Abstract
The leucocyte common antigen, protein tyrosine phosphatase receptor type C (PTPRC), also known as CD45, is a transmembrane glycoprotein, expressed on almost all haematopoietic cells except for mature erythrocytes, and is an essential regulator of T and B cell antigen receptor-mediated activation. Disruption of the equilibrium between protein tyrosine kinase and phosphatase activity (from CD45 and others) can result in immunodeficiency, autoimmunity, or malignancy. CD45 is normally present on the cell surface, therefore it works upstream of a large signalling network which differs between cell types, and thus the effects of CD45 on these cells are also different. However, it is becoming clear that CD45 plays an essential role in the innate immune system and this is likely to be a key area for future research. In this review of PTPRC (CD45), its structure and biological activities as well as abnormal expression of CD45 in leukaemia and lymphoma will be discussed.
Keywords: hematology, leukemia, leukemia, myeloid, myeloproliferative disorders
Introduction
Despite thousands of publications and more than 50 years of research, CD45 physiology and biology requires to be clarified to answer questions on CD45 phosphatase activity (PTA) regulation, the role of extracellular domain of CD45, alternative splicing of exons, the function of CD45 isoforms and many others.
CD45 is an essential protein on the surface of cells of the haematological and immunological systems. It has been used as a diagnostic tool to identify cells of haematological origin, but its function and structure are not widely understood. This review aims to update information on CD45
Structure of CD45
CD45 is a type 1 transmembrane protein tyrosine phosphatase (PTPase) expressed by all the haematopoietic stem cells (HSCs) except that of erythrocytes and platelets.1 CD45 is composed of two cytoplasmic domains, a transmembrane domain and an extracellular domain (figure 1). It is a large glycoprotein of 180–220 kDa and it forms about 10% of the surface antigens of positively expressing cells.2
Figure 1.
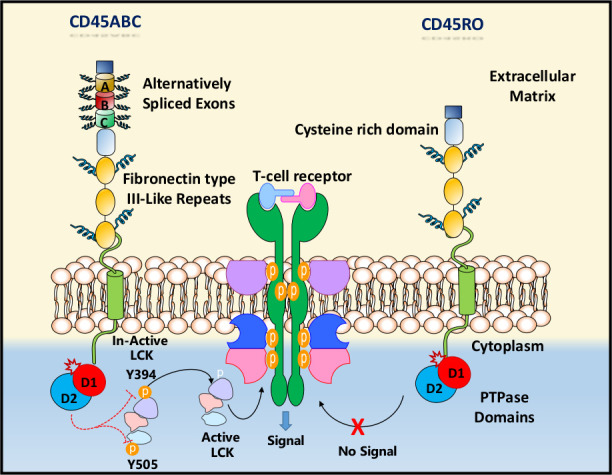
An illustration of CD45 structure. Different types of CD45 isoforms are expressed by haematopoietic stem cells (HSCs) based on alternative splicing of exons A, B and C. This results in modifications of the NH2-terminal domain of approximately 200 amino acids that contains many residues such as serine and threonine. This portion of CD45 structure is rich of O-glycosylation events which explains the distinctive glycosylation patterns of each CD45 isoform. Consequently, different lineages of HSCs express different types of CD45 isoforms based on different stages of differentiation and glycosylation events. The cysteine rich domain is connected by three fibronectin type III (FN III-like) repeats, a transmembrane domain followed by a cytoplasmic tail which contains two protein tyrosine phosphatase (PTPase) regions (D1 and D2). While D1 is characterised by PTPase activity, D2 has no enzymatic activity, but it is necessary for CD45 substrate recruitment and provide flexibility in folding of the molecule.
