Abstract
Introduction: CBD is a major phytocannabinoid in hemp (Cannabis sativa containing less than 0.3% THC). Hemp cigarettes are a combustible form of hemp consisting of dried and smokable flowers, which represent 2% of the overall CBD market, and the market is expected to grow. Combustion and pyrolysis of organic material are associated with the production of carbonyl compounds, which are known toxicants and are associated with adverse health outcomes. Concentrations of carbonyl compounds in mainstream hemp cigarette smoke are unknown.
Materials and Methods: We analyzed and compared carbonyl concentrations in the mainstream smoke produced by a hemp cigarette (Brand B), a premium hemp cigarette (Brand A), Marlboro Red tobacco cigarette, and a research reference tobacco cigarette using high-performance liquid chromatography. We measured carbonyl concentrations in μg per puff and mg per cigarette. Carbonyls investigated were formaldehyde, acetaldehyde, acetone, acrolein, propionaldehyde, crotonaldehyde, 2-butanone, and butyraldehyde. Significance was determined using Tukey's test.
Results: We observed that Brand B had significantly higher butyraldehyde than any cigarette. No significant differences were observed in crotonaldehyde concentration in the cigarettes. For the remaining carbonyls, Brand A had consistently lower concentrations in mainstream smoke than tobacco cigarettes. Hemp cigarettes emit carbonyls in a lower concentration in μg/puff than tobacco cigarettes, but the magnitude of significance generally decreases when normalized to mg/cigarette.
Conclusions: Smoke from hemp cigarettes contains carbonyls at biologically significant concentrations. Opportunities may exist to reduce carbonyl production in these products, and identified potential risks must be considered when balancing the harms and benefits of hemp cigarettes when used for therapeutic purposes.
Keywords: hemp, hemp cigarettes, smoking, carbonyls
Introduction
CBD is a major phytocannabinoid in the plant Cannabis sativa. CBD has been observed to exhibit anticonvulsant, anxiolytic, anti-inflammatory, antipsychotic, and neuroprotective properties.1–3 The legal landscape around CBD has changed substantially in the past several years. First, the Food & Drug Administration (FDA) approved oral plant-derived CBD for the treatment of severe forms of childhood epilepsy (Lennox-Gastaut and Dravet syndromes).4 As a result, CBD extracted from Cannabis (containing <0.1% THC) for specific use in an FDA-approved drug is classified by the Drug Enforcement Agency (DEA) as a schedule V substance, which is defined as having the lowest abuse potential.5,6 Second, the 2018 Farm Bill, also known as the Agricultural Improvement Act, excludes hemp, defined as Cannabis containing <0.3% THC on a dry weight basis, from DEA schedule I (i.e., no medical indication).7 As a result of these changes in the regulatory framework, it is expected that U.S. consumer sales of CBD will reach $1.8 billion by 2022.8 According to estimates from the Brightfield Group, CBD sales are expected to increase as high as $22 billion worldwide by 2022 with large chains such as CVS Pharmacy, Walgreens, and Rite Aid selling these products.8
CBD is well tolerated with few serious side effects.9,10 Consumers are adopting the use of this cannabinoid for a variety of reasons. In a survey assessing knowledge, attitudes, and use of CBD in 340 young adults, 55% reported use of at least one type of CBD product primarily for stress and pain relief, relaxation, and sleep improvement.11 Edibles (∼56%) were the most commonly used products, followed by tinctures (∼54%), vapes (∼39%), and topicals (∼30%). In a self-selected convenience sample of individuals conducted from October 25, 2017 to January 25, 2018 (n=2409), 62% of respondents used CBD to treat a medical condition (predominantly pain, anxiety, and depression), while 38% used it for general health and well-being.12 In this survey, the most common form of administration was sublingual (tinctures), and smoking was the fifth most common form after vaping, oral capsules, and liquids. However, the oral bioavailability of CBD is low.13 CBD total drug exposure and peak serum concentrations are reached more quickly with smoking/inhalation compared to oral/oromucosal routes, the absolute concentrations of which are dose dependent.14 These observations suggest that therapeutic efficacy and titration of CBD drug effect may be enhanced through inhalation.
Hemp cigarettes consisting of dried hemp flowers currently represent 2% of the overall CBD market but have been suggested to be one its fastest growing segments.15,16 However, this product requires combustion before inhalation, which poses potential adverse health risks. With combustible tobacco cigarettes, smoke contains more than 4000 chemicals, including carcinogenic and toxic carbonyl compounds such as formaldehyde.17,18 No previous publications exist regarding the concentrations of carbonyls present in the smoke produced through the combustion of hemp cigarettes.
To advance our understanding of the potential health risks of hemp cigarettes, concentrations of carbonyls in smoke produced by two brands of hemp cigarettes, a conventional cigarette, and a reference cigarette were analyzed.
