Abstract
Background:
Studies have observed associations between long-term air pollution and cardiovascular disease hospitalization. Little is known, however, about effect modification of these associations by greenness, temperature and humidity.
Methods:
We constructed an open cohort consisting of all fee-for-service Medicare beneficiaries, aged ≥ 65, living in the contiguous US from 2000 through 2016 (∼63 million individuals). We assigned annual average PM2.5, NO2 and ozone zip code concentrations. Cox-equivalent Poisson models were used to estimate associations with first cardiovascular disease (CVD), coronary heart disease (CHD) and cerebrovascular disease (CBV) hospitalization.
Results:
PM2.5 and NO2 were both positively associated with CVD, CHD and CBV hospitalization, after adjustment for potential confounders. Associations were substantially stronger at the lower end of the exposure distributions. For CVD hospitalization, the hazard ratio (HR) of PM2.5 was 1.041 (1.038, 1.045) per IQR increase (4.0 μg/m3) in the full study population and 1.327 (1.305, 1.350) per IQR increase for a subgroup with annual exposures always below 10 μg/m3 PM2.5. Ozone was only positively associated with CVD, CHD and CBV hospitalization for the low-exposure subgroup (<40 ppb). Associations of PM2.5 were stronger in areas with higher greenness, lower ozone and Ox, lower summer and winter temperature and lower summer and winter specific humidity.
Conclusion:
PM2.5 and NO2 were positively associated with CVD, CHD and CBV hospitalization. Associations were more pronounced at low exposure levels. Associations of PM2.5 were stronger with higher greenness, lower ozone and Ox, lower temperature and lower specific humidity.
Keywords: PM2.5, NO2, Ozone, Ox, Cardiovascular disease, Effect modification, Low-level air pollution
1. Introduction
Cardiovascular disease (CVD) is a leading cause of morbidity and mortality and produces immense economic burdens in the US [1]. About half of all US adults have a CVD [1]. Although the US death rate from CVD decreased from 2006 to 2016, in 2016 more than 840,000 people died from CVD [1].
Long-term exposure to air pollution, such as particulate matter less than 2.5 μm (PM2.5) and nitrogen dioxide (NO2), has been consistently associated with CVD mortality [2,3]. Evidence for associations with long-term exposure to ozone is more limited [4], but several recent studies reported positive associations with CVD mortality [5–7]. Fewer studies have evaluated associations between long-term air pollution and CVD hospitalization. Several studies that evaluated effects of short-term exposure to air pollution on CVD, indicated that temperature and humidity could modify the effects of air pollution [8–12]. However, less is known about effect modification of temperature and humidity on long-term exposure to air pollution. Furthermore, there is some evidence that greenness, or quantity of surrounding residential vegetation, may modify effects of long-term exposure to air pollution [13–16].
Our aim was to evaluate associations of long-term exposure to PM2.5, NO2, and ozone with CVD hospitalization in all fee-for-service Medicare beneficiaries in the contiguous US from 2000 through 2016 (∼63 million individuals). To identify susceptible sub-populations, we looked at effect modification by demographic characteristics (e.g. sex, age) and other environmental exposures (e.g. temperature, humidity, and greenness). We further evaluated associations at pollutant levels below international regulations.
2. Methods
2.1. Study population
We derived data from the Medicare denominator and Medicare Provider Analysis and Review (MEDPAR) files to construct an open cohort consisting of all fee-for-service Medicare beneficiaries, aged ≥ 65 years, living in the contiguous US from January 1, 2000 through December 31, 2016 (∼63 million individuals). Medicare is a national health insurance program in the US that provides health insurance for Americans aged 65 and older and for younger people with disability status. For each beneficiary, follow-up started on January 1st 2000 or January 1st of the year following entry into the cohort. Beneficiaries were followed until the first hospital admission for the outcome of interest, or until they died, were censored, or reached the end of the follow-up time.
2.2. Outcome definition
Data on hospital admissions were obtained from the MEDPAR dataset. This administrative dataset contains all hospital admissions for Medicare fee-for-service beneficiaries from 2000 through 2016. Hospital admissions were defined by ICD-9 codes from 2000 through the third quarter of 2015 and then switched to ICD-10 codes. We looked at first hospital admissions with a primary discharge diagnosis of cardiovascular disease (ICD-9 codes: 390–459, ICD-10 codes: I00-I99), coronary heart disease (ICD-9 code: 410–414, ICD-10 codes: I20-I25), and cerebrovascular disease (ICD-9 codes: 430–438, ICD-10 codes: I60-I69), hereafter referred to as CVD, CHD, and CBV, respectively. We created separate cohorts for CVD, CHD, and CBV hospitalizations.
2.3. Exposure assessment
Detailed information about air pollution models can be found elsewhere [17–19]. Briefly, annual PM2.5, NO2, and ozone concentrations and summer ozone concentrations across the contiguous US for 2000–2016 were estimated based on predictions from well-validated spatio-temporal ensemble models [17–19]. Daily ambient PM2.5, 1-hour daily maximum NO2 and ozone concentrations were estimated for each grid cell (1 km × 1 km) by combining predictions from three machine learning algorithms (random forest, gradient boosting, and neural network) in a geographically-weighted regression. The algorithms were based on multiple predictors, including satellite data, meteorological variables, land-use variables, and chemical transport model predictions. The overall cross-validated R2 for annual estimates was 0.89 for PM2.5, 0.84 for NO2, and 0.86 for ozone [17–19]. For each zip code, the annual average concentrations were estimated by averaging the estimations at grid cells whose centroids fall within the boundary of that ZIP code. Annual air pollution zip code level estimates were assigned to beneficiaries that live within that zip code.
2.4. Covariates
The Medicare beneficiary file provides information about age at year of Medicare entry, year of entry, sex, race, Medicaid eligibility (a proxy for low socioeconomic status), and zip code of residence for all Medicare beneficiaries. As we lack information about individual-level SES (except Medicaid eligibility) and because SES has multiple dimensions (e.g. income, occupation, education), we included multiple area-level SES variables. We linked zip code-level SES variables derived from the US Census and American Community Survey: median home value, median household income, population density, percent Hispanic, percent Black, percent of the population with less than a high school degree, percent below the poverty level, and percent of owner-occupied housing units). Two county-level variables (% population that were ever smokers and mean BMI) were acquired from the nationwide Behavioural Risk Factor Surveillance System (BRFSS). BRFSS is the nation’s premier system of surveys that collect information about health-related risk behaviours of US residents. US census (2000, 2009–2016) and BRFSS (2000–2011) variables were available for some years but not all. Temporal interpolation using a moving average algorithm within each zip code was performed for missing years, as described previously [20]. Further, we divided the US into 5 regions (Northeast, Southeast, Midwest, Southwest, and West) based on geographical position, climate, and cultural differences (Figure S1), similar to Shi et al. [21].
For each zip code, the Normalized Difference Vegetation Index (NDVI, an indicator of greenness) was estimated using satellite imagery. The NDVI is calculated as the ratio between the red and near infrared values, and ranges from − 1 to 1 [22]. Values close to 1 correspond to areas with complete coverage by live vegetation, values close to zero correspond to areas without much live vegetation (e.g. rocks, sand) and negative values correspond to water. We used Landsat 7 and Landsat 8 (Collection 1 Tier 1 DN values, representing scaled, calibrated at sensor radiance) images for the entire US from June 1, up to August 31 (summer), for each year (2000–2016). Landsat 7 and Landsat 8 images are generated every 16 days at 30 m resolution. Using Google Earth Engine (https://earthengine.google.com/), cloud-free Landsat composites were created for the US. We calculated the spatially weighted (cells that are partially included in a zip code are given a weight based on the overlap) mean summer NDVI for each zip code in the US for each year, after setting negative NDVI values to zero.
