Abstract
Millions of children are impacted by neurodevelopmental disorders (NDDs), which unfold early in life, have varying genetic etiologies and can involve a variety of specific or generalized impairments in social, cognitive and motor functioning requiring potentially lifelong specialized supports. While specific disorders vary in their domain of primary deficit (e.g. autism spectrum disorder (social), attention-deficit/hyperactivity disorder (attention), developmental coordination disorder (motor) and developmental language disorder (language)), comorbidities between NDDs are common. Intriguingly, many NDDs are associated with difficulties in skills related to rhythm, timing and synchrony though specific profiles of rhythm/timing impairments vary across disorders. Impairments in rhythm/timing may instantiate vulnerabilities for a variety of NDDs and may contribute to both the primary symptoms of each disorder as well as the high levels of comorbidities across disorders. Drawing upon genetic, neural, behavioural and interpersonal constructs across disorders, we consider how disrupted rhythm and timing skills early in life may contribute to atypical developmental cascades that involve overlapping symptoms within the context of a disorder's primary deficits. Consideration of the developmental context, as well as common and unique aspects of the phenotypes of different NDDs, will inform experimental designs to test this hypothesis including via potential mechanistic intervention approaches.
This article is part of the theme issue ‘Synchrony and rhythm interaction: from the brain to behavioural ecology’.
Keywords: neurodevelopmental disorders, rhythm, synchrony, language disorders, autism spectrum disorder, developmental coordination disorder
1. Introduction
Rhythm and timing are essential to successful development. Beginning in the neonatal period, infants are sensitive to rhythmic regularities present in music and speech, with electroencephalography (EEG) studies indicating detection of auditory changes occurring at rhythmically salient moments [1,2]. According to some theories, humans' sensitivity to rhythm to coordinate behaviour is essential to the forming of social bonds critical for survival [3]. The importance of rhythm is exemplified by its prominence in infant-directed communication, which provides a predictable, temporal structure to facilitate coordinated interaction between infant and carer (e.g. modulation of attention, turn-taking vocal exchanges) [4]. Rhythm is also salient to infants in part because it crosses modalities [5]: for example, a mother singing a nursery rhyme to her child provides integrated rhythmic information across auditory (vocal acoustics), visual (facial and body movements), vestibular (rocking/bouncing motion) and haptic (patting child) modalities. By supporting temporal predictions regarding when and what will occur across modalities, rhythm provides a foundation for individuals to attend to the world around them and plan and execute behaviours. Indeed, success in early temporal coordination such as measured, e.g. by the relative durations of interpersonal pauses during an adult-infant vocal interaction, predicts an array of socio-cognitive outcomes in language, cognition and attachment (e.g. [4]).
Given the fundamental role of rhythm in typical development, it is perhaps not surprising that impairments in rhythm, timing and synchrony/coordination occur across a variety of neurodevelopmental disorders (NDDs) [6–8]. NDDs, which impact millions of children and include both relatively common (e.g. attention-deficit/hyperactivity disorder (ADHD); autism spectrum disorder (ASD); developmental coordination disorder (DCD); language disorders) and rare (e.g. specific genetic syndromes) disorders, begin early in life and may require lifelong, specialized supports. NDDs are characterized by specific constellations of strengths and weaknesses that involve cognitive, sensory, motor and social functioning (table 1). While the aetiology and primary diagnostic deficit(s) of specific disorders vary, overlapping behavioural symptoms occur across NDDs and comorbidities between disorders are common [9]. Here, we explore an emerging framework which suggests that impairments in rhythm and time processing may be associated with comorbidities and common areas of divergence from typical development across different NDDs; moreover, individual differences in rhythm processing could explain the heterogeneity of skill development within disorders. In the current paper, we integrate knowledge from genetic, neuroscientific, behavioural and clinical perspectives to examine the development of rhythm in its phenotypic correlations and biological underpinnings across NDDs. We review cross-domain rhythm skills in a variety of NDDs and propose future directions that include rhythm in models of typical and atypical human development.
Table 1.
NDDs associated with impairments in rhythm/timing skills. (NDDs emerge early in development and involve impairments/delays in one or more functional domains.)
| NDD | primary characterization | estimated prevalence | common comorbidities (examples)a |
|---|---|---|---|
| attention-deficit/ hyperactivity disorder (ADHD) | persistent pattern of inattention and/or hyperactivity-impulsivity that interferes with functioning or development [9] | approximately 4–7% | learning disorders including dyslexia (18–45%) [10], DLD (45%) [11], other externalizing disorders (e.g. oppositional defiant disorder; 50–60%) [12], DCD (50%) [12] |
| autism spectrum disorder (ASD) | early emerging and persistent deficits in social communication and interaction and restricted, repetitive patterns of behaviour [9] | approximately 1.5% | ADHD (20–59%) [13,14], anxiety (20–40%) [14], intellectual disability (33%) [15] |
| developmental coordination disorder (DCD) | impairments in motor skills and coordination (e.g. clumsiness, slowness and inaccuracy of motor skills) that significantly and persistently interfere with everyday activities [9] | approximately 5–6% | ADHD (50%) [16,17], learning disorders including dyslexia, DLD [13,14], anxiety (16–48%) [18] |
| developmental language disorder (DLD) | difficulties in understanding and/or using language in one or multiple domains (e.g. expressive grammar) that cannot be explained by a known biomedical condition (such as a brain injury, hearing loss, intellectual disability, or ASD) [19] | approximately 3–7% | ADHD (46%) [20], DCD (20–75%) [21], dyslexia (17–67%) [22] |
| dyslexia | learning disorder involving difficulties in word decoding and identifying speech sounds, which impact the development of reading and spelling skills [23] | approximately 3–10% | ADHD (18–42%) [10], DLD (15–55%) [22], DCD (40–57%) [24] |
| stuttering | speech disorder characterized by frequent occurrences of repetitions or prolongations of sounds, syllables, or words that disrupt the rhythmic flow of speech [9] | approximately 0.3–5.6% | ADHD (6%), subclinical ADHD symptoms (approx. 50%) [25], language disorder (35%) [26] |
| Williams syndrome (WS) | neurodevelopmental disorder caused by the deletion of approximately 26 genes on chromosome 7; associated with mild to moderate intellectual deficits, attention problems, hypersociability, and auditory sensitivities [27] | approximately 0.01% | ADHD (65%) [28], anxiety/phobias (50%) [28], intellectual disability (43%) [29], ASD (10–20%) [30,31] |
aPrevalence rates of comorbidities may vary across studies in part owing to study design (e.g. population sample versus clinical cohort; screening surveys versus clinical interviews; self- versus parent-report) and changes in diagnostic criteria across time (including whether or not diagnosis of specific separate comorbid disorders was permitted).
2. Genetic and neural perspectives in rhythm/timing impairments: it is time for a transdiagnostic approach
Human rhythm and timing sensitivities emerge very early in development and contribute to adaptive behaviours including language and social communication. If (impaired) rhythm processing plays a role in the manifestation of NDDs, we must consider what genetic factors might play a role in the development of rhythm processing. Similar to most cognitive traits that vary across the population, there is a continuous, normal distribution of rhythm sensitivity/skill in the population, and these individual differences can provide a lens into the underlying biology. Rhythm-related skills such as rhythm discrimination and sensorimotor tapping show moderate heritability (i.e. a proportion of the variance in rhythm skills are owing to genetic factors), as demonstrated by twin studies (e.g. [32]). The molecular genetics underlying rhythm ability have also been recently investigated in a large-scale genome-wide association study (GWAS) [33]. Genetic associations with self-reported beat synchronization were examined in n = 606 825 (the phenotype was responses to the item ‘Can you clap in time with a musical beat?’, validated in relation to task-based synchronization to a musical beat in a separate sample). Findings indicated a highly polygenic genetic architecture, i.e. beat synchronization is associated with genetic variation at a large number of genomic loci occurring widely across the genome. These genetic associations showed enrichment for regions of the genome that regulate gene expression in the central nervous system, both in fetal and adult brain tissue, highlighting potential early neurodevelopmental processes. The study also uncovered genetic pleiotropy (shared genetic architecture among distinct traits [34]) between beat synchronization and two types of biological rhythms: respiration and circadian chronotypes.
These initial GWAS results for rhythm align with conceptual frameworks of ongoing work in the psychiatric genetics field showing that widespread pleiotropy among cognitive/neurological traits is the rule rather than exception [35]. Not only is there emerging evidence of pleiotropy between neurological and psychiatric traits [36,37], but recent work analysing the relationship between over 500 GWAS's shows that 90% of genomic loci were linked to multiple traits [38]. Indeed, pleiotropic genetic effects span clinical/developmental boundaries [35] and are reported for a growing number of clinically distinct disorders (see for example shared genetic liability of reading disability with several other NDD traits [39,40]). The atypical rhythm risk hypothesis (ARRH) predicts that genetic liability for atypical rhythm increases the risk of diverse developmental speech and language problems in part through genetic pleiotropy [6]. As evidence for this, ARRH points to atypical rhythm as a frequent feature among developmental speech and language disorders, with converging evidence of atypical rhythm in motor and attentional disorders that are often co-morbid with speech and language disorders [6]. Many different aspects of atypical rhythm task performance (e.g. rhythm discrimination, beat perception and synchronization) have been linked to these disorders (see below and the electronic supplementary material, table S1); there is not a clear, consistent pattern of a specific type of atypical rhythm being systemically linked only to a specific disorder. Indeed, performance on different rhythm tasks tends to be correlated [41], which may reflect some degree of underlying pleiotropy [34]. Per ARRH, some shared variance among genetics of atypical rhythm skills is expected to also overlap with developmental speech and language problems. Furthermore, frameworks such as ARRH are consistent with the development of transdiagnostic criteria [42], which focus on identifying behavioural and biological features co-occurring across diagnostically distinct conditions as an alternative to identifying single ‘core deficits’ of each diagnosis, (see [43] for discussion of the limitations of core-deficit hypotheses in NDDs, and table 1 for examples of cross-disorder comorbidities).
