Abstract
Background/Aims:
Phthalate exposure is associated with altered reproductive function, but little is known about associations between phthalate and hormone levels in midlife women.
Methods:
This cross-sectional analysis includes 45–54-year-old pre- and perimenopausal women from Baltimore, MD and its surrounding counties enrolled in the Midlife Women’s Health Study (n=718). Serum and urine samples were collected from participants once a week for four consecutive weeks to span the menstrual cycle. Serum samples were assayed for estradiol, testosterone, progesterone, sex hormone binding globulin (SHBG), follicle-stimulating hormone (FSH), and anti-Müllerian hormone (AMH), and geometric means were calculated for each hormone across all four weeks. Urine samples were analyzed for nine phthalate metabolites from pools of one-to-four urine samples. Phthalate metabolite concentrations were specific gravity-adjusted and assessed as individual metabolites or as molar sums of metabolites from common parents (di(2-ethylhexyl) phthalate metabolites, ∑DEHP), exposure sources (plastic, ∑Plastics; personal care products, ∑PCP), biological activity (anti-androgenic, ∑AA), and sum of all metabolites (∑Phthalates). We used linear regression models to assess overall associations of phthalate metabolites with hormones, controlling for important demographic, lifestyle, and health factors. We also explored whether associations differed by menopause status, body mass index (BMI), and race/ethnicity.
Results:
Most participants were non-Hispanic white (67%) or black (29%), college-educated (65%), employed (80%), and had somewhat higher mean urinary phthalate metabolite concentrations than other U.S. women. Overall, the following positive associations were observed between phthalate metabolites and hormones: ∑DEHP (%Δ: 4.9; 95%CI: 0.5, 9.6), ∑Plastics (%Δ: 5.1; 95%CI: 0.3, 10.0), and ∑AA (%Δ:7.8; 95%CI: 2.3, 13.6) with estradiol; MiBP (%Δ: 6.6; 95%CI: 1.5, 12.1) with testosterone; ∑DEHP (%Δ: 8.3; 95%CI: 1.5, 15.6), ∑Plastics (%Δ: 9.8; 95%CI: 2.4, 17.7), MEP (%Δ: 4.6; 95%CI: 0.1, 9.2), ∑PCP (%Δ: 6.0; 95%CI: 0.2, 12.2), ∑Phthalates (%Δ: 9.0; 95%CI: 2.1, 16.5), and ∑AA (%Δ: 12.9; 95%CI: 4.4, 22.1) with progesterone; and MBP (%Δ: 8.5; 95%CI: 1.2, 16.3) and ∑AA (%Δ: 9.0; 95%CI: 1.3, 17.4) with AMH. Associations of phthalate metabolites with hormones differed by menopause status (strongest in premenopausal women for estradiol, progesterone, and FSH), BMI (strongest in obese women for progesterone), and race/ethnicity (strongest in non-Hispanic white women for estradiol and AMH).
Conclusions:
We found that phthalate metabolites were positively associated with several hormones in midlife women, and that some demographic and lifestyle characteristics modified these associations. Future longitudinal studies are needed to corroborate these findings in more diverse midlife populations.
INTRODUCTION
Phthalates are commonly used to impart strength and flexibility to a variety of plastic products (1, 2). Additionally, low molecular weight phthalates are often used in personal care products to stabilize scents and colors (1, 2). Phthalates are non-covalently bound to the products in which they are used, allowing them to leach from products over time and resulting in human exposure on a daily basis (3, 4). Phthalates used in food and consumer good production can lead to human exposure by ingestion of foods contaminated with phthalates through processing or packaging and dermal absorption through use of phthalate-containing personal care products and clothing (2, 5, 6). Additionally, people undergoing medical procedures are exposed to phthalates directly via medical devices (7, 8). Further, humans are exposed through routes such as inhalation of house dust and air contaminated with phthalates (9). Although phthalate exposure is ubiquitous in humans, exposure levels vary between populations and even sex. Women have higher exposure to phthalates than men, potentially due to their greater use of personal care products compared to men (10, 11). In fact, studies often find that phthalate metabolites are detectable in 99–100% of samples submitted by women (12, 13), making women an especially vulnerable population.
Phthalate exposure is of concern because phthalates have been shown to have endocrine disrupting capabilities (14–18). Epidemiological studies have shown that phthalates are associated with altered hormone levels in both men and women (19–22). Although several epidemiological studies have focused on phthalate exposure in a variety of populations, few studies have investigated health outcomes associated with phthalate exposure in mid-life women. Some studies in older women have shown associations between phthalates and health outcomes such as bone mineral density, hot flash experience, and weight change (23–25). However, less is known about the relationship between phthalates and health outcomes during the menopausal transition (i.e. perimenopause) because most studies have thus far investigated women that classify as either pre- or postmenopausal.
The transition into the menopausal state is an event known for its hormonal fluctuations and discomforts. This transition begins when the ovaries undergo follicular exhaustion, which results in a shift in the hormonal milieu during the menopausal transition (26). In a cycling woman, the ovary is the primary source of the sex steroid hormones estradiol, progesterone, and testosterone (27). These sex steroid hormones interact with the hypothalamus and pituitary to affect the production of the gonadotropins, follicle stimulating hormone (FSH) and luteinizing hormone (LH), from the pituitary. As the ovary produces fewer sex steroid hormones with age, the negative feedback exerted by the ovarian hormones on the hypothalamus and pituitary is alleviated, leading to an increase in the release of FSH and LH (28). Additionally, in cycling women, anti-Mϋllerian hormone (AMH) is synthesized by cells within small, growing ovarian follicles, leading to high levels of AMH during prime reproductive years (29). Depletion of the ovarian reserve during aging leads to a loss of follicles that produce AMH, and subsequently, AMH levels decline (26). Thus, the hormonal profile of the non-cycling woman (i.e. postmenopausal) can generally be characterized as having lower levels of sex steroid hormones and AMH and higher levels of gonadotropins (30).
The primary objective of this study was to address a gap in previous knowledge about the associations between phthalate levels and hormones that fluctuate during the menopause transition. To do so, we investigated the overall associations of common urinary phthalate metabolites with reproductive hormones including estradiol, testosterone, progesterone, sex hormone binding globulin (SHBG), AMH, and FSH in the Midlife Women’s Health Study (MWHS). Because hormone levels may differ in women based on menopause status, midlife body mass index (BMI), and race/ethnicity, the secondary objective of this study was to evaluate differences in associations of phthalate metabolites with reproductive hormones by these characteristics (31–35).
METHODS
Midlife Women’s Health Study Cohort
The Midlife Women’s Health Study Cohort (MWHS) is a longitudinal population-based study that recruited women from Baltimore, MD (USA) and its surrounding counties between the ages of 45 and 54 in 2006–2015. The full study protocol has been previously published (34). Briefly, women were eligible if they had 3 or more periods within the past 12 months (pre- or perimenopausal), had no history of oophorectomy or hysterectomy, were not on hormone therapy, were not taking botanical supplements to alleviate menopausal symptoms, were not on oral contraceptives, were not pregnant, were not undergoing cancer treatment, and had no history of ovarian, breast, or endometrial cancer. The current study focused on year 1 of MWHS and included a total of 718 women who had information about reproductive hormones, urinary phthalate metabolite concentrations and/or specific gravity, and covariates (described in the statistical analysis section). All women gave written and informed consent according to procedures that were approved by the University of Illinois Review Board.
Demographic and lifestyle characteristics
At the baseline visit, participants filled out a detailed questionnaire about their demographic information, as well as lifestyle characteristics such as alcohol consumption, physical activity, and smoking status. Information on racial and ethnic background was obtained by asking women to choose their most representative race/ethnicity from the following options: Caucasian/white, African American/black, Hispanic, Asian, or other. Alcohol consumption was ascertained by asking women whether they consumed 12 alcoholic drinks in the past year (answer: yes, no). Women self-reported their leisure physical activity compared to others, and this was categorized as physically active much more or more than others, as much as others, or less or much less than others. Lifetime smoking status was self-reported as current, former, or never. Women who reported having at least 1 menstrual period within the last 3 months and at least 11 menstrual periods over the last year were considered premenopausal. Women were classified as perimenopausal if they experienced at least one menstrual period over the last year, but not the past 3 months, or if they experienced a menstrual period within the past 3 months, but had experienced 10 or fewer menstrual periods over the last year. At clinic visits, women were asked to list medications (over the counter and prescription) that they were currently taking. Additionally, at the first clinic visit, women had their height and weight measured by clinic staff and values rounded to the nearest 0.5 pound and 0.5 inch. Body mass index (BMI) in kg/m2 was calculated using the National Institutes of Health on-line BMI calculator.
Collection and measurement of hormones
Women visited the clinic once a week for up to four consecutive weeks for collection of serum samples. Visits to the clinic occurred in the morning to minimize fluctuation in hormones (36, 37). Levels of circulating hormones were measured in serum samples, which were stored at −20 °C prior to measurement. DRG® enzyme-linked immunosorbent assay (ELISA) kits were used to measure levels of estradiol, progesterone, testosterone, and SHBG. Lypocheck® from Bio-Rad Laboratories was used as a control with known values for estradiol, progesterone, testosterone, and SHBG for every assay of these hormones. All samples, controls, and standards were run in duplicates. The limits of detection (LODs) for estradiol, progesterone, testosterone, and SHBG were 9.714 pg/mL, 0.045 ng/mL, 0.083 ng/mL, and 0.77 nmol/L, respectively. The inter- and intra-assay %CVs were all ≤ 10.0, with the exception of estradiol which was ≤ 14.9.
Aliquots of serum from the first visit of each patient were submitted to the University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core for measurement of levels of AMH and FSH. AMH was assayed via ELISA, and FSH was measured via radioimmunoassay (RIA). The LODs for AMH and FSH were 0.2 ng/mL and 0.1 mIU/mL, respectively. The intra- and inter-assay %CVs for AMH were 3.9 and 6.2, respectively. The intra- and inter-assay %CVs for FSH were 3.2% and 4.9%, respectively.
Measurement of phthalate metabolites in urine
During the same visit in which women donated serum, spot urine samples were also collected. Each woman provided at least one and up to four urine samples (sample number was dependent on the number of clinic visits completed by each woman), which were pooled for each participant to measure phthalate metabolite concentrations. Due to the short half-lives of phthalates in the body and high within person variability of measured concentrations, previous studies have shown that a pooled sample better represents phthalate exposure compared to a single urine sample (38, 39). Urine samples were stored at −80 °C prior to measurement. Phthalates were measured in pooled urine samples via isotope dilution high-performance liquid chromatography negative-ion electrospray ionization-tandem mass spectrometry (HPLC-MS/MS) at the Metabolomics Center of the Roy J. Carver Biotechnology Center at the University of Illinois at Urbana-Champaign. Phthalate metabolites measured included: mono-2-ethylhexyl phthalate (MEHP), mono-(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP), mono-(2-ethyl-5-oxohexyl)phthalate (MEOHP), mono-(2-ethyl-5-carboxypentyl) phthalate (MECPP), mono-(3-carboxypropyl) phthalate (MCPP), mono-benzyl phthalate (MBzP), monoethyl phthalate (MEP), monobutyl phthalate (MBP), and mono-isobutyl phthalate (MiBP). The limits of detection (LOD) for each phthalate metabolite were as follows: MEHP: 0.2 ng/mL; MEHHP: 0.05 ng/mL; MEOHP: 0.1 ng/mL; MECPP: 0.05 ng/mL; MCPP: 0.05 ng/mL; MBzP: 0.05 ng/mL; MEP: 0.1 ng/mL; MBP: 0.05 ng/mL; and MiBP: 0.1 ng/mL. In addition, the intra-assay and inter-assay CVs were below 5%. Further, all standard curves had correlation coefficient values larger than 0.992 and all runs included internal standards.
Statistical Analysis
To evaluate associations of midlife urinary phthalate metabolite concentrations with hormone concentrations, covariates were chosen a priori and based on previous literature that informed a directed acyclic graph (Supplemental Figure 1). We assessed correlations among all covariates to evaluate potential multicollinearity issues and found that none of the covariates were strongly correlated with each other. Final statistical models evaluating overall associations of urinary phthalate metabolite concentrations with midlife hormones were adjusted for age, race/ethnicity, employment status, education, annual family income, marital status, menopausal status, alcohol consumption status, smoking status, physical activity, midlife BMI, and current medication use. Age and income were included as continuous variables, while the other covariates were categorized with reference groups set as shown in Table 1. For our secondary objective, we a priori stratified our analyses as follows: pre- versus perimenopausal women; under-/normal weight (BMI < 25.0 kg/m2), overweight (BMI ≥ 25.0 – 29.9 kg/m2), versus obese (BMI ≥ 30.0 kg/m2) women; and non-Hispanic white versus black/other women. All stratified models included the previously listed covariates.
