Abstract
Cancer is a devastating and aggressive disease that is globally ranked as the second-leading cause of deaths despite the relentless efforts being directed towards the discovery of novel chemotherapeutic drugs. Plants naturally produce a plethora of secondary metabolites that play a crucial role as effective therapeutic agents. Cancer treatment rely primarily on chemo- and radio-therapeutic strategies that suffers from known side effects. Recently, the strategy of controlling cancer progression by use of plant-derived natural products have extensively attracted research interests. In this study, the antioxidant and anticancer activities of the methanolic extract of Calligonum comosum (MeCc) fruit hairs were investigated. According to DPPH and ABTS assays, MeCc exhibited potent antioxidant capacity as it displayed significant free-radical scavenging activity. Results of the MTT cytotoxicity assay revealed that the MeCc exhibited potent anti-proliferation activity (IC50 = 10.4 µg/ml) that is specific against human hepatocarcinoma cells (HepG2), as only marginally harmful effect against non-cancerous control BJ-1 cells was detected. Results of the RT-qPCR gene expression analyses indicated that MeCc resulted in significant overexpression of mRNA transcript levels of the pro-apoptotic genes p53, caspase-3 and Bax, while downregulating the level of Bcl-2, an anti-apoptotic marker gene. Immunoblotting of the protein expression levels for the same markers showed similar pattern to that observed in RT-qPCR profiling. While the levels of p53, caspase-3 and Bax proteins exhibited significant increase, the protein level of Bcl-2 was significantly reduced. In conclusion, it is proposed that the observed specific anticancer activity of MeCc against HepG2 cells takes place via the engagement of apoptosis. This highlights the value of C. comosum as a source of potent natural anticancer agents and warrants further investigation to identify the active principals involved.
Keywords: Calligonum comosum, Natural products, Antioxidants, Cytotoxicity, Cancer, Apoptosis, P53
1. Introduction
Plants-derived compounds have been examined for their therapeutic and pharmaceutical properties over last decades. In addition to their affordability, they have been reported to exhibit remarkably potent antioxidant and anticancer activities with minimal or no observed side effects, and commercially available (Lü et al., 2010). In this context, herbal plants that are growing under very harsh biotic and abiotic stress conditions (e.g., pathogen infection, drought, salinity and heat stresses) which force plants to divert their main metabolic pathways to produce different secondary metabolites as an adaptation response to those conditions (Al Ameri et al., 2014). These metabolites belong to diverse chemical-structure families of compounds such as polysaccharides, amino acids, phenolics, flavonoids, alkaloids and tannins; collectively they contribute to the maintenance of human health and balanced nutrition (Azziz, 2015, 2016., Pinola et al., 2017). Moreover, these metabolites can be either concentrated in the whole plant or at specific parts such as leaves, stem, bark, root, flower, tuber and seed. These plant-derived natural secondary metabolites are considered a powerful alternative to synthetic chemo-therapeutic drugs for their antineoplastic activities against various stages of carcinogenesis, biologically-friendly effects and wide margins of safety to adjacent non-cancerous cells (Lemos et al., 2014).
The genus Calligonum is a member of Polygonaceae (smartweed) plant family which comprises about 40 genera and 80 different species distributed in the desert areas of North Africa, Gulf countries and Asia. C. comosum (L'Her) is a leafless, tall, woody and perennial shrub that is found throughout North Africa, central and eastern Arabia and developing widely in sandy deserts and arid habitats. Arta or Abal is its traditional Arabic name in the Gulf region. The plant has been reported to display several valuable medicinal properties including antioxidant, anti-inflammatory, anti-ulcer, anticancer, antifungal and antihelmentic, estrogenic, hypoglycemic and cardioprotective activities (Ahmed et al., 2020). Its aerial parts (i.e, leaves, flowers and stems) have traditionally several ethno-medicinal applications including chewing to relief toothache and treat gastric ulcers. Moreover, its roots decoction has been utilized to cure gum sores. Nutritionally, the fruits and shoots are used in some alimentary dishes such as green salad thanks to its rich content of sugar and nitrogenous compounds.
