Abstract
Aims
L-type amino acid transporter 1 (LAT1) is a major Na+-independent neutral amino acid transporter, forming a complex with CD98hc. The aim of this study is to investigate the significance of LAT1 and CD98hc in invasive breast cancer.
Methods
LAT1 and CD98hc expression was immunohistochemically assessed in 280 invasive breast cancers and analysed for association with clinicopathological features.
Results
High levels of LAT1 and CD98hc were observed in triple-negative breast cancers (TNBCs) possessing negative immunoreactivity with oestrogen receptor, progesterone receptor and human epidermal growth factor receptor 2, compared with non-TNBCs (NTNBCs), and were associated with lymph-node metastasis and higher nuclear grade. The high-LAT1-expression group showed a poor prognosis in NTNBC and TNBC, however, high-CD98hc-expression group showed a poor prognosis only in NTNBC. LAT1 and CD98hc expression could be the prognostic factors in univariate analyses, but not in multivariate analyses. Further, we found that invasive tumour components showed higher LAT1 and CD98hc expression than non-invasive tumour components.
Conclusions
LAT1 and CD98hc may possess prognostic values in invasive breast cancer. LAT1 may be linked with cancer cell activities and disease progression in breast cancer.
Keywords: breast cancer, breast pathology, histopathology
Introduction
Breast cancer is a second leading cause of death for women in the USA, and the incidence of breast cancer is gradually increasing.1 The most common type of breast cancer is ductal carcinoma, which is classified into four categories according to oestrogen receptor (ER), progesterone receptor (PgR) and human epidermal growth factor receptor 2 (HER2) expression. The first category, luminal A type, expresses ER and/or PgR without HER2 expression; the second category, luminal B type, expresses ER and/or PgR and HER2; the third category, HER2 type, expresses HER2 without hormone receptor expression; and the final category, triple-negative type, expresses none of these markers.2 ER-positive tumours are considered an indication for hormone therapy, whereas HER2-positive tumours are considered for the HER2-targeted agent trastuzumab.3 Triple-negative breast cancer (TNBC) accounts for 10%–20% of breast cancers and is characterised by clinically aggressive behaviour and limited therapeutic strategies compared with other types of breast cancers because of an ineffectiveness of HER2-targeted therapy and hormone therapy.4 5 Thus, TNBC has a poor prognosis.5 6
Amino acid transporters are required for cell growth and proliferation.7 8 L-type amino acid transporter 1 (LAT1/SLC7A5) is the first isoform of the system L amino acid transporter, which functions as an essential cellular component to provide large neutral amino acids through the cytoplasmic membrane.9 LAT1 forms a heterodimeric complex with the heavy chain of the 4F2 antigen (4F2hc or CD98hc). LAT1 is expressed in several organs including renal distal tubules, epithelia of the oesophagus and intestine, and endothelia of cerebral vessels in adults, as well as in the thymic epithelia, hepatic cells and haematopoietic cells in fetuses, whereas CD98hc is expressed ubiquitously in most organs in adults.10 11 In the last decade, upregulation of LAT1 has been reported in several solid tumours, such as brain tumour, gastric cancer, pancreas cancer, prostate cancer, lung cancer, cholangiocarcinoma, colorectal cancer and pulmonary pleomorphic carcinoma.12–19 Similar results for CD98hc have also been reported in non-small cell lung cancer, biliary tract cancer, hypopharyngeal squamous cell carcinoma and gastric cancer.19–23 In breast cancer, Furuya et al reported that TNBC showed positive LAT1 and CD98hc expression at a higher frequency than non-TNBC (NTNBC), and CD98hc expression, but not LAT1 expression, was associated with prognosis in TNBC.24
In this study, we investigated LAT1 and CD98hc expression in a large population of invasive ductal breast cancer and analysed their clinical significance. Our results indicate the prognostic values of LAT1 and CD98hc in invasive ductal breast cancer.
Materials and methods
Patients
We sought patients with invasive ductal carcinoma of the breast that had been surgically resected at Kitasato University Hospital and Toho University Medical Center Omori Hospital between 2006 and 2010. All 78 identified TNBC cases and 202 randomly selected NTNBC cases were enrolled in this study. Patients who received preoperative chemotherapy and/or irradiation were excluded. Information about the study and the option to opt-out at any time were posted on the bulletin board of each hospital and announced to all prospective participants. We used clinical data and pathological specimens from patients who had not opted-out. The day of surgery was determined as the starting day for postoperative disease free survival (DFS) and overall survival (OS) periods. The follow-up duration ranged from 1 to 90 months.
Immunohistochemical staining
Surgically resected specimens were fixed with 10% formalin and embedded in paraffin. The paraffin blocks were sliced into 4 μm-thick sections. The primary antibodies used are listed in table 1. Immunohistochemical staining for ER, PgR, Ki-67, LAT1 and CD98hc was performed using an Envision+ kit (DAKO, Glostrup, Denmark). De-paraffinised and re-hydrated sections were treated with 0.3% H2O2 in methanol for 30 min to block endogenous peroxidase activity. Antigen retrieval for ER and PgR was performed with a hot water bath, and for Ki-67 and LAT1 with a microwave oven. Then, the sections were incubated with the primary antibodies overnight at 4°C. After washing with phosphate-buffered salin (PBS), the sections were processed with the secondary antibody. I-VIEW DAB Universal Kit (Roche Diagnostic, Japan) were used for ER and PgR. LAT1, CD98hc and Ki-67 were used Histofine Simple Stain Max-PO MULTI (Nichirei Bioscience, Japan). The peroxidase reaction was performed with 3-3′-diaminobenzidine. Nuclear counter staining were performed with haematoxylin. HER2 was stained using the HercepTest (DAKO) according to the manufacturer’s manual.
