Abstract
Humans carry residues of multiple synthetic chemicals at any given point in time. Research has demonstrated that compounds with varying molecular initiating events (MIE) that disrupt common key events can act in concert to produce cumulative adverse effects. Congenital defects of the male reproductive tract are some of the most frequently diagnosed malformations in humans and chemical exposures in utero can produce these effects in laboratory animals and humans. Here, we hypothesized that in utero exposure to a mixture of pesticides and phthalates, each of which produce male reproductive tract defects individually, would produce cumulative effects even when each chemical is present at a no observed adverse effect level (NOAEL) specific for male reproductive effects. Pregnant Sprague-Dawley rats were exposed via oral gavage to a fixed-ratio dilution mixture of 5 pesticides (vinclozolin, linuron, procymidone, prochloraz, pyrifluquinazon), 1 pesticide metabolite (dichlorodiphenyldichloroethylene (DDE)), and 9 phthalates (dipentyl, dicyclohexyl, di-2-ethylhexyl, dibutyl, benzyl butyl, diisobutyl, diisoheptyl, dihexyl, and diheptyl) during the critical window of rat fetal masculinization (gestation day 14–18). The top dose (100% dose) contained each compound at a concentration 2-fold greater than the individual chemical NOAEL followed by a dilution series that represented each chemical at NOAEL, NOAEL/2, NOAEL/4, NOAEL/8, NOAEL/15, NOAEL/100, NOAEL/1000. Reduced fetal testis gene expression occurred at NOAEL/15, reduced fetal testis testosterone production occurred at NOAEL/8, reduced anogenital distance, increased nipple retention, and delayed puberty occurred at NOAEL/4, and severe effects including genital malformations and weight reductions in numerous reproductive tissues occurred at NOAEL/2. This study demonstrates that these phthalates and pesticides acted cumulatively to produce adverse effects at doses below which any individual chemical had been shown to produce an effect alone and even though they have different MIEs.
Keywords: chemical mixtures, dose addition, birth defect, phthalate, pesticide, male reproductive tract
1. INTRODUCTION
Synthetic compounds of diverse chemical structure and source are pervasive around the globe and advances in analytical chemistry continue to expand our understanding of the presence and concentrations of complex mixtures of xenobiotic compounds in environmental and biological matrices (Vermeulen et al. 2020). Concomitant advances in mixtures toxicology and cumulative risk assessment are needed to more comprehensively and accurately characterize hazard and estimate risks of co-exposure to multiple exogenous compounds (Kortenkamp and Faust 2018). Experimental toxicology research can address hypotheses related to mixture theory (e.g., models of additivity), provide empirical examples of how chemical co-exposure affects risk assessment relevant endpoints (e.g., benchmark dose points of departure), and provide biological context for coherent grouping of compounds for cumulative risk assessment (Monosson 2005).
The inception and adoption of Adverse Outcome Pathways (AOP) (Ankley et al. 2010) into both toxicology research and risk assessment frameworks has aided in the conceptual association of chemicals acting on a common pathway, albeit via dissimilar molecular initiating events (MIE) (Gray et al. 2006; Howdeshell et al. 2017; Rider et al. 2009; Wilson et al. 2008). Research from our group and others has demonstrated that diortho-phthalate esters, acting through an unknown MIE, can impair fetal testis testosterone and Insl3 production during the critical window of sex differentiation leading to birth defects and demasculinized male offspring in the rat (Parks et al. 2000; Wilson et al. 2004). Further, several pesticides have been identified that operate via a MIE of androgen receptor (AR) antagonism (Gray et al. 1994) and/or direct inhibition of steroidogenic enzymes thereby reducing testis testosterone (T) production (Lambright et al. 2000; McIntyre et al. 2000; Wilson et al. 2009). These three disparate MIEs alter AR signaling pathways in various male reproductive tissues during development and lead to similar male reproductive malformations when exposure occurs during the critical window in utero (Fig. 1) (Conley et al. 2018; Howdeshell et al. 2017; Kortenkamp 2020).
Figure 1.
Adverse Outcome Pathway (AOP) network for Molecular Initiating Events (MIEs) that disrupt androgen receptor (AR) mediated cellular signaling leading to adverse effects on development of the male reproductive tract resulting from in utero exposure during the masculinizing window of development. The MIE for phthalates is unknown but also involves suppression of insulin-like hormone 3 (Insl3) synthesis. Some compounds target more than one MIE, such as linuron and prochloraz.
Two of the defects that antiandrogenic phthalates and pesticides produce in rodent models are cryptorchidism (undescended testis) and hypospadias (malformation of penile urethral opening), which are among the most diagnosed birth defects in humans. In the United States, hypospadias occurs in ~0.3–0.8% of live births (Carmichael et al. 2012; Mai et al. 2015) and cryptorchidism occurs in ~1–3% of live births (Thonneau et al. 2003). Some studies have reported that the incidence rates for both have increased during the last 30–40 years in some countries (Paulozzi 1999; Paulozzi et al. 1997; Toppari et al. 2001; Yu et al. 2019), while others dispute the presence of increasing trends but not the overall common incidence of these effects (van der Horst and de Wall 2017). Anogenital distance (AGD) is also an androgen-dependent developmental biomarker that is reported in animal studies and clinically measured in humans and linked to adverse male reproductive effects (Dorman et al. 2018; Garcia-Villarino et al. 2020; Schwartz et al. 2019). Additional human health studies have reported declines in male sperm production leading to reduced fertility (Levine et al. 2017) and increases in testicular germ cell cancers (Nigam et al. 2015; Skakkebaek 2017). Regardless of temporal trends in incidence rates, exposure to environmental chemicals that alter AR signaling during fetal development have been implicated in the etiology of this collection of human health effects (termed Testicular Dysgenesis Syndrome (Skakkebaek et al. 2016)) due to similarity of effects in animal studies and overall weight of evidence.
Previously, we exposed pregnant rats to a mixture of 18 different “anti-androgenic” chemicals, including phthalates, pesticides, and pharmaceutical drugs (Conley et al. 2018). For each chemical we identified the lowermost lowest observed adverse effect level (LOAEL) from the literature for any adverse effect on development of the male reproductive system and the top dose contained each chemical at 20% of its respective LOAEL (i.e., LOAEL/5) followed by a 50% dilution series down to each chemical at LOAEL/80. The mixture was tested for both effects on fetal testis T production and testis gene expression, as well as on early postnatal malformations and permanent adverse effects in mature adults following in utero exposure. We found that male rat offspring displayed a variety of neonatal, pubertal, and permanent adult effects across all dose levels. Even at the lowest dose (LOAEL/80) there were permanent reductions in several reproductive tract tissue weights. In the top dose group (LOAEL/5), 100% of male offspring displayed permanent severe birth defects including genital malformations. While that study included the potent anti-androgenic drugs finasteride and flutamide, here we modified the approach to focus specifically on the environmental chemicals (phthalates and pesticides) and the doses were based upon no observed adverse effect levels (NOAELs) for F1 male reproductive tract development rather than LOAELs. For each of the 15 compounds we identified a NOAEL from our studies and/or the literature that produced no statistically significant adverse effects on F1 male reproductive development (i.e., reduced AGD, delayed puberty, reductions in reproductive tissue weights, and/or reproductive tract malformations or histopathological lesions) in individual experiments. Similar to the previous study we hypothesized that these phthalates and pesticides would act cumulatively to produce adverse effects at doses below which any individual chemical had been shown to produce an effect alone and despite the fact that they have different MIEs.
2. MATERIALS AND METHODS
2.1. Chemicals and dosing
Dosing solutions were prepared using laboratory-grade corn oil (Sigma-Aldrich, St. Louis, MO, USA). Vinclozolin ((VIN), CAS 50471-44-8; Lot 2296X) was purchased from Riedel-de Haen (Seelze, Germany). Linuron ((LIN), CAS 330-55-2; Lot 265-44A) was purchased from Crescent Chemical Company (Islandia, NY, USA). Procymidone ((PCD), CAS 32809-16-8; Lot 231-100A) and Pyrifluquinazon ((PFQ), CAS 337458-27-2; Lot 2110000) were purchased from Chem Services (West Chester, PA, USA). Prochloraz ((PCZ), CAS 67747-09-5; Lot SZBA112XV), Dicyclohexyl phthalate ((DCHP), CAS 84-61-7; Lot 17518JB), diisoheptyl phthalate ((DIHEP), CAS 71888-89-6; Batch 04516HJ), and 1,1’-(2,2-Dichloroethene-1,1-diyl)bis(4-chlorobenzene) ((DDE), CAS 72-55-9; Lot 10230AZ) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Dipentyl phthalate ((DPEP), CAS 131-18-0; Lot 1431420; RTI Log 040109-A-14), diethylhexyl phthalate ((DEHP), CAS 117-81-7; Lot 01514TH; RTI Log 121208-B-16), dibutyl phthalate ((DBP), CAS 84-74-2; Lot 91997PJ; RTI Log 031609-A-17), butyl benzyl phthalate ((BBP), CAS 85-68-7; Lot 03405JH; RTI Log 121208-A-15), diisobutyl phthalate ((DIBP), CAS 84-69-5; Lot 07425BJ; RTI Log 070208-A-18), dihexyl phthalate ((DHP), CAS 84-75-3; Lot 139AG; RTI Log 040809-A-14), and diheptyl phthalate ((DHEP), CAS 3648-21-3; Lot 125AG; RTI Log 031209-A-14) were gifted from the National Toxicology Program (Research Triangle Park, NC, USA). All purchased chemicals were ≥98% purity as reported by the supplier and gifted phthalates from NTP were independently verified for purity (>99%) by Research Triangle Institute (Durham, NC, USA) using gas chromatography with flame ionization detection.
Treatments were prepared as a fixed-ratio dilution series based on the individual chemical NOAELs for F1 male reproductive development (Table 1). The NOAEL values used for the present mixture study represent the judgement of the authors given the existing data for these chemicals specifically for low dose male reproductive effects and should not be conflated with NOAELs for these chemicals that consider all possible organ systems/effects typically used in formal risk assessments or regulatory frameworks. For each chemical we selected the highest oral dose that did not have any statistically significant adverse effects on F1 male reproductive tissues from studies conducted in our lab or others on pregnant rats with oral dosing that at least included the masculinizing window of gestation (GD14-18) (Table S1). We identified studies conducted on the same species and strain as used in the present study (Sprague-Dawley rat) for all but one chemical (vinclozolin, Wistar and Long-Evans rats). Effects evaluated included reduced AGD, delayed onset of puberty, reproductive tract malformations, reproductive tract organ weight reductions, and testis/epididymis histopathology. Reduction in fetal testis testosterone production (where available) and increased retention of female-like nipples/areolae were considered supportive evidence but not sufficient by themselves to set NOAELs for the current mixture study design. It is important to note that data does not exist for all possible male reproductive effects assessed here for all chemicals in the mixture.
Table 1.