Materials and Methods
Overall study design
Selected carbonyl content in mainstream smoke of two hemp cigarettes and two tobacco cigarettes was analyzed. Carbonyls were derivatized in a solution of 2,4-dinitrophenylhydrazine (DNPH), and the subsequent hydrazones were analyzed using high performance liquid chromatography with diode array detection (HPLC-DAD).
Cigarette products and chemicals
Two brands of hemp cigarettes were purchased from brick-and-mortar tobacco stores. The first brand, defined herein as Brand A, was deemed a premium product at ∼$2.50 per cigarette. Information regarding Brand A was obtained from the cigarette carton, as well as the product website. Brand A is the original blend of proprietary organic traditional smoking herbs in combination with organic CBD hemp flower devoid of stems and seeds. Each cigarette is noted to contain ∼1 g of material with 90 mg of CBD. Finally, Brand A utilizes biodegradable rolling paper and filters. The second brand, Brand B, was ∼$0.65 per cigarette. Brand B's product website details that the hemp cigarette is composed of U.S. grown hemp and utilizes the aerial components (stalks, stems, leaves, etc.) of the hemp plant. According to the website, there is ∼50 mg of CBD per cigarette. Brand B utilizes standard rolling paper; however, the filter has a star-shaped cutout through the center of the filter decreasing the integrity of the filter. Both brands boast third party testing for their products, although Brand A did not have a current report (expired September, 2019) and Brand B did not have a properly functioning hyperlink to their data.
In addition, two types of tobacco cigarettes were used. Marlboro Red cigarettes were purchased from a local convenience store at ∼$0.50 per cigarette and 1R6F reference cigarettes from Tobacco Laboratory Research at the University of Kentucky (Lexington, KY).19 All cigarettes tested were a standard king size of 84 mm.
Water with a resistance of 18.2 MΩ cm was generated by the Barnstead (Los Angeles, CA) Nanopure Diamond laboratory water purification system. Trizma™ base (>99.9%) titration grade, HPLC grade acetonitrile (ACN), tetrahydrofuran (THF), 2-propanol, and 85% phosphoric acid, DNPH (97%), analytical standards of dinitrophenylhydrazone carbonyls: formaldehyde, acetaldehyde, acetone, acrolein, propionaldehyde, crotonaldehyde, 2-butanone, and butyraldehyde were all purchased from Millipore-Sigma (St. Louis, MO).
Sample preparation
The following sample preparation was based upon the CORESTA Recommended Method No. 74 Determination of Selected Carbonyls in Mainstream Cigarette Smoke by HPLC20 with the following modifications. Briefly, 55/45 (% v/v) ACN/water DNPH trapping solution was used to aid in a greater solubility of DNPH.21 Twenty five milliliters of DNPH trapping solution was split between two impingers in series, and each sample consisted of the smoke extract from a single cigarette. A schematic of the carbonyl trapping apparatus is available in the Supplementary Fig. S1. Each cigarette was run in quadruplicate.
Cigarettes were smoked to the filter utilizing a Single Cigarette Smoking Machine (SCSM-STEP; CH Technologies, Westwood, NJ) with two additional clearing puffs, and the smoldering cigarette was removed from the collection apparatus.22 The smoking regiment implemented was the Health Canada Intense (HCI) regime characterized by a puff duration of 2 sec, puff volume of 55 mL, and an interpuff interval of 30 sec.23 Ventilation holes were blocked with Tygon® tubing. Flow of the program was checked at the beginning of each experimental day using a flowmeter (TSI, Inc., Shoreview, MN) to ensure proper functioning.
HPLC analysis
Ten carbonyl products were separated and quantified using HPLC-DAD, utilizing several aspects of the CORESTA No 74 method mentioned previously. The system consisted of an Agilent (Santa Clara, CA) 1260 Infinity II instrument equipped with an InfinityLab Poroshell 120 EC-C18 100 mm×3.0 mm×2.7 μm column and a corresponding InfinityLab Poroshell 120 EC-C18 5 mm guard column (Chrom Tech, Inc., Apple Valley, MN). Column compartment was held constant at 30°C,20 injection volume was 3.0 μL, and flow rate was 0.8 mL/min. Mobile phase A consisted of 30% ACN, 10% THF, and 1% 2-propanol in ultrapure water. Mobile phase B consisted of 65% ACN, 1% THF, and 1% 2-propanol in ultrapure water. The elution gradient was: 0.0 min, 55% A; 2.8 min, 50% A, 7.1 min, 45% A; 9.5 min, 40% A, 10.6 min, 35% A; 11.3 min, 25% A, 15.0 min, 10% A, method end at 16.0 min, 3.0 min postrun. The detector was set at 365 nm.20 Representative chromatograms are available in the Supplementary Data: calibration standard, Supplementary Figure S2; 1R6F, Supplementary Figure S3; Brand A, Supplementary Figure S4, sample blank, Supplementary Figure S5.
