To date, failure of the first-line medical management of Crohn’s disease (CD) in children and adolescents has been followed by escalation treatment, with administration of immunomodulators, e.g., azathioprine, or biologics, such as infliximab (1). The most recent revision of the international guideline proposes stratification of the therapy, with selection of either accelerated step-up or primary treatment with biologics on the basis of predictors of poor outcome (POPO) (2). The objective of this study was to evaluate this concept of early immunosuppression with the aid of data from the largest registry of children and adolescents with chronic inflammatory bowel disease (IBD) in Europe.
Acknowledgments
Translated from the original German by David Roseveare
CEDATA GPGE—list of contributors
Dr. med. Stephan Buderus, Bonn
Prof. Dr. med. Philip Bufler, Berlin
Dr. med. Martin Claßen, Bremen
Prof. Dr. med. Jan Däbritz, Rostock
Dr. med. Söhnke Dammann, Stuttgar
Dr. med. Jan de Laffolie, Giessen
Prof. Dr. med. Almuthe Christina Hauer, Graz, Austria
Prof. Dr. med. Klaus-Michael Keller, Wiesbaden
Prof. Dr. med. Sibylle Koletzko, Munich
Dr. med. Andreas Krahl, Darmstadt
Dr. med. Martin Laaß, Dresden
Dr. med. Thomas Lang, Regensburg
Dr. med. Carsten Posovszky, Ulm
PD Dr. med. Burkhard Rodeck, Osnabrück
Dr. med. Stefan Trenkel, Potsdam
Support and sponsorship
The CEDATA GPGE registry was/is supported by the Justus Liebig University of Giessen, Medical University Graz, and the Rudolf Chaudoire Research Fund for Switzerland, Germany, and Austria. It was/is sponsored by Abbvie, Dr. Falk Pharma, Vifor, A Heart for Children (BILD hilft e.V.), and Takeda.
Footnotes
Conflict of interest statement
PD Dr. de Laffolie has received consultancy honoraria (Advisory Board) from Abbvie.
Prof. Hauer has received consultancy honoraria from Abbvie, MSD, Nutricia, Shire, Milupa, and Hipp and has been the beneficiary of study support (third-party funds) from Janssen.
The remaining authors declare that no conflict of interest exists.
Patients and method
CEDATA GPGE is a multicenter registry in German-speaking countries that has accumulated data on more than 5000 children and adolescents with chronic inflammatory bowel disease and over 50 000 documented contacts. This study included all patients with CD who fulfilled the following criteria: age 1–18 years, diagnosis at a participating center in the period 2004–2018, addition to the registry within 3 months of diagnosis (to avoid recall bias), and at least two follow-up visits. Patients with at least one predictor of poor outcome were classified as POPO positive, while those without were classed as POPO negative. The following are considered as POPO: perianal disease; a stricturing/penetrating disease pattern; severe growth retardation; extensive panenteric disease; and persistent disease activity despite adequate induction therapy. On the one hand, we ascertained the frequency of occurrence of negative outcome parameters (especially extraintestinal manifestations [EIM]), and on the other hand, we compared patients in whom administration of azathioprine or infliximab was started early with those for whom that was not the case. Kaplan–Meier analysis and Cox regression were performed, excluding medications after occurrence of the endpoint prior to analysis.
Results
Of 2980 patients in the register, 1084 met the criteria specified above and were included. The median follow-up was 10 visits (interquartile range [IQR] 5–15 visits, maximum 94 visits over a period of 769 days (median; IQR 275–1595 days, maximum 3570 days. Seven hundred nine patients (65.4%) had at least one POPO (children under 6: 70%). Although there were no differences between POPO-positive and POPO-negative patients with regard to age, sex, or diagnostic latency, they did differ in disease activity at the time of diagnosis (Paediatric Crohn’s Disease Activity Index 23.3 versus 19.3; p = 0.05).
