Abstract
Background and Objectives: Preclinical studies have shown cannabidiol is protective in models of ischemic stroke. Based on results from our recent systematic review, we investigated the effects of two promising neuroprotective phytocannabinoids, cannabigerol (CBG) and cannabidivarin (CBDV), on cells of the blood–brain barrier (BBB), namely human brain microvascular endothelial cells (HBMECs), pericytes, and astrocytes.
Experimental Approach: Cultures were subjected to oxygen-glucose deprivation (OGD) protocol to model ischemic stroke and cell culture medium was assessed for cytokines and adhesion molecules post-OGD. Astrocyte cell lysates were also analyzed for DNA damage markers. Antagonist studies were conducted where appropriate to study receptor mechanisms.
Results: In astrocytes CBG and CBDV attenuated levels of interleukin-6 (IL-6) and lactate dehydrogenase (LDH), whereas CBDV (10 nM–10 μM) also decreased vascular endothelial growth factor (VEGF) secretion. CBDV (300 nM–10 μM) attenuated levels of monocyte chemoattractant protein (MCP)-1 in HBMECs. In astrocytes, CBG decreased levels of DNA damage proteins, including p53, whereas CBDV increased levels of DNA damage markers. Antagonists for CB1, CB2, PPAR-γ, PPAR-α, 5-HT1A, and TRPV1 had no effect on CBG (3 μM) or CBDV (1 μM)-mediated decreases in LDH in astrocytes. GPR55 and GPR18 were partially implicated in the effects of CBDV, but no molecular target was identified for CBG.
Conclusions: We show that CBG and CBDV were protective against OG mediated injury in three different cells that constitute the BBB, modulating different hallmarks of ischemic stroke pathophysiology. These data enhance our understanding of the protective effects of CBG and CBDV and warrant further investigation into these compounds in ischemic stroke. Future studies should identify other possible neuroprotective effects of CBG and CBDV and their corresponding mechanisms of action.
Keywords: blood–brain barrier, cannabidivarin, cannabigerol, cannabinoids, ischemia, neuroprotection
Introduction
The blood–brain barrier (BBB) is a unique interface that separates the central nervous system (CNS) and the periphery, protecting the brain from damaging components found in general circulation, namely peripheral leukocytes, macromolecules, and xenobiotics.1,2 The barrier itself is formed by microvascular endothelial cells, which are encompassed by pericytes, and altogether surrounded by astrocyte end feet, which cover 99% of BBB endothelia.3 Cerebral ischemia–reperfusion (IR) initiates a plethora of inflammatory signaling pathways, cytotoxic glutamate release, and oxidative stress, all of which contribute to increases in BBB permeability.4 This loss of BBB integrity ultimately causes uncontrolled immune infiltration into the CNS that perpetuates neuronal injury and hinders poststroke recovery. Although administration of tissue plasminogen activator (tPA) and mechanical thrombectomy are effective licensed therapies to dissolve or remove the culpable clot, at present, there are no available approved therapies that mitigate poststroke injury.5
Cannabidiol (CBD), one of the chemicals found in Cannabis sativa, has displayed a range of neuroprotective qualities, preventing neuronal loss,6,7 attenuating astrocyte reactivity,8 and dampening the neuroinflammatory response.9 Unlike delta9-tetrahydrocannabinol (Δ9-THC), CBD does not activate the central cannabinoid receptors, CB1 or CB2, but activates a plethora of other targets including PPAR-γ, TRPV1, and 5-HT1A receptors.10–13 CBD has formulations (alone and with Δ9-THC) licensed by GW pharmaceuticals to treat rare childhood epilepsies and spasticity associated with multiple sclerosis. The protective effects of CBD in stroke models has been well documented,14 specifically CBD has been shown to reduce infarct volume,15,16 reduce glutamate toxicity,9,17 attenuate mitochondrial dysfunction18 and glial activation.6,19 In a co-culture BBB model CBD preserved barrier integrity after oxygen-glucose deprivation (OGD), which was mediated at least in part by PPAR-γ and 5-HT1A receptors.12
Cannabigerol (CBG) and cannabidivarin (CBDV) are neutral cannabinoids present in cannabis and studies have found these compounds share similar pharmacological characteristics to CBD. Like CBD, they do not produce feelings of euphoria and display antioxidant and anti-inflammatory properties, as well as interacting with a range of target proteins including TRPV1,13 PPAR-γ,20 5-HT1A, and CB2.21 Recently our group conducted a systematic review focusing on the neuroprotective properties of minor phytocannabinoids (other than Δ9-THC or CBD) and found that CBG and CBDV show efficacy in models of Huntington's disease, Alzheimer's, and epilepsy, with CBG mediating its protective effects through PPAR-γ activation,22 the same mechanism by which we have shown that CBD protects BBB integrity.12 However, despite these compounds having neuroprotective effects in other models, no studies have been conducted to test whether CBG or CBDV are protective in IR injury.
In light of the above, we hypothesized these compounds may exhibit protective properties at the BBB in a stroke model. To test this, we treated cells of the BBB with CBG or CBDV in vitro before an OGD protocol and measured various proinflammatory cytokines, adhesion molecules, and cell damage markers.
Materials and Methods
Materials
CBG and CBDV were kindly gifted by STI pharmaceuticals. Both compounds were dissolved in 100% ethanol to 10 mM and were stored at −20°C. AM251, AM630, GW6471, GW9962, O1918, CID16020046, SB366791 (Tocris, United Kingdom) were dissolved in dimethyl sulfoxide as stock solutions of 10 mM. (S)-WAY100135 was dissolved in deionized water. Antagonists were stored at −20°C and dilutions were made fresh as required.
General cell culture
Human brain microvascular endothelial cells (HBMECs), astrocytes, and pericytes (passages 3–6) were grown in their respective medium and maintained at 37°C in a humidified incubator supplemented with 5% CO2. HBMECs were cultured on fibronectin-coated plasticware (2 μg/cm2), as per supplier recommendations. Primary cells and medium were purchased from ScienCell, United Kingdom.
OGD protocol
To simulate ischemic conditions, normal medium was replaced with glucose free RPMI medium (Gibco, United Kingdom) containing either CBG or CBDV (10 nM to 10 μM), alongside a vehicle control (0.01% ethanol). Cell culture plates were then placed in an anoxic bag (BD GasPak™, anaerobe) for 4 h (8 h for astrocyte experiments) plus an additional 20 min to ensure anaerobic conditions. For vehicle normoxia, ethanol (0.01%) was added to the respective medium of each cell type (ScienCell) and maintained in normal oxygenated conditions. After OGD, medium was aspirated and replaced with each cell types respective medium (ScienCell) containing the relevant concentrations of CBG or CBDV for a 20-h/16-h reperfusion period. At 24 h, the medium was sampled, and cells were lysed with RIPA buffer containing protease and phosphatase inhibitors (Sigma, United Kingdom; ThermoFisher, United Kingdom). Medium and lysates were stored at −80°C for future analysis.
