Abstract
West Nile virus (WNV) is a mosquito-borne flavivirus that can cause severe neurological disease in humans, for which there is no treatment or vaccine. From 2009 to 2018, California has reported more human disease cases than any other state in the United States. We sought to identify smaller geographic areas within the 10 California counties with the highest number of WNV cases that accounted for disproportionately large numbers of human cases from 2009 to 2018. Eleven areas, consisting of groups of high-burden ZIP codes, were identified in nine counties within southern California and California's Central Valley. Despite containing only 2% of California's area and 17% of the state's population, these high-burden ZIP codes accounted for 44% of WNV cases reported and had a mean annual incidence that was 2.4 times the annual state incidence. Focusing mosquito control and public education efforts in these areas would lower WNV disease burden.
Keywords: West Nile virus, California, arbovirus, epidemiology, public health
Introduction
West Nile virus (WNV) is the leading cause of mosquito-borne disease in the contiguous United States (Rosenberg et al. 2018, McDonald et al. 2019). WNV is maintained in an enzootic cycle between birds and mosquitoes and is transmitted tangentially to humans primarily by Culex mosquitoes (Petersen et al. 2013, Reisen 2013, Rochlin et al. 2019). This complex ecology results in high spatiotemporal variability in disease incidence, with seasonal summer peaks that vary in size and location (Lindsey et al. 2010, CDC 2020). Although most areas have only sporadic cases or periodic outbreaks, some have a consistently higher burden of WNV disease.
Most WNV infections are asymptomatic (Mostashari et al. 2001, Sejvar and Marfin 2006, Petersen et al. 2013). Approximately 20–30% of infections result in an acute systemic febrile illness and <1% lead to neuroinvasive disease (e.g., meningitis, encephalitis, or myelitis), with a higher incidence among older populations. Among patients with neuroinvasive disease, the case fatality rate is close to 10% (Sejvar 2007). Due to its severe clinical features, diagnosis and reporting of neuroinvasive disease are more consistent and complete than non-neuroinvasive disease (Lindsey et al. 2008, 2010). From 2009 to 2018, a total of 21,869 WNV disease cases were reported in the United States, including 12,835 (58.7%) cases of neuroinvasive disease and 1199 (5.5%) deaths (CDC 2020). California reported more cases of WNV disease than any other state, accounting for 4035 (18%) of all cases nationwide from 2009 to 2018 (California Department of Public Health 2019, Snyder et al. 2020a).
Since WNV was introduced in North America, there have been a number of studies exploring demographic, ecological, environmental, and community determinants of WNV disease. In Orange County, California, increased WNV incidence was associated with low income and high housing density from 2004 to 2008 (Harrigan et al. 2010). In Kern County, there was an association between human WNV cases and delinquent mortgages and neglected swimming pools (Reisen et al. 2008). A 2019 study in San Joaquin County, California, analyzing data from 2011 to 2015, indicated that WNV incidence was higher in communities with a high number of housing foreclosures, a higher proportion of non-White residents, and a higher proportion of the population over 50 years of age (Hernandez et al. 2019). In addition, although nationwide disease incidence is generally higher in rural areas compared with cities, most cases are reported from large metropolitan areas (Petersen 2019). Although temperature and humidity have been shown to be closely related to increased WNV transmission (Epstein 2001, Epstein and Defilippo 2001, Hernandez et al. 2019), the relationships between WNV disease and other environmental parameters are not as well described, but increased amounts of vegetation have been associated with WNV risk (Brownstein et al. 2002, Ruiz et al. 2007).
No WNV vaccine or specific treatment is currently available for use in humans (Beasley 2011, Kaiser and Barrett 2019). Reducing mosquito exposure through vector control, public education, and personal protective behaviors are the primary forms of prevention (Petersen et al. 2013). States report disease cases to the CDC by county of residence (CDC 2020), and California has already identified that >95% of all cases occur in southern California or Central Valley counties (Snyder et al. 2020b). However, California's counties are large, with 47 of the 58 having areas greater than 2000 km2, including San Bernardino County, the largest in the continental United States, covering 51,947 km2. Identifying subcounty areas with increased burden of WNV disease could help optimize interventions and resource allocation. In this descriptive retrospective study, we identified and characterized California counties and ZIP codes with disproportionately high numbers of WNV disease cases to direct further evaluation and prevention efforts.
