Abstract
It is now established that the gut microbiome influences human neurology and behavior, and vice versa. Distinct mechanisms underlying this bidirectional communication pathway, termed the gut-brain axis, are becoming increasingly uncovered. This review summarizes recent interkingdom signaling research focused on gamma-aminobutyric acid (GABA), a human neurotransmitter and ubiquitous signaling molecule found in bacteria, fungi, plants, invertebrates, and mammals. We detail how GABAergic signaling has been shown to be a crucial component of the gut-brain axis. We further describe how GABA is also being found to mediate interkingdom signaling between algae and invertebrates, plants and invertebrates, and plants and bacteria. Based on these emerging results, we argue that obtaining a complete understanding of GABA-mediated communication in the gut-brain axis will involve deciphering the role of GABA signaling and metabolism within bacterial communities themselves.
Keywords: gut-brain axis, microbial endocrinology, interkingdom signaling, GABA
Introduction
The gut microbiome is a complex consortium of bacterial species that colonize the gastrointestinal tract and play critical roles in human health and disease. In addition to its established roles in nutrient absorption, immunity, and metabolism, there is a growing appreciation of the extent to which the gut microbiome influences human neurology and behavior. This concept of an overlap between microbiology and neuroscience only began to gain traction in the 1990s, when the field was referred to as microbial endocrinology.1–3 Microbial endocrinology encompasses bidirectional interkingdom signaling through a range of neuroactive compounds, including neurotransmitters, steroids, neuropeptides, gasotransmitters, and endocannabinoids. In this context, variations in microbiome composition have been linked to major depressive disorder, anxiety, and autism. For example, psychiatric comorbidity has been cited to occur at rates as high as 60% with inflammatory gastrointestinal disorders.4,5 These findings, among others, have increased appreciation for the ability of the microbiome to impact human neurology and behavior, transforming the pioneering microbial endocrinology movement into the dynamic and emergent field of the microbiome gut-brain axis (Fig. 1).
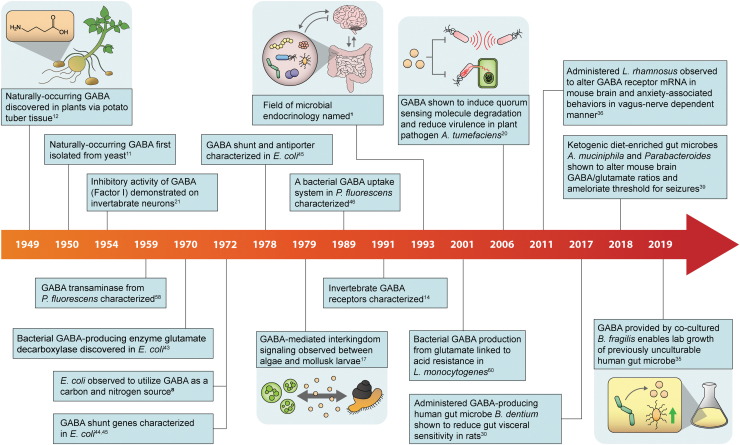
FIG. 1.
A timeline depicting major discoveries relating to the potential for GABA as an interkingdom signaling molecule and as a signaling molecule within bacterial communities. GABA, gamma-aminobutyric acid.
Animal models have indicated that communication along the gut-brain axis likely occurs through the immune system and the vagus nerve, and through bidirectional signaling through neuroactive compounds.6 One possibility is that neuroactive compounds are being produced, sensed, and responded to by the resident gut microbiota, influencing microbiome composition and biology and modulating host responses. Bacteria have been shown to produce and respond to compounds traditionally thought of as human neurotransmitters, including gamma-aminobutyric acid (GABA), serotonin, dopamine, and norepinephrine.6,7 However, while it is clear that microbial composition of the resident microbiota can affect behavior, the specific mechanisms by which this effect occurs are largely unknown in many cases. To fully understand how the microbiome impacts human neurology and behavior, and to leverage these impacts for therapeutic applications, we must understand the underlying bacterial mechanisms of neurotransmitter production and response.