Total protein
To quantify total protein, a bicinchoninic acid (BCA) protein assay was performed on cell lysates. A working reagent of copper II sulfate and BCA (Sigma-Aldrich) was prepared in a 1:50 ratio and added to wells. After a 30-min incubation at 37°C, plates were read at 562 nm. Unknowns were extrapolated from a standard curve of known concentrations of bovine serum album. Unless otherwise stated, all secreted and intracellular proteins were normalized to total protein.
Enzyme-linked immunosorbent assay
Medium samples were analyzed for various proinflammatory cytokines including interleukin (IL)-6, IL-8, and adhesion molecules including intracellular adhesion molecule (ICAM)-1, vascular endothelial growth factor (VEGF), monocyte chemoattractant protein (MCP)-1 using duo-set enzyme-linked immunosorbent assay (ELISA) by R&D systems, United Kingdom (DY206, DY208, DY720, DY293B, and DY279). Raw values at 570 nm were subtracted from values obtained at 450 nm, sample concentrations were determined by extrapolating unknowns from the 8-point standard curve (known concentrations).
Lactate dehydrogenase assay
A lactate dehydrogenase (LDH) assay was performed to determine nonspecific damage induced by the OGD protocol. A standard curve of known concentration of nicotinamide adenine dinucleotide was constructed as per manufacturer's instructions. Fifty microliters of standard or sample was aliquoted into a 96-well plate and 50 μL of assay mix was added. Plate absorbance was read at 450 nm and unknown values were obtained from a standard curve.
DNA damage/genotoxicity assay
Astrocyte lysates post-OGD were analyzed using the Milliplex DNA damage/Genotoxicity multiplex assay kit (Millipore, 48–621MAG) to detect changes in DNA damage markers ataxia-telangiectasia mutated (ATR-Total), checkpoint kinases 1, 2 (Chk1, Ser345 and Chk2, and Thr68), histone family member X (H2A.X, Ser139), mouse double minute 2 homolog (MDM2, total), cyclin-dependent kinase inhibitor 1 (p21, total), tumour protein (p53, Ser15). Kits were performed according to manufacturer's instructions.
Statistical analysis
All data are represented as the mean±standard error of the mean, data were assessed for normality using the D'Agostino–Pearson normality test and subsequently analyzed using one-way analysis of variance with Dunnett's post hoc analysis. All statistical analyses were conducted using GraphPad prism (7/8) (Version 7.01; GraphPad Software, Inc.), comparing either vehicle normoxia or vehicle OGD with all other treatments. A value of p<0.05 was considered significant.
Results
HBMEC monocultures
Protein levels from HBMEC lysates were significantly lower post-OGD compared with vehicle normoxia wells (p<0.001). This was not affected by pretreatment with CBDV or CBG (Supplementary Fig. S1C, F).
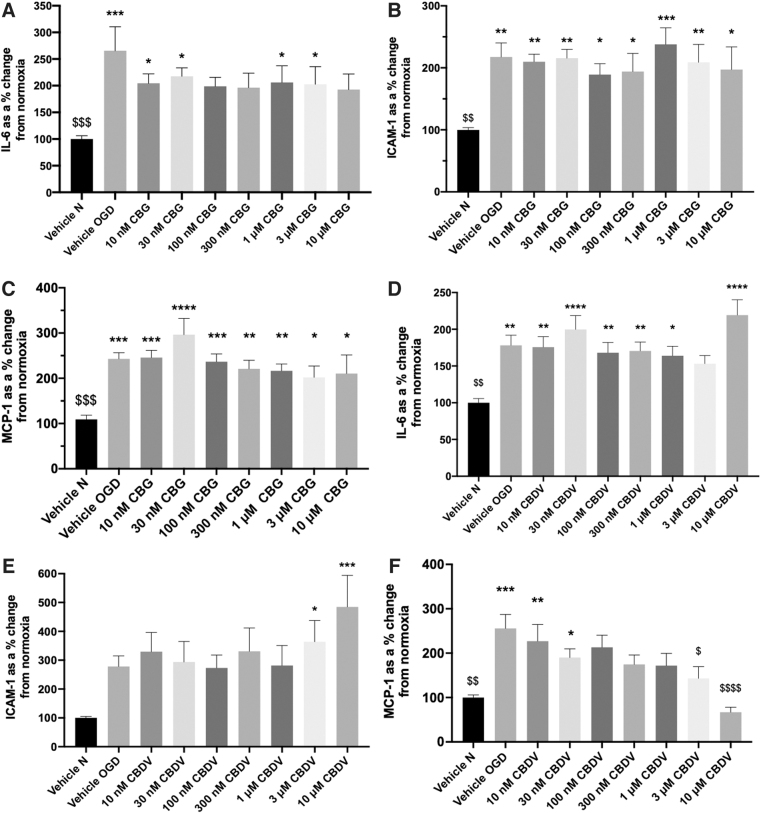
IL-6, ICAM-1, and MCP-1 were significantly increased in cell culture medium 24 h after 4-h OGD compared with normoxia vehicle (p<0.05; Fig. 1A–F). Pretreatment with CBG (10 nM–10 μM) displayed an overall trend to decrease IL-6 and 100 nM, 300 nM, and 10 μM CBG-treated wells were not statistically significant to vehicle normoxia (Fig. 1A). CBG pretreatment did not alter ICAM-1 and MCP-1 secretion in response to OGD (Fig. 1B, C).
FIG. 1.
The effects of CBG and CBDV on HBMEC monocultures. Medium was analyzed for IL-6 (A, D), ICAM-1 (B, E), and MCP-1 (C, F) 24 h after 4-h OGD. Data were normalized to total protein (calculated using a BCA assay) and are given as a % change from the normoxia vehicle presented as means with error bars representing SEM. n=6–9 from three experimental repeats. *, Significant difference compared with vehicle normoxia (vehicle N) (*p<0.05, **p<0.01, ***p<0.001, and ****p<0.0001). $p < 0.05, $$p < 0.01, $$$p < 0.001, and $$$$p < 0.0001) significant difference to vehicle OGD, one-way ANOVA with Dunnett's post hoc analysis. ANOVA, analysis of variance; BCA, bicinchoninic acid; CBDV, cannabidivarin; CBG, cannabigerol; HBMEC, human brain microvascular endothelial cell; ICAM-1, intracellular adhesion molecule-1; IL-6, interleukin-6; MCP-1, monocyte chemoattractant protein-1; OGD, oxygen-glucose deprivation; SEM, standard error of the mean.
Pretreatment with CBDV (10 nM–1 μM and 10 μM) did not attenuate IL-6 levels 24-h post-OGD. However, 3 μM CBDV was not significantly different from vehicle normoxia (Figure 1D). Pretreatment with 3 and 10 μM CBDV significantly increased levels of ICAM-1 24-h post-OGD (p<0.05, Fig. 1E). CBDV (100 nM–10 μM) concentration-dependently reduced levels of MCP-1, an effect that was significantly different to vehicle OGD at 3 and 10 μM (p<0.05; Fig. 1F).