The cytoplasmic domain has a C-terminal tail of 79 amino acids (AAs), a putative wedge-like domain and two tandem PTPase domains. Although both domains are important in the cell physiology, it is only the D1 domain which is enzymatically active. The D2 domain, composed of 19 AAs, can be phosphorylated by C-terminal Src-kinase.3 The extracellular portion of the CD45 consists of five regions (figure 1). The extended N-terminal region has alternatively spliced exons, with several regions for O-linked glycosylation and thus form the protein variable area. All species have a similar structure of the CD45 extracellular domain, the three membrane-proximal portion and the type III fibronectin (FnIII) domain. In mammals, between the O-glycan tract and FnIII domains, a globular domain exists with five conserved cysteine-rich domains (figure 1).4
The CD45 gene, protein tyrosine phosphatase receptor type C (PTPRC), consists of 35 exons, four of which act as alternative mRNA splicing (exons 4, 5, 6 and 7), affecting isoform expression options in different cell types. It is responsible for encoding A, B and C domains (figure 1) leading to the formation of different types of isoforms in the extracellular portion of the CD45 molecule.5
Isoforms of CD45
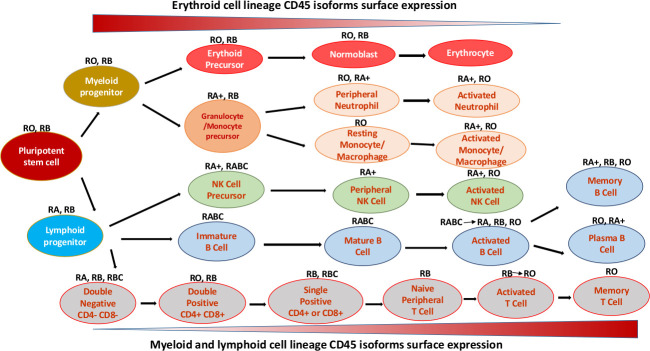
CD45 plays a vital role in HSCs proliferation and differentiation due to the alternative expression of different types of isoforms during their development.6 Human CD45 mRNA has six isoforms observed with no exon (RO), one exon (RA or RB), two exons (RAB or RBC), or all three exons (RABC) (figure 2).7
Figure 2.
CD45 isoform expression on human erythroid, lymphoid and myeloid haemopoietic cells at various stages of differentiation. RA, expressed on naïve T cells, has exon four with deficiency in exon 5 and 6. RB has only exon 5, expressed mainly on B cells, naïve T cells and thymocytes. RBC, has exons 5 and 6 of the CD45 gene. RABC, has all of the three exons 4, 6, and 6. RO, expressed on granulocytes, activated and memory T cells, some B subsets and activated monocytes/macrophages, has exon 3, 7 and 8 with deficiency in RA, RB and RC exons of the CD45 gene. ‘+’ means brighter expression (modified from Craig et al).8 NK, natural killer.
Encoding various types of CD45 isoforms due to the large number of alternatively spliced transcripts characterised by combinations of 0, 1, 2 or 3 exons, is a feature of CD45 molecule that has been used as a diagnostic tool to distinguish between different types of immune cells.5 Naive T cells, for instance, are well known to express CD45RA, while memory and activated T cells express the shortest isoform of CD45, CD45RO (figure 2).4
It is the extracellular domain of the CD45 molecule that forms heterogeneity of isoforms by glycosylation and alternative splicing of PTPRC gene exons and is also involved in monitoring the isoform expression by isoform-specific ligand interactions.8 However, all isoforms possess enzymatic activity and a common structure of Intracellular domain.8
It is challenging to define the various functions of variable CD45 isoforms which needs to be elucidated.
CD45 and the innate immune system
In the late eighties and nineties, the role of CD45 on T cell receptor (TCR) and BCR signalling was identified. The effects of CD45 on cytokine, NK receptor and Toll-like receptor (TLR) signalling and its role in mast cells, macrophages and DCs in leucocyte adhesion and migration have been explored. Soon after, the Janus kinases (JAKs) were identified as potential CD45 substrates. Here, we discuss the regulation of CD45 in the innate immunity and how CD45 affects different signalling pathways in leucocytes.9
While CD45 acts as a JAK phosphatase in mast cells to regulate antigen triggered Fc receptor and cytokine signalling, DCs require CD45 to regulate TLR signalling, thus widening the role of CD45 to recognise other receptors implicated in adaptive and innate immunity.9
Several CD45 substrates have been discovered including Transmembrane Immune Signalling Adaptor-(Dap12), Src-kinase-associated phosphoprotein (SkAP66 and SKAP55), JAKs, Transmembrane Adapter Protein/Transmembrane Phosphoprotein (PAG/Cbp), and TCR subunit (TCRζ).2 However, Src family kinases (SFKs) represent the best-defined substrate of CD45.2 CD45 can dephosphorylate the kinase-domain phosphotyrosine (Y394 in p56LCK) as well as the C-terminal phosphotyrosine (Y505 in p56LCK) which is antagonistic. The kinase is inactivated by dephosphorylation of kinase domain (Y394) and activated by dephosphorylation of C-terminal site (Y505) (figure 1).10 The activating and inhibitory phosphotyrosine in LYN is dephosphorylated by CD45 in B cells,11 indicating that CD45 has negative and positive regulating roles in T cells and B cells activation (table 1).