Different chemical constituents have been identified in several C. comosum plant parts including, catechin, isoquercetin, quercetin, and kaempferol-3-Oglucuronide and kaempferol from the ethanolic extract of the aerial parts; soprunetin, campesterol, genistein-6-C-glucoside and stigmasterol from the methanolic extract of the roots; (Shalabi et al., 2015, Badria et al., 2007, Ashour et al., 2012, Abdel-Sattar et al., 2014). In addition, the plant has been reported to be useful in other industrial applications such as firewood, flavouring, tanning of hides and as fodder for livestock animals in developing regions (Shalabi et al., 2015, Askari Jahromi et al., 2014). A circular single and hairy carpel is a characteristic morphological feature of its fruit. In terms of color, the hairy fruits are generally yellowish green, which turns into brownish hairs upon complete ripening. The fruit is intensely covered by long hairs originating from four narrow vertical ridges with wing-like structure. This feature facilitates the dispersion of the fruit over fast distances by wind.
Different plant parts have been demonstrated to possess potent biological activities (Ahmed et al., 2020). However, the knowledge on the bioactivity of its characteristic fruit hairs is lacking. Hence, this study investigates, for the first time, the antioxidant and anti-proliferation activities of the methanolic extract of C. comosum (MeCc) fruit hairs. The mode-of-action is further studied at the molecular level by gene and protein expression techniques of key pro- and anti-apoptotic markers.
2. Materials and methods
2.1. Chemicals and supplies
Unless otherwise stated, all chemicals and reagents were of molecular biology grade and have been purchased from Sigma-Aldrich (St Louis, MO, USA). Methanol was of analytical grade reagents (AR) procured from Merck Chemical Inc. (Darmstadt, Germany). All tissue culture media and materials including RPMI-1640, l-glutamine and penicillin/streptomycin, and FBS, were obtained from Gibco Inc. (NY, USA). Kits and reagents for total RNA extraction, cDNA synthesis, RT-qPCR were procured from Qiagen (Hilden, Germany). Cell culture flasks (75 cm2) with vent cap, Falcon® 15- and 50-mL polystyrene centrifuge tubes and sterile individually-wrapped Stripette™ serological polystyrene pipettes were purchased from Corning® USA. All protein chemistry reagents and buffers were obtained from Bio-Rad Laboratories GmbH (Munich, Germany).
2.2. Plant material and extract preparation
C. comosum hairy fruits were collected from the west of El-Dahnaa desert, in the road between Riyadh and Damam cities (Fig. 1). Air-dried hairy fruits of C. comosum were finely powdered with the help of a regular coffee grinder, and subsequently macerated in a glass jar with cold methanol. About 100 g fruit-hair powder was subjected to methanol extraction by placing it into 400 ml methanol under shaking for 48 h. The methanol extract of C. comosum (MeCc) fruit hairs was then filtered through Whatman no. 1 filter paper (Sigma) and the MeCc was subjected to evaporation by use of rotary evaporator. Then, the remaining residues were lyophilized in a freeze-drier at −50C° for another 24 h. With the help of vertexing, stock solutions of dimethyl sulfoxide (DMSO) at a concentration of 1 mg/ml were prepared for bioassays. The stock solution was stored at 4 °C in the dark until further use.
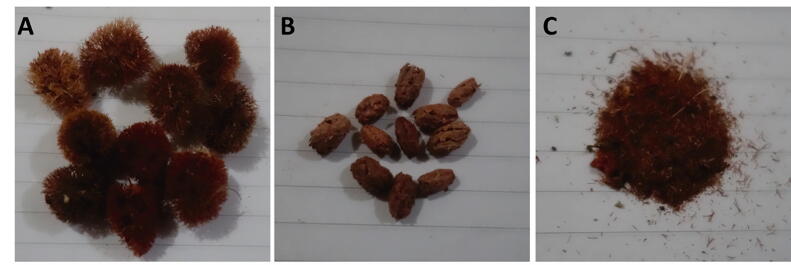
Fig. 1.
The morphological features of C. comosum fruit hairs. A) Intact hairy fruits. B) Fruits devoid of hairs. C) Ground fruit hairs that have been used in this study.
