Abstract
This review paper discusses rhythmic interactions and distinguishes them from non-rhythmic interactions. We report on communicative behaviours in social and sexual contexts, as found in dyads of humans, non-human primates, non-primate mammals, birds, anurans and insects. We discuss observed instances of rhythm in dyadic interactions, identify knowledge gaps and propose suggestions for future research. We find that most studies on rhythmicity in interactive signals mainly focus on one modality (acoustic or visual) and we suggest more work should be performed on multimodal signals. Although the social functions of interactive rhythms have been fairly well described, developmental research on rhythms used to regulate social interactions is still lacking. Future work should also focus on identifying the exact timing mechanisms involved. Rhythmic signalling behaviours are widespread and critical in regulating social interactions across taxa, but many questions remain unexplored. A multidisciplinary, comparative cross-species approach may help provide answers.
This article is part of the theme issue ‘Synchrony and rhythm interaction: from the brain to behavioural ecology’.
Keywords: animal communication, dyadic interaction, rhythm, signal timing, synchrony, multimodality
1. Introduction
Animals rely on effective signal transfer for communication with conspecifics. The sender must produce a clear signal that can be readily detected by the receiver [1]. A variety of sensory systems evolved to accommodate the production and reception of signals, and over time, these systems were fine-tuned by selective evolutionary pressures leading to remarkable species-specific adaptations (e.g. three-ossicle middle ear in mammals adapted for acoustic transmission of high-frequency signals [2]). Signals can be produced in several modalities, including acoustic, visual, chemical, tactile, etc.; each modality has its own (psycho)physical limitations that impose constraints on communication. For example, acoustic signals are effective for long-range information transfer but are affected by signal attenuation and degradation, especially at higher frequencies [3]. In the visual modality (e.g. signed languages), receivers are required to be in close contact with the signaller to accurately discriminate the signals because obstacles can block them. Regardless of the modality in which signalling occurs, interactive signalling will henceforth refer to a communicative exchange during which signals are transferred, back and forth, over a short time scale.
Timing is key in interactive signalling behaviour [4–6]. Individuals take turns in human conversation and entrain to the rhythms of other players while making music [7,8]. Individually timed behaviour in groups can lead to impressive collective phenomena such as synchronous movements to musical rhythms at concerts [9], cricket choruses [10] and the bioluminescent flashing of fireflies [11]. Unfortunately, natural interactions involving many agents are often difficult to study under controlled conditions. Hence, for practical reasons, most studies on interactive rhythms minimize the number of individuals by focusing on dyadic interactions. Some species can achieve a high degree of temporal coordination, and even synchronization, during conspecific interactions [10,12–14]. Therefore, we ask: how does rhythm shape dyadic interactions and how do dyadic interactions shape rhythm?
Here, we review stable interactions in dyads across species, modalities and contexts. This review covers humans and non-human animals—namely insects, anurans, birds, non-human primates and non-primate mammals—interacting mainly in the acoustic and visual modalities, and in social and sexual contexts. We (i) begin by proposing widely applicable definitions for relevant concepts (table 1); (ii) highlight rhythmic dyadic interactions in each group; and (iii) discuss the existing behaviours in the light of Tinbergen's four questions while proposing suggestions for future research.
Table 1.
Definitions of terms relating to dyadic interactions.
| term | definition |
|---|---|
| signals | ‘traits that (1) change another organism's behaviour while benefitting the sender, that (2) are evolved for this function, and that (3) have their effects through the evolved response of the receiver’ [15, p. 1011] |
| rhythm | ‘pattern of time intervals between the onset of events’ [16, p. 165] |
| rhythmic interaction | an interaction where two conspecifics adjust their timing behaviour to each other to create temporal regularities that facilitate the interaction |
| entrainment | ‘spatiotemporal coordination resulting from rhythmic responsiveness to a perceived rhythmic signal’ [17, p. 5] |
| duets | ‘joint […] displays where two [partners] coordinate their [signals] with a degree of temporal precision’ [18]; traditionally, duets occur in the acoustic modality, but we believe the use of the term should be extended to all other communicative modalities. |
| synchrony | ‘precise coincidence of events in time’ [19, p. 158] |
| turn-taking | ‘orderly exchange of purely communicative signals or behaviours (e.g. peek-a-boo games in humans) between individuals characterized by principles for the coordination of turn transfer, which result in observable temporal regularities’ [20, p. 2]; we subscribe to the idea that turn-taking is mostly rhythmic in its temporal dimension [6] |
| alternation | ‘where the regularly repeating signals of two […] individuals are broadcast such that they do not occur at the same time’ [21, p. 4] |
| antiphony | ‘when […] two animals transmit sounds among themselves in response to preceding signals’ [22, p. 155] |
We first need to distinguish between what constitutes a rhythmic versus non-rhythmic interaction. A rhythmic interaction is one where two conspecifics adjust their individual timing to each other to create temporal regularities that facilitate the interaction. The rhythmic structure that emerges can be measured and quantified along sequential and temporal dimensions [23]. For instance, in piano duets and dyadic finger tapping experiments, a statistical association, either synchronous and/or asynchronous, can be established between the temporal intervals of the two players [24,25]. Based on the concepts described in table 1, any interaction that requires timing adjustment between two conspecifics such as dyadic synchrony and turn-taking is considered rhythmic in this paper. A non-rhythmic interaction is one where there is no timing adjustment between the members of the dyad. Despite this, individual rhythms can still play an important role in regulating the interaction. For example, male northern elephant seals (Mirounga angustirostris) can recognize the rhythmic structure of their rivals' vocalizations; depending on the individual's status within the colony, males will either ignore or move away from the vocalizing male [26]. In the absence of a temporal relationship between the signals of two conspecifics, both alternation and antiphony are considered non-rhythmic. In the electronic supplementary material, readers can find examples of both interactions for all animal clades discussed in this review (electronic supplementary material, table S1) and a discussion on non-rhythmic interactions.
2. Humans
Humans are a highly social species and perform many types of duets; here, we focus on two: speech and music. In the rhythmic domain, speech generally involves turn-taking, whereas music is typically performed in synchrony, although people can chant speech together and call-and-response is used in music. Such behaviours involve complex social interactions and serve to communicate information, express emotion and socially bond [27,28]. These acoustic behaviours are performed with others in complex behavioural patterns involving cues including gesture, touch, body sway and dance, but also facial expressions and gaze direction [29–32].
(a) . Infancy and childhood
Human infants cannot survive without carers for a considerable period, and their early development and learning is done in a social context, often primarily with the mother [27]. Within this social context, movement behaviours [33], vocal behaviours [34], gaze [35], autonomic functioning [36] and hormone expression [37] are coordinated in time between infants and their carers. This early temporal coordination may enable infants to (i) regulate physiological and behavioural processes for survival [37], (ii) develop self-regulation [38], (iii) differentiate the emergence of their self from others [27], (iv) begin building social and empathetic relations with others [39], and (v) learn speech and music through continual reciprocal adjustments [40].
Carers provide rhythmic input to their infants in the forms of singing, patting and rocking, which helps infants to regulate their states [41]. The periodicities found in rhythms of infant-directed singing provide a context for bidirectional entrainment between infants and carers, which can be seen in the coordination of autonomic and brain responses between mothers and infants [35,42,43]. Synchronous movement to music during infancy also has important social consequences. Fourteen-month-old infants who are bounced in synchrony to music with an experimenter are much more likely to subsequently help that experimenter compared to infants who are bounced out of sync [39,44], suggesting that the origins of empathy and friendship may be found in coordinated rhythmic behaviours.
While the bidirectionality of interactions begins in infancy, carers initially have a broader role in structuring and scaffolding the interaction [45,46]. As children develop their advancing language, social and cognitive skills support and expand their active participation in complex, rhythmic verbal and non-verbal interactions [45]. In the verbal domain, children become increasingly proficient at dialogue and conversation [45,47]. Active turn-taking during conversation, which reflects partners' attunement to each other, is associated with children's language and social skill development [48,49]. Children who experience more appropriate turn-taking styles during conversations with their parents are more liked by their peers [50], and also exhibit more appropriate turn-taking with their peers even when the peers are novel acquaintances [51]. In the non-verbal domain, unfamiliar child peers who actively engage in coordinated rhythmic movement activities together show greater cooperation [52] and perceive that they are closer and more similar to each other, potentially due to increased intentional communication [53].
(b) . Adulthood
In adults, entrainment during dyadic conversation is observed through temporal adaptations within each individual's speech pattern that occur over multiple timescales [54]. The precise timing involved in conversational entrainment suggests the presence of underlying rhythmic processes that allow for accurate timing predictions [55]. During a conversation, dyads converge their individual speech rhythms as measured via speech rate, prosody and respiratory movement [56–58], as well as turn-taking timing (e.g. by minimizing silent gaps and avoiding overlap) [55,59,60]. The degree of entrainment in conversation is impacted by the interaction context (e.g. friendly versus unfriendly [54]). Rhythms are crucial in these interactions as their temporal regularity enables prediction and sampling of environmental stimuli [61].
