Abstract
E-cigarettes, which deliver vaporized nicotine, have dramatically risen in popularity in recent years, despite many unanswered questions about safety, efficacy in reducing dependence, and overall impact on public health. Other factors, such as sex, also play an important role in determining behavioral and neurochemical responses to drugs of abuse. In these studies, we sought to develop a protocol for vaporized e-cigarette nicotine self-administration in rats, as a foundation to better understand the differing effects of nicotine exposure routes on behavior and physiological function. We report a novel method that elicits robust nicotine vapor self-administration in male and female rats. Our findings indicate that 5 mg/ml nicotine vape solution provides a high level of consistency in lever-pressing behavior for both males and females. Moreover, in male rats, we find that such e-cigarette nicotine vapor induces similar blood levels of nicotine’s main metabolite, cotinine, as that found with intravenous nicotine self-administration. Therefore, the breathing pattern during vapor exposure in males leads to similar levels of titrated nicotine intake as with intravenous nicotine self-administration. Interestingly, a differential effect was found in the females, in which the same conditions of vapor exposure led to decreased cotinine levels with vapor compared to intravenous self-administration. Finally, differences in nicotine-mediated locomotion provide further support of the physiological effects of e-cigarette vapor inhalation. Taken together, our findings reveal important sex differences in nicotine intake based on the route of exposure, and we further establish a protocol for nicotine vapor self-administration in rats.
INTRODUCTION
Greater than 1.2 billion people use nicotine/tobacco products worldwide, resulting in more than 5 million deaths per year1. The positive reinforcing properties associated with nicotine intake underlie the addictive nature of the drug2. Humans consume nicotine through various routes, including tobacco cigarettes, electronic cigarettes (a.k.a., e-cigarettes or vapes), and chewing tobacco. The resulting bioavailability of nicotine is different based on the method of administration in consideration of the pharmacokinetics associated with absorption and distribution. Prior studies have found that nicotine is readily self-administered intravenously by animal models and humans3–7, supporting the reinforcing effects of this method of administration. Thus, intravenous nicotine self-administration has been traditionally accepted as having the highest translational validity to human drug use. However, with the recent emergence of e-cigarette products, it should be recognized that vaporized nicotine and constituents found in the vehicle solution may result in different neurochemical, physiological and behavioral alterations related to dependence, which could be different from those observed with other methods of administration8.
Cigarettes typically contain 10-14 mg of nicotine9. With tobacco smoking in humans, ~1-1.5 mg is absorbed systemically via the lungs for one cigarette, and with each inhalation, nicotine reaches the brain within 10-20 s, where the drug binds directly to nicotinic acetylcholine receptors (nAChRs)10–12. The nAChRs are ionotropic receptors that permit influx of Na+ and Ca2+, leading to membrane depolarization, Ca2+-dependent second messenger signaling, and/or presynaptic modulation of neurotransmitter release in many regions of the nervous system. Thus, variability in pharmacokinetics of vaporized nicotine would likely differentially affect the activation of these receptors, leading to important implications for processes mediating dependence. Nicotine is quickly metabolized by CYP450 enzymes in the liver, resulting in a relatively short half-life (~2 - 6 hrs in humans and ~45 min in rats) and subsequent formation of the main metabolite, cotinine, which exhibits a longer half-life (~16 hrs in humans and ~6 hrs in rats)13–15. Thus, nicotine exposure levels with self-administration methods can be evaluated by examining cotinine levels in blood plasma14.
E-cigarettes vaporize a solution typically composed of nicotine, propylene glycol, vegetable glycerin, and flavoring agents16. The spread of the e-cigarette market has been rapid, despite many unanswered questions about their safety, efficacy in reducing dependence, and overall impact on public health. At present, individuals across a wide range of ages utilize e-cigarettes, including individuals without a history of smoking tobacco16–18. Remarkably, e-cigarettes have become the most common drug product used by middle- and high-school students19–21. Compared to tobacco cigarettes, it is thought that e-cigarette consumption results in similar effects of nicotine concentration in the brain and nAChR activity, as well as greater compulsive behavior during intake8. Since e-cigarettes can be consumed more readily in various settings as they may be inhaled inconspicuously and typically do not emit a strong odor, the patterns of use may increase over time for the user. Given this, understanding the differing effects of vaporized nicotine on the brain and relevance to drug addiction is critical22–24. Indeed, recent studies have begun to examine nicotine vapor administration in rodent models8,25–29, but many questions still remain, including whether the level of intake is titrated similar to intravenous self-administration and whether sex-specific effects are present in the exposure and behavioral levels, factors which are addressed in the current studies.
Here, we first describe our developed model of a robust and reliable procedure for vaporized e-cigarette nicotine self-administration in rats. This protocol is an advance over other current methods, as we find selectivity in the behavioral responses for nicotine vapor on the active lever. To further validate this model, we then examined the effects of modifying the nicotine dose using passive vapor exposure to provide precise dosing across subjects. Next, we determined whether vaporized e-cigarette nicotine exposure results in a similar level of metabolized blood nicotine, as compared to volitional intake during intravenous nicotine self-administration. Subjects were also examined for nicotine-mediated change in locomotion as a measure of nicotine’s actions on the physiological measure. Finally, since women consume more tobacco products than men and studies suggest that women and female rodents are more sensitive to the rewarding effects of nicotine30–33, male and female subjects were examined in a within-sex manner. Together, the findings derived from these investigations establish a new procedural model for nicotine vapor exposure, thereby providing an important foundation for future studies.
MATERIALS AND METHODS
Animals
Adult male and female Wistar rats were purchased from Charles River. Subjects were maintained in an environmentally controlled vivarium on a 12h:12h reversed light:dark cycle. Food and water were provided ad libitum until behavioral training commenced. All testing was conducted in the dark phase of the light cycle, when rats are most active. During drug administration procedures, subjects were food restricted to 85-90% of their free-feeding body weight, and water was provided ad libitum. All procedures were conducted in strict accordance with the NIH Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of the University of California, Irvine.
Drugs
(−)-Nicotine hydrogen tartrate (Cat #0215355491 , MP Biomedicals) was dissolved in 0.9% sterile saline (intravenous self-administration) or 50:50 propylene glycol (PG) and vegetable glycerin (VG) solution (e-cigarette vapor) (free base, pH 7.4). Concentrations for intravenous solution are expressed as mg/kg and for aerosol solution as mg/ml.
Experimental Design
Food and vapor nicotine self-administration
Based on prior intravenous nicotine self-administration protocols7,34,35, initial food training was performed to operantly train the rats to press a lever to receive a reward under an effortful fixed ratio 5 schedule (FR5, in which 5 active lever presses elicits a reward). In the first set, male (n=8) and female (n=8) rats were initially trained to press the active lever in a sealed vapor chamber (340mm X 237mm X 198mm, LJARI) with regulated airflow (1 L/min) for liquid food infusion (50 μl/sec/infusion). Since it is essential that a constant airflow is maintained inside the vapor boxes, the design of the equipment did not allow for a food pellet hopper dispenser, and thus, food reward was provided in a liquid form to ensure consistent control of pressure and airflow.
Rewards were earned under the FR5TO20 sec schedule, in which 5 lever presses elicits food delivery and activation of a cue light above the active lever, followed by a 20 sec timeout period. Based on our preliminary studies, we found that the animals would highly respond for a solution of vegetable broth containing 5% sucrose. Once stable responding was achieved (>50 liquid rewards per session across 3 subsequent sessions), subjects were then transitioned to acquire e-cigarette vaporized nicotine during 1 h daily sessions for 7 days on each dose in ascending order (nicotine concentration: 2.5 mg/ml, 5 mg/ml or 7.5 mg/ml in VG:PG), and the mean of the last 3 days on each dose was used for data analysis. Of note, we did try to employ the higher nicotine dose of 10 mg/ml; however, the solution was cloudy and appeared to precipitate out of solution over time in the 50:50 PG/VG vehicle (pH 7.4), and thus, we did not employ this higher dose in these studies. It is recognized that altering the vehicle and/or pH may permit higher solubility for nicotine. Blood was drawn from the lateral tail vein to assess cotinine levels after final dose sessions. Each session was performed using 2 levers (1 active, 1 inactive). Completion of the response criteria on the active lever resulted in the delivery of a vapor nicotine puff in accordance with the FR5TO20 sec schedule. Responses on the inactive lever were recorded but had no scheduled consequences. Behavioral responses were automatically recorded by Med Associates software. The Med Associates custom computer interface allows for the delivery of vapor generated by a commercial e-cigarette vaporizer under specified controlled conditions (temperature 400°F; 5 sec programmed puff, total vapor time in chamber per puff ~100 sec) (La Jolla Alcohol Research, LJARI). The positive-pressure chamber air flow was vacuum controlled to maintain air delivery through the intake valve at 1 L/min. To ensure that the rats were specifically responding for nicotine, a separate control group (total n=13; 8 males, 5 females) was examined for their responding to vehicle-only vapor across 14 days following initial food training, under identical experimental conditions as above (but no nicotine). Given that we previously found initial exposure to a lower nicotine dose is necessary to establish reliable and consistent responding with intravenous nicotine self-administration34, a final self-administration group (total n=8; 4 males, 4 females) was examined for their initial responding on the moderate 5 mg/ml dose, without prior access to the lower dose of 2.5 mg/ml.