Composition of 15 chemical antiandrogen mixture based on individual male reproductive developmental no observed adverse effect levels (NOAEL).
| Chemical | Abb. | CASRN | NOAELx2 100% Dose (mg/kg/d | NOAEL 50% Dose (mg/kg/d | NOAEL/2 25% Dose (mg/kg/d | NOAEL/4 12.5% Dose (mg/kg/d | NOAEL/8 6.25% Dose (mg/kg/d | NOAEL/15 3.3% Dose (mg/kg/d | NOAEL/100 0.5% Dose (mg/kg/d | NOAEL/1000 0.05% Dose (mg/kg/d |
|---|---|---|---|---|---|---|---|---|---|---|
| Benzylbutyl phth. | BBP | 85-68-7 | 100 | 50 | 25 | 12.5 | 6.25 | 3.3 | 0.5 | 0.05 |
| Dibutyl phth. | DBP | 84-74-2 | 100 | 50 | 25 | 12.5 | 6.25 | 3.3 | 0.5 | 0.05 |
| Dicyclohexyl phth. | DCHP | 84-61-7 | 200 | 100 | 50 | 25 | 12.5 | 6.7 | 1 | 0.1 |
| Diethylhexyl phth. | DEHP | 117-81-7 | 9.6 | 4.8 | 2.4 | 1.2 | 0.6 | 0.32 | 0.048 | 0.0048 |
| Diheptyl phth. | DHEP | 3648-21-3 | 1000 | 500 | 250 | 125 | 62.5 | 33.3 | 5 | 0.5 |
| Dihexyl phth. | DHP | 84-75-3 | 100 | 50 | 25 | 12.5 | 6.25 | 3.3 | 0.5 | 0.05 |
| Diisobutyl phth. | DIBP | 84-69-5 | 250 | 125 | 62.5 | 31.3 | 15.6 | 8.3 | 1.25 | 0.125 |
| Diisoheptyl phth. | DIHEP | 71888-89-6 | 128 | 64 | 32 | 16 | 8 | 4.3 | 0.64 | 0.064 |
| Dipentyl phth. | DPEP | 131-18-0 | 22 | 11 | 5.5 | 2.8 | 1.38 | 0.73 | 0.11 | 0.011 |
| Linuron | LIN | 330-55-2 | 5 | 2.5 | 1.3 | 0.6 | 0.31 | 0.17 | 0.025 | 0.0025 |
| p,p’-DDE | DDE | 72-55-9 | 20 | 10 | 5 | 2.5 | 1.25 | 0.67 | 0.1 | 0.01 |
| Prochloraz | PCZ | 67747-09-5 | 62.5 | 31.3 | 15.6 | 7.8 | 3.9 | 2.1 | 0.313 | 0.0313 |
| Procymidone | PCD | 32809-16-8 | 25 | 12.5 | 6.3 | 3.1 | 1.56 | 0.83 | 0.125 | 0.0125 |
| Pyrifluquinazon | PFQ | 337458-27-2 | 25 | 12.5 | 6.3 | 3.1 | 1.56 | 0.83 | 0.125 | 0.0125 |
| Vinclozolin | VIN | 50471-44-8 | 12 | 6 | 3 | 1.5 | 0.75 | 0.4 | 0.06 | 0.006 |
|
| ||||||||||
| Theoretical total mixture dose (Total sum of NOAELs) * | 30 | 15 | 7.5 | 3.75 | 1.875 | 1 | 0.15 | 0.015 | ||
References and details on individual chemical NOAEL identification reported in Table S1
Assuming additivity, mixture predicted to produce male reproductive effects when total sum of NOAELs >~3
Generally, the NOAELs selected were the next lower dose than the LOAELs used in our prior mixture study (Conley et al. 2018). For VIN, Gray et al. (1999) reported significantly increased nipple retention and decreased AGD at 3.125 mg/kg/d from data pooled across four dosing blocks, which conflicts with data from Hellwig et al. (2000) that reported no significant effects at 6 mg/kg/d and Schneider et al. (2011) and Flick et al. (2017) who reported no significant effects at 4 mg/kg/d. Therefore, we used the NOAEL of 6 mg/kg/d consistent with USEPA (2000). Further, for linuron the lowest dose tested (12.5 mg/kg/d) produced testis and epididymis lesions in 2 of 56 F1 males (McIntyre et al. 2000), therefore we estimated a NOAEL for this chemical at LOAEL/5 (i.e., 2.5 mg/kg/d). Importantly, for each chemical the NOAEL we identified was experimentally shown to produce no permanent male reproductive tract malformations or reproductive organ weight reductions.
The 100% dose contained each chemical at 2-fold greater than the identified NOAEL (i.e., NOAELx2) for male reproductive effects, followed by a 50% dilution series down to each chemical at 1/15th of the NOAEL and two very low doses at 1/100th and 1/1000th of the NOAEL (8 total doses: 100, 50, 25, 12.5, 6.25, 3.3, 0.5, and 0.05% corresponding to NOAELx2, NOAEL, NOAEL/2, NOAEL/4, NOAEL/8, NOAEL/15, NOAEL/100, and NOAEL/1000 for each chemical in the mixture). The top dose (NOAELx2) was prepared first by weighing out chemical and corn oil and then sequential serial dilutions were performed to generate subsequent dosing solutions. Dosing solutions were prepared two days prior to the initiation of dosing in glass flasks and mixed until homogeneous on a stir plate using Teflon-coated stir bars. Solutions were stored at room temperature in the dark and restirred prior to exposure to ensure complete mixing. Pregnant dams were dosed via oral gavage with either vehicle control or treatment dilution at 2.5 mL kg−1 from GD14-18 between 0800-0930 EST daily.
Mixture dilution levels were selected by using the relative dose levels (e.g., NOAEL/15) to calculate a theoretical total mixture dose, which is the sum of all relative dose levels (Table 1 bottom). For example, at the 3.3% dose each chemical was present at NOAEL/15 (i.e., 1/15), therefore the sum of all relative doses=1 (i.e., 15 × 1/15) and indicates that we predicted this dose level to be the total mixture NOAEL (i.e., no male reproductive effects at this mixture dose), assuming additivity. For total mixture doses with sum NOAELs >~3 we predicted we would observe male reproductive effects with increasing severity as the total sum value increased. Similarly, we made a priori predictions of severe reproductive tract effects (i.e., hypospadias, cryptorchidism, epididymal agenesis) by calculating a reproductive malformation index ((RMI), Table S2). For this estimate we identified the lowest dose of each chemical that has been shown to produce severe effects and calculated a ratio of the individual chemical doses relative to LOAEL. The RMI is the sum of dose/LOAEL ratios for each chemical at each mixture dose level. For mixture doses with RMI ≥1 we predicted that dose would produce severe male reproductive effects with increasing incidence as the RMI metric increased.
2.2. Animals
Time-mated female Sprague Dawley rats (Crl:CD(SD)), approximately 90 days old, were purchased from Charles River Laboratories (Raleigh, NC) and shipped to USEPA (Research Triangle Park, NC) on gestation day 2 (GD2; day of breeding = GD0, plug positive = GD1). Animals are tested by the supplier to be negative for staphylococcus areus and group B streptococcus prior to shipping, and visually examined by laboratory staff upon arrival to be well groomed and in good condition. Dams were housed individually in clear polycarbonate cages (20 × 25 × 47 cm) with heat-treated, laboratory-grade pine shavings and fed NIH07 rodent diet and filtered (5 μm) municipal tap water ad libitum. Water provided to USEPA is tested every 4 months for a subset of metals, pesticides, and other chemical contaminants and tested monthly for Pseudomonas. Dams were weight-ranked and randomly assigned to treatment groups to produce similar mean weights and variances. This study was conducted in accordance with a protocol approved by the USEPA Center for Public Health and Environmental Assessment’s Institutional Animal Care and Use Committee. Animals were housed in a facility accredited by the Association for Assessment and Accreditation of Laboratory Animal Care and maintained at 20–22°C, 45–55% humidity, and a 12:12 h photoperiod (lights off 18:00).
2.3. Fetal testis screen
Specifics of this method have been previously reported (Furr et al. 2014). Briefly, dams were dosed via oral gavage with vehicle (n=12 dams), NOAELx2 (n=3), NOAEL (n=4), NOAEL/2 (n=7), NOAEL/4 (n=7), NOAEL/8 (n=7), NOAEL/15 (n=8), NOAEL/100 (n=6), or NOAEL/1000 (n=6) treatments from GD14-18. Late gestation (GD18) dams were euthanized by decapitation and fetuses were removed. Fetal testes were collected with a single testis from the first three males used for determination of testosterone production and the remaining testes were homogenized and preserved in TRI Reagent (Sigma) for gene expression analysis. Ex vivo testosterone production was quantified using radioimmunoassay (ALPCO, Cat. no. 72-TESTO-CT2) according to manufacturer specifications following 3 h incubation in 500 μL of M-199 media without phenol red (Gibco Life Technologies, Product #A31224DK), supplemented with 10% dextran-coated charcoal stripped fetal bovine serum (Hyclone Laboratories, Logan, UT). Testes were incubated for 3 hrs at 37°C on a rotating platform in a humidified atmosphere. Following incubation, media was removed and stored at −80°C until used for T measurement. The intra-assay coefficient of variation for this kit was 5.6% (based on variability of the standard curve) and the inter-assay coefficient of variation was 8%. Cross-reactivity of the T antibody with dihydrotestosterone (DHT) was 2.6%. The limit of detection was 0.08 ng testosterone/mL.
Gene expression was assessed using reverse transcriptase real-time PCR of cDNA synthesized from RNA extracted from testis homogenates. RNA extraction was performed according to TRI Reagent manufacturer specifications using chloroform and isopropanol. Following extraction, RNA was purified using the RNeasy Mini Kit (Qiagen). A 96-well gene array plate was previously custom designed to contain 89 target genes and 3 housekeeping genes, an intra-assay control, a genomic DNA control, a reverse transcriptase control, and a positive PCR control (SABioscience, Frederick, MD, USA; (Hannas et al. 2012)). Target genes represent several candidate pathways including male testis cell differentiation and signaling, gubernaculum development, steroidogenesis, androgen receptor signaling, and peroxisome proliferator-activated receptor signaling (complete list of genes in Table S3). PCR reaction was run on an iCycler iQ Real-Time Detection System (BioRad, Hercules, CA, USA) using RT2 SYBR Green qPCR Master Mix (SABioscience).