Coordination in both musical and conversational duets relies on visual and auditory cues [62,63] but, in contrast with speech which largely involves turn-taking, musical interactions typically involve synchrony. While the two interacting musicians may sing or play different pitches, their outputs must fit together both rhythmically and harmonically to create a single meaningful joint performance. The high real-time temporal demands of duet music-making require continual adjustments, anticipation and prediction (if an individual reacts to rather than anticipates their partner's output, they will be late and not in sync with them) [8]. The continual adaptation of two people tapping together can be seen in ‘lag-1 correlations’, whereby on a given tap, the individual who is slightly ahead will slow down on their next tap and the individual who is slightly behind will speed up on their next tap [64]. Studies of string quartets also show mutual adjustments of timing, with some quartets being more leader-driven and others more egalitarian [65–67]. However, asynchronies increase when one musician in a duet is replaced with a recording [68] and when tempo preferences of two musicians are divergent [69], highlighting the importance of bidirectional coordination. Musicians convey their upcoming intentions through body sway, similar to how people use hand gestures when they speak. Findings from string quartets show that the body sway of one musician predicts the upcoming body sway of another musician [70]. In short, the better overall communication is among group members, the higher rated is the quality of their performance [29]. Music affords an ideal context in which people can synchronize their movements, and when a person experiences even a short period of synchronization with another person, it has social consequences, leading to increased liking, cooperation and trust [71,72]. These social consequences may explain why music-making in groups is universal across human societies and likely an evolutionary adaptation [73].
3. Non-human primates
Dyadic interactions among primates are essential to determine hierarchies within stable groups [74], and establish alliances and partnerships [75]. In several monkey and ape species, where vocal communication follows precise temporal and social rules, strong social bonds shape interactive signalling patterns [76,77]. For instance, bonobos respond preferentially to conspecifics with whom they have close bonds, and take turns to avoid overlap when vocalizing with those [78]. Unfortunately, rhythmic features have generally been understudied in primate communication, with few exceptions.
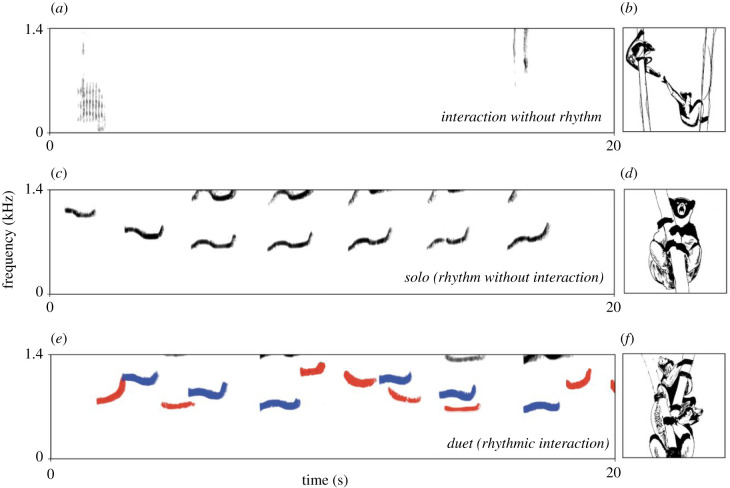
Primates may form long-lasting, socially monogamous pairs with opposite-sex conspecifics. Tarsiidae, Callicebinae, Hylobatidae and Indriidae include the main species of pair-living primates producing duets [79,80]. These pair-living primates face the need to defend a reduced home range [81] and males within these pairs struggle to protect mating exclusivity [82]. In both gibbons and indris, duets serve to inform neighbouring groups about the occupation of a territory and to defend it during group encounters [83]. In indris, duetting mediates group cohesiveness (see figure 1c, [86]), while in gibbons, it can inform about the presence of particular predators [87]. Duetting in siamang pairs (Hylobates syndactylus) is associated with a rhythmic swinging from branch to branch, which helps maintain entrained vocal displays between males and females [88]. Depending on the species, simultaneous singing or avoiding song overlap advertises the strength of a bond and may predict both behavioural coordination and grooming rates [89].
Figure 1.
Rhythm and social interactions in indris. (a) A spectrogram of a vocal interaction between two males from the same family group that compete for food; the adult male emits a low-pitched grunt [84] followed by a kiss and a wheeze from the younger male [85]. (c) A spectrogram of part of an unusual solo song by a male indri. (e) A spectrogram of part of a duet by a pair of indris (cohesion song). After dispersing within a territory, they emit a particular song type to regroup in a particular location [86]. Red shading denotes the fundamental frequency of the female's calls, and blue shading denotes the fundamental frequency of the male's units. (b), (d) and (f) are sketches of the animals represented in the adjacent spectrograms.
4. Non-primate mammals
Rhythmic interactions in other mammalian species allow individuals to identify conspecifics and maintain affiliations with other group members. We summarize findings of interactive exchanges in animal groups inhabiting different physical and socio-ecological environments including cetaceans, pinnipeds, bats, rodents, elephants, antelopes and meerkats.
Cetacean vocal interactions span alternation and synchrony. Bottlenose dolphins exchange signature whistles for individual recognition and group cohesion [90,91]. These exchanges are closely coordinated in time such that acoustic overlap is minimal (silent gaps < 1 s) [92]. Male bottlenose dolphins also engage in coordinated cooperative interactions when coercing females by synchronizing their threat vocalizations and matching tempos [93].
In pinnipeds, mother–pup recognition is a common dyadic interaction, where mothers and pups use vocal cues to identify and localize each other in the breeding colony, though species differ in whether mother, pup or both individuals vocalize [94,95]. Harbour seals may also adjust their call timing and call asynchronously relative to conspecific vocalizations [96]. Within a breeding colony, this turn-taking strategy may avoid acoustic overlap and make individual pup calls more conspicuous.
Several bat species engage in antiphonal exchanges of stereotyped calls, also for mother–pup recognition [97–99]. Moreover, adult white-winged vampire bats (Diaemus youngi) respond to contact calls in a duet-like fashion by temporally coordinating their reply (silent gaps < 500 ms), potentially to monitor the spatial positions of conspecifics [100].
Few studies reported on rhythmicity in rodent dyadic communication. In Alston's singing mice (Scotinomys teguina), depending on the social context, males adjust their signal timing to sing in turns and avoid acoustic overlap (silent gaps around 500 ms) [101]. Naked mole rats (Heterocephalus glaber) produce soft chirps antiphonally between two or more individuals to identify their social status and maintain affiliations (silent gaps < 400 ms) [102]. Middle East blind mole rats (Spalax ehrenbergi) communicate using vibratory signals by engaging in alternating head-drumming duets (silent gaps < 2 s) [103].
Data on interactive temporal coordination in dyads are limited for remaining mammal species. Female African elephants (Loxodonta africana) use antiphonal rumbling sequences between group members to maintain social distance [104]. An African antelope, the klipspringer (Oreotragus oreotragus), engages in alarm call duetting and calls are produced in alternation, with female calls closely following those of males [105]. Finally, meerkats (Suricata suricatta) avoid overlapping conspecifics in low-conflict group sunning calls by vocalizing in turns [106]. Here, group turn-taking is an outcome of dyadic interactions between group members and mechanistically relies on two alternating processes: call inhibition and call excitation.
5. Birds
Duets, which require a certain degree of temporal coordination (figure 1e), are also widespread across birds, especially in mating contexts. Vocal or dance duets occur in 18–20% of all avian species [107], which accounts for mostly song-duetting species among songbirds and dance-duetting species among non-songbirds (but see [108,109]). Avian duets are generally performed by paired partners or prospective mates, and serve different functions including mate-guarding, joint resource defence and/or mutual courtship [110,111].
Some barbet species (Capitonidae) [112] and the magpie lark (Grallina cyanoleuca) [113] perform multimodal duets, where the two birds simultaneously coordinate vocalizations and body movements. For singing, species-specific duet rules are well documented and show interspecific variation, even among closely related species. For example, in Thryothorus wrens, some species produce perfect antiphonal duets without overlap, while others sing in synchrony [114]. In avian song duetting, each of the sexes often produces a particular phrase at the precise onset or offset of its partner's singing, making it a perfectly timed collective display [115]. By contrast, rhythmicity in dance duets is still relatively unexplored. For example, in the red-crowned crane (Grus japonensis), males and females exhibit sequences of multiple dance elements for their joint display [116], but it is not known whether the paired cranes precisely time and synchronize their dancing with each other.