For each zip code for each year (2000–2016), annual (January-December), summer (June-August) and winter (December-February) daily maximum temperature and daily ambient specific humidity were estimated using data from the Gridded Surface Meteorological dataset [23]. The Gridded Surface Meteorological dataset provides daily surface fields of maximum temperature and daily ambient specific humidity (kg of water vapor / kg of dry air) at ∼ 4 km spatial resolution covering the contiguous US. We calculated the spatially weighted mean annual, summer and winter maximum temperature and daily ambient specific humidity, hereafter referred to as annual/summer/winter temperature and annual/summer/winter specific humidity. Correlations between zip-code level seasonal average temperature in different years were generally very strong (Pearson r > 0.90). This was also true for seasonal average specific humidity in different years.
We calculated the combined oxidant capacity (Ox) of NO2 and ozone, using a redox-weighted average (i.e. Ox = [(1.07 × NO2) + (2.075 × O3)]/3.145) [24]. The spatial variation of greenness, Ox, summer and winter temperature and specific humidity is shown in Figure S2.
2.5. Statistical analysis
Our large-scale cohort (∼63 million individuals) and the conventional Cox proportional hazard model led to computational challenges (e.g., inadequate memory size and lengthy computational time). To overcome these challenges, we applied a Cox-equivalent re-parameterized Poisson approach [21]. The key aspect of this approach was to collapse the individual-level records to a high-dimensional space of features, while keeping the integrity of stratum units for analysis. All people that live within the same zip code in a specific year, with the same sex, race, Medicaid eligibility, 2-year categories of age at study entry and year of follow-up were aggregated and treated as one single grid cell in this high-dimensional space, because they belonged to the same stratum and as such were treated as interchangeable in the analysis. Using this method, we considerably reduced the data size.
Specifically, we fitted a stratified quasi-Poisson model to estimate associations of time-varying annual mean PM2.5, NO2, and ozone with the rate of first CVD, CHD, and CBV hospitalizations. The dependent variable was the count of outcome-related first hospitalizations in each follow-up year, calendar year, and zip code location within strata specified by individual characteristics [sex, race, Medicaid eligibility, and age at study entry (2-year categories)], using the corresponding total person-time of Medicare beneficiaries as the offset. By stratifying on individual characteristics (sex, race, Medicaid eligibility, 2-year categories of age at study entry and year of follow-up), we allowed for flexible strata-specific baseline rates. Mathematically, this stratified Poisson model is equivalent to a time-varying Cox proportional hazard model under an Anderson-Gill representation. To account for within zip code correlated observations across years, we applied an m-out-n bootstrap method using zip code units to calculate statistically robust confidence intervals (CIs). Details about the Cox-equivalent re-parameterized Poisson approach can be found elsewhere [21].
We a priori specified four models with increasing degrees of covariate adjustment. Model 1 included all three pollutants, calendar year, an offset for total person-time, and strata for all possible combinations of sex, race, Medicaid Eligibility age at study entry (2-year categories), and follow-up year. In Model 2 we additionally adjusted for all US census covariates. In Model 3 we included BRFSS covariates and in Model 4 we included region. We evaluated the shape of the exposure–response curves for each pollutant by adding natural splines with 2, 3 or 4 degrees of freedom. In addition, we estimated associations at pollutant levels below international regulations, by restricting analyses to individuals with annual exposures always below 10 μg/m3 for PM2.5, 20 ppb for NO2, and 40 ppb for ozone. In all models, all three pollutants were included simultaneously unless otherwise stated.
To evaluate whether associations of PM2.5, NO2, and ozone were modified by greenness, temperature, and specific humidity, we performed stratified analyses. As there is some evidence that long-term summer and winter temperature are related to increased mortality in the US [25–27], we evaluated whether associations of air pollution were modified by summer and winter average temperature and summer and winter average specific humidity. As the literature suggests that health effects of greenness are stronger in urban areas [28], and types of green spaces likely differ between urban and rural areas, we also evaluated whether associations of PM2.5, NO2, and ozone differed across strata of greenness in urban areas (zip codes with a population density of ≥ 1000 persons/miles2). Further, we evaluated whether associations of PM2.5 were modified by the combined oxidant capacity (Ox) of NO2 and ozone, by stratified analyses (model including only PM2.5 and not NO2 and ozone). Further, we performed stratified analyses to assess potential effect modification by sex (male, female), age (<75 years, 75–84 years, 85 ≤ years), race (white, black, other/unknown), Medicaid eligibility (as an indicator for SES), and region (Northeast, Southeast, Midwest, Southwest, West).
We assessed several sensitivity analyses to test whether our results were robust. We excluded individuals who had their first hospital admission within the first year of their follow-up and all records in the year 2000, to exclude potential prevalent cases. We ran single-exposure models and we included summer average ozone (June-August) instead of annual average ozone. To evaluate the impact of adjustment for potential confounders on effect modification by greenness, temperature and specific humidity for CVD hospitalization, we ran models including calendar year, region, an offset for total person-time, and strata for all possible combinations of sex, race, Medicaid Eligibility age at study entry (2-year categories), and follow-up year (excluding US census covariates and BRFSS covariates). Further, we performed effect modification analyses by annual average temperature and annual average specific humidity for CVD hospitalization. All hazard ratios (HRs) were expressed per IQR increase (based on the CVD cohort).
All analyses were conducted on the Harvard Research Computing Environment, which is supported by the Institute for Quantitative Social Science at Harvard University. We used R software (R Project for Statistical Computing) version 3.6.1 for our analyses.
3. Results
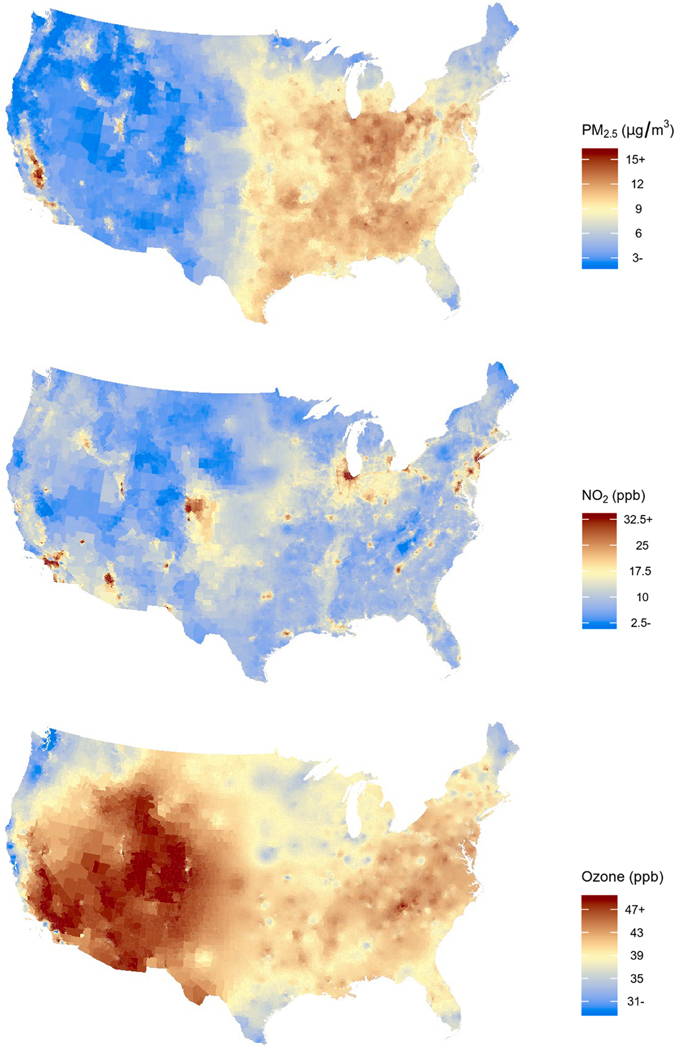
The full cohort consisted of 63,009,173 Medicare beneficiaries living in the contiguous US in 2000–2016. The vast majority of our cohort was white, between 65 and 74 years of age at study entry and not eligible for Medicaid (Table 1). We observed about 18.6 million first CVD hospital admissions, of which approximately 35% were CHD hospital admissions and 30% were CBV hospital admissions. The median follow-up period was 5 years for the CVD cohort, and slightly longer for the CHD and CBV cohort (Table 1). We observed the highest PM2.5 concentrations in the Southeast and Midwest of the US, while ozone levels were highest in the Southwest and West (Fig. 1). The highest NO2 concentrations were observed in urban areas. The variation (median/IQR) was largest for NO2 and lowest for ozone (Table S1). Descriptive statistics of the low-level cohort are shown in Table S2. PM2.5 and NO2 were moderately positively correlated (Pearson r = 0.43), ozone was very weakly negatively correlated with PM2.5 (Pearson r = −0.03) and NO2 (Pearson r = −0.12, Figure S3).
