1. Introduction
In daily life, heat, cold, and pressure at noxious intensities can elicit physical pain with different qualities. A vast clinical literature on human brain lesions giving rise to specific pain and temperature deficits supports the existence of specialized pain networks [3; 26; 27]. Modern functional MRI (fMRI), transcranial magnetic stimulation (TMS), and analysis of focal lesion studies in patients have linked intensity of pain sensations [20; 23; 37; 39; 40; 55] and the gating of painful and nonpainful information to high order brain regions [37; 51; 52]. There is also evidence supporting that brain regions engaged in painful and nonpainful sensation are spatially separated [6; 45; 51], such as in the anterior and posterior SII regions, respectively [51]. Consistently nociceptive fMRI responses were also detected in SII, the posterior granular insula and its adjacent medial operculum [1; 24; 25; 35; 36]. There have also been studies exploring how nociceptive, heat, cold, or mechanical inputs are represented in the operculum [2; 41-43; 63]. What is not completely clear is whether nociceptive pain areas in the operculo-insular cortex are spatially segregated or intermingled with other sensory modalities.
The present study aims to address this remaining question by using submillimeter resolution fMRI and intracranial electrophysiology in nonhuman primates. We have previously identified functionally discrete multiple heat nociceptive patches in the secondary somatosensory (SII) cortex, area 7b, retroinsula and posterior insula [6; 56], and spatially separated heat and touch patches in this region of the lateral sulcus [5; 6; 57]. New work analysis showed that the nociceptive SII and posterior insula serve as key hubs of the nociceptive networks that connect the somatosensory cortex to brain regions that are implicated in processing affective and cognitive aspects of pain in humans [56]. Consistent with these observations [6; 53; 55; 56], nociresponsive neurons were isolated in these deep cortical regions [10; 15; 16]. To date, however, there is limited knowledge as to how the neurons in the parietal operculum process the different sensory qualities and intensities of nociceptive inputs evoked by heat, cold, or mechanical noxious stimulation and whether these neurons are organized in functionally distinct cortical modules that are commonly observed in other sensory modalities (i.e., touch and vision). Nonhuman primate offers a unique experimental model that allows directly relating neuronal responses to fMRI observations. Here, we focus on studying the SII cortex because of its high functional importance and the paucity of functional neurophysiological studies of this area. Specifically, we first localized the nociceptive heat region in the upper bank of lateral sulcus (where S2 resides) by blood oxygenation level dependent (BOLD) fMRI, and then with microelectrode neurophysiology: (1) systemically characterized the receptive field features and firing properties of nociceptive neurons from this region in response to graded heat and cold temperatures in both noxious and innocuous ranges, and (2) examined their spatial somatotopic relationship to surrounding innocuous tactile neurons. We used “nociceptive stimuli “and “nociception” in the manuscript since our research subjects are studied under general anesthesia.
2. Materials and Methods
2.1. Animal preparation
Eight male squirrel monkeys (Saimiri sciureus) were included in this study. For the fMRI experiments, we used standard procedures published previously [7; 8]. Each animal was sedated with ketamine hydrochloride (10mg/kg)/atropine (0.05mg/kg) and maintained on mechanical ventilation with isoflurane anesthesia (0.6-1.0% during data collection) delivered in a 30:70 O2:N2O mixture. After intubation, the animal was placed in a custom-designed MR cradle and the head secured with ear and head bars. Dextrose (2.5%) in saline was infused intravenously (3ml/kg/hr) throughout the imaging session to prevent dehydration and provide caloric energy. Vital signs of SpO2 and heart rate (Nonin, Plymouth, MN), ECG, ET-CO2 (22-26 mmHg; Surgivet, Waukesha, WI), and respiratory pattern (SA instruments, Stony Brook, NY) were externally monitored. Rectal temperature was monitored (SA instruments) and maintained between 37.5 - 38.5 °C by means of a circulating water blanket (Gaymar Industries, Orchard Park, NY). Similar animal preparation practices were performed during the neurophysiological experimental procedures involving a craniotomy, except the animal was placed in a stereotaxic frame and provided for the surgery analgesia along with surgical levels of the isoflurane (1.2-1.5%). All procedures followed NIH guidelines on animal use and care in research and were approved by the Institutional Animal Care and Use Committee of Vanderbilt University.
2.2. MRI data acquisition
MRI scans were performed in a 9.4T 21-cm narrow-bore Varian Inova magnet (Varian Medical Systems, Palo Alto, CA) using either a 3 cm surface transmit-receive coil that was placed over the central and lateral sulci of the hemisphere contralateral to the stimulated hand or a 6 cm surface coil that was centered over the midline of the head to cover both hemispheres. For scans with the 3 cm coil, we acquired fMRI responses by placing four oblique image slices (red box in Fig. 1A and colored rectangles in Fig. 1B, only 3 slices shown) in parallel to the hand region of area 3b to maximize mapping precision around the central and lateral sulci. Figure 1C & 1D show activation maps on the third slice (green box and outline). A high resolution T2-weighted gradient-echo structural image [repetition time (TR) = 200 ms; echo time (TE) = 14 ms; 0.078 x 0.078 x 2 mm3 resolution] was acquired to visualize blood vessel features (e.g., Figs. 1E and 1F). These slices were used later for coregistration with MRI maps obtained in different imaging sessions and with the surface blood vessel photomicrographs (e.g., Fig 1G & 1H) used in microelectrode recording sessions. FMRI data were acquired from the same four slices using a gradient echo planar imaging (GE-EPI) sequence (TR=1.5 sec; TE=16 ms; 0.625 x 0.625 x 2 mm3 resolution). The ventilation rate was adjusted to match the TR of the fMRI scans to minimize respiration-induced signal variations in BOLD signal time courses.
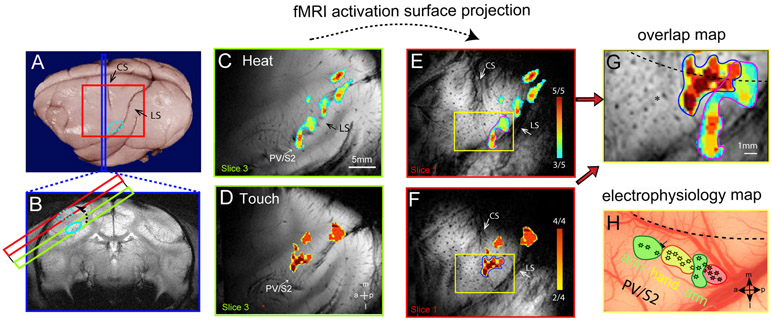
Figure 1. Image alignment method and spatial correspondence between fMRI activation maps and somatotopic map defined by microelectrode electrophysiology.
(A) The strategy for localizing and validating fMRI activations in deeper lateral sulcus areas involves the acquisition of two image planes, coronal (blue strip) and oblique (red square) MRI slices, shown on a post-mortem brain of a squirrel monkey. Both the central (CS) and lateral (LS) sulci (black arrows) are visible on the brain surface. (B) The T2*-weighted coronal MR image shows the location of the secondary somatosensory cortex (SII) on the third oblique image (light green outline). SII activation (light blue oval outline) is projected (dotted light blue oval outline) onto the top oblique image (red). (C & D) Nociceptive heat (47.5 °C, C) and innocuous touch (8 Hz vibration, D) stimulation of digits evoked fMRI activations on the third oblique image slice (light green slice in B). Activation maps are shown as probability maps thresholded at 60% (3 out of 5 runs) and 50% (2 out of 4 runs), see color bars in E and F. Locations of the activations (light blue circles) are confirmed to be at SII locations (the upper bank of lateral sulcus in B) on the corresponding coronal images. (E & F) Surface projected heat (E) and touch (F) fMRI activations from the third slice. The CS and LS are apparent on the surface slice (Slice 1), shown as dark strips. (G) The overlay map shows the spatial relationship between heat (E) and touch (F) activation maps. (H) The blood vessel map shows microelectrode mapping and recording in PV/S2 region in this subject. The yellow box (also in E, F, G) outlines the image aligned region over PV/S2. Stars indicate each microelectrode penetration site. The different colors indicate different receptive fields (see the label next to each patch). a: anterior. p: posterior. m: medial. l: lateral. Monkey SM-J.