Methods
Geographic area
California has a population of 39.6 million people and a cumulative area of 423,971 km2 (U.S. Census Bureau 2017). The state has 58 counties and 2597 ZIP codes.
Case identification and reporting
WNV disease is a nationally notifiable condition (NNDSS 2020). Cases that meet the national surveillance case definition, developed by the Council of State and Territorial Epidemiologists (CSTE 2015), and occur among California residents are reported to the California Department of Public Health (CDPH) by health care providers and diagnostic laboratories. WNV disease cases with symptom onset from January 1, 2009, to December 31, 2018, were extracted from CDPH records. Asymptomatic infections do not fulfill the case definition and therefore were excluded from analyses. The collection and analysis of human surveillance data are routine public health activities and are exempt from Institutional Review Board review and approval Project 2020-072-CDPH.
Defining high-burden counties and ZIP codes
We defined the 10 California counties with the most reported WNV infections from 2009 to 2018 as high-burden counties. Within each high-burden county, we divided ZIP codes into categories based on the cumulative number of WNV cases per ZIP code. Five categories were created using natural (Jenks) breaks, rounded to the nearest five (0, 1–5, 6–10, 11–20, and ≥21) to visualize the data. In each high-burden county, we identified one or more groups of adjacent ZIP codes with >21 cases, which collectively had a higher incidence than the entire county. If another ZIP code with less WNV disease (≤21) was geographically contained within the group of highest burden ZIP codes and was in the same county, it was also included to provide a contiguous area for analysis. All ZIP codes within these groups were defined as high burden, whereas all other ZIP codes within the county were defined as low burden, even those with >21 cases.
Comparing characteristics of high- and low-burden ZIP codes
Community-level demographic data for each ZIP code were excerpted from the U.S. Census Bureau 2017 American Community Survey, 5-year estimates (U.S. Census Bureau 2017). Potential risk factors for elevated WNV incidence included population, median age, proportion of population aged ≥60 years, proportion of the population that was White non-Hispanic, median household income, and proportion of the population with health insurance. Population density was estimated using the population size and the area of each ZIP code in square kilometers.
Mean elevations of each ZIP code, in meters, were estimated from the USGS 100-meter resolution raster file of the contiguous United States (U.S. Geological Survey 2012). To estimate land cover, we used the USGS GAP/LANDFIRE National Terrestrial Ecosystems raster file (U.S. Geological Survey Gap Analysis Program 2011). We extracted all raster points classified as low, moderate, or high intensity developed land cover, combined them into a single polygon, and calculated the proportion of each ZIP code that was covered by any of these land cover groups. We also identified which ZIP codes were within the jurisdictional boundaries of a local vector control agency (MVCAC 2017).
Data analyses
Average annual incidence per 100,000 residents was calculated by dividing the total number of cases in that time period within a geographic unit by the 2017 population estimate for that geographic area, multiplying by 100,000, and then dividing by 10. Continuous demographic and environmental characteristics of high- and low-burden ZIP codes were compared with two-sample t-tests. The only noncontinuous characteristic, presence or absence of a vector control agency in a ZIP code, was compared by chi-square analysis or by Fisher's exact test if an expected cell value was less than five. Due to making 10 comparisons, we applied a Bonferroni correction, reducing the threshold for statistical significance to p ≤ 0.005. Statistical analyses were conducted using SAS software, v.9.4 (SAS Institute 2016, Cary, NC), while all mapping and spatial calculations were conducted using ArcGIS Pro 2.3.15769 (Esri, Inc., 2018, Redlands, CA).
Results
A total of 4123 WNV disease cases were reported in California from 2009 to 2018, with an average statewide annual incidence of 1.1 cases per 100,000 population. The median number of cases each year was 425 (range, 112–843 cases). There were 2735 (66%) cases with symptom onset from July to September. The median age of case-patients was 59 years (interquartile range, 47–69 years), with 1978 (48%) cases aged ≥60 years; 2561 (62%) cases were male. Of the 4123 cases, 2899 (70%) were reported as neuroinvasive disease cases.
