observer.D1 using an AxioCamHRc and ZEN 2011 software. Adobe Photoshop 2020 version 21.1.2 was used to resize and adjust brightness and contrast, such that all images within a staining run and/or figure were modified in an identical manner.
Statistical analysis
Statistical analyses were done using GraphPad Prism version 8.0.0 for Windows or Mac (GraphPad Software, San Diego, California, USA, www.graphpad.com). The following statistical tests were used: Mantel–Cox log rank test (Fig 2A); Kruskal–Wallis test with Dunn’s multiple comparisons test (Figs 2C and S7); Welch’s ANOVA test with Dunnett’s multiple comparisons test (Fig 2D); two-way ANOVA with Tukey’s multiple comparisons test (Figs 2E and S3); unpaired t test (Figs 3A–C and S4); and Spearman’s correlation coefficient test (Figs 3D–F and S2). For data sets, normality was evaluated if possible, and if not, a non-parametric test was used. Otherwise, the appropriate statistical test was then selected for further analysis. All data is presented as mean ± SD unless otherwise indicated.
Study approval
All animal works were performed according to National Institutes of Health–approved animal care and use protocols.
Supplementary Material
Acknowledgements
We are grateful for the wealth of expertise and technical assistance regarding droplet digital PCR provided by Valery Bliskovsky, Steven Shema, and Liz Conner in the National Cancer Institute’s Genomics Core. The dedicated National Institutes of Health (NIH) animal care and veterinary staff is gratefully acknowledged for their commitment to the care and well-being of all animals in our facilities, especially the mice included in this study. Finally, and perhaps most importantly, the unwavering support and trust in research by NPC-affected patients and families is a powerful reminder of why our collective efforts as a research community must prevail. This research was funded by the Intramural Research Program of the National Human Genome Research Institute (NHGRI) at the NIH (1ZIAHG000068-15). Additional support was generously provided by Liferay, Inc. (Postbaccalaureate Intramural Research Training Award for AL Gibson) and Niemann-Pick Canada and Ara Parseghian Medical Research Fund at the University of Notre Dame. Support for CD Davidson was kindly provided by the Hide & Seek Foundation and Dana’s Angels Research Trust (both part of Support Of Accelerated Research for Niemann-Pick C). Work performed at the California Institute of Technology was supported by NIH Pioneer grant (DP1OD025535; V Gradinaru) and the Beckman Institute for CLARITY, Optogenetics and Vector Engineering Research (CLOVER) for technology development and dissemination (V Gradinaru). JL Rodriguez-Gil was supported by an NHGRI Intramural Research Training Award, the NIH Oxford-Cambridge Scholars Program, and the Medical Scientist Training Program from the School of Medicine and Public Health, University of Wisconsin-Madison (3T32GM008692).
Author Contributions
CD Davidson: conceptualization, formal analysis, investigation, visualization, project administration, and writing—original draft, review, and editing.
AL Gibson: investigation and writing—original draft, review, and editing.
T Gu: investigation and writing—original draft, review, and editing.
LL Baxter: formal analysis, visualization, and writing—original draft, review, and editing.
BE Deverman: conceptualization, resources, investigation, and writing—review and editing.
K Beadle: investigation and writing—review and editing.
AA Incao: investigation and writing—review and editing.
JL Rodriguez-Gil: formal analysis, investigation, and writing—review and editing.
H Fujiwara: formal analysis and writing—review and editing.
X Jiang: formal analysis, investigation, and writing—review and editing.
RJ Chandler: conceptualization and writing—review and editing.
DS Ory: resources and writing—review and editing.
V Gradinaru: resources, funding acquisition, and writing—review and editing.
CP Venditti: conceptualization, resources, supervision, funding acquisition, and writing—review and editing.
WJ Pavan: conceptualization, resources, supervision, funding acquisition, project administration, and writing—review and editing.
Conflict of Interest Statement
The NIH has filed patents on behalf of RJ Chandler, CP Venditti, and WJ Pavan related to NPC1 gene therapy and biomarkers. The California Institute of Technology has filed patents related to AAV-PHP.B with BE Deverman and V Gradinaru as inventors. BE Deverman also has a patent application filed by the Broad Institute and is a consultant for Voyager Therapeutics.