Table 1.
Antibodies used in this study
| Antigen | Antibody or kit | Dilution | Antigen retrieval methods | Supplier |
| ER | Monoclonal, 1D5 | Prediluted | Water bath, 99°C, 40 min | Nichirei (Tokyo, Japan) |
| PgR | mouse monoclonal, PgR636 | ×40 | Water bath, 99°C, 40 min | Thermo Fisher Scientific (Waltham, Massachusetts, USA) |
| HER2 | HercepTest | Kit | Water bath, 99°C, 20 min | DAKO (Glostrup, Denmark) |
| Ki-67 | Monoclonal, MIB-1 | ×100 | Microwave treatment for 15 min | DAKO (Glostrup, Denmark) |
| CD98hc | Polyclonal, H300 | ×200 | not done | Santa Cruz biotechnology (Santa Cruz, California, USA) |
| LAT1 | Monoclonal, 4A2 | Prediluted | Microwave treatment for 15 min | J Pharma (Yokohama, Japan) |
ER, estrogen receptor; HER2, human epidermal growth factor receptor-2; LAT1, L-type amino acid transporter 1; PgR, progesterone receptor.
Immunohistochemical staining results were judged as follows. For ER and PgR, cases were considered as positive when over 10% of cancer cells showed positive immunoreactivity. For HER2, cases were considered as positive when over 30% of cancer cells showed positive immunoreactivity, and cases were considered as negative when less than 10% of cancer cells showed positive immunoreactivity. In cases where between 10% and 30% of cancer cells were observed as positive, HER2 gene amplification was assessed using the FISH (Fluorescence In Situ Hybridization) method. In this study, FISH was performed in two cases, both of which did not show HER2 gene amplification. If these three markers were all negative, the cases were judged as TNBC. Evaluation of immunoreactivity for LAT1 and CD98hc was performed according to Sinicrope’s method with minor modifications.13–15 25 Based on the immunointensity of the carcinoma cell membrane, four categories of intensity were defined: 0, no staining; 1, weakly or patchily positive; 2, moderate staining; intensity 3, intense staining. The stained area was also evaluated as a percentage of the whole carcinoma area, and classified as follows: 0, none; 1, 1%–10%; 2, 11%–30%; 3, >30%. Immunoreactive scores were calculated by multiplication of the values for two parameters, intensity and area. The Ki-67 labelling index (LI) was assessed by counting at least 1000 cancer cells and indicated by the percentage value of positive cancer cells per total cells counted.
Statistical analyses
Statistical analyses were performed using StatView software (Abacus concepts, Berkeley, California, USA) and SPSS software V.22.0.0.0 (IBM SPSS). Spearman’s rank correlation coefficient and Wilcoxon-Mann-Whitney and Kruskal-Wallis tests were used to analyse associations and differences in locations for continuous variables. Categorical variables were analysed using χ2 tests.
Differences in DFS and OS periods were tested with the log-rank test. Univariable and multivariable Cox proportional hazard regression models were fitted to identify prognostic factors. All significance tests were carried out at the 0.05 level.
Results
Patients’ characteristics
Patients’ characteristics are shown in table 2. A total 280 patients with invasive ductal carcinoma of the breast were enrolled, with a mean age of 59 (range 32–92). Seventy-eight lesions were TNBC (28%) and 202 were NTNBC (72%) including 151 luminal A type (54%), 17 luminal B type (6%) and 34 HER2 type (12%). They were composed of 140 tumours with nuclear grade (NG) 1 or 2 and 140 with NG3, 91 tumours with lymph node metastasis and 189 without lymph node metastasis, and 94 tumours with pathological stage I, 174 with stage II and 12 with stage III. Between TNBC and NTNBC cases, statistical differences were observed for intraductal component, NG, Ki-67 LI and pathological stage (table 2).
Table 2.
Clinicopathological characteristics
| Patients | All (280) | TNBC (78) | NTNBC (202) | P value | |
| Age | Mean | 59 | 60 | 58 | 0.0585 |
| Range | 32–92 | 32–82 | 33–92 | ||
| Intraductal component | Present | 171 | 30 | 141 | 0.0004 |
| Absent | 109 | 48 | 61 | ||
| Nuclear grade | 1 or 2 | 140 | 12 | 128 | 0.0001 |
| 3 | 140 | 66 | 74 | ||
| Ki-67 LI (%) | Mean | 26.9 | 41.5 | 21.3 | 0.0001 |
| Range | 0–90.3 | 5.7–88.1 | 0–90.3 | ||
| pT | 1 | 134 | 26 | 108 | 0.0175 |
| 2 | 129 | 47 | 82 | ||
| 3 | 17 | 5 | 12 | ||
| 4 | 0 | 0 | 0 | ||
| lymph node metastasis | Positive | 91 | 30 | 61 | 0.1857 |
| Negative | 189 | 48 | 141 | ||
| pStage | I | 94 | 15 | 79 | 0.0049 |
| II | 174 | 59 | 115 | ||
| III | 12 | 4 | 8 | ||
| IV | 0 | 0 | 0 |
NTNBC, non-triple-negative breast cancer; TNBC, triple-negative breast cancer.
