Abstract
Background
Bullous autoimmune dermatoses are a clinically and immunopathologically heterogeneous group of diseases, characterized clinically by blisters or erosions of the skin and/or mucous membranes. In Germany, their prevalence is approximately 40 000 cases nationwide, and their incidence approximately 20 new cases per million people per year.
Methods
This review is based on publications that were retrieved by a selective search of the literature focusing on the current German and European guidelines.
Results
Recent years have seen the publication of guidelines, controlled prospective clinical trials, and multicenter diagnostic studies improving both diagnosis and therapy. Specific monovalent and multivariate serological test systems and pattern analysis of tissue-bound autoantibodies allow identification of the target antigens in 80–90% of patients. This enables the precise classification of disease entities, with implications for treatment selection and disease outcome. In 2019, the anti-CD20 antibody rituximab was approved by the European Medicines Agency for the treatment of moderate and severe pemphigus vulgaris, with an ensuing marked improvement in the care of the affected patients. To treat mild and moderate bullous pemphigoid, topical clobetasol proprionate is recommended, in severe disease, combined with systemic treatment, i.e. usually (a) prednisolone p.o. at an initial dose of 0.5mg/kg/d, (b) an immunomodulant, e.g. dapsone or doxycycline, or (c) prednisolone plus an immunomodulant.
Conclusion
The early recognition and precise diagnostic evaluation of bullous autoimmune dermatoses now enables improved, often interdisciplinary treatment, in accordance with the available guidelines. Current research projects are focused on new treatment approaches, an improved understanding of the underlying pathophysiology, and further refinements of diagnostic techniques.
cme plus
This article has been certified by the North Rhine Academy for Continuing Medical Education. Participation in the CME certification program is possible only over the internet: cme.aerzteblatt.de. The deadline for submissions is 17 June 2022.
Autoimmune bullous diseases (AIBD) are prototypical autoantibody-mediated autoimmune diseases in which the effects of the autoantibodies are directly visible on the skin and/or on mucous membranes. If left untreated, these diseases are potentially life-threatening due to superinfection, fluid loss, and severely restricted food intake (1– 4, e1, e2).
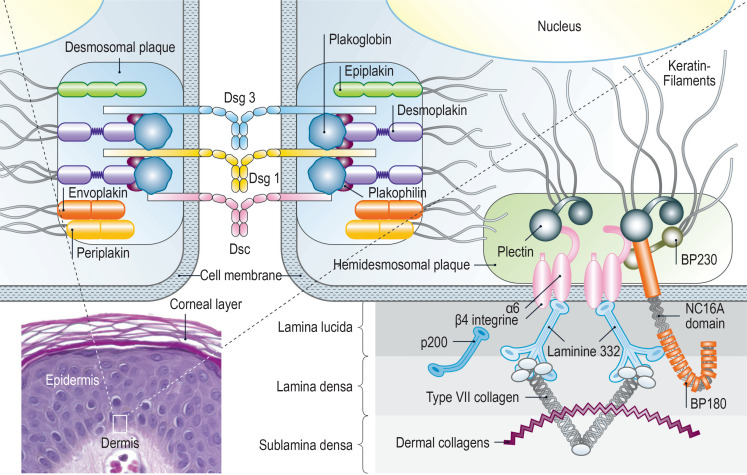
Clinically, depending on the disease entity, vesicles, blisters, pustules, erosions, excoriations, and erythema on the skin and mucous membranes can be seen. In AIBD, autoantibodies are directed against structural proteins of the skin; in pemphigus diseases, they are directed against desmosomal proteins, which connect neighboring keratinocytes/epithelial cells, and in pemphigoid diseases, against proteins of the basement membrane zone, which connect the epidermis/epithelium and the dermis/lamina propria (figure 1).
Figure 1.
Figure 1: Schematic diagram of the autoantigens in pemphigus and pemphigoid diseases. BP180, type XVII collagen; BP230, dystonin; Dsg, desmoglein; Dsc, desmocollin
Epidemiology
The frequency of AIBD differs significantly depending on the geographic region and population evaluated (2, e3, e4). In Germany and central Europe, bullous pemphigoid is by far the most common AIBD (5, e5– e10) (table 1), with an increasing incidence in recent decades (e8, e11– e13). Possible causes for the increasing incidence of bullous pemphigoid may include an aging population, the association with increasingly frequent neurological diseases and certain medications (see below), and a greater awareness of atypical variants without blistering (overview in [e4]).
Table 1. Incidence and Prevalence.
| Disease | Incidence/million inhabitants/year | Prevalence in Germany 2014/million (e9) |
| Pemphigus vulgaris | 0.5–6.8 (e5, 5, e88) Germany: 1.0 (e100) | 94.8 |
| Pemphigus foliaceus | < 1 (e89) | 10.0 |
| Bullous pemphigoid | 6.1–42.9 (5, e5– e8, e10) Germany: 19.6 (40) | 259.3 |
| Mucous membrane pemphigoid | 0.8–2 (e5, e90, e91) | 24.6 |
| Linear IgA disease | 0.25–1 (e5, e92) | 24.3 for children |
| Pemphigoid gestationis | 0.08–2 (e5, e92) Germany: 2.0 (e5) | 13.6 for women |
| Anti-p200 pemphigoid | Germany: 0.7 (40) | unknown |
| Epidermolysis bullosa acquisita | 0.2–0.5 (5, e5– e8, e10) | 2.8 |
The most common AIBDs in children are linear IgA dermatosis and pemphigus vulgaris (6, e14). An association with the human leukocyte antigens HLA-DRB1*04 and HLA-A*10 and a polymorphic variant in the ST18 gene have been described for pemphigus vulgaris, while an overrepresentation of HLA-DQB1*03:01 and polymorphism in the mitochondrial ATP8 gene has been described for bullous pemphigoid (1, 2, e3, e15, e16).
Clinical features
Pemphigus diseases
Pemphigus diseases can be classified in 4 main forms based on clincial and immunopathological features: pemphigus vulgaris, in about 70–80% of patients; pemphigus foliaceus, in about 20%; paraneoplastic pemphigus, in about 5%; and IgA pemphigus, in 1–3% (table 2) (2).
Table 2. Target antigens of autoimmune bullous dermatoses and serological diagnostics.
| Disease | Target antigen | Serological diagnostics*1 |
| Pemphigus diseases | ||
| Pemphigus vulgaris | Dsg 3 Dsg 1 |
IIF monkey esophagus: ICF IgG+ ELISA, IIF: Dsg3+ Dsg1± |
| Pemphigus foliaceus | Dsg 1 |
IIF monkey esophagus: ICF IgG+ ELISA, IIF: Dsg1+ |
| Paraneoplastic pemphigus | Envoplakin, periplakin, Dsg 3, desmoplakin I/II, plectin, epiplakin, BP230, BP180, Dsc 1, 2, 3, Dsg1, α2 macroglobulin-like 1 |
IIF monkey esophagus: ICF+, BMF± Monkey-/ rat bladder: urothelium + ELISA, IIF: Dsg3±, Dsg 1±, BP180±, BP230±; ELISA: envoplakin ± IB, IP, ELISA: all other target antigens ± |
| IgA pemphigus | Dsc 1, 2, 3 |
IIF monkey esophagus: ICF IgA ELISA, IIF: Dsc1, 2, 3 IgA±; Dsg3 IgA± |
| Pemphigoid diseases | ||
| Bullous pemphigoid | BP180, BP230 |
IIF monkey esophagus: BMF+ IIF salt-split skin: blister roof+ ELISA, IIF: BP180 (+ in 80–90%), BP230 (+ in 50–60%) |
| Mucous membrane pemphigoid | BP180, LAD-1, laminin332, BP230, (α4β6 integrin)*2 |
IIF monkey esophagus: BMF± IIF salt-split skin: blister roof± or blister floor± ELISA: BP180±, BP230± IB: LAD-1±; BP180±; IIF: laminin332± |
| Linear IgA disease | LAD-1, type VII collagen |
IIF monkey esophagus: BMF (IgA)+ IIF salt-split skin: blister roof (IgA) + IB, ELISA: BP180 (IgA) ±, LAD-1(IgA)+ |
| Pemphigoid gestationis | BP180 NC16A, BP230 |
IIF salt-split skin: blister roof± IIF complement binding test salt-split skin: blister roof + ELISA, IIF BP180+, BP230± |
| Anti-p200 pemphigoid | p200 protein, laminin γ1 |
IIF monkey esophagus: BMF+ IIF salt-split skin: blister roof+ IB: p200+, laminin γ1± |
| Epidermolysis bullosa acquisita | Type VII collagen |
IIF monkey esophagus: BMF± IIF salt-split skin: blister floor± ELISA, IIF: type VII collagen+ |
| Dermatitis herpetiformis | TG2, TG3 |
IIF monkey esophagus (IgA): endomysium + ELISA (IgA): TG2, TG3, deamidated gliadin + |
*1 commercially available tests are indicated in bold
*2 only described for individual patients
BMF, basement membrane fluorescence; Dsg, desmoglein; Dsc, desmocollin; ELISA, enzyme-linked immunosorbent assay; IIF, indirect immunofluorescence; ICF, intercellular fluorescence; IB, immunoblot; IP, immunoprecipitation; LAD-1, soluble ectodomain of BP 180; TG, transglutaminase
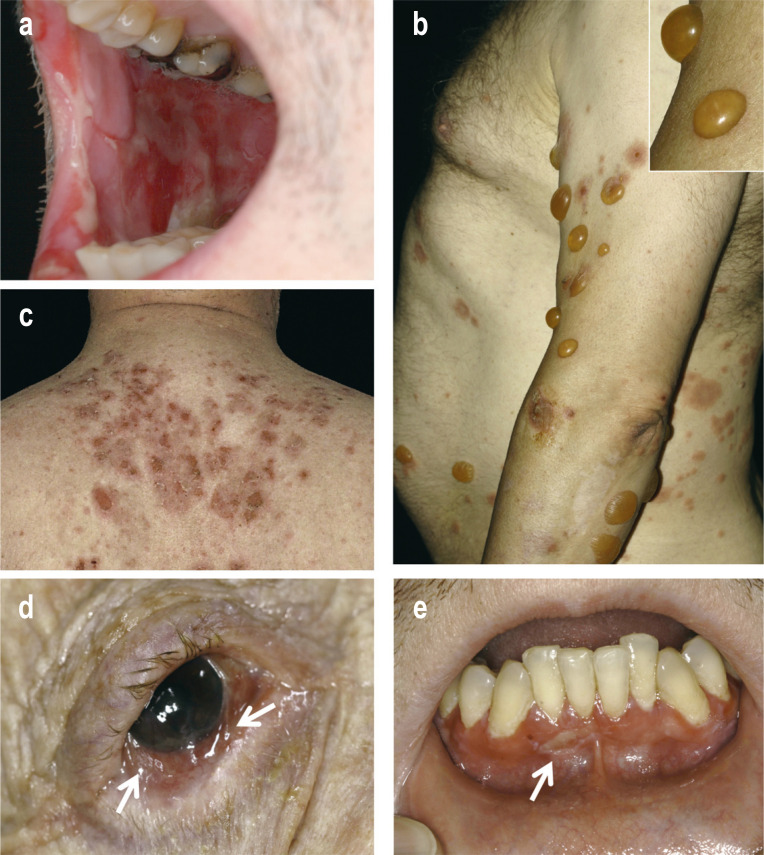
In pemphigus vulgaris, the mucous membranes close to the surface are always affected, including primarily the oral cavity (Figure 2a). Erosions predominate and can also manifest on the mucosa of the pharynx, larynx, esophagus, and genitalia (2, 3). In about half of the cases, flaccid blisters and erosions also appear on the skin, which may involve large areas. This led to a mortality of over 80% before the introduction of the corticosteroids (2, e3, 5). At present, the mortality of patients with pemphigus vulgaris is still two to three times higher than in the general population (e3, 5).