For a 6 cm coil scan, five 2-mm-thick coronal plan T2*-weighted gradient echo structural images (one such image is shown in Fig 1B) were acquired (TR, 200 ms; TE, 14 ms; 0.078 x 0.078 x 2 mm3 resolution) to visualize gray and white matter contrast, and to identify brain structural features for coregistration of MRI maps obtained across imaging sessions conducted on different days on the same animal, and across animals (example shown in Fig.1A). Five coronal slices were positioned to cover the posterior two-thirds of the lateral sulcus region, where SII, insular cortices, and thalamus reside. Coronal images were placed according to the stereotactic framework, in an effort to ensure alignment with MRI images acquired across animals. FMRI data were acquired from the same 5 slices by using a gradient echo planar imaging sequence (TE = 16 ms; 0.7 x 0.7 x 2 mm3 resolution).
Typically, two to four imaging runs for each stimulation condition (i.e., single temperature or multiple temperature series) were performed within one imaging session (day). MRI studies with 3 cm and 6 cm coils were done on separate days.
2.3. FMRI data analysis
All T2*-weighted fMRI data were converted into an analysis file format and processed using Statistical Parametric Mapping (SPM) (http://www.fil.ion.ucl.ac.uk/spm/) with Matlab (The Mathworks, Natick, MA, USA). The T2*-weighted images were 2D motion corrected and then analyzed with General Linear Models (GLMs). FMRI activation maps were created using a cross-correlation function between the fMRI signal time courses of each voxel and the boxcar predictor of the Hemodynamic Response Function (HRF) convolved with the stimulus presentation paradigm and were thresholded with a statistical t-value (p=0.05, FDR p<0.05 corrected) and a minimal in-plane cluster size of three voxels. The choice of three voxels was based on the fMRI voxel size of 0.625 x 0.612 x 2 mm3 and the size (~ 1.5x1.5 x 2 mm3) of the functional module we expected to detect with fMRI. To preserve the highest spatial resolution for mapping, no spatial smoothing was applied. FMRI activation maps were created for each MRI run and for each scan session (day). We also generated cross-run frequency maps (thresholded at > 50%) to determine the reliability of the detected fMRI activation in each animal. FMRI activation maps were spatially interpolated and then superimposed on the corresponding high resolution T2* weighted structural images for display (Fig. 1C and 1D).
To evaluate the fMRI responses, BOLD signal time courses were extracted from two to three adjoining peak voxels (with highest t values) within each activation cluster and then normalized as % signal changes and averaged for each run or session as appropriate. The % BOLD signal change during the stimulation period was calculated by: (post-stimulus BOLD signal amplitude – pre-stimulus BOLD signal amplitude)/ pre-stimulus BOLD signal amplitude. Two imaging volumes before stimulus onset was chosen as the baseline period. The % BOLD signal changes were later quantified across animals at the group level. To accurately estimate fMRI response properties, a double gamma-variate hemodynamic response function (HRF)
| (1) |
was employed to fit the averaged BOLD response, and the parameter of peak BOLD amplitude was derived [6; 60].
2.4. Stimulus presentation
2.4.1. Thermal stimulus
Fingers were secured by gluing small pegs to the fingernails and fixing these pegs firmly in plasticine, leaving the glabrous surfaces available for vibrotactile and thermal stimulation. For fMRI experiments, thermal stimuli were delivered via a 30x30 CHEPS thermode (Medoc Inc, Israel, ramp rate 70°C/s) positioned over two adjacent digits (D2 and D3). Thermal stimuli were presented in two different paradigms: single temperature mapping with nine alternating blocks of 47.5°C (21 sec duration) and 32°C (30 sec duration) for mapping purposes; and multiple interleaved temperatures of 38, 42, 46.5 and 47.5°C (each 21 sec duration), separated by 30 sec of 32°C baseline temperature, for quantifying the thermal stimulus-response function (temperature-dependent BOLD signal time courses) (Fig. 2). Each single temperature imaging run contained 9 stimulus blocks (epochs) whereas multiple temperature runs contained 7 stimulus blocks for each temperature condition. The thermode remained in contact with the skin during temperature changes. With this fMRI stimulation paradigm, thermal stimulation at 47.5 °C was found to elicit a strong burning pain sensation in human subjects in unpublished psychophysics observations. The MR scanner-controlled stimulus timing by sending trigger pulses to the Medoc system. For electrophysiological recording experiments, each thermal stimulation was presented as 5 sec duration blocks and interleaved with 30 sec duration of 32°C baseline blocks to maximize the number of data sets obtained within each recording session. Different temperatures were delivered in a pseudorandom manner.
Figure 2. The patchy functional organization of heat nociception in the lateral sulcus region and temperature-dependent fMRI responses in SII cortex.
(A & B) Probability maps of fMRI responses to 47.5°C heat stimulation on two coronal images (4 mm apart, and thresholded at 4/10 runs) from one representative monkey. Color scale indicates the probability of activation across runs. Left inserts are schematic illustrations of the cortical areas and interareal borders identified by cytoarchitectural features of the brain tissue in the squirrel monkey. The inserts are adapted and modified from Burton and Jones [4] and Jones and Burton [31]. (C & D) Percentage (color symbols) and two-gamma fitted (color lines) BOLD fMRI time courses to stimulation of the digits with four different temperatures (D) and in four representative areas (C). The orange bar indicates the stimulus duration of 30 sec. BOLD signal amplitude at each time point is presented as the mean ± standard deviation (SD). (E) Exponential fit of the fMRI response peaks to each temperature. fMRI data from eleven hemispheres (out of nine monkeys) are quantified. P1-P4: patches 1-4. AP2 & AP6: slices 2 and 6 in the anterior to posterior direction. These two slices are 4 mm apart. S2: secondary somatosensory cortex. 7b: area 7b. Ri: retro-insula. pIns: posterior insula.
2.4.2. Innocuous tactile stimulation
For fMRI experiments, an innocuous 8 Hz vibrotactile stimulus was delivered on single distal finger pads by indentation of a rounded plastic probe (2 mm diameter) mounted on a piezoceramic actuator (Noliac, Denmark) that was driven by a Grass stimulator. At a rate of 8 Hz, the probe was indented 0.48 mm for 20 ms in seven 30 sec on and off blocks. During off blocks, the probe was lightly touching the skin. The MR scanner controlled the stimulus timing by sending trigger pulses to the Grass stimulator to start each stimulus epoch within one run. For electrophysiological recording experiments, the duration of vibrotactile stimulation with varying frequencies was 3 sec with an interstimulus interval of 8 sec. Vibrotactile stimuli were presented in groups of 20 repeats. The presentation of different frequencies was delivered in a pseudorandom manner.
2.5. Intracortical microelectrode mapping and recording of multiunit firing activity
After fMRI activation maps were obtained in each animal, microelectrode receptive field mapping and stimulus-response electrophysiological recordings ensued in the hemisphere contralateral to the stimulated hand. Single tungsten microelectrodes with 1 MΩ impedance (FHC) were used for mapping, and U-Probes (Plexon Inc.) were used for recording. A 16 channel Plexon data acquisition system was used to collect the electrophysiological data. The neurophysiological experiment was performed in two phases: dense microelectrode receptive field mapping followed by targeted recordings of stimulus response properties. Mapping was used to establish the somatotopic map of digit/arm representation in the parietal operculum where S2 resides and to relate the fMRI activation focus to the known somatotopic organization of this region (Figs. 3A and 4A). Typically, 100 to 250 penetrations were made in the cortex around the central and upper bank of lateral sulci in each animal.
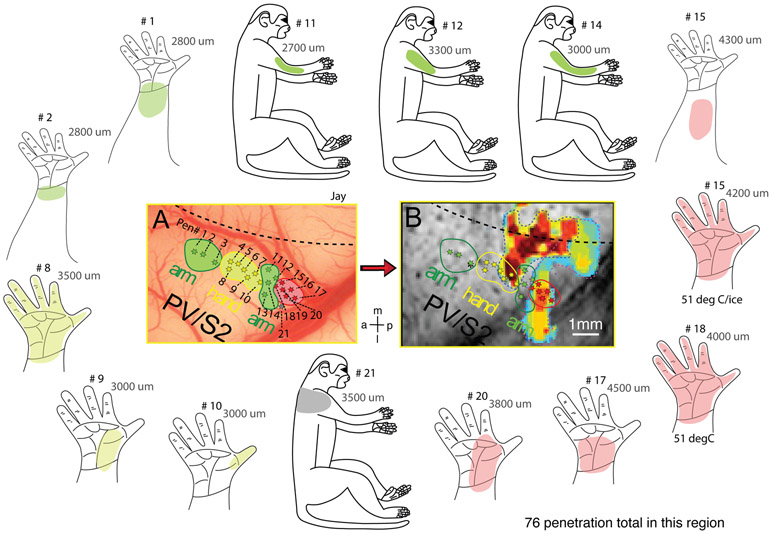
Figure 3. Receptive field maps of neurons isolated in PV/S2 region and their relationship to heat fMRI activation.