LAT1 expression in breast cancer
LAT1 immunoreactivity was detected in the cytoplasmic membrane of cancer cells, whereas it was not detected in non-neoplastic ductal epithelia. Representative LAT1 expression is shown in figure 1A–E. LAT1 scores are summarised in table 3. Median of LAT1 intensities and scores were significantly higher in TNBC than in NTNBC (figure 2A, B, table 3). LAT1 scores were also significantly high in tumours with lymph node metastasis or with high NG (NG3) compared with those in tumours without lymph node metastasis or with low NG (NG1 and NG2), respectively (figure 2C, D). Extraductal invasive regions exhibited enhanced LAT1 expression compared with intraductal non-invasive regions (figure 3A, B). When limited to NTNBC, cases with lymph node metastasis or with high NG showed significantly higher LAT1 scores than those without lymph node metastasis or with low NG, respectively (figure 2E, F). In comparison, when limited to TNBC, no significant differences in LAT1 scores were observed between cases with lymph node metastasis and those without lymph node metastasis, and between cases with high NG and those with low NG (data not shown). Statistical analysis was using Wilcoxon-Mann-Whitney tests.
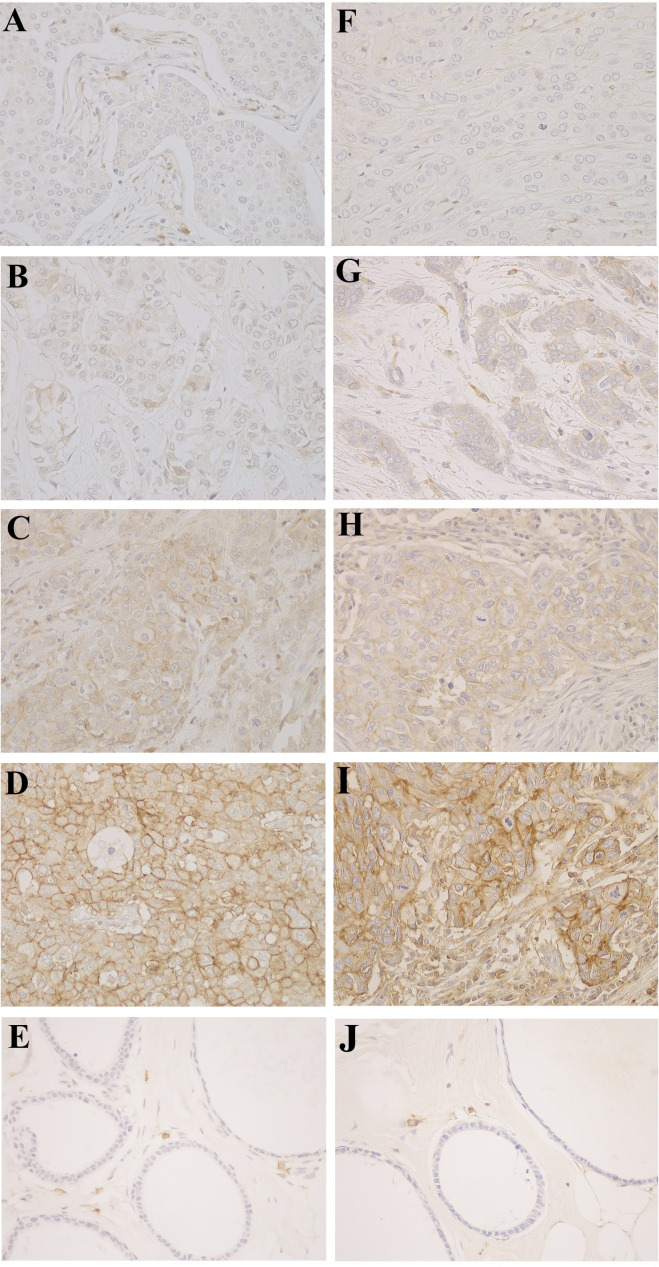
Figure 1.
Representative immunoreactivity of L-type amino acid transporter 1 (A–E) and CD98hc (F–J) in breast cancer tissues. Based on the immunointensity of the carcinoma cell membranes, four categories were defined. intensity 0, no immunoreactivity (A and F); intensity 1, weakly positive (B and G); intensity 2, moderately positive (C and H); intensity 3, strong expression (D and I). Normal epithelia with negative staining (E, J). Original magnifications ×400.
Table 3.
LAT1 and CD98hc scores in all cases and various category
| Subtype | LAT1 score | CD98hc score | |||||||||||||||
| N | 0 | 1 | 2 | 3 | 4 | 6 | 9 | Median (IQR) | 0 | 1 | 2 | 3 | 4 | 6 | 9 | Median (IQR) | |
| All cases | 280 | 54 | 21 | 20 | 52 | 5 | 47 | 81 | 3 (1–9) | 23 | 30 | 17 | 63 | 13 | 56 | 78 | 4 (2.8–9) |
| TNBC | 78 | 2 | 3 | 0 | 8 | 2 | 15 | 48 | 9 (6–9)* | 3 | 4 | 2 | 6 | 6 | 15 | 42 | 9 (4–9)* |
| NTNBC | 202 | 52 | 18 | 20 | 44 | 3 | 32 | 33 | 3 (0–6)* | 20 | 26 | 15 | 57 | 7 | 41 | 36 | 3 (2–6)* |
| Luminal A | 151 | 45 | 17 | 18 | 33 | 3 | 20 | 15 | 2 (0–3.5) | 17 | 24 | 12 | 50 | 7 | 25 | 16 | 3 (1–6) |
| Luminal B | 17 | 3 | 0 | 2 | 5 | 0 | 4 | 3 | 3 (2–6) | 1 | 1 | 2 | 3 | 0 | 4 | 6 | 6 (3–9) |
| HER2 | 34 | 4 | 1 | 0 | 6 | 0 | 8 | 15 | 6 (3–9) | 2 | 1 | 1 | 4 | 0 | 12 | 14 | 6 (6–9) |
*P value <0.0001.