Table 1.
Descriptive statistics of all US Medicare fee-for-service beneficiaries (n = 63,009,173) from 2000 through 2016 and in subsets of the study population including only Medicare beneficiaries with low exposure levels a,b.
| Covariate | Category | Full cohort | Low-level PM2.5 | Low-level NO2 | Low-level ozone |
|---|---|---|---|---|---|
|
|
|
|
|
||
| N (%) | N (%) | N (%) | N (%) | ||
|
| |||||
| Number of hospitalizations | CVD | 18,610,833 (29.5) | 3,755,279 (17.6) | 6,860,947 (25.1) | 8,362,303 (29.6) |
| CHD | 6,607,687 (10.5) | 1,323,950 (6.3) | 2,561,172 (9.4) | 2,914,271 (10.7) | |
| CBV | 5,551,735 (8.8) | 985,934 (4.7) | 1,933,203 (7.1) | 2,340,200 (8.7) | |
| Total person years (median per person) | CVD | 401,315,016 (5) | 99,144,014 (3) | 152,544,344 (4) | 146,294,509 (4) |
| CHD | 448,888,035 (6) | 105,830,022 (4) | 167,032,565 (5) | 158,088,646 (4) | |
| CBV | 460,574,345 (6) | 107,822,290 (4) | 171,669,892 (5) | 160,960,817 (4) | |
| Demographic characteristics at study entry | |||||
| Sex | Female | 34,725,534 (55.1) | 11,110,862 (52.1) | 14,518,670 (53.1) | 15,417,275 (54.6) |
| Age entry | 65–74 years | 48,240,802 (76.6) | 18,721,927 (87.8) | 23,089,599 (84.4) | 21,313,724 (75.5) |
| 75–84 years | 10,819,118 (17.2) | 1,850,369 (8.7) | 3,085,751 (11.3) | 4,877,852 (17.3) | |
| 85 + years | 3,949,253 (6.3) | 740,483 (3.5) | 1,183,791 (4.3) | 2,043,443 (7.2) | |
| Race | White | 53,262,938 (84.5) | 18,252,212 (85.6) | 24,220,341 (88.5) | 23,265,492 (82.4) |
| Black | 5,511,612 (8.7) | 1,290,529 (6.1) | 1,849,345 (6.8) | 2,696,881 (9.6) | |
| Other/unknown | 4,234,623 (6.7) | 1,770,038 (8.3) | 1,289,455 (4.7) | 2,272,646 (8.0) | |
| Medicaid eligibility | Not eligible | 55,164,043 (87.5) | 19,003,470 (89.2) | 24,236,200 (88.6) | 24,551,536 (87.0) |
| Eligible | 7,845,130 (12.5) | 2,309,309 (10.8) | 3,122,941 (11.4) | 3,683,483 (13.0) | |
| Region | Midwest | 15,254,270 (24.2) | 3,444,574 (16.1) | 6,238,743 (22.8) | 10,118,066 (35.8) |
| Northeast | 13,880,261 (22.0) | 4,461,736 (20.9) | 3,539,969 (12.9) | 8,319,138 (29.5) | |
| Southeast | 17,454,647 (27.7) | 5,406,735 (25.4) | 10,923,504 (39.9) | 4,437,350 (15.7) | |
| Southwest | 6,616,448 (10.5) | 2,566,482 (12.0) | 3,428,315 (12.5) | 1,625,681 (5.8) | |
| West | 9,803,547 (15.6) | 5,433,252 (25.5) | 3,228,610 (11.8) | 3,734,784 (13.2) | |
For PM2.5, Medicare beneficiaries with exposure levels always below 10 μg/m3 PM2.5 were included (for CVD n = 21,312,779; for CHD n = 21,099,651; for CBV n = 21,045,932). For NO2, Medicare beneficiaries with exposure levels always below 20 ppb NO2 were included (for CVD n = 27,359,141; for CHD n = 27,123,026; for CBV n = 27,073,535). For ozone, Medicare beneficiaries with exposure levels always below 40 ppb ozone were included (for CVD n = 28,235,019; for CHD n = 27,220,654; for CBV n = 26,992,359).
Demographic characteristics in subsets of the study population including only Medicare beneficiaries with low exposure levels are given for the CVD subsets.
Fig. 1.
The spatial variation of the mean annual PM2.5, NO2 and ozone concentration per zip code in the contiguous US (year = 2010).
In our minimally adjusted model, PM2.5 and ozone were positively associated with CVD, CHD, and CBV hospitalization (Figure S4). After adjustment for potential confounders, associations of PM2.5 attenuated but remained while associations of ozone became negative (Table 2). NO2 was inversely associated with CVD, CHD, and CBV hospitalization in our minimally adjusted model. After adjustment for potential confounders, associations of NO2 were positive. Associations of PM2.5 and NO2 were linear to supra-linear, while associations with ozone were positive at the low end of the distribution (Figure S5). Associations were substantially stronger for subgroups of the study population with annual exposures always below 10 μg/m3 for PM2.5 and with annual exposures always below 20 ppb for NO2 (Table 2). For ozone, we observed strong positive associations with all outcomes for individuals with ozone exposure always below 40 ppb. When restricting analyses to individuals in the low-level NO2 subgroup, associations of PM2.5 were fairly similar to associations of PM2.5 in the full cohort (Table S3). This also applies to associations of NO2 in the low-level PM2.5 subgroup. In the low-level subgroup of ozone, associations of PM2.5 were also substantially stronger, while NO2 was not associated with the outcomes.
Table 2.
Associations of PM2..5, NO2, and ozone with cardiovascular disease hospitalization (CVD), coronary heart disease hospitalization (CHD), and cerebrovascular disease hospitalization (CBV), in all US Medicare fee-for-service beneficiaries (Full, n =63,009,173) 2000–2016 and in subsets of the study population including only Medicare beneficiaries with low exposure levels (Low-exposure) a,b.
| Exposure (IQR) | CVD hospitalization | CHD hospitalization | CBV hospitalization | |||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| Full | Low-exposure | Full | Low-exposure | Full | Low-exposure | |
|
|
|
|
|
|
|
|
| HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | |
|
| ||||||
| PM 2.5 (4.0 μg/m 3 ) | 1.041 (1.038, 1.045) | 1.327 (1.305, 1.350) | 1.035 (1.030, 1.040) | 1.346 (1.321, 1.372) | 1.071 (1.066, 1.076) | 1.405 (1.380, 1.430) |
| NO 2 (13.9 ppb) | 1.033 (1.028, 1.037) | 1.226 (1.210, 1.242) | 1.023 (1.017, 1.029) | 1.209 (1.19, 1.229) | 1.025 (1.020, 1.031) | 1.237 (1.215, 1.259) |
| Ozone (4.4 ppb) | 0.992 (0.990, 0.994) | 1.375 (1.359, 1.391) | 0.997 (0.994, 1.001) | 1.46 (1.437, 1.482) | 0.990 (0.988, 0.993) | 1.407 (1.389, 1.426) |
For PM2.5, Medicare beneficiaries with exposure levels always below 10 μg/m3 PM2.5 were included (for CVD n = 21,312,779; for CHD n = 21,099,651; for CBV n = 21,045,932). For NO2, Medicare beneficiaries with exposure levels always below 20 ppb NO2 were included (for CVD n = 27,359,141; for CHD n = 27,123,026; for CBV n = 27,073,535). For ozone, Medicare beneficiaries with exposure levels always below 40 ppb ozone were included (for CVD n = 28,235,019; for CHD n = 27,220,654; for CBV n = 26,992,359).