Table 1.
CD45 biological functions and disorders associated with altered CD45 function
| CD45-dependant cellular physiology | CD45 function | Altered CD45 Expression consequences |
| T and B-lymphocytes activation, development, tolerance and survival2 | Regulation of SFK activity by dephosphorylating the C-terminal negative regulatory tyrosine2
Initiation of TCR signalling2 |
T- and B- cell dysfunction2
Affect immune cell adhesion and migration2 Autoimmune infectious diseases2 HIV2 |
| Downstream of JNK and P38 pathway41 | Modulation of cytokine and chemokine production and signalling14
Recruitment of DOK-1 to the proximal plasma membrane42 |
Severe combined immunodeficiency43 |
| Negatively regulator monocytic cell differentiation44
Regulate FLT3 signalling in vivo45 |
Inhibiting phorbol 12-myristate 13-acetate-dependent activation and tyrosine phosphorylation of protein kinase C44 | Acute myeloid leukaemia45
Myeloproliferative neoplasm45 Cortical porosity45 Ectopic bone formation45 |
| Increased cell movement15 | Regulation of calcium NF-AT TCA pathway46 | Decreased Insulin receptor signalling results in defective cellular motility due to reduction of matrix metalloproteinase secretion15 |
JNK, Janus kinases; NF-AT, nuclear factor of activated T cells; SFK, Src family kinase; TCA, T cell activation; TCR, T cell receptor.
CD45 regulates the interaction between T cells and macrophages via its ligand macrophage galactose-type lectin which binds to CD45 N-acetylgalactosamine and results in decreasing the level of T cell proliferation with proinflammatory cytokine production leading to T cell apoptosis.12
Furthermore, macrophage mannose receptor binds to the CD45 low molecular weight isoform on DCs, leads to inhibition of PTA of CD45 on CD8+ T cells and elevation of cytotoxic T lymphocyte-associated protein 4 with CD8+ T cell tolerance.12
The role of CD45 in nuclear apoptosis was studied by Desharnais et al.13 They concluded that CD45- cells cannot resist apoptosis induced by cycloheximide but are resistant to DNA fragmentation and chromatin condensation induced by tributyltin (TBT) and hydrogen peroxide (H2O2). Mediation of cleavage of DFF-45, a subunit of the heterodimeric caspase-activated DNase, and intracellular Ca2+ mobilisation following TBT exposure occurs in both CD45+ and CD45- T cells. This indicates that CD45 is vital in chromatin condensation and DNA fragmentation.13
Negative regulation of the JAK/STAT signalling pathway is another role of CD45 recruiting DOK-1 to the proximal plasma membrane to act as a downstream effector (table 1).14
A correlation between increased CD45 expression on bone marrow leucocytes and enhanced cell motility in response to stress signals has been proposed by Schivtiel et al.15 Immature CD45 deficient cells exhibit defective motility as a result of reduced matrix metalloproteinase 9 secretion and altered SFK activity which results in elevated activation of β 1 integrin, enhanced cell adhesion and reduced homing (table 1).15
Therefore, CD45 is an essential component to regulate the innate immunity signalling, however, specificity of regulation needs further studies.
CD45: gene polymorphisms and disease consequences
CD45 controls the immune function by regulating lymphocyte survival, cytokine responses, and TCR signalling.14 Altered CD45 could result in severe combined immunodeficiency (figure 3).
Figure 3.