(A) Microelectrode penetration map (selected penetrations) shows the spatial relationship of neurons with different receptive fields and preferred stimulus. Green stars: low threshold mechanoreceptive (tactile) neurons with receptive fields on arm (penetration numbers of 1-3 and 11-14). Yellow stars: tactile neurons with receptive fields on hand/digits (penetration numbers of 4-10). Red stars: high threshold heat nociceptive neurons (penetration numbers of 15-20). Gray star (p21): low threshold tactile neuron on the shoulder. Dotted black line: estimated hand-face border in area 3b based on the receptive field map. (B) Composite, zoomed-in view of the PV/S2 region shows the spatial correspondences between (a) touch fMRI activation of digits (dark red patch) and cortical regions containing touch neurons (yellow dots within the red outline), and (b) heat fMRI activation (yellow-blue patch) and the cluster of nociceptive heat neurons (red stars within the red outline in the left side field of view). Surrounding inserts: body maps showing the properties (the color of the patches) and location of the receptive fields (the colored the patches: heat red, hand thermal; vibrotactile arm; olive, vibrotactile hand; gray, vibrotactile, shoulder). Numbers on each map represent the penetration number (i.e., # 12) and the depth of the electrode where the neuron was isolated and tested (i.e., 3000 μm: 3000 μm below the surface of the cortex). A total of 76 and 55 penetrations were placed into this PV/S2 region on the upper bank of the lateral sulcus and around the central sulcus, respectively, in this subject (SM-J). For presentation purposes, only selected penetrations (21 out of 76) are shown in A and B. a: anterior. p: posterior. m: middle. l: lateral. Monkey SM-J.
Figure 4. Receptive field maps of neurons isolated in PV/S2 region and their relationship to heat fMRI activation in a second representative case.
Same format as Figure 3, except B is a multi-run fMRI activation map to 47.5°C stimulation. A total of 56 penetrations were placed into this PV/S2 region on the upper bank of the lateral sulcus in this subject. Receptive fields of neurons selected from 22 penetrations are shown in A and B. A total of 12 penetrations were placed around the central sulcus areas in this monkey. Monkey SM-S.
During mapping, electrodes were inserted into the upper bank of the lateral sulcus located 2500–5000 μm in depth from the brain surface where face representation in area 3b is located. The depth of the electrode tip was tracked and advanced in 300 μm increments through the cortex using a hydraulic microdrive. At each increment, the receptive fields of the neurons were qualitatively characterized by palpation or squeezing of the contralateral arm and hand while listening to an audio amplifier for spike activity to identify the responsive skin area of the cortical neurons at each electrode penetration site. When the neuronal activity was evoked with receptive fields on the hand or arm, subsequent stimulations were then applied. These included light tapings with a 2 mm diameter probe, stroking with a cotton wisp, moving joints of the fingers and wrist, contacting skin with noxious heat (51°C) or cold (4°C) via thermal probe or ice cube, and squeezing with fine-tip forceps, to qualitatively characterize a neurons’ stimulus preference and receptive field. Based on the established palm-digit-palm somatotopic organization of the traditionally defined SII (the secondary somatosensory) cortex in New World monkeys, we sub-divided SII into rostral parietal ventral (PV) and posterior S2 (see Figure 5 for map). Neuronal activity strength was qualitatively graded as no response, very weak, weak, good, very good, and excellent responses. The cortical depths that exhibited the strongest responses were logged for subsequent in-depth electrophysiological recordings. These recordings were to quantitatively characterize a neurons’ response to varying temperatures and vibration at different frequencies and were done in both tactile and nociceptive fMRI foci. Qualitative mapping results were used to generate the receptive field and somatotopic maps and report on the percentage of nociceptive neurons with different preferred stimuli. Quantitative recording data were used to generate stimulus-neuron response functions for both temperature and probe indentation frequency and record baseline firing rate.
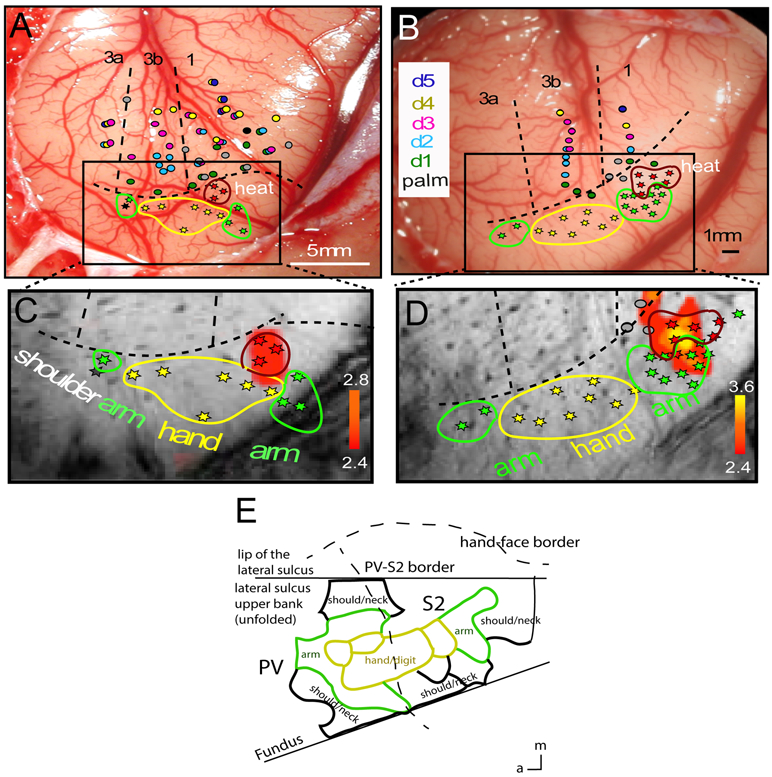
Figure 5. Nociceptive neuron clusters in nociceptive heat fMRI activation regions in PV/S2 in two more representative monkeys (SM-A and SM-H).
(A-B) Electrophysiology maps of S1 (areas 3a, 3b, 1, and 2) and PV/S2. Each colored dot indicates one microelectrode penetration site and the colors indicate the receptive field properties of neurons. Different colors indicate different digits (D1-D5, see color labels in B). Dotted black lines represent the approximate inter-areal borders among S1 sub-regions and the hand – face border. Body somatotopic maps in PV/S2 regions are highlighted by color stars and outlines (yellow: hand & digits; green: arm; shoulder: gray/black). Nociceptive neurons and clusters are indicated by red stars and outlines. a: anterior. m: middle. (C-D) Zoomed-in overlay of nociceptive heat fMRI response (red patches) and corresponding electrophysiological maps for each subject (SM-A, SM-H, respectively). (E) Adapted and modified illustration of the somatotopic map of the hand and its surrounding body parts of New World monkeys, as established by electrophysiological mapping and anatomy (Coq et al 2004).
2.6. Quantification of multi-unit spiking data
Multi-unit responses were analyzed with Spike 3 and NeuroExplorer software packages. Random and transient noise from the recordings were removed by amplitude thresholding and spike shape examination. Recordings from all cortical depths for each penetration within the same functional domain (i.e., heat or tactile) were pooled together for quantification at the group level. Peristimulus time histograms (PSTHs) were generated with a bin size of 250 ms for thermal neurons and 50 ms for tactile neurons. For nociceptive neurons, the 5 sec period prior to the stimulus onset was used as the baseline for quantifying % firing rate changes during stimulation. We calculated the mean firing rate in two temporal windows: baseline (5 sec prior to stimulus onset) and 0-25 sec after stimulus onset. For tactile neurons, firing rates during the stimulation period were used to calculate the mean firing rate. Across recordings, we normalized the firing rate by calculating (x-min/max-min). Spontaneous firing rates during the resting state period were also calculated for penetrations in both tactile and nociceptive regions.
3. Results
3.1. Spatially segregated fMRI activation foci to noxious heat versus touch stimuli
We first collected data with our MRI data acquisition strategy (see Methods and Figure 1) in two imaging planes (oblique images of contralateral hemisphere plus coronal images of bilateral hemispheres; Fig 1A & 1B) and compared fMRI activation patterns to nociceptive heat and innocuous touch stimulation along the lateral sulcus. Figure 1 shows that both noxious heat (47.5 °C, Fig. 1C) and innocuous vibrotactile stimulation (8 Hz, Fig. 1D) of digits (2 and 3) evoked multiple activation foci in the upper bank and caudal portion of the lateral sulcus in the contralateral hemisphere. We projected heat and tactile stimuli-evoked fMRI activation maps onto the top surface slice (Fig. 1E and 1F), on which rich blood vessel landmarks (visible as dark spots and stripes) are available for precise alignment and comparison with images obtained from different imaging sessions. After alignment, we overlaid the different maps to examine the spatial relationships of the functional activations (Fig. 1G) and used these maps in electrophysiology experiments (Fig 1H). The accuracy (> 0.5 mm) and rationale of this approach are as previously reported [8]. The heat fMRI activation locus was located posterior to tactile activation (compare patches outlined in magenta and blue in Fig. 1G) in the SII region in this animal.
