Abstract
The use of leadless trans-catheter pacemakers is increasing particularly in the elderly population. However, its indication for those with anatomical anomaly remains unknown. We had a 75-year-old woman with atrial standstill and ventricular escape. Micra leadless pacemaker (Medtronic, Inc, Minneapolis, MN, USA) failed to be deployed due to too enlarged right atrium accompanied by atrial septal deficiency, followed by successful implantation of transvenous pacemaker lead by using SelectSecure lead (Medtronic) with a C315 delivery catheter that enhanced back-up force toward the ventricular septum against significant tricuspid regurgitation. The Micra is a promising system, but we should understand its limitations as well as alternative systems particularly for such an anatomical anatomy.
<Learning objective: A Micra leadless trans-catheter pacemaker is a promising system with less invasiveness, particularly for elderly patients, but we should understand its technical limitation and consider alternative systems if necessary, particularly for those with enlarged right heart.>
Keywords: Heart failure, Arrhythmia, Bradycardia, Atrial fibrillation
Introduction
A leadless pacemaker is a unique technology that does not require any subcutaneous pocket formations for the generator implantation and any intra-vascular pacemaker leads, both of which are essential for conventional trans-venous pacemakers but are associated with various device-related complications [1]. The use of leadless pacemaker has been spreading given the high implant success rate (99.2%) and low one-year major complication rate (4%) in the original investigational device exemption study [2, 3], as well as 93.3% of 6-month freedom from serious adverse events in the LEADLESS II trial [4], followed by similar favorable outcomes in the post-approval multi-country registry (99.6% of implant success and 1.5% of 30-day major complications) [5].
Given these favorable outcomes, a leadless pacemaker might be preferred for all patients suitable for VVI mode pacemakers. However, an optimal strategy which system to use, i.e. a leadless pacemaker or a conventional one, remains unknown, particularly for those with anatomical anomaly such as an enlarged right heart [6]. In this case report, we present a patient with a giant right atrium accompanied by atrial septal deficiency, in whom a leadless pacemaker failed to be implanted, followed by the successful implantation of conventional trans-venous pacemaker using a unique delivery catheter.
Case report
On admission
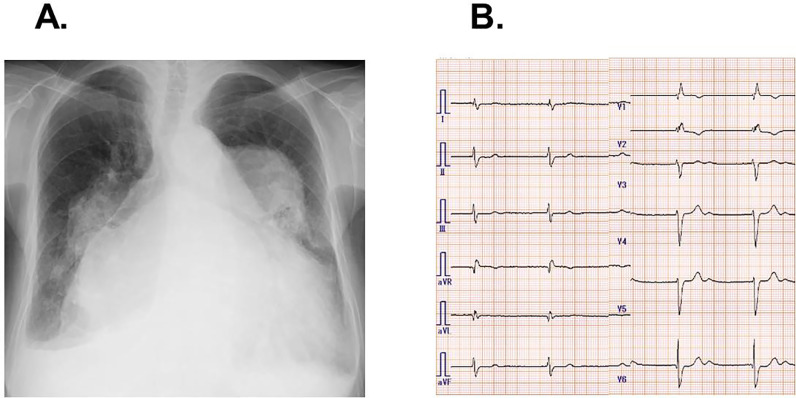
A 75-year-old woman with a history of untreated atrial septum deficiency was admitted to our institute complaining of worsening dyspnea refractory to medication including carvedilol 2.5 mg, azilsartan 20 mg, furosemide 80 mg, and tolvaptan 15 mg. Chest X-ray showed extreme cardiomegaly and dilated bilateral pulmonary artery (Fig. 1A). The electrocardiogram showed a heart rate of 46 bpm with persistent atrial standstill and ventricular escape (Fig. 1B). Transthoracic echocardiography showed left ventricular end-diastolic diameter of 44 cm, left ventricular ejection fraction of 65%, and moderate tricuspid regurgitation, as well as extremely enlarged bi-atrium and a jet through atrial septum deficiency with pulmonary to systemic blood flow ratio of 2.3 (Fig. 2A). Computed tomography showed an enlarged right atrium with antero-posterior dimension of 67.3 mm and transverse diameter of 104.1 mm (Fig. 2B).
Fig. 1.
Chest X-ray (A) and electrocardiogram (B) on admission.
Fig. 2.
Four-chamber trans-thoracic echocardiography (A) and chest computed tomography (B), showing enlarged right atrium.
Her plasma B-type natriuretic peptide level was 611 pg/mL. Euro score II was 3.5%, logistic Euro score was 11.8%, and STS score was 5.3%. Given a high risk of surgical intervention to atrial septum deficiency, we decided to intervene to her bradycardia to improve her symptoms. We preferred a leadless pacemaker (Micra trans-catheter pacing system; Medtronic, Inc, Minneapolis, MN, USA) instead of a conventional one with pacing leads, considering her moderate tricuspid regurgitation.