At the same time, it is becoming increasingly clear that the neuroactive compounds traditionally thought of as human neurotransmitters may play unappreciated signaling roles in bacterial communities themselves. This review will focus on GABA, a prominent human neurotransmitter as well as a ubiquitous signaling molecular in nature. GABA is produced by bacteria,8,9 fungi,10,11 plants,12,13 and invertebrates.14–16 GABA-mediated interkingdom signaling has been observed between algae and invertebrates,17 plants and fungi,18 plants and insects,19 and plants and bacteria.20 In one example, GABA, which is naturally produced by wounded plants, increases expression of a quorum-sensing molecule degradative enzyme and associated virulence in plant pathogen Agrobacterium tumefaciens.20 The goal of this review is to highlight emerging research, future directions, and potential signaling roles for GABA in bacterial communities. This knowledge will increase basic scientific understanding of the many fields that intersect with microbiology and further enhance the potential to leverage these findings for human therapeutic applications in the microbiome gut-brain axis.
Bacterial GABA Signaling and Human Neurological Functions
GABA is a primary inhibitory neurotransmitter in mammals, vertebrates, and invertebrate systems.21,22 Disruptions in GABA signaling have well-studied causative roles in neurological disorders like epilepsy, anxiety, autism, and depression.23–26 In addition to these established neurological roles, GABA can function as a regulator of immune function and blood pressure in mammals.27 Two major classes of GABA receptors, GABAA and GABAB, are present throughout the human and mouse GI tract, and have been shown to contribute to intestinal motility and gastric health.28,29 The positioning of these receptors may play a crucial role in gut-brain axis communication and mechanistic studies should take into account how GABA receptor distribution may contribute to host sensing of GABA produced by gut microbes.
There is growing evidence that enteric GABA production by the microbiota can impact a variety of disease outcomes, and the field is moving toward uncovering the specific mechanisms through which microbial GABA may influence human health, and vice versa. In one example, administration of a GABA-producing Bifidobacterium dentium species present in the human microbiome resulted in desensitized sensory neuron activity in a rat model of visceral sensitivity.30 Administration of Bifidobacterium breve, a genetically similar species that lacks glutamate decarboxylase (GAD), did not have this effect. This group also showed that microbial GAD expression is threefold enriched in the human GI tract, compared to other sites in the body, indicating that the gut microbiome is likely producing more GABA than the oral, nasal, skin, or vaginal microbiomes.30 Furthermore, administering a B. breve strain that recombinantly overexpresses a GABA-synthesizing enzyme from B. dentium resulted in a statistically significant increase in detectable GABA levels in the cecal content of these animals, on the order of 8–10 μg GABA/g cecum. This result supports a model wherein microbial GABA is not immediately degraded or converted in the intestinal lumen.30 As B. dentium is a component of what is considered a healthy human microbiome, this is mechanistic evidence that microbial GABA has the potential to lessen chronic abdominal pain.
In addition to neurological pain responses, there is evidence that microbial GABA regulates emotional behavior and an anxiety response. Disruptions in GABAergic signaling have been implicated in psychiatric anxiety disorders in humans, and GABAA receptors are the target of antianxiety pharmaceuticals like benzodiazapines.31 Germ-free mice have been shown to have nearly twofold lower levels of GABA in the stool and cardiac plasma, although the ability of any microbial GABA to cross the blood–brain barrier is unknown.32–34 The production of GABA microbiome Bacteroides genera is inversely correlated with the occurrence of major depressive disorder and there is evidence that alterations in GABA availability may fundamentally affect the composition of the microbiome.35 In one relevant example, feeding mice a common probiotic strain Lactobacillus rhamnosus reduced stress- and anxiety-related behaviors and modulated GABAA and GABAB mRNA expression in different areas of the brain.36 The effects depended on an intact vagus nerve, and did not occur is vagotomized mice, indicating direct evidence that a GABA-producing probiotic strain is capable of causing changes in the mouse brain and mouse behavior through the vagus nerve. Vagus nerve stimulation is a medical procedure that has been traditionally used to treat patients with epilepsy, another neurological disorder in which GABAergic signaling is known to be disrupted, in addition to some forms of treatment-resistant depression.37 Together, these data show that probiotic bacteria can modulate the GABAergic system in mice through the vagus nerve.