2.4. Postnatal reproductive development
Specifics for this methodology have been previously reported (Gray et al. 2009). Briefly, two blocks of 30 dams per block were dosed via oral gavage with vehicle (n=10 dams), NOAELx2 (n=5), NOAEL (n=10), NOAEL/2 (n=5), NOAEL/4 (n=5), NOAEL/8 (n=5), NOAEL/15 (n=10), NOAEL/100 (n=5), or NOAEL/1000 (n=5) treatments from GD14-18. One dam each in the NOAEL/15 and NOAELx2 dose groups were not pregnant, reducing the sample size to n=9 and n=4, respectively. One dam in the NOAEL dose group had a small litter that by PND2 consisted of 4 male pups and then no pups present on PND9 evaluation, reducing sample size to n=9. Dams gave birth naturally beginning on the morning of GD22 (i.e., PND0) and on PND2 and PND9 pups were sexed, weighed, and AGD was measured using a Leica MZ6 dissecting microscope with an ocular micrometer. On PND13 pups were again sexed, weighed, and nipple/areolae retention was visually scored and recorded as the number of nipples/areolae present per animal. For both AGD and nipple retention scoring the evaluations were performed by the same individual who was blinded to exposure group. Female pups in the NOAEL/1000 and NOAEL/100 doses were euthanized following evaluation of nipple retention on PND13 due to a lack of perinatal effects. On PND27 dams were euthanized, uterine implantation sites were scored, pups were weaned to two animals per cage by sex and treatment group, and food was changed to NTP2000 rodent diet. Beginning on PND31 for female offspring and PND41 for male offspring, individuals were evaluated at the same time every day for markers of pubertal onset, vaginal opening (VO) for females and balano-preputial separation (PPS) for males.
Beginning at PND90 female offspring were considered mature adults and were weighed, euthanized via decapitation, and examined for any gross reproductive tract abnormalities. Similarly, males were considered mature at PND120 and were weighed, euthanized, and examined for a range of reproductive tract malformations and weights were collected for all relevant reproductive tissues. Male necropsy included scoring nipple/areolae retention, external genital malformations (e.g., hypospadias), internal malformations (e.g., epididymal agenesis, testicular atrophy, undescended testes), and weights of glans penis, ventral prostate, seminal vesicles, testes, epididymides, levator ani-bulbocavernosus (LABC), and bulbourethral (Cowper’s) glands. After weighing, the left cauda and corpus/caput epididymis were separated and individually minced in M-199 media (Gibco, Cat. no. 94-0384DK) supplemented with Soybean Trypsin Inhibitor (Gibco 17075-011), Bovine Serum Albumin Fraction V (Gibco 11018-017), and HEPES (Invitrogen 15630-130). Sperm counts in epididymal sections were measured in males without epididymal lesions using a Multisizer 3 coulter counter (Beckman Coulter, Brea, CA, USA). Sperm counts were not analyzed from the NOAEL/1000 and NOAEL/100 doses due to lack of effects on all other endpoints. Both testes and the right epididymis were preserved in Bouin’s fixative for 24 h, then transferred to 70% ethanol and shipped to Experimental Pathology Laboratories, Inc. (Durham, NC, USA) where tissues were embedded, sectioned, stained with hematoxylin and eosin, and evaluated by a Diplomate of the American College of Veterinary Pathology for lesions.
2.5. Statistics
All reported values are mean ± standard error (SEM) and all statistical comparisons were conducted at α=0.05 significance level except for gene expression which utilized α=0.0001 to detect significant analysis of variance (ANOVA) trends. Data were analyzed using PROC GLM in SAS (v.9.4, SAS Institute, Cary, NC) using litter means as the statistical unit (i.e., endpoint measurements averaged across animals in a litter, then litter means averaged for statistical comparison). Sample sizes (i.e., number of litters per dose) were based on previously published power calculations which demonstrated 3–4 litters per dose group with 3 pups/litter for T production and remaining testes pooled for mRNA analyses was sufficient, and that for the majority of postnatal endpoints assessment of ≥4 pups from ≥5 litters provided adequate power (~0.8) to detect significant differences from control (Gray et al. 2009; Hannas et al. 2012). Pairwise comparison of significant ANOVA results was performed using the least squares means (LSMEANS) procedure in SAS. Data were evaluated untransformed and following log10 transformation. Male pup puberty (PPS) and weight at puberty were analyzed using weaning weight as a covariate in PROC GLM. To account for body weight, AGD was analyzed using body weight as a covariate and by calculating AGD index (AGDi = AGD/3√BW). Percent reduction in male pup AGD was calculated by subtracting mean control female AGD.
Fetal testis gene expression delta CT values were calculated using the equation 2-ΔΔCT and normalized to the mean CT value of the housekeeping genes Actb, Gusb, and Ldha. Delta CT values were then converted to fold-induction by dividing the treated replicate delta CT by the mean delta CT of the control replicates for each gene, followed by log10 transformation prior to ANOVA. Fetal testis testosterone production was normalized to the mean control concentration within a given block and analyzed as % of control values using PROC GLM with litter means as the statistical unit.
All dose response curve analyses were conducting using four-parameter logistic regression in GraphPad Prism v7.02 (GraphPad, Inc., La Jolla, CA, USA) with the following parameter constraints: bottom=0%, top=100%. Mixture model (dose addition (DA), response addition (RA), and integrated addition (IA)) calculations (Rider and LeBlanc 2005) were performed in Microsoft Excel using individual chemical data identified from the literature for the effects of anogenital distance, paired seminal vesicles weight, and hypospadias incidence (Table S8). For IA calculations, chemicals were grouped into cassettes based on mode of action as has been previously described (Rider and LeBlanc 2005). Cassette 1 included all phthalates (inhibit fetal testis testosterone and Insl3 production), cassette 2 included the compounds that are AR antagonists but do not affect fetal testis steroidogenesis (DDE, PFQ, PCD, VIN), and cassette 3 included the compounds that impair fetal testis steroidogenesis and are also AR antagonists (PCZ, LIN). To our knowledge, no data for seminal vesicle weight for DHEP or DIHEP, or hypospadias for DHEP was available in the literature (hypospadias data for DIHEP was available). To estimate the ED50 values and slopes for these compounds/effects we calculated relative potency factors (RPF) using data on anogenital distance reduction with DPEP as the index chemical (i.e., DPEP ED50/compound ED50). The DPEP ED50 for seminal vesicle weights were then converted to DHEP and DIHEP ED50s using the respective RPFs. Similarly, the DPEP ED50 for hypospadias was converted to DHEP ED50 using the DHEP RPF. For both effects the slope factors were estimated as the mean slope of the existing phthalate data for each effect.
3. RESULTS
3.1. Fetal testis screen - gene expression and testosterone production
Multiple genes in the fetal testis were significantly downregulated in a dose-dependent manner (Fig. 2A, Table S3). In the top two dose groups 12 genes were significantly affected, including: Cyp11b1, Cyp17a1, Cyp11b2, Star, Scarb1, Cyp11a1, Lhcgr, Insl3, Hsd3b, Inha, Dhcr7, and Nr0b1. Star (critical for conversion of cholesterol to pregnenolone) was the most sensitive gene with significantly lower expression at ≥NOAEL/15 dose. Cyp11b1 (adrenal CYP enzyme that catalyzes conversion of progesterone to cortisol) was the most highly affected gene with expression levels of 1.3% of control in the top dose group. No genes were significantly different from control in the NOAEL/100 and NOAEL/1000 dose groups.
Figure 2.
Effects of in utero exposure to 15 antiandrogen chemical mixture from GD14–18 on GD18 fetal testis. (A) Heatmap of significantly altered genes (ANOVA p<0.001) in fetal testis. All genes were down-regulated and values represent fold-change versus control for significantly (pairwise p<0.05) affected dose levels. (B) Reduction in ex vivo fetal testis testosterone production as percent of control. Data analyzed by ANOVA and asterisks represent statistically significant pairwise difference from controls (*, p<0.05; **, p<0.01; ***, p<0.001; ****, p<0.0001).
Absolute fetal testis testosterone production (ANOVA p<0.0001) and as percent of control (ANOVA p<0.0001) were both reduced at ≥ NOAEL/8 dose, with dramatic reductions in the NOAEL (90.3±4.3% reduction) and NOAELx2 (94.4±2.6% reduction) dose groups compared to controls (Fig. 2B).
3.2. Postnatal reproductive development - perinatal and pubertal effects
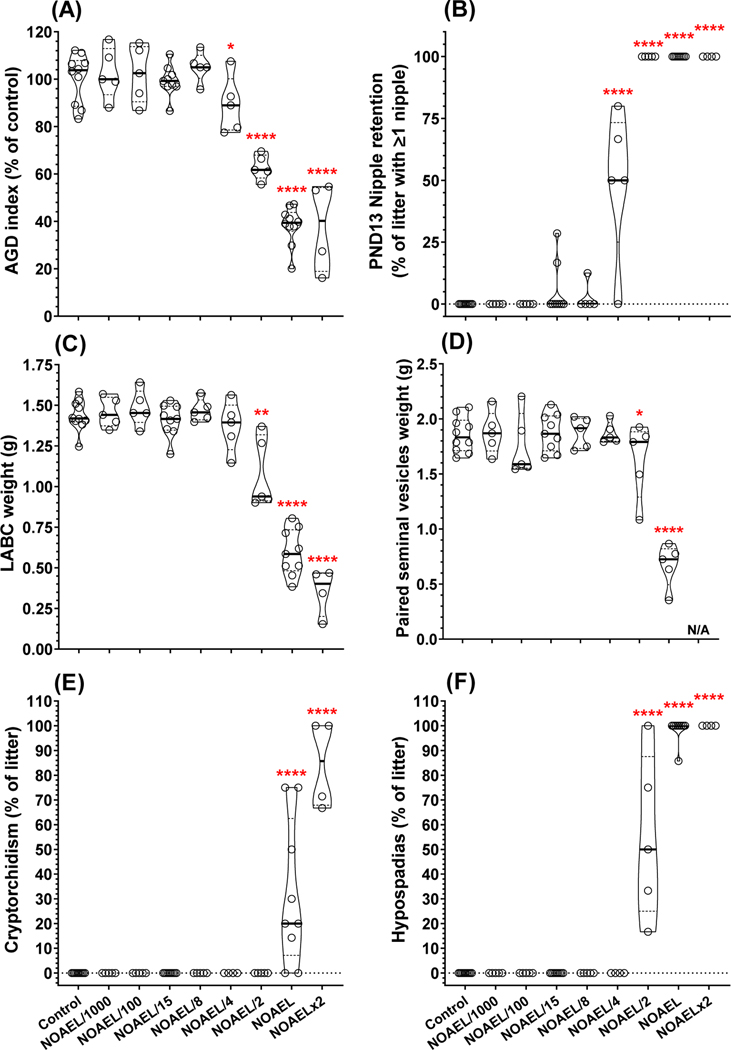
On PND2, male pup BW was reduced (ANOVA p=0.0478) in the NOAELx2 dose group (mean BW 7.06±0.18 g) compared to controls (8.13±0.17 g) (Table S5). Further, mean AGDi was reduced (ANOVA p<0.0001) in the NOAEL/4 – NOAELx2 doses by 11, 37, 62, and 62%, respectively (Fig. 3A). Absolute AGD (using body weight as covariate) was reduced similar to AGDi but the effect at the NOAEL/4 was marginally significant (p=0.08; Table S5). Pups were reevaluated on PND9 and BW was not affected, but AGDi was reduced (ANOVA p<0.0001) in the NOAEL/4 – NOAELx2 doses by 17, 48, 81, and 88%, respectively (Table S5).
