Abstract
Background:
Nicotine withdrawal syndrome is a major clinical problem. Animal models with sufficient predictive validity to support translation of pre-clinical findings to clinical research are lacking.
Aims:
We evaluated the behavioural and neurochemical alterations in zebrafish induced by short- and long-term nicotine withdrawal.
Methods:
Zebrafish were exposed to 1 mg/L nicotine for 2 weeks. Dependence was determined using behavioural analysis following mecamylamine-induced withdrawal, and brain nicotinic receptor binding studies. Separate groups of nicotine-exposed and control fish were assessed for anxiety-like behaviours, anhedonia and memory deficits following 2–60 days spontaneous withdrawal. Gene expression analysis using whole brain samples from nicotine-treated and control fish was performed at 7 and 60 days after the last drug exposure. Tyrosine hydroxylase (TH) immunoreactivity in pretectum was also analyzed.
Results:
Mecamylamine-precipitated withdrawal nicotine-exposed fish showed increased anxiety-like behaviour as evidenced by increased freezing and decreased exploration. 3H-Epibatidine labeled heteromeric nicotinic acethylcholine receptors (nAChR) significantly increased after 2 weeks of nicotine exposure while 125I-αBungarotoxin labelled homomeric nAChR remained unchanged. Spontaneous nicotine withdrawal elicited anxiety-like behaviour (increased bottom dwelling), reduced motivation in terms of no preference for the enriched side in a place preference test starting from Day 7 after withdrawal and a progressive decrease of memory attention (lowering discrimination index). Behavioural differences were associated with brain gene expression changes: nicotine withdrawn animals showed decreased expression of chrna 4 and chrna7 after 60 days, and of htr2a from 7 to 60 days.The expression of c-Fos was significantly increased at 7 days. Finally, Tyrosine hydroxylase (TH) immunoreactivity increased in dorsal parvocellular pretectal nucleus, but not in periventricular nucleus of posterior tuberculum nor in optic tectum, at 60 days after withdrawal.
Conclusions:
Our findings show that nicotine withdrawal induced anxiety-like behaviour, cognitive alterations, gene expression changes and increase in pretectal TH expression, similar to those observed in humans and rodent models.
Keywords: Nicotine dependence, emotion, memory, IEG, hyperkatifeia, TH
1. Introduction
The World Health Organization reported that tobacco claims the life of 50% of those that smoke, causing about six million deaths every year (WHO, 2015, 2016). The economic costs of tobacco use are substantial and include significant health care costs for treating the diseases caused by tobacco use as well as the lost human capital that results from tobacco-attributable morbidity and mortality Nicotine, the main ingredient of tobacco cigarette, is one of the most addictive drugs which strongly activate the reward pathway (Volkow and Morales, 2015). The negative state and dysphoria associated with nicotine withdrawal, are good predictors of smoking relapse (Ockene et al., 2000). A key problem for the development of medications to treat nicotine addiction is the lack of animal models with sufficient predictive validity to support translation of pre-clinical findings to clinical research (Lerman et al., 2007). Recently, zebrafish have become a species of interest for drug discovery programmes including in the field of drug addiction. Zebrafish have a conserved drug-responsive ‘reward’ or reinforcement pathway and show adaptive changes and behavioural correlates of addiction after prolonged exposure to addictive drugs including nicotine (Kily et al., 2008; Klee et al., 2011; Kedikian et al., 2013; Cousin et al., 2014; Stewart et al., 2015).
Zebrafish have homologues for all 16 human neuronal acetylcholine nicotinic receptors (nAChRs) genes although several are duplicated (Pedersen et al., 2019) and possess well-developed cholinergic neuronal pathways which are the main targets of nicotine action in the central nervous system (Edwards et al., 2007; Bencan and Levin, 2008; Ackerman et al., 2009; Petzold et al., 2009; Papke et al., 2012; Braida et al., 2014a; Menelaou et al., 2014; Ponzoni et al., 2014). These findings support the use of zebrafish as a pre-clinical model for nicotine research.
Withdrawal syndrome is a major clinical problem related to psychoactive drugs (Hodding et al., 1980). As in rodents, withdrawal syndrome in zebrafish is characterised by behavioural and physiological responses (Tran et al., 2015). For example, after chronic ethanol exposure, zebrafish showed altered motor responses (Gerlai et al., 2009; Cachat et al., 2010b; Tran and Gerlai, 2013), disrupted social behaviour (Müller et al., 2017), increased anxiety (Mathur and Guo, 2011; Pittman and Ichikawa, 2013; Benneh et al., 2017; Mocelin et al., 2019), reversed scototaxis (Holcombe et al., 2013), loss of reproductive fitness (Dewari et al., 2016) and change in neurotransmitter levels (Gerlai et al., 2009). Morphine withdrawal produced robust anxiogenic effects and impaired social interaction (Cachat et al., 2010a). Long-lasting (5 days) behavioural effects consistent with an anxiety-like state were observed in zebrafish exposed for 5 days to cocaine and then withdrawn at 72 h (López-Patiño et al., 2008). Further, immediately following 4 day exposure to nicotine zebrafish showed increased anxiety in the novel tank diving test with a significant increase in latency to reach the upper half of the tank, a decrease in transitions to the upper part of the tank and a decreased time spent there (Stewart et al., 2015). However, few studies evaluated, after a prolonged nicotine withdrawal, a correlation between withdrawal-related behavioural changes and nicotine receptor gene expression in zebrafish. Kily et al., (2008) demonstrated that repeated exposure in adult zebrafish to nicotine or ethanol led to a robust conditioned place preference that persisted following 3 weeks of abstinence and in the face of adverse stimuli, a behavioural indicator of the establishment of dependence. Microarray analysis using whole brain samples from drug-treated and control zebrafish identified changes in gene expression of members of pathways and processes implicated in drug dependence.
The aim of the present study was firstly to evaluate the suitability of zebrafish as a model for studying nicotine withdrawal for future development of therapeutics. To this end we assessed the ability of short term (1–2 week) exposure to nicotine to induce changes in nicotinic receptor expression consistent with those seen in humans and rodent models (for rev. see Melroy-Greif et al., 2016), and withdrawal related changes in behaviour on mecamylamine-induced withdrawal. Subsequently, the effects of spontaneous nicotine withdrawal on behaviours and gene expression associated with withdrawal in mammals were assessed. We focussed on anxiety-like behaviour, anhedonia, attention deficits and nicotinic and serotonergic system gene expression as these have previously been shown to be hallmarks of (nicotine) withdrawal in other species (Ribeiro-Carvalho et al., 2009; Cook et al., 2015; Abreu-Villaça et al., 2019). We also monitored these behaviours after short- (2 and 7 days) and long- (30 and 60-days) term withdrawal. Gene expression changes were evaluated at 7 and 60 days and Tyrosine hydroxylase (TH) expression in pretectum was analyzed at 60 days. Dopaminergic pretectal neurons (which are located in dorsal parvocellular pretectal nucleus) are crucially involved in contributing a cross-modal detection of visual preys during hunting (Shang et al., 2015), a behaviour which is dependent on emotional-like states (Speedie and Gerlai, 2008). Thus, we examined the distribution of dopaminergic neurons in the parvocellular pretectal nucleus in nicotine withdrawn and control animals to assess the potential role of alterations in dopaminergic pathways in emotion sensitive behaviours in withdrawn animals.
2. Methods
All protocols were approved by the National Ethic Committee for the care and use of laboratory animals and by the National Ministry of Health with the Italian Government Decree N° 323/2018 PR and 513/2018 PR. In addition, the number of animals used and their suffering was minimized in all experiments, in accordance with ARRIVE guidelines.
2.1. Animals and housing
A total of 237 (120 for behavioural experiments, 20 for gene expression, 17 for TH immunofluorescence and 80 for binding studies) adult (12 month old) male ‘wild type’ (short fin,) zebrafish (Danio rerio) were obtained from Università Politecnica delle Marche, Dipartimento di Scienze della Vita e dell’Ambiente, Ancona, Italy). Only males were used due to previously reported sex differences in anxiety-like behaviour according to Fontana et al., (2019).
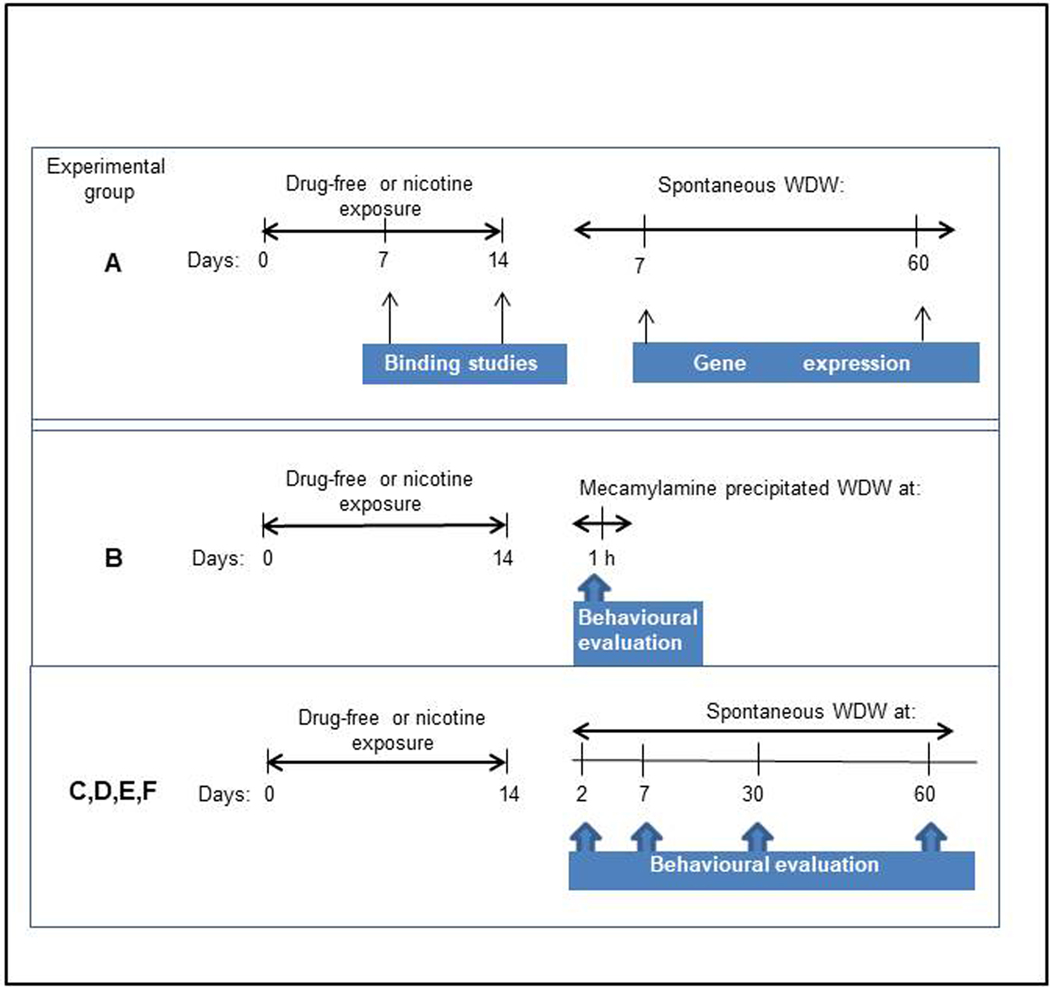
All fish were housed in groups of 30 in a 96 L home tank maintained in the home tank under standard conditions at 28 (± 2) °C), 14–10 h day/night cycle (lights on at 7:00 a.m.) for at least two weeks before the experiments. Tank water, containing sea salts (Instant Ocean, Aquarium System, Sarrebourg, France) at a concentration of 0.6 g/10L, was obtained by reverse osmosis filter system. Water quality was maintained at optimal levels and checked daily for pH (6.5–7.5) and every 3 days for nitrates (<0.02 ppm). Fish were fed twice a day with commercial flakes (tropical fish food, Consorzio G5, Italy) supplemented with live brine shrimp. The experimental design for the biochemical and behavioural analysis is reported in Figure 1. For behavioural analysis during spontaneous nicotine withdrawal, groups of 10 animals at a time of the Group C (10 for each group), were subjected to different behavioural tests (to avoid habituation to the test), according to the schedule reported in Supplementary Table 1. Animals were maintained in the home tank in groups of 10 each between tests.
