Summary
Background
Women of African ancestry have a higher proportion of early-onset and estrogen receptor (ER) negative cancers compared to women of European ancestry. Differences in risk factor distributions and associations by age at diagnosis and ER status might explain this disparity.
Methods
We analysed data from 1,126 women with invasive breast cancer and 2,106 population controls aged 18–74 years recruited from three hospitals in Ghana from 2013–2015. Odds ratios (OR) and 95% confidence intervals (CI) were estimated for menstrual and reproductive factors using polytomous logistic regression models adjusted for potential confounders.
Findings
Among controls, the medians for age at menarche, parity, age at first birth and breastfeeding/pregnancy were 15 years, 4 births, 20 years, and 18 months, respectively. For women ≥50 years, parity and extended breastfeeding were associated with decreased risks: >5 births vs. nulliparous, OR 0·40 (95% CI 0·20–0·83), and 0·71 (95% CI 0·51–0·98) for ≥19 vs. <13 breastfeeding months/pregnancy, which did not differ by ER. In contrast, for earlier onset cases (<50 years) parity was associated with increased risk for ER-negative tumours (p-heterogeneity by ER=0·02), which was offset by extended breastfeeding. Similar associations were observed by intrinsic-like subtypes. Less consistent relations were observed with ages at menarche and first birth.
Interpretation
Reproductive risk factor distributions was different from Western populations, but exhibited aetiologic heterogeneity by age at diagnosis and ER status similar to other populations. Differences in reproductive patterns and subtype heterogeneity are consistent with racial disparities in subtype distributions.
Introduction
Reproductive factors have been well documented as key breast cancer risk factors, with direct associations observed with early ages at menarche, nulliparity, late ages at first birth, and limited breastfeeding. Breast cancer is a heterogeneous disease, with differential aetiologic associations for tumour subtypes defined by oestrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor receptor 2 (HER2) status.1 Most of these results derive from studies of European ancestry populations. Similar investigations among African ancestry populations are crucial, given differences in demographic and risk factor distributions and their disproportionately high incidence of early-onset breast cancer and ER-negative aggressive subtypes.1–4
Analyses of risk factors by the African American Breast Cancer Epidemiology and Risk (AMBER) consortium have revealed differential risk factor associations by tumour subtypes defined by ER, PR and HER2 status.5,6 Parity was associated with a decreased risk for ER-positive cancers but an increased risk for triple-negative breast tumours; furthermore, ever breastfeeding in parous women was strongly inversely related to the risk of triple-negative tumours.6 Accumulating data support similar observations in other studies of African American and European ancestry women, although distributions of risk factors differ.1,7–11
With substantially increasing rates of breast cancer in sub-Saharan Africa, identifying risk factors and strategies for reducing incidence are essential.12,13 A population-based case-control study of breast cancer in Ghana aimed to overcome challenges of previous African studies that were unable to select population-based controls and properly classify hormone receptor-negative cases.3,12,14,15 Using a census-based sampling of controls16 and standardised protocols for collecting tumour biopsy samples for immunohistochemical (IHC) staining from cases prior to treatment17, we sought to determine the associations between reproductive risk factors and breast cancer subtypes.
Methods
Study population
A multi-disciplinary population-based case-control study in Ghana, recruited women presenting between 2013–2015 with lumps suspicious for breast cancer at three hospitals: Korle Bu Teaching Hospital (Accra), Komfo Anokye Teaching Hospital (Kumasi) and Peace and Love Hospital (Kumasi).17 Cases comprised women ages 18–74 years from defined catchment areas (chosen to be within restricted travel times from the study hospitals because the study also involved recruiting population controls from the same residential areas) who had resided in these areas for ≥1 year and were diagnosed with pathologically confirmed invasive breast cancer. Enumeration areas were randomly selected from the Ghana 2010 national census to select population controls.22 Controls were frequency matched to cases on age and districts of residence in Accra and Kumasi. Recent data cleaning efforts identified some duplicate subjects, leading to a few changes in eligibility status as previously described.17 Figure 1 details the 1,126 invasive breast cancer cases and 2,106 controls included in the present analysis.
Figure 1:
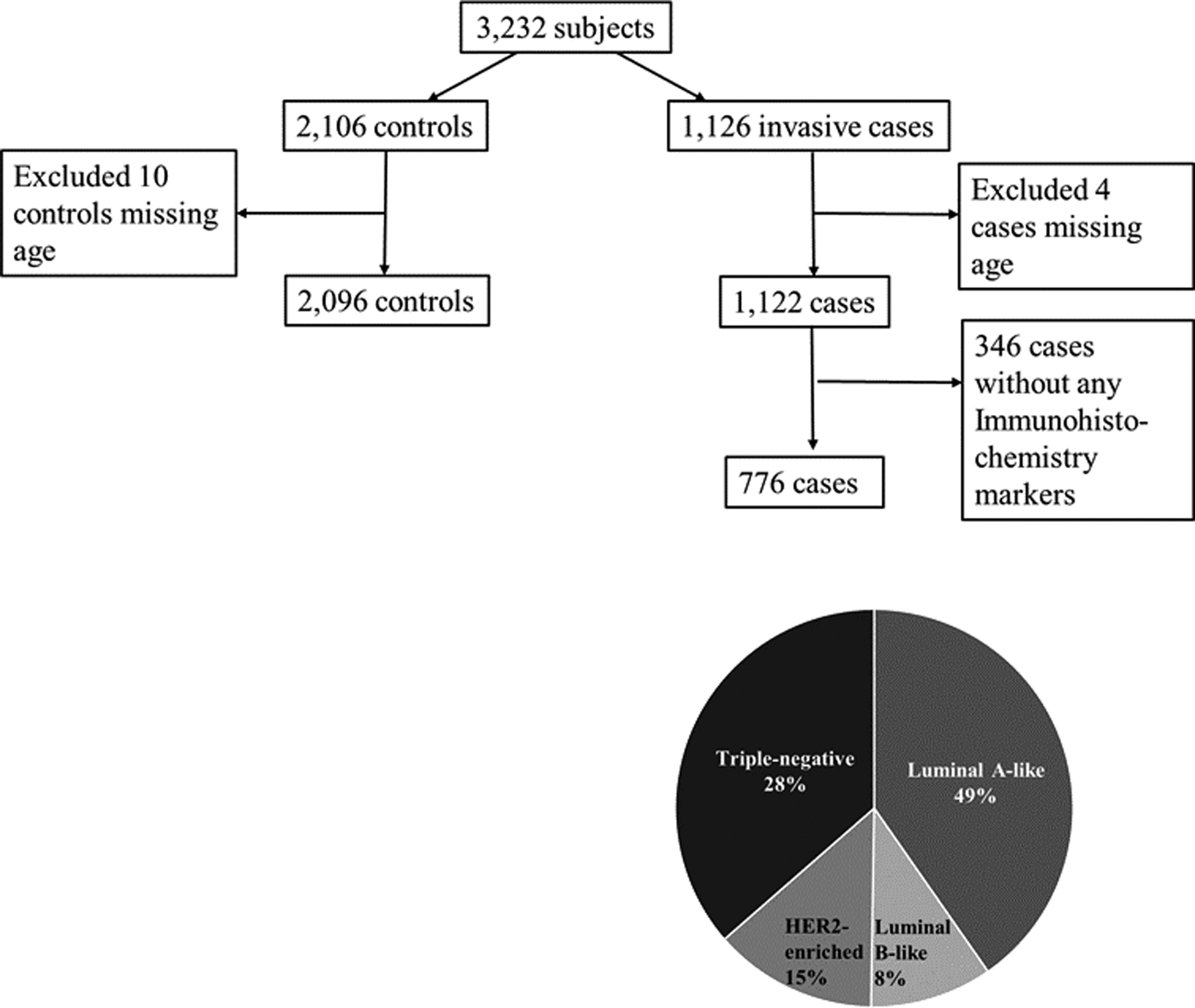
Details of cases and controls for analysis of reproductive factors and breast cancer risk by tumour characteristics in the Ghana Breast Health Study
Risk factor information
Subjects were asked about their pregnancies and outcomes, the month and year that each pregnancy was completed, whether the baby (or babies) was breastfed and for how many months they were breastfed. Women were also asked questions on age at first menstruation, whether they were still menstruating and, if no longer menstruating, the age at which menstrual periods stopped and the reason for stopping.
