Abstract
Background/Aim
To report the efficacy and tolerability of antitumour necrosis factor-alpha therapy (TNF inhibitors [TNFi]) in the management of non-infectious ocular inflammation, including uveitis and scleritis, in adult patients over an 8-year period.
Materials and methods
This is a prospective cohort study of infliximab and adalimumab in the treatment of non-infectious ocular inflammatory disease. 43 of 85 adult patients on TNFi (34 infliximab, 9 adalimumab) for ≥1 year with non-infectious uveitis or scleritis were followed from 2006 to 2014. Clinical assessments, medication, adverse events and history of steroid rescues were collected at 6 monthly intervals. General quality of life (Short Form Health Survey (SF-36)) and visual quality of life (Vision-related quality of life Core Measure (VCM1)) were assessed annually. Outcome measures included rate of sustained remission, rate of relapse, systemic corticosteroid reduction, adverse events, and VCM1 and SF-36 scores.
Results
The median time on infliximab was 3.2 years (IQR 4.3) and on adalimumab was 2.4 years (IQR 1.8). Sustained remission was induced in 39 patients (91%) (0.5 per patient year) after a median of 1.2 years on a TNFi. 22 (51%) experienced one relapse, and 5 (12%) had two relapses. 23 (54%) had at least one adverse event; serious adverse events necessitating hospitalisation or cessation of medication occurred in four (9%) patients. 10 patients (23%) switched from the initiation of TNFi, at 1.7 years after starting, to another TNFi or another class of biologic therapy.
Conclusion
TNFi treatment is associated with long-term drug-induced remission of ocular inflammation, visual stability and corticosteroid reduction. Adverse events were common and no new safety signals occurred. Relapse of inflammation occurs in half of the treated population.
Keywords: inflammation, immunology, drugs
Introduction
Sight-threatening non-infectious uveitis is responsible for up to 10%–15% of blindness in the developed world.1 2 The biologic agents, tumour necrosis factor-alpha (TNFα) inhibitors (TNFi)1 2 and interferon-α,3 4 are treatment alternatives to conventional immunosuppressants for uveitis and as early therapies in Behcet’s disease or for refractory uveitis.1 2 5 Infliximab is effective in inducing and maintaining remission in up to 86% of patients with uveitis and particularly in Behcet’s disease.5 6 The VISUAL I and II trials evidence the ability of adalimumab to reduce the number of uveitis flares compared with placebo (28% and 20% for VISUAL I and II, respectively) and enable corticosteroid reduction over an 80-week period.1 2 An open-label extension of the original VISUAL trials for active and inactive uveitis provides further evidence of efficacy7 and patient-reported measures inform visual functioning ability.8 In a post-hoc analysis of participants with active and inactive uveitis treated with adalimumab within the VISUAL trials, there was an improvement in at least 75% of the VQF25 visual quality of life (QoL) questionnaire subcomponents compared with placebo.9 Non-infectious scleritis is a typically painful inflammatory disorder which has also been treated with TNFi.10
TNFi has been used regularly in clinical practice in ocular inflammation for over a decade. This prospective immunosurveillance study aimed to capture clinical indices for patients on TNFi, to obtain insight into long-term patient response characteristics for the two most widely available TNFi therapies for ocular inflammation.
Patients and methods
Data collection
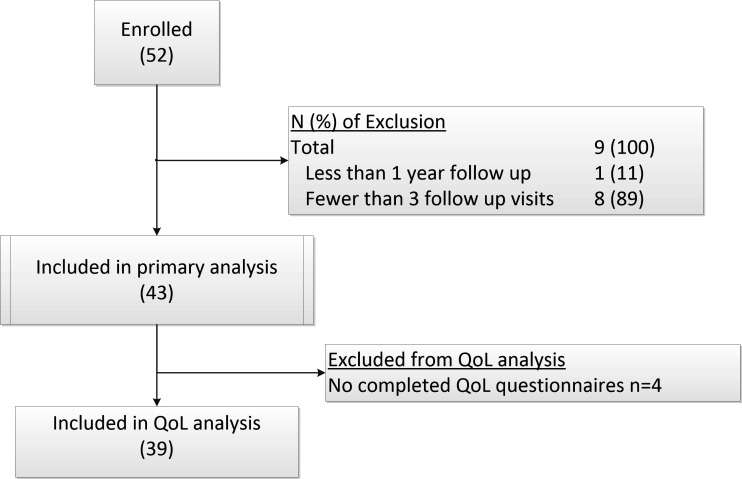
Longitudinal, cohort data for patients receiving TNFi were captured within an electronic database hosted on a National Health Service digital network. This analysis reports an 8-year outcome data and QoL parameters for patients over the age of 18 years old treated with TNFi for at least 1 year from October 2006 to May 2015 at Bristol Eye Hospital, UK (figure 1).
Figure 1.
