Abstract
Following injury, the oral mucosa undergoes a complex sequences of biological healing processes to restore homeostasis. While general similarities exist, there are marked differences in the genomics and kinetics of wound healing between the oral cavity and cutaneous epithelium. The lack of successful therapy for oral mucosal wounds has influenced clinicians to explore alternative treatments and potential autotherapies to enhance intraoral healing. The present in-depth review discusses current gold standards for oral mucosal wound healing and compares endogenous factors that dictate the quality of tissue remodeling. We conducted a review of the literature on in vivo oral wound healing models and emerging regenerative therapies published during the past twenty years. Studies were evaluated by injury models, therapy interventions, and outcome measures. The success of therapeutic approaches was assessed, and research outcomes were compared based on current hallmarks of oral wound healing. By leveraging therapeutic advancements, particularly within in cell-based biomaterials and immunoregulation, there is great potential for translational therapy in oral tissue regeneration.
Introduction
As the largest organs in the body, the skin and mucous membranes are the first line of defense against any invasion that may disrupt homeostasis. Chronic wound sites are those requiring a healing time greater than 12 weeks; these sites have increased predisposition to bacterial invasion and wound infection that can further inhibit proper wound healing[1]. While wound healing is well-characterized and treated in cutaneous wounds, there is limited knowledge in intraoral healing, which reduces the clinical translation of treatment alternatives. In the case of impaired wound healing, the oral cavity is susceptible to challenges arising from trauma-related injury, prolonged inflammation, and postoperative complications. As an example, in oral deformities such as cleft palate, successful wound healing is difficult due to a bacteria-laden environment that undergoes constant physical trauma, so chronic wounds are common. Regenerative approaches hold out promise to enhance oral wound healing and require targeted treatment options to effectively promote tissue re-epithelialization and extracellular matrix (ECM) remodeling.
The current in-depth review consolidates advanced research on oral mucosal wound healing models and compares endogenous factors that dictate the quality of tissue regeneration. We discuss the comprehensive wound healing phases and include the feasibility and disadvantages of current conventional methods to promote oral mucosal healing. There is a clear need for new, efficacious delivery systems and alternative approaches to promote intraoral healing and tissue remodeling. To address this gap in viable treatment routes, we conducted a review of the literature on oral wound healing published during the past twenty years by curating search criteria specific to in vivo studies of oral wound therapies. Studies were analyzed for injury location, species, strain, sample size, timeline, and defect size to gauge the effectiveness of treatment by each model. Studies were then compared by therapeutic interventions, delivery methods, and outcome measures. The success of therapeutic approaches was evaluated by comparing research outcomes to current hallmarks of oral wound healing. To conclude the review, we further delve into currently available modalities and immunotherapies for patients and provide discussion on prospective avenues for efficacious treatment alternatives. New therapies, particularly within cell-based biomaterials and immunoregulation, are making substantial progress and have the potential to translate into better healing outcomes in a wide array of oral wounds.
Structure and Function of the Oral Mucosa versus the Cutaneous Epithelium
The architectures of both the oral mucosa and cutaneous epithelium are primarily composed of superficial epithelium and an underlying basement membrane that act as a barrier against pathogens and mechanical stresses. Both tissue types consist of keratinocytes that are attached by desmosomes[2]. While general similarities exist, there are critical structural and functional differences between the oral mucosa and the skin (Fig. 1). The cutaneous skin is composed of keratinized epidermal layer, dermis, and hypodermis; whereas, the oral mucosa consists of stratified squamous epithelium followed by layers of the basal lamina, lamina propria, and the submucosa[3–5]. The palatal and gingival regions of the oral cavity routinely sustain greater mechanical forces and associated physical trauma from eating and chewing, and therefore have increased keratinized epithelium[6]. In contrast, elastic regions of the oral mucosa that undergo less physical stress, like the buccal tissue, are typically composed of nonkeratinized epithelium with loose ECM[3].
Figure 1:
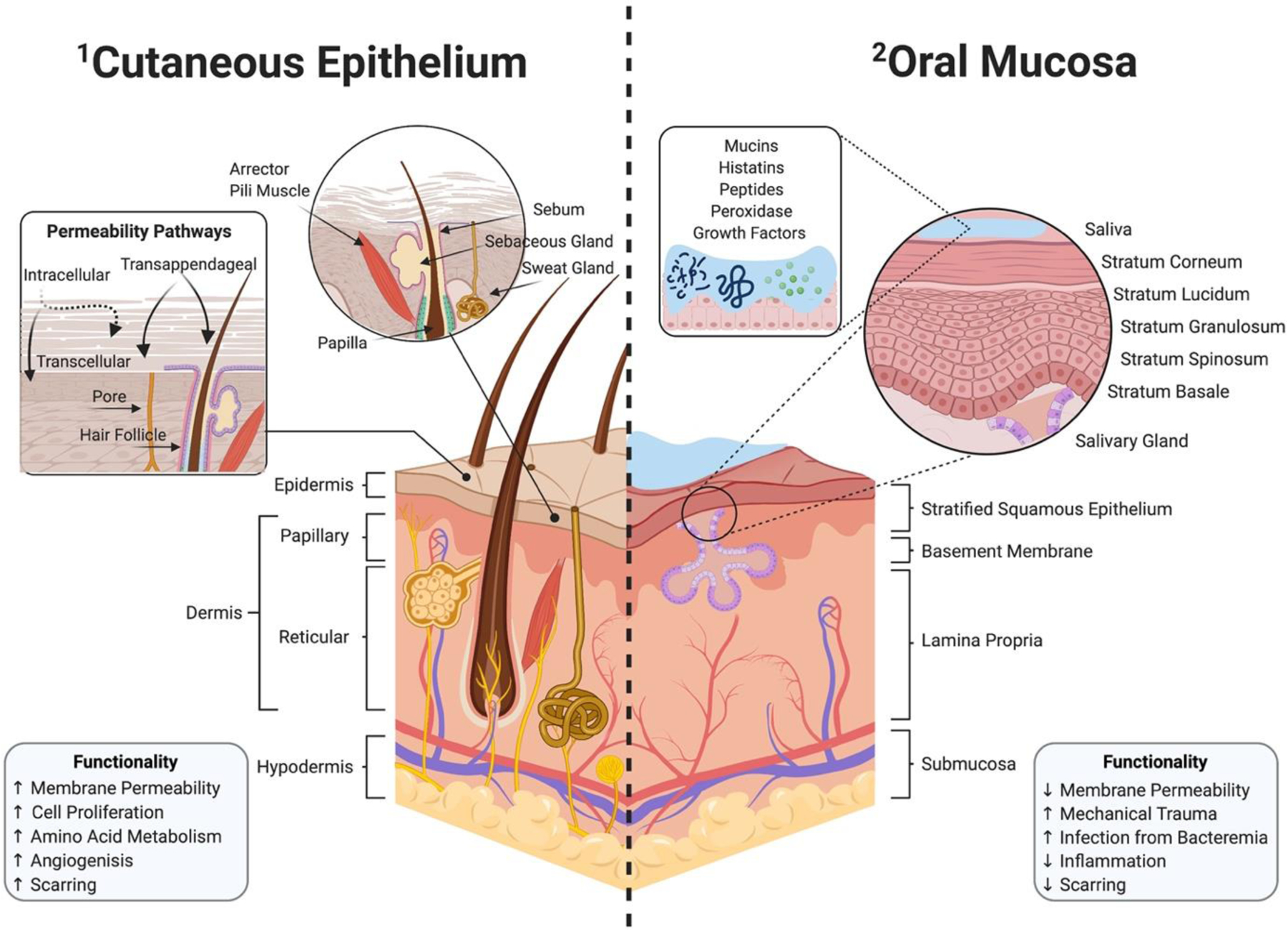
Graphical representation of the structural and functional differences in cutaneous epithelium versus oral mucosa. The cutaneous epithelium consists of three distinct layers: the epidermis, dermis, and hypodermis (1–1). The skin can utilize additional routes for enhanced transcutaneous permeability through intracellular, transcellular, and transappendageal pathways using pores and hair follicles. In contrast, the oral mucosa is composed of stratified squamous epithelium, followed by the basement membrane, lamina propria, and submucosa (1–2). Exclusive to the oral environment, saliva contains mucins, histatins, peptides, peroxidase, and growth factors that play a role in oral homeostasis. An injured oral mucosa is susceptible to infections caused by bacteremia, due to a complex oral microflora with an upward of millions of microorganisms.
Although, both the cutaneous epithelium and oral mucosa display similar healing patterns, there are distinct differences in the genomics and kinetics of wound healing between the two sites. Unlike the oral mucosa, the cutaneous epithelium contain hair follicles which have multi-potent stem cells found within the bulge region (Fig. 1–1)[7]. Since an injury can disrupt dermal homeostasis by cell depletion, stem cells within the hair bulge activate nearby epithelial cells to migrate to the injury and assist in tissue proliferation[8, 9]. While the exact contribution of hair follicles to dermal wound healing is unclear, clinicians have cited rapid healing in hair-bearing regions of wounds compared to areas lacking follicles, suggesting that bulge cells can help promote healing[7, 8, 10, 11]. The cutaneous epithelium can also utilize hair follicles and pores as additional routes for enhanced transcutaneous permeability and can provide transappendageal absorption routes from topical therapy (Fig. 1–1)[12–14]. In a 2008 study by Headon et al., investigators concluded that wounds are slower to heal on mice with a genetic mutation causing a lack of appendageal structure when compared to control mice[15]. This suggest that lack of hair follicles reduces the regenerative capacity that includes a migratory burst of immune cells from the appendage and its secretion of cytokines, and growth factors[7, 15, 16].
In contrast to cutaneous wounds, distinct genomic expression patterns demonstrate that the oral mucosa supports rapid healing with minimal scarring[17]. The oral mucosa is intrinsically less reactive to inflammation during the healing process, with lower infiltration from macrophages, T-cells, and neutrophils[18, 19]. Similarly compared to its counterpart, the oral epithelium has lower expression of transforming growth factor beta-1 (TGF-β1), a pro-fibrotic and pro-inflammatory cytokine recognized for its contribution to hypertrophic scars during wound healing[20]. Exclusive to the oral cavity, saliva, a weak buffer with pH ranging from 5.5 to 7, has shown to accelerate wound re-epithelialization while constantly providing hydration and warm temperature (Fig. 1–2)[21]. Saliva also contains histatins, antimicrobial peptides, and mucins that can aid in wound healing by assisting fibroblast proliferation and migration, increasing keratinocyte turnover, and releasing growth factors[21–24].
Infections following an injury to the oral cavity can increase the risk of bacteremia, previously researched in dental procedures like periodontal surgery and tooth extraction[25]. The oral microenvironment is associated with a complex microflora in which over 500 species exist in periodontitis alone and upwards of millions of microorganisms that can contribute to human endodontal and periodontal infections[26, 27]. A study by Debelian et al. (1998) traced microorganisms released into the bloodstream following root canals in twenty-six patients[28]. Blood was drawn from patients ten minutes after endodontal therapy, and results showed dissemination of anaerobic bacteria and other oral microorganisms in the blood, suggesting that bacteria from the infected site may have also reached the lungs, heart, and peripheral capillary system[25, 28]. In the case of oral mucosal infection, bacteremia can also lead to systemic inflammation and sepsis[29]. Systemic infection can ultimately lead to endocarditis, joint infections, Behçet’s syndrome, Crohn’s disease, etc[25, 30]. Therefore, further research is required to reduce or prevent oral infections and poor wound healing leading to systemic infections, which can compromise the body’s innate ability to heal. By understanding the marked differences between cutaneous and oral wounds, we can identify novel treatment options leveraging inherent biological healing mechanisms and influence effective tissue re-epithelialization through targeted therapy.
Timeline of Oral Wound Healing
Hemostasis
Following injury, there are four distinct, spatiotemporally overlapping stages of wound healing that are conserved across all tissue types: hemostasis; inflammation; proliferation; and maturation (Fig. 2–1). When the body is wounded, hemostasis occurs almost immediately to reduce blood loss. Within seconds, the immune system is activated as a result of the damage to the blood vessel endothelium[27]. The exposed ECM causes activation of local circulating platelets further initiating the hemostatic cascade[31, 32]. Platelets produce biologically active products such as vasoactive mediators and chemotactic signals-mediated release of proteases, cytokines, and growth factors[27, 33, 34]. Blood vessels constrict to prevent bleeding, and platelets adhere to form platelet plugs that are reinforced by fibrin polymerization to create a fibrin clot and seal the wound. Fibro-fibronectin clots provide support as a temporary ECM matrix and allow epithelial cells and fibroblasts to migrate into the wound site[27, 34].
