Abstract
Introduction: Published preclinical and clinical studies support the anti-inflammatory activity of CBD, but the molecular targets (e.g., genes and proteins) that are involved in its mechanisms of action remain unclear. Herein, a network-based pharmacology analysis was performed to aid in the identification of potential molecular targets for CBD's anti-inflammatory activity.
Materials and Methods: Target genes and proteins were obtained from several online databases, including Swiss target prediction, Online Mendelian Inheritance in Man, and the DrugBank database. A compound-target-disease network was constructed with Cytoscape tool, and a network of protein–protein interactions was established with the Search Tool for the Retrieval of Interacting Genes/Proteins database. Lead proteins identified from the compound-target-disease network were further studied for their interactions with CBD by computational docking. In addition, biological pathways involved in CBD's anti-inflammatory activity were identified with the Gene Ontology enrichment and the Kyoto Encyclopedia of Genes and Genomes analysis.
Results: A panel of proteins, including cellular tumor antigen p53, NF-kappa-B essential modulator, tumor necrosis factor (TNF) receptor, transcription factor p65, NF-kappa-B p105, NF-kappa-B inhibitor alpha, inhibitor of nuclear factor kappa-B kinase subunit alpha, and epidermal growth factor receptor, were identified as lead targets involved in CBD's anti-inflammatory activity. This finding was further supported by molecular docking, which showed interactions between the lead proteins and CBD. In addition, several signaling pathways, including TNF, toll-like receptor, mitogen-activated protein kinases, nuclear factor kappa-light-chain-enhancer of activated B cells, and nucleotide-binding oligomerization domain-like receptors, were identified as key regulators in the mediation of CBD's anti-inflammatory activity.
Conclusion: A network-based pharmacology analysis identified potential molecular targets and signaling pathways for CBD's anti-inflammatory activity. Findings from this study add to the growing body of data supporting the utilization of CBD as a promising anti-inflammatory natural product.
Keywords: cannabidiol (CBD), anti-inflammation, network pharmacology, NFκB, target identification, drug-diseases network
Introduction
Inflammation is a vital immune response to combat harmful stimuli, irritants, cell damage, and tissue injury.1 These stimuli trigger a series of physiological consequences, including increased blood flow, dilation of capillary blood vessels, and impaired vascular permeability, which can further lead to numerous cell and tissue dysfunctions. Inflammation-mediated pathological progressions are regulated by complex immune systems involving different immune cells and various signaling pathways.2 Among these various regulatory mechanisms, one of the most studied signaling pathway is the signal transduction of nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB).3 NFκB is a complex of proteins with five family members, including NFκB1 (subunit p50), NFκB2 (subunit p100), RelA (subunit p65), RelB, and c-Rel. These proteins are usually formed in dimers (e.g., p50-p65 dimer) to enable a wide range of biological functions and mediate the inflammation processes, such as coordinating the expression of multiple inflammatory genes. NFκB is considered as a central mediator for inflammation responses.
NFκB can be collectively regulated by its upstream signal transduction cascade, including inhibitors of nuclear factor kappa-B kinase subunit alpha, beta, and gamma (also known as IKK-α/β/γ), which are associated with the propagation of cellular response to inflammation.3 Activation of the NFκB pathway also leads to a cascade of downstream signaling, including the expressions of several proinflammatory cytokines, including interleukin-1β (IL-1β) and tumor necrosis factor alpha (TNF-α).
The NFκB pathway in the inflammation process is an example showing that the regulation of inflammation is orchestrated by complex mechanisms involving multiple genes, proteins, and signaling pathways. Therefore, identification of molecular targets for the development of anti-inflammatory agents as therapeutics for inflammatory-mediated diseases remains a challenge.
A promising preventive and/or therapeutic strategy against inflammatory-mediated disorders is immunomodulation by natural products from medicinal plants.4 Cannabis (Cannabis sativa) has been widely used in empirical and traditional remedies for various inflammation-related ailments.5,6 Notably, the anti-inflammatory activity and related molecular targets of a major phytocannabinoid in Cannabis, namely CBD, are supported by several reported studies. For instance, CBD showed anti-neuroinflammatory effects in murine microglial cells through the downregulation of the NFκB pathway and upregulation of transcription factors, including signal transducer and activator of transcription 3 (STAT3).7 CBD's anti-inflammatory effects in microglial cells are also supported by other studies, which showed that CBD's anti-inflammatory activity was associated with its modulation of downstream transcriptional events, including the regulation of gene expression and transcription.8,9
In addition, functional subsets of genes and gene networks, such as molecular and cellular functions, of CBD's anti-inflammatory effects, were identified by bioinformatic analysis tools, including Ingenuity Pathway Analysis, Database for Annotation, Visualization, and Integrated Discovery (DAVID) bioinformatics resources, and Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis.8,9 These findings suggest that CBD's anti-inflammatory activity is mediated by a complex combination of multiple molecular targets, rather than certain individual proteins.
In addition to experimental “omic” methods, computational modeling of biological pathways and molecular interactions has been developed as a “network” approach for a broad view of the interactions between ligands and their multiple targets.10,11 The network-based pharmacology approach has been used to predict molecular targets, including genes and proteins, protein–protein interactions (PPIs), and signaling pathways for drug candidates.10 A computational pharmacology network analysis has been reported to aid in the identification of CBD's pharmacological targets.12 This network analysis utilized a combination of chemogenomics-knowledgebase network analysis and integrated in silico modeling for the identification of three human neuro-related rhodopsin-like G-protein-coupled receptors as CBD's molecular targets.
However, to date, a network analysis approach has not been utilized for the prediction of potential molecular targets that are related to CBD's anti-inflammatory activity. Herein, the aims of the current study are to (1) identify putative genes and proteins that contribute to CBD's anti-inflammatory activity; (2) explore the interactions between CBD and inflammation-related proteins; and (3) analyze signaling pathways that are involved in CBD's anti-inflammatory activity.
