Abstract
During aging, mitochondrial membrane potential, a key indicator for bioenergetics of cells, depolarizes in a wide range of species—from yeasts, plants to animals. In humans, the decline of mitochondrial activities can impact the high-energy-consuming organs, such as the brain and heart, and increase the risks of age-linked diseases. Intriguingly, a mild depolarization of mitochondria has lifespan-extending effects, suggesting an important role played by bioelectricity during aging. However, the underpinning biophysical mechanism is not very well understood due in part to the difficulties associated with a multiscale process. Budding yeast Saccharomyces cerevisiae could provide a model system to bridge this knowledge gap and provide insights into aging. In this perspective, we overview recent studies on the yeast mitochondrial membrane electrophysiology and aging and call for more electrochemical and biophysical studies on aging.
Keywords: aging, mitochondrial membrane potential, yeast, electrophysiology
Further to being a model system for complex fundamental biological processes in eukaryotic organisms, the budding yeast Saccharomyces cerevisiae has been established in the past few decades as a powerful model system for studying aging.1 Even though some characteristics of yeast aging are specific to this organism, many of the modulators for vertebrates lifespans, such as calorie restriction, the nutrient-sensing signaling pathway target of rapamycin (TOR) and sirtuins, are conserved from yeast to human.1,2 S. cerevisiae was the fast model system revealing the molecular mechanisms by which calorie restriction extends lifespan.3 Building on this finding, it is now evident that calorie restriction is the most ubiquitous approach for extending lifespan in a wide range of species including humans.4
S. cerevisiae is particularly useful because the process of aging is heterogeneous and multiscale, especially regarding time; that is, a long-time-scale process such as aging emerges from faster dynamic molecular processes such as genomic instability, cellular senescence, and mitochondrial dysfunction.5 Their relatively short lifespans, compared with the other model organisms, such as Mus musculus (mice), Drosophila melanogaster (fruit fly), and Caenorhabditis elegans (worm), enable bridging the differences in time scales and characterizing cell-to-cell heterogeneity.6 In a typical cultivation condition, S. cerevisiae has a replicative lifespan (RLS) of ∼24 buds, although it varies between strains. RLS, defined as the number of buds produced by a single mother cell, is a model of mitotically active cells.1 Another aspect of aging is the chronological lifespan (CLS), which is a model of postmitotic cells. CLS is defined as the survival time of cells in the stationary phase (nondividing cells); it is generally measured by colony forming units after 3 days of the start of the culture (when the majority of cells stops dividing).7
Among the classic theories on cellular aging are the programmed aging theory and free-radical theory. The programmed aging theory proposes that aging is driven by genetic pathways where sequential switching on and off of certain genes occur over time. The free-radical theory proposes that free radicals, such as superoxide, cause accumulating damage to DNA and proteins and impair cell function.8
While the free-radical theory is consistent with many observations, not all damages are linked to radicals.9 Accounting for this, the damage-accumulation theory considers not only oxidative damages but also cumulative damages such as translational errors and transcriptional heterogeneity. Accumulation of oxidatively damaged proteins, extrachromosomal ribosomal DNA circles and defective mitochondria, in parallel to dysregulated nutrient sensing, are aspects of the aging process.1,2,5,10 Although an increasing amount of evidence suggests that these aspects are interconnected, for example, the emerging impact that defective mitochondria has on stem-cell pool decline during aging,11 a comprehensive understanding of the mechanisms underpinning the aging process is not yet achieved.