Table 1.
Demographic and lifestyle characteristics of women from MWHS (n=718).
| Demographic or Lifestyle Characteristic | n (%) |
|---|---|
| Age (years) | |
| 45 to 49 | 469 (65.3) |
| 50 to 54 | 249 (34.7) |
| Race/ethnicity | |
| Non-Hispanic white (ref) | 477 (66.4) |
| Black/Other1 | 241 (33.6) |
| Employment status | |
| Unemployed | 143 (19.9) |
| Employed (ref) | 575 (80.1) |
| Education | |
| Some college or less | 251 (35.0) |
| College graduate or higher (ref) | 467 (65.0) |
| Annual family income ($) | |
| <20,000 | 47 (6.5) |
| 20,000 to 39,999 | 117 (16.3) |
| 40,000 to 99,999 | 243 (33.8) |
| ≥100,000 | 311 (43.3) |
| Marital status | |
| Single | 129 (18.0) |
| Married/Living with Partner (ref) | 466 (64.9) |
| Widowed/divorced/separated | 123 (17.1) |
| Menopausal status | |
| Premenopausal (ref) | 461 (64.2) |
| Perimenopausal | 257 (35.8) |
| Alcohol consumption status | |
| No drinks or <12 drinks over past year | 246 (34.3) |
| At least 12 drinks over past year (ref) | 472 (65.7) |
| Smoking status | |
| Current | 72 (10.0) |
| Former | 258 (35.9) |
| Never (ref) | 388 (54.0) |
| Leisure physical activity compared to others | |
| Much more/more (ref) | 258 (35.9) |
| As much | 223 (31.1) |
| Less/much less | 237 (33.0) |
| Body mass index (kg/m 2 ) | |
| Under-/normal weight (<25.0) (ref) | 288 (40.1) |
| Overweight (≥25.0–29.9) | 187 (26.0) |
| Obese (≥30.0) | 243 (33.8) |
| Current medication use | |
| None | 306 (42.6) |
| Any (ref) | 412 (57.4) |
Other includes Hispanic, Asian, or other race/ethnicity. MWHS, Midlife Women’s Health Study.
Urinary phthalate metabolite concentrations and serum hormone concentrations below the LOD were converted to the LOD/√(2). Because estradiol, testosterone, progesterone, and SHBG concentrations were assessed in multiple samples per participant, the geometric means of these hormones were calculated and used in statistical analyses. To account for differences in urine dilution, phthalate metabolite measurements were adjusted for specific gravity (SG) using the following formula: Pc = P[(1.018 − 1)/(SGi − 1)], where Pc is the specific gravity adjusted concentration, P is the measured concentration (ng/mL), 1.018 is the median specific gravity of the overall MWHS population included in this analysis, and SGi is the specific gravity of each woman’s pooled urine sample (40). Specific gravity-adjusted phthalate metabolite concentrations were used to approximate exposure to common phthalate parent compounds. DEHP metabolites (MEHP, MEHHP, MEOHP, MECPP) were molar converted and summed (nmol/mL) to estimate exposure to DEHP (∑DEHP). The concentrations of the other major urinary phthalate metabolites (MCPP, MBzP, MEP, MBP, MiBP) were not molar converted (ng/mL). Additional phthalate sums (nmol/mL) were created to estimate phthalate exposure based on sources of exposure (personal care products, plastics) and potential biological mechanism (anti-androgenic). MEP, MBP, and MiBP were molar summed to estimate exposure to personal care product phthalates (∑PCP), while MCPP, MBzP, MEHP, MEHHP, MEOHP, MECPP were molar summed to estimate exposure to phthalates found in plastics (∑Plastics). Phthalate metabolites that were shown in in vitro and in vivo studies to have anti-androgenic properties (MBzP, MEHP, MEHHP, MEOHP, MECPP, MBP, MiBP) were molar summed to approximate exposure to anti-androgenic phthalates (∑AA) (17, 41, 42). All phthalate metabolites were also molar-covered and summed to estimate total phthalate metabolite concentrations (∑Phthalates).
We used linear regression models to assess overall and stratified associations of midlife urinary phthalate concentrations with midlife hormones. We first evaluated overall associations of continuous phthalates with hormones. Both phthalate and hormone concentrations were natural log-transformed to better approximate normality assumptions in these generalized linear regression models. Second, we evaluated dose-response relationships of phthalates with hormones by categorizing urinary phthalate concentrations into quartiles; hormones were transformed as previously described. For our second objective, linear regression models were stratified by menopause status, midlife BMI, and race/ethnicity to evaluate differences in associations between phthalate metabolites and hormones by these factors.
All statistical analyses were conducted in SAS 9.4 (version 14.3, SAS Institute) using PROC GLM. In models where phthalates were assessed as continuous measures (objectives 1 and 2), β-estimates and 95% confidence intervals (CIs) were back-transformed using the equation [((2.00)β – 1)*100] to represent a percent change in hormones for each two-fold increase in phthalate concentration. For models where phthalates were categorized in quartiles, β-estimates and 95% CIs were back-transformed using the equation [(eβ – 1)*100] to represent the percent change in hormones among women in quartiles two (Q2), three (Q3), and four (Q4) of urinary phthalate concentrations, compared to the lowest quartile (Q1). We tested for linear trends (Plinear trend) across quartiles by assessing separate linear regression models that treated the ordinal phthalate variables as continuous. For models evaluating stratified associations of phthalates with hormones, we formally tested for effect modification (Pint) in linear regression by including multiplicative interactions between phthalates and menopause status, phthalates and race/ethnicity, and phthalates and BMI. However, we reported all stratified associations regardless of Pint significance.
RESULTS
MWHS demographics and lifestyle characteristics
At the time of enrollment, all women were between the ages of 45 and 54 years, with 65% of women being 49 years or younger (Table 1). In terms of racial background, 66% were non-Hispanic white and 34% were black or of other racial/ethnic background. The majority of the women were employed (80%), had a college education or higher (65%), and were premenopausal (64%). Most women reported being at least occasional drinkers (66%) and over half had a midlife BMI ≥ 25 kg/m2 (60%). Over half of women were never smokers (54%), 36% were former smokers, and only 10% were current smokers.
MWHS urinary phthalate metabolite concentrations
Median (25th, 75th percentile) urinary concentrations of phthalate metabolites are presented in Table 2. Greater than 99% of women had detectable concentrations (≥ LOD) of all urinary phthalate metabolites (data not shown). Median phthalate metabolite concentrations from our study were generally higher than those in a nationally representative sample of 45–54-year-old women from the National Health and Nutrition Examination Survey (NHANES), likely due to different subpopulations of women in the MWHS and NHANES studies and measurements of urinary metabolites by different laboratories. However, it is important to note that the 25th and 75th percentiles overlapped in metabolite levels between the two studies.
Table 2.
Phthalate metabolite concentrations in MWHS and NHANES.
| Name | Abbreviation | MWHS (2006–2015) n=718 | NHANES (2005–2016) n=7571 |
|---|---|---|---|
| Phthalate metabolitev | Median (25th, 75th percentile) in ng/mL | ||
| Mono(2-ethylhexyl) phthalate | MEHP | 4.5 (2.7, 9.4) | 1.2 (0.6, 3.1) |
| Mono(2-ethyl-5-hydroxyhexyl) phthalate | MEHHP | 33.7 (20.3, 58.1) | 9.1 (3.4, 22.5) |
| Mono(2-ethyl-5-oxohexyl) phthalate | MEOHP | 12 (7.3, 22.3) | 5.6 (2.1, 13.2) |
| Mono(2-ethyl-5-carboxypentyl) phthalate | MECPP | 25.8 (15.8, 48.1) | 13.4 (5.6, 31.7) |
| Mono(3-carboxypropyl) phthalate | MCPP | 2.5 (1.3, 5.4) | 1.4 (0.6, 3.4) |
| Monobenzyl phthalate | MBzP | 9.4 (5.4, 16) | 4.1 (1.8, 10.4) |
| Monoethyl phthalate | MEP | 95.4 (47.4, 192) | 58.8 (20.0, 179.6) |
| Mono-n-butyl phthalate | MBP | 19.7 (12.9, 32.6) | 11.5 (5.4, 25.3) |
| Mono-isobutyl phthalate | MiBP | 16.3 (9.8, 26.1) | 5.7 (2.6, 13.1) |
| Phthalate molar-converted sum | Median (25 th , 75 th percentile) in nmol/mL | ||
| Sum of di(2-ethylhexyl) phthalate metabolites | ∑DEHP2 | 0.3 (0.2, 0.5) | 0.1 (0.04, 0.2) |
| Sum of all plastic phthalate metabolites | ∑Plastics3 | 0.3 (0.2, 0.6) | 0.1 (0.1, 0.3) |
| Sum of all personal care product phthalate metabolites | ∑PCP4 | 0.7 (0.4, 1.3) | 0.4 (0.2, 1.2) |
| Sum of all phthalate metabolites | ∑Phthalates5 | 1.1 (0.7, 2) | 0.7 (0.3, 1.8) |
| Sum of anti-androgenic phthalate metabolites | ∑AA6 | 0.5 (0.3, 0.8) | 0.2 (0.1, 0.5) |
Weighted phthalate concentrations for 45–54 year-old US women from combined NHANES cycles 2005–06, 2007–08, 2009–10, 2011–12, 2013–14, and 2015–16. MWHS, Midlife Women’s Health Study; NHANES, National Health and Nutrition Examination Survey.
∑DEHP: MEHP, MEHHP, MEOHP, MECPP.
∑PCP: MEP, MBP, and MiBP.
∑Plastics): MCPP, MBzP, MEHP, MEHHP, MEOHP, MECPP.
∑Phthalates: all phthalate metabolites.
∑AA: MBzP, MEHP, MEHHP, MEOHP, MECPP, MBP, MiBP.
MWHS plasma hormone concentrations
Plasma hormone concentrations from year 1 of the MWHS are presented in Figure 1. Median (range) hormone concentrations were as follows: estradiol, 49.9 pg/mL (6.9 – 349.3); testosterone, 0.3 ng/mL (0.1 – 4.3); progesterone, 0.6 ng/mL (0.05 – 17.7); SHBG, 64.4 nmol/L (9.0 – 264.8); FSH, 11.3 mIU/mL (0.1 – 161.0); and AMH, 0.1 ng/mL (0.1 – 8.3).
Figure 1. Hormone concentrations of women from MWHS (n=718).