Figure 3.
Perinatal and permanent adult effects of in utero exposure to 15 antiandrogen chemical mixture from GD14–18. (A) anogenital distance (AGD) index measured on postnatal day (PND) 2 and normalized to control. AGD index percent reduction based on relative change between control male (100% level) and control female (0% level) AGD index. (B) Female-like nipple/areolae retention in F1 male offspring on PND13. Permanent effects measured in adulthood included, but not limited to, (C) reduced weight of levator ani-bulbocavernosus (LABC) and (D) paired seminal vesicles (N/A in top dose indicates complete agenesis of this tissue in all adult males at this dose). A range of malformations were also identified in adult F1 animals including (E) cryptorchidism (i.e., undescended testis) and (F) hypospadias (i.e., improper location of urethral meatus on glans penis). Data analyzed by ANOVA and asterisks represent statistically significant pairwise difference from controls (*, p<0.05; **, p<0.01; ***, p<0.001; ****, p<0.0001).
On PND13, the number of female-like nipple/areola retained per male (ANOVA p<0.0001), percent of males with ≥1 retained nipple (ANOVA p<0.0001), and percent of maximum nipples retained (ANOVA p<0.0001) were all increased in the NOAEL/4 – NOAELx2 dose groups. The percent of males with ≥1 retained nipple was 49, 100, 100, and 100%, respectively (Fig. 3B). Further, the NOAEL/15 and NOAEL/8 dose groups had non-statistically significant nipple retention in 5.0% (p=0.51) and 2.5% (p=0.81) of males, respectively; while no males in the NOAEL/100, NOAEL/1000 or controls had any retained nipples (Table S5).
Pups were weaned on PND27 and there was no effect of treatment on male BW after correcting for litter size. PPS (marker of male puberty), could not be evaluated in the NOAEL and NOAELx2 dose groups due to external genital malformations in all animals (Table S5). In the remaining dose groups, day of PPS and BW at PPS were analyzed with weaning weight as a covariate and there was a delay in puberty (ANOVA p=0.0299) at ≥NOAEL/4 and increase in BW at puberty (ANOVA p<0.0001) at NOAEL/15. Increased BW was most likely a result of the trend in delayed onset of puberty (Table S5).
3.3. Postnatal reproductive development - permanent/adult postnatal male effects
All androgen-dependent male reproductive tissues displayed significant weight reductions as a result of in utero exposure to the 15-chemical mixture (Table S6, Fig. S2). The most sensitive endpoints were levator ani-bulbocavernosus (LABC, Fig. 3C), paired seminal vesicles (Fig. 3D), ventral prostate, and glans penis weights (Table S6), which all displayed significant reductions at ≥NOAEL/2 dose. The remaining reproductive tissues were significantly reduced in the NOAEL and NOAELx2 dose groups including: paired testes, paired epididymides, and paired Cowper’s glands. In general, the dose response curves for all the androgen-dependent tissues displayed similar ED50 and Hillslope values (Fig. S2). Gubernaculum lengths (both right and left) were also significantly increased in the NOAEL and NOAELx2 dose groups (Table S6). Adult male BW at necropsy significantly increased in a non-monotonic fashion (ANOVA p=0.0011) with males in the NOAEL/8 and NOAEL/4 dose groups (but not NOAEL/2, NOAEL, or NOAELx2) larger than controls (Table S6).
Permanent reproductive tract malformations of the external genitalia and internal sex accessory tissues (not including nipple retention) were elevated (ANOVA p<0.0001) in the NOAEL/2 – NOAELx2 dose groups. Malformations present at ≥NOAEL/2 doses included: cleft prepuce, cleft phallus, hypospadias (Fig. 3F), exposed os penis, vaginal pouch formation, seminal vesicle agenesis, epididymal agenesis, atrophic/fluid-filled testis (Table S6). A few malformations were only observed in the top two dose groups including: vas deferens agenesis, ventral prostate agenesis, and cryptorchidism (Fig. 3E and Table S6). Permanent retention of ≥1 female-like nipples/areolae was elevated (ANOVA p<0.0001) at ≥NOAEL/4 dose (Table S6). The percentage of adult males with one or more permanent reproductive tract malformations (not including nipples) was 6.3, 65, 100, and 100% in the NOAEL/4 – NOAELx2 dose groups, respectively (Table S6). A single NOAEL/4 male had an elongated gubernaculum (25mm vs <10mm in controls) and one control male was found to have an atrophic, fluid-filled left testis, otherwise there were no abnormalities of external genitalia or internal sex accessory tissues in any control males.
Sperm was collected from the adult F1 left epididymis and the corpus/caput and cauda were counted separately (Table S6). All males in the NOAELx2 dose group displayed epididymal agenesis and therefore no sperm could be counted. Cauda sperm counts were reduced in the NOAEL dose group (p=0.0089), corresponding to a 25% reduction compared to control. Corpus/caput sperm counts were reduced in the NOAEL dose group (p=0.0056), with a 22% reduction compared to control. Overall, there was a decrease in total epididymal sperm in the NOAEL dose group (23% reduction, p=0.0036) compared to control (Table S6).
Histopathological evaluation for each adult F1 male included both testes and the right epididymis. Generally, histopathological treatment-related findings were identified in the NOAEL and NOAELx2 dose groups (Table S7). All males from the NOAELx2 dose group displayed epididymal agenesis and therefore had no tissue to evaluate. Only 16 of 46 males from the NOAEL dose group had epididymal tissue to evaluate and these individuals displayed severe hypospermia (9 of 16 samples) and moderate to marked efferent duct dilation (4 of 16 samples). One of 49 controls displayed severe epididymal hypospermia (the male with the small testis), while no other epididymal findings were reported for the NOAEL/1000 – NOAEL/2 dose groups. Microscopic findings in testes included mineralization, fibrosis, interstitial hyperplasia, and atrophy. In the top dose group 14 of 15 samples displayed minimal to moderate interstitial hyperplasia and 15 of 15 samples displayed moderate to severe atrophy. In the NOAEL dose group 3 of 46 displayed minimal or severe mineralization, 3 of 46 displayed mild to severe fibrosis, 33 of 46 displayed minimal to moderate interstitial hyperplasia, and 44 of 46 displayed moderate to severe atrophy. Further, 1 of 20 males in the NOAEL/2 dose group displayed both mild interstitial hyperplasia and moderate atrophy, while 1 of 49 males in the control group displayed severe atrophy (male with small testis mentioned above). Testicular atrophy was typically bilateral, characterized by markedly decreased testicular size compared to controls and degenerative seminiferous tubules that exhibited partial or complete germ cell depletion. For the majority of animals with bilateral testicular atrophy, both testes appeared to be equally affected; however, when the degree of atrophy was more severe in one testis the greatest severity score was recorded (Table S7).
3.4. Maternal and female offspring evaluation
In the fetal testis screen experiments there was a reduction in dam absolute BW gain (ANOVA p=0.0012) and as percent of control (ANOVA p=0.0035) in the NOAELx2 (19.8±9.9 g, 59% of control) and NOAEL (22.5±6.6 g, 58% of control) dose groups compared to controls (39.4±2.2 g); however, across all treatments there were no significant differences in the number per litter of total fetuses, live fetuses, fetal resorptions, or mean dam BW at assay origination (GD14) or termination (GD18) (Table S4).
In the postnatal reproductive development study there was a significant reduction (ANOVA p=0.0024) in dam BW gain in the NOAELx2 (19.0±14.9 g) and NOAEL (21.3±4.3 g) dose groups compared to controls (38.7±1.3 g) (Table S5). One dam each in the NOAEL/15 and NOAELx2 dose groups was not pregnant, but the remaining dams produced similarly sized litters of viable pups (12.5 ± 0.4 pups) as scored on PND2. Dams averaged 14 ± 1 uterine implantation sites with 96.3 ± 1.8% pup viability to PND9, 94.9 ± 2% pup viability to PND13, and 92.4 ± 2.8% pup viability to weaning (PND27) (Table S5). Similar to male pups, female pup BW was significantly lower than control on PND2 in the NOAELx2 dose group, but there was no effect of treatment on female AGD or AGDi on PND2/9 or BW on PND9 (Table S5). There was no effect of treatment on female pup BW on PND13 or female nipple retention. There was no effect of treatment on female weaning weight or timing of vaginal opening (marker of female puberty) (Table S5). Adult F1 females displayed no gross malformations of female reproductive anatomy at necropsy; however, similar to adult F1 males, adult F1 females had higher BW in the NOAEL/4 (342.2±6 g) and NOAEL/8 (363.3±14.1 g) dose groups compared to controls (309.8±6.4 g) (Table S6).
3.5. Estimates of mixture effective doses
Dose response analyses were conducted for selected study endpoints representing a range of mechanistic events (fetal testis STAR gene expression and T production), biomarkers of effect (reduced anogenital distance, increased nipple retention), and overt adverse effects (reduced LABC weight, hypospadias incidence) (Fig. 4). Using 4-parameter logistic regression we estimated the ED10 (i.e., 10% effect level compared to control, modeled as 90% departure point from 100% control response in Fig. 4), 95% confidence intervals, and dose relative to NOAEL for each effect. The range of ED10 values spanned from 1.2% of the top dose (NOAEL/41.7) for STAR gene expression to 17.7% of the top dose (NOAEL/2.8) for hypospadias incidence. Clearly, numerous effects occurred in response to mixture exposure at levels considerably below individual chemical NOAELs.
Figure 4.
Dose response curves for multiple observed effects from in utero exposure to 15 antiandrogen chemical mixture. Effective dose 10% (ED10) levels modeled as 90% departure point from 100% control response. Table presents ED10 levels, 95% confidence intervals, and ED10 % of top dose converted to fold of NOAEL. Nipple retention and hypospadias incidence plotted as inverse relationships. Response curves modeled using sigmoidal four parameter least squares logistic regression. Points slightly offset along x-axis to avoid overlap.
3.6. Effect of co-exposure on individual chemical hypospadias effective dose estimates
Despite acting via multiple MIEs, all compounds in the mixture have previously been experimentally demonstrated to induce hypospadias following in utero exposure, except for DIHEP. To illustrate the impact of chemical co-exposure on individual chemical dose response outcomes we estimated the effect dose 10% (ED10; i.e., 10% incidence in hypospadias) for each chemical in the mixture based on previously published data, then the dose of each chemical at the mixture ED10 was estimated given the fixed ratios from Table 1 (Fig. 5). Individual chemical ED10 values ranged from 27.8 mg/kg for PFQ to 1987 mg/kg for DHEP. The mixture ED10 was 17.7% of the top dose (NOAEL/2.8), which corresponds to each chemical having an ED10 that was between 6.3 – 279.5-fold lower (geometric mean: 25.7-fold lower) in the mixture than in isolation.
Figure 5.