Figure 2:
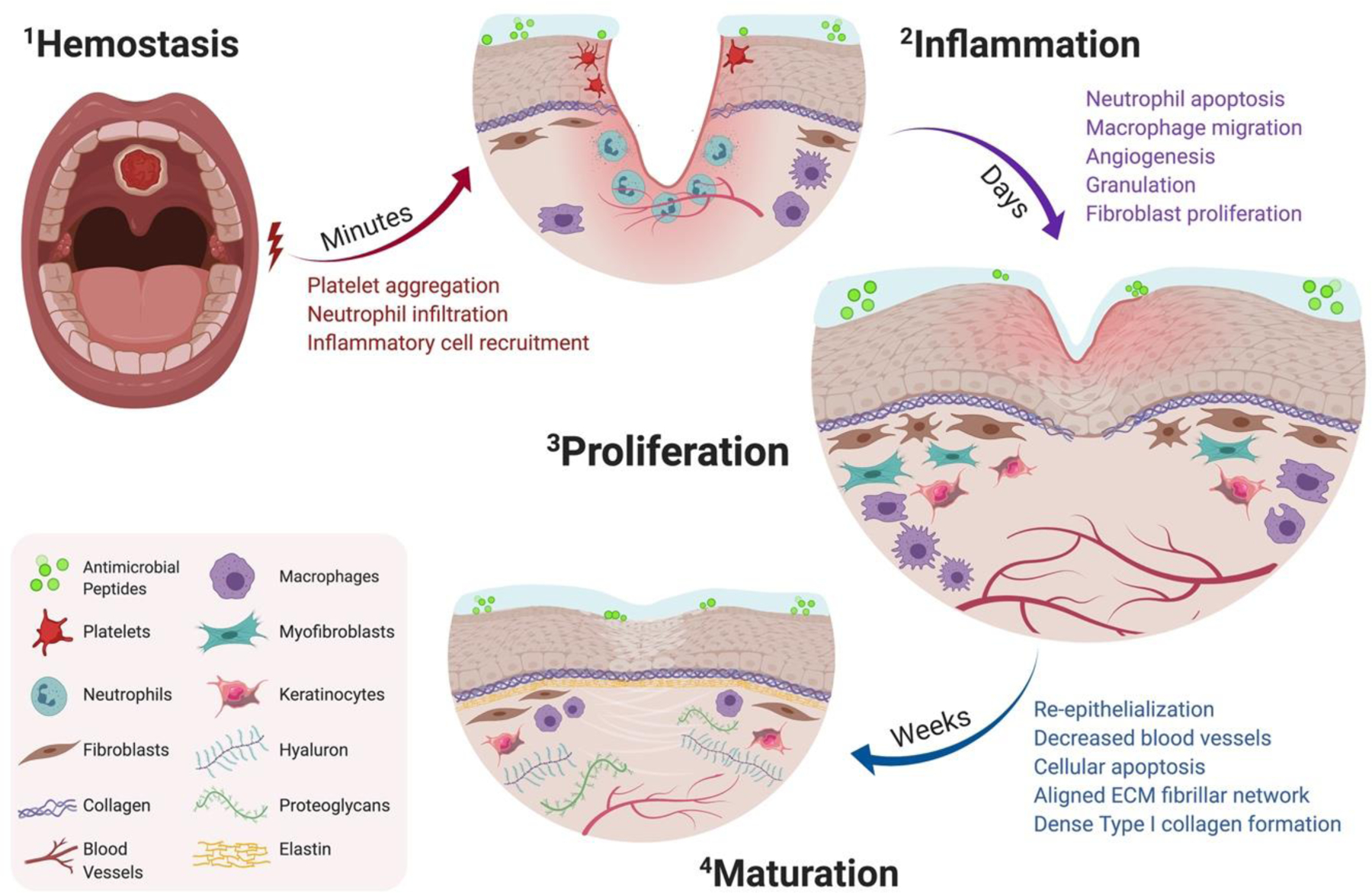
Timeline of oral wound healing and oral mucosal remodeling. Following injury, the hemostatic cascade is initiated to prevent excessive bleeding at the wound site (2–1). In the days following injury, inflammation peaks through neutrophil debridement and macrophage-mediated secretion of inflammatory cytokines (2–2). Within a week, the proliferation phase promotes fibroblast migration, increases vascular networks by angiogenesis, and enhances macrophage migration (2–3). Following fibroblast migration, the tissue surrounding the defect begins to re-epithelialize and mature by aligned fibrillar and dense collagen networks (2–4).
Inflammation
Following the initial hemostasis phase, the wound undergoes immediate inflammatory infiltration in response to chemokines at the site of the injury (Fig. 2–2). Inflammatory response peaks at 24 to 48 hours post-injury and can lasts for up to a week[31]. In the early onset phase of inflammation, there are fewer resident cytokines, reduced blood vessels, and rapid local fibroblast formation at the wound bed[27, 35, 36]. Though, in order to remodel the matrix into new tissue, the early inflammatory phase first promotes immune cell-mediated removal of debris and pathogens. Neutrophils are the first to migrate to the wound site to debride damaged ECM components and to secrete protease like matrix metalloproteinase (MMP)[37]. Subsequently, during the early inflammatory phase neutrophils initiate a cascade of cytokine secretion and growth factors to recruit other immune cells, including monocytes, which help initiate re-epithelialization[38]. After the wound bed is clear of microbes, neutrophils exit the wound bed through extrusion, apoptosis, and phagocytosis. In the case of impaired or prolonged wound healing, neutrophils abnormally persist during the prolonged inflammatory phase, creating a chronic wound setting through continued protease production[39, 40]. Approximately 48 to 72 hours post-injury, monocytes migrate to the wound and differentiate to become macrophages, serving as the dominant cell type during the inflammatory phase of wound healing—primarily through “pro-inflammatory” M1 macrophage polarization[41]. Macrophages secrete cytokines including interleukin-1, interleukin-6, fibroblast growth factor (FGF), platelet-derived growth factor (PDGF), epidermal growth factor (EGF), and TGF-β which orchestrate cell migration of keratinocytes and fibroblasts to the wound bed[27, 42]. During the late inflammatory phase, macrophages lead proliferative healing through “anti-inflammatory” M2 macrophage polarization and continue to secrete regenerative cytokines like interleukin-10; M2 macrophages help to upregulate endogenous “anti-inflammatory” cytokines and downregulate previously secreted “pro-inflammatory” cytokines near the wound[43]. Following the immune cell-mediated removal of pathogens, there is an increase of blood vessel permeability and transudate leakage from capillaries, leading up to the proliferation phase[34, 44].
Proliferation
The proliferation phase begins in the days after wounding and lasts up to three weeks in response to regenerative cytokines and growth factors; during this stage re-epithelialization begins to occur from the wound edges (Fig. 2–3)[31]. The reestablishment of existing vascular networks and formation of new blood vessels are hallmarks of successful wound healing. Angiogenesis is the process by which new blood vessels sprout from existing vascular networks to restore tissue perfusion, establish microcirculation, and increase oxygenation to support collagen crosslinking and wound maturation[45, 46]. One of the most well-characterized pro-angiogenic regulators is vascular endothelial growth factor (VEGF), a protein that stimulates the formation of blood vessels and aid in endothelial cell proliferation, differentiation, and migration[45, 47]. However, some scenarios of impaired wound healing can occur due to an imbalance of angiogenic mediators (e.g. diabetic venous stasis ulcers) associated with aberrant angiogenesis[45, 48]. As the wound progresses through the proliferation phase, there is an increase in capillaries near the healing edge delivering nutrients and cells to heal the wound. Additionally, the provisional fibrin-fibronectin ECM formed by the temporary platelet plugs is replaced by highly vascularized stroma leading to granulation tissue formation[31]. Remodeling of the granulation tissue occurs by M2 macrophages that provide pro-regenerative growth factors like FGF, EGF, and VEGF[34, 46]. Fibroblasts migrate to the provisional matrix and are integral for ECM remodeling; these cells lay down matrix proteins, including collagen and fibronectin, to provide structural integrity of the healing tissue[31, 49, 50]. Migratory fibroblasts differentiate into myofibroblasts, beginning the process of wound contraction to support wound edge healing and lead into the maturation phase of wound healing[51].
Maturation and Remodeling
In the last phase of wound healing, the repaired tissue goes through a remodeling and maturation phase which can begin around three weeks after injury and can last up to two years post-injury (Fig. 2–4)[52]. Protease activity, particularly MMPs, further aid healing during the maturation phase by providing a balance between deposition and degradation of the ECM[53]. However, in the case of prolonged healing, pro-inflammatory cytokines may induce elevated levels of MMP production, causing imbalance towards excessive ECM degradation[54]. Therefore, local delivery of protease inhibitors has been shown to aid in healing progress by regulating protease expression at the wound site and promoting ECM remodeling[55]. During wound maturation, activated myofibroblasts stop providing matrix, and granulation tissue begins to remodel the wound site as it gradually returns to homeostasis. The wound bed becomes less cellular as cells like fibroblasts and macrophages undergo apoptosis[56]. The previous provisional ECM transitions from loose fibronectin tissue networks to larger and denser collagen bundles[27, 34]. Large networks of blood vessels begin to be pruned and the fibrillar network of the ECM becomes a more aligned structure[52]. Overtime, resident cells like keratinocytes and macrophages continue to remodel the remaining permanent ECM as the repaired tissue return to homeostasis[52, 57].
Causes of Poor Wound Healing in the Oral Cavity
When the body is unsuccessful in achieving homeostasis after injury, the previously described phases of wound healing are disrupted and result in impaired tissue regeneration. For example, the inflammatory phase can abnormally persist when inflammation extends greater than seven days and is characterized by delayed epithelialization and tissue necrosis[27]. Impaired wound healing can occur from continued secretion of pro-inflammatory mediators and can be characterized by granuloma formation, fistula occurrence, wound dehiscence, ulcers, and excessive bleeding[27, 58]. One of the most severe forms of post-surgical healing abnormalities is fistula formation, an improper passage between different body compartments. Following cleft palate surgery and tumor resection, oronasal fistulas (ONF) can occur in up to ~60% of cases and result from infection, flap necrosis, hematoma formation, and constant tension[59]. ONF as small as 4.5mm can diminish speech quality by causing audible nasal air escape, hypernasal resonance, and nasal regurgitation of fluids[60]. Unattended fistulas can enlarge and later inhibit palatal growth, eventually requiring orthodontic intervention to expand alveolar arch[27, 61, 62]. Current therapy for ONF and similar palatal defects involves corrective palatoplasty in which multiple techniques like straight line closure and geometric repair are utilized to restore function and anatomic contour[63]. However, with high rate of ONF re-occurrences despite revision surgery, many of the predisposing factors to ONF occurrence are attributed to variability in surgical techniques, patient healing capability, age of palatoplasty, and severity of the original cleft[64]. Unsuccessful healing can lead to scar formation and impaired growth of the palate and dento-maxillary complex, reducing the mechanical integrity of formed tissue; persistent ONF can cause nasal bacterial accumulation and mucosal inflammation[65–67]. The lack of success in oral wound healing has motivated research towards translational delivery systems and alternative strategies in order to promote oral wound healing and tissue regeneration in conjunction with surgical intervention.