References
- Alam MS, Getz M, Haldar K (2016) Chronic administration of an HDAC inhibitor treats both neurological and systemic Niemann-Pick type C disease in a mouse model. Sci Transl Med 8: 326ra23. 10.1126/scitranslmed.aad9407 [DOI] [PubMed] [Google Scholar]
- Al-Zaidy S, Pickard AS, Kotha K, Alfano LN, Lowes L, Paul G, Church K, Lehman K, Sproule DM, Dabbous O, et al. (2019) Health outcomes in spinal muscular atrophy type 1 following AVXS-101 gene replacement therapy. Pediatr Pulmonol 54: 179–185. 10.1002/ppul.24203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arteaga-Bracho EE, Gulinello M, Winchester ML, Pichamoorthy N, Petronglo JR, Zambrano AD, Inocencio J, De Jesus CD, Louie JO, Gokhan S, et al. (2016) Postnatal and adult consequences of loss of huntingtin during development: Implications for Huntington’s disease. Neurobiol Dis 96: 144–155. 10.1016/j.nbd.2016.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batista AR, King OD, Reardon CP, Davis C, Shankaracharya, Philip V, Aronin N, Lutz C, Landers J, et al. (2020) Ly6a differential expression in blood-brain barrier is responsible for strain specific central nervous system transduction profile of AAV-PHP.B. Hum Gene Ther 31: 90–102. 10.1089/hum.2019.186 [DOI] [PubMed] [Google Scholar]
- Bevan AK, Duque S, Foust KD, Morales PR, Braun L, Schmelzer L, Chan CM, McCrate M, Chicoine LG, Coley BD, et al. (2011) Systemic gene delivery in large species for targeting spinal cord, brain, and peripheral tissues for pediatric disorders. Mol Ther 19: 1971–1980. 10.1038/mt.2011.157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carstea ED, Morris JA, Coleman KG, Loftus SK, Zhang D, Cummings C, Gu J, Rosenfeld MA, Pavan WJ, Krizman DB, et al. (1997) Niemann-Pick C1 disease gene: Homology to mediators of cholesterol homeostasis. Science 277: 228–231. 10.1126/science.277.5323.228 [DOI] [PubMed] [Google Scholar]
- Castle MJ, Turunen HT, Vandenberghe LH, Wolfe JH (2016) Controlling AAV tropism in the nervous system with natural and engineered capsids. Methods Mol Biol 1382: 133–149. 10.1007/978-1-4939-3271-9_10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler RJ, Williams IM, Gibson AL, Davidson CD, Incao AA, Hubbard BT, Porter FD, Pavan WJ, Venditti CP (2017) Systemic AAV9 gene therapy improves the lifespan of mice with Niemann-Pick disease, type C1. Hum Mol Genet 26: 52–64. 10.1093/hmg/ddw367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cologna SM, Rosenhouse-Dantsker A (2019) Insights into the molecular mechanisms of cholesterol binding to the NPC1 and NPC2 proteins. Adv Exp Med Biol 1135: 139–160. 10.1007/978-3-030-14265-0_8 [DOI] [PubMed] [Google Scholar]
- Davidson J, Molitor E, Moores S, Gale SE, Subramanian K, Jiang X, Sidhu R, Kell P, Zhang J, Fujiwara H, et al. (2019) 2-Hydroxypropyl-β-cyclodextrin is the active component in a triple combination formulation for treatment of Niemann-Pick C1 disease. Biochim Biophys Acta Mol Cell Biol Lipids 1864: 1545–1561. 10.1016/j.bbalip.2019.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deverman BE, Pravdo PL, Simpson BP, Kumar SR, Chan KY, Banerjee A, Wu WL, Yang B, Huber N, Pasca SP, et al. (2016) Cre-dependent selection yields AAV variants for widespread gene transfer to the adult brain. Nat Biotechnol 34: 204–209. 10.1038/nbt.3440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elrick MJ, Pacheco CD, Yu T, Dadgar N, Shakkottai VG, Ware C, Paulson HL, Lieberman AP (2010) Conditional Niemann-Pick C mice demonstrate cell autonomous Purkinje cell neurodegeneration. Hum Mol Genet 19: 837–847. 