Materials and Methods
Target prediction
Potential targets for CBD's anti-inflammatory effects were obtained from several online databases, including the Swiss Target Prediction database (www.swisstargetprediction.ch), the Online Mendelian Inheritance in Man (OMIM, www.omim.org), and the DrugBank database (https://www.drugbank.ca/). Human target connexins were obtained from the interactive protein database (DIP; http://dip.doe-mbi.ucla.edu). All the tested targets were transformed into the UniProt database in the protein ID format using the Retrieve/ID mapping tool (www.uniprot.org).
Construction of network and topology analysis
To elucidate the relationship between CBD and its anti-inflammatory targets, CBD and target genes and enriched pathways were imported into Cytoscape 3.6.1 software (www.cytoscape.org) to build a “component-target-disease” network. Then the topological parameters of the network analysis were calculated to identify key nodes. Among the topologic parameters, closeness centrality (a measure of how close a molecule is to other nodes)13 and betweenness centrality (the number of nonredundant shortest paths traveling through a node in the network)14 were used as crucial factors to describe the most influential nodes in the network. Thus, the nodes of degree and betweenness centrality that are two times higher than the median value of the total nodes, and the nodes of closeness centrality are higher than the median value of the total nodes were selected as the hubs for the anti-inflammatory activity.
Molecular docking
The three-dimensional structural coordinates of CBD were obtained from the human metabolome database (www.HMDB.ca), and the PDB files were generated with BIOVIA Discovery Studio Visualizer 4.5. The structural coordinates of target proteins were retrieved in PDB format from the RCSB protein data bank (www.rcsb.org). Chimera 1.11.2 was applied to delete the solvent and ligands from the target proteins. AutoDockTools 1.5.6 was used to perform molecular docking with AutoDock 4.2 algorithm. BIOVIA Discovery Studio Visualizer 4.5 was applied to analyze the interactions between CBD and target proteins.
Binding energies of CBD and its target proteins were obtained from parameters, including intermolecular energy (1), internal energy (2), torsional free energy (3), and unbound system's energy (4). The total binding energy was calculated as energy of binding=[(1) + (2) + (3) − (4)].
PPI network and cluster analysis
The hub targets from the aforementioned screening process were further imported into the Search Tool for the Retrieval of Interacting Genes/Proteins (STRING) database (http://string-db.org/) to explore the interactions between the known and predicted proteins. The topological parameters of mean and maximum degrees of freedom in PPI network were analyzed with Cytoscape 3.6.1 software, and then the network was analyzed with the MCODE plug-in for cluster analysis.
Enrichment analysis for target proteins
The plug-in ClueGO in Cytoscape 3.6.1 software was used to perform the Gene Ontology (GO), including cell component, molecular function, and biological process enrichment and the KEGG pathway annotation. The localization of the biological and molecular functions of the proteins was identified based on high confidence with a p value (<0.01) calculated by the two-side hypergeometric test method.
Results and Discussion
Protein targets and topological parameters
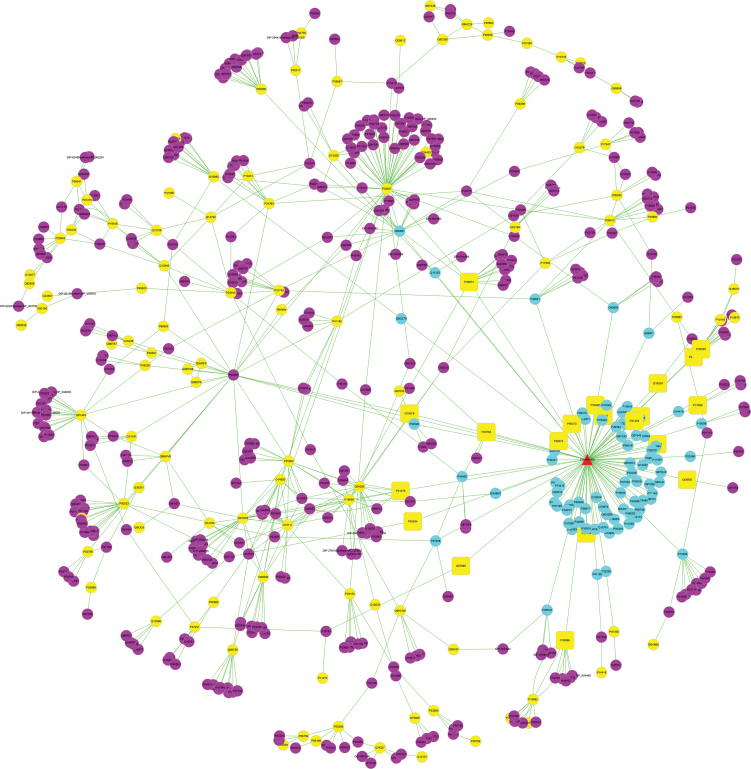
Potential targets for CBD's anti-inflammatory activity were obtained from several databases. CBD's protein targets were identified through the Swiss Target Prediction database and inflammation-related genes and protein targets were obtained through the OMIM and DrugBank databases. An interactive network of CBD's anti-inflammatory activity was constructed through network pharmacological analysis (Fig. 1). The red triangle represents CBD and the circles are proteins for pharmacological targets of CBD (in yellow), inflammation (in violet), and PPI (in cyan-blue). The yellow rectangles represent protein targets that are directly involved with CBD and inflammation pathways. Through the analysis of this “compound-target-disease” interactive network, 100 protein targets are affected by CBD and 570 protein targets are affected by inflammation. Among these protein targets, 20 proteins are common targets shared by CBD and inflammation-related pathways (as shown in yellow triangles; Fig. 1). Analysis of the topological parameters of all nodes in the network showed that the median of degree, betweenness centrality, and closeness centrality values are 1, 0, and 0.19641626, respectively. The thresholds of topological parameters were setup as degree value (>2), betweenness centrality (>0), and closeness centrality values (>0.19641626) and a total of 44 potential targets for CBD's anti-inflammatory activity were obtained (Supplementary Table S1). Targets with degree value greater than 10 are listed in Table 1. The protein target with the highest degree was cellular tumor antigen p53 (p53; 58 degree), followed by NF-kappa-B essential modulator (as known as IKK-γ), TNF receptor-associated factor 6 (TRAF6), transcription factor subunit p65 (NFκB p65), nuclear factor NF-kappa-B p105 subunit (NFκB p105), NF-kappa-B inhibitor alpha (IκBα), IKKα, and epidermal growth factor receptor with comparable degrees (21, 20, 19, 19, 18, 18, and 17, respectively).