Robust and reproducible nociceptive heat-elicited fMRI activation was detected in a posteriorly located S2 locus in all animals (n=8) studied. Sample cases from four animals are shown in Figures 2-5. To illustrate the activation patterns in the lateral sulcus, heat-activation reliability maps on the coronal imaging plane from one representative monkey are shown in Figure 2A and 2B. Multiple patches of fMRI activation were detected, and their relative locations with respect to the architectonically-defined regions around the lateral sulcus are presented as a reference for selected patches. BOLD time courses extracted from four patches showed strong and typical HRF and responded robustly to 47.5°C stimuli (Fig. 2C) at the group level (data were averaged from 20 runs from 8 monkeys). We labeled fMRI activations detected around the posterior portion of the lateral sulcus as the conventional secondary somatosensory cortex (SII) (e.g., Fig 2B). SII contains at least 3 subdivisions; S2 and PV are found on the upper bank of the lateral sulcus and the ventral somatosensory area (VS) resides in the most medial part of the operculum adjacent to the posterior insula. We focused on the fMRI cluster located on the upper bank of the lateral sulcus in the follow-up microelectrode mapping and recording experiments.
3.2. Temperature-dependent fMRI BOLD signal changes to heat ranging from innocuous to noxious intensity in SII cortex
To understand the functional properties of this nociceptive region in SII cortex in the upper bank of the lateral sulcus, we examined the stimulus-response function by quantifying the magnitude of fMRI signal changes as a function of temperature ranging from innocuous to noxious levels (38, 42, 46.5 and 47.5°C; Fig. 2D and 2E). The group (n=8 animals) averaged BOLD time courses, and the two-gamma function fittings (color-coded symbols and lines in Fig. 2D) showed two distinct response magnitudes. Percent BOLD signal changes to noxious 46.5 and 47.5 °C stimuli were robust with normalized peaks of 0.67 ± 0.08 and 0.73 ± 0.07, respectively. Signal changes to innocuous warm stimuli at 38 and 42°C were markedly weaker (compare red and black with blue and green lines in Fig. 2D). Figure 2E shows a clear preferential response to noxious levels of heat stimuli.
3.3. Electrophysiological mapping of heat nociceptive fMRI activation in SII region
We performed fMRI-guided dense microelectrode mapping studies to understand the functional organization features of the SII nociceptive heat responsive region identified with fMRI. The overlay map of heat and tactile fMRI activation in one monkey (Fig. 1G and Fig. 3B) showed that there was a partial overlap at the medial portion but a clear separation of heat (magenta outline) and tactile (blue outline) activation centers. Subsequently, 76 electrode penetrations were placed in this region; 21 are shown in Figure 3A-B. At each penetration, response properties of neurons were tested with heat (51°C), warm (40°C), cold (application of an ice cube for 5 sec), and tactile stroking and tapping (with hand and cotton swab stick) stimuli. The receptive fields (colored patches on body maps) and preferred stimuli (the color of the patch: thermal, red; tactile, either green, olive, gray) of neurons isolated are shown in the schematics in Figure 3. Figure 4 shows another case and the dense neurophysiological mapping results.
Heat and tactile activation foci were localized in the core S2/PV region of SII where low threshold mechanoreceptive neurons with receptive fields on the hand and arm were organized in a somatotopic arm-hand-arm mirror representation (see green-yellow-green patches and outlines in Figs. 3A-B and 4A-B). Low threshold mechanoreceptive neurons with receptive fields on hand/digits co-localized with the fMRI activation focus (see overlapping yellow stars and the dark-red fMRI activation foci in Fig. 3B). At the heat fMRI activation locus posterior to the tactile activation, we isolated a cluster of nociceptive neurons (red stars in Figs 3AB and 4AB). This cluster, however, was located outside of the core hand representations in the S2/PV where low threshold mechanoreceptive neurons predominantly reside (compare locations of red and green-yellow stars in Figs. 3A and 4A). Such a posteriorly located noxious heat fMRI activation locus containing a cluster of nociceptive neurons was confirmed in all monkeys that received dense microelectrode mapping. Figure 5 shows two more examples. Based on the receptive field properties (i.e., multi-digit receptive fields) of low-threshold tactile neurons and their somatotopic organization (i.e., anterior to posterior presentation of arm-hand/digit-palm) in the region, we determined that the heat-responsive neurons were located in the S2 region of SII cortex.
3.4. Somatotopy of neurons in the nociceptive heat cluster
In five out of the eight monkeys, we successfully isolated nociceptive units from 25 out of 224 penetrations placed around the upper bank of the lateral sulcus. Nociceptive neurons identified in PV/S2 regions exhibited their own unique somatotopic organization. Across animals, the nociceptive neuron clusters were located posterior or posterior-medial to the core of low threshold mechanical touch neurons. The precise spatial relationships between the nociceptive and tactile neuron clusters, however, varied across animals (Figs. 3-5, compare the red stars/outlines of nociceptive heat neurons with the yellow stars/outlines of low threshold tactile neurons). The nociceptive neurons with receptive fields of the hand or forearm were surrounded by low threshold touch neurons with comparable receptive fields on the hand, shoulder, or arm, indicating that clustered nociceptive neurons were located at appropriate regions as a somatotopically homogeneous neuronal patch. As a reference, Figure 5E shows the anatomically and electrophysiologically determined somatotopic representation of the hand and surrounding arm and shoulder/neck in PV/S2 of a similar type of New World monkey [12]. We did not isolate nociceptive neurons with corresponding shoulder or trunk receptive fields within this small SII hand region where dense mapping was performed. We speculate that these regions exist outside of our mapping territory.
