Abstract
Heterozygous familial hypercholesterolemia (HeFH) is a common, autosomal dominant, genetic disease that results in premature atherosclerotic cardiovascular disease secondary to high-level low-density lipoprotein cholesterol (LDL-C) exposure. We present a 68-year-old male patient with HeFH who was diagnosed with acute coronary syndrome at 9 months after coronary artery bypass grafting, although his LDL-C level was decreased to 77 mg/dL from 213 mg/dL. The emergency coronary angiography revealed that all bypass grafts were occluded, and the large atherosclerotic plaque burden was observed even in right internal thoracic artery (RITA) by intravascular ultrasound examination. Emergency percutaneous coronary intervention (PCI) was performed to his RITA bypass graft. After strict LDL-C management with proprotein convertase subtilisin/kexin 9 (PCSK-9) inhibitors, re-stenosis was not observed at the PCI site and the atherosclerotic plaque burden in his graft drastically disappeared. The high-risk HeFH patients, including those suffering from coronary bypass graft stenosis despite receiving medical therapy, might need stricter management of lipid profile with PCSK-9 inhibitors.
<Learning objective: Heterozygous familial hypercholesterolemia (HeFH) is one of the most common genetic disease causes of premature coronary disease. High-risk HeFH patients with multiple risk factors, including those suffering from coronary bypass graft stenosis despite receiving medical therapy, might need stricter management of lipid profile with proprotein convertase subtilisin/kexin 9inhibitors than that recommended in the guideline.>
Keywords: Familial hypercholesterolemia, Proprotein convertase subtilisin/kexin 9 inhibitor, Acute coronary syndrome, Coronary artery bypass grafting, Strict management for high-risk familial hypercholesterolemia
Introduction
Heterozygous familial hypercholesterolemia (HeFH) is one of the most common, autosomal dominant, genetic diseases caused by mutations in the low-density lipoprotein-receptor (LDLR), proprotein convertase subtilisin/kexin 9 (PCSK-9), or apolipoprotein B (ApoB) genes. FH results from premature atherosclerotic cardiovascular disease due to exposure to high levels of low-density lipoprotein cholesterol (LDL-C) [1]. Therefore, patients with HeFH have a high risk for cardiovascular disease (CVD) without any intervention, with approximately 50% and 30% of men and women experiencing CVD by the age of 50 and 60 years, respectively. The prevalence of HeFH has been estimated at 1/200–1/500. However, the estimated rate of diagnosed patients with FH is <1% in Japan [2]. According to the Japanese Atherosclerosis Society FH guidelines 2012 for the management of dyslipidemias in patients with HeFH, LDL-C should be controlled at <100 mg/dL or patients must have a 50% reduction from the baseline level without medications. Furthermore, the Japanese Atherosclerosis Society FH guidelines 2017 were revised in the patients with HeFH requiring secondary prevention for acute coronary syndrome, LDL-C should be controlled at <70 mg/dL [1]. PCSK9 is a secreted protein that binds to the LDLR, preventing it from recycling to the cell surface. This results in less available LDLR and higher circulating LDL-C levels. PCSK9 inhibitors increase hepatic LDLR recycling, which enhances LDL-C clearance from the serum [3]. PCSK9 inhibitors produce incremental LDL-C lowering in statin-treated patients. Given that we can control the patients’ LDL-C levels more strictly with PCSK-9 inhibitors, the extent of LDL-C reduction is the most important question.
We present a case of HeFH in a patient diagnosed with acute coronary syndrome 9 months after coronary artery bypass grafting (CABG); the patient's atherosclerotic plaque in the graft improved drastically by the strict LDL-C management with the PCSK-9 inhibitor treatment.