FIG. 1.
CBD-target-inflammation network created by software Cytoscape (www.cytoscape.org). All target proteins are represented by their UniProt IDs. Red triangle: CBD; yellow circles: CBD's pharmacological target proteins; violet circle: proteins related to inflammation; cyan-blue circle: protein–protein interaction; yellow rectangles: proteins targets that are directly involved with CBD and inflammation pathways. Color images are available online.
Table 1.
Topological Parameters of Potential Targets for CBD's Anti-Inflammatory Activity in the “Compound-Target-Disease” Network
| UniProt ID | Protein name | Betweenness centrality | Closeness centrality | Degree |
|---|---|---|---|---|
| P04637 | Cellular tumor antigen p53 | 0.3046 | 0.3012 | 58 |
| Q9Y6K9 | NF-kappa-B essential modulator | 0.0648 | 0.2502 | 21 |
| Q9Y4K3 | TNF receptor-associated factor 6 | 0.0643 | 0.2377 | 20 |
| Q04206 | Transcription factor p65 | 0.0323 | 0.2513 | 19 |
| P19838 | Nuclear factor NF-kappa-B p105 subunit | 0.0472 | 0.2468 | 19 |
| P25963 | NF-kappa-B inhibitor alpha | 0.0426 | 0.2471 | 18 |
| O15111 | Inhibitor of nuclear factor kappa-B kinase subunit alpha | 0.0196 | 0.2340 | 18 |
| P00533 | Epidermal growth factor receptor | 0.0591 | 0.2427 | 17 |
| P31749 | RAC-alpha serine/threonine-protein kinase | 0.0457 | 0.2653 | 13 |
| O14920 | Inhibitor of nuclear factor kappa-B kinase subunit beta | 0.0241 | 0.2456 | 13 |
| P05412 | Transcription factor AP-1 | 0.0404 | 0.2321 | 13 |
| Q99558 | Mitogen-activated protein kinase 14 | 0.0246 | 0.2300 | 13 |
| P09874 | Poly [ADP-ribose] polymerase 1 | 0.1020 | 0.2864 | 12 |
| Q16665 | Hypoxia-inducible factor 1-alpha | 0.0332 | 0.2254 | 12 |
| P24385 | G1/S-specific cyclin-D1 | 0.0347 | 0.2441 | 10 |
| Q99759 | Mitogen-activated protein kinase 3 | 0.0279 | 0.1993 | 10 |
Notably, several proteins among the identified CBD's anti-inflammatory targets, namely, IκBα, IKKα, IKKβ, and KKγ are the key regulators of the upstream NFκB signal cascade.3 NFκB is in the inactivated state when IκBα masks the nuclear localization signals of NFκB and sequesters NFκB in the cytoplasm. Phosphorylation of IκBα proteins by IκB kinase, including IKKα, IKKβ, and KKγ, leads to the activation of NFκB via allowing it to enter into the cell nucleus from the cytoplasm. Therefore, given that IκB and IκB kinase are the top predicted protein targets, it is possible that CBD's anti-inflammatory effects are primarily associated with the NFκB signal cascade. This is in agreement with in vitro experimental data from several previously reported studies. In these studies, CBD showed anti-inflammatory effects by the inhibition of NFκB pathways in BV-2 microglial cells,7 PC12 neuronal cells,15 and RAW264.7 murine macrophage cells.16 To further validate the prediction of CBD's target proteins, computational docking was performed to evaluate the interactions between CBD and lead target proteins (top 16 proteins in Table 1).
Computational docking analysis
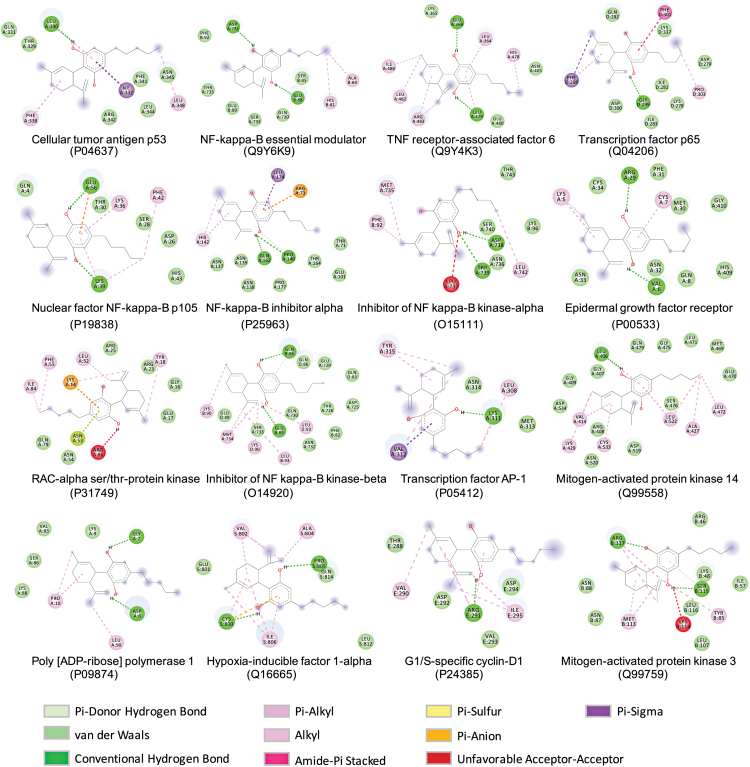
Computational docking revealed the interactions between CBD and the lead target proteins (top 16 from the protein targets analysis in Table 1). CBD can fit into the binding pocket of the target proteins and interact with protein amino acid residues by forming interactions, including van der Waals force, conventional hydrogen bonding, carbon hydrogen bonding, pi-donor hydrogen bonding, and pi-sigma, pi-alkyl, pi-anion, amide-pi stacked, and pi-sulfur forces (Fig. 2). In addition, the binding affinities between CBD and lead target proteins were ranked by their predicted free energy of binding and inhibition constant (Table 2). Proteins IKKβ and mitogen-activated protein kinase (MAPK) 14 had the lowest binding energy (−7.99 and −7.35 kcal/mol, respectively) and inhibition constant (1.4 and 4.1 μM, respectively). Comparably, p53, which had the highest degree in the “compound-target-disease” network, also had a third ranked low-binding energy and inhibition constant (−6.08 kcal/mol and 35.0 μM, respectively).