Pericyte monocultures
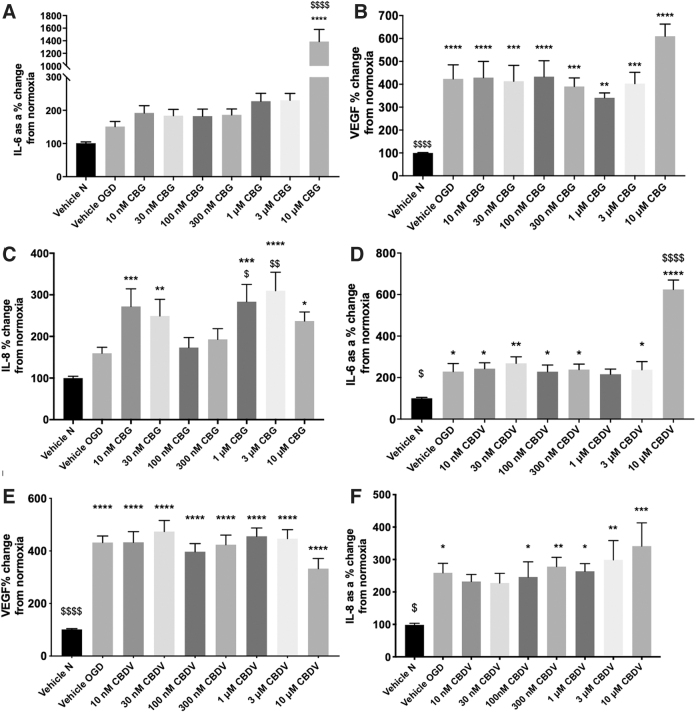
Protein levels from pericyte monocultures were not significantly altered by the OGD protocol or drug treatment (Supplementary Fig. S1A, D). A 4-h OGD increased levels of IL-6, VEGF, and IL-8 measured in cell culture medium 24-h post-OGD (Fig. 2A–F).
FIG. 2.
The effects of CBDV and CBG on pericyte monocultures. Medium 24 h after 4-h OGD was analyzed for IL-6, VEGF, and IL-8 (A–F). Data were normalized to total protein and are given as a % change from the normoxia vehicle, presented as means with error bars representing SEM. n=6–9 from 3 experimental repeats. *, Significant difference compared with vehicle normoxia (vehicle N) (*p<0.05, **p<0.01, ***p<0.001, and ****p<0.0001). $p < 0.05, $$p < 0.01, $$$p < 0.001, and $$$$p < 0.0001) significant difference to vehicle OGD, one-way ANOVA with Dunnett's post hoc analysis. VEGF, vascular endothelial growth factor.
In pericyte monocultures, neither CBG nor CBDV (10 nM–3 μM) altered IL-6 levels post-OGD; however, both compounds increased IL-6 levels at 10 μM (p<0.0001; Fig. 2A, D). Pretreatment with CBG and CBDV (10 nM–10 μM) did not alter levels of VEGF (Fig. 2B, E).
At the lowest and highest concentrations tested, CBG pretreatment increased IL-8 levels compared with vehicle normoxia and vehicle OGD (p<0.05; Fig. 2C). At 100 and 300 nM, CBG did not alter increased levels of IL-8 produced by OGD (Fig. 2C). CBDV did not affect IL-8 levels post-OGD, although there was a trend to produce an increase in IL-8 at 10 μM (Fig. 2F).
Astrocyte monocultures
IL-6 levels were not statistically different to vehicle normoxia 24 h after 4-h OGD (70.03 pg·mL normoxia vs. 65.29 pg·mL OGD, data not shown), but levels of IL-6 were significantly increased 24 h after 8-h OGD (p<0.01; Figure 3A and C). Therefore, subsequent experiments in astrocytes were conducted using an 8-h OGD protocol.
FIG. 3.
The effects of CBG and CBDV on astrocyte monocultures. (A-D) Medium 24 h after 8-h OGD were analyzed for IL-6 and VEGF. Data were normalized to total protein and are given as a % change from the normoxia vehicle, presented as means with error bars representing SEM. n=5–9 from 3 experimental repeats. *, Significant difference compared with vehicle normoxia (vehicle N) (*p<0.05, **p<0.01, ***p<0.001, and ****p<0.0001). $p < 0.05, $$p < 0.01, $$$p < 0.001, and $$$$p < 0.0001) significant difference to vehicle OGD, one-way ANOVA with Dunnett's post hoc analysis.
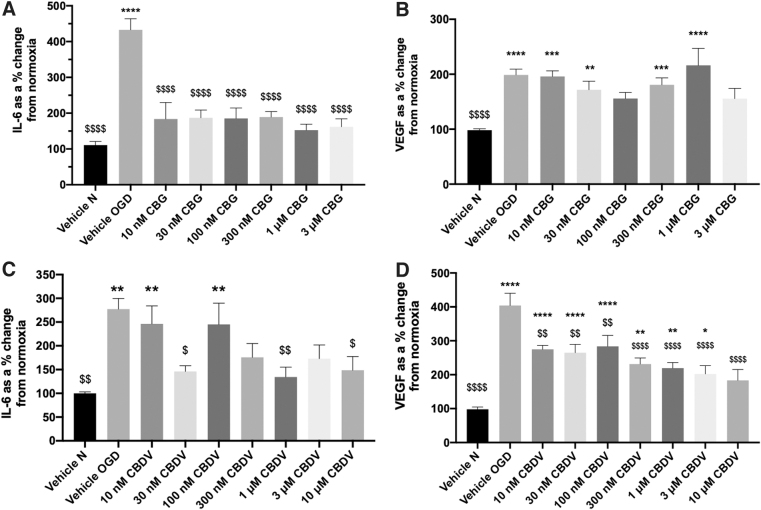
An 8-h protocol significantly decreased protein levels in astrocyte cell lysates (p<0.01 versus vehicle normoxia; Supplementary Fig. S1B, E). Treatment with 10 μM CBG decreased protein content compared with both vehicle OGD and vehicle normoxia (p<0.0001; Supplementary Fig. S1B). Pretreatment with CBDV did not prevent the decrease in protein content caused by the 8-h OGD protocol (p<0.05 vs. vehicle normoxia); however, 30 nM, 1 and 10 μM CBDV did not exhibit a significant difference compared with vehicle normoxia (Supplementary Figure S1E).
Pretreatment with CBG 10 nM–3 μM attenuated astrocytic IL-6 levels (p>0.0001 vs. vehicle OGD; Fig. 3A); however, at 10 μM CBG significantly increased IL-6 (Supplementary Fig. S2A). CBDV reduced levels of IL-6 compared with vehicle OGD at 30 nM (p<0.05), 1 μM (p<0.01), and 10 μM (p<0.05; Fig. 3C). CBDV at 300 nM and 3 μM also appeared to decrease IL-6 levels, exhibiting no statistical difference to vehicle normoxia.