Case report
A 68-year-old man visited a local doctor due to a complaint of shortness of breath during exertion in March 2016. He was diagnosed with coronary ischemia because his electrocardiogram (ECG) revealed abnormal Q wave in V1–3 and negative T wave in V4, and he was referred to our hospital. He had not received treatment for hypercholesterolemia, since his diagnosis at 58 years old. His-smoking history was 20 pack years, but he quit smoking at 40 years old. He took two cups of shochu per day. Physical examination revealed that he had a cornea wheel in his eyes, but no Achilles tendon growths were noted. He was diagnosed with HeFH because his mother had premature CVD and his LDL-C level at the first visit was 213 mg/dl without medications, which were according to the Japanese Atherosclerosis Society FH guideline 2012. In addition, he had a high level of triglyceride (TG, 355 mg/dL), oxidized LDL-C (246 U/L), ApoB (165 mg/dL), and remnant-like lipoprotein particles-cholesterol (RLP, 17 mg/dL) and a low level of high-density lipoprotein-cholesterol (HDL-C, 31 mg/dL) without therapy (Online Table 1). The results of the exercise stress myocardial scintigraphy showed severe ischemia (Fig. 1A); thus, he was hospitalized to undergo coronary angiography (CAG) in April 2016 (Fig. 1B). He had two-vessel disease (#1 total occlusion, #6 99% stenosis, and #9 99% stenosis) and underwent CABG with the right internal thoracic artery (RITA) to the left anterior descending artery (LAD), the left internal thoracic artery (LITA) to the first diagonal branch (D1), and the gastrointestinal artery (GEA) to the posterior descending artery (4PD). Medications for hypercholesterolemia included pitavastatin 1 mg initially and finally pitavastatin 4 mg and ezetimibe 10 mg. His-LDL-C level decreased to 77 mg/dL from 213 mg/dL at 7 months after CABG. However, oxidized LDL-C and high sensitivity C-reactive protein (hsCRP) were still elevated (from 246 U/L to 97 U/L, from 732 mg/dL to 1210 mg/dL, respectively). At 9 months after CABG, he was re-admitted owing to a heart attack due to an unstable angina pectoris (uAP), and emergency CAG was performed (Fig. 2). All bypass grafts were occluded, and the atherosclerotic plaque was observed even in RITA by intravascular ultrasound (IVUS) examination (Fig. 3A). The emergency percutaneous coronary intervention (PCI) was performed to his RITA bypass graft. The gene analytical results showed that he had no significant FH gene mutation except for PCSK-9 (V4I and A53V) and ApoA5(G185C) by next generation sequencing (NGS) on the Illumina Mi-seq sequencer (Illumina, San Diego, CA, USA) using custom-made HaloPlex (Agilent Technologies, Santa Clara, CA, USA). For clinically severe HeFH, we thought that his lipid profile should be controlled more strictly; thus, we decided to start the treatment with PCSK-9 inhibitors.
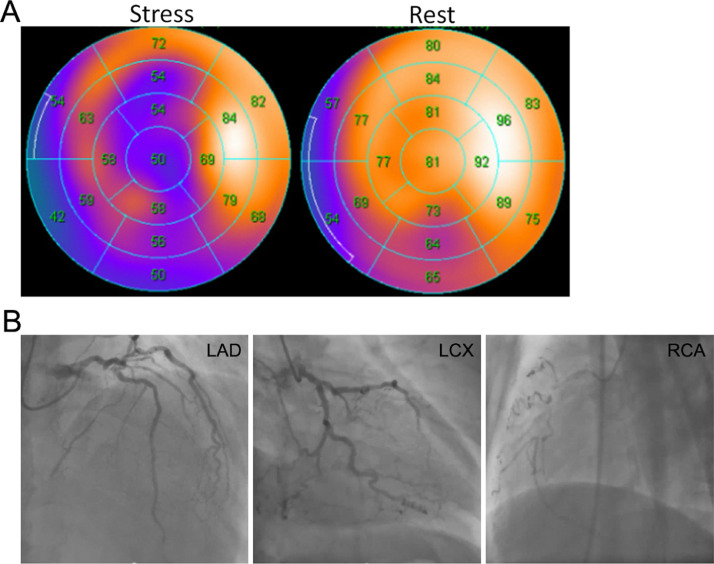
Fig. 1.
(A) Exercise stress myocardial scintigraphy showed severe transient ischemia of the left anterior descending artery (LAD) and right coronary artery (RCA) region. (B) Coronary angiography revealed that the RCA #1 had total occlusion, the LAD #6 had 99% stenosis, and the left circumflex artery (LCX) #9 had 99% stenosis.