HER2, human epidermal growth factor receptor-2; LAT1, L-type amino acid transporter1; NTNBC, non-triple-negative breast cancer; TNBC, triple-negative breast cancer.
Figure 2.
Association of LAT1 and CD98hc expression with clinicopathological parameters. (A–D) Analyses of LAT1 expression in all cases. (E and F) Analyses of LAT1 expression in NTNBC cases. (G–J) Analyses of CD98 expression in all cases. (K and L) Analyses of CD98hc expression in NTNBC cases. Number of cases in each group is indicated in parentheses. *P value <0.05. LAT1, L-type amino acid transporter 1; LN(+), with lymph node metastasis; LN(−), without lymph node metastasis; NG, nuclear grade; NTNBC, non-triple-negative breast cancer; TNBC, triple-negative breast cancer.
Figure 3.
L-type amino acid transporter 1 (LAT1) and CD98hc expression is upregulated in invasive lesions compared with non-invasive lesions. A representative area for both invasive and non-invasive lesions is shown. (A) H&E staining. (B) Immunohistochemical staining for LAT1. (C) Immunohistochemical staining for CD98hc. The right lower part indicates invasive lesions (I), whereas the left upper part indicates non-invasive lesions (N–I). Original magnifications ×100.
CD98hc expression in breast cancer
Similar to LAT1, CD98hc immunoreactivity was also detected in the cytoplasmic membrane of cancer cells, but not in normal ductal epithelia (figure 1F–J), and means of CD98hc intensities and scores were significantly higher in TNBC than in NTNBC (figure 2G, H, table 3). The mean CD98hc score was also significantly higher in cases with lymph node metastasis or with high NG (figure 2I, J). Invasive regions exhibited more enhanced CD98hc expression than non-invasive regions (figure 3A, C). High CD98hc score was significantly correlated with lymph node metastasis or with high NG in NTNBC cases (figure 2K, L), but not in TNBC cases (data not shown). Statistical analysis were using Wilcoxon-Mann-Whitney tests.
Correlation among LAT1 expression, CD98hc expression and Ki-67 LI
We investigated correlation among LAT1 expression, CD98hc expression and Ki-67 LI with the Spearman’s rank correlation coefficient test. A strong positive correlation was observed between LAT1 and CD98hc scores (ρ=0.729, p<0.0001) in all cases. Relatively weak but significant correlations were also observed between LAT1 score and Ki-67 LI (ρ=0.436, p<0.0001), and between CD98hc score and Ki-67 LI (ρ=0.411, p<0.0001).
Prognosis analysis
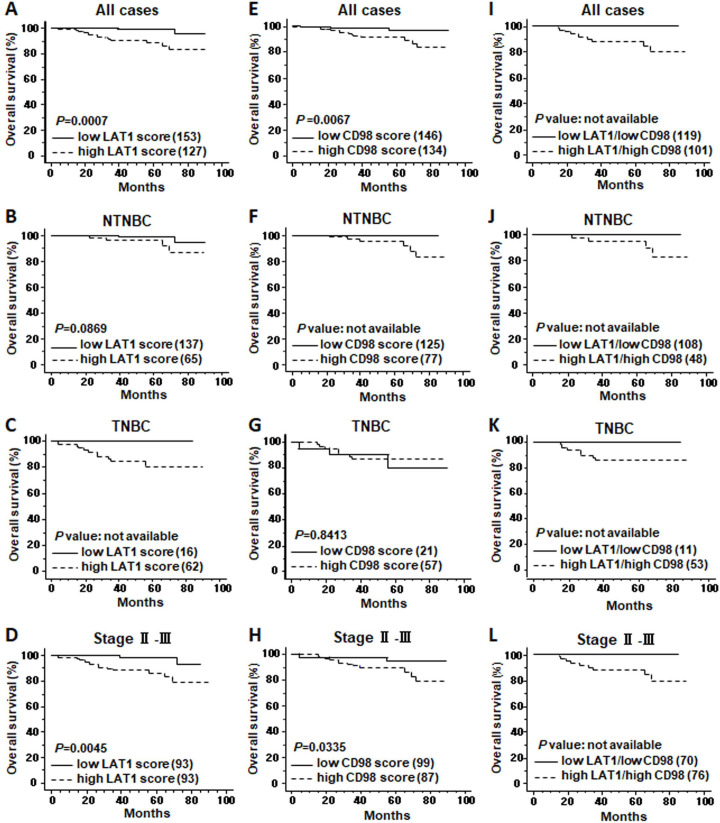
We tested OS and DFS in association with LAT1 expression with a log-rank test. Cases were divided into low-LAT1-expression and high-LAT1-expression groups according to the LAT1 score (0–4 as low, 6–9 as high). The high-LAT1-expression group showed significantly poor prognosis in both OS and DFS compared with the low-LAT1-expression group analysed in all cases (OS, p=0.0007, DFS, p=0.0004) (figure 4A and online supplementary figure S1A). When cases were limited to NTNBC, there was a significant difference in DFS, but not in OS, between low-LAT1-expression and high-LAT1-expression groups (OS, p=0.0869, DFS, p=0.0172) (figure 4B and online supplementary figure S1B). When limited to TNBC, no significant difference was observed in DFS between the two groups (p=0.3710) (online supplementary figure S1C), however, an apparent difference was observed in OS between the two groups, although the p value could not be calculated (figure 4C). In cases with advanced stage (stage II or III), high LAT1 expression was linked to a poor prognosis (OS, p=0.0045, DFS, p=0.0048) (figure 4D and online supplementary figure S1D).