High-burden counties
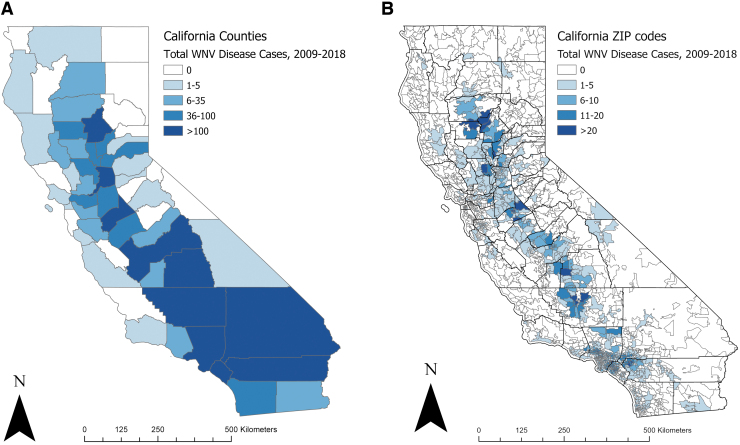
Each of the 10 (17%) counties in California with the highest number of WNV cases had >100 total cases reported from 2009 to 2018 (Fig. 1A). All 10 counties were located in southern California (Los Angeles, Orange, Riverside, and San Bernardino) or California's Central Valley (Stanislaus, Kern, Fresno, Butte, Sacramento, and Tulare). Combined, these 10 counties accounted for 3338 (81%) of the WNV disease cases reported in the state during this time frame (Table 1). Los Angeles County accounted for 1414 (34%) of all cases reported statewide. The median number of annual cases in these high-burden counties during the 10-year period ranged from 11 in Butte, San Bernardino, and Tulare to 151 in Los Angeles. Eight of the counties had an average annual incidence ≥1.1 per 100,000 residents.
FIG. 1.
WNV disease cases by county (A) and ZIP code (B) of residence—California, 2009–2018 (n = 4123 cases). Detailed regional maps of ZIP code-level data are shown in Fig. 2. (B) Includes 3864 (94%) cases with known ZIP code of residence. WNV, West Nile virus.
Table 1.
California Counties with the Most Reported West Nile Virus Disease Cases, 2009–2018
| County | Region | Cumulative cases [N = 4123] |
Annual cases |
Average annual incidence |
|---|---|---|---|---|
| n (%) | Median, (IQR) | Per 100,000 populationa | ||
| Los Angeles | Southern California | 1414 (34) | 151 (37–263) | 1.4 |
| Orange | Southern California | 502 (12) | 22 (4–35) | 1.6 |
| Riverside | Southern California | 271 (7) | 16 (6–33) | 1.1 |
| San Bernardino | Southern California | 206 (5) | 11 (5–38) | 1.0 |
| Stanislaus | Central Valley | 206 (5) | 18 (14–27) | 3.8 |
| Kern | Central Valley | 186 (5) | 18 (13–25) | 2.1 |
| Fresno | Central Valley | 169 (4) | 14 (9–23) | 1.7 |
| Butte | Central Valley | 155 (4) | 11 (3–24) | 6.8 |
| Sacramento | Central Valley | 124 (3) | 13 (5–18) | 0.8 |
| Tulare | Central Valley | 105 (3) | 11 (7–12) | 2.3 |
Ten (17%) of California's 58 counties accounted for 3338 (81%) of the 4123 cases reported from 2009 to 2018.
Incidence calculated using the 2017 Census-estimated population.
IQR, interquartile range.
High-burden ZIP codes
Of the 2597 ZIP codes in California, 30 (1.2%) had >20 WNV disease cases (maximum: 43) and 94 (3.6%) had 11–20 cases reported from 2009 to 2018; 1766 (68.0%) ZIP codes had no WNV disease cases reported during the 10-year period (Fig. 1B). Within the 10 counties with the most cases, we identified 11 geographical groupings of ZIP codes with a higher incidence of WNV disease than the county as a whole, including two high-burden ZIP code clusters each in Los Angeles and Orange counties and one cluster each in Riverside, San Bernardino, Stanislaus, Kern, Fresno, Butte, and Tulare counties (Fig. 2 and Table 2). These 11 ZIP code clusters included 151 (5.8%) of the 2597 ZIP codes in California, contained 6.5 million (16.6%) of the state's 39.1 million population, and covered 3444 miles2 (2.3%) of the state's total area, but accounted for 1710 (44.3%) of the state's WNV disease cases reported from 2009 to 2018. Combined, the 11 clusters had an average annual incidence of 2.6 cases per 100,000 persons per year, almost 2.5 times the state's average annual incidence.