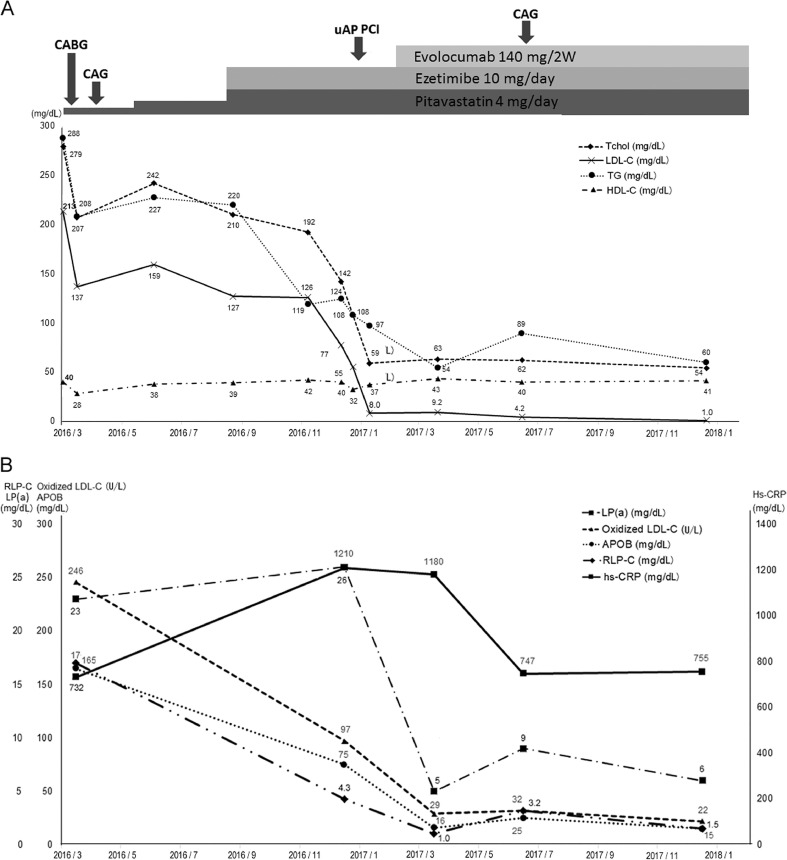
Fig. 2.
The lipid profile and hs-CRP changes in the clinical course. (A) T-chol, total cholesterol; LDL-C, low-density lipoprotein cholesterol; TG, triglycerides; HDL-C, high-density lipoprotein cholesterol; PCI, percutaneous coronary intervention. (B) LP(a), lipoprotein(a); oxidized LDL-C, oxidized low-density lipoprotein cholesterol; APOB, apolipoprotein B; RLP-C, remnant-like lipoprotein particles-cholesterol; hs-CRP, high-sensitivity C-reactive protein; CABG, coronary artery bypass grafting; CAG, coronary angiography; uAP, unstable angina pectoris.
Fig. 3.
Coronary angiography (CAG) and intravascular ultrasound (IVUS) images. (A) CAG image on the left side shows that the right internal thoracic artery had 99% stenosis, as shown by the arrow. IVUS image on the right side shows the atherosclerotic plaque and the plaque ratio in the right internal thoracic artery (where the 4 white line points on the left side of the image). (B) CAG image on the left side shows that the right internal thoracic artery had no re-stenosis. Arrows point to the region where we performed percutaneous coronary intervention. IVUS image on the right side shows atherosclerotic plaque decrease in the right internal thoracic artery (where the 4 white line points on the left side of the image). IVUS images in panels A and B are almost on the same region.
The patient received evolocumab 140 mg twice in a month. After two times of PCSK-9 inhibitor administration, his total cholesterol (T-chol) and LDL-C levels decreased to 59 and 8.0 mg/dL, respectively. His-oxidized LDL-C, lipoprotein(a) [LP(a)], ApoB, and RLP-C levels were also decreased (22 U/L, 15 mg/dL, and 1.5 mg/dL, respectively). His-LDL-C level remained at <10 U/L without any side effects (Fig. 2A, B). We performed follow-up CAG at 5 months after the uAP event and started evolocumab. No re-stenosis at the region where we performed PCI and obvious atherosclerotic plaque decrease in the RITA graft were noted in the IVUS examination (Fig. 3B, the average of cross-section plaque ratio from 38.7 to 19.4%). We continued to strictly control his LDL-C level, and the patient has had no coronary event after the uAP event.