Passive vapor nicotine administration
Subjects (n=16 males, n=6 females) were exposed to vaporized e-cigarette nicotine during 1 h daily sessions for 7 days (nicotine concentration: 5mg/ml) in the sealed LJARI chambers (340mm X 237mm X 198mm) described above. As an additional control, females were examined for differences in blood cotinine with the same 1 h session under single or dual nicotine vapor exposure conditions (n=4). For each daily session, animals were exposed to nicotine or PG:VG vehicle at a rate of one puff every 5 min (consistent with above: 5 sec programmed puff allowing for ~100 s of vapor presence in chamber); this resulted in 12 total puffs per 1 hr session. The rationale for the number of administrations per 1 hr session was based on the average number of infusions at the 0.03 mg/kg/infusion dose intravenously self-administered by rats in prior studies7,36.
Food and intravenous nicotine self-administration
A separate group of subjects (n=8 male, n=6 female) were trained to press a lever in an operant chamber (Med Associates) for food pellets (45 mg; TestDiet) under a fixed-ratio 5, timeout 20 sec (FR5TO20 sec) schedule of reinforcement, as described previously7,37. For the intravenous self-administration rats, the food was provided in pellet form given the design of the chambers with food hoppers. Once stable responding was achieved (>75 pellets per session across 3 subsequent sessions), subjects were surgically catheterized7,37. Briefly, rats were anesthetized with an isoflurane (1-3%)/oxygen vapor mixture and prepared with intravenous catheters. Catheters consisted of a 6 cm length of silastic tubing fitted to guide cannula (Plastics One) bent at a curved right angle and encased in dental acrylic. The catheter tubing was passed subcutaneously from the animal’s back to the right jugular vein, and a 2 cm length of the catheter tip was inserted into the vein and tied with surgical silk suture. Following intravenous surgery, animals were allowed ≥48 h to recover from surgery, then permitted again to respond for food reward. Subjects were then transitioned to acquire intravenous nicotine self-administration during 1 h daily sessions for 7 days. Nicotine was delivered through the tubing into the intravenous catheter by a Razel syringe pump (Med Associates). Each session was performed using 2 retractable levers (1 active, 1 inactive). Completion of the response criteria on the active lever resulted in the delivery of an intravenous nicotine infusion (0.03 ml infusion volume; FR5TO20 sec schedule). Responses on the inactive lever were recorded but had no scheduled consequences. Catheters were flushed daily with physiological sterile saline solution containing heparin. Catheter integrity was tested with the ultra-short-acting barbiturate anesthetic Brevital (methohexital sodium, Eli Lilly), and all subjects had catheter patency at the end of the experiment. Behavioral responses were automatically recorded by Med Associates software.
Locomotor assessment
Subjects were examined for nicotine-mediated locomotor effects in an open field chamber prior to any drug exposure (baseline) and immediately after the nicotine exposure sessions on Day 1 and Day 7. This assessment was included in a subset of the rats tested in later cohorts, and thus, the group number included: males (n=6 passive nicotine vapor, n=6 intravenous nicotine self-administration) and females (n=6 passive nicotine vapor, n=5 intravenous nicotine self-administration). The chamber was composed of plexiglass (35 cm L x 35 cm W x 31 cm H) as described previously38, with a shielded white light lamp ~90 cm above the apparatus for consistent lighting. Prior to testing, animals were habituated by handling for at least 5 mins per day for 2 days. On the first testing day (baseline), they were individually placed into the open field and recorded for a 15 min test. On Day 1 and Day 7, rats were individually placed into the chamber for the 15 min period and then returned to their home cage at the end of each session. Activity was recorded with a video camera and scored with ANYmaze software that divided the field into center and outer edge zones.
Blood cotinine analysis
Collection of blood samples for cotinine measurement was performed from the tail vein. For the first set with vapor self-administration, blood was collected 30 min after the final session on each dose. To limit the potential stress-related impact of repeated blood draws, each subject had blood drawn ≤2 times, with at least 6 days in between each blood collection. For the intravenous nicotine self-administration and passive vapor exposure, blood collection was scheduled as follows: (1) 30 min after chamber-only exposure on Day 0 (baseline), (2) 30 min after the first vapor session (Day 1), and (3) 30 min after the final vapor session (Day 7). On Day 1, a second sample was collected at the post-session 6 hr time point. Serum was separated by centrifugation at 3000 × g for 20 min at 4°C and then stored at −80 °C. The concentration of cotinine was determined with the cotinine ELISA kit (OriGene Technologies, Inc) according to the manufacturer’s instructions.
Approach for unbiased data collection
For the studies, each subject was provided with a subject ID number that did not denote group assignment. This information was retained in a secure database. The animals were randomly assigned into experimental groups for testing. Blood samples were coded during analysis to provided blinded conditions, and group identification was revealed after the analysis was completed. To provide further confidence in the findings, behavior was scored by two different experimenters who were blinded to the experimental conditions.
Statistical Analyses
All data were analyzed by a t-test, or one-way or two-way analysis of variance (ANOVA) using Graphpad Prism software (La Jolla, CA), as appropriate. Significant main or interaction effects were followed by Bonferroni post-hoc comparison with correction for multiple comparisons. The criterion for significance was set at α=0.05.
RESULTS
Male and female rats self-administer nicotine vapor across doses
Subjects were first examined for their self-administration behavior to earn nicotine vapor puffs according to the fixed ratio 5, timeout 20 schedule of reinforcement. Compared to vehicle vapor, we found that males exhibited a significant increase in their level of responding for nicotine vapor at the 5.0 and 7.5 mg/ml doses (One-way ANOVA, F(3,28)=4.692, p=0.0089, R2=0.3345; post-hoc, 0 vs. 5 mg/ml dose p=0.0106, 0 vs. 7.5 mg/ml dose p=0.0432) (Figure 1A). In contrast, females exhibited a statistically significant increase from control levels only at the 5.0 mg/ml dose (One-way ANOVA, F(3,25)=3.072, p=0.0461, R2=0.2693; post-hoc 0 vs. 5 mg/ml dose p=0.0410) (Figure 1B). To verify that these responses were nicotine vapor-directed, the number of lever presses was examined between the active and inactive levers. We found a statistically significant preference for responding on the active lever across all nicotine doses in both males (Two-way ANOVA, Lever F(1,56)=129.2, p<0.0001, Dose F(3,56)=4.156, p=0.0100, Interaction F(3,56)=5.887, p=0.0014; post-hoc, active vs. inactive lever at 2.5 mg/ml p<0.0001, 5 mg/ml p<0.0001, 7.5 mg/ml p<0.0001) (Figure 1C) and females (Two-way ANOVA, Lever F(1,50)=152.6, p<0.0001, Dose F(3,50)=7.691, p=0.0003, Interaction F(3,50)=4.821, p=0.0050; post-hoc, active vs. inactive lever at 2.5 mg/ml p<0.0001, 5 mg/ml p<0.0001, 7.5 mg/ml p<0.0001) (Figure 1D). Importantly, no significant differences in lever pressing on the active and inactive lever were found in subjects only exposed to vehicle vapor (e.g., 0 mg/kg dose). This demonstrates a selective responding for nicotine vapor in both males and females across a range of doses, with preference in both sexes at the 5.0 mg/ml dose.
Figure 1. Nicotine vapor self-administration in male and female rats.
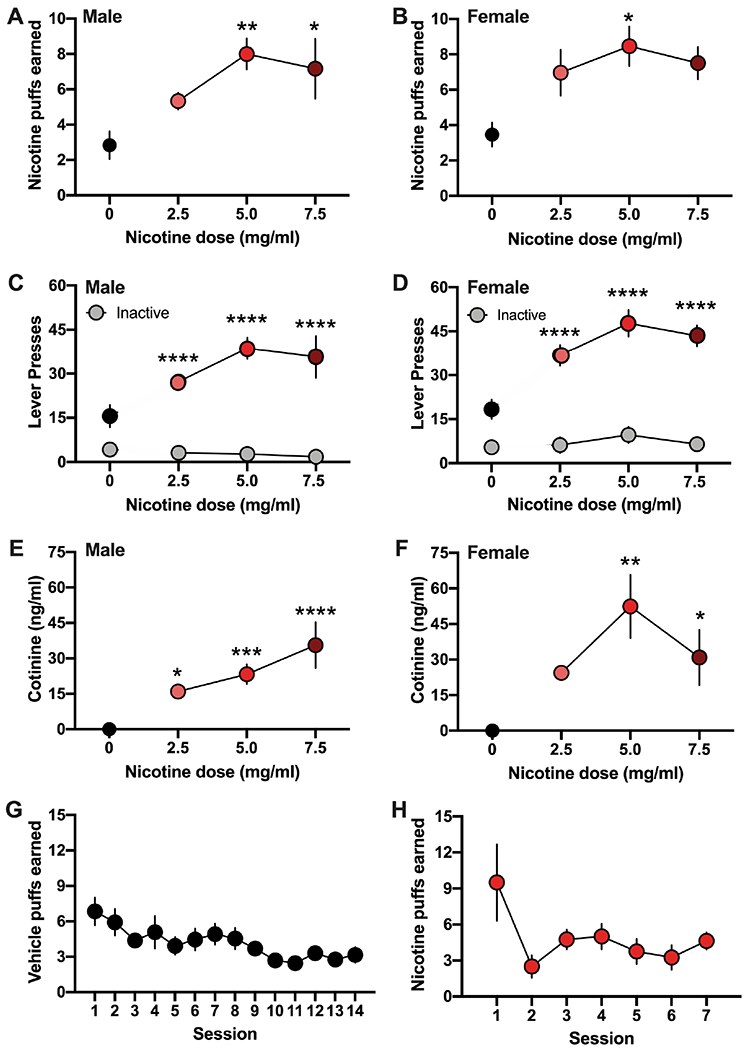
Subjects were examined for self-administration behavior at baseline (0 mg/ml, vehicle) and across a range of nicotine doses, which progressively increased from 2.5 to 5.0 to 7.5 mg/ml. (A) Male rats (n=8/group) self-administered a significantly increased number of vapor puffs at the 5 and 7.5 mg/ml doses compared to vehicle vapor. *p<0.05, **p<0.01 vs. vehicle control. (B) Female rats (n=5-8/group) exhibited increased responding for the delivery of nicotine vapor puffs at the 5.0 mg/kg dose compared to vehicle control. *p<0.05 vs. vehicle control. (C-D) When the number of presses on the active and inactive levers were examined, both males (C) and females (D) exhibited a clear dissociation between the active (colored circles) and inactive (grey circles) levers. This preference in responding on the active lever was present for all nicotine doses examined, but not present for the vehicle control. ****p<0.0001 active vs. inactive lever. (E-F) Blood samples were examined for cotinine levels 30 min after the final session of vapor treatment at each dose. (E) Cotinine levels progressively increased in male rats as the unit dose increased. *p<0.05, ***p<0.001, ****p<0.0001 vs. vehicle control. (F) In females, significant levels of cotinine were found in rats self-administering at the 5 and 7.5 mg/ml concentrations of nicotine. *p<0.05, **p<0.01 vs. vehicle control. (G) When rats (n=13 males/females combined) were only provided access to vehicle vapor, their behavioral lever pressing decreased ~3 puffs per hour session, indicating a low baseline level of responding. (H) When initially provided access to the higher 5 mg/ml dose, subjects (n=8 males/females combined) decreased their responding across sessions to near baseline levels, indicative of an aversive behavioral response when presented with this higher dose initially. Data are presented as mean ± SEM.
