Abstract
A large number of studies have examined the association between advanced paternal age (APA) and risk of schizophrenia in offspring. Here we present an overview of epidemiological studies on this subject published since 2000, and systematically summarize their methodologies and results. Next, we discuss evidence to elucidate the potential mechanisms contributing to the association between APA and offspring schizophrenia, considering paternal psychiatric morbidity and genetic liability, maternal factors, and findings from family design studies. We propose that multiple mechanisms, including causal and non-causal pathways, contribute to the observed relationship between APA and schizophrenia in offspring, and conclude by highlighting the need for multi-disciplinary studies in disentangling these complex, non-mutually exclusive mechanisms.
Keywords: Advanced paternal age, paternal age, schizophrenia, de novo mutations, delayed fatherhood, psychiatric epidemiology
1. Introduction
Johanson (1958) was the first to report an association between APA and schizophrenia in 1958. Since then, several other studies (e.g., Hare et al., 1979; Kinnell, 1983; Zammit et al., 2003; Sørensen et al., 2014) found a relationship between APA and risk of schizophrenia in offspring, in parallel with experimental studies supporting these observations (García-Palomares et al., 2009; Milekic et al., 2015). Despite the increase in average parental age in the past decades (Matthews et al., 2009, 2014; Khandwala et al., 2017) and growing interest in the topic, the findings from studies regarding the pattern and magnitude of the positive association between paternal age and offspring schizophrenia are heterogenous and the literature lacks consensus.
To advance our understanding of the association between APA and risk of schizophrenia, research has also focused on possible mechanisms underlying this association, proposing several hypotheses and evidence in support of them (Malaspina et al., 2015; Gratten et al., 2016; Taylor et al., 2019). Nevertheless, the conclusions about the underpinning mechanisms of APA and offspring schizophrenia remain divided (Janecka et al., 2017).
In this review, we begin with discussing findings from key epidemiological studies on APA and the risk of schizophrenia in offspring published from 2000 through 2020, systematically summarizing their methodology and results. Next, we present evidence regarding the hypothesized mechanisms contributing to the association between APA and schizophrenia in offspring. Finally, we discuss the implications of the lack of unequivocal evidence about the underpinning mechanisms, and potential directions for future studies on the topic.
2. Epidemiological studies
In a seminal birth cohort study in Israel, Malaspina et al. (2001) was first to demonstrate a positive dose-response-like association between APA and risk of schizophrenia in the offspring, reporting about 190% higher risk of schizophrenia among offspring of fathers ≥ 50 years of age compared to their counterparts born to fathers 20–24 years of age. These findings supported the plausibility of paternal age effects and were shortly after replicated in a cohort study in the US (Brown et al., 2002) and case-control studies in Sweden (Dalman et al., 2002) and Denmark (Byrne et al., 2003). In the cohort study conducted in the US, Brown et al. reported an 89% increase in the risk of schizophrenia associated with every 10-year increase in paternal age. The dose-response pattern was also observed in the population-based case-control studies from Sweden (Dalman et al., 2002) and Denmark (Byrne et al., 2003). Several other studies have assessed the association between APA and offspring risk of schizophrenia, and except for a few studies (Torrey et al., 2009; Ek et al., 2015; Byars et al., 2016), the majority have consistently pointed to a positive association between APA and risk of schizophrenia (Figure 1 and Table S1). Table S1 provides a summary of the original studies (including study design, sample size, and crude and adjusted measures of association) and their findings.
Figure 1 –
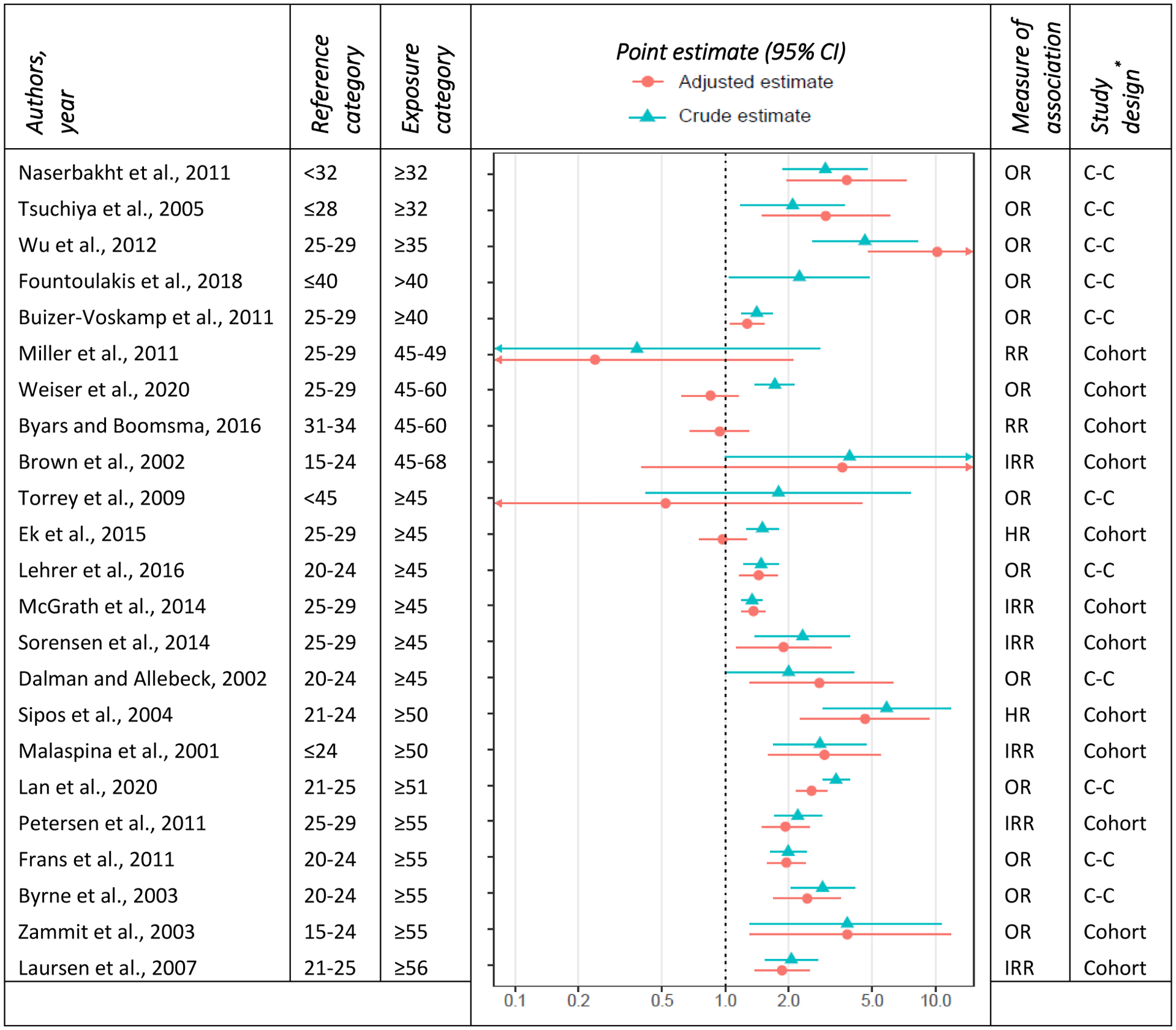
forest plot of the association between paternal age and schizophrenia. The estimates are based on the highest age category in each study and studies are ordered ascendingly according to the exposure age category.