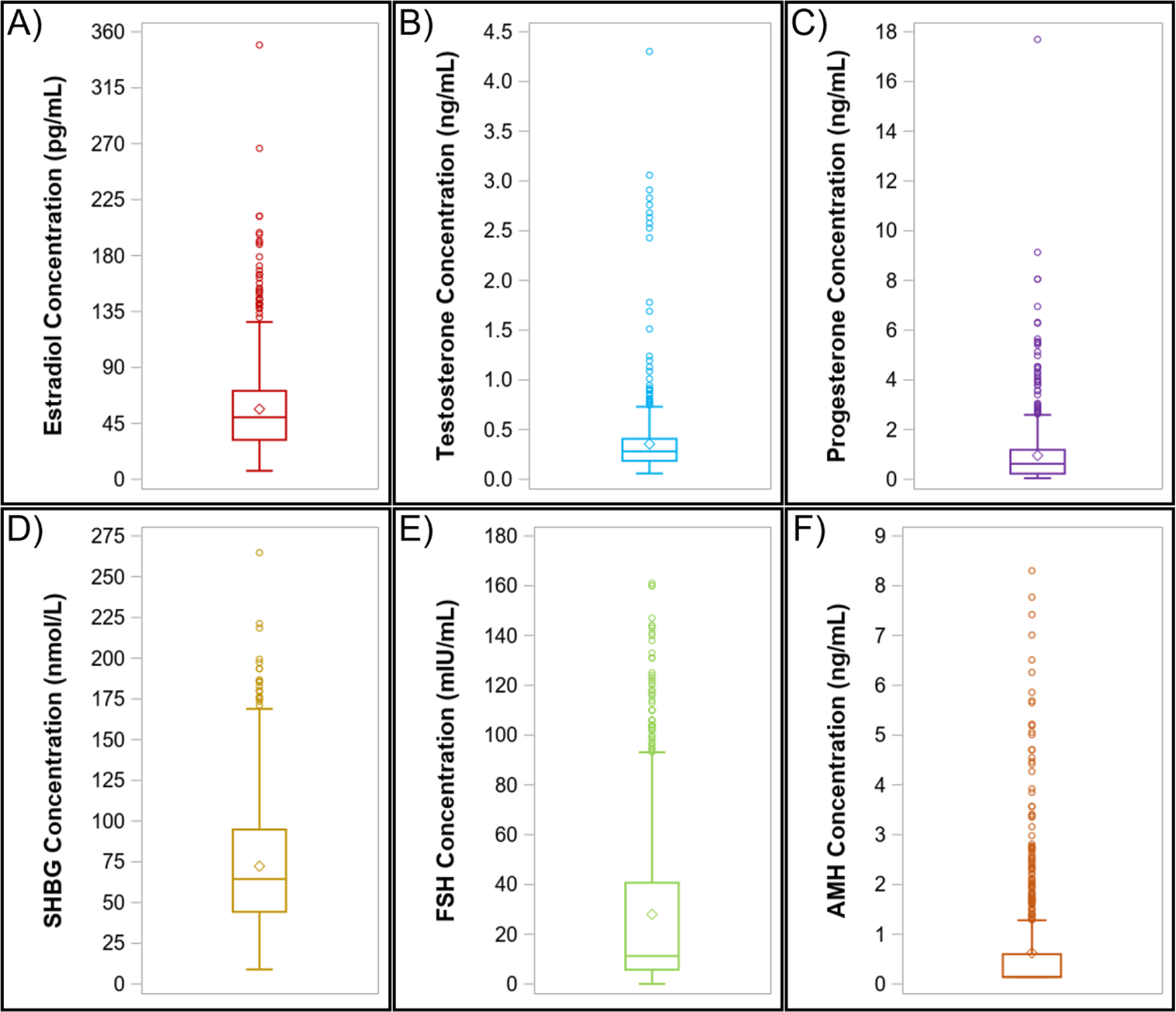
Mid-life A) estradiol, B) testosterone, C) progesterone, D) sex hormone binding globulin (SHBG), E) follicle stimulating hormone (FSH), and F) anti-Mullerian hormone (AMH) concentrations. Results are presented as 1.5 times the interquartile range below and above the 25th and 75th percentiles (lower and upper endpoints of whisker), the 25th and 75th percentiles (lower and upper edges of box), median (line inside box), and mean (diamond). MWHS, Midlife Women’s Health Study.
Overall associations of urinary phthalates with hormones
In linear regression models where phthalate metabolites were modeled continuously, select phthalates were positively associated with estradiol, testosterone, progesterone, and AMH, but not with SHBG or FSH (Table 3). Specifically, 2-fold increases in ∑DEHP, ∑Plastics, and ∑AA were associated with 4.9% (95%CI: 0.5, 9.6), 5.1% (95%CI: 0.3, 10.0), and 7.8% (95%CI: 2.3, 13.6) higher estradiol concentrations, respectively. Additionally, each 2-fold increase in MiBP was associated with 6.6% (95%CI: 1.5, 12.1) higher testosterone concentrations, whereas 2-fold increases in MBP and ∑AA were associated with 8.5% (95%CI: 1.2, 16.3) and 9.0% (95%CI: 1.3, 17.4) higher AMH concentrations, respectively. Lastly, 2-fold increases in ∑DEHP, ∑Plastics, MEP, ∑PCP, ∑Phthalates, and ∑AA were associated with 4.6 – 12.9% higher progesterone concentrations.
Table 3.
Overall linear associations of phthalate metabolites with hormones (n=718).
| Exposure | Estradiol | Testosterone | Progesterone | SHBG | FSH | AMH |
|---|---|---|---|---|---|---|
| % change in hormones (95%CI) | ||||||
| MCPP | 1.2 (−1.7, 4.3) | 0.1 (−3.0, 3.3) | 2.4 (−2.1, 7.2) | −2.2 (−4.5, 0.2) | −1.9 (−6.9, 3.5) | 4.2 (−0.1, 8.7) |
| MBzP | 1.2 (−2.8, 5.4) | −1.3 (−5.4, 3.0) | 3.5 (−2.6, 10.0) | 1.6 (−1.7, 4.9) | −4.4 (−10.9, 2.7) | 5.4 (−0.4, 11.6) |
| ∑DEHP | 4.9 (0.5, 9.6) | 1.4 (−3.1, 6.1) | 8.3 (1.5, 15.6) | 0.9 (−2.5, 4.5) | −3.7 (−10.8, 4.0) | 4.1 (−2.1, 10.7) |
| ∑Plastics | 5.1 (0.3, 10.0) | 1.6 (−3.2, 6.6) | 9.8 (2.4, 17.7) | 0.9 (−2.8, 4.7) | −4.7 (−12.2, 3.4) | 5.4 (−1.3, 12.5) |
| MEP | −0.2 (−3.1, 2.7) | 0.0 (−3.0, 3.0) | 4.6 (0.1, 9.2) | 0.3 (−2.1, 2.6) | 4.2 (−1.0, 9.7) | −1.8 (−5.7, 2.3) |
| MBP | 2.0 (−3.0, 7.1) | 2.9 (−2.3, 8.3) | 6.2 (−1.4, 14.4) | 1.1 (−2.8, 5.2) | −5.2 (−13.1, 3.4) | 8.5 (1.2, 16.3) |
| MiBP | 4.0 (−0.8, 9.0) | 6.6 (1.5, 12.1) | 5.3 (−2.0, 13.1) | 1.5 (−2.3, 5.5) | −2.9 (−10.7, 5.6) | 4.9 (−1.9, 12.1) |
| ∑PCP | 0.2 (−3.5, 4.1) | 0.8 (−3.1, 4.9) | 6.0 (0.2, 12.2) | 0.9 (−2.1, 4.0) | 3.7 (−2.9, 10.9) | −0.1 (−5.3, 5.4) |
| ∑Phthalates | 2.3 (−2.1, 6.9) | 1.1 (−3.4, 5.9) | 9.0 (2.1, 16.5) | 1.0 (−2.5, 4.6) | 2.1 (−5.5, 10.4) | 2.0 (−4.2, 8.5) |
| ∑AA | 7.8 (2.3, 13.6) | 3.5 (−2.0, 9.3) | 12.9 (4.4, 22.1) | 2.0 (−2.2, 6.4) | −6.9 (−15.1, 2.1) | 9.0 (1.3, 17.4) |
Data are presented as the % change in hormones for every 2-fold increase in phthalate metabolite (ng/mL or nmol/mL). Linear regression models adjusted for age, race/ethnicity employment status, education, income, marital status, alcohol consumption, smoking status, physical activity, medication use, menopausal status, and BMI. CI, confidence interval; AMH, anti-Mullerian hormone; BMI, body mass index; FSH, follicle stimulating hormone; SHBG, sex hormone binding globulin.
In analyses where phthalate metabolites were modeled in quartiles, phthalates were associated with all hormones, except for SHBG (Figure 2, Supplemental Table 1). Specifically, compared to those in Q1, estradiol concentrations were 18.7% (95%CI: 4.3, 35.0) higher in women at ∑AA Q4 (Plinear trend = 0.07; Figure 2a), whereas progesterone concentrations were 24.4% (95%CI: 1.8, 51.9) and 26.1% (95%CI: 3.8, 53.2) higher, respectively, in women in the highest quartile of ∑Phthalates (Plinear trend = 0.05) and ∑AA (Plinear trend = 0.04; Figure 2c). Compared to those in the lowest quartile, testosterone concentrations were 14.7% (95%CI: 0.0, 31.6) higher in women at MEP Q3 (Plinear trend = 0.81), as well as 18.5% (95%CI: 3.7, 35.5) and 23.0% (95%CI: 7.3, 41.0) higher, respectively, in women at MiBP Q2 and Q4 (Plinear trend = 0.05; Figure 2b). However, testosterone concentrations were 13.1% (95%CI: 0.5, 24.0) lower in women at ∑DEHP Q2 (Plinear trend = 0.28) compared to those in Q1. Compared to those in the lowest quartile, AMH concentrations were 23.1% (95%CI: 2.6, 47.7) and 19.9% (95%CI: 0.1, 43.7) higher in women at MBP Q2 and Q4 (Plinear trend = 0.09), and 20.7% (95%CI: 0.7, 44.6) higher in women at ∑AA Q3 (Plinear trend = 0.03; Figure 2f). Lastly, FSH concentrations were 31.3% higher (95%CI: 4.1, 65.5) higher in MEP Q3 compared to Q1 (Plinear trend = 0.88; Figure 2e).
Figure 2. Associations of phthalate metabolites in quartiles with hormones (n=718).
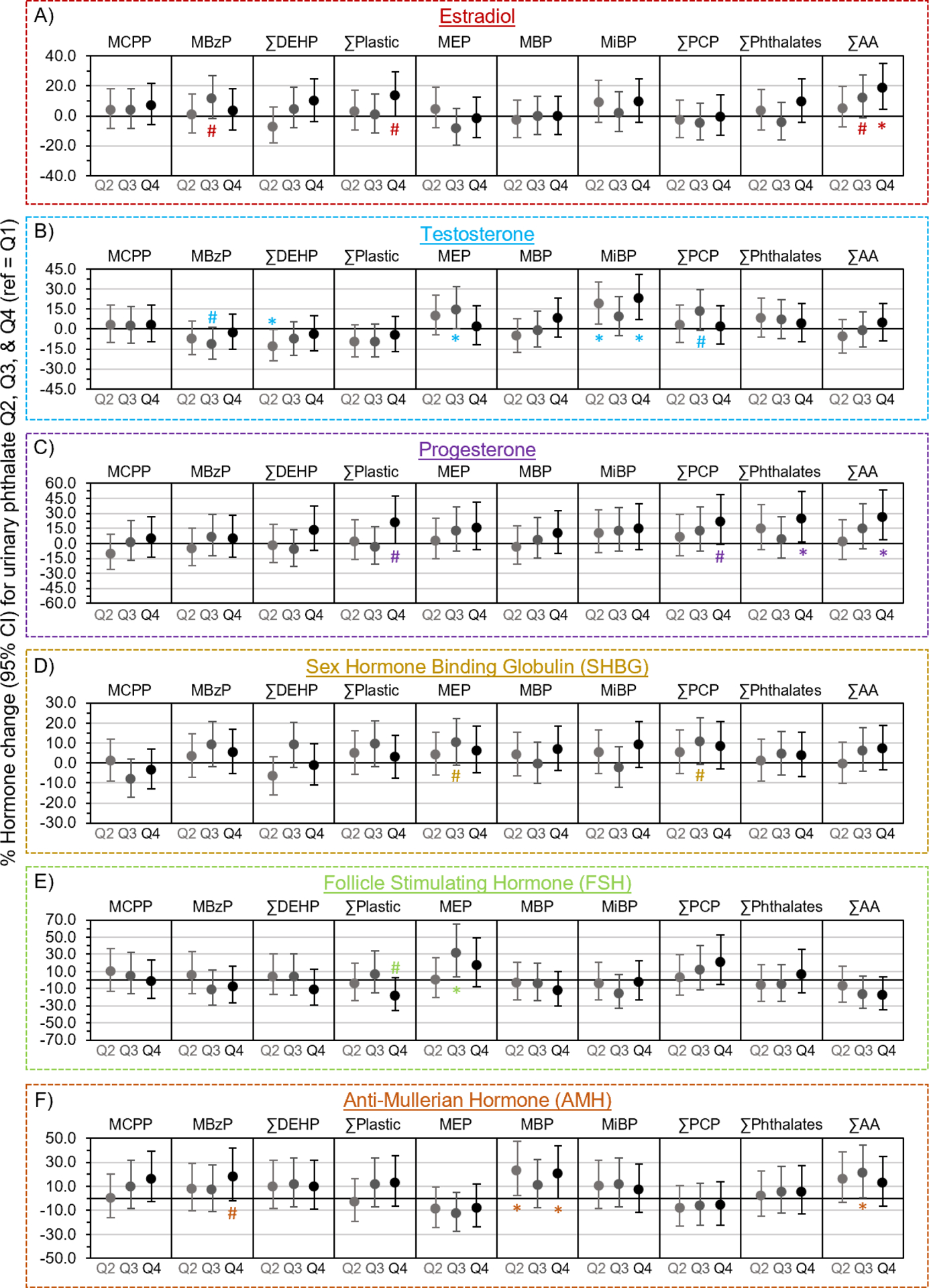
Multivariable generalized linear models evaluated associations of urinary phthalate concentrations with A) estradiol, B) testosterone, C) progesterone, D) sex hormone binding globulin (SHBG), E) follicle stimulating hormone (FSH), and F) anti-Mullerian hormone (AMH). Data are presented as % change (filled circles) and 95% confidence interval (solid lines) comparing phthalate quartiles 2 (Q2), 3 (Q3), and 4 (Q4) to quartile 1 (Q1). Models were adjusted for age, race, employment status, education, income, marital status, alcohol consumption, smoking status, physical activity, medication use, menopausal status, and body mass index. Confidence intervals that do not cross the null are significantly different from quartile 1 at #P<0.10 and *P<0.05.