Tumour characteristics
Prior to treatment, 4–8 core-needle biopsies (14-gauge) were processed into formalin-fixed paraffin embedded blocks for diagnosis using standardised protocols.17 Blocks that were not required for diagnosis were sent to the National Cancer Institute (NCI) for additional pathology review (80% of the 1,126 invasive cases). Because organised mammography screening is not routine in Ghana, 96% of tumours presented as lumps >2 cm based on clinical examination.18 We obtained information on key IHC ER, PR and HER2 markers from pathology departments in Ghana for 776 cases (69%).
Our primary analyses focused on ER status because this was the key marker of aetiologic heterogeneity demonstrated in previous studies.19 ER and PR status were considered positive if ≥10% of tumour cells stained positive. The proportion of cases that were classified as 1–9% ER positive cells was minimal (1.8%). For HER2, tumours were considered positive if they demonstrated a homogeneous, dark pattern of staining in ≥10% of tumour cells. Indeterminate and negative cases were combined and considered HER2 negative.
We assessed the agreement of IHC assays performed in pathology departments in Ghana with those performed at an NCI laboratory in 87 cases, using two tumour tissue samples from the same patient. We observed good agreement for ER and HER2 (79% for ER, n=87, p < 0·0001 and 78% for HER2, n = 76, p < 0·0001). PR showed a 65% agreement (n=86, p=0·002). To determine if associations differed by proxies for intrinsic subtypes based on IHC data, we further classified tumours as luminal A-like (ER+ or PR+ and HER2−), luminal B-like (ER+ or PR+ and HER2+), HER2-enriched-like (ER−, PR− and HER2+) or triple-negative/basal-like (ER−, PR− and HER2−).
Statistical analysis
Odds ratios (ORs) and 95% confidence intervals (95% CIs) were estimated for reproductive factors using logistic models adjusted for study site and age (as a categorical variable) as well as key risk factors: education, a family history of breast cancer, body size17 and menopausal status or age at menopause. We observed a high correlation between total breastfeeding years and number of births (rho=0·87 among controls) and a lower correlation with median breastfeeding months per pregnancy (rho=0·15 among controls), the latter of which was used to avoid collinearity in models. Polytomous logistic regression estimated the OR and 95% CI for each breast cancer subtype (comparing case IHC-defined subtypes with controls). Heterogeneity between reproductive risk factors was assessed using polytomous logistic regression analyses restricted to cases (case-only analyses) with tumour characteristics and IHC as the outcome variable. To test differences in ORs by age, a likelihood-ratio test was performed by fitting the logistic regression models with and without interaction terms. For all analyses, p-values <0·05 were considered statistically significant, and all statistical tests were two-sided. Analyses were performed using STATA/MP 14.2 (StataCorp, College Station, TX). Plots on the means and standard deviations of a 3-point running average were performed using R version 3.4.4.
Role of the funding source
The funders had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
Descriptive characteristics of cases and controls
Cases were slightly older than controls, reflecting that controls were initially frequency matched to all women with a suspicion of breast cancer prior to diagnosis confirmation. Approximately half of these women had non-malignant breast diseases and tended to be younger than those with malignant breast disease.17 Cases more often than controls reported late ages at menarche, few births, late ages at first birth and low median breastfeeding months (Table 1).
Table 1:
Demographic and reproductive characteristics of 1,126 diagnosed invasive breast cancer cases and 2,106 controls from the Ghana Breast Health Study
| Controls N = 2,106 | Cases N = 1,126 | |||
|---|---|---|---|---|
| Study Population Characteristics | N | % | N | % |
| Age (years) | ||||
| <35 | 435 | 21 | 114 | 10 |
| 35–44 | 561 | 27 | 277 | 25 |
| 45–55 | 554 | 26 | 330 | 29 |
| ≥55 | 546 | 26 | 401 | 36 |
| Unknown | 10 | 4 | ||
| Study site | ||||
| Accra | 736 | 35 | 384 | 34 |
| Kumasi | 1370 | 65 | 742 | 66 |
| Age at menarche (years) | ||||
| Median age at menarche (IQR) | 15 (15–15) | 15 (15–15) | ||
| <15 | 568 | 30 | 266 | 27 |
| 15 | 548 | 29 | 255 | 26 |
| 16 | 383 | 20 | 223 | 23 |
| ≥17 | 395 | 21 | 228 | 23 |
| Unknown | 212 | 154 | ||
| Parity | ||||
| Median parity (IQR) | 4 (2–5) | 3 (2–5) | ||
| Nulliparous | 228 | 11 | 107 | 10 |
| 1–2 | 533 | 25 | 319 | 28 |
| 3–4 | 685 | 33 | 365 | 33 |
| ≥5 | 652 | 31 | 331 | 30 |
| Unknown | 8 | 4 | ||
| Age at first birth (years) | ||||
| Median age (IQR) | 20 (18–24) | 21 (19–25) | ||
| <19 | 555 | 31 | 235 | 25 |
| 19–21 | 510 | 28 | 265 | 28 |
| 22–25 | 412 | 23 | 260 | 27 |
| ≥26 | 322 | 18 | 197 | 21 |
| Unknown | 79 | 62 | ||
| Median breastfeeding per pregnancy (months) | ||||
| Median months(IQR) | 18 (15–24) | 18 (12–24) | ||
| <13 | 352 | 20 | 239 | 26 |
| 13–18 | 692 | 39 | 341 | 37 |
| ≥19 | 747 | 42 | 347 | 37 |
| Unknown | 87 | 92 | ||
| ER status | ||||
| Positive | 393 | 50 | ||
| Negative | 387 | 50 | ||
| Unknown | 346 | |||
| PR status | ||||
| Positive | 402 | 52 | ||
| Negative | 374 | 48 | ||
| Unknown | 350 | |||
| HER2 status | ||||
| Positive | 181 | 23 | ||
| Negative | 544 | 70 | ||
| Inconclusive | 54 | 7 | ||
| Unknown | 347 | |||
IQR = interquartile range. ER = oestrogen receptor. PR = progesterone receptor. HER2 = human epidermal growth factor receptor 2.