Figure 2.
Figure 2: Clinical presentation of selected cases of autoimmune bullous dermatoses
Pronounced erosions of the buccal oral mucosa in pemphigus vulgaris.
Tense blisters, erythema, and erosions in bullous pemphigoid.
Erosions and crusts on the upper back in pemphigus foliaceus.
Conjunctival injection and synechiae (indicated by arrows) in MMP.
Gingivitis with erosions (indicated by arrow) in MMP.
In pemphigus foliaceus, only the skin is affected, with erosions and scaly crusts, predominantly in seborrheic areas on the trunk and head (Figure 2c) (2, 3).
Paraneoplastic pemphigus is associated with neoplasia and may clinically resemble pemphigus vulgaris. Characteristic features are pronounced stomatitis, lip involvement, and polymorphic, often lichenoid, skin changes (2, 7, e17). With a mortality of 75–90%, the prognosis is unfavorable, primarily due to neoplasm and bronchiolitis obliterans, which occurs in 5–20% of cases (e3, e18, e19).
In IgA pemphigus, pustules and erosions are the most prominent lesions (e20– e22) (table 2). Furthermore, neonatal pemphigus, pemphigus herpetiformis, and endemic pemphigus foliaceus are described as separate entities; pemphigus vegetans is considered a clinical variant of pemphigus vulgaris with predominant involvement of the axillary and inguinal areas. (2, 3, e23).
The differential diagnoses of pemphigus vulgaris and paraneopalstic pemphigus are severe drug reactions, such as Steven–Johnson syndrome, toxic epidermal necrolysis, stomatitis due to herpes simplex virus, hereditary epidermolysis, mucosal lichen planus, and mucous membrane pemphigoid (MMP). Pemphigus foliaceus must be differentiated from seborrheic dermatitis and impetigo, and IgA pemphigus, from pustular psoriasis as well as from pustular reactions to drugs.
Pemphigoid diseases
Bullous pemphigoid presents with tense blisters (Figure 2b), erosions, and urticarial erythema. Non-bullous forms are found in around 20% of cases (e24, e25). Characteristic features are the often severe pruritus and manifestation in old age (mean age of onset, 78 years). Therefore, bullous pemphigoid should be excluded in case of chronic pruritus in old age. Mucosal involvement can be seen in 10–20% of patients (8– 10, e26).
Associated diseases that have been described include cardiovascular diseases, psoriasis, diabetes mellitus, hematological malignancies, and degenerative neurological diseases, the latter mostly preceding the skin disease and affecting 30–50% of patients (11, 12, e27– e29). Associations with the use of dipeptidyl peptidase IV inhibitors have also been observed, particularly with vildagliptin, as well as (although to a lesser degree) with spironolactone, loop diuretics, and drugs for Parkinson’s disease (13, e27, e29– e33). Gliptins should be replaced by other antidiabetic drugs in any case, and the other drugs switched to alternatives when possible. The 1-year mortality rates have been reported to range between 8% and 41% (1, e7, e10, e13, e34, e35). Differential diagnoses are bullous erysipelas, impetigo contagiosa, adverse drug reactions, herpes zoster, urticarial eczema, bullous reactions to insect stings, artifactual changes, hereditary epidermolysis, and other pemphigoid diseases.
Predominant involvement of mucous membranes supports the clinical diagnosis of MMP (Figures 2d, Figures e). The mucous membranes of the mouth and the conjunctiva are particularly affected, as well as (less frequently) mucous membranes of the nose, pharynx, anogenital region, larynx, esophagus, and trachea. About 25–30% of patients present with additional erosions and blisters on the skin (1, 14).
Lesions of the conjunctiva, nose, larynx, esophagus and trachea in particular heal with scarring, which can lead to blindness, chronic hoarseness, difficulties in breathing and dysphagia, respectively. The main autoantigens are BP180 (in around 75% of patients) and laminin 332 (in up to 25%). Anti-laminin 332 MMP is associated with malignancy in 25–30% of cases, and in these patients, a tumor search is required (14, 15, e36). MMP has a differential diagnosis similar to that of pemphigus vulgaris.
Pemphigoid gestationis usually occurs in the third trimester of pregnancy, with severe pruritus and urticarial erythematous plaques, initially mainly in the periumbilical region. The disease resolves postpartum but usually recurs in subsequent pregnancies (1, e2, e37). As main differential diagnoses, polymorphic eruption of pregnancy and urticaria are to be distinguished. Linear IgA disease is characterized by tense vesicles and blisters, often arranged in an annular pattern, but may also resemble bullous pemphigoid and is a common AIBD in childhood (6, e14). In adults, induction by drugs should be considered; notably, about half of the drug-induced cases are caused by vancomycin (e38). Anti-p200 pemphigoid clinically resembles bullous pemphigoid but shows more palmoplantar involvement (e39). In epidermolysis bullosa acquisita, the inflammatory variant mimics bullous pemphigoid, MMP, or linear IgA disease. In the mechanobullous variant, which is present in a third of patients, blisters appear on areas most stressed by mechanical forces, such as elbows, knees, and feet. Involvement of the mucous membranes and healing with scarring are common in this variant (16, 17, e40); the most important differential diagnosis is porphyria cutanea tarda.
Dermatitis herpetiformis, which is the cutaneous manifestation of celiac disease, is characterized by severe pruritus, excoriated papules, and vesicles with predilection for knees, elbows, and buttocks (4, 18).
Diagnosis
AIBD cannot be diagnosed on the basis of the clinical picture alone. Rather, detection of tissue-bound and/or circulating autoantibodies is required (10).
Direct immunofluorescence
Tissue-bound autoantibodies (primarily IgG and IgA) and complement deposits are detected using direct immunofluorescence (IF) in a perilesional skin/mucosal biopsy and continue to represent the gold standard in AIBD diagnostics (9, 10, 17, 18– 21). Direct IF allows a differentiation between pemphigoid diseases with linear deposits on the basement membrane (Figure 3a, Figure b), pemphigus diseases with intercellular fluorescence in the epithelium (Figure 3c), and dermatitis herpetiformis with granular deposits of IgA along the basement membrane and/or in the tips of the dermal papillae. Linear and intercellular fluorescence together indicate paraneoplastic pemphigus (7, e17).
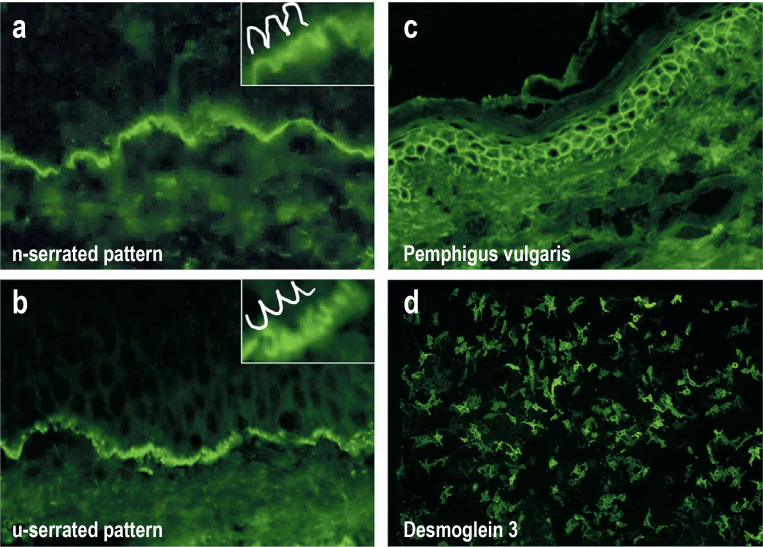
Figure 3.
Figure 3: Direct immunofluorescence of perilesional biopsies for the detection of tissue-bound autoantibodies (a–c) and indirect immunofluorescence of the desmoglein 3–specific biochip (d).
Linear deposits of IgG on the dermal–epidermal junction in bullous pemphigoid. At 400 x magnification, an n-serrated pattern can be seen (inset), which is found in all pemphigoid diseases with the exception of epidermolysis bullosa acquisita.
Linear deposits of IgG on the dermal–epidermal junction zone, with a u-serrated pattern, in epidermolysis bullosa acquisita.
Epidermal intercellular deposits of C3 with a reticular pattern, consistent with pemphigus vulgaris
IgG reactivity to Dsg3-expressing HEK293 cells of a biochip, typical for pemphigus vulgaris
Of the pemphigoid diseases, linear IgA disease can be differentiated based on predominant IgA deposits along the basement membrane, and epidermolysis bullosa acquisita, based on serration pattern analysis (1). Almost all pemphigoid diseases show an n-serrated pattern (Figure 3a); except epidermolysis bullosa acquisita and bullous lupus erythematosus which reveal a u-serrated pattern (Figure 3b) (22, e41, e42).
Serological diagnostics
Circulating autoantibodies can be detected in the serum of about 90% of AIBD patients. In contrast, this is only possible for about half of the patients with epidermolysis bullosa acquisita or MMP (16, 17, e43). A serological diagnosis in combination with the clinical picture allows an exact assignment to the individual entities and thus a tailored therapy and a more precise prognosis. Anti-laminin 332 MMP and paraneoplastic pemphigus are both facultative and obligatory paraneoplasia, respectively, and a search for an underlying malignancy is indicated (7, 15, e36, e44).
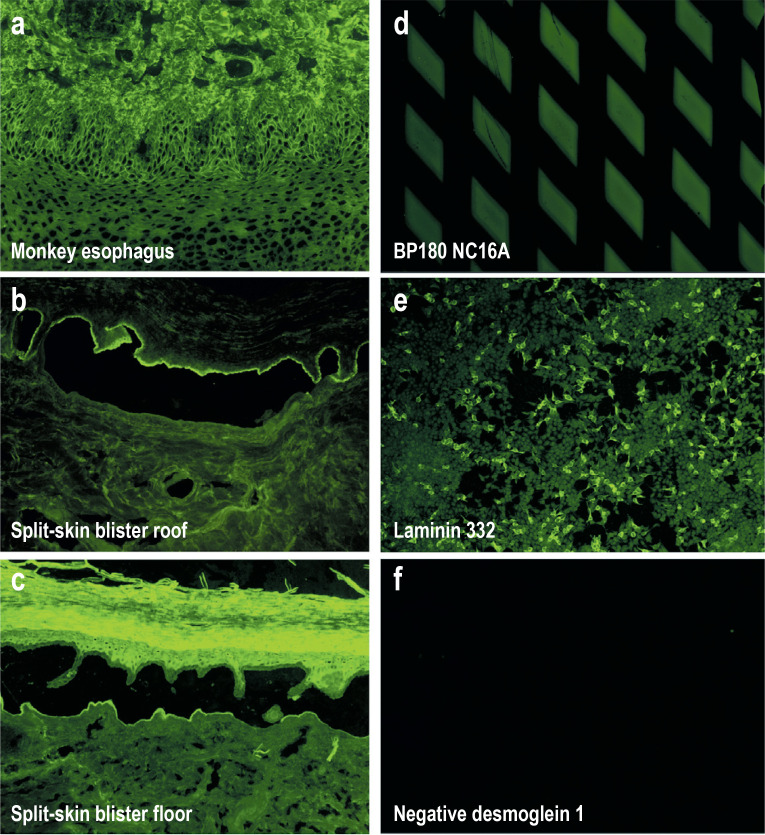
To screen for suspected AIBD, an indirect IF on monkey esophagus and 1 M NaCl–split skin is carried out, which enables a differentiation into pemphigus and pemphigoid diseases (Figure 4a – Figurec). The salt-split skin allows a subdivision of binding to the epidermal roof (in the case of autoantibodies against BP180 and BP230) or to the floor (autoantibodies against p200 antigen, laminin 332, and type VII collagen) of the artificial split (Figures 1 and 4a – c, Table 2) (1, 2, 10, 18, 19, e45).