Astrocytic VEGF levels were significantly increased post-OGD (p<0.0001; Fig. 3B, D). CBG pretreatment appeared to attenuate VEGF levels at 100 nM and 3 μM, but this did not reach significance to vehicle OGD (Fig. 3B). Conversely, 10 μM CBG significantly increased VEGF compared with both vehicle normoxia and vehicle OGD (p<0.001; Supplementary Fig. S2B). Pretreatment with CBDV (10 nM–10 μM) decreased VEGF levels in a concentration-dependent manner. At 10 μM this was not significantly different to vehicle normoxia and significantly different to vehicle OGD (p<0.0001; Fig. 3D).
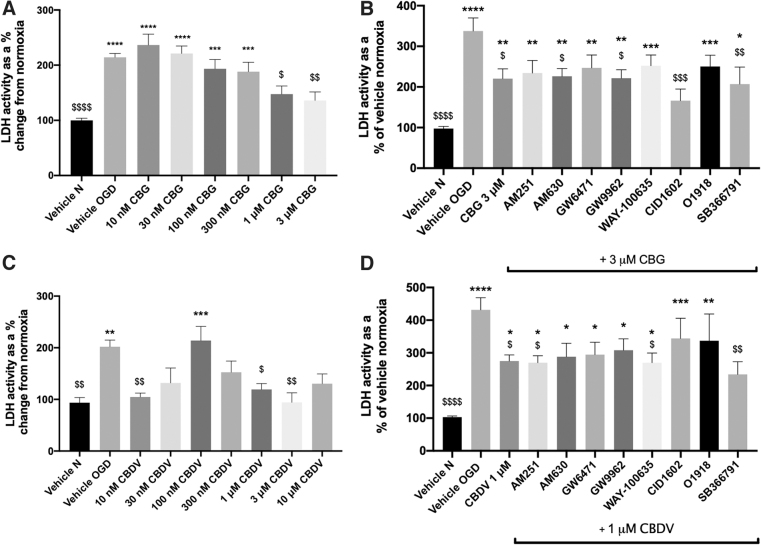
LDH was significantly elevated in astrocyte medium post-OGD (p<0.01; Fig. 4A, C). Pretreatment with 1 and 3 μM CBG significantly attenuated LDH activity (p<0.05; Fig. 4A); however, at 10 μM CBG significantly increased LDH activity (Supplementary Fig. S2C). CBDV exhibited a biphasic concentration response, decreasing LDH activity at lower (10 nM; p<0.01) and higher concentrations (p<0.05; 1 and 3 μM), but increasing levels at 100 nM (p<0.001; Fig. 4C).
FIG. 4.
The effects of CBG and CBDV treatment alone (A, B) and with antagonists (C, D) on LDH release from astrocyte monocultures. Medium 24 h after 8-h OGD were analyzed LDH. Data were normalized to total protein and are given as a % change from the normoxia vehicle, presented as means with error bars representing SEM. n=5–6 from 3 experimental repeats. *, Significant difference compared with vehicle normoxia (vehicle N) (*p<0.05, **p<0.01, ***p<0.001, and ****p<0.0001). $p < 0.05, $$p < 0.01, $$$p < 0.001, and $$$$p < 0.0001) significant difference to vehicle OGD, one-way ANOVA with Dunnett's post hoc analysis. LDH, lactate dehydrogenase.
None of the antagonists tested blocked CBG (3 μM)-mediated decreases in LDH; however, application of CID1602 (antagonist for GPR55) appeared to potentiate the effects of CBG (p<0.001; 3 μM CBG+CID1602 vs. vehicle OGD; Fig. 4B). In the presence of antagonists for GPR55, CID1602 and O1918, CBDV (1 μM)-mediated decreases in LDH were no longer significantly different to vehicle OGD (Fig. 4D). In addition, SB366791 appeared to potentiate the LDH-reducing effects of CBDV (p<0.01; 1 μM CBDV SB366791 vs. vehicle OGD, Fig. 4D).
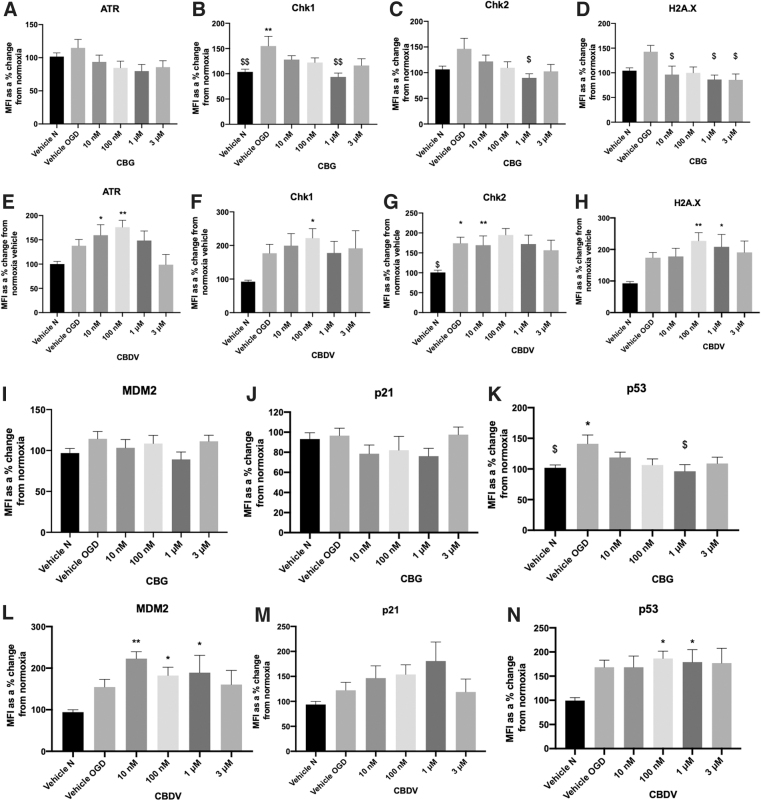
As CBDV and CBG reduced cell damage in astrocytes, we next investigated whether these compounds (at the most efficacious lower and higher concentrations tested) influenced levels of DNA damage proteins. Levels of DNA damage proteins, ATR, Chk1, Chk2, H2A.X, and p53 were increased in astrocyte cell lysates 24 h after 8-h OGD. MDM2 showed a trend for increasing post-OGD, but levels of p21 were not affected (Fig. 5A–N). Application of CBG (1 μM) before OGD significantly reduced levels of Chk1 and Chk2 compared with OGD vehicle (p>0.01, p<0.05; Fig. 5B, C). In addition, CBG pretreatment at 10 nM, 1 μM, and 3 μM decreased H2A.X levels that displayed trend for increasing post-OGD (p>0.05; Fig. 5D). Levels of p53 were also increased post-OGD (p>0.05) and attenuated by CBG in a concentration-dependent manner that was significant at 1 μM (p>0.05; Fig. 5K). By contrast, CBDV (10 nM, 100 nM) increased levels of ATR (p > 0.0001, p < 0.01; Fig. 5E) as well as increasing levels of Chk1 at 100 nM (p < 0.05) and Chk2 at 10 nM (p < 0.01; Fig.5F). CBDV (100 nM and 1 μM) also increased levels of H2A.X, p53 and MDM2 (p < 0.05; Fig. 5H, L, N).