Associations are expressed per IQR increase of the cardiovascular disease hospitalization cohort. Models included PM2.5, NO2 and ozone and were adjusted for calendar year, US census covariates, BRFSS covariates, US regions, an offset for total person-time and strata for all possible combinations of sex, race, Medicaid Eligibility, age at study entry (2-year categories), and follow-up year.
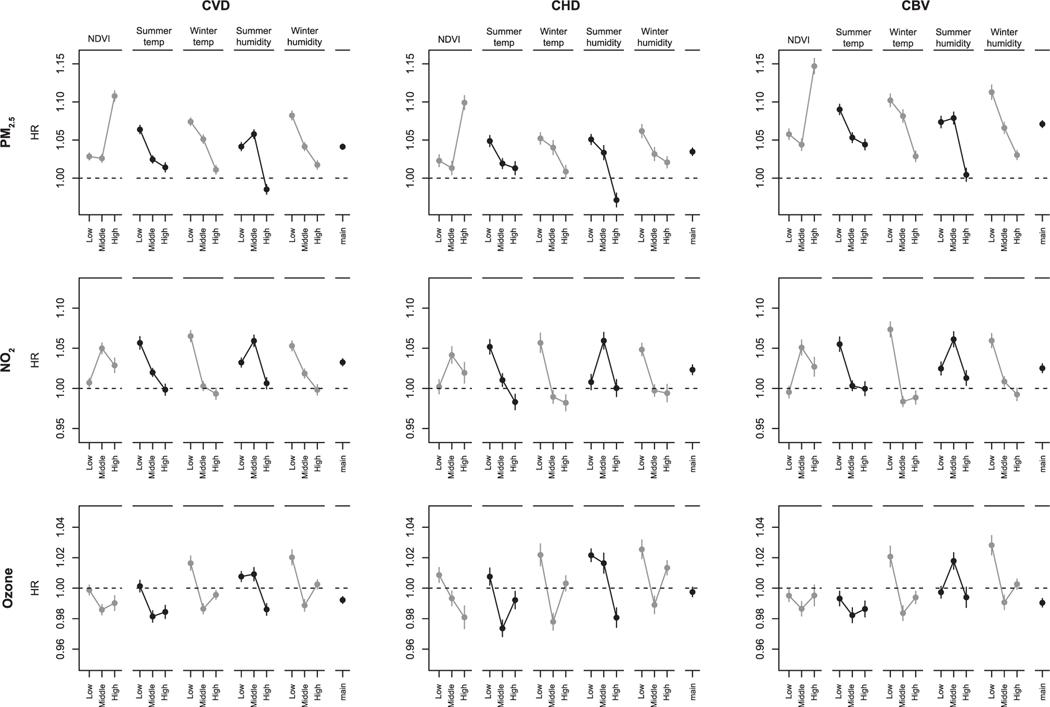
Associations with PM2.5, NO2, and ozone were modified by greenness, temperature, and specific humidity (Fig. 2). In general, we observed stronger associations of PM2.5 with lower summer and winter temperature and lower summer and winter specific humidity. Associations of PM2.5 were similar in the lowest and middle greenness tertile and strongest in the highest greenness tertile. For NO2 and ozone, patterns of effect modification were less clear. In general, the strongest associations of NO2 were observed with lower temperature and specific humidity, but we also observed negative associations in the highest summer and winter temperature tertiles. For ozone, associations were generally negative, except for associations in the lowest temperature and specific humidity tertiles. In urban areas, associations of PM2.5 and NO2 were strongest in the highest greenness tertile (Table S4). Stratified analyses by Ox showed that associations of PM2.5 were stronger with lower Ox concentrations (Table S5).
Fig. 2.
Associations of PM2.5, NO2 and ozone with cardiovascular disease (CVD), coronary heart disease (CHD) and cerebrovascular disease (CBV) hospitalization in stratified analyses by tertiles of NDVI, summer temperature (summer temp), winter temperature (winter temp), summer specific humidity (summer humidity) and winter specific humidity (winter humidity) a, b. a Associations are expressed per IQR increase (IQR PM2.5 = 4.0 μg/m3, IQR NO2 = 13.9 ppb, IQR Ozone = 4.4 ppb) of the cardiovascular disease hospitalization cohort. Models included PM2.5, NO2 and ozone and were adjusted for calendar year, US census covariates, BRFSS covariates, US regions, an offset for total person-time and strata for all possible combinations of sex, race, Medicaid Eligibility, age at study entry (2-year categories), and follow-up year. b To define strata, we used the following quantiles (q33.3, q66.7) for the CVD cohort: summer temp (°C): 28.2, 31.7; winter temp (°C): 4.8, 13.1; summer humidity (g of water vapor / kg of dry air): 10.9, 13.4; winter humidity (g of water vapor / kg of dry air): 2.7, 4.1; NDVI: 0.41, 0.60. For the CHD cohort: summer temp (°C): 28.2, 31.7; winter temp (°C): 4.8, 13.1; summer humidity (g of water vapor / kg of dry air): 11.0, 13.4; winter humidity (g of water vapor / kg of dry air): 2.7, 4.1; NDVI: 0.42, 0.60. For the CBV cohort: summer temp (°C): 28.2, 31.7; winter temp (°C): 4.8, 13.1; summer humidity (g of water vapor / kg of dry air): 11.0, 13.4, winter humidity (g of water vapor / kg of dry air): 2.7, 4.1; NDVI: 0.42, 0.60.
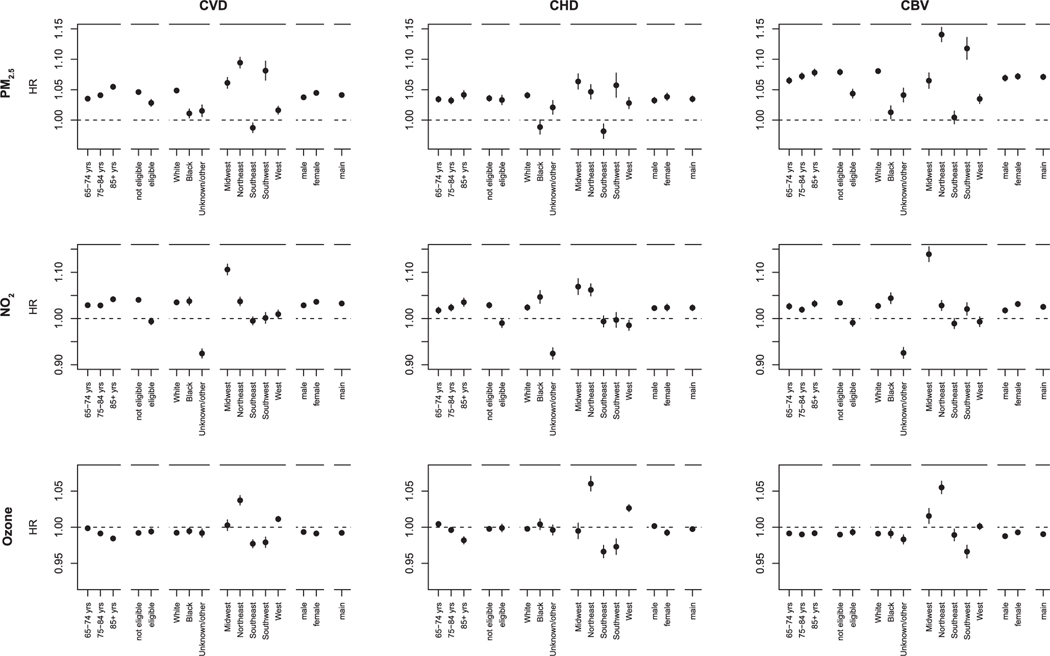
Effect modification by demographics was most pronounced for Medicaid eligibility, race, and region (Fig. 3). Associations of PM2.5 were weaker for individuals eligible for Medicaid compared to individuals not eligible for Medicaid. For NO2, we found positive associations for individuals not eligible for Medicaid but not for individuals that were eligible. For PM2.5, we observed stronger associations for white individuals, while for NO2 associations were slightly stronger for black individuals. We found no or positive associations of ozone in the northern regions (Northeast, Midwest, and West), and negative associations in the southern regions (Southeast and Southwest).