Figure 4.
Figure 4: Indirect immunofluorescence on monkey esophagus, salt-split skin, and selected biochips
IgG reactivity on monkey esophagus epithelium, with an intercellular pattern typical of pemphigus vulgaris/foliaceus
IgG reactivity along the epidermal side of human salt-split skin (using 1M NaCl), fitting to binding of autoantibodies to BP180 and BP230
IgG reactivity along the dermal side of human salt-split skin (using 1M NaCl), fitting to binding of autoantibodies to p200, laminin 332, and type VII collagen
IgG reactivity to recombinant BP180 NC16A on a biochip, indicative of bullous pemphigoid, pemphigoid gestationis, or mucous membrane pemphigoid
IgG reactivity to laminin 332-expressing HEK293 cells of a biochip, indicative of anti-laminin 332 mucous membrane pemphigoid
Lack of fluorescence with Dsg1-expressing HEK293 cells of a biochip after incubation with a negative serum
For the detection of autoantibodies against the most important target antigens of AIBD, sensitive and specific enzyme-linked immunosorbent assays (ELISA) using the recombinant immunodominant regions of the target antigens are available (Euroimmun, Lübeck; MBL, Nagoya, Japan; Table 2) (10, e46– e52). For instance, ELISA can detect circulating antibodies against desmoglein 3 in the sera of patients with pemphigus vulgaris, and circulating antibodies against desmoglein 1 in patients with pemphigus foliaceus, in >95% of the cases (3, 23, e47, e52). Serum IgG antibodies against BP180 NC16A can be detected in 80–90% of the sera from patients with bullous pemphigoid.
The diagnostic sensitivity of bullous pemphigoid can be increased by 5–8% by the additional use of the BP230 ELISA, with which 50–60% of patients react (e46, e53). Serum autoantibodies against BP180 NC16A are also found in patients with pemphigoid gestationis as well as in 30–50% of patients with MMP who have serum antibodies against the epidermal side of human split skin (e54– e57).
For circulating autoantibodies against desmoglein 1, desmoglein 3, BP180 NC16A, and type VII collagen, a correlation with disease activity has been shown (3, e47, e48, e58, e59); their determination via ELISA during the course of the disease is recommended to be indcluded in therapeutic decisions (17, 21, 24). Instead of a step-by-step diagnostic approach, multivariate ELISA systems can be used in which autoantibodies against several target antigens are analyzed in parallel (e51, e60). The indirect IF-based BIOCHIP technology offers a comparable option. It assembles several substrates in so-called BIOCHIP mosaics in a single incubation field of a standard laboratory slide (15, 23, e61 – e63) (Figures 3d and 4d – f).
The detection of specific autoantibodies that are not (yet) included in commercial assays is carried out in some specialized laboratories (Table 2 and Box).
BOX. Dermatology departments in Germany that carry out more than 500 non-commercial test systems/year*.
Department of Dermatology, Venereology, and Allergology, University Medical Center of Schleswig-Holstein, Lübeck Campus, Lübeck, Germany
Department of Dermatology and Allergology, University Hospital Gießen and Marburg, Marburg, Germany
Department of Dermatology, Venereology, and Allergology and Skin Cancer Center, University Hospital Würzburg, Würzburg, Germany.
* in alphabetical order of the location (e93)
Pathophysiology
The pathophysiology of pemphigus and pemphigoid diseases has been presented in detail in recent reviews (1– 3, 25, e1, e64– e67). A common feature of all AIBDs is the presence of T cells and pathogenetically relevant autoantibodies against the respective autoantigens in genetically susceptible individuals (e68– e75). The trigger factors that lead to a breach of tolerance are still largely unknown.
In pemphigus, autoantibody binding is followed by the desmogleins being depleted from the cell surface and further signal transducing events, among others via the p38MAP kinase. Both lead to a weakening of the cell–cell interactions and to the separation of the keratinocytes/epithelial cells called acantholysis (3, 25, e1, e64).
In pemphigoid diseases, the binding of the autoantibodies leads to the local activation of complement and subsequently to the infiltration of inflammatory cells, such as eosinophils, neutrophils, macrophages, and T cells, into the upper dermis. The release of specific proteases from granulocytes, macrophages, and activated mast cells ultimately results in degradation of the proteins of the dermal–epidermal junction, which appears histologically as subepidermal clefts and clinically as tense blisters and erosions (1, e76). C5aR1, leukotriene B4, the neonatal Fc receptor, eotaxin, the IL-5 receptor, and IL-17A have been identified as key mediators of pemphigoid diseases; clinical studies are currently underway in which some of these are investigated (26– 29, e67, e77– e83).
Therapy
German and/or European guidelines have been formulated for bullous pemphigoid, pemphigus vulgaris/foliaceus, MMP, and dermatitis herpetiformis (9, 18, 21, 24, 30, 31, e84) (eTables 1 and 2). In addition to an interdisciplinary approach with ENTs, ophthalmologists, gynecologists, general practitioners, infectiologists, paediatricians, and, if necessary, other specialist disciplines, cooperation with patient support groups is recommended, for example with the German Pemphigus und Pemphigoid Selbsthilfegruppe (www.pemphigus-pemphigoid-selbsthilfe.de) or the International Pemphigus and Pemphigoid Foundation (www.pemphigus.org).
eTable 1. Treatment options for pemphigoid diseases and dermatitis herpetiformis.
| Disease/ severity | Treatment | |
| Medication*2 | Adverse drug reactions*11 | |
| Bullous pemphigoid*1 (24) | ||
| Mild (< 10% body surface area) | Topical 0.05% clobetasol propionate (level B) | Skin atrophy |
| Moderate (10–30% body surface area) | Topical 0.05% clobetasol propionate (level B) ± systemic treatment Systemic treatment Prednisolone*3 p.o. 0.5 mg/kg/day (level B) or Dapsone*7 ± prednisolone*3 p.o. 0.5 mg/kg/day (level C) or Doxycycline (200 mg/day) (level B) ± nicotinamide (level D) ± prednisolone*3 p.o. 0.5 mg/kg/day or Azathioprine*5 (level C) + prednisolone*3 p.o. 0.5 mg/kg/day or Methotrexate (≤ 20 mg /week) (level D) ± prednisolone*3 p.o. 0.5 mg/kg/day or Mycophenols*6 (level C) + prednisolone*3 p.o. 0.5 mg/kg/day |
Skin atrophy Hypertension, diabetes, osteoporosis, AI, infections Dapsone: hemolytic anemia, GI complaints, methemoglobinemia, agranulocytosis Doxycycline: GI complaints, sensitivity to light Azathioprine: myelo- and hepatotoxicity, arthralgias, infections Methotrexate: myelo- and hepatotoxicity, infections, kidney dysfunction, possible reactivation Mycophenols: GI complaints, infections, (rarely) myelotoxicity |
| Severe (> 30% body surface area) | Topical 0.05% clobetasol propionate + systemic treatment (see above) | (see above) |
| Refractory to treatment | IVIg (level D)*9 Immunadsorption (level D)*10 Rituximab*8 (level D) Cyclophosphamide (level E) Omalizumab (level E) |
Headache, chills, flushing, fever, hypertension, nausea Hypotension, thrombosis, bradycardia, anaphylaxis, herpes zoster infection, sepsis Infusion reaction, infections, reactivation of hepatitis B, PML Myelotoxicity, hemorrhagic cystitis, carcinogenicity, infertility, infections |
| MMP*1 (30, e84) | ||
| Mild/moderate (only oral cavity and skin are affected) |
Topical corticosteroids class III/IV and/or dapsone*7 (level D), methotrexate (≤ 20 mg/week) (level E) or tetracycline (level E) |
(ADRs of these drugs are described above) GI complaints, dizziness |
| Mild, refractory to treatment | + Prednisolone*3 p.o. 0.5–1.5 mg/kg/day (level C) and/or azathioprine*5 or mycophenols*6 (level D) | (ADRs of these drugs are described above) |
| Severe (involvement of conjunctiva, nasopharynx, larynx, trachea or esophagus) | Dapsone*7 (level C) + cyclophosphamide p.o./i.v. and/or prednisolone*3 p.o. 0.5–1.5 mg/kg/day (or i.v. steroid pulse*4) (all: level D) | (ADRs of these drugs are described above) |
| Severe, refractory to treatment | Dapsone*7 + rituximab 2 × 1 g*8 (level D) If still refractory: + IVIg*9 (level D) |
(ADRs of these drugs are described above) |
| Linear IgA disease (e96, e97) | Topical corticosteroids class III/IV or prednisolone*3 p.o. 0.25–0.5 mg/kg/day ± dapsone*7 (all: level D) | (ADRs of these drugs are described above) |
| Refractory to treatment | Prednisolone*3 p.o. 0.5 mg/kg/day + Sulfasalazine/-pyridine (level D) Doxycycline (200 mg/day) (level E) Cholchicine (level E) IVIg*9, azathioprine*5, or mycophenols*6 (all, level E) |
(see above) GI complaints, hepato- and nephrotoxicity, myelotoxicity (see above) GI complaints, hepato- and nephrotoxicity, myelotoxicity (see above) |
| Pemphigoid gestationis (e37) | Topical 0.05% clobetasol proprionate or prednisolone*3 p.o. 0.25–0.5 mg/kg/day (level D) | (ADRs of these drugs are described above) |
| Refractory to treatment | Immunadsorption*10, rituximab*8 (only postpartum) (level E) | (ADRs of these drugs are described above) |
| Anti-p200 pemphigoid (e39) | Topical 0.05% clobetasol proprionate ± prednisolone*3 p.o. 0.25–0.5 mg/kg/day ± dapsone*7 or doxycycline 200 mg/day (level E) | (ADRs of these drugs are described above) |
| Epidermolysis bullosa acquisita (39, e98, e99) | ||
| Mild (< 10% body surface area) | Topical 0.05% clobetasol proprionate + dapsone*7 or colchizine (level E) | (ADRs of these drugs are described above) |
| Moderate/severe | Prednisolone*3 p.o. 1.0–2.0 mg/kg/day or i.v. steroid pulses*4, tapering over course+ azathioprine*5, mycophenols*6 or dapsons*7 (all: level E) | (ADRs of these drugs are described above) |
| Refractory to treatment | + Rituximab*8 and/or IVIg*9 (both level E) | (ADRs of these drugs are described above) |
| Dermatitis herpetiformis*1(18) | Gluten-free diet (life-long) ± dapsone*7 (until skin lesions have healed) (level D) | (ADRs of these drugs are described above) |
*1 German and/or European therapy guidelines are available for these diseases
*2 Level of evidence: level A, meta-analyses of prospective, controlled trials; level B, high-quality prospective, controlled trials; level C, lower-quality prospective controlled trials; level D, larger case studies; level E, small case studies, case reports
*3 or prednisolone equivalent
*4 dexamethasone 100 mg/day for three consecutive days, or prednisolone 500–1000 mg/day, every 3–4 weeks, eventually every 6–8 weeks
*5 2.0–2.5 mg/kg/day with normal thiopurine methyltransferase levels
*6 mycophenolate mofetil (2 g/day), mycophenolate sodium (1440 mg/day)
*7 1.0–1.5 mg/kg/day with normal glucose 6-phosphate levels
*8 or biosimilar
*9 2 g/kg for 2–5 days every four weeks; after six months, interval extension (37)
*10 on 3–4 consecutive days with regenerable adsorbers every 3–4 weeks, for 8–16 weeks
*11 important adverse drug reactions (ADRs) are indicated; note that this list is not comprehensive
ADR, adverse drug reactions; AI, adrenal insufficiency; GI, gastrointestinal; IVIg, intravenous immunoglobulins; MMP, mucous membrane pemphigoid; PDAI, Pemphigus Disease Activity Index; PML, progressive multifocal leukoencephalopathy.