Discussion
We experienced a patient who suffered from occlusion of the bypass graft even if his LDL-C level was 77 mg/dL and he was taking statin and ezetimibe. After starting PCSK-9 inhibitors, his LDL-C has been strictly controlled, and the atherosclerotic plaque drastically disappeared.
This patient was diagnosed with clinical HeFH based on his familial medical history and his high LDL-C level without medication. Age (male > 35 years and female > 45 years), LDL-C > 260 mg/dL, TG > 150 mg/dL, HDL-C < 40 mg/dL, LP (a) > 50 mg/dL, Achilles tendon thickening > 15 mm, male sex, hypertension, diabetes, body mass index, smoking, chronic kidney disease, and a family history of CVD are known risk factors for coronary events in patients with HeFH [4]. The following risk factors were present in our patient: age, male sex, smoking, LDL-C level, and family history of CVD. In addition, the oxidized LDL-C, RLP-C, and hsCRP were elevated. Given these findings, he was a very high-risk HeFH patient and should be treated strictly as a secondary prevention. However, the LDL-C level was not controlled well before and after CABG; thus, unstable angina occurred at 9 months after CABG. The detection and diagnosis rates of FH are low in Japan. In fact, our patient was not diagnosed with HeFH before undergoing CABG, and continuous high LDL-C levels led to failure to prevent secondary coronary events.
As reported in a secondary analysis of the FOURIER trial, there was a monotonic relationship between achieved LDL-C and major cardiovascular outcomes down to LDL-C concentration of < 0.2 mmol/L (7.7 mg/dL) without safety concerns [5]. This result suggested that the lower the LDL-C level, the better the outcome, if it is controlled under the guideline-recommended level. Two randomized, double-blind studies (ODYSSEY FH I and FH II) that assessed the outcomes of long-term (78 weeks) PCSK-9 inhibitor (alirocumab) treatment in patients with HeFH and those with inadequate LDL-C control after undergoing maximally tolerated lipid-lowering therapy indicated that alirocumab treatment was well tolerated and resulted in significant LDL-C reduction and greater achievement of LDL-C target levels [6]. In this case, coronary bypass graft arteriosclerosis progressed for only 8 months, despite taking pitavastatin 4 mg and ezetimibe 10 mg and LDL-C level of 77 mg/dL, according to the Japanese Atherosclerosis Society FH guidelines 2012. After starting treatment with PCSK-9 inhibitors, his LDL-C was controlled at < 10 mg/dL and the plaque in RITA drastically disappeared.
Previous papers reported that LP (a) and ApoB increase the risk of cardiovascular disease [7, 8]. Oxidized LDL-C plays an important role in the atherogenesis by promoting an inflammatory environment and lipid deposition in the arterial wall [9]. This patients’ lipid profile improvement including oxidized-LDL, Lp(a), and ApoB by PCSK-9 inhibitors contributed to plaque disappearance of the coronary bypass graft. A sub-analysis of the FOURIER trial proved that lowering of LDL-C levels with evolocumab reduced the risk of major adverse limb events among patients with peripheral artery disease [5]. Our case is among those cases showing that strict management of LDL-C is an effective treatment strategy for coronary bypass graft stenosis. According to the GLAGOV randomized clinical trial, among patients with angiographic coronary disease treated with statins, the addition of evolocumab, compared with a placebo, resulted in a greater decrease in percent atheroma volume after 76 weeks of treatment [10]. We performed IVUS to the RITA bypass graft before and after treatment with PCSK-9 inhibitors and found drastically atherosclerotic plaque decrease in the RITA graft.
In conclusion, high-risk HeFH patients, including those suffering from coronary bypass graft stenosis despite receiving medical therapy, might need stricter management of lipid profile with PCSK-9 inhibitors than that recommended in the guidelines.
Declaration of Competing Interest
The authors declare that there is no conflict of interest.
Acknowledgments
None.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jccase.2021.02.012.