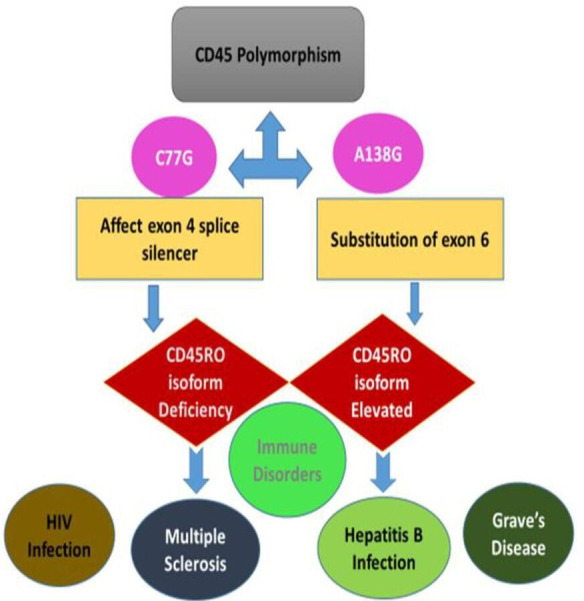
CD45 gene polymorphism and disease consequences. C77G and A138G are the two CD45 polymorphisms affecting PTPRC gene in human. While C77G target exon four splice silencer which affects the expression of CD45RO and leads to immune disorders such as multiple sclerosis and HIV infection, A138G polymorphism is a substitution of exon six and results in high expression of CD45RO with subsequent immune disorders such as hepatitis B infection and Grave’s disease.
Two CD45 polymorphisms have been found in PTPRC gene in humans, C77G and A138G. C77G is rare and affects the exon 4 splice silencer, leading to CD45RO isoform deficiency and immune disorders including autoimmune hepatitis, HIV infection and multiple sclerosis.16 The A138G mutation results in altered CD45 isoform expression with increased numbers of memory activated lymphocytes and increased interferon-gamma production (figure 3).17
The A138G polymorphism is found in up to 20% of the Japanese population. It occurs by substitution of exon 6, resulting in elevated production of CD45RO isoform (figure 3). A138G carriers have shown to suffer from Hepatitis B and Graves’ disease. This reflects the association between the increased distributions of this allele in population where these diseases are common.18
Abnormal expression of CD45 in leukaemia and lymphoma
CD45 deficiency or altered expression is associated with various diseases including leukaemia and lymphoma.2 Immunophenotyping studies of CD45 have shown that lymphocytes exhibit the brightest intensity among the other types of leucocytes. This has led to the use of CD45 as a diagnostic tool to distinguish between immature lymphoblasts found in acute lymphoblastic leukaemia (ALL) and plasma cell neoplasms which show weaker expression of CD45 than mature lymphoid neoplasms.19 Therefore, the differentiation and maturation stage determines the intensity of CD45 expression on leucocytes.
In diffuse large B cell lymphoma, Suzuki et al 20 have found that interaction between galectin-1 and CD45 glycans could enhance cell death of lymphoma cells. Therefore, CD45 plays an important role in stimulating DLBCL cells to secrete large amounts of galectin-3, an anti-apoptotic factor, and on binding to glycan receptors on CD45, DLBCL cells becoming more sensitive to apoptosis.20
CD45 expression on non-Hodgkin’s lymphoma cells is higher than found on chronic lymphoblastic leukaemia (CLL) cells. Keeney et al 21 showed a considerable reduction of CD45 expression in CLL patients associated with an elevated number of smudge cells, a prognostic monitor for disease management. Therefore, determining the CD45 percentages on these cells can be useful to monitor disease development.21
Paediatric ALL and Hodgkin lymphoma cells have shown significant disappearance of CD45 expression.22 23In comparison, adult ALL patients with elevated CD45 have shown reduced rate of survival. Patients with high levels of CD45 expression on multiple myeloma cells have shown a favourable prognosis, but cells are more vulnerable to oxidative stress (an apoptotic factor) with activated JAK/STAT pathway initiated by IL-6.24
Saint-Paul et al 25 found that CD45 had a role in the progression of acute myeloid leukaemia (AML), as the disease develops, there is a modification in the position of CD45 within lipid rafts, cholesterol and glycosphingo lipid enriched patches located in the plasma membrane. The localisation of CD45 within or outside lipid rafts is crucial for dephosphorylation of SFKs which results in accelerating the granulocyte monocyte colony stimulating factor (GM-CSF) signal, essential for proliferation of leukaemic cells. This discovery has shed light on targeting the position of CD45 in lipid rafts as a potential method of treatment for AML.25
Therapeutic role of CD45
CD45 controls the signalling pathways of the immune system, thus it has been studied as a therapeutic target for many immune diseases including autoimmunity and organ transplantation.2 A number of CD45 ligands have been discovered such as PUL11, placental protein 14 (PP14) and different types of lectins which regulate CD45 isoforms. Therefore, it might be useful to target those ligands in order to develop potential pharmaceutical therapeutic options.2
PUL11 is constructed from the UL11 found in cytomegalovirus infected human cells.26 CD45RA and CD45R0 interaction with the extracellular portion of pUL11 results in down-regulation of T cell proliferation and alteration in TCR signalling pathway.27High PUL11 concentration phosphorylates the inhibitory Y505 residue of LCK, while the activating Y394 residue of LCK showed comparatively increased phosphorylation at all concentrations of PUL11.28 Thus, CD45 PTA is influenced by PUL11 concentration.