Genetic liabilities may link to impairments across disorders through neural endophenotypes for rhythm and time processing [6]. Rhythm and time perception and production involve interactions between the auditory and motor systems. Auditory rhythm processing involves a network of cortical and subcortical motor areas including basal ganglia, cerebellum, premotor cortex and supplementary motor area (see [44] for a review). Neurologically, listening to auditory rhythms without movement activates motor regions of the brain (e.g. [44,45]) and both transient brain stimulation and neurological disorders affecting motor regions impair time perception (e.g. [46,47]). People's rhythm perception is impacted by how they move to the music [48]—or, in the case of infants, how the infants are moved to the music [49]—as by vestibular stimulation [50]. Rhythm processing is subserved by neural oscillatory activity that synchronizes to external stimuli. Under frameworks such as Dynamic Attending Theory [51], neural entrainment to rhythmic stimuli involves extracting regularities from incoming sensory input to develop expectancies (predictions) regarding the timing and content of upcoming events. Neural entrainment to rhythmic signals such as music and speech is also modulated by attention and experience [52,53]. Structural abnormalities and atypical functional activity for rhythm and time processing are observed across a variety of NDDs including speech/language disorders, DCD, ASD and rare genetic syndromes (see below).
3. Rhythm and timing profiles in neurodevelopmental disorders
We next review profiles of NDDs with a focus on the common theme of atypical rhythm skills and their association with a variety of functional impairments on tasks targeting attention, language, communication and social functioning. We included disorders for which extant research has considered multiple aspects of rhythm and timing skills and because of their patterns of comorbidity with each other in these different domains of functioning.
4. Speech/language disorders
Speech/language disorders are common in childhood (3–16%) though many children are not formally diagnosed or treated [54]. As summarized in the ARRH [6], accumulating evidence demonstrates impaired timing skills across a variety of speech/language disorders, which exhibit frequent comorbidities among each other and with other disorders such as ADHD and DCD. The majority of rhythm research in speech/language disorders thus far has focused on dyslexia, a developmental disorder affecting approximately 3–10% of children with characteristic difficulties in word decoding, which impacts individuals' spelling performance and the development of reading fluency [23]. A few studies have investigated timing skills in children with developmental language disorder (DLD; previously known as specific language impairment), a similarly common developmental disorder with an approximately 3–7% prevalence rate, that is characterized by weak language abilities in one or multiple domains that cannot be explained by a known biomedical condition [19] (such as a brain injury, hearing loss or intellectual disability or ASD). Timing skills have also been examined in individuals with stuttering, which is a speech disorder appearing in approximately 0.3–5.6% of children [55]. The speech of children who stutter shows frequent occurrences of repetitions or prolongations of sounds, syllables or words that disrupt the rhythmic flow of speech [9].
Rhythm skills are often assessed with tapping tasks and rhythm discrimination tasks. Tapping tasks measure the ability to temporally coordinate with a predictable event by asking participants to tap to the beat of music or together with an isochronous metronome [56]. Performance is typically described by the consistency/variability of the taps, the difference between the expected and the actual tapping rate, or the difference between the expected and the actual times of the taps. In rhythm discrimination tasks, participants are typically presented with two rhythms that are either the same or different as a result of changing the duration of one or more tones or the interval between two tones. Compared to age-matched typically developing (TD) peers, tapping rates of children with DLD and with dyslexia have been reported to deviate more from the expected tapping rate, and taps of children who stutter to occur significantly earlier than the expected time according to the stimulus [57–59]. There is some inconsistency across studies in regard to behavioural performance, however, and between behavioural and neural measures (e.g. some studies report reduced tapping consistency in dyslexia [57,60], while others observe intact consistency of synchronized tapping [61,62] but atypical neural responses [61]). Rhythm discrimination has been found to be impaired in dyslexia, DLD and stuttering [58,63,64]. Processing of low-level cues related to rhythm processing, such as amplitude rise time or duration, has also frequently been found to be weaker in individuals with DLD [65] and dyslexia [57,60,66,67] compared to their TD peers, though one study did not observe differences in duration discrimination in dyslexia [66].
Impairments appear in the processing of timing-related aspects of spoken language as well. Children with dyslexia and DLD demonstrate impaired performance on tasks measuring word stress or sentence-level prosody processing [68,69]. In [68], word stress sensitivity was measured with two tasks in children with dyslexia and TD peers. At 9 years of age, children were presented with stimuli that had the same segmental information (the syllable dee presented repeatedly) but differed in their stress pattern, and had to choose the stimulus that matched a picture (e.g. the matching stimulus for a picture of Harry Potter was DEE-dee DEE-dee). Children with dyslexia demonstrated reduced sensitivity to word stress compared to age-matched and younger, reading-matched peers. At 13 years, stress sensitivity was measured with a stress discrimination task in which the same word was presented twice either with the same stress pattern or with different stress patterns (e.g. DIfficulty (SWWW) and diFFIculty (WSWW), and children had to decide if they are the same or different; children with dyslexia performed worse than age-matched peers [68]. Sentence-level prosody processing was found to be impaired in children with DLD in an artificial language learning task with and without prosodic cues (i.e. frequency, intensity and duration) to the underlying language structure. In contrast to children with TD, children with DLD could not take advantage of the prosodic cues in acquiring the rules of the artificial language [69].
Some of the structural and functional brain characteristics of children with speech/language disorders have been proposed to underlie atypical timing skills in these populations. Functional connectivity measured with resting-state functional magnetic resonance imaging in brain areas associated with rhythm processing (basal ganglia-thalamocortical network) as well as structural connectivity in areas supporting internal timing (putamen, supplementary motor area) appears to be weaker in individuals who stutter [70]. Interestingly, stuttering is significantly reduced if individuals are exposed to a regular external rhythm like a metronome and otherwise atypical brain activation patterns become similar to those in individuals who do not stutter in parallel with improved speech fluency [71]. Oscillatory activity in the beta frequency band, which has been proposed to reflect functional coordination between auditory and motor systems and involved in timing, has also been found to be atypical in adults who stutter [70]. In dyslexia, the degree of neural entrainment to the speech envelope [72] as well as to non-speech stimuli [73] is less when compared to TD children. Neural entrainment to the regularities of auditory stimuli has been proposed to be impaired in DLD as well, but to our knowledge, this hypothesis has not been tested yet, nor have other neural correlates of impaired timing skills in DLD.
Performance on various measures of rhythm as described above (speech rhythm, rhythm discrimination, tapping to the beat) has been associated with language skills in children with language impairments in multiple studies (e.g. [74]) indicating that a common mechanism underlies the development of both rhythm and language processing. This idea is supported by a recent meta-analysis of neuroimaging studies showing that shared neural structures underlying temporal hierarchical processing and predictive coding are involved in both rhythm and spoken language processing [75].
Motivated by findings on (i) weak timing skills in children with speech/language disorders and (ii) their associations with speech/language skills, it has been proposed that improving rhythm processing with music training could also benefit speech/language skills. In a randomized controlled trial, children with dyslexia performed better on rhythm reproduction, phonological awareness and reading tasks following 30 weeks of music training focusing on rhythm skills, compared to a control group that completed a painting training [76]. Improvements in rhythm skills and phonological awareness were associated suggesting that mechanisms involved in rhythm-based processing support language acquisition and phonological development.
The short-term effect of rhythm on subsequent speech/language processing of children with DLD and/or dyslexia has also been investigated with rhythmic priming paradigms: for instance, Przybylski et al. [77] found that children with dyslexia and DLD (as well as children with TD) perform better on a grammaticality judgement task following an exposure to a rhythm with a regular versus an irregular beat, indicating shared mechanisms underlying rhythm and speech/language processing.
5. Attention-deficit/hyperactivity disorder
ADHD is a common NDD (approx. 4–7% of children) characterized by inattention and/or hyperactivity-impulsivity, which interferes with everyday functioning [9]. Children may exhibit ADHD symptoms independent of other disorders though ADHD also occurs comorbidly with other NDDs (e.g. language disorders, DCD and ASD) [78–81]. Children with ADHD have difficulties with perceiving, reproducing or comparing durations [82] as well as with beat perception in music that cannot be accounted for by duration coding problems [83]. Beat perception is, however, comparable to TD children's performance when stimuli consist of simple sound patterns instead of music with a complex structure [83].
A recent study [83] found lower synchronization consistency in children with ADHD than in TD children in a finger tapping task, both when children were asked to synchronize their taps with tones and with the beat of music. Children with ADHD had more difficulty tapping to real music than simple tonal stimuli. Children with comorbid ADHD and DCD demonstrated even lower performance than children with only ADHD. The authors suggest that the greater difficulty with synchronizing to music may indicate difficulties with internal generation and maintenance of the beat in ADHD because the beat in music must be inferred based on the rhythmic patterns (versus it being provided by a periodic, acoustic cue as with a metronomic tone series) [83]. Interestingly, children with ADHD showing better beat tracking abilities also performed better on tasks measuring flexibility and inhibition, which are characteristic impairments in ADHD [83].
Structural brain abnormalities described in ADHD appear to be associated with these timing deficits. Duration discrimination impairments were related to atypical function of corticocerebellar and cortico-striatal pathways [84]. The basal ganglia and their connectivity with cortical areas (e.g. supplementary motor area, premotor cortex, auditory cortex) have been suggested to be associated with internal beat generation and maintenance (i.e. perceiving and maintaining the beat from the hierarchical rhythmic structure even without the onset of a physical acoustic cue at each beat time) [85]; abnormalities in these areas and pathways may underlie rhythmic difficulties with this process in individuals with ADHD, who often show structural basal ganglia abnormalities. In addition, research has revealed atypical resting-state oscillatory activity in children with ADHD, which is proposed to play a crucial role in temporal processing according to several theories (see [86] for a review).
While several studies have found impaired timing skills as well as structural/functional abnormalities in timing-related brain areas/functions in children with ADHD on the group level, these characteristics are not general across all children with ADHD. These results highlight individual differences within an NDD and suggest that timing impairments may be a feature of a subgroup of children with ADHD (see [83,86]).