Associations between phthalate metabolites and hormones stratified by menopause status
Associations of phthalates with estradiol, FSH, and AMH were only observed in premenopausal women (Table 4), in whom ∑DEHP, ∑Plastics, and ∑AA were positively associated with estradiol concentrations, MBzP, ∑Plastics, and ∑AA were negatively associated with FSH, while ∑AA was positively associated with AMH. Conversely, MiBP was positively associated with testosterone only in perimenopausal women (Table 4). Associations of phthalates with progesterone were observed in both pre- and perimenopausal women, but they differed depending on the phthalate metabolite (Table 4). ∑DEHP, ∑Plastics, and ∑AA were positively associated with progesterone in premenopausal women, whereas ∑Phthalates was positively associated with progesterone in perimenopausal women.
Table 4.
Associations of phthalate metabolites with hormones stratified by menopause status.
| Menopause Status | Phthalate | Estradiol | P int | Testosterone | P int | Progesterone | P int | SHBG | P int | FSH | P int | AMH | P int |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % change in hormones for every 2-fold increase in phthalate concentrations | |||||||||||||
| Pre | MCPP | 1.2 (−1.8, 4.2) | 0.77 | 0.3 (−3.4, 4.2) | 0.43 | 3.7 (−1.6, 9.3) | 0.35 | −2.5 (−5.2, 0.3) | 0.70 | −3.7 (−8.6, 1.5) | 0.21 | 3.8 (−2.3, 10.2) | 0.50 |
| Peri | 2.4 (−4.4, 9.7) | −1.7 (−7.4, 4.4) | 1.3 (−7.4, 10.9) | −1.5 (−6.3, 3.5) | 2.3 (−10.0, 16.3) | −0.4 (−3.9, 3.2) | |||||||
| Pre | MBzP | 1.8 (−2.2, 6.0) | 0.96 | 0.4 (−4.5, 5.6) | 0.26 | 4.9 (−2.2, 12.5) | 0.65 | 1.0 (−2.7, 4.9) | 0.59 | -9.0 (−15.0, −2.5) | 0.08 | 7.5 (−0.8, 16.5) | 0.15 |
| Peri | 1.4 (−7.6, 11.3) | −4.7 (−12.1, 3.2) | 0.0 (−11.4, 12.9) | 3.8 (−2.9, 10.9) | 7.3 (−9.7, 27.5) | 1.9 (−2.8, 6.9) | |||||||
| Pre | ∑DEHP | 6.0 (1.5, 10.8) | 0.99 | 0.0 (−5.4, 5.7) | 0.59 | 9.4 (1.4, 18.2) | 0.83 | 1 (−3.1, 5.2) | 0.76 | −7.1 (−13.9, 0.3) | 0.30 | 6.7 (−2.3, 16.5) | 0.08 |
| Peri | 2.0 (−7.2, 12.1) | 2.9 (−5.1, 11.6) | 5.0 (−7.2, 18.8) | 1.3 (−5.3, 8.4) | 4.2 (−12.5, 24.1) | −1.4 (−6.1, 3.5) | |||||||
| Pre | ∑Plastics | 5.8 (1.0, 10.8) | 0.77 | 0.8 (−4.9, 6.8) | 0.87 | 10.8 (2.2, 20.1) | 0.89 | 0.7 (−3.5, 5.2) | 0.69 | -8.8 (−15.8, −1.2) | 0.20 | 8.2 (−1.4, 18.8) | 0.06 |
| Peri | 3.0 (−7.0, 14.2) | 1.8 (−6.8, 11.3) | 6.6 (−6.8, 21.9) | 1.7 (−5.5, 9.5) | 5.5 (−12.8, 27.7) | −1.1 (−6.2, 4.2) | |||||||
| Pre | MEP | 1.3 (−1.6, 4.3) | 0.60 | −1.2 (−4.8, 2.5) | 0.13 | 4.2 (−1.1, 9.7) | 0.41 | 0.5 (−2.2, 3.3) | 0.71 | 1.7 (−3.4, 7.0) | 0.37 | −2.1 (−7.7, 3.9) | 0.99 |
| Peri | −1.4 (−7.4, 5.0) | 2.4 (−3.0, 8.1) | 7.8 (−0.6, 17) | 0.4 (−4.0, 5.0) | 7.0 (−4.7, 20.2) | −2.5 (−5.6, 0.7) | |||||||
| Pre | MBP | 3.5 (−1.7, 8.9) | 0.68 | 1.4 (−4.9, 8.2) | 0.69 | 8.4 (−0.9, 18.5) | 0.45 | −0.5 (−5.1, 4.4) | 0.15 | −7.5 (−15.3, 1.1) | 0.43 | 10.2 (−0.5, 22.1) | 0.32 |
| Peri | 1.8 (−8.3, 13.1) | 3.8 (−5.2, 13.6) | 1.6 (−11.4, 16.6) | 5.8 (−1.8, 14.1) | −0.1 (−17.8, 21.4) | 2.5 (−2.9, 8.2) | |||||||
| Pre | MiBP | 3.4 (−1.5, 8.6) | 0.51 | 2.7 (−3.4, 9.1) | 0.04 | 7.1 (−1.6, 16.5) | 0.60 | −0.2 (−4.6, 4.4) | 0.14 | −1.2 (−9.2, 7.5) | 0.56 | 2.6 (−6.9, 13.1) | 0.72 |
| Peri | 9.3 (−1.2, 20.9) | 14.8 (5.4, 25.2) | 4.3 (−8.6, 19.1) | 7.3 (−0.2, 15.4) | −7.3 (−23.3, 11.9) | 3.3 (−1.9, 8.9) | |||||||
| Pre | ∑PCP | 1.8 (−2.3, 6.1) | 0.70 | −0.7 (−5.6, 4.6) | 0.22 | 5.3 (−2.0, 13.1) | 0.54 | 1.0 (−2.8, 4.9) | 0.67 | 1.2 (−5.7, 8.7) | 0.59 | 1.0 (−7.0, 9.7) | 0.68 |
| Peri | −0.1 (−7.2, 7.5) | 3.0 (−3.3, 9.8) | 9.3 (−0.7, 20.3) | 1.3 (−3.9, 6.8) | 5.0 (−8.4, 20.3) | −2.0 (−5.6, 1.8) | |||||||
| Pre | ∑Phthalates | 4.2 (−0.6, 9.2) | 0.64 | −0.8 (−6.5, 5.3) | 0.27 | 7.9 (−0.7, 17.1) | 0.52 | 1.0 (−3.3, 5.5) | 0.72 | −1.9 (−9.6, 6.4) | 0.34 | 5.4 (−4.1, 15.9) | 0.20 |
| Peri | 0.7 (−7.8, 9.9) | 3.7 (−3.9, 11.8) | 12.5 (0.4, 26.1) | 1.1 (−5.1, 7.7) | 6.3 (−9.7, 25.1) | −2.4 (−6.7, 2.1) | |||||||
| Pre | ∑AA | 7.7 (2.2, 13.4) | 0.44 | 0.7 (−5.7, 7.5) | 0.29 | 12.6 (2.8, 23.3) | 0.81 | 0.4 (−4.3, 5.4) | 0.15 | -9.8 (−17.6, −1.3) | 0.38 | 12.3 (1.2, 24.7) | 0.07 |
| Peri | 9.2 (−2.9, 22.7) | 7.7 (−2.7, 19.2) | 12.2 (−3.8, 30.7) | 6.8 (−1.8, 16.2) | 0.8 (−18.9, 25.5) | 0.3 (−5.6, 6.5) | |||||||
Data are presented as % change in hormones for every 2-fold increase in phthalate metabolite (ng/mL or nmol/mL) in pre- and peri-menopausal women from linear regression models adjusted for age, race/ethnicity, employment status, education, income, marital status, alcohol consumption, smoking status, physical activity, medication use, and BMI. In separate models, an interaction between phthalate and menopause status was included to formally test for effect modification by menopause status, and the resulting P-value (Pint) is provided in the table. CI, confidence interval; AMH, anti-Mullerian hormone; BMI, body mass index; FSH, follicle stimulating hormone; SHBG, sex hormone binding globulin. n = 461 and 257 for pre- and peri-menopausal women, respectively.
Associations between phthalate metabolites and hormones stratified by BMI
Associations of phthalate metabolites with estradiol were only observed in under-/normal weight women (Table 5). Specifically, ∑AA was positively associated with estradiol. Associations of phthalate metabolites with SHBG were only observed in overweight women, in whom MCPP was negatively associated with SHBG (Table 5). Associations of phthalates with progesterone were only observed in obese women, in whom ∑DEHP, ∑Plastics, MEP, ∑PCP, ∑Phthalates, and ∑AA were positively associated with progesterone (Table 5). Associations of phthalates with FSH and AMH were observed in both under-/normal weight and obese women, but they differed depending on the phthalate metabolite (Table 5). Specifically, ∑DEHP and ∑AA were negatively associated with FSH in obese women, but MBzP was negatively associated, while MEP and ∑PCP were positively associated with FSH in under-/normal weight women. Additionally, MBzP was positively associated with AMH in under-/normal weight women, while MBP was positively associated with AMH in obese women.
Table 5.
Associations of phthalate metabolites with hormones stratified by mid-life BMI.