10.1093/hmg/ddp552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan M, Sidhu R, Fujiwara H, Tortelli B, Zhang J, Davidson C, Walkley SU, Bagel JH, Vite C, Yanjanin NM, et al. (2013) Identification of Niemann-Pick C1 (NPC1) disease biomarkers through sphingolipid profiling. J Lipid Res 54: 2800–2814. 10.1194/jlr.M040618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederick A, Sullivan J, Liu L, Adamowicz M, Lukason M, Raymer J, Luo Z, Jin X, Rao KN, O’Riordan C (2020) Engineered capsids for efficient gene delivery to the retina and cornea. Hum Gene Ther 31: 756–774. 10.1089/hum.2020.070 [DOI] [PubMed] [Google Scholar]
- Gao Y, Geng L, Chen VP, Brimijoin S (2017) Therapeutic delivery of butyrylcholinesterase by brain-wide viral gene transfer to mice. Molecules 22: 1145. 10.3390/molecules22071145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garver WS, Jelinek D, Oyarzo JN, Flynn J, Zuckerman M, Krishnan K, Chung BH, Heidenreich RA (2007) Characterization of liver disease and lipid metabolism in the Niemann-Pick C1 mouse. J Cell Biochem 101: 498–516. 10.1002/jcb.21200 [DOI] [PubMed] [Google Scholar]
- Geberhiwot T, Moro A, Dardis A, Ramaswami U, Sirrs S, Marfa MP, Vanier MT, Walterfang M, Bolton S, Dawson C, et al. (2018) Consensus clinical management guidelines for Niemann-Pick disease type C. Orphanet J Rare Dis 13: 50. 10.1186/s13023-018-0785-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin E, Roff CF, Miller SP, Rodriguez-Lafrasse C, Vanier MT, Brady RO, Pentchev PG (1992) Type C niemann-pick disease: A murine model of the lysosomal cholesterol lipidosis accumulates sphingosine and sphinganine in liver. Biochim Biophys Acta 1127: 303–311. 10.1016/0005-2760(92)90236-o [DOI] [PubMed] [Google Scholar]
- Gray SJ, Nagabhushan Kalburgi S, McCown TJ, Jude Samulski R (2013) Global CNS gene delivery and evasion of anti-AAV-neutralizing antibodies by intrathecal AAV administration in non-human primates. Gene Ther 20: 450–459. 10.1038/gt.2012.101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulinello M, Acquarone M, Kim JH, Spray DC, Barbosa HS, Sellers R, Tanowitz HB, Weiss LM (2010) Acquired infection with Toxoplasma gondii in adult mice results in sensorimotor deficits but normal cognitive behavior despite widespread brain pathology. Microbes Infect 12: 528–537. 10.1016/j.micinf.2010.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet SJ, Furrer SA, Damian VM, Baughan TD, La Spada AR, Garden GA (2010) A simple composite phenotype scoring system for evaluating mouse models of cerebellar ataxia. J Vis Exp 1787. 10.3791/1787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon KS, Meltzer JC, Buzhdygan T, Cheng MJ, Sena-Esteves M, Bennett RE, Sullivan TP, Razmpour R, Gong Y, Ng C, et al. (2019) Selection of an efficient AAV vector for robust CNS transgene expression. Mol Ther Methods Clin Dev 15: 320–332. 10.1016/j.omtm.2019.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havlik LP, Simon KE, Smith JK, Klinc KA, Tse LV, Oh DK, Fanous MM, Meganck RM, Mietzsch M, Kleinschmidt J, et al. (2020) Coevolution of adeno-associated virus capsid antigenicity and tropism through a structure-guided approach. J Virol 94: 495. 10.1128/JVI.00976-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinderer C, Katz N, Buza EL, Dyer C, Goode T, Bell P, Richman LK, Wilson JM (2018) Severe toxicity in nonhuman primates and piglets following high-dose intravenous administration of an adeno-associated virus vector expressing human SMN. Hum Gene Ther 29: 285–298. 10.1089/hum.2018.