Demonstration of the effect of chemical co-exposure from 15 antiandrogen chemical mixture on individual chemical effective dose estimates. The individual effective dose 10% (ED10), which is a 10% incidence in hypospadias, was estimated for each chemical in the mixture using previously published data (see Fig. S1). Similarly, the ED10 was estimated for the current mixture (see Fig. 4), then the dose of each chemical at the mixture ED10 was estimated given the fixed-ratios from Table 1. Finally, the individual chemical ED10 values were compared to the mixture ED10 values (as fold difference). In general, the effect of chemical co-exposure resulted in hypospadias ED10 levels at least an order of magnitude lower than the ED10 levels for the chemicals in isolation.
3.7. Mixture model analyses
Mixture models form the basis of mixtures toxicity assessment and rely most commonly on three established models: dose addition (DA), response addition (RA), and integrated addition (IA). A prerequisite of model fitting and predictions is appropriate individual chemical parameter data including ED50 and slope. We evaluated mixture model predictions for three effects (AGD reduction, paired seminal vesicle weight, and hypospadias incidence in F1 offspring) using data from individual chemical studies from our lab or the literature, or based on reasonable estimates of ED50 and slope parameters (see Section 2.5 and Table S8). For AGD reduction, DA was the only model that produced an estimated ED50 that fell within the 95% CI of the observed data; however, RA and IA only slightly underestimated mixture potency (Fig. 6A). All models underpredicted the effect of reduced paired seminal vesicle weight, but DA produced the estimate (46%) which was closest to the observed 95% CI (32–38%), with IA underpredicting potency ~2-fold (67%), and RA underpredicted mixture potency by ~4-fold (121%) (Fig. 6B). For incidence of hypospadias, all mixture models underestimated mixture potency, however the DA estimate (29%) was only slightly outside of the observed 95% CI (23–26%), whereas IA and RA underpredicted mixture potency by ~2-fold (52%) and ~8-fold (174%), respectively (Fig. 6C).
Figure 6.
Comparison of observed 15 antiandrogen chemical mixture study data to predictions using dose addition, response addition, and integrated addition mixture models for (A) reduction in PND2 anogenital distance, (B) reduction in adult paired seminal vesicles weight, and (C) increase in incidence of hypospadias measured in F1 adult male rats. Table below figures reports the ED50 and 95% confidence intervals for the observed data and the point estimates for each effect from each mixture model. Response curves modeled using sigmoidal four parameter least squares logistic regression.
Similarly, our a priori predictions of all male reproductive effects (theoretical total mixture dose, Table 1) and severe effects (reproductive malformation index, Table S2) were reasonably accurate compared to the observed data. At the NOAEL/15 dose we only observed significant reduction of a single fetal testis gene but no alterations of any male phenotypic reproductive endpoints; whereas at NOAEL/8 fetal T production was reduced, and at NOAEL/4 AGD was reduced and nipple retention was increased. The RMI approach predicted severe effects to be possible but very low incidence at the NOAEL/4 dose; whereas, we observed significant induction of severe effects at the NOAEL/2 dose. The approach of using ratios between chemical dose and effect level summed across the mixture was effective at informing mixture design and making semi-quantitative predictions of various male reproductive effects.
4. DISCUSSION
We found that a complex mixture of 15 anti-androgenic phthalates and pesticides with at least three different MIEs produced permanent malformations in F1 male reproductive tissues when each chemical was present at a dose below which had been individually shown to produce no adverse male reproductive effects. Adverse outcomes including hypospadias and weight deficits in multiple accessory sex tissues (LABC, seminal vesicles, ventral, prostate, glans penis) occurred at doses 2-fold below individual NOAELs. Delayed puberty and biomarkers of effect including feminized AGD and retention of female-like nipples/areolae occurred at doses 4-fold below NOAELs. Critical key events like reduced fetal testis testosterone production and gene expression, although not universally affected by all mixture chemicals, were affected at doses 8-fold and 15-fold below individual NOAELs, respectively. Chemical co-exposure reduced risk assessment-relevant ED10 estimates for hypospadias incidence ~25-fold, on average, for each chemical, demonstrating the potential importance of cumulative assessment approaches when estimating points-of-departure. Dose addition modeling more accurately predicted the dose response curves for three observed effects (reduced AGD, reduced seminal vesicle weight, and increased hypospadias), whereas response addition and integrated addition mildly or substantially underpredicted mixture potency.
Given the expanding breadth of coverage of chemical space in analytical chemical approaches it is common, if not universal, for multiple compounds to be detected in monitoring studies of drinking water, foods, and/or in human serum and tissues (Bradley et al. 2018; Dixon et al. 2019; Mitro et al. 2015; Vermeulen et al. 2020). The synthetic organic chemical milieu that humans experience is most commonly a complex assortment of persistent, bioaccumulative compounds (e.g., per- and polyfluoroalkyl substances and polychlorinated biphenyls) with long biological half-life, along with compounds that are non-persistent or bioaccumulative, but are commonly utilized and thus frequently detected despite relatively rapid human clearance (e.g., bisphenols and phthalates) (Dereumeaux et al. 2016; Pumarega et al. 2016; Qian et al. 2015). The particular assortments of chemicals, relative ratios, and exposure levels are impacted by variations due to life stage, geographic, cultural, and occupational factors, among others (Vermeulen et al. 2020). Further, there can be aggregate exposure from multiple sources including drinking water, food, dermal contact, and inhalation.
For protection of human health, one challenge is to scientifically determine groupings of environmental chemicals for cumulative risk assessment that toxicologically share an AOP network and could have some expectation of co-exposure in human populations (Kortenkamp 2020). There is some discrepancy as to which compounds should be grouped with phthalates (as well as some discrepancy as to which phthalates should be grouped) for mixtures assessment. Kortenkamp (2020) and Christiansen et al. (2020) have reported on the importance of inclusion of analgesics including paracetamol (i.e., acetaminophen), non-steroidal anti-inflammatory drugs (NSAIDs) including aspirin and ibuprofen, and phenolics including bisphenol A (BPA) and butylparaben. However, research from our group did not identify any effects of in utero BPA exposure on male reproductive developmental outcomes (Howdeshell et al. 2008) nor any effect of paracetamol exposure on fetal testis testosterone production (Gray et al. 2021). Butylparaben was rigorously studied by the National Institute of Environmental Health Sciences, National Toxicology Program and no effects on male reproductive tissues, puberty, or fertility were identified (Hubbard et al. 2020). Further, (Ramhoj et al. 2018) reported antiandrogenic effects of in utero PFHxS exposure, however we recently reported on the developmental toxicity of the emerging PFAS hexafluoropropylene oxide-dimer acid (GenX) and found no effects on fetal testis testosterone production (Conley et al. 2019) or male pup AGD (Conley et al. 2021). Thus, there is considerable debate regarding which compounds are antiandrogenic in utero and should be considered for cumulative assessment; however, there is general agreement regarding the antiandrogenic effects of certain pesticides including those studied here, particularly linuron, prochloraz, procymidone, and vinclozolin, and we argue for the relevance of pyrifluquinazon (Gray et al. 2019), as well. Grouping chemicals that are “toxicologically similar” based on specific chemical classes that share a common specific MIE (e.g., organophosphates) potentially excludes compounds that may be toxicologically relevant for cumulative risk assessment. As has been presented elsewhere for in utero antiandrogens (Christiansen et al. 2020; Conley et al. 2018; Howdeshell et al. 2017; Kortenkamp 2020) and demonstrated here, converging AOPs with distinct MIEs but that share one or more critical key events and/or the ultimate adverse outcomes can be combined into an AOP network that can form the basis for identifying chemicals that are toxicologically similar and adversely affect a common biological system (Boberg et al. 2021; Escher et al. 2017).
The mixture utilized here was not intended to reflect an “environmentally relevant” mixture, but instead to test what has been referred to as the “something from nothing” hypothesis, whereby a mixture of compounds present at doses that “do nothing” produce adverse effects due to cumulative alteration of an AOP network (Silva et al. 2002). The likelihood of human exposure to the specific mixture tested here is very low. However, importantly, biomonitoring data from NHANES indicates that humans are exposed to numerous phthalates with high rates of detection of multiple phthalate metabolites in serum (CDC 2009). The 2013/2014 NHANES dataset, for example, reported 62%, 94%, and 98% detection of urinary metabolites for DEHP, DBP, and BBP, respectively, in women aged 16 to 49 years. Further, the majority of people in the US carry some burden of the DDT metabolite, p,p’-DDE, due to the heavy use of the parent compound decades ago and long human and environmental half-lives (100% detection in 2013/2014 NHANES dataset). The other pesticides included here represent a range of compounds that either are currently listed in registered use in the US (linuron, pyrifluquinazon) (USEPA 2020), were never registered in the US but are registered and used elsewhere globally (procymidone, prochloraz) (PPDB 2020) with EPA tolerance limits for imported products, or are no longer found on the US market (vinclozolin). Thus, co-exposure to multiple phthalates, DDE, and one or more of the pesticides included here is not explicitly unlikely and may occur for some individuals, albeit likely at different exposure levels and relative ratios.
The present study is the second of two studies we designed to examine the effects of fixed-ratio dilutions of defined mixtures of 18 (Conley et al. 2018) and 15 (herein) antiandrogenic chemicals with diverse MIEs at levels well below LOAELs (Conley et al. 2018) or NOAELs to identify BMDs and to determine if any low-dose non-monotonic dose response effects were displayed. Both of these studies specifically expanded on the studies of (Rider et al. 2008; Rider et al. 2010) who tested a 7-chemical mixture (vinclozolin, procymidone, linuron, prochloraz and benzyl butyl, dibutyl, and diethyl hexyl phthalates) and a 10-chemical mixture (7 chemical mixture plus diisobutyl, diisoheptyl, and dipentyl phthalate), respectively. In both studies, dose additive effects on male reproductive tract development were observed when each chemical in the mixture was present at dose levels near the individual chemical NOAELs. In these two low dose studies, in addition to the larger number of chemicals, we structured the top doses upon LOAELs and NOAELs for male reproductive toxicity; whereas, in previous studies (Howdeshell et al. 2015; Howdeshell et al. 2008; Rider et al. 2008; Rider et al. 2010) the chemical mixtures were designed such that each chemical was administered at equipotent levels and therefore would contribute to the effects equally if the mixture behaved in a dose-additive manner. We included two very low doses (i.e., NOAEL/100 and NOAEL/1000) due to widespread reports of very low dose non-monotonic effects of endocrine disruptors (Vandenberg et al. 2012); however, there was no difference in any measured endpoint for these lower doses as compared to control and response curves were monotonic. Overall, numerous laboratory studies have now demonstrated that in utero exposure to chemicals that disrupt androgen signaling via common and diverse mechanisms of toxicity produce cumulative effects on male reproductive tract development and that the doses of individual chemicals needed to produce effects are inversely related to the number of chemicals in the mixture (Axelstad et al. 2014; Axelstad et al. 2018; Beverly et al. 2014; Christiansen et al. 2012; Christiansen et al. 2008; Christiansen et al. 2009; Conley et al. 2018; Hannas et al. 2011; Hass et al. 2012; Hass et al. 2007; Hotchkiss et al. 2004; Hotchkiss et al. 2010; Howdeshell et al. 2007; Howdeshell et al. 2015; Howdeshell et al. 2008; Isling et al. 2014; Jacobsen et al. 2010; Metzdorff et al. 2007; Ramhoj et al. 2018; Rider et al. 2008; Rider et al. 2010; Rider et al. 2009; Schneider et al. 2017).