PP14, also known as PAEP (progesterone-associated endometrial protein), a glycoprotein produced during pregnancy causes dimerisation and modification in CD45 PTA resulting in reduction of T cell activity required during the pregnancy. Thus PP14 can act as natural immunosuppressive CD45 ligand.29
Lectins bind to CD45 as well as many other molecules. While CD22 is a lectin B cell surface molecule that inhibits BCR signalling when it binds to CD45, galectin-1 binds and inhibits CD45 PTA, important to enhance T cell apoptosis.30
Furthermore, Galectin-3 binds to glycans on CD45 causing reduction in CD45 PTA which contributes to the pathophysiology of several diseases such as DLBCL,31 heart failure,32renal fibrosis33 and cancer.34
CD45 antibodies with isoform specificity and selective phosphatase inhibitors have been developed to alter CD45 physiology.7 These are immunosuppressive and used to treat different types of leukaemias35 and in stem cell transplantation.36 Development of CD45RB antibodies have been successfully applied in the treatment of modified CD45 isoform expression and prevention of rejection of allografts with associated inflammation caused by allergic pulmonary reactions.37
Alzheimer disease is characterised by the activation of microglial cells which play an essential role in the disease pathophysiology and are highly expressing CD45. Cross-linking of CD45 leads to inactivation of microglial cells.38
It is essential to validate the specificity of CD45 inhibitor to avoid adverse effects considering its wide distribution. Researchers should propose to inhibit the CD45 enzymatic activity, but not the CD45 protein as leukaemic cells are highly proliferating and need CD45 activity, while normal cells are resting and do not require CD45 activity. Thus, it would be benefit to target inhibiting SFK-dependent growth cancer cells to produce high selectivity of CD45 inhibitors.
PTP inhibitor XIX (PI‐19) is one potential CD45 inhibitor drug used for the treatment of organ graft rejection and autoimmunity.39
Perron et al 40 discovered 211 (2-[(4-acetylphenyl) amino]−3-chloronaphthoquinone), a selective CD45 inhibitor effective in suppressing T cell responses in a delayed hypersensitivity inflammatory model in vivo with minor toxicity to the normal immune system. This agent could be used as a potential drug to prevent the dissemination of metastasis of lymphoid tumours by targeting CD45 enzymatic activity for cancer therapy.40
This approach would protect normal non-proliferating cells that express CD45 but do not depend on CD45 activity, while clearance of the inhibitor from the body enables normal cells to resume their ability to form new enzymatically active CD45 protein and activate a normal immune response when required.
Summary
CD45 is vital for the normal physiology of haematopoiesis. This review has discussed the structure and biological activities of CD45 to better understand the contribution of CD45 to the regulation of the immunity signalling.
Take home message.
CD45 has many modes of action including in in apoptosis and cell survival. This molecule may be a potentially useful drug target. However, further studies are required to help fully understand all functions of CD45.
Footnotes
Handling editor: Des Richardson.
Contributors: As a PhD student at the Queen’s University Belfast, Patrick G Johnston Centre for Cancer Research (PGJCCR); previously known as CCRCB; MAAB’s first supervisor, KM provided assistance in reviewing the grammar; second supervisor, MFM participated in the overall revision of content in terms of grammar, paraphrasing and connectivity of sentences. AA is part of our research group as a postdoctoral research fellow helping in figure construction.