Calibration curves
Eight point calibration curves were created according to CORESTA No 7420 (Supplementary Fig. S6). Lower limit of detection (LLOD) and lower limit of quantification (LLOQ) were defined as LLOD=3.0(Sy/m) and LLOQ=10(Sy/m), where Sy is the standard deviation of the response and m is the slope of the calibration curve. Linearity was represented by the coefficient of determination (R2); all analytes showed excellent linearity. R2, LLOD, and LLOQ values for each analyte are presented in Table 1.
Table 1.
Calibration Curve Linearity and Values of Lower Limit of Detection and Lower Limit of Quantification
| Analyte | R2 | LLOD (μg/mL) | LLOQ (μg/mL) |
|---|---|---|---|
| Formaldehyde | 0.9999 | 0.024 | 0.079 |
| Acetaldehyde | 0.9999 | 0.243 | 0.809 |
| Acetone | 0.9999 | 0.056 | 0.187 |
| Acrolein | 0.9999 | 0.012 | 0.041 |
| Propionaldehyde | 0.9999 | 0.024 | 0.079 |
| Crotonaldehyde | 0.9999 | 0.050 | 0.166 |
| 2-butanone | 0.9999 | 0.060 | 0.199 |
| Butyraldehyde | 0.9999 | 0.104 | 0.348 |
LLOD, lower limit of detection; LLOQ, lower limit of quantification.
Quality controls
Quality control (QC) samples were prepared by generating blank matrix samples and spiking with three different concentrations for each analyte. Low, medium, and high concentrations were ∼0.5, 1.0, and 1.5 times the expected concentration of each carbonyl, respectively. Precision was determined by percent relative standard deviation (%RSD) within each day. Accuracy is determined through spike recovery; dilution due to spike volume was accounted for in calculations. Percent recovery is defined with the following equation: [(measured concentration)/(nominal concentration×dilution factor)]×100, where dilution factor is equivalent to: [(sample volume)/(sample volume + spike volume)]. Standards and QC samples were run in triplicate on three consecutive days to determine interday accuracy and precision (Supplementary Table S1). There is sample degradation past day 1, emphasizing the importance of analyzing samples immediately preceding generation. Recovery is generally similar to recoveries reported in a collaborative study compiled from 15 participating laboratories.24
Data analysis
Significance was determined by Tukey adjustments for multiple comparisons; a p-value <0.05 was considered significant in all cases. Formaldehyde and acetone were detected in blank samples at low concentrations and have been subtracted out of the data presented. Peak identities were confirmed with spiking. Carbonyl concentrations were calculated in units of μg/puff (Table 2) and mg/cigarette (Supplementary Table S2). p-Values for pairwise comparisons in μg/puff are available in Supplementary Table S3.
Table 2.
Mean and Standard Deviation of Selected Carbonyls in Microgram per Puff
| Mean±SD (μg/puff) |
Puffs/cigarette |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Formaldehyde | Acetaldehyde | Acetone | Acrolein | Propionaldehyde | Crotonaldehyde | 2-butanone | Butyraldehyde | Average of n=4 | |
| 1R6F | 8.98±0.68 | 113.26±15.37 | 41.13±3.06 | 12.30±0.67 | 8.77±0.81 | 4.26±0.18 | 13.01±1.49 | 5.92±0.56 | 11.25 |
| Marlboro | 7.76±0.98 | 123.47±11.54 | 46.12±5.23 | 16.89±1.95 | 10.22±1.46 | 4.82±2.13 | 13.12±2.88 | 6.81±1.03 | 11.25 |
| Brand A | 3.71±1.01 | 63.65±8.08 | 18.80±1.65 | 9.03±4.01 | 4.69±0.48 | 3.68±0.47 | 4.11±0.33 | 7.03±0.75 | 16.5 |
| Brand B | 4.94±0.63 | 117.77±6.19 | 37.08±1.82 | 11.71±0.85 | 8.42±0.30 | 3.64±0.36 | 8.02±0.25 | 10.87±0.37 | 14.25 |
SD, standard deviation.