Figure 1.
Experimental design for binding, gene expression, immunofluorescence and behavioural studies evaluated in zebrafish drug-free or exposed to nicotine. WDW= withdrawal.
2.2. Exposure to nicotine
Chronic exposure to nicotine bitartrate (1mg/L of tank water) (Sigma-Aldrich, St. Louis, MO, USA) was performed, in groups of 20 fish at a time, in the home tank before inducing withdrawal. Nicotine concentration was chosen according to Stewart et al. (2015). The duration of exposure (two weeks) was chosen according to our previous findings (Ponzoni et al, 2020). Three times a week (Monday, Wednesday, Friday) fresh nicotine was added to the water of the tank. Only on Day 7 the water tank was completely changed. Animals were monitored during nicotine chronic treatment to evaluate possible toxic/sedative effects of nicotine and biochemical analysis of water nicotine content was performed to assess possible nicotine build up and degradation rates. The choice to use a paradigm predicted to result in variable nicotine exposure was based on previous work in mice submitted to cigarettes withdrawal (Ponzoni et al., 2015) and in zebrafish exposed to nicotine (Ponzoni et al., 2020). After the last exposure (Day 14), all fish were put in fresh drug free tank water. Those of Group B (see Supplementary Table 1), to be used for mecamylamine-induced withdrawal analysis, were individually placed in tanks containing novel fresh, drug-free tank water. All other fish were placed in fresh, drug-free water in their home tanks in groups of 10. Control fish, exposed only to tank water, were subjected to the same handling regime as nicotine-exposed fish and evaluated simultaneously to with nicotine-exposed fish.
2.3. Biochemical studies
2.3.1. Determination of nicotine in tank water.
To determine if, when adding nicotine to the tank (Monday, Wednesday, Friday for two weeks), there was an accumulation of nicotine, total nicotine, present in the tank water was evaluated according to a spectrophotometric method described in Asthana et al. (2004). The absorbance values of the samples were interpolated on a calibration curve generated using known concentrations from 0.25 to 4 μg/ml diluted in a final solution volume of 25 ml. To assess a possible nicotine accumulation the samples were collected 5 min before or 1 h after adding 1 mg/L of nicotine to the tank water. The determination of nicotine in the water was also performed 24 and 48 h after nicotine addition.
2.3.2. Nicotinic receptor binding
Seven and 14 days after exposure, zebrafish of Group A (40 after 7 days and 40 after 14 days of nicotine cessation) were euthanized in a tricaine bath, (300 mg/L), decapitated, and their brains quickly removed and frozen at −80 °C until later use. In each experiment, the tissues from 5 zebrafish for each replicate (4) were pooled and homogenized in 1 ml of 10 mM Na phosphate pH 7.4, 137 mM NaCl, 4 mM KCl and 2 mM phenylmethylsulfonylfluoride (PMSF) with a Potter homogenizer. The homogenates were then diluted and centrifuged for 1.5 h at 60,000×g. The pellets were rapidly rinsed and then re-suspended in the same buffer containing a mixture of 10 μg/ml of each of the following protease inhibitors: leupeptin, bestatin, pepstatin A and aprotinin. The procedures of homogenization, dilution, and centrifugation of the total membranes were performed five times, after which the pellets were collected, rapidly rinsed, and then resuspended in the same buffer containing a mixture of 10 μg/ml of each of the following protease inhibitors: leupeptin, bestatin, pepstatin A, and aprotinin.
[3H]-Epibatidine binding.
In order to ensure that the α7-nAChRs did not contribute to [3H]-Epibatidine (Epi) binding to the membrane, the binding was performed in the presence of 1 μM αBungarotoxin (αBgtx), which specifically binds to α7*-nAChR and thus prevents [3H]-Epi binding to these sites. Saturation binding to the membrane homogenates was carried out for 3 h at RT or overnight at 4 °C by incubating aliquots of the membrane with increasing concentration of [3H]-Epi (0.005–10 nM). Nonspecific binding (averaging 5–10 % of total binding) was determined in parallel samples containing 100 nM unlabeled Epi. At the end of the incubation, the samples were filtered on a GFC filter soaked in 0.5 % polyethylenimine, washed with 15 ml of buffer (NaH2PO4 and Na2HPO410 mM, pH 7.4; NaCl, 50 mM), and counted in a γ counter.
[125I]-αBungarotoxin binding.
Binding experiments were performed by incubating zebrafish membranes with increasing concentration (0.01–10 nM) of [125I]-αbungarotoxin (αBgtx) overnight at 20 °C in the presence of 2 mg/ml bovine serum albumin (BSA). Specific radioligand binding was defined as total binding minus nonspecific binding determined in the presence of 1 μM cold αBgtx. After incubation, the samples were filtered as described above and the bound radioactivity directly counted in a γ counter. [3H]-Epibatidine and [125I]-αBgtx binding were performed in four separated experiment after 7 and 14 days exposure to nicotine and in each experiment brains from 10 zebrafish were pooled.
2.4. Behavioural studies
Behavioural testing took place during the light phase between 09:00 and 14:00 h. For each test the tank was positioned in front of the webcam for optimal video recording for later video-aided analysis. Each video was evaluated by three observers who, independently, processed and scored behaviours blinded to treatment condition. There was > 80% congruency between observers.
2.4.1. Injection method
Each fish was injected intramuscularly (i.m.) in the caudal musculature along the posterior axis (as previously described (Ponzoni et al., 2014). The fish were placed in anaesthetic solution of tricaine methanesulfonate (Sigma Aldrich, St. Louis, MO, USA) (150 mg/L), a dose reported to be anaesthetic (Matthews and Varga, 2012). Upon loss of response to touch, fish were injected with a volume depending on the weight of the fish (2 μl/g) using a Hamilton syringe (Hamilton Bonaduz AG, Bonaduz, Switzerland). Considering that the mean body weight was approximately 500 mg the final volume was 1μl. The site of injection was constantly maintained at the area below the caudal fin on the left side of each fish. Briefly, with the use of a net fish were individually pulled out of the water tank Then, each fish was kept immobilized through the net with two fingers of the left hand. Immediately after, the needle was positioned at a 45° angle in relation to the back of the fish with the needle pointing towards the head. The injection was in the largest portion of the caudal muscle, immediately posterior to the caudal fin. The needle was inserted into the muscle just beyond the bevel of the needle. Total time out of water was approximately 10 s. Each fish was removed from the net and immediately dipped in the tank water.
2.4.2. Mecamylamine-precipitated nicotine withdrawal
In the Group B to assess mecamylamine-induced withdrawal the non-selective nAChR antagonist mecamylamine hydrochloride (Sigma-Aldrich, MO, USA) was injected intramuscularly (i.m.) at a dose of (1 mg/kg i.m.) dissolved in its vehicle (sterile saline, 0.9%). The dose was chosen based on previous studies on nicotine withdrawal in mice (Ponzoni et al., 2015). On day 14, fish (20 for each group) were divided into four groups: exposure to nicotine +vehicle (saline), exposure to nicotine + mecamylamine (1 mg/kg, i.m.); drug-free exposure +vehicle; drug-free exposure+ mecamylamine. Precipitated nicotine withdrawal assessment was performed immediately after treatment.
Swimming activity and freezing behaviour (physical signs of nicotine withdrawal) were evaluated in a swimming tank divided into ten equal-sized 2 × 10 cm rectangles marked with permanent marker on the floor (Swain et al. 2004). The number of crossed lines was measured counting the number of lines crossed in a 30-s observation period, every min for a total of 5 observation bins, over 5 min, according to Ponzoni et al. (2014). Freezing behaviour, sign of anxiety was defined as a total absence of movement, except for the gills and eyes for > 2 sec, as reported by Stewart et al. (2015). At the end of the behavioural experiments zebrafish were killed.
2.4.3. Spontaneous withdrawal
Two, 7, 30 and 60 days after the last nicotine exposure, different groups (C,D,E F, G) of fish underwent the following behavioural tasks:
2.4.4. Novel tank diving test.
Anxiety-like behaviour, evoked by novelty was examined using the novel tank test, a commonly used standardized protocol for neurophenotypic screening in adult zebrafish (Demin et al., 2017; Kolesnikova et al., 2017). On the day of testing, the fish were individually and gently caught with the net, removed from their home tanks and individually placed for 5-min in a 1.5 L transparent tank filled with water to the height of 12 cm. The tank was divided into 2 equal virtual horizontal portions, marked by a dividing line (with a marker on the outside walls).The time spent (s) in the upper and bottom portion, the latency to top and the number of transitions, into the upper half, were evaluated manually over a period of 5 min.
2.4.5. Compartment preference test.
In the wild, zebrafish prefer to swim in areas with vegetation where they are protected from predators (Engeszer et al., 2007; Spence et al., 2006;). Preference for an environment rich with vegetation was evaluated according to Kistler et al. (2011) using a plexiglass three-chamber apparatus filled with tank water to a level of 10 cm. The left and right compartments had the same dimensions (13 cm x 14 cm x 29 cm h) while the middle compartment was smaller (9 cm x 14 cm x 29 cm h). The compartments were delimited by lines marked externally by a permanent marker pen. To make the compartment enriched, plants, shells and stones were placed only in one of the lateral compartments. To minimize procedural novelty stress, the day before the test animals were individually habituated to the test tank for 15 min without enrichment. The day after, two semitransparent plates were put in the tank to divide it into three compartments. Fish were placed individually in the centre of the apparatus and 5 min later the plates were removed and each zebrafish had the opportunity to choose between the empty and the enriched compartment for 6 min. The first minute was not included in the evaluation. All the tasks were videotaped and then the latency to reach the enriched compartment, the time spent in the empty and enriched compartment and the number of transitions from the central area to the lateral compartments were scored by an experimenter blind to treatment.
2.4.6. Virtual Object Recognition (VORT).
VORT was carried out according to Braida et al. (2014b) in a transparent Plexiglas tank, filled with tank water to a level of 10 cm. In order to minimize procedural novelty stress, zebrafish were habituated to the apparatus for 10 min a day for one week. Then, they were restricted for 5 min in a 20 cm central area delimited by two opaque barriers after which, each animal was submitted to a familiarization trial (acquisition phase during T1) followed by a shape recognition trial (test phase during T2). Five min intervals separated the acquisition phase from the test phase. T1 consisted of a 10-min session, during which two identical white geometrical shapes on a black background were shown on two 3.5-inch widescreen displays (iPod screens). After T1 the fish were returned to the home tank. During T2 each animal was placed again in the same tank (test phase) in which one of the two identical familiar shapes was replaced by a novel one. The two iPods were located externally to the opposite 10-cm-wide walls of the tank. To reduce shape preference effect, the nature of the stimuli (familiar or new shape) was counterbalanced from animal to animal. Shape recognition was manually scored with a stopwatch by an experimenter blind to the treatment, in terms of exploration time whenever the zebrafish approached the iPod area (10 cm, marked on the bottom of the tank) and directed its head towards the shape. Simple geometrical shapes (square, triangle, circle, cross, etc.) were used.
2.4.7. Swimming activity
Swimming activity, in terms of crossed lines, evaluated at 2, 7, 30 and 60 days after nicotine cessation was evaluated in 30 min every 5 min for 1 min.
2.5. RNA extraction, microarray analysis and quantitative real-time PCR
Five brains from water- or nicotine-exposed fish of Group A were extracted 7 or 60 days after nicotine cessation and snap frozen at −80 °C until later use. Total RNA from brains was extracted using TRIzol reagent (Thermo Scientific) as suggested by the manufacturer. Briefly, samples were homogenized and lysated with TRizol reagent, and chloroform, and RNA separated by centrifugation. RNA was precipitated with isopropanol, washed with 70% ethanol, air dried and solubilized with RNase-free water. RNA yield and quality were tested with a nanodrop. Then, RNA extracts were treated with DNase I (ThermoFisher) and cDNA libraries created using the ProtoScript II First Strand cDNA Synthesis Kit (New England Biolabs) as suggested by the fabricant. Resulting cDNA yield and quality were also evaluated using nanodrop (ThermoFisher. Relative qPCR assays were performed using the LightCycler 480 qpcr system from Roche Diagnostics, Ltd. with all reactions carried out in triplicates. Accession numbers and primers sequences for all the reference and target genes (Tang et al., 2007; Parker et al., 2016). can be found in Supplementary Table 2.