To further confirm the inhalation of nicotine vapor and blood bioavailability, subjects were examined for blood cotinine levels 30 min after each dose. Nicotine’s metabolite, cotinine, was detected across all doses of vapor nicotine self-administration. In males, a high level of blood cotinine was detected at the 2.5, 5.0 and 7.5 mg/ml doses (One-way ANOVA, F(3,18)=13.93, p<0.0001, R2 =0.6990; post-hoc, 0 vs. dose 2.5 mg/ml p=0.0437, 5.0 mg/ml p=0.0009, 7.5 mg/ml p<0.0001) (Figure 1E). In females, a high level of detectable cotinine was found at both the 5.0 and 7.5 mg/ml doses (One-way ANOVA, F(3,14)=7.516, p=0.0031, R2=0.6169; post-hoc, 0 vs. dose 5.0 mg/ml p=0.0011, 7.5 mg/ml p=0.0457) (Figure 1F). Since significant differences were not found in the level of cotinine with post-hoc comparisons among the different nicotine doses, the animals appear to have titrated their intake via modified breathing patterns with exposure and by adjusting their rate of lever pressing behavior to receive nicotine vapor.
Next, to further demonstrate that the rats were responding for nicotine vapor specifically, subjects were examined for self-administration of vehicle vapor alone across 14 sessions following food training. We found that both males and females extinguished their responding to low lever-pressing behavior levels. Given the similar behavioral response for males and females in this control condition, their data are combined into one graph (Figure 1G). This supports the notion that the higher levels of self-administration with nicotine vapor were due to the presence of nicotine. Finally, since prior protocols for intravenous nicotine self-administration found that access to a lower dose is necessary to support self-administration behavior prior to access at a moderate preferred dose, likely due to nicotine’s initial aversive effects34,39, we examined whether this would also occur for nicotine vapor inhalation. Therefore, after food training, subjects were given immediate access to the moderate 5 mg/ml dose of nicotine vapor (e.g., preferred dose in findings above). Under these conditions, the lever pressing behavior for vapor reward was more variable and extinguished across time to low levels (Figure 1H). These findings provide evidence of nicotine’s initial aversive effects at this moderate vapor dose and the need to provide a lower dose for initial acquisition, prior to access to a moderate/high dose, to subsequently allow for sustained self-administration behavior.
Comparison of nicotine metabolite level with different exposure routes
To ascertain a better understanding of the level of nicotine intake with vapor administration, we next examined passive vapor exposure to provide a controlled dosing schedule across the 1 hr session. This paradigm was compared to intravenous nicotine self-administration, the common technique in the field that provides an exact measure of nicotine bioavailability with volitional intake. After the 1 hr session in males, we found a significant increase in cotinine levels at the 30 min post-session time-point and 5.5 hrs thereafter (Repeated-measures, two-way ANOVA, Nicotine method F(1,22)=3.57, p=0.072; Time F(3,66)=56.78, p<0.0001; Interaction F(3,66)=2.24, p=0.092) (Figure 2A). Since the immediate effects on Day 1 could be due to stress-related interaction effects with the novel experimental conditions and/or since nicotine metabolism may change with repeated nicotine exposure across time, we further examined the same subjects after seven consecutive daily nicotine sessions. At the 30 min post-session time-point on Day 7, passive vapor subjects did not differ in the level of cotinine from those that self-administered intravenous nicotine, and further, these values did not significantly differ from Day 1 in male subjects (Figure 2A). In an initial cohort of males, we also examined whether differences would be present with passive vapor exposure if subjects were placed in the chambers individually or in pairs with a conspecific partner. We found no differences between conditions in the level of cotinine at the various time points, indicating that similar inhalation rates were maintained under the single- vs. dual-exposure conditions (Repeated-measures, two-way ANOVA, Nicotine method F(1,14)=0.0721, p=0.7922; Time F(2,28)=1.138, p=0.3349; Interaction F(2,28)=1.128, p=0.3379) (Figure 2B). Thus, these data were compiled with additional cohorts for the passive exposure group shown in Figure 2A, and subsequent exposure sessions in females were performed with two subjects per chamber. As a further analysis of the intravenous nicotine self-administration data, we examined whether the rats differed in the amount of nicotine self-administered on Day 1 or Day 7. There was an overall difference in the amount (mg/kg) of nicotine self-administered (Paired t-test, t(7)=2.366, p=0.0499, R2=0.4444) (Figure 2C). Therefore, we also examined the amount of nicotine self-administered across the 15-min increments of the session, and we found a significant increase only in the first 15 min epoch on Day 1 compared to Day 7 (Repeated measures, two-way ANOVA, Session Day F(1,56)=8.811, p=0.0044; Time F(3,56)=1.098, p=0.3575; Interaction F(3,56)=2.477, p=0.0707; post-hoc, Day 1 vs. Day 7 at 0-15’ p=0.0177) (Figure 2D).
Figure 2. Comparison of nicotine metabolite levels following passive vapor or intravenous nicotine self-administration (IVSA) in male rats.
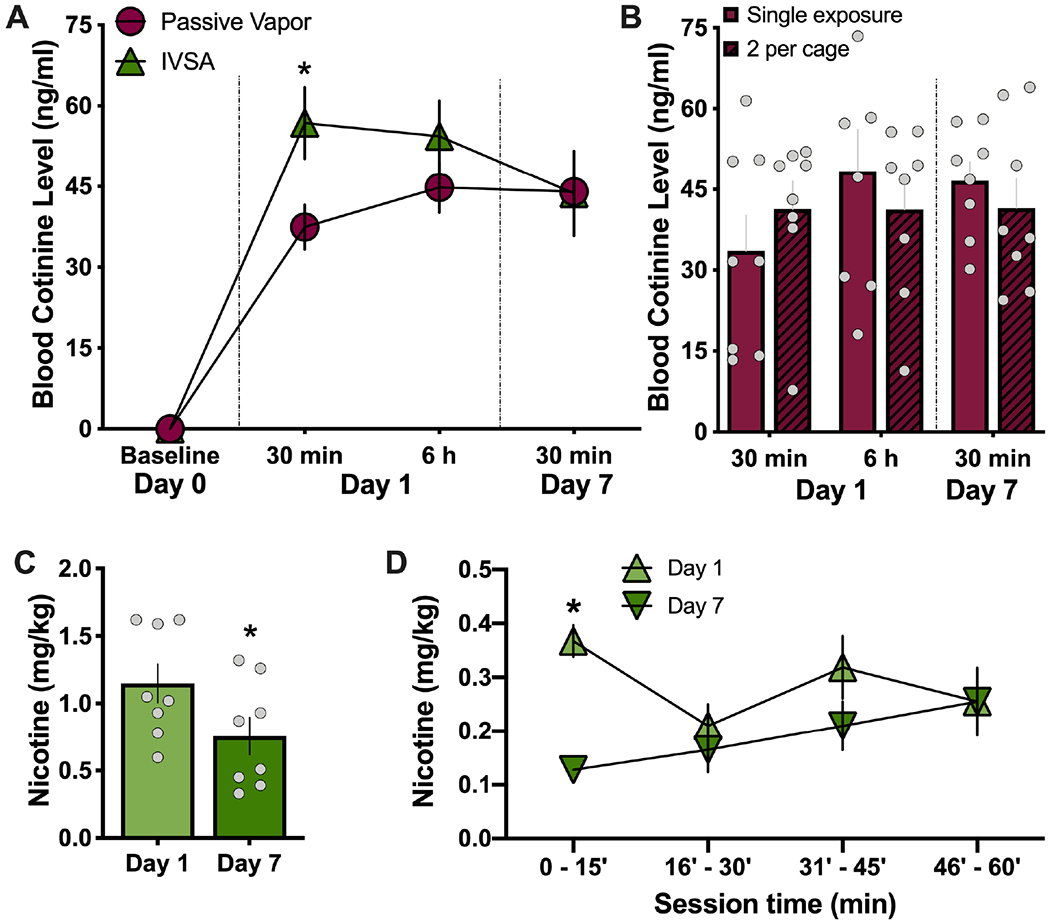
Blood samples were collected at baseline (pre-nicotine) or following 1 hr sessions of either nicotine vapor (5 mg/ml) or IVSA (0.03 mg/ml/infusion) from male rats. (A) Cotinine levels (n=8-16/group) were decreased with passive vapor exposure 30 min after the first session, as compared to IVSA subjects. However, these differences were not maintained at the later Day 1 time-point of 6 hr, or on Day 7 at the 30 min post-session time-point. *p<0.05 passive vapor vs. IVSA. (B) Male rats (n=8/group) exposed to nicotine vapor did not differ in their cotinine levels if placed in the chamber individually (single exposure) or with a cagemate (dual exposure) at all time-points assessed. (C) During the nicotine IVSA session (n=8), the rats self-administered a greater net amount of nicotine on Day 1 than on Day 7, which is consistent with the differences found in blood cotinine levels. *p<0.05 Day 1 vs. Day 7. (D) When IVSA nicotine intake was examined in 15-min intervals across the 1 hr session, it was found that the males exhibited a significant increase in responding only during the first 15 min interval, and thereafter, they maintained a consistent level of responding. *p<0.05 Day 1 vs. Day 7. Data are presented as mean ± SEM.