FIG. 5.
The effects of CBG and CBDV on DNA damage markers (ATR [A,E], Chk1 [B,F], Chk2 [C,G], H2A.X [D,H], MDM2 [I,L], p21 [J,M], and p53 [K,N] from astrocyte cell lysates, 24 h after 8-h OGD. Data were normalized to total protein and are given as MFI as a % change from the normoxia vehicle (vehicle N); means with error bars represent SEM. n=6–8 from 3 experimental repeats. *, Significant difference compared with vehicle normoxia (vehicle N) (*p<0.05, **p<0.01). $p<0.05, $$p<0.01 significant difference to vehicle OGD, one-way ANOVA with Dunnett's post hoc analysis. MFI, mean fluorescent intensity.
Discussion
In this study we assessed whether non-euphoric phytocannabinoids CBG and CBDV protected cells of the BBB in a cellular model of ischemic stroke. Despite promising preclinical data, drugs developed for one or more of the hallmarks of stroke have failed once they have reached clinical trials.23,24 Poor translational efficacy is likely to stem from the multifactorial pathophysiology of ischemic stroke and complicating factors among elderly patients, which are often overlooked in ischemic stroke modeling.25 These points emphasize the need to generate new, effective therapies for patients, which target multiple aspects of stroke pathogenesis.26
CBD has been widely studied as a neuroprotectant, partly because of its promiscuous pharmacology, tolerable safety profile in humans and absence of euphoric effects.10,27,28 However, other phytocannabinoids are beginning to gain significant interest as therapeutic agents. CBG has displayed prominent anti-inflammatory and antioxidant capabilities20,29,30 and the antiepileptic properties of CBDV have been well documented.31–33 Recently, CBDV has been shown to reduce inflammatory cytokine release in a model of intestinal inflammation.34 Our results demonstrate that CBDV and CBG exhibit protective properties against OGD-induced damage in astrocytes and HBMECs, modulating a range of biochemical parameters measured post-OGD. For CBDV, its cytoprotective effects appeared to partially involve GPR55, but a target for CBG was not identified. These data warrant their further investigation into these compounds as neuroprotectants and to assess their clinical applicability, specifically, their efficacy in in vivo models of ischemic stroke and whether they are protective when applied post-OGD.
Post-cerebral ischemia and elevated levels of proinflammatory cytokine IL-6 are associated with increased neuronal cell necrosis and are correlated with stroke severity, increases in mortality rate, poor performance, and functional disability.35–38 In this study, CBG and CBDV significantly decreased levels of IL-6 in astrocytes, suggesting that like CBD, CBDV and CBG may offer protection against inflammation caused by ischemic stroke.12 Increases in IL-6 post-ischemia have also been implicated in BBB breakdown and tight junction remodeling, including reduced expression of VE-cadherin, occludin, and claudin-5.39 Although there was a trend for CBDV and CBG to attenuate IL-6 levels in HBMECs, more pronounced reductions in IL-6 were observed in astrocytes. Astrocytes provide biochemical and mechanical support that help to maintain the BBB, as well as providing neurovascular crosstalk between neurons and cerebral blood vessels.40 Unlike in monoculture, in vivo, astrocyte endfeet are in direct contact with endothelial cells; thus, modulating the astrocyte inflammatory response in situ may act to preserve BBB integrity indirectly by soluble factors secreted by astrocytes or by preserving normal astrocyte function.
Mice lacking the receptor for adhesion molecule, MCP-1 (CCR2), have significantly reduced infarct sizes together with reduced BBB permeability and similarly, MCP-1 knockout mice have a reduced influx of hematogenic cells from systemic circulation and improved neurological outcome.41,42 Bonifačić et al. found a relationship between patients with poor outcomes 90 days after stroke and elevated levels of MCP-1 and a recent meta-analysis revealed that higher baseline circulating levels of MCP-1 correlated with a higher risk of ischemic stroke.43,44 Our data show that CBDV concentration dependently decreased levels of MCP-1 secreted by HBMECs when applied at the same time as initiating OGD, suggesting that CBDV might offer protection against MCP-1-related damage post-stroke and/or offer protection in individuals at a higher risk of ischemic stroke. These data are also consistent with that of a recent study showing that CBDV treatment attenuated MCP-1 mRNA levels in colonic tissue post-colitis.34 Of interest, this study also showed that CBDV was able to reduce intestinal permeability, an effect that may be replicated at the BBB, but this has yet to be investigated.
We also measured VEGF secreted by pericytes and astrocytes post-OGD reperfusion as elevations in VEGF are correlated with increased endothelial barrier permeability post-ischemia.45–47 Li and co-authors found that astrocyte-derived VEGF mediated endothelial barrier disruption, which was associated with decreases in occludin and claudin-5.47 Interestingly, whilst CBG and CBDV did not affect pericyte-derived VEGF, CBDV decreased VEGF secretion in astrocytes in a concentration-dependent manner and CBG exhibited a trend for decreasing VEGF at 100 nM and 3 μM. As VEGF is known to facilitate BBB opening these compounds may offer protection against BBB breakdown post-ischemia; however, the mechanisms in which these compounds decrease VEGF remains to be elucidated.
During IR injury cells undergo a combination of apoptosis and necrosis, causing various cellular components to be released into the extracellular space. One of these components, LDH, is often used as a marker of cell damage. Previous studies have shown that IR models cause LDH leakage into cell culture medium48,49 and clinically, LDH has been trialled as a marker of ischemic severity.50,51 Pretreatment with CBDV and CBG offset increases in LDH, suggesting both compounds mitigate cellular damage produced by OGD reperfusion. Application of receptor antagonists revealed that CBDV appeared to mediate its effects on LDH levels by GPR55; however, none of the antagonists tested blocked the effect of CBG. This could be explained by the nonspecific antioxidant properties of cannabinoids, namely owing to their phenolic rings and hydroxyl moieties.17,52 Indeed, previous studies have shown that CBD increases antioxidant enzymes in BV2 microglial cells,53 as well as attenuating oxidative stress and increasing mitochondrial bioenergetics in OGD reperfusion-damaged neurons.18 Similarly, CBD, CBDV, and CBG were able to prevent oxytosis in a preclinical drug screen for Alzheimer's disease and CBG exhibited antioxidant capacity in neuroblastoma cells.54,55 More data are clearly needed on the specific and nonspecific mechanisms in which these compounds mediate their protective effects, particularly whether their antioxidant status is responsible for reducing cell damage in the context of ischemia.
Ischemia is a pathophysiological stressor and as a consequence, nonspecific single- and double-strand DNA breaks (ssDNA/dsDNA breaks) and replication-associated DNA damage responses (DDRs) occur. DNA damage can activate the DDR pathway and DDR response proteins ATR, Chk1, Chk2, H2A.X, MDM2, p21 and p53 that govern elements of DNA repair, cell cycle arrest, apoptotic and necrotic cell death.56–59 These processes are central in IR injury and early studies found that neurons are the first to exhibit signs of DNA damage (0.5–8 h reperfusion) followed by astrocytes (24 h reperfusion).60 Thus, we next investigated the effect of CBDV and CBG on DDR proteins post-OGD in astrocytes.