Fifty percent, 52% and 23% of cases were ER positive, PR positive and HER2 positive, respectively (Table 1). Luminal A-like breast cancer was the most common subtype (49%) followed by triple-negative/basal-like (28%), HER2-enriched (15%) and luminal B-like breast (8%) cancers (Figure 1). There were no significant differences in cases missing ER, PR and HER2 status by risk factor data (data not shown).
Descriptive analyses of reproductive factors by birth cohort (1945–1975) are shown in Figure 2. Mean number of births was lower in later birth cohorts compared to earlier birth cohorts, with cases having fewer births on average compared to controls (1945, mean 4·1 for cases and 6·4 for controls; 1975, mean 2·5 for cases and 3·3 for controls). Mean age at first birth increased by approximately 1 year in the later compared with earlier birth cohorts for both cases and controls (21·7 in 1945 and 22·3 in 1975 among controls). Mean age at menarche showed no apparent trends, hovering around 15 years across the birth cohorts. Mean breastfeeding months per pregnancy among controls declined until the 1960s and steadily increased until 1975. Among the cases, breastfeeding months per pregnancy increased over time by one month per pregnancy from 17 to 18.
Figure 2: Temporal trends of menstrual and reproductive risk factors for cases and controls in the Ghana Breast Health Study by birth cohorts from 1945 to 1975.
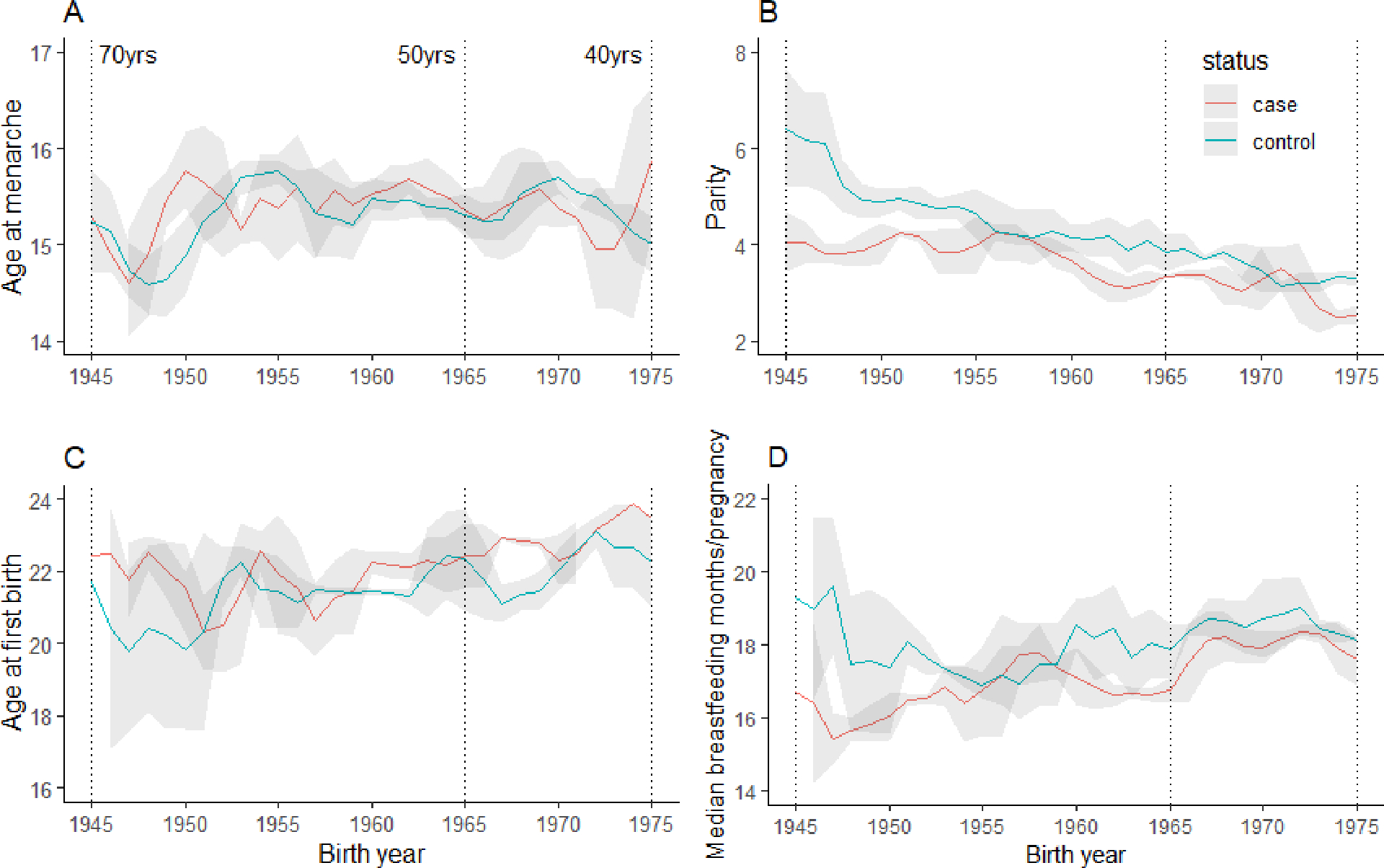
(A) Age at menarche, (B) parity, (C) age at first birth and (D) median breastfeeding months per pregnancy. The means and standard deviations plotted are the results of a 3-point running average. Grey indicates standard deviation.
Associations with reproductive factors overall and stratified by age
Associations for age at menarche, number of births, age at first birth and median breastfeeding months per pregnancy overall and stratified by age (Table 2). Analyses of all cases combined showed number of births as the only risk factor with a statistically significant risk association (p-trend=0·005). Among women aged <50 years, we observed an inverse association with parity (≥5 vs 0 births: OR 0·70, 95% CI 0·42–1·18, p-trend=0·06) and an increased risk with older ages at first birth (≥26 vs <19 years: OR 1·40, 95% CI 0·97–2·01, p-trend=0·05). In more discrete categories of age we observed a significant trend (p=0·01) with advancing age at first birth among women aged <40 years (Supplemental Table 1). Age at menarche and median breastfeeding months were not significantly associated with breast cancer risk among younger women. Among women aged ≥50 years, a strong inverse association was observed with parity (≥5 vs 0 births: OR 0·40, 95% CI 0·20–0·83), and a test for interaction with age was significant (p=0·02). Similarly, median breastfeeding months among older women were inversely associated with risk (≥19 vs <13 months: OR 0·71, 95% CI 0·51–0·98) and demonstrated a significant interaction with age (p=0·01). Age at menarche was unrelated to risk among the older women (Table 2). Evaluation of these associations with more detailed categories of age revealed a significant interaction by age for parity and median breastfeeding months per pregnancy, with the strongest inverse associations of parity and extended breastfeeding among women aged ≥60 years (Supplemental Table 1).