For the females, cotinine levels were also assessed following passive nicotine vapor or intravenous nicotine self-administration. Surprisingly, females demonstrated a dramatically different effect based on nicotine exposure route. Across all time points examined in females, passive nicotine vapor exposure resulted in a significantly lower level of cotinine than that found with intravenous nicotine self-administration (Repeated-measures, two-way ANOVA, Nicotine method F(1,10)=51.87, p<0.0001; Time F(3,30)=13.89, p<0.0001; Interaction F(3,30)=3.568, p=0.0255; post-hoc, passive vs. self-administration at time Day 1 - 30 min p=0.0002, 6 hr p=0.0488, Day 7 - 30 min p=0.0017) (Figure 3A). For intravenous nicotine self-administration, the females self-administered nicotine at a consistent rate, with no differences in nicotine intake between Day 1 and Day 7 (Repeated-measures, two-way ANOVA, Day session F(1,40)=3.843, p=0.0570; Time F(3,40)=0.5799, p=0.6317; Interaction F(3,40)=0.3305, p=0.8033) (Figure 3B), which corresponded to the similar cotinine levels at the varying blood sampling time-points. Given these unexpected differences between the two administration routes in females, we conducted an additional control study to exclude the possibility that the low levels of cotinine found in females was potentially due to the dual exposure condition. Following single or dual exposure in the chambers, blood was collected at the 30 min post-session time point. Females exposed to passive nicotine vapor alone in the chamber exhibited a similar level of blood cotinine as that found when two females were in the chamber together (Single exposure (n=4) 21.72 ± 5.923 (mean ± SEM); Dual exposure (n=4) 23.30 ± 3.071 (mean ± SEM); t(6) = 0.2372, p = 0.8204). Thus, these findings further support the striking differences in cotinine levels found between the intravenous nicotine self-administration and nicotine vapor exposure conditions for females.
Figure 3. Comparison of nicotine metabolite levels following passive vapor or intravenous nicotine self-administration (IVSA) in female rats.
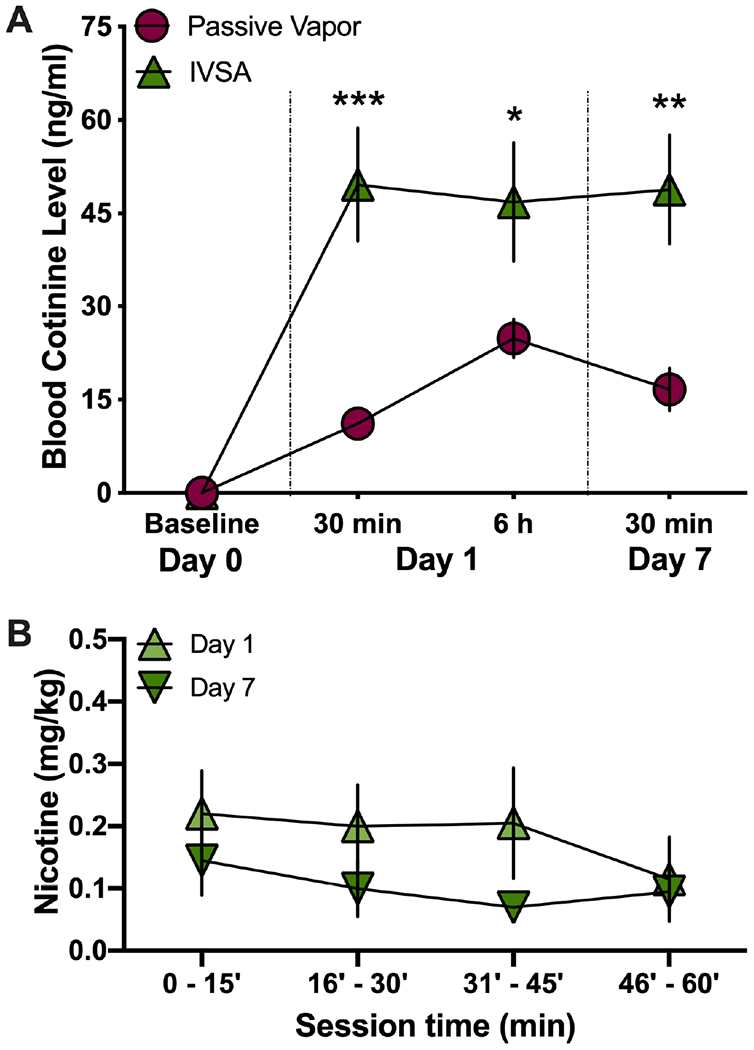
Blood samples were collected at baseline (pre-nicotine) or following 1 hr sessions of either nicotine vapor (5 mg/ml) or IVSA (0.03 mg/ml/infusion) from female rats (n=6/group). (A) Female cotinine levels were decreased following passive nicotine vapor exposure for all time-points, as compared to IVSA nicotine. *p<0.05, **p<0.01, ***p<0.001 passive vapor vs. IVSA. (B) Females did not differ in the amount of nicotine self-administered between Day 1 and Day 7. Data are presented as mean ± SEM.
Effect of nicotine administration route on behavioral locomotion
Peripheral experimenter-administered injections of nicotine at high doses are known to induce hypolocomotion, whereas conversely, lower doses result in hyperlocomotion. However, it has not been previously demonstrated what effect self-administered nicotine has on locomotor behavior in rats. Thus, the goal of this study was two-fold: (1) to examine whether intravenous nicotine self-administration alters locomotor behavior, consistent with prior results from experimenter-administered dosing and (2) to determine whether vapor and intravenous nicotine result in comparable locomotor effects given the similar cotinine levels noted in the above studies. Therefore, we examined whether the male and female rats would differ in their locomotor behavior immediately following the nicotine sessions on Day 1 and Day 7. In males, passive vapor exposure resulted in a significant increase in total distance travelled on the last day of nicotine exposure (Day 7), whereas the intravenous nicotine self-administration resulted in increased locomotion at both the acute and chronic session time points (Day 1 and Day 7) (Repeated measures, two-way ANOVA, Nicotine method F(1,10)=10.37, p=0.0092; Time F(2,20)=12.64, p=0.0003; Interaction F(2,20)=12.69, p=0.0003; post-hoc, passive - Day 0 vs. Day 7 p=0.0028, D1 vs. D7 p=0.0055, self-administration - Day 0 vs. Day 1 p<0.0001, Day 1 vs. Day 7 p=0.0148) (Figure 4A). To further understand these behavioral differences, we compared the locomotor behavior between groups across 5-min epochs during each 15-min locomotor test. Prior to drug exposure, the subjects did not differ in their distance travelled (Repeated measures two-way ANOVA, Nicotine method F(1,10)=3.930, p=0.0756; Time F(2,20)=9.011; p=0.0016; Interaction F(2,20)=0.0812, p=0.9224) (Figure 4B), which demonstrates no difference in the baseline level of responding for subjects between groups. On Day 1, the intravenous nicotine self-administration group displayed a significant increase in distance traveled at all time points assessed (Two-way ANOVA, Nicotine method F(1,10)=17.55, p=0.0019; Time F(2,20)=14.21; p=0.0001; Interaction F(2,20)=1.636, p=0.2197; post-hoc, passive vs. self-administration 0-5’ p=0.0008, 5-10’ p=0.0040) (Figure 4C). These findings in males indicate that self-administration of reinforcing doses of nicotine results in hyperlocomotion, which is greater than that found with passive vapor exposure at this timepoint. On Day 7, the groups displayed no difference in their increased locomotor effect across the 15-min session (Two-way ANOVA Nicotine method F(1,10)=1.823, p=0.2067; Time F(2,20)=11.78, p=0.0004; Interaction F(2,20)=0.1741, p=0.8415) (Figure 4D). Together, these findings suggest that a stress-related effect may have been imposed on Day 1 of the passive vapor exposure, and subsequently, habituation with chronic nicotine vapor sessions allowed for the unmasking of nicotine’s behavioral locomotor effect.