*C–C: case-control
The measures of association for different age groups vary across studies and indicate up to a fivefold increase in the likelihood of schizophrenia in offspring of men 50 years of age or older (Sipos et al., 2004). For instance, Zammit et al. (Zammit et al., 2003) reported a 280% increase in the risk of offspring schizophrenia of fathers 55 years of age or older whereas Laursen et al. (2007) found that paternal age of 56 or older was associated with an 86% increase in the risk of offspring schizophrenia. Despite these variations in the effect sizes, the positive association in most studies emerges from as early as mid-to-late 30s and is the strongest for the oldest paternal age category (Malaspina et al., 2001; Dalman et al., 2002; Zammit et al., 2003; Sipos et al., 2004; Laursen et al., 2007; Frans et al., 2011; Wu et al., 2012; McGrath et al., 2014; Sørensen et al., 2014; Lan et al., 2020). Nevertheless, the pattern of the relationship between paternal age and risk of schizophrenia is not consistent across studies. Several studies have pointed to a monotonic dose-response pattern (Malaspina et al., 2001; A. S. Brown et al., 2002; Tsuchiya et al., 2005; Laursen et al., 2007; Wu et al., 2012; Sørensen et al., 2014), while others have found an increase in the highest age categories (≥45), suggesting a potential threshold effect (Byrne et al., 2003; Lehrer et al., 2016). A few studies have also shown an increased risk in both extremes of paternal age, depicting a possible U-shaped relationship between paternal age and risk of offspring schizophrenia (Wohl et al., 2007; Buizer-Voskamp et al., 2011; Miller, Messias, et al., 2011; McGrath et al., 2014).
The heterogeneity of the measures of association and their patterns could partly be attributable to the study methodology, including study design, study population, and analytical approach. For instance, studies have reported that the association between APA and schizophrenia is modified by sex (Byrne et al., 2003; Miller, Suvisaari, et al., 2011). However, this pattern is not consistent across all studies (e.g., Sipos et al., 2004; Wu et al., 2012). If, in fact, such a pattern exists, then possible differences in the gender distribution between study samples (especially in ascertained, case-control studies) could lead to variation in results observed between studies. The choice of covariates varied significantly across studies, which could also have contributed to the heterogeneity of findings. In fact, the substantial differences between crude and adjusted measures of association observed in most studies (Figure 1) highlight the effects of covariate adjustment on the estimated associations. Moreover, even for the commonly adjusted variables (e.g., maternal age), the operationalization of the variable, including its functional form, had significant variations contributing to the heterogeneity of results across studies.
Another study design factor with implications on the magnitude of estimated association is the reference age category. Most studies published before 2010 used the age category of 20–24 as the reference, whereas recent studies have most commonly used 25–29, perhaps following the trend of increased age at parenthood (Matthews et al., 2009, 2014; Khandwala et al., 2017), or acknowledging the possible excess risk associated with younger age of the father (Wohl et al., 2007; Buizer-Voskamp et al., 2011; Miller, Messias, et al., 2011; McGrath et al., 2014). Despite that, we did not observe a substantial difference in study results by reference age category (Figure 1). Nevertheless, the potential impact of categorizing paternal age is not limited to the reference category. The cut-off values used for age categories could potentially account for some variation between studies. Even studies using the same cut-off values are not guaranteed to have a similar distribution of individual observations within the defined age categories. This is of particular importance for broad age categories and categories with no lower or upper limit (e.g., <25 or ≥45 years of age).
Notwithstanding these variations across studies, overall findings are suggestive of a robust, positive association between APA and risk of offspring schizophrenia, which have been replicated through diverse study designs and populations. This conclusion is also supported by meta-analyses (Wohl et al., 2007; Torrey et al., 2009; Miller, Messias, et al., 2011; Oldereid et al., 2018) such as Torrey et al. who, analyzing results from 10 studies, found a 122% increase in odds of schizophrenia among offspring of fathers 55 years and older. Similarly, analyzing results from 12 studies, Miller et al. (2011) found APA to be associated with a significant increase in the risk of schizophrenia in the offspring. This analysis also indicated an increased risk of schizophrenia associated with young paternal age (<25). These meta-analyses by Torrey et al. (2009) and Miller et al. had 7 studies in common. The most recent meta-analysis included a total of 19 original studies and reached the same conclusion (Oldereid et al., 2018). Resulting from the consistent evidence supporting an association between APA and offspring schizophrenia, a growing number of studies have been investigating underlying mechanisms to explain this relationship; however, evidence remains equivocal. The next section probes commonly discussed hypotheses regarding the possible mechanisms contributing to the association between APA and offspring schizophrenia.
3. Mechanisms
There are several hypotheses regarding the mechanisms underpinning the association between APA and offspring risk of schizophrenia, and debates around them continue (Janecka et al., 2017). These hypotheses can be broadly classified into two main categories: i) mechanisms that are age-related (time-dependent), e.g., de novo mutations and morbidities, and ii) mechanisms that are age-independent (time-invariant), e.g., inherited effects. Figure 2 illustrates the patterns of various potential acting mechanisms. Importantly, while these mechanisms may differ for a group of largely monogenic congenital disorders associated with APA (e.g. achondroplasia (Orioli et al., 1995)), they likely generalize to other neuropsychiatric conditions with complex etiology (e.g., autism (Reichenberg et al., 2006; Janecka et al., 2017)).