The dose addition modeling approach has received broad support as the most appropriate default model for estimating mixture effects of anti-androgens in a component-based approach (EFSA 2019; European Commission 2012; NRC 2008). As depicted here, the three established mixture models produce similar dose response curve predictions when the target-effect Hill slope parameter is ~1, such as with AGD reduction (Fig. 6A). Endpoints that are reported as percent incidence (e.g., hypospadias, presence of female-like nipples/areolae) or organ weight reductions frequently display steep Hill slope parameters (e.g., ~5–10) and have disparate dose addition, response addition, and integrated addition mixture model predictions (Fig. 6B,C), with the dose addition model being the most accurate in predicting the observed ED50.
It is widely recognized that exposure to multiple chemicals, not just one chemical at a time, may present a risk to human health. Based on the results of this and other mixtures studies, there is growing support for the idea that cumulative assessment groups need to include all chemicals that produce common adverse effects in an assessment of chemical risk and not narrowly limited to those with identical modes of action. Clearly, adverse effects can occur at exposure levels below either individual chemical effect levels (i.e., LOAEL, NOAEL) or effect levels (i.e., ED10) when mixed with additional compounds that share key events in an AOP network.
Supplementary Material
ACKNOWLEGEMENTS
We would like to thank Drs. Anthony Luz (USEPA/OPPT), Cynthia Rider (NIEHS/DNTP), and Erin Yost (USEPA/ORD) for reviewing and providing comments on prior drafts. The manuscript has been subjected to review by the USEPA Center for Public Health and Environmental Assessment and approved for publication, but the views expressed do not necessarily reflect the views or policy of the USEPA.
FUNDING INFORMATION
This work was supported by the U.S. Environmental Protection Agency Chemical Safety for Sustainability Research Action Program under the Adverse Outcome Pathway Discovery and Development task.
ABBREVIATIONS:
- NOAEL
No observed adverse effect level
- AOP
Adverse outcome pathway
- MIE
Molecular initiating event
- AR
Androgen receptor
- T
Testosterone
- AGD
Anogenital distance
- GD
Gestation day
- PND
Postnatal day
- LOAEL
Lowest observed adverse effect level
- RMI
Reproductive malformation index
- DA
Dose addition
- RA
Response addition
- IA
Integrated addition
- ED
Effective dose
Footnotes
SUPPLEMENTAL DATA
Includes summary data for all study endpoints and figures, complete assortment of fetal testis genes investigated, histopathology lesion incidence, data sources and effect estimates for mixture model endpoints (AGD, SV weight, hypospadias).
REFERENCES
- Ankley GT, Bennett RS, Erickson RJ, Hoff DJ, Hornung MW, Johnson RD, Mount DR, Nichols JW, Russom CL, Schmieder PK, Serrrano JA, Tietge JE and Villeneuve DL (2010). “Adverse outcome pathways: a conceptual framework to support ecotoxicology research and risk assessment.” Environ Toxicol Chem 29(3): 730–741. doi: 10.1002/etc.34. [DOI] [PubMed] [Google Scholar]
- Axelstad M, Christiansen S, Boberg J, Scholze M, Jacobsen PR, Isling LK, Kortenkamp A. and Hass U. (2014). “Mixtures of endocrine-disrupting contaminants induce adverse developmental effects in preweaning rats.” Reproduction 147(4): 489–501. doi: 10.1530/REP-13-0447. [DOI] [PubMed] [Google Scholar]
- Axelstad M, Hass U, Scholze M, Christiansen S, Kortenkamp A. and Boberg J. (2018). “EDC IMPACT: Reduced sperm counts in rats exposed to human relevant mixtures of endocrine disrupters.” Endocr Connect 7(1): 139–148. doi: 10.1530/EC-17-0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beverly BE, Lambright CS, Furr JR, Sampson H, Wilson VS, McIntyre BS, Foster PM, Travlos G. and Gray LE Jr. (2014). “Simvastatin and dipentyl phthalate lower ex vivo testicular testosterone production and exhibit additive effects on testicular testosterone and gene expression via distinct mechanistic pathways in the fetal rat.” Toxicol Sci 141(2): 524–537. doi: 10.1093/toxsci/kfu149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boberg J, Bredsdorff L, Petersen A, Lobl N, Jensen BH, Vinggaard AM and Nielsen E. (2021). “Chemical Mixture Calculator - A novel tool for mixture risk assessment.” Food Chem Toxicol: 112167. doi: 10.1016/j.fct.2021.112167. [DOI] [PubMed] [Google Scholar]
- Bradley PM, Kolpin DW, Romanok KM, Smalling KL, Focazio MJ, Brown JB, Cardon MC, Carpenter KD, Corsi SR, DeCicco LA, Dietze JE, Evans N, Furlong ET, Givens CE, Gray JL, Griffin DW, Higgins CP, Hladik ML, Iwanowicz LR, Journey CA, Kuivila KM, Masoner JR, McDonough CA, Meyer MT, Orlando JL, Strynar MJ, Weis CP and Wilson VS (2018). “Reconnaissance of Mixed Organic and Inorganic Chemicals in Private and Public Supply Tapwaters at Selected Residential and Workplace Sites in the United States.” Environ Sci Technol 52(23): 13972–13985. doi: 10.1021/acs.est.8b04622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael SL, Shaw GM and Lammer EJ (2012). “Environmental and genetic contributors to hypospadias: a review of the epidemiologic evidence.” Birth Defects Res A Clin Mol Teratol 94(7): 499–510. doi: 10.1002/bdra.23021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC (2009). “Fourth national report on human exposure to environmental chemicals.” Department of Health and Human Services. [Google Scholar]
- Christiansen S, Axelstad M, Scholze M, Johansson HKL, Hass U, Mandrup K, Frandsen HL, Frederiksen H, Isling LK and Boberg J. (2020). “Grouping of endocrine disrupting chemicals for mixture risk assessment - Evidence from a rat study.” Environ Int 142: 105870. doi: 10.1016/j.envint.2020.105870. [DOI] [PubMed] [Google Scholar]
- Christiansen S, Kortenkamp A, Axelstad M, Boberg J, Scholze M, Jacobsen PR, Faust M, Lichtensteiger W, Schlumpf M, Burdorf A. and Hass U. (2012). “Mixtures of endocrine disrupting contaminants modelled on human high end exposures: an exploratory study in rats.” Int J Androl 35(3): 303–316. doi: 10.1111/j.1365-2605.2011.01242.x. [DOI] [PubMed] [Google Scholar]
- Christiansen S, Scholze M, Axelstad M, Boberg J, Kortenkamp A. and Hass U. (2008). “Combined exposure to anti-androgens causes markedly increased frequencies of hypospadias in the rat.” Int J Androl 31(2): 241–248. doi: 10.1111/j.1365-2605.2008.00866.x. [DOI] [PubMed] [Google Scholar]
- Christiansen S, Scholze M, Dalgaard M, Vinggaard AM, Axelstad M, Kortenkamp A. and Hass U. (2009). “Synergistic disruption of external male sex organ development by a mixture of four antiandrogens.” Environ Health Perspect 117(12): 1839–1846. doi: 10.1289/ehp.0900689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conley JM, Lambright CS, Evans N, Cardon M, Furr J, Wilson VS and Gray LE Jr. (2018). “Mixed “Antiandrogenic” Chemicals at Low Individual Doses Produce Reproductive Tract Malformations in the Male Rat.” Toxicol Sci 164(1): 166–178. doi: 10.1093/toxsci/kfy069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conley JM, Lambright CS, Evans N, McCord J, Strynar MJ, Hill D, Medlock-Kakaley E, Wilson VS and Gray LE Jr. (2021). “Hexafluoropropylene oxide-dimer acid (HFPO-DA or GenX) alters maternal and fetal glucose and lipid metabolism and produces neonatal mortality, low birthweight, and hepatomegaly in the Sprague-Dawley rat.” Environ Int 146: 106204. doi: 10.1016/j.envint.2020.106204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conley JM, Lambright CS, Evans N, Strynar MJ, McCord J, McIntyre BS, Travlos GS, Cardon MC, Medlock-Kakaley E, Hartig PC, Wilson VS and Gray LE Jr. (2019). “Adverse Maternal, Fetal, and Postnatal Effects of Hexafluoropropylene Oxide Dimer Acid (GenX) from Oral Gestational Exposure in Sprague-Dawley Rats.” Environ Health Perspect 127(3): 37008. doi: 10.1289/EHP4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dereumeaux C, Saoudi A, Pecheux M, Berat B, de Crouy-Chanel P, Zaros C, Brunel S, Delamaire C, le Tertre A, Lefranc A, Vandentorren S. and Guldner L. (2016). “Biomarkers of exposure to environmental contaminants in French pregnant women from the Elfe cohort in 2011.” Environ Int 97: 56–67. doi: 10.1016/j.envint.2016.10.013. [DOI] [PubMed] [Google Scholar]
- Dixon HM, Armstrong G, Barton M, Bergmann AJ, Bondy M, Halbleib ML, Hamilton W, Haynes E, Herbstman J, Hoffman P, Jepson P, Kile ML, Kincl L, Laurienti PJ, North P, Paulik LB, Petrosino J, Points GL 3rd, Poutasse CM, Rohlman D, Scott RP, Smith B, Tidwell LG, Walker C, Waters KM and Anderson KA (2019). “Discovery of common chemical exposures across three continents using silicone wristbands.” R Soc Open Sci 6(2): 181836. doi: 10.1098/rsos.181836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorman DC, Chiu W, Hales BF, Hauser R, Johnson KJ, Mantus E, Martel S, Robinson KA, Rooney AA, Rudel R, Sathyanarayana S, Schantz SL and Waters KM (2018). “Systematic reviews and meta-analyses of human and animal evidence of prenatal diethylhexyl phthalate exposure and changes in male anogenital distance.” J Toxicol Environ Health B Crit Rev 21(4): 207–226. doi: 10.1080/10937404.2018.1505354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (2019). “Guidance on harmonised methodologies for human health, animal health and ecological risk assessment of combined exposure to multiple chemicals.” EFSA J 17(3): e05634. doi: 10.2903/j.efsa.2019.5634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escher BI, Hackermuller J, Polte T, Scholz S, Aigner A, Altenburger R, Bohme A, Bopp SK, Brack W, Busch W, Chadeau-Hyam M, Covaci A, Eisentrager A, Galligan JJ, Garcia-Reyero N, Hartung T, Hein M, Herberth G, Jahnke A, Kleinjans J, Kluver N, Krauss M, Lamoree M, Lehmann I, Luckenbach T, Miller GW, Muller A, Phillips DH, Reemtsma T, Rolle-Kampczyk U, Schuurmann G, Schwikowski B, Tan YM, Trump S, Walter-Rohde S. and Wambaugh JF (2017). “From the exposome to mechanistic understanding of chemical-induced adverse effects.” Environ Int 99: 97–106. doi: 10.1016/j.envint.2016.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Commission (2012). SCHER, SCCS, SCENIHR, Opinion on the Toxicity and Assessment of Chemical Mixtures. doi: 10.2772/21444. [DOI] [Google Scholar]
- Flick B, Schneider S, Melching-Kollmuss S, Fussell KC, Groters S, Buesen R, Strauss V. and van Ravenzwaay B. (2017). “Investigations of putative reproductive toxicity of low-dose exposures to vinclozolin in Wistar rats.” Arch Toxicol 91(4): 1941–1956. doi: 10.1007/s00204-016-1811-y. [DOI] [PubMed] [Google Scholar]
- Furr JR, Lambright CS, Wilson VS, Foster PM and Gray LE Jr. (2014). “A short-term in vivo screen using fetal testosterone production, a key event in the phthalate adverse outcome pathway, to predict disruption of sexual differentiation.” Toxicol Sci 140(2): 403–424. doi: 10.1093/toxsci/kfu081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Villarino M, Riano-Galan I, Rodriguez-Dehli AC, Freire C, Vizcaino E, Grimalt JO, Tardon A. and Fernandez-Somoano A. (2020). “Association between pre/perinatal exposure to POPs and children’s anogenital distance at age 4 years: A study from the INMA-Asturias cohort.” Int J Hyg Environ Health 229: 113563. doi: 10.1016/j.ijheh.2020.113563. [DOI] [PubMed] [Google Scholar]