3.5. Temperature-dependent multi-unit activity of nociceptive heat neurons
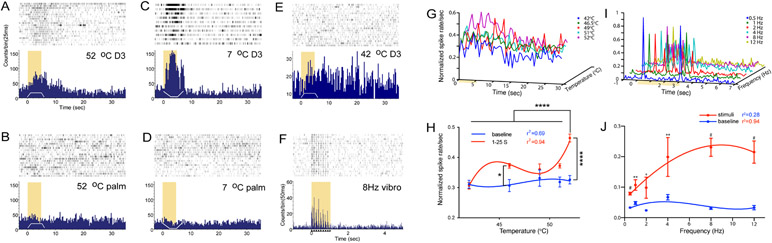
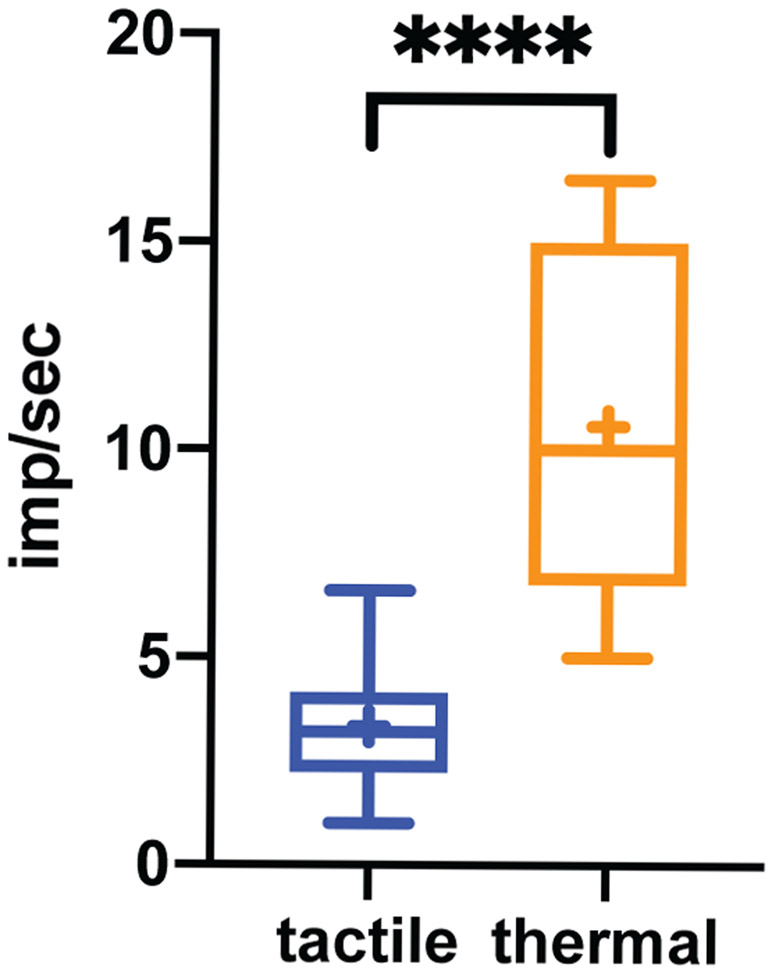
In S2/PV, the strongest response typically occurred at a depth between 2700-4500 μm from the cortical surface of area 3b. Figure 6A-E show raster plots (n=20 trials) and PSTHs of multiunit responses to different temperatures presented on either digits or palm from one representative recording site. Heat nociceptive neurons had relatively restricted receptive fields on the hand. Temperatures of 52°C or 7°C on digit 3 (D3) elicited the strongest firing activity (Fig. 6A and 6C), whereas the same stimulus on the palm evoked no response (Fig. 6B and 6D). The firing rate to a 5-sec 52°C stimulation peaked in about 5 sec after stimulus onset and returned to baseline in about 10 sec after stimulus offset. Noxious cold (7°C) stimulation of the same digit (D3) evoked strong firing activity, which peaked around 3 sec and lasted about 8 sec (Fig. 6C). In contrast, low-threshold units isolated from a neighboring D3 tactile patch responded to epochs of 8 Hz vibrotactile stimulation and stopped firing quickly after the stimulus ended (Fig. 6F). Similarly, 3D plots of the normalized mean firing rates to different temperatures showed similar temperature-dependent responses, and the most robust firing was to 52°C stimulation (Fig. 6G). Across all five heat temperatures (Fig. 6H), the firing rate for the 52°C stimuli was significantly higher than those from temperatures of 42, 46.5, 49, and 51°C (p<0.0001, independent t-tests). Firing rates for 46.5 and 52°C stimuli were also significantly higher than those at baseline. The mean firing latency (determined by the time point when the firing rate was one standard deviation above the baseline rate) for 52°C was 5.125 ± 0.95 sec (mean ± standard error). At the group level, the firing rate increased in a non-linear manner best fit by a two-exponential curve (red dots and curve in Fig. 6H). It was evident that one of the cortical patches that showed fMRI responses to nociceptive heat stimuli contained clusters of solely high threshold thermal nociceptive neurons, supporting that heat fMRI activations are reflective of underlying neuronal activity. In contrast, the firing rates of tactile neurons isolated from neighboring tactile patches followed closely to vibrotactile stimulation onset and offset (Fig. 6I). The spontaneous baseline firing was low (blue dots and line in Fig. 6J) where the mean firing rate increased from 0.5 to 8 Hz but decreased slightly at 12 Hz (red dots and line in Fig. 6J). The mean baseline firing rate (10.58 ± 0.77 imp/sec) of heat nociceptive neurons was significantly higher (p<0.0001, t-test) than the rate (3.35 ± 0.24 imp/sec) of tactile neurons (Fig. 7).
Figure 6. Raster and PSTH plots of multi-unit activity to temperature stimulation ranging from innocuous to nociceptive or to innocuous touch in PV/S2.
(A-E) Raster plots (top) and peristimulus histograms (PSTHs) (bottom) of multi-unit electrophysiological activity to different temperatures (see panel labels, 20 trials per condition) at 3090 μm depth from the brain surface in one representative subject, SM-J. (F) Raster plots and PSTH to 8Hz vibrotactile stimulation of one representative low threshold mechanoreceptive neuron isolated in adjacent touch core region. Yellow background shading indicates the stimulus presentation period. (G). 3-D plot of normalized PSTHs of multiunit spiking activity of nociceptive neurons (n=24) to the stimulation with temperatures of 42, 46.5, 49, 51, and 52°C in 5-sec on and 60-sec off cycles. (H) Fitting of normalized spiking rate (mean ± standard derivation) as a function of stimulation temperature during and after the stimulation (red dots and best curve fit line) and prior to stimulation (blue dots and line). *p<0.05; **p<0.01; ****p<0.0001 (independent t-test). (I) 3-D plot of normalized PSTH of the multiunit spiking activity of tactile neurons (n=20) to indentation (in 0.48 mm vertical displacement and 3-sec stimulus on and 8-sec off cycles) of single digits at frequencies of 0.5, 1, 2, 4, 8, 12 Hz. (J) Fitting of percentage firing rate as a function of vibrotactile stimulus frequencies during stimulation (red dots and line) and non-stimulation baseline periods (blue dots and line). *p<0.05; **p< 0.01; # p< 0.001. The goodness of the fit is indicated by R2 values in H and J.
Figure 7. Baseline firing rates of heat nociceptive neurons and tactile neurons.
In the plots, firing rate is shown as mean ± standard error. **** p<0.0001 (student t-test).
3.5. Electrophysiological properties of heat nociceptive neurons
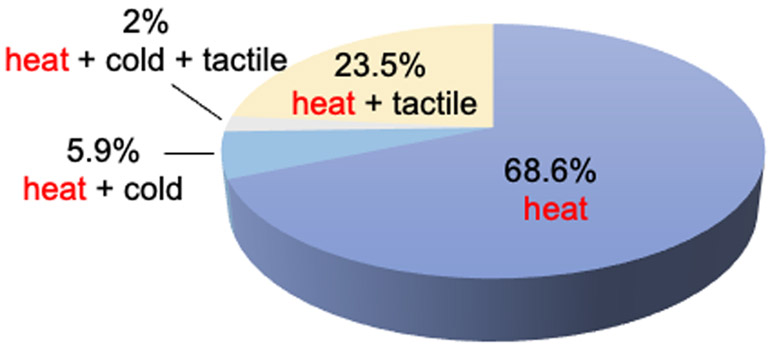
Within each fMRI and electrophysiologically confirmed heat nociceptive patch, the neurons isolated robustly responded to 47°C and 51 °C stimulation. Out of the 51 fully characterized heat nociceptive neuron clusters (out of 25 penetrations) from five monkeys, 74.5% responded to thermal stimulation only. Of those, 68.6% (35/51) were heat only and 5.9% (3/51) were heat and cold (7 °C) nociceptive neurons. Additionally, 23.5% (12/51) of the neurons were both heat and tactile sensitive, and only one neuron cluster (2%) responded to heat, cold, and tactile stimuli. The pie chart illustrates their proportional composition (Fig. 8).
Figure 8.
Composition of S2 heat nociceptive neurons with different preferred stimuli.
Thermal nociceptive neurons isolated in the S2 region exhibited unique firing properties, summarized as follows. First, all neurons isolated from this particular patch are nociceptive heat specific neurons that only responded to thermal stimulus in the noxious range (all to 42°C-51°C heat, and a few to 7°C cold) (Figure 6A-6E). Second, they had high baseline spontaneous firing rates that were significantly higher than those of low threshold mechanoreceptive neurons isolated from a neighboring tactile patch (Fig. 7; compare Fig 6B with 6F). Third, their receptive fields were comparable to those of tactile neurons and often represented parts of the hand, digits, or palm (Fig. 3 and 4). Fourth, responses to thermal stimuli were slow (exhibiting a delay of several seconds) and long-lasting, continuing several seconds after stimulation offset (Figure 6A and 6G). Fifth, along the cortical depth there seemed to be no apparent changes in their preferred stimulus types and receptive fields sizes. Finally, the size of the nociceptive clusters identified in each animal in this posterior S2 region was comparable (Figs. 3-5) and was estimated to be about 1.5x1.5 mm2 in size on the cortical sheet. In the cortex surrounding the cluster, abundant low threshold tactile neurons were isolated in intermingled touch patches. These tactile neurons typically showed easily defined receptive fields on multiple digits, palm, or forearm, with low spontaneous baseline firing (Figs 3-5, 6F, 6I, 6J) and did not respond to innocuous warm or nociceptive heat stimulation of their receptive fields.
To summarize, at cortical S2 regions that showed nociceptive-heat evoked fMRI responses, we isolated a nociceptive heat selective neuron cluster in the posterior portion of the classical PV/S2 region. Firing rate changes of the nociceptive neurons appeared to be similar to those of fMRI responses, which is a non-linear increase of response to high noxious temperatures (>46°C). This region is adjacent to regions containing somatotopically appropriate low threshold mechanoreceptive neurons, indicating the nociceptive neurons cluster formed a functionally distinct patch. Figure 9 schematically illustrates the novel finding of a parallel modular organization of tactile and thermal nociceptive neurons in the S2 cortex of the primate brain.