Figure 4. In male rats, nicotine vapor exposure and intravenous selfadministration (IVSA) both induce a hyperlocomotor effect in male rats.
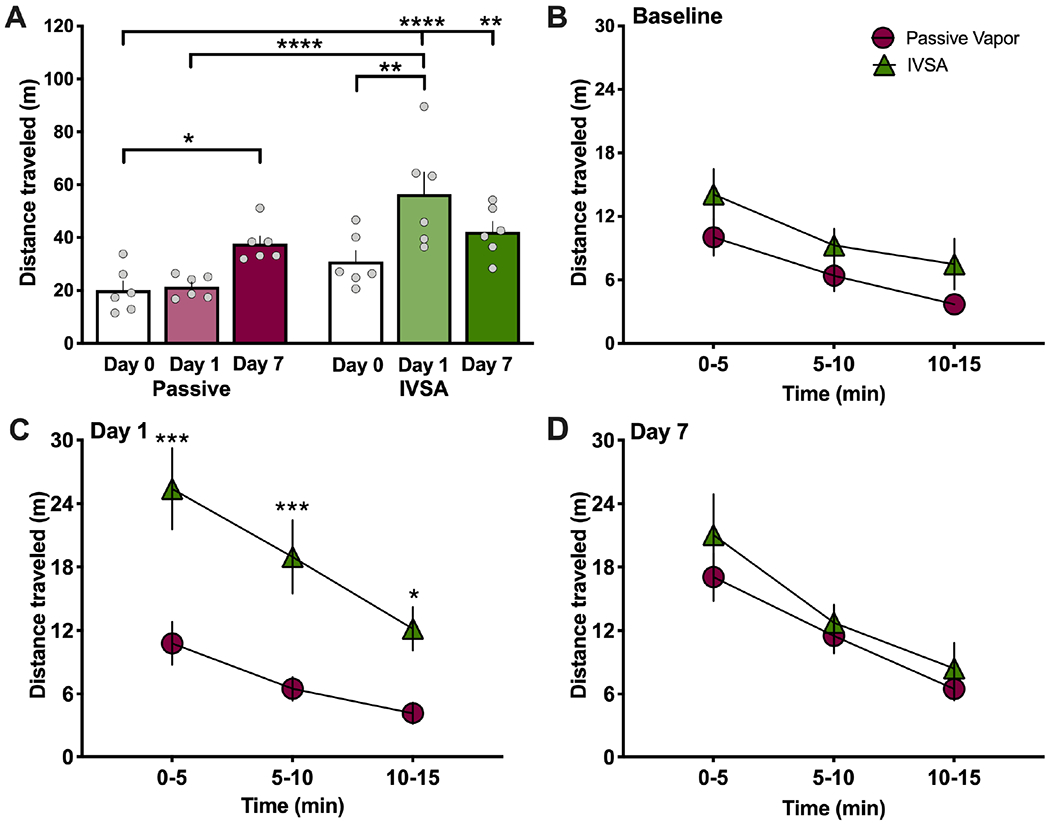
Male rats (n=6/group) were tested for nicotine-mediated locomotor effects in an open field chamber at baseline (pre-nicotine) and immediately after each nicotine sessions. (A) Passive nicotine vapor induced a significant increase in locomotor activity on Day 7 of exposure, whereas nicotine IVSA induced a significant increase on Day 1 of exposure, as compared to baseline levels. *p<0.05, **p<0.01, ****p<0.0001. (B-D) Locomotor behavior was analyzed in 5 min intervals for each session to compare the level of responding between nicotine passive vapor and IVSA. (B) At baseline, there were no differences between groups in distance traveled. (C) On Day 1, the IVSA nicotine group exhibited an increase in their distance traveled across the first two 5 min epochs. **p<0.01, ***p<0.001. (D) No significant differences were found between groups during the last day of treatment (Day 7). Data are presented as mean ± SEM.
Interestingly, while females exhibited a similar hyperlocomotor effect with intravenous nicotine self-administration, passive vapor exposure did not result in a significant change in behavior (Repeated measures, two-way ANOVA, Nicotine method F(1,9)=7.695, p=0.0216; Time F(2,18)=9.663, p=0.0014; Interaction F(2,18)=4.277, p=0.0302; post-hoc, self-administration Day 0 vs. Day 1 p=0.0017, Day 0 vs. Day 7 p=0.0012) (Figure 5A). When comparing the groups across the 5-min session epochs, the subjects did not differ at baseline levels (Repeated measures, two-way ANOVA, Nicotine method F(1,9)=0.0056, p=0.9421; Time F(2,18)=9.751, p=0.0014; Interaction F(2,18)=0.9168, p=0.4177) (Figure 5B). Differences were found between groups on Day 1 with increased locomotion for self-administration compared to passive nicotine vapor (Repeated measures, two-way ANOVA, Nicotine method F(1,9)=9.896, p=0.0118; Time F(2,18)=27.92, p<0.0001; Interaction F(2,18)=0.6084, p=0.5550; post-hoc, passive vs. IVSA 0-5’ p=0.0093, 5-10’ p=0.0311) (Figure 5C). However, on Day 7, no differences were found between the groups (Repeated measures, two-way ANOVA, Nicotine method F(1,9)=4.489, p=0.0551; Time F(2,18)=0.7410, p=0.4906; Interaction F(2,18)=1.313, p=0.2935) (Figure 5D).
Figure 5. In female rats, intravenous nicotine self-administration (IVSA), but not passive vapor exposure, induces a hyperlocomotor effect.
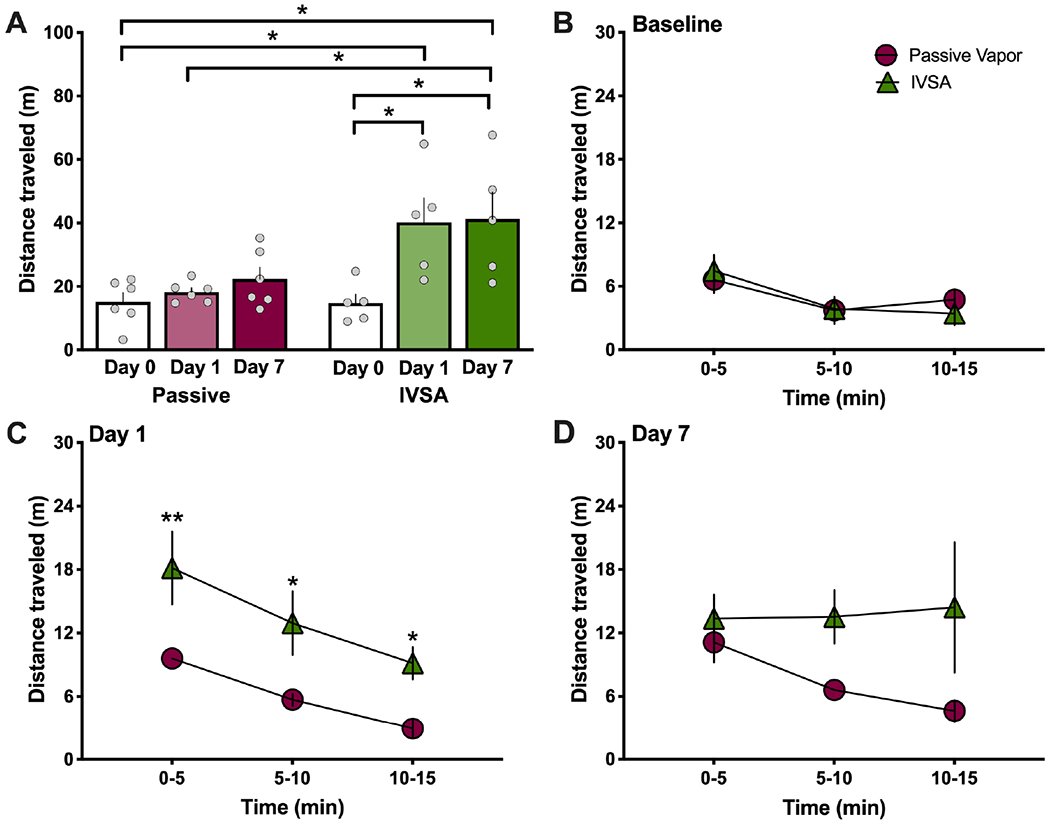
Female rats (n=5-6/group) were tested for nicotine-mediated locomotor effects in an open field chamber at baseline (pre-nicotine) and immediately after the nicotine sessions. (A) Passive nicotine vapor did not significantly alter locomotion, whereas nicotine IVSA induced a significant increase on Day 1 and Day 7 of exposure, as compared to baseline levels. **p<0.01. (B-D) Locomotor behavior was analyzed in 5 min intervals for each session to compare the level of responding between nicotine passive vapor and IVSA. (B) At baseline, there were no differences between groups in distance traveled. (C) On Day 1, the IVSA nicotine group exhibited an increase in their distance traveled across the first two 5 min epochs. *p<0.05, **p<0.01. (D) No significant differences were found between groups during the last day of treatment (Day 7). Data are presented as mean ± SEM.