Study Diagram.
For six patients who started TNFi before the study, baseline visit data were collected retrospectively from patients’ notes (table 1). We prospectively recorded clinical activity parameters, adverse events (AEs) and medications at 6 monthly dedicated clinics. QoL was assessed annually. Clinical assessment included a full systemic history, logarithm of the minimum angle of resolution (logMAR) visual acuity with current spectacle correction, Goldmann tonometry, slit-lamp examination, and fundal examination including biomicroscopic indirect ophthalmoscopy (BIO) to score vitreous haze. Intraocular inflammation was graded according to Standardised Uveitis Nomenclature.11 The presence or absence of vasculitis and macular oedema was scored by clinical examination and ocular imaging. Scleritis was scored clinically as active or inactive.
Table 1.
Patient demographics and disease characteristics at baseline (prior to commencement of TNFi)
| Demographics | % | |
| Cohort size | 43 | – |
| Age at study entry (SD) | 38.9 (11.6) | – |
| Female gender | 27 | 62.8 |
| Ethnicity | ||
| Caucasian | 42 | 97.7 |
| Asian | 1 | 2.3 |
| Afro-Caribbean | 1 | 2.3 |
| Bilateral disease | 31 | 72.3 |
| Median duration of disease prior to biologic therapy, years (IQR) | 3.0 (7.0) | |
| Anatomical classification of ocular disease | ||
| Anterior uveitis | 3 | 7 |
| Intermediate uveitis | 8 | 18.6 |
| Panuveitis | 13 | 30.2 |
| Posterior uveitis | 15 | 34.9 |
| Scleritis | 4 | 9.3 |
| Aetiology | ||
| Idiopathic | 19 | 44.2 |
| Systemic vasculitis | 1 | 2.3 |
| Ankylosing spondylitis | 1 | 2.3 |
| Behcet’s | 15 | 34.8 |
| Juvenile idiopathic arthritis | 2 | 4.6 |
| Juvenile sarcoidosis | 1 | 2.3 |
| Psoriatic arthropathy | 2 | 4.6 |
| Sarcoidosis | 1 | 2.3 |
| Undifferentiated arthritis | 1 | 2.3 |
| Visual acuity logMAR score | ||
| ≤0 | 14 | 33 |
| >0.1, ≤0.3 | 18 | 41.8 |
| >0.3, ≤1 | 9 | 20.9 |
| >1 | 2 | 4.6 |
| Clinical disease activity | ||
| Anterior chamber inflammation ≥1+ | 7 | 16.3 |
| Cystoid macular oedema (active inflammation) | 9* | 20.9 |
| Vitreous haze score ≥1 | 5 | 11.6 |
| Retinal vasculitis | 23 | 53.5 |
| Choroidal inflammation | 2 | 4.6 |
| Missing data | 0 | – |
| Comorbidities/Complications | ||
| Glaucoma | 6 | 14.0 |
| Ocular hypertension | 2 | 4.6 |
| Epiretinal membrane | 14 | 32.6 |
| Cataract | 12 | 27.9 |
| Retinal ischaemia within the macula | 8 | 18.6 |
| Retinal ischaemia within peripheral retina | 12 | 27.9 |
*Missing data for one patient.
TNFi, tumour necrosis factor-alpha inhibitor; logMAR, logarithm of the minimum angle of resolution.
Immunosuppressant medication, corticosteroid doses and corticosteroid ‘rescue’ treatments were recorded. Treatment decisions for immunomodulatory agents followed a paradigm contemporary for uveitis practice prior to current trial data. Patients on biologic therapy were maintained on a single immunomodulatory therapy in order to diminish the formation of human antichimeric antibodies. Typical immunomodulatory agents and doses were infliximab 3–5 mg/kg 4–8 weekly via intravenous administration, adalimumab 40 mg alternate weeks via subcutaneous route, mycophenolate mofetil 1–3 g/day and tacrolimus titrated to whole blood trough levels of 5−10 ng/L. If there was a failure to respond to TNFi, we adopted a strategy to optimise background immunosuppression to tolerated therapeutic levels, followed by in-class switching and lastly, out-of-class switching. Treatment cessation was immediate where serious adverse events (SAEs) occurred. Steroid rescue treatment was defined as a short escalation of corticosteroids given either intravenously as methylprednisolone (500 mg–1 g/day for 3 days), a tapering course of high-dose oral prednisolone (starting ≥40 mg, tapering to ≤7.5 mg/day), periorbital corticosteroid injection (triamcinolone 40 mg), intravitreal triamcinolone injection (4 mg) or a dexamethasone (Ozurdex) intravitreal implant. Data on steroid rescue events prior to biologic therapy were collected retrospectively from patients’ clinical records.