The degree to which two individuals coordinate their signalling during a duet varies even among dyads of the same species. Although temporal coordination can greatly influence reproductive fitness as better-coordinated signals are more effective for securing mates or reproductive resources [117], not every pair will be able to achieve the same degree of coordination. In fact, high-level coordination can only be achieved by pairs with longer partnerships [118]. Indeed, newly established pairs of canebrake wrens (Cantorchilus zeledoni) improve the coordination of their duets over time, suggesting that learning enables fine duetting [119].
Multimodal signal coordination in dyadic interactions is not restricted to species known for their duetting. The zebra finch has been intensively studied and disproportionately so relative to other songbirds, but lacks duetting as only males sing [120]. Despite this, mated pairs display tight temporal coordination in both visual and auditory modalities. Zebra finches form strong dyads with a lifelong mate [118] where coordinated behaviours serve as an honest signal of the pair's coalition quality. Indeed, the strength of the bond in zebra finch pairs predicts the degree of coordination of movements [121] and call exchanges [122], which mechanistically rely on predictive timing [123]. Pairs use such vocal exchanges to maintain a joint behavioural state [124] and coordinate shared parenting duties [125].
6. Anurans
Dyadic interactions in anurans occur in mating contexts, where the same-sex duets serve to compete for access to mates and opposite-sex duets serve to attract potential mates [126]. Anurans show a precedence effect, where calls of leading individuals are preferred when two identical calls are presented in close succession [21], but exceptions exist [127]. Moreover, calls that alternate in precise phase relationships are preferred by females of some species (e.g. the midwife toad Alytes obstetricans) [127]. Precise patterns of signal timing largely influence mate choice and are thus under strong sexual selection in anurans [126].
Males typically form large groups and produce loud alternating advertisement calls to attract females for mating [128]. Instances of non-random call timing in males were studied in larger groups, but also in dyads [129]. In duetting male pairs of the Neotropical toad (Rhinella ocellata), males call in alternation and avoid overlap with each other [130]. Moreover, the call delay of the responding male varies depending on the call duration of the male that initiated the interaction. This alternation pattern shows a high degree of temporal coordination within bouts. Call timing adjustment between two males of the European tree frog (Hyla arborea) can result in near-perfect antiphonal calling with the mean relative phase angles distributed around 180° [131]. The onset of vocalizations can even be adjusted based on the distance separating individuals, as evidenced in bullfrogs (Rana catesbeiana) [132]. Bullfrog males call more frequently following calls of distant neighbours than of those nearby; nearby neighbours may inhibit the vocal response of the focal individual. In sum, males try to avoid signal overlap in an effort to increase their conspicuousness by paying selective attention to their close neighbours and vary their call timing accordingly [131]. However, precise alternation of vocalizations is not always the norm. A few species signal using overlapped calls. For example, males of the American toad (Bufo americanus) signal in synchrony or near-perfect synchrony [12]. This timing strategy may help reduce predation risk [133] and/or increase the chorus' audibility in order to attract more females to the breeding area [129].
Even though they are rarely described, duets between males and females do occur at the beginning of courtship. In the South African clawed frog (Xenopus laevis), receptive females produce a vocalization named ‘call rapping’, which is composed of a rapid series of loud clicks, in response to the male's advertising call [134]. The female call spurs the male to move towards the sound source and produce an intense bout of calling within 1–2 s of the female's signal. A possible explanation for the evolution of female courtship vocalizations in anurans could be found by looking at similarities in terms of breeding biology [134].
7. Insects
Observations of non-random timing suggest that insects can adjust the onset of their signals. Temporal relationships of interactive signals are described for the acoustic modality in bush-crickets, grasshoppers, mosquitoes and flies, for the vibratory modality in planthoppers, leafhoppers, stoneflies, stink bugs and lacewings, and for the bioluminescent modality in fireflies [10,135]. Generally, males initiate insect duets and females respond with a fixed latency relative to the male signal [135]. The temporal pattern of the duet and the time window of the female reply are species-specific [136], hence allowing species recognition [135]. The duration and complexity of the male signal affects the reply latency of the female [137]. The longer and more complex the male signal is, the more time is needed to process the information encoded in the call and assess the male's quality. However, the latency of the reply may also depend on the female's readiness to mate [137].
In the leafhopper Aphrodes makarovi, sexual communication and mate recognition are mediated via species-specific and sex-specific vibrational signals. During duetting, the female's response overlaps with the last portion of the male's signal [138]. Males can only evaluate the non-overlapped part of the female call, and consequently adjust their signal period length to the duration of the female's reply to obtain a longer non-overlapped segment of the female's call [139]. While competing for access to females in the presence of rivals, male broad-winged bush katydids (Scudderia pistillata) produce an acoustic tick that mimics the female's tick signal and response timing [140]. This indicates to the female when the call has concluded and also confounds eavesdropping males by making it difficult for them to accurately move towards the true female's sound source [137]. Not only does this reduce the risk of competing males interrupting the established duet, but it also increases the coordination of the duet itself.
Some insects perform multimodal duets. For temporal coordination to occur in duets of the fruit fly Drosophila virilis, females need to detect the male's sound cues and be in close physical proximity [141]. During courtship, males tap the female's abdomen and lick the genitalia in a precisely timed manner to coordinate the duet. Females may choose to mate only with males that provide multiple timing cues during the duet. In the katydid Onomarchus uninotatus, male and female signals alternate in a clear phase relationship. Males produce a low-pitched call which receives a female vibrational signal in response [142] (see electronic supplementary material, figure S1). This unique multimodal duet may have evolved in response to predation; silent flying females were predated at a higher rate than calling males. With females using vibrational signals, the roles are now reversed: males must search for females by moving towards the vibration source and face increased risk of predation by bats.
The same-sex dyadic interactions in insects occur in highly competitive environments, hence the resulting temporal patterns are an outcome of competition between signalling males. In species where females prefer to mate with males leading the call sequence, males alternate calls to avoid losing their leader role [10,21]. In tarbush grasshoppers (Ligurotettix planum), males engage in acoustic ‘fights’ to defend or conquer a mating territory. The male that cannot match the signalling rate and length of the signal of its opponent gives up the fight [143]. The aggressive calls emitted by competing males thus represent an honest signal of male fitness.
8. Ultimate causes of dyadic interactions: function and phylogeny
Dyads can consist of mates, siblings, parent and offspring, or any two individuals from the same group. Dyadic interactions serve a variety of functions including social bonding, sharing emotion, establishing hierarchies and partnerships, mate-guarding, courtship, joint resource defence, etc. [14,27,28,40,74,75,110,111]. Studying dyads means that experiments possibly neglect relevant group dynamics, especially in highly social species. However, it also enables researchers to study semi-natural behavioural interactions under more controlled experimental conditions.
In this review, we identified two settings in which dyadic behavioural interactions can occur: social and sexual. Helping behaviour, learning behaviour and parent–offspring recognition were classified as occurring in social settings. Dyadic interactions in sexual settings mostly pertain to opposite-sex conspecifics that are potential mates or already form an established pair, but they can also involve same-sex conspecifics during intrasexual competition occurring during mate search and attraction [127]. Rhythmic behaviours have been reported for both contexts in mammals and birds, but in anurans and insects, they have only been reported for sexual settings.
Animals produce many different types of rhythms; hence, they could entrain to conspecific rhythms in ways that can be difficult to observe. For instance, individual rhythms do not need to occur in the same modality to interact rhythmically (i.e. coordination of an infant's brain responses to its mother's singing [42]). Moreover, the dynamics of the interaction can be both unidirectional and bidirectional [29,65–67]. In music, bidirectional interaction improves coordination, while unidirectional interactions create larger asynchronies [68]. Does this finding in humans carry over to interactions in other species? One could test whether the degree of coordination is affected by unidirectionality in songbird species in which one of the sexes does not sing (e.g. the Java sparrow or zebra finch). It seems that several species overcome such asynchrony by signalling in more than one modality to achieve higher levels of temporal coordination.
Even though multimodal signals are widespread across numerous taxa [62,88,112,113,141], their modalities are often studied separately [144]. Rhythmic interactive signalling has been reported mainly for the acoustic and visual modalities (table 2). However, birds can communicate using vibratory signals [147], but avian rhythmic interactions in the tactile modality have not been described. Similarly, anurans have visual signals [148], but rhythmicity in this modality has not been described during conspecific interactions. Future work on interactive rhythms should investigate the unexplored modalities in these animal groups. In addition, analysing rhythmic patterns of multimodal signals during interactive communication will provide more insight into the functions of signal timing.
Table 2.
