Abstract
Rhythmic behaviour is ubiquitous in both human and non-human animals, but it is unclear whether the cognitive mechanisms underlying the specific rhythmic behaviours observed in different species are related. Laboratory experiments combined with highly controlled stimuli and tasks can be very effective in probing the cognitive architecture underlying rhythmic abilities. Rhythmic abilities have been examined in the laboratory with explicit and implicit perception tasks, and with production tasks, such as sensorimotor synchronization, with stimuli ranging from isochronous sequences of artificial sounds to human music. Here, we provide an overview of experimental findings on rhythmic abilities in human and non-human animals, while critically considering the wide variety of paradigms used. We identify several gaps in what is known about rhythmic abilities. Many bird species have been tested on rhythm perception, but research on rhythm production abilities in the same birds is lacking. By contrast, research in mammals has primarily focused on rhythm production rather than perception. Many experiments also do not differentiate between possible components of rhythmic abilities, such as processing of single temporal intervals, rhythmic patterns, a regular beat or hierarchical metrical structures. For future research, we suggest a careful choice of paradigm to aid cross-species comparisons, and a critical consideration of the multifaceted abilities that underlie rhythmic behaviour.
This article is part of the theme issue ‘Synchrony and rhythm interaction: from the brain to behavioural ecology’.
Keywords: rhythm, beat, temporal processing, cross-species, non-human animals, music
1. Introduction
Rhythmic behaviour is ubiquitous in both human and non-human animals. To understand the origin and function of rhythmic behaviour and the cognitive mechanisms underlying it, cross-species comparisons can be informative [1,2]. However, the specific rhythmic behaviours exhibited by different species vary wildly, from humans dancing to a regular musical beat, to rhythmic katydid calls, to bird vocalizations containing precisely timed rhythmic patterns. It is currently unclear which behaviours exhibited by different species result from similar underlying rhythmic abilities and cognitive mechanisms, and which can be considered qualitatively different.
One of the challenges in cross-species comparisons of rhythmic abilities lies in the definition of what constitutes rhythmic behaviour. First, to compare rhythmic abilities across species, we must decompose these abilities into components, rather than considering them as one entity [2–4]. Indeed, specific components of rhythmic abilities may differ between human and non-human animals, such as the ability to perceive a regular beat [5], and the ability to perceive hierarchical rhythmical structure [6]. Second, while many important insights about rhythmic behaviour result from observations in the natural environment (see [7,8], this volume), rhythmic features of natural behaviour may have evolved in a specific functional context, or may be emergent from group behaviour [9]. Such rhythmic behaviours may not be related to a general ability in an individual to perceive and/or produce arbitrary rhythmic patterns, and the cognitive architecture underlying this ability.
Laboratory studies using arbitrary and highly controlled stimuli and tasks (e.g. rhythms and rhythmic behaviours not found in the natural environment or behaviour of a species) can be very effective to study the cognitive mechanisms underlying the production and perception of rhythms. First, by using arbitrary stimuli in the laboratory, the various components of rhythms and rhythmic behaviour that co-occur in the natural environment can be studied in isolation. For example, rhythmic calls often contain multiple types of structure, both in time and in order. The use of artificial stimuli in which only one or a few carefully controlled components are present allows for testing exactly which rhythmic aspects are perceived or drive a particular response. Second, humans have the ability to perceive and produce arbitrary rhythmic stimuli outside of a functional context. By focusing on the processing of artificially constructed sequences of simple tones or pulses that are not necessarily tied to a specific function, laboratory studies are well suited to probe whether other animal species can also apply rhythmic abilities flexibly across different contexts, stimuli and motor patterns. Related to this, a human or non-human animal may never show a certain rhythmic behaviour in its natural environment if it is not functionally relevant (e.g. tapping to a non-metric rhythm), but laboratory studies may show that the capability to do so is present [10]. Finally, by using tasks that probe individuals, rather than groups, laboratory studies can focus on rhythmic abilities of the individual, rather than rhythms that are emergent from group interactions.
Thus, highly controlled laboratory studies with arbitrary stimuli and tasks provide several advantages and possibilities for studying rhythmic abilities. However, rhythmic behaviour has also been associated with social ([8,11], this volume), and emotional [12] factors that are hard to reproduce in a laboratory setting. Moreover, the lack of functional meaning in arbitrary stimuli that are unlike real music or vocalizations may affect the motivation to attend to such stimuli [13], making it necessary to artificially elicit the motivation to engage with rhythm in the laboratory (i.e. by using food as a reward). Finally, responses required in the laboratory may be far removed from rhythmic behaviour in the natural environment (e.g. for humans, finger tapping may not have much to do with dancing in a social setting, and for a songbird, detecting isochrony may not be relevant to mate choice). Ultimately, understanding the full breadth of rhythmic abilities in human and non-human animals, therefore, requires both types of approaches: laboratory-based experiments with arbitrary stimuli and tasks, on which we focus here, as well as observations on and experiments with more natural stimuli and tasks, as discussed elsewhere ([7,14], this volume).
In laboratory experiments, a great heterogeneity of paradigms has been used to probe rhythmic abilities, making cross-species comparisons difficult. To better understand which components of rhythmic abilities are similar across species, a comparison should be made between studies using similar methodology and probing similar aspects of rhythmic behaviour. Here, as a starting point in this endeavour, we provide an overview of experimental findings on rhythmic abilities in human and non-human animals, and what these reveal about similarities and differences in these abilities. We critically consider the tasks and stimuli used, to arrive at recommendations for future research that aims to determine the cognitive mechanisms underlying rhythmic abilities across species.
2. Components of rhythmic abilities
Rhythm is often defined as ‘a sequence of events in time’ [15], or a pattern of multiple time intervals demarcated by the onsets of those events [16]. While processing of one single duration (e.g. duration discrimination) is considered a very fundamental part of timing abilities in the broader sense [3], it can be dissociated from processing a sequence of multiple intervals [5]. Therefore, we focus on the perception and production of rhythmic sequences, spanning multiple events. For an overview of single interval timing, we refer to several excellent reviews (e.g. [17,18]).
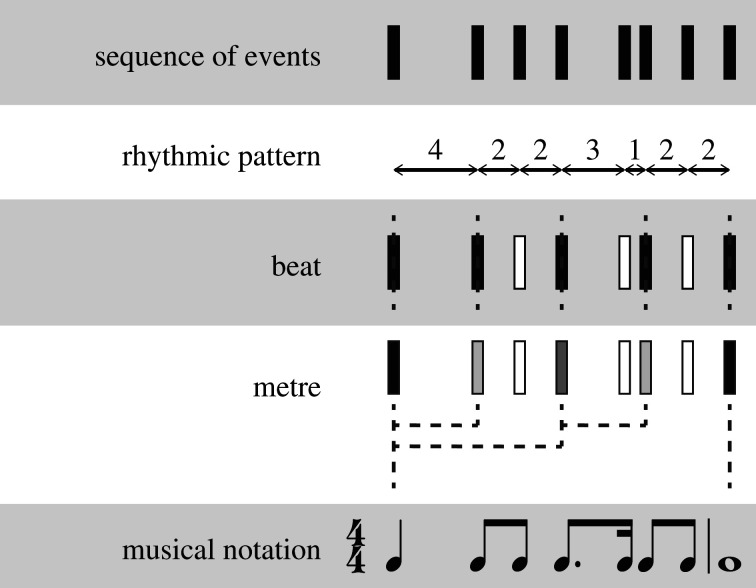
In rhythmic sequences, several types of structure can be discerned (figure 1). First, humans can perceive and produce rhythmic patterns of longer and shorter temporal intervals. Second, when a sequence contains accented events with a regular temporal spacing, humans can perceive a regular, periodic beat in response to a rhythm, and synchronize movement to the beat [19]. The beat is not always directly associated with the structure of the rhythmic pattern: a regular beat can be extracted from time-varying, non-isochronous rhythms, highlighting that it is a perceptual rather than a stimulus feature [15]. Finally, rhythms can contain hierarchical metrical structure, with the salience of events depending on the hierarchical ordering of beats (e.g. ‘metre’, such as alternating strong and weak beats in a march).
Figure 1.
Structure in rhythm. Colouring of events (black and white) indicates the perceived salience when a beat (dotted line) is present or when a hierarchical metrical structure (dotted tree structure) creates more and less salient beats (grey shades: darker indicates more salient, with black being the most salient beats, and white subdivisions of the beat).
The processes involved in perceiving rhythmic patterns and beats can be distinguished somewhat based on behavioural [20,21] and neural data [21]. However, it has also been proposed that the mechanisms used to process patterns and beats may be similar, both relying on oscillatory entrainment [22,23] and on probabilistic processes [24]. Similarly, while some consider metre to be a property of rhythm that is emergent from the presence of a beat in a dynamical systems perspective [25], others consider the hierarchical perception of metre as distinct from beat perception, and more related to language processing [6]. Thus, the precise relationship and interdependence of processing of rhythmic patterns, a beat and metre remains a topic for future research [26,27]. It is also often not clear which type of structure elicits rhythmic behaviour. For example, frequently used stimuli in rhythm studies—isochronous sequences—conceptually contain all three types of structure. Isochronous sequences have regularly spaced events, allowing for beat processing. Also, the pattern consisting of repeating single intervals can be learned [28]. Finally, even in isochronous sequences, humans perceive hierarchical structure in the form of alternating more and less salient tones [29]. Despite the relatedness of rhythmic patterns, beats and metre, the division of rhythmic structure into these types provides a useful framework to compare paradigms and behaviours [26,30], and where possible, we will therefore consider whether rhythmic behaviour is pattern-based, beat-based or (hierarchical) metre-based.