6. Developmental coordination disorder
DCD is a NDD with a prevalence of around 5–6% that involves deficits in fine and/or gross motor skills, postural control, balance and motor learning that are significant enough to affect self-care and activities of daily living. While different criteria for diagnosis have been used (see [87]), in the Diagnostic and statistical manual of mental disorders, 5th edition [9], the definition of DCD includes early-developing very poor coordinated motor skills for age (e.g. scores less than 16th percentile on the Motor Assessment Battery for Children, MACB-2) that affect activities of leisure and play, impact academic and vocation performance, and that cannot be explained by intellectual impairment or other neurological conditions affecting motor function. DCD is heterogenous, involving fine and/or gross motor skills. Children with DCD may have trouble with tasks from running to throwing and catching a ball to tying their shoes to using pencils and scissors. For more than half of children diagnosed with DCD, the impairments continue into adolescence, suggesting that it is not simply a question of delayed motor development [88].
DCD has high co-morbidity with other developmental disorders involving difficulties with timing and rhythm [8]. It has been estimated that 50% of children with DCD also have a diagnosis of ADHD, and vice versa [78,79]. DCD has also been linked to language disorders, including DLD and dyslexia [89,90]. While children with ASD frequently exhibit DCD symptoms (up to 79%; [91]), less than 10% of children with DCD have ASD [92]. There are few genetic studies to date, but heritability estimates for DCD have been as high as 70% [93].
There is relatively little research in general on DCD compared to other developmental disorders, and the majority of studies have focused on motor or visuomotor impairments. However, several studies using questionnaires indicate that children with DCD also have general sensory processing difficulties, including in the domains of audition, balance (vestibular) and body awareness [94].
Motor deficits in DCD critically involve deficits in motor and sensorimotor timing [95], and poor timing (often conceptualized as poor predictive internal modelling) can explain their less accurate, slower and more variable performance on a number of motor tasks [8,96], including rhythmic multi-joint coordination, motor sequencing, motor learning, use of feedback, automatization and anticipation [95,96], as well as visual tracking of objects [97], visual-motor coincident timing [98] and tapping along to rhythmic sequences of visual [99] or auditory [100] events. Neuroimaging studies show general abnormalities including reduced cortical thickness, reduced white matter in sensorimotor tracts, and compromised connectome distributed networks (for a review, see [95]). The timing and rhythm deficits in DCD are particularly consistent with reported abnormalities in cerebellar function [95,101].
While the auditory and motor systems are intimately connected, little research has investigated auditory time and rhythm perception in DCD in the absence of a motor task. Chang et al. [102] hypothesized that children with DCD would show deficits in both auditory duration perception and auditory rhythm perception given evidence that the auditory system connects with the motor system to accomplish time perception. Using psychophysical methods, they found that, compared to TD children, those with DCD had poorer discrimination thresholds for detecting changes in the duration of single time intervals, and detecting the presence of non-isochrony in an otherwise isochronous tone sequence, while their pitch discrimination thresholds were relatively unaffected. Furthermore, EEG showed delayed event-related potential responses in the DCD children in response to change detection for duration and rhythm, but not pitch.
7. Autism spectrum disorder
Individuals with ASD, a heterogeneous but highly heritable NDD [93], constitute approximately 1.5% of the general population, and exhibit impairments in social engagement, communication and restricted/repetitive behaviours, which includes sensory atypicalities [9]. Individuals with ASD often exhibit comorbidities with various other psychiatric disorders and conditions, including epilepsy, ADHD, motor coordination deficits, anxiety and cognitive impairment [80]. Speech and language problems may also occur with some individuals not developing spoken language and others exhibiting fluent speech [103]. As for motor development and function, children and adults with ASD often show poor lower limb coordination during tasks requiring balance, agility and speed, and poor upper limb coordination during visuomotor and manual dexterity tasks [104]. Sensory and motor difficulties may contribute to and/or reflect social communication impairments in ASD [105] including in regard to rhythm and timing [106].
Attention problems are commonly observed in individuals with ASD [93]. Challenges in modulating attention to others (e.g. joint attention) may contribute to language impairments in individuals with ASD [107]. Reduced attention to social audio-visual speech synchrony is observed in children with ASD compared to children without ASD [108] and sensitivity to audio-visual synchrony is associated with language skills in children with ASD [109]. In regard to speech skills, some individuals exhibit repetitive, stereotyped language and/or atypical speech prosody [9,110]. Impairments in vocal turn-taking occur beginning in infancy [111] while difficulties maintaining appropriate conversational turn-taking are observed in some older children and adults [112].
In regard to nonverbal social coordination, children with ASD exhibit different and less stable forms of social synchronization, or the ability to synchronize one's body together with a partner [113]. For example, individuals with ASD show reduced synchronization to others during rhythm interaction activities such as swinging a pendulum [114], rocking in a rocking chair [115] and rhythmic clapping or drumming games [113,116,117]. Difficulties with behavioural imitation are also common [118]. Some studies relate social synchronization difficulties to performance on other social tasks though findings are somewhat mixed at this time perhaps owing to task and population heterogeneity [113,116,117]. A growing body of research seeks to enhance social-emotional capacities such as joint attention, eye contact, emotion inference and empathy via supporting interpersonal coordination and synchrony in both children and adults with ASD [119–121].
A few studies have investigated musical rhythm skills in ASD. Studies indicate age-appropriate musical rhythm perception (same/difference judgements of musical rhythm patterns) [122] and rhythm production (tapping rhythmic patterns with an example) [123], with performance increasing with age and degree of metric structure (i.e. better performance for strongly metrical versus weakly metric rhythms). By contrast, impairments are observed for rhythmic sensorimotor synchronization on tasks requiring speaking or tapping to a beat, particularly in regard to lower consistency of synchronization in individuals with ASD [62,106,124]. Impairments are also observed in discriminating auditory durations, especially for shorter (subsecond) intervals [125]. Neuroimaging studies in individuals with ASD reveal reduced connectivity of fronto-temporal and cortical-subcortical networks [126,127], as well as structural abnormalities in the cerebellum and basal ganglia [127,128]. Children with ASD who participated in a music therapy intervention demonstrated increased resting-state functional connectivity between auditory, striatal and motor regions post-intervention compared to children in a non-music control intervention, which was related to children's communication skills on a parent questionnaire [129].
8. Williams syndrome
In comparison to the previously reviewed disorders, Williams syndrome (WS) is a rare (approx. 1 in 10 000) NDD with a known genetic aetiology, the deletion of approximately 26–28 genes on chromosome 7 [27]. Nevertheless, WS is included here because of its unique cognitive-behavioural phenotype, which presents both overlaps with, and differences from, several of the disorders discussed above, as well as the existence of a robust literature into musical and rhythm processing in WS.
The WS phenotype includes mild to moderate cognitive impairment with deficits in visuospatial construction skills [130]. Common comorbidities include ADHD (approx. 65%; particularly in childhood), as well as anxiety and phobias (approx. 50%) [28]. While initial profiles of WS focused on their strengths in verbal and social skills, more recent research from the last decade highlights a more nuanced profile of strengths and difficulties within these domains [130].
Language development is delayed in WS, though areas such as concrete vocabulary, verbal short-term memory and grammatical skills become relative strengths later in development (at mental-age expected levels) [130]. Of relevance to rhythm and timing skills, reduced sensitivity to word stress patterns are observed, as well as atypical speech prosody (such as observed in ASD) [30,31,131]. While WS is associated with hypersociability, difficulties with social pragmatics are common and ASD-related symptomatology is elevated in WS even compared to other non-ASD developmental disabilities [132]. Social coordination difficulties are observed in WS across the lifespan such as impairments in joint attention and integrating social gaze during the early years (before verbal skills develop), and impairments in initiating and maintaining conversations in older (verbal) individuals [30,31,130].
Rhythm and timing skills have also been assessed in WS through music perception and production tasks. Many individuals with WS experience auditory sensitivities and heightened emotional responsiveness to music, but there are substantial individual differences in musical skills. In general, rhythm skills in WS are reduced compared to chronological age-matched peers but commensurate with mental-age abilities. For example, individuals with WS perform worse than age-matched peers on tasks involving same/difference judgements of rhythmic patterns, detecting beat alignment to real music and reproducing rhythmic patterns by clapping or singing [133–136]. Motor difficulties are often common including coordination of rhythmic motor movements and disrupted perception-action coupling [137,138].
Only one study has assessed neural correlates of rhythm skills in WS using EEG [139]. During passive listening to rhythmic patterns, adults with WS exhibited the canonical oscillatory activity in beta and gamma bands in response to beat onsets. However, individuals with WS also exhibited greater alpha activity, as well as the increased amplitude of auditory evoked potentials. This suggests that rhythm and beat processing may connect with their atypical auditory attention profile (e.g. increased responsiveness, poor inhibition and attention disengagement). Neuroimaging studies in WS also reveal differences in brain structure and connectivity in areas important for beat and rhythm processing (e.g. reduced basal ganglia volume) [140].
9. Summary and future directions
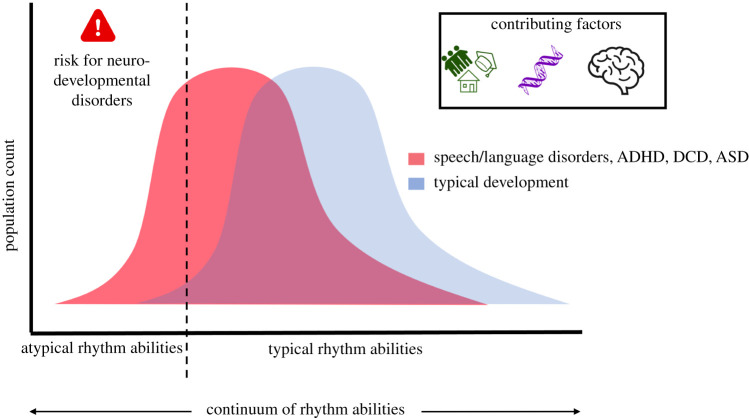
Rhythm is an early emerging and essential component of human development and interactions; yet, as reviewed here, impairments in rhythm are associated with a variety of NDDs including relatively common disorders with complex and varying genetic etiologies (e.g. ADHD, language disorders, DCD and ASD) as well as rare genetic syndromes of known aetiology (e.g. WS). Indeed, as reviewed above, the available research finds that many types of rhythm impairments are evident across multiple NDDs (see the electronic supplementary material, table S1). Rhythm impairments may be associated with particular facets of individual NDDs, the common comorbidities across NDDs and broader social-emotional/behavioural difficulties frequently observed (figure 1). Indeed, regardless of the ‘primary’ domain of a given disorder, profiles of impairments that include increased timing and rhythm deficits in children with various NDDs increase the risk of mental health problems, social problems and poor academic performance [28,80,141,142].