Table 2:
ORs and 95% CIs for select reproductive risk factors and breast cancer risk overall, stratified by women younger and older than 50 years among 1,122 cases and 2,096 controls
| All women | Women <50 years old (N = 564) | Women ≥50 years old (N = 558) | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | p | p-trend | Controls | Cases | OR | 95% CI | p | p-trend | Controls | Cases | OR | 95% CI | p | p-trend | p-int (LRT) | ||||
| Age at menarche (years) | ||||||||||||||||||||
| <15 | 1·00 | 391 | 148 | 1·00 | 174 | 118 | 1·00 | |||||||||||||
| 15 | 0·88 | 0·70 | 1·10 | 0·25 | 323 | 121 | 0·88 | 0·65 | 1·20 | 0·42 | 225 | 134 | 0·81 | 0·57 | 1·14 | 0·22 | ||||
| 16 | 1·13 | 0·89 | 1·44 | 0·31 | 238 | 114 | 1·17 | 0·86 | 1·61 | 0·31 | 143 | 108 | 0·94 | 0·64 | 1·38 | 0·75 | ||||
| ≥17 | 1·08 | 0·85 | 1·37 | 0·53 | 0·30 | 249 | 117 | 1·08 | 0·79 | 1·48 | 0·63 | 0·46 | 146 | 111 | 0·97 | 0·66 | 1·42 | 0·87 | 0·82 | 0·34 |
| Parity | ||||||||||||||||||||
| Nulliparous | 1·00 | 209 | 73 | 1·00 | 19 | 34 | 1·00 | |||||||||||||
| 1–2 | 1·04 | 0·72 | 1·51 | 0·83 | 406 | 204 | 0·93 | 0·58 | 1·50 | 0·78 | 122 | 115 | 0·59 | 0·28 | 1·24 | 0·16 | ||||
| 3–4 | 0·80 | 0·55 | 1·15 | 0·23 | 429 | 187 | 0·79 | 0·49 | 1·27 | 0·32 | 254 | 176 | 0·41 | 0·20 | 0·84 | 0·01 | ||||
| ≥5 | 0·73 | 0·50 | 1·07 | 0·10 | 0·005 | 246 | 99 | 0·70 | 0·42 | 1·18 | 0·18 | 0·06 | 403 | 230 | 0·40 | 0·20 | 0·83 | 0·01 | 0·01 | 0·02 |
| Age at first birth (years) | ||||||||||||||||||||
| <19 | 1·00 | 310 | 103 | 1·00 | 242 | 132 | 1·00 | |||||||||||||
| 19–21 | 1·14 | 0·90 | 1·43 | 0·28 | 290 | 121 | 1·18 | 0·85 | 1·64 | 0·32 | 219 | 143 | 1·15 | 0·82 | 1·60 | 0·42 | ||||
| 22–25 | 1·27 | 1·00 | 1·62 | 0·05 | 247 | 127 | 1·42 | 1·01 | 2·00 | 0·04 | 164 | 133 | 1·15 | 0·81 | 1·65 | 0·43 | ||||
| ≥26 | 1·18 | 0·91 | 1·54 | 0·22 | 0·135 | 204 | 121 | 1·40 | 0·97 | 2·01 | 0·07 | 0·05 | 118 | 76 | 1·03 | 0·68 | 1·56 | 0·88 | 0·74 | 0·28 |
| Median months breastfeeding per pregnancy (among parous women) | ||||||||||||||||||||
| <13 | 1·00 | 189 | 89 | 1·00 | 163 | 150 | 1·00 | |||||||||||||
| 13–18 | 0·85 | 0·68 | 1·06 | 0·16 | 416 | 182 | 0·98 | 0·71 | 1·36 | 0·92 | 272 | 158 | 0·74 | 0·54 | 1·03 | 0·07 | ||||
| ≥19 | 0·84 | 0·67 | 1·05 | 0·12 | 0·159 | 434 | 184 | 1·04 | 0·75 | 1·44 | 0·82 | 0·77 | 308 | 160 | 0·71 | 0·51 | 0·98 | 0·04 | 0·05 | 0·01 |
Logistic regression models were adjusted for age, study site, body size, family history of breast cancer, menopausal status, age at menopause, and all reproductive factors listed above. P-values <0·05 were considered statistically significant. OR=odds ratio. CI=confidence interval, LRT=Likelihood ratio test for interaction term.
Associations with reproductive factors by ER overall and stratified by age
Analyses for all cases combined did not show statistically significant differences for ER-negative compared with ER-positive cases (Supplemental Table 2). When we evaluated associations by ER status among women aged <50 years (Table 3), we observed a strong inverse association with parity for ER-positive tumours and a positive association for ER-negative tumours, with the test for heterogeneity being statistically significant (p-het=0·02). Among women <50 years, older ages at first birth showed a slightly stronger association with increased risk for ER-positive than ER-negative breast tumours, but the test for heterogeneity was not statistically significant. Extended breastfeeding only showed an inverse association among ER-negative tumours, with evidence of significant heterogeneity compared to ER-positive tumours (≥19 vs <13 months: ER-positive tumours OR 1·39, 95% CI 0·82–2·34; ER-negative tumours 0·71, 0·45–1·12; p-het=0·04). There was no additional relation for ≥19 breastfeeding months, and when we compared women with ≥13 breastfeeding months per pregnancy to <13 months, the resultant OR for ER-negative tumours was OR 0·69 (95% CI 0·45–1·03). There was a suggestion of a positive association with older ages at menarche for ER-positive breast tumours that was not apparent for ER-negative breast tumours.