Funding: This study was funded by Queen's University Belfast.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Not required.
References
- 1.Barford D, Flint A, Tonks N. Crystal structure of human protein tyrosine phosphatase 1B. Science 1994;263:1397–404. 10.1126/science.8128219 [DOI] [PubMed] [Google Scholar]
- 2.Rheinländer A, Schraven B, Bommhardt U. Cd45 in human physiology and clinical medicine. Immunol Lett 2018;196:22–32. 10.1016/j.imlet.2018.01.009 [DOI] [PubMed] [Google Scholar]
- 3.Hermiston ML, Zikherman J, Zhu JW. Cd45, CD148, and Lyp/Pep: critical phosphatases regulating Src family kinase signaling networks in immune cells. Immunol Rev 2009;228:288–311. 10.1111/j.1600-065X.2008.00752.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holmes N. Cd45: all is not yet crystal clear. Immunology 2006;117:145–55. 10.1111/j.1365-2567.2005.02265.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hall LR, Streuli M, Schlossman SF, et al. Complete exon-intron organization of the human leukocyte common antigen (CD45) gene. J Immunol 1988;141:2781–7. [PubMed] [Google Scholar]
- 6.Dornan S, Sebestyen Z, Gamble J, et al. Differential association of CD45 isoforms with CD4 and CD8 regulates the actions of specific pools of p56lck tyrosine kinase in T cell antigen receptor signal transduction. J Biol Chem 2002;277:1912–8. 10.1074/jbc.M108386200 [DOI] [PubMed] [Google Scholar]
- 7.Hermiston ML, Xu Z, Weiss A. Cd45: a critical regulator of signaling thresholds in immune cells. Annu Rev Immunol 2003;21:107–37. 10.1146/annurev.immunol.21.120601.140946 [DOI] [PubMed] [Google Scholar]
- 8.Craig W, Poppema S, Little M-T, et al. Cd45 isoform expression on human haemopoietic cells at different stages of development. Br J Haematol 1994;88:24–30. 10.1111/j.1365-2141.1994.tb04972.x [DOI] [PubMed] [Google Scholar]
- 9.Saunders AE, Johnson P. Modulation of immune cell signalling by the leukocyte common tyrosine phosphatase, CD45. Cell Signal 2010;22:339–48. 10.1016/j.cellsig.2009.10.003 [DOI] [PubMed] [Google Scholar]
- 10.Alexander DR. The CD45 tyrosine phosphatase: a positive and negative regulator of immune cell function. Semin Immunol 2000;12:349–59. 10.1006/smim.2000.0218 [DOI] [PubMed] [Google Scholar]
- 11.Katagiri T, Ogimoto M, Hasegawa K, et al. Cd45 negatively regulates Lyn activity by dephosphorylating both positive and negative regulatory tyrosine residues in immature B cells. J Immunol 1999;163:1321–6. [PubMed] [Google Scholar]
- 12.Schuette V, Embgenbroich M, Ulas T, et al. Mannose receptor induces T-cell tolerance via inhibition of CD45 and up-regulation of CTLA-4. Proc Natl Acad Sci U S A 2016;113:10649–54. 10.1073/pnas.1605885113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Desharnais P, Dupéré-Minier G, Hamelin C, et al. Involvement of CD45 in DNA fragmentation in apoptosis induced by mitochondrial perturbing agents. Apoptosis 2008;13:197–212. 10.1007/s10495-007-0162-9 [DOI] [PubMed] [Google Scholar]
- 14.Tchilian EZ, Beverley PCL. Altered CD45 expression and disease. Trends Immunol 2006;27:146–53. 10.1016/j.it.2006.01.001 [DOI] [PubMed] [Google Scholar]
- 15.Shivtiel S, Kollet O, Lapid K, et al. Cd45 regulates retention, motility, and numbers of hematopoietic progenitors, and affects osteoclast remodeling of metaphyseal trabecules. J Exp Med 2008;205:2381–95. 10.1084/jem.20080072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rong-jun HE, Zhi-hong YU, Ruo-yu Z, et al. Protein tyrosine phosphatases as potential therapeutic targets. Acta Pharmacol Sin 2014;35:1227–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boxall S, Stanton T, Hirai K, et al. Disease associations and altered immune function in CD45 138G variant carriers. Hum Mol Genet 2004;13:2377–84. 10.1093/hmg/ddh276 [DOI] [PubMed] [Google Scholar]