2.3. DPPH antioxidant assay
Total free radical scavenging potencies of the MeCc was evaluated by two different methods using the stable 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical and 2,2′-Azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS) as previously reported (Askari Jahromi et al., 2014, El-Hallouty et al., 2020) with slight modification. In DPPH assay, 100 µl containing (2.5, 5, 7.5, 10, 15 and 20 μg/ml) extract were added to 900 µl of 0.1 mM DPPH in methanol. The mixture was vortexed and incubated under dark at room temperature for 30 min. The absorbance of the reaction mixture was measured at 517 nm using Shimadzu UV-1280 UV–vis spectrophotometer (Nakagyo-ku, Kyoto, Japan). The absorbance of DPPH solution was used as blank and each concentration was measured in triplicates. The concentration causing 50% DPPH scavenging (SC50) was calculated using the following equation. Antioxidant activity %= (Acontrol–Asample)/Acontrol) × 100; where, Acontrol is the absorbance of the DPPH solution without sample; Asample is the absorbance of the DPPH incubated with an exact concentration of the methanol extract for 30 min. Standard butylated hydroxytoluene (BHT) was used as a positive antioxidant control.
2.4. ABTS antioxidant assay
The ABTS radical assay was carried out according to a previously published report with slight modifications (El-Hallouty et al., 2020). In brief, a mixture of 1 ml of 7 mM ABTS and 1 ml of 2.45 mM potassium persulfate was incubated in a dark place at room temperature. After 16 h of incubation, the mixture was adjusted with water to an absorbance of 0.7 at 734 nm. Then, 100 μl of (2.5, 5, 7.5, 10, 15 and 20 μg/ml of MeCc μg/ml) was mixed with 900 μl of ABTS mixture and left for incubation at room temperature in dark place for 10 min. The absorbance of the reaction mixture was measured at 734 nm. Trolox was used as positive antioxidant control. The ABTS scavenging effect was measured using the following equation: Antioxidant activity %= (0.700–Asample)/0.700) × 100; where, 0.700 is the absorbance of the ABTS radical without extract; Asample is the absorbance of each concentration of the extract at 10 min. The concentrations that cause 50% of ABTS radicals scavenging (IC50) were obtained by use of regression analysis.
2.5. Cell lines and culturing conditions
Cancerous and non-cancerous cell lines used in this study were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA). The anticancer activity of MeCc fruit hairs was tested against human hepatocellular carcinoma (HepG2, ATCC® HB-8065™) compared to noncancerous skin fibroblast BJ-1 (ATCC® CRL-2522™). The cells were subcultured in RPMI-1640 medium supplemented with 10% FBS, 100 U/ml penicillin and 100 μg/ml penicillin/streptomycin at 37 °C, 5% CO2 in 75 cm2 tissue culture flask. For the cytotoxicity assessment, cells were trypsinized (Trypsin 0.05%/0.53 mM EDTA). Then, cells were seeded (2 × 103 cell/well in 100 μl of medium) into 96-well plates (Gibco, USA) for 24 h before treated with different concentrations of MeCc (2.5, 5, 7.5, 10, 15, 20 and μg/ml) for another 24 h (Aboul-Soud et al., 2020, Abo-Salem et al., 2020). To analyze the gene expression level and western blot of pro- and anti-apoptotic markers (p53, Bax, Caspase 3 and Bcl-2), HepG-2 and BJ-1 cells were seeded (6 × 106 cell/well in 3 ml of medium) into in a 6-well plate. After 24 h, the medium was replaced with a fresh medium containing 15 μg/ml of MeCc and incubated for another 24 h.
2.6. Cell viability MTT assay
After 24 h of incubation with different MeCc concentrations, the media were discarded, and adherent cells were incubated with 100 µl/well MTT at a concentration of 0.5 mg/ml prepared in PBS and subsequently incubated at 37 °C for additional 3 h at 37 °C under dark condition (Aboul-Soud et al., 2020, Mosmann, 1983). Then, 100 μl isopropyl alcohol was added per well in order to dissolve the purple formazan crystals by the help of shaking for another 2 h at room temperature. Subsequently, the absorbance was measured at 549 nm using ELX 800 BioTek microplate reader (BioTek Instruments, Winooski, VT, USA). The results were analyzed in triplicates and the viability percentage were calculated. The MeCc concentrations resulting in 50% growth inhibition of HepG2 cells (IC50) were calculated by use of Microsoft Excel trendline equation (Aboul-Soud et al., 2020).