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hordeaux J, Wang Q, Katz N, Buza EL, Bell P, Wilson JM (2018) The neurotropic properties of AAV-PHP.B are limited to C57BL/6J mice. Mol Ther 26: 664–668. 10.1016/j.ymthe.2018.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hordeaux J, Yuan Y, Clark PM, Wang Q, Martino RA, Sims JJ, Bell P, Raymond A, Stanford WL, Wilson JM (2019) The GPI-linked protein LY6A drives AAV-PHP.B transport across the blood-brain barrier. Mol Ther 27: 912–921. 10.1016/j.ymthe.2019.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Q, Chan KY, Tobey IG, Chan YA, Poterba T, Boutros CL, Balazs AB, Daneman R, Bloom JM, Seed C, et al. (2019) Delivering genes across the blood-brain barrier: LY6A, a novel cellular receptor for AAV-PHP.B capsids. PLoS One 14: e0225206. 10.1371/journal.pone.0225206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudry E, Vandenberghe LH (2019) Therapeutic AAV gene transfer to the nervous system: A clinical reality. Neuron 102: 263–862. 10.1016/j.neuron.2019.03.020 [DOI] [PubMed] [Google Scholar]
- Hudry E, Andres-Mateos E, Lerner EP, Volak A, Cohen O, Hyman BT, Maguire CA, Vandenberghe LH (2018) Efficient gene transfer to the central nervous system by single-stranded Anc80L65. Mol Ther Methods Clin Dev 10: 197–209. 10.1016/j.omtm.2018.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes MP, Smith DA, Morris L, Fletcher C, Colaco A, Huebecker M, Tordo J, Palomar N, Massaro G, Henckaerts E, et al. (2018) AAV9 intracerebroventricular gene therapy improves lifespan, locomotor function and pathology in a mouse model of Niemann-Pick type C1 disease. Hum Mol Genet 27: 3079–3098. 10.1093/hmg/ddy212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Infante RE, Wang ML, Radhakrishnan A, Kwon HJ, Brown MS, Goldstein JL (2008) NPC2 facilitates bidirectional transfer of cholesterol between NPC1 and lipid bilayers, a step in cholesterol egress from lysosomes. Proc Natl Acad Sci U S A 105: 15287–15292. 10.1073/pnas.0807328105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanchenko MV, Hanlon KS, Devine MK, Tenneson K, Emond F, Lafond JF, Kenna MA, Corey DP, Maguire CA (2020) Preclinical testing of AAV9-PHP.B for transgene expression in the non-human primate cochlea. Hear Res 394: 107930. 10.1016/j.heares.2020.107930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson KL, Dayton RD, Deverman BE, Klein RL (2016) Better targeting, better efficiency for wide-scale neuronal transduction with the synapsin promoter and AAV-PHP.B. Front Mol Neurosci 9: 116. 10.3389/fnmol.2016.00116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly DA, Portmann B, Mowat AP, Sherlock S, Lake BD (1993) Niemann-Pick disease type C: Diagnosis and outcome in children, with particular reference to liver disease. J Pediatr 123: 242–247. 10.1016/s0022-3476(05)81695-6 [DOI] [PubMed] [Google Scholar]
- Ko DC, Milenkovic L, Beier SM, Manuel H, Buchanan J, Scott MP (2005) Cell-autonomous death of cerebellar purkinje neurons with autophagy in Niemann-Pick type C disease. PLoS Genet 1: 81–95. 10.1371/journal.pgen.0010007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Samulski RJ (2020) Engineering adeno-associated virus vectors for gene therapy. Nat Rev Genet 21: 255–272. 10.1038/s41576-019-0205-4 [DOI] [PubMed] [Google Scholar]