In humans, performance on perception and production tests of rhythmic abilities is correlated [3,4]. However, some individuals show normal perceptual and impaired production abilities, with normal performance in detecting small timing perturbations in isochronous sequences (anisochrony detection) and on a rhythm discrimination test, but an inability to synchronize their tapping to music [31]. Complementing this, two ‘beat-deaf’ individuals were shown to have retained the capability to synchronize their tapping to a rhythm, while displaying perceptual deficits in anisochrony detection [32]. These findings suggest that perception and production of rhythm can be at least partially dissociated. Moreover, studying perceptual abilities in isolation provides the advantage of being able to test populations or animal species lacking the capability (or motivation) to perform certain rhythmic actions. For example, new-born human babies have yet to learn how to synchronize movement to rhythm [33], but may be able to perceptually differentiate between sounds on and off the beat in musical rhythm [34] (but see [35]). Finally, perceptual tasks allow for perception to be probed without the possibly confounding factor of body movement [36]. Thus, in this overview, we will consider tasks probing perception and production abilities separately.
3. Rhythm perception
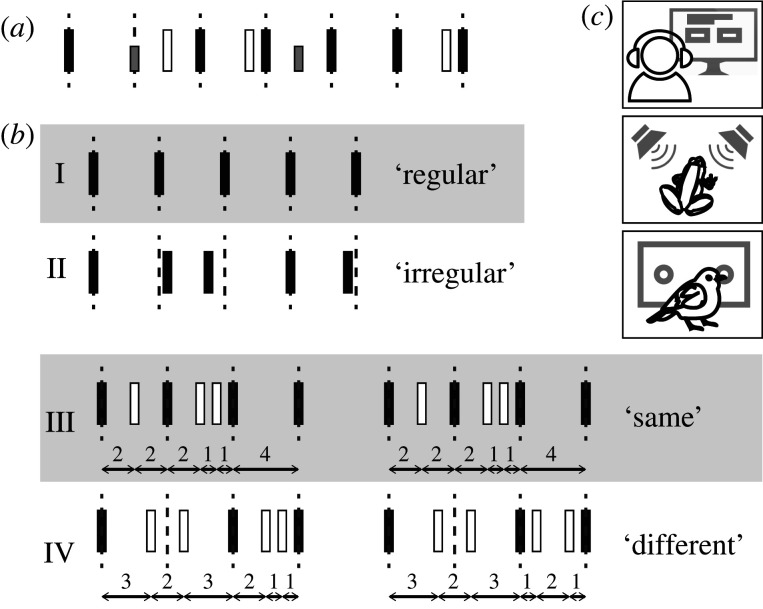
Perceptual tasks that require an overt estimation of time are considered explicit [37], such as discriminating between two rhythms (figure 2c), or rating the rhythmicity of a sequence. Explicit tasks often require some training or instruction targeting the rhythm. In implicit tasks, a rhythm can be leveraged to enhance performance, but the task itself is unrelated to the rhythm [37]. For example, participants may detect pitch or intensity changes embedded in a rhythmic stream (figure 2a), with better performance for events in metrically strong than in metrically weak positions [38]. Thus, in implicit tasks, processing of the task-relevant aspect of the stimulus is expected to depend on the perceived rhythmic structure, but the rhythmic aspect of the stimulus itself is not task relevant nor explicitly trained. Implicit and explicit processing of rhythm rely to some extent on different neural networks [37], and have a different developmental trajectory in humans [39].
Figure 2.
Rhythm perception tasks. (a) In implicit tasks, participants perform a task unrelated to the rhythm, such as detecting intensity changes. Performance is associated with the rhythmic structure, with better performance in more salient metrical positions. (b) In explicit tasks, participants may, for example, discriminate between different categories of rhythms (I and II), or may judge whether two rhythmic patterns are the same (III) or different (IV). (c) Variants of perceptual tasks have been done both in humans and non-human animals. For example, frogs' mating preferences, measured as approach to a stimulus, can be used as a proxy for discrimination performance, and birds can be trained in 2AFC tasks.
While implicit rhythm tasks have rarely been used in non-human animals, explicit rhythm tasks were used in birds, rats, crickets and frogs. Birds and rats are usually first trained to discriminate two categories of rhythms (e.g. regular and irregular sequences, figure 2b). Successful training indicates that the animal can differentiate between the sequences. However, this may not reveal whether the animals do this by attending to the rhythm or to some lower level feature, such as the presence of a specific interval in a specific position of a sequence. This requires subsequent presentation of test stimuli from the trained categories, but physically different (e.g. a different tempo) to probe generalization. Tasks include variants of Go/No-Go tasks, in which non-human animals get a food reward for responding to one but not the other type of rhythm, and two-alternative forced choice (2AFC) tasks, in which rewards are given when the correct response is chosen out of two responses each associated with a specific type of rhythm, such as pressing a different lever for each rhythm type (figure 2c). These tasks are similar to discrimination tasks often used in humans. While non-human animals, of course, do not provide explicit responses similar to humans, we consider these tasks explicit since the rhythm itself is task-relevant. Below we discuss findings from both explicit and implicit tasks in more detail.
(a) . Explicit rhythm perception tasks
Humans can differentiate between non-isochronous rhythms with regular accents (‘strictly metrical rhythms', with a strong beat, figure 2b(III) for an example) and without regular accents (‘weakly or non-metrical rhythms’, with a weaker beat, figure 2b(IV) for an example), by explicitly rating expected ease of tapping along [40,41], and beat presence [42]. Also, human ratings of rhythmicity show sensitivity to hierarchical structure [43,44].
In discrimination tasks, humans can judge whether rhythms are in duple (march) or triple (waltz) metre [4], whether a metronome or non-isochronous metrical rhythm is speeding up or slowing down [4], and whether a beep track overlain on music is ‘on’ or ‘off’ the beat (Beat Alignment Test, BAT; [3,45,46]). Stimuli on these tasks vary in intensity, pattern and metrical structure, and often include samples of real music, so it is not entirely clear what aspects of rhythmic abilities are probed. Also, while the BAT has been used to probe rhythm perception abilities in the general population [46], some of these explicit tasks use musical terminology and may, therefore, be less suitable for musical novices [40].
A discrimination task of which variants have been used in non-human animals is anisochrony detection (figure 2b). Here, subjects are presented an isochronous sequence, in which one or multiple events can be displaced in time, and judge whether the sequence is ‘regular’ (figure 2b(I)) or ‘irregular’ (figure 2b(II)). Humans are generally capable of detecting irregularities around 10% the size of the original isochronous interval, and many can detect irregularities that are much smaller [3,47–49]. Interestingly, while this task uses isochronous sequences—making it impossible to know whether the abilities probed are beat-based, pattern-based or both—the time difference detected by humans is typically approximately twice as small in anisochrony detection than in tasks probing single duration discrimination [50]. This can be explained either by assuming that humans leverage the regularity of the stimulus (beat-based) or that they benefit from the repeated presentation of the single interval [48].
Like humans, non-human animals can discriminate between regular and irregular sequences. Both rats (Rattus norvegicus) [51] and starlings (Sturnus vulgaris) [52,53] show tempo generalization on these tasks (e.g. they will differentiate between regular and irregular test sequences at rates that were not trained), suggesting that they base discrimination on the overall rhythmic structure, not just on learning one interval. This is in contrast with zebra finches (Taeniopigea guttata) [54] and pigeons (Columba livia) [55]. Zebra finches can discriminate between regular and irregular sequences, but generalize less to tempo changes than starlings (but see [56] for evidence of somewhat more flexible rhythmic abilities in zebra finches). Pigeons have trouble not just in tempo generalization but also in discriminating regular and irregular sequences during training.
In addition to discriminating regular and irregular sequences, humans can discern tempo differences between sequences with inter-onset intervals differing by as little as 2% in duration [48], but, like for anisochrony detection, whether this is beat-based or based on processing absolute durations (or both) is not clear. Similarly, several species of birds are sensitive to rate differences in isochronous sequences. This sensitivity may depend on different mechanisms in different species. Starlings can be trained to differentiate between sequences with rates of 4 (slow) and 8 (fast) events per second, and will generalize to comparisons of sequences at double tempo, with 8 events per second now eliciting ‘slow’ responses, and 16 ‘fast’ responses, showing sensitivity to relative rate [57]. By contrast, pigeons base discrimination of different rates on the absolute lengths of events and intervals, rather than the sequence tempo [55]. Both canaries (Serinus canaria) and budgerigars (Melopsittacus undulatus) can also differentiate between sequences of different tempi, though it is unclear whether this is based on comparing absolute intervals or rates [58]. In crickets and frogs, mate preference has been used to study whether they differentiate between calls with different calling rates (figure 2c). Calls or chirps are often large repetitive units made up of smaller units called pulses or syllables, and both calls and pulses have characteristic species-specific rates. Preferences for calls/chirps are often for faster rates, but preferences for pulse rates are often species-specific [59–61], indicating that these species are sensitive to rate differences. In crickets, this preference may be driven by differences in instantaneous stimulation [62], and/or by neurons that are tuned to a specific pulse rate [63], suggesting possible pre-existing preferences for a particular call rate.
While discrimination of rate and regularity in isochronous patterns can be achieved by multiple mechanisms, including single duration perception, some studies have explicitly targeted pattern-based and beat-based rhythm perception. Using a 2AFC task, humans were shown to recognize whether two rhythmic patterns are the same (figure 2b(III)) or different (figure 2b(IV), see [10,19,64,65]). Typically, humans perform better for patterns with than without a beat (‘beat-based advantage’, see [19]). Thus, while these tasks explicitly test perception of the rhythmic pattern, implicitly, beat-based perception can be probed by comparing performance on metrical and non-metrical rhythms [66]. Of note, auditory short-term memory may affect task performance, especially for longer rhythms [67].