eTable 2. Treatment options for pemphigus diseases.
| Disease/ severity | Treatment | |
| Medication*2 | Adverse drug reactions*11 | |
| Pemphigus vulgaris/Pemphigus foliaceus*1 (24) | ||
| Mild (PDAI ≤ 15) | Prednisolone*3 p.o. 1.0–1.5 mg/kg/day or i.v. steroid pulses*4 (level C) ± azathioprine*5 (level C) mycophenoles*6 (level C) dapsone*7 (only for pemphigus foliaceus) (level E) |
Hypertension, diabetes, osteoporosis, AI, infections Myelo- and hepatotoxicity, arthralgias, infections GI complaints, infections, (rarely) myelotoxicity Hemolytic anemia, GI complaints |
| Moderate, severe (PDAI > 15) | Rituximab 2 × 1 g*8 (level B)+ prednisolone*3 p.o. 1.0 mg/kg/day or i.v. steroid pulses*4 (tapering over 3–6 months; level B) ± azathioprine*5 or mycophenols*6 (level C) prednisolone*3 p.o. 1.0–1.5 mg/kg/day or i.v. steroid pulses*4 + azathioprine*5 or mycophenols*6 (level C) |
Infusion reaction, infections, reactivation of hepatitis B, PML (ADRs of these drugs are described above) |
| Refractory to treatment | IVIg*9 (level D) Immunadsorption*10 (level D) |
Headache, chills, flushing, fever, hypertension, nausea Hypotension, thrombosis, bradycardia, anaphylaxis, herpes zoster infection |
| Paraneoplastic pemphigus (2, 3) | Treatment of neoplasm + prednisolone*3 p.o. 0.5–1.0 mg/kg/day or i. v. steroid pulses*4 eventually in combination with rituximab*8, IVIg*9, immunadsorption*10 (all: level E) | (ADRs of these drugs are described above) |
| IgA pemphigus (2, 3, e94, e95) | Dapsone*7 and/or acitretine + Prednisolone*3 p.o. 0.5–1.0 mg/kg/day (all: level E) |
Hemolytic anemia, GI complaints, teratogenicity Hypertension, diabetes, osteoporosis, AI, infections |
*1 German and/or European therapy guidelines are available for these diseases
*2 Level of evidence: level A, meta-analyses of prospective, controlled trials; level B, high-quality prospective, controlled trials; level C, lower-quality prospective controlled trials; level D, major case studies; level E, small case studies, case reports
*3 or prednisolone equivalent
*4 dexamethasone 100 mg/day for three consecutive days, or prednisolone 500–1 000 mg/day, every 3–4 weeks, eventually every 6–8 weeks
*5 2.0–2.5 mg/kg/day with normal thiopurine methyltransferase levels
*6 mycophenolate mofetil (2 g/day), mycophenolate sodium (1 440 mg/day)
*7 1.0–1.5 mg/kg/day with normal glucose 6-phosphate
*8 or biosimilar
*9 2 g/kg for 2–5 days every four weeks; after six months, interval extension (37)
*10 on 3–4 consecutive days with regenerable adsorbers every 3–4 weeks, for 8–16 weeks
*11 not a comprehensive list
ADR, adverse drug reactions; AI, adrenal insufficiency; GI, gastrointestinal; IVIg, intravenous immunoglobulins; PDAI, Pemphigus Disease Activity Index; PML, progressive multifocal leukoencephalopathy.
Pemphigus diseases
First-line therapy for pemphigus vulgaris/foliaceus has changed significantly following the approval of the anti-CD20 antibody rituximab for the treatment of moderate and severe pemphigus vulgaris by the European Medicines Agency (EMA) and the US American Food and Drug Administration (FDA). Joly et al. demonstrated that treatment of patients with newly diagnosed pemphigus vulgaris/foliaceus with rituximab (2× 1g plus 0.5 g each, in months 12 and 18) plus prednisolone (0.5–1.0mg/kg/day p.o. for three to six months) was significantly more effective and safer than therapy with oral prednisolone 1.0–1.5mg/kg/day for 12–18 months (55% difference, 95% confidence interval: [38.4; 71, 7]; p<0.0001) (32). For moderate and severe pemphigus vulgaris/foliaceus, administration of rituximab (2× 1g at an interval of 2–3 weeks) is recommended in combination with systemic corticosteroids (tapering over 3–6 months). Alternatively, conventional therapy with prednisolone p.o. 1.0mg/kg/day plus azathioprine or mycophenols can be applied (etable 2) (24). As an alternative to oral corticosteroids, intravenous corticosteroid pulses can be used (24, 31). The guideline recommends another infusion of rituximab (1 g) after six months in the event of relapse or incomplete remission; in refractory patients, the guideline also recommends high-dose intravenous immunoglobulins (IVIg) or immune apheresis (etable 2) (24, 31).
Currrent clinical trials for treatment of pemphigus are evaluating efficacy and safety of inhibition of the Bruton tyrosine kinase or the neonatal Fc receptor, depletion of desmoglein 3–specific B cells using chimeric autoantibody receptor T cells (CAART), and tolerance induction by nanoparticles (27, 33, e1, e85, e86).
Pemphigoid diseases
For bullous pemphigoid, the current German AWMF guideline recommends a whole-body application of topical 0.05% clobetasol propionate (40 g/day), a superpotent glucocorticosteroid of class IV, for mild as well as moderate cases, if necessary; for severe cases, this is usually recommended in combination with systemic treatment (24). In a controlled randomized study, topical 0.05% clobetasol propionate (40g/day) had a comparable effect in patients with bullous pemphigoid as prednisolone (0.5mg/kg/day) (disease control in moderate cases, topical 100% [95; 100]) versus oral 95% [87; 99], p=0.06; in severe cases, topical 99% [94; 100] versus oral 91% [83; 96], p=0.02) (34). As a systemic treatment, prednisolone is given orally at 0.5 mg/kg/day, possibly in combination with the (potentially steroid-sparing) agents azathioprine, dapsone, doxycycline, methotrexate, mycophenolate mofetil, or mycophenolate sodium. Alternatively, dapsone, doxycycline, or methotrexate can also be used as the only systemic treatment without oral corticosteroid (24) (see further details, eTable 1). In randomized controlled trials in patients with bullous pemphigoid, doxycycline was associated with significantly fewer serious adverse events than oral prednisolone (difference 19.0% [7.9; 30.1], p=0.001), and dapsone was associated with a lower cumulative corticosteroid dose than azathioprine (p=0.06) (35, 36). IVIg, immunoadsorption, rituximab, cyclophosphamide, or omalizumab can be used in refractory patients (etable 1) (24, 37– 39, e87).
The severity of MMP is distinguished on the basis of the risk of scarring, as mild/moderate with exclusive involvement of the skin and oral mucosa, or as severe, with involvement of the eyes, nasal mucosa, pharynx, larynx, esophagus, or trachea (30, e84). In the case of mild/moderate MMP, topical treatment with highly potent topical glucocorticoids, possibly in combination with immunomodulators, is often sufficient. For severe MMP, treatment with dapsone combined with systemic corticosteroid (prednisolone, either orally 0.5–1.5 mg/kg/day or as an intravenous pulse therapy) or cyclophosphamide (orally or intravenously) is recommended (e84). In the case of eye involvement, topical treatments that can be used in addition to lubricants include corticosteroids, tetracyclines, and cyclosporine (e84). Timely interdisciplinary treatment of inflammation is crucial before irreversible scarring occurs, especially in the eyes.
The systemic treatments for refractory MMP and other pemphigoid diseases and dermatitis herpetiformis are summarized in etable 1.
Questions on the article in the issue 24/2021:
Bullous Autoimmune Dermatoses – Clinical Features, Diagnostic Evaluation, and Treatment Options
The submission deadline is 17 June 2022.
Only one answer is possible per question. Please select the answer that is most appropriate.
Question 1
Which type of autoimmune bullous diseases mainly has antibodies targeted against the desmoglein 3 antigen?
IgA pemphigus
Bullous pemphigoid
Linear IgA disease
Pemphigus vulgaris
Mucous membrane pemphigoid (MMP)
Question 2
Which autoimmune bullous disease has the highest prevalence in Germany?
Pemphigus vulgaris
Pemphigoid gestationis
Bullous pemphigoid
Epidermolysis bullosa acquisita
Pemphigus foliaceus
Question 3
Which is characteristic of bullous pemphigoid?
Scaly crusts, manifestation in middle age
Manifestation mostly in childhood, severe pruritus
Erosion strictly limited to the skin, severe pruritus
Manifestation at a young age, usually without pruritus
Manifestation in old age, severe pruritus
Question 4
Which autoimmune bullous disease almost always occurs in connection with celiac disease?
Epidermolysis bullosa acquisita
Dermatitis herpetiformis
Linear IgA disease
Anti-p200 pemphigoid
IgA pemphigus
Question 5
Detection of autoantibodies is part of the serological diagnosis of patients with autoimmune bullous diseases. In what percentage of these patients can circulating autoantibodies be detected in the serum?
ca. 90 %
ca. 70 %
ca. 50 %
ca. 20 %
ca. 10 %
Question 6
Which technology is frequently used to detect autoantibodies against the most important target antigens in autoimmune bullous dermatoses?
Polymerase chain reaction (PCR)
Immunoprecipitation
Fluorescence In Situ Hybridization (FISH)
Matrix-Assisted Laser-Desorption-Ionization (MALDI)
Enzyme-linked immunosorbent assay (ELISA)
Question 7
In addition to conventional therapy, which treatment is recommended for moderate and severe pemphigus vulgaris?