In support of previous studies, our OGD protocol (and subsequent reperfusion period) increased levels of almost all measured DDR proteins in astrocyte monoculture lysates.61 In stroke patients, Huttner and colleagues found evidence of ATM/ATR activity in the penumbra of cortical neurons 7–10 days post-ischemia.62 Studies have also shown p53 activation is implicated in ischemia-induced neuronal cell death, with elevated levels of p53 also present in reactive astrocytes and microglia.63,64 Ahn and colleagues found that inhibition of p53 by pifithrin-α reduced OGD-induced cell death in cultured astrocytes, and as a secondary effect reduced elevated levels of glutamate and glial fibrillary acidic protein (GFAP), which were also increased post-OGD.65 To our knowledge, this is the first study to show that CBG pretreatment reduced levels of Chk1, Chk2, H2A.X, and p53 in astrocytes post-OGD. It is likely that these decreases in DNA damage proteins were caused indirectly, possibly because of the overall reductions in cellular damage and inflammation, as well as the known antioxidant properties of CBG that have both been demonstrated in other studies.20,66 Nevertheless, direct modulation of these proteins should not be ruled out particularly as PPAR-γ, a known target for phytocannabinoids, has been implicated in ATM signaling and the DDR.67
Pretreatment with CBDV significantly increased expression of the majority of DNA damage proteins in astrocytes and exhibited a trend for increasing p21. CBD was recently found to increase protein expression of ATM and p21, but not p53 in an in vitro model of gastric cancer, suggesting CBD promotes cell cycle arrest at the G0–G1 phase.68 Our data suggest that CBDV acts in a similar manner; however, it is important to emphasize that p21 has roles in both enhancing and inhibiting apoptosis depending on the type of stressor; thus, generating this response in a cancer cell model will be different to responses of astrocytes subjected to OGD. Low dose N-methyl-d-aspartate (NMDA) to simulate ischemic preconditioning was shown to increase MDM2 protein expression, preventing p53 stabilization in mouse cortical neurons and ischemia-induced apoptotic cell death.69 CBDV significantly increased levels of MDM2, which is a key protein involved in p53 degradation and thus promotion of cell survival. Future studies should clarify the implications of CBDVs ability to increase levels of DNA damage proteins in ischemia and establish whether modulating DNA damage and repair in astrocytes can influence post-stroke injury and recovery.
Conclusions
This study provides novel data on the neuroprotective and anti-inflammatory properties of CBG and CBDV in an in vitro model of IR. These data, together with evidence from other studies, corroborate the protective properties of these compounds and further studies are needed to elucidate the mechanism of action of CBG and CBDV and whether they can modulate BBB permeability in more clinically relevant in vivo models of ischemic stroke. There is lack of effective treatments for ischemic stroke, a condition that will increase in prevalence in coming years, to which cannabinoids may offer a unique therapeutic strategy.
Supplementary Material
Acknowledgments
The authors specially thank STI Pharmaceuticals for providing the test compounds CBDV and CBG.
Abbreviations Used
- Δ9-THC
delta9-tetrahydrocannabinol
- ANOVA
analysis of variance
- BBB
blood–brain barrier
- BCA
bicinchoninic acid
- CBDV
cannabidivarin
- CBG
cannabigerol
- CNS
central nervous system
- DDR
DNA damage response
- ELISA
enzyme-linked immunosorbent assay
- GFAP
glial fibrillary acidic protein
- HA
human astrocytes
- HBMECs
human brain microvascular endothelial cells
- ICAM-1
intracellular adhesion molecule-1
- IR
ischaemia–reperfusion
- LDH
lactate dehydrogenase
- MCP-1
monocyte chemoattractant protein-1
- MFI
mean fluorescent intensity
- NMDA
N-methyl-d-aspartate
- OGD
oxygen-glucose deprivation
- TEER
trans epithelial resistance
- VEGF
vascular endothelial growth factor
- VCAM-1
vascular adhesion molecule
Authors' Contributions
S.O.S. and N.S. designed the experiments that were carried out by N.S. N.S. wrote the article with input from S.O.S. and T.J.E. T.J.E. and S.O.S. supervised the project.
Author Disclosure Statement
S.O.S. is director of CanPharmaConsulting, scientific advisor to pharmaceutical companies developing cannabinoid-based medicines. None of these companies were involved in this research.
Funding Information
This work was supported by the Biotechnology and Biological Sciences Research Council (Grant No. BB/M008770/1).
Supplementary Material
Cite this article as: Stone NL, England TJ, O'Sullivan SE (2021) Protective effects of cannabidivarin and cannabigerol on cells of the blood-brain barrier under ischemic conditions, Cannabis and Cannabinoid Research 6:4, 315–326, DOI: 10.1089/can.2020.0159
References
- 1.Abbott NJ, Patabendige AAK, Dolman DEM, et al. Structure and function of the blood-brain barrier. Neurobiol Dis. 2010;37:13–25 [DOI] [PubMed] [Google Scholar]
- 2.McConnell HL, Kersch CN, Woltjer RL, et al. The translational significance of the neurovascular unit. J Biol Chem. 2017;292:762–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sosunov AA, Wu X, Tsankova NM, et al. Phenotypic heterogeneity and plasticity of isocortical and hippocampal astrocytes in the human brain. J Neurosci. 2014;34:2285–2298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Q, Tang XN, Yenari MA. The inflammatory response in stroke. J Neuroimmunol. 2007;184:53–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Douglas DR, Luoma V, Reddy U. Acute management of ischaemic stroke. Anaesth Intensive Care Med. 2020;21:1–7 [Google Scholar]
- 6.Ceprián M, Jiménez-Sánchez L, Vargas C, et al. Cannabidiol reduces brain damage and improves functional recovery in a neonatal rat model of arterial ischemic stroke. Neuropharmacology. 2017;116:151–159 [DOI] [PubMed] [Google Scholar]
- 7.Rodríguez-Muñoz M, Onetti Y, Cortés-Montero E, et al. Cannabidiol enhances morphine antinociception, diminishes NMDA-mediated seizures and reduces stroke damage via the sigma 1 receptor. Mol Brain. 2018;11:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lafuente H, Alvarez FJ, Pazos MR, et al. Cannabidiol reduces brain damage and improves functional recovery after acute hypoxia-ischemia in newborn pigs. Pediatr Res. 2011;70:272–277 [DOI] [PubMed] [Google Scholar]
- 9.Castillo A, Tolón MR, Fernández-Ruiz J, et al. The neuroprotective effect of cannabidiol in an in vitro model of newborn hypoxic-ischemic brain damage in mice is mediated by CB2 and adenosine receptors. Neurobiol Dis. 2010;37:434–440 [DOI] [PubMed] [Google Scholar]