FIG. 2.
WNV disease cases by ZIP code in (A) southern California; (B) southern Central Valley; and (C) northern Central Valley—California, 2009–2018.
Table 2.
Characteristics of California ZIP Code Clusters with High Burden of West Nile Virus Disease, 2009–2018
| ZIP code clusters | ZIP codes; [N = 2597] |
Population; [N = 39,071,323] |
Area (km2); [N = 423,971 km2] |
Cumulative cases; [N = 3864] |
Average annual incidence (1.1) |
|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | Per 100,000 population | |
| Los Angeles—West | 40 (1.5) | 1,489,910 (3.8) | 523 (0.1) | 417 (10.8) | 3.0 |
| Los Angeles—East | 17 (0.7) | 628,593 (1.6) | 218 (0.1) | 134 (3.5) | 2.1 |
| Orange—North | 14 (0.5) | 682,856 (1.8) | 218 (0.1) | 178 (4.6) | 2.6 |
| Orange—South | 18 (0.7) | 880,417 (2.3) | 293 (0.1) | 214 (5.5) | 2.4 |
| Riverside | 12 (0.5) | 652,435 (1.7) | 593 (0.1) | 170 (4.4) | 2.6 |
| San Bernardino | 10 (0.4) | 493,390 (1.3) | 329 (0.1) | 99 (2.6) | 2.0 |
| Stanislaus | 8 (0.3) | 278,486 (0.7) | 566 (0.1) | 116 (3.0) | 4.2 |
| Fresno | 9 (0.3) | 349,781 (0.9) | 199 (<0.1) | 77 (2.0) | 2.2 |
| Tulare | 5 (0.2) | 226,646 (0.6) | 668 (0.2) | 55 (1.4) | 2.4 |
| Kern | 15 (0.6) | 674,425 (1.7) | 4128 (1.0) | 164 (4.2) | 2.4 |
| Butte | 3 (0.1) | 111,302 (0.3) | 1186 (0.3) | 86 (2.2) | 7.7 |
| All ZIP code clusters | 151 (5.8) | 6,468,241 (16.6) | 8921 (2.1) | 1710 (44.3) | 2.6 |
Percentages calculated as proportion of California's total reported cases.
The largest cluster comprised 40 adjacent ZIP codes in western Los Angeles County, which contained 3.8% of the state's population and 0.1% of the state's total area, but accounted for 10.8% of the reported WNV disease cases. Two clusters that straddled the border between southeastern Los Angeles County and northwestern Orange County (i.e., Los Angeles County—east, and Orange County—north) included a total of 31 ZIP codes, containing 3.4% of the state's population and covering 0.2% of the state's total area, but accounting for 8.1% of the state's WNV disease cases. In the northern Central Valley, the only high-burden cluster was identified in Butte County. Although Sacramento County had 124 reported cases from 2009 to 2018, they were relatively evenly distributed throughout the county with no high-burden ZIP codes identified.
Characteristics of high-burden ZIP codes
When aggregated, the 151 ZIP codes in the 11 high-burden clusters had a higher mean population, higher population density, younger median age, and a smaller proportion of the population aged 60 years and older than the 2446 low-burden ZIP codes in the same nine counties (Table 3). The higher burden ZIP codes were also lower in elevation, more developed, and more likely to be within the service area of a local vector control agency. These trends were relatively consistent across counties. However, unlike the high-burden ZIP codes in rest of the nine counties, high-burden ZIP codes in Los Angeles County had a similar number of residents and population density compared with low-burden ZIP codes in the county.
Table 3.