2.6. Quantitative confocal immunofluorescence analysis
The fish (8 exposed to water and 9 exposed to nicotine for 14 days) and then withdrawn for 60 days were placed in a tricaine solution (150 mg/l). When opercular movements stopped and all reflex reactivity was lost, the fish were placed in a sponge in a Petri dish that contained 150 mg/l chilled tricaine solution. The heart was exposed, and a smoothed 30 Gauge needle inserted into the ventricle. A saline solution was flushed (1–2 ml, at 1 ml/min) and when fish gills whitened, a fixative solution (2% paraformaldehyde, pH 7.4; 10 ml, at 1 ml/min) was used to perfuse. The brains were then carefully removed and placed overnight in the same fixative solution on a shaker (at 4°C). The brains were subsequently washed in PBS, cryoprotected in 20% sucrose, placed in embedding medium-containing mold, frozen and 10 μm thick coronal sections cut at cryostat and processed for immunofluorescence and confocal analysis as already described (Rossetti et al., 2018). In preliminary experiments with immersion fixation, moderate-strong autofluorescent structures were detected throughout the brain that were easily identifiable as brain vessels: however, perfusion fixation eliminates this strong autofluorescence. Images for quantitative analysis were obtained at Zeiss Meta 510 confocal microscope by using a 40x/1.4 objective. For TH immunofluorescence, anti-TH rabbit antiserum (AB152, Millipore; 1/2000) and Alexa Fluor 594 goat anti-rabbit antiserum (A11012, Molecular Probes; 1/200) were used. Following immunofluorescence staining, adjacent sections were Nissl stained for histological analysis.
For quantitative analysis, the images were processed for background subtraction by MosaicSuite plugin of Fiji free software (Rossetti et al., 2019). The cleaned images were then segmented by automatic Image J thresholding methods, normalized (using the median levels of the background) and automatically analyzed by a home-made macro in Fiji. Structures were identified and named according to the zebrafish brain atlas (ZeBrain) by Kawakami Laboratory (https://zebrain.nig.ac.jp/zebrain/page.do?p=welcome).
2.7. Statistical analysis
All parametric data were expressed as mean ± standard error of the mean (SEM). Pair-wise comparisons were assessed with Student’s t-test. Different groups were assessed by one- or two-way analysis of variance (ANOVA) for multiple comparisons followed by Tukey’s for one-way ANOVA or Bonferroni’s for two-way ANOVA post-hoc test. When the normal distribution was not met (checked by mean, median and symmetry) data were analyzed by non-parametric tests, Mann-Whitney or Kruskal–Wallis, followed by Dunn’s post hoc test. For the frequency of the number of animals showing freezing behaviour Contigency Table was adopted and then Fisher’s exact probability test was used. Data of VORT experiments were expressed as discrimination index [(time spent exploring novel shape − time exploring familiar shape) /(time spent exploring novel shape + time exploring familiar shape)], as previously described (Braida et al., 2014b). To objectively and reliably quantify zebrafish behaviour parameters, inter-observer differences in scoring were tested with three observers who independently, through the videos, processed the behaviour. The results were statistically analyzed using the appropriate statistical test and if no significance (p > 0.05) was obtained data were pulled together. There was > 80% congruency between observers. Relative mRNA expression in qPCR was calculated against reference gene cycle-threshold (Ct) values, and then subjected to one-way ANOVA. To account for multiple testing a Bonferroni correction was applied, and significance was declared at a threshold of p ≤0.05. For quantitative immunofluorescence analysis, 8/9 animals/group (drug-free or nicotine withdrawn at 60 days) were used. For each animal, we obtained 3–4 scan in each TH-positive region. Distribution of continuous variables showing departure from normality was assessed by Shapiro-Wilk test. The analysis (the tests were two-tailed) was performed by Student’s t-test. Data were analysed using Prism 7 software (GraphPad, USA).
3. Results
3.1. Biochemical studies
3.1.1. Determination of nicotine in the tank water
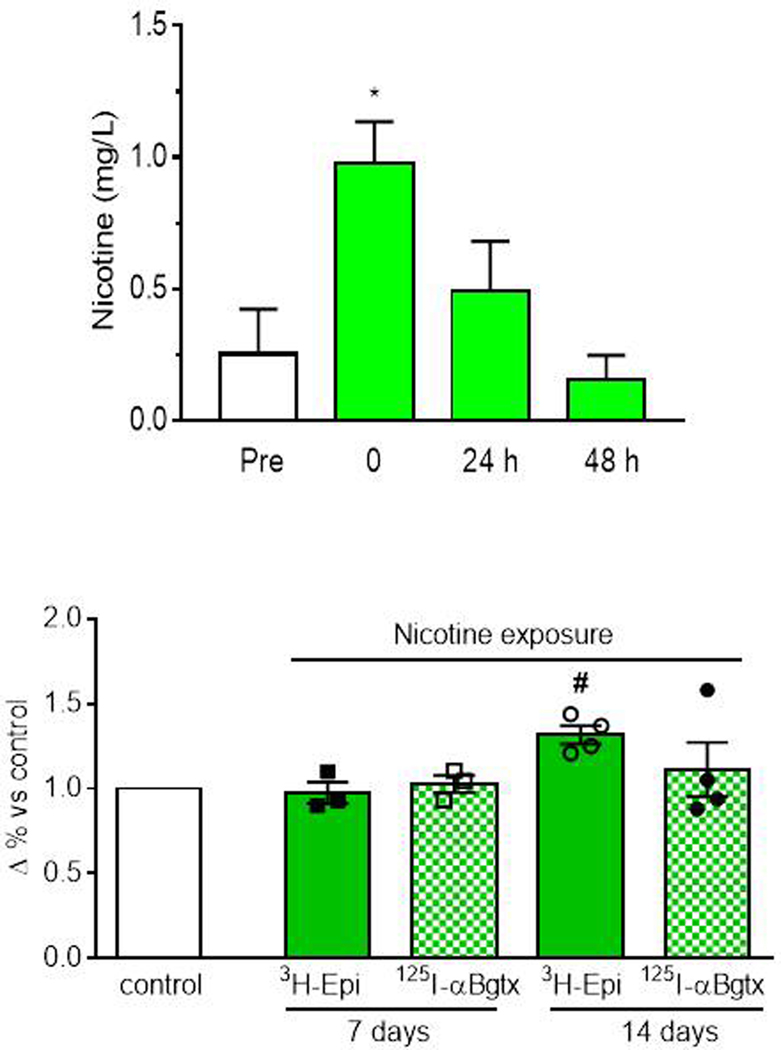
Nicotine level was determined as mean ± SEM of 3 determinations across the 14 days at 5 min before (pre), 1 h, 24 h and 48 h after every nicotine application. There was a difference among groups (F(3,8) = 5.45; p=0.002). Post-hoc comparisons indicated that nicotine level significantly increased only in the samples taken from the water tank 1 hour after adding nicotine (1mg/L) compared to the pre-exposure and gradually decreased over the following 48 hours (Fig. 2a). These results indicate that nicotine concentration decreased across the three days before the subsequent nicotine addition, suggesting no accumulation of the drug. Accordingly, no death or side effects were observed in zebrafish during nicotine exposure.
3.1.2. Binding studies
Studies to examine the time course of nicotine effects on the expression of [3H]-Epi labeled heteromeric receptors and [125I]-αBgtx labeled homomeric receptors in total brain homogenates revealed no difference after one or two weeks in the [125I]-αBgtx receptors, as depicted in Figure 2b. There was a slight non-significant increase in the number of [3H]-Epi receptors after one week. However, the difference was significant after two weeks (χ2= 9.82; p = 0.04), thus this time interval was chosen for further behavioural and gene expression studies.
3.2. Behavioural studies
Mecamylamine-precipitated nicotine withdrawal
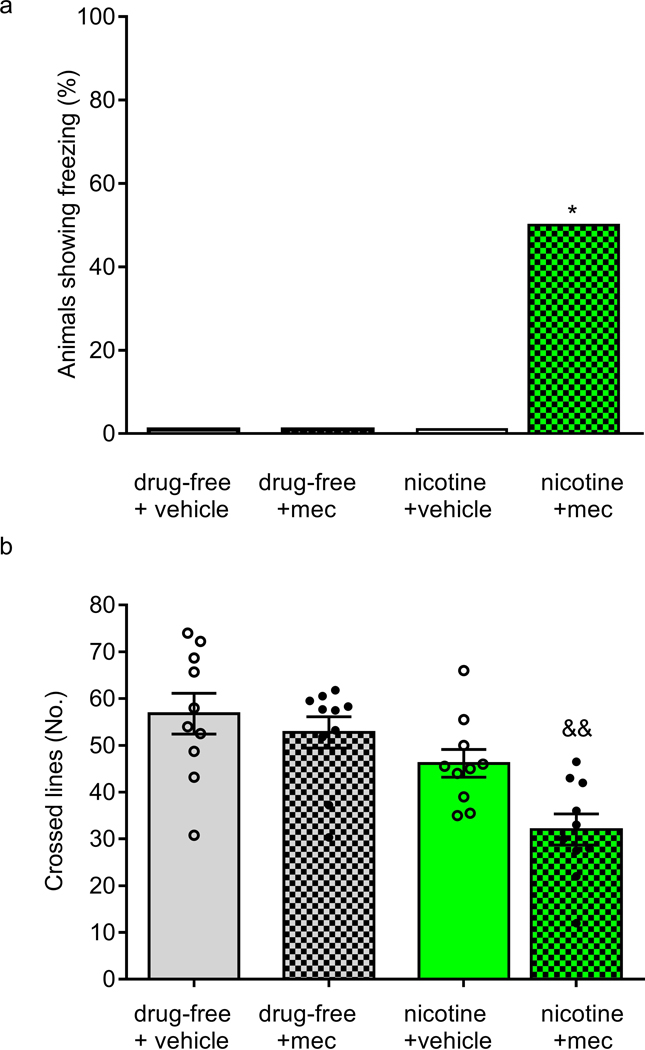
Swimming activity and Freezing behaviour (physical signs of nicotine withdrawal) were evaluated in a swimming tank calculating the number of lines crossed by each fish in a 30-s observation period. On Day 14, 1 h after the last nicotine exposure, animals received mecamylamine hydrochloride (1mg/kg i.m.) or vehicle, and were individually assessed for behavioural signs of withdrawal. A significant increase in the number of animals showing freezing was observed in nicotine exposed fish (p < 0.05) (Fig. 3a). Regarding the swimming activity, there was a difference among groups (F(3,36) = 9.194, p < 0.0001). The group previously exposed to nicotine and treated with mecamylamine showed a significantly lower number of mean crossed lines compared to the drug-free + vehicle group and drug-free + mecamylamine group (Fig. 3b).
Figure 3.
Nicotine withdrawal evaluated in drug-free fish or fish that had been nicotine pre-exposed to nicotine (1 mg/L/), 10 min after treatment with the non-selective nAChR antagonist mecamylamine (mec) (1 mg/kg i.m.) or vehicle (saline). Behaviour was evaluated in the swimming tank for 5 min, every min for 30 s, in terms of the percentage of animals showing freezing (a) and the number of crossed lines (b). Data are mean ± SEM except for freezing (%). Comparisons were performed using Fisher’s exact probability test or unpaired Student’s t test. WDW=withdrawal. Mec= mecamylamine. N = 10 animals for each group. *p < 0.05vs all the remaining groups; &&p <0.02 vs drug-free vehicle and drug-free mec.