Patients
Patients with non-infectious uveitis and scleritis receiving TNFi for 1 year, who were over the age of 18 years, had a negative or treated tuberculosis (TB) status, had been on no other biologic therapy, and had at least two recorded attendances in the biologics registry clinic were eligible for inclusion (figure 1). Informed consent was obtained from patients prior to participation.
Data analysis and statistics
The principal outcome measure was the rate of achieving sustained remission defined as anterior chamber inflammation and vitreous haze scores of ≤0.5+ on two successive visits, absence of retinal vasculitis or worsening cystoid macular oedema. The event rate is defined as the total number of events as a proportion of the time from TNFi initiation to the event to census, reported as incidence rates per patient year (PPY).
The secondary objectives included the following:
Relapse rate (requirement for steroid rescue therapy, two-step increase in anterior chamber (AC) activity or vitreous haze score, new cystoid macular oedema, retinal vasculitis on fluorescein angiography, or a doubling of the visual angle).
Reduction of daily oral prednisolone to ≤10 mg for two successive visits.
Doubling or halving of the visual angle (loss or gain of 15 letters on the ETDRS chart) compared with baseline on two successive visits.
Rate of AEs.
Vision-related quality of life Core Measure (VCM1) and Short Form Health Survey -36 (SF-36) QoL scores.
Relapse and remission rates during TNFi treatment are presented as cumulative incidence curves using the product limit method of Kaplan and Meier and calculated using the statistical package ‘survival’ in ‘R’ (where patients who did not experience the endpoint before the end of their follow-up were censored).
QoL scores were compared annually using the UK standard version of the SF-36.12 Vision-related QoL was measured using VCM1, which is validated for uveitis.13 14 To account for within-hospital clustering, generalised estimating equations were used to generate models using sex, BIO score, duration of treatment and visual acuity as potential predictive variables. For VCM1 a binomial distribution was used to determine which variables were predictive of a VCM1 score of more than 2.0 (which reflected a patient experiencing ‘more than a little’ concern about their vision).13
Results
Study population
Of 85 patients within the registry, 43 met the inclusion criteria (table 1). Patients attended 1.5 (SD 0.4) clinic visits per year for a mean of 5.4 years (SD 2.2; range 1.2–11.3 years) to the census date (May 2015). Thirty-four (79%) patients were treated with a TNFi for over 2 years and 23 (54%) were treated for over 4 years. Thirty-eight completed at least two QoL questionnaires. Thirty-four patients commenced treatment with infliximab and nine with adalimumab. The median time on infliximab was 3.2 years (IQR 4.3) and on adalimumab was 2.4 years (IQR 1.8).
The clinical indications for TNFi were grouped into three non-mutually exclusive categories: non-infectious uveitis or scleritis refractory to therapeutic doses of at least one immunosuppressive therapy (n=30), patients who were intolerant of therapeutic doses of conventional immunosuppression (n=15), and patients who were experiencing rapid deterioration in vision (n=10). (For detailed baseline disease characteristics, see online supplementary table 1.
bjophthalmol-2018-312767supp001.pdf (90.4KB, pdf)
Efficacy of anti-TNFα therapy: remission and relapse
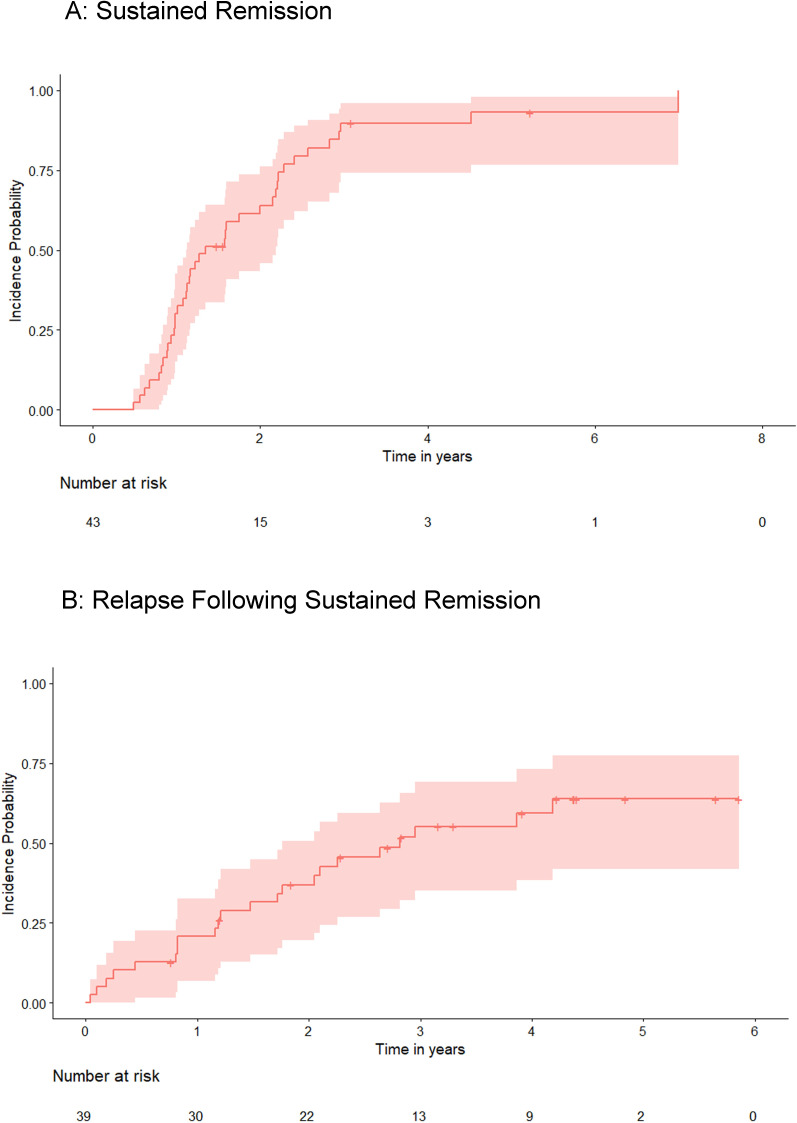