There is also direct evidence that GABA availability may alter microbiome composition, and that genes associated with GABA production and consumption are widespread throughout the human microbiome. In seeking to understand the biological roles of species within the microbiome consortia, some researchers have underscored the importance of understanding how unculturable species contribute to the microbiome organ. In one such study, a new genus of Gram-positive bacterium belonging to the Ruminococcaceae family and the group named Evtepia gabavorous could only be cultured in the presence of the human microbiome component Bacteroides fragilis.35 Surprisingly, GABA was revealed to be the sole nutrient component B. fragilis was providing to this previously unculturable isolate. Further metabolic research into this organism revealed that it lacks the ability to utilize common sugars and amino acids as a carbon source, and instead utilizes GABA as a carbon energy source. This is the first report of GABA as an essential bacterial nutrient. Genomic analyses searching for GABA metabolic gene homologs revealed the presence of hundreds of putative sole GABA producers, sole consumers, and combination consumer-producers throughout the human microbiome. This research implies that GABA availability may change the composition of the microbiome, as some nonproducer species require it for growth. In addition, it shows the extent to which GABA availability and bacterial metabolism may affect interspecies competition, colonization, and the potential ability of probiotic strains to colonize the GI tract.
Aside from gut-brain axis communication occurring through alterations in microbial-produced GABA, diet-induced alterations in microbiome composition may impact GABAergic signaling. The translational benefit of manipulating microbial GABA production is perhaps best exemplified through the success of treating epilepsy with a ketogenic (high fat and low carbohydrate) diet. A ketogenic diet has been used for decades to treat epileptic seizures that do not respond to conventional anticonvulsants,38 although the mechanism underlying this phenomenon was unknown. A recent study used two different mouse models of epilepsy to show that a ketogenic diet enriches for Akkermansia muciniphila and Parabacteroides, which are together necessary and sufficient to ameliorate the threshold for seizures.39 This effect is correlated with decreased levels of gamma-glutamylated (GG) amino acids in the sera, which are human neurotransmitter precursors thought to have increased transport capacity across the blood–brain barrier compared to non-GG counterparts. In addition to approximately twofold decreased circulating GG amino acids, the tested animals also had approximately threefold increased GABA/glutamate ratios in the hippocampus. This work expands on previous studies showing microbiota and diet can affect GABA levels in the brain,40,41 and provide a causal mechanism by which the ketogenic diet lowers seizure susceptibility. As different diets are known to variably affect microbiome composition between people, this work also provides a baseline for investigating whether enrichment for different microbial taxa in patients may be functionally redundant. In the case of the ketogenic diet, for example, different enriched taxa between patients may result in the same physiological effect of decreased circulating GG amino acids.
Bacterial GABA Signaling Within Bacterial Communities
While neuroactive compounds like GABA are commonly known as human neurotransmitters, many neurotransmitters exist across disparate biological kingdoms and take part in adaptive and signaling mechanisms outside human physiology. In addition to functioning as a synaptic neurotransmitter, GABA is a ubiquitous signaling molecule in nature that is produced by bacteria, fungi, plants, and invertebrates. Archaea contain GABA-producing enzyme homologs, although their function remains unclear.42 Due to their universal occurrence across biological kingdoms and ecosystems, it has been argued that these compounds should more accurately be thought of as ubiquitous biomediators as opposed to specifically synaptic neurotransmitters.7 Acquiring a complete understanding of microbial GABA signaling will therefore require understanding bacterial GABA signaling, which may have evolved outside the human body.