Table 3:
Reproductive risk factors and breast cancer risk by ER status among women <50 years of age among 378 cases and 1,294 controls
| ER-positive N = 185 | ER-negative N = 193 | ER-negative/ER-positive | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Controls | ER-positive | ER-negative | OR | 95% CI | p-trend | OR | 95% CI | p-trend | p-het | |||
| Age at menarche (years) | ||||||||||||
| <15 | 391 | 40 | 54 | 1·00 | 1·00 | |||||||
| 15 | 323 | 44 | 37 | 1·21 | 0·76 | 1·93 | 0·69 | 0·43 | 1·09 | 0·08 | ||
| 16 | 238 | 37 | 43 | 1·38 | 0·85 | 2·25 | 1·21 | 0·77 | 1·90 | 0·79 | ||
| ≥17 | 249 | 47 | 38 | 1·61 | 1·00 | 2·58 | 0·05 | 0·97 | 0·61 | 1·55 | 0·81 | 0·09 |
| Parity | ||||||||||||
| Nulliparous | 209 | 23 | 19 | 1·00 | 1·00 | |||||||
| 1–2 | 406 | 62 | 64 | 0·57 | 0·26 | 1·22 | 1·70 | 0·82 | 3·51 | 0·06 | ||
| 3–4 | 429 | 66 | 67 | 0·51 | 0·24 | 1·09 | 1·62 | 0·78 | 3·36 | 0·04 | ||
| ≥5 | 246 | 34 | 43 | 0·46 | 0·20 | 1·06 | 0·19 | 1·80 | 0·82 | 3·95 | 0·32 | 0·02 |
| Age at first birth (years) | ||||||||||||
| <19 | 310 | 31 | 40 | 1·00 | 1·00 | |||||||
| 19–21 | 290 | 45 | 40 | 1·43 | 0·86 | 2·36 | 1·08 | 0·66 | 1·76 | 0·54 | ||
| 22–25 | 247 | 37 | 52 | 1·34 | 0·78 | 2·30 | 1·64 | 1·01 | 2·65 | 0·49 | ||
| ≥26 | 204 | 45 | 32 | 1·72 | 0·99 | 2·97 | 0·08 | 1·15 | 0·66 | 1·99 | 0·30 | 0·29 |
| Median months breastfeeding per pregnancy (among parous women) | ||||||||||||
| <13 | 189 | 24 | 39 | 1·00 | 1·00 | |||||||
| 13–18 | 416 | 65 | 57 | 1·37 | 0·82 | 2·29 | 0·67 | 0·42 | 1·05 | 0·02 | ||
| ≥19 | 434 | 64 | 61 | 1·39 | 0·83 | 2·34 | 0·29 | 0·71 | 0·45 | 1·12 | 0·25 | 0·04 |
Polytomous logistic regression models were adjusted for age, education, study site, body size, family history of breast cancer, menopausal status, and all reproductive factors listed above. P-values <0·05 were considered statistically significant. ER=oestrogen receptor. OR=odds ratio. CI=confidence interval. P-het=p-heterogeneity test.
Among the women aged ≥50 years (Table 4), parity was inversely associated with risk for both ER-negative and ER-positive tumours (although there were few nulliparous women, p-het=0·33). Although extended breastfeeding showed an inverse associations regardless of ER status, a stronger association was observed among ER-positive tumours (p-het=0·07). Age at first birth did not demonstrate any consistent associations with risk.
Table 4:
Risk factor associations by ER status among women ≥50 years of age among 398 cases and 802 controls
| ER-positive N = 205 | ER-negative N = 193 | ER-negative/ER-positive | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Controls | ER-positive | ER-negative | OR | 95% CI | p-trend | OR | 95% CI | p-trend | p-het | |||
| Age at menarche (years) | ||||||||||||
| <15 | 177 | 51 | 39 | 1·00 | 1·00 | |||||||
| 15 | 225 | 50 | 46 | 0·69 | 0·43 | 0·85 | 0·85 | 0·51 | 1·39 | 0·60 | ||
| 16 | 145 | 37 | 27 | 0·73 | 0·43 | 0·69 | 0·69 | 0·39 | 1·22 | 0·84 | ||
| ≥17 | 146 | 42 | 43 | 0·84 | 0·50 | 1·13 | 0·74 | 1·13 | 0·67 | 1·92 | 0·77 | 0·36 |
| Parity | ||||||||||||
| Nulliparous | 19 | 12 | 14 | 1·00 | 1·00 | |||||||
| 1–2 | 127 | 48 | 36 | 0·82 | 0·31 | 2·16 | 0·34 | 0·13 | 0·88 | 0·13 | ||
| 3–4 | 256 | 72 | 53 | 0·58 | 0·23 | 1·49 | 0·23 | 0·09 | 0·57 | 0·83 | ||
| ≥5 | 406 | 76 | 91 | 0·49 | 0·19 | 1·26 | 0·24 | 0·28 | 0·11 | 0·70 | 0·004 | 0·33 |
| Age at first birth (years) | ||||||||||||
| <19 | 245 | 46 | 50 | 1·00 | 1·00 | |||||||
| 19–21 | 220 | 49 | 56 | 1·10 | 0·67 | 1·78 | 1·26 | 0·80 | 2·00 | 0·55 | ||
| 22–25 | 165 | 48 | 42 | 1·10 | 0·66 | 1·83 | 1·06 | 0·64 | 1·75 | 1·00 | ||
| ≥26 | 118 | 32 | 24 | 1·09 | 0·61 | 1·93 | 0·72 | 1·02 | 0·57 | 1·84 | 0·95 | 0·84 |
| Median months breastfeeding per pregnancy (among parous women) | ||||||||||||
| <13 | 163 | 68 | 42 | 1·00 | 1·00 | |||||||
| 13–18 | 276 | 54 | 53 | 0·61 | 0·39 | 0·95 | 0·81 | 0·50 | 1·30 | 0·35 | ||
| ≥19 | 313 | 51 | 66 | 0·54 | 0·34 | 0·85 | 0·01 | 0·89 | 0·56 | 1·42 | 0·82 | 0·07 |
Polytomous logistic regression models were adjusted for age, education, study site, body size, family history of breast cancer, menopausal status, age at menopause, and all reproductive factors listed above. P-values <0·05 were considered statistically significant. ER=oestrogen receptor. OR=odds ratio. CI=confidence index. P-het=p-heterogeneity test.
We further assessed the joint effects of parity and breastfeeding per pregnancy (Figure 3). Among women aged ≥50 years, increasing parity and breastfeeding were associated with reduced risks for both ER-negative and ER-positive tumours, with the lowest risks observed among women with ≥3 births who breastfed for ≥13 months/pregnancy compared with nulliparous women (ER-negative cases: OR 0·45, 95% CI 0·21–0·95; ER-positive cases: OR 0·31, 95% CI 0·13–0·75). This trend was less apparent among women aged <50 years with ER-positive tumours (≥3 births who breastfed for ≥13 months/pregnancy compared with nulliparous women (OR 0·69, 95% CI 0·36–1·30). In contrast, among women aged <50 years with ER-negative tumours, compared with nulliparous women, the highest relative risk was for those with ≥3 births who breastfed <13 months/pregnancy (OR 1·91, 95% CI 0·89–4.10). Women with ≥3 births who breastfeed, on average, ≥13 months per pregnancy were not at increased risk (OR 1·09, 95% CI 0·56–2·10), due to the multiplicative joint association of two factors associated with risk in opposite directions.
Figure 3: ORs and 95% CIs for joint effects of parity and breastfeeding (vs nulliparous) by ER status and age of onset.