| BMI Category | Phthalate | Estradiol | P int | Testosterone | P int | Progesterone | P int | SHBG | P int | FSH | P int | AMH | P int |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % change in hormones for every 2-fold increase in phthalate concentrations | |||||||||||||
| Under/Normal | MCPP | 0.8 (−3.5, 5.4) | 0.90 | −2.3 (−7.3, 2.9) | 0.60 | −1.9 (−8.8, 5.6) | 0.27 | −0.3 (−3.6, 3.1) | 0.36 | 0.5 (−7.7, 9.4) | 0.44 | 1.0 (−6.1, 8.7) | 0.56 |
| Overweight | 1.6 (−4.6, 8.2) | −0.1 (−6.3, 6.6) | 4.7 (−4.5, 14.7) | -5.1 (−9.7, −0.3) | −7.8 (−18.8, 4.7) | 8.4 (−0.2, 17.7) | |||||||
| Obese | 1.1 (−4.4, 6.9) | 3.0 (−2.2, 8.5) | 5.9 (−2.2, 14.7) | −1.8 (−6.4, 3.1) | −1.7 (−9.3, 6.6) | 5.7 (−1.5, 13.4) | |||||||
| Under/Normal | MBzP | 3.0 (−3.0, 9.3) | 0.18 | 0.8 (−6.1, 8.1) | 0.32 | 7.1 (−2.9, 18.2) | 0.52 | 3.5 (−1.0, 8.3) | 0.63 | -11.6 (−21.1, −1.0) | 0.20 | 10.5 (0.2, 21.9) | 0.34 |
| Overweight | 5.9 (−3.4, 16.1) | 0.7 (−8.4, 10.7) | 4.9 (−8.3, 20.1) | −0.8 (−7.9, 6.8) | 6.4 (−11.7, 28.2) | 1.9 (−9.8, 15.2) | |||||||
| Obese | −2.7 (−9.4, 4.5) | −5.2 (−11.3, 1.2) | −3.1 (−12.5, 7.4) | 1.0 (−5.1, 7.4) | 2.0 (−8.0, 13.0) | 3.4 (−5.5, 13.1) | |||||||
| Under/Normal | ∑DEHP | 5.7 (−1.3, 13.3) | 0.95 | 0.8 (−7.0, 9.2) | 0.52 | 4 (−7.1, 16.5) | 0.11 | 2.5 (−2.7, 7.9) | 0.86 | −2.7 (−14.7, 11.0) | 0.24 | 5.9 (−5.4, 18.7) | 0.80 |
| Overweight | 5.1 (−3.3, 14.2) | 4.5 (−4.1, 13.8) | 4.3 (−7.7, 17.8) | 0.2 (−6.3, 7.1) | 2.2 (−13.7, 21.0) | 1.9 (−8.8, 13.8) | |||||||
| Obese | 3.7 (−4.4, 12.6) | −1.6 (−8.8, 6.3) | 17.9 (5.1, 32.4) | 1.1 (−5.8, 8.6) | -11.0 (−20.9, 0.0) | 5.4 (−5, 16.8) | |||||||
| Under/Normal | ∑Plastics | 6.8 (−0.6, 14.7) | 0.78 | 1.6 (−6.7, 10.5) | 0.61 | 4.9 (−6.9, 18.1) | 0.15 | 3.1 (−2.3, 8.9) | 0.73 | −5.0 (−17.2, 9.1) | 0.45 | 7.5 (−4.6, 21) | 0.82 |
| Overweight | 5.9 (−3.3, 15.9) | 3.7 (−5.5, 13.8) | 6.7 (−6.6, 21.8) | 0.2 (−6.9, 7.7) | 0.7 (−16.2, 21.1) | 3.8 (−7.9, 17.1) | |||||||
| Obese | 2.6 (−6.0, 12.0) | −1.0 (−8.7, 7.4) | 19.0 (5.2, 34.6) | 0.6 (−6.8, 8.5) | −9.9 (−20.5, 2.1) | 6.2 (−4.9, 18.5) | |||||||
| Under/Normal | MEP | −4.2 (−9.1, 0.9) | 0.21 | 1.6 (−4.5, 8) | 0.81 | 1.0 (−7.4, 10.2) | 0.21 | 0.8 (−3.2, 4.8) | 0.01 | 12.7 (2, 24.5) | 0.06 | −4.2 (−12.2, 4.5) | 0.11 |
| Overweight | 2.3 (−2.7, 7.6) | 2.8 (−2.4, 8.2) | 1.8 (−5.4, 9.6) | −2.7 (−6.5, 1.3) | 3.4 (−6.6, 14.5) | −4.2 (−10.3, 2.4) | |||||||
| Obese | 1.2 (−3.7, 6.4) | −2.8 (−7.2, 1.9) | 9.7 (2.1, 17.7) | 2.6 (−1.7, 7.2) | −3.3 (−10.1, 3.9) | 4.0 (−2.4, 10.8) | |||||||
| Under/Normal | MBP | 5.5 (−2.3, 13.9) | 0.50 | 8.3 (−1.0, 18.5) | 0.32 | 5.1 (−7.4, 19.3) | 0.19 | 2.6 (−3.2, 8.7) | 0.94 | −8.7 (−21.1, 5.8) | 0.25 | 14.6 (1.1, 30.0) | 0.12 |
| Overweight | −4.2 (−14.2, 6.9) | 0.3 (−10.4, 12.4) | −5.3 (−19.4, 11.3) | −1.5 (−9.8, 7.6) | 13.8 (−8.9, 42.2) | −6.1 (−18.8, 8.7) | |||||||
| Obese | 1.0 (−7.0, 9.7) | −1.6 (−8.9, 6.3) | 12.3 (−0.2, 26.3) | 0.8 (−6.2, 8.3) | −8.7 (−18.9, 2.8) | 12.8 (1.7, 25.1) | |||||||
| Under/Normal | MiBP | 2.6 (−5.0, 10.7) | 0.83 | 7.6 (−1.5, 17.6) | 0.97 | 0.0 (−11.8, 13.4) | 0.36 | 0.2 (−5.4, 6.1) | 0.18 | 0.0 (−13.5, 15.7) | 0.63 | 8.5 (−4.3, 22.9) | 0.10 |
| Overweight | 6.4 (−2.8, 16.5) | 7.3 (−2.2, 17.8) | 9.9 (−3.7, 25.4) | 0.5 (−6.5, 8.1) | −2.0 (−18.4, 17.9) | −6.0 (−16.6, 6.0) | |||||||
| Obese | 3.4 (−5.0, 12.5) | 3.6 (−4.2, 12.1) | 8.4 (−4.0, 22.3) | 3.9 (−3.4, 11.8) | −5.9 (−16.7, 6.2) | 9.1 (−1.9, 21.3) | |||||||
| Under/Normal | ∑PCP | −3.2 (−9.1, 3.0) | 0.34 | 3 (−4.3, 10.8) | 0.84 | 1.5 (−8.4, 12.6) | 0.41 | 1.1 (−3.6, 6.0) | 0.09 | 12.6 (0, 26.9) | 0.12 | −2.2 (−11.8, 8.5) | 0.24 |
| Overweight | 2.9 (−3.9, 10.3) | 4.1 (−3.0, 11.7) | 5.2 (−4.9, 16.4) | −1.4 (−6.7, 4.3) | 1.5 (−11.8, 16.8) | −2.5 (−11.1, 6.9) | |||||||
| Obese | 2.3 (−4.3, 9.4) | −2.6 (−8.4, 3.6) | 10.4 (0.4, 21.3) | 3.0 (−2.8, 9.1) | −4.7 (−13.4, 4.9) | 6.2 (−2.4, 15.5) | |||||||
| Under/Normal | ∑Phthalates | 0.7 (−6.5, 8.5) | 0.78 | 2.5 (−6.1, 11.8) | 0.80 | 3.0 (−8.9, 16.4) | 0.18 | 2.7 (−2.9, 8.6) | 0.38 | 9.9 (−4.6, 26.6) | 0.14 | 0.7 (−10.9, 13.8) | 0.55 |
| Overweight | 4.6 (−3.2, 13.0) | 5.2 (−2.8, 13.9) | 6.5 (−4.9, 19.3) | −0.4 (−6.5, 6.0) | 1.0 (−13.7, 18.2) | 1.1 (−8.8, 12.1) | |||||||
| Obese | 3.2 (−4.7, 11.9) | −2.7 (−9.7, 4.9) | 17.2 (4.6, 31.3) | 1.6 (−5.3, 8.9) | −7.3 (−17.4, 4.1) | 6.8 (−3.5, 18.2) | |||||||
| Under/Normal | ∑AA | 11.0 (2.1, 20.6) | 0.83 | 6 (−3.9, 16.9) | 0.42 | 8.5 (−5.5, 24.5) | 0.25 | 4.4 (−2.0, 11.2) | 0.84 | −7.4 (−21.1, 8.7) | 0.32 | 12.7 (−1.8, 29.3) | 0.33 |
| Overweight | 7.0 (−3.4, 18.5) | 5.2 (−5.3, 16.8) | 9.4 (−5.7, 27.0) | 1.9 (−6.1, 10.6) | 2.1 (−17, 25.7) | 1.0 (−11.9, 15.7) | |||||||
| Obese | 5.5 (−3.9, 15.8) | −1.0 (−9.2, 8.0) | 20.8 (5.9, 37.8) | 1.0 (−6.9, 9.5) | -13.2 (−24.0, −0.8) | 14.3 (1.7, 28.4) | |||||||
Data are presented as the % change in hormones for every 2-fold increase in phthalate metabolite (ng/mL or nmol/mL) in under-/normal weight and overweight/obese women from linear regression models adjusted for adjusted for age, race/ethnicity, employment status, education, income, marital status, alcohol consumption, smoking status, physical activity, medication use, and menopausal status. In separate models, an interaction between phthalate and BMI was included to formally test for effect modification by mid-life BMI, and the resulting P-value (Pint) is provided in the table. CI, confidence interval; AMH, anti-Mullerian hormone; BMI, body mass index; FSH, follicle stimulating hormone; SHBG, sex hormone binding globulin. n = 288, 187, and 243 for under-/normal weight, overweight, and obese women, respectively.
Associations between phthalate metabolites and hormones stratified by race/ethnicity
Associations of phthalate metabolites with estradiol, testosterone, and AMH were only observed in non-Hispanic white women (Table 6). Specifically, ∑DEHP, ∑Plastics, and ∑AA were positively associated with estradiol, MiBP was positively associated with testosterone, and MCPP and ∑AA were positively associated with AMH. However, associations of phthalate metabolites with progesterone were observed in both non-Hispanic white and black/other women (Table 6). Specifically, MCPP and ∑Plastics were positively associated with progesterone in black/other women, while ∑Phthalates was positively associated with progesterone in non-Hispanic white women.
Table 6.
Associations of phthalate metabolites with hormones stratified by race/ethnicity.
| Race/Ethnicity | Phthalate | Estradiol | P int | Testosterone | P int | Progesterone | P int | SHBG | P int | FSH | P int | AMH | P int |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % change in hormones for every 2-fold increase in phthalate concentrations | |||||||||||||
| Black/Other | MCPP | 4.1 (−1.6, 10.1) | 0.25 | −0.1 (−5.1, 5.1) | 0.89 | 13.1 (4.1, 22.9) | 0.005 | −2.2 (−6.4, 2.1) | 0.97 | −5.9 (−13.5, 2.5) | 0.36 | 1.0 (−6.3, 9.0) | 0.19 |
| Non-Hispanic white | 0.1 (−3.4, 3.8) | 0.0 (−4.0, 4.1) | −2.6 (−7.8, 2.8) | −2.0 (−4.8, 1.0) | −0.2 (−6.8, 6.9) | 6.3 (0.9, 11.9) | |||||||
| Black/Other | MBzP | 4.0 (−3.9, 12.6) | 0.25 | 4.4 (−2.9, 12.2) | 0.23 | 7.0 (−4.9, 20.5) | 0.45 | 1.9 (−4.2, 8.2) | 0.87 | −1.6 (−12.7, 11.0) | 0.96 | 4.8 (−5.7, 16.5) | 0.57 |
| Non-Hispanic white | −0.9 (−5.5, 3.9) | −3.9 (−8.9, 1.3) | 1.3 (−5.7, 8.9) | 1.0 (−2.8, 5.0) | −4.2 (−12.5, 4.8) | 5.6 (−1.4, 13.1) | |||||||
| Black/Other | ∑DEHP | 3.4 (−5.0, 12.6) | 0.49 | −3.5 (−10.7, 4.3) | 0.08 | 12.1 (−1.2, 27.3) | 0.56 | −1.1 (−7.4, 5.6) | 0.52 | −1.2 (−13.1, 12.4) | 0.80 | 2.3 (−8.7, 14.7) | 0.49 |
| Non-Hispanic white | 5.9 (0.7, 11.4) | 3.9 (−1.8, 9.9) | 6.7 (−1.1, 15.1) | 2.2 (−2.0, 6.5) | −4.5 (−13.3, 5.1) | 5.2 (−2.2, 13.2) | |||||||
| Black/Other | ∑Plastics | 4.6 (−4.5, 14.6) | 0.75 | −2.5 (−10.2, 6.0) | 0.13 | 17.1 (2.3, 34.1) | 0.29 | −1.1 (−7.8, 6.1) | 0.57 | −2.3 (−14.9, 12.2) | 0.90 | 2.2 (−9.6, 15.5) | 0.31 |
| Non-Hispanic white | 5.5 (0.0, 11.3) | 3.6 (−2.5, 10.0) | 6.7 (−1.7, 15.7) | 2.0 (−2.4, 6.6) | −5.2 (−14.4, 5.0) | 7.2 (−0.8, 15.9) | |||||||
| Black/Other | MEP | 1.6 (−3.8, 7.4) | 0.47 | 0.6 (−4.4, 5.8) | 0.62 | 4.2 (−4.1, 13.2) | 0.91 | −0.4 (−4.5, 4.0) | 0.85 | 1.5 (−6.7, 10.3) | 0.58 | 0.7 (−6.5, 8.4) | 0.45 |
| Non-Hispanic white | −1.1 (−4.4, 2.3) | −0.4 (−4.1, 3.5) | 4.9 (−0.4, 10.4) | 0.6 (−2.2, 3.4) | 5.2 (−1.4, 12.2) | −2.6 (−7.2, 2.4) | |||||||
| Black/Other | MBP | 0.5 (−9.0, 11.0) | 0.92 | 0.6 (−8.1, 10.2) | 0.86 | 6.1 (−8.6, 23.2) | 0.83 | −0.7 (−8.0, 7.2) | 1.00 | −5.5 (−18.7, 9.9) | 0.71 | 8.3 (−5.2, 23.7) | 0.96 |
| Non-Hispanic white | 1.6 (−4.1, 7.6) | 2.3 (−4.1, 9.1) | 4.8 (−3.9, 14.4) | 1.0 (−3.6, 5.9) | −3.7 (−13.7, 7.5) | 7.8 (−0.8, 17.1) | |||||||
| Black/Other | MiBP | 3.9 (−4.8, 13.4) | 0.81 | 0.4 (−7.3, 8.8) | 0.19 | 0.1 (−12.3, 14.3) | 0.47 | 3.8 (−3.0, 11.1) | 0.22 | 4.6 (−8.5, 19.5) | 0.29 | 6.5 (−5.4, 19.8) | 0.80 |
| Non-Hispanic white | 3.2 (−2.5, 9.2) | 9.0 (2.3, 16.1) | 7.2 (−1.7, 16.9) | 0.1 (−4.5, 4.9) | −5.7 (−15.4, 5.2) | 3.2 (−5.0, 12.1) | |||||||
| Black/Other | ∑PCP | 2.5 (−3.8, 9.3) | 0.43 | 0.5 (−5.2, 6.5) | 0.92 | 5.0 (−4.6, 15.5) | 0.76 | −0.2 (−5.0, 4.8) | 0.64 | 0.8 (−8.5, 11.0) | 0.61 | 1.9 (−6.5, 11.0) | 0.68 |
| Non-Hispanic white | −1.2 (−5.8, 3.6) | 0.8 (−4.5, 6.3) | 6.9 (−0.5, 14.9) | 1.8 (−2.1, 5.9) | 5.3 (−3.8, 15.3) | −1.0 (−7.6, 6.1) | |||||||
| Black/Other | ∑Phthalates | 3.9 (−3.5, 11.9) | 0.74 | −0.2 (−6.7, 6.8) | 0.77 | 8.7 (−2.7, 21.4) | 0.91 | −1.2 (−6.7, 4.5) | 0.38 | 0.7 (−10.0, 12.7) | 0.86 | 2.6 (−7.1, 13.3) | 0.85 |
| Non-Hispanic white | 1.4 (−4.2, 7.2) | 1.7 (−4.5, 8.3) | 9.5 (0.6, 19.1) | 3.0 (−1.6, 7.9) | 2.8 (−7.6, 14.4) | 2.2 (−5.8, 10.8) | |||||||
| Black/Other | ∑AA | 8.4 (−2.5, 20.6) | 0.93 | −1.1 (−10.3, 9.1) | 0.26 | 18.3 (0.9, 38.7) | 0.44 | 0.0 (−7.9, 8.6) | 0.79 | −3.7 (−18.1, 13.2) | 0.90 | 8.9 (−5.6, 25.7) | 0.75 |
| Non-Hispanic white | 7.2 (1.0, 13.8) | 4.9 (−1.9, 12.1) | 10.3 (0.8, 20.8) | 2.9 (−2.1, 8.1) | −7.2 (−17.3, 4.0) | 9.1 (0.0, 19.0) | |||||||
Data are presented as % change in hormones for every 2-fold increase in phthalate metabolite (ng/mL or nmol/mL) in White and Black/Other women from linear regression models adjusted for age, employment status, education, income, marital status, alcohol consumption, smoking status, physical activity, medication use, menopausal status, and BMI. In separate models, an interaction between phthalate and race/ethnicity was included to formally test for effect modification by race/ethnicity, and the resulting P-value (Pint) is provided in the table. CI, confidence interval; AMH, anti-Mullerian hormone; BMI, body mass index; FSH, follicle stimulating hormone; SHBG, sex hormone binding globulin. n = 241 and 477 for black/other and white women, respectively.