Fig. 3.
Associations of PM2.5, NO2 and ozone with cardiovascular disease, coronary heart disease and cerebrovascular disease hospitalization in stratified analyses by age (65–74, 75–84, 85 + years), Medicaid eligibility (not eligible, eligible), race (White, Black, unknown/other), US region (Midwest, Northeast, Southeast, Southwest and West) and sex (male, female) a. a Associations are expressed per IQR increase (IQR PM2.5 = 4.0 μg/m3, IQR NO2 = 13.9 ppb, IQR Ozone = 4.4 ppb) of the cardiovascular disease hospitalization cohort. Models included PM2.5, NO2 and ozone and were adjusted for calendar year, US census covariates, BRFSS covariates, US regions, an offset for total person-time and strata for all possible combinations of sex, race, Medicaid Eligibility, age at study entry (2-year categories), and follow-up year.
Single-exposure models showed stronger associations for PM2.5 and especially NO2 compared to models including all three pollutants (Table S6). In the multi-pollutant models, associations of ozone were similar to associations of ozone in single-exposure models. Associations of all three pollutants were similar in sensitivity analyses including summer ozone instead of annual average ozone. Associations were robust to exclusion of potential prevalent cases. For PM2.5, patterns of effect modification by greenness, temperature and specific humidity were similar in models adjusted for area-level SES indicators compared to models not adjusted for area-level SES indicators (Table S7). For NO2 and ozone, associations in strata of greenness, temperature and specific humidity differed between models, in line with differences in associations between Model 1 and Model 4 in the full population (Figure S4). For CVD hospitalization, patterns of effect modification by annual temperature and annual specific humidity were similar to patterns effect modification by summer and winter temperature and summer and winter specific humidity, respectively (Table S8).
4. Discussion
We found positive associations of PM2.5 and NO2 with cardiovascular disease, coronary heart disease, and cerebrovascular disease hospitalization. Our results are in line with previous studies about PM2.5 and CVD incidence [29–31]. Evidence for associations of NO2 with CVD incidence is mixed [30,32,33]. Both pollutants may induce oxidative stress, vascular dysfunction, and autonomic nervous system imbalance, thereby contributing to the development of CVD [34,35]. Associations of PM2.5 and NO2 were substantially stronger at the lower end of the exposure distribution. This is consistent with other studies that evaluated associations of low level PM2.5 with mortality [20,36,37].
We observed supra-linear associations of PM2.5 and NO2 with all three outcomes. The supra-linear curves could be due to changes in composition of the air pollution mixture across the air pollution distribution or a potential saturation effect [36,38]. An increase in measurement error at high concentrations could also have affected the shape of the association. This seems unlikely, given the strong performance of the PM2.5 and NO2 models at high concentrations [18,19]. However, our zip code level exposures were assessed by averaging the model estimations. In urban zip codes, there may be more variation in air pollution concentrations due to the presence of multiple sources, which may result in an increase in measurement error compared to more rural zip codes. This is especially true for NO2, as NO2 is primarily emitted by local traffic and has a shorter atmospheric lifetime than PM2.5 [18]. However, urban zip codes generally cover much smaller geographic areas than rural zip codes.
In the full study population, ozone was very weakly negatively associated with CVD, CHD, and CBV hospitalization. A previously published study reported a positive association of ozone with CVD hospitalization in the Southeast of the US [29]. Other studies about effects of long-term exposure to ozone on CVD mortality in North America also showed positive associations [5–7]. However, recent reviews of associations of ozone reported no associations with all-cause mortality (HR 0.97, 95% CI: 0.93, 1.02 per 10 μg/m3) and cardiovascular mortality (HR = 1.01, 95% CI: 0.99, 1.03 per 10 μg/m3) [4,39]. Further, only a limited number of studies evaluated the shape of the exposure–response curve [39]. We found positive associations of ozone with CVD, CHD, and CBV hospitalizations in northern regions of the US and in subgroups of individuals that were exposed to levels always below 40 ppb. In zip codes with high ozone levels, personal exposure may be affected by adaptive strategies and ozone alerts. Ozone is generally higher in rural areas, where access to health care may be limited which could have affected the associations.
Patterns of effect modification by greenness, temperature, specific humidity and oxidant capacity are likely due to a combination of differences in air pollution composition and population characteristics. Stronger associations for PM2.5 were seen in areas with higher greenness. In urban areas, associations of PM2.5 and NO2 were also stronger with higher greenness. Several studies have showed that greenness was associated with better health outcomes, especially in urban areas [28,40], and therefore may reduce susceptibility to air pollution. However, evidence about effects of greenness on CVD is mixed [28].
Two studies that evaluated effect modification by greenness showed no or weaker effects of air pollution on CVD mortality in green areas compared to less green areas [13,14]. Kioumourtzoglou et al., on the other hand, showed stronger associations between PM2.5 and all-cause mortality with increasing greenness in US cities [15]. The supra-linear curve may have affected the strength of the association of NO2 across greenness tertiles, as NO2 concentrations were lower with increasing greenness (Table S9). However, we did not observe this trend for PM2.5. PM2.5 is a mixture of particles from various sources, such as traffic emissions, biomass burning and organic dust, and the composition might differ between tertiles of greenness, which could affect the toxicity of PM2.5. Further, the spatial contrast in NO2 and PM2.5 might be limited in (non-green) urban areas, which makes it hard to capture effects of both pollutants.
Decreases in summer and winter temperature and specific humidity were associated with stronger PM2.5 associations and to a lesser extent NO2 associations. Our results are in contrast with a study that reported stronger associations between long-term PM2.5 and mortality in cities with high temperatures in the US [15]. However, several studies reported stronger associations of short-term exposure to air pollution with cardiovascular hospitalization on cold winter days [10,12,41–43]. Moreover, some studies showed stronger associations for short-term air pollution with decreasing humidity [10,44]. As there is considerable overlap between summer and winter temperature and specific humidity, it is difficult to disentangle the impact of each modifier on the associations. Exposure to cold and dry air might impact the cardiovascular system and therefore increases susceptibility to air pollution exposure. Dry air could dry the mucosal surface [45] and in turn impair the airway clearance processes that help to protect the lungs [46,47]. However, effects of long-term exposure to warm and cold temperatures and high and low humidity levels on the cardiovascular system are not yet well understood. Temperature and specific humidity also play a role in composition of the air pollution mixture. A possible explanation for the stronger associations is that in more humid conditions, the size of particles may increase by moisture absorption [48]. This might impact the effect of the air pollution mixture as smaller particles penetrate deeper into the lungs and could enter the bloodstream [49]. Warm temperatures and sunlight intensity may accelerate photochemical aging of particles which affects the chemical properties of the air pollution mixture [50,51]. Secondary aerosols formed by photochemical formation may be less toxic than their precursors. Furthermore, the impact of home heating emissions (from coal and wood burning) on the air composition is likely stronger in the lowest summer and winter temperature tertiles. We also note that in the lowest temperature and specific humidity tertiles, ozone concentrations were generally lowest (Table S9). Hence, the supra-linear associations of ozone may have affected the effect modification pattern.