Figure 4.
Overall survival curves in association with LAT1 and CD98hc expression. Cases were divided into high-expression and low-expression groups according to LAT1 and CD98hc scores (0–4 as low and 6–9 as high). Kaplan-Meier curves of the groups in all cases (A, E, I), in NTNBC cases (B, F, J), in TNBC (C, G, K) and incases with pathological stage II and III (D, H, L) are shown. LAT1, L-type amino acid transporter 1; NTNBC, non-triple-negative breast cancer; TNBC, triple-negative breast cancer.
jclinpath-2020-206457supp001.pdf (427.4KB, pdf)
Similar results were obtained in the analyses of prognostic significance of CD98hc. High CD98hc expression was linked to poor OS (p=0.0067) and DFS (p=0.0112) in all cases (figure 4E and online supplementary figure S1E), poor OS (p value was not available) and DFS (p=0.0056) in NTNBC cases (figure 4F and online supplementary figure S1F) and poor OS (p=0.0335) in cases with advanced stages (figure 4H), however, it was not linked to OS (p=0.8413) or DFS (p=0.3548) in TNBC (figure 4G and online supplementary figure S1G), or to DFS (p=0.0851) in cases with advanced stages (online supplementary figure S1H).
When combined positive and negative expression of LAT1 and CD98hc was analysed for OS and DFS, high-LAT1/high-CD98hc groups showed apparently poor prognosis compared with low-LAT1/low-CD98hc groups in all the patients categories except for DFS in TNTBC (p=0.8435) (figure 4I–L and online supplementary figure S1I–L).
The results indicate that both LAT1 and CD98hc possess prognostic values in invasive breast cancer, and suggest that their expression is likely more useful for a prognosis predictive factor in NTNBC than in TNBC.
Cox proportional hazard regression models
We confirmed proportionality of hazards in the Cox models before the analyses. Univariable Cox proportional hazard methods revealed that poor prognosis was significantly associated with TNBC (DFS, p=0.0013; OS, p=0.0029), high NG (DFS, p=0.0209; OS, p=0.0199), positive lymph-node metastasis (DFS, p=0.0014; OS, p=0.0047), high pathological stage (stage II and III; DFS, p=0.0157; OS, p=0.0461) and high Ki-67 LI (>20%, DFS, p=0.0026; OS, p=0.0263) (table 4). LAT1 score could be one of the prognostic factors in univariate analyses (score 0–4 vs 6–9; DFS, p=0.0011; OS, p=0.0046) as well as CD98hc score (score 0–4 vs 6–9; DFS, p=0.0146; OS, p=0.0143) (table 4). We analysed the multivariable models only those covariates which were significant in the univariable models.
Table 4.
Cox hazard analysis of cause-specific survival in all cases
| Variable | Univariate analysis | P value | Multivariate analysis | P value |
| HR (95% CI) | HR (95% CI) | |||
| (A) Disease free survival | ||||
| Age | 1.015 (0.985 to 1.046) | 0.3295 | ||
| Size | 1.445 (1.265 to 1.650) | <0.0001 | 1.422 (1.221 to 1.657) | <0.0001 |
| Nuclear grade (1–2 vs 3) | 0.413 (0.195 to 0.874) | 0.0209 | 1.255 (0.506 to 3.112) | 0.6233 |
| TNBC or NTNBC | 3.129 (1.560 to 6.276) | 0.0013 | 2.490 (1.068 to 5.806) | 0.0346 |
| LAT1 score (0–4 vs 6–9) | 3.798 (1.706 to 8.457) | 0.0011 | 0.989 (0.844 to 1.159) | 0.887 |
| CD98hc score (0–4 vs 6–9) | 2.538 (1.201 to 5.362) | 0.0146 | 1.153 (0.435 to 3.056) | 0.7743 |
| Ki-67 LI (≤20% vs >20%) | 3.641 (1.572 to 8.432) | 0.0026 | 2.274 (1.014 to 5.097) | 0.046 |
| lymph node metastasis | 3.170 (1.565 to 6.420) | 0.0014 | 2.708 (1.177 to 6.233) | 0.0192 |
| pStage (I vs II-III) | 3.640 (1.276 to 10.380) | 0.0157 | 0.810 (0.231 to 2.845) | 0.7424 |
| (B) Overall survival | ||||
| Age | 1.00 (0.959 to 1.043) | 0.997 | ||
| Size | 1.561 (1.302 to 1.870) | <0.0001 | 1.485 (1.206 to 1.830) | 0.0002 |
| Nuclear grade 1–2 vs 3 | 0.225 (0.64 to 0.790) | 0.0199 | 0.825 (0.102 to 3.535) | 0.7951 |
| TNBC or NTNBC | 4.655 (1.690 to 12.826) | 0.0029 | 3.150 (0.988 to 10.037) | 0.0523 |
| LAT1score 0–4 vs 6–9 | 8.502 (1.932 to 37.420) | 0.0046 | 2.339 (0.352 to 15.555) | 0.3792 |
| CD98hc score 0–4 vs 6–9 | 4.804 (1.368 to 16.869) | 0.0143 | 1.167 (0.238 to 5.728) | 0.8489 |
| Ki-67 LI (≤20% vs >20%) | 4.151 (1.182 to 14.575) | 0.0263 | 1.952 (0.442 to 8.626) | 0.3778 |
| lymph node metastasis | 4.594 (1.596 to 13.227) | 0.0047 | 3.015 (0.881 to 10.318) | 0.0788 |
| pStage (I vs II-III) | 7.845 (1.036 to 59.408) | 0.0461 | 1.202 (0.119 to 12.142) | 0.876 |
HR, Hazard ratio; LAT1, L-type amino acid transporter 1; LI, labelling index; NTNBC, non-triple-negative breast cancer; TNBC, triple-negative breast cancer.