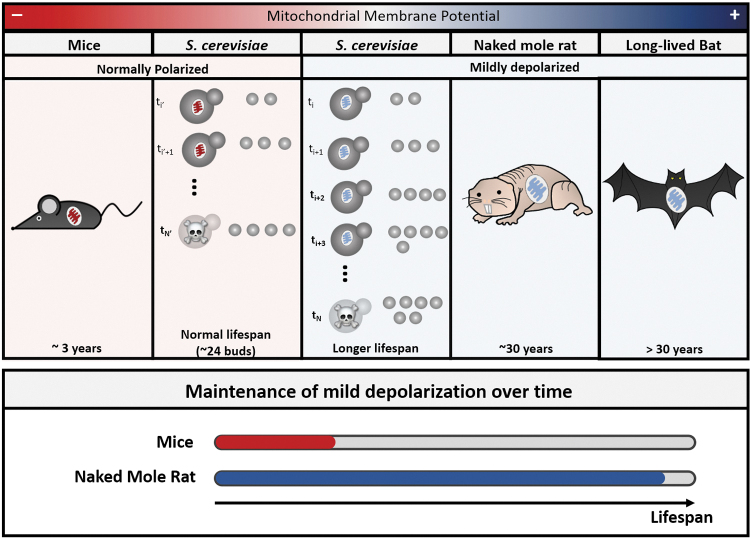
During aging, mitochondrial adenosine triphosphate (ATP) production—a process, driven by an electrochemical potential gradient of proton12—declines in yeast, as well as in mammalian cells.10,11,13 In animals, mitochondrial dysfunction, in part due to changes in mitochondrial DNA (mtDNA) integrity and production of reactive oxygen species (ROS), appears to be associated with age-related disabilities and diseases, such as reduced heart function, neurodegeneration, and even cancer progression.14 Intriguingly, naturally long-lived animals, such as naked mole rats and long-living bats, possess a mechanism to sustain a mild depolarized mitochondrial membrane potential during life15 (Fig. 1). These results suggest that there is a fundamental link between mitochondrial activity and aging. Multiscale analysis bridging the fast-time-scale activities of mitochondria (e.g., ATP synthesis) and the long-time-scale process of aging is a key to elucidate this link.
FIG. 1.
Membrane potential and lifespan. Recent evidence shows that mitochondrial membrane potential is inversely correlated to lifespan, to an extent that does not impair energy production. Yeast cultivated in lifespan extending calorie restriction conditions have a mildly depolarized mitochondrial membrane potential, whereas yeast cells cultivated in regular conditions have a more polarized mitochondria and shorter lifespan. Long-lived bat and naked mole rat, naturally longevous organisms, have a mildly depolarized membrane potential sustained through life. Mice, which have a 10-fold shorter lifespan, loses its mild mitochondrial depolarization early in life.
Given that aging-associated mitochondrial dysfunction is observed in yeasts,10,16,17 S. cerevisiae could provide a useful model system for investigating the dynamic crosstalk between fast and slow dynamics and elucidate the underpinning mechanisms of age-linked mitochondrial function loss. With the hope of facilitating more research into this direction, this perspective overviews S. cerevisiae studies highlighting the fundamental link between aging and bioelectricity—more specifically, mitochondrial membrane potential.
Mitochondrial Membrane Depolarizes During Aging
Analyzing the function and structure of mitochondria by measuring oxygen consumption in yeasts, Volejnikova et al. demonstrated that respiration increases in the first 4 days, and decreases over the remaining period.10 During the course of a 10-day experiment, the routine oxygen consumption by uncoupled respiration increases from ∼10 to ∼30 pmol O2/s·106 cells during the first 4 days, which then decline to near zero on day 10. This change in oxygen consumption is accompanied by the fragmentation of mitochondria and depolarization of their inner membrane, which may reflect the cell-cycle arrest in the G1 phase. Coinciding with the start of oxygen-consumption decline, the membrane potential sharply depolarizes after day 4. Before the mitochondrial depolarization during aging, the vacuolar pH increases.16 By performing a screen for genes whose overexpression prevented mitochondrial dysfunction, Hughes et al. identify VMA1 and VPH2, genes that are required for the function of the vacuolar H+-ATPase, which acidifies the vacuole. Overexpression of VMA1 and VPH2 not only prevents the age-associated vacuolar acidity decline but also inhibits mitochondrial membrane depolarization and contributes to lifespan extension by also maintaining adequate amino acid import by the vacuole.
The age-linked mitochondrial dysfunction and depolarization are also associated with genome instability and increased loss of heterozygosity.17 Deletion of the mtDNA induces a reduction in respiration capacity, depolarization of the inner membrane and defective synthesis or transport of iron–sulfur clusters (ISC), core components of several proteins, including those responsible for DNA repair. One mitochondrial function, besides energy production, is synthesis and export of ISC, and Veatch et al. report that more than respiration capacity, the mitochondrial inner membrane potential is essential for correct production and transport of ISC. Although fluorescent reporters for mitochondrial membrane potential demonstrate the depolarization, quantitative measurements of mitochondrial membrane potential are yet to be done. The biophysical mechanism by which mtDNA degradation, calorie restriction, and vacuolar pH increase induce mitochondrial membrane depolarization is still not entirely clear.