Figure 1.
Impaired rhythm processing skills may be a risk factor for developing a variety of NDDs and may help explain high levels of comorbidities across disorders and broader patterns of behavioural/emotional problems. Genetic vulnerabilities, environmental factors (e.g. education, training, social interactions) and neural processing contribute to rhythm skills, development of NDDs and profiles within and across NDDs. (Online version in colour.)
In this summary section, we consider how transdiagnostic investigations into rhythm and timing skills across domains may be fruitful for elucidating underlying biology, characterizing psychological/behavioural sequelae and designing effective interventions for individuals with various NDDs. Frameworks that consider heterogeneity within and across NDDs dovetail with ongoing efforts to identify the aetiology of features and function that are common across multiple mental traits and disorders [143], using phenotypic heterogeneity and comorbidities as an important source of biological covariation (e.g. as in the National Institutes of Mental Health Research Domain Criteria (RDoC) Project). Although rhythm is not currently formally included as an RDoC domain, motor processes and sensory domains (important components of rhythm processing) have recently been incorporated into RDoC [144,145]. Increasing evidence suggests that transdiagnostic investigations across NDDs may be more powerful for revealing links between underlying mechanisms and functional outcomes than comparisons of specific NDDs and typical controls [43,146].
Critical to NDDs is the focus on development; these are disorders that unfold over time and reflect interactions among genetic vulnerabilities, neural development and a variety of environmental factors (figure 1). Overlapping difficulties as well as the degree to which different domains are impacted across different NDDs may reflect similarities and differences in the timing, amount and how widespread (e.g. across neural systems) are the instantiation of atypical developmental processes [147]. Additionally, development is a dynamic process and any vulnerabilities in rhythm processing will both impact and be shaped by other (common and unique) vulnerabilities as well as experiences (e.g. as in neuroconstructivist approaches [147]). For example, rhythm is integrally connected with the motor system suggesting potential feedback loops between vulnerabilities in rhythm perception and motor skills; as summarized above, motor impairments of varying degrees commonly occur across NDDs [6,78,89,91]. Another example comes from rhythms in social contexts. Rhythm and timing deficits may impair attention shifting and modulation that contribute to successful social interactions; at the same time, atypical (decreased or increased) interest in social information may reduce opportunities to develop and refine rhythm and timing skills needed for social coordination [148].
Given the developmental context of NDDs, one important and growing direction for future research includes longitudinal designs of rhythm sensitivity early in life in children with or at risk for various NDDs (owing to family risk status or other health risk factors) in order to investigate trajectories of rhythm sensitivity development, as well as relationships between early rhythm sensitivity and later functional language, social, emotional and motor skills. While some studies with infants at risk for ADHD, ASD and/or language disorders, as well as children with genetic syndromes such as WS, have investigated early motor, language, sensory or attentional functioning, rhythm processing has generally not been a focus of these studies [81,147]. Yet the evidence presented in the current paper suggests theoretical support for such future work. One recent study reported associations between infants' temporal sensitivity (amplitude envelope rise time discrimination, important for synchronization of neural oscillatory activity to the temporal signal and reflective of the speech rhythm) and later vocabulary development [149]. Ladányi et al. [6] suggest that rhythm may be a powerful means of early identification of children at risk for language disorders owing to shared underlying biology. Large-scale population-wide epidemiological studies are needed to test the prediction that atypical rhythm increases the risk of the presence of developmental speech and language problems, as well as DCD and ADHD. Furthermore, specific familial influences (genetic and environment factors) accounting for the hypothesized shared risk will need to be disentangled and may point to specific convergent or divergent biological pathways.
From an assessment standpoint, in order to better conceptualize common and unique rhythm difficulties across different NDDs, transdiagnostic approaches should thoroughly assess participants for comorbid conditions in order to appropriately characterize their samples (e.g. DCD + DLD versus DCD alone). Additionally, studies should use task batteries that incorporate a variety of rhythm and temporal processing activities (e.g. beat perception and production, interval timing, simple versus complex rhythms). Tasks must also consider different domains (e.g. visual, auditory, tactile and multisensory) and consider tasks with and without motor components, as well as tasks with and without social contexts. Parsing specific rhythm and timing skills across domains may reveal rhythm profiles across different disorders and/or profiles of comorbidities. For example, some children may be particularly impaired in tasks that involve explicit motor components (i.e. rhythm production) while others may perform similarly across perception and production tasks; some children may be supported by rhythm tasks embedded in linguistic/speech or social stimuli while others may be particularly impaired in such contexts. More nuanced studies of the processes underlying tasks would also clarify profiles across disorders or comorbidities. For example, impairments in rhythmic synchronization to beat-based stimuli are observed across nearly all NDD disorders (see the electronic supplementary material, table S1). However, it is unknown if difficulties across disorders involve the same or overlapping components (e.g. beat finding, beat maintenance, beat adjustment, error correction). Comparisons across disorders with different types of beat synchronization tasks may be informative for parsing specific impairment profiles (e.g. tapping at different tempi, fixed versus variable tempi, manipulating the metrical strength, specific acoustic characteristics of stimuli).
It is also critical to identify mechanisms by which rhythm processing contributes to individual differences in phenotypic expressions across NDDs and whether overlapping behavioural difficulties are subserved by the same neural mechanisms across different NDDs or if different (potentially compensatory) profiles are observed. Rhythmic processing involves temporal predictability (e.g. Dynamic Attending Theory [51]), impairments in which are associated with multiple downstream effects including attention to (or attentional disengagement from) sensory stimuli. For example, the temporal sampling framework proposes that impairments in processing slow rhythms (caused by inefficient phase-locking of neural oscillatory activity at low frequencies) are predictive of reduced phonological development in language disorders [150]. Another potential mechanism may involve reward processing, which is impacted in multiple NDDs [151]. Reward processing shares neural pathways (e.g. caudate) with rhythmic sensorimotor synchronization and connects synchrony with subsequent prosocial behaviour [152]. In addition to assessing neural activity (e.g. EEG, magnetoencephalography), other methodological approaches that allow for assessing how behaviours unfold over time (e.g. movement tracking, eye-tracking) may elucidate mechanistic processes underlying rhythm skills across NDDs, as well as links to functional skills across domains.
As noted in the reviews of different NDDs, there is substantial interest and growing research in rhythm-based interventions for children with NDDs [76,120,121]. Rhythm-based intervention studies take a variety of forms and target a range of outcome measures. The general principle of these interventions is that providing structured, predictable, rhythmic stimuli increases relevant neural activity to support behavioural task performance (e.g. increasing oscillatory power at the stimulus frequency). As proof-of-concept designs, several studies have used rhythmic priming activities, in which regular rhythmic stimuli precede or co-occur with target stimuli (e.g. hearing or reading sentences, completing a motor task), to investigate rhythm facilitation of task performance [77]. Other studies involve single or multi-session auditory-motor training activities, often using music/music and movement activities or interventions [120,129]. While there is promising evidence from several studies, larger studies with appropriate comparison conditions are needed, as well as a broadening of the types of symptoms and NDDs being targeted. As with all interventions, clearly elucidated theories of change are needed in order to optimize intervention design; indeed, intervention studies can be used to test connections between proposed mechanisms and outcome behaviours. Mechanistic intervention research across and within NDDs will support intervention development and help determine for whom interventions are most appropriate and for what target outcomes.
10. Conclusion
Rhythm and timing underlie a variety of human behaviours including attentional, sensory, motor, linguistic and social domains. There is considerable evidence that rhythm and timing deficits are common across a variety of NDDs with overlapping profiles of rhythm impairments. The presence of timing and rhythm processing deficits across NDDs suggests there may be overlapping genetic and neural vulnerabilities for developing NDDs and potentially explains one facet of the high comorbidity across disorders. Individual differences in rhythm skills in different domains may also help to explain heterogeneity within disorders in regard to the magnitude and scope of co-occurring challenges. Transdiagnostic approaches that include rhythm impairments in models of NDDs may point to novel mechanisms of action for characterizing NDDs and the design of intervention strategies to support children with NDDs.
Acknowledgements
We would like to thank the organizers of the Lorentz Center workshop: Synchrony and Rhythmic Interaction: From Neurons to Ecology.
Data accessibility
This article has no additional data.
Authors' contributions
All authors conceived of the article, contributed to writing and revising of the manuscript, and gave final approval for publication.
Competing interests
We declare we have no competing interests.
Funding
This work was supported in part by the National Institute of Mental Health (NIMH) and National Center for Complementary and Integrative Health (NCCIH) (R61MH123029) and National Institute on Deafness and Other Communication Disorders (NIDCD) (R21DC016710) awarded to M.D.L.; the Office of the Director of the NIH (DP2HD098859) to R.L.G.; the Natural Sciences and Engineering Research Council of Canada (NSERC) (RGPIN-2019–05416), the Canadian Institutes of Health Research (CIHR) (RTI-2017-00643) and the Canadian Institute for Advanced Research to L.J.T. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health (NIH), NSERC, CIHR or Canadian Institute for Advanced Research.