Rituximab (1 g/day for 3 months) as a monotherapy
Azathioprine as monotherapy
Rituximab in combination with systemic corticosteroids
Mycophenolate mofetil (500 mg/day) as a monotherapy
Azathioprine combined with mycophenolate mofetil
Question 8
Which types of autoimmune bullous diseases are most common in children?
Linear IgA disease and pemphigus vulgaris
Bullous pemphigoid and mucous membrane pemphigoid
Epidermolysis bullosa acquisita and pemphigus foliaceus
IgA pemphigus and dermatitis herpetiformis Duhring
Dermatitis herpetiformis Duhring and bullous pemphigoid
Question 9
Which pattern is typically seen in the direct immunofluorescence of perilesional biopsies in epidermolysis bullosa acquisita?
An n-serrated pattern
A u-serrated pattern
An a-serrated pattern
An o-serrated pattern
A y-serrated pattern
Question 10
What is known about the occurrence of pemphigoid gestationis?
It is usually most pronounced at the beginning of pregnancy
The disease stops immediately postpartum
After recovery from the disease, it will not occur in subsequent pregnancies
Urticarial erythematous plaques do not appear on the abdomen
It usually occurs in the third trimester of pregnancy
Acknowledgments
Translated from the original German by Veronica A. Raker, PhD
Acknowledgements
We would like to thank Carolin Mahlerwein (Lübeck) for the schematic overview figure, Ingeborg Atefi and Marina Kongsback-Reim (Lübeck) for help in preparing the flourescent images, and the patients for the clinical images. This work was supported by the strukturelle Förderung des Exzellenz Cluster Precision Medicine in Chronic Inflammation (EXC2167) This work was supported by structural funding from the Cluster of Excellence Precision Medicine in Chronic Inflammation (PMI) (grant number EXC 2167) from the German Research Foundation.
Footnotes
Conflict of Interest Statement
Dr. van Beek has received reimbursement of meeting participation fees and travel expenses from Actelion, speaking honoraria from Infinite Science, and support for shared research projects from Euroimmun.
Prof. Zillikens has received consulting honoraria from Almirall, arGEN-X, Pincell, Roche Pharma, and UCB, speaking honoraria and reimbursement of travel expenses and conference fees from Novartis, Roche Pharma, Abbvie, UCB, Janssen, Almirall, and Fresenius, and support for shared research and development projects from Dompe, Euroimmun, and Fresenius.
Prof. Schmidt has received consulting honoraria from Argen X, UCB, AstraZeneca, Roche, Topas, Almirall, and Thermo Fischer, reimbursement of meeting participation fees and travel expenses as well as speaking honoraria from Biotest, Novartis, and Fresenius, and research support (third-party funds) from UCB, Biotest, Incyte, Novartis, Euroimmun, Argen X, AstraZeneca, Dompe, Admirx, Synthon/Biondis, and Fresenius.
References
- 1.Schmidt E, Zillikens D. Pemphigoid diseases. Lancet. 2013;381:320–332. doi: 10.1016/S0140-6736(12)61140-4. [DOI] [PubMed] [Google Scholar]
- 2.Schmidt E, Kasperkiewicz M, Joly P. Pemphigus. Lancet. 2019;394:882–894. doi: 10.1016/S0140-6736(19)31778-7. [DOI] [PubMed] [Google Scholar]
- 3.Kasperkiewicz M, Ellebrecht CT, Takahashi H, et al. Pemphigus. Nat Rev Dis Primers. 2017;3 doi: 10.1038/nrdp.2017.26. 17026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sardy M, Karpati S, Merkl B, Paulsson M, Smyth N. Epidermal transglutaminase (TGase 3) is the autoantigen of dermatitis herpetiformis. J Exp Med. 2002;195:747–757. doi: 10.1084/jem.20011299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Langan SM, Smeeth L, Hubbard R, Fleming KM, Smith CJ, West J. Bullous pemphigoid and pemphigus vulgaris—incidence and mortality in the UK: population based cohort study. Bmj. 2008;337 doi: 10.1136/bmj.a180. a180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hubner F, Konig IR, Holtsche MM, Zillikens D, Linder R, Schmidt E. Prevalence and age distribution of pemphigus and pemphigoid diseases among pediatric patients in Germany. J Eur Acad Dermatol Venereol. 2020;34:2600–2605. doi: 10.1111/jdv.16467. [DOI] [PubMed] [Google Scholar]
- 7.Anhalt GJ. Paraneoplastic pemphigus. J Investig Dermatol Symp Proc. 2004;9:29–33. doi: 10.1111/j.1087-0024.2004.00832.x. [DOI] [PubMed] [Google Scholar]
- 8.Murrell DF, Daniel BS, Joly P, et al. Definitions and outcome measures for bullous pemphigoid: recommendations by an international panel of experts. J Am Acad Dermatol. 2012;66:479–485. doi: 10.1016/j.jaad.2011.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feliciani C, Joly P, Jonkman MF, et al. Management of bullous pemphigoid: the European Dermatology Forum consensus in collaboration with the European Academy of Dermatology and Venereology. Br J Dermatol. 2015;172:867–877. doi: 10.1111/bjd.13717. [DOI] [PubMed] [Google Scholar]
- 10.Schmidt E, Goebeler M, Hertl M, et al. S2k guideline for the diagnosis of pemphigus vulgaris/foliaceus and bullous pemphigoid. J Dtsch Dermatol Ges. 2015;13:713–727. doi: 10.1111/ddg.12612. [DOI] [PubMed] [Google Scholar]
- 11.Kibsgaard L, Rasmussen M, Lamberg A, Deleuran M, Olesen AB, Vestergaard C. Increased frequency of multiple sclerosis among patients with bullous pemphigoid: a population-based cohort study on comorbidities anchored around the diagnosis of bullous pemphigoid. Br J Dermatol. 2017;176:1486–1491. doi: 10.1111/bjd.15405. [DOI] [PubMed] [Google Scholar]
- 12.Schulze F, Neumann K, Recke A, Zillikens D, Linder R, Schmidt E. Malignancies in pemphigus and pemphigoid diseases. J Invest Dermatol. 2015;135:1445–1447. doi: 10.1038/jid.2014.547. [DOI] [PubMed] [Google Scholar]
- 13.Kridin K, Cohen AD. Dipeptidyl-peptidase IV inhibitor-associated bullous pemphigoid: a systematic review and meta-analysis. J Am Acad Dermatol. 2018 doi: 10.1016/j.jaad.2018.09.048. S0190 9622(18)32660-4. [DOI] [PubMed] [Google Scholar]
- 14.Murrell DF, Marinovic B, Caux F, et al. Definitions and outcome measures for mucous membrane pemphigoid: recommendations of an international panel of experts. J Am Acad Dermatol. 2015;72:168–174. doi: 10.1016/j.jaad.2014.08.024. [DOI] [PubMed] [Google Scholar]
- 15.Goletz S, Probst C, Komorowski L, et al. A sensitive and specific assay for the serological diagnosis of antilaminin 332 mucous membrane pemphigoid. Br J Dermatol. 2019;180:149–156. doi: 10.1111/bjd.17202. [DOI] [PubMed] [Google Scholar]
- 16.Vorobyev A, Ludwig RJ, Schmidt E. Clinical features and diagnosis of epidermolysis bullosa acquisita. Expert Rev Clin Immunol. 2017;13:157–169. doi: 10.1080/1744666X.2016.1221343. [DOI] [PubMed] [Google Scholar]
- 17.Prost-Squarcioni C, Caux F, Schmidt E, et al. International Bullous Diseases Group: consensus on diagnostic criteria for epidermolysis bullosa acquisita. Br J Dermatol. 2018;179:30–41. doi: 10.1111/bjd.16138. [DOI] [PubMed] [Google Scholar]
- 18.Görög A, Antiga E, Caproni M, et al. S2k guideline (consensus statement) for diagnosis and therapy of dermatitis herpetiformis initiated by the European Academy of Dermatology and Venereology (EADV) J Eur Acad Dermatol Venereol. 2021;351:251–277. doi: 10.1111/jdv.17183. [DOI] [PubMed] [Google Scholar]
- 19.van Beek N, Zillikens D, Schmidt E. Diagnostik blasenbildender Autoimmundermatosen. J Dtsch Dermatol Ges. 2018;16:1077–1092. doi: 10.1111/ddg.13637_g. [DOI] [PubMed] [Google Scholar]
- 20.Harman KE, Brown D, Exton LS, et al. British Association of Dermatologists‘ guidelines for the management of pemphigus vulgaris 2017. Br J Dermatol. 2017;177:1170–1201. doi: 10.1111/bjd.15930. [DOI] [PubMed] [Google Scholar]
- 21.Hertl M, Jedlickova H, Karpati S, et al. Pemphigus. S2 Guideline for diagnosis and treatment—guided by the European Dermatology Forum (EDF) in cooperation with the European Academy of Dermatology and Venereology (EADV). J Eur Acad Dermatol Venereol. 2015;29:405–414. doi: 10.1111/jdv.12772. [DOI] [PubMed] [Google Scholar]
- 22.Terra JB, Meijer JM, Jonkman MF, Diercks GF. The n- vs. u-serration is a learnable criterion to differentiate pemphigoid from epidermolysis bullosa acquisita in direct immunofluorescence serration pattern analysis. Br J Dermatol. 2013;169:100–105. doi: 10.1111/bjd.12308. [DOI] [PubMed] [Google Scholar]
- 23.van Beek N, Kruger S, Fuhrmann T, et al. Multicenter prospective —study on multivariant diagnostics of autoimmune bullous dermatoses using the BIOCHIP(TM) technology. J Am Acad Dermatol. 2020;83:1315–1322. doi: 10.1016/j.jaad.2020.01.049. [DOI] [PubMed] [Google Scholar]
- 24.Schmidt E, Sticherling M, Sardy M, et al. S2k guidelines for the treatment of pemphigus vulgaris/foliaceus and bullous pemphigoid: 2019 update. J Dtsch Dermatol Ges. 2020;18:516–526. doi: 10.1111/ddg.14097. [DOI] [PubMed] [Google Scholar]
- 25.Spindler V, Eming R, Schmidt E, et al. Mechanisms causing loss of keratinocyte cohesion in pemphigus. J Invest Dermatol. 2018;138:32–37. doi: 10.1016/j.jid.2017.06.022. [DOI] [PubMed] [Google Scholar]
- 26.Chakievska L, Holtsche MM, Kunstner A, et al. IL-17A is functionally relevant and a potential therapeutic target in bullous pemphigoid. J Autoimmun. 2019;96:104–112. doi: 10.1016/j.jaut.2018.09.003. [DOI] [PubMed] [Google Scholar]
- 27.Kasprick A, Hofrichter M, Smith B, et al. Treatment with anti-neonatal Fc receptor (FcRn) antibody ameliorates experimental epidermolysis bullosa acquisita in mice. Br J Pharmacol. 2020;177:2381–2392. doi: 10.1111/bph.14986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Izumi K, Bieber K, Ludwig RJ. Current clinical trials in pemphigus and pemphigoid. Front Immunol. 2019;10 doi: 10.3389/fimmu.2019.00978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maglie R, Hertl M. Pharmacological advances in pemphigoid. Curr Opin Pharmacol. 2019;46:34–43. doi: 10.1016/j.coph.2018.12.007. [DOI] [PubMed] [Google Scholar]