Mild Mitochondrial Depolarization as a Possible Lifespan-Extending Mechanism
A mildly depolarized mitochondrial membrane, while keeping all of this organelle's functionalities, appears to have an antiaging effect in S. cerevisiae—reminiscent of the way in naturally long-lived organisms.15 S. cerevisiae cells cultivated in caloric restriction conditions, an intervention capable of increasing lifespan, displayed reduced mitochondrial membrane potential. Among cells cultivated on nonrestrictive conditions, those that display depolarized membrane potential have a longer lifespan, compared with the cells with more polarized potential.18
Miceli et al. also demonstrate this correlation between mildly reduced mitochondrial membrane potential and extended longevity.19 Through activation of the retrograde process, an inter-organelle signal that modulates nuclear gene expression, cells that had their mtDNA deleted display reduced mitochondrial membrane potential and longer lifespan. Yet, in cells with deleted mtDNA, Miceli et al. observed augmented levels of extrachromosomal rDNA circles (ERC). And in a trend similar to that observed in the study by Veatch et al.,17 an increase in mitochondrial membrane potential by the introduction of mutant allele of ATP1-111, encoding a hyperactive F1 ATP synthase, results in levels of ERC comparable with wild-type cells. Interestingly, reduction of mitochondrial membrane potential by deletion of COX4, which encodes a subunit of cytochrome oxidase, leads to a longer lifespan, while maintaining the cells mtDNA and keeping ERC similar or lower to that of wild-type cells.19 Exposing yeast cells to the uncoupler dinitrophenol (DNP), which is capable of depolarizing mitochondria,20 decreases ROS, and increases yeast CLS.21 However, whether the effect of DNP treatments causes mitochondrial depolarization is not yet demonstrated directly.
Interventions for Increasing Lifespan Reduce Mitochondrial Membrane Potential in Yeast
Interventions capable of increasing lifespan, such as caloric restriction, induce a mild depolarization of the mitochondria. As demonstrated by Delaney et al.,18 cultivation of yeast cells in caloric restriction reduces mitochondrial membrane potential, as does buffer of cultured media, another intervention capable of extending lifespan in yeast. Furthermore, it has been observed that yeast cells receiving lithocholic bile acid (LCA) have a delayed aging process and, for young cells, LCA lowers mitochondrial membrane potential and sustains it in S. cerevisiae.22,23 It is worth noting that the pathways modulated by the addition of LCA do not overlap with nutrient-sensing signaling pathways such as TOR and cAMP/protein kinase A (cAMP/PKA), as LCA is able to extend the lifespan of yeast cells cultivated in calorie-restriction conditions.22,23 The mitochondrial membrane depolarization and antiaging effects, caused by the administration of LCA, also involve the activation of the retrograde signaling pathway, which is in agreement with a previous study.19
Inhibiting the TOR signaling pathways results in polarized mitochondrial membrane potential.24 Pan et al. show that the mutant yeast strain Δtor1, lacking a gene encoding a protein subunit unique to TORC1, has higher respiration activity, a more polarized mitochondrial membrane potential and a longer CLS compared with isogenic wild-type control. However, when entering the stationary phase, Δtor1 cells have reduced ROS production and reduced mitochondrial membrane potential to levels below wild type, which promoted a longer lifespan.24
Adding to the complexity of how the modulation of mitochondrial electrophysiology affects longevity, supplementing DNP to wild-type cultures increases CLS, as also observed by Barros et al.,21 but the same treatment with Δtor1 cultures severely impairs CLS extension. Δtor1 cells have a metabolism shifted toward respiration, in comparison with wild type, which might provide an explanation to why dissipating mitochondrial membrane potential in Δtor1 cells by the addition of DNP does not extend CLS, but impairs its extension. The facts that Δtor1 cells received DNP in the first 24 h of cultivation and were transferred to the media lacking this compound might have had an impact on the observed results, as the CLS extension for WT cells was observed in culture with DNP for the whole cultivation period (growth and stationary phases).
Future Perspectives: Is There a Bioelectric Code of Aging?