Thirty-nine patients (91%) achieved sustained remission (0.5 PPY) at a median time of 1.2 years from starting TNFi (figure 2). Twenty-two (51%) of the cohort had a relapse (0.2 PPY) at 2.9 years (median) (range 0.9–7.2 years) from commencing a TNFi (index). In five patients (12%) a second relapse occurred at 6.6 years (range 2.9–9.2 years) from index. No patient had more than two relapses. Six out of nine (67%) patients who started on adalimumab achieved sustained remission; all six later experienced a relapse (median 2.0 years from index; IQR: 1.6). Thirty-three of the 34 (97%) patients receiving infliximab achieved sustained remission. Relapse occurred in 18 patients (53%) (median 3.4 years from index; IQR: 2.7). Twenty-six patients received a steroid rescue treatment during follow-up (high-dose oral prednisolone [n=21, intravenous methylprednisolone n=11, intravitreal triamcinolone n=7], periocular steroid injection n=5). The rate of steroid rescue prior to commencing biologic therapy was 0.8 PPY for 33 of 43 patients in whom these data were available; the introduction of TNFi was associated with a reduction in steroid rescue (0.1 PPY; n=43) to census or cessation of the TNFi.
Figure 2.
Sustained remission and relapse during follow up. Kaplan Meier estimator curve of the probability ofpatients (y-axis) experiencing (A) sustained remission and (B) relapse following remission on eitheradalimumab or infliximab. The x-axis shows the time to either outcome.
Visual acuity
At baseline, 32 (74%) patients met the driving licence standard for distance central visual acuity logMAR 0.3 (6/12 Snellen) (table 1). In 14 patients the visual acuity was better than or equal to logMAR 0 (6/6 Snellen). Two patients were legally blind in the better eye (logMAR 1, 6/60 Snellen). Overall, 79% subjects (n=34) maintained visual stability. This included 81% (n=32) of those with vision of logMAR 0.3 or better at baseline and 86% (n=12) of those with baseline visual acuity of logMAR 0.
An improvement in vision (a gain in 15 ETDRS letters or more) occurred in three (7%) patients (0.01/PPY) at a mean of 0.9 years from starting TNFi. The visual angle doubled (a loss of 15 ETDRS letters or more) in nine patients (0.05 PPY) at a mean time of 1.8 years from index, although in one patient vision later recovered (online supplementary table 3).
New visual loss was attributed mainly to cataract (n=5), vitritis plus epiretinal membrane (n=2) and chronic cystoid macular oedema (n=2). In seven patients who lost vision during treatment, there was pre-existing visual loss due to structural complications, either due to retinal vasculitis involving the macula (n=5) or chronic cystoid macular oedema (n=2) (online supplementary table 3). Of those who lost vision, six patients had posterior or panuveitis, two had intermediate uveitis and one had anterior uveitis. Over 50% of the patients who lost vision on treatment had Behcet’s disease (n=5). Five out of nine were smokers or ex-smokers, three had never smoked, and in another the smoking status was unknown.
Corticosteroid-sparing effect of TNFi
Prior to starting anti-TNFα therapy, 21 of 43 patients (49%) were on >10 mg prednisolone per day, 32 of 43 patients (74%) were on >7.5 mg per day Online supplementary table 2), and 25 of 32 patients (78%) reduced to less than 7.5 mg at a rate of 0.3 PPY by 1.9 years (IQR: 1.7). Thirty-nine per cent (n=15) of patients were able to withdraw steroids completely at a rate of 0.09 PPY by 2 years (IQR 1.7). (For changes in concomitant immunomodulatory therapies, see (online supplementary table 2).