Bacterial genes for GABA metabolism and uptake were first described in Escherichia coli and Pseudomonas fluorescens in the 1970s and 1980s.8,43–46 It is now known that many species of bacteria produce, transport, and utilize GABA as a carbon and nitrogen source.6 While systems for GABA transport vary between organisms, these genes have been rigorously characterized in E. coli and Listeria monocytogenes.43,47–49 These bacteria metabolize GABA through a conserved pathway of enzymes known as the GABA shunt.48 The components of the GABA shunt generally consist of a group of enzymes that convert GABA to succinate for use in the tricarboxylic acid cycle (TCA) and energy production. In both microbial species and mammals, GABA is most commonly synthesized from L-glutamate by a GAD enzyme.43,48 However, GABA can also be synthesized from ornithine, arginine, and putrescine, and homologous biosynthesis enzymes have been found in many human gut bacteria.35 Glutamate decarboxylation is the first step in the GABA shunt, which ultimately catabolizes GABA to succinate, feeding into the TCA cycle. After GABA is produced from glutamate by a GAD enzyme, it is converted to succinic semialdehyde by a GABA aminotransferase, and then to succinate by a succinic semialdehyde dehydrogenase. Some bacteria contain multiple GAD enzymes with redundant function, and some GABA-producing bacteria contain no known GAD homologs. In general, the components and activity of the GABA shunt vary even between different species within the same genus, as is the case with Bacillus species.48
The crucial role of GABAergic signaling in many human diseases has resulted in increased commercial interest in supplementing human diets with GABA as part of healthy lifestyle branding.50 GABA production by bacteria is often studied in the context of food production, as GABA is found in foods like cheeses, kimchi, and yogurt that often contain probiotic strains of lactic acid-producing bacteria.9 Food scientists and engineers have sought to increase the levels of GABA in various foods by recombinant engineering of microbial GABA-producing enzymes and optimization of microbial fermentation conditions during food production.51–53 Regulation of pH during fermentation has been shown to be the most effective way of increasing microbial GABA production, although the specific optimal pH range varies greatly (pH 4.5–8) from species to species. The variation in GABA production by different microbial species is thought to result from different biochemical properties of the GAD enzyme, among other differences in enzyme activity within the GABA shunt. Taking into account a large amount of variation by species, many microbial species have demonstrated maximum GABA production, a proxy for optimal GAD enzyme activity, in a weak acidic pH range near pH 5.54–56 In contrast, many microbial GABA-consuming enzymes in the GABA shunt, like GABA transaminase and succinic semialdehyde dehydrogenase, have shown optimal activity in the more weak alkaline pH range closer to 8.57–59
In agreement with these findings, in the context of food production, the primary known function of the GABA shunt, aside from linking carbon and nitrogen metabolism, is maintaining pH homeostasis in acidic environments.49,60,61 In this context, it follows that so many GABA-producing and GABA-consuming bacterial homologs would be present in the acidic portions (pH 5.7–7.4) of the human GI tract.35 Acid tolerance is thought to occur when a molecule of glutamate is converted to GABA by the GAD enzyme, consuming a proton and causing the intracellular pH to rise. The resulting GABA is either transported out of the cell or catabolized to succinate by the GABA shunt. In one example, in gastric pathogen L. monocytogenes, a double mutant in the bacterium's two GAD homologs is unable to survive when exposed to acidic porcine and synthetic human gastric fluid, pH 2.5.60 In this organism, the GAD enzyme and GABA shunt are thought to contribute to survival within the acidic GI tract environment. The reduced tolerance for acidic conditions that gad mutants exhibit is thought to be caused by a reduced ability to regulate intracellular pH, although this model has been debated.48
Aside from its role in acid tolerance, there is growing evidence that GABA may play roles in bacteria-plant signaling. For over a decade, plant biologists have argued that extracellular GABA may be a ubiquitous signaling molecule mediating plant interactions with a variety of nonplant eukaryotes and prokaryotes.19,62 In turn, it is possible that GABA mediates bacteria-bacteria interactions as well as bacteria-plant interactions, aside from its role as a metabolite. GABA production by plants has been implicated in plant development,13 immunity,63 and abiotic stress tolerance to low light and high salinity.64–66 Wounded plants increase GABA synthesis and wounding is required for tumor formation during Agrobacterium tumefaciens infection and subsequent crown gall disease.20 Notably, GABA-mediated signaling has been observed to decrease virulence in plant pathogens A. tumefaciens and Pseudomonas syringae20,67 and affect quorum-sensing molecule production in opportunistic pathogen Pseudomonas aeruginosa.68 One study found that imported GABA caused upregulation of a quorum sensing hormone degradative enzyme in A. tumefaciens, thereby reducing quorum sensing. In transgenic plants with elevated GABA concentrations, A. tumefaciens showed decreased virulence. GABA-mediated signaling between host plant and bacterial pathogen is complex, as a bacterial small RNA tightly controls GABA import in A. tumefaciens, the first example of a bacterial sRNA controlling a plant-derived signaling molecule.69 In addition, proline, which also accumulates in crown gall-diseased plant tumors, antagonizes GABA quenching of A. tumefaciens quorum sensing.70 The complex nature of plant pathogen GABA-mediated signaling implies there are likely unknown GABA-mediated signaling pathways in many bacteria. If bacteria have evolved unknown signaling mechanisms in response to GABA, it is likely that those mechanisms could play a role in complex microbial consortia like the human gut microbiome, in addition to the rhizome of plant roots.