As outlined above, qualitative evidence demonstrates both that mitochondrial depolarization is a characteristic of aging, and that a sustained mildly depolarized mitochondria throughout life extends longevity. Evidence also highlights the association between metabolism and the aging process, and how changes in metabolic pathways can impact lifespan, emphasizing how cell metabolism is an interconnected network, linking metabolic states (fermentative or respiratory in yeast), electron flow (membrane potential) and the aging process.18,24,25 However, it is not clear yet if mitochondrial depolarization is a consequence of aging or if it is a compensatory mechanism to regulate the accumulation of damage (e.g., a mildly depolarized mitochondrial membrane potential decreases ROS production15,24). Some hallmarks of aging are considered as compensatory or antagonistic responses to damage.5 Even though it is still an open question if this is the case for mitochondrial membrane potential, it could provide an explanation of why mitochondrial depolarization is observed in old cells and as a lifespan-extending strategy.
Thus, an important point for investigation is whether bioelectrical dynamics has a causal (or circular causal26) effect on aging, or if it is only correlational. If it is causal, it opens up a possibility that decoding the fast and slow dynamics of mitochondrial membrane potential may lead to new biomedical applications for aging. For example, dynamically controlling the permeability of ion channels may allow accelerating or slowing aging. To this end, it is interesting that the permeability of voltage-dependent anion channel is modified during aging in Drosophila and in rat cardiomyocytes.27,28 Another important regulator of permeability in mitochondrial is the Permeability Transition Pore (mPTP), which has its activity altered during aging in the brain and heart. mPTP opening can be altered by variations in membrane potential,29 hence being an additional target for dynamic control as a means to modulate lifespan.
Another interesting point is that aging is generally associated with reduced regenerative capacity. Intriguingly, it has been shown that the plasma membrane potential and ionic changes can act as a signal for regeneration.30 The possible bioelectric code for reprogramming cancer and aging has also been discussed.31 However, a thorough multiscale understanding of bioelectrical dynamics during aging is still lacking. To this, a multiscale computational model integrating biophysical dynamics of the plasma and mitochondrial membrane potential is needed.
Toward this, we believe that S. cerevisiae could be an ideal model system for biophysical experimental characterization due to their short lifespan and our ability to control their growth and manipulate their genes and genomes. Furthermore, yeast could be useful for a high-throughput investigation of different drugs on mitochondrial activity and membrane potential regarding their impacts on aging, which may allow drug repurposing for antiaging and health span extending interventions. Among several potential drug candidates for screening, one worth mentioning is metformin, a drug used to treat diabetes, but which also provides mitochondrial protective properties.27,32 We hope this perspective facilitates more bioelectrical research and bioelectrical understanding of yeast aging.
Authors' Contributions
T.C.d.S.-G. and M.A. equally contributed to writing this article.
Disclaimer
This review has been submitted solely to this journal and is not published, in press, or submitted elsewhere.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
This study was supported by funding from Biotechnology and Biological Sciences Research Council (BBSRC)/Engineering and Physical Sciences Research Council (EPSRC) grant to the Warwick Integrative Synthetic Biology Centre (Grant No. BB/M017982/1).
References
- 1.Kaeberlein M. Lessons on longevity from budding yeast. Nature 2010;464:513–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Denoth Lippuner A, Julou T, Barral Y. Budding yeast as a model organism to study the effects of age. FEMS Microbiol Rev 2014;38:300–325 [DOI] [PubMed] [Google Scholar]
- 3.Lin SJ, Kaeberlein M, Andalis AA, et al. Calorie restriction extends Saccharomyces cerevisiae lifespan by increasing respiration. Nature 2002;418:344–348 [DOI] [PubMed] [Google Scholar]
- 4.Fontana L, Partridge L, Longo VD. Extending healthy life span-from yeast to humans. Science 2010;328:321–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.López-Otín C, Blasco MA, Partridge L, et al. The hallmarks of aging. Cell;153:1194–1217. DOI: 10.1016/j.cell.2013.05.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Souza-Guerreiro TC, Meng X, Dacheux E, et al. Translational control of gene expression noise and its relationship to ageing in yeast. FEBS J 2020. [Epub ahead of print]; DOI: 10.1111/febs.15594 [DOI] [PubMed] [Google Scholar]
- 7.Longo VD, Fabrizio P, Author SB. Chronological aging in Saccharomyces cerevisiae yeast as a model for aging research: Two aging paradigms NIH Public Access Author Manuscript. Subcell Biochem 2012;57:101–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jin K. Modern biological theories of aging. Aging and Disease 2010;1:72–74 [PMC free article] [PubMed] [Google Scholar]