Adverse events
Twenty-three (54%) patients had at least one AE (0.35 PPY) (table 2). There were more side effects on infliximab (n=28) compared with adalimumab (n=15) but with a similar rate (0.38 PPY vs 0.34 PPY). These included hypersensitivity reactions (n=9) and headache (n=8). One patient had an anaphylactic response to infliximab, seven experienced an infection (six infliximab, one adalimumab), and one developed miliary TB on infliximab (see the Discussion section). One patient was diagnosed with non-Hodgkin’s lymphoma (see the Discussion section). There were no cardiovascular events, demyelinating episodes and no deaths.
Table 2.
Number of patients experiencing side effects during TNFi treatment
| Adalimumab | Infliximab | Total | |
| Infusion-related reaction | |||
| Mild | 0 (0) | 6 (18) | 6 (14) |
| Severe | 0 (0) | 3 (9) | 3 (7) |
| Anaphylaxis | |||
| Anaphylaxis | 0 (0) | 1 (3) | 1 (2) |
| Infection | |||
| Respiratory | 1 (11) | 3 (9) | 4 (9) |
| Abscesses | 0 (0) | 1 (3) | 1 (2) |
| Miliary tuberculosis | 0 (0) | 1 (3) | 1 (2) |
| Cellulitis with septicaemia | 0 (0) | 1 (3) | 1 (2) |
| Neoplasia | |||
| Non-Hodgkin’s lymphoma | 1 (11) | 0 (0) | 1 (2) |
| Cutaneous | |||
| Injection site cutaneous | 1 (11) | 0 (0) | 1 (2) |
| Psoriasis | 0 (0) | 1 (3) | 1 (2) |
| Alopecia | 1 (11) | 0 (0) | 1 (2) |
| Other rash | 0 (0) | 4 (12) | 4 (9) |
| Other | |||
| Lupus | 0 (0) | 2 (6) | 2 (5) |
| Myalgia | 2 (22) | 0 (0) | 2 (5) |
| Fatigue | 1 (11) | 3 (9) | 4 (9) |
| Headache | 2 (22) | 0 (0) | 2 (5) |
| Arthralgia | 2 (22) | 1 (3) | 3 (7) |
| Back pain | 0 (0) | 1 (3) | 1 (2) |
| Nausea | 2 (22) | 0 (0) | 2 (5) |
| Haematuria | 1 (11) | 0 (0) | 1 (2) |
| Night sweats | 1 (11) | 0 (0) | 1 (2) |
| Gout | 1 (11) | 0 (0) | 1 (2) |
| Mood disturbance | 1 (11) | 0 (0) | 1 (2) |
| Total | 17 | 28 | 45 |
Percentage proportions are shown in brackets.
TNFi, tumour necrosis factor-alpha inhibitor.
Stopping TNFi and switching biologic therapies
Twenty-three per cent (10 patients) changed biologic agent (0.04 PPY, median time from index of 1.7 years). Eight patients were switched from infliximab to adalimumab (0.04 PPY) for convenience of administration (n=4, 0.02 PPY), primary lack of efficacy (n=2, 0.01 PPY) or an AE related to infliximab use (n=2, 0.01 PPY); all were followed by remission. No patients were switched from adalimumab to infliximab.
TNFi was changed to a different class of biologic therapy in two patients (infliximab to tocilizumab; one switched from adalimumab to interferonα-2α after failing two TNFi). Ten patients (26%) stopped treatment (0.05 PPY), including those who stopped due to poor efficacy (2 infliximab [0.02 PPY]) or AEs (0.02 PPY) (2 infliximab [0.04 PPY], 2 adalimumab [0.01 PPY]).
Quality of life
There was a non-statistically significant improvement in the VCM1 score, SF-36 Physical Component Score (PCS) and SF-36 Mental Component Score (MCS) summary scores following the start of TNFi by a median of 1.7, 10 and 16 points, respectively, in year 1 (figure 3). Visual acuity alone was independently predictive of a VCM1 score above 2.0. (p=4×10−5). The VCM1 and SF-36 scores were maintained throughout treatment. Better visual acuity (p=2×10−8) and longer duration of treatment (p=0.03) contributed to general QoL scores.
Figure 3.