However, in multivariate analyses, both LAT1 and CD98hc were revealed not to be independent prognostic factors (LAT1, DFS, p=0.887; OS, p=0.3792; CD98, DFS, p=0.7743; OS, p=0.8489) (table 4).
Discussion
Breast cancer is classified into four subtypes according to the expression of hormone receptors and HER2, and the classification is important to determine the therapeutic strategy. NTNBC, which is positive for at least one of the three markers, has an indication for hormone therapy or HER2-targeted therapy, whereas TNBC, which is negative for all of the three markers, has an indication for neither hormone therapy nor HER2-targeted therapy. Therefore, NTNBC is supposed to have a favourable prognosis compared with TNBC, however, not all cases show adequate therapeutic responses.
LAT1 is a tumour type-amino acid transporter that shows high levels of expression in several malignant tumours but a limited expression in normal tissues, and is reported to be an independent prognostic factor in some tumours.13–16 LAT1 requires a binding partner to form a heterodimer, CD98hc, for its sufficient transporting ability.9 In this study, we investigated the expression of LAT1 and CD98hc in a large population of patients with invasive breast cancer, focusing on the correlation between their expression and prognosis-related factors. We showed that TNBC exhibited higher LAT1 and CD98hc expression than NTNBC. Tumours with lymph node metastasis, high NG, or high Ki-67 LI showed enhanced LAT1 and CD98 expression compared with tumours without lymph node metastasis, with low NG, or with low Ki-67 LI, respectively. These observations are compatible with a previous publication.24 In contrast, we found that invasive tumour components showed higher LAT1 and CD98hc expression than non-invasive tumour components, whereas no obvious differences were detected between the invasive and non-invasive components in the previous study.24
It was reported that positive rates of LAT1 and CD98hc in metastatic sites were significantly high compared with primary sites in several human neoplasms, and that gastric carcinomas with lymph node metastasis showed higher LAT1 expression than those without lymph node metastasis.13 26 Therefore, LAT1 expression, as well as CD98hc expression, may be linked with cancer cell activities and disease progression, such as invasion and metastasis, in breast cancer. It is possible that invading carcinoma cells in the stroma have a higher demand for nutrition than non-invading cells, and the LAT1 complex might be required for its supply. To clarify this point, further in vitro studies should be necessary.
The previous report showed that high-level expression of CD98hc was correlated with poor prognosis in TNBC, but that of LAT1 was not.24 In this study, high-LAT1-expression or high-CD98hc-expression groups showed significantly poor prognosis in all cases and in NTNBC cases. In TNBC cases, there was no association between CD98hc expression and prognosis, whereas a positive association between high-level expression of LAT1 and poor OS was observed. In addition, in advanced cases, high CD98hc expression was weakly associated with poor OS, whereas high LAT1 expression showed a relatively strong association with poor DFS and OS. These results suggest that LAT1 might be more useful as a prognosis predictive factor than CD98hc in invasive breast cancer, especially in TNBC and advanced cases. One possible reason for the discrepancy between the two studies might be the methods used for evaluation of LAT1 and CD98hc immunoreactivity (see the Materials and methods section). In the previous study, 64% and 44% of TNBCs were classified as LAT1- or CD98hc-positive, respectively, whereas in this study, 79% and 73% of TNBCs were classified into high-LAT1-score and high-CD98hc-score groups, respectively. Another possible reason might be the number of participants in each study.
It has been reported that LAT1 and CD98hc expression are independent prognostic factors in some tumours, including gastric cancer, lung cancer, bile duct cancer, hypopharyngeal squamous cell carcinoma and breast cancer.13 16 17 22–24 27 28 However, LAT1 and CD98hc were not found to be independent prognostic factors in this study. CD98hc can bind with another transporter such as xCT or ASCT2 besides LAT1 and form a CD98 complex. It is possible that CD98 signal does not overlap with LAT1 signal well. In tongue cancer, it was reported that the expression of LAT1 is a significant independent factor for predicting poor prognosis and positive expression of ASCT2 yielded a significant relationship with worse prognosis, but not xCT.29
Up to now, expression of LAT1 and CD98hc has been correlated with cancer cell survival and proliferation in several malignant tumours, and the development of anticancer therapy targeting LAT1 has been studied. Inhibition of system L by 2-aminobicyclo-(2,2,1)-hepatane-2-carboxylic acid, a broad system L inhibitor, suppressed cell growth and promoted cell cycle arrest and apoptosis in several cancer cells.30 31 JPH203, an LAT1-selective compound, also induced apoptosis in human cancer cells.32 33 Further, there was a report JPH203 reduces growth of thyroid carcinoma in mouse model.34
However, the precise mechanism of the relationship between LAT1 and cell proliferation is still unknown. In lung cancer, it has been reported that LAT1 is related to hypoxic marker expression and mammalian target of rapamycin (mTOR) pathway, and LAT1 is thought to play important roles in multiple cellular activities including tumour cell proliferation.35 There might be a possibility of a similar relationship between the mTOR pathway and LAT1 expression in breast cancer.
In conclusion, in invasive ductal carcinoma of the breast, LAT1 and CD98hc are possible candidates for prognosis predictive factors. LAT1 offers a potential target for anticancer therapy in invasive breast cancer.
Take home messages.