Effect of nicotine on anxiety-related behaviors in the open field
As additional measures of behavior in the open field, we analyzed two parameters that have been associated with anxiety-related effects in rodents: the time spent in the center of the arena and freezing time. We found no differences in the time spent in the center of the open field between passive nicotine vapor and intravenous nicotine self-administration for males (Repeated measures, two-way ANOVA, Nicotine method F(1,10)=1.080, p=0.3231; Time F(2,20)=0.5051, p=0.6109; Interaction F(2,20)=0.0609, p=0.9411) (Figure 6A) and females (Repeated measures, two-way ANOVA, Nicotine method F(1,9)=1.978, p=0.1932; Time F(2,18)=0.1878, p=0.8304; Interaction F(2,18)=0.6601, p=0.5289) (Figure 6B). Similarly, no differences were found between the groups in freezing behavior for males (Repeated measures, two-way ANOVA, Nicotine method F(1,10)=0.1805, p=0.6799; Time F(2,20)=0.3168, p=0.7321, Interaction F(2,20)=0.4652, p=0.6347) (Figure 6C) and females (Repeated measures, two-way ANOVA, Nicotine method F(1,9)=0.8220, p=0.3882; Time F(2,18)=1.041, p=0.3733, Interaction F(2,18)=0.6236, p=0.5472) (Figure 6D).
Figure 6. No effect of nicotine on anxiety-related behaviors in the open field.
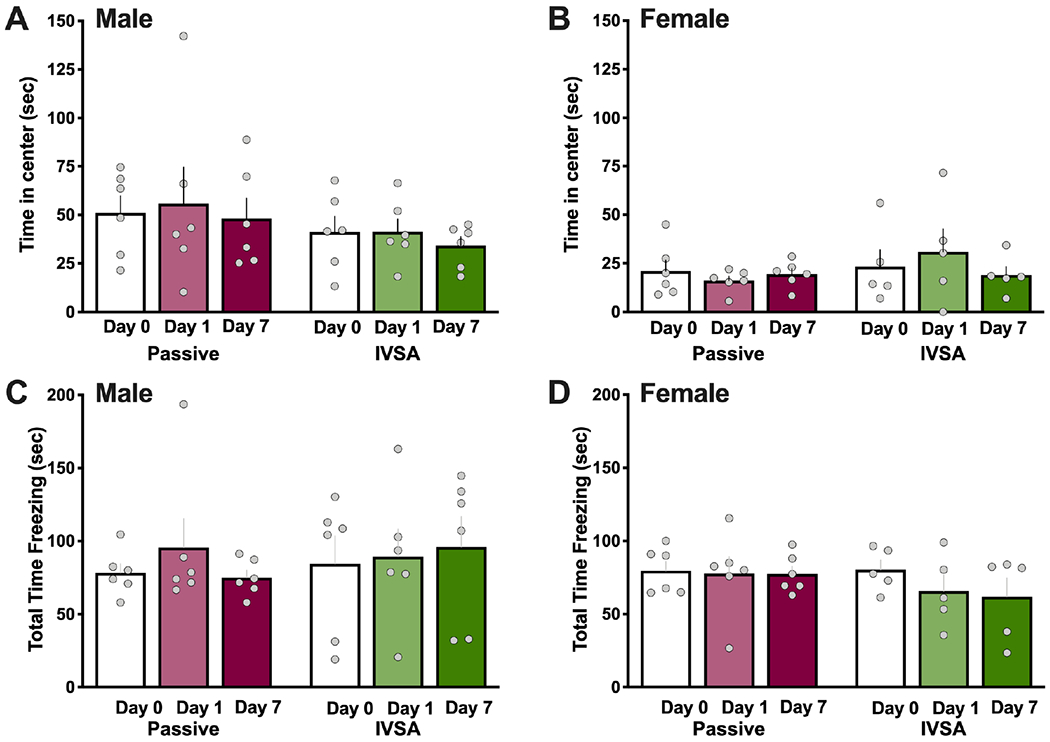
Male (n=6/group) and female (n=5-6/group) subjects were further examined for indicators of behaviors associated with anxiety on Day 0 (baseline, no nicotine), and immediately after nicotine sessions on Day 1 and Day 7. (A-B) Time in the center of the open field did not differ across all exposures or time-points for both males (A) and females (B). (C-D) Freezing time was examined but no differences were found across all exposures or time-points for both males (C) and females (D). Data are presented as mean ± SEM.
Discussion
In these studies, we developed and validated a reliable procedure for vapor self-administration of nicotine in rats. In establishing this method, we compared to the ‘gold-standard’ procedure of voluntary drug intake in animal models - intravenous nicotine self-administration. Both males and females exhibited robust and selective responding for vaporized nicotine at the dose of 5 mg/ml, a dose which also elicited a high level of nicotine’s metabolite cotinine in blood plasma within both sexes. These effects were specific to the presence of nicotine, as behavioral responding was not maintained when subjects were provided access to vehicle puffs alone. Similar levels of cotinine were observed following passive vapor or intravenous drug administration in males, indicating vapor exposure can elicit comparable levels of nicotine as that found with intravenous self-administration. Interestingly, lower levels of cotinine were detected in females with passive vapor exposure as compared to intravenous self-administration, suggesting either differential inhalation rates for vapor or altered nicotine metabolism with lung inhalation.
Importantly, the current studies provide an advance over prior investigations in the field. For instance, a recent study from Smith et al.28 employed a vapor self-administration method in rats. However, in their studies, they were not able to observe dissociative responding between the active and inactive lever, suggesting that the rats did not discriminate the drug-delivering lever. In self-administration protocols, a significant difference between the active and inactive levers supports the contention of directed drug-seeking behavior, consistent with what we found in the current studies. The reasons for such a difference may be either (1) different chamber environments, or (2) effort required to elicit drug delivery. While Smith and colleagues28 utilized a low-effort fixed-ratio 1 (FR1) schedule of reinforcement, our studies employed a more effortful fixed ratio 5 (FR5) schedule. Thus, by imposing a higher effort to receive vaporized nicotine delivery, the rats may have been able to more clearly dissociate the contributions of pressing each individual lever. This is similar to our prior findings with intravenous nicotine self-administration in mice34 and a recent study with self-administration of vaporized cannabis extracts under increasing schedules of reinforcement in rats40.
The presence of nicotine following systemic administration is commonly assessed by measuring its main metabolite, cotinine14,41. After inhalation, nicotine is absorbed rapidly into the lungs, resulting in a high blood nicotine concentration that quickly declines due to absorption into tissues, including the brain. An average cigarette delivers roughly 10–30 μg/kg, typically resulting in 10–50 ng/ml peak nicotine plasma levels11,15. In our study, cotinine concentrations in the plasma after vapor and intravenous administration were in the range of ~10-60 ng/ml for both males and females, similar also to that found in prior passive vapor exposure studies27,29.
Approximately 70– 80% of nicotine is converted to cotinine42,43, which supports the level of vapor nicotine inhalation occurring with the current protocol conditions. Interestingly, pulmonary tissue appears to function as a short-term depot for nicotine binding44, which may in part explain the sustained levels of cotinine found at the 6 h time point in our current investigations.
The lower levels of cotinine found at the 30-min post-session time point in males with passive vapor and at all time points in females may be further explained by differences in inhalation and/or stress-related behavioral effects. First, prior studies have found that nicotine detection in the arterial blood is slower if through the lung tissue, as compared to systemic injection45. Second, the pH of smoke particulate may modulate pulmonary absorption46; although the nicotine solution pH was adjusted to 7.4 in these studies, heating of the solution for vapor delivery may alter the pH upon smoke delivery. Third, male and female rats have been shown to exhibit differences in nicotine metabolism47, and compared to men, women exhibit a shorter half-life and a quicker elimination of nicotine to yield a significantly higher elimination rate value48,49. Thus, nicotine metabolism differences may have partially contributed to the effects across doses. Finally, stress-related behavioral effects may alter breathing patterns, thereby modulating the net amount of nicotine inhaled through the lungs. In particular, this effect may explain the differences found between day 1 and day 7 of exposure, as the animals became habituated to the passive vapor puffs across 7 consecutive sessions and thus were likely experiencing lower stress levels. Interestingly, a recent study has demonstrated that females exhibit an increase in α5 nicotinic receptor subunit expression in the interpeduncular nucleus following chronically administered nicotine, which was also correlated with anxiety-like behavior50. Thus, differential effects related to an interaction between nicotine modulation of brain function and anxiety-related behaviors may underlie the effects found in females.
We also found increased locomotion in males with both passive inhalation and intravenous nicotine self-administration. These findings are similar to that previously found with passive vapor exposure in male rats29. In contrast, females only exhibited increased locomotion following intravenous nicotine self-administration. Importantly, these behavioral findings are supported by the similar levels of blood cotinine found in males with both methods of nicotine exposure. Nicotine administration has been shown to increase plasma adrenocorticotropic hormone (ACTH) and corticosterone51, leading to an increase in locomotion through dopamine release in the nucleus accumbens15,52,53. In contrast, females exhibited lower levels of cotinine with passive exposure, which is reflective of the lack of locomotor effect. Given that experimenter-administered nicotine may induce hypolocomotion at high doses, or hyperlocomotion at lower doses, these findings support the notion that lower doses in the hyperlocomotion range are more reinforcing. It should also be noted that with experimenter administered nicotine, hyperlocomotor effects are more likely to occur with repeated exposure54, which is also consistent with our findings in males for passive vapor exposure and further supports the notion of potential stress-related effects in the first vapor session.