Current Treatment Approaches to Improve Oral Wound Healing
Surgical intervention after injury can prevent prolonged inflammation by enhancing the body’s innate healing capabilities towards a pro-regenerative phenotype. Following oral cavity surgery or tumor resection, cellular grafts can be used to provide structural and functional support in the wound area. The gold standard treatment involves using mucosal or allogenic grafts and can be categorized into two groups, allograft or decellularized tissue graft. For example, AlloDerm™ or de-cellularized donor-derived dermis are acellular scaffolds that are commonly supplemented as barrier grafts and have had promising results with reduced scarring, immediate wound coverage, and enhanced functional performance[68]. Treatment with allogeneic tissue grafts, such as Integra® and PriMatrix®, are beneficial when there is an inadequate supply of local tissue for autografts, especially in large or severe oral wounds requiring a restoration of mucosal surface lining[69]. In the case of surgical oral palate repair, allogenic grafts are used to help restore tissue to the injured area; however, the use of grafts are off-label uses as they do not have FDA-approval for mucosal replacement. Furthermore, using skin substitutes can lead to infection and give rise to greater complications with immunological rejection, as studied in diabetic chronic wounds and vascular insufficiency[70, 71]. Although the mechanism involved in chronic graft rejection is not well understood, one of the major disadvantageous features include narrowing of graft vessels, which limits proper integration to the host site and compromises blood flow, fibrosis, cell death, and ultimately graft failure[72].
In a 2018 retrospective analysis of Medicare beneficiaries, approximately 8.2 million people had clinically-diagnosed wounds that cost upwards of 96.8 billion dollars for treatment of acute or chronic wounds[73, 74]. It is projected that the annual wound care products market is expected to reach 22 billion dollars by 2024 due to rising technological advancements in wound healing therapy and incidences of chronic wounds with the rising geriatric population[74]. In recent years, there is increasing support for using in vitro engineered, cell-based alternatives to assist in intraoral healing and to replace current standard methods. Tissue regeneration can be approached using cell-based therapy, biomaterials-alone therapy, or a combination of the two with biomaterials seeded with cells, matrices, and growth factors. Stem cells, such as embryonic stem cells, induced pluripotent cells, and mesenchymal stem cells, are common sources of autologous reservoirs for cell-based therapy and pro-regenerative medicine[75]. Gintuit™ is the first FDA-approved cell-based therapy using allogenic human cells and bovine collagen and has shown promising results when administered topically in adults with mucogingival defects[76, 77]. However, a current limitation within the field of cell-based therapy is that implanted cells have trouble grafting, low viability, and hampered transmucosal permeability[23, 78]. Poor grafting causes drugs or small molecules to clear quickly from the wound site, delivering only a short burst of therapy rather than sustained release to the wound[23]. To our knowledge, there are limited FDA-approved immunomodulatory therapies that provide a pro-regenerative approach that harnesses the body’s natural ability to heal oral wounds post-surgery. By utilizing technological advancements in cell-based biomaterials, there is great potential to create better healing outcomes in a wide array of oral wounds.
In Vivo Models for Oral Wound Healing
To address the gap in current potential therapy options for oral wound healing, we conducted a review of the literature on oral wound healing published during the past twenty years by curating search criteria specific to in vivo studies of oral wound healing therapies. These studies distinctly used an oral injury model to evaluate the efficacy of their treatment modality for wound healing in pre-clinical models and clinical applications. Shown in Table 1, studies were analyzed for injury location, species, strain, sample size, timeline, and defect size to evaluate the effectiveness of therapies. Studies were then compared by therapeutic approaches, delivery methods (Table 2), and outcome measures were evaluated by comparison of research outcomes to current hallmarks of oral wound healing (Table 3). To conclude the review, we delve into current modalities or immunotherapies investigated in the studies and provide discussion on promising regenerative therapies for oral wound healing.
Table 1: In Vivo Oral Wound Healing Models.
A review of the literature on oral wound healing published in the past twenty years. Studies were limited to in vivo models that were using therapeutic approaches for oral wound healing. Oral wound healing models were analyzed by injury location, species, strain, sample size, timeline, and defect size. Injury location were primarily in the palatal, buccal, and gingival regions of the oral cavity. Species used to model studies ranged from small animals, as in mice and rat, to larger species like dogs and human. Timeline indicated days or weeks when healing outcomes were recorded, and defect details the injury size and methods by which the injury was created.
| Wound Model | Species | Strain | Sample Size | Timeline | Defect Details | Studies |
|---|---|---|---|---|---|---|
| Palatal | Rat | Sprague Dawley | 20 18 |
8 weeks 2, 4, 7 days |
1.3mm by 7mm surgical excise 3mm biopsy punch |
Li et al. [2019]
Zhu et al. [2015] |
| Wistar | 28 72 20 |
7, 14 days 3, 5, 7 days 0, 3, 7, 14, 21 days |
5mm biopsy punch 4mm biopsy punch 4.5mm biopsy punch |
Maeda et al. [2013]
Kim et al. [2013] Suragimath et al. [2010] |
||
| Mouse | C57BL/6 | 11 174 |
3, 5, 7, 10 days 1, 3, 5, 7 days |
1.5mm biopsy punch 1.5 mm cautery excise |
Keswani et al. [2013]
Ballestas et al. [2019] |
|
| Dog | Beagle | 6 16 |
1, 3, 12 weeks 7, 14 days |
6mm biopsy punch 6mm biopsy punch |
Ophof et al. [2008]
Ayvazyan et al. [2011] |
|
| Minipig | Berlin | 7 | 40 days | 15mm biopsy punch | Kesting et al. [2010] | |
| Piglet | Yorkshire Hybrid |
6 18 |
5 weeks 3, 7, 10, 76 days |
10mm biopsy punch 15mm biopsy punch |
Kirschner et al. [2006]
Rohleder et al. [2013] |
|
| Human | N/A | 15 | 4, 8, 15, 29 days | 6mm biopsy punch | Thoma et al. [2012] | |
| Buccal | Rat | Sprague Dawley | 56 | 12, 28 days | 75mm2 by iris scissors | Roh et al. [2018] |
| Wistar | 60 36 |
1, 3, 7 days 3, 5 days |
7mm biopsy forceps 5mm biopsy punch |
Couto et al. [2016]
Priprem et al. [2018] |
||
| Mouse | Institute of Cancer Research | 36 | 1, 3, 5 days | 1.5mm biopsy punch | Shim et al. [2007] | |
| Rabbit | New Zealand White | 36 | 1, 3, 7, 14 days | 10mm surgical excise | Lis et al. [2012] | |
| Gingival | Rabbit | New Zealand White | 20 22 36 |
0, 7, 14 days 5 days 3, 6, 9, 12 days |
6mm by 50% acetic acid 15mm surgical excise 5mm by 50% acetic acid |
Lim et al. [2016]
Kılıç et al. [2013] Karavana et al. [2011] |
| Japanese White | 24 24 |
13 days 7, 14 days |
5mm by 50% acetic acid 6mm by 50% acetic acid |
Umeki et al. [2014]
Fujisawa et al. [2003] |
Table 2: Regenerative Therapies for Oral Wound Healing.
Oral wound healing models were organized by therapeutic approaches. Studies were analyzed by therapy vehicles, treatment methods, cell/drug used (if any), and outcome measures. Treatments were either synthetic polymer scaffolds, biological-synthetic combination scaffolds, biologically derived grafts, or fluid-like gel/topical ointment. Outcome measures ranged from qualitative analysis through gross observations and histology to quantitative analysis using flow cytometry and qPCR.
| Therapy Vehicle | Treatment Matrix | Cell/Drug/Growth Factor | Outcome Measures | Studies |
|---|---|---|---|---|
| Polymer Scaffold | Chitosan disc | Glutathione | Histology, GSH levels, malondialdehyde levels, nitric oxide levels | Kılıç et al. [2013] |
| PLGA/PCL electrospun nanofiber | FTY720 | Histology, IHC, flow cytometry, ELISA, serum sodium, qPCR | Ballestas et al. [2019] | |
| Carboxyvinyl polymer and trolamine | Curcumin | Gross observation, histology | Lim et al. [2016] | |
|
| ||||
| Biopolymer Scaffold | Decellularized amnion membrane with POC | Gross observation, histology, body weight, CT scanning, IHC | Li, et al [2019] | |
|
| ||||
| Biological Graft | Collagen-gelatin sponge | bFGF | Gross observation, histology; IHC | Ayvazyan et al. [2011] |
| Oral mucosal cell sheets | Keratinocytes, Fibroblasts | Gross observation, histology, IHC | Roh et al. [2018] | |
| Collagen matrix (Mucograft®) | Gross observation, somatosensory measurements | Thoma et al. [2012] | ||
| Human amnion membrane; Collagen-based dermal matrix (Integra®) | Gross observation, histology, IHC | Kesting et al. [2010] | ||
| Amniotic membrane allograft; small intestinal submucosa; autofetal amniotic membrane | Gross observation, histology | Rohleder et al. [2013] | ||
| Dermal substrates (DED and AlloDerm™) | Keratinocytes | Histology, IHC, heparan sulphate | Ophof et al. [2008] | |
| Acellular human dermal graft (AlloDerm™) | Gross observation, histology | Kirschner et al. [2006] | ||
| Cellmatrix | Leptin | IHC, RT-PCR, wound healing assay, ELISA | Umeki et al. [2014] | |
| Fibrin glue | Keratinocyte | Gross observation, histology | Lis et al. [2012] | |
|
| ||||
| Gel/ Topical Ointment | 10% niosome gel | Anthocyanin | Gross observation, histology, XANES, EXAFS | Priprem et al. [2018] |
| Hydrochloride gel | Benzydamine | Gross observation, histology | Karavana et al. [2011] | |
| Lectin Artin M gel | Histology, IHC, ELISA | Kim et al. [2013] | ||
| Saline solution | Bismuth subgallate | Histology, IHC | Couto et al. [2016] | |
| Hyaluronan ointment | Dimethyloxalylglycine | Gross observation, histology | Zhu et al. [2015] | |
|
| ||||
| Injection | N/A | EGF, bFGF | Gross observation, histology | Fujisawa et al, [2003] |
| N/A | Aucubin | Gross observation, histology | Shim et al. [2007] | |
| N/A | VEGF | Histology, IHC, ELISA | Keswani et al. [2013] | |
|
| ||||
| Alternative Therapy | ||||
|
| ||||
| Carbonated drink | Gross observation, histology | Suragimath et al. [2010] | ||
| Low-intensity pulsed ultrasound | Gross observation, histology | Maeda et al. [2013] | ||
Table 3: Analyzing the Signatures of Oral Wound Healing.
Oral wound healing studies were organized by injury model, therapeutic approach, and main treatment method. Success of regenerative therapy was evaluated by comparison of research outcomes to current hallmarks of oral wound healing. Studies were assessed by wound closure, angiogenesis, cell/gene proliferation, epithelial thickness, inflammatory response, and collagen deposition. Positive sign denotes a reported increase in respective category, and negative sign represents a decrease in outcome following main treatment.
| Studies | Model | Therapy Vehicle | Main Treatment | Wound Closure | Blood Vessel/Angiogenesis | Cell / Gene Proliferation | Epithelial Thickness | Inflammatory Response | Collagen Deposition |
|---|---|---|---|---|---|---|---|---|---|
|
Ballestas et al. [2019]
Ayvazyan et al. [2011] Li et al. [2019] Ophof et al. [2008] Kesting et al. [2010] Kirschner et al. [2006] Rohleder et al. [2013] Thoma et al. [2012] Zhu et al. [2015] Kim et al. [2013] Keswani et al. [2013] Maeda et al. [2013] Suragimath et al. [2010] Roh et al. [2018] Lis et al. [2012] Couto et al. [2016] Priprem et al. [2018] Shim et al. [2007] Lim et al. [2016] Kılıç et al. [2013] Umeki et al. [2014] Karavana et al. [2011] Fujisawa et al. [2003] |
Palatal | Polymer Scaffold | PLGA/PCL electrospun nanofibers with FTY720 Collagen-gelatin sponge with bFGF |
+ + |
+ |
+ |
+ |
− | |
| Biopolymer Scaffold | Decellularized amnion membrane and POC | + | − | + | + | + | |||
| Biological Graft | Dermal substrates (DED and AlloDerm™) Human amnion membrane (Integra®) Acellular human dermal graft (AlloDerm™) Amniotic membrane allograft Collagen matrix (Mucograft®) |
+ + + + |
+ |
+ + |
+ + |
||||
| Gel/Ointment | Hyaluronan ointment with dimethyloxalylglycine Lectin Artin M gel |
+ + |
+ |
+ + |
+ |
− |
+ |
||
| Injection | VEGF | + | + | + | |||||
| Other | Low-intensity pulsed ultrasound Carbonated drink |
+ − |
− |
− |
|||||
| Buccal | Biological Graft | Oral mucosal cell sheets with keratinocyte and fibroblasts Fibrin glue with keratinocyte |
+ + |
||||||
| Gel/Ointment | Bismuth subgallate 10% niosome gel with anthocyanin |
+ |
− | + | = | ||||
| Injection | Aucubin | + | − | + | |||||
| Gingival | Polymer Scaffold | Carboxyvinyl polymer and trolamine with curcumin Chitosan disc with glutathione |
+ |
+ |
+ |
+ |
|||
| Biological Graft | Cellmatrix with leptin | + | + | = | |||||
| Gel | Hydrochloride gel with Benzydamine | + | + | ||||||
| Injection | bFGF | + | + |
+ Analyzed and Increase
− Analyzed and Decrease
= Analyzed and Inconclusive
Blank Did Not Analyze
Palatal Wound Healing Models
Cleft lip with or without cleft palate is the most common congenital defect, occurring in 1 in 940 live births[79]. Despite surgical repair, a high degree of patients has persistent ONF formation[80–82]. Palatal wound models are often used as pre-clinical in vivo models of orofacial clefts and oral wound healing. The palate is made up of three distinct areas: the anterior and posterior hard palatal mucosa covering bone, and the soft palatal mucosa covering muscle in the pharynges[83]. The heterogenous structure of palatal mucosa can cause wounds to vary in laterality, completeness, severity, and tissue architecture[63].