Figure 9. Schematic summary of the main finding of the study.
(A) top: location of the secondary somatosensory cortex (SII) projected onto the surface of a post-mortem squirrel monkey brain. bottom: modular organizations of touch and heat nociceptive neurons clusters in the S2 sub-region of SII cortex. (B) Schematic illustration of the topographic map of cortical areas around the sub-regions (PV, S2, and VS) of the secondary somatosensory cortex (SII) and its surrounding cortical areas of 3a, 3b, 1, and 2 of the first somatosensory cortex (SI), area 7b (7b), retro-insula (Ri), posterior insula, located at the caudal end of lateral sulcus. Green and red circles indicate the locations of touch and heat nociceptive neuron modules identified by electrophysiology in the current study. Light orange circles represent the fine-scale heat nociception network identified in the operculum region in a previous fMRI study [6].
4. Discussion
4.1. Discrete representation of thermal nociception in the primate cortex
Using a combined submillimeter fMRI and microelectrode mapping and recording approach, we confirmed that one particular nociceptive heat responsive fMRI focus (a functional patch) is located on the upper bank of lateral sulcus that belongs to an S2 subregion of SII cortex contained a cluster of solely nociceptive heat neurons. These findings are significant. First, it provides first-time evidence supporting that nociceptive stimulation evoked fMRI signal changes are reflective of underlying activity of nociceptive neurons and the existence of a functionally distinct millimeter-sized modular patch for thermal nociception in primate operculum. Second, the modality preferred nociceptive heat patch is spatially separated and intermingled with touch patches containing neurons with comparable receptive fields and forms functionally distinct mini-networks at mesoscale (defined as in the millimeter to centimeter range).
We and others have found that S2 and posterior insula cortices serve as key hubs in interconnecting the sensory/motor network to emotional, cognitive, and subcortical (including modulatory) networks [11; 19; 56]. The electrophysiological validation of the thermal nociceptive patch presented herein let us further propose that, S2 cortex likely functions as a key hub for interconnecting cortical regions and circuits engaged in the representations of different dimensions of pain perception, akin to the role V2 visual cortex serves by interconnecting the ventral and dorsal visual pathways. Specifically, we hypothesize that there are discrete reigns and pathways for heat and cold nociception which are separated from classical touch, affective touch, and proprioception pathways. Human studies shown that cortical areas encoding heat (possibly cold) pain, and touch sensation are spatially separated in the corresponding opercula-insular region [1; 2; 24; 25; 29; 35; 36; 41; 42; 63]. At high MRI field, identified a more complex seven-hub mini-network, containing PV and S2 subregions, and possibly VS (ventral somatosensory subregion) of SII cortex, area 7b (the equivalent of parietal operculum in humans), posterior insula, and retro-insula [6], for heat nociception in the opercula-insular region in squirrel monkeys. In our NHP fMRI studies, all three SII subregions (S2/PV and the ventral somatosensory (VS) area, in the most medial part of the operculum adjacent to the posterior insula (e.g., Figure 2B)) responded to nociceptive heat stimulation [6]. The VS area is distinct from S2/PV, contains both nociceptive and non-nociceptive neurons, and has a clear counterpart in humans, labeled Opercular-3, or "OP3" [17; 18]. S2/PV equivalents in humans (OP1 & OP4) are considered relevant to different aspects of pain processing than the medial operculum (OP2-3, VS) [26-28]. Intracortical stimulation of the operculum in humans also supports this segregation [43]. The mini-networks detected in monkeys contain more regions (hubs) than those reported in the human opercula-insular cortex [22; 24; 41; 46]. More studies are needed to determine these distinct opercular-insula subareas' functional differences in pain processing and sensation in primates.
4.2. Nociceptive stimulus induced fMRI signal changes reflect the underlying activity of nociceptive neurons
We observed high correspondences between nociceptive heat stimulus evoked fMRI signal changes and activity of thermal nociceptive neurons in both locations and in stimulus-response properties in the S2 subdivision of SII cortex. Neuronal firing properties of S2 thermal nociceptive neurons differed significantly from previous observations in the parietal operculum and SI cortex sub-regions of monkeys [5; 7; 15; 16; 49; 50], indicating each cortical area plays a different role in the processing and integration of nociceptive information. For example, thermal and mechanical nociceptive neurons isolated in areas 3b and 1 of SI cortex typically respond to stimulus intensities ranging from innocuous to noxious level and intermingle with innocuous sensory neurons [5; 7; 33; 50]. The firing followed closely with the physical aspects of stimulation, supporting their roles in representing the physical contact and intensity of information originating at the periphery. Area 3a neurons in SI however showed preferred responses to noxious stimuli [49; 50]. The magnitude of spike firing increased linearly as probe temperature increased from 49-51°C. Neuronal sustained responses were longer than those from neurons isolated in areas 3b and 1, which typically stop immediately after stimulus presentation [7; 33; 34].
S2 nociceptive neurons we isolated exhibited a coherent preference for one particular stimulus property – nociceptive heat. Only a small portion (7.9%) also responded to nociceptive cold stimulation. This feature supports the separation of functional processing pathways for different sensory modalities of nociceptive information in the high-order SII cortex. The feature of slow onset and offset firing after stimulation indicates that S2 nociceptive neurons likely engage in representing the slow-developing burning sensation that builds after a thermal nociceptive stimulus [9; 44; 58]. These electrophysiological features also differed significantly from those isolated from other lateral sulcus regions. Area 7b neurons exhibited multisensory properties (somatosensory and visual) [15; 16]. They either responded exclusively to noxious thermal stimuli or differentially to noxious and innocuous thermal stimuli [16; 54; 62].
Moreover, the existence of a modular thermal nociceptive neuron patch supports the notion that nociception is an independent sensory modality that is processed and integrated in SI, SII cortices, and beyond. Clustered neurons with similar functional features are commonly observed in sensory cortices, and spatially isolated cortical patches carrying discrete functions are considered as fundamental building blocks of the cerebral cortex, particularly in early sensory cortices. It is believed that clustered assembly of functionally similar neurons in spatially segregated domains, such as those slow-adapting and fast-adapting low threshold mechanoreceptive modules in somatosensory areas 3b and 1, ocular dominance columns in V1 and motion sensitive modules in V2 cortices [21; 32; 38; 47; 48], permits faster information processing and integration while preserving functional specificity. Here we provided evidence that a similar modular structure is present for thermal nociception. One limitation of the current study is that we are not able to determine whether the SII heat nociceptive neural responses have any hemisphere preference (contralateral versus the ipsilateral side of the body) because we focused on electrophysiology validation on the contralateral hemisphere.
4.3. High tonic firing rate and slow temporal response of nociceptive neurons: implication for functional imaging studies
The high-tonic firing feature of cortical nociceptive neurons poses challenges in detecting spontaneous pain-associated fMRI signal changes. Assessment of pain networks associated with a particular pain stimulus (or a condition) relies on contrasting two experimental conditions in fMRI studies, such as no-pain versus pain or low-pain versus high-pain conditions. Such an fMRI experimental design, by nature, could fail to detect alterations in tonic firing rates that could reflect a critical aspect of nociceptive processing and integration. As the spontaneous firing rate provides a noisy background, it is conceivable that such clusters may be characterized as 'nociceptive specific' simply because only nociceptive stimuli have enough driving energy to supersede the metabolic/ionic processes underlying spontaneous activity and thus have neuronal firing consequences [24]. Our data do not support this possibility because the isolated high tonic firing neurons exhibited preferred responses to nociceptive heat but not nociceptive cold, which presumably have comparative energy drive or demand. Thus, these neurons’ preference for heat-nociceptive stimuli cannot solely be explained by energy demand. Nevertheless, the role of high-tonic firing on the response selectivity of cortical heat nociceptive neurons requires further investigation.
Cortical nociceptive neurons in S2 also exhibited slow and variable temporal responses to nociceptive heat stimuli, which also has significant implications for human fMRI studies involving painful heat stimulation. The first implication relates to the design of HRF (hemodynamic response function) models in detecting pain-related fMRI signals. In our experiment, there was about 3-5 sec delay in nociceptive neuronal activity that is several-fold longer than the activity of typical tactile neurons [30; 59]. A standard HRF convolution procedure may only capture a small portion of fMRI responses in those pain regions. Characterization of response profiles of modality-distinct nociceptive neurons will no doubt help refine HRF models for maximal detection of fMRI signals associated with various types of pain sensation. In support of this idea, our unpublished data and other studies have shown that using perceptual pain ratings in the HRF model often led to increased detection of pain-related fMRI signals in the human brain [13; 14]. Further work is needed to fully characterize the HRF model in the context of different nociceptive processing schemes.