Results and Discussion
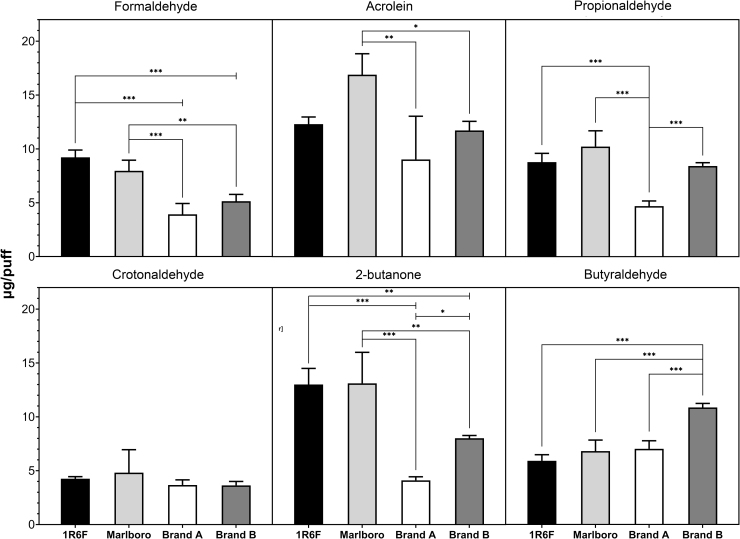
Our results are consistent with previous publications analyzing carbonyl content in smoke produced from conventional and reference tobacco cigarettes using the HCI regime.19,25–28 In all pairwise comparisons of the Brand A cigarette, the smoke contained significantly lower yields of acetaldehyde, acetone, propionaldehyde, and 2-butanone (Figs. 1 and 2). With regards to formaldehyde, the Brand A and B were not significantly different from each other, but both significantly differed from the Marlboro and reference cigarettes (Fig. 2). Brand B had significantly more butyraldehyde than any other cigarette tested (Fig. 2). The pairwise comparisons between the two tobacco cigarettes never significantly differed per puff. The results suggest that product quality, defined here as cost per cigarette, has potential to indicate trends in carbonyl output.
FIG. 1.
Mainstream smoke concentrations (μg/puff) of acetaldehyde and acetone in reference cigarette (1R6F), Marlboro, and of two brands of hemp cigarettes. Columns with vertical bars indicate the mean + SD (n=4); significance is expressed as **p≤0.01 and ***p<0.001.
FIG. 2.
Mainstream smoke concentrations (μg/puff) of formaldehyde, acrolein, propionaldehyde, crotonaldehyde, 2-butanone, and butyraldehyde in reference cigarette (1R6F), Marlboro, and of two brands of hemp cigarettes. Columns with vertical bars express the mean + SD (n=4); significance is shown as *p<0.05, **p≤0.01, and ***p<0.001.
Significance in units of mg/cigarette differed slightly (Supplementary Table S2); the magnitude of significance in Brand A decreased in some carbonyl species but remained nonetheless with the exception of acrolein, where in mg/cigarette no significant relationships were identified. Broadly, differences between Brand B and tobacco cigarettes were also accentuated. Finally, Brand A had significantly more butyraldehyde than tobacco cigarettes in mg/cigarette. Butyraldehyde has been previously observed to have higher titers in Cannabis than in tobacco cigarettes.29
Of note, Brand A cigarettes were rolled in an unbleached biodegradable paper, while the Brand B, Marlboro, and 1R6F cigarettes all were wrapped in conventional rolling paper. Differences in physical characteristics of manufactured cigarettes such as cigarette length, paper type, paper porosity, and filter have been shown to influence mainstream smoke.30–32 The use of recycled, less-robust wrapping may influence the quantity of carbonyls in the smoke of the Brand A hemp cigarette. In addition, Brand B had a star-shaped cutout through the center of the filter, providing a pathway for unfiltered mainstream smoke. Physical characteristics of the cigarettes may account for some of the variation in the measured quantities of carbonyls detected in the smoke.
Available literature suggests that potentially therapeutic components (i.e., terpenoids) vary by hemp variety, while rolling paper, filter, and additives used in hemp cigarette manufacturing have the greatest impact on toxicant exposure. Studies have identified terpenoids as the primary variable in Cannabis smoke across plant varieties, presenting a 40-fold range of total terpenoid content.33 In tobacco cigarettes, differences in filters, which have been used to reduce harmful volatile constituents in mainstream smoke, impact observed carbonyl yields.34 In addition, fast-burning, bleached, and flavored papers have been observed to contribute to higher levels of aerosol toxicants.30 Formaldehyde is increased in cigarettes containing higher levels of sugar, and other carbonyls are formed by the pyrolysis of cellulosic and other polysaccharide materials.35 Variation in filter, additives, and rolling paper may account for observed differences in carbonyls between the two hemp products.
Formaldehyde, acetaldehyde, acetone, acrolein, propionaldehyde, crotonaldehyde, and 2-butanone (methyl ethyl ketone) are listed on the FDA's list of “harmful and potentially harmful constituents (HPHCs) in tobacco products and tobacco smoke” (Table 3).18 This list includes any chemical or chemical compound in a tobacco product or in tobacco smoke that is inhaled, ingested, or absorbed into the body that causes or has the potential to cause direct or indirect harm to product users or nonusers.