Both starlings [68] and zebra finches [69] can also discriminate between different rhythmic patterns, as can female crickets and frogs, who selectively move towards certain calling rhythms—demonstrating the ability to discriminate different rhythms [70]. Using a similar paradigm, budgerigars were shown to preferentially move towards metrical (females) or non-metrical (males) patterns [71]. Likewise, rats differentiate between ‘Happy Birthday’ and a rhythmically scrambled version of the song [72]. While these studies explicitly targeted rhythmic pattern structure, performance could be based on memorizing only the first temporal interval [72]. Interestingly, jackdaws (Corvus monedula) were shown to not only distinguish two rhythmic patterns, but to maintain discrimination with tempo changes, suggesting more advanced abilities based on the pattern, or even the beat [73], in contrast with zebra finches, budgerigars [74] and starlings [75], who seem to have limited ability to use the beat to distinguish stimuli, but rather attend to absolute durations.
(b) . Implicit rhythm perception tasks
Humans implicitly leverage rhythmic structure in isochronous sequences for the detection and discrimination of pitch differences [20,76], temporal shifts [77], sounds at hearing threshold [78] and silent gaps [79,80]. Humans also perform better at detecting timbre and intensity differences in salient metrical positions (on the beat) than in non-salient metrical positions (off the beat) in non-isochronous metrical rhythms (figure 2a), possibly showing some sensitivity to hierarchical structure in rhythm [21,81–83]. Finally, humans can learn structure from patterns without a beat, and leverage this when detecting intensity or pitch changes in rhythmic sequences [20,21].
The implicit influence of rhythm on performance can also be probed after a rhythm ceases, based on the idea that entrainment to a beat outlasts physical stimulation [84]. In humans, the effects of rhythmic stimulation last up to two cycles after input ceases, with better performance in phase than out of phase with the previous rhythmic context when detecting threshold sounds in noise [84], and when performing a pitch comparison task [85]. Note, however, that the latter study could not be replicated [86], possibly due to only a proportion of humans showing lasting effects of a beat [27,86].
Surprisingly, studies using equivalent implicit paradigms in non-human animals are rare. Some work has been done in macaques, but with scalp EEG instead of behavioural responses as an outcome measure [87,88]. A rare study looking at how rhythm implicitly affects behaviour in zebra finches found that these birds more readily learned a novel song sequence in an isochronous than in a jittered context [89], akin to humans showing improved sequence learning when rhythmic structure is present [90], and suggesting that these birds can leverage regularity in a sequence to optimize perception.
To sum up, in both explicit and implicit perceptual tasks, humans have been shown to be able to detect a beat, rhythmic patterns and hierarchical metrical structure. These rhythmic abilities were shown in both musically trained and untrained subjects, though especially for explicit tasks [40] and the perception of hierarchical structure [43], training seems to improve performance. Using discrimination tasks akin to explicit timing tasks in humans, some species of birds, rats, crickets and frogs were shown to discern different rhythmic patterns, but several of these findings can be explained by simple discrimination of single absolute temporal intervals. Rats, jackdaws, starlings and possibly zebra finches show behaviour (e.g. tempo generalization) that can only be explained by assuming they perceive relations between different intervals in a pattern. While this may appear to suggest that the ability for relative timing is somewhat rare, the overall paucity of non-human data makes it difficult to draw meaningful conclusions on why it is present in some, but not other species.
4. Rhythm production
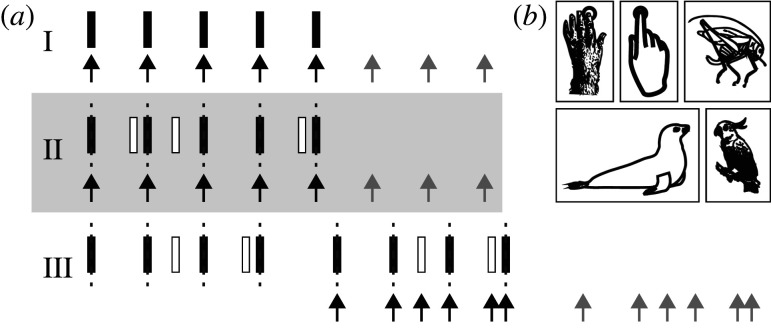
The workhorse of rhythm production ability tests is sensorimotor synchronization (SMS, figure 3): the coordination of movement with an external rhythm [91]. While the movement can be with any effector, in humans, most studies involve finger tapping. Variants of SMS probe whether subjects can maintain an internal representation of rhythm in the absence of a pacing signal, like in the synchronization-continuation task, which has an initial pacing signal (usually isochronous), after which a subject is required to continue tapping at the same rate. (For excellent and comprehensive overviews of the tapping literature, we refer to [91,92].) Here, we highlight a few findings of interest to cross-species comparisons.
Figure 3.
Rhythm production tasks. (a) SMS involves synchronizing movement to a rhythm, and varies from 1 : 1 synchronization to an isochronous sequence (I), to tapping the beat to a non-isochronous pattern (II) and tapping the pattern itself (III). In unpaced tasks, tapping is continued after the input ceases. (b) Different movements can be used in SMS, such as finger taps in primates and humans, head bobs in sea lion and parrots, and chirps in katydids.
In SMS, measures of performance include the mean and variability of the inter-tap interval, and the asynchrony between movement and the pacing rhythm [92]. Humans tend to tap earlier than the pacing signal, a phenomenon known as negative mean asynchrony (NMA). NMA has been related to rhythmic anticipation abilities in SMS. However, the usefulness of NMA as a marker of rhythmic abilities can be questioned. First, any asynchrony shorter than the shortest possible reaction time (approx. 150 ms) can be considered evidence of predictive behaviour [92]. Second, with musical or instrument-specific training, NMA generally decreases to values close to zero [93], while rhythmic abilities likely do not become worse with training. Finally, while synchronization with different effectors has been suggested to originate from shared underlying rhythmic abilities [94], NMA depends on the effector used for synchronizing, with larger NMA for foot than finger tapping [95], and no NMA for other movements, such as walking on the spot [96]. Thus, the origin of NMA and its relation to rhythmic abilities is not yet clear [92]. In SMS, when a pacing rhythm is perturbed (e.g. deviates from perfect periodicity), humans will adapt their tapping using two mechanisms: phase and period correction. The former is considered automatic, and subjects may be unaware of adjusting their taps to phase perturbations, while the latter requires intentional effort [91,92].
Most humans are capable of synchronizing their movement to an external rhythm, but synchronization precision and range are affected by musical experience and age. Musicians outperform non-musicians on various tapping tasks [10,97–100], and tapping variability is larger in children and older adults [101]. Also, while humans are generally capable of tapping regular intervals between 150 and 2000 ms, the range of tapping in children and non-musicians is more restricted [91,102]. Synchronization accuracy also depends on the effector used, with reports of more coupled synchronization (i.e. less variable relative phase difference between the pacing signal and movement) to a beat with foot, hip and torso movement than with head and hand movement [103,104], but also slower adjustment to perturbations in the pacing signal for lower limb movement than finger tapping [105,106], the latter possibly caused by increased biomechanical constraints on maintaining postural balance.
Non-human animals in rhythm production studies are often trained with operant methods to synchronize an arbitrary motor action, like pressing a lever or pecking a key, with auditory (and occasionally visual) stimuli, akin to SMS in humans. Some initial research tested the timing abilities of humans and three rhesus macaques (Macaca mulatta) with synchronization-continuation [107]. Subjects were presented with a visual or auditory isochronous pattern and required to push a button synchronously with the stimulus for six intervals: three with the stimulus present, and three after the stimulus stopped. The macaques took an average of 16 months to reach stable performance. Unlike humans, they never tapped with an NMA (e.g. preceding the time of the stimulus). However, their asynchronies were faster than simple reaction times, suggesting some anticipatory behaviour [92].
In three subsequent studies, rhesus macaques [108] and Japanese macaques (Macaca fuscata) [109,110] achieved much higher performance than previously reported in SMS tasks, showing adjustment to tempo changes akin to humans [108], NMA and generalization over different rates [108–110]. In all cases, the monkeys synchronized best to a visual metronome, contrary to the auditory advantage often reported for synchronization in humans. Importantly, in these monkey studies, anticipation was specifically rewarded, and feedback was given for every movement, showing the importance of motivational factors for rhythmic abilities ([7], this volume). Interestingly, budgerigars can be trained to synchronize their pecking to a metronome without requiring such substantial reward and feedback, showing both adaptation to tempo changes and occasional NMA [111]. Rats were shown to synchronize lever pressing with an isochronous audio-visual stimulus, with some tendency for anticipatory behaviour as apparent from smaller asynchronies in response to regular than random sequences. However, only a few individuals managed the task, and only in a very limited range of tempi [112].
Some production studies have focused on spontaneous movement synchronization to rhythm. Japanese macaque pairs were individually trained to perform a button-pressing task and, when paired with a partner, spontaneously synchronized their button presses [113]. Similarly, spontaneous and cooperative synchronization of drumming was observed in a bonobo (Pan paniscus) subject when a human partner drummed near the subject's preferred tempo, though it was unclear whether the bonobo relied on the rhythm or the visual input [114]. Likewise, one chimpanzee (Pan troglodites) (out of three tested) synchronized responses to an isochronous stimulus [115], and budgerigars adjusted their pecking to an isochronous sequence without being prompted to do so [116]. Note that while spontaneous synchronization can be shown in humans, the occurrence of this phenomenon depends strongly on contextual factors [116,117].