Female and male rats have been previously shown to differ in nicotine-mediated reward and reinforcement. For these studies, we sought to establish and validate a vapor self-administration protocol, but given that females and males have been shown to differ in various assessments and with nicotine metabolism, we sought to examine each sex independently. However, it is still worth considering potential sex differences as a foundation for future studies. For instance, compared to male rats, nicotine’s rewarding effects are enhanced in females in the conditioned place preference paradigm55, females acquire intravenous nicotine self-administration quicker at a low nicotine dose56, females demonstrate higher elasticity in demand for nicotine in a behavioral economics assessment57, and females exhibit higher motivation to obtain nicotine infusions with the progressive ratio schedule or under extended access conditions (23 hr)56,58. Age-related effects have also been found in the reinforcing effects of nicotine on brain reward thresholds, with adolescent females exhibiting a higher sensitivity to nicotine compared to males59. Interestingly, with specific relevance to nicotine vapor inhalation, a recent study in humans has found that an e-cigarette puff results in a higher concentration of brain nicotine in women compared to men, as demonstrated with 11C-nicotine PET60. In our study, although the males and females exhibited a similar rate of nicotine vapor self-administration across the dose response function, it is therefore possible that differences were present in brain nicotine concentration between sexes. Thus, it will be interesting in future studies to directly compare males and females under varying experimental conditions.
In addiction-related processes, stress is known to be a key factor mediating drug intake and relapse-related behaviors61, and among these factors, social isolation represents a well-established model of chronic stress, which has been shown to interfere with the mechanisms underlying dependence for different drugs, such as amphetamine62, cocaine63, ethanol64 and nicotine65. Here, we found similar levels of blood cotinine with one or two rats present during vapor delivery in the chamber. This is an important finding since it establishes the validity of group housing for vapor drug exposure, thereby overcoming the issue of single administration necessary with intravenous self-administration paradigms due to the presence of the tubing attachment within the operant chamber. Future studies will be of great interest that focus on the impact of social environment on drug-related effects with this vapor exposure method.
In conclusion, we have developed a robust and reliable method of vapor nicotine selfadministration that has high translational relevance to human e-cigarette and tobacco intake. The protocol for vapor exposure was shown to result in comparable blood levels of nicotine’s metabolite cotinine, compared to intravenous nicotine selfadministration. We have also found intriguing differences within each sex based on the route of nicotine intake, and such differences will be important to investigate in future studies to ascertain a better understanding of the underlying mechanisms for lung and/or brain function. Further, it will be important in future studies to employ the vapor protocol under long-term exposure conditions and with solid vape cartridges that contain a higher dose of vapor nicotine to better elucidate behavioral and metabolic changes occurring with vaporized nicotine inhalation. Since vapor exposure requires no surgical manipulations, this delivery method will also be optimal to examine a range of ages (e.g., young, adolescent, young adult, adult, aged) and interactive effects with disease models (e.g., Alzheimer’s disease, COVID-19 viral infection, etc.). Through these efforts, the scientific findings ascertained may better inform tobacco/nicotine regulatory science for effective control polices66 and may also lead to more efficacious therapeutic interventions for nicotine dependence.
Acknowledgements
This work was supported by the National Institutes of Health NIDA grants DA039658 and DA051831 (C.D.F.), NIDA Summer Research Internship Program with DA039658 (M.M.R., C.D.F.), Tobacco-Related Disease Research (TRDRP) program grant T30FT0967 (V.L.), Irvine Center for Addiction Neuroscience (ICAN) Track2 funding from the Office of Research at UC Irvine, Initiative for Maximizing Student Development (MBRS-IMSD) Program NIH Grant GM-055246 (A.C.) and Broadening Research Achievement in Neurosciences (BRAiN) for a Diverse Workforce NIH Grant NS-112136 (A.C.).
References
- 1.Jha PCF, Moore J, et al. Tobacco Addiction. Jamison DT, Breman JG, Measham AR, et al, editors Disease Control Priorities in Developing Countries 2nd edition Washington (DC): The International Bank for Reconstruction and Development / The World Bank; 2006. Chapter 46. [PubMed] [Google Scholar]
- 2.Henningfield JE, Miyasato K, Jasinski DR. Abuse liability and pharmacodynamic characteristics of intravenous and inhaled nicotine. The Journal of pharmacology and experimental therapeutics . 1985;234(1):1–12. [PubMed] [Google Scholar]
- 3.Johnson LM. Tobacco smoking and nicotine. Lancet . 1942;2:742. [Google Scholar]
- 4.Henningfield JE, Miyasato K, Jasinski DR. Cigarette smokers self-administer intravenous nicotine. Pharmacology, biochemistry, and behavior . 1983; 19(5):887–890. [DOI] [PubMed] [Google Scholar]
- 5.Rose JE, Behm FM, Westman EC, Bates JE. Mecamylamine acutely increases human intravenous nicotine self-administration. Pharmacology, biochemistry, and behavior . 2003;76(2):307–313. [DOI] [PubMed] [Google Scholar]
- 6.Goldberg SR, Spealman RD, Goldberg DM. Persistent behavior at high rates maintained by intravenous self-administration of nicotine. Science . 1981. ;214(4520):573–575. [DOI] [PubMed] [Google Scholar]
- 7.Fowler CD, Lu Q, Johnson PM, Marks MJ, Kenny PJ. Habenular alpha5 nicotinic receptor subunit signalling controls nicotine intake. Nature . 2011. ;471(7340):597–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ponzoni L, Moretti M, Sala M, et al. Different physiological and behavioural effects of e-cigarette vapour and cigarette smoke in mice. European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology . 2015;25(10):1775–1786. [DOI] [PubMed] [Google Scholar]
- 9.Kozlowski LT, Mehta NY, Sweeney CT, et al. Filter ventilation and nicotine content of tobacco in cigarettes from Canada, the United Kingdom, and the United States. Tobacco control . 1998;7(4):369–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Armitage AK, Dollery CT, George CF, Houseman TH, Lewis PJ, Turner DM. Absorption and metabolism of nicotine from cigarettes. Br Med J . 1975;4(5992):313–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benowitz NL, Jacob P 3rd. Daily intake of nicotine during cigarette smoking. Clinical pharmacology and therapeutics . 1984;35(4):499–504. [DOI] [PubMed] [Google Scholar]
- 12.Benowitz NL, Porchet H, Sheiner L, Jacob P 3rd. Nicotine absorption and cardiovascular effects with smokeless tobacco use: comparison with cigarettes and nicotine gum. Clinical pharmacology and therapeutics . 1988;44(1):23–28. [DOI] [PubMed] [Google Scholar]
- 13.Li P, Beck WD, Callahan PM, Terry AV Jr., Bartlett MG. Pharmacokinetics of cotinine in rats: a potential therapeutic agent for disorders of cognitive function. Pharmacol Rep . 2015;67(3):494–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen YC, Fowler JP, Wang J, et al. The Novel CYP2A6 Inhibitor, DLCI-1, Decreases Nicotine Self-Administration in Mice. The Journal of pharmacology and experimental therapeutics . 2020;372(1):21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matta SG, Balfour DJ, Benowitz NL, et al. Guidelines on nicotine dose selection for in vivo research. Psychopharmacology . 2007;190(3):269–319. [DOI] [PubMed] [Google Scholar]
- 16.Grana R, Benowitz N, Glantz SA. E-cigarettes: a scientific review. Circulation . 2014;129(19):1972–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adkison SE, O’Connor RJ, Bansal-Travers M, et al. Electronic nicotine delivery systems: international tobacco control four-country survey. American journal of preventive medicine . 2013;44(3):207–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pearson JL, Richardson A, Niaura RS, Vallone DM, Abrams DB. e-Cigarette awareness, use, and harm perceptions in US adults. Am J Public Health . 2012;102(9):1758–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arrazola RA, Neff LJ, Kennedy SM, et al. Tobacco use among middle and high school students--United States, 2013. MMWR Morbidity and mortality weekly report . 2014;63(45):1021–1026. [PMC free article] [PubMed] [Google Scholar]
- 20.Litt MD, Duffy V, Oncken C. Cigarette smoking and electronic cigarette vaping patterns as a function of e-cigarette flavourings. Tobacco control . 2016;25(Suppl 2):ii67–ii72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alsharari sD, King JR, Nordman JC, et al. Effects of Menthol on Nicotine Pharmacokinetic, Pharmacology and Dependence in Mice. PloS one . 2015;10(9):e0137070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clearing the smoke. Nat Neurosci . 2014;17(8):1013. [DOI] [PubMed] [Google Scholar]