DISCUSSION
In the present study, we found that several phthalate metabolites were positively associated with both sex steroid and protein hormones. This particular trend was unexpected due to previous in vitro and in vivo studies, as well as observational studies suggesting that phthalates inhibit steroidogenesis (11, 17, 21, 43, 44). Previous observational studies evaluated these associations in men and women during their reproductive life, as well as in children, which may account for these discrepancies given that our study population is in midlife. We also found that some associations of phthalate metabolites with hormones differed by menopause status, midlife BMI, and race/ethnicity, which may provide critical information as to which midlife populations may be more susceptible to the endocrine disrupting effects of phthalates. Overall, our results suggest that phthalates may disrupt steroidogenesis through different mechanisms involving more than simple inhibition.
Overall associations of phthalates with hormones
We found that phthalates primarily found in plastic food packaging (i.e. ∑DEHP and ∑Plastics) and those shown to have anti-androgenic activity (i.e. ∑AA) share positive, linear associations with estradiol. ∑AA displayed positive relationships with estradiol in women in the third and fourth quartiles as well, further demonstrating the strength of this positive association. These results are consistent with a study in U.S. pregnant women (45–47) and some studies conducted in pregnant women and women between the ages of 16 and 45 that have found positive associations between some phthalate metabolites such as MiBP, MBzP (46), and MBP and estradiol (48), all of which are components of the ∑AA measurement used in our study. However, some of our results are inconsistent with some experimental studies showing that phthalate exposure decreases estradiol levels in rodents (17, 49). Our results also differ from a study in Japanese pregnant women and a recent study in pre- and postmenopausal women from NHANES, which showed that DEHP was associated with lower serum estradiol concentrations (22).
We also observed associations of phthalates with testosterone and progesterone. While we did not observe an overall linear association between ∑DEHP and testosterone, our findings from quartile analyses showing negative associations between ∑DEHP and testosterone are consistent with experimental studies showing that DEHP has anti-androgenic properties (59, 63–65). However, we also found that MEP (quartile) and MiBP (linear and quartile) were positively associated with testosterone, which is not consistent with most studies (66–68). Although one observational study showed that prenatal MiBP exposure was associated with increased peripubertal testosterone in girls (69), a cross-sectional study using data from NHANES cycles 2013–2016 found that MEP, MiBP, and ∑DEHP were associated with reduced testosterone, and these associations were strongest in 40–60 year old females (22). Our study population acutely targeted women within a narrow age range to capture the menopausal transition, which may also account for discrepancies in our findings. Most notably, we found that ∑DEHP and MEP were positively associated with progesterone, and these were driving the associations observed for ∑Plastics, ∑PCP, ∑Phthalates, and ∑AA with progesterone. However, previous studies in animals and humans found equivocal results regarding these associations as those studies have reported positive and negative associations of phthalates with progesterone (60, 70).
Overall associations of phthalates with non-steroid hormones (i.e. AMH, FSH, and SHBG) were less frequent. We found that MBP and ∑AA were positively associated with AMH in both linear and quartile analyses. Few studies have investigated associations between phthalates and AMH, but one research group found inverse associations between concentrations of MBP and AMH in follicular fluid (50), but also reported in an earlier study in the same group of women that MBP shared a positive association with serum AMH, similar to what we observed in our population (51). We also observed that MEP was positively associated with FSH in quartile analyses. However, two studies, one in healthy 16–45 year old women and the other in healthy 11–88 year old men found that some phthalate metabolites (but not MEP) were positively associated with FSH (48, 52). Lastly, we observed no associations between phthalates and SHBG. These results are consistent with studies in peripubertal girls and pregnant women (53–55). Overall, our results and those from previous studies further illustrate the complex relationships that phthalates can share with different hormones and that these associations may also differ across populations. However, additional studies, especially in midlife, are needed to corroborate our findings.
Differences in associations by menopause status
We found that associations of phthalates with estradiol, progesterone, and FSH were strongest in premenopausal women. Namely, ∑AA, ∑Plastics, and ∑DEHP were all positively associated with estradiol and progesterone in premenopausal women. Coinciding with this finding is that ∑AA and ∑Plastics were also negatively associated with FSH in premenopausal women. Inverse relationships between estradiol and FSH are expected due to the negative feedback loop wherein FSH stimulates estradiol production and estradiol in turn suppresses FSH production. Studies have shown that phthalates are capable of modulating steroidogenic enzymes responsible for rate-limiting steps in the steroidogenesis pathway (56–58). Thus, it is possible that these effects may be due to direct phthalate-induced alterations of steroidogenic enzyme and/or activity. We speculate that these effects may be muted or not present in perimenopausal women because the entire hypothalamic-pituitary-gonadal (HPG) axis in perimenopausal women may be less sensitive to phthalate-induced changes or that the ovary itself is less sensitive to phthalate-induced changes due to the transition into menopause.
Differences in associations by midlife BMI
While we found that associations of phthalates with most hormones differed by midlife BMI, the most consistent associations were observed with progesterone. Most notably, positive associations of ∑DEHP, ∑Plastics, MEP, ∑PCP, ∑Phthalates, and ∑AA were positively associated with progesterone in obese women only. Adipose tissue is metabolically active with the capability to synthesize and metabolize sex steroid hormones (59). Additionally, the link between phthalates and obesity broadens the possibilities for the relationships that may exist between phthalates, adiposity, and hormone levels (60, 61). It is possible that phthalate-induced disruption in one steroidogenic organ (i.e., the ovary or the adipose tissue) can lead to compensatory action by the other. Alternatively, it is possible that subtle actions on both the ovary and the adipose tissue in women with less adipose mass are less detectable than when in overweight and obese women, thus leading to relationships being observed in overweight and obese women only. The complex relationships that are likely to exist between phthalates, adipose tissue, and hormone levels merit further investigation.
Differences in associations by race/ethnicity
In race/ethnicity stratified analyses, positive associations of phthalate metabolites with estradiol and AMH were consistently strongest in non-Hispanic white women. Comparison to existing literature is difficult due to the lack of studies that investigate the interaction of hormones, race/ethnicity, and phthalates. However, one study that investigated the changes in hormones in different races found that African American women had a more rapid decline in estradiol concentrations during the menopausal transition than non-Hispanic white women (62). Although not a direct comparison, the study partially supports our findings in that we observed many different positive associations between phthalate measures and hormone levels, but we did not observe that black/other women had positive relationships between any phthalate measures and estradiol. However, this finding contrasts somewhat with other studies that have found that African American women have higher estradiol levels than non-Hispanic white women pre- and post-menopause (63, 64). Circulating hormone concentrations can be influenced by body composition and stress, which could also contribute to racial/ethnic differences in measured hormone levels, as well as result in differential impacts of phthalates on hormones in non-Hispanic white versus black women (65, 66). This highlights the need for further investigation into the complex relationships between race/ethnicity, phthalate exposure, and hormones to fully appreciate the vulnerability of certain populations.
Strengths and limitations
Our study has limitations and strengths. Due to the cross-sectional nature of our analyses, we are unable to make conclusions about temporality of associations between phthalates and hormones. Further, it is possible that some women in our study experienced irregular menstrual cycles, which could impact hormone levels. However, to counterbalance the variability of menstrual cycles and timing during the cycle for collection of samples, we collected four blood samples that represent each week of a woman’s menstrual cycle for hormone assessment and averaged these hormone concentrations for a more stable outcome measure. Further, the majority of the women in our study were either non-Hispanic white or black, leaving other races and ethnicities underrepresented in our study. While we a priori identified and adjusted for important confounders (i.e. sociodemographic characteristics, behavioral factors, and menopausal status), there may be unobserved or unmeasured confounding not accounted for in our statistical models, which could bias our observed associations. For example, diet is an important source of phthalate exposure and may also influence circulating hormone concentrations (67, 68)(69, 70). Given that we were unable to control for diet, we may be overestimating associations between phthalates and hormones levels. Selection bias is also possible if participants with higher phthalate levels had certain characteristics that would impact their hormones. If selection bias exists, it could potentially lead to an under- or overestimation of the strength of our observed associations.
Major strengths of our study included the use of a pooled sample for assessing urinary phthalate metabolites, which is important given the short half-lives phthalates have in the body. Additionally, we were also powered enough to detect some differences in associations of phthalates metabolites with hormones by menopausal status, BMI, and race/ethnicity, revealing populations that are potentially more susceptible to the endocrine disrupting effects of phthalates. In addition, this was a multi-racial cohort of midlife women and one of the first studies to provide evidence of associations between urinary phthalate metabolites and hormone levels during a time period of rapid hormonal changes for women—midlife.
CONCLUSION
Our study found that some phthalates were associated with several critical hormones in midlife women. Specifically, the following positive associations were observed: ∑DEHP, ∑Plastics, and ∑AA with estradiol; MiBP with testosterone; ∑DEHP, ∑Plastics, MEP, ∑PCP, ∑Phthalates, and ∑AA with progesterone; MBP and ∑AA with AMH. Additionally, associations of phthalate metabolites differed by menopausal status, BMI, and race/ethnicity. Specifically, associations of phthalate metabolites with estradiol, progesterone, and FSH were strongest in premenopausal women, with progesterone were strongest in obese women, and with estradiol and AMH were strongest in non-Hispanic white women. Although some of our findings were corroborated by previous studies, many contrasted with the current literature. The variability in strength and direction of association between phthalates and reproductive hormones highlights the need for future studies to investigate a wide range of exposure windows and to elucidate the mechanism(s) through which phthalates may act to disrupt the HPG-axis.