Similar to spontaneous SMS in humans, primates and birds, males of several insect and frog species interact with each other to achieve synchronized signals, although studies of this do not use arbitrary stimuli and motor responses but rather naturally evolved (and hence likely context-specific) behavioural synchronization [118–128]. This synchronization capacity has been studied not only through field observations, but also by examining individual responses to playback or visual presentation of artificial stimuli in the laboratory [119,121,124,125,128]. Signalling frogs and insects demonstrate a form of SMS by maintaining a fixed phase relationship with such stimuli, often either close to 0° (synchrony) or 180° (out of phase; ‘alternation’). These patterns have been shown in both acoustic (katydids, frogs, cicadas) and visual (fireflies) modalities. Katydids were shown to adjust their responses differently to stimuli heard at different phases during their calling cycle—demonstrating a form of phase correction [121,124,128–130]. This has been studied using phase response curves, which describe how katydids lengthen or shorten their calling periods in response to stimuli presented at different points in their calling cycle. Models and simulations based on these curves demonstrate how different forms of phase-locking to external stimuli are enabled. In addition to showing phase correction, katydids and frogs can entrain their calls to stimuli presented at a (limited) range of rates. Playback experiments have been particularly useful in demonstrating that different species of katydids and fireflies entrain to external stimuli using different mechanisms [121,124,127–130]. These include adjustment of calling periods and resetting their calling cycles or a combination of both. In almost all frog and katydid species studied so far, SMS involves simple acoustic units repeated at regular intervals. However, at least one species of katydid demonstrates SMS of multiple components of a complex call, consisting of a trill and chirps [130]. This suggests that future work might be called for investigating the possibility of more complex rhythm abilities in frogs and katydids with complex calls. The SMS-like behaviour observed in insects and frogs is reminiscent of SMS in humans, primates and birds, with the calls of other individuals serving as the pacing signal for the synchronizing animal. However, it must be noted that this behaviour typically occurs within the context of natural or simulated signal exchanges, and may, therefore, be more comparable with studies of interpersonal synchronization in humans, as discussed elsewhere (see [131], this volume).
Many rhythm production studies, both in human and non-human animals, have used isochronous stimuli. However, SMS can range from synchronizing to a metronome (1 : 1 mapping between movement and sound) to synchronization to (the beat of) non-isochronous metrical rhythms (figure 3a) and real music. Humans are even capable of tapping the pattern of non-metrical rhythms, though synchronization is more precise when a beat is present [100], highlighting that humans leverage the beat to improve tapping a rhythmic pattern, similar to improved performance for metrical rhythms in discrimination tasks. Humans can also tap multiple hierarchical levels of regularity in rhythm [132], and with training and maturation, tend to tap to higher levels [102]. The ability of producing movement synchronized to hierarchical structure was also shown in studies looking at whole body movement, with different effectors being synchronized to different levels of regularity [133].
Few non-human animals have been shown to be able to synchronize to the beat of real music, which requires a more complex mapping of movement to sound than synchronizing to a metronome. A California sea lion (Zalophus californianus) was trained to entrain her head-bobbing not only to a metronome, but also to real (human) music, showing generalization over different rates and stimuli, and phase and period correction mechanisms akin to humans [134,135]. Two parrots, a sulfur-crested cockatoo (Cacatua sulphurea) and a grey parrot (Psittacus erithacus), were also shown to be capable of moving on the beat of real music, maintaining synchronization at varying tempos [136,137]. This behaviour is suggestive of beat perception, though real music contains regularities based on patterns and hierarchy as well.
Additional production tasks have been used in humans, like synchronization-continuation tasks with a rate change just before the continuation phase starts, to probe tapping flexibility [3], completely unpaced tapping to measure spontaneous motor tempo and variability [102], and rhythm reproduction tasks (figure 3c(III)), which require subjects to repeat a pattern. Tapping back a pattern can be somewhat dissociated from tapping a beat [138], suggesting partly separate mechanisms underlying rhythmic abilities based on patterns and beats. In all these tasks, tapping variability can be diminished through musical training [10,100,102]. While the motor component in unpaced tapping is comparable to that in SMS, neuroimaging evidence suggests a dissociation between externally and internally generated rhythms [139], in line with some humans showing impaired unpaced but intact paced tapping [140]. Also, whereas paced tapping tasks generally have good test–retest reliability, unpaced tasks were found to be unreliable on an individual level [47].
In summary, humans can show rhythmic movement related to the beat, the rhythmic pattern and hierarchical structure. While primates can produce rhythmic movement synchronized to a metronome, this behaviour strongly depends on motivational factors, such as a food reward that can be introduced in the laboratory setting. Also, like for insects and frogs, synchronization was only shown for a metronome, which could result from anticipation based on absolute intervals. Two parrots and a sea lion showed flexible movement synchronization to real music reminiscent of human behaviour, which may result from beat-based processing.
5. Discussion and outlook
To better understand the function and cognitive underpinnings of rhythmic behaviour, cross-species comparisons of experimental findings can be valuable. To arrive at a clear picture of which rhythmic abilities are shared between different species, rhythmic abilities must be subdivided, separating between perception and production of rhythmic patterns and taking into account the multiple components of rhythmic structure. A summary of the rhythmic abilities discussed here can be found in table 1.
Table 1.
Overview of rhythmic abilities shown in different species, with (non-exhaustive) associated references.
| task/ability | rhythmic component | species |
|---|---|---|
| implicit perception: leverage rhythmic structure for task performance | isochrony | humans [20,76–80]; zebra finches [89] |
| pattern | humans [20,21] | |
| beat; metrical structure | humans [21,81–83] | |
| explicit perception: discriminate differences in rhythmic structure | pattern similarity | humans [10,19,64,65]; starlings [68]; zebra finches [69]; crickets [70]; katydids [70]; frogs [70]; budgerigars [71]; rats [72]; jackdaws [73] |
| rate (isochronous) | humans [4,48]; starlings [57]; pigeons [55]; canaries [58]; budgerigars [58]; crickets [61]; frogs [59] | |
| rate (non-isochronous) | humans [4] | |
| isochrony | humans [3,47–49]; rats [51]; starlings [52,53]; zebra finches [54,56] | |
| beat; metrical structure | humans [4,40–44]; budgerigars [71] | |
| synchrony between rhythm and metronome | humans [45,46] | |
| paced production: synchronize movement to pacing signal | isochronous | humans [91,92]; macaques [107–110]; budgerigars [111]; rats [112]; sea lion [136,137] |
| beat (non-isochronous) | humans [91,92]; parrots [136,137]; sea lion [134,135] | |
| pattern | humans [91,92,100] | |
| metrical hierarchy | humans [91,92,102,132] | |
| spontaneous (isochronous) | humans [116,117]; macaques [113]; bonobos [114]; chimpanzees [115]; budgerigars [116]; katydids [121,124]; frogs [119] | |
| unpaced production: rhythmic movement after pacing signal ends | pattern reproduction | humans [100,140] |
| isochronous (continuation) | humans [91,92]; macaques [107] |
Concerning strict perception, many bird species, rats, frogs and insects were shown to distinguish rhythmic patterns. However, in most species, pattern recognition does not generalize to different tempi, suggestive of an absolute, duration-based, rather than a relative, beat-based mechanism involved. Beat-based perception, present in most humans, was only tentatively shown in jackdaws [73]. The absence of beat-based perception and limited flexibility fits into frameworks of rhythmic abilities coined elsewhere [2,141]. Concerning rhythm production, many diverse species are capable of predictive synchronization to an isochronous sequence. While at odds with the idea that beat-based synchronization is specific to a select number of species [1], the question is whether synchronization with a metronome necessarily evidences beat-based processing. First, the human capability to synchronize to variable, non-isochronous and hierarchically organized rhythms has not been shown in other species, with the exception of two parrots and a sea lion, that showed entrainment to music. Thus, like for perceptual abilities, rhythm production in humans seems exceptionally flexible when compared with most other species. Also, while some species show trained responses to arbitrary stimuli (humans, monkeys, some birds), others have only been shown to synchronize in the context of the natural behaviour they show in rhythmic interactions (frogs), suggesting different underlying mechanisms. In summary, the research discussed here hints at beat-based processing, hierarchical processing and tempo flexibility as being features of rhythm ability that are especially pronounced in humans (as suggested elsewhere—[2]).
Notably, we identify several clear gaps in experimental research probing rhythmic abilities. First, hardly any perceptual rhythm tasks are attempted in primates and other mammals, nor in the bird species (cockatoos and parrots) that showed synchronization to real music. Although successful SMS implies detection of underlying rhythms, perceptual tasks can be more precisely controlled and allow investigation into what sound features synchronization may be based on. Second, implicit perceptual tasks, which can reliably show beat-based, pattern-based and hierarchical rhythmic abilities in humans, are rarely used in non-human animals, but may provide an interesting addition to the non-human animal experiments. Third, motor synchronization tasks are lacking in some bird species that were tested on their perceptual rhythmic abilities (e.g. zebra finches). A clearer picture of both rhythm production and perception abilities may shed light on whether these are related, or can be dissociated in some species. Fourth, in production tasks in parrots, stimuli were real, acoustically rich music, making it hard to discern exactly which information the birds used to synchronize. In insects and frogs, (semi)natural calls were used, which may be linked to interindividual synchronization, rather than to individual synchronization ability. A more standardized approach to stimulus and task selection might allow better comparability between species and experiments. Fifth, in both perception and production tasks, (isochronous) stimuli often do not allow for a clear differentiation between different types of structure processed in rhythm. Sixth, the range of non-human animals tested on rhythmic abilities in the laboratory is very limited and does not include species from all clades. While understandably related to practical limitations, this leaves large gaps in our understanding of rhythmic abilities across species. Related to this, while there is little evidence for some abilities in non-human animals (like hierarchical processing of rhythm), there is just as little evidence for the absence of these abilities: they have simply not been tested. Finally, future research may also differentiate between what is usually observed, and what is possible given the cognitive and neural constraints of a species. Testing the limits of rhythmic abilities—not only in non-human animals, but also in humans (for example, in experts [10])—may shed further light on the rhythmic abilities that give rise to rhythmic interactions across species and cultures.