2.7. RT-qPCR analysis
HepG2 cells were treated with 15 µg/ml MeCc for 24 h prior to RNA extraction. Total RNA was extracted from collected cells using the “RNeasy mini kit” (Qiagen) following the manufacturer's recommendations. The RNA obtained was quantified using NanoDropTM OneC Microvolume UV–vis Spectrophotometer (Thermo Scientific). RNA purity was evaluated by the ratio of the absorbance at OD260/OD280. In order to determine RNA integrity (RIN) values, Agilent 2100 Bioanalyzer (Agilent Technologies GmbH, Waldbronn, Germany) was employed, which were ≥ 8.0 indicative of high integrity. Next, 2 μg of total RNA were used for cDNA synthesis, using transcription with Fastlane® Cell cDNA kit (Qiagen, Germany). The “FastStart Universal SYBR Green Master mix” (Roche) was used for amplification, using specific primers for p53, Bax, Caspase 3 and Bcl-2; β-actin gene was used as an internal control (Table 1). The RT-qPCR reactions were performed in a thermal cycler 7500 Real-Time PCR system (Applied Biosystems) and the amplification data was analyzed by the 7500-software version 2.0. PCR conditions were: 2 min at 50° C, 10 min at 95° C, and 40 cycles of 15 s at 95° C and 30 s at 60° C. The 2−ΔΔCt method was used to determine the relative mRNA expression level for a specific gene and were represented as fold change compared to β-actin, as a house-keeping gene (Aboul-Soud et al., 2020).
Table 1.
Nucleotide sequences of oligonucleotide primers used in RT-qPCR analysis.
| Gene symbol | Primer sequences (5′-- -->3′) |
|---|---|
| p53 | F: CCTCCTGGCCCCTGTCATCTT R: ACCTCCGTCATGTGCTGTGAC |
| Caspase-3 | F: GAGTGCTCGCAGCTCATACCT R: CCTCACGGCCTGGGATTT |
| BAX | F: GCCCTTTTCTACTTTGCCAGC R: TCAGCCCATCTTCTTCCAGAT |
| Bcl-2 | F: GGCCTTCTTTGAGTTCGGTGG R: GATAGGCACCCAGGGTGATGC |
| β-Actin | F: GGGTCAGAAGGATTCCTATG R: GGTCTCAAACATGATCTGGG |
2.8. Immunoblotting of apoptosis markers
Cells were treated with 15 µg/ml MeCc for 24 h prior to protein extraction. Collected cells were extracted by a protein extraction buffer composed of 150 mM NaCl, 50 mM Tris-HCl pH 7.5, 10 mM MgCl2, 1 mM PMSF, 0.1% NP-40 and 1x complete protease inhibitor (Roche Diagnostics GmbH, Mannheim, Germany) using a stick “pellet pestle blue” (Sigma Aldrich, St Louis, MO, USA). The total proteins were quantified using Bradford assay method (Bio-Rad® Laboratories GmbH, Munich, Germany). Forty µg protein of each treatment were loaded into 7–12% SDS-PAGE gels depending on the size of target protein. Then the gels were transferred to a PVDF membrane using a semidry transfer blot system. The membranes were blocked using 5% non-fat milk diluted in PBS buffer 0.1% Tween-20 (PBS-T) and subjected to immunodetection by the anti-Caspase-3 polyclonal antibody (ab13847; Abcam UK), anti-p53 monoclonal antibody (PAb 240; ab26; Abcam UK), anti-Bcl-2 monoclonal antibody (E17; ab32124; Abcam UK) and anti-Bax monoclonal antibody (E63, ab32503; Abcam UK). anti-β-Actin monoclonal antibody (SP124; ab115777; Abcam UK) was used as an internal control, whereas anti-Mouse and anti-Rabbit were used as secondary antibodies. Dilution rates were either 1:1000 or 1:2000 dependging on the respective antibody used (El-Hallouty et al., 2020, Aboul-Soud et al., 2020).