FIG. 2.
Computational docking for interactions between CBD and potential target proteins for CBD's anti-inflammatory activity. Color images are available online.
Table 2.
Interactions Between CBD and Target Proteins
| UniProt-PBD ID | Protein name | Free energy of binding (kcal/mol) | Inhibition constant (Ki; μM) |
|---|---|---|---|
| O14920-3BRT | Inhibitor of nuclear factor kappa-B kinase subunit β | −7.99 | 1.4 |
| Q99558-4IDT | Mitogen-activated protein kinase 14 | −7.35 | 4.1 |
| P04637-IAIE | Cellular tumor antigen p53 | −6.08 | 35.0 |
| P25963-1IKN | NF-kappa-B inhibitor alpha | −5.82 | 53.7 |
| Q9Y4K3-1IB6 | TNF receptor-associated factor 6 | −5.74 | 62.3 |
| Q04206-1NFI | Transcription factor p65 | −5.66 | 71.5 |
| P00533-1MOX | Epidermal growth factor receptor | −5.64 | 73.9 |
| Q9Y6K9-3BRV | NF-kappa-B essential modulator | −5.36 | 117.8 |
| P31749-1UNQ | RAC-alpha serine/threonine-protein kinase | −5.34 | 121.0 |
| Q99579-2O2V | Mitogen-activated protein kinase 3 | −5.34 | 122.6 |
| P09874-2COK | Poly [ADP-ribose] polymerase 1 | −4.88 | 264.4 |
| Q16665-1H2K | Hypoxia-inducible factor 1-alpha | −4.41 | 581.3 |
| O15111-3BRT | Inhibitor of nuclear factor kappa-B kinase subunit α | −4.32 | 686.9 |
| P19838-IMDI | Nuclear factor kappa-B p105 subunit | −4.23 | 799.9 |
| P24385- 5VZU | G1/S-specific cyclin-D1 | −3.44 | 3010 |
| P05412-1JUN | Transcription factor AP-1 | −3.41 | 3180 |
Free energy of binding and inhibition constant predicted by computational docking.
The utilization of computational docking as a complementary approach to validate the prediction of target proteins for CBD's neurophysiological effects has been reported.12 In addition, molecular docking has also been used to evaluate the interactions between lupenone, a natural anti-inflammatory agent, and its predicted target proteins.17 In the current study, data from the computational docking study supported the prediction of CBD's anti-inflammatory target proteins. Four of the top 6 proteins, including p53, TRAF6, p65, and IκBα, with lower binding affinity, matched within the top 6 lead target proteins predicted in Table 1. In addition, findings from computational docking are supported by reported experimental studies. For instance, CBD had the second lowest free binding energy and inhibition constant with protein MAPK 14 (also known as p38-α), which is a protein known as a mediator for cellular responses to external proinflammatory signals.18 Animal experimental studies have reported that CBD exerted anti-inflammatory effects by the regulation of p38-α pathway in mouse models of type I diabetic cardiomyopathy19 and alcohol-induced steatosis.16 Nevertheless, further biological investigations are warranted to validate other predicted target proteins for CBD's anti-inflammatory activity.
PPI network analysis and cluster analysis of hub targets
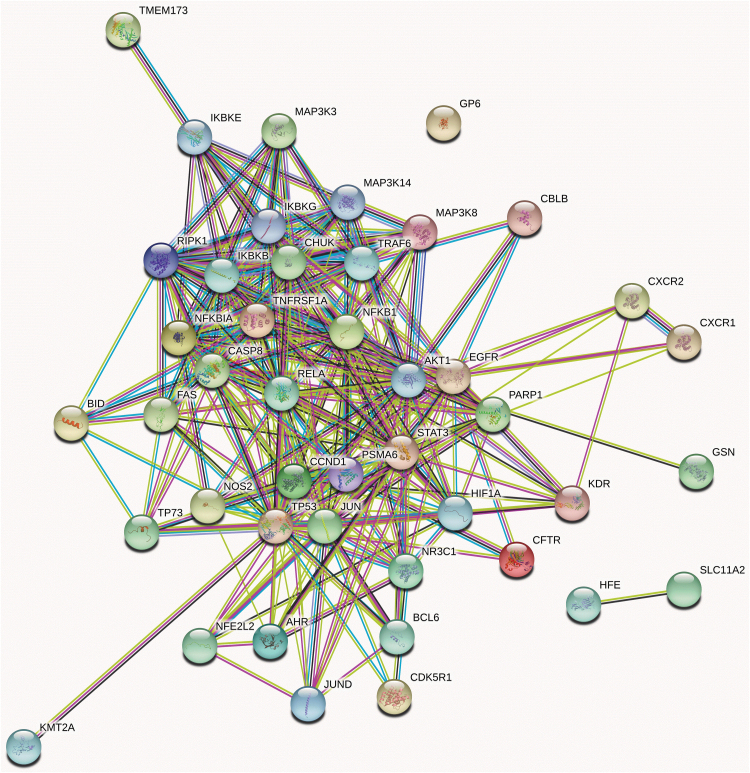
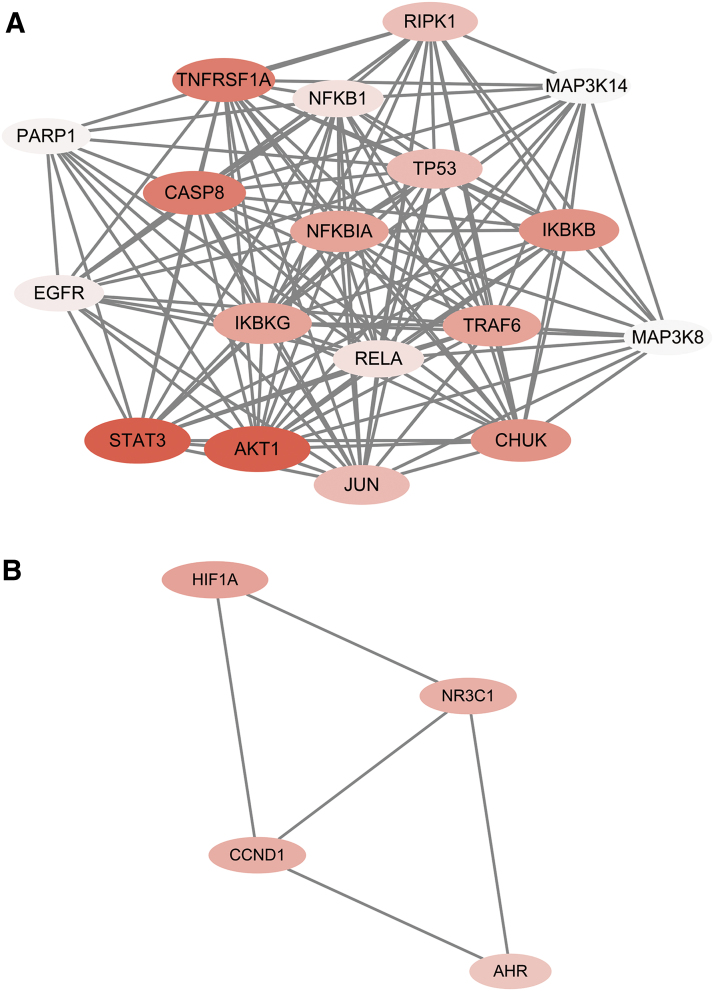
The identified potential targets were imported into the STRING database. A PPI network of protein targets for CBD's inflammation activity was obtained with 44 nodes (target protein; shown as circles) and 288 edges (PPI; shown as lines; Fig. 3). In addition, the clustering of the target interaction network was further analyzed by the Cytoscape software MCODE plug-in to obtain two subnetworks (Fig. 4A, B), which represent possible independent pathways that contributed to the overall anti-inflammatory effects of CBD. In this PPI network, target proteins, including STAT3, RAC-alpha serine/threonine-protein kinase (also known as AKT1), tumor necrosis factor receptor superfamily member 1A (TNFRSF1A), and cysteine-aspartic acid protease-8 (CASP8), had greater number of degrees (as shown in a darker color) suggesting that they may play a pivotal role in the anti-inflammatory effects of CBD. This is in agreement with reported experimental studies. For instance, CBD was reported to exert anti-inflammatory effects by the downregulation of the proinflammatory STAT1 pathway and the activation of anti-inflammatory STAT3 pathway in murine microglial BV-2 cells.7 In addition, CBD's anti-inflammatory effects were reported to be associated with the upregulation of AKT phosphorylation in a mouse model of experimental autoimmune encephalomyelitis.20 However, further studies are needed to elucidate the roles of other pathways in the predicted PPI network.