Acknowledgements
We would like to thank the organizers and all participants of the Lorentz Center workshop ‘Synchrony and Rhythmic Interaction: From Neurons to Ecology’ (July 2019) for the inspiring discussions that led to this paper.
Data accessibility
This article has no additional data.
Authors' contributions
F.L.B., C.t.C., V.N. and A.A.R. conceived of the paper; F.L.B. drafted and revised the manuscript; C.t.C., V.N. and A.A.R. critically revised the manuscript; all authors gave final approval for publication and agree to be held accountable for the work performed therein.
Competing interest
We declare we have no competing interests.
Funding
F.L.B. is supported by a Veni grant (VI.Veni.201G.066) awarded by the Dutch Research Council (NWO) and an ABC Talent Grant awarded by Amsterdam Brain and Cognition. V.N. is supported by a BBSRC David Phillips fellowship (BB/S009760/1). A.A.R. is supported by an NIH grant (R21NS114682) awarded to Dr Mimi H. Kao and Dr Aniruddh D. Patel.
References
- 1.Honing H, ten Cate C, Peretz I, Trehub SE. 2015. Without it no music: cognition, biology and evolution of musicality. Phil. Trans. R. Soc. B 370, 20140088. ( 10.1098/rstb.2014.0088) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kotz SA, Ravignani A, Fitch WT. 2018. The evolution of rhythm processing. Trends Cogn. Sci. 22, 896-910. ( 10.1016/J.TICS.2018.08.002) [DOI] [PubMed] [Google Scholar]
- 3.Dalla BS, Farrugia N, Benoit C-E, Bégel V, Verga L, Harding E, Kotz SA. 2017. BAASTA: battery for the assessment of auditory sensorimotor and timing abilities. Behav. Res. Methods 49, 1128-1145. ( 10.3758/s13428-016-0773-6) [DOI] [PubMed] [Google Scholar]
- 4.Fujii S, Schlaug G. 2013. The Harvard Beat Assessment Test (H-BAT): a battery for assessing beat perception and production and their dissociation. Front. Hum. Neurosci. 7, 771. ( 10.3389/fnhum.2013.00771) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Merchant H, Honing H. 2014. Are non-human primates capable of rhythmic entrainment? Evidence for the gradual audiomotor evolution hypothesis. Front. Neurosci. 7, 274. ( 10.3389/fnins.2013.00274) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fitch WT. 2013. Rhythmic cognition in humans and animals: distinguishing meter and pulse perception. Front. Syst. Neurosci. 7, 68. ( 10.3389/fnsys.2013.00068) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Henry MJ, Cook PF, de Reus K, Nityananda V, Rouse AA, Kotz SA. 2021. An ecological approach to measuring synchronization abilities across the animal kingdom. Phil. Trans. R. Soc. B 376, 20200336. ( 10.1098/rstb.2020.0336) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Greenfield MD, Aihara I, Amichay G, Anichini M, Nityananda V. 2021. Rhythm interaction in animal groups: selective attention and social networks. Phil. Trans. R. Soc. B 376, 20200338. ( 10.1098/rstb.2020.0338) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buck J, Buck E. 1966. Biology of synchronous flashing of fireflies. Nature 211, 562-564. ( 10.1038/211562a0) [DOI] [Google Scholar]
- 10.Cameron DJ, Grahn JA. 2014. Enhanced timing abilities in percussionists generalize to rhythms without a musical beat. Front. Hum. Neurosci. 8, 1003. ( 10.3389/fnhum.2014.01003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cirelli LK, Trehub SE, Trainor LJ. 2018. Rhythm and melody as social signals for infants. Ann. N. Y. Acad. Sci. 1423, 66-72. ( 10.1111/nyas.13580) [DOI] [PubMed] [Google Scholar]
- 12.Matthews TE, Witek MAG, Lund T, Vuust P, Penhune VB. 2020. The sensation of groove engages motor and reward networks. Neuroimage 214, 116768. ( 10.1016/J.NEUROIMAGE.2020.116768) [DOI] [PubMed] [Google Scholar]
- 13.Wilson M, Cook PF. 2016. Rhythmic entrainment: why humans want to, fireflies can't help it, pet birds try, and sea lions have to be bribed. Psychon. Bull. Rev. 23, 1647-1659. ( 10.3758/s13423-016-1013-x) [DOI] [PubMed] [Google Scholar]
- 14.Hoeschele M, Merchant H, Kikuchi Y, Hattori Y, ten Cate C. 2015. Searching for the origins of musicality across species. Phil. Trans. R. Soc. B 370, 20140094. ( 10.1098/rstb.2014.0094) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Honing H, Bouwer FL. 2019. Rhythm. In Foundations in music psychology: theory and research (eds Rentfrow PJ, Levitin D), pp. 33-69. Cambridge, MA: MIT Press. [Google Scholar]
- 16.Leow L, Grahn JA. 2014. Neural mechanisms of rhythm perception: present findings and future directions. In Neurobiology of interval timing (eds Merchant H, de Lafuente V), pp. 325– 338. New York, NY: Springer. [DOI] [PubMed] [Google Scholar]
- 17.Grondin S. 2010. Timing and time perception: a review of recent behavioral and neuroscience findings. Atten. Percept. Psychophys. 72, 561-582. ( 10.3758/APP) [DOI] [PubMed] [Google Scholar]
- 18.Merchant H, Harrington DL, Meck WH. 2013. Neural basis of the perception and estimation of time. Annu. Rev. Neurosci. 36, 313-336. ( 10.1146/annurev-neuro-062012-170349) [DOI] [PubMed] [Google Scholar]
- 19.Grahn JA, Brett M. 2007. Rhythm and beat perception in motor areas of the brain. J. Cogn. Neurosci. 19, 893-906. ( 10.1162/jocn.2007.19.5.893) [DOI] [PubMed] [Google Scholar]
- 20.Morillon B, Schroeder CE, Wyart V, Arnal LH. 2016. Temporal prediction in lieu of periodic stimulation. J. Neurosci. 36, 2342-2347. ( 10.1523/JNEUROSCI.0836-15.2016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bouwer FL, Honing H, Slagter HA. 2020. Beat-based and memory-based temporal expectations in rhythm: similar perceptual effects, different underlying mechanisms. J. Cogn. Neurosci. 32, 1221-1241. ( 10.1162/jocn_a_01529) [DOI] [PubMed] [Google Scholar]
- 22.Rimmele JM, Morillon B, Poeppel D, Arnal LH. 2018. Proactive sensing of periodic and aperiodic auditory patterns. Trends Cogn. Sci. 22, 870-882. ( 10.1016/J.TICS.2018.08.003) [DOI] [PubMed] [Google Scholar]
- 23.Tichko P, Large EW. 2019. Modeling infants' perceptual narrowing to musical rhythms: neural oscillation and Hebbian plasticity. Ann. N. Y. Acad. Sci. 1453, 125-139. ( 10.1111/nyas.14050) [DOI] [PubMed] [Google Scholar]
- 24.Cannon JJ, Patel AD. 2021. How beat perception co-opts motor neurophysiology. Trends Cogn. Sci. 25, 137-150. ( 10.1016/j.tics.2020.11.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Large EW. 2008. Resonating to musical rhythm: theory and experiment. In Psychology of time (ed. Grondin S), pp. 189-231. Bingley, UK: Emerald Group Publishing. [Google Scholar]
- 26.Nobre AC, van Ede F. 2018. Anticipated moments: temporal structure in attention. Nat. Rev. Neurosci. 19, 34-48. ( 10.1038/nrn.2017.141) [DOI] [PubMed] [Google Scholar]
- 27.Bouwer FL, Fahrenfort JJ, Millard SK, Slagter HA. 2020. A silent disco: persistent entrainment of low-frequency neural oscillations underlies beat-based, but not memory-based temporal expectations. bioRxiv, 2020.01.08.899278. ( 10.1101/2020.01.08.899278) [DOI]
- 28.Keele SW, Nicoletti R, Ivry RI, Pokorny RA. 1989. Mechanisms of perceptual timing: beat-based or interval-based judgements? Psychol. Res. 50, 251-256. ( 10.1007/BF00309261) [DOI] [Google Scholar]
- 29.Potter DD, Fenwick M, Abecasis D, Brochard R. 2009. Perceiving rhythm where none exists: event-related potential (ERP) correlates of subjective accenting. Cortex 45, 103-109. ( 10.1016/j.cortex.2008.01.004) [DOI] [PubMed] [Google Scholar]
- 30.Breska A, Ivry RB. 2016. Taxonomies of timing: where does the cerebellum fit in? Curr. Opin. Behav. Sci. 8, 282-288. ( 10.1016/J.COBEHA.2016.02.034) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sowiński J, Dalla Bella S. 2013. Poor synchronization to the beat may result from deficient auditory-motor mapping. Neuropsychologia 51, 1952-1963. ( 10.1016/j.neuropsychologia.2013.06.027) [DOI] [PubMed] [Google Scholar]
- 32.Bégel V, Benoit C-E, Correa Á, Cutanda D, Kotz SA, Dalla Bella S. 2017. ‘Lost in time’ but still moving to the beat. Neuropsychologia 94, 129-138. ( 10.1016/J.NEUROPSYCHOLOGIA.2016.11.022) [DOI] [PubMed] [Google Scholar]