- 30.Rashid H, Lambert A, Alberti-Violetti S, et al. European guidelines (S3) on diagnosis and management of mucous membrane pemphigoid, initiated by the European Academy of Dermatology and Venereology (EADV) - part I: Clinical presentation and outcome measurements for disease assessment. J Eur Acad Dermatol Venereol (Epub ahead of print) doi: 10.1111/jdv.17397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Joly P, Horwath B, Patsatsi A, et al. Updated S2K guidelines on the management of pemphigus vulgaris and foliaceus initiated by the european academy of dermatology and venereology (EADV) J Eur Acad Dermatol Venereol. 2020;34:1900–1913. doi: 10.1111/jdv.16752. [DOI] [PubMed] [Google Scholar]
- 32.Joly P, Maho-Vaillant M, Prost-Squarcioni C, et al. First-line rituximab combined with short-term prednisone versus prednisone alone for the treatment of pemphigus (Ritux 3): a prospective, multicentre, parallel-group, open-label randomised trial. Lancet. 2017;389:2031–2040. doi: 10.1016/S0140-6736(17)30070-3. [DOI] [PubMed] [Google Scholar]
- 33.Ellebrecht CT, Bhoj VG, Nace A, et al. Reengineering chimeric antigen receptor T cells for targeted therapy of autoimmune disease. Science. 2016;353:179–184. doi: 10.1126/science.aaf6756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Joly P, Roujeau JC, Benichou J, et al. A comparison of oral and topical corticosteroids in patients with bullous pemphigoid. N Engl J Med. 2002;346:321–327. doi: 10.1056/NEJMoa011592. [DOI] [PubMed] [Google Scholar]
- 35.Sticherling M, Franke A, Aberer E, et al. An open, multicentre, randomized clinical study in patients with bullous pemphigoid comparing methylprednisolone and azathioprine with methylprednisolone and dapsone. Br J Dermatol. 2017;177:1299–1305. doi: 10.1111/bjd.15649. [DOI] [PubMed] [Google Scholar]
- 36.Williams HC, Wojnarowska F, Kirtschig G, et al. Doxycycline versus prednisolone as an initial treatment strategy for bullous pemphigoid: a pragmatic, non-inferiority, randomised controlled trial. Lancet. 2017;389:1630–1638. doi: 10.1016/S0140-6736(17)30560-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Enk A, Hadaschik E, Eming R, et al. European Guidelines (S1) on the use of high-dose intravenous immunoglobulin in dermatology. J Dtsch Dermatol Ges. 2017;15:228–241. doi: 10.1111/ddg.13013. [DOI] [PubMed] [Google Scholar]
- 38.Kremer N, Snast I, Cohen ES, et al. Rituximab and omalizumab for the treatment of bullous pemphigoid: a systematic review of the literature. Am J Clin Dermatol. 2019;20:209–216. doi: 10.1007/s40257-018-0401-6. [DOI] [PubMed] [Google Scholar]
- 39.Ujiie H, Iwata H, Yamagami J, et al. Japanese guidelines for the management of pemphigoid (including epidermolysis bullosa acquisita) J Dermatol. 2019;46:1102–1135. doi: 10.1111/1346-8138.15111. [DOI] [PubMed] [Google Scholar]
- 40.van Beek N, Weidinger A, Schneider SW, et al. Incidence of pemphigoid diseases in Northern Germany in 2016—first data from the Schleswig-Holstein Registry of autoimmune bullous diseases. J Eur Acad Dermatol Venereol. 2021;35:1197–1202. doi: 10.1111/jdv.17107. [DOI] [PubMed] [Google Scholar]
- E1.Pollmann R, Schmidt T, Eming R, Hertl M. Pemphigus: a comprehensive review on pathogenesis, clinical presentation and novel therapeutic approaches. Clin Rev Allergy Immunol. 2018;54:1–25. doi: 10.1007/s12016-017-8662-z. [DOI] [PubMed] [Google Scholar]
- E2.Amber KT, Murrell DF, Schmidt E, Joly P, Borradori L. Autoimmune subepidermal bullous diseases of the skin and mucosae: clinical features, diagnosis, and management. Clin Rev Allergy Immunol. 2018;54:26–51. doi: 10.1007/s12016-017-8633-4. [DOI] [PubMed] [Google Scholar]
- E3.Kridin K. Pemphigus group: overview, epidemiology, mortality, and comorbidities. Immunol Res. 2018;66:255–270. doi: 10.1007/s12026-018-8986-7. [DOI] [PubMed] [Google Scholar]
- E4.Kridin K, Ludwig RJ. The growing incidence of bullous pemphigoid: overview and potential explanations. Front Med (Lausanne) 2018;5 doi: 10.3389/fmed.2018.00220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E5.Bertram F, Brocker EB, Zillikens D, Schmidt E. Prospective analysis of the incidence of autoimmune bullous disorders in Lower Franconia, Germany. J Dtsch Dermatol Ges. 2009;7:434–440. doi: 10.1111/j.1610-0387.2008.06976.x. [DOI] [PubMed] [Google Scholar]
- E6.Marazza G, Pham HC, Scharer L, et al. Incidence of bullous pemphigoid and pemphigus in Switzerland: a 2-year prospective study. Br J Dermatol. 2009;161:861–868. doi: 10.1111/j.1365-2133.2009.09300.x. [DOI] [PubMed] [Google Scholar]
- E7.Joly P, Baricault S, Sparsa A, et al. Incidence and mortality of bullous pemphigoid in France. J Invest Dermatol. 2012;132:1998–2004. doi: 10.1038/jid.2012.35. [DOI] [PubMed] [Google Scholar]
- E8.Forsti AK, Jokelainen J, Timonen M, Tasanen K. Increasing incidence of bullous pemphigoid in Northern Finland: a retrospective database study in Oulu University Hospital. Br J Dermatol. 2014;171:1223–1226. doi: 10.1111/bjd.13189. [DOI] [PubMed] [Google Scholar]
- E9.Hubner F, Recke A, Zillikens D, Linder R, Schmidt E. Prevalence and age distribution of pemphigus and pemphigoid diseases in Germany. J Invest Dermatol. 2016;136:2495–2498. doi: 10.1016/j.jid.2016.07.013. [DOI] [PubMed] [Google Scholar]
- E10.Jelti L, Cordel N, Gillibert A, et al. Incidence and mortality of pemphigus in France. J Invest Dermatol. 2019;139:469–473. doi: 10.1016/j.jid.2018.07.042. [DOI] [PubMed] [Google Scholar]
- E11.Joly P. Incidence of bullous pemphigoid and pemphigus vulgaris. BMJ. 2008;337 doi: 10.1136/bmj.a209. a209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E12.Loget J, Barbe C, Duvert-Lehembre S, et al. The regibul register: a tool for monitoring the distribution and incidence of autoimmune bullous dermatoses in three french regions, 2010 to 2015. Acta Derm Venereol. 2018;98:380–381. doi: 10.2340/00015555-2848. [DOI] [PubMed] [Google Scholar]
- E13.Persson MSM, Harman KE, Vinogradova Y, et al. Incidence, prevalence and mortality of bullous pemphigoid in England 1998-2017: a population-based cohort study. Br J Dermatol. 2021;184:68–77. doi: 10.1111/bjd.19022. [DOI] [PubMed] [Google Scholar]
- E14.van Beek N, Schmidt E. Autoimmune bullous diseases. In: Höger P, Kinsler V, Yan A, editors. Harper’s Textbook of Pediatric Dermatology, chapter 73, Wiley-Blackwell. 4. Chichester: 2020. pp. 868–897. [Google Scholar]
- E15.Sarig O, Bercovici S, Zoller L, et al. Population-specific association between a polymorphic variant in ST18, encoding a pro-apoptotic molecule, and pemphigus vulgaris. J Invest Dermatol. 2012;132:1798–1805. doi: 10.1038/jid.2012.46. [DOI] [PubMed] [Google Scholar]
- E16.Hirose M, Schilf P, Benoit S, et al. Polymorphisms in the mitochondrially encoded ATP synthase 8 gene are associated with susceptibility to bullous pemphigoid in the German population. Exp Dermatol. 2015;24:715–717. doi: 10.1111/exd.12732. [DOI] [PubMed] [Google Scholar]
- E17.Zimmermann J, Bahmer F, Rose C, Zillikens D, Schmidt E. Clinical and immunopathological spectrum of paraneoplastic pemphigus. J Dtsch Dermatol Ges. 2010;8:598–606. doi: 10.1111/j.1610-0387.2010.07380.x. [DOI] [PubMed] [Google Scholar]
- E18.Yong AA, Tey HL. Paraneoplastic pemphigus. Australas J Dermatol. 2013;54:241–250. doi: 10.1111/j.1440-0960.2012.00921.x. [DOI] [PubMed] [Google Scholar]
- E19.Leger S, Picard D, Ingen-Housz-Oro S, et al. Prognostic factors of paraneoplastic pemphigus. Arch Dermatol. 2012;148:1165–1172. doi: 10.1001/archdermatol.2012.1830. [DOI] [PubMed] [Google Scholar]
- E20.Hashimoto T, Kiyokawa C, Mori O, et al. Human desmocollin 1 (Dsc1) is an autoantigen for the subcorneal pustular dermatosis type of IgA pemphigus. J Invest Dermatol. 1997;109:127–131. doi: 10.1111/1523-1747.ep12319025. [DOI] [PubMed] [Google Scholar]
- E21.Muller R, Heber B, Hashimoto T, et al. Autoantibodies against desmocollins in European patients with pemphigus. Clin Exp Dermatol. 2009;34:898–903. doi: 10.1111/j.1365-2230.2009.03241.x. [DOI] [PubMed] [Google Scholar]
- E22.Hashimoto T, Teye K, Ishii N. Clinical and immunological studies of 49 cases of various types of intercellular IgA dermatosis and 13 cases of classical subcorneal pustular dermatosis examined at Kurume University. Br J Dermatol. 2017;176:168–175. doi: 10.1111/bjd.14780. [DOI] [PubMed] [Google Scholar]
- E23.Kasperkiewicz M, Kowalewski C, Jablonska S. Pemphigus herpetiformis: from first description until now. J Am Acad Dermatol. 2014;70:780–787. doi: 10.1016/j.jaad.2013.11.043. [DOI] [PubMed] [Google Scholar]
- E24.della Torre R, Combescure C, Cortes B, et al. Clinical presentation and diagnostic delay in bullous pemphigoid: a prospective nationwide cohort. Br J Dermatol. 2012;167:1111–1117. doi: 10.1111/j.1365-2133.2012.11108.x. [DOI] [PubMed] [Google Scholar]
- E25.Lamberts A, Meijer JM, Jonkman MF. Nonbullous pemphigoid: a systematic review. J Am Acad Dermatol. 2018;78:989–995. doi: 10.1016/j.jaad.2017.10.035. e2. [DOI] [PubMed] [Google Scholar]
- E26.Kridin K, Bergman R. Assessment of the prevalence of mucosal involvement in bullous pemphigoid. JAMA Dermatol. 2019;155:166–171. doi: 10.1001/jamadermatol.2018.5049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E27.Forsti AK, Huilaja L, Schmidt E, Tasanen K. Neurological and psychiatric associations in bullous pemphigoid-more than skin deep? Exp Dermatol. 2017;26:1228–1234. doi: 10.1111/exd.13401. [DOI] [PubMed] [Google Scholar]