Presented in Table 1, 13 of the 23 oral wound studies utilized palatal models of wounding and varied widely in species, strain, sample size, timeline, and defect type. The most commonly used model organism was the rat (5/13), followed by mouse (2/13), dog (2/13), piglet (2/12), minipig (1/13), and human (1/13, observational). Study timelines ranged widely by organism, from as short as 7 days to as long as 12 weeks. As an example, Li et al. (2019) performed an 8-week study using Sprague Dawley rats to test the efficacy of polymer-integrated amnion scaffold in cleft palate repair[81]. The study used surgical excision to create a 1.3mm by 7mm full-thickness defect at the palatal midline by surgically removing the mucosa, periosteum, and bone. The 8-week timespan of the study allowed researchers to evaluate whether healing was complete and the subsequent quality of healing using histological analyses. Other studies ranged as short as 7 days, such as in the study by Ballestas et al. (2019)[84]. In that study, a 1.5mm full thickness hard palatal mucosal wound was created in C57BL/6 mice via cauterization. The relatively short 7-day timespan of this study was used to assess the initial immune and cytokine profile of the wound site following treatment.
The majority of palatal wounding studies (11/13) used a biopsy punch to create palatal wounds. The biopsy punch approach provides consistency and reproducibility across studies which vary by organism, sample size, and timeline. According to Oliver et al. (2004), small diameter wounds, which classifies almost all wounds in small animal models, may not require suturing post-excision, however, also state that palatal and gingival sites are not as suited for punch biopsy[85]. One limitation of these surgically created wounds is that they do not fully represent the variation in human palatal wounds, and therefore may not be extrapolatable to human oral wounds. Human oral wounds, in general, are irregular as they occur due to trauma, cancer surgery, or poor post-surgical healing, and thus are asymmetrical in height, width, depth, and ratios of different tissues. ONF and other palatal wounds continue to range in severity and can grow in diameter over time, making it challenging to model the human phenotype of post-surgical oral wounds in an animal model and limiting suitable treatment alternatives.
Buccal Wound Healing Models
Buccal wounds occur in the cheek mucosa and can have a range of causes from superficial tears while chewing to deep laceration post-oral surgery. Within buccal tissue (5/23), models varied in species used, sample size, timeline, and defect type (Table 1). Compared to palatal models, buccal tissue was used in fewer types of model organisms; the majority of studies used rats (3/5), while other studies used rabbits (1/5) or mice (1/5) models. Study lengths were relatively shorter than palate studies, ranging from 5 to 28 days. When compared to palatal models, defect types were created with a wider array of instruments, utilizing iris scissors, biopsy forceps, biopsy punch, and surgical excision. For example, Shim et al. (2007) examined a biopsy punch mice model to test the healing effects of the small molecule plant derivative, aucubin, on buccal wounds[86]. The heterogeneity in buccal injury models suggests that further study is needed to consider the utility of these models prior to consideration of translational experiments.
Gingival Wound Healing Models
Gingival tissue, or gums, differs markedly from buccal and soft palate tissue as the submucosa is not present, so the lamina instead attaches directly to the mucoperiosteum or periosteum of bone[2]. Gingival tissue has a relatively shorter healing timeline and disparate phases of healing compared to hard palatal and buccal wounds. Gingival injury can vary from minor sports-related trauma to dental surgery for periodontitis. Five out of the 23 studies identified used a gingival model, all of which used rabbits as a model organism: New Zealand White (NZW) and Japanese White rabbits (Table 1). These studies were also the shortest in timeline, ranging from 5 to 14 days. Four out of five of the studies created the oral wound through application of 50% acetic acid, phenotyping an oral ulcer. Umeki et al. (2014) examined the role of leptin, a naturally occurring hormone in saliva, for the treatment of oral wounds by topically applying it to gingival wounds in Japanese White rabbits[87]. These wounds were 5mm in diameter and created by the application of filter paper soaked in 50% acetic acid to gingival tissue in the mandible. Similarly, Lim et al. (2016) studied the topical treatment of 1% curcumin in NZW rabbits with a 6mm gingival wound created using 15µL of 50% acetic acid[88]. In contrast, Kiliç et al. (2013) was the only study to perform a 15mm injury via surgical excision of gingival tissue[89]. They performed a 5-day study using NZW rabbits to test the effectiveness of local glutathione and chitosan application on reducing oxidation and adverse healing at the wound site.
Developing Therapies in Oral Wound Healing Models
Current therapies for oral wound healing lack efficacious treatment outcomes during oral wound management and tissue regeneration. To reduce the occurrence of impaired healing following oral surgery, there is ongoing research to improve the off-label use of acellular human donor dermal tissue, as demonstrated by Kirschner et al. (2006), Thoma et al. (2012), Kesting et al. (2010), among others discussed in Table 1[90–92]. Furthermore, FDA-approved drugs and therapeutic agents are being investigated as potential treatments for oral wound healing delivering fibroblasts and/or VEGF as a pro-regenerative therapy, FTY720 as an immune modulatory drug, or nonsteroidal anti-inflammatory drug like Benzydamine[84, 93].
Current oral wound healing models have examined the use of a delivery vehicle to facilitate healing by promoting a pro-regenerative environment over time. Emerging therapies have seen promising therapeutic efficacy from polymeric scaffolds that are coupled with drugs, cells, tissue, or growth factors to enhance oral wound healing. Most of the studies analyzed in Table 2 utilized four major types of treatment delivery vehicles: biological scaffolds (9/23), gel-like or topical ointment (5/23), synthetic polymeric scaffolds (3/23), and direct delivery of growth factors and plant derivative (3/23). The remaining studies used alternative therapies like ultrasound (2/23) or a hybrid biological-synthetic polymer scaffold (1/23). The studies then evaluated the success of oral wound healing therapy primarily using histology for tissue re-epithelialization and microscopy images for wound closure (Table 3).
Polymer and Biopolymer Scaffolds
Synthetic polymeric scaffolds (SPS) are commonly used as drug delivery vehicles due to their biocompatibility in clinical settings, predictable material properties, and tunable size and rate of biodegradation[94]. SPS are widely used in the literature to facilitate wound healing and can vary in polymers such as poly(lactic-co-glycolic acid) (PLGA) used in Dermagraft® skin substitute or polyurethane-based Omiderm® dressing[95–97]. Other polymeric matrices that are commercially available for wound healing include hydrogels, alginate, and hydrocolloid[97]. Presented in Table 2, 4 out of 23 studies utilized SPS strategies for drug delivery as treatment for oral wound healing. Ballestas et al. (2019), for example, tested the efficacy of hybrid polymeric scaffolds loaded with FTY720 drug in a murine palatal wound healing model[84]. FTY720, an immunomodulating drug that sequesters lymphocytes in the lymph nodes to prevent an autoimmune reaction, was loaded into a degradable nanofiber scaffold to modulate the inflammatory phase of wound healing and reduced ONF formation through an immunoregenerative approach[98]. Drug-embedded nanofibers were electrospun using PLGA and polycaprolactone (PCL) biodegradable polymers to deliver sustained release of FTY720 and to enhance tissue-scaffold integration while decreasing fibrosis around the wound site during healing[99, 100]
Combination scaffolds with biologically derived therapeutic agents and SPS have also been used in oral wound healing applications. Li et al. (2019), created a polymer-integrated amnion scaffold to treat palatal injury in Sprague Dawley rats[81]. The decellularized amnion membrane (DAM) was derived from placental tissues and contains collagen, hyaluronan, fibronectin, and growth factors shown to help reduce inflammation and facilitate epithelialization, possibly improving healing outcomes[101]. The synthetic polymer was poly(1,8-octamethylene-citrate) (POC), a low-cost, biodegradable elastomer widely used to coat medical devices [102, 103]. Coupled together, the bioengineered synthetic and biological scaffold, DAM-POC, contained endogenous healing molecules as well as a synthetic polymer to provide strength, biocompatibility, and increased resistance to enzyme digestion of the graft.
Biological Grafts
Biological scaffolds, composed of allogeneic or xenogeneic ECM, were the most common type of scaffolds[95]. Biological scaffolds are beneficial because they are intrinsically biocompatible, biomimetic, and can promote cell attachment; some are FDA-approved to clinically use in wound healing for the repair and restore function through the regeneration of injured and missing tissue[94, 104]. These scaffolds can be composed of natural components found in the ECM such as collagen, laminin, chitosan, elastin, or fibronectin, among other natural polymers[96]. Although biological scaffolds have relatively poor mechanical strength and biostability compared to synthetic scaffolds, they are favorable in treatment options for low inflammatory response, low toxicity, and enhanced cell-environment interaction[96, 105, 106]. Of the studies included in Table 2, natural polymer-based scaffolds were among the most common including collagen-gelatin sponge used by Ayvazyan et al. (2011), extracellular matrix as used by Kesting et al. (2010), and acellular dermal grafts as used by Kirschner et al. (2006)[90, 92, 107]. Additionally, Ayvazyan et al. (2011) performed a 2-week study using Beagles to test the efficacy of a collagen-gelatin sponge as a scaffold to provide sustained release of bFGF, a fibroblast growth factor that is known to accelerate wound healing[108]. Furthermore, Thoma et al. (2012) performed a randomized, controlled clinical trial to test the efficacy of using collagen matrix, Mucograft®, in human subjects with 6mm palatal defects[91]. Treatment with amnion membrane was also popular, as shown with Rohleder et al. (2013), who used porcine amniotic membrane to treat a 15mm palatal defect in hybrid piglets[109]. The use of biological scaffolds was pivotal in providing structural support in almost all tissues and was combined with embedded cells or growth factors to enhanced tissue regeneration.
Pre-vascularized oral mucosal cell sheets were also frequently researched in intraoral healing. Recent research by Lesman et al. (2010) suggest that pre-formation of cell sheets can enhance oxygen and nutrient supply, accelerate neovascularization, and improve cell survival and integration with the host environment[110]. For example, shown in Table 2, Roh et al. (2018) produced oral mucosal cell sheets seeded with keratinocytes and fibroblasts as a biological-based scaffold to treat 75mm2 buccal injury in Sprague Dawley rats[111]. Keratinocytes, fibroblasts, and endothelial progenitor cells are all critical mediators of oral wound healing, thereby assisting in the inflammatory and proliferative phases of healing. Although not utilized in the studies included in this review, in vitro studies revealed that Gintuit™ worked to increase keratinized tissue through secretion of human growth factors and cytokines— a promising biologic-based treatment for cutaneous wounds that can be expanded to a broad range of oral wound healing models[77, 112].