3.2.1. Spontaneous withdrawal
Novel tank diving test and swimming behaviour
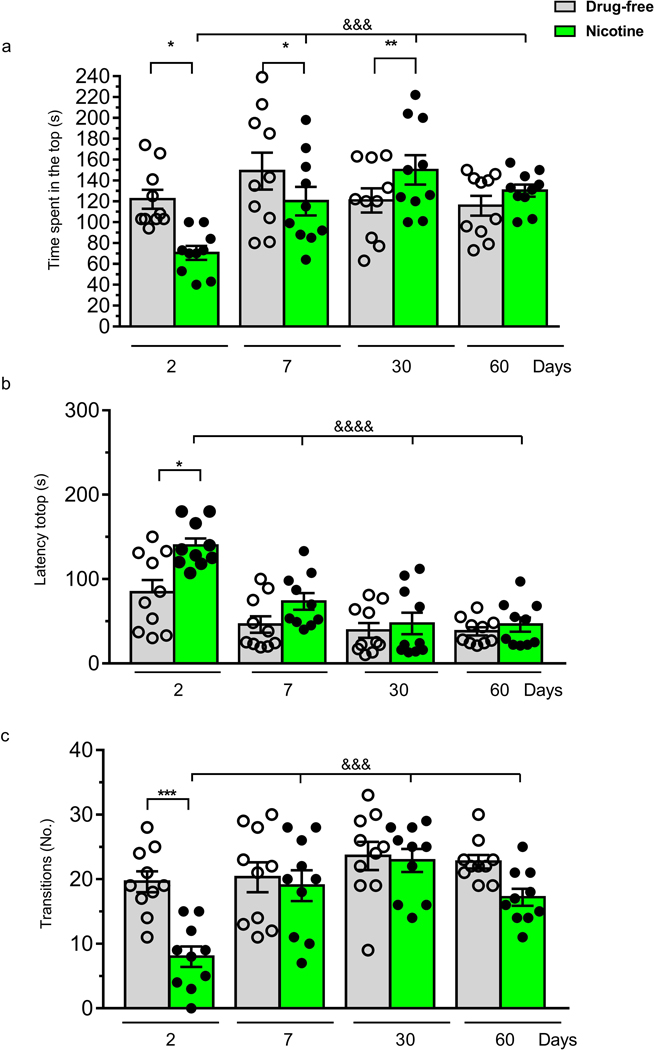
In the novel tank test nicotine exposed fish showed a reduced time spent in the top and a longer latency to explore the upper portion of the tank (Fig. 4a,b) compared to drug-free fish at 2 and 7 days after nicotine cessation. A two-way ANOVA of the top time showed significant effect of days of withdrawal (F (1, 72) = 4.45; p <0.001), and a days of withdrawal x treatment interaction (F(3, 72) = 5.10; p < 0.001) but no treatment effect (F(3, 72) = 0.05; p > 0.05). Post-hoc test showed, on Day 2 and Day 7, a significant decrease in the time spent in the top and an increase at 30 days (Fig. 4a). Analysis of the latency to top revealed a significant time effect (F (3, 72) = 21.42; p < 0.0001), treatment effect (F(1, 72) = 12.15, p < 0.001 but not treatment x days of withdrawal interaction (F (3, 72) = 2.50; p > 0.05). A significant increase in the latency to reach the upper portion of the tank was observed in the nicotine group on Day 2 (Fig. 4b). Post-hoc analysis revealed that there was an increase of latency on Day 2 in the nicotine group, which progressively decreased through the days. A two-way ANOVA of the number of transitions to top showed significant treatment effect (F(1, 72) = 13.56; p <0.001), a days of withdrawal effect F(3, 72) = 9.18; p < 0.0001) and a treatment x days of withdrawal interaction (F(3, 72) = 3.756; p < 0.01) (Fig. 4c). A significant decrease in the transitions was shown only on Day 2.
Figure 4.
Effect of nicotine (1 mg/L) pre-exposure for 2 weeks in the Novel diving tank test. Behaviour was evaluated for 5 min on Day 2, 7, 30 and 60 after nicotine cessation, during spontaneous withdrawal, in terms of (a) time spent in the top,(b) latency to top and (c) the number of transitions to top. Data are mean ± SEM. Comparisons were performed using two-way ANOVA followed by post-hoc Bonferroni test. N = 10 animals for each group. *p < 0.05,**p < 0.01,***p < 0.001 compared to the corresponding drug-free zebrafish; $ $ $p < 0.001, &&&&p < 0.0001 compared to the corresponding nicotine group, Day 2.
3.2.2. Compartment preference test
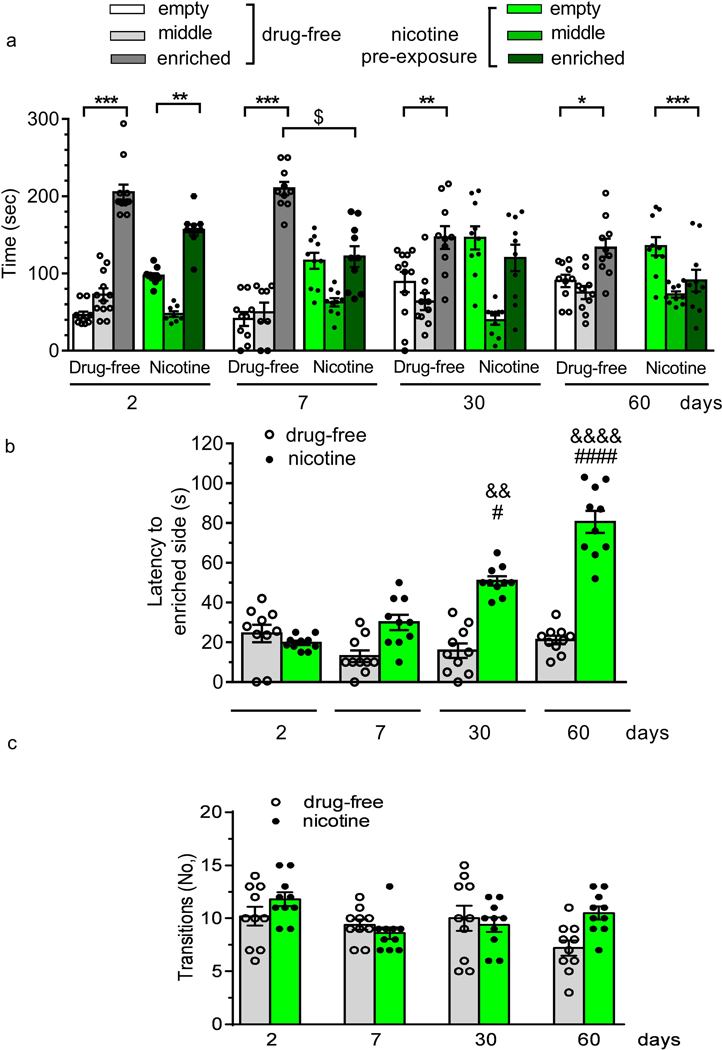
There was a difference among groups in compartment preference time (treatment effect:F(5,216) = 16.31, p < 0.0001; interaction treatment x days: F(15,216) = 8.13, p < 0.0001) and days (F(3,216) = 2.699, p < 0.05) (Fig. 5a). Post-hoc comparison revealed a significant preference for the enriched compartment in drug-free zebrafish at all the tested days. On Day 7 and Day 30 of withdrawal, zebrafish pre-exposed to nicotine spent the same time in the empty and enriched compartment. A significant preference for the empty compartment in nicotine treated zebrafish was shown on Day 60. Nicotine pre-exposure resulted in a longer latency to explore the enriched compartment compared to drug-free fish, as depicted in Figure 5b. Two-way ANOVA showed a significant treatment effect (F(1, 72)= 22.59; p<0.0001), days of withdrawal effect (F(3, 72) = 6.72; p < 0.05) and treatment x days of withdrawal interaction (F(1, 72) = 6.19; p < 0.01). Drug-free animals did not change the latency to reach the enriched compartment over time. In contrast, nicotine-exposed zebrafish showed a significant increase in the time to explore the enriched side on Day 30 and Day 60 of abstinence. The number of transitions was not affected by nicotine pre-exposure (treatment effect: F(1, 72) = 0.0003; p > 0.05; days of withdrawal effect: F(3, 72) = 0.40 p > 0.05; treatment x days of withdrawal interaction: F(3, 72) = 0.62 p > 0.05) As (Fig. 5c).
Figure 5.
Effect of nicotine (1 mg/L) pre-exposure (for 2 weeks) in the compartment preference test. Behaviour was evaluated for 5 min on Day 2, 7, 30 and 60 in terms of (a) time spent in three different compartments (enriched, empty, middle), (b) latency to the enriched side and (c) total number of transitions to the enriched side. Data are mean ± SEM. Comparisons were performed using two-way ANOVA followed by post-hoc Bonferroni test. WDW = withdrawal. N = 10 animals for each group. *p < 0.05, **p < 0.01,***p < 0.001, ****p < 0.0001 compared to the corresponding empty compartment; $p < 0.05 compared to the corresponding drug-free enriched side; &&p < 0.01 and &&&& p < 0.0001 compared to the corresponding drug-free group; #p < 0.05, ####p < 0.0001 compared to nicotine group, Day 2.
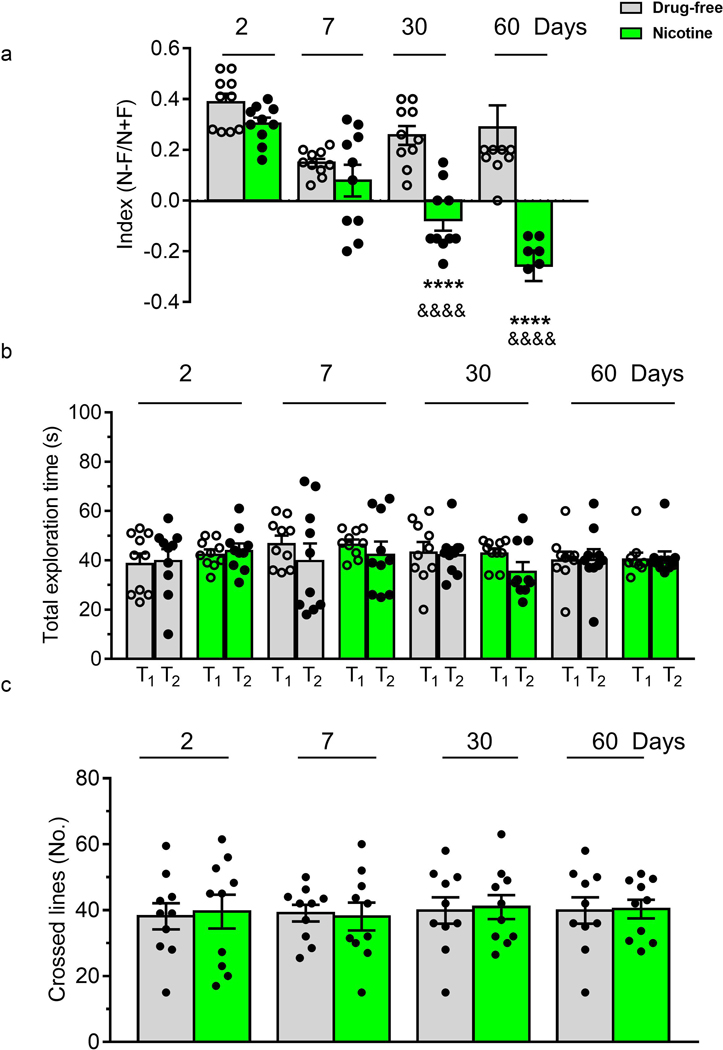
3.2.3. VORT
Figure 6 shows the memory attention ability of zebrafish submitted to VORT at different withdrawal intervals. A two-way ANOVA of discrimination index showed significant treatment effect (F(1, 72) = 61.45; p < 0.0001), a days of withdrawal effect (F(3, 72) = 18.37; p = 0.0005) and treatment x time interaction (F(3,72) = 11.61; p<0.0001) as depicted in Figure 6a. Post-hoc tests showed a significant decrease in the discrimination index on Day 30 and on Day 60 in the nicotine exposed group. In Figure 6b) total exploration time is depicted. Two-way ANOVA revealed no significant differences among groups for days of withdrawal (F(1,144) = 0.76; p > 0.05) treatment (F(3,144) =0.474; p > 0.05) and treatment x days of withdrawal interaction (F(9,144) = 0.66; p > 0.05) (Fig. 6b). Swimming activity (Fig. 6c), in terms of crossed lines, did not significantly vary during nicotine cessation: treatment effect (F(1,72) = 0.029 p > 0.05), days of withdrawal effect F(3, 72) = 0.11; p > 0.05 and treatment x days of withdrawal interaction (F(3, 72) = 0.039 p > 0.05).
Figure 6.