- Liguore WA, Domire JS, Button D, Wang Y, Dufour BD, Srinivasan S, McBride JL (20192018–2037) AAV-PHP.B administration results in a differential pattern of CNS biodistribution in non-human primates compared with mice. Mol Ther 27: 2018–2037. 10.1016/j.ymthe.2019.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim JA, Yi H, Gao F, Raben N, Kishnani PS, Sun B (2019) Intravenous injection of an AAV-PHP.B vector encoding human acid α-glucosidase rescues both muscle and CNS defects in murine Pompe disease. Mol Ther Methods Clin Dev 12: 233–245. 10.1016/j.omtm.2019.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissa D, Ishigame T, Noro R, Tucker MJ, Bliskovsky V, Shema S, Beck JA, Bowman ED, Harris CC, Robles AI (2018) HOXA9 methylation and blood vessel invasion in FFPE tissues for prognostic stratification of stage I lung adenocarcinoma patients. Lung Cancer 122: 151–159. 10.1016/j.lungcan.2018.05.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowes LP, Alfano LN, Arnold WD, Shell R, Prior TW, McColly M, Lehman KJ, Church K, Sproule DM, Nagendran S, et al. (2019) Impact of age and motor function in a phase 1/2A study of infants with SMA type 1 receiving single-dose gene replacement therapy. Pediatr Neurol 98: 39–45. 10.1016/j.pediatrneurol.2019.05.005 [DOI] [PubMed] [Google Scholar]
- Luoni M, Giannelli S, Indrigo MT, Niro A, Massimino L, Iannielli A, Passeri L, Russo F, Morabito G, Calamita P, et al. (2020) Whole brain delivery of an instability-prone Mecp2 transgene improves behavioral and molecular pathological defects in mouse models of Rett syndrome. Elife 9: E7287. 10.7554/eLife.52629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lykken EA, Shyng C, Edwards RJ, Rozenberg A, Gray SJ (2018) Recent progress and considerations for AAV gene therapies targeting the central nervous system. J Neurodev Disord 10: 16. 10.1186/s11689-018-9234-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma C-C, Wang Z-L, Xu T, He Z-Y, Wei Y-Q (2019) The approved gene therapy drugs worldwide: From 1998 to 2019. Biotechnol Adv 40: 107502. 10.1016/j.biotechadv.2019.107502 [DOI] [PubMed] [Google Scholar]
- Markmann S, J Christie-Reid J, Rosenberg JB, De BP, Kaminsky SM, Crystal RG, Sondhi D (2018) Attenuation of the Niemann-Pick type C2 disease phenotype by intracisternal administration of an AAVrh.10 vector expressing Npc2. Exp Neurol 306: 22–33. 10.1016/j.expneurol.2018.04.001 [DOI] [PubMed] [Google Scholar]
- Matsuzaki Y, Konno A, Mochizuki R, Shinohara Y, Nitta K, Okada Y, Hirai H (2018) Intravenous administration of the adeno-associated virus-PHP.B capsid fails to upregulate transduction efficiency in the marmoset brain. Neurosci Lett 665: 182–188. 10.1016/j.neulet.2017.11.049 [DOI] [PubMed] [Google Scholar]
- Morabito G, Giannelli SG, Ordazzo G, Bido S, Castoldi V, Indrigo M, Cabassi T, Cattaneo S, Luoni M, Cancellieri C, et al. (2017) AAV-PHP.B-Mediated global-scale expression in the mouse nervous system enables GBA1 gene therapy for wide protection from synucleinopathy. Mol Ther 25: 2727–2742. 10.1016/j.ymthe.2017.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris MD, Bhuvaneswaran C, Boothe AD (1977) Tissue cholesterol storage disorder in BALB/c mice. Fed Proc 36: 1158. [Google Scholar]
- Morris MD, Bhuvaneswaran C, Shio H, Fowler S (1982) Lysosome lipid storage disorder in NCTR-BALB/c mice. I. Description of the disease and genetics. Am J Pathol 108: 140–149. [PMC free article] [PubMed] [Google Scholar]
- Naureckiene S, Sleat DE, Lackland H, Fensom A, Vanier MT, Wattiaux R, Jadot M, Lobel P (2000) Identification of HE1 as the second gene of Niemann-Pick C disease. Science 290: 2298–2301. 10.1126/science.290.5500.2298 [DOI] [PubMed] [Google Scholar]