Figure 2 –
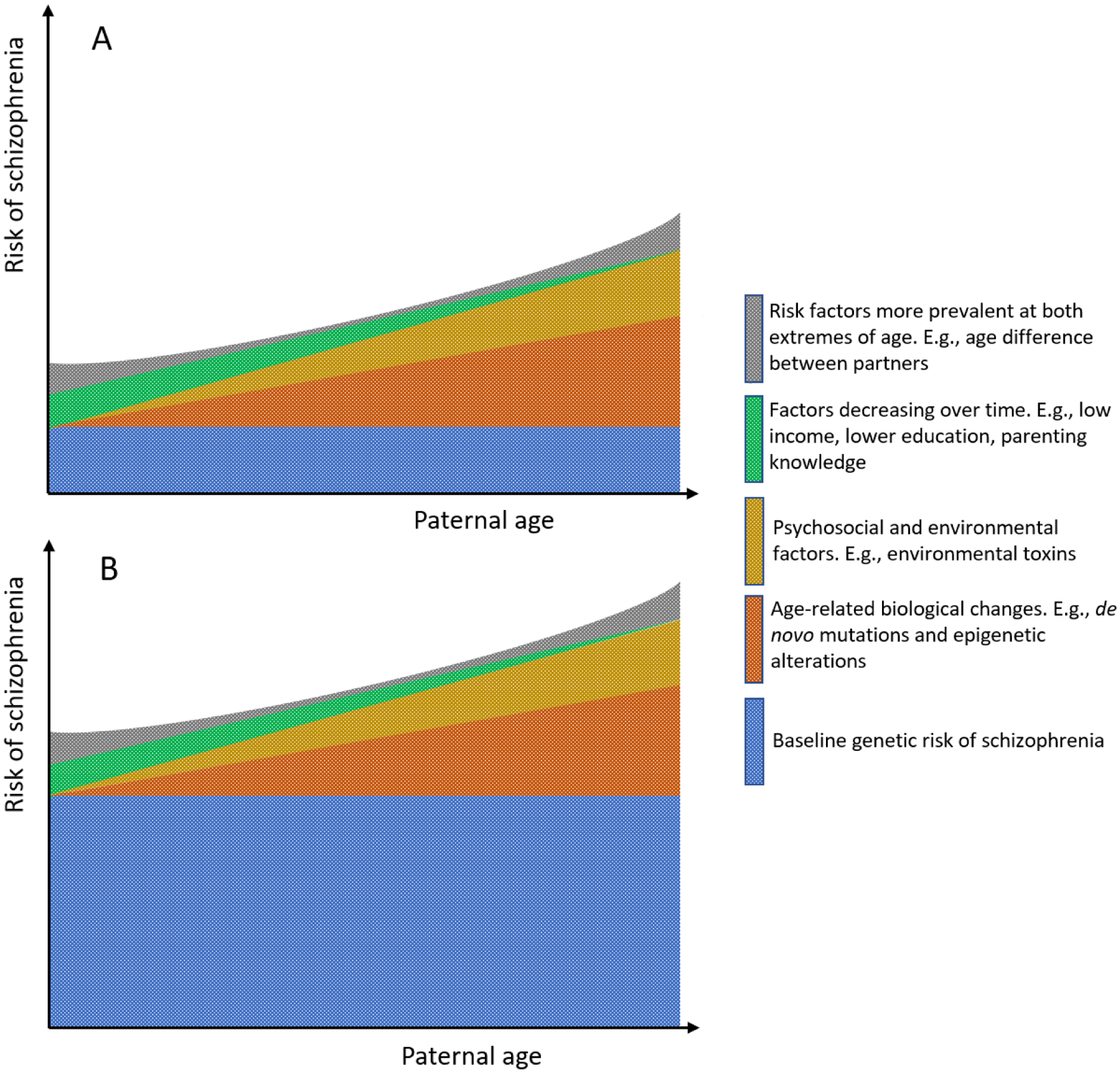
Multifactorial origins of schizophrenia risk in offspring in relation to paternal age at conception. A) among those with extended fatherhood, B) among those with delayed fatherhood
Note: Extended fatherhood includes fathers who have had their first child not at an advanced age, however, one or more of their subsequent children was born at an advanced age. Delayed fatherhood includes fathers who have had their first child at an advanced age.
Figures depict the qualitative aspect of the trajectory for these factors at a population level and does not intend to capture the heterogeneity and complexity of these trajectories on the individual level
De novo mutations (Malaspina, 2001) and epigenetic changes (Malaspina, 2001; Rutten et al., 2009) are two of the common hypotheses that postulate that biological changes associated with advancing age contribute to the relationship between APA and offspring schizophrenia. De novo mutation hypothesis stems from the evidence about the increasing number of mutations in the male germline by age (Crow, 1997, 2000; Kong et al., 2012; Goldmann et al., 2016) and suggests that these changes are associated with increased risk of schizophrenia in the offspring (Malaspina, 2001; Malaspina et al., 2002; Sipos et al., 2004). The epigenetic alteration hypothesis draws evidence from animal (Smith et al., 2013) and human studies (Jenkins et al., 2013) (e.g., loss of DNA methylation over time in the spermatogonia), proposing that such epigenetic alterations in the germline contribute to the association between APA and schizophrenia in offspring (Malaspina, 2001; Perrin et al., 2006).
Several hypotheses postulate that other factors, not the biological changes correlated with age per se, contribute to the association between paternal age and risk of schizophrenia (Gratten et al., 2016). The leading hypothesis in this category is selection into late fatherhood (Petersen et al., 2011; Ek et al., 2015). One of the plausible scenarios supporting this hypothesis is the possibility of a genetic profile among one or both parents that is associated with both APA as well as a higher risk of schizophrenia in offspring (Ek et al., 2015; Janecka et al., 2017) (Figure 2, A vs B). Other proposed mechanisms for the association between paternal age and risk of schizophrenia in offspring focus on parental psycho-social factors and maternal conditions (Miller, Suvisaari, et al., 2011).
Of the various hypotheses, de novo mutations and inherited effects related to selection into late fatherhood have received the most attention and support in the literature (Janecka et al., 2017). In the next section, we review evidence for de novo and inherited effects of APA from epidemiological studies. Subsequently, we highlight other possible mechanisms underpinning the association between paternal age and schizophrenia in offspring, focusing on the role of reproductive function. Although beyond the focus of this review, molecular and biological studies also provide support for other possible age-related mechanisms (e.g., DNA methylation changes (Malaspina, 2001; Perrin et al., 2006)) contributing to the association between paternal age and risk of schizophrenia in the offspring (see Janecka et al., 2017).
3.1. De novo and inherited APA effects
The de novo mutations hypothesis regarding APA has mechanistic molecular support and is consistent with the existing evidence on the contribution of de novo mutations to the complex etiology of schizophrenia (McClellan et al., 2007; Howrigan et al., 2020). As discussed by Crow (1997 and 2000) and supported with further molecular evidence (Kong et al., 2012), there is a positive association between paternal age and increasing rates of de novo mutations in the germline with about three-quarters of all de novo mutations being attributable to paternal origin (Goldmann et al., 2016; Goriely, 2016). Epidemiological studies have also evaluated the robustness of the de novo mutations hypothesis, signaling some support in favor (Malaspina, 2001; Malaspina et al., 2002; Sipos et al., 2004). However, unlike molecular and biological studies, the spectrum of findings from epidemiological studies is less conclusive and warrants consideration of other mechanisms.
Inherited APA effects related to late selection into fatherhood can emerge if the genetic profile of the father is associated with behaviors that are correlated with delayed or extended fatherhood (e.g., social withdrawal and aloofness), which is also correlated with offspring psychiatric risk. Similarly, such patterns can occur if mothers with increased genetic liability for schizophrenia are more likely to have children with men at a more advanced age. Assortative mating with regard to genetic vulnerability to psychiatric illnesses can further increase the role of such a mechanism of action (de Kluiver et al., 2017). Of note, the inherited effects hypothesis is not attributable to genetic or environmental changes related to advancing age and can merely be a non-causal mechanism contributing to the association between APA and schizophrenia. Therefore, in the presence of genetic liability from parents associated with advanced schizophrenia risk, the predisposition of the child to develop schizophrenia would not be modified by age of the father at conception (Janecka et al., 2017).
Studies have leveraged family designs to disentangle the contribution of possible mechanisms and pathways to the APA and risk of offspring schizophrenia association. This has been a motive for studies comparing the role of APA in sporadic vs familial forms of schizophrenia. The key assumption in these studies is that a stronger association with sporadic cases would suggest a relatively higher contribution of de novo mutations compared to inherited factors. Findings from these studies have been inconclusive, adding mixed signals regarding the contribution of de novo and inherited effects (Malaspina et al., 2002; Zammit et al., 2003; El-Saadi et al., 2004; Pulver et al., 2004; Sipos et al., 2004; Tsuchiya et al., 2005; Kollias et al., 2019). For instance, Malaspina et al. (2002) found that schizophrenia cases without a family history for the disease had a higher paternal age than schizophrenia cases with positive family history. In line with that, Sipos et al. (2004) found a positive association between APA and offspring schizophrenia among those with no family history of this illness. Nevertheless, several studies (Zammit et al., 2003; El-Saadi et al., 2004; Pulver et al., 2004; Tsuchiya et al., 2005) did not find evidence about family history of schizophrenia modifying the association between APA and offspring schizophrenia. It is worth noting that the accuracy of these studies relies heavily on ascertainment and phenotyping relatives of cases, which are subject to potential biases and underdiagnosis (Janecka et al., 2017). Hence, the findings of these studies are as reliable as their underlying methodology and should be interpreted in light of those limitations.