Characteristics of ZIP Codes in Contiguous Groups with High Burden of Reported West Nile Virus Disease Cases Compared with All Other ZIP Codes in Nine California Counties (Los Angeles, Orange, Riverside, San Bernardino, Stanislaus, Kern, Fresno, Butte, and Tulare)
| Characteristic | High-burden ZIP codes (IQR) [N = 151] | Low-burden ZIP codes (IQR) [N = 607] | pa | ||
|---|---|---|---|---|---|
| Total population | 6,468,241 | 14,818,550 | |||
| Mean population | 42,836 | 27,233–55,869 | 25,245 | 3962–38,304 | <0.001 |
| Population density per km2 | 2369 | 1140–3379 | 1756 | 43–2725 | 0.004 |
| Median age in years | 36 years | 32–39 | 39 years | 32–43 | <0.001 |
| Proportion aged ≥60 years | 18% | 14–20 | 21% | 14–25 | <0.001 |
| Average elevation | 160 meters | 39–249 | 421 meters | 65–583 | <0.001 |
| Proportion of developed land cover | 71% | 56–95 | 43% | 38–88 | <0.001 |
| Proportion with vector control agency | 99% | 88% | <0.001 | ||
| Proportion of White non-Hispanic residents | 37% | 20–51 | 42% | 16–66 | 0.01 |
| Median household income | $67,436 | 51,961–84,714 | $63,558 | 41,276–80,230 | 0.08 |
| Proportion of medically insured people | 89% | 86–92 | 88% | 85–94 | 0.61 |
Bold text indicates statistical significance.
Due to making 10 comparisons, the Bonferroni adjustment was applied, resulting in statistical significance at p ≤ 0.005.
Discussion
California has the highest WNV disease burden in the United States, but that burden is not uniformly distributed in the state. From 2009 to 2018, the 10 counties with the highest WNV disease burden in California were all in southern California or the Central Valley. Among these 10 counties, there were clusters of ZIP codes with a disproportionately higher burden of human WNV disease. These 11 high-burden groups of ZIP codes collectively contained 17% of California's population and covered 2% of its area, but accounted for 44% of the state's WNV disease cases reported during the 10-year study period. In Los Angeles County alone, two of these high-burden ZIP code clusters accounted for 14.3% of all cases reported in the state despite being home to 5.4% of the population and comprising 0.2% of state's area.
By identifying contiguous ZIP codes within already high-burden California counties with elevated burden of WNV disease, we have potentially found areas that could benefit the most from targeted public education campaigns, where vector control agencies should focus mosquito control efforts, particularly when resources, such as time and staffing, are limited. Not only is there no effective treatment for patients with WNV disease but also medical care, particularly for those with neuroinvasive disease, is expensive. National estimates found an average annual cost of $56 million in medical expenses due to WNV disease from 1999 to 2016 (Ronca et al. 2019). In Sacramento County in 2005, an epidemic year of WNV disease and environmental activity, it was estimated to cost ∼$33,000 to treat each patient with WNV neuroinvasive disease and >$700,000 to provide emergency vector control to treat the county's 2570-km2 area (Barber et al. 2010). If vector control efforts prevented just 15 WNV neuroinvasive disease cases, it became more cost-effective than providing medical care (Barber et al. 2010). A more recent study in California found that from 2004 to 2017, there was a median charge of $142,321 per patient with WNV disease; an average of almost $60 million in charges per year (Snyder et al. 2020a). Based on these latter estimates, vector control efforts are even more cost-effective. By treating these smaller, densely populated high-disease burden areas, resources would be used most efficiently to reduce the current disease burden.
We also described the common demographic and environmental characteristics of these high-burden ZIP codes. When compared with the rest of the county, they tended to have higher populations and population densities than other parts of the same county. Residents in high-burden ZIP codes were, on average, younger than residents of lower burden ZIP codes. They also tended to have a higher proportion of White non-Hispanic residents, although that difference was not significant (p > 0.005). These clusters of high-burden ZIP codes were at a lower elevation, had more of their land area classified as developed, and were more likely to be within the service area of a vector control agency.
There were several differences between the population at risk of WNV and the demographic characteristics of these high-burden ZIP code groups. It is well established that older persons are more at risk of severe WNV disease (O'Leary et al. 2004, Hayes et al. 2005, Martinez et al. 2017, McDonald et al. 2019, Petersen 2019), and in at least one part of California's Central Valley, incidence was reported to be higher in areas where the population was older (Hernandez et al. 2019). However, we focused on the raw number of cases, which has previously been observed to be higher in metropolitan areas (Petersen 2019) where populations tend to be younger (Parker et al. 2018). The WNV incidence in these areas is not the highest in California (Snyder et al. 2020b), but by sheer numbers and density of people, there were more cases.