- Ory DS, Ottinger EA, Farhat NY, King KA, Jiang X, Weissfeld L, Berry-Kravis E, Davidson CD, Bianconi S, Keener LA, et al. (2017) Intrathecal 2-hydroxypropyl-β-cyclodextrin decreases neurological disease progression in Niemann-Pick disease, type C1: A non-randomised, open-label, phase 1-2 trial. Lancet 390: 1758–1768. 10.1016/S0140-6736(17)31465-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson MC, Mengel E, Wijburg FA, Muller A, Schwierin B, Drevon H, Vanier MT, Pineda M (2013) Disease and patient characteristics in NP-C patients: Findings from an international disease registry. Orphanet J Rare Dis 8: 12. 10.1186/1750-1172-8-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pentchev PG, Gal AE, Booth AD, Omodeo-Sale F, Fouks J, Neumeyer BA, Quirk JM, Dawson G, Brady RO (1980) A lysosomal storage disorder in mice characterized by a dual deficiency of sphingomyelinase and glucocerebrosidase. Biochim Biophys Acta 619: 669–679. 10.1016/0005-2760(80)90116-2 [DOI] [PubMed] [Google Scholar]
- Percie du Sert N, Hurst V, Ahluwalia A, Alam S, Avey MT, Baker M, Browne WJ, Clark A, Cuthill IC, Dirnagl U, et al. (2020) The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. J Physiol 598: 3793–3801. 10.1113/JP280389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer SR (2019) NPC intracellular cholesterol transporter 1 (NPC1)-mediated cholesterol export from lysosomes. J Biol Chem 294: 1706–1709. 10.1074/jbc.TM118.004165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praggastis M, Tortelli B, Zhang J, Fujiwara H, Sidhu R, Chacko A, Chen Z, Chung C, Lieberman AP, Sikora J, et al. (2015) A murine Niemann-Pick C1 I1061T knock-in model recapitulates the pathological features of the most prevalent human disease allele. J Neurosci 35: 8091–8106. 10.1523/JNEUROSCI.4173-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Gil JL, Watkins-Chow DE, Baxter LL, Elliot G, Harper UL, Wincovitch SM, Wedel JC, Incao AA, Huebecker M, Boehm FJ, et al. (2020) Genetic background modifies phenotypic severity and longevity in a mouse model of Niemann-Pick disease type C1. Dis Model Mech 13: dmm.042614. 10.1242/dmm.042614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaranch L, Salegio EA, San Sebastian W, Kells AP, Foust KD, Bringas JR, Lamarre C, Forsayeth J, Kaspar BK, Bankiewicz KS (2012) Adeno-associated virus serotype 9 transduction in the central nervous system of nonhuman primates. Hum Gene Ther 23: 382–389. 10.1089/hum.2011.200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraiva J, Nobre RJ, Pereira de Almeida L (2016) Gene therapy for the CNS using AAVs: The impact of systemic delivery by AAV9. J Control Release 241: 94–109. 10.1016/j.jconrel.2016.09.011 [DOI] [PubMed] [Google Scholar]
- Shahryari A, Saghaeian Jazi M, Mohammadi S, Razavi Nikoo H, Nazari Z, Hosseini ES, Burtscher I, Mowla SJ, Lickert H (2019) Development and clinical translation of approved gene therapy products for genetic disorders. Front Genet 10: 868. 10.3389/fgene.2019.00868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan JA, Stanek LM, Lukason MJ, Bu J, Osmond SR, Barry EA, O’Riordan CR, Shihabuddin LS, Cheng SH, Scaria A (2018) Rationally designed AAV2 and AAVrh8R capsids provide improved transduction in the retina and brain. Gene Ther 25: 205–219. 10.1038/s41434-018-0017-8 [DOI] [PubMed] [Google Scholar]