Gel Scaffolds and Topical Ointment
Gel scaffolds are semi-solid materials made of hydrophilic polymers, which can be a potential candidate for intraoral wound healing[113]. A common type of gel scaffold includes hyaluronan (HA), a glycosaminoglycan and an integral constituent of the ECM that is responsible for stabilizing and organizing the ECM, mediating cell proliferation and differentiation, and regulating cell motility during tissue healing[114]. To test the efficacy and biocompatibility of a HA-based gel, Zhu et al. (2015) used a Sprague Dawley rat model of palatal wound healing and delivered dimethyloxalylglycine (DMOG) in a HA ointment(Table 2)[115]. DMOG is a small molecule inhibitor of prolyl hydroxylases which participates in the degradation pathway of HIF-1a, and thereby upregulates angiogenesis via VEGF production, as previously studied in diabetic mice[116, 117]. Therapy with DMOG complexed in HA gel ointment allowed for a treatment with high biocompatibility and low risk for inflammatory or allergic affects.
Another example of gel-based therapy, Priprem et al. (2018) topically delivered anthocyanin via niosome gel to promote buccal wound healing in Wistar rats (Table 2)[118]. Anthocyanin is a plant compound with anti-inflammatory and anti-cancer effects on oral lesions; however, it is relatively sensitive to pH change, light, oxygen, and is poorly absorbed through the oral mucosa[119–121]. When synthesized with zinc, the anthocyanin complex improved in stability and additional anti-inflammatory activity through TNF-α-induced inflamed human gingival fibroblast[121, 122]. The anthocyanin-zinc compound was encapsulated in niosome, a non-ionic bilayer vesicle allowing controlled delivery of active compound that has shown to enhance mucosal interaction and facilitate prolonged release[123, 124]. Gel scaffolds have demonstrated controlled release of therapeutic agents as well as provided a liquid-like adaptable and re-appliable substitute for intraoral wound healing.
Other Therapeutic Approaches
Alternative tissue regenerative approaches include the use of ultrasound as studied by Maeda et al. (2013) (Table 2)[125]. Maeda et al. were motivated to evaluate the effects of low-intensity pulsed ultrasound (LIPUS) on palatal wound healing because it previously demonstrated efficacy following dental extraction and implant placement; it also stimulates fracture healing and possibly soft tissue healing through the release of bFGF and TGF-β[126–129]. Maeda et al. performed a 2-week longitudinal study with 5mm hard palate injury in Wistar rats that were exposed to LIPUS at 160mW for 15 min every day until the end of the study.
Discussion: Characterizing Oral Wound Healing in Emerging Therapies
Wound Closure and Epithelial Thickness
Oral wound healing can be characterized by both physical and molecular changes within the injury site and surrounding tissue. Through gross observation using microscopy images, the majority of studies measured wound closure or significant growth of tissue at the defect site. Of the 23 studies analyzed, 19 models concluded overall closure at the wound site by microscopy images of the transverse length of the defect (Table 3). The remaining four studies did not quantify changes or noted a greater wound area following treatment, as with Suragimath et al. (2010)[130]. Ballestas et al. (2019) reported complete ONF closure as early as Day 5 post-palatal injury in mice with treatment of FTY720-loaded PLGA/PCL nanofiber scaffolds[84]. Similarly, Priprem et al. (2018) identified partial wound closure at Day 3 post-buccal injury and complete closure by Day 5 using a 10% niosome gel with anthocyanin[118]. With systemic administration of bFGF, as demonstrated by Fujisawa et al. (2003), all gingival ulcers in Japanese White Rabbits completely healed by Day 18 in bFGF-treated group when compared to Day 24 and Day 29 in EGF-treated group and control, respectively[131]. Although many of the studies analyzed wound healing from the surface, only ten studies analyzed marginal epithelialization, of which nine studies concluded growth in epithelium thickness. Li et al. (2019) utilized Hematoxylin and Eosin (H&E) stains for re-epithelization, Masson’s Trichrome stains for ECM, and von Willebrand factor (vWF) for vascularity. Results conclude that rats treated with DAM had a greater number of blood vessels than treatment group DAM-POC, yet saw a significant increase in epithelial thickness after treatment of DAM-POC compared to controls[81]. In addition, Lis et al. (2012) studied the effect of using autologous keratinocytes suspended in fibrin glue in the buccal wounds of NZW Rabbits. Following H&E staining, wounds treated with keratinocytes in fibrin glue displayed significantly higher epithelization area of the wound surface, when compared to treatment with fibrin glue alone and untreated wounds[132].
Blood Vessel Formation and Angiogenesis
Angiogenesis and neovascularization are hallmarks of the proliferative phase of wound healing, accounting for nearly 60% of granulation tissue mass during the early stages of wound healing[133, 134]. Of the 23 studies analyzed, 9 of them evaluated changes in angiogenesis primarily using histological techniques. Ayvazyan et al. (2011) assessed the efficacy of using a collagen-gelatin sponge impregnated with bFGF at concentrations of 1µg/cm, 7µg/cm, and 14µg/cm. Fourteen days following injury, immunohistochemistry (IHC) for vWF confirmed neoformed capillaries in all groups, with greatest number of vessels in the group receiving 7µg/cm of bFGF[107]. Kim et al. (2013) revealed elevated expression of VEGF using ELISA and IHC analyses in wounds treated with Artin M gel, a lectin previously shown to accelerate wound healing by acting on neutrophils and intracellular tyrosine phosphorylation[135–138]. Similarly, Zhu et al. (2015) investigated the effects of DMOG through HA ointment but only saw upregulation of VEGF in fibroblast-like cells in in vitro experiments[115]. Keswani et al. (2013) administered oral VEGF protein in drinking water and liquid chow and showed that salivary VEGF level was inversely correlated to epithelial gap and positively correlated to neovascularization in palatal wounds of mice[139]. Lastly, Karavana et al. (2011) investigated the effects of benzydamine hydrochloride (Bnz HCL) bioadhesive gel in 5mm gingival wound of NZW Rabbits[140, 141]. H&E assessment from Day 12 post-wounding indicated that rabbits treated with Bnz HCL showed new capillary proliferation reaching the wound surface when compared to control.
Inflammatory Cell Response
The recruitment of inflammatory immune cell subsets during wound healing is pivotal to the success of tissue re-epithelialization. As immune cells release cytokines that induce a systemic response for regeneration, only 5 of the 23 studies included in this review commented on inflammatory changes at the defect site largely using semi-quantitative histology (Table 3). For example, Shim et al. (2007) analyzed the number of inflammatory cells near the wound area using H&E and Giemsa stains. Results demonstrated a significant decrease in inflammatory cells at Day 5 in Aucubin-treated mice compared to control; however, they did not specify the immune cell subsets quantified during the histological analysis[86]. On the other hand, Kiliç et al. (2013) showed general increase in macrophage infiltration on Day 5 post-treatment with glutathione; however, this study lacked quantitative evidence besides H&E staining on the epidermis and lamina propria[89]. Couto et al. (2016) studied the effects of applying bismuth subgallate in the buccal wounds of Wistar rat but results found a chronic inflammation status and a possible negative effect leading to delayed healing[142]. Similarly, Ophof et al. (2008) stated that treatment with autologous mucosa using de-epidermized dermis or AlloDerm® decreased overall degree of inflammation of the experimental wound through semi-quantitative analysis using H&E staining, yet did not support the data with additional quantitative measures[143]. Though most studies analyzed inflammatory infiltrates through histological staining, Ballestas et al. (2019) additionally included flow cytometric data for quantitative analysis of immune infiltration around the ONF injury in the hard palate. Ballestas et al. (2019) observed that treatment with FTY720-loaded nanofibers increased pro-regenerative monocytes Ly6Clo subpopulation at Day 3 when compared to blank nanofibers. Furthermore, flow cytometric data showed significant increase in total number of M2 pro-regenerative macrophages in wounds at Day 3 post-FTY720 treatment. Ballestas et al. (2019) also used quantitative polymerase chain reaction to characterize the overall inflammatory response following the delivery of FTY720, revealing decreased levels of IL-1, IL-4, and IL-6 pro-inflammatory cytokines and an increase in anti-inflammatory IL-10 cytokines[84].
Collagen Deposition
Collagen synthesis plays a pivotal role in dictating quality of tissue regeneration, structural integrity of healed tissue, and matrix remodeling. Collagen fibers act as foundation for intracellular matrix formation and helps protect the wound from mechanical stresses and pressure, indicating matrix maturation[52, 144]. Wounded granulation tissue primarily expresses 40% of Type III collagen, which gradually begins to transition to Type I collagen that makes up nearly 80% of fibers in unwounded tissue[52, 145]. Of the studies presented in Table 3, only 8 of them provided qualitative results, mainly using histological assessment on collagen deposition following treatment. For example, Shim et al. (2007) reported an overall increased in collagen synthesis in the buccal wound of mice injected with aucubin. Specimens were stained Masson’s Trichrome and picrosirius red, and results showed a significant increase in newly accumulated collagen near the healed area of aucubin-treated mice compared to control at Day 5 post-injury[86]. Similarly, Ophof et al. (2008) revealed a semi-quantitative analysis for the presence of Type III collagen following treatment with dermal substrates, de-epidermized dog dermis and Alloderm®. Following a 6mm palatal injury in Beagles, H&E and Sirius red stains on palatal mucosa suggested that collagen fibers gradually transitioned from thin fibers with transverse orientation at Week 3 to a clearly aligned collagen fiber networks at Week 12 post-implantation. Ophof et al. (2008) also concluded that all experimental wounds at Week 1 showed intense staining of Type III collagen networks at the surface of the wound and by Week 12, the intensity of the collagen stains in the lamina propria and the periosteum was comparable to normal palate; yet, the amount of Type III collagen in the submucosa remained higher than that of the control[143].
Conclusion
The present review offers an in-depth analysis of current oral wound healing models that are being investigated with the potential for translational therapy to treat oral wounds and enhance mucosal regeneration (Fig. 3). Primarily in vivo palatal, buccal, and gingival injuries were utilized to model the human phenotype of post-surgical oral wounds. The injuries varied across model organisms ranging from mice to humans receiving therapeutic intervention for intraoral healing. Similarly, the therapeutic approaches investigated in the review supported a vast array of treatment options with the use of synthetic polymers, biological grafts, gel-like ointments, hybrid scaffolds, and alternative ultrasound-based therapy. Majority of the treatment methods coupled both synthetic and biologics to produce a treatment delivery vehicle, showing promising results and a potential of harnessing engineered systems with biological components. However, there is still an absence of proper, comprehensive analyses to effectively evaluate the success of treatment methods using both qualitative and quantitative evidence. Many of the studies discussed here lacked a quantitative approach to assess overall wound remodeling when compared to current hallmarks of the biological wound healing processes. Ironically, very few of the studies concluded significant changes in immune response following treatment, as disturbed healing is often attributed to prolonged or impaired immunoregulation [39]. Future avenues of oral mucosal treatment can be expanded to mast cells and their role as effector cells of the immune system, for example [146]. Mature mast cells can help stimulate angiogenesis through increased vascular permeability during the inflammatory phase and can upregulate collagen synthesis to promote re-epithelialization[147–149]. Similarly, therapies being developed for dental pulp regeneration and its immunoregulation of various immune cell subsets can also be explored as potential therapeutic agents for oral wound healing [150]. By modulating the inflammatory response and thereby the proliferative phase of mucosal healing, suitable treatment methods can advance towards an enhanced remodeling phase with a pro-regenerative phenotype. Therefore, translational therapies employing an immunomodulatory approach, while harnessing synthetic and biological systems, can provide breakthrough treatment options for overall oral wound healing and mucosal tissue regeneration.