- E28.Bech R, Kibsgaard L, Vestergaard C. Comorbidities and treatment strategies in bullous pemphigoid: an appraisal of the existing litterature. Front Med (Lausanne) 2018;5 doi: 10.3389/fmed.2018.00238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E29.Bastuji-Garin S, Joly P, Lemordant P, et al. Risk factors for bullous pemphigoid in the elderly: a prospective case-control study. J Invest Dermatol. 2011;131:637–643. doi: 10.1038/jid.2010.301. [DOI] [PubMed] [Google Scholar]
- E30.Lloyd-Lavery A, Chi CC, Wojnarowska F, Taghipour K. The associations between bullous pemphigoid and drug use: a UK case-control study. JAMA Dermatol. 2013;149:58–62. doi: 10.1001/2013.jamadermatol.376. [DOI] [PubMed] [Google Scholar]
- E31.Varpuluoma O, Forsti AK, Jokelainen J, et al. Vildagliptin significantly increases the risk of bullous pemphigoid: a finnish nationwide registry study. J Invest Dermatol. 2018;138:1659–1661. doi: 10.1016/j.jid.2018.01.027. [DOI] [PubMed] [Google Scholar]
- E32.Plaquevent M, Tetart F, Fardet L, et al. Higher frequency of dipeptidyl peptidase-4 inhibitor intake in bullous pemphigoid patients than in the french general population. J Invest Dermatol. 2019;139:835–841. doi: 10.1016/j.jid.2018.10.045. [DOI] [PubMed] [Google Scholar]
- E33.Liu SD, Chen WT, Chi CC. Association between medication use and bullous pemphigoid: a systematic review and meta-analysis. JAMA Dermatol. 2020;156:891–900. doi: 10.1001/jamadermatol.2020.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E34.Holtsche MM, Goletz S, van Beek N, et al. Prospective study in bullous pemphigoid: association of high serum anti-BP180 IgG levels with increased mortality and reduced Karnofsky score. Br J Dermatol. 2018;179:918–924. doi: 10.1111/bjd.16553. [DOI] [PubMed] [Google Scholar]
- E35.Kridin K, Shihade W, Bergman R. Mortality in patients with bullous pemphigoid: a retrospective cohort study, systematic review and meta-analysis. Acta Derm Venereol. 2019;99:72–77. doi: 10.2340/00015555-2930. [DOI] [PubMed] [Google Scholar]
- E36.Egan CA, Lazarova Z, Darling TN, Yee C, Cote T, Yancey KB. Anti-epiligrin cicatricial pemphigoid and relative risk for cancer. Lancet. 2001;357:1850–1851. doi: 10.1016/S0140-6736(00)04971-0. [DOI] [PubMed] [Google Scholar]
- E37.Huilaja L, Makikallio K, Tasanen K. Gestational pemphigoid. Orphanet J Rare Dis. 2014;9 doi: 10.1186/s13023-014-0136-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E38.Lammer J, Hein R, Roenneberg S, Biedermann T, Volz T. Drug-induced linear IgA bullous dermatosis: a case report and review of the literature. Acta Derm Venereol. 2019;99:508–515. doi: 10.2340/00015555-3154. [DOI] [PubMed] [Google Scholar]
- E39.Goletz S, Hashimoto T, Zillikens D, Schmidt E. Anti-p200 pemphigoid. J Am Acad Dermatol. 2014;71:185–191. doi: 10.1016/j.jaad.2014.02.036. [DOI] [PubMed] [Google Scholar]
- E40.Ludwig RJ. Clinical presentation, pathogenesis, diagnosis, and treatment of epidermolysis bullosa acquisita. ISRN dermatology. 2013;2013 doi: 10.1155/2013/812029. 812029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E41.Meijer JM, Atefi I, Diercks GFH, et al. Serration pattern analysis for differentiating epidermolysis bullosa acquisita from other pemphigoid diseases. J Am Acad Dermatol. 2018;78:754–759. doi: 10.1016/j.jaad.2017.11.029. e6. [DOI] [PubMed] [Google Scholar]
- E42.Buijsrogge JJ, Diercks GF, Pas HH, Jonkman MF. The many faces of epidermolysis bullosa acquisita after serration pattern analysis by direct immunofluorescence microscopy. Br J Dermatol. 2011;165:92–98. doi: 10.1111/j.1365-2133.2011.10346.x. [DOI] [PubMed] [Google Scholar]
- E43.Holtsche MM, Zillikens D, Schmidt E. [Mucous membrane pemphigoid] Hautarzt. 2018;69:67–83. doi: 10.1007/s00105-017-4089-y. [DOI] [PubMed] [Google Scholar]
- E44.Kaplan I, Hodak E, Ackerman L, Mimouni D, Anhalt GJ, Calderon S. Neoplasms associated with paraneoplastic pemphigus: a review with emphasis on non-hematologic malignancy and oral mucosal manifestations. Oral Oncol. 2004;40:553–562. doi: 10.1016/j.oraloncology.2003.09.020. [DOI] [PubMed] [Google Scholar]
- E45.Sardy M, Kostaki D, Varga R, Peris K, Ruzicka T. Comparative study of direct and indirect immunofluorescence and of bullous pemphigoid 180 and 230 enzyme-linked immunosorbent assays for diagnosis of bullous pemphigoid. J Am Acad Dermatol. 2013;69:748–753. doi: 10.1016/j.jaad.2013.07.009. [DOI] [PubMed] [Google Scholar]
- E46.Blocker IM, Dahnrich C, Probst C, et al. Epitope mapping of BP230 leading to a novel enzyme-linked immunosorbent assay for autoantibodies in bullous pemphigoid. Br J Dermatol. 2012;166:964–970. doi: 10.1111/j.1365-2133.2012.10820.x. [DOI] [PubMed] [Google Scholar]
- E47.Schmidt E, Dahnrich C, Rosemann A, et al. Novel ELISA systems for antibodies to desmoglein 1 and 3: correlation of disease activity with serum autoantibody levels in individual pemphigus patients. Exp Dermatol. 2010;19:458–463. doi: 10.1111/j.1600-0625.2010.01069.x. [DOI] [PubMed] [Google Scholar]
- E48.Kobayashi M, Amagai M, Kuroda-Kinoshita K, et al. BP180 ELISA using bacterial recombinant NC16a protein as a diagnostic and monitoring tool for bullous pemphigoid. J Dermatol Sci. 2002;30:224–232. doi: 10.1016/s0923-1811(02)00109-3. [DOI] [PubMed] [Google Scholar]
- E49.Saleh MA, Ishii K, Kim YJ, et al. Development of NC1 and NC2 domains of type VII collagen ELISA for the diagnosis and analysis of the time course of epidermolysis bullosa acquisita patients. J Dermatol Sci. 2011;62:169–175. doi: 10.1016/j.jdermsci.2011.03.003. [DOI] [PubMed] [Google Scholar]
- E50.Sitaru C, Dahnrich C, Probst C, et al. Enzyme-linked immunosorbent assay using multimers of the 16th non-collagenous domain of the BP180 antigen for sensitive and specific detection of pemphigoid autoantibodies. Exp Dermatol. 2007;16:770–777. doi: 10.1111/j.1600-0625.2007.00592.x. [DOI] [PubMed] [Google Scholar]
- E51.van Beek N, Dahnrich C, Johannsen N, et al. Prospective studies on the routine use of a novel multivariant enzyme-linked immunosorbent assay for the diagnosis of autoimmune bullous diseases. J Am Acad Dermatol. 2017;76:889–894. doi: 10.1016/j.jaad.2016.11.002. e5. [DOI] [PubMed] [Google Scholar]
- E52.Ishii K, Amagai M, Hall RP, et al. Characterization of autoantibodies in pemphigus using antigen-specific enzyme-linked immunosorbent assays with baculovirus-expressed recombinant desmogleins. J Immunol. 1997;159:2010–2017. [PubMed] [Google Scholar]
- E53.Charneux J, Lorin J, Vitry F, et al. Usefulness of BP230 and BP180-NC16a enzyme-linked immunosorbent assays in the initial diagnosis of bullous pemphigoid: a retrospective study of 138 patients. Arch Dermatol. 2011;147:286–291. doi: 10.1001/archdermatol.2011.23. [DOI] [PubMed] [Google Scholar]
- E54.Sadik CD, Pas HH, Bohlmann MK, et al. Value of BIOCHIP technology in the serological diagnosis of pemphigoid gestationis. Acta Derm Venereol. 2017;97:128–130. doi: 10.2340/00015555-2460. [DOI] [PubMed] [Google Scholar]
- E55.Murakami H, Nishioka S, Setterfield J, et al. Analysis of antigens targeted by circulating IgG and IgA autoantibodies in 50 patients with cicatricial pemphigoid. J Dermatol Sci. 1998;17:39–44. doi: 10.1016/s0923-1811(97)00067-4. [DOI] [PubMed] [Google Scholar]
- E56.Calabresi V, Carrozzo M, Cozzani E, et al. Oral pemphigoid autoantibodies preferentially target BP180 ectodomain. Clin Immunol. 2007;122:207–213. doi: 10.1016/j.clim.2006.10.007. [DOI] [PubMed] [Google Scholar]
- E57.Schmidt E, Skrobek C, Kromminga A, et al. Cicatricial pemphigoid: IgA and IgG autoantibodies target epitopes on both intra- and extracellular domains of bullous pemphigoid antigen 180. Br J Dermatol. 2001;145:778–783. doi: 10.1046/j.1365-2133.2001.04471.x. [DOI] [PubMed] [Google Scholar]
- E58.Schmidt E, Obe K, Brocker EB, Zillikens D. Serum levels of autoantibodies to BP180 correlate with disease activity in patients with bullous pemphigoid. Arch Dermatol. 2000;136:174–178. doi: 10.1001/archderm.136.2.174. [DOI] [PubMed] [Google Scholar]
- E59.Kim JH, Kim YH, Kim S, et al. Serum levels of anti-type VII collagen antibodies detected by enzyme-linked immunosorbent assay in patients with epidermolysis bullosa acquisita are correlated with the severity of skin lesions. J Eur Acad Dermatol Venereol. 2012;27:e224–e230. doi: 10.1111/j.1468-3083.2012.04617.x. [DOI] [PubMed] [Google Scholar]
- E60.Horvath ON, Varga R, Kaneda M, Schmidt E, Ruzicka T, Sardy M. Diagnostic performance of the „MESACUP anti-Skin profile TEST“. Eur J Dermatol. 2016;26:56–63. doi: 10.1684/ejd.2015.2692. [DOI] [PubMed] [Google Scholar]
- E61.van Beek N, Rentzsch K, Probst C, et al. Serological diagnosis of autoimmune bullous skin diseases: prospective comparison of the BIOCHIP mosaic-based indirect immunofluorescence technique with the conventional multi-step single test strategy. Orphanet J Rare Dis. 2012;7 doi: 10.1186/1750-1172-7-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E62.Yang A, Xuan R, Melbourne W, Tran K, Murrell DF. Validation of the BIOCHIP test for the diagnosis of bullous pemphigoid, pemphigus vulgaris and pemphigus foliaceus. J Eur Acad Dermatol Venereol. 2020;34:153–160. doi: 10.1111/jdv.15770. [DOI] [PubMed] [Google Scholar]
- E63.Komorowski L, Muller R, Vorobyev A, et al. Sensitive and specific assays for routine serological diagnosis of epidermolysis bullosa acquisita. J Am Acad Dermatol. 2012;68:e89–e95. doi: 10.1016/j.jaad.2011.12.032. [DOI] [PubMed] [Google Scholar]