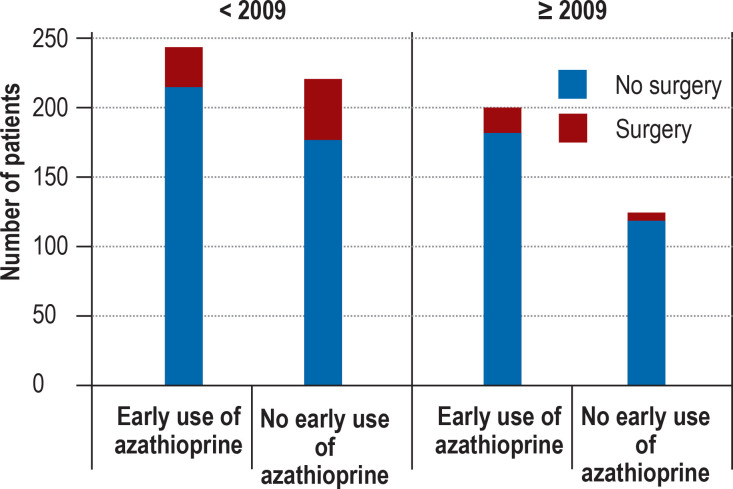
The use of azathioprine and infliximab increased over the study period (azathioprine: 45% in 2004, 62% in 2018; infliximab: 3% in 2004, 35% in 2018). In the first year following diagnosis, POPO-positive patients were somewhat more likely than POPO-negative patients to receive infliximab (11.1% versus 7.3%; p = 0.047) and azathioprine (64.4% versus 61.5%; p = 0.35). The POPO-positive patients with abscesses, fistulas, or stenoses were given infliximab more frequently than those without such findings (37.1% versus 22.1%, p = 0.06). A similar relationship was seen between these two groups with regard to the need for surgery (17.1% versus 7.2%, p = 0.054). In patients diagnosed before 2009, early initiation of azathioprine was associated with a lower risk of surgery (odds ratio [OR] 0.59; 95% confidence interval [0.35; 0.98]; p = 0.032; Figure 1).
Figure 1.
Association of early treatment with azathioprine with the need for surgical procedures before and after 2009
Before 2009, patients with early azathioprine treatment had a lower risk of surgery (12.3% versus 19.1%, p = 0.041). This effect is no longer present after 2009, with fewer operations and a higher proportion of patients with early administration of azathioprine and other interventions (including early administration of infliximab).
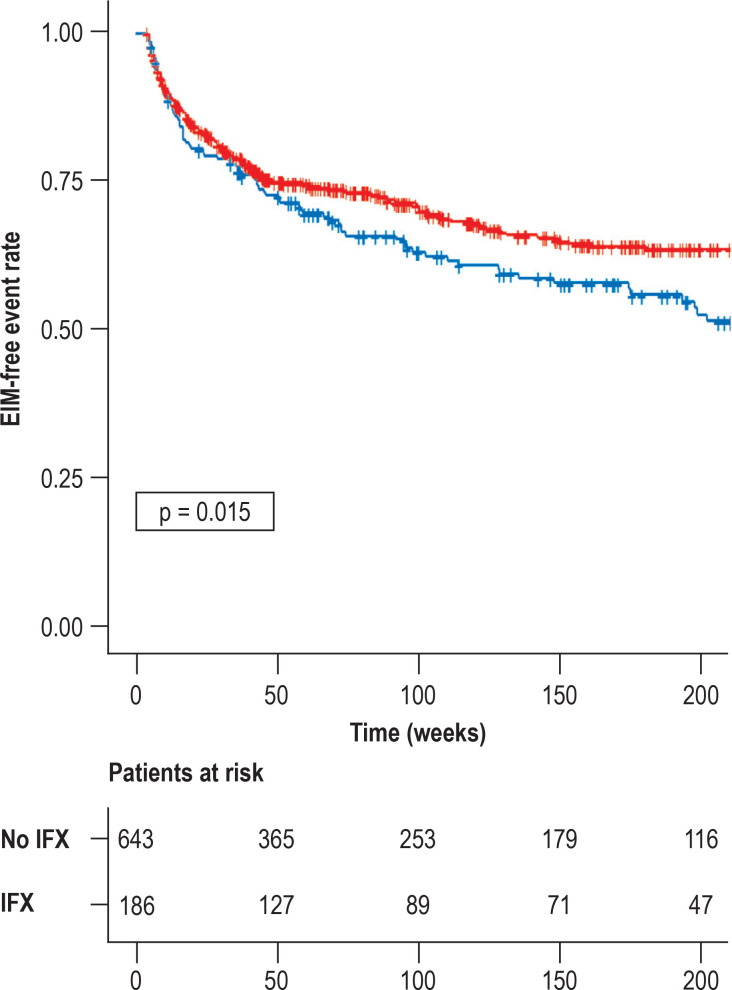
Cox regression showed that diagnosis after 2009 was the parameter associated strongly with a reduced risk of EIM (hazard ratio [HR] 0.32 [0.2; 0.51]). When the time of diagnosis (before or after 2009) was ignored, initiation of infliximab within 3 months after diagnosis was associated with a lower risk of EIM than later administration (HR = 0.27 [0.036; 2.0]). However, no such association was found for azathioprine (HR = 1.45 [0.6; 3.1]). In Kaplan–Meier analysis, the trend was also apparent at the end of the observation period for infliximab (OR 0.17 [0.05–0.61]), but not for azathioprine (OR 1.00 [0.70; 1.44]; Figure 2).