Supplementary Material
Highlights.
Phthalate metabolites were positively associated with estradiol levels in midlife women
Phthalate metabolites were positively associated with testosterone levels in midlife women
Phthalate metabolites were positively associated with progesterone levels in midlife women
Phthalate metabolites were positively associated with anti-Müllerian hormone levels in midlife women
Some demographic and lifestyle characteristics modify the associations between phthalate metabolites and hormones
ACKNOWLEDGMENTS
This work was supported by the National Institute of Environmental Health Sciences (R01 ES026956 and T32 007326). The University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core is supported by the Eunice Kennedy Shriver NICHD/NIH (NCTRI) Grant P50-HD28934. The authors thank the MWHS participants for their time, information, and biological samples and the staff members at Johns Hopkins University and the University of Illinois at Urbana-Champaign who helped with this study. Thank you to Yonatan (Yoni) Segev and Tyler Beers for their help with aliquoting samples.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
REFERENCES
- 1.Benjamin S, Masai E, Kamimura N, Takahashi K, Anderson RC, Faisal PA. Phthalates impact human health: Epidemiological evidences and plausible mechanism of action. J Hazard Mater. 2017;340:360–83. Epub 06/19. doi: 10.1016/j.jhazmat.2017.06.036. [DOI] [PubMed] [Google Scholar]
- 2.Schettler T Human exposure to phthalates via consumer products. International journal of andrology. 2006;29(1):134–9; discussion 81–5. Epub 2006/02/10. doi: 10.1111/j.1365-2605.2005.00567.x. [DOI] [PubMed] [Google Scholar]
- 3.Weiss JM, Gustafsson Å, Gerde P, Bergman Å, Lindh CH, Krais AM. Daily intake of phthalates, MEHP, and DINCH by ingestion and inhalation. Chemosphere. 2018;208:40–9. doi: 10.1016/j.chemosphere.2018.05.094. [DOI] [PubMed] [Google Scholar]
- 4.Ding M, Kang Q, Zhang S, Zhao F, Mu D, Zhang H, Yang M, Hu J. Contribution of phthalates and phthalate monoesters from drinking water to daily intakes for the general population. Chemosphere. 2019;229:125–31. doi: 10.1016/j.chemosphere.2019.05.023. [DOI] [PubMed] [Google Scholar]
- 5.Garcia Ibarra V, Rodriguez Bernaldo de Quiros A, Paseiro Losada P, Sendon R. Identification of intentionally and non-intentionally added substances in plastic packaging materials and their migration into food products. Anal Bioanal Chem. 2018;410(16):3789–803. Epub 2018/05/08. doi: 10.1007/s00216-018-1058-y. [DOI] [PubMed] [Google Scholar]
- 6.Rastkari N, Zare Jeddi M, Yunesian M, Ahmadkhaniha R. The Effect of Storage Time, Temperature and Type of Packaging on the Release of Phthalate Esters into Packed Acidic Liquids. Food Technol Biotechnol. 2017;55(4):562–9. doi: 10.17113/ftb.55.04.17.5128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marie C, Hamlaoui S, Bernard L, Bourdeaux D, Sautou V, Lémery D, Vendittelli F, Sauvant-Rochat M-P. Exposure of hospitalised pregnant women to plasticizers contained in medical devices. BMC Womens Health. 2017;17(1):45-. doi: 10.1186/s12905-017-0398-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Calafat AM, Needham LL, Silva MJ, Lambert G. Exposure to di-(2-ethylhexyl) phthalate among premature neonates in a neonatal intensive care unit. Pediatrics. 2004;113(5):e429–34. Epub 2004/05/04. doi: 10.1542/peds.113.5.e429. [DOI] [PubMed] [Google Scholar]
- 9.Martine B, Marie-Jeanne T, Cendrine D, Fabrice A, Marc C. Assessment of Adult Human Exposure to Phthalate Esters in the Urban Centre of Paris (France). Bulletin of Environmental Contamination and Toxicology. 2013;90(1):91–6. doi: 10.1007/s00128-012-0859-5. [DOI] [PubMed] [Google Scholar]
- 10.Craig ZR, Ziv-Gal A. Pretty Good or Pretty Bad? The Ovary and Chemicals in Personal Care Products. Toxicological Sciences. 2017;162(2):349–60. doi: 10.1093/toxsci/kfx285. [DOI] [PubMed] [Google Scholar]
- 11.Meeker JD, Ferguson KK. Urinary phthalate metabolites are associated with decreased serum testosterone in men, women, and children from NHANES 2011–2012. J Clin Endocrinol Metab. 2014;99(11):4346–52. Epub 2014/08/14. doi: 10.1210/jc.2014-2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.EPA US. Biomonitoring: Phthalates. America's Children and the Environment. 2017(3rd). [Google Scholar]
- 13.Woodruff TJ, Zota AR, Schwartz JM. Environmental chemicals in pregnant women in the United States: NHANES 2003–2004. Environmental health perspectives. 2011;119(6):878–85. Epub 2011/01/14. doi: 10.1289/ehp.1002727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brehm E, Rattan S, Gao L, Flaws JA. Prenatal Exposure to Di(2-Ethylhexyl) Phthalate Causes Long-Term Transgenerational Effects on Female Reproduction in Mice. Endocrinology. 2018;159(2):795–809. Epub 2017/12/12. doi: 10.1210/en.2017-03004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang WH, Wu MH, Pan HA, Guo PL, Lee CC. Semen quality and insulin-like factor 3: Associations with urinary and seminal levels of phthalate metabolites in adult males. Chemosphere. 2017;173:594–602. Epub 2017/02/06. doi: 10.1016/j.chemosphere.2017.01.056. [DOI] [PubMed] [Google Scholar]
- 16.Chiang C, Flaws JA. Subchronic Exposure to Di(2-ethylhexyl) Phthalate and Diisononyl Phthalate During Adulthood Has Immediate and Long-Term Reproductive Consequences in Female Mice. Toxicological sciences : an official journal of the Society of Toxicology. 2019;168(2):620–31. Epub 2019/01/17. doi: 10.1093/toxsci/kfz013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hannon PR, Flaws JA. The effects of phthalates on the ovary. Frontiers in endocrinology. 2015;6:8. Epub 2015/02/24. doi: 10.3389/fendo.2015.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoyer BB, Lenters V, Giwercman A, Jonsson BAG, Toft G, Hougaard KS, Bonde JPE, Specht IO. Impact of Di-2-Ethylhexyl Phthalate Metabolites on Male Reproductive Function: a Systematic Review of Human Evidence. Current environmental health reports. 2018;5(1):20–33. Epub 2018/02/23. doi: 10.1007/s40572-018-0174-3. [DOI] [PubMed] [Google Scholar]
- 19.Cathey AL, Watkins D, Rosario ZY, Vélez C, Alshawabkeh AN, Cordero JF, Meeker JD. Associations of Phthalates and Phthalate Replacements With CRH and Other Hormones Among Pregnant Women in Puerto Rico. J Endocr Soc. 2019;3(6):1127–49. doi: 10.1210/js.2019-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duty SM, Calafat AM, Silva MJ, Ryan L, Hauser R. Phthalate exposure and reproductive hormones in adult men. Hum Reprod. 2005;20(3):604–10. Epub 12/09. doi: 10.1093/humrep/deh656. [DOI] [PubMed] [Google Scholar]
- 21.Meeker JD, Calafat AM, Hauser R. Urinary Metabolites of Di(2-ethylhexyl) Phthalate Are Associated With Decreased Steroid Hormone Levels in Adult Men. Journal of Andrology. 2009;30(3):287–97. doi: 10.2164/jandrol.108.006403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Long SE K L T L, Jacobson MH. Urinary phthalate metabolites and alternatives and serum sex steroid hormones among pre- and postmenopausal women from NHANES, 2013–16. The Science of the total environment. 2021( 10.1016/j.scitotenv.2020.144560). Epub 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diaz Santana MV, Hankinson SE, Bigelow C, Sturgeon SR, Zoeller RT, Tinker L, Manson JAE, Calafat AM, Meliker JR, Reeves KW. Urinary concentrations of phthalate biomarkers and weight change among postmenopausal women: a prospective cohort study. Environ Health. 2019;18(1):20. Epub 2019/03/15. doi: 10.1186/s12940-019-0458-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ziv-Gal A, Gallicchio L, Chiang C, Ther SN, Miller SR, Zacur HA, Dills RL, Flaws JA. Phthalate metabolite levels and menopausal hot flashes in midlife women. Reproductive toxicology (Elmsford, NY). 2016;60:76–81. Epub 2016/02/13. doi: 10.1016/j.reprotox.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DeFlorio-Barker SA, Turyk ME. Associations between bone mineral density and urinary phthalate metabolites among post-menopausal women: a cross-sectional study of NHANES data 2005–2010. Int J Environ Health Res. 2016;26(3):326–45. Epub 2015/11/21. doi: 10.1080/09603123.2015.1111312. [DOI] [PubMed] [Google Scholar]
- 26.Gore AC, Hall JE, Hayes FJ. Chapter 37 - Aging and Reproduction. In: Plant TM, Zeleznik AJ, editors. Knobil and Neill's Physiology of Reproduction (Fourth Edition). San Diego: Academic Press; 2015. p. 1661–93. [Google Scholar]
- 27.McKenna NJ. Chapter 9 - Gonadal Steroid Action. In: Plant TM, Zeleznik AJ, editors. Knobil and Neill's Physiology of Reproduction (Fourth Edition). San Diego: Academic Press; 2015. p. 313–33. [Google Scholar]
- 28.McArdle CA, Roberson MS. Chapter 10 - Gonadotropes and Gonadotropin-Releasing Hormone Signaling. In: Plant TM, Zeleznik AJ, editors. Knobil and Neill's Physiology of Reproduction (Fourth Edition). San Diego: Academic Press; 2015. p. 335–97. [Google Scholar]
- 29.Pangas SA, Rajkovic A. Chapter 21 - Follicular Development: Mouse, Sheep, and Human Models. In: Plant TM, Zeleznik AJ, editors. Knobil and Neill's Physiology of Reproduction (Fourth Edition). San Diego: Academic Press; 2015. p. 947–95. [Google Scholar]
- 30.Bacon JL. The Menopausal Transition. Obstet Gynecol Clin North Am. 2017;44(2):285–96. Epub 2017/05/14. doi: 10.1016/j.ogc.2017.02.008. [DOI] [PubMed] [Google Scholar]
- 31.Gallicchio L, Flaws JA, Smith RL. Age at menarche, androgen concentrations, and midlife obesity: findings from the Midlife Women's Health Study. Menopause (New York, NY). 2016;23(11):1182–8. Epub 2016/10/26. doi: 10.1097/gme.0000000000000691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller SR, Gallicchio LM, Lewis LM, Babus JK, Langenberg P, Zacur HA, Flaws JA. Association between race and hot flashes in midlife women. Maturitas. 2006;54(3):260–9. Epub 2006/01/21. doi: 10.1016/j.maturitas.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 33.Smith RL, Gallicchio LM, Flaws JA. Understanding the complex relationships underlying hot flashes: a Bayesian network approach. Menopause (New York, NY). 2018;25(2):182–90. Epub 2017/08/02. doi: 10.1097/gme.0000000000000959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ziv-Gal A, Smith RL, Gallicchio L, Miller SR, Zacur HA, Flaws JA. The Midlife Women's Health Study - a study protocol of a longitudinal prospective study on predictors of menopausal hot flashes. Womens Midlife Health. 2017;3:4. Epub 2017/08/17. doi: 10.1186/s40695-017-0024-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gallicchio L, Schilling C, Miller SR, Zacur H, Flaws JA. Correlates of depressive symptoms among women undergoing the menopausal transition. J Psychosom Res. 2007;63(3):263–8. Epub 2007/08/28. doi: 10.1016/j.jpsychores.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 36.Bao A-M, Liu R-Y, van Someren EJW, Hofman MA, Cao Y-X, Zhou J-N. Diurnal rhythm of free estradiol during the menstrual cycle. Eur J Endocrinol. 2003;148(2):227–32. doi: 10.1530/eje.0.1480227. [DOI] [PubMed] [Google Scholar]