2.9. Statistical analysis
Statistical analyses of data were carried by use of Statistical Package for Social Sciences (SPSS; IL, USA) for Windows version 18.0. All data points were represented as means of three replicates in each group (mean ± SD) and values of (p < 0.05) and (p < 0.01) were considered to be statistically significant (Yuan et al., 2006, Duncan, 1957).
3. Results
3.1. Antioxidant activity of C. Comosum
Fig. 2 depicts the radical-scavenging potencies of MeCc using DPPH and ABTS. The antioxidant activity results indicated that the methanol extract under investigation exhibited radical-scavenging activity against both DPPH and ABTS radicals. It is worth noting that the values were remarkably higher in ABTS compared to DPPH assays. The concentration that scavenges 50% of radicals (SC50) for C. comosum seed-hair methanol extract antioxidants IC50 were 14.0 and 18.3 µg/ml (p < 0.05) against ABTS and DPPH, respectively. This result suggests that MeCc harbors bioactive compounds that are essentially required for the enhancement cellular capacity to neutralize reactive oxygen species (ROS), thereby protecting it from any oxidative stress-mediated damage.
Fig. 2.
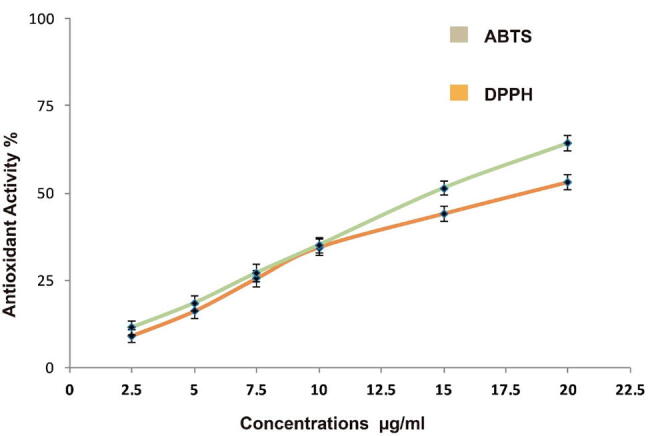
Antioxidant activity of different concentration of the methanol extract of C. comosum fruit hairs (2.5, 5, 7.5, 10, 15 and 20 μg/ml) using DPPH and ABTS assays. Histograms represent mean Antioxidant activity % ± SEM for 3 technical and 2 biological replicas.
3.2. Effect of C. Comosum methanolic extract on the proliferation HepG2 cells
After 24 h of incubation, the viable number of HepG-2 cells was significantly decreased in response to different concentration (2.5, 5, 7.5, 10, 15, 20 and 25 μg/ml) of MeCc compared to BJ-1 normal cell (Fig. 2). The MTT results revealed a clear dose-dependent anti-proliferation response against liver cancer cells 24-hour post-treatment with MeCc. Marginal cytotoxic effects were observed for the C. comosum against the non-cancerous BJ-1 control cells as only antiproliferative effect of 12.45 ± 2.42% was obtained (Fig. 2). The concentration of MeCc that inhibits 50% of HepG2 cell population (IC50) was calculated by use of trendline equation. In this context, the IC50 of MeCc against HepG2 cells was 10.4 µg/ml (Fig. 2). Therefore, 15 µg/ml of C. comosum extract was selected as the optimum concentration for the subsequent in vitro assays.