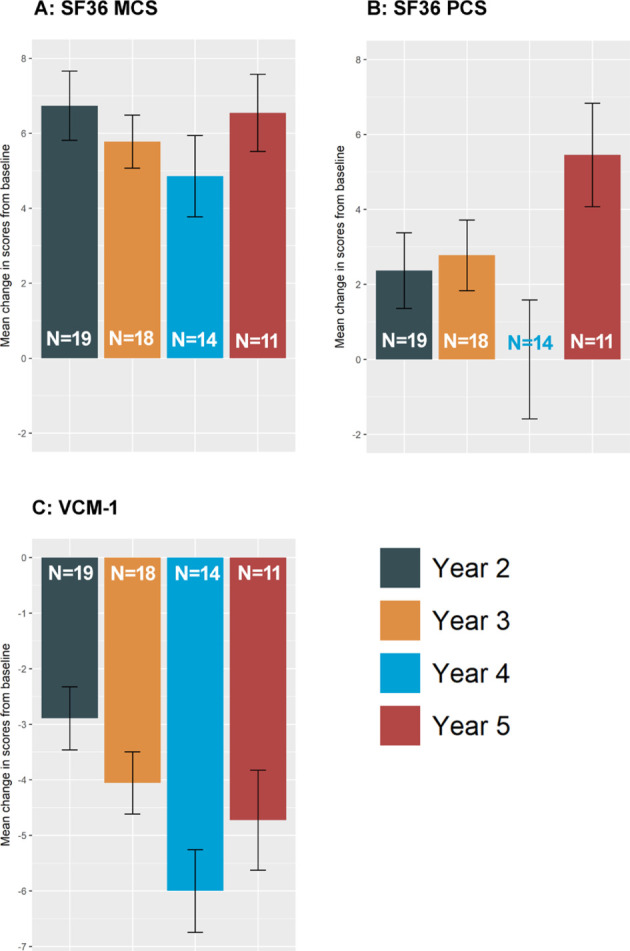
General quality of life and visual quality of life Mean change in General QoL (SF36)* and Visual QoL* (VCM1) scores from the baseline visit to each year of treatment on either adalimumab or infliximab. (A) SF36 mental component, (B) SF36 physical component and (C) VCM1 Visual QoL. (N indicates the number of participants for each year of treatment. Error bars represent the standard error. For SF36, a positive change indicates improvement of QoL. For VCM, a negative change indicates improvement of visual QoL. *The SF36 summarises 8 dimensions of general health into a Physical Component Score (PCS) and a Mental Component score (MCS), ranging from 0 to 100 (best score).12 The VCM1 is a 10 point visual QoL which captures patient–reported measures of visual health on emotional, physical, social and psychological aspects of visual quality of life.8 13 14 23
Discussion
In a real-world prospective analysis, we found that regular, scheduled dosing with TNFi was successful in inducing sustained remission in 91% of adult patients, and a sustained corticosteroid-sparing effect was observed in three-quarters of the patients. There were no new safety signals and QoL was maintained during treatment. At the census date, 77% had quiescent inflammation. In eight patients who had active inflammation at census, cystoid macular oedema (CMO) and vitritis were the main features. This is comparable with 65% of patients who were quiescent during the VISUAL III trial after 78 weeks’ follow-up.7
A key goal of treatment of ocular inflammation is maintenance of remission after achieving disease control. We observed that 51% of patients experience at least one relapse of inflammation. An initial relapse occurring 2.7 years from commencing a biologic was followed by a second nearly 4 years later in 12% of patients. Supporting the results of the VISUAL II trial, our data imply that a single relapse, adequately treated, may be followed by a long period of remission and does not predict treatment cessation.2
Sustained remission as an endpoint is a surrogate of long-term disease control. A previous report using a sustained remission endpoint demonstrated 60% of patients on TNFi achieved remission after 6 months of treatment.15 In our cohort, the median time to a sustained remission was 1.2 years. The difference may be due to overestimation of time to remission in our data due to missed study clinic appointments and the fact that six patients had started TNFi in excess of a year before the prospective arm of the study, where no retrospective data informed index to remission time.
Switching biologics is a means of restoring disease control. In a study of juvenile idiopathic arthritis, switching from infliximab to adalimumab resulted in disease remission in 75% of patients.16 In Behcet’s disease, switching TNFi led to drug-induced remission of uveitis in 11 out of 13 patients.17 Here we show that for 10 patients, changing TNFi does not negatively impact clinical outcomes. However, patient preference directed the switch in 4 out of the 10 patients, and the sample size is too small to draw firm conclusions.
During treatment 79% (n=34) experienced stability or improvement of vision, while 19% (8 patients) lost vision. Over half of those with loss of vision also had a history of smoking, which has been linked to visual loss in retinal vasculitis.18 A multicentre study of adalimumab in chronic uveitis reported a gain in vision in 21% of patients and a loss of vision in 3.7% of patients over a 6-month period of follow-up. One may anticipate that the refractory, advanced nature of disease in this cohort and the presence of structural complications are highly likely to be factors in the failure to arrest further visual loss.