Effect of nicotine (1 mg/L) pre-exposure (for 2 weeks) on (a) mean discrimination index, (b) total exploration time evaluated in VORT. and (c) total number of crossed lines on Day 2, 7, 30 and 60 after nicotine withdrawal. In VORT the performance was assessed using highly discriminated shapes. N= time spent in the novel shape, F= time spent in the familiar shape. N = 10 animals per group. Data are mean ± SEM. ****p < 0.0001, compared to the corresponding drug-free group; &&&& p < 0.0001 compared to the corresponding nicotine group, Day 2. Comparisons were performed using two-way ANOVA followed by post-hoc Bonferroni test.
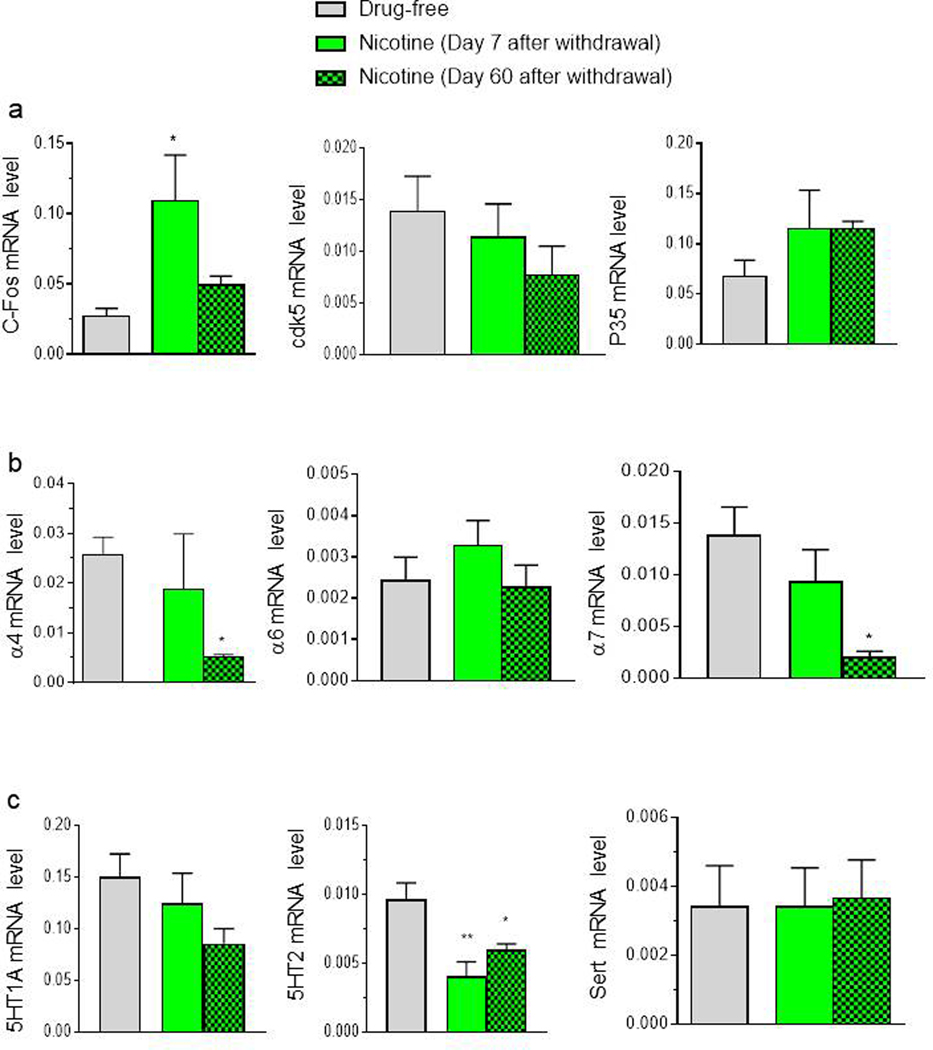
3.3. mRNA levels
Some significant changes in gene expression between controls and nicotine pre-exposed zebrafish were found (Fig. 7). A significant increase was found for c-Fos expression after 7 days (F(2,12) = 4.77, p < 0.02). No difference among groups was found for Cdk5 mRNA level (F(2,12) = 1.13, p > 0.05) or p35 gene expression (F(2,12) = 1.28, p > 0.05) (Fig.7a). There was a significant decrease in nicotine exposed zebrafish compared to the free water group for chrnα7 expression after 60 days from withdrawal (chrnα7: F (2,12) = 5.15, p < 0.02. No significant change in chrnα4 (F(2,12) = 3.85, p > 0.05) or chrnα6 nicotinic subtype receptors mRNA expression was found (F(2,12) = 0.87, p > 0.05) (Fig. 7b). A significant reduction in htr2a receptors was observed after 7 and 60 days of withdrawal in nicotine pre-exposed fish (F(2,12) = 7.93, p < 0.001). No difference was found for htr1a (F(2,12) = 1.81, p > 0.05) or slc6a4a (F(2,12) = 0.01, p > 0.05) mRNA expression (Fig. 7c).
Figure 7.
Quantitative real-time PCR analysis of c-Fos, cdk5, p35, nicotinic subtype receptors and serotonergic signalling pathway genes evaluated 1 h after the test on day 7 and 60 after nicotine cessation. Data are mean ± SEM of 5 zebrafish per group. *p < 0.05, **p < 0.01 compared to vehicle group (one-way ANOVA followed by Tukey’s test).
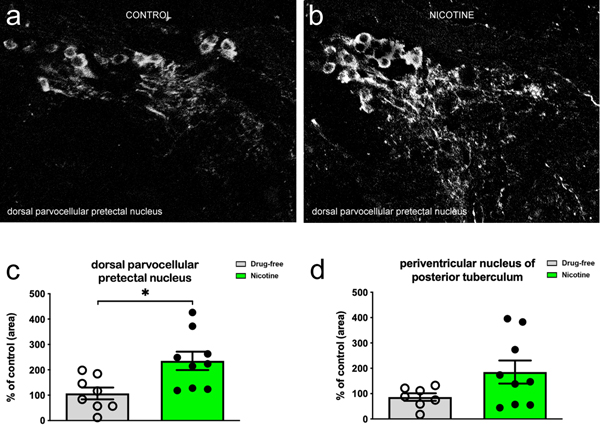
3.4. TH expression
The analysis of TH expression focused on dorsal parvocellular pretectal nucleus (also known as periventricular pretectum) and periventricular posterior tuberculum. In both nuclei, cell bodies as well as fibers and terminals were labelled (Figure 8). In fish that underwent spontaneous withdrawal at 60 days, TH fluorescence intensity was found to be increased in dorsal parvocellular pretectal nucleus (p < 0.05) (Fig. 8a), but not in periventricular posterior tuberculum (p > 0.05) (Fig. 8b). Quantitative analysis revealed a significant increase of TH expression only in dorsal parvocellular pretectal nucleus (Fig. 8c,d).
Figure 8.
TH expression evaluated in the dorsal parvocellular pretectal nucleus (dPPN) (aka periventricular pretectum) 60 days after spontaneous nicotine withdrawal. Coronal sections through the dorsal parvocellular pretectal nucleus show TH immunofluorescence in control (drug-free) (a) and nicotine exposed (b) fish at 60 days from withdrawal. Quantitative analysis shows that TH increases in dPPN (c) (p < 0.05), but not in periventricular nucleus of posterior tuberculum (d) (p > 0.05). Data are mean ± SEM of 8/9 zebrafish/group (expressed as percentage of mean control value). The analysis was conducted by Student’s t test.
4. Discussion
The present study demonstrates that zebrafish, like rodents and humans, have altered behaviour in terms of anxiety, motivation and cognition during prolonged withdrawal following chronic exposure to nicotine. These findings confirm that zebrafish, as suggested by Müller et al., (2020), is a promising tool for assessing drug-abuse related effects. The behavioural alteration was accompanied by changes in expression of nicotinic receptors, serotonergic receptors, the immediate early gene cFos and TH.
In humans, smoking cessation produces withdrawal which is characterized by affective symptoms (anxiety, depression, anhedonia, dysphoria, and hyperalgesia) (Jackson et al., 2003; Markou, 2008), which collectively give rise to the state of hyperkatifeia, defined as the manifestation of a negative emotional state during withdrawal from drugs of abuse (Koob, 2021). Similarly, increased anxiety is an affective component of nicotine withdrawal in rodents (Damaj et al., 2003; Jonkman et al., 2005; Ponzoni et al., 2015). Here nicotine-treated zebrafish showed behavioural alterations consistent with an increased anxiety-like state on either mecamylamine-induced injection or spontaneous withdrawal: Zebrafish that had been exposed to nicotine for 2 weeks showed increased freezing behaviour after mecamylamine injection, and spontaneously withdrawn fish showed increased bottom-dwelling time as well as increased latency to the top in the Novel Tank Diving test 2 and 7 days post-withdrawal. Our findings are consistent with those of others where zebrafish show increased freezing (Tran et al., 2015) and increased anxiety-like behaviour (López-Patiño et al., 2008; Cachat et al., 2010a) during withdrawal from ethanol or psychostimulants, respectively. The decreased anxiety-like behaviour found here at 30 and 60 days from withdrawal is in accordance with Morud et al. (2018) who found that anxiety–like behaviour evaluated with elevated plus maze and marble burying test spontaneously decreased in rats after prolonged nicotine withdrawal (3 months).
Although increased freezing is a recognized measure of fear/anxiety in zebrafish (Blaser et al., 2012), decreased motor function is also seen following mecamylamine injection in nicotinedependent mice (Castanê et al., 2002) suggesting direct locomotor effects may contribute to the reduction in zebrafish motility seen here. It is also possible that nicotine degradation products and/or metabolites influenced the results. As previously reported (Müller et al., 2020), little is known about pharmacokinetics in this species and the drug concentration which reaches the brain is poorly investigated. Our water analysis showed nicotine concentration in the tank water decreased over 48 hours consistent with the aim of a variable exposure schedule. However, as we did not assess brain nicotine concentration or breakdown products we cannot be sure of the dosing regimen. Studies in embryonic zebrafish (Thomas et al., 2009) suggest nicotine is rapidly taken up from the water and rapidly equilibrates within the embryos. On removal from nicotine solutions, tissue nicotine concentrations rapidly fall. Although these kinetic studies are in embryos, they suggest that the observed rise and fall of nicotine in the water would be reflected by an increase and decrease in brain nicotine concentration in the fish. The lack of accumulation of nicotine in the fish water and absence of signs of toxicity in zebrafish exposed to nicotine, suggest behavioural and physiological effects on withdrawal reflect adaptive responses to the presence of nicotine rather than toxicity.
Despite many studies showing anxiety-like states in zebrafish during withdrawal from drugs of abuse (López-Patiño et al., 2008; Cachat et al., 2010a, Tran et al., 2015), there are few studies describing models of withdrawal-induced depression in zebrafish. Depression-like behaviours can be assessed in zebrafish by motor phenotypes (e.g., the novel tank test motor retardation-like behaviours restored by chronic antidepressants) and amotivation states (e.g., reduced preference for food, conspecifics and sexual interactions) (Nguyen et al., 2014). However, no study has examined depression-like behaviour in zebrafish following drug withdrawal. Here we used reduction in motivation to seek a complex environment as a measure of depression-like/anhedonic behaviour.
Previous studies indicated that either in a group context or singly, zebrafish show clear preferences for substrates and plants over barren conditions (Kistler et al., 2011; Schroeder et al., 2014). Therefore, we used a compartment preference procedure to study the motivational state of nicotine withdrawn fish. Thus, the preference for an enriched side was taken as evidence for a hedonic component (Kistler et al., 2011), while its loss was taken as a depression-like state. Using this interpretation, the finding that nicotine withdrawn fish showed a significant decrease in preference for the enriched compartment at 7 days post-nicotine withdrawal is consistent with the withdrawal-induced depression seen in mammals (Le Foll and Goldberg, 2009). As there was no difference in locomotion, as determined by number of transitions, it is unlikely that differences in time spent in the enriched compartment are due to locomotor effects.