3.3. C. Comosum methanolic extract induces the expression of apoptotic gene markers
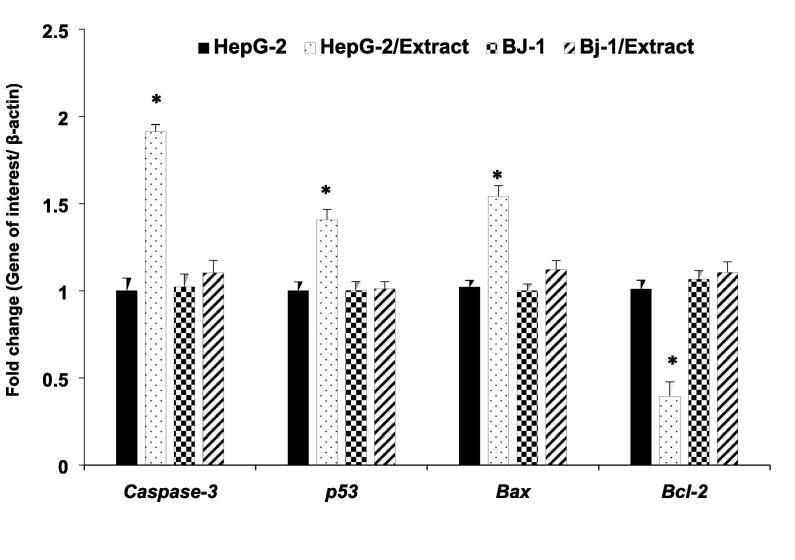
RT-qPCR analysis was conducted to determine whether the observed antiproliferation effect of MeCc against human hepatocarcinoma cells (HepG2) is due to the engagement of apoptotic gene markers at the level of transcript abundance or via a posttranscriptional regulatory mechanism. RT-qPCR analysis for the expression levels of tumor suppression-related genes (p53) and apoptosis-related genes (Bax, Caspase-3, Bcl-2) were carried out. As shown in Fig. 3, the expression levels of p53, Bax, Caspase 3 markers genes exhibited significant (p < 0.01) increase 24-hour post-treatment showing fold-change values of 1.9, 1. 4 and 1.5, respectively. By contrast, exposure to MeCc resulted in significant (p < 0.05) reduction in the expression of the anti-apoptotic marker gene Bcl-2 compared to untreated cells, with a fold change of 0.4 (Fig. 3). Whereas, no significant differences were observed in the same set of marker gene expression in non-cancerous BJ-1 control cells following the same treatment. The results indicated that apoptosis marker gene expression is altered in response to the treatment of MeCc at the transcriptional level, and not the posttranscriptional regulatory stage, culminating into death of cancer cells by apoptosis.
Fig. 3.
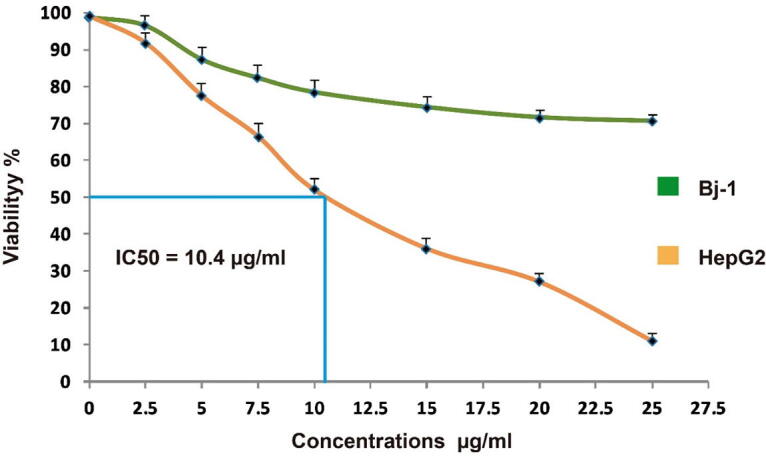
Anti-proliferation effect of different concentration of methanol extract of C. comosum fruit hairs (2.5, 5, 7.5, 10, 15, 20 and 25 μg/ml) on HepG-2 and BJ-1 cells using MTT assay. Histograms represent mean viability % ± SEM for 3 technical and 2 biological replicas.