Table 3.
Abbreviated Food & Drug Administration List of Harmful and Potentially Harmful Constituents in Tobacco Products and Tobacco Smoke
| Carbonyl | Carcinogen | Respiratory toxicant | Cardiovascular toxicant | Addictive agent |
|---|---|---|---|---|
| Formaldehyde | X | X | ||
| Acetaldehyde | X | X | X | |
| Acetone | X | |||
| Acrolein | X | X | ||
| Propionaldehyde | X | X | ||
| Crotonaldehyde | X | |||
| 2-butanone | X | X | ||
| Butyraldehyde |
We observed no significant differences between concentrations of crotonaldehyde, a carcinogen, in any hemp and tobacco cigarettes (Fig. 2). A similar relationship is seen with acrolein in mg/cigarette. In a neutral red uptake assay for cytotoxicity acrolein, crotonaldehyde, and formaldehyde comprised 90% of the gas vapor phase of reference cigarette toxicity.36 Despite lower levels of carbonyls in a portion of hemp cigarettes, they are still at biologically significant levels that can cause oxidative stress and apoptosis; especially notable is the delivery of a mixture of carbonyls producing synergistic apoptotic effects.37,38 Consumers elect to use hemp cigarettes for their advertised therapeutic effects, and our data suggest that inhaled products of combustion and pyrolysis could potentially mitigate these beneficial effects.
Longitudinal studies evaluating the carcinogenic potential and other health hazards associated with hemp cigarettes have not been conducted. However, comparisons can be made with combusted Cannabis for a similar generic smoke profile. Although reports have not generated conclusive evidence to determine that smoking Cannabis causes cancer,39,40 biological plausibility exists for cancer development. For example, Ghasemiesfe et al.39 observed low strength evidence with the development of testicular germ cell cancer, and the Centers for Disease Control and Prevention suggest that smoking Cannabis may lead to an increased risk of stroke and heart disease. With respect to lung health, Cannabis smoke causes damage and scarring to small blood vessels.41
Our data emphasize the importance of understanding the relative potential risks associated with different forms of CBD delivery. Although clear evidence of medical benefits of CBD has been demonstrated in a few diseases such as Parkinson's disease, seizures, and social anxiety disorder,1,42–44 available studies indicate that people instead use CBD to self-manage other physical and psychiatric symptoms. In a survey of CBD users, 62% sought to treat a medical condition. Among these respondents, 36% reported that CBD treated their condition “very well by itself” or “moderately well by itself” for chronic pain, arthritis and/or joint pain, and anxiety.12 Unclear dosing is a common issue, highlighted by Wheeler et al. reporting that a minority of respondents used a CBD product according to the label dose recommendation and a mere 9.6% of users reported discussing CBD use with their health care provider.11
CBD may potentially be inhaled in less toxic forms while exhibiting similar therapeutic effects. For example, electronic nicotine delivery systems demonstrate lower levels of formaldehyde and other carbonyls compared to smoked conventional tobacco cigarettes.45–47 Similar findings of lower toxicant yields were identified in a whole plant Cannabis vaporizer device48 and electronic cigarette-like devices,49 termed electronic drug-delivery systems (EDDS), compared to combusted whole plant material. However, a report in 2017 stated that both self-identified recreational cannabis and medicinal users preferred to smoke Cannabis, but medicinal users were much more likely to vaporize or consume it in food.50 Vaporized pure CBD has been shown to incite significantly greater subjective drug effect, pleasant drug effect, dry mouth, and throat irritated compared to oral CBD and placebo.51 This indicates that aerosolizing or vaporizing hemp with an EDDS or vaporizer may provide a lower-risk alternative to smoking hemp cigarettes with similar efficacy, but this is yet to be supported by scientific literature.
This study provides novel data regarding the carbonyl concentrations in mainstream smoke from combusted hemp cigarettes. Future studies should aim to provide data regarding long-term smoking patterns for hemp cigarettes to determine an accurate exposure and risk assessment over an extended period of product use. Only two brands of hemp cigarettes were chosen, which does not indicate the absolute carbonyl levels for hemp cigarettes. To properly assess the relative risk of hemp cigarettes, levels of metals, polycyclic aromatic hydrocarbons, volatile organic compounds, and other HPHCs as identified by the FDA18 should be measured. Future work should characterize the deposition pattern of CBD delivery along the respiratory path to determine efficacy. Other CBD delivery devices should be tested for similar constituents and relevant efficacy measurements to provide consumers with the information required to make an informed decision. Conventional cigarette manufacturing is highly automated52,53; QC measures and regulation should be developed for hemp-derived products.