It is now accepted that the difficulty of smoking cessation is, in part, due to the cognitive deficits associated with withdrawal (Hall et al. 2015). Accordingly (Gilbert et al., 2004), the number of correct detections of target sequences on the Rapid Visual Information Processing task, a measure of sustained attention, decreased significantly in an abstinent smokers group compared with those who had never smoked with the decrease in performance persisting across days 3,10,17 and 31 of abstinence (Gilbert et al., 2004). In addition, a slower visual search speed during smoke cessation compared to those who had never smoked (Richards et al., 2003) and an attentional impairment using a modified visual probe task from 1 to 6 months of smoke withdrawal, were found (Begh et al., 2015). Effects on performance of cognitive tasks have also been reported in rodents. For example, rats, withdrawn from chronic nicotine show a deficit in sustained attention deficits 16 hours post-withdrawal when assessed using 5-choice serial reaction time task (5-CSRTT) (Shoaib and Bizarro, 2005; Semenova et al., 2007), and mice submitted for 7 weeks to standard cigarette or e-cigarette inhalation showed a significant impairment in the spatial version of the novel object recognition task that persisted from 1 to 30 days of withdrawal (Ponzoni et al., 2015). Learning and memory tasks in humans are predominantly based on visual stimuli (Fei-Fei et al., 2007; He et al., 2011). Here we used a visual object recognition task (VORT) to assess memory recognition deficit in zebrafish following nicotine withdrawal. Although no effects on VORT were seen at 2 or 7 days post withdrawal, withdrawn fish showed a significant reduction in VORT at 30 and 60 days consistent with the findings in rodents and humans.
To further explore zebrafish withdrawal response we examined the persistence of behavioural responses at 30 and 60 days of withdrawal. Long term effects on anxiety declined but effects on depression-like behaviour and attention (as mentioned above) persisted or even got worse. Differences in behaviour may reflect gene expression changes or may result from nicotine withdrawal effecting the fish’s ‘emotional’ response to housing conditions.
The persistent lack of motivation/anhedonia and cognitive deficits is consistent with findings in humans following long term withdrawal (Martinotti et al., 2008; Cook et al., 2015). However, it is difficult to explain why the attention deficit we found, in terms of reduced VORT discrimination index, exhibited a progressive deterioration from 7 to 60 days of withdrawal. Previous studies have suggested anxiety levels influence cognitive performance such that increased anxiety leads to decreased performance (Barbot and Carrasco, 2018). Thus, one possibility is that prolonged withdrawal leads to increased anxiety in the VORT assay. However, the fact that anxiety levels as assessed by the Novel Tank Diving assay return to control levels after 30 and 60 days of withdrawal argue against this. Alternatively, it may be that prolonged withdrawal and continued housing in a small group lead to reduced motivation to attend to reduced interest in the environment. Our finding that zebrafish show reduced motivation to explore a complex environment that is greater at 30 and 60 days than at 7 days is consistent with this suggestion.
Our behavioural data are supported by changes in expression of genes involved with drug-abuse related processes (Müller et al., 2020). We found an increase in 3H -Epibatidine binding sites in nicotine-exposed versus unexposed zebrafish brains following 2 weeks of nicotine exposure. The up-regulation is in agreement with studies in rodents (Moretti et al.,2018; Nguyen et al., 2004; Ponzoni et al., 2015) and in humans in which nicotine and tobacco smoke exposure robustly upregulates α4β2*-nAChRs that bind 3H-Epibatidine with high affinity in brain cortex and cerebellum (Moretti et al., 2014; Perry et al.,1999). Notably, previous data revealed that zebrafish brain express α7-containing receptors that bind [125 I]-αBungarotoxin with high affinity and heteromeric receptors that bind [3H]-Epibatidine with high affinity (Ponzoni et al., 2014).
At 7 and 60 days from withdrawal, we observed in the whole brain changes in the nicotinic and serotonergic systems and the immediate early gene, c-Fos. These alterations could explain why residual behavioural alterations persisted long after nicotine cessation. Chrna mRNA gene expression was reduced at 60 days from withdrawal in nicotine-exposed animals compared to controls. This decrease could account for the reduced memory function observed (Nikiforuk A, et al.,2015). No previous studies have examined the gene expression of nicotinic receptors after 7 or 60 days from withdrawal. Mugnaini et al (2002) found that nicotine-induced chrna7 receptor upregulation is not paralleled by change in the level of the chrna mRNA. We found a dysregulation of the serotonergic system in terms of decreased mRNA expression of htr2aa at 7 and 60 days from withdrawal. Similarly, a significant decrease in htr2aa receptor mRNA level in the hippocampus and ventral tegmental areas of rats was observed during nicotine withdrawal after 5-day nicotine treatment (Zaniewska et al., 2010). Blockade of htr2A receptors and activation of htr2C receptors has been found to be crucial for counteracting the depression-like behaviour observed in rats experiencing withdrawal from chronic nicotine exposure. The conclusion of that study is that the htr2A receptor antagonism and htr2C receptor agonism can facilitate tobacco cessation by relieving nicotine withdrawal symptoms. Our findings are consistent with this hypothesis.
In zebrafish (as in mammals) dopamine system also contributes to the modulation of emotional-like behaviour, as, e.g., anxiety (Kacprzak et al., 2017), attention and hyperactive behaviour (Huang et al., 2015). Surprisingly, we did not find significant changes in gene expression of dopaminergic system (data not shown) but we cannot exclude that the evaluation of gene expression in the whole brain could have masked some alterations in specific areas/nuclei. It was found that the increased TH expression in the dorsal parvocellular pretectal nucleus was parallel to decreased exploration, increased anxiety-like behaviour, reduced motivation and memory attention.
The analysis of immediate early genes (IEG expression) i.e. c-Fos, is used for investigating activated circuits involved in behavioural or physiological functions in fish. The expression of IEGs such as, c-Fos, Egr1 and arc are selectively and promptly up-regulated in learning and memory among neuronal subpopulations in regions associated with these processes (Gallo et al., 2018). We found an incease in c-Fos expression in nicotine–exposed fish at 7 days of withdrawal. We found an increase in c-Fos expression in nicotine–exposed fish at 7 days of withdrawal. Fos family proteins are transcription factors, believed to act as initiators of long-term cellular changes in response to different kinds of stimulation. Thus the increase of c-Fos is consistent with withdrawal inducing enhanced neuronal activity and long term changes affecting neurotransmission and behaviour. Further studies are required to determine the brain regions and specific neuronal circuits that may be involved. However, Following exposure to nicotine, adult zebrafish have been found previously to preferentially up-regulate Fos expression in the interpeduncular nucleus (IPN) following exposure to nicotine (Hong et al., 2013). Similarly, mecamylamine-precipitated withdrawal in mice, chronically treated with nicotine four times daily for 7 days led to an increase of c-Fos expression within the IPN indicating enhanced neuronal activity in this region during nicotine withdrawal (Varani et al., 2014 ).
Taken together, our findings show for the first time that, two weeks nicotine exposure induced behavioural alterations during short- and long-term withdrawal. Increased anxiety, cognitive impairment and reduced motivation were accompanied by changes in the expression of nicotinic receptor, serotonergic receptor and c-Fos mRNA levels and TH increase in pretectal neurons. The evaluation of gene expression in specific brain regions will be the subject of future studies together with binding studies on nicotinic receptors. Our findings provide evidence for nicotine-induced long-lasting neuroadaptations which may underlie the observed long-term behavioural changes.
Supplementary Material
Treatment paradigm and experimental timeline
Primer pairs for the genes examined in quantitative real-time PCR analysis.
Figure 2.
Biochemical determination of nicotine in the tank water during 14 days exposure (a) and binding studies (b) evaluated at 7 and 14 days after nicotine exposure in the whole brain. 3H-Epi = Epibatidine,125αBgtx = Bungarotoxin. CTR= drug-free group. Data are mean ± SEM of three replicate analyses for nicotine determination and 4 replicates (10 fish each) for binding studies. WDW=withdrawal. **p < 0.01 vs pre and 48 h, #p < 0.05 vs CTR, $ $p < 0.01 vs 1h (one–way ANOVA, Tukey’s test).
Highlights.
Mecamylamine-precipitated withdrawal increases freezing and decreased exploration
3H-Epibatidine labeled nAChR significantly increases after nicotine exposure
Anxiety, reduced motivation, decreased memory attention is shown after withdrawal
mRNA expression of chrnα7 and htr2A receptors decreases while that of c-Fos increases
TH fluorescence intensity increases in dorsal parvocellular pretectal nucleus
Acknowledgements
We thank Zardi-Gori Foundation for providing fellowship to L.P. and Dr Ike Olivotto for providing us with zebrafish from his facility.
Funding
NIH programme grant 1 U01 DA044400–01A1 to CHB. We thank Zardi-Gori Foundation for providing fellowship to L.P. and Dr Ike Olivotto for providing us with zebrafish from his facility.
Footnotes
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Statement
All protocols were approved by the National Ethic Committee for the care and use of laboratory animals and by the National Ministry of Health with the Italian Government Decree N° 323/2018 PR and 513/2018 PR.