Appendix. Supplementary materials
References
- 1.Kinoshita M., Yokote K., Arai H., Iida M., Ishigaki Y., Ishibashi S., Umemoto S., Egusa G., Ohmura H., Okamura T., Kihara S., Koba S., Saito I., Shoji T., Daida H. Japan Atherosclerosis society (JAS) guidelines for prevention of Atherosclerotic Cardiovascular diseases 2017. J Atheroscler Thromb. 2018;25:846–984. doi: 10.5551/jat.GL2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nordestgaard B.G., Chapman M.J., Humphries S.E., Ginsberg H.N., Masana L., Descamps O.S., Wiklund O., Hegele R.A., Raal F.J., Defesche J.C., Wiegman A., Santos R.D., Watts G.F., Parhofer K.G., Hovingh G.K. Familial hypercholesterolaemia is underdiagnosed and undertreated in the general population: guidance for clinicians to prevent coronary heart disease: consensus statement of the European Atherosclerosis Society. Eur Heart J. 2013;34:3478. doi: 10.1093/eurheartj/eht273. 90a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reiner Z. Management of patients with familial hypercholesterolaemia. Nat Rev Cardiol. 2015;12:565–575. doi: 10.1038/nrcardio.2015.92. [DOI] [PubMed] [Google Scholar]
- 4.Akioyamen L.E., Genest J., Chu A., Inibhunu H., Ko D.T., Tu J.V. Risk factors for cardiovascular disease in heterozygous familial hypercholesterolemia: a systematic review and meta-analysis. J Clin Lipidol. 2019;13:15–30. doi: 10.1016/j.jacl.2018.10.012. [DOI] [PubMed] [Google Scholar]
- 5.Bonaca M.P., Nault P., Giugliano R.P., Keech A.C., Pineda A.L., Kanevsky E. Low-density lipoprotein cholesterol lowering with evolocumab and outcomes in patients with peripheral artery disease: insights from the FOURIER trial (Further Cardiovascular Outcomes Research With PCSK9 Inhibition in Subjects With Elevated Risk) Circulation. 2018;137:338–350. doi: 10.1161/CIRCULATIONAHA.117.032235. [DOI] [PubMed] [Google Scholar]
- 6.Kastelein J.J., Ginsberg H.N., Langslet G., Hovingh G.K., Ceska R., Dufour R., Blom D., Civeira F., Krempf M., Lorenzato C., Zhao J., Pordy R., Baccara-Dinet M.T., Gipe D.A., Geiger M.J. Odyssey fh i and fh ii: 78 week results with alirocumab treatment in 735 patients with heterozygous familial hypercholesterolaemia. Eur Heart J. 2015;36:2996–3003. doi: 10.1093/eurheartj/ehv370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsimikas S., Viney N.J., Hughes S.G., Singleton W., Graham M.J., Baker B.F., Burkey J.L., Yang Q., Marcovina S.M., Geary R.S., Crooke R.M., Witztum J.L. Antisense therapy targeting apolipoprotein(a): a randomised, double-blind, placebo-controlled phase 1 study. Lancet. 2015;386:1472–1483. doi: 10.1016/S0140-6736(15)61252-1. [DOI] [PubMed] [Google Scholar]
- 8.Clarke R., Peden J.F., Hopewell J.C., Kyriakou T., Goel A., Heath S.C., Parish S., Barlera S., Franzosi M.G., Rust S., Bennett D., Silveira A., Malarstig A., Green F.R., Lathrop M. Genetic variants associated with Lp(a) lipoprotein level and coronary disease. N Engl J Med. 2009;361:2518–2528. doi: 10.1056/NEJMoa0902604. [DOI] [PubMed] [Google Scholar]
- 9.Trpkovic A., Resanovic I., Stanimirovic J., Radak D., Mousa S.A., Cenic-Milosevic D., Jevremovic D., Isenovic E.R. Oxidized low-density lipoprotein as a biomarker of cardiovascular diseases. Crit Rev Clin Lab Sci. 2015;52:70–85. doi: 10.3109/10408363.2014.992063. [DOI] [PubMed] [Google Scholar]
- 10.Nicholls S.J., Puri R., Anderson T., Ballantyne C.M., Cho L., Kastelein J.J., Koenig W., Somaratne R., Kassahun H., Yang J., Wasserman S.M., Scott R., Ungi I., Podolec J., Ophuis A.O. Effect of evolocumab on progression of coronary disease in statin-treated patients: the GLAGOV randomized clinical trial. JAMA. 2016;316:2373–2384. doi: 10.1001/jama.2016.16951. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.