A few studies have used sibling design to examine the association between birth order and the risk of schizophrenia. Considering that siblings share family-level, time-invariant factors, an association between birth order and risk of schizophrenia might be suggestive of time-dependent (i.e., age-related) mechanisms such as de novo mutations. Findings from these studies, although mixed, have been predominantly negative, suggesting lack of strong support for the role of de novo mutations and signaling the relatively greater importance of time-invariant factors (e.g., inherited effects). Sham et al. (1993) observed a positive association between birth order and the risk of schizophrenia. Nevertheless, several other studies (Malama et al., 1988; Kemppainen et al., 2001; Tsuchiya et al., 2005) did not yield support for such a relationship.
Time dependent factors can impact the estimation of the association between APA and risk of schizophrenia as well as its underlying mechanisms. Maternal age is an example of a time dependent factor that can potentially contribute to the association between APA and offspring risk of schizophrenia. Maternal age is strongly correlated with paternal age (Croen et al., 2007; Carslake et al., 2017) and, considering the evidence suggesting a positive association between maternal age and risk of schizophrenia (Mehta et al., 2016; Ni et al., 2018), it can strengthen the association between APA and offspring schizophrenia. Although most studies have adjusted for maternal age (Table S1), assumptions regarding the functional form of the relationship or aggregate age categorization of maternal age in the adjusted models may result in inadequate control for this spurious correlation, biasing (in this case often upwardly) the association of APA and offspring schizophrenia. Residual confounding from time dependent factors can result in overestimation of the actual contribution of time dependent mechanisms, such as de novo mutations, to the association between APA and risk of schizophrenia.
The evidence about the possibility of a higher psychiatric liability associated with APA is limited, and debates around it remain unsettled (Malaspina, 2001; Malaspina et al., 2002; Jaffe et al., 2014; Janecka et al., 2017; Taylor et al., 2019). Nilsen et al. (2013) found that fathers with advanced age at fatherhood had a higher number of health problems, and were more likely to report risky health behaviors (e.g., smoking or consuming alcohol) compared to younger fathers. Nevertheless, it is unclear if the observed associations are due to an underlying genetic risk correlated with advanced age and poor health outcomes, or represent only the expected increase in health problems attributable to advancing age. Moreover, it is also unknown if the possible underlying genetic risk or the health effects themselves are associated with risk of offspring schizophrenia.
In support of the possible role of maternal contribution to the observed associations, Miller et al. (Miller, Suvisaari, et al., 2011) found a positive correlation between APA and diagnosis of schizophrenia in the offspring’s mother, but not father. Although most of the studies examining the association between APA and offspring schizophrenia have accounted for parental psychiatric history, the adjustment is often limited to certain illnesses. Moreover, likely underdiagnoses and ascertainment biases for psychiatric conditions could result in further residual confounding, biasing the estimated measures of association. Therefore, the correlation of maternal schizophrenia with APA and possible enrichment of the child’s risk of schizophrenia attributable to maternal genetics can contribute to the observed patterns, despite statistical adjustment. Nonetheless, in a recent study, Wang et. al (2019) showed that even after directly controlling for the parental genetic vulnerability to schizophrenia, through adjustment for polygenic risk score, APA remained significantly associated with an earlier onset of schizophrenia among sporadic cases.
A few studies have used a family design and compared the effect of APA among those with delayed fatherhood (age at first child) and those with advanced age at the conception of a later-born child in order to advance our understanding about inherited vs age-related (e.g., de novo mutations) mechanisms. In a population-based sample from Denmark, Petersen et al. (2011) found that age at fatherhood, but not age at the conception of later children, is associated with the risk of schizophrenia. Weiser et al. demonstrated a similar pattern in a population-based sample from Israel, where after controlling for age at fatherhood, paternal age was no longer associated with risk of schizophrenia in the offspring, concluding that the observed association between APA and schizophrenia was likely attributable to other factors such as common genetic profile associated with delayed fatherhood (Weiser et al., 2020) (Figure 2.B). In line with these findings, studies of age at first birth among mothers (Mehta et al., 2016; Ni et al., 2018) have found that both extremes of age at motherhood (early and late motherhood) are correlated with a higher polygenic risk score for schizophrenia; however, to our knowledge, the literature lacks genetic studies directly investigating this pattern among fathers.
Although findings from individual studies often provide support for one or few mechanisms, none of the proposed mechanisms alone can fully explain the APA “effect” (Gratten et al., 2016). Therefore, evidence supporting a certain mechanism is not to be perceived as an indication of the absence of other mechanisms. In fact, as we discussed here and previously (Janecka et al., 2017), the true association is likely attributable to a combination of age-related (e.g., de novo mutations) and age-independent (e.g., inherited effects) mechanisms. Nevertheless, quantification of the contribution of each of these mechanisms remains uncertain and further studies are required to disentangle these effects.
3.2. Other mechanisms
Along with the ongoing debates about the role of de novo mutations and inherited effect mechanisms, several other mechanisms have also been suggested to contribute to the association between APA and schizophrenia in offspring. These mechanisms predominantly include factors associated with reproductive function and health and are briefly summarized below.
Studies have demonstrated a positive association between unwanted pregnancy and risk of schizophrenia in the offspring (Myhrman et al., 1996; Herman et al., 2006; McNeil et al., 2009). Since unintended pregnancies are more common at both extremes of the reproductive age (Brown et al., 1995), unwanted pregnancies can not only partly account for the increase in the risk of schizophrenia associated with APA, but can also explain the excess risk of schizophrenia in younger fathers observed in a few studies (Wohl et al., 2007; Buizer-Voskamp et al., 2011; Miller, Messias, et al., 2011; McGrath et al., 2014). Nevertheless, the mechanisms that could link the pregnancy intent with offspring risk of neuropsychiatric conditions have not been established, and background confounding remains plausible.
Alternatively, maternal immune intolerance of fetal antigens (paternal origin antigens) has been suggested to be partly attributable to the short duration of vaginal exposure to the semen of the offspring’s father before fertilization (Thaler, 1989; Kelly et al., 1997; Robertson et al., 2001) and to be associated with adverse maternal and child health outcomes (Dekker et al., 1998; Arck et al., 2013). If such immune intolerance of the fetus was also related to schizophrenia risk, it could serve as a mediator between the duration of sexual cohabitation and schizophrenia. This was the motive behind a study by Malaspina et al. where the duration of marriage, a proxy for the history of sexual cohabitation, was found to be associated with risk of schizophrenia in the offspring, after controlling for paternal age , paternal age at marriage, and parental history of psychiatric illness (Malaspina et al., 2019). The study also showed that the duration of marriage explained much of the increased risk in schizophrenia ascribed to paternal age at marriage. Although the duration of vaginal pre-pregnancy exposure to the semen of the offspring’s father is unlikely to explain the association between APA and risk of schizophrenia in offspring, the excess risk of schizophrenia observed with young paternal age (Wohl et al., 2007; Buizer-Voskamp et al., 2011; Miller, Messias, et al., 2011; McGrath et al., 2014), as well as in unwanted pregnancies (Myhrman et al., 1996; Herman et al., 2006; McNeil et al., 2009), could partly be attributable to the duration of pre-pregnancy “sexual” cohabitation.