FIG. 3.
Interaction network (created by software Cytoscape) of potential target proteins for CBD's anti-inflammatory activity. Color images are available online.
FIG. 4.
Interaction network (created by software Cytoscape) of two protein core subnetworks (A, B) of target proteins for CBD's anti-inflammatory activity. Darker color indicates greater number of degrees. Color images are available online.
Enrichment analysis for GO biological processes and KEGG signaling pathway analysis
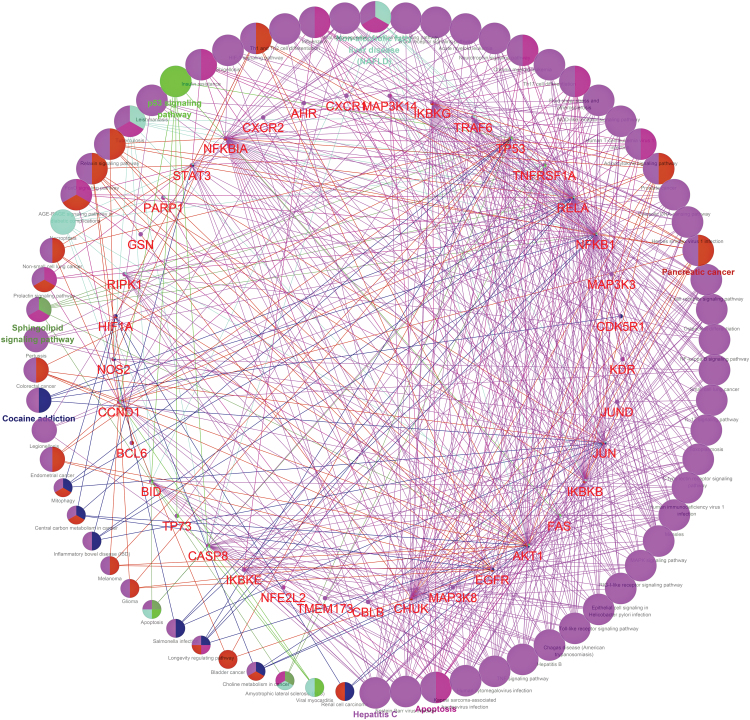
A panel of 44 potential targets was analyzed by the ClueGO plug-in for systematical analysis of the enrichment of biological functions and biological processes. There were 155 biological processes that were enriched (p value ≤0.01) and they were related to cellular response to the interleukin-1 (IL-1) and IL1-mediated signaling pathways (Table 3). For the molecular functions, 11 biological processes (p value ≤0.01) were related to histone deacetylase binding and enhancer binding (Table 3). For the cell components, five biological processes (p value ≤0.01) were strongly related to IκB kinase complex and CD40 receptor complex (Table 3). In addition, KEGG analysis revealed that a total of 71 pathways related to CBD's anti-inflammatory activity were found to be enriched with the protein targets (p value ≤0.01; shown in Fig. 5). The KEGG analysis of CBD's anti-inflammatory activity revealed several related signaling pathways, including TNF, toll-like receptor (TLR), retinoic acid-inducible gene-I-like receptors (RLRs), MAPK, C-type lectin receptors (CLRs), IL-17, NFκB, T cell receptor (TCR), cytosolic DNA-sensing, adipocytokine, nucleotide-binding oligomerization domain-like receptors (NLRs), neurotrophin, B cell receptor, chemokine, and advance glycation end products (AGEs)-receptor for AGEs (RAGE). Notably, some of these signaling pathways are known to be associated with a specific anti-inflammation mechanism, namely the inflammasome pathway, which is responsible for the maturation and secretion of proinflammatory cytokines IL-1β.21 Our group has previously reported that the inhibitory effect of CBD on NLRP3 inflammasome activation is associated with its modulation of a purinergic receptor, namely, P2X7 receptor, which regulates several signaling pathways to release proinflammatory cytokines.22 Published studies have also reported that the activation of signaling pathways, including TLR,23 TCR,24 NLRs,25 and RAGE,26 are closely related to the regulation of inflammasome activation. Therefore, multiple signaling pathways may be involved in CBD's anti-inflammasome activity, which contribute to CBD's overall anti-inflammatory activity. In addition, the drug-target association network also suggested that CBD had significant correlations with several inflammatory-mediated diseases, including hepatitis B and C, herpesviruses infection, various types of cancers (e.g., lung cancer, pancreatic cancer, prostate cancer, bladder cancer, myeloid leukemia, colorectal cancer, and melanoma), nonalcoholic fatty liver diseases, inflammatory bowel disease (IBD), and amyotrophic lateral sclerosis (ALS) (Fig. 5). These findings are supported by several preclinical investigations of CBD as interventions for the aforementioned inflammatory diseases, including hepatitis,27 viral infections,28 