Conclusion and Future Directions
Understanding how bacterial GABA impacts human health will require discovering how GABA signaling operates within bacterial communities themselves. It is becoming increasing clear that the neuroactive compounds traditionally thought of as human neurotransmitters play unappreciated signaling roles in bacterial communities. Traditionally known as a human neurotransmitter, GABA is now appreciated to be ubiquitous signaling molecular in nature that is produced by bacteria, archaea, fungi, plants, and invertebrates. For example, it is already known that wounded plants release large concentrations of GABA that cause decreased quorum sensing and subsequent reduced virulence in the associated bacterial communities. Work in this emergent field will continue to transform the pioneering microbial endocrinology movement of the 1990s into the dynamic and emergent field of the microbiome gut-brain axis. This knowledge will increase basic scientific understanding of the many fields that intersect with microbiology and further enhance the potential to leverage these findings for human therapeutic applications.
Acknowledgments
The authors apologize to our many colleagues whose work we could not include due to space constraints.
Authors' Contributions
S.J.Q. and A.P. authored and edited the article. P.T. authored the article figure. All co-authors have reviewed and approved the article before submission.
Disclaimer
The article has been submitted solely to Bioelectricity and is not published, in press, or submitted elsewhere.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
We are grateful for generous support from the Burroughs Wellcome Fund (1015883.01), the David and Lucile Packard Foundation (2018-68055), the Army Research Office (W911NF-19-1-0136), Pew Charitable Trusts (2019-A-06953), the Hartwell Biomedical Research Fellowship, and the Biotechnology Training Program at Northwestern University.
References
- 1.Lyte M. The role of microbial endocrinology in infectious disease. J Endocrinol 1993;137:343–345 [DOI] [PubMed] [Google Scholar]
- 2.Neuman H, Debelius JW, Knight R, et al. Microbial endocrinology: The interplay between the microbiota and the endocrine system. FEMS Microbiol Rev 2015;39:509–521 [DOI] [PubMed] [Google Scholar]
- 3.Lyte M. Probiotics function mechanistically as delivery vehicles for neuroactive compounds: Microbial endocrinology in the design and use of probiotics. Bioessays 2011;33:574–581 [DOI] [PubMed] [Google Scholar]
- 4.Whitehead WE, Palsson O, Jones KR. Systematic review of the comorbidity of irritable bowel syndrome with other disorders: What are the causes and implications? Gastroenterology 2002;122:1140–1156 [DOI] [PubMed] [Google Scholar]
- 5.Gros DF, Antony MM, McCabe RE, et al. Frequency and severity of the symptoms of irritable bowel syndrome across the anxiety disorders and depression. J Anxiety Disord 2009;23:290–296 [DOI] [PubMed] [Google Scholar]
- 6.Strandwitz P. Neurotransmitter modulation by the gut microbiota. Brain Res 2018;1693:128–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roshchina VV. New trends and perspectives in the evolution of neurotransmitters in microbial, plant, and animal cells. Adv Exp Med Biol 2016;874:25–77 [DOI] [PubMed] [Google Scholar]
- 8.Dover S, Halpern YS. Utilization of -aminobutyric acid as the sole carbon and nitrogen source by Escherichia coli K-12 mutants. J Bacteriol 1972;109:835–843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dhakal R, Bajpai VK, Baek KH. Production of gaba (gamma-Aminobutyric acid) by microorganisms: A review. Braz J Microbiol 2012;43:1230–1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coleman ST, Fang TK, Rovinsky SA, et al. Expression of a glutamate decarboxylase homologue is required for normal oxidative stress tolerance in Saccharomyces cerevisiae. J Biol Chem 2001;276:244–250 [DOI] [PubMed] [Google Scholar]