Furthermore, given the decline in fertility with increasing maternal and paternal age (Hassan et al., 2003; Buizer-Voskamp et al., 2011; Liu et al., 2011), several studies have investigated these effects on the risk of schizophrenia (Hutchinson et al., 1999; Opler et al., 2010). For example, Opler et al. (2010) found a positive association between time to pregnancy and risk of schizophrenia in the offspring. The results were robust to adjustment for paternal age, parental history of psychiatric illness, and child’s year of birth. Although the study did not separate the potential contribution of maternal vs paternal characteristics to time to pregnancy, the observed association between time to pregnancy and risk of schizophrenia was stronger in the sub-sample with a paternal age of 30 years and older, indicating that the observed association could contribute to the association between APA and risk of offspring schizophrenia.
Several other mechanisms proposed to contribute to the associations between paternal age and other psychiatric illnesses in offspring could hold relevance for the role of APA in schizophrenia risk. For instance, a large population-based study combining samples from Denmark, Israel, Norway, Sweden, and Western Australia found that parents with a greater age difference were more likely to have offspring diagnosed with autism spectrum disorder (Sandin et al., 2016). Byars and Boomsma (2016), in a partially overlapping sample from Denmark, replicated the results and additionally observed that a greater parental age difference is also associated with an increased risk of schizophrenia in the offspring. The association between parental age difference and offspring schizophrenia held regardless of whether the father was the older parent or the mother, suggesting that age difference between parents can potentially contribute to the association between paternal age and schizophrenia in offspring. However, further studies are warranted to examine before drawing conclusions.
4. Conclusion
This review presented evidence from epidemiological studies about the association between APA and schizophrenia in offspring. It elucidated the literature and debates regarding the mechanisms underpinning this association. In contrast to the clear and robust evidence supporting APA effects, there is still no consensus regarding the mechanisms to explain these effects, especially the role of de novo mutation and inherited effects. The mixed findings suggest the involvement of multiple pathways, including age-related and age-independent mechanisms. To date, most of the studies evaluating mechanistic underpinning of APA effects have considered only one hypothesis or treated different hypotheses as competing alternatives. Comprehensive studies drawing from molecular, biological, and epidemiological methodologies to concurrently test multiple mechanisms and potential interactions between pathways can provide further insight into the underpinning mechanisms of APA effects.
Importantly, in light of the low baseline risk of schizophrenia in the population, even a many-fold relative increase in risk due to advanced paternal age translates into a measurable increase in the absolute risk and thus should be taken into account when counseling and advising on family planning. Formally distinguishing between causal (e.g., age-related changes) vs. non-causal (e.g., inherited effects) pathways between paternal age and schizophrenia, and disentangling the potential contribution of these pathways will have critical implications for genetic counseling and family planning.
Supplementary Material
Acknowledgements
We would like to acknowledge the generous support of the Seaver Foundation. VK was supported by National Institute of Mental Health Award T32 MH122394. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the authors’ employers.
Role of the funding source
The funding sources had no involvement in the study design, in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of competing interest
None.
References
- Arck PC, & Hecher K (2013). Fetomaternal immune cross-talk and its consequences for maternal and offspring’s health. Nature Medicine, 19(5), 548–556. 10.1038/nm.3160 [DOI] [PubMed] [Google Scholar]
- Brown AS, Schaefer CA, Wyatt RJ, Begg MD, Goetz R, Bresnahan MA, Harkavy-Friedman J, Gorman JM, Malaspina D, & Susser ES (2002). Paternal age and risk of schizophrenia in adult offspring. American Journal of Psychiatry. 10.1176/appi.ajp.159.9.1528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SS, & Eisenberg L (1995). The best intentions: Unintended pregnancy and the well-being of children and families. National Academy Press. [PubMed] [Google Scholar]
- Buizer-Voskamp JE, Laan W, Staal WG, Hennekam EAM, Aukes MF, Termorshuizen F, Kahn RS, Boks MPM, & Ophoff RA (2011). Paternal age and psychiatric disorders: Findings from a Dutch population registry. Schizophrenia Research, 129(2–3), 128–132. 10.1016/j.schres.2011.03.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byars SG, & Boomsma JJ (2016). Opposite differential risks for autism and schizophrenia based on maternal age, paternal age, and parental age differences. Evolution, Medicine, and Public Health, 2016(1), 286–298. 10.1093/emph/eow023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne M, Agerbo E, Ewald H, Eaton WW, & Mortensen PB (2003). Parental Age and Risk of Schizophrenia. Archives of General Psychiatry, 60(7), 673. 10.1001/archpsyc.60.7.673 [DOI] [PubMed] [Google Scholar]
- Carslake D, Tynelius P, van den Berg G, Davey Smith G, & Rasmussen F (2017). Associations of parental age with health and social factors in adult offspring. Methodological pitfalls and possibilities. Scientific Reports, 7(1), 45278. 10.1038/srep45278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croen LA, Najjar DV, Fireman B, & Grether JK (2007). Maternal and Paternal Age and Risk of Autism Spectrum Disorders. Archives of Pediatrics & Adolescent Medicine, 161(4), 334. 10.1001/archpedi.161.4.334 [DOI] [PubMed] [Google Scholar]
- Crow JF (1997). The high spontaneous mutation rate: Is it a health risk? Proceedings of the National Academy of Sciences, 94(16), 8380–8386. 10.1073/pnas.94.16.8380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow JF (2000). The origins, patterns and implications of human spontaneous mutation. Nature Reviews Genetics, 1(1), 40–47. 10.1038/35049558 [DOI] [PubMed] [Google Scholar]
- Dalman C, & Allebeck P (2002). Paternal Age and Schizophrenia: Further Support for an Association. American Journal of Psychiatry, 159(9), 1591–1592. 10.1176/appi.ajp.159.9.1591 [DOI] [PubMed] [Google Scholar]