References
- 1.Suppanen E, Huotilainen M, Ylinen S. 2019. Rhythmic structure facilitates learning from auditory input in newborn infants. Infant Behav. Dev. 57, 101346. ( 10.1016/j.infbeh.2019.101346) [DOI] [PubMed] [Google Scholar]
- 2.Winkler I, Háden GP, Ladinig O, Sziller I, Honing H.. 2009. Newborn infants detect the beat in music. Proc. Natl Acad. Sci. USA 106, 2468-2471. ( 10.1073/pnas.0809035106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Savage PE, Loui P, Tarr B, Schachner A, Glowacki L, Mithen S, Fitch WT. 2020. Music as a coevolved system for social bonding. Behav. Brain Sci. 1-42. ( 10.1017/s0140525x20000333) [DOI] [PubMed] [Google Scholar]
- 4.Jaffe J, Beebe B, Feldstein S, Crown CL, Jasnow MD. 2001. Rhythms of dialogue in infancy: coordinated timing in development. Monogr. Soc. Res. Child Dev. 66, i-viii, 1–132. ( 10.2307/3181589) [DOI] [PubMed] [Google Scholar]
- 5.Lewkowicz DJ. 2003. Learning and discrimination of audiovisual events in human infants: the hierarchical relation between intersensory temporal synchrony and rhythmic pattern cues. Dev. Psychol. 39, 795-804. ( 10.1037/0012-1649.39.5.795) [DOI] [PubMed] [Google Scholar]
- 6.Ladányi E, Persici V, Fiveash A, Tillmann B, Gordon RL. 2020. Is atypical rhythm a risk factor for developmental speech and language disorders? Wiley Interdiscip. Rev. Cogn. Sci. 11, e1528. ( 10.1002/wcs.1528) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peretz I, Vuvan DT. 2017. Prevalence of congenital amusia. Eur. J. Hum. Genet. 25, 625-630. ( 10.1038/ejhg.2017.15) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trainor L, Chang A, Cairney J, Li Y-C. 2018. Is auditory perceptual timing a core deficit of developmental coordination disorder? Ann. N. Y. Acad. Sci. 1423, 30-39. ( 10.1111/nyas.13701) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.American Psychiatric Association. 2013. Diagnostic and statistical manual of mental disorders, fifth edition: DSM-5. 5th edn. Arlington, VA: American Psychiatric Pub. [Google Scholar]
- 10.Germanò E, Gagliano A, Curatolo P. 2010. Comorbidity of ADHD and dyslexia. Dev. Neuropsychol. 35, 475-493. ( 10.1080/87565641.2010.494748) [DOI] [PubMed] [Google Scholar]
- 11.Tirosh E, Cohen A. 1998. Language deficit with attention-deficit disorder: a prevalent comorbidity. J. Child Neurol. 13, 493-497. ( 10.1177/088307389801301005) [DOI] [PubMed] [Google Scholar]
- 12.Gillberg C, Gillberg IC, Rasmussen P, Kadesjö B, Söderström H, Råstam M, Johnson M, Rothenberger A, Niklasson L. 2004. Co-existing disorders in ADHD - implications for diagnosis and intervention. Eur. Child Adolesc. Psychiatry Suppl. 13, 80-92. ( 10.1007/s00787-004-1008-4) [DOI] [PubMed] [Google Scholar]
- 13.Stevens T, Peng L, Barnard-Brak L. 2016. The comorbidity of ADHD in children diagnosed with autism spectrum disorder. Res. Autism Spectr. Disord. 31, 11-18. ( 10.1016/j.rasd.2016.07.003) [DOI] [Google Scholar]
- 14.Lai MC, Kassee C, Besney R, Bonato S, Hull L, Mandy W, Szatmari P, Ameis SH. 2019. Prevalence of co-occurring mental health diagnoses in the autism population: a systematic review and meta-analysis. Lancet Psychiatry 6, 819-829. ( 10.1016/S2215-0366(19)30289-5) [DOI] [PubMed] [Google Scholar]
- 15.Baio J, et al. 2018. Prevalence of autism spectrum disorder among children aged 8 years—autism and developmental disabilities monitoring network, 11 sites, United States, 2014. MMWR Surveill. Summ. 67, 1-23. ( 10.15585/mmwr.ss6706a1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blank R, Smits-Engelsman B, Polatajko H, Wilson P. 2012. European Academy for Childhood Disability (EACD): recommendations on the definition, diagnosis and intervention of developmental coordination disorder (long version). Dev. Med. Child Neurol. 54, 54-93. ( 10.1111/j.1469-8749.2011.04171.x) [DOI] [PubMed] [Google Scholar]
- 17.Harris SR, Mickelson ECR, Zwicker JG. 2015. Diagnosis and management of developmental coordination disorder. Canadian Med. Assoc. J. 187, 659-665. ( 10.1503/cmaj.140994) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Missiuna C, et al. 2014. Psychological distress in children with developmental coordination disorder and attention-deficit hyperactivity disorder. Res. Dev. Disabil. 35, 1198-1207. ( 10.1016/j.ridd.2014.01.007) [DOI] [PubMed] [Google Scholar]
- 19.Bishop DVM, et al. 2017. Phase 2 of CATALISE: a multinational and multidisciplinary Delphi consensus study of problems with language development: terminology. J. Child Psychol. Psychiatry Allied Discip. 58, 1068-1080. ( 10.1111/jcpp.12721) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cohen NJ, Vallance DD, Barwick M, Im N, Menna R, Horodezky NB, Isaacson L. 2000. The interface between ADHD and language impairment: an examination of language, achievement, and cognitive processing. J. Child Psychol. Psychiatry 41, 353-362. ( 10.1111/1469-7610.00619) [DOI] [PubMed] [Google Scholar]
- 21.Flapper BCT, Schoemaker MM. 2013. Developmental coordination disorder in children with specific language impairment: co-morbidity and impact on quality of life. Res. Dev. Disabil. 34, 756-763. ( 10.1016/j.ridd.2012.10.014) [DOI] [PubMed] [Google Scholar]
- 22.Catts HW, Adlof SM, Hogan TP, Weismer SE. 2005. Are specific language impairment and dyslexia distinct disorders? J. Speech Lang. Hear. Res. 48, 1378-1396. ( 10.1044/1092-4388(2005/096)) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Snowling MJ. 2013. Early identification and interventions for dyslexia: a contemporary view. J. Res. Spec. Educ. Needs 13, 7-14. ( 10.1111/j.1471-3802.2012.01262.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chaix Y, Albaret JM, Brassard C, Cheuret E, de Castelnau P, Benesteau J, Karsenty C, Démonet JF.. 2007. Motor impairment in dyslexia: the influence of attention disorders. Eur. J. Paediatr. Neurol. 11, 368-374. ( 10.1016/j.ejpn.2007.03.006) [DOI] [PubMed] [Google Scholar]
- 25.Druker K, Hennessey N, Mazzucchelli T, Beilby J. 2019. Elevated attention deficit hyperactivity disorder symptoms in children who stutter. J. Fluency Disord. 59, 80-90. ( 10.1016/j.jfludis.2018.11.002) [DOI] [PubMed] [Google Scholar]
- 26.Arndt J, Schools AP, Healey EC. 2001. School-age children who stutter. Lang. Speech. Hear. Serv. Sch. 32, 68-78. ( 10.1044/0161-1461(2001/006)) [DOI] [PubMed] [Google Scholar]
- 27.Martens MA, Wilson SJ, Reutens DC. 2008. Research review: Williams syndrome: a critical review of the cognitive, behavioral, and neuroanatomical phenotype. J. Child Psychol. Psychiatry Allied Discip. 49, 576-608. ( 10.1111/j.1469-7610.2008.01887.x) [DOI] [PubMed] [Google Scholar]
- 28.Leyfer OT, Woodruff-Borden J, Klein-Tasman BP, Fricke JS, Mervis CB. 2006. Prevalence of psychiatric disorders in 4 to 16-year-olds with Williams syndrome. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 141, 615-622. ( 10.1002/ajmg.b.30344) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pitts CH, Mervis CB. 2016. Performance on the Kaufman brief intelligence test-2 by children with Williams syndrome. Am. J. Intellect. Dev. Disabil. 121, 33-47. ( 10.1352/1944-7558-121.1.33) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klein-Tasman BP, Mervis CB, Lord C, Phillips KD. 2007. Socio-communicative deficits in young children with Williams syndrome: performance on the autism diagnostic observation schedule. Child Neuropsychol. 13, 444-467. ( 10.1080/09297040601033680) [DOI] [PubMed] [Google Scholar]
- 31.Klein-Tasman BP, van der Fluit F, Mervis CB.. 2018. Autism spectrum symptomatology in children with Williams syndrome who have phrase speech or fluent language. J. Autism Dev. Disord. 48, 3037-3050. ( 10.1007/s10803-018-3555-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mosing MA, Verweij KJH, Madison G, Ullén F. 2016. The genetic architecture of correlations between perceptual timing, motor timing, and intelligence. Intelligence 57, 33-40. ( 10.1016/j.intell.2016.04.002) [DOI] [Google Scholar]
- 33.Niarchou M, et al. 2019. Unravelling the genetic architecture of musical rhythm: a large-scale genome-wide association study of beat synchronization. BioRxiv, 836197. ( 10.1101/836197) [DOI]
- 34.Solovieff N, Cotsapas C, Lee PH, Purcell SM, Smoller JW. 2013. Pleiotropy in complex traits: challenges and strategies. Nat. Rev. Genet. 14, 483-495. ( 10.1038/nrg3461) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smoller JW. 2019. Psychiatric genetics begins to find its footing. Am. J. Psychiatry 176, 609-614. ( 10.1176/appi.ajp.2019.19060643) [DOI] [PubMed] [Google Scholar]
- 36.Anttila V, et al. 2018. Analysis of shared heritability in common disorders of the brain. Science 360, eaap8757. ( 10.1126/science.aap8757) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee PH, et al. 2019. Genomic relationships, novel loci, and pleiotropic mechanisms across eight psychiatric disorders. Cell 179, 1469-1482.e11. ( 10.1016/j.cell.2019.11.020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Watanabe K, et al. 2019. A global overview of pleiotropy and genetic architecture in complex traits. Nat. Genet. 51, 1339-1348. ( 10.1038/s41588-019-0481-0) [DOI] [PubMed] [Google Scholar]