- 10.Russo EB, Marcu J. Cannabis pharmacology: the usual suspects and a few promising leads. Adv Pharmacol. 2017;80:67–134 [DOI] [PubMed] [Google Scholar]
- 11.Russo EB, Burnett A, Hall B, et al. Agonistic properties of cannabidiol at 5-HT1a receptors. Neurochem Res. 2005;30:1037–1043 [DOI] [PubMed] [Google Scholar]
- 12.Hind WH, England TJ, O'Sullivan SE. Cannabidiol protects an in vitro model of the blood-brain barrier from oxygen-glucose deprivation via PPARγ and 5-HT1A receptors. Br J Pharmacol. 2016;173:815–825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iannotti FA, Hill CL, Leo A, et al. Nonpsychotropic plant cannabinoids, cannabidivarin (CBDV) and cannabidiol (CBD), activate and desensitize transient receptor potential vanilloid 1 (TRPV1) channels in vitro: potential for the treatment of neuronal hyperexcitability. ACS Chem Neurosci. 2014;5:1131–1141 [DOI] [PubMed] [Google Scholar]
- 14.England TJ, Hind WH, Rasid NA, et al. Cannabinoids in experimental stroke: a systematic review and meta-analysis. J Cereb Blood Flow Metab. 2015;35:348–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khaksar S, Bigdeli MR. Correlation between cannabidiol-induced reduction of infarct volume and inflammatory factors expression in ischemic stroke model. Basic Clin Neurosci. 2017;8:139–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pazos MR, Cinquina V, Gómez A, et al. Cannabidiol administration after hypoxia-ischemia to newborn rats reduces long-term brain injury and restores neurobehavioral function. Neuropharmacology. 2012;63:776–783 [DOI] [PubMed] [Google Scholar]
- 17.Hampson AJ, Grimaldi M, Axelrod J, et al. Cannabidiol and (-)Δ9-tetrahydrocannabinol are neuroprotective antioxidants. Proc Natl Acad Sci U S A. 1998;95:8268–8273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun S, Hu F, Wu J, et al. Cannabidiol attenuates OGD/R-induced damage by enhancing mitochondrial bioenergetics and modulating glucose metabolism via pentose-phosphate pathway in hippocampal neurons. Redox Biol. 2017;11:577–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mori MA, Meyer E, Soares LM, et al. Cannabidiol reduces neuroinflammation and promotes neuroplasticity and functional recovery after brain ischemia. Prog Neuropsychopharmacol Biol Psychiatry. 2017;75:94–105 [DOI] [PubMed] [Google Scholar]
- 20.Gugliandolo A, Pollastro F, Grassi G, et al. In vitro model of neuroinflammation: efficacy of cannabigerol, a non-psychoactive cannabinoid. Int J Mol Sci. 2018;19:1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giacoppo S, Gugliandolo A, Trubiani O, et al. Cannabinoid CB2 receptors are involved in the protection of RAW264.7 macrophages against the oxidative stress: an in vitro study. Eur J Histochem. 2017;61:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stone NL, Murphy AJ, England TJ, et al. A systematic review of minor phytocannabinoids with promising neuroprotective potential. Br J Pharmacol. 2020;177:4330–4352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O'Collins VE, Macleod MR, Donnan GA, et al. 1,026 Experimental treatments in acute stroke. Ann Neurol. 2006;59:467–477 [DOI] [PubMed] [Google Scholar]
- 24.Schmidt-Pogoda A, Bonberg N, Koecke MHM, et al. Why most acute stroke studies are positive in animals but not in patients: a systematic comparison of preclinical, early phase, and phase 3 clinical trials of neuroprotective agents. Ann Neurol. 2020;87:40–51 [DOI] [PubMed] [Google Scholar]
- 25.Cai W, Zhang K, Li P, et al. Dysfunction of the neurovascular unit in ischemic stroke and neurodegenerative diseases: an aging effect. Ageing Res Rev. 2017;34:77–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suzman R, Beard JR, Boerma T, Chatterji S. Health in an ageing world—what do we know? Lancet. 2015;385:484–486 [DOI] [PubMed] [Google Scholar]
- 27.Ibeas Bih C, Chen T, Nunn AVW, et al. Molecular targets of cannabidiol in neurological disorders. Neurotherapeutics. 2015;12:699–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Millar SA, Stone NL, Bellman ZD, et al. A systematic review of cannabidiol dosing in clinical populations. Br J Clin Pharmacol. 2019;85:1888–1900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Borrelli F, Fasolino I, Romano B, et al. Beneficial effect of the non-psychotropic plant cannabinoid cannabigerol on experimental inflammatory bowel disease. Biochem Pharmacol. 2013;85:1306–1316 [DOI] [PubMed] [Google Scholar]
- 30.Echeverry C, Prunell G, Narbondo C, et al. A comparative in vitro study of the neuroprotective effect induced by cannabidiol, cannabigerol, and their respective acid forms: relevance of the 5-HT1A receptors. Neurotox Res. 2021;39:335–348 [DOI] [PubMed] [Google Scholar]
- 31.Hill TDM, Cascio MG, Romano B, et al. Cannabidivarin-rich cannabis extracts are anticonvulsant in mouse and rat via a CB1 receptor-independent mechanism. Br J Pharmacol. 2013;170:679–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hill AJ, Mercier MS, Hill TDM, et al. Cannabidivarin is anticonvulsant in mouse and rat. Br J Pharmacol. 2012;167:1629–1642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Amada N, Yamasaki Y, Williams CM, et al. Cannabidivarin (CBDV) suppresses pentylenetetrazole (PTZ)-induced increases in epilepsy-related gene expression. PeerJ. 2013;1:e214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pagano E, Romano B, Iannotti FA, et al. The non-euphoric phytocannabinoid cannabidivarin counteracts intestinal inflammation in mice and cytokine expression in biopsies from UC pediatric patients. Pharmacol Res. 2019;149:104464. [DOI] [PubMed] [Google Scholar]
- 35.Shaafi S, Sharifipour E, Rahmanifar R, et al. Interleukin-6, a reliable prognostic factor for ischemic stroke. Iran J Neurol. 2014;13:70–76 [PMC free article] [PubMed] [Google Scholar]
- 36.Miao Y, Liao JK. Potential serum biomarkers in the pathophysiological processes of stroke. Expert Review of Neurother. 2014;14:173–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Armstead WM, Hekierski H, Pastor P, et al. Release of IL-6 after stroke contributes to impaired cerebral autoregulation and hippocampal neuronal necrosis through NMDA receptor activation and upregulation of ET-1 and JNK. Transl Stroke Res. 2019;10:104–111 [DOI] [PubMed] [Google Scholar]