Summary table of rhythmic interactive signalling in dyads across taxa. Although many signalling modalities have been proposed, the distinctions between them are often murky, hence, in the spirit of simplicity and to avoid confusion, we only include the main ones: auditory (A), visual (V) and tactile (T). Bioluminescent signals enhance visual perception [145] and are grouped under the visual modality. Vibratory signals are substrate-borne signals that are perceived using mechanoreceptors [146], similar to touch, hence they are considered part of the tactile modality. Cases where no empirical evidence was found are represented by a long dash (—).
| composition of dyad | interaction context | taxonomic group |
|||
|---|---|---|---|---|---|
| mammals (including humans) | birds | anurans | insects | ||
| kin | |||||
| parent–offspring | social | A, V | A, V | — | — |
| social group member | social | A, V | A, V | — | — |
| non-kin | |||||
| social group member | social | A, V | A, V | — | — |
| male–female | sexual | A, V | A, V | A | A, V, T |
| male–male | sexual | A, V | A, V | A | A, V, T |
9. Proximate causes of dyadic interactions: ontogeny and mechanisms
A wide repertoire of signals is needed to become an active, grown-up participant in dyadic interactions and precise timing of signals requires extensive practice. The social environment during development plays a crucial role in shaping adult signals. Human children benefit from structured bidirectional interactions scaffolded by their carers [45,46]. Similarly, young birds learn to participate in adult interactions. Male zebra finches learn their courtship song from their father, but can also receive feedback from other individuals within their social environment [149]. It has even been argued that learning of species-specific rhythms starts before birth, with possible long-term effects on vocal and social development [150]. The social context experienced during development modulates male signal features and female mate preferences in insects which will constrain the interaction [151]. Future work on dyadic interactions should accurately describe the social environment in which animals have been raised as it may influence both rhythm perception and production. Moreover, longitudinal studies should aim to follow developing individuals to investigate the effect of development on rhythmic interactions.
Coordinated signal exchanges in dyadic interactions show reply latencies ranging from the order of milliseconds to seconds [92,100,101]. The precise temporal coordination of behaviours must be regulated by the presence of underlying rhythmic processes, and many timing mechanisms proposed in the literature are based on the concept of coupled oscillators [55]. Human behaviours such as conversational entrainment [54] or the synchronous playing of music [8] rely on mechanisms that allow us to make accurate timing predictions about the onset of upcoming signals. Pinnipeds and birds also show some affinity with predictive timing. Similar to conversational turn-taking, seal pups may adapt the timing of their calls [96], and some songbirds attempt to simultaneously synchronize their vocalizations and body movements [112,121,122]. Insects are also capable of synchronous displays in larger groups, but the neural processes that regulate signal timing are different from mammals and birds. Instead of being able to predict the exact onsets of events, signalling in anurans and insects is reactive [152]. Similar observable and interactive behaviours can thus be produced using different mechanisms, but we do not know whether these mechanisms are learned and whether they change across modalities.
10. Conclusion
Rhythmic behaviours are widespread among animal clades, and crucial in regulating social interactions in dyads and larger groups. Unfortunately, in the temporal domain of animal communication, many species remain unexplored and several questions unanswered. Within species and across contexts, which features of signal timing are stable, and which are constrained, and to what degree? How are timing mechanisms learned and do they change from one modality to the next? Lines of investigation that integrate ecological and neuroethological perspectives have begun to resituate rhythmic behaviours within animal communication systems (see [153]), but they have not been described in this paper. Such a multidisciplinary approach would allow researchers to design species-specific experiments to infer how rhythm functions during interactive signalling [154]. We thus strongly encourage future studies on interactive rhythmic behaviours in the hope of ultimately developing an integrative cross-species framework [4]. The comparative method could then provide crucial insights into the evolution and adaptive functions of interactive rhythmic behaviour across taxa [5,6].
Acknowledgements
We thank the organizers of the Lorentz workshop on Synchrony and Rhythmic Interaction: From Neurons to Ecology, the Lorentz Center, and the NIAS, ILLC and ANU.
Contributor Information
Koen de Reus, Email: kdereus95@gmail.com.
Andrea Ravignani, Email: andrea.ravignani@mpi.nl.
Data accessibility
This article has no additional data.
Authors' contributions
K.d.R. conceived the manuscript. All authors contributed in writing and revising of the manuscript, and gave final approval for publication.
Competing interests
The authors declare no competing or financial interests.
Funding
This work was supported by the Research Foundation Flanders (FWO) [G034720N] to Bart de Boer.; JSPS Grant-in-Aid for Young Scientist [16H06177] and for Scientific Research [20K06809] to M.S.; Junior Fellowship of the Hanse-Wissenschaftskolleg (Institute for Advanced Study) to M.A.; NIMH and NCCIH [R61MH123029] and NIDCD [R21DC016710] to M.L.; the Natural Sciences and Engineering Research Council of Canada (NSERC) [RGPIN-2019-05416], the Canadian Institutes of Health Research (CIHR) [RTI-2017-00643] and the Canadian Institute for Advanced Research to L.J.T. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
References
- 1.Dusenbery DB. 1992. Sensory ecology: how organisms acquire and respond to information. New York, NY: WH Freeman. [Google Scholar]
- 2.Tucker AS. 2017. Major evolutionary transitions and innovations: the tympanic middle ear. Phil. Trans. R. Soc. B 372, 20150483. ( 10.1098/rstb.2015.0483) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wiley RH, Richards DG. 1978. Physical constraints on acoustic communication in the atmosphere: implications for the evolution of animal vocalizations. Behav. Ecol. Sociobiol. 3, 69-94. ( 10.1007/BF00300047) [DOI] [Google Scholar]
- 4.Anichini M, de Heer Kloots M, Ravignani A. 2020. Interactive rhythms in the wild, in the brain, and in silico. Can. J. Exp. Psychol. 74, 170-175. ( 10.1037/cep0000224) [DOI] [PubMed] [Google Scholar]
- 5.Ravignani A, Bowling D, Fitch WT. 2014. Chorusing, synchrony, and the evolutionary functions of rhythm. Front. Psychol. 5, 1118. ( 10.3389/fpsyg.2014.01118) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ravignani A, Verga L, Greenfield MD. 2019. Interactive rhythms across species: the evolutionary biology of animal chorusing and turn-taking. Ann. N. Y. Acad. Sci. 1453, 12-21. ( 10.1111/nyas.14230) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nguyen T, Schleihauf H, Kayhan E, Matthes D, Vrtička P, Hoehl S. 2020. Neural synchrony in mother–child conversation: exploring the role of conversation patterns. Soc. Cogn. Affect. Neurosci. 2020, 1-10. ( 10.1093/scan/nsaa079) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keller PE, Novembre G, Hove MJ. 2014. Rhythm in joint action: psychological and neurophysiological mechanisms for real-time interpersonal coordination. Phil. Trans. R. Soc B 369, 20130394. ( 10.1098/rstb.2013.0394) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trehub SE, Becker J, Morley I. 2015. Cross-cultural perspectives on music and musicality. Phil. Trans. R. Soc. B 370, 20140096. ( 10.1098/rstb.2014.0096) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greenfield MD. 1994. Cooperation and conflict in the evolution of signal interactions. Annu. Rev. Ecol. Syst. 25, 97-126. ( 10.1146/annurev.es.25.110194.000525) [DOI] [Google Scholar]