L-type amino acid transporter 1 (LAT1) and CD98hc expression was analysed in invasive breast cancer.
High levels of both LAT1 and CD98hc expression were observed in triple-negative breast cancer.
Both LAT1 and CD98hc possess prognostic values in invasive breast cancer.
LAT1 may be linked with cancer cell activities and disease progression in breast cancer.
Acknowledgments
The authors are grateful to Ms Tsukiko Sato and Ms Fusako Nose for their expert technical assistance. The authors also thank Dr Hisashi Eguchi for statistical analysis advise, medical students Mr Thun Leewiboonsilp and Miss Gryn Kateryna for helping us to collect the data.
Footnotes
Handling editor: Cheok Soon Lee.
Contributors: MI: conceptualisation, investigation, data collection, data analysis writing; TM: conceptualisation, data collection, data analysis; NY: resource, investigation; TY: investigation; KH: technical assistance; HE: technical assistance; IO: investigation; NS: investigation; HO: investigation; MS: investigation; KS: investigation; YM: conceptualisation, writing-review and editing, supervision, funding acquisition.
Funding: This work was supported by the grant 'Practical use of a diagnostic kit for triple negative breast cancer' from the Special Economic Ward Project of City of Yokohama in 2014–2015. This work was also partly supported by research funding from J-Pharma.
Competing interests: HE is the Chief Executive Officer (CEO) and KH is an employee of J-Pharma, and both participated in this study with technical assistance.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
No data are available.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
Tissue samples were used with written informed consent of the patients. The study was approved by the Kitasato University Medical Ethics Organization (B05-34) and the Ethics Organization of Toho University School of Medicine (#25077).
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin 2017;67:7–30. 10.3322/caac.21387 [DOI] [PubMed] [Google Scholar]
- 2.Heaphy CM, Subhawong AP, Gross AL, et al. Shorter telomeres in luminal B, HER-2 and triple-negative breast cancer subtypes. Mod Pathol 2011;24:194–200. 10.1038/modpathol.2010.198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Honma N, Horii R, Ito Y, et al. Differences in clinical importance of Bcl-2 in breast cancer according to hormone receptors status or adjuvant endocrine therapy. BMC Cancer 2015;15:1–11. 10.1186/s12885-015-1686-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vona-Davis L, Rose DP, Hazard H, et al. Triple-negative breast cancer and obesity in a rural Appalachian population. Cancer Epidemiol Biomarkers Prev 2008;17:3319–24. 10.1158/1055-9965.EPI-08-0544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boyle P. Triple-negative breast cancer: epidemiological considerations and recommendations. Ann Oncol 2012;23 Suppl 6:vi7–12. 10.1093/annonc/mds187 [DOI] [PubMed] [Google Scholar]
- 6.Lund MJ, Trivers KF, Porter PL, et al. Race and triple negative threats to breast cancer survival: a population-based study in Atlanta, GA. Breast Cancer Res Treat 2009;113:357–70. 10.1007/s10549-008-9926-3 [DOI] [PubMed] [Google Scholar]
- 7.Christensen HN. Role of amino acid transport and countertransport in nutrition and metabolism. Physiol Rev 1990;70:43–77. 10.1152/physrev.1990.70.1.43 [DOI] [PubMed] [Google Scholar]
- 8.McGivan JD, Pastor-Anglada M. Regulatory and molecular aspects of mammalian amino acid transport. Biochem J 1994;299:321–34. 10.1042/bj2990321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kanai Y, Segawa H, Miyamoto Ki, et al. Expression cloning and characterization of a transporter for large neutral amino acids activated by the heavy chain of 4F2 antigen (CD98). J Biol Chem 1998;273:23629–32. 10.1074/jbc.273.37.23629 [DOI] [PubMed] [Google Scholar]
- 10.Nakada N, Mikami T, Hana K, et al. Unique and selective expression of L-amino acid transporter 1 in human tissue as well as being an aspect of oncofetal protein. Histol Histopathol 2014;29:217–27. 10.14670/HH-29.217 [DOI] [PubMed] [Google Scholar]
- 11.Yanagida O, Kanai Y, Chairoungdua A, et al. Human L-type amino acid transporter 1 (LAT1): characterization of function and expression in tumor cell lines. Biochim Biophys Acta 2001;1514:291–302. 10.1016/S0005-2736(01)00384-4 [DOI] [PubMed] [Google Scholar]
- 12.Haining Z, Kawai N, Miyake K, et al. Relation of LAT1/4F2hc expression with pathological grade, proliferation and angiogenesis in human gliomas. BMC Clin Pathol 2012;12:4. 10.1186/1472-6890-12-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ichinoe M, Mikami T, Yoshida T, et al. High expression of L-type amino-acid transporter 1 (LAT1) in gastric carcinomas: comparison with non-cancerous lesions. Pathol Int 2011;61:281–9. 10.1111/j.1440-1827.2011.02650.x [DOI] [PubMed] [Google Scholar]
- 14.Yanagisawa N, Ichinoe M, Mikami T, et al. High expression of L-type amino acid transporter 1 (LAT1) predicts poor prognosis in pancreatic ductal adenocarcinomas. J Clin Pathol 2012;65:1019–23. 10.1136/jclinpath-2012-200826 [DOI] [PubMed] [Google Scholar]