The environmental characteristics of higher burden groups of ZIP codes were largely consistent across high-burden counties. The primary vectors of WNV in California, Culex tarsalis, Culex pipiens, and Culex quinquefasciatus (Goddard et al. 2002), are found at lower elevations in the state (Bohart and Washino 1978) where most of the cases were reported. Metropolitan areas, which have higher populations and subsequently higher population densities, tend to have more area covered with low to high developed land cover, defined as 20–100% of the area covered with impervious surfaces (MRLCC 2011). In the northeastern United States, WNV incidence was higher in urban counties than those with more forest cover (Brown et al. 2008). Although several prior studies focused on vegetation abundance, as opposed to coverage by impervious surfaces, they only looked at a single year of disease in New York City, Chicago, and Detroit (Brownstein et al. 2002, Ruiz et al. 2007). However, our study covered a period of 10 years, including 2012–2016, when California experienced a drought of historic severity (USGS 2020). To simplify the variation in vegetation during a prolonged time period, we chose to focus on impervious land cover.
There were several limitations to this study. Available surveillance data included only reported cases. However, <5% of WNV non-neuroinvasive disease cases are likely to be diagnosed and reported (Lindsey et al. 2010, McDonald et al. 2019). Reported cases could differ from unreported cases in their geographic distribution or key demographic characteristics that would have biased these results. For example, low-burden ZIP codes tended to be lower income areas, although we did not observe a significant difference (p > 0.005) in income, where residents often have limited access to medical care, resulting in fewer reported cases (Shi et al. 2010). This may have been due to the ecological nature of our analysis or the inadequacy of using a measure such as median household income to describe poverty. Furthermore, this study was based on the residential address of reported cases, but this may have been different than the location where infection occurred. We visualized the geographic distribution of cases to identify high-burden areas instead of more robust methods such as geographically weighted regression or hot spot analysis, using Getis-Ord G. A previous exploration of those methods consistently identified Los Angeles County as a high-burden area (unpublished data), which is not surprising given that Los Angeles County reports more cases than any other county and has a population of >10 million people, 25% of the state's total (U.S. Census Bureau 2017). However, we sought to explore the potential for high-burden areas in other parts of the state. There is also the risk that ZIP codes in the high-burden groups were similar to each other simply due to spatial proximity. Autocorrelation is likely in any geospatial analysis and methods to address it tend to reduce statistical power. However, we set a very high threshold for statistical significance (p ≤ 0.005) so as to reduce the potential impact of autocorrelation.
All 10 counties that reported the most WNV cases had at least one vector control agency that services most or all of the county's area. As a result, 99% of our high-burden ZIP codes fell within the service area of a vector control agency. Those agencies collect environmental data, such as mosquito abundance and infection rates, to estimate risk of human WNV transmission (Snyder 2020b). We hope to quantify how those environmental indicators were associated with the number and timing of human cases in a given area. In response to surveillance results, the vector control agencies routinely conduct mosquito control, including the application of pesticides to reduce mosquito abundance (Barber et al. 2010). It is feasible that their ongoing mosquito control activities have already substantially reduced WNV transmission in California, particularly in lower burden areas.
Conclusion
There is no vaccine to prevent or specific therapy to treat WNV disease (Beasley 2011, Kaiser and Barrett 2019). Comprehensive mosquito control, public education, and personal protective behaviors remain the primary tools for prevention and control. Targeting enhanced efforts to a limited number of areas that account for a disproportionate number of cases could provide substantial reductions in disease burden and lead to a more efficient and effective deployment of public health resources.
Acknowledgments
The authors thank the local public health jurisdictions in California who investigated and reported human WNV disease, as well as the state's local vector control agencies that conducted environmental WNV surveillance and control. The authors also acknowledge the contributions of Tina Feiszli and other members of the CDPH Vector-Borne Disease Section. Finally, they thank their partners in the CDC Emerging Infections Program Arbovirus Project from the Colorado Department of Public Health and Environment, specifically Jennifer House, Tracy Woodall, and Juan Vazquez.