4.4. Influences of anesthesia
We attribute our success in mapping and isolating nociceptive neurons to the use of fMRI activation maps for targeting. One potential issue is the effects of anesthesia on nociceptive responses and the function of pain networks. With careful maintenance of the anesthesia at a relatively low level (0.8 - 1.0% isoflurane), we were able to obtain robust and reproducible nociceptive heat-induced changes in fMRI signals as well as single- and multi-unit activity. The level of isoflurane anesthesia is known to suppress neuronal activity and has been reported to affect the high frequency or burst component of spontaneous EEG electrophysiological signals [61]. In our experiments, successful isolation of nociceptive neurons with robust firing activity achieved in the current study at least indicates that isoflurane affected only minimally the noxious stimulus-response properties of nociceptive cortical neurons.
Acknowledgments
The work is supported by the National Institutes of Health (NS069909 to LMC). We thank Dr. Feng Wang and Mr. Fuxue Xin for their assistance on fMRI data collection, Ms. Chaohui Tang for her technical support on animal preparation, and Drs. Nellie Byun and Jamie Reed for language editing of the manuscript. All authors declare no conflict of interest in publishing the manuscript.
References
- [1].Bastuji H, Frot M, Perchet C, Magnin M, Garcia-Larrea L. Pain networks from the inside: Spatiotemporal analysis of brain responses leading from nociception to conscious perception. Hum Brain Mapp 2016;37(12):4301–4315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Baumgartner U, Iannetti GD, Zambreanu L, Stoeter P, Treede RD, Tracey I. Multiple somatotopic representations of heat and mechanical pain in the operculo-insular cortex: a high-resolution fMRI study. J Neurophysiol 2010;104(5):2863–2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Biemond A The conduction of pain above the level of the thalamus opticus. AMA Arch Neurol Psychiatry 1956;75(3):231–244. [DOI] [PubMed] [Google Scholar]
- [4].Burton H, Jones EG. The posterior thalamic region and its cortical projection in New World and Old World monkeys. J Comp Neurol 1976;168(2):249–301. [DOI] [PubMed] [Google Scholar]
- [5].Chen LM, Dillenburger BC, Wang F, Friedman RM, Avison MJ. High-resolution functional magnetic resonance imaging mapping of noxious heat and tactile activations along the central sulcus in New World monkeys. Pain 2011;152(3):522–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Chen LM, Dillenburger BC, Wang F, Tang CH. Differential fMRI activation to noxious heat and tactile stimuli in parasylvian areas of new world monkeys. Pain 2012;153(1):158–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Chen LM, Friedman RM, Roe AW. Area-specific representation of mechanical nociceptive stimuli within SI cortex of squirrel monkeys. Pain 2009;141(3):258–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Chen LM, Turner GH, Friedman RM, Zhang N, Gore JC, Roe AW, Avison MJ. High-resolution maps of real and illusory tactile activation in primary somatosensory cortex in individual monkeys with functional magnetic resonance imaging and optical imaging. J Neurosci 2007;27(34):9181–9191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Chery-Croze S. Painful sensation induced by a thermal cutaneous stimulus. Pain 1983;17(2):109–137. [DOI] [PubMed] [Google Scholar]
- [10].Chudler EH, Anton F, Dubner R, Kenshalo DR Jr. Responses of nociceptive SI neurons in monkeys and pain sensation in humans elicited by noxious thermal stimulation: effect of interstimulus interval. J Neurophysiol 1990;63(3):559–569. [DOI] [PubMed] [Google Scholar]
- [11].Cipolloni PB, Pandya DN. Cortical connections of the frontoparietal opercular areas in the rhesus monkey. J Comp Neurol 1999;403(4):431–458. [PubMed] [Google Scholar]
- [12].Coq JO, Qi H, Collins CE, Kaas JH. Anatomical and functional organization of somatosensory areas of the lateral fissure of the New World titi monkey (Callicebus moloch). J Comp Neurol 2004;476(4):363–387. [DOI] [PubMed] [Google Scholar]
- [13].Davis KD. Neurophysiological and anatomical considerations in functional imaging of pain. Pain 2003;105(1-2):1–3. [DOI] [PubMed] [Google Scholar]
- [14].Davis KD, Kwan CL, Crawley AP, Mikulis DJ. Functional MRI study of thalamic and cortical activations evoked by cutaneous heat, cold, and tactile stimuli. J Neurophysiol 1998;80(3):1533–1546. [DOI] [PubMed] [Google Scholar]
- [15].Dong WK, Chudler EH, Sugiyama K, Roberts VJ, Hayashi T. Somatosensory, multisensory, and task-related neurons in cortical area 7b (PF) of unanesthetized monkeys. J Neurophysiol 1994;72(2):542–564. [DOI] [PubMed] [Google Scholar]
- [16].Dong WK, Salonen LD, Kawakami Y, Shiwaku T, Kaukoranta EM, Martin RF. Nociceptive responses of trigeminal neurons in SII-7b cortex of awake monkeys. Brain Res 1989;484(1-2):314–324. [DOI] [PubMed] [Google Scholar]
- [17].Eickhoff SB, Amunts K, Mohlberg H, Zilles K. The human parietal operculum. II. Stereotaxic maps and correlation with functional imaging results. Cereb Cortex 2006;16(2):268–279. [DOI] [PubMed] [Google Scholar]
- [18].Eickhoff SB, Schleicher A, Zilles K, Amunts K. The human parietal operculum. I. Cytoarchitectonic mapping of subdivisions. Cereb Cortex 2006;16(2):254–267. [DOI] [PubMed] [Google Scholar]
- [19].Fauchon C, Meunier D, Faillenot I, Pomares FB, Bastuji H, Garcia-Larrea L, Peyron R. The modular organization of pain brain networks-an fMRI graph analysis informed by intracranical EEG. Cereb Cortex Communications 2020;1:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ferretti A, Babiloni C, Gratta CD, Caulo M, Tartaro A, Bonomo L, Rossini PM, Romani GL. Functional topography of the secondary somatosensory cortex for nonpainful and painful stimuli: an fMRI study. Neuroimage 2003;20(3):1625–1638. [DOI] [PubMed] [Google Scholar]
- [21].Friedman RM, Chen LM, Roe AW. Modality maps within primate somatosensory cortex. Proc Natl Acad Sci U S A 2004;101(34):12724–12729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Frot M, Faillenot I, Mauguiere F. Processing of nociceptive input from posterior to anterior insula in humans. Hum Brain Mapp 2014;35(11):5486–5499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Frot M, Garcia-Larrea L, Guenot M, Mauguiere F. Responses of the supra-sylvian (SII) cortex in humans to painful and innocuous stimuli. A study using intra-cerebral recordings. Pain 2001;94(1):65–73. [DOI] [PubMed] [Google Scholar]
- [24].Frot M, Magnin M, Mauguiere F, Garcia-Larrea L. Human SII and posterior insula differently encode thermal laser stimuli. Cereb Cortex 2007;17(3):610–620. [DOI] [PubMed] [Google Scholar]
- [25].Frot M, Mauguiere F. Dual representation of pain in the operculo-insular cortex in humans. Brain : a journal of neurology 2003;126(Pt 2):438–450. [DOI] [PubMed] [Google Scholar]
- [26].Garcia-Larrea L, Mauguiere F. Pain syndromes and the parietal lobe. Handb Clin Neurol 2018;151:207–223. [DOI] [PubMed] [Google Scholar]
- [27].Garcia-Larrea L, Perchet C, Creac'h C, Convers P, Peyron R, Laurent B, Mauguiere F, Magnin M. Operculo-insular pain (parasylvian pain): a distinct central pain syndrome. Brain : a journal of neurology 2010;133(9):2528–2539. [DOI] [PubMed] [Google Scholar]
- [28].Greenspan JD, Lee RR, Lenz FA. Pain sensitivity alterations as a function of lesion location in the parasylvian cortex. Pain 1999;81(3):273–282. [DOI] [PubMed] [Google Scholar]
- [29].Greenspan JD, Ohara S, Franaszczuk P, Veldhuijzen DS, Lenz FA. Cold stimuli evoke potentials that can be recorded directly from parasylvian cortex in humans. J Neurophysiol 2008;100(4):2282–2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Hsiao SS, O'Shaughnessy DM, Johnson KO. Effects of selective attention on spatial form processing in monkey primary and secondary somatosensory cortex. J Neurophysiol 1993;70(1):444–447. [DOI] [PubMed] [Google Scholar]