Leadless pacemaker implantation
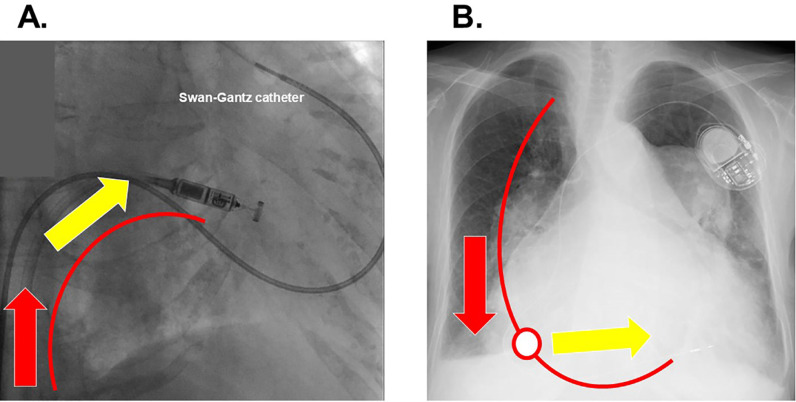
We performed leadless pacemaker implantation under general anesthesia via right femoral vein approach. Both transesophageal echocardiography and intra-cardiac echocardiography failed to let us observe whole right heart anatomy due to enlarged right atrium, and a Swan-Gantz catheter remained in the left pulmonary artery to guide the catheter (Fig. 3A). We could direct the delivery catheter tangentially to the septum by adjusting the tip of the catheter, but the delivery catheter was not deeply advanced enough into the right ventricle due to extremely enlarged right heart. We could not make an appropriate “gooseneck shape” by sufficiently the attaching catheter on the interventricular septum, and eventually decided not to proceed.
Fig. 3.
Micra implantation procedure under the swan-gantz catheter guidance (A) and chest X-ray following the implantation of conventional trans-venous pacemaker (B). When we pushed the Micra catheter via the femoral approach (red arrow; A), the power did not direct tangentially toward the ventricular septum (yellow arrow; A) because of the lack of support point. This is the rationale why we attempted to make a gooseneck shape. On the contrary, when we pushed the lead via the jugular or subclavian approach (red arrow; B), the power was translated into the appropriate direction toward interventricular septum (yellow arrow; B) due to the support point (red circle; B).
Conventional pacemaker implantation
Four days later, we performed conventional VVI pacemaker implantation via left subclavian vein approach. SelectSecure™ (Medtronic) using a C315 delivery catheter could be successfully screwed in the interventricular septum against moderate tricuspid regurgitation, obtaining an appropriate electrical condition (V lead impedance 711 Ω, R wave 10.1 mV, and V lead threshold 0.5 V/0.40 msec; Fig. 3B). The procedure was completed without any complications and the pacing rate was set at 80 bpm.
Post-procedural course
Post-procedural course has passed without any comorbidities. Congestion has improved with body weight reduction from 48 kg on admission down to 43 kg. Plasma B-type natriuretic peptide decreased to 127 pg/mL on discharge.
Discussion
Indication of Micra versus conventional trans-venous pacemaker
Given that the only available mode of Micra system is VVI thus far, the dominant arrhythmic indication is atrioventricular block with concurrent atrial fibrillation as in our patient, whereas its indication is expanding to atrioventricular block and sinus node disease if they require less frequent pacing [7].
Given less invasiveness, low infection rate, and preserved physical activity, Micra system is preferred particularly in the elderly population [8]. Those with a superior venous obstruction or advanced tricuspid regurgitation are also good candidates for the Micra system rather than the conventional trans-venous pacemaker [9]. Given relatively high age and moderate tricuspid regurgitation, we preferred the Micra system in this case.
On the contrary, Micra has several limitations including unclarified long-term evidence and battery depletion. Young patients or those requiring frequent pacing may not be good candidates for the Micra system [7].
Micra system and anatomical features
Another concern is an anatomical anomaly as we experienced. The literature proposes several implantation techniques including advancement of the delivery catheter to make gooseneck appearance and contrast guiding, both of which failed in our case due to too enlarged right atrium. A double snare technique that Alyesh and colleagues proposed might have been an effective technique for improving the reach of the delivery system [6].
It would have been an appropriate decision to decline Micra implantation before encountering serious complications in our case. A conventional trans-superior vena cava pacemaker could be instead successfully deployed when we used SelectSecure lead with a C315 delivery catheter that enhanced back-up force toward the ventricular septum against significant tricuspid regurgitation. The existence of tricuspid regurgitation might not be a contraindication to conventional trans-venous pacemaker implantation if appropriate delivery systems are used. Of note, trans-superior vena cava approach might have an advantage over the trans-femoral approach in receiving sufficient back-up force from the right ventricular wall and directing the device tangentially toward the interventricular septum [10].