Publisher's Disclaimer: This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abreu-Villaça Y, Guimarães VMS, Nunes-Freitas A, Dutra-Tavares AC, Manhães AC, Filgueiras CC, Ribeiro-Carvalho A, 2019.Tobacco smoke and ethanol during adolescence: Both combined- and single-drug exposures lead to short- and long-term disruption of the serotonergic system in the mouse brain. Brain Res. Bull 146,94–103. 10.1016/j.brainresbull.2018.12.007. [DOI] [PubMed] [Google Scholar]
- Ackerman KM, Nakkula R, Zirger JM, Beattie CE, Boyd RT, 2009. Cloning and spatiotemporal expression of zebrafish neuronal nicotinic acetylcholine receptor alpha 6 and alpha 4 subunit RNAs. Dev. Dyn 238(4):980–992. 10.1002/dvdy.21912238:980992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asthana A, Rastogi R, Sunita G, Gupta VK, 2004. A Simple Spectrophotometric Method for the Determination of Nicotine in Environmental Samples. J. Chin. Chem. Soc 51:949–953. 10.1002/jccs.200400141. [DOI] [Google Scholar]
- Barbot A, Carrasco M, 2018. Emotion and anxiety potentiate the way attention alters visual appearance. Sci. Rep 8(1):5938. 10.1038/s41598-018-23686-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begh R, Munafò MR, Shiffman S, Ferguson SG, Nichols L, Mohammed MA, et al. , 2015. Lack of attentional retraining effects in cigarette smokers attempting cessation: a proof of concept double-blind randomised controlled trial. Drug Alcohol Depend. 149:158–165. 10.1016/j.drugalcdep.2015.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bencan Z, Levin ED, 2008.The role of alpha7 and alpha4beta2 nicotinic receptors in the nicotine-induced anxiolytic effect in zebrafish. Physiol. Behav 95(3):408–412. 10.1016/j.physbeh.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braida D, Ponzoni L, Martucci R, Sparatore F, Gotti C, Sala M, 2014a. Role of neuronal nicotinic acetylcholine receptors (nAChRs) on learning and memory in zebrafish. Psychopharmacology (Berl) 23:1975–1985. 10.1007/s00213-013-3340-1. [DOI] [PubMed] [Google Scholar]
- Braida D, Ponzoni L, Martucci R, Sala M, 2014b. A new model to study visual attention in zebrafish. Progr. Neuropsychopharmacol. Biol. Psychiatry 5:80–86. 10.1016/j.pnpbp.2014.03.010. [DOI] [PubMed] [Google Scholar]
- Cachat J, Canavello P, Elegante M, Bartels B, Hart P, Bergner C,et al. , 2010a. Modeling withdrawal syndrome in zebrafish. Behav. Brain Res 208:371–376. 10.1016/j.bbr.2009.12.004. [DOI] [PubMed] [Google Scholar]
- Cachat J, Kyzar EJ, Collins C, Gaikwad S, Green J, Roth A, et al. , 2013. Unique and potent effects of acute ibogaine on zebrafish: the developing utility of novel aquatic models for hallucinogenic drug research. Behav. Brain Res 236:258–269. 10.1016/j.bbr.2012.08.04.1 [DOI] [PubMed] [Google Scholar]
- Cachat J, Stewart A, Grossman L, Gaikwad S, Kadri F, Chung KM, et al. , 2010b. Measuring behavioral and endocrine responses to novelty stress in adult zebrafish. Nat. Protoc 5: 1786–1799. 10.1038/nprot.2010.140. [DOI] [PubMed] [Google Scholar]
- Castanê A, Valjent E, Ledent C, Parmentier M, Maldonado R, Valverde O, 2002. Lack of CB1 cannabinoid receptors modifies nicotine behavioral responses, but not nicotine abstinence. Neuropharmacology 43:857–867. 10.1016/s0028-3908(02)00118-1. [DOI] [PubMed] [Google Scholar]
- Cook JW, Piper ME,, Leventhal AM, et al. , 2015. Anhedonia as a component of the tobacco withdrawal syndrome. J. Abnorm. Psychol 124:215–225. 10.1037/abn0000016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damaj MJ, Kao W, Martin BR, 2003. Characterization of spontaneous and precipitated nicotine withdrawal in the mouse. J. Pharmacol. Exp Ther 307(2):526–534. 10.1124/jpet.103.054908. [DOI] [PubMed] [Google Scholar]
- Demin KA, Kolesnikova TO, Khatsko SL, Meshalkina DA, Efimova EV, Morzherin YY, Kalueff AV, 2017. Acute effects of amitriptyline on adult zebrafish: Potential relevance to antidepressant drug screening and modeling human toxidromes. Neurotoxicology and Teratology 62: 27–33. 10.1016/j.ntt.2017.04.002. [DOI] [PubMed] [Google Scholar]
- Dewari PS, Ajani F, Kushawah G, Kumar DS, Mishra RK, 2016. Reversible loss of reproductive fitness in zebrafish on chronic alcohol exposure. Alcohol 50:83–89. Alcohol 50:83–9. 10.1016/j.alcohol.2015.11.006. [DOI] [PubMed] [Google Scholar]
- Edwards JG, Greig A, Sakata Y, Elkin D, Michel WC, 2007. Cholinergic innervation of the zebrafish olfactory bulb. J. Comp. Neurol 504:631–645. 10.1002/cne.21480. [DOI] [PubMed] [Google Scholar]
- Egan RJ, Bergner CL, Hart PC, Cachat JM, Canavello PR, Elegante MF, et al. , 2009. Understanding behavioral and physiological phenotypes of stress and anxiety in zebrafish. Behav.Brain Res 205:38–44. 10.1016/j.bbr.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engeszer RE, Patterson LB, Rao AA, Parichy DM, 2007. Zebrafish in the wild: a review of natural history and new notes from the field. Zebrafish 4:21–40. 10.1089/zeb.2006.9997. [DOI] [PubMed] [Google Scholar]
- Epping-Jordan MP, Watkins SS, Koob GF, Markou A, 1998. Dramatic decreases in brain reward function during nicotine withdrawal. Nature 393:76–79. 10.1038/30001. [DOI] [PubMed] [Google Scholar]
- Fei-Fei L, Fergus R, Perona P, 2007. Learning generative visual models from few training examples: an incremental Bayesian approach tested on 101 object categories. Computer Vision and Image Understanding 106:59–70. 10.1016/j.cviu.2005.09.012 [DOI] [Google Scholar]
- Fontana BD, Cleal M, Parker MO, 2019. Female adult zebrafish (Danio rerio) show higher levels of anxiety-like behavior than males, but do not differ in learning and memory capacity. Eur J Neurosci. 52(1):2604–2613. 10.1111/ejn.14588. [DOI] [PubMed] [Google Scholar]
- Gallo FT, Katche C, Morici JF, Medina JH, Weisstaub NV, 2018. Immediate Early Genes, memory and psychiatric Disorders: Focus on c-Fos, Egr1 and Arc. Front. Behav Neurosci 12;79. eCollection 2018. 10.3389/fnbeh.2018.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-González J, Brock AJ, Parker MO, Riley RJ, DavidJoliffe D, Sudwarts A, et al. , 2020. Identification of slit3 as a locus affecting nicotine preference in zebrafish and human smoking behaviour. Elife. Mar 25;9. pii: e51295. 10.7554/eLife.51295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlai R, Chatterjee D, Pereira T, Sawashima T, Krishnannair R, 2009. Acute and chronic alcohol dose:population differences in behaviour and neurochemistry of zebrafish. Genes, Brain Behav. 8:586–599. 10.1111/j.1601-183X.2009.00488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert D, McClernon J, Rabinovich N, Sugai C, Plath L, Asgaard G, et al. , 2004. Effects of quitting smoking on EEG activation and attention last for more than 31 days and are more severe with stress, dependence, DRD2 A1 allele, and depressive traits. Nicotine Tob. Res 6: 249–267. 10.1080/14622200410001676305. [DOI] [PubMed] [Google Scholar]
- Hall FS, Der-Avakian A, Gould TJ, Markou A, Shoaib M, Young JW, 2015. Negative affective states and cognitive impairments in nicotine dependence. Neurosci. Biobehav. Rev 168–185. 10.1016/j.neubiorev.2015.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X, Yang Z, Tsien JZ, 2011. A hierarchical probabilistic model for rapid object categorization in natural scenes. PLoS One 2011;6:e20002. 10.1371/journal.pone.0020002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holcombe A, Howorko A, Powell RA, Schalomon M, Hamilton TJ, 2013. Reversed scototaxis during withdrawal after daily-moderate, but not weekly-binge, administration of ethanol in zebrafish. PLoS One 8: e63319. 10.1371/journal.pone.0063319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong E, Santhakumar K, Akitake CA, Ahn SJ, Thisse C, Thisse B. et al. , 2013. Cholinergic left-right asymmetry in the habenulo-interpeduncular pathway. Proc. Natl. Acad. Sci. (U S A) 110:21171–21176. 10.1073/pnas.1319566110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson AB, Toma W, Contreras KM, 2003. The β3 subunit of the nicotinic acetylcholine receptor is required for nicotine withdrawal-induced affective but not physical signs or nicotine reward in mice. Pharmacol. Biochem. Behav 183:1–5. 10.1016/j.pbb.2019.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonkman S, Henry B, Semenova S, Markou A, 2005. Mild anxiogenic effects of nicotine withdrawal in mice. Eur J Pharmacol. 516(1):40–45. 10.1016/j.ejphar.2005.04.032. [DOI] [PubMed] [Google Scholar]
- Kacprzak V, Patel NA, Riley E, Yu L, Yeh J-RJ, Zhdanova IV, 2017. Dopaminergic control of anxiety in young and aged zebrafish. Pharmacol. Biochem. Behav 157, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedikian X, Faillace MP, Bernabeu R, 2013. Behavioral and molecular a nalysis of nicotine-conditioned place preference in zebrafish. PLoS One 8:e69453. 10.1371/journal.pone.0069453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny PJ, Gasparini F, Markou A, 2003. Group II metabotropic and alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionate (AMPA)/kainate glutamate receptors regulate the deficit in brain reward function associated with nicotine withdrawal in rats. J Pharmacol. Exp. The.r 306(3):1068–1076. 10.1124/jpet.103.052027. [DOI] [PubMed] [Google Scholar]
- Kily LJ, Cowe YC, Hussain O, Patel S, McElwaine S, Cotter FE, Brennan CH, 2008. Gene expression changes in a zebrafish model of drug dependency suggest conservation of neuro-adaptation pathways. J. Exp. Biol 211(Pt 10):1623–1634. 10.1242/jeb.014399. [DOI] [PubMed] [Google Scholar]
- Kistler C, Hegglinb D, Würbel H, König B, 2011. Preference for structured environment in zebrafish (Danio rerio) and checker barbs (Puntius oligolepis). Appl. Anim. Behav. Sci 135: 318–327. 10.1016/j.applanim.2011.10.014. [DOI] [Google Scholar]
- Klee EW, Ebbert JO, Schneider H, Hurt RD, Ekker SC, 2011. Zebrafish for the study of the biological effects of nicotine. Nicotine Tob. Res 13(5):301–312. 10.1093/ntr/ntr010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolesnikova TO, Khatsko SL, Shevyrin VA, Morzherin YY, Kalueff AV, 2017. Effects of a non-competitive N-methyl-d-aspartate (NMDA) antagonist, tiletamine, in adult zebrafish. Neurotoxicol. Teratol 59: 62–67. 10.1016/j.ntt.2016.11.009. [DOI] [PubMed] [Google Scholar]
- Koob GF, 2021. Drug Addiction: Hyperkatifeia/Negative Reinforcement as a Framework for Medications Development. Pharmacol. Rev 73(1):163–201. 10.1124/pharmrev.120.000083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kysil EV, Meshalkina DA, Frick EE, Echevarria DJ, Rosemberg DB, Maximino C, et al. , 2017. Comparative analyses of zebrafish anxiety-like behavior using conflict-based novelty tests. Zebrafish 14(3):197–208. 10.1089/zeb.2016.1415. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Goldberg SR, 2009. Effects of nicotine in experimental animals and humans: an update on addictive properties. Handb. Exp. Pharmacol 192: 335–367. 10.1007/978-3-540-69248_512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerman C, LeSage MG, Perkins KA, O’Malley SS, Siegel SJ, Benowitz NL, Corrigall WA, 2007. Translational research in medication development for nicotine dependence. Nat. Rev. Drug Discov 6:746–762. 10.1038/nrd2361. [DOI] [PubMed] [Google Scholar]
- López-Patiño MA, Yu L, Cabral H, Zhdanova IV, 2008. Anxiogenic effects of cocaine withdrawal in zebrafish. Physiol. Behav 93:160–171. 10.1016/j.physbeh.2007.08.013. [DOI] [PubMed] [Google Scholar]
- Markou A, (2008). Review. Neurobiology of nicotine dependence. Philos. Trans. R. Soc. Lond. B. Biol. Sci 363:3159–3168. 63(1507):3159–68. 10.1098/rstb.2008.0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinotti G, Di Nicola M,, Reina D, Andreoli S, Focà F, Cunniff A, Tonioni F, Bria P, Janiri L, 2008. Alcohol protracted withdrawal syndrome: the role of anhedonia. Subst. Use Misuse 43(3–4):271–84. 10.1080/10826080701202429. [DOI] [PubMed] [Google Scholar]
- Mathur P. Guo S, 2011. Differences of acute versus chronic ethanol exposure on anxiety-like behavioral responses in zebrafish. Behav. Brain Res. 1;219(2):234–239. 10.1016/j.bbr.2011.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews M, Varga ZM, 2012.Anesthesia and euthanasia in zebrafish ILAR J 53:192–204. 10.1093/ilar.53.2.192. [DOI] [PubMed] [Google Scholar]