- 11.Reed L. The occurrence of γ-Aminobutyric acid in yeast extract; its isolation and identification. J Biol Chem 1949;183:451–458 [Google Scholar]
- 12.Steward FC, et al. γ-aminobutyric acid: A constituent of the potato tuber? Science 1949;110:439–440 [Google Scholar]
- 13.Bouche N, Lacombe B, Fromm H. GABA signaling: A conserved and ubiquitous mechanism. Trends Cell Biol 2003;13:607–610 [DOI] [PubMed] [Google Scholar]
- 14.Ffrench-Constant RH, Mortlock DP, Shaffer CD, et al. Molecular cloning and transformation of cyclodiene resistance in Drosophila: An invertebrate gamma-aminobutyric acid subtype A receptor locus. Proc Natl Acad Sci U S A 1991;88:7209–7213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harvey RJ, Vreugdenhil E, Zaman SH, et al. Sequence of a functional invertebrate GABAA receptor subunit which can form a chimeric receptor with a vertebrate alpha subunit. EMBO J 1991;10:3239–3245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lunt GG. GABA and GABA receptors in invertebrates. Semin Neurosci 1991;3:251–258 [Google Scholar]
- 17.Morse DE, Hooker N, Duncan H, et al. ggr-Aminobutyric acid, a neurotransmitter, induces planktonic abalone larvae to settle and begin metamorphosis. Science 1979;204:407–410 [DOI] [PubMed] [Google Scholar]
- 18.Solomon PS, Oliver RP. The nitrogen content of the tomato leaf apoplast increases during infection by Cladosporium fulvum. Planta 2001;213:241–249 [DOI] [PubMed] [Google Scholar]
- 19.Bown AW, Macgregor KB, Shelp BJ. Gamma-aminobutyrate: Defense against invertebrate pests? Trends Plant Sci 2006;11:424–427 [DOI] [PubMed] [Google Scholar]
- 20.Chevrot R, Rosen R, Haudecoeur E, et al. GABA controls the level of quorum-sensing signal in Agrobacterium tumefaciens. Proc Natl Acad Sci U S A 2006;103:7460–7464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Florey E. An inhibitory and an excitatory factor of mammalian central nervous system, and their action of a single sensory neuron. Arch Int Physiol 1954;62:33–53 [DOI] [PubMed] [Google Scholar]
- 22.Bazemore A, Elliott KA, Florey E. Factor I and gamma-aminobutyric acid. Nature 1956;178:1052–1053 [DOI] [PubMed] [Google Scholar]
- 23.Wallace RH, Marini C, Petrou S, et al. Mutant GABA(A) receptor gamma2-subunit in childhood absence epilepsy and febrile seizures. Nat Genet 2001;28:49–52 [DOI] [PubMed] [Google Scholar]
- 24.Wong CG, Bottiglieri T, Snead OC, 3rd. GABA, gamma-hydroxybutyric acid, and neurological disease. Ann Neurol 2003;54Suppl 6:S3–S12 [DOI] [PubMed] [Google Scholar]
- 25.Braat S, Kooy RF. The GABAA receptor as a therapeutic target for neurodevelopmental disorders. Neuron 2015;86:1119–1130 [DOI] [PubMed] [Google Scholar]
- 26.Luscher B, Shen Q, Sahir N. The GABAergic deficit hypothesis of major depressive disorder. Mol Psychiatry 2011;16:383–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jin Z, Mendu SK, Birnir B. GABA is an effective immunomodulatory molecule. Amino Acids 2013;45:87–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Auteri M, Zizzo MG, Serio R. GABA and GABA receptors in the gastrointestinal tract: From motility to inflammation. Pharmacol Res 2015;93:11–21 [DOI] [PubMed] [Google Scholar]
- 29.Beaumont H, Smout A, Aanen M, et al. The GABA(B) receptor agonist AZD9343 inhibits transient lower oesophageal sphincter relaxations and acid reflux in healthy volunteers: A phase I study. Aliment Pharmacol Ther 2009;30:937–946 [DOI] [PubMed] [Google Scholar]
- 30.Pokusaeva K, Johnson C, Luk B, et al. GABA-producing Bifidobacterium dentium modulates visceral sensitivity in the intestine. Neurogastroenterol Motil 2017;29:e12904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haefely W. Benzodiazepine interactions with GABA receptors. Neurosci Lett 1984;47:201–206 [DOI] [PubMed] [Google Scholar]