- 37.Vermeulen A The hormonal activity of the postmenopausal ovary. J Clin Endocrinol Metab. 1976;42(2):247–53. Epub 1976/02/01. doi: 10.1210/jcem-42-2-247. [DOI] [PubMed] [Google Scholar]
- 38.Calafat AM, Longnecker MP, Koch HM, Swan SH, Hauser R, Goldman LR, Lanphear BP, Rudel RA, Engel SM, Teitelbaum SL, Whyatt RM, Wolff MS. Optimal Exposure Biomarkers for Nonpersistent Chemicals in Environmental Epidemiology. Environ Health Perspect. 2015;123(7):A166–8. Epub 2015/07/02. doi: 10.1289/ehp.1510041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shin HM, Bennett DH, Barkoski J, Ye X, Calafat AM, Tancredi D, Hertz-Picciotto I. Variability of urinary concentrations of phthalate metabolites during pregnancy in first morning voids and pooled samples. Environ Int. 2019;122:222–30. doi: 10.1016/j.envint.2018.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meeker JD, Hu H, Cantonwine DE, Lamadrid-Figueroa H, Calafat AM, Ettinger AS, Hernandez-Avila M, Loch-Caruso R, Tellez-Rojo MM. Urinary phthalate metabolites in relation to preterm birth in Mexico city. Environmental health perspectives. 2009;117(10):1587–92. Epub 2009/12/19. doi: 10.1289/ehp.0800522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marsee K, Woodruff TJ, Axelrad DA, Calafat AM, Swan SH. Estimated daily phthalate exposures in a population of mothers of male infants exhibiting reduced anogenital distance. Environ Health Perspect. 2006;114(6):805–9. doi: 10.1289/ehp.8663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Parks LG, Ostby JS, Lambright CR, Abbott BD, Klinefelter GR, Barlow NJ, Gray LE, Jr. The plasticizer diethylhexyl phthalate induces malformations by decreasing fetal testosterone synthesis during sexual differentiation in the male rat. Toxicol Sci. 2000;58(2):339–49. doi: 10.1093/toxsci/58.2.339. [DOI] [PubMed] [Google Scholar]
- 43.Reinsberg J, Wegener-Toper P, van der Ven K, van der Ven H, Klingmueller D. Effect of mono-(2-ethylhexyl) phthalate on steroid production of human granulosa cells. Toxicology and applied pharmacology. 2009;239(1):116–23. Epub 2009/06/09. doi: 10.1016/j.taap.2009.05.022. [DOI] [PubMed] [Google Scholar]
- 44.Araki A, Mitsui T, Miyashita C, Nakajima T, Naito H, Ito S, Sasaki S, Cho K, Ikeno T, Nonomura K, Kishi R. Association between maternal exposure to di(2-ethylhexyl) phthalate and reproductive hormone levels in fetal blood: the Hokkaido study on environment and children's health. PloS one. 2014;9(10):e109039. Epub 2014/10/09. doi: 10.1371/journal.pone.0109039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rattan S, Brehm E, Gao L, Niermann S, Flaws JA. Prenatal exposure to di(2-ethylhexyl) phthalate disrupts ovarian function in a transgenerational manner in female mice. Biology of reproduction. 2018;98(1):130–45. Epub 2017/11/23. doi: 10.1093/biolre/iox154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sathyanarayana S, Butts S, Wang C, Barrett E, Nguyen R, Schwartz SM, Haaland W, Swan SH, Team T. Early Prenatal Phthalate Exposure, Sex Steroid Hormones, and Birth Outcomes. J Clin Endocrinol Metab. 2017;102(6):1870–8. doi: 10.1210/jc.2016-3837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chiang C, Lewis LR, Borkowski G, Flaws JA. Exposure to di(2-ethylhexyl) phthalate and diisononyl phthalate during adulthood disrupts hormones and ovarian folliculogenesis throughout the prime reproductive life of the mouse. Toxicology and applied pharmacology. 2020;393:114952. doi: 10.1016/j.taap.2020.114952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cao M, Pan W, Shen X, Li C, Zhou J, Liu J. Urinary levels of phthalate metabolites in women associated with risk of premature ovarian failure and reproductive hormones. Chemosphere. 2020;242:125206. Epub 2019/11/05. doi: 10.1016/j.chemosphere.2019.125206. [DOI] [PubMed] [Google Scholar]
- 49.Hannon PR, Brannick KE, Wang W, Gupta RK, Flaws JA. Di(2-ethylhexyl) phthalate inhibits antral follicle growth, induces atresia, and inhibits steroid hormone production in cultured mouse antral follicles. Toxicology and applied pharmacology. 2015;284(1):42–53. Epub 2015/02/24. doi: 10.1016/j.taap.2015.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Du Y, Guo N, Wang Y, Teng X, Hua X, Deng T, Yao Y, Yuan X, Li Y. Follicular fluid concentrations of phthalate metabolites are associated with altered intrafollicular reproductive hormones in women undergoing in vitro fertilization. Fertility and sterility. 2019;111(5):953–61. Epub 2019/03/19. doi: 10.1016/j.fertnstert.2019.01.021. [DOI] [PubMed] [Google Scholar]
- 51.Du Y-Y, Guo N, Wang Y-X, Hua X, Deng T-R, Teng X-M, Yao Y-C, Li Y-F. Urinary phthalate metabolites in relation to serum anti-Müllerian hormone and inhibin B levels among women from a fertility center: a retrospective analysis. Reprod Health. 2018;15(1):33-. doi: 10.1186/s12978-018-0469-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang J, Yin W, Li P, Hu C, Wang L, Li T, Gao E, Hou J, Wang G, Wang X, Wang L, Yu Z, Yuan J. Interaction between diet- and exercise-lifestyle and phthalates exposure on sex hormone levels. J Hazard Mater. 2019;369:290–8. Epub 2019/02/20. doi: 10.1016/j.jhazmat.2019.02.011. [DOI] [PubMed] [Google Scholar]
- 53.Watkins DJ, Sánchez BN, Téllez-Rojo MM, Lee JM, Mercado-García A, Blank-Goldenberg C, Peterson KE, Meeker JD. Phthalate and bisphenol A exposure during in utero windows of susceptibility in relation to reproductive hormones and pubertal development in girls. Environ Res. 2017;159:143–51. Epub 2017/08/12. doi: 10.1016/j.envres.2017.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Johns LE, Ferguson KK, Soldin OP, Cantonwine DE, Rivera-González LO, Del Toro LVA, Calafat AM, Ye X, Alshawabkeh AN, Cordero JF, Meeker JD. Urinary phthalate metabolites in relation to maternal serum thyroid and sex hormone levels during pregnancy: a longitudinal analysis. Reprod Biol Endocrinol. 2015;13:4-. doi: 10.1186/1477-7827-13-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hart R, Doherty DA, Frederiksen H, Keelan JA, Hickey M, Sloboda D, Pennell CE, Newnham JP, Skakkebaek NE, Main KM. The influence of antenatal exposure to phthalates on subsequent female reproductive development in adolescence: a pilot study. Reproduction (Cambridge, England). 2013. Epub 2013/09/13. doi: 10.1530/rep-13-0331. [DOI] [PubMed] [Google Scholar]
- 56.Li N, Liu T, Guo K, Zhu J, Yu G, Wang S, Ye L. Effect of mono-(2-ethylhexyl) phthalate (MEHP) on proliferation of and steroid hormone synthesis in rat ovarian granulosa cells in vitro. J Cell Physiol. 2018;233(4):3629–37. Epub 2017/10/17. doi: 10.1002/jcp.26224. [DOI] [PubMed] [Google Scholar]
- 57.Meling DD, Warner GR, Szumski JR, Gao L, Gonsioroski AV, Rattan S, Flaws JA. The effects of a phthalate metabolite mixture on antral follicle growth and sex steroid synthesis in mice. Toxicology and applied pharmacology. 2020;388:114875. Epub 2019/12/31. doi: 10.1016/j.taap.2019.114875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ma Y, Zhang J, Zeng R, Qiao X, Cheng R, Nie Y, Luo Y, Li S, Zhang J, Xu W, Xu L, Hu Y. Effects of the Dibutyl Phthalate (DBP) on the Expression and Activity of Aromatase in Human Granulosa Cell Line KGN. Ann Clin Lab Sci. 2019;49(2):175–82. Epub 2019/04/28. [PubMed] [Google Scholar]
- 59.Kershaw EE, Flier JS. Adipose Tissue as an Endocrine Organ. The Journal of Clinical Endocrinology & Metabolism. 2004;89(6):2548–56. doi: 10.1210/jc.2004-0395. [DOI] [PubMed] [Google Scholar]
- 60.Kim SH, Park MJ. Phthalate exposure and childhood obesity. Ann Pediatr Endocrinol Metab. 2014;19(2):69–75. Epub 2014/08/01. doi: 10.6065/apem.2014.19.2.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zettergren A, Andersson N, Larsson K, Kull I, Melén E, Georgelis A, Berglund M, Lindh C, Bergström A. Exposure to environmental phthalates during preschool age and obesity from childhood to young adulthood. Environ Res. 2021;192:110249. Epub 2020/09/28. doi: 10.1016/j.envres.2020.110249. [DOI] [PubMed] [Google Scholar]
- 62.Manson JM, Sammel MD, Freeman EW, Grisso JA. Racial differences in sex hormone levels in women approaching the transition to menopause. Fertility and sterility. 2001;75(2):297–304. Epub 2001/02/15. doi: 10.1016/s0015-0282(00)01723-4. [DOI] [PubMed] [Google Scholar]
- 63.Pinheiro SP, Holmes MD, Pollak MN, Barbieri RL, Hankinson SE. Racial differences in premenopausal endogenous hormones. Cancer Epidemiol Biomarkers Prev. 2005;14(9):2147–53. Epub 2005/09/21. doi: 10.1158/1055-9965.Epi-04-0944. [DOI] [PubMed] [Google Scholar]
- 64.Setiawan VW, Haiman CA, Stanczyk FZ, Le Marchand L, Henderson BE. Racial/ethnic differences in postmenopausal endogenous hormones: the multiethnic cohort study. Cancer Epidemiol Biomarkers Prev. 2006;15(10):1849–55. Epub 2006/10/13. doi: 10.1158/1055-9965.Epi-06-0307. [DOI] [PubMed] [Google Scholar]
- 65.Schilling C, Gallicchio L, Miller SR, Langenberg P, Zacur H, Flaws JA. Relation of body mass and sex steroid hormone levels to hot flushes in a sample of mid-life women. Climacteric. 2007;10(1):27–37. Epub 2007/03/17. doi: 10.1080/13697130601164755. [DOI] [PubMed] [Google Scholar]
- 66.Schliep KC, Mumford SL, Vladutiu CJ, Ahrens KA, Perkins NJ, Sjaarda LA, Kissell KA, Prasad A, Wactawski-Wende J, Schisterman EF. Perceived stress, reproductive hormones, and ovulatory function: a prospective cohort study. Epidemiology. 2015;26(2):177–84. Epub 2015/02/03. doi: 10.1097/ede.0000000000000238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Serrano SE, Braun J, Trasande L, Dills R, Sathyanarayana S. Phthalates and diet: a review of the food monitoring and epidemiology data. Environmental Health. 2014;13(1):43. doi: 10.1186/1476-069X-13-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Varshavsky JR, Morello-Frosch R, Woodruff TJ, Zota AR. Dietary sources of cumulative phthalates exposure among the U.S. general population in NHANES 2005–2014. Environment international. 2018;115:417–29. Epub 2018/03/29. doi: 10.1016/j.envint.2018.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Carruba G, Granata OM, Pala V, Campisi I, Agostara B, Cusimano R, Ravazzolo B, Traina A. A traditional Mediterranean diet decreases endogenous estrogens in healthy postmenopausal women. Nutr Cancer. 2006;56(2):253–9. Epub 2007/05/04. doi: 10.1207/s15327914nc5602_18. [DOI] [PubMed] [Google Scholar]
- 70.Chang VC, Cotterchio M, Boucher BA, Jenkins DJA, Mirea L, McCann SE, Thompson LU. Effect of Dietary Flaxseed Intake on Circulating Sex Hormone Levels among Postmenopausal Women: A Randomized Controlled Intervention Trial. Nutr Cancer. 2019;71(3):385–98. Epub 2018/10/31. doi: 10.1080/01635581.2018.1516789. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