- 9.Gladyshev VN. The free radical theory of aging is dead. Long live the damage theory! Antioxidants Redox Signal 2014;20:727–731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Volejníková A, Hlousková J, Sigler K, et al. Vital mitochondrial functions show profound changes during yeast culture ageing. FEMS Yeast Res 2013;13:7–15 [DOI] [PubMed] [Google Scholar]
- 11.Jang JY, Blum A, Liu J, et al. The role of mitochondria in aging. J Clin Invest 2018;128:3662–3670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zorova LD, Popkov VA, Plotnikov EY, et al. Mitochondrial membrane potential. Anal Biochem 2018;552:50–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hagen TM, Yowe DL, Bartholomew JC, et al. Mitochondrial decay in hepatocytes from old rats: Membrane potential declines, heterogeneity and oxidants increase. Proc Natl Acad Sci 1997;94:3064–3069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Srivastava S. The mitochondrial basis of aging and age-related disorders. Genes (Basel) 2017;8:398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vyssokikh MY, Holtze S, Averina OA, et al. Mild depolarization of the inner mitochondrial membrane is a crucial component of an anti-aging program. Proc Natl Acad Sci 2020;117:6491–6501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hughes AL, Gottschling DE. An early age increase in vacuolar pH limits mitochondrial function and lifespan in yeast. Nature 2012;492:261–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Veatch JR, McMurray MA, Nelson ZW, et al. Mitochondrial dysfunction leads to nuclear genome instability via an iron-sulfur cluster defect. Cell 2009;137:1247–1258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Delaney JR, Murakami C, Chou A, et al. Dietary restriction and mitochondrial function link replicative and chronological aging in Saccharomyces cerevisiae. Exp Gerontol 2013;48:1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miceli MV, Jiang JC, Tiwari A, et al. Loss of mitochondrial membrane potential triggers the retrograde response extending yeast replicative lifespan. Front Genet 2012;2:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buckler KJ, Vaughan-Jones RD. Effects of mitochondrial uncouplers on intracellular calcium, pH and membrane potential in rat carotid body type I cells. J Physiol 1998;513:819–833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barros MH, Bandy B, Tahara EB, et al. Higher respiratory activity decreases mitochondrial reactive oxygen release and increases life span in Saccharomyces cerevisiae. J Biol Chem 2004;279:49883–49888 [DOI] [PubMed] [Google Scholar]
- 22.Beach A, Richard VR, Bourque S, et al. Lithocholic bile acid accumulated in yeast mitochondria orchestrates a development of an anti-aging cellular pattern by causing age-related changes in cellular proteome. Cell Cycle 2015;14:1643–1656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Medkour Y, Dakik P, McAuley M, et al. Mechanisms underlying the essential role of mitochondrial membrane lipids in yeast chronological aging. Oxid Med Cell Longev 2017;2017:2916985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pan Y, Schroeder EA, Ocampo A, et al. Regulation of yeast chronological life span by TORC1 via adaptive mitochondrial ROS signaling. Cell Metab 2011;13:668–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zerfaß C, Asally M, Soyer OSOS, et al. Interrogating metabolism as an electron flow system. Curr Opin Syst Biol 2018;13:59–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Noble R, Tasaki K, Noble PJ, et al. Biological relativity requires circular causality but not symmetry of causation: So, where, what and when are the boundaries? Front Physiol 2019;10:827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Strickland M, Yacoubi-Loueslati B, Bouhaouala-Zahar B, et al. Relationships between ion channels, mitochondrial functions and inflammation in human aging. Front Physiol 2019;10:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Groebe K, Klemm-Manns M, Schwall GP, et al. Age-dependent posttranslational modifications of voltage-dependent anion channel 1. Exp Gerontol 2010;45:632–637 [DOI] [PubMed] [Google Scholar]
- 29.Panel M, Ghaleh B, Morin D. Mitochondria and aging: A role for the mitochondrial transition pore? Aging Cell 2018;17:e12793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mclaughlin KA, Levin M. Bioelectric signaling in regeneration: Mechanisms of ionic controls of growth and form. Dev Biol 2018;433:177–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Silver BB, Nelson CM. The bioelectric code: Reprogramming cancer and aging from the interface of mechanical and chemical microenvironments. Front Cell Dev Biol 2018;6:1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chanthammachat P, Dharmasaroja P. Metformin restores the mitochondrial membrane potentials in association with a reduction in TIMM23 and NDUFS3 in MPP+-induced neurotoxicity in SH-SY5Y cells. EXCLI J 2019;18:812–823 [DOI] [PMC free article] [PubMed] [Google Scholar]