- 11.Buck J. 1988. Synchronous rhythmic flashing of fireflies. II. Q. Rev. Biol. 63, 265-289. ( 10.1086/415929) [DOI] [PubMed] [Google Scholar]
- 12.Grafe TU. 1999. A function of synchronous chorusing and a novel female preference shift in an anuran. Proc. R. Soc. Lond. B 266, 2331-2336. ( 10.1098/rspb.1999.0927) [DOI] [Google Scholar]
- 13.Backwell P, Jennions M, Passmore N, Christy J. 1998. Synchronized courtship in fiddler crabs. Nature 391, 31-32. ( 10.1038/34076) [DOI] [Google Scholar]
- 14.Hall ML. 2009. A review of vocal duetting in birds. Adv. Study Behav. 40, 67-121. ( 10.1016/S0065-3454(09)40003-2) [DOI] [Google Scholar]
- 15.Stegmann UE. 2005. John Maynard Smith's notion of animal signals. Biol. Phil. 20, 1011-1025. ( 10.1007/s10539-005-9020-8) [DOI] [Google Scholar]
- 16.van den Bosch der Nederlanden CM, Taylor JET, Grahn JA. 2019. Neural basis of rhythm perception. In The Oxford handbook of music and the brain (eds Thaut M, Hodges D), pp. 165-186. Oxford, UK: Oxford University Press. [Google Scholar]
- 17.Phillips-Silver J, Aktipis CA, Bryant GA. 2010. The ecology of entrainment: foundations of coordinated rhythmic movement. Music Percept. 28, 3-14. ( 10.1525/mp.2010.28.1.3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Farabaugh SM. 1982. The ecological and social significance of duetting. In Acoustic communication in birds, vol. 2 (eds Kroodsma DE, Miller EH), pp. 85-124. New York, NY: Academic Press. [Google Scholar]
- 19.Ravignani A. 2017. Interdisciplinary debate: agree on definitions of synchrony. Nature 545, 158. ( 10.1038/545158c) [DOI] [PubMed] [Google Scholar]
- 20.Pika S, Wilkinson R, Kendrick KH, Vernes SC. 2018. Taking turns: bridging the gap between human and animal communication. Proc. R. Soc. B 285, 20180598. ( 10.1098/rspb.2018.0598) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greenfield MD. 2005. Mechanisms and evolution of communal sexual displays in arthropods and anurans. Adv. Study Behav. 35, 1-62. ( 10.1016/S0065-3454(05)35001-7) [DOI] [Google Scholar]
- 22.Yoshida S, Okanoya K. 2005. Evolution of turn-taking: a bio-cognitive perspective. Cogn. Stud. 12, 153-165. [Google Scholar]
- 23.Ravignani A, Norton P. 2017. Measuring rhythmic complexity: a primer to quantify and compare temporal structure in speech, movement, and animal vocalizations. J. Lang. Evol. 2, 4-19. ( 10.1093/jole/lzx002) [DOI] [Google Scholar]
- 24.Goebl W, Palmer C. 2009. Synchronization of timing and motion among performing musicians. Music Percept. 26, 427-438. ( 10.1525/mp.2009.26.5.427) [DOI] [Google Scholar]
- 25.Loehr JD, Palmer C. 2011. Temporal coordination between performing musicians. Q. J. Exp. Psychol. 64, 2153-2167. ( 10.1080/17470218.2011.603427) [DOI] [PubMed] [Google Scholar]
- 26.Mathevon N, Casey C, Reichmuth C, Charrier I. 2017. Northern elephant seals memorize the rhythm and timbre of their rivals' voices. Curr. Biol. 27, 2352-2356.e2. ( 10.1016/j.cub.2017.06.035) [DOI] [PubMed] [Google Scholar]
- 27.Feldman R. 2007. Parent–infant synchrony: biological foundations and developmental outcomes. Curr. Dir. Psychol. Sci. 16, 340-345. ( 10.1111/j.1467-8721.2007.00532.x) [DOI] [Google Scholar]
- 28.Juslin PN, Sloboda JA. 2013. Music and emotion. In The psychology of music (ed. Deutsch D), pp. 583-645. Amsterdam, The Netherlands: Elsevier Inc. [Google Scholar]
- 29.Chang A, Kragness HE, Livingstone SR, Bosnyak DJ, Trainor LJ. 2019. Body sway reflects joint emotional expression in music ensemble performance. Sci. Rep. 9, 1-11. ( 10.1038/s41598-018-36358-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chauvigné LAS, Walton A, Richardson MJ, Brown S. 2019. Multi-person and multisensory synchronization during group dancing. Hum. Mov. Sci. 63, 199-208. ( 10.1016/j.humov.2018.12.005) [DOI] [PubMed] [Google Scholar]
- 31.Goldin-Meadow S. 1999. The role of gesture in communication and thinking. Trends Cogn. Sci. 3, 419-429. ( 10.1016/S1364-6613(99)01397-2) [DOI] [PubMed] [Google Scholar]
- 32.Holler J, Schubotz L, Kelly S, Hagoort P, Schuetze M, Özyürek A. 2014. Social eye gaze modulates processing of speech and co-speech gesture. Cognition 133, 692-697. ( 10.1016/j.cognition.2014.08.008) [DOI] [PubMed] [Google Scholar]
- 33.Tronick EZ, Cohn JF. 1989. Infant–mother face-to-face interaction: age and gender differences in coordination and the occurrence of miscoordination. Child Dev. 60, 92. ( 10.2307/1131074) [DOI] [PubMed] [Google Scholar]
- 34.Beebe B, Alson D, Jaffe J, Feldstein S, Crown C. 1988. Vocal congruence in mother-infant play. J. Psycholinguist. Res. 17, 245-259. ( 10.1007/BF01686358) [DOI] [PubMed] [Google Scholar]
- 35.Leong V, Byrne E, Clackson K, Georgieva S, Lam S, Wass S. 2017. Speaker gaze increases information coupling between infant and adult brains. Proc. Natl Acad. Sci. USA 114, 13 290-13 295. ( 10.1073/pnas.1702493114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leclère C, Viaux S, Avril M, Achard C, Chetouani M, Missonnier S, Cohen D. 2014. Why synchrony matters during mother–child interactions: a systematic review. PLoS ONE 9, e113571. ( 10.1371/journal.pone.0113571) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Feldman R. 2017. The neurobiology of human attachments. Trends Cogn. Sci. 21, 80-99. ( 10.1016/j.tics.2016.11.007) [DOI] [PubMed] [Google Scholar]
- 38.Feldman R, Greenbaum CW, Yirmiya N. 1999. Mother–infant affect synchrony as an antecedent of the emergence of self-control. Dev. Psychol. 35, 223-231. ( 10.1037/0012-1649.35.1.223) [DOI] [PubMed] [Google Scholar]
- 39.Trainor LJ, Cirelli L. 2015. Rhythm and interpersonal synchrony in early social development. Ann. N. Y. Acad. Sci. 1337, 45-52. ( 10.1111/nyas.12649) [DOI] [PubMed] [Google Scholar]
- 40.Lytle SR, Kuhl PK. 2018. Social interaction and language acquisition: toward a neurobiological view. In The handbook of psycholinguistics (eds Fernández EM, Cairns HS), pp. 615-634. Hoboken, NJ: Wiley Blackwell. [Google Scholar]
- 41.Trehub SE, Ghazban N, Corbeil M. 2015. Musical affect regulation in infancy. Ann. N. Y. Acad. Sci. 1337, 186-192. ( 10.1111/nyas.12622) [DOI] [PubMed] [Google Scholar]
- 42.Cirelli LK, Jurewicz ZB, Trehub SE. 2020. Effects of maternal singing style on mother–infant arousal and behavior. J. Cogn. Neurosci. 32, 1213-1220. ( 10.1162/jocn_a_01402) [DOI] [PubMed] [Google Scholar]
- 43.Patel AD. 2010. Music, language, and the brain. Oxford, UK: Oxford University Press. [Google Scholar]
- 44.Cirelli LK, Trehub SE, Trainor LJ. 2018. Rhythm and melody as social signals for infants. Ann. N. Y. Acad. Sci. 1423, 66-72. ( 10.1111/nyas.13580) [DOI] [PubMed] [Google Scholar]
- 45.Harrist AW, Waugh RM. 2002. Dyadic synchrony: its structure and function in children's development. Dev. Rev. 22, 555-592. ( 10.1016/S0273-2297(02)00500-2) [DOI] [Google Scholar]
- 46.Maccoby, E. E. 2004. The role of parents in the socialization of children: an historical overview. In A century of developmental psychology (eds Parke RD, Ornstein PA, Rieser JJ, Zahn-Waxler C), pp. 589-615. Washington, DC: American Psychological Association. [Google Scholar]
- 47.Casillas M, Frank MC. 2017. The development of children's ability to track and predict turn structure in conversation. J. Mem. Lang. 92, 234-253. ( 10.1016/j.jml.2016.06.013) [DOI] [Google Scholar]
- 48.Tomasello M, Farrar MJ. 1986. Joint attention and early language. Child Dev. 57, 1454. ( 10.2307/1130423) [DOI] [PubMed] [Google Scholar]
- 49.Stanton-Chapman TL, Snell ME. 2011. Promoting turn-taking skills in preschool children with disabilities: the effects of a peer-based social communication intervention. Early Childhood Res. Q. 26, 303-319. ( 10.1016/j.ecresq.2010.11.002) [DOI] [Google Scholar]
- 50.Black B, Logan A. 1995. Links between communication patterns in mother-child, father-child, and child-peer interactions and children's social status. Child Dev. 66, 255-271. ( 10.1111/j.1467-8624.1995.tb00869.x) [DOI] [Google Scholar]
- 51.Black B, Hazen NL. 1990. Social status and patterns of communication in acquainted and unacquainted preschool children. Dev. Psychol. 26, 379-387. ( 10.1037/0012-1649.26.3.379) [DOI] [Google Scholar]