Figure 3:
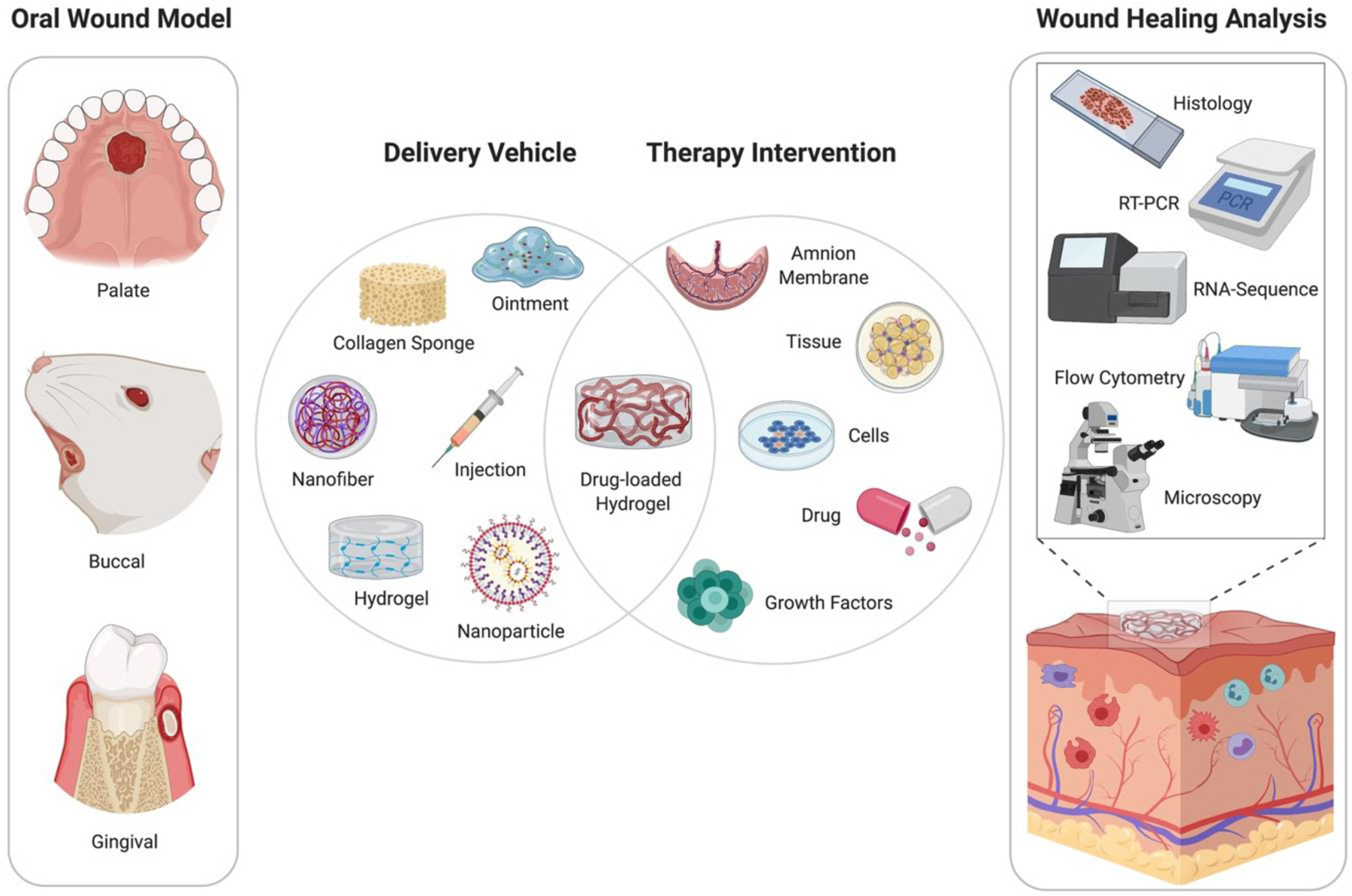
Schematic of current models and therapeutics to promote oral wound healing. Injury models are primarily studied in the palatal, buccal, and gingival regions of the oral cavity. Current modalities for oral wound healing include a wide variety of delivery vehicles, such as polymeric scaffolds, biological matrices, or gel-like ointment. The delivery vehicles can also be coupled with drugs, cells, tissue, or growth factors to enhance therapeutic efficacy. Following treatment, studies evaluated the success of oral wound healing therapy using both qualitative and quantitative approaches, such as histology for tissue re-epithelialization, flow cytometry for immune cell infiltration, and microscopy images for wound closure.
Background:
The Orofacial Tissue Regeneration Lab, located at Emory Children’s Center, Atlanta, GA, focuses on understanding the biological mechanisms involved in facial bone and soft tissue regeneration. These regenerative therapies include hydrogel-delivery of growth factors to repair bone and delivery of immunomodulatory factors on nanofiber scaffolds to repair oral mucosa.
Translational Significance:
The present in-depth review consolidates emerging regenerative therapies that have the potential to reduce post-operative complications and restore function in intraoral wounds. By evaluating oral injury models and promising therapeutic approaches, researchers can make substantial progress for translational, clinically relevant treatment options to enhance oral wound healing.
Acknowledgements
Figures and tables were created by AIT using BioRender.com. Drafting manuscript: AIT, JMF, and SLG. Revising and approving final version of manuscript: All authors. All authors have read the journal’s authorship agreement and declare no conflict of interest. Research reported in this publication was supported by the Oral Maxillofacial Surgery Foundation (Funding ID: 2591) and the National Institute of Dental and Craniofacial Research of the National Institutes of Health under award number R01DE028905. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This manuscript has not previously been copyrighted or published and is not under consideration for publication elsewhere.
Abbreviations:
- ECM
extracellular matrix
- TGF-β1
transforming growth factor beta-1
- EGF
epidermal growth factor
- PDGF
platelet-derived growth factor
- FGF
fibroblast growth factor
- VEGF
vascular endothelial growth factor
- ONF
oronasal fistula
- NZW
New Zealand White Rabbit
- SPS
synthetic polymeric scaffolds
- PLGA
poly(lactic-co-glycolic acid)
- PCL
polycaprolactone
- DAM
decellularized amnion membrane
- POC
poly(1,8-octamethylene-citrate)
- DMOG
dimethyloxalylglycine
- HA
hyaluronan
- LIPUS
low-intensity pulsed ultrasound
- H&E
Hematoxylin and Eosin
- vWF
von Willebrand factor
- IHC
immunohistochemistry
- Bnz HCL
benzydamine hydrochloride
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Uncategorized References
- 1.Cho SK, et al. , Development of a Model to Predict Healing of Chronic Wounds Within 12 Weeks. Adv Wound Care (New Rochelle), 2020. 9(9): p. 516–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu J., et al. , Skin and oral mucosa equivalents: construction and performance. Orthod Craniofac Res, 2010. 13(1): p. 11–20. [DOI] [PubMed] [Google Scholar]
- 3.Squier CA and Kremer MJ, Biology of oral mucosa and esophagus. J Natl Cancer Inst Monogr, 2001(29): p. 7–15. [DOI] [PubMed]
- 4.Arda O, Goksugur N, and Tuzun Y, Basic histological structure and functions of facial skin. Clin Dermatol, 2014. 32(1): p. 3–13. [DOI] [PubMed] [Google Scholar]
- 5.Losquadro WD, Anatomy of the Skin and the Pathogenesis of Nonmelanoma Skin Cancer. Facial Plast Surg Clin North Am, 2017. 25(3): p. 283–289. [DOI] [PubMed] [Google Scholar]
- 6.Squier CA, Kremer MJ, and Wertz PW, Effect of ethanol on lipid metabolism and epithelial permeability barrier of skin and oral mucosa in the rat. J Oral Pathol Med, 2003. 32(10): p. 595–9. [DOI] [PubMed] [Google Scholar]
- 7.Ito M and Cotsarelis G, Is the hair follicle necessary for normal wound healing? J Invest Dermatol, 2008. 128(5): p. 1059–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ito M., et al. , Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nat Med, 2005. 11(12): p. 1351–4. [DOI] [PubMed] [Google Scholar]
- 9.Levy V., et al. , Epidermal stem cells arise from the hair follicle after wounding. FASEB J, 2007. 21(7): p. 1358–66. [DOI] [PubMed] [Google Scholar]
- 10.Martinot V., et al. , Comparative study of split thickness skin grafts taken from the scalp and thigh in children. Burns, 1994. 20(2): p. 146–50. [DOI] [PubMed] [Google Scholar]
- 11.Brown JB and McDowell F, Epithelial Healing and the Transplantation of Skin. Ann Surg, 1942. 115(6): p. 1166–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mitragotri S, Modeling skin permeability to hydrophilic and hydrophobic solutes based on four permeation pathways. J Control Release, 2003. 86(1): p. 69–92. [DOI] [PubMed] [Google Scholar]
- 13.Wosicka H and Cal K, Targeting to the hair follicles: current status and potential. J Dermatol Sci, 2010. 57(2): p. 83–9. [DOI] [PubMed] [Google Scholar]
- 14.Todo H., et al. , Permeation pathway of macromolecules and nanospheres through skin. Biol Pharm Bull, 2010. 33(8): p. 1394–9. [DOI] [PubMed] [Google Scholar]
- 15.Langton AK, Herrick SE, and Headon DJ, An extended epidermal response heals cutaneous wounds in the absence of a hair follicle stem cell contribution. J Invest Dermatol, 2008. 128(5): p. 1311–8. [DOI] [PubMed] [Google Scholar]
- 16.Martin P, Wound healing--aiming for perfect skin regeneration. Science, 1997. 276(5309): p. 75–81. [DOI] [PubMed] [Google Scholar]
- 17.Whitby DJ and Ferguson MW, The extracellular matrix of lip wounds in fetal, neonatal and adult mice. Development, 1991. 112(2): p. 651–68. [DOI] [PubMed] [Google Scholar]
- 18.Chen L., et al. , Positional differences in the wound transcriptome of skin and oral mucosa. BMC Genomics, 2010. 11: p. 471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Szpaderska AM, Zuckerman JD, and DiPietro LA, Differential injury responses in oral mucosal and cutaneous wounds. J Dent Res, 2003. 82(8): p. 621–6. [DOI] [PubMed] [Google Scholar]
- 20.Turabelidze A., et al. , Intrinsic differences between oral and skin keratinocytes. PLoS One, 2014. 9(9): p. e101480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brand HS, Ligtenberg AJ, and Veerman EC, Saliva and wound healing. Monogr Oral Sci, 2014. 24: p. 52–60. [DOI] [PubMed] [Google Scholar]
- 22.Boink MA, et al. , Different wound healing properties of dermis, adipose, and gingiva mesenchymal stromal cells. Wound Repair Regen, 2016. 24(1): p. 100–9. [DOI] [PubMed] [Google Scholar]
- 23.Sattar M, Sayed OM, and Lane ME, Oral transmucosal drug delivery--current status and future prospects. Int J Pharm, 2014. 471(1–2): p. 498–506. [DOI] [PubMed] [Google Scholar]
- 24.Nagy G, [Role of saliva, salivary glands and epidermal growth factor (EGF) on oral wound healing]. Fogorv Sz, 2003. 96(1): p. 17–20. [PubMed] [Google Scholar]
- 25.Li X., et al. , Systemic diseases caused by oral infection. Clin Microbiol Rev, 2000. 13(4): p. 547–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baltch AL, et al. , Bacteremia in patients undergoing oral procedures. Study following parenteral antimicrobial prophylaxis as recommended by the American Heart Association, 1977. Arch Intern Med, 1988. 148(5): p. 1084–8. [DOI] [PubMed] [Google Scholar]
- 27.Politis C., et al. , Wound Healing Problems in the Mouth. Front Physiol, 2016. 7: p. 507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Debelian GJ, Olsen I, and Tronstad L, Anaerobic bacteremia and fungemia in patients undergoing endodontic therapy: an overview. Ann Periodontol, 1998. 3(1): p. 281–7. [DOI] [PubMed] [Google Scholar]
- 29.Thoden van Velzen SK, Abraham-Inpijn L, and Moorer WR, Plaque and systemic disease: a reappraisal of the focal infection concept. J Clin Periodontol, 1984. 11(4): p. 209–20. [DOI] [PubMed] [Google Scholar]
- 30.Okuda K and Ebihara Y, Relationships between chronic oral infectious diseases and systemic diseases. Bull Tokyo Dent Coll, 1998. 39(3): p. 165–74. [PubMed] [Google Scholar]
- 31.desJardins-Park HE, et al. , The Spectrum of Scarring in Craniofacial Wound Repair. Front Physiol, 2019. 10: p. 322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eming SA, Martin P, and Tomic-Canic M, Wound repair and regeneration: mechanisms, signaling, and translation. Sci Transl Med, 2014. 6(265): p. 265sr6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Broughton G 2nd, Janis JE, and Attinger CE, The basic science of wound healing. Plast Reconstr Surg, 2006. 117(7 Suppl): p. 12S–34S. [DOI] [PubMed] [Google Scholar]
- 34.Broughton G 2nd, Janis JE, and Attinger CE, Wound healing: an overview. Plast Reconstr Surg, 2006. 117(7 Suppl): p. 1e-S–32e-S. [DOI] [PubMed] [Google Scholar]
- 35.Glim JE, et al. , The number of immune cells is lower in healthy oral mucosa compared to skin and does not increase after scarring. Arch Oral Biol, 2015. 60(2): p. 272–81. [DOI] [PubMed] [Google Scholar]
- 36.Funato N., et al. , Evidence for apoptosis induction in myofibroblasts during palatal mucoperiosteal repair. J Dent Res, 1999. 78(9): p. 1511–7. [DOI] [PubMed] [Google Scholar]
- 37.Olczyk P, Mencner L, and Komosinska-Vassev K, The role of the extracellular matrix components in cutaneous wound healing. Biomed Res Int, 2014. 2014: p. 747584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schroder JM, The role of keratinocytes in defense against infection. Curr Opin Infect Dis, 2010. 23(2): p. 106–10. [DOI] [PubMed] [Google Scholar]
- 39.Eming SA, et al. , Interrelation of immunity and tissue repair or regeneration. Semin Cell Dev Biol, 2009. 20(5): p. 517–27. [DOI] [PubMed] [Google Scholar]
- 40.Beidler SK, et al. , Inflammatory cytokine levels in chronic venous insufficiency ulcer tissue before and after compression therapy. J Vasc Surg, 2009. 49(4): p. 1013–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koh TJ and DiPietro LA, Inflammation and wound healing: the role of the macrophage. Expert Rev Mol Med, 2011. 13: p. e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Singer AJ and Clark RA, Cutaneous wound healing. N Engl J Med, 1999. 341(10): p. 738–46. [DOI] [PubMed] [Google Scholar]