We found substantially stronger associations of PM2.5 in the low-level ozone subgroup and also observed stronger associations of PM2.5 with lower Ox concentrations. A Canadian study found no clear pattern of effect modification by Ox or ozone of the associations between PM2.5 and cardiovascular disease mortality [52]. Positive (significant) associations of PM2.5 were found in the highest and lowest tertiles of Ox and ozone, but not in the middle tertiles [52]. Another study in Canada showed positive associations of PM2.5 with mortality in the high Ox group and negative (insignificant) associations in the low Ox group [53]. We have no clear explanation for the stronger associations of PM2.5 with lower Ox and ozone concentrations in our study, but note that associations of ozone with CVD mortality were positive at lower levels but not across the full distribution range. Little is known about exposure–response curves of Ox.
The stronger associations of PM2.5 for white individuals and NO2 for black individuals could indicate differences in susceptibility to both pollutants. The difference could also be due to variations in exposure levels and air pollution composition, as black individuals generally live in more urban areas. The weak or null associations of PM2.5 and NO2 for individuals eligible for Medicaid (an indicator of a low SES) is in contrast to other studies that generally found stronger associations with decreasing SES [15,30]. We speculate that Medicaid eligible individuals might have higher rates of pre-existing conditions and are more susceptible to other risk factors which could attenuate the associations of air pollution, as differences in incidence rates across the exposure distribution might be limited. Associations of PM2.5 and NO2 by region were in line with results of effect modification by temperature and humidity; associations were generally stronger in the Northeast and Midwest (regions with low temperatures and specific humidity) and weaker in the Southeast (a region with high temperatures and specific humidity). Further, we note that in the Southeast, isoprene and monoterpene emissions from trees during warm conditions are likely higher compared to other regions [19,53]. Isoprene and monoterpene may play a role in the formation of aerosols and in turn affect the air pollution mixture [54,55], which may have resulted in the absence of associations of PM2.5 in the Southeast. Associations of ozone by region, were in line with the exposure–response curves, with positive associations in regions with low ozone levels and vice versa.
This study has several strengths. Our cohort consists of approximately 63 million Medicare FFS beneficiaries living in the contiguous US. The large cohort made it possible to perform stratified analyses by demographics, greenness, temperature and specific humidity. We included PM2.5, NO2, and ozone simultaneously in our models, to estimate associations for each exposure while accounting for the other exposures. Use of Medicare data also allowed us to have a fairly representative sample of individuals aged 65 + years across the US. However, we note that the Medicare FFS population does not include all Medicare beneficiaries. The portion of Medicare FFS beneficiaries in the total Medicare population differed over time and by region. Medicare-fee-for-service beneficiaries may have switched to Medicare managed care plan and back during our follow-up period, which could have resulted in some missed cases in our data, as we have no information on Medicare-HMO hospitalization claims. Our study also has some limitations. Exposures were assigned on zip code level which resulted in some measurement error. We believe this measurement error was likely non-differential and would only bias the associations towards the null. We were unable to adjust for individual-level SES (other than Medicaid eligibility) and lifestyle factors, such as income, education, smoking and BMI, which may have resulted in an overestimation of the associations. However, a previously published study reported no associations between air pollutants and either smoking or body mass index in the Medicare Current Beneficiary Survey, a representative subsample of Medicare enrollees [20]. We included various zip code SES factors, that are likely related to individual SES.
5. Conclusion
Long-term exposure to PM2.5 and NO2 were associated with an increased risk of cardiovascular disease, coronary heart disease and cerebrovascular disease hospitalization in a nationwide study in the US. Associations were substantially stronger at low exposure levels. Ozone was only associated with an increased risk for all outcomes at low exposure levels. Associations of PM2.5 were stronger with higher greenness levels, lower ozone and Ox, lower summer and winter temperature and lower summer and winter specific humidity.
Supplementary Material
Acknowledgement:
This study was supported by National Institute of Environmental Health Sciences (R01ES028033, R01 ES024332, P30ES000002) National Institute on Aging (R01 AG066793-01), and the National Heart, Lung and Blood Institute (R01HL150119). The funders had no role in the in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication. This study was approved by the institutional review board at the Harvard T H Chan School of Public Health and was exempt from informed consent requirements as a study of previously collected administrative data.
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.envint.2021.106715.
References
- [1].Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Das SR, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Jordan LC, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, Lichtman JH, Longenecker CT, Loop MS, Lutsey PL, Martin SS, Matsushita K, Moran AE, Mussolino ME, O’Flaherty M, Pandey A, Perak AM, Rosamond WD, Roth GA, Sampson UKA, Satou GM, Schroeder EB, Shah SH, Spartano NL, Stokes A, Tirschwell DL, Tsao CW, Turakhia MP, VanWagner LB, Wilkins JT, Wong SS, Virani SS, 2019. Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation 139 (10). 10.1161/CIR.0000000000000659. [DOI] [PubMed] [Google Scholar]
- [2].Atkinson RW, Butland BK, Anderson HR, Maynard RL, 2018. Long-term Concentrations of Nitrogen Dioxide and Mortality: A Meta-analysis of Cohort Studies. Epidemiology 29 (4), 460–472. 10.1097/EDE.0000000000000847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Chen J, Hoek G, 2020. Long-term exposure to PM and all-cause and cause-specific mortality: A systematic review and meta-analysis. Environ. Int 143, 105974. 10.1016/j.envint.2020.105974. [DOI] [PubMed] [Google Scholar]
- [4].Atkinson RW, Butland BK, Dimitroulopoulou C, Heal MR, Stedman JR, Carslaw N, Jarvis D, Heaviside C, Vardoulakis S, Walton H, Anderson HR, 2016. Long-term exposure to ambient ozone and mortality: A quantitative systematic review and meta-analysis of evidence from cohort studies. BMJ Open. 6 (2), e009493. 10.1136/bmjopen-2015-009493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Lim CC, Hayes RB, Ahn J, Shao Y, Silverman DT, Jones RR, Garcia C, Bell ML, Thurston GD, 2019. Long-term exposure to ozone and cause-specific mortality risk in the United States. Am J Respir Crit Care Med 200 (8), 1022–1031. 10.1164/rccm.201806-1161OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Cakmak S, Hebbern C, Vanos J, Crouse DL, Burnett R, 2016. Ozone exposure and cardiovascular-related mortality in the Canadian Census Health and Environment Cohort (CANCHEC) by spatial synoptic classification zone. Environ Pollut 214, 589–599. 10.1016/j.envpol.2016.04.067. [DOI] [PubMed] [Google Scholar]