- 52.Kirschner Sebastian S, Tomasello M. 2010. Joint music making promotes prosocial behavior in 4-year-old children. Evol. Hum. Behav. 31, 354-364. ( 10.1016/j.evolhumbehav.2010.04.004) [DOI] [Google Scholar]
- 53.Rabinowitch T-C, Knafo-Noam A. 2015. Synchronous rhythmic interaction enhances children's perceived similarity and closeness towards each other. PLoS ONE 10, e0120878. ( 10.1371/journal.pone.0120878) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Abney DH, Paxton A, Dale R, Kello CT. 2014. Complexity matching in dyadic conversation. J. Exp. Psychol. Gen. 143, 2304-2315. ( 10.1037/xge0000021) [DOI] [PubMed] [Google Scholar]
- 55.Wilson M, Wilson TP. 2005. An oscillator model of the timing of turn-taking. Psychon. Bull. Rev. 12, 957-968. ( 10.3758/BF03206432) [DOI] [PubMed] [Google Scholar]
- 56.Buder EH, Warlaumont AS, Kimbrough D, Chorna LB. 2010. Dynamic indicators of mother-infant prosodic and illocutionary coordination. In Speech Prosody 2010, 5th Int. Conf. Chicago, IL, May 10–14. ISCA Archive, https://www.isca-speech.org/archive/sp2010/papers/sp10_433.pdf. [Google Scholar]
- 57.Schultz BG, O'Brien I, Phillips N, McFarland DH, Titone D, Palmer C. 2016. Speech rates converge in scripted turn-taking conversations. Appl. Psycholinguist. 37, 1201-1220. ( 10.1017/S0142716415000545) [DOI] [Google Scholar]
- 58.McFarland DH. 2001. Respiratory markers of conversational interaction. J. Speech. Lang. Hear. Res. 44, 128-143. ( 10.1044/1092-4388(2001/012)) [DOI] [PubMed] [Google Scholar]
- 59.O'Dell M, Nieminen T, Lennes M. 2012. Modeling turn-taking rhythms with oscillators. Linguistica Ural. 48, 218-227. ( 10.3176/lu.2012.3.08) [DOI] [Google Scholar]
- 60.Stivers T, et al. 2009. Universals and cultural variation in turn-taking in conversation. Proc. Natl Acad. Sci. USA 106, 10587. ( 10.1073/pnas.0903616106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Haegens S, Zion Golumbic E. 2018. Rhythmic facilitation of sensory processing: a critical review. Neurosci. Biobehav. Rev. 86, 150-165. ( 10.1016/j.neubiorev.2017.12.002) [DOI] [PubMed] [Google Scholar]
- 62.Bishop L, Goebl W. 2015. When they listen and when they watch: pianists' use of nonverbal audio and visual cues during duet performance. Music. Sci. 19, 84-110. ( 10.1177/1029864915570355) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Moran N, Hadley LV, Bader M, Keller PE. 2015. Perception of ‘back-channeling’ nonverbal feedback in musical duo improvisation. PLoS ONE 10, e0130070. ( 10.1371/journal.pone.0130070) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Repp BH, Su YH. 2013. Sensorimotor synchronization: a review of recent research (2006–2012). Psychon. Bull. Rev. 20, 403-452. ( 10.3758/s13423-012-0371-2) [DOI] [PubMed] [Google Scholar]
- 65.Gilboa A, Tal-Shmotkin M. 2012. String quartets as self-managed teams: an interdisciplinary perspective. Psychol. Music 40, 19-41. ( 10.1177/0305735610377593) [DOI] [Google Scholar]
- 66.Timmers R, Endo S, Bradbury A, Wing AM. 2014. Synchronization and leadership in string quartet performance: a case study of auditory and visual cues. Front. Psychol. 5, 645. ( 10.3389/fpsyg.2014.00645) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wing AM, Endo S, Bradbury A, Vorberg D. 2014. Optimal feedback correction in string quartet synchronization. J. R. Soc. Interface 11, 20131125. ( 10.1098/rsif.2013.1125) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Demos AP, Carter DJ, Wanderley MM, Palmer C. 2017. The unresponsive partner: roles of social status, auditory feedback, and animacy in coordination of joint music performance. Front. Psychol. 8, 149. ( 10.3389/fpsyg.2017.00149) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zamm A, Wellman C, Palmer C. 2016. Endogenous rhythms influence interpersonal synchrony. J. Exp. Psychol. Hum. Percept. Perform. 42, 611-616. ( 10.1037/xhp0000201) [DOI] [PubMed] [Google Scholar]
- 70.Chang A, Livingstone SR, Bosnyak DJ, Trainor LJ. 2017. Body sway reflects leadership in joint music performance. Proc. Natl Acad. Sci. USA 114, E4134-E4141. ( 10.1073/pnas.1617657114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hove MJ, Risen JL. 2009. It's all in the timing: interpersonal synchrony increases affiliation. Soc. Cogn. 27, 949-960. ( 10.1521/soco.2009.27.6.949) [DOI] [Google Scholar]
- 72.Wiltermuth SS, Heath C. 2009. Synchrony and cooperation. Psychol. Sci. 20, 1-5. ( 10.1111/j.1467-9280.2008.02253.x) [DOI] [PubMed] [Google Scholar]
- 73.Savage PE, Loui P, Tarr B, Schachner A, Glowacki L, Mithen S, Fitch WT. 2020. Music as a coevolved system for social bonding. Behav. Brain Sci. online, 1-36. ( 10.1017/S0140525X20000333) [DOI] [PubMed] [Google Scholar]
- 74.Cheney DL, Silk JB, Seyfarth RM. 2016. Network connections, dyadic bonds and fitness in wild female baboons. R. Soc. Open Sci. 3, 160255. ( 10.1098/rsos.160255) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Silk JB, Alberts SC, Altmann J. 2003. Social bonds of female baboons enhance infant survival. Science 302, 1231-1234. ( 10.1126/science.1088580) [DOI] [PubMed] [Google Scholar]
- 76.Lemasson A, Ouattara K, Petit EJ, Zuberbühler K. 2011. Social learning of vocal structure in a nonhuman primate? BMC Evol. Biol. 11, 1-7. ( 10.1186/1471-2148-11-362) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lemasson A, Hausberger M. 2004. Patterns of vocal sharing and social dynamics in a captive group of Campbell's monkeys (Cercopithecus campbelli campbelli). J. Comp. Psychol. 118, 347-359. ( 10.1037/0735-7036.118.3.347) [DOI] [PubMed] [Google Scholar]
- 78.Levréro F, Touitou S, Frédet J, Nairaud B, Guéry JP, Lemasson A. 2019. Social bonding drives vocal exchanges in bonobos. Sci. Rep. 9, 1-11. ( 10.1038/s41598-018-36024-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Geissmann T. 2000. Gibbon songs and human music from an evolutionary perspective. In The origins of music (eds Wallin N, Merker B, Brown S), pp. 103-123. Cambridge, MA: MIT Press. [Google Scholar]
- 80.Torti V, Bonadonna G, De Gregorio C, Valente D, Randrianarison RM, Friard O, Pozzi L, Gamba M, Giacoma C. 2017. An intra-population analysis of the indris' song dissimilarity in the light of genetic distance. Sci. Rep. 7, 1-12. ( 10.1038/s41598-017-10656-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bonadonna G, Torti V, Sorrentino V, Randrianarison RM, Zaccagno M, Gamba M, Tan CL, Giacoma C. 2017. Territory exclusivity and intergroup encounters in the indris (Mammalia: Primates: Indridae: Indri indri) upon methodological tuning. Eur. Zool. J. 84, 238-251. ( 10.1080/24750263.2017.1318184) [DOI] [Google Scholar]
- 82.Bonadonna G, Torti V, Gregorio C, Valente D, Randrianarison RM, Pozzi L, Gamba M, Giacoma C. 2019. Evidence of genetic monogamy in the lemur Indri (Indri indri). Am. J. Primatol. 81, e22993. ( 10.1002/ajp.22993) [DOI] [PubMed] [Google Scholar]
- 83.Gamba M, Torti V, Estienne V, Randrianarison RM, Valente D, Rovara P, Bonadonna G, Friard O, Giacoma C. 2016. The indris have got rhythm! Timing and pitch variation of a primate song examined between sexes and age classes. Front. Neurosci. 10, 249. ( 10.3389/fnins.2016.00249) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Maretti G, Sorrentino V, Finomana A, Gamba M, Giacoma C. 2010. Not just a pretty song: an overview of the vocal repertoire of Indri indri. J. Anthropol. Sci. 88, 151-165. [PubMed] [Google Scholar]
- 85.Valente D, De Gregorio C, Torti V, Miaretsoa L, Friard O, Randrianarison RM, Giacoma C, Gamba M. 2019. Finding meanings in low dimensional structures: stochastic neighbor embedding applied to the analysis of Indri indri vocal repertoire. Animals 9, 243. ( 10.3390/ani9050243) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Torti V, Gamba M, Rabemananjara ZH, Giacoma C. 2013. The songs of the indris (Mammalia: Primates: Indridae): contextual variation in the long-distance calls of a lemur. Ital. J. Zool. 80, 596-607. ( 10.1080/11250003.2013.845261) [DOI] [Google Scholar]