- Gray LE, Furr JR, Conley JM, Lambright CS, Evans N, Cardon MC, Wilson VS, Foster PM and Hartig PC (2019). “A Conflicted Tale of Two Novel AR Antagonists In Vitro and In Vivo: Pyrifluquinazon Versus Bisphenol C.” Toxicol Sci 168(2): 632–643. doi: 10.1093/toxsci/kfz010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray LE Jr., Barlow NJ, Howdeshell KL, Ostby JS, Furr JR and Gray CL (2009). “Transgenerational effects of Di (2-ethylhexyl) phthalate in the male CRL:CD(SD) rat: added value of assessing multiple offspring per litter.” Toxicol Sci 110(2): 411–425. doi: 10.1093/toxsci/kfp109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray LE Jr., Ostby J, Monosson E. and Kelce WR (1999). “Environmental antiandrogens: low doses of the fungicide vinclozolin alter sexual differentiation of the male rat.” Toxicology and Industrial Health 15: 48–64. [DOI] [PubMed] [Google Scholar]
- Gray LE Jr., Wilson VS, Stoker T, Lambright C, Furr J, Noriega N, Howdeshell K, Ankley GT and Guillette L. (2006). “Adverse effects of environmental antiandrogens and androgens on reproductive development in mammals.” Int J Androl 29(1): 96–104; discussion 105–108. doi: 10.1111/j.1365-2605.2005.00636.x. [DOI] [PubMed] [Google Scholar]
- Gray LE, Lambright C, Conley JM, Evans N, Furr J, Hannas BR, Wilson VS and Foster PM (2021). “Genomic and Endocrine Biomarkers of Phthalate-Induced Male Rat Reproductive Developmental Toxicity Part II: A Targeted RT-qPCR Array Approach that Defines a Unique Adverse Outcome Pathway.” Toxicol Sci Accepted [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray LE, Ostby JS and Kelce WR (1994). “Developmental effects of an environmental antiandrogen: the fungicide vinclozolin alters sex differentiation of the male rat.” Toxicol Appl Pharmacol 129: 46–52. [DOI] [PubMed] [Google Scholar]
- Hannas BR, Lambright CS, Furr J, Evans N, Foster PM, Gray EL and Wilson VS (2012). “Genomic biomarkers of phthalate-induced male reproductive developmental toxicity: a targeted RT-PCR array approach for defining relative potency.” Toxicol Sci 125(2): 544–557. doi: 10.1093/toxsci/kfr315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannas BR, Lambright CS, Furr J, Howdeshell KL, Wilson VS and Gray LE Jr. (2011). “Dose-response assessment of fetal testosterone production and gene expression levels in rat testes following in utero exposure to diethylhexyl phthalate, diisobutyl phthalate, diisoheptyl phthalate, and diisononyl phthalate.” Toxicol Sci 123(1): 206–216. doi: 10.1093/toxsci/kfr146. [DOI] [PubMed] [Google Scholar]
- Hass U, Boberg J, Christiansen S, Jacobsen PR, Vinggaard AM, Taxvig C, Poulsen ME, Herrmann SS, Jensen BH, Petersen A, Clemmensen LH and Axelstad M. (2012). “Adverse effects on sexual development in rat offspring after low dose exposure to a mixture of endocrine disrupting pesticides.” Reprod Toxicol 34(2): 261–274. doi: 10.1016/j.reprotox.2012.05.090. [DOI] [PubMed] [Google Scholar]
- Hass U, Scholze M, Christiansen S, Dalgaard M, Vinggaard AM, Axelstad M, Metzdorff SB and Kortenkamp A. (2007). “Combined exposure to anti-androgens exacerbates disruption of sexual differentiation in the rat.” Environ Health Perspect 115 Suppl 1: 122–128. doi: 10.1289/ehp.9360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellwig J, van Ravenzwaay B, Mayer M. and Gembardt C. (2000). “Pre- and postnatal oral toxicity of vinclozolin in Wistar and Long-Evans rats.” Regul Toxicol Pharmacol 32(1): 42–50. doi: 10.1006/rtph.2000.1400. [DOI] [PubMed] [Google Scholar]
- Hotchkiss AK, Parks-Saldutti LG, Ostby JS, Lambright C, Furr J, Vandenbergh JG and Gray LE Jr. (2004). “A mixture of the “antiandrogens” linuron and butyl benzyl phthalate alters sexual differentiation of the male rat in a cumulative fashion.” Biol Reprod 71(6): 1852–1861. doi: 10.1095/biolreprod.104.031674. [DOI] [PubMed] [Google Scholar]
- Hotchkiss AK, Rider CV, Furr J, Howdeshell KL, Blystone CR, Wilson VS and Gray LE Jr. (2010). “In utero exposure to an AR antagonist plus an inhibitor of fetal testosterone synthesis induces cumulative effects on F1 male rats.” Reprod Toxicol 30(2): 261–270. doi: 10.1016/j.reprotox.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howdeshell KL, Furr J, Lambright CR, Rider CV, Wilson VS and Gray LE Jr. (2007). “Cumulative effects of dibutyl phthalate and diethylhexyl phthalate on male rat reproductive tract development: altered fetal steroid hormones and genes.” Toxicol Sci 99(1): 190–202. doi: 10.1093/toxsci/kfm069. [DOI] [PubMed] [Google Scholar]
- Howdeshell KL, Furr J, Lambright CR, Wilson VS, Ryan BC and Gray LE Jr. (2008). “Gestational and lactational exposure to ethinyl estradiol, but not bisphenol A, decreases androgen-dependent reproductive organ weights and epididymal sperm abundance in the male long evans hooded rat.” Toxicol Sci 102(2): 371–382. doi: 10.1093/toxsci/kfm306. [DOI] [PubMed] [Google Scholar]
- Howdeshell KL, Hotchkiss AK and Gray LE Jr. (2017). “Cumulative effects of antiandrogenic chemical mixtures and their relevance to human health risk assessment.” Int J Hyg Environ Health 220(2 Pt A): 179–188. doi: 10.1016/j.ijheh.2016.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howdeshell KL, Rider CV, Wilson VS, Furr JR, Lambright CR and Gray LE Jr. (2015). “Dose Addition Models Based on Biologically Relevant Reductions in Fetal Testosterone Accurately Predict Postnatal Reproductive Tract Alterations by a Phthalate Mixture in Rats.” Toxicol Sci 148(2): 488–502. doi: 10.1093/toxsci/kfv196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howdeshell KL, Wilson VS, Furr J, Lambright CR, Rider CV, Blystone CR, Hotchkiss AK and Gray LE Jr. (2008). “A mixture of five phthalate esters inhibits fetal testicular testosterone production in the sprague-dawley rat in a cumulative, dose-additive manner.” Toxicol Sci 105(1): 153–165. doi: 10.1093/toxsci/kfn077. [DOI] [PubMed] [Google Scholar]
- Hubbard TD, Brix A, Blystone CR, McIntyre BS, Shockley K, Cunny H, Waidyanatha S, Turner KJ, McBride S. and Roberts GK (2020). “Butylparaben multigenerational reproductive assessment by continuous breeding in Hsd:Sprague Dawley SD rats following dietary exposure.” Reprod Toxicol 96: 258–272. doi: 10.1016/j.reprotox.2020.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isling LK, Boberg J, Jacobsen PR, Mandrup KR, Axelstad M, Christiansen S, Vinggaard AM, Taxvig C, Kortenkamp A. and Hass U. (2014). “Late-life effects on rat reproductive system after developmental exposure to mixtures of endocrine disrupters.” Reproduction 147(4): 465–476. doi: 10.1530/REP-13-0448. [DOI] [PubMed] [Google Scholar]
- Jacobsen PR, Christiansen S, Boberg J, Nellemann C. and Hass U. (2010). “Combined exposure to endocrine disrupting pesticides impairs parturition, causes pup mortality and affects sexual differentiation in rats.” Int J Androl 33(2): 434–442. doi: 10.1111/j.1365-2605.2009.01046.x. [DOI] [PubMed] [Google Scholar]
- Kortenkamp A. (2020). “Which chemicals should be grouped together for mixture risk assessments of male reproductive disorders?” Mol Cell Endocrinol 499: 110581. doi: 10.1016/j.mce.2019.110581. [DOI] [PubMed] [Google Scholar]
- Kortenkamp A. and Faust M. (2018). “Regulate to reduce chemical mixture risk.” Science 361(6399): 224–226. doi: 10.1126/science.aat9219. [DOI] [PubMed] [Google Scholar]
- Lambright C, Ostby J, Bobseine K, Wilson VS, Hotchkiss AK, Mann PC and Gray J, L. E. (2000). “Cellular and molecular mechanisms of action of linuron: An antiandrogenic herbicide that produces reproductive malformations in male rats.” Toxicol Sci 56: 389–399. [DOI] [PubMed] [Google Scholar]
- Levine H, Jørgensen N, Martino-Andrade A, Mendiola J, Weksler-Derri D, Mindlis I, Pinotti R. and Swan SH (2017). “Temporal trends in sperm count: a systematic review and meta-regression analysis.” Human Reproduction Update: 1–14. doi: 10.1093/humupd/dmx022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mai CT, Isenburg J, Langlois PH, Alverson CJ, Gilboa SM, Rickard R, Canfield MA, Anjohrin SB, Lupo PJ, Jackson DR, Stallings EB, Scheuerle AE and Kirby RS (2015). “Population-based birth defects data in the United States, 2008 to 2012: Presentation of state-specific data and descriptive brief on variability of prevalence.” Birth Defects Res A Clin Mol Teratol 103(11): 972–993. doi: 10.1002/bdra.23461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre BS, Barlow NJ, Wallace DG, Maness SC, Gaido KW and Foster PM (2000). “Effects of in utero exposure to linuron on androgen-dependent reproductive development in the male Crl:CD(SD)BR rat.” Toxicol Appl Pharmacol 167(2): 87–99. doi: 10.1006/taap.2000.8998. [DOI] [PubMed] [Google Scholar]
- Metzdorff SB, Dalgaard M, Christiansen S, Axelstad M, Hass U, Kiersgaard MK, Scholze M, Kortenkamp A. and Vinggaard AM (2007). “Dysgenesis and histological changes of genitals and perturbations of gene expression in male rats after in utero exposure to antiandrogen mixtures.” Toxicol Sci 98(1): 87–98. doi: 10.1093/toxsci/kfm079. [DOI] [PubMed] [Google Scholar]
- Mitro SD, Johnson T. and Zota AR (2015). “Cumulative Chemical Exposures During Pregnancy and Early Development.” Curr Environ Health Rep 2(4): 367–378. doi: 10.1007/s40572-015-0064-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monosson E. (2005). “Chemical mixtures: considering the evolution of toxicology and chemical assessment.” Environ Health Perspect 113(4): 383–390. doi: 10.1289/ehp.6987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigam M, Aschebrook-Kilfoy B, Shikanov S. and Eggener S. (2015). “Increasing incidence of testicular cancer in the United States and Europe between 1992 and 2009.” World J Urol 33(5): 623–631. doi: 10.1007/s00345-014-1361-y. [DOI] [PubMed] [Google Scholar]
- NRC (2008). Committee on the Health Risks of Phthalates - Phthalates and cumulative risk assessment: The task ahead. Washington, D.C., The National Academies Press. [PubMed] [Google Scholar]
- Parks LG, Ostby JS, Lambright CR, Abbott BD, Klinefelter GR, Barlow NJ and Gray LE (2000). “The plasticizer diethylhexyl phthalate induces malformations by decreasing fetal testosterone synthesis during sexual differentiation in the male rat.” Toxicol Sci 58: 339–349. [DOI] [PubMed] [Google Scholar]
- Paulozzi LJ (1999). “International trends in the rates of hypospadias and cryptorchidism.” Environ Health Perspect 107: 297–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulozzi LJ, Erickson JD and Jackson RJ (1997). “Hypospadias trends in two US surveillance systems.” Pediatrics 100(5): 831–834. [DOI] [PubMed] [Google Scholar]
- PPDB (2020). “Pesticide Properties Database.” Online available - Accessed December 17, 2020 http://sitem.herts.ac.uk/aeru/ppdb/en/index.htm.