- 18.Lynch KW. Consequences of regulated pre-mRNA splicing in the immune system. Nat Rev Immunol 2004;4:931–40. 10.1038/nri1497 [DOI] [PubMed] [Google Scholar]
- 19.Gitte SJ, Poppema S, Mant MJ, et al. Transition in CD45 isoform expression during differentiation of normal and abnormal B cells. Int Immunol 1989;1:229–36. [DOI] [PubMed] [Google Scholar]
- 20.Suzuki O, Nozawa Y, Abe M. Regulatory roles of altered N- and O-glycosylation of CD45 in galectin-1-induced cell death in human diffuse large B cell lymphoma. Int J Oncol 2005;26:1063–8. [PubMed] [Google Scholar]
- 21.Keeney M, Hedley BD, Chin-Yee IH. Flow cytometry-Recognizing unusual populations in leukemia and lymphoma diagnosis. Int J Lab Hematol 2017;39:86–92. 10.1111/ijlh.12666 [DOI] [PubMed] [Google Scholar]
- 22.Ozdemirli M, Mankin HJ, Aisenberg AC, et al. Hodgkin’s disease presenting as a solitary bone tumor: A report of four cases and review of the literature. Cancer 1996;77:79–88. [DOI] [PubMed] [Google Scholar]
- 23.Ratei R, Sperling C, Karawajew L, et al. Immunophenotype and clinical characteristics of CD45-negative and CD45-positive childhood acute lymphoblastic leukemia. Ann Hematol 1998;77:107–14. 10.1007/s002770050424 [DOI] [PubMed] [Google Scholar]
- 24.Ishikawa H, Mahmoud MS, Fujii R, et al. Proliferation of immature myeloma cells by interleukin-6 is associated with CD45 expression in human multiple myeloma. Leuk Lymphoma 2000;39:51–5. 10.3109/10428190009053538 [DOI] [PubMed] [Google Scholar]
- 25.Saint-Paul L, Nguyen C-H, Buffière A, et al. Cd45 phosphatase is crucial for human and murine acute myeloid leukemia maintenance through its localization in lipid rafts. Oncotarget 2016;7:64785–97. 10.18632/oncotarget.11622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elbasani GE, Ameres S, Steinbruck L, et al. Expression of the human cytomegalovirus UL11 glycoprotein in viral infection and evaluation of its effect on virus-specific CD8T cells. J Virol 2014;88:14326–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gabaev I, Steinbrück L, Pokoyski C, et al. The human cytomegalovirus UL11 protein interacts with the receptor tyrosine phosphatase CD45, resulting in functional paralysis of T cells. PLoS Pathog 2011;7:e1002432. 10.1371/journal.ppat.1002432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zischke J, Mamareli P, Pokoyski C, et al. The human cytomegalovirus glycoprotein pUL11 acts via CD45 to induce T cell IL-10 secretion. PLoS Pathog 2017;13:e100645. 10.1371/journal.ppat.1006454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Julkunen M, Raikar RS, Joshi SG, et al. Placental protein 14 and progestagen-dependent endometrial protein are immunologically indistinguishable. Hum Reprod 1986;1:7–8. 10.1093/oxfordjournals.humrep.a136349 [DOI] [PubMed] [Google Scholar]
- 30.Walzel H, Schulz U, Neels P, et al. Galectin-1, a natural ligand for the receptor-type protein tyrosine phosphatase CD45. Immunol Lett 1999;67:193–202. 10.1016/S0165-2478(99)00012-7 [DOI] [PubMed] [Google Scholar]