- 39.Willcutt EG, McGrath LM, Pennington BF, Keenan JM, DeFries JC, Olson RK, Wadsworth SJ. 2019. Understanding comorbidity between specific learning disabilities. New Dir. Child Adolesc. Dev. 2019, 91-109. ( 10.1002/cad.20291) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gialluisi A, et al. 2020. Genome-wide association study reveals new insights into the heritability and genetic correlates of developmental dyslexia. Mol. Psychiatry ( 10.1038/s41380-020-00898-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tierney A, White-Schwoch T, MacLean J, Kraus N. 2017. Individual differences in rhythm skills: links with neural consistency and linguistic ability. J. Cogn. Neurosci. 29, 855-868. ( 10.1162/jocn_a_01092) [DOI] [PubMed] [Google Scholar]
- 42.Sauer-Zavala S, Gutner CA, Farchione TJ, Boettcher HT, Bullis JR, Barlow DH. 2017. Current definitions of ‘Transdiagnostic’ in treatment development: a search for consensus. Behav. Ther. 30, 35-51. ( 10.1016/j.beth.2016.09.004) [DOI] [PubMed] [Google Scholar]
- 43.Astle DE, Fletcher-Watson S. 2020. Beyond the core-deficit hypothesis in developmental disorders. Curr. Dir. Psychol. Sci. 29, 431-437. ( 10.1177/0963721420925518) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grahn JA. 2012. Neural mechanisms of rhythm perception: current findings and future perspectives. Top. Cogn. Sci. 4, 585-606. ( 10.1111/j.1756-8765.2012.01213.x) [DOI] [PubMed] [Google Scholar]
- 45.Fujioka T, Trainor LJ, Large EW, Ross B. 2012. Internalized timing of isochronous sounds is represented in neuromagnetic beta oscillations. J. Neurosci. 32, 1791-1802. ( 10.1523/JNEUROSCI.4107-11.2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cope T, Grube M, Singh B, Burn D, Griffiths T. 2014. The basal ganglia in perceptual timing: timing performance in multiple system atrophy and Huntington's disease. Neuropsychologia 52, 73-81. ( 10.1016/j.neuropsychologia.2013.09.039) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ross JM, Iversen JR, Balasubramaniam R. 2018. The role of posterior parietal cortex in beat-based timing perception: a continuous theta burst stimulation study. J. Cogn. Neurosci. 30, 633-643. ( 10.1162/jocn_a_01237) [DOI] [PubMed] [Google Scholar]
- 48.Phillips-Silver J, Trainor LJ. 2007. Hearing what the body feels: auditory encoding of rhythmic movement. Cognition 105, 533-546. ( 10.1016/j.cognition.2006.11.006) [DOI] [PubMed] [Google Scholar]
- 49.Phillips-Silver J, Trainor LJ. 2005. Feeling the beat: movement influences infant rhythm perception. Science 308, 1430. ( 10.1126/science.1110922) [DOI] [PubMed] [Google Scholar]
- 50.Trainor L, Gao X, Lei J, Lehtovaara K, Harris L. 2009. The primal role of the vestibular system in determining musical rhythm. Cortex 45, 35-43. ( 10.1016/j.cortex.2007.10.014) [DOI] [PubMed] [Google Scholar]
- 51.Large EW, Jones MR. 1999. The dynamics of attending: how people track time-varying events. Psychol. Res. 106, 119-159. ( 10.1037/0033-295X.106.1.119) [DOI] [Google Scholar]
- 52.Herrojo Ruiz M, Maess B, Altenmüller E, Curio G, Nikulin VV. 2017. Cingulate and cerebellar beta oscillations are engaged in the acquisition of auditory-motor sequences. Hum. Brain Mapp. 38, 5161-5179. ( 10.1002/hbm.23722) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cirelli LK, Spinelli C, Nozaradan S, Trainor LJ. 2016. Measuring neural entrainment to beat and meter in infants: effects of music background. Front. Neurosci. 10, 1-11. ( 10.3389/fnins.2016.00229) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.National Academies of Sciences, Engineering and Medicine 2016. Speech and language disorders in children: implications for the social security administration's supplemental security income program. Washington, DC: National Academic Press. [PubMed] [Google Scholar]
- 55.Yairi E, Ambrose N. 2013. Epidemiology of stuttering: 21st century advances. J. Fluency Disord. 38, 66-87. ( 10.1016/j.jfludis.2012.11.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Repp BH. 2005. Sensorimotor synchronization: a review of the tapping literature. Psychon. Bull. Rev. 12, 969-992. ( 10.3758/BF03206433) [DOI] [PubMed] [Google Scholar]
- 57.Thomson JM, Goswami U. 2008. Rhythmic processing in children with developmental dyslexia: auditory and motor rhythms link to reading and spelling. J. Physiol. Paris 102, 120-129. ( 10.1016/j.jphysparis.2008.03.007) [DOI] [PubMed] [Google Scholar]
- 58.Cumming R, Wilson A, Leong V, Colling LJ, Goswami U. 2015. Awareness of rhythm patterns in speech and music in children with specific language impairments. Front. Hum. Neurosci. 9, 672. ( 10.3389/fnhum.2015.00672) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Falk S, Müller T, Dalla Bella S. 2015. Non-verbal sensorimotor timing deficits in children and adolescents who stutter. Front. Psychol. 6, 847. ( 10.3389/fpsyg.2015.00847) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thomson JM, Fryer B, Maltby J, Goswami U. 2006. Auditory and motor rhythm awareness in adults with dyslexia. J. Res. Read. 29, 334-348. ( 10.1111/j.1467-9817.2006.00312.x) [DOI] [Google Scholar]
- 61.Colling LJ, Noble HL, Goswami U. 2017. Neural entrainment and sensorimotor synchronization to the beat in children with developmental dyslexia: an EEG study. Front. Neurosci. 11, 360. ( 10.3389/fnins.2017.00360) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vishne G, Jacoby N, Malinovitch T, Epstein T, Frenkel O, Ahissar M. 2020. Impaired online error-correction disrupts synchronization to external events in autism. bioRxiv, 2020.09.28.316828. ( 10.1101/2020.09.28.316828) [DOI] [PMC free article] [PubMed]
- 63.Overy K, Nicolson RI, Fawcett AJ, Clarke EF. 2003. Dyslexia and music: measuring musical timing skills. Dyslexia 9, 18-36. ( 10.1002/dys.233) [DOI] [PubMed] [Google Scholar]
- 64.Chang S-E, Chow HM, Wieland EA, McAuley JD. 2016. Relation between functional connectivity and rhythm discrimination in children who do and do not stutter. NeuroImage Clin. 12, 442-450. ( 10.1016/j.nicl.2016.08.021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Corriveau K, Pasquini E, Goswami U. 2007. Basic auditory processing skills and specific language impairment: a new look at an old hypothesis. J. Speech Lang. Hear. Res. 50, 647-666. ( 10.1044/1092-4388(2007/046)) [DOI] [PubMed] [Google Scholar]
- 66.Huss M, Verney JP, Fosker T, Mead N, Goswami U. 2011. Music, rhythm, rise time perception and developmental dyslexia: perception of musical meter predicts reading and phonology. Cortex 47, 674-689. ( 10.1016/J.CORTEX.2010.07.010) [DOI] [PubMed] [Google Scholar]
- 67.Wang HLS, Huss M, Hämäläinen JA, Goswami U. 2012. Basic auditory processing and developmental dyslexia in Chinese. Read. Writ. 25, 509-536. ( 10.1007/s11145-010-9284-5) [DOI] [Google Scholar]
- 68.Goswami U, Mead N, Fosker T, Huss M, Barnes L, Leong V. 2013. Impaired perception of syllable stress in children with dyslexia: a longitudinal study. J. Mem. Lang. 69, 1-17. ( 10.1016/j.jml.2013.03.001) [DOI] [Google Scholar]
- 69.Weinert S. 1992. Deficits in acquiring language structure: the importance of using prosodic cues. Appl. Cogn. Psychol. 6, 545-571. ( 10.1002/acp.2350060607) [DOI] [Google Scholar]
- 70.Chang S-E, Garnett EO, Etchell A, Chow HM. 2019. Functional and neuroanatomical bases of developmental stuttering: current insights. Neuroscientist 25, 566-582. ( 10.1177/1073858418803594) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Toyomura A, Fujii T, Kuriki S. 2015. Effect of an 8-week practice of externally triggered speech on basal ganglia activity of stuttering and fluent speakers. Neuroimage 109, 458-468. ( 10.1016/j.neuroimage.2015.01.024) [DOI] [PubMed] [Google Scholar]
- 72.Power AJ, Colling LJ, Mead N, Barnes L, Goswami U. 2016. Neural encoding of the speech envelope by children with developmental dyslexia. Brain Lang. 160, 1-10. ( 10.1016/j.bandl.2016.06.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cutini S, Szucs D, Mead N, Huss M, Goswami U. 2016. Atypical right hemisphere response to slow temporal modulations in children with developmental dyslexia. Neuroimage 143, 40-49. ( 10.1016/j.neuroimage.2016.08.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Corriveau KH, Goswami U. 2009. Rhythmic motor entrainment in children with speech and language impairments: tapping to the beat. Cortex 45, 119-130. ( 10.1016/j.cortex.2007.09.008) [DOI] [PubMed] [Google Scholar]
- 75.Heard M, Lee YS. 2020. Shared neural resources of rhythm and syntax: an ALE meta-analysis. Neuropsychologia 137, 107284. ( 10.1016/j.neuropsychologia.2019.107284) [DOI] [PubMed] [Google Scholar]
- 76.Flaugnacco E, Lopez L, Terribili C, Montico M, Zoia S, Schön D. 2015. Music training increases phonological awareness and reading skills in developmental dyslexia: a randomized control trial. PLoS ONE 10, 1-17. ( 10.1371/journal.pone.0138715) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Przybylski L, Bedoin N, Krifi-Papoz S, Herbillon V, Roch D, Léculier L, Kotz SA, Tillmann B. 2013. Rhythmic auditory stimulation influences syntactic processing in children with developmental language disorders. Neuropsychology 27, 121-131. ( 10.1037/a0031277) [DOI] [PubMed] [Google Scholar]
- 78.Piek JP, Pitcher TM, Hay DA. 2007. Motor coordination and kinaesthesis in boys with attention deficit-hyperactivity disorder. Dev. Med. Child Neurol. 41, 159-165. ( 10.1111/j.1469-8749.1999.tb00575.x) [DOI] [PubMed] [Google Scholar]
- 79.Kadesjo B, Gillberg C. 2001. The comorbidity of ADHD in the general population of Swedish school-age children. J. Child Psychol. Psychiatry 42, 487-492. ( 10.1111/1469-7610.00742) [DOI] [PubMed] [Google Scholar]