- de Kluiver H, Buizer-Voskamp JE, Dolan CV, & Boomsma DI (2017). Paternal age and psychiatric disorders: A review. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics, 174(3), 202–213. 10.1002/ajmg.b.32508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekker GA, Robillard PY, & Hulsey TC (1998). Immune Maladaptation in the Etiology of Preeclampsia. Obstetrical & Gynecological Survey, 53(6), 377–382. 10.1097/00006254-199806000-00023 [DOI] [PubMed] [Google Scholar]
- Ek M, Wicks S, Svensson AC, Idring S, & Dalman C (2015). Advancing Paternal Age and Schizophrenia: The Impact of Delayed Fatherhood. Schizophrenia Bulletin, 41(3), 708–714. 10.1093/schbul/sbu154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Saadi O, Pedersen CB, McNeil TF, Saha S, Welham J, O’Callaghan E, Cantor-Graae E, Chant D, Mortensen PB, & McGrath J (2004). Paternal and maternal age as risk factors for psychosis: findings from Denmark, Sweden and Australia. Schizophrenia Research, 67(2–3), 227–236. 10.1016/S0920-9964(03)00100-2 [DOI] [PubMed] [Google Scholar]
- Fountoulakis KN, Gonda X, Siamouli M, Panagiotidis P, Moutou K, Nimatoudis I, & Kasper S (2018). Paternal and maternal age as risk factors for schizophrenia: a case–control study. International Journal of Psychiatry in Clinical Practice, 22(3), 170–176. 10.1080/13651501.2017.1391292 [DOI] [PubMed] [Google Scholar]
- Frans EM, McGrath JJ, Sandin S, Lichtenstein P, Reichenberg A, Långström N, & Hultman CM (2011). Advanced paternal and grandpaternal age and schizophrenia: A three-generation perspective. Schizophrenia Research, 133(1–3), 120–124. 10.1016/j.schres.2011.09.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Palomares S, Pertusa JF, Miñarro J, García-Pérez MA, Hermenegildo C, Rausell F, Cano A, & Tarín JJ (2009). Long-Term Effects of Delayed Fatherhood in Mice on Postnatal Development and Behavioral Traits of Offspring1. Biology of Reproduction, 80(2), 337–342. 10.1095/biolreprod.108.072066 [DOI] [PubMed] [Google Scholar]
- Goldmann JM, Wong WSW, Pinelli M, Farrah T, Bodian D, Stittrich AB, Glusman G, Vissers LELM, Hoischen A, Roach JC, Vockley JG, Veltman JA, Solomon BD, Gilissen C, & Niederhuber JE (2016). Parent-of-origin-specific signatures of de novo mutations. Nature Genetics, 48(8), 935–939. 10.1038/ng.3597 [DOI] [PubMed] [Google Scholar]
- Goriely A (2016). Decoding germline de novo point mutations. Nature Genetics, 48(8), 823–824. 10.1038/ng.3629 [DOI] [PubMed] [Google Scholar]
- Gratten J, Wray NR, Peyrot WJ, McGrath JJ, Visscher PM, & Goddard ME (2016). Risk of psychiatric illness from advanced paternal age is not predominantly from de novo mutations. Nature Genetics, 48(7), 718–724. 10.1038/ng.3577 [DOI] [PubMed] [Google Scholar]
- Hare EH, & Moran PAP (1979). Raised Parental Age in Psychiatric Patients: Evidence for the Constitutional Hypothesis. British Journal of Psychiatry, 134(2), 169–177. 10.1192/bjp.134.2.169 [DOI] [PubMed] [Google Scholar]
- Hassan MA ., & Killick SR (2003). Effect of male age on fertility: evidence for the decline in male fertility with increasing age. Fertility and Sterility, 79, 1520–1527. 10.1016/S0015-0282(03)00366-2 [DOI] [PubMed] [Google Scholar]
- Herman DB, Brown AS, Opler MG, Desai M, Malaspina D, Bresnahan M, Schaefer CA, & Susser ES (2006). Does unwantedness of pregnancy predict schizophrenia in the offspring? Social Psychiatry and Psychiatric Epidemiology, 41(8), 605–610. 10.1007/s00127-006-0078-7 [DOI] [PubMed] [Google Scholar]
- Howrigan DP, Rose SA, Samocha KE, Fromer M, Cerrato F, Chen WJ, Churchhouse C, Chambert K, Chandler SD, Daly MJ, Dumont A, Genovese G, Hwu H-G, Laird N, Kosmicki JA, Moran JL, Roe C, Singh T, Wang S-H, … Neale BM (2020). Exome sequencing in schizophrenia-affected parent–offspring trios reveals risk conferred by protein-coding de novo mutations. Nature Neuroscience, 23(2), 185–193. 10.1038/s41593-019-0564-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson G, Bhugra D, Mallett R, Burnett R, Corridan B, & Leff J (1999). Fertility and marital rates in first-onset schizophrenia. Social Psychiatry and Psychiatric Epidemiology, 34(12), 617–621. 10.1007/s001270050183 [DOI] [PubMed] [Google Scholar]
- Jaffe AE, Eaton WW, Straub RE, Marenco S, & Weinberger DR (2014). Paternal age, de novo mutations and schizophrenia. Molecular Psychiatry, 19(3), 274–275. 10.1038/mp.2013.76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janecka M, Mill J, Basson MA, Goriely A, Spiers H, Reichenberg A, Schalkwyk L, & Fernandes C (2017). Advanced paternal age effects in neurodevelopmental disorders—review of potential underlying mechanisms. Translational Psychiatry, 7(1), e1019–e1019. 10.1038/tp.2016.294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins TG, Aston KI, Cairns BR, & Carrell DT (2013). Paternal aging and associated intraindividual alterations of global sperm 5-methylcytosine and 5-hydroxymethylcytosine levels. Fertility and Sterility, 100(4), 945–951.e2. 10.1016/j.fertnstert.2013.05.039 [DOI] [PubMed] [Google Scholar]
- Johanson E (1958). A study of schizophrenia in the male: a psychiatric and social study based on 138 cases with follow up. Acta Psychiatrica et Neurologica Scandinavica. Supplementum, 125, 1–132. [PubMed] [Google Scholar]
- Kelly RW, & Critchley HO (1997). Immunomodulation by human seminal plasma: a benefit for spermatozoon and pathogen? Human Reproduction, 12(10), 2200–2207. 10.1093/oxfordjournals.humrep.a019559 [DOI] [PubMed] [Google Scholar]
- Kemppainen L, Veijola J, Jokelainen J, Hartikainen A-L, Järvelin M-R, Jones P, Croudace T, & Isohanni M (2001). Birth order and risk for schizophrenia: a 31-year follow-up of the Northern Finland 1966 Birth Cohort. Acta Psychiatrica Scandinavica, 104(2), 148–152. 10.1034/j.1600-0447.2001.00258.x [DOI] [PubMed] [Google Scholar]
- Khandwala YS, Zhang CA, Lu Y, & Eisenberg ML (2017). The age of fathers in the USA is rising: an analysis of 168 867 480 births from 1972 to 2015. Human Reproduction, 32(10), 2110–2116. 10.1093/humrep/dex267 [DOI] [PubMed] [Google Scholar]
- Kinnell HG (1983). Parental Age in Schizophrenia. British Journal of Psychiatry, 142(2), 204–204. 10.1192/bjp.142.2.204a [DOI] [PubMed] [Google Scholar]
- Kollias C, Dimitrakopoulos S, Xenaki L-A, Stefanis N, & Papageorgiou C (2019). Evidence of advanced parental age linked to sporadic schizophrenia. Psychiatriki, 30(1), 24–31. 10.22365/jpsych.2019.301.24 [DOI] [PubMed] [Google Scholar]