- 31.Clark MC, Pang M, Hsu DK, et al. Galectin-3 binds to CD45 on diffuse large B-cell lymphoma cells to regulate susceptibility to cell death. Blood 2012;120:4635–44. 10.1182/blood-2012-06-438234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lok DJA, Van Der Meer P, de la Porte PWB-A, et al. Prognostic value of galectin-3, a novel marker of fibrosis, in patients with chronic heart failure: data from the DEAL-HF study. Clin Res Cardiol 2010;99:323–8. 10.1007/s00392-010-0125-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Henderson NC, Mackinnon AC, Farnworth SL, et al. Galectin-3 expression and secretion links macrophages to the promotion of renal fibrosis. Am J Pathol 2008;172:288–98. 10.2353/ajpath.2008.070726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu F-T, Rabinovich GA. Galectins as modulators of tumour progression. Nat Rev Cancer 2005;5:29–41. 10.1038/nrc1527 [DOI] [PubMed] [Google Scholar]
- 35.Ostergaard H, Trowbridge I. Negative regulation of CD45 protein tyrosine phosphatase activity by ionomycin in T cells. Science 1991;253:1423–5. 10.1126/science.1654595 [DOI] [PubMed] [Google Scholar]
- 36.Autero M, Saharinen J, Pessa-Morikawa T, et al. Tyrosine phosphorylation of CD45 phosphotyrosine phosphatase by p50csk kinase creates a binding site for p56lck tyrosine kinase and activates the phosphatase. Mol Cell Biol 1994;14:1308–21. 10.1128/MCB.14.2.1308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang Z, Lazarovits A, Gao Z, et al. Prolongation of xenograft survival using monoclonal antibody CD45RB and cyclophosphamide in RAT-TO-MOUSE kidney and heart transplant MODELS1. Transplantation 2000;69:1137–46. 10.1097/00007890-200003270-00019 [DOI] [PubMed] [Google Scholar]
- 38.Jen KY, Campo M, He H, et al. CD45RB ligation inhibits allergic pulmonary inflammation by inducing CTLA4 transcription. J Immunol 2007;179:4212–8. 10.4049/jimmunol.179.6.4212 [DOI] [PubMed] [Google Scholar]
- 39.Le HTT, Cho Y-C, Cho S. Inhibition of protein tyrosine phosphatase non-receptor type 2 by PTP inhibitor XIX: its role as a multiphosphatase inhibitor. BMB Rep 2017;50:329–34. 10.5483/bmbrep.2017.50.6.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Perron M, Saragovi HU. Inhibition of CD45 Phosphatase Activity Induces Cell Cycle Arrest and Apoptosis of CD45 + Lymphoid Tumors Ex Vivo and In Vivo. Mol Pharmacol 2018;93:575–80. 10.1124/mol.117.110908 [DOI] [PubMed] [Google Scholar]
- 41.Huntington ND, Xu Y, Nutt SL, et al. A requirement for CD45 distinguishes Ly49D-mediated cytokine and chemokine production from killing in primary natural killer cells. J Exp Med 2005;201:1421–33. 10.1084/jem.20042294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu L, Bijian K, Shen S-S. Cd45 recruits adapter protein Dok-1 and negatively regulates JAK–STAT signaling in hematopoietic cells. Mol Immunol 2009;46:2167–77. 10.1016/j.molimm.2009.04.032 [DOI] [PubMed] [Google Scholar]
- 43.Cale CM, Klein NJ, Novelli V. Severe combined immunodeficiency with abnormalities in expression of the common leucocyte. [DOI] [PMC free article] [PubMed]
- 44.Eric L, Deszo EL, Danett K, et al. Cd45 negatively regulates monocytic cell differentiation by inhibiting phorbol 12-myristate 13-acetate-dependent activation and tyrosine phosphorylation of protein kinase. J Biol Chem 2001;276:10212–7. [DOI] [PubMed] [Google Scholar]
- 45.Kresinsky A, Schnöder TM, Jacobsen ID, et al. Lack of CD45 in FLT3-ITD mice results in a myeloproliferative phenotype, cortical porosity, and ectopic bone formation. Oncogene 2019;38:4773–87. 10.1038/s41388-019-0757-y [DOI] [PubMed] [Google Scholar]
- 46.Fric J, Lim CXF, Mertes A, et al. Calcium and calcineurin-NFAT signaling regulate granulocyte-monocyte progenitor cell cycle via Flt3-L. Stem Cells 2014;32:3232–44. 10.1002/stem.1813 [DOI] [PMC free article] [PubMed] [Google Scholar]