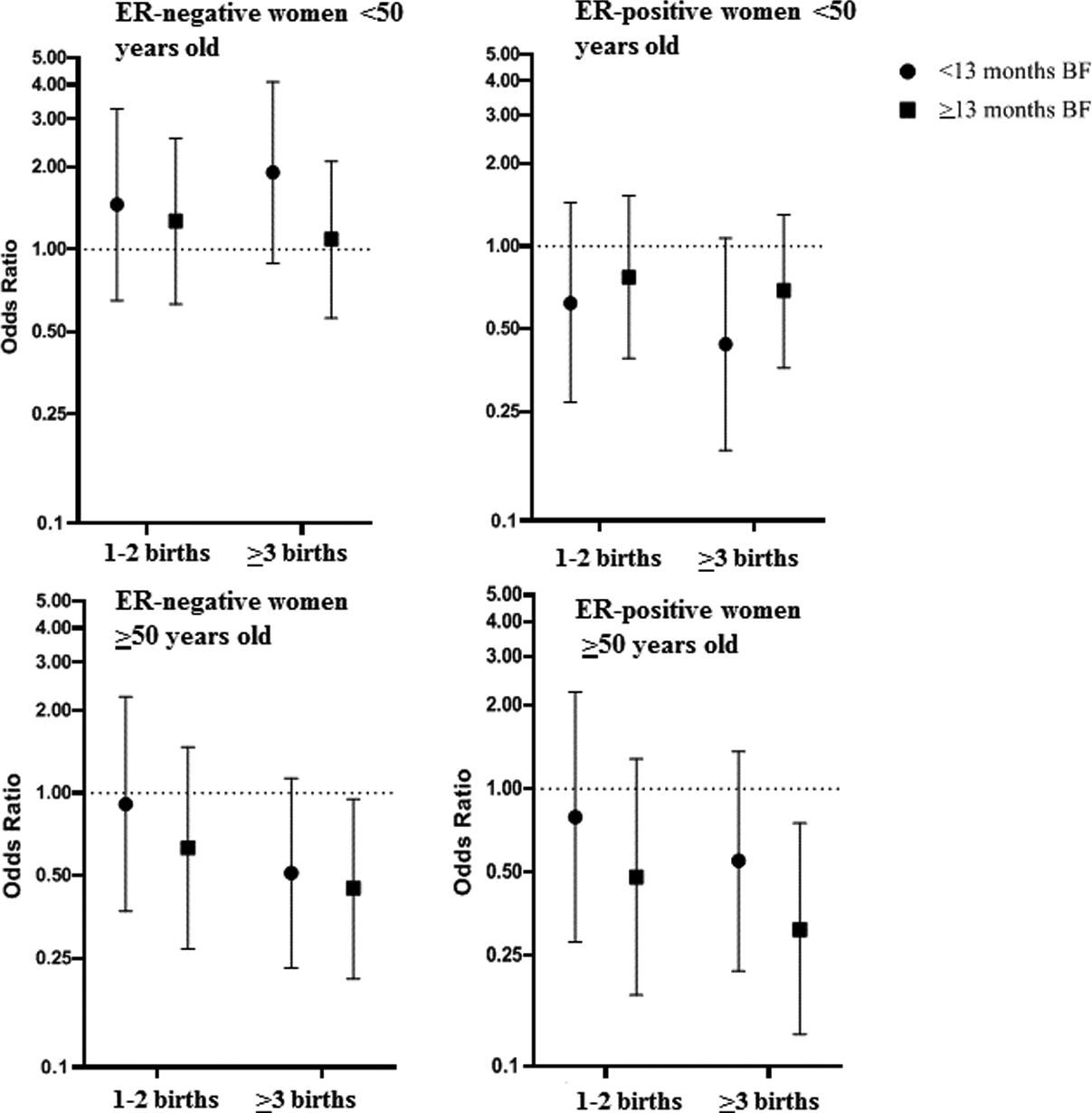
Polytomous logistic regression models were used to calculate ORs and 95% CI, adjusted for age, education, study site, body size, family history of breast cancer, age at menarche, age at first birth, menopausal status and age at menopause. Error bars indicate standard deviations = breastfeeding. ER = Oestrogen receptor.
Associations with reproductive factors by ER, PR, HER2 status overall, and stratified by age
We evaluated if associations with parity and breastfeeding differed using the IHC proxy for intrinsic subtypes. We focused our analyses on triple-negative compared with luminal A-like cases, since previous studies have shown differences between these two groups6–9,20,21 and these were also the two most common tumour subtypes (Supplemental Table 3–4). Parity was inversely related to the risk of luminal A-like tumours regardless of age, as well as with risk of triple-negative tumours among women aged ≥50 years (Supplemental Table 3–4). In contrast, a positive association was observed for triple-negative tumours among women aged <50 years (p-het=0·03). Among younger women with triple-negative tumours, extended breastfeeding was inversely associated with risk, a relationship not observed for luminal A-like tumours. In contrast, among older women, we observed a strong inverse association of breastfeeding with luminal A-like tumours (OR 0·52, 95% CI 0·33–0·82) that was not observed for triple-negative tumours (p-het=0·04) (Supplemental Table 4).
Discussion
Among Ghanaian women, we observed substantial heterogeneity of the parity association with breast cancer risk by age at diagnosis and ER, with strong inverse associations for all tumour subtypes in older (≥50 years) women and for younger-onset ER-positive tumours, but an opposite association for younger-onset ER-negative tumours (i.e. increased risk with increasing birth numbers). Median breastfeeding months per pregnancy was strongly inversely associated with later-onset breast tumour risk (particularly ER-positive or luminal A-like tumours); among younger women, it was an apparent protective factor for ER-negative tumours only. Expanding on previous reports,6–9,20,21 our study population allowed the evaluation of associations for a wide range of number of births and breastfeeding months per pregnancy.
Few studies have addressed the relation of reproductive risk factors in women of African ancestry. The largest dataset derives from the African Breast Cancer Study (ABCS),22 a hospital-based case-control study in Nigeria, Cameroon and Uganda, comprising 1,995 cases and 2,631 controls (with 81% of the cases from Nigeria). Analyses from this study showed changing reproductive patterns over time (particularly numbers of births) and an inverse association of risk with parity; however it did not show statistically significant heterogeneity of risk associations by menopausal status or age at diagnosis.22,23 Of note, in contrast to our study, ABCS was not population-based and lacked information on hormone receptor status of the tumours, thus limiting the comparability of findings. Data from the AMBER consortium, a pooled analysis of four studies of African American women with available tumour IHC data, found that among 1,252 ER-negative breast tumours, parous women were at elevated risk compared with nulliparous women, increasing to 1·60 among those aged <40 years.6 Our data are consistent with AMBER and other recent studies,9,11 supporting a cross-over association of parity on breast cancer risk that is dependent on age at onset and ER status.