- Pumarega J, Gasull M, Lee DH, Lopez T. and Porta M. (2016). “Number of Persistent Organic Pollutants Detected at High Concentrations in Blood Samples of the United States Population.” PLoS One 11(8): e0160432. doi: 10.1371/journal.pone.0160432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian H, Chen M, Kransler KM and Zaleski RT (2015). “Assessment of chemical coexposure patterns based upon phthalate biomonitoring data within the 2007/2008 National Health and Nutrition Examination Survey.” J Expo Sci Environ Epidemiol 25(3): 249–255. doi: 10.1038/jes.2014.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramhoj L, Hass U, Boberg J, Scholze M, Christiansen S, Nielsen F. and Axelstad M. (2018). “Perfluorohexane Sulfonate (PFHxS) and a Mixture of Endocrine Disrupters Reduce Thyroxine Levels and Cause Antiandrogenic Effects in Rats.” Toxicol Sci 163(2): 579–591. doi: 10.1093/toxsci/kfy055. [DOI] [PubMed] [Google Scholar]
- Rider CV, Furr J, Wilson VS and Gray LE Jr. (2008). “A mixture of seven antiandrogens induces reproductive malformations in rats.” Int J Androl 31(2): 249–262. doi: 10.1111/j.1365-2605.2007.00859.x. [DOI] [PubMed] [Google Scholar]
- Rider CV, Furr JR, Wilson VS and Gray LE Jr. (2010). “Cumulative effects of in utero administration of mixtures of reproductive toxicants that disrupt common target tissues via diverse mechanisms of toxicity.” Int J Androl 33(2): 443–462. doi: 10.1111/j.1365-2605.2009.01049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rider CV and LeBlanc GA (2005). “An integrated addition and interaction model for assessing toxicity of chemical mixtures.” Toxicol. Sci 87(2): 520–528. doi: 10.1093/toxsci/kfi247. [DOI] [PubMed] [Google Scholar]
- Rider CV, Wilson VS, Howdeshell KL, Hotchkiss AK, Furr JR, Lambright CR and Gray LE Jr. (2009). “Cumulative effects of in utero administration of mixtures of “antiandrogens” on male rat reproductive development.” Toxicol Pathol 37(1): 100–113. doi: 10.1177/0192623308329478. [DOI] [PubMed] [Google Scholar]
- Schneider S, Fussell KC, Melching-Kollmuss S, Buesen R, Groters S, Strauss V, Jiang X. and van Ravenzwaay B. (2017). “Investigations on the dose-response relationship of combined exposure to low doses of three anti-androgens in Wistar rats.” Arch Toxicol. doi: 10.1007/s00204-017-2053-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider S, Kaufmann W, Strauss V. and van Ravenzwaay B. (2011). “Vinclozolin: a feasibility and sensitivity study of the ILSI-HESI F1-extended one-generation rat reproduction protocol.” Regul Toxicol Pharmacol 59(1): 91–100. doi: 10.1016/j.yrtph.2010.09.010. [DOI] [PubMed] [Google Scholar]
- Schwartz CL, Christiansen S, Vinggaard AM, Axelstad M, Hass U. and Svingen T. (2019). “Anogenital distance as a toxicological or clinical marker for fetal androgen action and risk for reproductive disorders.” Arch Toxicol 93(2): 253–272. doi: 10.1007/s00204-018-2350-5. [DOI] [PubMed] [Google Scholar]
- Silva E, Rajapakse N. and Kortenkamp A. (2002). “Something from “nothing” - eight weak estrogenic chemicals combined at concentrations below NOECs produce significant mixture effects.” Environ Sci Technol 36: 1751–1756. doi: 10.1021/es0101227. [DOI] [PubMed] [Google Scholar]
- Skakkebaek NE (2017). “Sperm counts, testicular cancers, and the environment.” BMJ 359: j4517. doi: 10.1136/bmj.j4517. [DOI] [PubMed] [Google Scholar]
- Skakkebaek NE, Rajpert-De Meyts E, Buck Louis GM, Toppari J, Andersson AM, Eisenberg ML, Jensen TK, Jorgensen N, Swan SH, Sapra KJ, Ziebe S, Priskorn L. and Juul A. (2016). “Male Reproductive Disorders and Fertility Trends: Influences of Environment and Genetic Susceptibility.” Physiol Rev 96(1): 55–97. doi: 10.1152/physrev.00017.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thonneau PF, Candia P. and Mieusset R. (2003). “Cryptorchidism: Incidence, risk factors, and potential role of environment; an update.” J Androl 24(2): 155–162. [DOI] [PubMed] [Google Scholar]
- Toppari J, Kaleva M. and Virtanen HE (2001). “Trends in the incidence of cryptorchidism and hypospadias, and methodological limitations of registry-based data.” Human Reprod Update 7(3): 282–286. [DOI] [PubMed] [Google Scholar]
- USEPA (2000). “Registration Eligibility Decision (RED): Vinclozolin.” EPA 738-R-00–023. [Google Scholar]
- USEPA (2020). “Pesticide Product and Label System.” Online available - Accessed December 17, 2020 https://www.epa.gov/safepestcontrol/search-registered-pesticide-products.
- van der Horst HJ and de Wall LL (2017). “Hypospadias, all there is to know.” Eur J Pediatr 176(4): 435–441. doi: 10.1007/s00431-017-2864-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg LN, Colborn T, Hayes TB, Heindel JJ, Jacobs DR Jr., Lee DH, Shioda T, Soto AM, vom Saal FS, Welshons WV, Zoeller RT and Myers JP (2012). “Hormones and endocrine-disrupting chemicals: low-dose effects and nonmonotonic dose responses.” Endocr Rev 33(3): 378–455. doi: 10.1210/er.2011-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeulen R, Schymanski EL, Barabasi A. and Miller GW (2020). “The exposome and health: Where chemistry meets biology.” Science 367(6476): 392–396. doi: 10.1126/science.aay3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson VS, Blystone CR, Hotchkiss AK, Rider CV and Gray LE Jr. (2008). “Diverse mechanisms of anti-androgen action: impact on male rat reproductive tract development.” Int J Androl 31(2): 178–187. doi: 10.1111/j.1365-2605.2007.00861.x. [DOI] [PubMed] [Google Scholar]
- Wilson VS, Lambright C, Furr J, Ostby J, Wood C, Held G. and Gray LE (2004). “Phthalate esterinduced gubernacular lesions are associated with reduced insl3 gene expression in the fetal rat testis.” Toxicology Letters 146(3): 207–215. doi: 10.1016/j.toxlet.2003.09.012. [DOI] [PubMed] [Google Scholar]
- Wilson VS, Lambright CR, Furr JR, Howdeshell KL and Earl Gray L Jr. (2009). “The herbicide linuron reduces testosterone production from the fetal rat testis during both in utero and in vitro exposures.” Toxicol Lett 186(2): 73–77. doi: 10.1016/j.toxlet.2008.12.017. [DOI] [PubMed] [Google Scholar]
- Yu X, Nassar N, Mastroiacovo P, Canfield M, Groisman B, Bermejo-Sanchez E, Ritvanen A, Kiuru-Kuhlefelt S, Benavides A, Sipek A, Pierini A, Bianchi F, Kallen K, Gatt M, Morgan M, Tucker D, Canessa MA, Gajardo R, Mutchinick OM, Szabova E, Csaky-Szunyogh M, Tagliabue G, Cragan JD, Nembhard WN, Rissmann A, Goetz D, Bower C, Baynam G, Lowry RB, Leon JA, Luo W, Rouleau J, Zarante I, Fernandez N, Amar E, Dastgiri S, Contiero P, Martinez-de-Villarreal LE, Borman B, Bergman JEH, de Walle HEK, Hobbs CA, Nance AE and Agopian AJ (2019). “Hypospadias Prevalence and Trends in International Birth Defect Surveillance Systems, 1980–2010.” Eur Urol 76(4): 482–490. doi: 10.1016/j.eururo.2019.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.