- 15.Sakata T, Ferdous G, Tsuruta T, et al. L-type amino-acid transporter 1 as a novel biomarker for high-grade malignancy in prostate cancer. Pathol Int 2009;59:7–18. 10.1111/j.1440-1827.2008.02319.x [DOI] [PubMed] [Google Scholar]
- 16.Imai H, Kaira K, Oriuchi N, et al. L-type amino acid transporter 1 expression is a prognostic marker in patients with surgically resected stage I non-small cell lung cancer. Histopathology 2009;54:804–13. 10.1111/j.1365-2559.2009.03300.x [DOI] [PubMed] [Google Scholar]
- 17.Yanagisawa N, Hana K, Nakada N, et al. High expression of L-type amino acid transporter 1 as a prognostic marker in bile duct adenocarcinomas. Cancer Med 2014;3:1246–55. 10.1002/cam4.272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hayase S, Kumamoto K, Saito K, et al. L-type amino acid transporter 1 expression is upregulated and associated with cellular proliferation in colorectal cancer. Oncol Lett 2017;14:7410–6. 10.3892/ol.2017.7148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaira K, Kawashima O, Endoh H, et al. Expression of amino acid transporter (LAT1 and 4F2hc) in pulmonary pleomorphic carcinoma. Hum Pathol 2019;84:142–9. 10.1016/j.humpath.2018.09.020 [DOI] [PubMed] [Google Scholar]
- 20.Kaira K, Oriuchi N, Imai H, et al. CD98 expression is associated with poor prognosis in resected non-small-cell lung cancer with lymph node metastases. Ann Surg Oncol 2009;16:3473–81. 10.1245/s10434-009-0685-0 [DOI] [PubMed] [Google Scholar]
- 21.Kaira K, Sunose Y, Oriuchi N, et al. CD98 is a promising prognostic biomarker in biliary tract cancer. Hepatobiliary Pancreat Dis Int 2014;13:654–7. 10.1016/S1499-3872(14)60278-2 [DOI] [PubMed] [Google Scholar]
- 22.Toyoda M, Kaira K, Shino M, et al. CD98 as a novel prognostic indicator for patients with stage III/IV hypopharyngeal squamous cell carcinoma. Head Neck 2015;37:1569–74. 10.1002/hed.23797 [DOI] [PubMed] [Google Scholar]
- 23.Satoh T, Kaira K, Takahashi K, et al. Prognostic significance of the expression of CD98 (4F2hc) in gastric cancer. Anticancer Res 2017;37:631–6. 10.21873/anticanres.11357 [DOI] [PubMed] [Google Scholar]
- 24.Furuya M, Horiguchi J, Nakajima H, et al. Correlation of L-type amino acid transporter 1 and CD98 expression with triple negative breast cancer prognosis. Cancer Sci 2012;103:382–9. 10.1111/j.1349-7006.2011.02151.x [DOI] [PubMed] [Google Scholar]
- 25.Sinicrope FA, Ruan SB, Cleary KR, et al. bcl-2 and p53 oncoprotein expression during colorectal tumorigenesis. Cancer Res 1995;55:237–41. [PubMed] [Google Scholar]
- 26.Kaira K, Oriuchi N, Imai H, et al. L-Type amino acid transporter 1 and CD98 expression in primary and metastatic sites of human neoplasms. Cancer Sci 2008;99:2380–6. 10.1111/j.1349-7006.2008.00969.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaira K, Oriuchi N, Imai H, et al. Prognostic significance of L-type amino acid transporter 1 (LAT1) and 4F2 heavy chain (CD98) expression in stage I pulmonary adenocarcinoma. Lung Cancer 2009;66:120–6. 10.1016/j.lungcan.2008.12.015 [DOI] [PubMed] [Google Scholar]
- 28.Kaira K, Oriuchi N, Imai H, et al. Prognostic significance of L-type amino acid transporter 1 (LAT1) and 4F2 heavy chain (CD98) expression in surgically resectable stage III non-small cell lung cancer. Exp Ther Med 2010;1:799–808. 10.3892/etm.2010.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Toyoda M, Kaira K, Ohshima Y, et al. Prognostic significance of amino-acid transporter expression (LAT1, ASCT2, and xCT) in surgically resected tongue cancer. Br J Cancer 2014;110:2506–13. 10.1038/bjc.2014.178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim CS, Cho S-H, Chun HS, et al. BCH, an inhibitor of system L amino acid transporters, induces apoptosis in cancer cells. Biol Pharm Bull 2008;31:1096–100. 10.1248/bpb.31.1096 [DOI] [PubMed] [Google Scholar]
- 31.Imai H, Kaira K, Oriuchi N, et al. Inhibition of L-type amino acid transporter 1 has antitumor activity in non-small cell lung cancer. Anticancer Res 2010;30:4819–28. [PubMed] [Google Scholar]
- 32.Oda K, Hosoda N, Endo H, et al. L-type amino acid transporter 1 inhibitors inhibit tumor cell growth. Cancer Sci 2010;101:173–9. 10.1111/j.1349-7006.2009.01386.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yun D-W, Lee SA, Park M-G, et al. JPH203, an L-type amino acid transporter 1-selective compound, induces apoptosis of YD-38 human oral cancer cells. J Pharmacol Sci 2014;124:208–17. 10.1254/jphs.13154FP [DOI] [PubMed] [Google Scholar]
- 34.Häfliger P, Graff J, Rubin M, et al. The LAT1 inhibitor JPH203 reduces growth of thyroid carcinoma in a fully immunocompetent mouse model. Exp Clin Cancer Res 2018;21:234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaira K, Oriuchi N, Takahashi T, et al. LAT1 expression is closely associated with hypoxic markers and mTOR in resected non-small cell lung cancer. Am J Transl Res 2011;3:468–78. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jclinpath-2020-206457supp001.pdf (427.4KB, pdf)
Data Availability Statement
No data are available.