- 87.Clarke E, Reichard UH, Zuberbühler K. 2006. The syntax and meaning of wild gibbon songs. PLoS ONE 1, e73. ( 10.1371/journal.pone.0000073) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Badraun JC, Mootnick AR, Deaner RO, Agoramoorthy G, McNeese KM. 1998. Hand modulation of vocalization in Siamangs Hylobates syndactylus. Int. Zoo. Yearb. 36, 84-89. ( 10.1111/j.1748-1090.1998.tb02888.x) [DOI] [Google Scholar]
- 89.Geissmann T, Orgeldinger M. 2000. The relationship between duet songs and pair bonds in siamangs, Hylobates syndactylus. Anim. Behav. 60, 805-809. ( 10.1006/anbe.2000.1540) [DOI] [PubMed] [Google Scholar]
- 90.Lilly JC, Miller AM. 1961. Sounds emitted by the bottlenose dolphin. Science 133, 1689-1693. ( 10.1126/science.133.3465.1689) [DOI] [PubMed] [Google Scholar]
- 91.Caldwell CA. 1968. Vocalisation of native dolphins in small groups. Science 159, 1121-1123. [DOI] [PubMed] [Google Scholar]
- 92.Nakahara F, Miyazaki N. 2011. Vocal exchanges of signature whistles in bottlenose dolphins (Tursiops truncatus). J. Ethol. 29, 309-320. ( 10.1007/s10164-010-0259-4) [DOI] [Google Scholar]
- 93.Moore BL, Connor RC, Allen SJ, Krützen M, King SL. 2020. Acoustic coordination by allied male dolphins in a cooperative context. Proc. R. Soc. B 287, 20192944. ( 10.1098/rspb.2019.2944) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Insley SJ, Phillips A, Charrier I. 2003. A review of social recognition in pinnipeds. Aquat. Mamm. 29, 181-201. ( 10.1578/016754203101024149) [DOI] [Google Scholar]
- 95.Charrier I. 2020. Mother–offspring vocal recognition and social system in pinnipeds. In Coding strategies in vertebrate acoustic communication (eds Aubin T, Mathevon N), pp. 231-246. Cham, Switzerland: Springer. [Google Scholar]
- 96.Ravignani A. 2019. Timing of antisynchronous calling: a case study in a harbor seal pup (Phoca vitulina). J. Comp. Psychol. 133, 272-277. ( 10.1037/com0000160) [DOI] [PubMed] [Google Scholar]
- 97.Esser K-H, Schmidt U. 2010. Mother-infant communication in the lesser spear-nosed bat Phyllostomus discolor (Chiroptera, Phyllostomidae)—evidence for acoustic learning. Ethology 82, 156-168. ( 10.1111/j.1439-0310.1989.tb00496.x) [DOI] [Google Scholar]
- 98.Balcombe JP, McCracken GF. 1992. Vocal recognition in Mexican free-tailed bats: do pups recognize mothers? Anim. Behav. 43, 79-87. ( 10.1016/S0003-3472(05)80073-9) [DOI] [Google Scholar]
- 99.De Fanis E, Jones G. 1996. Allomaternal care and recognition between mothers and young in pipistrelle bats (Pipistrellus pipistrellus). J. Zool. 240, 781-787. ( 10.1111/j.1469-7998.1996.tb05324.x) [DOI] [Google Scholar]
- 100.Carter GG, Fenton MB, Faure PA. 2009. White-winged vampire bats (Diaemus youngi) exchange contact calls. Can. J. Zool. 87, 604-608. ( 10.1139/Z09-051) [DOI] [Google Scholar]
- 101.Okobi DE, Banerjee A, Matheson AMM, Phelps SM, Long MA. 2019. Motor cortical control of vocal interaction in neotropical singing mice. Science 363, 983-988. ( 10.1126/science.aau9480) [DOI] [PubMed] [Google Scholar]
- 102.Yosida S, Okanoya K. 2009. Naked mole-rat is sensitive to social hierarchy encoded in antiphonal vocalization. Ethology 115, 823-831. ( 10.1111/j.1439-0310.2009.01677.x) [DOI] [Google Scholar]
- 103.Rado R, Levi N, Hauser H, Witcher J, Alder N, Intrator N, Wollberg Z, Terkel J. 1987. Seismic signalling as a means of communication in a subterranean mammal. Anim. Behav. 35, 1249-1251. ( 10.1016/S0003-3472(87)80183-5) [DOI] [Google Scholar]
- 104.Leighty KA, Soltis J, Wesolek CM, Savage A. 2008. Rumble vocalizations mediate interpartner distance in African elephants, Loxodonta africana. Anim. Behav. 76, 1601-1608. ( 10.1016/j.anbehav.2008.06.022) [DOI] [Google Scholar]
- 105.Tilson RL, Norton PM. 1981. Alarm duetting and pursuit deterrence in an African antelope. Am. Nat. 118, 455-462. ( 10.1086/283840) [DOI] [Google Scholar]
- 106.Demartsev V, Strandburg-Peshkin A, Ruffner M, Manser M. 2018. Vocal turn-taking in meerkat group calling sessions. Curr. Biol. 28, 3661-3666.e3. ( 10.1016/j.cub.2018.09.065) [DOI] [PubMed] [Google Scholar]
- 107.Tobias JA, Sheard C, Seddon N, Meade A, Cotton AJ, Nakagawa S. 2016. Territoriality, social bonds, and the evolution of communal signaling in birds. Front. Ecol. Evol. 4, 74. ( 10.3389/fevo.2016.00074) [DOI] [Google Scholar]
- 108.Malacarne G, Cucco M, Camanni S. 1991. Coordinated visual displays and vocal duetting in different ecological situations among western palearctic non-passerine birds. Ethol. Ecol. Evol. 3, 207-219. ( 10.1080/08927014.1991.9525369) [DOI] [Google Scholar]
- 109.Soma M, Garamszegi LZ. 2015. Evolution of courtship display in Estrildid finches: dance in relation to female song and plumage ornamentation. Front. Ecol. Evol. 3, 4. ( 10.3389/fevo.2015.00004) [DOI] [Google Scholar]
- 110.Hall ML. 2004. A review of hypotheses for the functions of avian duetting. Behav. Ecol. Sociobiol. 55, 415-430. ( 10.1007/s00265-003-0741-x) [DOI] [Google Scholar]
- 111.Langmore NE. 1998. Functions of duet and solo songs of female birds. Trends Ecol. Evol. 13, 136-140. ( 10.1016/S0169-5347(97)01241-X) [DOI] [PubMed] [Google Scholar]
- 112.Short LL, Horne JFM. 1983. A review of duetting, sociality and speciation in some African barbets (Capitonidae). Condor 85, 323. ( 10.2307/1367069) [DOI] [Google Scholar]
- 113.Ręk P, Magrath RD. 2016. Multimodal duetting in magpie-larks: how do vocal and visual components contribute to a cooperative signal's function? Anim. Behav. 117, 35-42. ( 10.1016/j.anbehav.2016.04.024) [DOI] [Google Scholar]
- 114.Mann NI, Dingess KA, Barker KF, Graves JA, Slater PJB. 2009. A comparative study of song form and duetting in neotropical Thryothorus wrens. Behaviour 146, 1-43. ( 10.1163/156853908X390913) [DOI] [Google Scholar]
- 115.Brumm H, Slater P. 2007. Animal communication: timing counts. Curr. Biol. 17, R521-R523. ( 10.1016/j.cub.2007.04.053) [DOI] [PubMed] [Google Scholar]
- 116.Takeda KF, Hiraiwa-Hasegawa M, Kutsukake N. 2019. Uncoordinated dances associated with high reproductive success in a crane. Behav. Ecol. 30, 101-106. ( 10.1093/beheco/ary159) [DOI] [Google Scholar]
- 117.Re¸k P. 2018. Multimodal coordination enhances the responses to an avian duet. Behav. Ecol. 29, 411-417. ( 10.1093/beheco/arx174) [DOI] [Google Scholar]
- 118.Hall ML, Magrath RD. 2007. Temporal coordination signals coalition quality. Curr. Biol. 17, R406-R407. ( 10.1016/j.cub.2007.04.022) [DOI] [PubMed] [Google Scholar]
- 119.Rivera-Cáceres KD, Quirós-Guerrero E, Araya-Salas M, Searcy WA. 2016. Neotropical wrens learn new duet rules as adults. Proc. R. Soc. B 283, 20161819. ( 10.1098/rspb.2016.1819) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Griffith SC, Buchanan KL. 2010. The zebra finch: the ultimate Australian supermodel. Emu 110, v-xii. ( 10.1071/MUv110n3_ED) [DOI] [Google Scholar]
- 121.Prior NH, Smith E, Dooling RJ, Ball GF. 2020. Familiarity enhances moment-to-moment behavioral coordination in zebra finch (Taeniopygia guttata) dyads. J. Comp. Psychol. 134, 135-148. ( 10.1037/com0000201) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.D'Amelio PB, Trost L, ter Maat A. 2017. Vocal exchanges during pair formation and maintenance in the zebra finch (Taeniopygia guttata). Front. Zool. 14, 1-12. ( 10.1186/s12983-017-0197-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Benichov JI, Benezra SE, Vallentin D, Globerson E, Long MA, Tchernichovski O. 2016. The forebrain song system mediates predictive call timing in female and male zebra finches. Curr. Biol. 26, 309-318. ( 10.1016/j.cub.2015.12.037) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Perez EC, Elie JE, Boucaud ICA, Crouchet T, Soulage CO, Soula HA, Theunissen FE, Vignal C. 2015. Physiological resonance between mates through calls as possible evidence of empathic processes in songbirds. Horm. Behav. 75, 130-141. ( 10.1016/j.yhbeh.2015.09.002) [DOI] [PubMed] [Google Scholar]
- 125.Boucaud ICA, Perez EC, Ramos LS, Griffith SC, Vignal C. 2017. Acoustic communication in zebra finches signals when mates will take turns with parental duties. Behav. Ecol. 28, 645-656. ( 10.1093/beheco/arw189) [DOI] [Google Scholar]
- 126.Grafe TU. 2005. Anuran choruses as communication networks. In Animal communication networks (ed. PK McGregor), pp. 277–299. Cambridge, UK: Cambridge University Press.