- 32.Boonstra E, de Kleijn R, Colzato LS, et al. Neurotransmitters as food supplements: The effects of GABA on brain and behavior. Front Psychol 2015;6:1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pardridge WM. Blood-brain barrier delivery. Drug Discov Today 2007;12:54–61 [DOI] [PubMed] [Google Scholar]
- 34.Matsumoto M, Kibe R, Ooga T, et al. Cerebral low-molecular metabolites influenced by intestinal microbiota: A pilot study. Front Syst Neurosci 2013;7:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Strandwitz P, Kim KH, Terekhova D, et al. GABA-modulating bacteria of the human gut microbiota. Nat Microbiol 2019;4:396–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bravo JA, Forsythe P, Chew MV, et al. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci U S A 2011;108:16050–16055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nemeroff CB, Mayberg HS, Krahl SE, et al. VNS therapy in treatment-resistant depression: Clinical evidence and putative neurobiological mechanisms. Neuropsychopharmacology 2006;31:1345–1355 [DOI] [PubMed] [Google Scholar]
- 38.Kwan P, Brodie MJ. Early identification of refractory epilepsy. N Engl J Med 2000;342:314–319 [DOI] [PubMed] [Google Scholar]
- 39.Olson CA, Vuong HE, Yano JM, et al. The gut microbiota mediates the anti-seizure effects of the ketogenic diet. Cell 2018;173:1728–1741e1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Frost GS, Walton GE, Swann JR, et al. Impacts of plant-based foods in ancestral hominin diets on the metabolism and function of gut microbiota in vitro. mBio 2014;5:e00853–00814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Singh RK, Chang HW, Yan D, et al. Influence of diet on the gut microbiome and implications for human health. J Transl Med 2017;15:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tomita H, Yokooji Y, Ishibashi T, et al. An archaeal glutamate decarboxylase homolog functions as an aspartate decarboxylase and is involved in beta-alanine and coenzyme A biosynthesis. J Bacteriol 2014;196:1222–1230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Strausbauch PH, Fischer EH. Chemical and physical properties of Escherichia coli glutamate decarboxylase. Biochemistry 1970;9:226–233 [DOI] [PubMed] [Google Scholar]
- 44.Dover S, Halpern YS. Control of the pathway of -aminobutyrate breakdown in Escherichia coli K-12. J Bacteriol 1972;110:165–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kahane S, Levitz R, Halpern YS. Specificity and regulation of gamma-aminobutyrate transport in Escherichia coli. J Bacteriol 1978;135:295–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guthrie GD, Nicholson-Guthrie CS. gamma-Aminobutyric acid uptake by a bacterial system with neurotransmitter binding characteristics. Proc Natl Acad Sci U S A 1989;86:7378–7381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smith DK, Kassam T, Singh B, et al. Escherichia coli has two homologous glutamate decarboxylase genes that map to distinct loci. J Bacteriol 1992;174:5820–5826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Feehily C, Karatzas KA. Role of glutamate metabolism in bacterial responses towards acid and other stresses. J Appl Microbiol 2013;114:11–24 [DOI] [PubMed] [Google Scholar]
- 49.Feehily C, O'Byrne CP, Karatzas KA. Functional gamma-Aminobutyrate Shunt in Listeria monocytogenes: Role in acid tolerance and succinate biosynthesis. Appl Environ Microbiol 2013;79:74–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li H, Cao Y. Lactic acid bacterial cell factories for gamma-aminobutyric acid. Amino Acids 2010;39:1107–1116 [DOI] [PubMed] [Google Scholar]
- 51.Park KB, Oh SH. Production of yogurt with enhanced levels of gamma-aminobutyric acid and valuable nutrients using lactic acid bacteria and germinated soybean extract. Bioresour Technol 2007;98:1675–1679 [DOI] [PubMed] [Google Scholar]