cancers,29,30 liver diseases,31 IBD,32,33 and ALS.34 Further experimental studies using “-omic” approaches (e.g., genomics, proteomics, and metabolomics) with in vitro and in vivo models are warranted to verify the biological effects of predicted protein targets.35 Several challenges still remain before CBD's therapeutic applications for inflammatory-mediated diseases can be fully explored, due to (1) only limited data from clinical trials are available;36–39 (2) CBD may exert anti-inflammatory activity via multiple pharmacological targets (e.g., the endocannabinoid system)40,41; and (3) further mechanistic studies of CBD's pharmacological effects are warranted. It should be noted that the current study solely focused on the analysis of CBD's network for anti-inflammatory effects. However, there are numerous other phytochemicals, including over a hundred phytocannabinoids, such as THC, present in cannabis extracts. Similar to CBD, these phytochemicals (phytocannabinoids and nonphytocannabinoids) may also be involved in the modulation of the pharmacological targets identified herein and contribute to the overall anti-inflammatory effects of cannabis extracts. Therefore, it is possible that CBD and other phytochemicals, including THC in cannabis extracts, exert pharmacological effects in a complementary, additive, and/or synergistic manner, but further studies are warranted to confirm this.
Table 3.
The Functional Analysis of Identified Compound-Related Targets by Gene Ontology Analysis and Kyoto Encyclopedia of Genes and Genomes Signaling Pathway Analysis
| Ontology source | GOID | GOTerm | Term p value corrected with Bonferroni step down |
|---|---|---|---|
| GO_BiologicalProcess | GO:0070498 | Interleukin-1-mediated signaling pathway | 2.56E-13 |
| GO_BiologicalProcess | GO:0071347 | Cellular response to interleukin-1 | 7.48E-12 |
| GO_BiologicalProcess | GO:0043123 | Positive regulation of I-kappaB kinase/NF-kappaB signaling | 1.83E-11 |
| GO_BiologicalProcess | GO:0070555 | Response to interleukin-1 | 4.43E-11 |
| GO_BiologicalProcess | GO:0009612 | Response to mechanical stimulus | 1.57E-09 |
| GO_BiologicalProcess | GO:0033209 | Tumor necrosis factor-mediated signaling pathway | 6.06E-09 |
| GO_BiologicalProcess | GO:0038061 | NIK/NF-kappaB signaling | 6.28E-09 |
| GO_BiologicalProcess | GO:1902895 | Positive regulation of pri-miRNA transcription by RNA polymerase II | 3.38E-08 |
| GO_BiologicalProcess | GO:0035666 | TRIF-dependent toll-like receptor signaling pathway | 6.22E-08 |
| GO_BiologicalProcess | GO:0038095 | Fc-epsilon receptor signaling pathway | 8.02E-08 |
| GO_MolecularFunction | GO:0042826 | Histone deacetylase binding | 2.81E-06 |
| GO_MolecularFunction | GO:0035326 | Enhancer binding | 1E-05 |
| GO_MolecularFunction | GO:0000980 | RNA polymerase II distal enhancer sequence-specific DNA binding | 3.95E-05 |
| GO_MolecularFunction | GO:0051879 | Hsp90 protein binding | 0.000357 |
| GO_MolecularFunction | GO:0097110 | Scaffold protein binding | 0.000576 |
| GO_MolecularFunction | GO:0032813 | Tumor necrosis factor receptor superfamily binding | 0.00121 |
| GO_MolecularFunction | GO:0033613 | Activating transcription factor binding | 0.001913 |
| GO_MolecularFunction | GO:0005123 | Death receptor binding | 0.001915 |
| GO_MolecularFunction | GO:0051059 | NF-kappaB binding | 0.002589 |
| GO_MolecularFunction | GO:0001102 | RNA polymerase II activating transcription factor binding | 0.002872 |
| GO_CellularComponent | GO:0035631 | CD40 receptor complex | 0.000252 |
| GO_CellularComponent | GO:0008385 | IkappaB kinase complex | 0.000297 |
| GO_CellularComponent | GO:1902554 | Serine/threonine protein kinase complex | 0.000316 |
| GO_CellularComponent | GO:0031264 | Death-inducing signaling complex | 0.000402 |
| GO_CellularComponent | GO:0045178 | Basal part of cell | 0.001531 |
GO, Gene Ontology.