- [31].Jones EG, Burton H. Areal differences in the laminar distribution of thalamic afferents in cortical fields of the insular, parietal and temporal regions of primates. J Comp Neurol 1976;168(2):197–247. [DOI] [PubMed] [Google Scholar]
- [32].Kaskan PM, Lu HD, Dillenburger BC, Roe AW, Kaas JH. Intrinsic-signal optical imaging reveals cryptic ocular dominance columns in primary visual cortex of New World owl monkeys. Front Neurosci 2007;1(1):67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Kenshalo DR, Iwata K, Sholas M, Thomas DA. Response properties and organization of nociceptive neurons in area 1 of monkey primary somatosensory cortex. J Neurophysiol 2000;84(2):719–729. [DOI] [PubMed] [Google Scholar]
- [34].Kenshalo DR Jr., Chudler EH, Anton F, Dubner R. SI nociceptive neurons participate in the encoding process by which monkeys perceive the intensity of noxious thermal stimulation. Brain Res 1988;454(1-2):378–382. [DOI] [PubMed] [Google Scholar]
- [35].Lenz FA, Krauss G, Treede RD, Lee JL, Boatman D, Crone N, Minahan R, Port J, Rios M. Different generators in human temporal-parasylvian cortex account for subdural laser-evoked potentials, auditory-evoked potentials, and event-related potentials. Neurosci Lett 2000;279(3):153–156. [DOI] [PubMed] [Google Scholar]
- [36].Lenz FA, Rios M, Chau D, Krauss GL, Zirh TA, Lesser RP. Painful stimuli evoke potentials recorded from the parasylvian cortex in humans. J Neurophysiol 1998;80(4):2077–2088. [DOI] [PubMed] [Google Scholar]
- [37].Lockwood PL, Iannetti GD, Haggard P. Transcranial magnetic stimulation over human secondary somatosensory cortex disrupts perception of pain intensity. Cortex 2013;49(8):2201–2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Lu HD, Chen G, Tanigawa H, Roe AW. A motion direction map in macaque V2. Neuron 2010;68(5):1002–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Maihofner C, Herzner B, Otto Handwerker H. Secondary somatosensory cortex is important for the sensory-discriminative dimension of pain: a functional MRI study. Eur J Neurosci 2006;23(5):1377–1383. [DOI] [PubMed] [Google Scholar]
- [40].Mauguiere F. The role of secondary somatosensory cortex and insula in pain. Suppl Clin Neurophysiol 2004;57:62–71. [DOI] [PubMed] [Google Scholar]
- [41].Mazzola L, Faillenot I, Barral FG, Mauguiere F, Peyron R. Spatial segregation of somato-sensory and pain activations in the human operculo-insular cortex. Neuroimage 2012;60(1):409–418. [DOI] [PubMed] [Google Scholar]
- [42].Mazzola L, Isnard J, Mauguiere F. Somatosensory and pain responses to stimulation of the second somatosensory area (SII) in humans. A comparison with SI and insular responses. Cereb Cortex 2006;16(7):960–968. [DOI] [PubMed] [Google Scholar]
- [43].Mazzola L, Isnard J, Peyron R, Mauguiere F. Stimulation of the human cortex and the experience of pain: Wilder Penfield's observations revisited. Brain : a journal of neurology 2012;135(Pt 2):631–640. [DOI] [PubMed] [Google Scholar]
- [44].Morin C, Bushnell MC. Temporal and qualitative properties of cold pain and heat pain: a psychophysical study. Pain 1998;74(1):67–73. [DOI] [PubMed] [Google Scholar]
- [45].Niddam DM, Yeh TC, Wu YT, Lee PL, Ho LT, Arendt-Nielsen L, Chen AC, Hsieh JC. Event-related functional MRI study on central representation of acute muscle pain induced by electrical stimulation. Neuroimage 2002;17(3):1437–1450. [DOI] [PubMed] [Google Scholar]
- [46].Pomares FB, Faillenot I, Barral FG, Peyron R. The 'where' and the 'when' of the BOLD response to pain in the insular cortex. Discussion on amplitudes and latencies. Neuroimage 2013;64:466–475. [DOI] [PubMed] [Google Scholar]
- [47].Sur M, Wall JT, Kaas JH. Modular segregation of functional cell classes within the postcentral somatosensory cortex of monkeys. Science 1981;212(4498):1059–1061. [DOI] [PubMed] [Google Scholar]
- [48].Sur M, Wall JT, Kaas JH. Modular distribution of neurons with slowly adapting and rapidly adapting responses in area 3b of somatosensory cortex in monkeys. J Neurophysiol 1984;51(4):724–744. [DOI] [PubMed] [Google Scholar]
- [49].Tommerdahl M, Delemos KA, Favorov OV, Metz CB, Vierck CJ Jr., Whitsel BL. Response of anterior parietal cortex to different modes of same-site skin stimulation. J Neurophysiol 1998;80(6):3272–3283. [DOI] [PubMed] [Google Scholar]
- [50].Tommerdahl M, Delemos KA, Vierck CJ Jr., Favorov OV, Whitsel BL. Anterior parietal cortical response to tactile and skin-heating stimuli applied to the same skin site. J Neurophysiol 1996;75(6):2662–2670. [DOI] [PubMed] [Google Scholar]
- [51].Torquati K, Pizzella V, Babiloni C, Del Gratta C, Della Penna S, Ferretti A, Franciotti R, Rossini PM, Romani GL. Nociceptive and non-nociceptive sub-regions in the human secondary somatosensory cortex: an MEG study using fMRI constraints. Neuroimage 2005;26(1):48–56. [DOI] [PubMed] [Google Scholar]
- [52].Torquati K, Pizzella V, Della Penna S, Franciotti R, Babiloni C, Romani GL, Rossini PM. "Gating" effects of simultaneous peripheral electrical stimulations on human secondary somatosensory cortex: a whole-head MEG study. Neuroimage 2003;20(3):1704–1713. [DOI] [PubMed] [Google Scholar]
- [53].Treede RD, Apkarian AV, Bromm B, Greenspan JD, Lenz FA. Cortical representation of pain: functional characterization of nociceptive areas near the lateral sulcus. Pain 2000;87(2):113–119. [DOI] [PubMed] [Google Scholar]
- [54].Vogel H, Port JD, Lenz FA, Solaiyappan M, Krauss G, Treede RD. Dipole source analysis of laser-evoked subdural potentials recorded from parasylvian cortex in humans. J Neurophysiol 2003;89(6):3051–3060. [DOI] [PubMed] [Google Scholar]
- [55].Wager TD, Atlas LY, Lindquist MA, Roy M, Woo CW, Kross E. An fMRI-based neurologic signature of physical pain. N Engl J Med 2013;368(15):1388–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Wu R, Wang F, Yang PF, Chen LM. High-resolution functional MRI identified distinct global intrinsic functional networks of nociceptive posterior insula and S2 regions in squirrel monkey brain. Neuroimage 2017;155:147–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Yang PF, Wu R, Wu TL, Shi Z, Chen LM. Discrete Modules and Mesoscale Functional Circuits for Thermal Nociception within Primate S1 Cortex. J Neurosci 2018;38(7):1774–1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Yarnitsky D, Ochoa JL. Studies of heat pain sensation in man: perception thresholds, rate of stimulus rise and reaction time. Pain 1990;40(1):85–91. [DOI] [PubMed] [Google Scholar]
- [59].Zhang HQ, Murray GM, Coleman GT, Turman AB, Zhang SP, Rowe MJ. Functional characteristics of the parallel SI- and SII-projecting neurons of the thalamic ventral posterior nucleus in the marmoset. J Neurophysiol 2001;85(5):1805–1822. [DOI] [PubMed] [Google Scholar]
- [60].Zhang N, Gore JC, Chen LM, Avison MJ. Dependence of BOLD signal change on tactile stimulus intensity in SI of primates. Magn Reson Imaging 2007;25(6):784–794. [DOI] [PubMed] [Google Scholar]
- [61].Zhang Z, Cai DC, Wang Z, Zeljic K, Wang Z, Wang Y. Isoflurane-Induced Burst Suppression Increases Intrinsic Functional Connectivity of the Monkey Brain. Front Neurosci 2019;13:296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Zhang ZH, Dougherty PM, Oppenheimer SM. Monkey insular cortex neurons respond to baroreceptive and somatosensory convergent inputs. Neuroscience 1999;94(2):351–360. [DOI] [PubMed] [Google Scholar]
- [63].zu Eulenburg P, Baumgartner U, Treede RD, Dieterich M. Interoceptive and multimodal functions of the operculo-insular cortex: tactile, nociceptive and vestibular representations. Neuroimage 2013;83:75–86. [DOI] [PubMed] [Google Scholar]