3.4. C. Comosum methanolic extract induces the expression of apoptotic protein markers
Immunoblotting was employed to study the expression patterns of pro- (p53, Caspase-3 and Bax) and anti-apoptotic (Bcl-2) marker proteins in both human hepatocellular carcinoma (HepG-2) and non-cancerous fibroblast (BJ-1) control cells. The obtained immunoblotting results indicated that HepG2 cells treated with MeCc exhibited significant accumulation in the proteins levels of pro-apoptotic markers (p53, Caspase-3 and Bax) relative to untreated cells, whereas Bcl-2 protein level was significantly reduced (Fig. 4). By contrast, MeCc-treated BJ-1 cells did not exhibit any significant alterations in the expression levels of the examined marker proteins relative to controls (Fig. 4). Hence, RT-qPCR and immunoblotting results clearly suggest that the MeCc modulates essential markers of programmed cell death in HepG2 human hepatocarcinoma cells, at both the mRNA transcriptional and protein translational levels, culminating into their commitment towards apoptosis.
Fig. 4.
Gene expression of apoptosis markers (Casp3, p53 and Bax and Bcl-2) genes in HepG-2 and Bj-1 cells treated with 15 µg/ml methanol extract of C. comosum fruit hairs for 24 h. Gene expression levels were quantified by RT-qPCR using β-actin as a housekeeping gene. Significant differences between the means of individual treatment and control were analyzed by one-side Student’s t-test. The mean of expression levels represents as fold change ± SEM for 3 technical and 2 biological replicas.
4. Discussion
Plants are sessile organisms that produce a unique set of ubiquitous phytochemicals in order to cope with diverse harsh conditions. Specifically, desert plants are surrounded by different biotic and abiotic stresses, which forces them to modulate their regulatory metabolic pathways to achieve better adaptation responses against severe climatic and water shortage conditions (Jahan et al., 2018). These highly orchestrated adaptative metabolic strategies lead to the enrichment of plant parts with unique bioactive phytochemicals that belong to several chemical groups including phenolics, flavonoids, alkaloids and terpenoids (Ahmed et al., 2020). Extracts of different plant organs including Calligonum comosum are enriched in their contents of these phytochemicals that exhibit diverse therapeutic properties against various malignant cancer types (Aboul-Soud et al., 2020, Ashour et al., 2016, Aboul-Soud et al., 2016, Alehaideb et al., 2020). However, knowledge on the bioactivity of C. comosum fruit hairs is scarce. In this context, the antioxidants and anticancer activities were evaluated, exploring the spectrum and modes-of-action of anticancer potential mediated of methanol extract of Calligonum comosum (MeCc) fruit hairs.
The MeCc of fruit hairs exhibited potent antioxidant activity using both DPPH and ABTS radical scavenging assays (Fig. 2). This potent antioxidant capacity could be explained by the rich contents of phenolic and flavonoid compounds present in fruit hairs. Recently, the in vitro antioxidant potency as well as total flavonoid and total phenolic contents of different C. comosum organs, namely barks, leaves and fruits, have been reported (Ahmed et al., 2020, Cheruth et al., 2016). Leaves and bark have been shown to exhibit the highest radical scavenging potencies (ascorbic acid equivalent), total phenolic content (gallic acid equivalent), as well as total flavonoid content (rutin equivalent). Moreover, while flowers and fruits have the highest contents of flavonols, other organs (i.e., bark, leaves, and stems), have been found to have the highest concentrations of taxifolin and catechin (Ahmed et al., 2020).
In this study, the MeCC of fruit hairs exhibited a potent anticancer activity against HepG2 cells as indicated by a very low IC50 value of 10.4 µg/ml, as reported by the MTT anti-proliferation assay (Fig. 3). The observed anticancer activity against HepG-2 was specific as marginal growth inhibition was observed on BJ-1 normal cells. Similar IC50 values (9.60 ± 0.01) have been reported in support of our findings but with the MeCC of its aerial parts against the same HepG-2 hepatocarcinoma cell line under investigation in this study (Shalabi et al., 2015).