- 80.Hawks Z, Constantino J. 2020. Neuropsychiatric ‘Comorbidity’ as causal influence in autism. J. Am. Acad. Child Adolesc. Psychiatry 59, 229-235. ( 10.1016/j.jaac.2019.07.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Johnson MH, Gliga T, Jones E, Charman T. 2015. Annual research review: infant development, autism, and ADHD - early pathways to emerging disorders. J. Child Psychol. Psychiatry Allied Discip. 56, 228-247. ( 10.1111/jcpp.12328) [DOI] [PubMed] [Google Scholar]
- 82.Noreika V, Falter CM, Rubia K. 2013. Timing deficits in attention-deficit/hyperactivity disorder (ADHD): evidence from neurocognitive and neuroimaging studies. Neuropsychologia 51, 235-266. ( 10.1016/j.neuropsychologia.2012.09.036) [DOI] [PubMed] [Google Scholar]
- 83.Puyjarinet F, Bégel V, Lopez R, Dellacherie D, Dalla Bella S. 2017. Children and adults with attention-deficit/hyperactivity disorder cannot move to the beat. Sci. Rep. 7, 11550. ( 10.1038/s41598-017-11295-w) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Valera EM, Spencer RMC, Zeffiro TA, Makris N, Spencer TJ, Faraone SV, Biederman J, Seidman LJ. 2010. Neural substrates of impaired sensorimotor timing in adult attention-deficit/hyperactivity disorder. Biol. Psychiatry 68, 359-367. ( 10.1016/j.biopsych.2010.05.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Grahn JA, Rowe JB. 2013. Finding and feeling the musical beat: striatal dissociations between detection and prediction of regularity. Cereb. Cortex 23, 913-921. ( 10.1093/cercor/bhs083) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Slater JL, Tate MC. 2018. Timing deficits in ADHD: insights from the neuroscience of musical rhythm. Front. Comput. Neurosci. 12, 51. ( 10.3389/fncom.2018.00051) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Blank R, et al. 2019. International clinical practice recommendations on the definition, diagnosis, assessment, intervention, and psychosocial aspects of developmental coordination disorder. Dev. Med. Child Neurol. 61, 242-285. ( 10.1111/dmcn.14132) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Visser J, Geuze R, Kalverboer A. 1998. The relationship between physical growth, the level of activity and the development of motor skills in adolescence: differences between children with DCD. Hum. Mov. Sci. 17, 573-608. ( 10.1016/S0167-9457(98)00014-1) [DOI] [Google Scholar]
- 89.Visscher C, Houwen S, Scherder E, Moolenaa B, Hartman E. 2007. Motor profile of children with developmental speech and language disorders. Pediatrics 120, e158-e163. ( 10.1542/peds.2006-2462) [DOI] [PubMed] [Google Scholar]
- 90.Gomez A, Sirigu A. 2015. Developmental coordination disorder: core sensori-motor deficits, neurobiology and etiology. Neuropsychologia 79, 272-287. ( 10.1016/j.neuropsychologia.2015.09.032) [DOI] [PubMed] [Google Scholar]
- 91.Green D, Charman T, Pickles A, Chandler S, Loucas T, Simonoff E, Baird G. 2009. Impairment in movement skills of children with autistic spectrum disorders. Dev. Med. Child Neurol. 51, 311-316. ( 10.1111/j.1469-8749.2008.03242.x) [DOI] [PubMed] [Google Scholar]
- 92.Lingam R, Hunt L, Golding J, Jongmans M, Emond A. 2009. Prevalence of developmental coordination disorder using the DSM-IV at 7 years of age: a UK population–based study. Pediatrics 123, e693-e700. ( 10.1542/peds.2008-1770) [DOI] [PubMed] [Google Scholar]
- 93.Lichtenstein P, Carlström E, Råstam M, Gillberg C, Anckarsäter H. 2010. The genetics of autism spectrum disorders and related neuropsychiatric disorders in childhood. Am. J. Psychiatry 167, 1357-1363. ( 10.1176/appi.ajp.2010.10020223) [DOI] [PubMed] [Google Scholar]
- 94.Allen S, Casey J. 2017. Developmental coordination disorders and sensory processing and integration: incidence, associations and co-morbidities. Br. J. Occup. Ther. 80, 549-557. ( 10.1177/0308022617709183) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wilson PH, Smits-Engelsman B, Caeyenberghs K, Steenbergen B, Sugden D, Clark J, Mumford N, Blank R. 2017. Cognitive and neuroimaging findings in developmental coordination disorder: new insights from a systematic review of recent research. Dev. Med. Child Neurol. 59, 1117-1129. ( 10.1111/dmcn.13530) [DOI] [PubMed] [Google Scholar]
- 96.Debrabant J, Gheysen F, Caeyenberghs K, Van Waelvelde H, Vingerhoets G.. 2013. Neural underpinnings of impaired predictive motor timing in children with developmental coordination disorder. Res. Dev. Disabil. 34, 1478-1487. ( 10.1016/j.ridd.2013.02.008) [DOI] [PubMed] [Google Scholar]
- 97.Adams ILJ, Lust JM, Wilson PH, Steenbergen B. 2014. Compromised motor control in children with DCD: a deficit in the internal model? A systematic review. Neurosci. Biobehav. Rev. 47, 225-244. ( 10.1016/j.neubiorev.2014.08.011) [DOI] [PubMed] [Google Scholar]
- 98.Caçola P, Ibana M, Ricard M, Gabbard C. 2016. Children with developmental coordination disorder demonstrate a spatial mismatch when estimating coincident-timing ability with tools. Res. Dev. Disabil. 48, 124-131. ( 10.1016/j.ridd.2015.10.021) [DOI] [PubMed] [Google Scholar]
- 99.Castelnau P de, Albaret J, Chaix Y, Zanone P-G. 2007. Developmental coordination disorder pertains to a deficit in perceptuo-motor synchronization independent of attentional capacities. Hum. Mov. Sci. 26, 477-490. ( 10.1016/j.humov.2007.03.001) [DOI] [PubMed] [Google Scholar]
- 100.Whitall J, Chang T-Y, Horn C, Jung-Potter J, McMenamim S, Wilms-Floet A, Clark JE. 2008. Auditory-motor coupling of bilateral finger tapping in children with and without DCD compared to adults. Hum. Mov. Sci. 27, 914-931. ( 10.1016/j.humov.2007.11.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zwicker JG, Missiuna C, Harris SR, Boyd LA. 2011. Brain activation associated with motor skill practice in children with developmental coordination disorder: an fMRI study. Int. J. Dev. Neurosci. 29, 145-152. ( 10.1016/j.ijdevneu.2010.12.002) [DOI] [PubMed] [Google Scholar]
- 102.Chang A, Li Y-C, Chan J, Dotov D, Cairney J, Trainor L. 2020. Inferior auditory time perception in children with motor difficulties. Child Dev. 1-7. [DOI] [PubMed] [Google Scholar]
- 103.Brignell A, Williams K, Jachno K, Prior M, Reilly S, Morgan AT. 2018. Patterns and predictors of language development from 4 to 7 years in verbal children with and without autism spectrum disorder. J. Autism Dev. Disord. 48, 3282-3295. ( 10.1007/s10803-018-3565-2) [DOI] [PubMed] [Google Scholar]
- 104.Bhat A, Landa R, Galloway JC. 2011. Current perspectives on motor functioning in infants, children, and adults with autism spectrum disorders. Phys. Ther. 91, 1116-11129. ( 10.2522/ptj.20100294) [DOI] [PubMed] [Google Scholar]
- 105.Thye MD, Bednarz HM, Herringshaw AJ, Sartin EB, Kana RK. 2018. The impact of atypical sensory processing on social impairments in autism spectrum disorder. Dev. Cogn. Neurosci. 29, 151-167. ( 10.1016/j.dcn.2017.04.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Franich K, Wong HY, Yu ACL, To CKS. 2021. Temporal coordination and prosodic structure in autism spectrum disorder: timing across speech and non-speech motor domains. J. Autism Dev. Disord. 51, 2929-2949. ( 10.1007/s10803-020-04758-z) [DOI] [PubMed] [Google Scholar]
- 107.Bruinsma Y, Koegel RL, Koegel LK. 2004. Joint attention and children with autism: a review of the literature. Ment. Retard. Dev. Disabil. Res. Rev. 10, 169-175. ( 10.1002/mrdd.20036) [DOI] [PubMed] [Google Scholar]
- 108.Grossman RB, Steinhart E, Mitchell T, Mcilvane W. 2015. ‘Look who's talking!’ gaze patterns for implicit and explicit audio-visual speech synchrony detection in children with high-functioning autism. Autism Res. 8, 307-316. ( 10.1002/aur.1447) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Patten E, Watson LR, Baranek GT. 2014. Temporal synchrony detection and associations with language in young children with ASD. Autism Res. Treat. 2014, 1-8. ( 10.1155/2014/678346) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Paul R, Augustyn A, Klin A, Volkmar FR. 2005. Perception and production of prosody by speakers with autism spectrum disorders. J. Autism Dev. Disord. 35, 205-220. ( 10.1007/s10803-004-1999-1) [DOI] [PubMed] [Google Scholar]
- 111.Northrup JB, Iverson JM. 2015. Vocal coordination during early parent – infant interactions predicts language outcome in infant siblings of children with autism spectrum disorder. Infancy 20, 1-25. ( 10.1111/infa.12090) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Charlop MH, Milstein JP. 1989. Teaching autistic children conversational speech using video modeling. J. Appl. Behav. Anal. 22, 275-285. ( 10.1901/jaba.1989.22-275) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fitzpatrick P, Romero V, Amaral JL, Duncan A, Barnard H, Richardson MJ, Schmidt RC. 2017. Social motor synchronization: insights for understanding social behavior in autism. J. Autism Dev. Disord. 47, 2092-2107. ( 10.1007/s10803-017-3124-2) [DOI] [PubMed] [Google Scholar]
- 114.Fitzpatrick P, Frazier JA, Cochran DM, Mitchell T, Coleman C, Schmidt RC. 2016. Impairments of social motor synchrony evident in autism spectrum disorder. Front. Psychol. 7, 1323. ( 10.3389/fpsyg.2016.01323) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Marsh KL, Isenhower RW, Richardson MJ, Helt M, Verbalis AD, Schmidt RC, Fein D. 2013. Autism and social disconnection in interpersonal rocking. Front. Integr. Neurosci. 7, 4. ( 10.3389/fnint.2013.00004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Fitzpatrick P, Romero V, Amaral JL, Duncan A, Barnard H, Richardson MJ, Schmidt RC. 2017. Evaluating the importance of social motor synchronization and motor skill for understanding autism. Autism Res. 10, 1687-1699. ( 10.1002/aur.1808) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kaur M, Srinivasan SM, Bhat AN. 2018. Comparing motor performance, praxis, coordination, and interpersonal synchrony between children with and without autism spectrum disorder (ASD). Res. Dev. Disabil. 72, 79-95. ( 10.1016/j.ridd.2017.10.025) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ingersoll B. 2008. The social role of imitation in autism: implications for the treatment of imitation deficits. Infants Young Child. 21, 107-119. ( 10.1097/01.IYC.0000314482.24087.14) [DOI] [Google Scholar]