- 33.Zentner M, Eerola T. 2010. Rhythmic engagement with music in infancy. Proc. Natl Acad. Sci. USA 107, 5768-5773. ( 10.1073/pnas.1000121107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Winkler I, Háden GP, Ladinig O, Sziller I, Honing H. 2009. Newborn infants detect the beat in music. Proc. Natl Acad. Sci. USA 106, 2468-2471. ( 10.1073/pnas.0809035106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bouwer FL, Van Zuijen TL, Honing H.. 2014. Beat processing is pre-attentive for metrically simple rhythms with clear accents: an ERP study. PLoS ONE 9, e97467. ( 10.1371/journal.pone.0097467) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Su Y-H, Pöppel E. 2012. Body movement enhances the extraction of temporal structures in auditory sequences. Psychol. Res. 76, 373-382. ( 10.1007/s00426-011-0346-3) [DOI] [PubMed] [Google Scholar]
- 37.Coull JT, Nobre AC. 2008. Dissociating explicit timing from temporal expectation with fMRI. Curr. Opin. Neurobiol. 18, 137-144. ( 10.1016/j.conb.2008.07.011) [DOI] [PubMed] [Google Scholar]
- 38.Honing H, Bouwer FL, Háden GP. 2014. Perceiving temporal regularity in music: the role of auditory event-related potentials (ERPs) in probing beat perception. In Neurobiology of interval timing (eds Merchant H, de Lafuente V), pp. 305-323. New York, NY: Springer. [DOI] [PubMed] [Google Scholar]
- 39.Droit-Volet S, Coull JT. 2016. Distinct developmental trajectories for explicit and implicit timing. J. Exp. Child Psychol. 150, 141-154. ( 10.1016/j.jecp.2016.05.010) [DOI] [PubMed] [Google Scholar]
- 40.Bouwer FL, Burgoyne JA, Odijk D, Honing H, Grahn JA. 2018. What makes a rhythm complex? The influence of musical training and accent type on beat perception. PLoS ONE 13, e0190322. ( 10.1371/journal.pone.0190322) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kung S-JS, Chen JLJ, Zatorre RJR, Penhune VBV. 2013. Interacting cortical and basal ganglia networks underlying finding and tapping to the musical beat. J. Cogn. Neurosci. 25, 401-420. ( 10.1162/jocn_a_00325) [DOI] [PubMed] [Google Scholar]
- 42.Araneda R, Renier L, Ebner-Karestinos D, Dricot L, De Volder AG.. 2017. Hearing, feeling or seeing a beat recruits a supramodal network in the auditory dorsal stream. Eur. J. Neurosci. 45, 1439-1450. ( 10.1111/ejn.13349) [DOI] [PubMed] [Google Scholar]
- 43.Nave-Blodgett JE, Hannon E, Snyder JS. 2021. Hierarchical beat perception develops throughout childhood and adolescence and is enhanced in those with musical training. J. Exp. Psychol. Gen. 150, 314-339. ( 10.1037/xge0000903) [DOI] [PubMed] [Google Scholar]
- 44.Grube M, Griffiths TD. 2009. Metricality-enhanced temporal encoding and the subjective perception of rhythmic sequences. Cortex 45, 72-79. ( 10.1016/j.cortex.2008.01.006) [DOI] [PubMed] [Google Scholar]
- 45.Iversen JR, Patel AD. 2008. The Beat Alignment Test (BAT): surveying beat processing abilities in the general population. In 10th Int. Conf. on music perception and cognition (eds Miyazaki K, Hiraga Y, Adachi M, Nakajima Y, Tsuzaki M), pp. 465-468. Adelaide, South Australia: Causal Productions. [Google Scholar]
- 46.Müllensiefen D, Gingras B, Musil J, Stewart L. 2014. The musicality of non-musicians: an index for assessing musical sophistication in the general population. PLoS ONE 9, e89642. ( 10.1371/journal.pone.0089642) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bégel V, Verga L, Benoit C-E, Kotz SA, Dalla Bella S. 2018. Test-retest reliability of the battery for the assessment of auditory sensorimotor and timing abilities (BAASTA). Ann. Phys. Rehabil. Med. 61, 395-400. ( 10.1016/j.rehab.2018.04.001) [DOI] [PubMed] [Google Scholar]
- 48.Miller NS, McAuley JD. 2005. Tempo sensitivity in isochronous tone sequences: the multiple-look model revisited. Percept. Psychophys. 67, 1150-1160. ( 10.3758/BF03193548) [DOI] [PubMed] [Google Scholar]
- 49.Friberg A, Sundberg J. 1995. Time discrimination in a monotonic, isochronous sequence. J. Acoust. Soc. Am. 98, 2524-2531. ( 10.1121/1.413218) [DOI] [Google Scholar]
- 50.Cope TE, Grube M, Singh B, Burn DJ, Griffiths TD. 2014. The basal ganglia in perceptual timing: timing performance in multiple system atrophy and Huntington's disease. Neuropsychologia 52, 73-81. ( 10.1016/j.neuropsychologia.2013.09.039) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Celma-Miralles A, Toro JM. 2020. Discrimination of temporal regularity in rats (Rattus norvegicus) and humans (Homo sapiens). J. Comp. Psychol. 134, 3-10. ( 10.1037/com0000202) [DOI] [PubMed] [Google Scholar]
- 52.Humpal J, Cynx J. 1984. Discrimination of temporal components of acoustic patterns by birds. Ann. N. Y. Acad. Sci. 423, 600-602. ( 10.1111/j.1749-6632.1984.tb23466.x) [DOI] [Google Scholar]
- 53.Hulse SH, Humpal J, Cynx J. 1984. Processing of rhythmic sound structures by birds. Ann. N. Y. Acad. Sci. 423, 407-419. ( 10.1111/j.1749-6632.1984.tb23449.x) [DOI] [PubMed] [Google Scholar]
- 54.van der Aa J, Honing H, ten Cate C. 2015. The perception of regularity in an isochronous stimulus in zebra finches (Taeniopygia guttata) and humans. Behav. Process. 115, 37-45. ( 10.1016/j.beproc.2015.02.018) [DOI] [PubMed] [Google Scholar]
- 55.Hagmann CE, Cook RG. 2010. Testing meter, rhythm, and tempo discriminations in pigeons. Behav. Process. 85, 99-110. ( 10.1016/j.beproc.2010.06.015) [DOI] [PubMed] [Google Scholar]
- 56.Rouse AA. 2019. Developing an animal model for human rhythm perception. Medford, MA: Tufts University. [Google Scholar]
- 57.Hulse SH, Kline CL. 1993. The perception of time relations in auditory tempo discrimination. Anim. Learn. Behav. 21, 281-288. ( 10.3758/BF03197992) [DOI] [Google Scholar]
- 58.Fishbein AR, Lawson SL, Dooling RJ, Ball GF. 2019. How canaries listen to their song: species-specific shape of auditory perception. J. Acoust. Soc. Am. 145, 562-574. ( 10.1121/1.5087692) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gerhardt HC. 1991. Female mate choice in treefrogs: static and dynamic acoustic criteria. Anim. Behav. 42, 615-635. ( 10.1016/S0003-3472(05)80245-3) [DOI] [Google Scholar]
- 60.Wagner WE. 1996. Convergent song preferences between female field crickets and acoustically orienting parasitoid flies. Behav. Ecol. 7, 279-285. ( 10.1093/beheco/7.3.279) [DOI] [Google Scholar]
- 61.Doherty JA. 1985. Phonotaxis in the cricket, Gryllus bimaculatus de Geer: comparisons of choice and no-choice paradigms. J. Comp. Physiol. A 157, 279-289. ( 10.1007/BF00618118) [DOI] [Google Scholar]
- 62.Hedwig B, Poulet JFA. 2004. Complex auditory behaviour emerges from simple reactive steering. Nature 430, 781-785. ( 10.1038/nature02723.1.) [DOI] [PubMed] [Google Scholar]
- 63.Schildberger K. 1984. Temporal selectivity of identified auditory neurons in the cricket brain. J. Comp. Physiol. A 155, 171-185. ( 10.1007/BF00612635) [DOI] [Google Scholar]
- 64.Wallentin M, Nielsen AH, Friis-Olivarius M, Vuust C, Vuust P. 2010. The musical ear test, a new reliable test for measuring musical competence. Learn. Individ. Differ. 20, 188-196. ( 10.1016/j.lindif.2010.02.004) [DOI] [Google Scholar]
- 65.Law LNC, Zentner M. 2012. Assessing musical abilities objectively: construction and validation of the profile of music perception skills. PLoS ONE 7, e52508. ( 10.1371/journal.pone.0052508) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cameron DJ, Pickett KA, Earhart GM, Grahn JA. 2016. The effect of dopaminergic medication on beat-based auditory timing in Parkinson's disease. Front. Neurol. 7, 19. ( 10.3389/fpls.2015.00830) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Grahn JA, Schuit D. 2012. Individual differences in rhythmic ability: behavioral and neuroimaging investigations. Psychomusicol. Music Mind Brain 22, 105-121. ( 10.1037/a0031188) [DOI] [Google Scholar]
- 68.Hulse SH, Humpal J, Cynx J. 1984. Discrimination and generalization of rhythmic and arrhythmic sound patterns by European starlings (Sturnus vulgaris). Music Percept. 1, 442-464. ( 10.2307/40285272) [DOI] [Google Scholar]
- 69.Spierings M, ten Cate C. 2014. Zebra finches are sensitive to the prosodic features of human speech. Proc. R. Soc. B 281, 20140480. ( 10.1098/rspb.2014.0480) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gerhardt HC, Huber F. 2002. Acoustic communication in insects and anurans: common problems and diverse solutions. Chicago, IL: University of Chicago Press. [Google Scholar]