FIG. 5.
Network diagram (created by software Cytoscape) of putative signaling pathways that are involved in CBD's anti-inflammatory activity and related diseases. Colors of nodes reflect the enrichment of biological function and classification of diseases associated with inflammation. Color images are available online.
In summary, a network-based pharmacological analysis was utilized to predict the potential molecular targets for CBD's anti-inflammatory activity, which revealed the NFκB cascade as one of its primary anti-inflammatory mechanism of action. In addition, target proteins, including p53, IκBα, IKKs, and MAP kinases, as well as signaling pathways, including STAT3, AKT1, TNF, TLR, RLRs, and MAPK, were linked to CBD's anti-inflammatory activity. These molecular targets may contribute to CBD's overall anti-inflammatory activity and its potential therapeutic applications for several inflammatory-mediated diseases. Although further biological experiments are warranted to validate these molecular targets, our findings add to the growing body of data supporting the utilization of CBD as a promising anti-inflammatory natural product.
Supplementary Material
Abbreviations Used
- AGEs
advance glycation end products
- ALS
amyotrophic lateral sclerosis
- CASP8
cysteine-aspartic acid protease-8
- CBD
cannabidiol
- CLRs
C-type lectin receptors
- DAVID
Database for Annotation, Visualization, and Integrated Discovery
- GO
Gene Ontology
- IBD
inflammatory bowel disease
- IKK-α/β/γ
inhibitors of nuclear factor kappa-B kinase subunit alpha/beta/gamma
- IL-1
interleukin-1
- IκBα
NF-kappa-B inhibitor alpha
- KEGG
Kyoto Encyclopedia of Genes and Genomes
- MAPK
mitogen-activated protein kinase
- NFκB
nuclear factor kappa-light-chain-enhancer of activated B cells
- NFκB p105
nuclear factor NF-kappa-B p105 subunit
- NFκB p65
transcription factor p65
- NLRs
nucleotide-binding oligomerization domain-like receptors
- OMIM
Online Mendelian Inheritance in Man
- PPI
protein–protein interaction
- RAGE
receptor for advance glycation end products
- RLRs
retinoic acid-inducible gene-I-like receptors
- STAT3
signal transducer and activator of transcription 3
- STRING
Search Tool for the Retrieval of Interacting Genes/Proteins
- TCR
T cell receptor
- THC
tetrahydrocannabinol
- TLR
toll-like receptor
- TNF
tumor necrosis factor
- TNFRSF1A
tumor necrosis factor receptor superfamily member 1A
- TNFα
tumor necrosis factor alpha
- TRAF6
TNF receptor-associated factor 6
Author Disclosure Statement
N.P.S. serves on the Advisory Board of Alluvion Brands, LLC (Warwick, RI, USA) as a consultant for the biological evaluations of phytocannabinoids. Alluvion Brands did not influence the design of this study nor had any financial contributions to this work. The other authors declare no conflicts of interest.
Funding Information
No funding was received for this study.
Supplementary Material
Cite this article as: Ma H, Xu F, Liu C, Seeram NP (2021) A network pharmacology approach to identify potential molecular targets for cannabidiol's anti-inflammatory activity, Cannabis and Cannabinoid Research 6:4, 288–299, DOI: 10.1089/can.2020.0025.
References
- 1.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mantovani A. Molecular pathways linking inflammation and cancer. Curr Mol Med. 2010;10:369–373 [DOI] [PubMed] [Google Scholar]
- 3.Liu T, Zhang L, Joo D, et al. NF-κB signaling in inflammation. Signal Transduct Target Ther. 2017;2:17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arulselvan P, Fard MT, Tan WS, et al. Role of antioxidants and natural products in inflammation. Oxid Med Cell Longev. 2016;2016:5276130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ryz NR, Remillard DJ, Russo EB. Cannabis roots: a traditional therapy with future potential for treating inflammation and pain. Cannabis Cannabinoid Res. 2017;2:210–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zlas J, Stark H, Seligman J, et al. Early medical use of cannabis. Nature. 1993;363:215. [DOI] [PubMed] [Google Scholar]
- 7.Kozela E, Pietr M, Juknat A, et al. Cannabinoids Δ9-tetrahydrocannabinol and cannabidiol differentially inhibit the lipopolysaccharide-activated NF-κB and interferon-β/STAT proinflammatory pathways in BV-2 microglial cells. J Biol Chem. 2010;285:1616–1626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Juknat A, Pietr M, Kozela E, et al. Differential transcriptional profiles mediated by exposure to the cannabinoids cannabidiol and Δ 9-tetrahydrocannabinol in BV-2 microglial cells. Br J Pharmacol. 2012;165:2512–2528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Juknat A, Rimmerman N, Levy R, et al. Cannabidiol affects the expression of genes involved in zinc homeostasis in BV-2 microglial cells. Neurochem Int. 2012;61:923–930 [DOI] [PubMed] [Google Scholar]
- 10.Zhao S, Iyengar R. Systems pharmacology: network analysis to identify multiscale mechanisms of drug action. Annu Rev Pharmacol Toxicol. 2012;52:505–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berger SI, Iyengar R. Network analyses in systems pharmacology. Bioinformatics. 2009;25:2466–2472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bian Y min, He X bing, Jing Y kang, et al. Computational systems pharmacology analysis of cannabidiol: a combination of chemogenomics-knowledgebase network analysis and integrated in silico modeling and simulation. Acta Pharmacol Sin. 2019;40:374–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berger SI, Iyengar R. Network analyses in systems pharmacology. Bioinformatics. 2009;25:2466–2472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hopkins AL. Network pharmacology: the next paradigm in drug discovery. Nat Chem Biol. 2008;4:682–690 [DOI] [PubMed] [Google Scholar]