- 43.Zhang JM and An J, Cytokines, inflammation, and pain. Int Anesthesiol Clin, 2007. 45(2): p. 27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Diegelmann RF and Evans MC, Wound healing: an overview of acute, fibrotic and delayed healing. Front Biosci, 2004. 9: p. 283–9. [DOI] [PubMed] [Google Scholar]
- 45.Wietecha MS and DiPietro LA, Therapeutic Approaches to the Regulation of Wound Angiogenesis. Adv Wound Care (New Rochelle), 2013. 2(3): p. 81–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barrientos S., et al. , Growth factors and cytokines in wound healing. Wound Repair Regen, 2008. 16(5): p. 585–601. [DOI] [PubMed] [Google Scholar]
- 47.Neufeld G., et al. , Vascular endothelial growth factor (VEGF) and its receptors. FASEB J, 1999. 13(1): p. 9–22. [PubMed] [Google Scholar]
- 48.Martin A, Komada MR, and Sane DC, Abnormal angiogenesis in diabetes mellitus. Med Res Rev, 2003. 23(2): p. 117–45. [DOI] [PubMed] [Google Scholar]
- 49.Glim JE, et al. , Detrimental dermal wound healing: what can we learn from the oral mucosa? Wound Repair Regen, 2013. 21(5): p. 648–60. [DOI] [PubMed] [Google Scholar]
- 50.Darby IA and Hewitson TD, Fibroblast differentiation in wound healing and fibrosis. Int Rev Cytol, 2007. 257: p. 143–79. [DOI] [PubMed] [Google Scholar]
- 51.Darby IA, et al. , Fibroblasts and myofibroblasts in wound healing. Clin Cosmet Investig Dermatol, 2014. 7: p. 301–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Velnar T, Bailey T, and Smrkolj V, The wound healing process: an overview of the cellular and molecular mechanisms. J Int Med Res, 2009. 37(5): p. 1528–42. [DOI] [PubMed] [Google Scholar]
- 53.McCarty SM and Percival SL, Proteases and Delayed Wound Healing. Adv Wound Care (New Rochelle), 2013. 2(8): p. 438–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schultz GS and Wysocki A, Interactions between extracellular matrix and growth factors in wound healing. Wound Repair Regen, 2009. 17(2): p. 153–62. [DOI] [PubMed] [Google Scholar]
- 55.Lobmann R, Schultz G, and Lehnert H, Proteases and the diabetic foot syndrome: mechanisms and therapeutic implications. Diabetes Care, 2005. 28(2): p. 461–71. [DOI] [PubMed] [Google Scholar]
- 56.Greenhalgh DG, The role of apoptosis in wound healing. Int J Biochem Cell Biol, 1998. 30(9): p. 1019–30. [DOI] [PubMed] [Google Scholar]
- 57.Xue M and Jackson CJ, Extracellular Matrix Reorganization During Wound Healing and Its Impact on Abnormal Scarring. Adv Wound Care (New Rochelle), 2015. 4(3): p. 119–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Guo S and Dipietro LA, Factors affecting wound healing. J Dent Res, 2010. 89(3): p. 219–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cohen SR, et al. , Cumulative operative procedures in patients aged 14 years and older with unilateral or bilateral cleft lip and palate. Plast Reconstr Surg, 1995. 96(2): p. 267–71. [DOI] [PubMed] [Google Scholar]
- 60.Isberg A and Henningsson G, Influence of palatal fistulas on velopharyngeal movements: a cineradiographic study. Plast Reconstr Surg, 1987. 79(4): p. 525–30. [DOI] [PubMed] [Google Scholar]
- 61.Wolford LM and Stevao EL, Correction of jaw deformities in patients with cleft lip and palate. Proc (Bayl Univ Med Cent), 2002. 15(3): p. 250–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Motamedi MH, A Textbook of Advanced Oral and Maxillofacial Surgery. Vol. 3. 2016: Books on Demand. 836. [Google Scholar]
- 63.Crockett DJ and Goudy SL, Cleft lip and palate. Facial Plast Surg Clin North Am, 2014. 22(4): p. 573–86. [DOI] [PubMed] [Google Scholar]
- 64.Rohrich RJ and Gosman AA, An update on the timing of hard palate closure: a critical long-term analysis. Plast Reconstr Surg, 2004. 113(1): p. 350–2. [DOI] [PubMed] [Google Scholar]
- 65.Moore P., et al. , Competence and physical impairment of pediatric survivors of burns of more than 80% total body surface area. J Burn Care Rehabil, 1996. 17(6 Pt 1): p. 547–51. [DOI] [PubMed] [Google Scholar]
- 66.Molsted K, Treatment outcome in cleft lip and palate: issues and perspectives. Crit Rev Oral Biol Med, 1999. 10(2): p. 225–39. [DOI] [PubMed] [Google Scholar]
- 67.Ross RB, Growth of the facial skeleton following the Malek repair for unilateral cleft lip and palate. Cleft Palate Craniofac J, 1995. 32(3): p. 194–8. [DOI] [PubMed] [Google Scholar]
- 68.Vyas KS and Vasconez HC, Wound Healing: Biologics, Skin Substitutes, Biomembranes and Scaffolds. Healthcare (Basel), 2014. 2(3): p. 356–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rhee PH, et al. , The use of processed allograft dermal matrix for intraoral resurfacing: an alternative to split-thickness skin grafts. Arch Otolaryngol Head Neck Surg, 1998. 124(11): p. 1201–4. [DOI] [PubMed] [Google Scholar]
- 70.Rosenberg AS, et al. , Cellular basis of skin allograft rejection across a class I major histocompatibility barrier in mice depleted of CD8+ T cells in vivo. J Exp Med, 1991. 173(6): p. 1463–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Frykberg RG and Banks J, Challenges in the Treatment of Chronic Wounds. Adv Wound Care (New Rochelle), 2015. 4(9): p. 560–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kloc M and Ghobrial RM, Chronic allograft rejection: A significant hurdle to transplant success. Burns Trauma, 2014. 2(1): p. 3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nussbaum SR, et al. , An Economic Evaluation of the Impact, Cost, and Medicare Policy Implications of Chronic Nonhealing Wounds. Value Health, 2018. 21(1): p. 27–32. [DOI] [PubMed] [Google Scholar]
- 74.Sen CK, Human Wounds and Its Burden: An Updated Compendium of Estimates. Adv Wound Care (New Rochelle), 2019. 8(2): p. 39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nauta A, Gurtner G, and Longaker MT, Wound healing and regenerative strategies. Oral Dis, 2011. 17(6): p. 541–9. [DOI] [PubMed] [Google Scholar]
- 76.New drugs/drug news. P T, 2012. 37(4): p. 202–11. [Google Scholar]
- 77.Yang R., et al. , Progress in studies of epidermal stem cells and their application in skin tissue engineering. Stem Cell Res Ther, 2020. 11(1): p. 303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Naumova EA, et al. , The oral mucosal surface and blood vessels. Head Face Med, 2013. 9: p. 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mai CT, et al. , Birth defects data from population-based birth defects surveillance programs in the United States, 2007 to 2011: highlighting orofacial clefts. Birth Defects Res A Clin Mol Teratol, 2014. 100(11): p. 895–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Parker SE, et al. , Updated National Birth Prevalence estimates for selected birth defects in the United States, 2004–2006. Birth Defects Res A Clin Mol Teratol, 2010. 88(12): p. 1008–16. [DOI] [PubMed] [Google Scholar]
- 81.Li W., et al. , Polymer-integrated amnion scaffold significantly improves cleft palate repair. Acta Biomater, 2019. 92: p. 104–114. [DOI] [PubMed] [Google Scholar]
- 82.Jeffery SL, Boorman JG, and Dive DC, Use of cartilage grafts for closure of cleft palate fistulae. Br J Plast Surg, 2000. 53(7): p. 551–4. [DOI] [PubMed] [Google Scholar]
- 83.Zheng J., et al. , Presurgical nasoalveolar molding with 3D printing for a patient with unilateral cleft lip, alveolus, and palate. Am J Orthod Dentofacial Orthop, 2019. 156(3): p. 412–419. [DOI] [PubMed] [Google Scholar]
- 84.Ballestas SA, et al. , Improving hard palate wound healing using immune modulatory autotherapies. Acta Biomater, 2019. 91: p. 209–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Oliver RJ, Sloan P, and Pemberton MN, Oral biopsies: methods and applications. Br Dent J, 2004. 196(6): p. 329–33; quiz 362. [DOI] [PubMed] [Google Scholar]
- 86.Shim KM, et al. , Effects of aucubin on the healing of oral wounds. In Vivo, 2007. 21(6): p. 1037–41. [PubMed] [Google Scholar]
- 87.Umeki H., et al. , Leptin promotes wound healing in the oral mucosa. PLoS One, 2014. 9(7): p. e101984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lim YS, et al. , Enhanced mucosal healing with curcumin in animal oral ulcer model. Laryngoscope, 2016. 126(2): p. E68–73. [DOI] [PubMed] [Google Scholar]
- 89.Kilic C., et al. , Investigation of the effects of local glutathione and chitosan administration on incisional oral mucosal wound healing in rabbits. Colloids Surf B Biointerfaces, 2013. 112: p. 499–507. [DOI] [PubMed] [Google Scholar]
- 90.Kirschner RE, et al. , Repair of oronasal fistulae with acellular dermal matrices. Plast Reconstr Surg, 2006. 118(6): p. 1431–40. [DOI] [PubMed] [Google Scholar]
- 91.Thoma DS, et al. , Impact of a collagen matrix on early healing, aesthetics and patient morbidity in oral mucosal wounds - a randomized study in humans. J Clin Periodontol, 2012. 39(2): p. 157–65. [DOI] [PubMed] [Google Scholar]
- 92.Kesting MR, et al. , Repair of oronasal fistulas with human amniotic membrane in minipigs. Br J Oral Maxillofac Surg, 2010. 48(2): p. 131–5. [DOI] [PubMed] [Google Scholar]
- 93.Karavana SY, Guneri P, and Ertan G, Benzydamine hydrochloride buccal bioadhesive gels designed for oral ulcers: preparation, rheological, textural, mucoadhesive and release properties. Pharm Dev Technol, 2009. 14(6): p. 623–31. [DOI] [PubMed] [Google Scholar]
- 94.Wubneh A., et al. , Current state of fabrication technologies and materials for bone tissue engineering. Acta Biomater, 2018. 80: p. 1–30. [DOI] [PubMed] [Google Scholar]
- 95.Costa A., et al. , Biologic Scaffolds. Cold Spring Harb Perspect Med, 2017. 7(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Debels H., et al. , Dermal matrices and bioengineered skin substitutes: a critical review of current options. Plast Reconstr Surg Glob Open, 2015. 3(1): p. e284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mir M., et al. , Synthetic polymeric biomaterials for wound healing: a review. Prog Biomater, 2018. 7(1): p. 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Awojoodu AO, et al. , Sphingosine 1-phosphate receptor 3 regulates recruitment of anti-inflammatory monocytes to microvessels during implant arteriogenesis. Proc Natl Acad Sci U S A, 2013. 110(34): p. 13785–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cao H., et al. , The topographical effect of electrospun nanofibrous scaffolds on the in vivo and in vitro foreign body reaction. J Biomed Mater Res A, 2010. 93(3): p. 1151–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ambekar RS and Kandasubramanian B, Advancements in nanofibers for wound dressing: A review. European Polymer Journal, 2019. 117: p. 304–336. [Google Scholar]