Authors' Contributions
All authors met the definition of authorship, as defined by the International Committee of Medical Journal Editors.
Author Disclosure Statement
The authors report no real or perceived conflicts of interest.
Funding Information
This work was supported by the U.S. Centers for Disease Control and Prevention through the Emerging Infections Program Cooperative Agreement (grant CK17–1701).
References
- Barber LM, Schleier JJ, 3rd, Peterson RK. Economic cost analysis of West Nile virus outbreak, Sacramento County, California, USA, 2005. Emerg Infect Dis 2010; 16:480–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beasley DW. Vaccines and immunotherapeutics for the prevention and treatment of infections with West Nile virus. Immunotherapy 2011; 3:269–285 [DOI] [PubMed] [Google Scholar]
- Bohart RM, Washino RK. Mosquitoes of California, 3rd ed. Berkeley, California: University of California Division of Agricultural Sciences, 1978 [Google Scholar]
- Brown HE, Childs JE, Diuk-Wasser MA, Fish D. Ecologic factors associated with West Nile virus transmission, northeastern United States. Emerg Infect Dis 2008; 14:1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownstein JS, Rosen H, Purdy D, Miller JR, et al. Spatial analysis of West Nile virus: Rapid risk assessment of an introduced vector-borne zoonosis. Vector Borne Zoonotic Dis 2002; 2:157–164 [DOI] [PubMed] [Google Scholar]
- California Department of Public Health V.B.D.S. Reported Incidence of West Nile Virus. California, 2019 [Google Scholar]
- Centers for Disease Control and Prevention. West Nile Virus Disease Cases and Deaths Reported to CDC By Year and Clinical Presentation, Atlanta, Georgia, 1999–2018. 2020
- Council of State and Territorial Epidemiologists. CSTE Position Statement 14-ID-04: Arboviral Diseases, Neuroinvasive and Non-neuroinvasive 2015 Case Definition, Atlanta, Georgia, 2018
- Epstein PR. West Nile virus and the climate. J Urban Health 2001; 78:367–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein PR, Defilippo C. West Nile virus and drought. Global Change Hum Health 2001; 2:105–107 [Google Scholar]
- Goddard LB, Roth AE, Reisen WK, Scott TW. Vector competence of California mosquitoes for West Nile virus. Emerg Infect Dis 2002; 8:1385–1391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrigan RJ, Thomassen HA, Buermann W, Cummings RF, et al. Economic conditions predict prevalence of West Nile virus. PLoS One 2010; 5:e15437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes EB, Komar N, Nasci RS, Montgomery SP, et al. Epidemiology and transmission dynamics of West Nile virus disease. Emerg Infect Dis 2005; 11:1167–1173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez E, Torres R, Joyce AL. Environmental and sociological factors associated with the incidence of West Nile virus cases in the Northern San Joaquin Valley of California, 2011–2015. Vector Borne Zoonotic Dis 2019; 11:851–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser JA, Barrett AD. Twenty years of progress toward west nile virus vaccine development. Viruses 2019; 11:823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsey NP, Kuhn S, Campbell GL, Hayes EB. West Nile virus neuroinvasive disease incidence in the United States, 2002–2006. Vector Borne Zoonotic Dis 2008; 8:35–40 [DOI] [PubMed] [Google Scholar]
- Lindsey NP, Staples JE, Lehman JA, Fischer M, et al. Surveillance for human West Nile virus disease—United States, 1999–2008. MMWR Morb Mortal Wkly Rep 2010; 59:1–17 [PubMed] [Google Scholar]
- Martinez D, Murray KO, Reyna M, Arafat R, et al. West Nile virus outbreak in Houston and Harris County, Texas, USA, 2014. Emerg Infect Dis 2017; 23:1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald E, Martin SW, Landry K, Gould CV, et al. West Nile virus and other domestic nationally notifiable arboviral diseases—United States, 2018. MMWR Morb Mortal Wkly Rep 2019; 68:673–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosquito and Vector Control Association of California. MVCAC Webmap, Sacromento, California, 2017 [Google Scholar]