Conclusion
Mainstream smoke from hemp cigarettes contains an array of carbonyls in biologically concerning levels. However, brand may determine whether or not the smoke has significantly lower concentration of carbonyls compared to conventional tobacco cigarettes. Importantly, all smoke samples contained easily detectable quantities of carbonyls, solidifying the assertion that hemp cigarettes are not risk free. A complete understanding of the risk profile of CBD products should be understood before recommending them for therapeutic purposes.
Supplementary Material
Abbreviations Used
- %RSD
percent relative standard deviation
- ACN
acetonitrile
- CBD
cannabidiol
- DAD
diode array detection
- DEA
Drug Enforcement Agency
- DNPH
2,4-dinitrophenylhydrazine
- EDDS
electronic drug-delivery systems
- FDA
Food & Drug Administration
- HCI
Health Canada Intense
- HPHC
harmful and potentially harmful constituent
- HPLC
high performance liquid chromatography
- LLOD
lower limit of detection
- LLOQ
lower limit of quantification
- m
slope
- QC
quality control
- R2
coefficient of determination
- SCSM-STEP
single cigarette smoking machine
- SD
standard deviation
- Sy
standard deviation of the response
- THC
Δ9-tetrahydrocannabinol
- THF
tetrahydrofuran
Author Disclosure Statement
J.O.E. is consultant to Nesmah. A.M.W. does not have any competing financial interests.
Funding Information
This work is made possible through benefactor support of the Inhaled Particle Aerosol Laboratory (IPAL) and Community Internal Medicine at Mayo Clinic Rochester, MN. Funders had no role in study design, data collection and analysis, decision to publish, or preparation of the article.
Supplementary Material
Cite this article as: Ward AM, Ebbert JO (2021) Carbonyl compounds in mainstream smoke of hemp cigarettes, Cannabis and Cannabinoid Research 6:4, 349–357, DOI: 10.1089/can.2020.0039.
References
- 1.National Academies of Sciences Engineering and Medicine (U.S.). Committee on the Health Effects of Marijuana: an Evidence Review and Research Agenda. The health effects of cannabis and cannabinoids: the current state of evidence and recommendations for research. The National Academies Press: Washington, DC, 2017 [PubMed] [Google Scholar]
- 2.Solowij N, Broyd SJ, van Hell HH, et al. A protocol for the delivery of cannabidiol (CBD) and combined CBD and 9-tetrahydrocannabinol (THC) by vaporisation. BMC Pharmacol Toxicol. 2014;15:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morgan CJ, Curran HV. Effects of cannabidiol on schizophrenia-like symptoms in people who use cannabis. Br J Psychiatry. 2008;192:306–307 [DOI] [PubMed] [Google Scholar]
- 4.FDA approves first drug comprised of an active ingredient derived from marijuana to treat rare, severe forms of epilepsy. https://www.fda.gov/news-events/press-announcements/fda-approves-first-drug-comprised-active-ingredient-derived-marijuana-treat-rare-severe-forms. Published 2018 (last accessed July20, 2020)
- 5.Andres C.Important legislative and regulatory changes impacting the commercialization of cannabis, hemp, and CBD. Altern Ther Health Med. 2019;25:36–38 [PubMed] [Google Scholar]
- 6.Schedules of Controlled Substances: Placement in Schedule V of Certain FDA-Approved Drugs Containing Cannabidiol; Corresponding Change to Permit Requirements. Drug Enforcement Administration. https://www.govinfo.gov/content/pkg/FR-2018-09-28/pdf/2018-21121.pdf (last accessed July20, 2020) [PubMed]
- 7.House - Agriculture. The Agriculture Improvement Act of 2018. https://www.congress.gov/bill/115th-congress/house-bill/2/text. Published 2018. (last accessed July20, 2020) [Google Scholar]
- 8.Williams S.9 Major Retailers That Are Selling CBD Products. https://www.fool.com/investing/2019/06/03/9-major-retailers-that-are-selling-cbd-products.aspx. Published 2019. (last accessed July20, 2020) [Google Scholar]
- 9.Dos Santos RG, Guimaraes FS, Crippa JAS, et al. Serious adverse effects of cannabidiol (CBD): a review of randomized controlled trials. Expert Opin Drug Metab Toxicol. 2020;16:517–526 [DOI] [PubMed] [Google Scholar]
- 10.Chesney E, Oliver D, Green A, et al. Adverse effects of cannabidiol: a systematic review and meta-analysis of randomized clinical trials. Neuropsychopharmacology. 2020. [Epub ahead of print]; DOI: 10.1038/s41386-020-0667-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wheeler M, Merten JW, Gordon BT, et al. CBD (Cannabidiol) product attitudes, knowledge, and use among young adults. Subst Use Misuse. 2020;55:1138–1145 [DOI] [PubMed] [Google Scholar]