Adverse events
Twenty-three patients (54%; 0.35 PPY) experienced at least one side effect and SAEs occurring at a rate of 0.02 PPY. A similar rate of SAEs (0.02 PPY) but a higher overall AE rate (4.2 PPY) was noted in VISUAL III.7 In a French study of long-term outcomes with TNFi, 28% of patients experienced AEs and the frequency of neoplastic events (2.5%) was similar to ours (2%).19 In a 2-year follow-up study of a prospective, open-label trial of infliximab, 3 out of 31 (10%) participants reported malignancies, while 29% experienced AEs overall.20 A review of several study designs showed that discontinuation of TNFi due to AEs was 0.02 PPY for adalimumab and 0.06 PPY for infliximab.21
These AE data demonstrate continued need for vigilance. In our cohort, two patients required hospitalisation for infections and temporary cessation of therapy. A case of miliary TB despite previously negative tuberculin test (Purified Protein Derivative (PPD)) and normal chest X-ray highlights the risk of acquiring TB while on TNFi. There was one case of a non-fatal non-Hodgkin’s lymphoma in a patient presenting with a new abdominal mass 8 months after starting adalimumab for idiopathic panuveitis in whom a causal relationship with adalimumab is possible.
Limitations
Study limitations include the use of retrospective baseline data for six patients. Although there may be disease-specific differences in response to TNFi, these differences are likely to be small in in such a heterogeneous cohort. Our data suggest that patients are more likely to achieve a sustained remission with infliximab but experience a similar rate of relapse to patients on adalimumab. A report of 164 patients showed no differential response to either TNFi agent19 and similar results were observed in children.22
Conclusion
A contemporary approach to uveitis treatment includes biologic therapy. This prospective report supports long-term TNFi associated with no attrition of QoL despite the requirement for several years of treatment. Side effects were frequent, and although SAEs are infrequent they remain an appreciable risk. Patients may require inclass switching or change in biologic due to AEs or loss of efficacy. This emphasises that, despite successful TNFi, wider treatment options are necessary, as well as further assessment of which uveitic entities respond best, in order to optimise outcomes.
Acknowledgments
We gratefully recognise the contribution to this study of the clinical fellows and registrars participating in the Regional Ocular Inflammatory Service at Bristol Eye Hospital, Bristol, UK between 2006 and 2014. We are grateful to Professor Kate Tilling (University of Bristol) for statistical advice.
Footnotes
Contributors: CDA: Statistical analysis and assistance in preparation of the manuscript. ED: assisted in conducting the study, data collection and contributed to analysis. ADD: chief investigator of the study, contributed to the design of the study and edited the manuscript. AEH: coordinated the study. KM: quality of life data collation and assistance in preparation of the manuscript. RL: contributed to the design, collection of data and review of the manuscript. SMS: designed and conducted the study, was the PI of the study, interpreted the data and prepared the manuscript.
Funding: This work was funded by Above & Beyond Charity, Bristol, UK.
Competing interests: SMS has participated in advisory boards for AbbVie, outside the submitted work. ADD has been an external advisor for AbbVie, Novartis and Roche, outside the submitted work. ADD and SMS have participated in educational initiatives on behalf of AbbVie. SMS and ADD have received honoraria from AbbVie. All other authors have no competing interests to report.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: To our knowledge, there are no unpublished data from this data set which may be made available to any other party.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
The Ocular Inflammation Biologics Registry received a favourable Research Ethics Committee opinion in the UK (07/Q2006/3).
References
- 1.Jaffe GJ, Dick AD, Brézin AP, et al. Adalimumab in patients with active noninfectious uveitis. N Engl J Med 2016;375:932–43. 10.1056/NEJMoa1509852 [DOI] [PubMed] [Google Scholar]
- 2.Nguyen QD, Merrill PT, Jaffe GJ, et al. Adalimumab for prevention of uveitic flare in patients with inactive non-infectious uveitis controlled by corticosteroids (visual II): a multicentre, double-masked, randomised, placebo-controlled phase 3 trial. Lancet 2016;388:1183–92. 10.1016/S0140-6736(16)31339-3 [DOI] [PubMed] [Google Scholar]
- 3.Plskova J, Greiner K, Forrester JV. Interferon-alpha as an effective treatment for noninfectious posterior uveitis and panuveitis. Am J Ophthalmol 2007;144:55–61. 10.1016/j.ajo.2007.03.050 [DOI] [PubMed] [Google Scholar]