- Mostashari F, Bunning ML, Kitsutani PT, Singer DA, et al. Epidemic West Nile encephalitis, New York, 1999: Results of a household-based seroepidemiological survey. Lancet 2001; 358:261–264 [DOI] [PubMed] [Google Scholar]
- Multi-Resolution Land Characteristics Consortium. National Land Cover Database 2011 (NLCD2011) Legend, Sioux Falls, South Dakota, 2011 [Google Scholar]
- National Notifiable Diseases Surveillance System. Centers for Disease Control and Prevention, Atlanta, Georgia, 2020 [Google Scholar]
- O'Leary DR, Marfin AA, Montgomery SP, Kipp AM, et al. The epidemic of West Nile virus in the United States, 2002. Vector Borne Zoonotic Dis 2004; 4:61–70 [DOI] [PubMed] [Google Scholar]
- Parker K, Menasce Horowitz J, Brown A, Fry R, et al. Demographic and economic trends in urban, suburban and rural communities. Pew Research Center 2018 [Google Scholar]
- Petersen L, Carson P, Biggerstaff B, Custer B, et al. Estimated cumulative incidence of West Nile virus infection in US adults, 1999–2010. Epidemiol Infect 2013; 141:591–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen LR. Epidemiology of West Nile virus in the United States: Implications for arbovirology and public health. J Med Entomol 2019;56:1456–1462 [DOI] [PubMed] [Google Scholar]
- Reisen WK. Ecology of West Nile virus in North America. Viruses 2013; 5:2079–2105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisen WK, Takahasi RM, Carroll BD, et al. Delinquent mortgages, neglected swimming pools, and West Nile virus, California. Emerg Inf Dis 2008; 14:1747–1749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochlin I, Faraji A, Healy K, Andreadis TG. West Nile virus mosquito vectors in North America. J Med Entomol 2019;56:1475–1490 [DOI] [PubMed] [Google Scholar]
- Ronca SE, Murray KO, Nolan MS. Cumulative incidence of West Nile virus infection, Continental United States, 1999–2016. Emerg Infect Dis 2019; 25:325–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg R, Lindsey NP, Fischer M, Gregory CJ, et al. Vital signs: Trends in reported vectorborne disease cases—United States and Territories, 2004–2016. MMWR Morb Mortal Wkly Rep 2018; 67:496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz MO, Walker ED, Foster ES, Haramis LD, et al. Association of West Nile virus illness and urban landscapes in Chicago and Detroit. Int J Health Geogr 2007; 6:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sejvar JJ. The long-term outcomes of human West Nile virus infection. Clin Infect Dis 2007; 44:1617–1624 [DOI] [PubMed] [Google Scholar]
- Sejvar JJ, Marfin AA. Manifestations of West Nile neuroinvasive disease. Rev Med Virol 2006; 16:209–224 [DOI] [PubMed] [Google Scholar]
- Shi L, Lebrun LA, Tsai J. Access to medical care, dental care, and prescription drugs: The roles of race/ethnicity, health insurance, and income. South Med J 2010; 103:509. [DOI] [PubMed] [Google Scholar]
- Snyder RE, Cooksey G, Kramer V, Jain S, et al. West Nile virus-associated hospitalizations, California, USA, 2004–2017. Clin Infect Dis 2020a [DOI] [PubMed] [Google Scholar]
- Snyder RE, Feiszli T, Foss L, Messenger S, et al. West Nile virus in California, 2003–2018: A persistent threat. PLoS Negl Trop Dis 2020b; 14:e0008841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Census Bureau. American Community Survey 5-Year Estimates, Washington, DC, 2017 [Google Scholar]
- U.S. Geological Survey. 100-Meter Resolution Elevation of the Conterminous United States. Rolla, MO: National Atlas of the United States, 2012 [Google Scholar]
- U.S. Geological Survey Gap Analysis Program. GAP/LANDFIRE National Terrestrial Ecosystems. U.S. Geological Survey, 2011 [Google Scholar]
- United States Geological Survey. n.d. 2012. –2016 California Drought: Historical Perspective. U.S. Department of the Interior. https://ca.water.usgs.gov/california-drought/california-drought-comparisons.html, accessed April19, 2020