- 15.Esposito G, De Filippis D, Maiuri MC, et al. Cannabidiol inhibits inducible nitric oxide synthase protein expression and nitric oxide production in β-amyloid stimulated PC12 neurons through p38 MAP kinase and NF-κB involvement. Neurosci Lett. 2006;399:91–95 [DOI] [PubMed] [Google Scholar]
- 16.Huang Y, Wan T, Pang N, et al. Cannabidiol protects livers against nonalcoholic steatohepatitis induced by high-fat high cholesterol diet via regulating NF-κB and NLRP3 inflammasome pathway. J Cell Physiol. 2019;234:21224–21234 [DOI] [PubMed] [Google Scholar]
- 17.Xu F, Yang L, Huang X, et al. Lupenone is a good anti-inflammatory compound based on the network pharmacology. Mol Divers. 2020;24:21–30 [DOI] [PubMed] [Google Scholar]
- 18.She H, He Y, Zhao Y, et al. Release the autophage brake on inflammation: The MAPK14/p38α-ULK1 pedal. Autophagy. 2018;14:1097–1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rajesh M, Mukhopadhyay P, Btkai S, et al. Cannabidiol attenuates cardiac dysfunction, oxidative stress, fibrosis, and inflammatory and cell death signaling pathways in diabetic cardiomyopathy. J Am Coll Cardiol. 2010;56:2115–2125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Giacoppo S, Pollastro F, Grassi G, et al. Target regulation of PI3K/Akt/mTOR pathway by cannabidiol in treatment of experimental multiple sclerosis. Fitoterapia. 2017;116:77–84 [DOI] [PubMed] [Google Scholar]
- 21.Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140:821–832 [DOI] [PubMed] [Google Scholar]
- 22.Liu C, Ma H, Slitt AL, et al. Inhibitory effect of cannabidiol on the activation of NLRP3 inflammasome is associated with its modulation of the P2X7 receptor in human monocytes. J Nat Prod. 2020. [Epub ahead of print]; DOI: 10.1021/acs.jnatprod.0c00138 [DOI] [PubMed] [Google Scholar]
- 23.Hanamsagar R, Hanke ML, Kielian T. Toll-like receptor (TLR) and inflammasome actions in the central nervous system. Trends Immunol. 2012;33:333–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dostert C, Ludigs K, Guarda G. Innate and adaptive effects of inflammasomes on T cell responses. Curr Opin Immunol. 2013;25:359–365 [DOI] [PubMed] [Google Scholar]
- 25.Mason DR, Beck PL, Muruve DA. Nucleotide-binding oligomerization domain-like receptors and inflammasomes in the pathogenesis of non-microbial inflammation and diseases. J Innate Immun. 2011;4:16–30 [DOI] [PubMed] [Google Scholar]
- 26.Kang R, Chen R, Xie M, et al. The receptor for advanced glycation end products activates the AIM2 inflammasome in acute pancreatitis. J Immunol. 2016;196:4331–4337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hegde VL, Nagarkatti PS, Nagarkatti M. Role of Myeloid-derived suppressor cells in amelioration of experimental autoimmune hepatitis following activation of TRPV1 receptors by cannabidiol. PLoS One. 2011;6:e18281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reiss CS. Cannabinoids and viral infections. Pharmaceuticals (Basel). 2010;3:1873–1886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramer R, Hinz B. Cannabinoids as anticancer drugs. Adv Pharmacol. 2017;80:397–436 [DOI] [PubMed] [Google Scholar]
- 30.Massi P, Solinas M, Cinquina V, et al. Cannabidiol as potential anticancer drug. Br J Clin Pharmacol. 2013;75:303–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patsenker E, Stickel F. Cannabinoids in liver diseases. Clin Liver Dis. 2016;7:21–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Naftali T, Mechulam R, Lev LB, et al. Cannabis for inflammatory bowel disease. Dig Dis. 2014;32:468–474 [DOI] [PubMed] [Google Scholar]
- 33.Esposito G, De Filippis D, Cirillo C, et al. Cannabidiol in inflammatory bowel diseases: a brief overview. Phyther Res. 2013;27:633–636 [DOI] [PubMed] [Google Scholar]
- 34.Elliott DM, Singh N, Nagarkatti M, et al. Cannabidiol attenuates experimental autoimmune encephalomyelitis model of multiple sclerosis through induction of myeloid-derived suppressor cells. Front Immunol. 2018;9:1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Horgan RP, Kenny LC. ‘Omic’ technologies: genomics, transcriptomics, proteomics and metabolomics. Obstet Gynaecol. 2011;13:189–195 [Google Scholar]
- 36.Samanta D. Cannabidiol: a review of clinical efficacy and safety in epilepsy. Pediatr Neurol. 2019;96:24–29 [DOI] [PubMed] [Google Scholar]
- 37.Hill KP, Palastro MD, Johnson B, et al. Cannabis and pain: a clinical review. Cannabis Cannabinoid Res. 2017;2:96–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Iseger TA, Bossong MG. A systematic review of the antipsychotic properties of cannabidiol in humans. Schizophr Res. 2015;162:153–161 [DOI] [PubMed] [Google Scholar]
- 39.Pisanti S, Malfitano AM, Ciaglia E, et al. Cannabidiol: state of the art and new challenges for therapeutic applications. Pharmacol Ther. 2017;175:133–150 [DOI] [PubMed] [Google Scholar]
- 40.Talevi A. Multi-target pharmacology: possibilities and limitations of the “skeleton key approach” from a medicinal chemist perspective. Front Pharmacol. 2015;6:205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kaur R, R. Ambwani S, Singh S. Endocannabinoid system: a multi-facet therapeutic target. Curr Clin Pharmacol. 2016;11:110–117 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.