- Melroy-Greif WE, Stitzel JA, Ehringer MA, 2016. Nicotinic acetylcholine receptors: upregulation, age-related effects and associations with drug use. Genes Brain Behav. 15: 89–107. 10.1111/gbb.12251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menelaou E, Udvadia AJ, Tanguay RL, Svoboda KR, 2014. Activation of α2A-containing nicotinic acetylcholine receptors mediates nicotine-induced motor output in embryonic zebrafish. Eur. J. Neurosci 40:2225–2240. 10.1111/ejn.12591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mocelin R, Marcon M, da Rosa Araujo AS, Herrmann AP, Piato A, 2019. Withdrawal effects following repeated ethanol exposure are prevented by N-acetylcysteine in zebrafish. Prog. Neuropsychopharmacol. Biol. Psychiatry 93:161–170. 10.1016/j.pnpbp.2019.03.014. [DOI] [PubMed] [Google Scholar]
- Moretti M, Fasoli F, Gotti C, Marks MJ, 2018. Reduced α4 subunit expression in α4+- and α4+- /β2+- nicotinic acetylcholine receptors alters α4β2 subtype up-regulation following chronic nicotine treatment. Br. J. Pharmacol 175(11):1944–1956. 10.1111/bph.13896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretti M, Zoli M, George AA, Lukas RJ, Pistillo F, Maskos U, et al. , 2014. The novel alpha7beta2-nicotinic acetylcholine receptor subtype is expressed in mouse and human basal forebrain: biochemical and pharmacological characterization. Mol. Pharmacol 86(3):306–317. 10.1124/mol.114.093377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morud J, Strandberg J, Andrén A, Ericson M, Söderpalm B, Adermark L, 2018. Progressive modulation of accumbal neurotransmission and anxiety-like behavior following protracted nicotine withdrawal. Neuropharmacology 128:86–95. 10.1016/j.neuropharm.2017.10.002. [DOI] [PubMed] [Google Scholar]
- Mugnaini M, Tessari M, Tarter G, Pich EM, Chiamulera C, Bunnemann B, 2002. Upregulation of [3H]methyllycaconitine binding sites following continuous infusion of nicotine, without changes of alpha7 or alpha6 subunit mRNA: an autoradiography and in situ hybridization study in rat brain. Eur. J. Neurosci 16(9):1633–1646. 10.1046/j.14609568.2002.02220.x. [DOI] [PubMed] [Google Scholar]
- Müller TE, Nunes SZ, Silveira A, Loro VL, Rosemberg DB, 2017. Repeated ethanol exposure alters social behavior and oxidative stress parameters of zebrafish. Prog. Neuropsychopharmacol. Biol. Psychiatry 79 (Pt B):105–111. 10.1016/j.pnpbp.2017.05.026. [DOI] [PubMed] [Google Scholar]
- Müller TE, Fontana BD, Bertoncello KT, Franscescon F, Mezzomo NJ, Canzian J, Stefanello FV, Parker MO, Gerlai R, Rosemberg DB,2020. Understanding the neurobiological effects of drug abuse: Lessons from zebrafish models. Prog Neuropsychopharmacol Biol. Psychiatry 8;100:109873. doi: 10.1016/j.pnpbp.2020.109873. [DOI] [PubMed] [Google Scholar]
- Nguyen HN, Rasmussen BA, Perry DC, 2004. Binding and functional activity of nicotinic cholinergic receptors in selected rat brain regions are increased following long-term but not short-term nicotine treatment. J. Neurochem 90(1):40–49. 10.1111/j.1471-4159.2004.02482.x. [DOI] [PubMed] [Google Scholar]
- Nguyen M, Stewart AM, Kalueff AV 2014. Aquatic blues: modeling depression and antidepressant action in zebrafish. Prog. Neuropsychopharmacol. Biol. Psychiatry 55:26–39. 10.1016/j.pnpbp.2014.03.003. [DOI] [PubMed] [Google Scholar]
- Nikiforuk A, Kos T, Potasiewicz A, Popik P, 2015. Positive allosteric modulation of alpha 7 nicotinic acetylcholine receptors enhances recognition memory and cognitive flexibility in rats. Eur. Neuropsychopharmacol 25(8):1300–13. 10.1016/j.euroneuro.2015.04.018. [DOI] [PubMed] [Google Scholar]
- Ockene JK, Emmons KM, Mermelstein RJ, Bonollo DS, Voorhees CC, Hollis JF, 2000. Relapse and maintenance issues for smoking cessation. Health Psychol. 19(1S):17–31. 10.1037/0278-6133.19.suppl1.17. [DOI] [PubMed] [Google Scholar]
- Papke RL, Ono F, Stokes C, Urban JM, Boyd RT, 2012. The nicotinic acetylcholine receptors of zebrafish and an evaluation of pharmacological tools used for their study. Biochem. Pharmacol 84(3):352–365. 10.1016/j.bcp.2012.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker MO, Evans AM-D, Brock AJ, Combe FJ, The MT, Brennan CH, 2016. Moderate alcohol exposure during early brain development increases stimulus-response habits in adulthood. Addict. Biol 21(1):49–60. 10.1111/adb.12176. [DOI] [PubMed] [Google Scholar]
- Pedersen JE, Bergqvist CA, Larhammar D, 2019. Evolution of vertebrate nicotinic acetylcholine receptors. BMC Evol Biol. 19(1):38. 10.1186/s12862-018-1341-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry DC, Dávila-García MI, Stockmeier CA, Perry DC, Kellar KJ, 1999. Increased nicotinic receptors in brains from smokers: membrane binding and autoradiography studies. J. Pharma. Exp. Ther 289:1545–1552. [PubMed] [Google Scholar]
- Petzold AM, Balciunas D, Sivasubbu S, Clark KJ, Bedell VM, Westcot SE, et al. , 2009. Nicotine response genetics in the zebrafish. Proc. Natl. Acad. Sci. (U S A) 106(44):18662–18667. 10.1073/pnas.0908247106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittman JT Ichikawa KM, 2013. iPhone® applications as versatile video tracking tools to analyze behavior in zebrafish (Danio rerio) Pharmacol. Biochem. Behav. 106:137–142. 10.1016/j.pbb.2013.03.013. [DOI] [PubMed] [Google Scholar]
- Ponzoni L, Braida D, Pucci L, Donzelli A, Fasoli F, Manfredi I, et al. , 2014. The cytisine derivatives, CC4 and CC26, reduce nicotine-induced conditioned place preference in zebrafish by acting on heteromeric neuronal nicotinic acetylcholine receptors. Psychopharmacology (Berl) 231(24):4681–4693. 10.1007/s00213-014-3619-x. [DOI] [PubMed] [Google Scholar]
- Ponzoni L, Moretti M, Sala M, Fasoli F, Mucchietto V, Lucini V, et al. , 2015. Different physiological and behavioural effects of e-cigarette vapour and cigarette smoke in mice. Eur. Neuropsychopharmacol 25(10):1775–86. 10.1016/j.euroneuro.2015.06.010. [DOI] [PubMed] [Google Scholar]
- Ponzoni L, Teh MT, Torres-Perez JV, Brennan CH, Braida D, Sala M, 2020. Increased response to 3,4-methylenedioxymethamphetamine (MDMA) reward and altered gene expression in zebrafish during short- and long-term nicotine withdrawal. Mol. Neurobiol 10.1007/s12035-020-02225-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro-Carvalho A, Lima CS, Medeiros AH, Siqueira NR, Filgueiras CC, Manhães AC, Abreu-Villaça Y, 2009. Combined exposure to nicotine and ethanol in adolescent mice: effects on the central cholinergic systems during short and long term withdrawal. Neuroscience 162(4):1174–1186. 10.1016/j.neuroscience.2009.05.032. [DOI] [PubMed] [Google Scholar]
- Richards M, Jarvis MJ, Thompson N, Wadsworth MEJ, 2003.Cigarette smoking and cognitive decline in midlife: evidence from a prospective birth cohort study. Am. J. Public Health 93(6):994–8. 10.2105/ajph.93.6.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossetti I, Zambusi L, Finardi A, Bodini A, Provini L, Furlan R, Morara S, 2018. Calcitonin gene-related peptide decreases IL-1beta, IL-6 as well as Ym1, Arg1, CD163 expression in a brain tissue context-dependent manner while ameliorating experimental autoimmune encephalomyelitis. J. Neuroimmunol 323,94–104. 10.1016/j.jneuroim.2018.07.005. [DOI] [PubMed] [Google Scholar]
- Rossetti I, Zambusi L, Maccioni P, Sau R, Provini L, Castelli MP, Gonciarz K, Colombo G, Morara S, 2019. Predisposition to alcohol drinking and alcohol consumption alter expression of calcitonin gene-related peptide, neuropeptide y, and microglia in bed nucleus of stria terminalis in a subnucleus-specific manner. Front. Cell Neurosci 13:158. 10.3389/fncel.2019.00158.eCollection2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang C-f., Li X-q., Yin C, Liu B, Wang Y-f., Zhou Z, Du J-l., 2015. Amperometric Monitoring of Sensory-Evoked Dopamine Release in Awake Larval Zebrafish. J. Neurosci 35(46):15291–4. 10.1523/JNEUROSCI.3050-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder P, Jones S, Young IS, Sneddon LU, 2014. What do zebrafish want? Impact of social grouping, dominance and gender on preference for enrichment. Lab Anim. 48(4):328–337. 10.1177/0023677214538239. [DOI] [PubMed] [Google Scholar]
- Semenova S, Stolerman IP, Markou A, 2007. Chronic nicotine administration improves attention while nicotine withdrawal induces performance deficits in the 5-choice serial reaction time task in rats. Pharmacol. Biochem. Behav 87(3):360–3568. 10.1016/j.pbb.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoaib M. Bizarro L, 2005. Deficits in a sustained attention task following nicotine withdrawal in rats. Psychopharmacology (Berl) 178(2–3):211–222. 10.1007/s00213-004-2004-6. [DOI] [PubMed] [Google Scholar]
- Skjei KL Markou A, 2003. Effects of repeated withdrawal episodes, nicotine dose, and duration of nicotine exposure on the severity and duration of nicotine withdrawal in rats. Psychopharmacology (Berl) 168(3):280–292. 10.1007/s00213-003-1414-1. [DOI] [PubMed] [Google Scholar]
- Speedie N, Gerlai R, Alarm substance induced behavioral responses in zebrafish (Danio rerio). Behav. Brain Res 188 (2008) 168–177. 10.1016/j.bbr.2007.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence R, Fatema MK, Reichard M, Huq KA, Wahab MA, Ahmed ZF, Smith C, 2006. The distribution and habitat preferences of the zebrafish in Bangladesh. J. Fish Biology 69:1435–1448. 10.1111/j.1095-8649.2006.01206.x. [DOI] [Google Scholar]
- Stewart AM, Grossman L, Collier AD, Echevarria DJ, Kalueff AV, 2015. Anxiogenic-like effects of chronic nicotine exposure in zebrafish. Pharmacol. Biochem. Behav 139 Pt B:112–120. 10.1016/j.pbb.2015.01.016. [DOI] [PubMed] [Google Scholar]
- Swain HA, Sigstad C, Scalzo FM, 2004. Effects of dizocilpine (MK801) on circling behavior, swimming activity and place preference in zebrafish (Danio rerio). Neurotoxicol. Teratol 26(6):725–729. 10.1016/j.ntt.2004.06.009. [DOI] [PubMed] [Google Scholar]
- Tang R, Dodd A, Lai D, McNabb WC, Love DR, 2007. Validation of zebrafish (Danio rerio) reference genes for quantitative real-time RT-PCR normalization. Acta Biochim. Biophys. Sin. (Shanghai) 39(5):384–390. 10.1111/j.1745-7270.2007.00283.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas LT, Welsh L, Galvez F, Svoboda KR, (2009.) Acute nicotine exposure and modulation of a spinal motor circuit in embryonic zebrafish. Toxicol. Appl. Pharmacol 239(1):1–12. 10.1016/j.taap.2008.08.023. [DOI] [PubMed] [Google Scholar]
- Tran S, Chatterjee D, Gerlai R. 2015. An integrative analysis of ethanol tolerance and withdrawal in zebrafish (Danio rerio). Behav. Brain Res 276:161–70. 10.1016/j.bbr.2014.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran S, Gerlai R,. 2013. Time-course of behavioural changes induced by ethanol in zebrafish (Danio rerio) Behav. Brain Res. 252:204–213. 10.1016/j.bbr.2013.05.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varani AP, Moutinho Machado L, Balerio GN, 2014. Baclofen prevented the changes in c-Fos and brain-derived neutrophic factor expressions during mecamylamine-precipitated nicotine withdrawal in mice. Synapse 68(11):508–17. 10.1002/syn.21763. [DOI] [PubMed] [Google Scholar]
- Vlachou S, Paterson NE, Guery S, Kaupmann K, Froestl W, Banerjee D, et al. , 2011. Both GABA(B) receptor activation and blockade exacerbated anhedonic aspects of nicotine withdrawal in rats. Eur. J. Pharmacol 655(1–3):52–58. 10.1016/j.ejphar.2011.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow N, Morales M, 2015. The brain on drugs: from reward to addiction. Cell 162(4):712–725. 10.1016/j.cell.2015.07.046. [DOI] [PubMed] [Google Scholar]
- World Health Organization (WHO). Global Health Observatory (GHO) data 2015. Prevalence of tobacco smoking. 2015. Retrieved fromhttp://www.who.int/gho/tobacco/use/en/.
- World Health Organization Tobacco. (2016) Available from: http://www.who.int/mediacentre/factsheets/fs339/en/
- Woods IG, Schoppik D, Shi VJ, Zimmerman S, Coleman HA, Greenwood J, et al. , 2014. Neuropeptidergic signaling partitions arousal behaviors in zebrafish. J. Neurosci 34(9):31423160. 10.1523/JNEUROSCI.3529-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaniewska M, McCreary AC, Wydra K, Małgorzata F, 2010. Effects of serotonin (5-HT2) receptor ligands on depression-like behavior during nicotine withdrawal. Neuropharmacology 58(7):1140–1146. 10.1016/j.neuropharm.2010.02.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Treatment paradigm and experimental timeline
Primer pairs for the genes examined in quantitative real-time PCR analysis.