- 119.Landa RJ, Holman KC, O'Neill AH, Stuart EA. 2011. Intervention targeting development of socially synchronous engagement in toddlers with autism spectrum disorder: a randomized controlled trial. J. Child Psychol. Psychiatry 52, 13-21. ( 10.1111/j.1469-7610.2010.02288.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Srinivasan SM, Kaur M, Park IK, Gifford TD, Marsh KL, Bhat AN. 2015. The effects of rhythm and robotic interventions on the imitation/praxis, interpersonal synchrony, and motor performance of children with autism spectrum disorder (ASD): a pilot randomized controlled trial. Autism Res. Treat. 2015, 1-18. ( 10.1155/2015/736516) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Srinivasan SM, Bhat AN. 2013. A review of ‘music and movement’ therapies for children with autism: embodied interventions for multisystem development. Front. Integr. Neurosci. 7, 22. ( 10.3389/fnint.2013.00022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Jamey K, Foster NEV, Sharda M, Tuerk C, Nadig A, Hyde KL. 2019. Evidence for intact melodic and rhythmic perception in children with autism spectrum disorder. Res. Autism Spectr. Disord. 64, 1-12. ( 10.1016/j.rasd.2018.11.013) [DOI] [Google Scholar]
- 123.Tryfon A, Foster NE, Ouimet T, Doyle-Thomas K, Anagnostou E, Sharda M, Hyde KL. 2017. Auditory-motor rhythm synchronization in children with autism spectrum disorder. Res. Autism Spectr. Disord. 35, 51-61. ( 10.1016/j.rasd.2016.12.004) [DOI] [Google Scholar]
- 124.Morimoto C, Hida E, Shima K, Okamura H. 2018. Temporal processing instability with millisecond accuracy is a cardinal feature of sensorimotor impairments in autism spectrum disorder: analysis using the synchronized finger-tapping task. J. Autism Dev. Disord. 48, 351-360. ( 10.1007/s10803-017-3334-7) [DOI] [PubMed] [Google Scholar]
- 125.Isaksson S, Salomäki S, Tuominen J, Arstila V, Falter-Wagner CM, Noreika V. 2018. Is there a generalized timing impairment in autism spectrum disorders across time scales and paradigms? J. Psychiatr. Res. 99, 111-121. ( 10.1016/j.jpsychires.2018.01.017) [DOI] [PubMed] [Google Scholar]
- 126.Hahamy A, Behrmann M, Malach R. 2015. The idiosyncratic brain: distortion of spontaneous connectivity patterns in autism spectrum disorder. Nat. Neurosci. 18, 302-309. ( 10.1038/nn.3919) [DOI] [PubMed] [Google Scholar]
- 127.Langen M, Leemans A, Johnston P, Ecker C, Daly E, Murphy CM, dell'Acqua F, Durston S, Murphy DGM. 2012. Fronto-striatal circuitry and inhibitory control in autism: findings from diffusion tensor imaging tractography. Cortex 48, 183-193. ( 10.1016/j.cortex.2011.05.018) [DOI] [PubMed] [Google Scholar]
- 128.Sussman D, et al. 2015. The autism puzzle: diffuse but not pervasive neuroanatomical abnormalities in children with ASD. NeuroImage Clin. 8, 170-179. ( 10.1016/j.nicl.2015.04.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sharda M, Tuerk C, Chowdhury R, Jamey K, Foster N, Custo-Blanch M, Tan M, Nadig A, Hyde K. 2018. Music improves social communication and auditory-motor connectivity in children with autism. Transl. Psychiatry 8, 231. ( 10.1038/s41398-018-0287-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mervis CB, John AE. 2010. Cognitive and behavioral characteristics of children with Williams syndrome: implications for intervention approaches. Am. J. Med. Genet. Part C Semin. Med. Genet. 154, 229-248. ( 10.1002/ajmg.c.30263) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Nazzi T, Paterson S, Karmiloff-Smith A. 2003. Early word segmentation by infants and toddlers with Williams syndrome. Infancy 4, 251-271. ( 10.1207/S15327078IN0402_06) [DOI] [Google Scholar]
- 132.Klein-Tasman BP, Phillips KD, Lord C, Mervis CB, Gallo FJ. 2009. Overlap with the autism spectrum in young children with Williams syndrome. J. Dev. Behav. Pediatr. 30, 289-299. ( 10.1097/DBP.0b013e3181ad1f9a) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lense MD, Dykens EM. 2016. Beat perception and sociability: evidence from Williams syndrome. Front. Psychol. 7, 886. ( 10.3389/fpsyg.2016.00886) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Don A, Schellenberg G, Rourke B. 1999. Music and language skills of children with Williams syndrome. Child Neuropsychol. 5, 154-170. ( 10.1076/chin.5.3.154.7337) [DOI] [Google Scholar]
- 135.Martens MA, Reutens DC, Wilson SJ. 2010. Auditory cortical volumes and musical ability in Williams syndrome. Neuropsychologia 48, 2602-2609. ( 10.1016/j.neuropsychologia.2010.05.007) [DOI] [PubMed] [Google Scholar]
- 136.Hopyan T, Dennis M, Weksberg R, Cyrtrynbaum C. 2001. Music skills and the expressive interpretation of music in children with Williams–Beuren syndrome: pitch, rhythm, melodic imagery, phrasing, and musical affect. Child Neuropsychol. 7, 42-53. ( 10.1076/chin.7.1.42.3147) [DOI] [PubMed] [Google Scholar]
- 137.Mayall LA, D'Souza H, Hill EL, Karmiloff-Smith A, Tolmie A, Farran EK. 2020. Motor abilities and the motor profile in individuals with Williams syndrome. Adv. Neurodev. Disord. 5, 46-60. ( 10.1007/s41252-020-00173-8) [DOI] [Google Scholar]
- 138.Vivanti G, Dissanayake C, Fanning PAJ, Hocking DR. 2018. Reduced motor interference in preschoolers with autism spectrum disorder and Williams syndrome. Dev. Neuropsychol. 43, 751-763. ( 10.1080/87565641.2018.1531289) [DOI] [PubMed] [Google Scholar]
- 139.Kasdan A, Gordon RL, Lense MD. 2020. Neurophysiological correlates of dynamic beat tracking in individuals with Williams syndrome. Biol. Psychiatry Cogn. Neurosci. Neuroimaging. ( 10.1016/j.bpsc.2020.10.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Campbell LE, Daly E, Toal F, Stevens A, Azuma R, Karmiloff-Smith A, Murphy DGM, Murphy KC. 2009. Brain structural differences associated with the behavioural phenotype in children with Williams syndrome. Brain Res. 1258, 96-107. ( 10.1016/j.brainres.2008.11.101) [DOI] [PubMed] [Google Scholar]
- 141.Cairney J. 2015. Developmental coordination disorder and its consequences: an introduction to the problem. In Developmental coordination disorder and its consequences (ed. J Cairney), pp. 5-32. Toronto, Canada: Toronto Press. [Google Scholar]
- 142.Law J, Rush R, Schoon I, Parsons S. 2009. Modeling developmental language difficulties from school entry into adulthood: literacy, mental health, and employment outcomes. J. Speech Lang. Hear. Res. 52, 1401-1416. ( 10.1044/1092-4388(2009/08-0142)) [DOI] [PubMed] [Google Scholar]
- 143.Simmons JM, Quinn KJ. 2014. The NIMH research domain criteria (RDoC) project: implications for genetics research. Mamm. Genome 25, 23-31. ( 10.1007/s00335-013-9476-9) [DOI] [PubMed] [Google Scholar]
- 144.Garvey MA, Cuthbert BN. 2017. Developing a motor systems domain for the NIMH RDoC program. Schizophr. Bull. 43, 935-936. ( 10.1093/schbul/sbx095) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Harrison LA, Kats A, Williams ME, Aziz-Zadeh L. 2019. The importance of sensory processing in mental health: a proposed addition to the research domain criteria (RDoC) and suggestions for RDoC 2.0. Front. Psychol. 10, 103. ( 10.3389/fpsyg.2019.00103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Choi EJ, et al. 2020. Beyond diagnosis: cross-diagnostic features in canonical resting-state networks in children with neurodevelopmental disorders. NeuroImage Clin. 28, 102476. ( 10.1016/j.nicl.2020.102476) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Karmiloff-Smith A. 2015. An alternative to domain-general or domain-specific frameworks for theorizing about human evolution and ontogenesis. AIMS Neurosci. 2, 91-104. ( 10.3934/Neuroscience.2015.2.91) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lense MD, Jones W. 2016. Beat-based entrainment during infant-directed singing supports social engagement. Paper presented at 14th biennial meeting of the International Conference on Music Perception & Cognition, San Francisco, CA, USA. [Google Scholar]
- 149.Kalashnikova M, Goswami U, Burnham D. 2019. Sensitivity to amplitude envelope rise time in infancy and vocabulary development at 3 years: a significant relationship. Dev. Sci. 22, e12836. ( 10.1111/desc.12836) [DOI] [PubMed] [Google Scholar]
- 150.Goswami U. 2011. A temporal sampling framework for developmental dyslexia. Trends Cogn. Sci. 15, 3-10. ( 10.1016/j.tics.2010.10.001) [DOI] [PubMed] [Google Scholar]
- 151.Dichter GS, Damiano CA, Allen JA. 2012. Reward circuitry dysfunction in psychiatric and neurodevelopmental disorders and genetic syndromes: animal models and clinical findings. J. Neurodev. Disord. 4, 19. ( 10.1186/1866-1955-4-19) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kokal I, Engel A, Kirschner S, Keysers C. 2011. Synchronized drumming enhances activity in the caudate and facilitates prosocial commitment - if the rhythm comes easily. PLoS ONE 6, 1-12. ( 10.1371/journal.pone.0027272) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.