- 127.Bosch J, Márquez R. 2001. Call timing in male-male acoustical interactions and female choice in the midwife toad Alytes obstetricans. Copeia 2001, 169-177. ( 10.1643/0045-8511(2001)001[0169:CTIMMA]2.0.CO;2) [DOI] [Google Scholar]
- 128.Wells KD, Schwartz JJ. 2006. The behavioral ecology of anuran communication. In Hearing and sound communication in amphibians (eds PM Narins, AS Feng, RR Fay, AN Popper), Springer Handbook of Auditory Research, vol. 28, pp. 44-86. New York, NY: Springer. [Google Scholar]
- 129.Wells KD. 1977. The social behaviour of anuran amphibians. Anim. Behav. 25, 666-693. ( 10.1016/0003-3472(77)90118-X) [DOI] [Google Scholar]
- 130.Caldwell JP, Shepard DB. 2007. Calling site fidelity and call structure of a neotropical toad, Rhinella ocellata (Anura: Bufonidae). J. Herpetol. 41, 611-621. ( 10.1670/07-025.1) [DOI] [Google Scholar]
- 131.Klump GM, Gerhardt HC. 1992. Mechanisms and function of call-timing in male-male interactions in frogs. In Playback and studies of animal communication (ed. PK McGregor), NATO ASI Series A, vol. 228, pp. 153-174. Boston, MA: Springer US. [Google Scholar]
- 132.Boatright-Horowitz SL, Horowitz SS, Simmons AM. 2000. Patterns of vocal interactions in a bullfrog (Rana catesbeiana) chorus: preferential responding to far neighbors. Ethology 106, 701-712. ( 10.1046/j.1439-0310.2000.00580.x) [DOI] [Google Scholar]
- 133.Tuttle MD, Ryan MJ. 1982. The role of synchronized calling, ambient light, and ambient noise, in anti-bat-predator behavior of a treefrog. Behav. Ecol. Sociobiol. 11, 125-131. ( 10.1007/BF00300101) [DOI] [Google Scholar]
- 134.Tobias ML, Viswanathan SS, Kelley DB. 1998. Rapping, a female receptive call, initiates male-female duets in the South African clawed frog. Proc. Natl Acad. Sci. USA 95, 1870-1875. ( 10.1073/pnas.95.4.1870) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bailey WJ. 2003. Insect duets: underlying mechanisms and their evolution. Physiol. Entomol. 28, 157-174. ( 10.1046/j.1365-3032.2003.00337.x) [DOI] [Google Scholar]
- 136.Zimmermann U, Rheinlaender J, Robinson D. 1989. Cues for male phonotaxis in the duetting bushcricket Leptophyes punctatissima. J. Comp. Physiol. A 164, 621-628. [Google Scholar]
- 137.Bailey WJ, Hammond TJ. 2003. Duetting in insects does call length influence reply latency? J. Zool. 260, 267-274. ( 10.1017/S0952836903003728) [DOI] [Google Scholar]
- 138.Kuhelj A, de Groot M, Blejec A, Virant-Doberlet M. 2015. The effect of timing of female vibrational reply on male signalling and searching behaviour in the leafhopper Aphrodes makarovi. PLoS ONE 10, e0139020. ( 10.1371/journal.pone.0139020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kuhelj A, de Groot M, Blejec A, Virant-Doberlet M. 2016. Sender-receiver dynamics in leafhopper vibrational duetting. Anim. Behav. 114, 139-146. ( 10.1016/j.anbehav.2016.02.001) [DOI] [Google Scholar]
- 140.Villarreal SM, Gilbert C. 2014. Male Scudderia pistillata katydids defend their acoustic duet against eavesdroppers. Behav. Ecol. Sociobiol. 68, 1669-1675. ( 10.1007/s00265-014-1775-y) [DOI] [Google Scholar]
- 141.LaRue KM, Clemens J, Berman GJ, Murthy M. 2015. Acoustic duetting in Drosophila virilis relies on the integration of auditory and tactile signals. eLife 4, 1-23. ( 10.7554/eLife.07277) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Rajaraman K, Godthi V, Pratap R, Balakrishnan R. 2015. A novel acoustic-vibratory multimodal duet. J. Exp. Biol. 218, 3042-3050. ( 10.1242/jeb.122911) [DOI] [PubMed] [Google Scholar]
- 143.Greenfield MD, Minckley RL. 1993. Acoustic dueling in tarbush grasshoppers: settlement of territorial contests via alternation of reliable signals. Ethology 95, 309-326. ( 10.1111/j.1439-0310.1993.tb00480.x) [DOI] [Google Scholar]
- 144.Halfwerk W, Varkevisser J, Simon R, Mendoza E, Scharff C, Riebel K. 2019. Toward testing for multimodal perception of mating signals. Front. Ecol. Evol. 7, 124. ( 10.3389/fevo.2019.00124) [DOI] [Google Scholar]
- 145.Jeng M-L. 2019. Biofluorescence in terrestrial animals, with emphasis on fireflies: a review and field observation. In Bioluminescence: analytical applications and basic biology (ed. H Suzuki). London, UK: IntechOpen. [Google Scholar]
- 146.Tuthill JC, Wilson RI. 2016. Mechanosensation and adaptive motor control in insects. Curr. Biol. 26, R1022-R1038. ( 10.1016/j.cub.2016.06.070) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ota N, Gahr M, Soma M. 2015. Tap dancing birds: the multimodal mutual courtship display of males and females in a socially monogamous songbird. Sci. Rep. 5, 1-6. ( 10.1038/srep16614) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Grafe TU, Wanger TC. 2007. Multimodal signaling in male and female foot-flagging frogs Staurois guttatus (Ranidae): an alerting function of calling. Ethology 113, 772-781. ( 10.1111/j.1439-0310.2007.01378.x) [DOI] [Google Scholar]
- 149.Carouso-Peck S, Menyhart O, DeVoogd TJ, Goldstein MH. 2020. Contingent parental responses are naturally associated with zebra finch song learning. Anim. Behav. 165, 123-132. ( 10.1016/j.anbehav.2020.04.019) [DOI] [Google Scholar]
- 150.Lampen J, McAuley JD, Chang SE, Wade J. 2019. Neural activity associated with rhythmicity of song in juvenile male and female zebra finches. Behav. Process. 163, 45-52. ( 10.1016/j.beproc.2017.12.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Desjonquères C, Maliszewski J, Lewandowski EN, Speck B, Rodríguez RL. 2019. Social ontogeny in the communication system of an insect. Anim. Behav. 148, 93-103. ( 10.1016/j.anbehav.2018.12.002) [DOI] [Google Scholar]
- 152.Greenfield MD. 1994. Synchronous and alternating choruses in insects and anurans: common mechanisms and diverse functions. Integr. Comp. Biol. 34, 605-615. ( 10.1093/icb/34.6.605) [DOI] [Google Scholar]
- 153.Hoffmann S, et al. 2019. Duets recorded in the wild reveal that interindividually coordinated motor control enables cooperative behavior. Nat. Commun. 10, 1-11. ( 10.1038/s41467-019-10593-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Henry MJ, Cook PF, de Reus K, Nityananda V, Rouse AA, Kotz SA. 2021. An ecological approach to measuring synchronization abilities across the animal kingdom. Phil. Trans. R. Soc. B 376, 20200336. ( 10.1098/rstb.2020.0336) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.