- 71.Hoeschele M, Bowling DL. 2016. Sex differences in rhythmic preferences in the budgerigar (Melopsittacus undulatus): a comparative study with humans. Front. Psychol. 7, 1543. ( 10.3389/fpsyg.2016.01543) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Celma-Miralles A, Toro JM. 2020. Non-human animals detect the rhythmic structure of a familiar tune. Psychon. Bull. Rev. 27, 694-699. ( 10.3758/s13423-020-01739-2) [DOI] [PubMed] [Google Scholar]
- 73.Reinert J. 1965. Takt- und Rhythmusunderscheid bei Dohlen. Z. Tierpsychol. 22, 623-671. ( 10.1111/j.1439-0310.1965.tb01683.x) [DOI] [PubMed] [Google Scholar]
- 74.ten Cate C, Spierings M, Hubert J, Honing H. 2016. Can birds perceive rhythmic patterns? A review and experiments on a songbird and a parrot species. Front. Psychol. 7, 730. ( 10.3389/fpsyg.2016.00730) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Samuels B, Grahn J, Henry MJ, Macdougall-Shackleton SA. 2021. European starlings (Sturnus vulgaris) discriminate rhythms by rate, not temporal patterns. J. Acoust. Soc. Am. 149, 2546. ( 10.1121/10.0004215) [DOI] [PubMed] [Google Scholar]
- 76.Chang A, Bosnyak DJ, Trainor LJ. 2019. Rhythmicity facilitates pitch discrimination: differential roles of low and high frequency neural oscillations. Neuroimage 198, 31-43. ( 10.1016/j.neuroimage.2019.05.007) [DOI] [PubMed] [Google Scholar]
- 77.Heynckes M, De Weerd P, Valente G, Formisano E, De Martino F.. 2020. Behavioral effects of rhythm, carrier frequency and temporal cueing on the perception of sound sequences. PLoS ONE 15, e0234251. ( 10.1371/journal.pone.0234251) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lawrance ELA, Harper NS, Cooke JE, Schnupp JWH. 2014. Temporal predictability enhances auditory detection. J. Acoust. Soc. Am. 135, 357-363. ( 10.1121/1.4879667) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Henry MJ, Herrmann B, Obleser J. 2014. Entrained neural oscillations in multiple frequency bands comodulate behavior. Proc. Natl Acad. Sci. USA 111, 14 935-14 940. ( 10.1073/pnas.1408741111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bauer A-KR, Bleichner MG, Jaeger M, Thorne JD, Debener S. 2018. Dynamic phase alignment of ongoing auditory cortex oscillations. Neuroimage 167, 396-407. ( 10.1016/j.neuroimage.2017.11.037) [DOI] [PubMed] [Google Scholar]
- 81.Bolger D, Trost WJ, Schön D. 2013. Rhythm implicitly affects temporal orienting of attention across modalities. Acta Psychol. 142, 238-244. ( 10.1016/j.actpsy.2012.11.012) [DOI] [PubMed] [Google Scholar]
- 82.Bolger D, Coull JT, Schön D. 2014. Metrical rhythm implicitly orients attention in time as indexed by improved target detection and left inferior parietal activation. J. Cogn. Neurosci. 26, 593-605. ( 10.1162/jocn_a_00511) [DOI] [PubMed] [Google Scholar]
- 83.Bouwer FL, Honing H. 2015. Temporal attending and prediction influence the perception of metrical rhythm: evidence from reaction times and ERPs. Front. Psychol. 6, 1094. ( 10.3389/fpsyg.2015.01094) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hickok G, Farahbod H, Saberi K. 2015. The rhythm of perception: entrainment to acoustic rhythms induces subsequent perceptual oscillation. Psychol. Sci. 26, 1006-1013. ( 10.1177/0956797615576533) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jones MR, Moynihan H, MacKenzie N, Puente J. 2002. Temporal aspects of stimulus-driven attending in dynamic arrays. Psychol. Sci. 13, 313-319. ( 10.1111/1467-9280.00458) [DOI] [PubMed] [Google Scholar]
- 86.Bauer A-KR, Jaeger M, Thorne JD, Bendixen A, Debener S. 2015. The auditory dynamic attending theory revisited: a closer look at the pitch comparison task. Brain Res. 1626, 198-210. ( 10.1016/j.brainres.2015.04.032) [DOI] [PubMed] [Google Scholar]
- 87.Honing H, Bouwer FL, Prado L, Merchant H. 2018. Rhesus monkeys (Macaca mulatta) sense isochrony in rhythm, but not the beat: additional support for the gradual audiomotor evolution hypothesis. Front. Neurosci. 12, 475. ( 10.3389/fnins.2018.00475) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Honing H, Merchant H, Háden GP, Prado L, Bartolo R.. 2012. Rhesus monkeys (Macaca mulatta) detect rhythmic groups in music, but not the beat. PLoS ONE 7, e51369. ( 10.1371/journal.pone.0051369) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bruno JH. 2017. Song rhythm development in zebra finches. CUNY Academic Works. See https://academicworks.cuny.edu/gc_etds/2354. [Google Scholar]
- 90.Selchenkova T, et al. 2014. Metrical presentation boosts implicit learning of artificial grammar. PLoS ONE 9, e112233. ( 10.1371/journal.pone.0112233) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Repp BH. 2005. Sensorimotor synchronization: a review of the tapping literature. Psychon. Bull. Rev. 12, 969-992. ( 10.3758/BF03206433) [DOI] [PubMed] [Google Scholar]
- 92.Repp BH, Su YH. 2013. Sensorimotor synchronization: a review of recent research (2006–2012). Psychon. Bull. Rev. 20, 403-452. ( 10.3758/s13423-012-0371-2) [DOI] [PubMed] [Google Scholar]
- 93.Stoklasa J, Liebermann C, Fischinger T. 2012. Timing and synchronization of professional musicians: a comparison between orchestral brass and string players. In 12th Int. Conf. on Music Perception and Cognition, Thessaloniki, Greece, vol. 12, 2005. [Google Scholar]
- 94.Keele SW, Pokorny RA, Corcos DM, Ivry R. 1985. Do perception and motor production share common timing mechanisms: a correlational analysis. Acta Psychol. 60, 173-191. ( 10.1016/0001-6918(85)90054-X) [DOI] [PubMed] [Google Scholar]
- 95.Aschersleben G, Prinz W. 1995. Synchronizing actions with events. Percept. Psychophys. 57, 305-317. ( 10.3758/BF03213056) [DOI] [PubMed] [Google Scholar]
- 96.Rose D, Delevoye-Turrell Y, Ott L, Annett LE, Lovatt PJ. 2019. Music and metronomes differentially impact motor timing in people with and without Parkinson's disease: effects of slow, medium, and fast tempi on entrainment and synchronization performances in finger tapping, toe tapping, and stepping on the spot tasks. Parkinsons Dis. 2019, 6530838. ( 10.1155/2019/6530838) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bailey JA, Penhune VB. 2010. Rhythm synchronization performance and auditory working memory in early- and late-trained musicians. Exp. Brain Res. 204, 91-101. ( 10.1007/s00221-010-2299-y) [DOI] [PubMed] [Google Scholar]
- 98.Bailey JA, Penhune VB. 2013. The relationship between the age of onset of musical training and rhythm synchronization performance: validation of sensitive period effects. Front. Neurosci. 7, 227. ( 10.3389/fnins.2013.00227) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chen JL, Penhune VB, Zatorre RJ. 2008. Listening to musical rhythms recruits motor regions of the brain. Cereb. Cortex 18, 2844-2854. ( 10.1093/cercor/bhn042) [DOI] [PubMed] [Google Scholar]
- 100.Matthews TE, Thibodeau JNL, Gunther BP, Penhune VB. 2016. The impact of instrument-specific musical training on rhythm perception and production. Front. Psychol. 7, 69. ( 10.3389/fpsyg.2016.00069) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Thompson EC, White-Schwoch T, Tierney AT, Kraus N. 2015. Beat synchronization across the lifespan: intersection of development and musical experience. PLoS ONE 10, e0128839. ( 10.1371/journal.pone.0128839) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Drake C, Jones MR, Baruch C. 2000. The development of rhythmic attending in auditory sequences: attunement, referent period, focal attending. Cognition 77, 251-288. ( 10.1016/S0010-0277(00)00106-2) [DOI] [PubMed] [Google Scholar]
- 103.Burger B, London J, Thompson MR, Toiviainen P. 2018. Synchronization to metrical levels in music depends on low-frequency spectral components and tempo. Psychol. Res. 82, 1195-1211. ( 10.1007/s00426-017-0894-2) [DOI] [PubMed] [Google Scholar]
- 104.Witek MAG, Popescu T, Clarke EF, Hansen M, Konvalinka I, Kringelbach ML, Vuust P. 2017. Syncopation affects free body-movement in musical groove. Exp. Brain Res. 235, 995-1005. ( 10.1007/s00221-016-4855-6) [DOI] [PubMed] [Google Scholar]