In our Ghanaian population, number of births and breastfeeding years were highly correlated. Our data showed a significant inverse risk relationship with median breastfeeding months per pregnancy, with a 15% reduced risk for those with 13–18 vs <13 months/pregnancy. In pooled analyses of populations of European ancestry, breastfeeding has been shown to have a weak inverse association with breast cancer risk. However, recent data that includes molecular subtyping information provides evidence of a possible stronger inverse association for hormone-negative breast tumours.6–9,20,21 In the AMBER study, the inverse association of breastfeeding was most pronounced for younger-onset ER-negative and triple-negative breast tumours. In fact, for such tumours, analyses demonstrated that extended breastfeeding could reduce the adverse risks associated with parity, which has also been seen in other studies that included African-American women.9,11 Our results revealed similar associations given that extended breastfeeding appeared to largely counteract the adverse relationship with multiparity among younger women with ER-negative tumours.
More recent studies assessing associations by molecular subtypes using IHC and mRNA expression profiling have shown that the increased risk with parity may predominate for triple-negative or basal-like breast tumours.20,24 In our study, the modifications of the risk associations for parity and breastfeeding by age mirrored different temporal trend patterns by birth cohort in cases and controls: the rate of decrease in number of births was faster for controls than cases in early birth cohorts (i.e. older women), and a decreasing trend of breastfeeding months per pregnancy in early birth cohorts was seen in controls but not in cases. Given that multiparity and increased breastfeeding are inversely associated with later-onset breast cancers (with somewhat stronger associations with ER-positive tumours), if the observed temporal trends of decreasing parity and breastfeeding continue, they are likely to result in an increased incidence of later onset breast cancer.13 This indicates the importance of public health measures to maintain high rates of breastfeeding,25 which could potentially attenuate the projected increase in risk due to changes in reproductive patterns and demographics.13,26
Older age at first birth has been associated with increases in breast cancer risk in numerous studies, particularly for ER-positive tumours.8,10,20,27,28 The AMBER consortium also found increased risks for older ages at first birth for ER-positive but not for ER-negative tumours. Our data was consistent with these findings, suggesting that this association might be stronger or limited to early onset ER-positive breast cancer cases.6 However, in African populations, this is a difficult exposure to assess given that few women actually delay their first births until truly late ages. With increasing adoption of westernised lifestyles and access to birth control, continued monitoring of maternity data are needed to determine if ages at first birth continue to increase.
In spite of the observed trends in reproductive patterns towards westernization, our study population still maintained higher parity and breastfeeding frequencies compared to other populations. The strong inverse associations of these factors with late onset, mostly ER-positive tumours, together with a lack of population-based screening, are likely important factors contributing to historically low incidence of late onset ER-positive breast cancers. In contrast, for early onset cancers, higher parity was directly associated with ER-negative disease. It is doubtful, however, that high parity explains the higher incidence of ER-negative early onset cancers in our population given the high prevalence of breastfeeding, which appeared to offset the higher risk from multiparity. Instead, the younger demographics in Ghana and other sub-Saharan African countries probably explains the higher proportion of these early onset cancers compared to populations of European ancestry.3 It may be that rather than a population with an “excess” of early onset ER-negative cancers, there could be fewer diagnoses of late onset ER-positive breast cancer, compared to other populations, as suggested in other studies.29 To specifically address this, further studies comparing age- incidence rates of breast cancer subtypes in Africa are needed as have been done addressing racial differences by age in the US.30
Age at menarche has been inversely associated with risk in European ancestry populations.31 In the studies of African American women, later ages at menarche were inversely associated with breast cancer regardless of hormone receptor status.5,9 In contrast, we observed no such relationship. The median age of menarche of 15 years in Ghanaian women is quite different from the reported age of 12 years among African American women, with our study having limited variation in ages at menarche. Increased nutrition has been suggested to lower the age at menarche, and this variable could reflect early exposures that may differ between populations (e.g. early adolescent weight).32 In addition, a substantial number of women in our study could not recall their ages at menarche, suggesting that measurement error could have impacted our ability to assess relationships reliably.
Strengths of this study are the population-based design, detailed risk factor assessment and tissue collection for quality assessment of IHC markers to examine aetiologic heterogeneity in sub-Saharan Africa. A limitation is that although IHC data can be used as a proxy for molecular subtypes, mRNA expression assays is required to classify previously described intrinsic molecular subtypes, especially HER2-enriched and luminal B subtypes. Although our study is one of the largest breast cancer epidemiological studies conducted in sub-Saharan Africa, analyses by age and subtypes resulted in small numbers within strata of these critical factors.
Our study indicates that while reproductive factors showed important temporal trends and distinct distributions compared with African American or European ancestry populations, their associations with breast cancer risk were generally consistent with those observed in these populations. Our data support the importance of breastfeeding to prevent early-onset ER-negative breast cancer associated with multiparty, and the longer-term protection of parity and breastfeeding for later-onset breast tumours, irrespective or their ER status. Further studies including more detailed molecular characterisation of tumours and additional risk factors might provide additional insights into breast cancer aetiology in sub-Saharan Africa.
Supplementary Material
Research in context.
Evidence before this study
Although breast cancer incidence among women from sub-Saharan Africa has been historically lower compared to populations of European descent, incidence is rising. Women of African ancestry also have a higher proportion of clinically aggressive breast cancers than other populations, including a preponderance of early onset (diagnosed <50 years of age) and oestrogen receptor (ER)-negative breast cancers. Data from European and African American populations have observed discrepant risk factors according to ER status and molecular breast cancer subtypes, particularly for reproductive factors, but similar large population-based epidemiology studies in sub-Saharan African women are lacking.
Added value of this study
In this first population-based study of breast cancer ever conducted in sub-Saharan Africa, tumour tissue samples collected from biopsy prior to treatment enabled accurate pathologic assessment and derivation of molecular subtypes that could be evaluated in relation to menstrual and reproductive risk factors. The differing prevalence of these risk factors in comparison with other populations, including women of European ancestry and African Americans, allowed an important comparison of findings according to ages at diagnosis and oestrogen-receptor status. This was of particular interest given the reported preponderance of clinically aggressive cancers among young African women. The joint assessment of parity and breastfeeding, the two major identified risk factors in this population, in relation to breast cancer subgroups provided clues as to why there may be discrepant proportion of subtypes among African women as compared to other populations.
Implications of the available evidence
Public health prevention strategies are needed to address the increasing burden of breast cancer incidence in sub-Saharan Africa, projected to double to 250,000 cases by 2030. In our study, extended breastfeeding showed an inverse association for all subtypes of later onset tumours, which are likely to rise, given trends of fewer births. Extended breastfeeding also appeared to offset a direct association that we observed of multiparity with early-onset ER-negative breast cancers, which has also been observed in African American populations. While the number of births and breastfeeding duration are much higher in Ghanaian women compared to women of European ancestry and African Americans, the relationships with risk are consistent. Promotion of breastfeeding could be beneficial not only for maternal and child health but also in reducing incidence of multiple types of breast cancer.
Funding
Intramural program of the National Cancer Institute
Footnotes
Declaration of interests
Authors have no conflicts of interest to declare.