- 23.Correa JB, Ariel I, Menzie NS, Brandon TH. Documenting the emergence of electronic nicotine delivery systems as a disruptive technology in nicotine and tobacco science. Addictive behaviors . 2017;65:179–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Glasser AM, Collins L, Pearson JL, et al. Overview of Electronic Nicotine Delivery Systems: A Systematic Review. American journal of preventive medicine . 2017;52(2):e33–e66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.George O, Grieder TE, Cole M, Koob GF. Exposure to chronic intermittent nicotine vapor induces nicotine dependence. Pharmacology, biochemistry, and behavior . 2010;96(1):104–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith D, Aherrera A, Lopez A, et al. Adult Behavior in Male Mice Exposed to E-Cigarette Nicotine Vapors during Late Prenatal and Early Postnatal Life. PloS one . 2015;10(9):e0137953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lefever TW, Lee YO, Kovach AL, et al. Delivery of nicotine aerosol to mice via a modified electronic cigarette device. Drug and alcohol dependence . 2017;172:80–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith LC, Kallupi M, Tieu L, et al. Validation of a nicotine vapor selfadministration model in rats with relevance to electronic cigarette use. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology . 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Javadi-Paydar M, Kerr TM, Harvey EL, Cole M, Taffe MA. Effects of nicotine and THC vapor inhalation administered by an electronic nicotine delivery system (ENDS) in male rats. Drug and alcohol dependence . 2019;198:54–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perkins KA, Donny E, Caggiula AR. Sex differences in nicotine effects and self-administration: review of human and animal evidence. Nicotine & tobacco research : official journal of the Society for Research on Nicotine and Tobacco . 1999;1(4):301–315. [DOI] [PubMed] [Google Scholar]
- 31.Kota D, Martin BR, Damaj MI. Age-dependent differences in nicotine reward and withdrawal in female mice. Psychopharmacology . 2008;198(2):201–210. [DOI] [PubMed] [Google Scholar]
- 32.Perkins KA. Smoking cessation in women. Special considerations. Cns Drugs . 2001;15(5):391–411. [DOI] [PubMed] [Google Scholar]
- 33.O’Dell LE, Torres OV. A mechanistic hypothesis of the factors that enhance vulnerability to nicotine use in females. Neuropharmacology . 2014;76 Pt B:566–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fowler CD, Kenny PJ. Intravenous nicotine self-administration and cueinduced reinstatement in mice: effects of nicotine dose, rate of drug infusion and prior instrumental training. Neuropharmacology . 2011;61(4):687–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kenny PJ, Markou A. Nicotine self-administration acutely activates brain reward systems and induces a long-lasting increase in reward sensitivity. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology . 2006;31 (6):1203–1211. [DOI] [PubMed] [Google Scholar]
- 36.Tuesta LM, Chen Z, Duncan A, et al. GLP-1 acts on habenular avoidance circuits to control nicotine intake. Nat Neurosci . 2017;20(5):708–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lallai V, Grimes N, Fowler JP, et al. Nicotine Acts on Cholinergic Signaling Mechanisms to Directly Modulate Choroid Plexus Function. eNeuro . 2019;6(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen E, Lallai V, Sherafat Y, et al. Altered Baseline and Nicotine-Mediated Behavioral and Cholinergic Profiles in ChAT-Cre Mouse Lines. The Journal of neuroscience : the official journal of the Society for Neuroscience . 2018;38(9):2177–2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fowler CD, Kenny PJ. Nicotine aversion: Neurobiological mechanisms and relevance to tobacco dependence vulnerability. Neuropharmacology . 2014;76 Pt B:533–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Freels TG, Baxter-Potter LN, Lugo JM, et al. Vaporized cannabis extracts have reinforcing properties and support conditioned drug-seeking behavior in rats. The Journal of neuroscience : the official journal of the Society for Neuroscience . 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hukkanen J, Jacob P 3rd, Benowitz NL. Metabolism and disposition kinetics of nicotine. Pharmacological reviews . 2005;57(1):79–115. [DOI] [PubMed] [Google Scholar]
- 42.Benowitz NL, Hukkanen J, Jacob P 3rd. Nicotine chemistry, metabolism, kinetics and biomarkers. Handb Exp Pharmacol . 2009(192):29–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Benowitz NL, Jacob P 3rd, Fong I, Gupta S. Nicotine metabolic profile in man: comparison of cigarette smoking and transdermal nicotine. J Pharmacol Exp Ther . 1994;268(1):296–303. [PubMed] [Google Scholar]
- 44.Brewer BG, Roberts AM, Rowell PP. Short-term distribution of nicotine in the rat lung. Drug and alcohol dependence . 2004;75(2):193–198. [DOI] [PubMed] [Google Scholar]
- 45.Rose JE, Behm FM, Westman EC, Coleman RE. Arterial nicotine kinetics during cigarette smoking and intravenous nicotine administration: implications for addiction. Drug and alcohol dependence . 1999;56(2):99–107. [DOI] [PubMed] [Google Scholar]
- 46.Pankow JF. A consideration of the role of gas/particle partitioning in the deposition of nicotine and other tobacco smoke compounds in the respiratory tract. Chem Res Toxicol . 2001;14(11):1465–1481. [DOI] [PubMed] [Google Scholar]
- 47.Kyerematen GA, Owens GF, Chattopadhyay B, deBethizy JD, Vesell ES. Sexual dimorphism of nicotine metabolism and distribution in the rat. Studies in vivo and in vitro. Drug Metab Dispos . 1988;16(6):823–828. [PubMed] [Google Scholar]
- 48.Benowitz NL. Nicotine addiction. Prim Care . 1999;26(3):611–631. [DOI] [PubMed] [Google Scholar]
- 49.Prather RD, Tu TG, Rolf CN, Gorsline J. Nicotine pharmacokinetics of Nicoderm (nicotine transdermal system) in women and obese men compared with normal-sized men. J Clin Pharmacol . 1993;33(7):644–649. [DOI] [PubMed] [Google Scholar]
- 50.Correa VL, Flores RJ, Carcoba LM, Arreguin MC, O’Dell LE. Sex differences in cholinergic systems in the interpeduncular nucleus following nicotine exposure and withdrawal. Neuropharmacology . 2019;158:107714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Matta SG, Beyer HS, McAllen KM, Sharp BM. Nicotine elevates rat plasma ACTH by a central mechanism. The Journal of pharmacology and experimental therapeutics . 1987;243(1):217–226. [PubMed] [Google Scholar]
- 52.Caggiula AR, Epstein LH, Antelman SM, et al. Conditioned tolerance to the anorectic and corticosterone-elevating effects of nicotine. Pharmacology, biochemistry, and behavior . 1991;40(1):53–59. [DOI] [PubMed] [Google Scholar]
- 53.Rouge-Pont F, Deroche V, Le Moal M, Piazza PV. Individual differences in stress-induced dopamine release in the nucleus accumbens are influenced by corticosterone. The European journal of neuroscience . 1998;10(12):3903–3907. [DOI] [PubMed] [Google Scholar]
- 54.Acute Ksir C. and chronic nicotine effects on measures of activity in rats: a multivariate analysis. Psychopharmacology . 1994;115(1–2):105–109. [DOI] [PubMed] [Google Scholar]
- 55.Torres OV, Natividad LA, Tejeda HA, Van Weelden SA, O’Dell LE. Female rats display dose-dependent differences to the rewarding and aversive effects of nicotine in an age-, hormone-, and sex-dependent manner. Psychopharmacology . 2009;206(2):303–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Donny EC, Caggiula AR, Rowell PP, et al. Nicotine self-administration in rats: estrous cycle effects, sex differences and nicotinic receptor binding. Psychopharmacology . 2000;151 (4):392–405. [DOI] [PubMed] [Google Scholar]
- 57.Chellian R, Wilson R, Polmann M, Knight P, Behnood-Rod A, Bruijnzeel AW. Evaluation of Sex Differences in the Elasticity of Demand for Nicotine and Food in Rats. Nicotine & tobacco research : official journal of the Society for Research on Nicotine and Tobacco . 2020;22(6):925–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Flores RJ, Uribe KP, Swalve N, O’Dell LE. Sex differences in nicotine intravenous self-administration: A meta-analytic review. Physiology & behavior . 2019;203:42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xue S, Behnood-Rod A, Wilson R, Wilks I, Tan S, Bruijnzeel AW. Rewarding Effects of Nicotine in Adolescent and Adult Male and Female Rats as Measured Using Intracranial Self-stimulation. Nicotine & tobacco research : official journal of the Society for Research on Nicotine and Tobacco . 2020;22(2):172–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Solingapuram Sai KK, Zuo Y, Rose JE, et al. Rapid Brain Nicotine Uptake from Electronic Cigarettes. J Nucl Med . 2020;61(6):928–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Koob GF, Buck CL, Cohen A, et al. Addiction as a stress surfeit disorder. Neuropharmacology . 2014;76 Pt B:370–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Whitaker LR, Degoulet M, Morikawa H. Social deprivation enhances VTA synaptic plasticity and drug-induced contextual learning. Neuron . 2013;77(2):335–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Howes SR, Dalley JW, Morrison CH, Robbins TW, Everitt BJ. Leftward shift in the acquisition of cocaine self-administration in isolation-reared rats: relationship to extracellular levels of dopamine, serotonin and glutamate in the nucleus accumbens and amygdala-striatal FOS expression. Psychopharmacology . 2000;151(1):55–63. [DOI] [PubMed] [Google Scholar]
- 64.Lallai V, Manca L, Dazzi L. Social Isolation Blunted the Response of Mesocortical Dopaminergic Neurons to Chronic Ethanol Voluntary Intake. Front Cell Neurosci . 2016;10:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Matta SG, Foster CA, Sharp BM. Selective administration of nicotine into catecholaminergic regions of rat brainstem stimulates adrenocorticotropin secretion. Endocrinology . 1993;133(6):2935–2942. [DOI] [PubMed] [Google Scholar]
- 66.Jensen KP, DeVito EE, Sofuoglu M. How Intravenous Nicotine Administration in Smokers Can Inform Tobacco Regulatory Science. Tobacco regulatory science . 2016;2(4):452–463. [DOI] [PMC free article] [PubMed] [Google Scholar]