- 4.Deuter CME, Zierhut M, Möhle A, et al. Long-term remission after cessation of interferon-α treatment in patients with severe uveitis due to Behçet's disease. Arthritis Rheum 2010;62:2796–805. 10.1002/art.27581 [DOI] [PubMed] [Google Scholar]
- 5.Sakai T, Watanabe H, Kuroyanagi K, et al. Health- and vision-related quality of life in patients receiving infliximab therapy for Behcet uveitis. Br J Ophthalmol 2013;97:338–42. 10.1136/bjophthalmol-2012-302515 [DOI] [PubMed] [Google Scholar]
- 6.Suhler EB, Smith JR, Wertheim MS, et al. A prospective trial of infliximab therapy for refractory uveitis: preliminary safety and efficacy outcomes. Arch Ophthalmol 2005;123:903–12. 10.1001/archopht.123.7.903 [DOI] [PubMed] [Google Scholar]
- 7.Suhler EB, Adán A, Brézin AP, et al. Safety and efficacy of adalimumab in patients with noninfectious uveitis in an ongoing open-label study: visual III. Ophthalmology 2018;125:1075–87. 10.1016/j.ophtha.2017.12.039 [DOI] [PubMed] [Google Scholar]
- 8.Gardiner AM, Armstrong RA, Dunne MCM, et al. Correlation between visual function and visual ability in patients with uveitis. Br J Ophthalmol 2002;86:993–6. 10.1136/bjo.86.9.993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sheppard J, Joshi A, Betts KA, et al. Effect of adalimumab on visual functioning in patients with noninfectious intermediate uveitis, posterior uveitis, and panuveitis in the VISUAL-1 and VISUAL-2 trials. JAMA Ophthalmol 2017;135:511–8. 10.1001/jamaophthalmol.2017.0603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ragam A, Kolomeyer AM, Fang C, et al. Treatment of chronic, noninfectious, nonnecrotizing scleritis with tumor necrosis factor alpha inhibitors. Ocul Immunol Inflamm 2014;22:469–77. 10.3109/09273948.2013.863944 [DOI] [PubMed] [Google Scholar]
- 11.Jabs DA, Nussenblatt RB, Rosenbaum JT. Standardization of uveitis Nomenclature (SUN) Working Group. standardization of uveitis Nomenclature for reporting clinical data. Results of the First International Workshop. AJOPHT 2005;140:509–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brazier JE, Harper R, Jones NM, et al. Validating the SF-36 health survey questionnaire: new outcome measure for primary care. BMJ 1992;305:160–4. 10.1136/bmj.305.6846.160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frost NA, Sparrow JM, Durant JS, et al. Development of a questionnaire for measurement of vision-related quality of life. Ophthalmic Epidemiol 1998;5:185–210. 10.1076/opep.5.4.185.4191 [DOI] [PubMed] [Google Scholar]
- 14.Murphy CC, Greiner K, Plskova J, et al. Validity of using vision-related quality of life as a treatment end point in intermediate and posterior uveitis. Br J Ophthalmol 2007;91:154–6. 10.1136/bjo.2006.105528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martel JN, Esterberg E, Nagpal A, et al. Infliximab and adalimumab for uveitis. Ocul Immunol Inflamm 2012;20:18–26. 10.3109/09273948.2011.633205 [DOI] [PubMed] [Google Scholar]
- 16.Simonini G, Katie D, Cimaz R, et al. Does switching anti-TNFα biologic agents represent an effective option in childhood chronic uveitis: the evidence from a systematic review and meta-analysis approach. Semin Arthritis Rheum 2014;44:39–46. 10.1016/j.semarthrit.2014.03.001 [DOI] [PubMed] [Google Scholar]
- 17.Calvo-Río V, Blanco R, Beltran E, et al. SAT0149 biologic therapy: switching and dose modification in refractory uveitis of behcet’s syndrome. Multicenter Study of 108 Patients 2014;72. [Google Scholar]
- 18.Ali A, Ku JH, Suhler EB, et al. The course of retinal vasculitis. Br J Ophthalmol 2014;98:785–9. 10.1136/bjophthalmol-2013-303443 [DOI] [PubMed] [Google Scholar]
- 19.Vallet H, Seve P, Biard L, et al. Infliximab versus adalimumab in the treatment of refractory inflammatory uveitis: a multicenter study from the French uveitis network. Arthritis Rheumatol 2016;68:1522–30. 10.1002/art.39667 [DOI] [PubMed] [Google Scholar]
- 20.Suhler EB, Smith JR, Giles TR, et al. Infliximab therapy for refractory uveitis: 2-year results of a prospective trial. Arch Ophthalmol 2009;127:819–22. 10.1001/archophthalmol.2009.141 [DOI] [PubMed] [Google Scholar]
- 21.Cordero-Coma M, Yilmaz T, Onal S. Systematic review of anti-tumor necrosis factor-alpha therapy for treatment of immune-mediated uveitis. Ocul Immunol Inflamm 2013;21:19–27. 10.3109/09273948.2012.723107 [DOI] [PubMed] [Google Scholar]
- 22.Simonini G, Taddio A, Cattalini M, et al. Prevention of flare recurrences in childhood-refractory chronic uveitis: an open-label comparative study of adalimumab versus infliximab. Arthritis Care Res 2011;63:612–8. 10.1002/acr.20404 [DOI] [PubMed] [Google Scholar]
- 23.Frost NA, Sparrow JM, Hopper CD, et al. Reliability of the VCM1 questionnaire when administered by post and by telephone. Ophthalmic Epidemiol 2001;8:1–11. 10.1076/opep.8.1.1.1539 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bjophthalmol-2018-312767supp001.pdf (90.4KB, pdf)