References
- 1.Anderson KN, Schwab RB, Martinez ME. Reproductive risk factors and breast cancer subtypes: a review of the literature. Breast Cancer Res Treat 2014; 144(1): 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ademuyiwa FO, Tao Y, Luo J, , et al. Differences in the mutational landscape of triple-negative breast cancer in African Americans and Caucasians. Breast Cancer Res Treat 2017; 161(3): 491–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brewster AM, Chavez-MacGregor M, Brown P. Epidemiology, biology, and treatment of triple-negative breast cancer in women of African ancestry. Lancet Oncol 2014; 15(13): e625–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Newman LA, Kaljee LM. Health Disparities and Triple-Negative Breast Cancer in African American Women: A Review. JAMA Surg 2017; 152(5): 485–93. [DOI] [PubMed] [Google Scholar]
- 5.Ambrosone CB, Zirpoli G, Hong CC, et al. Important Role of Menarche in Development of Estrogen Receptor-Negative Breast Cancer in African American Women. J Natl Cancer Inst 2015; 107(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Palmer JR, Viscidi E, Troester MA, et al. Parity, lactation, and breast cancer subtypes in African American women: results from the AMBER Consortium. J Natl Cancer Inst 2014; 106(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fortner RT, Sisti J, Chai B, et al. Parity, breastfeeding, and breast cancer risk by hormone receptor status and molecular phenotype: results from the Nurses’ Health Studies. Breast Cancer Res 2019; 21(1): 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gaudet MM, Gierach GL, Carter BD, et al. Pooled Analysis of Nine Cohorts Reveals Breast Cancer Risk Factors by Tumor Molecular Subtype. Cancer Res 2018; 78(20): 6011–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ma H, Ursin G, Xu X, et al. Reproductive factors and the risk of triple-negative breast cancer in white women and African-American women: a pooled analysis. Breast Cancer Res 2017; 19(1): 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lambertini M, Santoro L, Del Mastro L, et al. Reproductive behaviors and risk of developing breast cancer according to tumor subtype: A systematic review and meta-analysis of epidemiological studies. Cancer Treat Rev 2016; 49: 65–76. [DOI] [PubMed] [Google Scholar]
- 11.John EM, Hines LM, Phipps AI, et al. Reproductive history, breast-feeding and risk of triple negative breast cancer: The Breast Cancer Etiology in Minorities (BEM) study. Int J Cancer 2018; 142(11): 2273–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Akarolo-Anthony SN, Ogundiran TO, Adebamowo CA. Emerging breast cancer epidemic: evidence from Africa. Breast Cancer Res 2010; 12Suppl 4: S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Torre LA, Bray F, Siegel RL, , et al. Global cancer statistics, 2012. CA Cancer J Clin 2015; 65(2): 87–108. [DOI] [PubMed] [Google Scholar]
- 14.Bird PA, Hill AG, Houssami N. Poor hormone receptor expression in East African breast cancer: evidence of a biologically different disease? Ann Surg Oncol 2008; 15(7): 1983–8. [DOI] [PubMed] [Google Scholar]
- 15.Brinton LA, Figueroa JD, Awuah B, et al. Breast cancer in Sub-Saharan Africa: opportunities for prevention. Breast Cancer Res Treat 2014; 144(3): 467–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nyante SJ, Biritwum R, Figueroa J, et al. Recruiting population controls for case-control studies in sub-Saharan Africa: The Ghana Breast Health Study. PLoS One 2019; 14(4): e0215347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brinton LA, Awuah B, Nat Clegg-Lamptey J, et al. Design considerations for identifying breast cancer risk factors in a population-based study in Africa. Int J Cancer 2017; 140(12): 2667–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brinton L, Figueroa J, Adjei E, et al. Factors contributing to delays in diagnosis of breast cancers in Ghana, West Africa. Breast Cancer Res Treat 2017; 162(1): 105–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Allred DC, Brown P, Medina D. The origins of estrogen receptor alpha-positive and estrogen receptor alpha-negative human breast cancer. Breast Cancer Res 2004; 6(6): 240–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holm J, Eriksson L, Ploner A, et al. Assessment of Breast Cancer Risk Factors Reveals Subtype Heterogeneity. Cancer Res 2017; 77(13): 3708–17. [DOI] [PubMed] [Google Scholar]
- 21.Work ME, John EM, Andrulis IL, et al. Reproductive risk factors and oestrogen/progesterone receptor-negative breast cancer in the Breast Cancer Family Registry. Br J Cancer 2014; 110(5): 1367–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sighoko D, Ogundiran T, Ademola A, et al. Breast cancer risk after full-term pregnancies among African women from Nigeria, Cameroon, and Uganda. Cancer 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huo D, Adebamowo CA, Ogundiran TO, et al. Parity and breastfeeding are protective against breast cancer in Nigerian women. Br J Cancer 2008; 98(5): 992–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun X, Nichols HB, Tse CK, et al. Association of Parity and Time since Last Birth with Breast Cancer Prognosis by Intrinsic Subtype. Cancer Epidemiol Biomarkers Prev 2016; 25(1): 60–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Victora CG, Bahl R, Barros AJ, et al. Breastfeeding in the 21st century: epidemiology, mechanisms, and lifelong effect. Lancet 2016; 387(10017): 475–90. [DOI] [PubMed] [Google Scholar]
- 26.Tamimi RM, Spiegelman D, Smith-Warner SA, et al. Population Attributable Risk of Modifiable and Nonmodifiable Breast Cancer Risk Factors in Postmenopausal Breast Cancer. Am J Epidemiol 2016; 184(12): 884–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma H, Wang Y, Sullivan-Halley J, et al. Use of four biomarkers to evaluate the risk of breast cancer subtypes in the women’s contraceptive and reproductive experiences study. Cancer Res 2010; 70(2): 575–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tamimi RM, Colditz GA, Hazra A, et al. Traditional breast cancer risk factors in relation to molecular subtypes of breast cancer. Breast Cancer Res Treat 2012; 131(1): 159–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dickens C, Duarte R, Zietsman A, et al. Racial comparison of receptor-defined breast cancer in Southern African women: subtype prevalence and age-incidence analysis of nationwide cancer registry data. Cancer Epidemiol Biomarkers Prev 2014; 23(11): 2311–21. [DOI] [PubMed] [Google Scholar]
- 30.Clarke CA, Keegan TH, Yang J, et al. Age-specific incidence of breast cancer subtypes: understanding the black-white crossover. J Natl Cancer Inst 2012; 104(14): 1094–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Collaborative Group on Hormonal Factors in Breast C. Menarche, menopause, and breast cancer risk: individual participant meta-analysis, including 118 964 women with breast cancer from 117 epidemiological studies. Lancet Oncol 2012; 13(11): 1141–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheng G, Buyken AE, Shi L, et al. Beyond overweight: nutrition as an important lifestyle factor influencing timing of puberty. Nutr Rev 2012; 70(3): 133–52. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