- 12.Corroon J, Phillips JA. A cross-sectional study of cannabidiol users. Cannabis Cannabinoid Res. 2018;3:152–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mechoulam R, Parker LA, Gallily R. Cannabidiol: an overview of some pharmacological aspects. J Clin Pharmacol. 2002;42:11S–19S [DOI] [PubMed] [Google Scholar]
- 14.Millar SA, Stone NL, Yates AS, et al. A systematic review on the pharmacokinetics of cannabidiol in humans. Front Pharmacol. 2018;9:1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beck O.Cannabinoids in exhaled breath. In: Preedy V (ed). Handbook of cannabis and related pathologies: biology, pharmacology, diagnosis, and treatment. Elsevier, Inc.: Amsterdam, Netherlands, 2017, pp. 1018–1024 [Google Scholar]
- 16.Corwin E. Yes, You Can Smoke Hemp. And Yes, It's Gaining Popularity. Vermont Public Radio. https://www.vpr.org/post/yes-you-can-smoke-hemp-and-yes-its-gaining-popularity#stream/0. Published 2019. Updated August 22 (last accessed July20, 2020)
- 17.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Tobacco smoke and involuntary smoking. IARC Monogr Eval Carcinog Risks Hum. 2004;83:1–1438 [PMC free article] [PubMed] [Google Scholar]
- 18.FDA. Harmful and Potentially Harmful Constituents in Tobacco Products and Tobacco Smoke. https://www.fda.gov/tobacco-products/rules-regulations-and-guidance/harmful-and-potentially-harmful-constituents-tobacco-products-and-tobacco-smoke-established-list. Published 2012. (last accessed July20, 2020) [Google Scholar]
- 19.Jaccard G, Djoko DT, Korneliou A, et al. Mainstream smoke constituents and in vitro toxicity comparative analysis of 3R4F and 1R6F reference cigarettes. Toxicol Rep. 2019;6:222–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.CORESTA Recommended Method No. 74: Determination of Selected Carbonyls in Mainstream Cigarette Smoke by HPLC. Coorperation Centre for Scientific Research Relative to Tobacco: Paris, France, 2019 [Google Scholar]
- 21.Jablonski JJ, Maines JH, Cheetham AG, et al. Comparative levels of carbonyl delivery between mass-market cigars and cigarettes. Regul Toxicol Pharmacol. 2019;108:104453. [DOI] [PubMed] [Google Scholar]
- 22.Salamanca JC, Meehan-Atrash J, Vreeke S, et al. E-cigarettes can emit formaldehyde at high levels under conditions that have been reported to be non-averse to users. Sci Rep. 2018;8:7559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.World Health Organization. Standard operating procedure for intense smoking of cigarettes. https://apps.who.int/iris/bitstream/handle/10665/75261/9789241503891_eng.pdf. Published 2012. (last accessed July20, 2020) [Google Scholar]
- 24.Intorp M, Purkis S, Wagstaff W. Determination of carbonyl compounds in cigarette mainstream smoke. The CORESTA 2010 Collaborative Study and Recommended Method. Beitr Tabforsch Int. 2012;25:361–374 [Google Scholar]
- 25.Reilly SM, Goel R, Bitzer Z, et al. Little cigars, filtered cigars, and their carbonyl delivery relative to cigarettes. Nicotine Tob Res. 2018;20:S99–S106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Certificate of Analysis 1R6F Certified Reference Cigarette. University of Kentucky: Lexington, KY, 2016. https://www.ecigstats.org/docs/research/CoA_1R6F.pdf (last accessed July20, 2020)
- 27.Jain V, Alcheva A, Huang D, et al. Comprehensive chemical characterization of natural American spirit cigarettes. Tob Regul Sci. 2019;5:381–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pazo DY, Moliere F, Sampson MM, et al. Mainstream smoke levels of volatile organic compounds in 50 U.S. domestic cigarette brands smoked with the ISO and Canadian Intense Protocols. Nicotine Tob Res. 2016;18:1886–1894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Russo EB.Current therapeutic cannabis controversies and clinical trial design issues. Front Pharmacol. 2016;7:309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zumbado M, Luzardo OP, Rodriguez-Hernandez A, et al. Differential exposure to 33 toxic elements through cigarette smoking, based on the type of tobacco and rolling paper used. Environ Res. 2019;169:368–376 [DOI] [PubMed] [Google Scholar]
- 31.Geiss O, Kotzias D. Tobacco, cigarettes and cigarette smoke: an overview. Institute for Health and Consumer Protection: Ispra, Italy, 2007 [Google Scholar]