- [7].Turner MC, Jerrett M, Pope CA, Krewski D, Gapstur SM, Diver WR, Beckerman BS, Marshall JD, Su J, Crouse DL, Burnett RT, 2016. Long-Term Ozone Exposure and Mortality in a Large Prospective Study. Am J Respir Crit Care Med 193 (10), 1134–1142. 10.1164/rccm.201508-1633OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Chen K, Wolf K, Breitner S, Gasparrini A, Stafoggia M, Samoli E, Andersen ZJ, Bero-Bedada G, Bellander T, Hennig F, Jacquemin B, Pekkanen J, Hampel R, Cyrys J, Peters A, Schneider A, 2018. Two-way effect modifications of air pollution and air temperature on total natural and cardiovascular mortality in eight European urban areas. Environ Int 116, 186–196. 10.1016/j.envint.2018.04.021. [DOI] [PubMed] [Google Scholar]
- [9].Li J, Woodward A, Hou X-Y, Zhu T, Zhang J, Brown H, Yang J, Qin R, Gao J, Gu S, Li J, Xu L, Liu X, Liu Q, 2017. Modification of the effects of air pollutants on mortality by temperature: A systematic review and meta-analysis. Sci Total Environ 575, 1556–1570. 10.1016/j.scitotenv.2016.10.070. [DOI] [PubMed] [Google Scholar]
- [10].Qiu H, Yu I-S, Wang X, Tian L, Tse LA, Wong TW, 2013. Cool and dry weather enhances the effects of air pollution on emergency IHD hospital admissions. Int J Cardiol 168 (1), 500–505. 10.1016/j.ijcard.2012.09.199. [DOI] [PubMed] [Google Scholar]
- [11].Aga E, Samoli E, Touloumi G, Anderson HR, Cadum E, Forsberg B, Goodman P, Goren A, Kotesovec F, Kriz B, Macarol-Hiti M, Medina S, Paldy A, Schindler C, Sunyer J, Tittanen P, Wojtyniak B, Zmirou D, Schwartz J, Katsouyanni K, 2003. Short-term effects of ambient particles on mortality in the elderly: Results from 28 cities in the APHEA2 project. Eur. Respir. J. Suppl. European Respiratory Society21 (Supplement 40), 28S–33S. 10.1183/09031936.03.00402803. [DOI] [PubMed] [Google Scholar]
- [12].Yitshak-Sade M, Bobb JF, Schwartz JD, Kloog I, Zanobetti A, 2018. The association between short and long-term exposure to PM2.5 and temperature and hospital admissions in New England and the synergistic effect of the short-term exposures. Sci Total Environ 639, 868–875. 10.1016/j.scitotenv.2018.05.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Crouse DL, Pinault L, Balram A, Brauer M, Burnett RT, Martin RV, van Donkelaar A, Villeneuve PJ, Weichenthal S, 2019. Complex relationships between greenness, air pollution, and mortality in a population-based Canadian cohort. Environ Int 128, 292–300. 10.1016/j.envint.2019.04.047. [DOI] [PubMed] [Google Scholar]
- [14].Kim S, Kim H, Lee J-T, 2019. Interactions between Ambient Air Particles and Greenness on Cause-specific Mortality in Seven Korean Metropolitan Cities, 2008–2016. Int J Environ Res Public Health 16, 1866. 10.3390/ijerph16101866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kioumourtzoglou M-A, Schwartz J, James P, Dominici F, Zanobetti A, 2016. PM2.5 and mortality in 207 US cities: Modification by temperature and city characteristics. Epidemiology 1. 10.1097/EDE.0000000000000422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Yitshak-Sade M, James P, Kloog I, Hart J, Schwartz J, Laden F, Lane K, Fabian M, Fong K, Zanobetti A, 2019. Neighborhood greenness attenuates the adverse effect of PM2.5 on cardiovascular mortality in Neighborhoods of lower socioeconomic status. Int J Environ Res Public Health 16 (5), 814. 10.3390/ijerph16050814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Requia WJ, Di Q, Silvern R, Kelly JT, Koutrakis P, Mickley LJ, Sulprizio MP, Amini H, Shi L, Schwartz J, 2020. An ensemble learning approach for estimating high spatiotemporal resolution of ground-level ozone in the contiguous United States. Environ Sci Technol 54 (18), 11037–11047. 10.1021/acs.est.0c0179110.1021/acs.est.0c01791.s001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Di Q, Amini H, Shi L, Kloog I, Silvern R, Kelly J, Sabath MB, Choirat C, Koutrakis P, Lyapustin A, Wang Y, Mickley LJ, Schwartz J, 2020. Assessing NO 2 Concentration and Model Uncertainty with High Spatiotemporal Resolution across the Contiguous United States Using Ensemble Model Averaging. ACS Publ 54 (3), 1372–1384. 10.1021/acs.est.9b0335810.1021/acs.est.9b03358.s001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Di Q, Amini H, Shi L, et al. An ensemble-based model of PM2.5 concentration across the contiguous United States with high spatiotemporal resolution. Environ Int 2019;130:104909. doi: 10.1016/j.envint.2019.104909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Di Q, Wang Y, Zanobetti A, Wang Y, Koutrakis P, Choirat C, Dominici F, Schwartz JD, 2017. Air Pollution and Mortality in the Medicare Population. N Engl J Med 376 (26), 2513–2522. 10.1056/NEJMoa1702747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Shi L, Wu X, Danesh Yazdi M, Braun D, Abu Awad Y, Wei Y, Liu P, Di Q, Wang Y, Schwartz J, Dominici F, Kioumourtzoglou M-A, Zanobetti A, 2020. Long-term effects of PM2⋅5 on neurological disorders in the American Medicare population: a longitudinal cohort study. Lancet Planet Health 4 (12), e557–e565. 10.1016/S2542-5196(20)30227-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Measuring Vegetation (NDVI & EVI). https://earthobservatory.nasa.gov/features/MeasuringVegetation/measuring_vegetation_2.php (accessed 16 Jun 2020).
- [23].Abatzoglou JT, 2013. Development of gridded surface meteorological data for ecological applications and modelling. Int J Climatol 33 (1), 121–131. 10.1002/joc.v33.110.1002/joc.3413. [DOI] [Google Scholar]
- [24].Bratsch SG, 1989. Standard electrode potentials and temperature coefficients in water at 298.15 K. J Phys Chem Ref Data 18 (1), 1–21. 10.1063/1.555839. [DOI] [Google Scholar]
- [25].Shi L, Kloog I, Zanobetti A, Liu P, Schwartz JD, 2015. Impacts of temperature and its variability on mortality in New England. Nat Clim Chang 5 (11), 988–991. 10.1038/nclimate2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Shi L, Liu P, Wang Y, Zanobetti A, Kosheleva A, Koutrakis P, Schwartz J, 2016. Chronic effects of temperature on mortality in the Southeastern USA using satellite-based exposure metrics. Sci Rep 6 (1). 10.1038/srep30161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Zanobetti A, O’Neill MS, Gronlund CJ, Schwartz JD, 2012. Summer temperature variability and long-term survival among elderly people with chronic disease. Proc. Natl. Acad. Sci 109 (17), 6608–6613. 10.1073/pnas.1113070109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Fong KC, Hart JE, James P. A Review of Epidemiologic Studies on Greenness and Health: Updated Literature Through 2017 Current environmental health reports. 2018;5(1):77–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Danesh Yazdi M, Wang Y, Di Q, et al. Long-term exposure to PM2.5 and ozone and hospital admissions of Medicare participants in the Southeast USA. Environ Int 2019;130:104879. doi: 10.1016/j.envint.2019.05.