- E64.Waschke J, Spindler V. Desmosomes and extradesmosomal adhesive signaling contacts in pemphigus. Med Res Rev. 2014;34:1127–1145. doi: 10.1002/med.21310. [DOI] [PubMed] [Google Scholar]
- E65.Hammers CM, Stanley JR. Mechanisms of disease: pemphigus and bullous pemphigoid. Annu Rev Pathol. 2016;11:175–197. doi: 10.1146/annurev-pathol-012615-044313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E66.Sadik CD, Schmidt E, Zillikens D, Hashimoto T. Recent progresses and perspectives in autoimmune bullous diseases. J Allergy Clin Immunol. 2020;145:1145–1147. doi: 10.1016/j.jaci.2020.02.020. [DOI] [PubMed] [Google Scholar]
- E67.Sadik CD, Zillikens D. Current treatments and developments in pemphigoid diseases as paradigm diseases for autoantibody-driven, organ-specific autoimmune diseases. Semin Hematol. 2016;53(Suppl 1):S51–S53. doi: 10.1053/j.seminhematol.2016.04.015. [DOI] [PubMed] [Google Scholar]
- E68.Anhalt GJ, Labib RS, Voorhees JJ, Beals TF, Diaz LA. Induction of pemphigus in neonatal mice by passive transfer of IgG from patients with the disease. N Engl J Med. 1982;306:1189–1196. doi: 10.1056/NEJM198205203062001. [DOI] [PubMed] [Google Scholar]
- E69.Amagai M, Tsunoda K, Suzuki H, Nishifuji K, Koyasu S, Nishikawa T. Use of autoantigen-knockout mice in developing an active autoimmune disease model for pemphigus. J Clin Invest. 2000;105:625–631. doi: 10.1172/JCI8748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E70.Eming R, Hennerici T, Backlund J, et al. Pathogenic IgG antibodies against desmoglein 3 in pemphigus vulgaris are regulated by HLA-DRB1*04:02-restricted T cells. J Immunol. 2014;193:4391–4399. doi: 10.4049/jimmunol.1401081. [DOI] [PubMed] [Google Scholar]
- E71.Liu Z, Diaz LA, Troy JL, et al. A passive transfer model of the organ-specific autoimmune disease, bullous pemphigoid, using antibodies generated against the hemidesmosomal antigen, BP180. J Clin Invest. 1993;92:2480–2488. doi: 10.1172/JCI116856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E72.Nishie W, Sawamura D, Goto M, et al. Humanization of autoantigen. Nat Med. 2007;13:378–383. doi: 10.1038/nm1496. [DOI] [PubMed] [Google Scholar]
- E73.Sitaru C, Mihai S, Otto C, et al. Induction of dermal-epidermal separation in mice by passive transfer of antibodies specific to type VII collagen. J Clin Invest. 2005;115:870–878. doi: 10.1172/JCI21386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E74.Haeberle S, Wei X, Bieber K, et al. Regulatory T-cell deficiency leads to pathogenic bullous pemphigoid antigen 230 autoantibody and autoimmune bullous disease. J Allergy Clin Immunol. 2018;142:1831–1842. doi: 10.1016/j.jaci.2018.04.006. e7. [DOI] [PubMed] [Google Scholar]
- E75.Heppe EN, Tofern S, Schulze FS, et al. Experimental laminin 332 mucous membrane pemphigoid critically involves C5aR1 and reflects clinical and immunopathological characteristics of the human disease. J Invest Dermatol. 2017;137:1709–1718. doi: 10.1016/j.jid.2017.03.037. [DOI] [PubMed] [Google Scholar]
- E76.Sadik CD, Schmidt E. Resolution in bullous pemphigoid. Semin Immunopathol. 2019;41:645–654. doi: 10.1007/s00281-019-00759-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E77.Karsten CM, Pandey MK, Figge J, et al. Anti-inflammatory activity of IgG1 mediated by Fc galactosylation and association of FcgammaRIIB and dectin-1. Nat Med. 2012;18:1401–1406. doi: 10.1038/nm.2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E78.Koga H, Kasprick A, Lopez R, et al. Therapeutic effect of a novel phosphatidylinositol-3-kinase delta inhibitor in experimental epidermolysis bullosa acquisita. Front Immunol. 2018;9 doi: 10.3389/fimmu.2018.01558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E79.Samavedam UK, Mitschker N, Kasprick A, et al. Whole-genome expression profiling in skin reveals SYK as a key regulator of inflammation in experimental epidermolysis bullosa acquisita. Front Immunol. 2018;9 doi: 10.3389/fimmu.2018.00249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E80.Stussel P, Dieckhoff KS, Kunzel S, et al. Propranolol is an effective topical and systemic treatment option for experimental epidermolysis bullosa acquisita. J Invest Dermatol. 2020;140:2408–2420. doi: 10.1016/j.jid.2020.04.025. [DOI] [PubMed] [Google Scholar]
- E81.Gunther C, Wozel G, Meurer M, Pfeiffer C. Up-regulation of CCL11 and CCL26 is associated with activated eosinophils in bullous pemphigoid. Clin Exp Immunol. 2011;166:145–153. doi: 10.1111/j.1365-2249.2011.04464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E82.Shrikhande M, Hunziker T, Braathen LR, Pichler WJ, Dahinden CA, Yawalkar N. Increased coexpression of eotaxin and interleukin 5 in bullous pemphigoid. Acta Derm Venereol. 2000;80:277–280. doi: 10.1080/000155500750012162. [DOI] [PubMed] [Google Scholar]
- E83.Wakugawa M, Nakamura K, Hino H, et al. Elevated levels of eotaxin and interleukin-5 in blister fluid of bullous pemphigoid: correlation with tissue eosinophilia. Br J Dermatol. 2000;143:112–116. doi: 10.1046/j.1365-2133.2000.03599.x. [DOI] [PubMed] [Google Scholar]
- E84.Schmidt E, Lambert A, Marzano A, et al. S3 guideline on the diagnosis and management of mucous membrane pemphigoid initiated by the European Academy of Dermatology and Venereology (EADV) J Eur Acad Dermatol Venereol (Epub ahead of print) doi: 10.1111/jdv.17397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E85.Lee A, Sandhu S, Imlay-Gillespie L, Mulligan S, Shumack S. Successful use of Bruton‘s kinase inhibitor, ibrutinib, to control paraneoplastic pemphigus in a patient with paraneoplastic autoimmune multiorgan syndrome and chronic lymphocytic leukaemia. Australas J Dermatol. 2017;58:e240–e242. doi: 10.1111/ajd.12615. [DOI] [PubMed] [Google Scholar]
- E86.Hofrichter M, Dworschak J, Emtenani S, et al. Immunoadsorption o f desmoglein-3-specific IgG abolishes the blister-inducing capacity of pemphigus vulgaris IgG in neonatal mice. Front Immunol. 2018;9 doi: 10.3389/fimmu.2018.01935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E87.Hubner F, Kasperkiewicz M, Knuth-Rehr D, et al. Adjuvant treatment of severe/refractory bullous pemphigoid with protein A immunoadsorption. J Dtsch Dermatol Ges. 2018;16:1109–1118. doi: 10.1111/ddg.13642. [DOI] [PubMed] [Google Scholar]
- E88.Bastuji-Garin S, Souissi R, Blum L, et al. Comparative epidemiology of pemphigus in Tunisia and France: unusual incidence of pemphigus foliaceus in young Tunisian women. J Invest Dermatol. 1995;104:302–305. doi: 10.1111/1523-1747.ep12612836. [DOI] [PubMed] [Google Scholar]
- E89.Stanley J Pemphigus, Stanley J. Fitzpatrick’s dermatology in general medicine. In: Wolff J K GL, Katz SI, et al., editors. McGraw-Hill. New York : 2008. pp. 459–468. [Google Scholar]
- E90.Bernard P, Vaillant L, Labeille B, et al. Incidence and distribution of subepidermal autoimmune bullous skin diseases in three French regions. Bullous Diseases French Study Group. Arch Dermatol. 1995;131:48–52. [PubMed] [Google Scholar]
- E91.Radford CF, Rauz S, Williams GP, Saw VP, Dart JK. Incidence, presenting features, and diagnosis of cicatrising conjunctivitis in the United Kingdom. Eye (Lond) 2012;26:1199–1208. doi: 10.1038/eye.2012.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E92.Milinkovic MV, Jankovic S, Medenica L, et al. Incidence of autoimmune bullous diseases in Serbia: a 20-year retrospective study. J Dtsch Dermatol Ges. 2016;14:995–1005. doi: 10.1111/ddg.13081. [DOI] [PubMed] [Google Scholar]
- E93.van Beek N, Knuth-Rehr D, Altmeyer P, et al. Diagnostics of autoimmune bullous diseases in German dermatology departments. J Dtsch Dermatol Ges. 2012;10:492–499. doi: 10.1111/j.1610-0387.2011.07840.x. [DOI] [PubMed] [Google Scholar]
- E94.Tsuruta D, Ishii N, Hamada T, et al. IgA pemphigus. Clin Dermatol. 2011;29:437–442. doi: 10.1016/j.clindermatol.2011.01.014. [DOI] [PubMed] [Google Scholar]
- E95.Kridin K, Patel PM, Jones VA, Cordova A, Amber KT. IgA pemphigus: a systematic review. J Am Acad Dermatol. 2020;82:1386–1392. doi: 10.1016/j.jaad.2019.11.059. [DOI] [PubMed] [Google Scholar]
- E96.Wojnarowska F, Kirtschig G, Khumalo N. Treatment of subepidermal immunobullous diseases. Clin Dermatol. 2001;19:768–777. doi: 10.1016/s0738-081x(00)00189-9. [DOI] [PubMed] [Google Scholar]
- E97.Kasperkiewicz M, Meier M, Zillikens D, Schmidt E. Linear IgA disease: successful application of immunoadsorption and review of the literature. Dermatology. 2010;220:259–263. doi: 10.1159/000279318. [DOI] [PubMed] [Google Scholar]
- E98.Iwata H, Vorobyev A, Koga H, et al. Meta-analysis of the clinical and immunopathological characteristics and treatment outcomes in epidermolysis bullosa acquisita patients. Orphanet J Rare Dis. 2018;13 doi: 10.1186/s13023-018-0896-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E99.Santi CG, Gripp AC, Roselino AM, et al. Consensus on the treatment of autoimmune bullous dermatoses: bullous pemphigoid, mucous membrane pemphigoid and epidermolysis bullosa acquisita— Brazilian Society of Dermatology. An Bras Dermatol. 2019;94:33–47. doi: 10.1590/abd1806-4841.2019940207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E100.Hahn-Ristic K, Rzany B, Amagai M, Bröcker E-B, Zillikens D. Increased incidence of pemphigus vulgaris in southern Europeans living in Germany compared with native Germans. Eur Acad Dermatol Venereol. 2002;166:8–71. doi: 10.1046/j.1468-3083.2002.00384.x. [DOI] [PubMed] [Google Scholar]