The enlarged right heart due to congenital anomaly or any other secondary right heart failure might not be a good indication for Micra implantation, although further large-scale study is warranted to propose any index or cut-off of right heart size that is associated with procedure failure. Another challenging anatomy might be a relatively horizontal tricuspid valve line. In this case, it was challenging to advance the catheter transiently toward the septum. Micra implantation via right jugular vein might overcome such a disadvantage, although this has not yet been a standard procedure [9]. Micra is a promising system, but we should also understand its limitations and alternative procedures particularly for those with anatomical anomalies.
Declaration of Competing Interest
TI receives grant support from JSPS KAKENHI: JP20K17143. Other authors have no disclosures.
References
- 1.Kirkfeldt R.E., Johansen J.B., Nohr E.A., Jorgensen O.D., Nielsen J.C. Complications after cardiac implantable electronic device implantations: an analysis of a complete, nationwide cohort in Denmark. Eur Heart J. 2014;35:1186–1194. doi: 10.1093/eurheartj/eht511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Duray G.Z., Ritter P., El-Chami M., Narasimhan C., Omar R., Tolosana J.M., Zhang S., Soejima K., Steinwender C., Rapallini L., Cicic A., Fagan D.H., Liu S., Reynolds D. Micra transcatheter pacing study group. Long-term performance of a transcatheter pacing system: 12-month results from the Micra transcatheter pacing study. Heart Rhythm. 2017;14:702–709. doi: 10.1016/j.hrthm.2017.01.035. [DOI] [PubMed] [Google Scholar]
- 3.Reynolds D., Duray G.Z., Omar R., Soejima K., Neuzil P., Zhang S., Narasimhan C., Steinwender C., Brugada J., Lloyd M., Roberts P.R., Sagi V., Hummel J., Bongiorni M.G., Knops R.E. A leadless intracardiac transcatheter pacing system. N Engl J Med. 2016;374:533–541. doi: 10.1056/NEJMoa1511643. [DOI] [PubMed] [Google Scholar]
- 4.Reddy V.Y., Exner D.V., Cantillon D.J., Doshi R., Bunch T.J., Tomassoni G.F., Friedman P.A., Estes N.A.M., Ip J., Niazi I., Plunkitt K., Banker R., Porterfield J., Ip J.E., Dukkipati S.R. Percutaneous implantation of an entirely intracardiac leadless pacemaker. N Engl J Med. 2015;373:1125–1135. doi: 10.1056/NEJMoa1507192. 3rd. [DOI] [PubMed] [Google Scholar]
- 5.Roberts P.R., Clementy N., Al Samadi F., Garweg C., Martinez-Sande J.L., Iacopino S., Johansen J.B., Prat X.V., Kowal R.C., Klug D., Mont L., Steffel J., Li S., Van Osch D., El-Chami M.F. A leadless pacemaker in the real-world setting: the Micra transcatheter pacing system post-approval registry. Heart Rhythm. 2017;14:1375–1379. doi: 10.1016/j.hrthm.2017.05.017. [DOI] [PubMed] [Google Scholar]
- 6.Alyesh D., Cunnane R. Successful leadless pacemaker deployment in a patient with challenging right heart anatomy using a double snare technique. HeartRhythm Case Rep. 2019;5:399–401. doi: 10.1016/j.hrcr.2018.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Piccini J.P., Stromberg K., Jackson K.P., Kowal R.C., Duray G.Z., El-Chami M.F., Crossley G.H., Hummel J.D., Narasimhan C., Omar R., Ritter P., Roberts P.R., Soejima K., Reynolds D., Zhang S. Patient selection, pacing indications, and subsequent outcomes with de novo leadless single-chamber VVI pacing. Europace. 2019;21:1686–1693. doi: 10.1093/europace/euz230. [DOI] [PubMed] [Google Scholar]
- 8.El-Chami M.F., Soejima K., Piccini J.P., Reynolds D., Ritter P., Okabe T., Friedman P.A., Cha Y.-.M., Stromberg K., Holbrook R., Faga D.H., Roberts P.R. Incidence and outcomes of systemic infections in patients with leadless pacemakers: data from the Micra IDE study. Pacing Clin Electrophysiol. 2019;42:1105–1110. doi: 10.1111/pace.13752. [DOI] [PubMed] [Google Scholar]
- 9.Kolek M.J., Crossley G.H., Ellis C.R. Implantation of a MICRA leadless pacemaker via right internal jugular vein. JACC Clin Electrophysiol. 2018;4:420–421. doi: 10.1016/j.jacep.2017.07.014. [DOI] [PubMed] [Google Scholar]
- 10.Imamura T., Kinugawa K., Nitta D., Fujino T., Inaba T., Maki H., Hatano M., Kinoshita O., Nawata K., Yao A., Kyo S., Ono M. Is the internal jugular vein or femoral vein a better approach site for endomyocardial biopsy in heart transplant recipients? Int Heart J. 2015;56:67–72. doi: 10.1536/ihj.14-156. [DOI] [PubMed] [Google Scholar]