- 52.Kim JY, Lee MY, Ji GE, et al. Production of gamma-aminobutyric acid in black raspberry juice during fermentation by Lactobacillus brevis GABA100. Int J Food Microbiol 2009;130:12–16 [DOI] [PubMed] [Google Scholar]
- 53.Briguglio M, Dell'Osso B, Panzica G, et al. Dietary neurotransmitters: A narrative review on current knowledge. Nutrients 2018;10:591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kimura NKJSSKHMT. Production ofg-aminobutyric acid (GABA) byLactobacillusparacaseiisolated from traditional fermented foods. Food Microbiol 2005;22:497–504 [Google Scholar]
- 55.Yang SY, Lu FX, Lu ZX, et al. Production of gamma-aminobutyric acid by Streptococcus salivarius subsp. thermophilus Y2 under submerged fermentation. Amino Acids 2008;34:473–478 [DOI] [PubMed] [Google Scholar]
- 56.Siragusa S, De Angelis M, Di Cagno R, et al. Synthesis of gamma-aminobutyric acid by lactic acid bacteria isolated from a variety of Italian cheeses. Appl Environ Microbiol 2007;73:7283–7290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Voellym R, Leisinger T. Role of 4-aminobutyrate aminotransferase in the arginine metabolism of Pseudomonas aeruginosa. J Bacteriol 1976;128:722–729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Scott EM, Jakoby WB. Soluble gamma-aminobutyric-glutamic transaminase from Pseudomonas fluorescens. J Biol Chem 1959;234:932–936 [PubMed] [Google Scholar]
- 59.Jakoby WB, Scott EM. Aldehyde oxidation. III. Succinic semialdehyde dehydrogenase. J Biol Chem 1959;234:937–940 [PubMed] [Google Scholar]
- 60.Cotter PD, Gahan CG, Hill C. A glutamate decarboxylase system protects Listeria monocytogenes in gastric fluid. Mol Microbiol 2001;40:465–475 [DOI] [PubMed] [Google Scholar]
- 61.Capitani G, De Biase D, Aurizi C, et al. Crystal structure and functional analysis of Escherichia coli glutamate decarboxylase. EMBO J 2003;22:4027–4037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shelp BJ, Bown AW, Faure D. Extracellular gamma-aminobutyrate mediates communication between plants and other organisms. Plant Physiol 2006;142:1350–1352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tarkowski LP, Signorelli S, Hofte M. Gamma-Aminobutyric acid and related amino acids in plant immune responses: Emerging mechanisms of action. Plant Cell Environ 2020;43:1103–1116 [DOI] [PubMed] [Google Scholar]
- 64.Michaeli S, Fait A, Lagor K, et al. A mitochondrial GABA permease connects the GABA shunt and the TCA cycle, and is essential for normal carbon metabolism. Plant J 2011;67:485–498 [DOI] [PubMed] [Google Scholar]
- 65.Renault H, El Amrani A, Berger A, et al. Gamma-Aminobutyric acid transaminase deficiency impairs central carbon metabolism and leads to cell wall defects during salt stress in Arabidopsis roots. Plant Cell Environ 2013;36:1009–1018 [DOI] [PubMed] [Google Scholar]
- 66.Michaeli S, Fromm H. Closing the loop on the GABA shunt in plants: Are GABA metabolism and signaling entwined? Front Plant Sci 2015;6:419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Deng X, Xu X, Liu Y, et al. Induction of gamma-aminobutyric acid plays a positive role to Arabidopsis resistance against Pseudomonas syringae. J Integr Plant Biol 2020;62:1797–1812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dagorn A, Hillion M, Chapalain A, et al. Gamma-aminobutyric acid acts as a specific virulence regulator in Pseudomonas aeruginosa. Microbiology (Reading) 2013;159:339–351 [DOI] [PubMed] [Google Scholar]
- 69.Wilms I, Voss B, Hess WR, et al. Small RNA-mediated control of the Agrobacterium tumefaciens GABA binding protein. Mol Microbiol 2011;80:492–506 [DOI] [PubMed] [Google Scholar]
- 70.Haudecoeur E, Planamente S, Cirou A, et al. Proline antagonizes GABA-induced quenching of quorum-sensing in Agrobacterium tumefaciens. Proc Natl Acad Sci U S A 2009;106:14587–14592 [DOI] [PMC free article] [PubMed] [Google Scholar]