- Tordo J, O’Leary C, Antunes ASLM, Palomar N, Aldrin-Kirk P, Basche M, Bennett A, D’Souza Z, Gleitz H, Godwin A, et al. (2018) A novel adeno-associated virus capsid with enhanced neurotropism corrects a lysosomal transmembrane enzyme deficiency. Brain 141: 2014–2031. 10.1093/brain/awy126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tortelli B, Fujiwara H, Bagel JH, Zhang J, Sidhu R, Jiang X, Yanjanin NM, Shankar RK, Carillo-Carasco N, Heiss J, et al. (2014) Cholesterol homeostatic responses provide biomarkers for monitoring treatment for the neurodegenerative disease Niemann-Pick C1 (NPC1). Hum Mol Genet 23: 6022–6033. 10.1093/hmg/ddu331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanier MT (2010) Niemann-Pick disease type C. Orphanet J Rare Dis 5: 16. 10.1186/1750-1172-5-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanier MT (2013) Niemann-Pick diseases. Handb Clin Neurol 113: 1717–1721. 10.1016/b978-0-444-59565-2.00041-1 [DOI] [PubMed] [Google Scholar]
- Vanier MT, Latour P (2015) Laboratory diagnosis of Niemann-Pick disease type C: The filipin staining test. Methods Cell Biol 126: 357–375. 10.1016/bs.mcb.2014.10.028 [DOI] [PubMed] [Google Scholar]
- Wang D, Li S, Gessler DJ, Xie J, Zhong L, Li J, Tran K, Van Vliet K, Ren L, Su Q, et al. (2018) A rationally engineered capsid variant of AAV9 for systemic CNS-directed and peripheral tissue-detargeted gene delivery in neonates. Mol Ther Methods Clin Dev 9: 234–246. 10.1016/j.omtm.2018.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie C, Gong XM, Luo J, Li BL, Song BL (2017) AAV9-NPC1 significantly ameliorates Purkinje cell death and behavioral abnormalities in mouse NPC disease. J Lipid Res 58: 512–518. 10.1194/jlr.M071274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Slone J, Li Z, Lou X, Hu YC, Queme LF, Jankowski MP, Huang T (2020) Systemic administration of AAV-Slc25a46 mitigates mitochondrial neuropathy in Slc25a46-/- mice. Hum Mol Genet 29: 649–661. 10.1093/hmg/ddz277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon SY, Hunter JE, Chawla S, Clarke DL, Molony C, O’Donnell PA, Bagel JH, Kumar M, Poptani H, Vite CH, et al. (2020) Global CNS correction in a large brain model of human alpha-mannosidosis by intravascular gene therapy. Brain 143: 2058–2072. 10.1093/brain/awaa161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu T, Shakkottai VG, Chung C, Lieberman AP (2011) Temporal and cell-specific deletion establishes that neuronal Npc1 deficiency is sufficient to mediate neurodegeneration. Hum Mol Genet 20: 4440–4451. 10.1093/hmg/ddr372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelikowsky M, Hui M, Karigo T, Choe A, Yang B, Blanco MR, Beadle K, Gradinaru V, Deverman BE, Anderson DJ (2018) The neuropeptide Tac2 controls a distributed brain state induced by chronic social isolation stress. Cell 173: 1265–1279.e19. 10.1016/j.cell.2018.03.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Phenotype scores and balance beam: two-way ANOVA. (90.6KB, docx)
Table S2 Genotype, sample size, and gender of mice used in study. (14.6KB, docx)
This video demonstrates the enhanced therapeutic benefit of adeno-associated virus-PHP.B-NPC1 (left cage, mouse with red tail) versus adeno-associated virus 9-NPC1 (right cage, mouse with no tail color). An Npc1+/+ littermate is also present for comparison (left cage, mouse with no tail color) and all mice are age matched (15-wk-old). Download video (7.2MB, mp4)
This video shows the longest surviving adeno-associated virus-PHP.B-NPC1–treated Npc1m1N/m1N mouse (faint green tail; survived to 66.1 wk) with an age-matched Npc1+/+ littermate at 37 wk of age. Video 2 This adeno-associated virus-PHP.B-NPC1–treated Npc1m1N/m1N mouse exhibits relatively good overall body condition and modest motor impairment, although moderate kyphosis and ruffled fur persist. Download video (11.7MB, mp4)
Source Data for Figure 4LSA-2021-01040_SdataF4.pdf (526.5KB, pdf)
Table S3 Antibodies and dilutions for immunofluorescent staining. (13.8KB, docx)