- Kong A, Frigge ML, Masson G, Besenbacher S, Sulem P, Magnusson G, Gudjonsson SA, Sigurdsson A, Jonasdottir A, Jonasdottir A, Wong WSW, Sigurdsson G, Walters GB, Steinberg S, Helgason H, Thorleifsson G, Gudbjartsson DF, Helgason A, Magnusson OT, … Stefansson K (2012). Rate of de novo mutations and the importance of father’s age to disease risk. Nature, 488(7412), 471–475. 10.1038/nature11396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan K-C, Chiang H-J, Huang T-L, Chiou Y-J, Hsu T-Y, Ou Y-C, & Yang Y-H (2020). Association between paternal age and risk of schizophrenia: a nationwide population–based study. Journal of Assisted Reproduction and Genetics. 10.1007/s10815-020-01936-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laursen TM, & Munk-Olsen T (2007). A Comparison of Selected Risk Factors for Unipolar Depressive Disorder, Bipolar Affective Disorder, Schizoaffective Disorder, and Schizophrenia From a Danish Population-Based Cohort. The Journal of Clinical Psychiatry, 68(11), 1673–1681. 10.4088/JCP.v68n1106 [DOI] [PubMed] [Google Scholar]
- Lehrer DS, Pato MT, Nahhas RW, Miller BR, Malaspina D, Buckley PF, Sobell JL, Walsh-Messinger J, & Pato CN (2016). Paternal age effect: Replication in schizophrenia with intriguing dissociation between bipolar with and without psychosis. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics, 171(4), 495–505. 10.1002/ajmg.b.32334 [DOI] [PubMed] [Google Scholar]
- Liu K, Case A, Cheung AP, Sierra S, AlAsiri S, Carranza-Mamane B, Case A, Dwyer C, Graham J, Havelock J, Hemmings R, Lee F, Liu K, Murdock W, Senikas V, Vause TDR, & Wong BC-M (2011). Advanced Reproductive Age and Fertility. Journal of Obstetrics and Gynaecology Canada, 33(11), 1165–1175. 10.1016/S1701-2163(16)35087-3 [DOI] [PubMed] [Google Scholar]
- Malama IM, Papaioannou DJ, Kaklamani EP, Katsouyanni KM, Koumantaki IG, & Trichopoulos DV (1988). Birth Order Sibship Size and Socio-Economic Factors in Risk of Schizophrenia in Greece. British Journal of Psychiatry, 152(4), 482–486. 10.1192/bjp.152.4.482 [DOI] [PubMed] [Google Scholar]
- Malaspina D (2001). Paternal Factors and Schizophrenia Risk: De Novo Mutations and Imprinting. Schizophrenia Bulletin, 27(3), 379–393. 10.1093/oxfordjournals.schbul.a006882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaspina D, Corcoran C, Fahim C, Berman A, Harkavy-Friedman J, Yale S, Goetz D, Goetz R, Harlap S, & Gorman J (2002). Paternal age and sporadic schizophrenia: Evidence for de novo mutations. American Journal of Medical Genetics, 114(3), 299–303. 10.1002/ajmg.1701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaspina D, Gilman C, & Kranz TM (2015). Paternal age and mental health of offspring. Fertility and Sterility, 103(6), 1392–1396. 10.1016/j.fertnstert.2015.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaspina D, Harlap S, Fennig S, Heiman D, Nahon D, Feldman D, & Susser ES (2001). Advancing Paternal Age and the Risk of Schizophrenia. Archives of General Psychiatry, 58(4), 361. 10.1001/archpsyc.58.4.361 [DOI] [PubMed] [Google Scholar]
- Malaspina D, Kranz T, Kleinhaus K, Daboul S, Rothman K, Gilman C, Getz M, Harlap S, & Friedlander Y (2019). Short duration of marriage at conception as an independent risk factor for schizophrenia. Schizophrenia Research, 208, 190–195. 10.1016/j.schres.2019.03.001 [DOI] [PubMed] [Google Scholar]
- Matthews TJ, & Hamilton BE (2009). Delayed childbearing: more women are having their first child later in life. NCHS Data Brief, Aug(21), 1–8. [PubMed] [Google Scholar]
- Matthews TJ, & Hamilton BE (2014). First births to older women continue to rise. NCHS Data Brief, May(152), 1–8. [PubMed] [Google Scholar]
- McClellan JM, Susser E, & King M-C (2007). Schizophrenia: a common disease caused by multiple rare alleles. British Journal of Psychiatry, 190(3), 194–199. 10.1192/bjp.bp.106.025585 [DOI] [PubMed] [Google Scholar]
- McGrath JJ, Petersen L, Agerbo E, Mors O, Mortensen PB, & Pedersen CB (2014). A Comprehensive Assessment of Parental Age and Psychiatric Disorders. JAMA Psychiatry, 71(3), 301. 10.1001/jamapsychiatry.2013.4081 [DOI] [PubMed] [Google Scholar]
- McNeil TF, Schubert EW, Cantor-Graae E, Brossner M, Schubert P, & Henriksson KM (2009). Unwanted pregnancy as a risk factor for offspring schizophrenia-spectrum and affective disorders in adulthood: a prospective high-risk study. Psychological Medicine, 39(6), 957–965. 10.1017/S0033291708004479 [DOI] [PubMed] [Google Scholar]
- Mehta D, Tropf FC, Gratten J, Bakshi A, Zhu Z, Bacanu S-A, Hemani G, Magnusson PKE, Barban N, Esko T, Metspalu A, Snieder H, Mowry BJ, Kendler KS, Yang J, Visscher PM, McGrath JJ, Mills MC, Wray NR, … Wu JQ (2016). Evidence for Genetic Overlap Between Schizophrenia and Age at First Birth in Women. JAMA Psychiatry, 73(5), 497. 10.1001/jamapsychiatry.2016.0129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milekic MH, Xin Y, O’Donnell A, Kumar KK, Bradley-Moore M, Malaspina D, Moore H, Brunner D, Ge Y, Edwards J, Paul S, Haghighi FG, & Gingrich JA (2015). Age-related sperm DNA methylation changes are transmitted to offspring and associated with abnormal behavior and dysregulated gene expression. Molecular Psychiatry, 20(8), 995–1001. 10.1038/mp.2014.84 [DOI] [PubMed] [Google Scholar]
- Miller B, Messias E, Miettunen J, Alaräisänen A, Järvelin M-R, Koponen H, Räsänen P, Isohanni M, & Kirkpatrick B (2011). Meta-analysis of Paternal Age and Schizophrenia Risk in Male Versus Female Offspring. Schizophrenia Bulletin, 37(5), 1039–1047. 10.1093/schbul/sbq011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller B, Suvisaari J, Miettunen J, Järvelin M-R, Haukka J, Tanskanen A, Lönnqvist J, Isohanni M, & Kirkpatrick B (2011). Advanced paternal age and parental history of schizophrenia. Schizophrenia Research, 133(1–3), 125–132. 10.1016/j.schres.2011.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myhrman A, Rantakallio P, Isohanni M, Jones P, & Partanen U (1996). Unwantedness of a Pregnancy and Schizophrenia in the Child. British Journal of Psychiatry, 169(5), 637–640. 10.1192/bjp.169.5.637 [DOI] [PubMed] [Google Scholar]
- Naserbakht M, Ahmadkhaniha H-R, Mokri B, & Smith CL (2011). Advanced paternal age is a risk factor for schizophrenia in Iranians. Annals of General Psychiatry, 10(1), 15. 10.1186/1744-859X-10-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni G, Gratten J, Wray NR, & Lee SH (2018). Age at first birth in women is genetically associated with increased risk of schizophrenia. Scientific Reports, 8(1), 10168. 10.1038/s41598-018-28160-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsen ABV, Waldenström U, Rasmussen S, Hjelmstedt A, & Schytt E (2013). Characteristics of first-time fathers of advanced age: a Norwegian population-based study. BMC Pregnancy and Childbirth, 13(1), 29. 10.1186/1471-2393-13-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldereid NB, Wennerholm U-B, Pinborg A, Loft A, Laivuori H, Petzold M, Romundstad LB, Söderström-Anttila V, & Bergh C (2018). The effect of paternal factors on perinatal and paediatric outcomes: a systematic review and meta-analysis. Human Reproduction Update, 24(3), 320–389. 10.1093/humupd/dmy005 [DOI] [PubMed] [Google Scholar]