- 32.Darrall KG, Figgins JA. Roll-your-own smoke yields: theoretical and practical aspects. Tob Control. 1998;7:168–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sheehan TJ, Hamnett HJ, Beasley R, et al. Chemical and physical variations of cannabis smoke from a variety of cannabis samples in New Zealand. Forensic Sci Res. 2019;4:168–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cecil TL, Brewer TM, Young M, et al. Acrolein yields in mainstream smoke from commercial cigarette and little cigar tobacco products. Nicotine Tob Res. 2017;19:865–870 [DOI] [PubMed] [Google Scholar]
- 35.Baker RR, Pereira da Silva JR, Smith G. The effect of tobacco ingredients on smoke chemistry. Part I: flavourings and additives. Food Chem Toxicol. 2004;42:3–37 [DOI] [PubMed] [Google Scholar]
- 36.Stabbert R, Dempsey R, Diekmann J, et al. Studies on the contributions of smoke constituents, individually and in mixtures, in a range of in vitro bioactivity assays. Toxicol In Vitro. 2017;42:222–246 [DOI] [PubMed] [Google Scholar]
- 37.Zhang S, Zhang J, Chen H, et al. Combined cytotoxicity of co-exposure to aldehyde mixtures on human bronchial epithelial BEAS-2B cells. Environ Pollut. 2019;250:650–661 [DOI] [PubMed] [Google Scholar]
- 38.Zhang S, Zhang J, Cheng W, et al. Combined cell death of co-exposure to aldehyde mixtures on human bronchial epithelial BEAS-2B cells: molecular insights into the joint action. Chemosphere. 2020;244:125482. [DOI] [PubMed] [Google Scholar]
- 39.Ghasemiesfe M, Barrow B, Leonard S, et al. Association Between Marijuana Use and Risk of Cancer: a systematic review and meta-analysis. JAMA Netw Open. 2019;2:e1916318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tomar RS, Beaumount J, Hsieh JCY. Evidence on the carcinogenicity of marijuana smoke. California Environmental Protection Agency, August 2009: Sacramento, CA [Google Scholar]
- 41.Marijuana and Public Health. Centers for Disease Control and Prevention. https://www.cdc.gov/marijuana/health-effects.html. Updated February 27, 2018. (last accessed July20, 2020) [Google Scholar]
- 42.Chagas MH, Zuardi AW, Tumas V, et al. Effects of cannabidiol in the treatment of patients with Parkinson's disease: an exploratory double-blind trial. J Psychopharmacol. 2014;28:1088–1098 [DOI] [PubMed] [Google Scholar]
- 43.Lattanzi S, Brigo F, Trinka E, et al. Efficacy and safety of cannabidiol in epilepsy: a systematic review and meta-analysis. Drugs. 2018;78:1791–1804 [DOI] [PubMed] [Google Scholar]
- 44.Larsen C, Shahinas J. Dosage, Efficacy and safety of cannabidiol administration in adults: a systematic review of human trials. J Clin Med Res. 2020;12:129–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Farsalinos KE, Voudris V, Spyrou A, et al. E-cigarettes emit very high formaldehyde levels only in conditions that are aversive to users: a replication study under verified realistic use conditions. Food Chem Toxicol. 2017;109:90–94 [DOI] [PubMed] [Google Scholar]
- 46.Farsalinos KE, Yannovits N, Sarri T, et al. Carbonyl emissions from a novel heated tobacco product (IQOS): comparison with an e-cigarette and a tobacco cigarette. Addiction. 2018;113:2099–2106 [DOI] [PubMed] [Google Scholar]
- 47.Stephens WE.Comparing the cancer potencies of emissions from vapourised nicotine products including e-cigarettes with those of tobacco smoke. Tob Control. 2018;27:10–17 [DOI] [PubMed] [Google Scholar]
- 48.Gieringer D, St. Laurent J, Goodrich S. Cannabis vaporizer combines efficient delivery of THC with effective suppression of pyrolytic compounds. J Cannabis Ther. 2004;4:7–27 [Google Scholar]
- 49.Varlet V, Concha-Lozano N, Berthet A, et al. Drug vaping applied to cannabis: is “Cannavaping” a therapeutic alternative to marijuana? Sci Rep. 2016;6:25599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pacula RL, Jacobson M, Maksabedian EJ. In the weeds: a baseline view of cannabis use among legalizing states and their neighbours. Addiction. 2016;111:973–980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Spindle TR, Cone EJ, Goffi E, et al. Pharmacodynamic effects of vaporized and oral cannabidiol (CBD) and vaporized CBD-dominant cannabis in infrequent cannabis users. Drug Alcohol Depend. 2020;211:107937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Making Cigarettes. Philip Morris International. https://www.pmi.com/investor-relations/overview/how-cigarettes-are-made (last accessed July20, 2020)
- 53.Final Engineering Report: Tobacco Products Processing Detailed Study. U.S. Environmental Protection Agency, November 2006: Washington, D.C [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.