- 38.Beridze M, Sanikidze T, Shakarishvili R, et al. Selected acute phase CSF factors in ischemic stroke: findings and prognostic value. BMC Neurol. 2011;11:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rochfort KD, Collins LE, Murphy RP, et al. Downregulation of blood-brain barrier phenotype by proinflammatory cytokines involves NADPH oxidase-dependent ROS generation: consequences for interendothelial adherens and tight junctions. PLoS One. 2014;9:e101815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu Z, Chopp M. Astrocytes, therapeutic targets for neuroprotection and neurorestoration in ischemic stroke. Prog Neurobiol. 2016;144:103–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Strecker JK, Minnerup J, Gess B, et al. Monocyte chemoattractant protein-1-deficiency impairs the expression of IL-6, IL-1β and G-CSF after transient focal ischemia in mice. PLoS One. 2011;6:e25863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stamatovic SM, Shakui P, Keep RF, et al. Monocyte chemoattractant protein-1 regulation of blood-brain barrier permeability. J Cereb Blood Flow Metab. 2005;25:593–606 [DOI] [PubMed] [Google Scholar]
- 43.Georgakis MK, Malik R, Björkbacka H, et al. Circulating monocyte chemoattractant protein-1 and risk of stroke meta-analysis of population-based studies involving 17180 individuals. Circ Res. 2019;125:773–782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bonifačić D, Toplak A, Benjak I, et al. Monocytes and monocyte chemoattractant protein 1 (MCP-1) as early predictors of disease outcome in patients with cerebral ischemic stroke. Wien Klin Wochenschr. 2016;128:20–27 [DOI] [PubMed] [Google Scholar]
- 45.Bai Y, Zhu X, Chao J, et al. Pericytes contribute to the disruption of the cerebral endothelial barrier via increasing VEGF expression: implications for stroke. PLoS One. 2015;10:1–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Argaw AT, Asp L, Zhang J, et al. Astrocyte-derived VEGF-A drives blood-brain barrier disruption in CNS inflammatory disease. J Clin Invest. 2012;122:2454–2468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li YN, Pan R, Qin XJ, et al. Ischemic neurons activate astrocytes to disrupt endothelial barrier via increasing VEGF expression. J Neurochem. 2014;129:120–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang L, Zhang Y, Asakawa T, et al. Neuroprotective effect of neuroserpin in oxygen-glucose deprivation- and reoxygenation-treated rat astrocytes in vitro. PLoS One. 2015;10:e0123932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang QY, Wang ZJ, Sun DM, et al. Novel therapeutic effects of leonurine on ischemic stroke: new mechanisms of BBB integrity. Oxid Med Cell Longev. 2017;2017:7150376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lampl Y, Paniri Y, Eshel Y, et al. Cerebrospinal fluid lactate dehydrogenase levels in early stroke and transient ischemic attacks. Stroke. 1990;21:854–857 [DOI] [PubMed] [Google Scholar]
- 51.Karlsson M, Wiberg-Itzel E, Chakkarapani E, et al. Lactate dehydrogenase predicts hypoxic ischaemic encephalopathy in newborn infants: a preliminary study. Acta Paediatr Int J Paediatr. 2010;99:1139–1144 [DOI] [PubMed] [Google Scholar]
- 52.Borges RS, Batista J, Viana RB, et al. Understanding the molecular aspects of tetrahydrocannabinol and cannabidiol as antioxidants. Molecules. 2013;18:12663–12674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Juknat A, Pietr M, Kozela E, et al. Microarray and pathway analysis reveal distinct mechanisms underlying cannabinoid-mediated modulation of LPS-induced activation of BV-2 microglial cells. PLoS One. 2013;8:e61462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schubert D, Kepchia D, Liang Z, et al. Efficacy of cannabinoids in a pre-clinical drug-screening platform for Alzheimer's disease. Mol Neurobiol. 2019;56:7719–7730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Granja AG, Carrillo-Salinas F, Pagani A, et al. A cannabigerol quinone alleviates neuroinflammation in a chronic model of multiple sclerosis. J Neuroimmune Pharmacol. 2012;7:1002–1016 [DOI] [PubMed] [Google Scholar]
- 56.Liu Q, Guntuku S, Cui XS, et al. Chk1 is an essential kinase that is regulated by Atr and required for the G2/M DNA damage checkpoint. Genes Dev. 2000;14:1448–1459 [PMC free article] [PubMed] [Google Scholar]
- 57.Matsuoka S, Huang M, Elledge SJ. Linkage of ATM to cell cycle regulation by the Chk2 protein kinase. Science (80-). 1998;282:1893–1897 [DOI] [PubMed] [Google Scholar]
- 58.Savic V, Yin B, Maas NL, et al. Formation of dynamic γ-H2AX domains along broken DNA strands is distinctly regulated by ATM and MDC1 and dependent upon H2AX densities in chromatin. Mol Cell. 2009;34:298–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Maréchal A, Zou L. DNA damage sensing by the ATM and ATR kinases. Cold Spring Harb Perspect Biol. 2013;5:a012716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen J, Jin K, Chen M, et al. Early detection of DNA strand breaks in the brain after transient focal ischemia: implications for the role of DNA damage in apoptosis and neuronal cell death. J Neurochem. 1997;69:232–245 [DOI] [PubMed] [Google Scholar]
- 61.Hammond EM, Dorie MJ, Giaccia AJ. ATR/ATM targets are phosphorylated by ATR in response to hypoxia and ATM in response to reoxygenation. J Biol Chem. 2003;278:12207–12213 [DOI] [PubMed] [Google Scholar]
- 62.Huttner HB, Bergmann O, Salehpour M, et al. The age and genomic integrity of neurons after cortical stroke in humans. Nat Neurosci. 2014;17:801–803 [DOI] [PubMed] [Google Scholar]
- 63.Chung YH, Shin CM, Kim MJ, et al. Enhanced expression of p53 in reactive astrocytes following transient focal ischemia. Neurol Res. 2002;24:324–328 [DOI] [PubMed] [Google Scholar]
- 64.Leker RR, Aharonowiz M, Greig NH, et al. The role of p53-induced apoptosis in cerebral ischemia: effects of the p53 inhibitor pifithrin α. Exp Neurol. 2004;187:478–486 [DOI] [PubMed] [Google Scholar]
- 65.Ahn KC, MacKenzie EM, Learman CR, et al. Inhibition of p53 attenuates ischemic stress-induced activation of astrocytes. Neuroreport. 2015;26:862–869 [DOI] [PubMed] [Google Scholar]
- 66.Di Giacomo V, Chiavaroli A, Recinella L, et al. Antioxidant and neuroprotective effects induced by cannabidiol and cannabigerol in rat CTX-TNA2 astrocytes and isolated cortexes. Int J Mol Sci. 2020;21:3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li CG, Mahon C, Sweeney NM, et al. PPARγ interaction with UBR5/ATMIN promotes DNA repair to maintain endothelial homeostasis. Cell Rep. 2019;26:1333–1343.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang X, Qin Y, Pan Z, et al. Cannabidiol induces cell cycle arrest and cell apoptosis in human gastric cancer SGC-7901 cells. Biomolecules. 2019;9:302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vecino R, Burguete MC, Jover-Mengual T, et al. The MDM2–p53 pathway is involved in preconditioning-induced neuronal tolerance to ischemia. Sci Rep. 2018;8:1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.