- Opler MGA, Harlap S, Ornstein K, Kleinhaus K, Perrin M, Gangwisch JE, Lichtenberg P, Draiman B, & Malaspina D (2010). Time-to-pregnancy and risk of schizophrenia. Schizophrenia Research, 118(1–3), 76–80. 10.1016/j.schres.2010.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orioli IM, Castilla EE, Scarano G, & Mastroiacovo P (1995). Effect of paternal age in achondroplasia, thanatophoric dysplasia, and osteogenesis imperfecta. American Journal of Medical Genetics, 59(2), 209–217. 10.1002/ajmg.1320590218 [DOI] [PubMed] [Google Scholar]
- Perrin MC, Brown AS, & Malaspina D (2006). Aberrant Epigenetic Regulation Could Explain the Relationship of Paternal Age to Schizophrenia. Schizophrenia Bulletin, 33(6), 1270–1273. 10.1093/schbul/sbm093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen L, Mortensen PB, & Pedersen CB (2011). Paternal Age at Birth of First Child and Risk of Schizophrenia. American Journal of Psychiatry, 168(1), 82–88. 10.1176/appi.ajp.2010.10020252 [DOI] [PubMed] [Google Scholar]
- Pulver AE, McGrath JA, Liang K-Y, Lasseter VK, Nestadt G, & Wolyniec PS (2004). An indirect test of the new mutation hypothesis associating advanced paternal age with the etiology of schizophrenia. American Journal of Medical Genetics, 124B(1), 6–9. 10.1002/ajmg.b.20066 [DOI] [PubMed] [Google Scholar]
- Reichenberg A, Gross R, Weiser M, Bresnahan M, Silverman J, Harlap S, Rabinowitz J, Shulman C, Malaspina D, Lubin G, Knobler HY, Davidson M, & Susser E (2006). Advancing Paternal Age and Autism. Archives of General Psychiatry, 63(9), 1026. 10.1001/archpsyc.63.9.1026 [DOI] [PubMed] [Google Scholar]
- Robertson SA, & Sharkey DJ (2001). The role of semen in induction of maternal immune tolerance to pregnancy. Seminars in Immunology, 13(4), 243–254. 10.1006/smim.2000.0320 [DOI] [PubMed] [Google Scholar]
- Rutten BPF, & Mill J (2009). Epigenetic Mediation of Environmental Influences in Major Psychotic Disorders. Schizophrenia Bulletin, 35(6), 1045–1056. 10.1093/schbul/sbp104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandin S, Schendel D, Magnusson P, Hultman C, Surén P, Susser E, Grønborg T, Gissler M, Gunnes N, Gross R, Henning M, Bresnahan M, Sourander A, Hornig M, Carter K, Francis R, Parner E, Leonard H, Rosanoff M, … Reichenberg A (2016). Autism risk associated with parental age and with increasing difference in age between the parents. Molecular Psychiatry, 21(5), 693–700. 10.1038/mp.2015.70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sham PC, Maclean CJ, & Kendler KS (1993). Risk of Schizophrenia and Age Difference with Older Siblings. British Journal of Psychiatry, 163(5), 627–633. 10.1192/bjp.163.5.627 [DOI] [PubMed] [Google Scholar]
- Sipos A, Rasmussen F, Harrison G, Tynelius P, Lewis G, Leon DA, & Gunnell D (2004). Paternal age and schizophrenia: a population based cohort study. BMJ, 329(7474), 1070. 10.1136/bmj.38243.672396.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RG, Reichenberg A, Kember RL, Buxbaum JD, Schalkwyk LC, Fernandes C, & Mill J (2013). Advanced paternal age is associated with altered DNA methylation at brain-expressed imprinted loci in inbred mice: implications for neuropsychiatric disease. Molecular Psychiatry, 18(6), 635–636. 10.1038/mp.2012.88 [DOI] [PubMed] [Google Scholar]
- Sørensen HJ, Pedersen CB, Nordentoft M, Mortensen PB, Ehrenstein V, & Petersen L (2014). Effects of paternal age and offspring cognitive ability in early adulthood on the risk of schizophrenia and related disorders. Schizophrenia Research, 160(1–3), 131–135. 10.1016/j.schres.2014.09.035 [DOI] [PubMed] [Google Scholar]
- Taylor JL, Debost J-CPG, Morton SU, Wigdor EM, Heyne HO, Lal D, Howrigan DP, Bloemendal A, Larsen JT, Kosmicki JA, Weiner DJ, Homsy J, Seidman JG, Seidman CE, Agerbo E, McGrath JJ, Mortensen PB, Petersen L, Daly MJ, & Robinson EB (2019). Paternal-age-related de novo mutations and risk for five disorders. Nature Communications, 10(1), 3043. 10.1038/s41467-019-11039-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaler CJ (1989). Immunological Role for Seminal Plasma in Insemination and Pregnancy. American Journal of Reproductive Immunology, 21(3–4), 147–150. 10.1111/j.1600-0897.1989.tb01020.x [DOI] [PubMed] [Google Scholar]
- Torrey EF, Buka S, Cannon TD, Goldstein JM, Seidman LJ, Liu T, Hadley T, Rosso IM, Bearden C, & Yolken RH (2009). Paternal age as a risk factor for schizophrenia: How important is it? Schizophrenia Research, 114(1–3), 1–5. 10.1016/j.schres.2009.06.017 [DOI] [PubMed] [Google Scholar]
- Tsuchiya KJ, Takagai S, Kawai M, Matsumoto H, Nakamura K, Minabe Y, Mori N, & Takei N (2005). Advanced paternal age associated with an elevated risk for schizophrenia in offspring in a Japanese population. Schizophrenia Research, 76(2–3), 337–342. 10.1016/j.schres.2005.03.004 [DOI] [PubMed] [Google Scholar]
- Wang S-H, Hsiao P-C, Yeh L-L, Liu C-M, Liu C-C, Hwang T-J, Hsieh MH, Chien Y-L, Lin Y-T, Huang Y-T, Chen C-Y, Chandler SD, Faraone SV, Neale B, Glatt SJ, Tsuang MT, Hwu H-G, & Chen WJ (2019). Advanced Paternal Age and Early Onset of Schizophrenia in Sporadic Cases: Not Confounded by Parental Polygenic Risk for Schizophrenia. Biological Psychiatry, 86(1), 56–64. 10.1016/j.biopsych.2019.01.023 [DOI] [PubMed] [Google Scholar]
- Weiser M, Fenchel D, Frenkel O, Fruchter E, Burshtein S, Yehuda A. Ben, Yoffe R, Bergman-Levi T, Reichenberg A, Davidson M, & Sandin S (2020). Understanding the association between advanced paternal age and schizophrenia and bipolar disorder. Psychological Medicine, 50(3), 431–437. 10.1017/S0033291719000242 [DOI] [PubMed] [Google Scholar]
- Wohl M, & Gorwood P (2007). Paternal ages below or above 35 years old are associated with a different risk of schizophrenia in the offspring. European Psychiatry, 22(1), 22–26. 10.1016/j.eurpsy.2006.08.007 [DOI] [PubMed] [Google Scholar]
- Wu Y, Liu X, Luo H, Deng W, Zhao G, Wang Q, Zhang L, Ma X, Liu X, Murray RA, Collier DA, & Li T (2012). Advanced paternal age increases the risk of schizophrenia and obsessive–compulsive disorder in a Chinese Han population. Psychiatry Research, 198(3), 353–359. 10.1016/j.psychres.2012.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zammit S, Allebeck P, Dalman C, Lundberg I, Hemmingson T, Owen MJ, & Lewis G (2003). Paternal age and risk for schizophrenia. British Journal of Psychiatry, 183(5), 405–408. 10.1192/bjp.183.5.405 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