- 105.Wright RL, Spurgeon LC, Elliott MT. 2014. Stepping to phase-perturbed metronome cues: multisensory advantage in movement synchrony but not correction. Front. Hum. Neurosci. 8, 1-7. ( 10.3389/fnhum.2014.00724) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chen HY, Wing AM, Pratt D. 2006. The synchronisation of lower limb responses with a variable metronome: the effect of biomechanical constraints on timing. Gait Posture 23, 307-314. ( 10.1016/j.gaitpost.2005.04.001) [DOI] [PubMed] [Google Scholar]
- 107.Zarco W, Merchant H, Prado L, Mendez JC. 2009. Subsecond timing in primates: comparison of interval production between human subjects and rhesus monkeys. J. Neurophysiol. 102, 3191-3202. ( 10.1152/jn.00066.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gámez J, Yc K, Ayala YA, Dotov D, Prado L, Merchant H. 2018. Predictive rhythmic tapping to isochronous and tempo changing metronomes in the nonhuman primate. Ann. N. Y. Acad. Sci. 1423, 396-414. ( 10.1111/nyas.13671) [DOI] [PubMed] [Google Scholar]
- 109.Takeya R, Kameda M, Patel AD, Tanaka M. 2017. Predictive and tempo-flexible synchronization to a visual metronome in monkeys. Sci. Rep. 7, 6127. ( 10.1038/s41598-017-06417-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Takeya R, Patel AD, Tanaka M. 2018. Temporal generalization of synchronized saccades beyond the trained range in monkeys. Front. Psychol. 9, 2172. ( 10.3389/fpsyg.2018.02172) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hasegawa A, Okanoya K, Hasegawa T, Seki Y. 2011. Rhythmic synchronization tapping to an audio-visual metronome in budgerigars. Sci. Rep. 1, 1-8. ( 10.1038/srep00120) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Katsu N, Yuki S, Okanoya K. 2021. Production of regular rhythm induced by external stimuli in rats. Anim. Cogn. ( 10.1007/s10071-021-01505-4) [DOI] [PubMed] [Google Scholar]
- 113.Nagasaka Y, Chao ZC, Hasegawa N, Notoya T, Fujii N. 2013. Spontaneous synchronization of arm motion between Japanese macaques. Sci. Rep. 3, 1151. ( 10.1038/srep01151) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Large EW, Gray PM. 2015. Spontaneous tempo and rhythmic entrainment in a bonobo (Pan paniscus). J. Comp. Psychol. 129, 317-328. ( 10.1037/com0000011) [DOI] [PubMed] [Google Scholar]
- 115.Hattori Y, Tomonaga M, Matsuzawa T. 2013. Spontaneous synchronized tapping to an auditory rhythm in a chimpanzee. Sci. Rep. 3, 1566. ( 10.1038/srep01566) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Seki Y, Tomyta K. 2018. Effects of metronomic sounds on a self-paced tapping task in budgerigars and humans. Curr. Zool. 65, 121-128. ( 10.1093/cz/zoy075) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Dotov DG, Cochen De Cock V, Geny C, Ihalainen P, Moens B, Leman M, Bardy B.. 2019. The role of interaction and predictability in the spontaneous entrainment of movement. J. Exp. Psychol. Gen. 148, 1041-1057. ( 10.1037/xge0000609.supp) [DOI] [PubMed] [Google Scholar]
- 118.Greenfield MD, Snedden WA. 2003. Selective attention and the spatio-temporal structure of orthopteran choruses. Behaviour 140, 1-26. ( 10.1163/156853903763999863) [DOI] [Google Scholar]
- 119.Greenfield MD, Rand AS. 2000. Frogs have rules: selective attention algorithms regulate chorusing in Physalaemus pustulosus (Leptodactylidae). Ethology 106, 331-347. ( 10.1046/j.1439-0310.2000.00525.x) [DOI] [Google Scholar]
- 120.Buck J, Buck E. 1968. Mechanism of rhythmic synchronous flashing of fireflies. Science 159, 1319-1327. ( 10.1126/science.159.3821.1319) [DOI] [PubMed] [Google Scholar]
- 121.Hartbauer M, Kratzer S, Steiner K, Römer H. 2005. Mechanisms for synchrony and alternation in song interactions of the bushcricket Mecopoda elongata (Tettigoniidae: Orthoptera). J. Comp. Physiol. A 191, 175-188. ( 10.1007/s00359-004-0586-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Schwartz JJ, Buchanan BW, Gerhardt HC. 2002. Acoustic interactions among male gray treefrogs, Hyla versicolor, in a chorus setting. Behav. Ecol. Sociobiol. 53, 9-19. ( 10.1007/s00265-002-0542-7) [DOI] [Google Scholar]
- 123.Jones DL, Jones RL, Ratnam R. 2014. Calling dynamics and call synchronization in a local group of unison bout callers. J. Comp. Physiol. A 200, 93-107. ( 10.1007/s00359-013-0867-x) [DOI] [PubMed] [Google Scholar]
- 124.Nityananda V, Balakrishnan R. 2007. Synchrony during acoustic interactions in the bushcricket Mecopoda ‘Chirper’ (Tettigoniidae: Orthoptera) is generated by a combination of chirp-by-chirp resetting and change in intrinsic chirp rate. J. Comp. Physiol. A 193, 51-65. ( 10.1007/s00359-006-0170-1) [DOI] [PubMed] [Google Scholar]
- 125.Buck J, Buck E, Case JF, Hanson FE. 1981. Comparative control of flashing in fireflies: V. Pacemaker synchronization in Pteroptyx cribellata. J. Comp. Physiol. A 144, 287-298. ( 10.1007/BF00612560) [DOI] [Google Scholar]
- 126.Buck J, Buck E, Hanson FE, Case JF, Mets L, Atta GJ. 1981. Control of flashing in fireflies: IV. Free run pacemaking in a synchronic Pteroptyx. J. Comp. Physiol. A 144, 277-286. ( 10.1007/BF00612559) [DOI] [Google Scholar]
- 127.Hanson FE. 1978. Comparative studies of firefly pacemakers. Fed. Proc. 37, 2158-2164. [PubMed] [Google Scholar]
- 128.Sismondo E. 1990. Synchronous, alternating, and phase-locked stridulation by a tropical katydid. Science 249, 55-58. ( 10.1126/science.249.4964.55) [DOI] [PubMed] [Google Scholar]
- 129.Greenfield MD, Roizen I. 1993. Katydid synchronous chorusing is an evolutionary stable outcome of female choice. Nature 364, 618-620. ( 10.1038/364618a0) [DOI] [Google Scholar]
- 130.Nityananda V, Balakrishnan R. 2021. Synchrony of complex signals in an acoustically communicating katydid. J. Exp. Biol. 224, 241877. ( 10.1242/jeb.241877) [DOI] [PubMed] [Google Scholar]
- 131.de Reus K, Soma M, Anichini M, Gamba M, de Heer Kloots M, Lense M, Bruno JH, Trainor L, Ravignani A. 2021. Rhythm in dyadic interactions. Phil. Trans. R. Soc. B 376, 20200337. ( 10.1098/rstb.2020.0337) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hammerschmidt D, Wöllner C. 2020. Sensorimotor synchronization with higher metrical levels in music shortens perceived time. Music Percept. 37, 263-277. ( 10.1525/MP.2020.37.4.263) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Burger B, Thompson MR, Luck G, Saarikallio SH, Toiviainen P. 2014. Hunting for the beat in the body: on period and phase locking in music-induced movement. Front. Hum. Neurosci. 8, 903. ( 10.3389/fnhum.2014.00903) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Rouse AA, Cook PF, Large EW, Reichmuth C. 2016. Beat keeping in a sea lion as coupled oscillation: implications for comparative understanding of human rhythm. Front. Neurosci. 10, 257. ( 10.3389/fnins.2016.00257) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Cook PF, Rouse A, Wilson M, Reichmuth C. 2013. A California sea lion (Zalophus californianus) can keep the beat: motor entrainment to rhythmic auditory stimuli in a non vocal mimic. J. Comp. Psychol. 127, 412-427. ( 10.1037/a0032345) [DOI] [PubMed] [Google Scholar]
- 136.Patel AD, Iversen JR, Bregman MR, Schulz I. 2009. Experimental evidence for synchronization to a musical beat in a nonhuman animal. Curr. Biol. 19, 827-830. ( 10.1016/j.cub.2009.03.038) [DOI] [PubMed] [Google Scholar]
- 137.Schachner A, Brady TF, Pepperberg IM, Hauser MD. 2009. Spontaneous motor entrainment to music in multiple vocal mimicking species. Curr. Biol. 19, 831-836. ( 10.1016/j.cub.2009.03.061) [DOI] [PubMed] [Google Scholar]
- 138.Bonacina S, Krizman J, White-Schwoch T, Nicol T, Kraus N. 2019. How rhythmic skills relate and develop in school-age children. Glob. Pediatr. Health 6, 1-7. ( 10.1177/2333794X19852045) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Grahn JA, Rowe JB. 2009. Feeling the beat: premotor and striatal interactions in musicians and nonmusicians during beat perception. J. Neurosci. 29, 7540-7548. ( 10.1523/JNEUROSCI.2018-08.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Launay J, Grube M, Stewart L. 2014. Dysrhythmia: a specific congenital rhythm perception deficit. Front. Psychol. 5, 18. ( 10.3389/fpsyg.2014.00018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ravignani A, Dalla Bella S, Falk S, Kello CT, Noriega F, Kotz SA. 2019. Rhythm in speech and animal vocalizations: a cross-species perspective. Ann. N. Y. Acad. Sci. 1453, 79-98. ( 10.1111/nyas.14166) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.