Figure 2.
Risk of extraintestinal manifestations (EIM) after administration of infliximab and azathioprine
Patients with administration of infliximab (IFX) showed a lower risk of developing EIM (red) than patients without administration of infliximab (blue). This effect could not be demonstrated for azathioprine (data not shown).
The predictor “persistent disease activity despite adequate induction therapy” was associated with an elevated risk of failing to achieve a steroid-free remission lasting more than 1 year (OR 1.49 [1.07; 2.07], p = 0.02).
Discussion
The data presented here indicate that the risk is reduced by early administration of infliximab. They lend emphasis to recent calls for early escalation or initial treatment with biologics in selected cases (3), with the aim of achieving adequate control of persistent disease. Our findings are confirmed by a cohort study of 825 patients in which early initiation of infliximab treatment was associated with a low rate of surgery (4). It has to be taken into account that other factors have also contributed to the decrease in operative interventions over the past 15–20 years. The reduction in the risk of extraintestinal manifestations after administration of infliximab, but not of azathioprine, indicates that early treatment with anti-tumor necrosis factor (anti-TNF) alpha-antibodies is more effective than early use of immunomodulators, as was described in a smaller group (5).
Our observation study with analysis of data from a large cohort supports the treatment concept of the international guideline. The findings may contribute to science-based, patient-centered, and needs-oriented treatment of children and adolescents with IBD.
Conclusion
The increased and early use of azathioprine or infliximab over the past 15–20 years is associated with a decrease in the need for surgical procedures in children and adolescents with Crohn’s disease. Early disease control is particularly important in patients with risk factors to minimize long-term complications and achieve remission.
References
- 1.Buderus S, Scholz D, Behrens R, et al. Inflammatory bowel disease in pediatric patients—characteristics of newly diagnosed patients from the CEDATA-GPGE registry. Dtsch Arztebl Int. 2015;112:121–127. doi: 10.3238/arztebl.2015.0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rheenen PF, Aloi M, Assa A, Bronsky J, et al. The medical management of paediatric Crohn‘s disease: an ECCO-ESPGHAN guideline update. J Crohns Colitis. 2020 doi: 10.1093/ecco-jcc/jjaa161. jjaa161. doi: 10.1093/ecco-jcc/jjaa161. Epub ahead of print. PMID: 33026087. [DOI] [PubMed] [Google Scholar]
- 3.Brückner A, Werkstetter KJ, de Laffolie J, et al. Incidence and risk factors for perianal disease in pediatric Crohn disease patients followed in CEDATA-GPGE Registry. J Pediatr Gastroenterol Nutr. 2018;66:73–78. doi: 10.1097/MPG.0000000000001649. [DOI] [PubMed] [Google Scholar]
- 4.Ashton JJ, Borca F, Mossotto E, et al. Increased prevalence of anti-TNF therapy in paediatric inflammatory bowel disease is associated with a decline in surgical resections during childhood. Aliment Pharmacol Ther. 2019;49:398–407. doi: 10.1111/apt.15094. [DOI] [PubMed] [Google Scholar]
- 5.Walters TD, Kim MO, Denson LA, et al. Increased effectiveness of early therapy with anti-tumor necrosis factor-alpha vs an immunomodulator in children with Crohn‘s disease. Gastroenterology. 2014;146:383–391. doi: 10.1053/j.gastro.2013.10.027. [DOI] [PubMed] [Google Scholar]