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Stafoggia M, Cesaroni G, Peters A, Andersen ZJ, Badaloni C, Beelen R, Caracciolo B, Cyrys J, de Faire U, de Hoogh K, Eriksen KT, Fratiglioni L, Galassi C, Gigante B, Havulinna AS, Hennig F, Hilding A, Hoek G, Hoffmann B, Houthuijs D, Korek M, Lanki T, Leander K, Magnusson PK, Meisinger C, Migliore E, Overvad K, Östenson C-G, Pedersen NL, Pekkanen J, Penell J, Pershagen G, Pundt N, Pyko A, Raaschou-Nielsen O, Ranzi A, Ricceri F, Sacerdote C, Swart WJR, Turunen AW, Vineis P, Weimar C, Weinmayr G, Wolf K, Brunekreef B, Forastiere F, 2014. Long-Term Exposure to Ambient Air Pollution and Incidence of Cerebrovascular Events: Results from 11 European Cohorts within the ESCAPE Project. Environ Health Perspect 122 (9), 919–925. 10.1289/ehp.1307301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Cesaroni G, Forastiere F, Stafoggia M, et al. Long term exposure to ambient air pollution and incidence of acute coronary events: Prospective cohort study and meta-analysis in 11 european cohorts from the escape project. BMJ 2014;348. doi: 10.1136/bmj.f7412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Atkinson RW, Carey IM, Kent AJ, van Staa TP, Anderson HR, Cook DG, 2013. Long-term exposure to outdoor air pollution and incidence of cardiovascular diseases. Epidemiology 24 (1), 44–53. 10.1097/EDE.0b013e318276ccb8. [DOI] [PubMed] [Google Scholar]
- [33].Sørensen M, Lühdorf P, Ketzel M, Andersen ZJ, Tjønneland A, Overvad K, Raaschou-Nielsen O, 2014. Combined effects of road traffic noise and ambient air pollution in relation to risk for stroke? Environ Res 133, 49–55. 10.1016/j.envres.2014.05.011. [DOI] [PubMed] [Google Scholar]
- [34].Münzel T, Sørensen M, Gori T, et al. Environmental stressors and cardio-metabolic disease: part II–mechanistic insights. Eur Heart J 2016;38:ehw294. doi: 10.1093/eurheartj/ehw294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Franklin BA, Brook R, Arden Pope C, 2015. Air pollution and cardiovascular disease. Curr Probl Cardiol 40 (5), 207–238. 10.1016/j.cpcardiol.2015.01.003. [DOI] [PubMed] [Google Scholar]
- [36].Pinault LL, Weichenthal S, Crouse DL, Brauer M, Erickson A, Donkelaar AV, Martin RV, Hystad P, Chen H, Finès P, Brook JR, Tjepkema M, Burnett RT, 2017. Associations between fine particulate matter and mortality in the 2001 Canadian Census Health and Environment Cohort. Environ Res 159, 406–415. 10.1016/j.envres.2017.08.037. [DOI] [PubMed] [Google Scholar]
- [37].Wu X, Braun D, Schwartz J, Kioumourtzoglou MA, Dominici F, 2020. Evaluating the impact of long-term exposure to fine particulate matter on mortality among the elderly. Sci. Adv 6 (29), eaba5692. 10.1126/sciadv.aba5692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Pope CA, Burnett RT, Turner MC, Cohen A, Krewski D, Jerrett M, Gapstur SM, Thun MJ, 2011. Lung cancer and cardiovascular disease mortality associated with ambient air pollution and cigarette smoke: Shape of the exposure-response relationships. Environ Health Perspect 119 (11), 1616–1621. 10.1289/ehp.1103639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Huangfu P, Atkinson R, 2020. Long-term exposure to NO2 and O3 and all-cause and respiratory mortality: A systematic review and meta-analysis. Environ. Int 144, 105998. 10.1016/j.envint.2020.105998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Nieuwenhuijsen MJ, Khreis H, Triguero-Mas M, Gascon M, Dadvand P, 2017. Fifty Shades of Green. Epidemiology 28 (1), 63–71. 10.1097/EDE.0000000000000549. [DOI] [PubMed] [Google Scholar]
- [41].Bell ML, Ebisu K, Peng RD, et al. Seasonal and regional short-term effects of fine particles on hospital admissions in 202 US counties, 1999–2005. Am J Epidemiol 2008;168:1301–10. doi: 10.1093/aje/kwn252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Hsu W-H, Hwang S-A, Kinney PL, Lin S, 2017. Seasonal and temperature modifications of the association between fine particulate air pollution and cardiovascular hospitalization in New York state. Sci Total Environ 578, 626–632. 10.1016/j.scitotenv.2016.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Duan Y, Liao Y.i., Li H, Yan S, Zhao Z, Yu S, Fu Y, Wang Z, Yin P, Cheng J, Jiang H, 2019. Effect of changes in season and temperature on cardiovascular mortality associated with nitrogen dioxide air pollution in Shenzhen. China. Sci Total Environ 697, 134051. 10.1016/j.scitotenv.2019.134051. [DOI] [PubMed] [Google Scholar]
- [44].Leitte AM, Petrescu C, Franck U, Richter M, Suciu O, Ionovici R, Herbarth O, Schlink U, 2009. Respiratory health, effects of ambient air pollution and its modification by air humidity in Drobeta-Turnu Severin. Romania. Sci Total Environ 407 (13), 4004–4011. 10.1016/j.scitotenv.2009.02.042. [DOI] [PubMed] [Google Scholar]
- [45].Reinikainen L, Air JJ-I, 2003U. Significance of humidity and temperature on skin and upper airway symptoms. Indoor Air 2003;13:344–52. [DOI] [PubMed] [Google Scholar]
- [46].Cao Y.u., Chen M, Dong D, Xie S, Liu M, 2020. Environmental pollutants damage airway epithelial cell cilia: Implications for the prevention of obstructive lung diseases. Thorac Cancer 11 (3), 505–510. 10.1111/tca.v11.310.1111/1759-7714.13323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Kudo E, Song E, Yockey LJ, Rakib T, Wong PW, Homer RJ, Iwasaki A, 2019. Low ambient humidity impairs barrier function and innate resistance against influenza infection. Proc. Natl. Acad. Sci 116 (22), 10905–10910. 10.1073/pnas.1902840116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Davis RE, McGregor GR, Enfield KB, 2016. Humidity: A review and primer on atmospheric moisture and human health. Environ. Res 144, 106–116. 10.1016/j.envres.2015.10.014. [DOI] [PubMed] [Google Scholar]
- [49].HEI Perspectives 3., 2013www.healtheffects.org (accessed 28 Oct 2020).
- [50].Xu J, Hu W, Liang D, Gao P, 2020. Photochemical impacts on the toxicity of PM2. 5. Critical Reviews in Environmental Science and Technology. 1–27. [Google Scholar]
- [51].Zhang R, Wang G, Guo S, Zamora ML, Ying Q.i., Lin Y, Wang W, Hu M, Wang Y, 2015. Formation of urban fine particulate matter. Chem. Rev 115 (10), 3803–3855. [DOI] [PubMed] [Google Scholar]
- [52].Weichenthal S, Pinault LL, Burnett RT, 2017. Impact of oxidant gases on the relationship between outdoor fine particulate air pollution and nonaccidental, cardiovascular, and respiratory mortality. Sci. Rep 7 (1), 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Christidis T, Erickson AC, Pappin AJ, Crouse DL, Pinault LL, Weichenthal SA, Brook JR, van Donkelaar A, Hystad P, Martin RV, Tjepkema M, Burnett RT, Brauer M, 2019. Low concentrations of fine particle air pollution and mortality in the Canadian Community Health Survey cohort. Environmental Health 18 (1). 10.1186/s12940-019-0518-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Zhang H, Yee LD, Lee BH, Curtis MP, Worton DR, Isaacman-VanWertz G, Offenberg JH, Lewandowski M, Kleindienst TE, Beaver MR, Holder AL, Lonneman WA, Docherty KS, Jaoui M, Pye HOT, Hu W, Day DA, Campuzano-Jost P, Jimenez JL, Guo H, Weber RJ, de Gouw J, Koss AR, Edgerton ES, Brune W, Mohr C, Lopez-Hilfiker FD, Lutz A, Kreisberg NM, Spielman SR, Hering SV, Wilson KR, Thornton JA, Goldstein AH, 2018. Monoterpenes are the largest source of summertime organic aerosol in the southeastern United States. Proc. Natl. Acad. Sci 115 (9), 2038–2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Sharkey TD, Wiberley AE, Donohue AR, 2008January1. Isoprene emission from plants: why and how. Ann. Bot 101 (1), 5–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.