- 101.Niknejad H., et al. , Properties of the amniotic membrane for potential use in tissue engineering. Eur Cell Mater, 2008. 15: p. 88–99. [DOI] [PubMed] [Google Scholar]
- 102.Webb AR, Yang J, and Ameer GA, Biodegradable polyester elastomers in tissue engineering. Expert Opin Biol Ther, 2004. 4(6): p. 801–12. [DOI] [PubMed] [Google Scholar]
- 103.Ma C., et al. , In vitro cytocompatibility evaluation of poly(octamethylene citrate) monomers toward their use in orthopedic regenerative engineering. Bioact Mater, 2018. 3(1): p. 19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Filippi M., et al. , Natural Polymeric Scaffolds in Bone Regeneration. Front Bioeng Biotechnol, 2020. 8: p. 474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Vats A., et al. , Scaffolds and biomaterials for tissue engineering: a review of clinical applications. Clin Otolaryngol Allied Sci, 2003. 28(3): p. 165–72. [DOI] [PubMed] [Google Scholar]
- 106.Ono I, Tateshita T, and Inoue M, Effects of a collagen matrix containing basic fibroblast growth factor on wound contraction. J Biomed Mater Res, 1999. 48(5): p. 621–30. [DOI] [PubMed] [Google Scholar]
- 107.Ayvazyan A., et al. , Collagen-gelatin scaffold impregnated with bFGF accelerates palatal wound healing of palatal mucosa in dogs. J Surg Res, 2011. 171(2): p. e247–57. [DOI] [PubMed] [Google Scholar]
- 108.Gospodarowicz D, Neufeld G, and Schweigerer L, Molecular and biological characterization of fibroblast growth factor, an angiogenic factor which also controls the proliferation and differentiation of mesoderm and neuroectoderm derived cells. Cell Differ, 1986. 19(1): p. 1–17. [DOI] [PubMed] [Google Scholar]
- 109.Rohleder NH, et al. , Repair of oronasal fistulae by interposition of multilayered amniotic membrane allograft. Plast Reconstr Surg, 2013. 132(1): p. 172–81. [DOI] [PubMed] [Google Scholar]
- 110.Lesman A., et al. , Transplantation of a tissue-engineered human vascularized cardiac muscle. Tissue Eng Part A, 2010. 16(1): p. 115–25. [DOI] [PubMed] [Google Scholar]
- 111.Roh JL, et al. , Use of oral mucosal cell sheets for accelerated oral surgical wound healing. Head Neck, 2018. 40(2): p. 394–401. [DOI] [PubMed] [Google Scholar]
- 112.Schmidt C, Gintuit cell therapy approval signals shift at US regulator. Nat Biotechnol, 2012. 30(6): p. 479. [DOI] [PubMed] [Google Scholar]
- 113.El-Sherbiny IM and Yacoub MH, Hydrogel scaffolds for tissue engineering: Progress and challenges. Glob Cardiol Sci Pract, 2013. 2013(3): p. 316–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Shu XZ, et al. , Attachment and spreading of fibroblasts on an RGD peptide-modified injectable hyaluronan hydrogel. J Biomed Mater Res A, 2004. 68(2): p. 365–75. [DOI] [PubMed] [Google Scholar]
- 115.Zhu T., et al. , Effects of dimethyloxalylglycine on wound healing of palatal mucosa in a rat model. BMC Oral Health, 2015. 15: p. 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Agis H, Watzek G, and Gruber R, Prolyl hydroxylase inhibitors increase the production of vascular endothelial growth factor by periodontal fibroblasts. J Periodontal Res, 2012. 47(2): p. 165–73. [DOI] [PubMed] [Google Scholar]
- 117.Botusan IR, et al. , Stabilization of HIF-1alpha is critical to improve wound healing in diabetic mice. Proc Natl Acad Sci U S A, 2008. 105(49): p. 19426–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Priprem A., et al. , Topical Niosome Gel Containing an Anthocyanin Complex: a Potential Oral Wound Healing in Rats. AAPS PharmSciTech, 2018. 19(4): p. 1681–1692. [DOI] [PubMed] [Google Scholar]
- 119.Castañeda-Ovando A., et al. , Chemical studies of anthocyanins: A review. Food Chemistry, 2009. 113(4): p. 859–871. [Google Scholar]
- 120.Kay CD, Aspects of anthocyanin absorption, metabolism and pharmacokinetics in humans. Nutr Res Rev, 2006. 19(1): p. 137–46. [DOI] [PubMed] [Google Scholar]
- 121.Shumway BS, et al. , Effects of a topically applied bioadhesive berry gel on loss of heterozygosity indices in premalignant oral lesions. Clin Cancer Res, 2008. 14(8): p. 2421–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kim J., et al. , Anti-inflammatory effects of zinc in PMA-treated human gingival fibroblast cells. Med Oral Patol Oral Cir Bucal, 2015. 20(2): p. e180–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bartelds R., et al. , Niosomes, an alternative for liposomal delivery. PLoS One, 2018. 13(4): p. e0194179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Priprem A., et al. , Topical Niosome Gel of Zingiber cassumunar Roxb. Extract for Anti-inflammatory Activity Enhanced Skin Permeation and Stability of Compound D. AAPS PharmSciTech, 2016. 17(3): p. 631–9. [DOI] [PubMed] [Google Scholar]
- 125.Maeda T., et al. , Low-intensity pulsed ultrasound enhances palatal mucosa wound healing in rats. J Prosthodont Res, 2013. 57(2): p. 93–8. [DOI] [PubMed] [Google Scholar]
- 126.Sant’Anna EF, et al. , Effect of low intensity pulsed ultrasound and BMP-2 on rat bone marrow stromal cell gene expression. J Orthop Res, 2005. 23(3): p. 646–52. [DOI] [PubMed] [Google Scholar]
- 127.Liu Q., et al. , The effect of low-intensity pulsed ultrasound on the osseointegration of titanium dental implants. Br J Oral Maxillofac Surg, 2012. 50(3): p. 244–50. [DOI] [PubMed] [Google Scholar]
- 128.Pilla AA, et al. , Non-invasive low-intensity pulsed ultrasound accelerates bone healing in the rabbit. J Orthop Trauma, 1990. 4(3): p. 246–53. [DOI] [PubMed] [Google Scholar]
- 129.Ikai H., et al. , Low-intensity pulsed ultrasound accelerates periodontal wound healing after flap surgery. J Periodontal Res, 2008. 43(2): p. 212–6. [DOI] [PubMed] [Google Scholar]
- 130.Suragimath G., et al. , Effect of carbonated drink on excisional palatal wound healing: a study on Wistar rats. Indian J Dent Res, 2010. 21(3): p. 330–3. [DOI] [PubMed] [Google Scholar]
- 131.Fujisawa K, Miyamoto Y, and Nagayama M, Basic fibroblast growth factor and epidermal growth factor reverse impaired ulcer healing of the rabbit oral mucosa. J Oral Pathol Med, 2003. 32(6): p. 358–66. [DOI] [PubMed] [Google Scholar]
- 132.Lis GJ, et al. , Effect of cultured autologous oral keratinocyte suspension in fibrin glue on oral wound healing in rabbits. Int J Oral Maxillofac Surg, 2012. 41(9): p. 1146–52. [DOI] [PubMed] [Google Scholar]
- 133.Arnold F and West DC, Angiogenesis in wound healing. Pharmacol Ther, 1991. 52(3): p. 407–22. [DOI] [PubMed] [Google Scholar]
- 134.Byrne M., et al. , Mathematical modelling of angiogenesis in wound healing comparison of theory and experiment. Journal of Theoretical Medicine, 2000. 2(3): p. 175–197. [Google Scholar]
- 135.Toledo KA, et al. , Neutrophil activation induced by ArtinM: release of inflammatory mediators and enhancement of effector functions. Immunol Lett, 2009. 123(1): p. 14–20. [DOI] [PubMed] [Google Scholar]
- 136.Ganiko L., et al. , Lectin KM+-induced neutrophil haptotaxis involves binding to laminin. Biochim Biophys Acta, 2005. 1721(1–3): p. 152–63. [DOI] [PubMed] [Google Scholar]
- 137.Chahud F., et al. , The lectin KM+ induces corneal epithelial wound healing in rabbits. Int J Exp Pathol, 2009. 90(2): p. 166–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kim YJ, et al. , Topical application of the lectin Artin M accelerates wound healing in rat oral mucosa by enhancing TGF-beta and VEGF production. Wound Repair Regen, 2013. 21(3): p. 456–63. [DOI] [PubMed] [Google Scholar]
- 139.Keswani SG, et al. , Role of salivary vascular endothelial growth factor (VEGF) in palatal mucosal wound healing. Wound Repair Regen, 2013. 21(4): p. 554–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Karavana Hizarcioğlu SY, et al. , Efficacy of topical benzydamine hydrochloride gel on oral mucosal ulcers: an in vivo animal study. Int J Oral Maxillofac Surg, 2011. 40(9): p. 973–8. [DOI] [PubMed] [Google Scholar]
- 141.Karavana Hizarcioglu SY, et al. , Efficacy of topical benzydamine hydrochloride gel on oral mucosal ulcers: an in vivo animal study. Int J Oral Maxillofac Surg, 2011. 40(9): p. 973–8. [DOI] [PubMed] [Google Scholar]
- 142.Couto EV, et al. , Experimental study on the effects of bismuth subgallate on the inflammatory process and angiogenesis of the oral mucosa. Braz J Otorhinolaryngol, 2016. 82(1): p. 17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ophof R., et al. , Implantation of tissue-engineered mucosal substitutes in the dog palate. Eur J Orthod, 2008. 30(1): p. 1–9. [DOI] [PubMed] [Google Scholar]
- 144.Baum CL and Arpey CJ, Normal cutaneous wound healing: clinical correlation with cellular and molecular events. Dermatol Surg, 2005. 31(6): p. 674–86; discussion 686. [DOI] [PubMed] [Google Scholar]
- 145.Robson MC, Steed DL, and Franz MG, Wound healing: biologic features and approaches to maximize healing trajectories. Curr Probl Surg, 2001. 38(2): p. 72–140. [DOI] [PubMed] [Google Scholar]
- 146.Kamal R., et al. , Mast cells and oral pathologies: A Review. J Nat Sci Biol Med, 2015. 6(1): p. 35–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Egozi EI, et al. , Mast cells modulate the inflammatory but not the proliferative response in healing wounds. Wound Repair Regen, 2003. 11(1): p. 46–54. [DOI] [PubMed] [Google Scholar]
- 148.Tellechea A., et al. , Mast Cells Regulate Wound Healing in Diabetes. Diabetes, 2016. 65(7): p. 2006–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ud-Din S, Wilgus TA, and Bayat A, Mast Cells in Skin Scarring: A Review of Animal and Human Research. Front Immunol, 2020. 11: p. 552205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Morotomi T, Washio A, and Kitamura C, Current and future options for dental pulp therapy. Jpn Dent Sci Rev, 2019. 55(1): p. 5–11. [DOI] [PMC free article] [PubMed] [Google Scholar]