In this study, RT-qPCR gene expression profiling and immunoblotting results revealed that the MeCc-mediated cytotoxic effect against HepG-2 cells proceeds via the engagement of apoptosis; as judged from the significant upregulation of pro-apoptotic marker genes and proteins (p53, Caspase-3 and Bax) (Fig. 4, Fig. 5). Apoptosis is a genetically-controlled process that is vital for selective cellular elimination implicated in cell turnover and normal developmental processes. It is subjected under tight regulatory mechanisms through the expression of a battery of genes; the most important of which is the tumor suppressor gene (P53) that is regarded as the guardian of the genome. Apoptosis can be triggered by either an intrinsic or extrinsic pathway, both of which culminate into the proteolytic activation of caspase-3, the last enzyme that executes the characteristic hallmarks of apoptosis including DNA fragmentation, phosphatidylserine exposure causing reversion of the plasma membrane and degradation of nuclear protein (Aboul-Soud et al., 2016, Alehaideb et al., 2020). That main mode-of-action underlying the anticancer therapeutic potential of plant-derived phytochemicals has been suggested to be mediated by the induction of apoptosis and cell cycle arrest (Bailón-Moscoso et al., 2017, Fujita et al., 2014, Abaza et al., 2013, Gheena and Ezhilarasan, 2019). In agreement to our findings, previous studies employing FACS analysis with FITC-Annexin V/Propidium Iodide dual staining, , showed that methanolic extracts of C. comosum have triggered apoptosis induction, with all the associated morphological features, and cell cycle arrest against breast and liver cancer cell lines (Shalabi et al., 2015, Alehaideb et al., 2020); via the intracellular generation of membrane/protein/nucleic acids-damaging reactive oxygene species (ROS) (Shalabi et al., 2015).
Fig. 5.
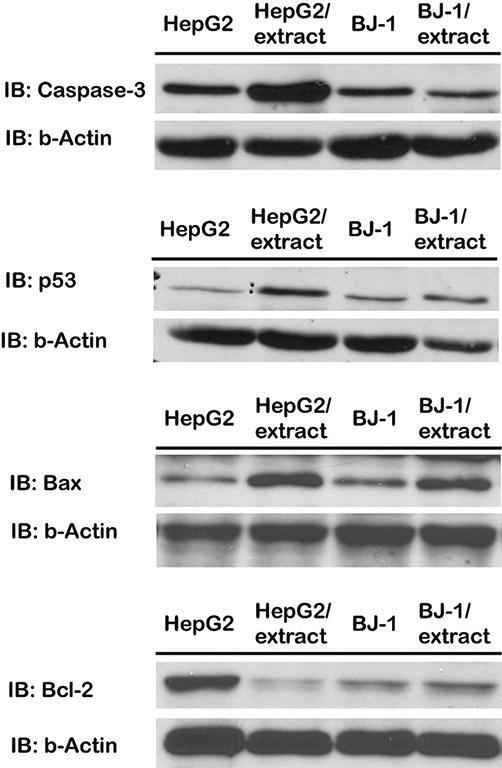
Immunoblotting of apoptosis protein markers (Casp3, p53 and Bax and Bcl-2) in HepG-2 and BJ-1 control non-cancerous cells treated with 15 µg/ml methanol extract of C. comosum fruit hairs for 24 h. Approximately, 40 µg protein extracts from HepG-2 or BJ-1 cells lysate were separated on 7–12% SDS-PAGE. anti-β actin was employed as loading control.
In conclusion, the current study provided evidence underscoring a potent antioxidant and anti-proliferation activities of the methanolic extract of C. comosum fruit hairs against human hepatocellular carcinoma (HepG2) that is dependent on the engagement of apoptosis.
Declaration of Competing Interest
The author declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
This work was funded by the Institutional Fund Program by the Saudi Ministry of Education, Hafr Al Batin University.
Footnotes
Peer review under responsibility of King Saud University.
References
- Lü J.M., Lin P.H., Yao Q., Chen C. Chemical and molecular mechanisms of antioxidants: experimental approaches and model systems. J Cell Mol Med. 2010;14(4):840–860. doi: 10.1111/j.1582-4934.2009.00897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Ameri S.A., Al Shaibani F.Y., Cheruth A.J., Al-Awad A.I., Al-Yafei M.A.S., Karthishwaran K., Kurup S.S. Comparative phytochemical analysis of Moringa oleifera and Moringa peregrina. Pharmacologyonline. 2014;3:216–221. [Google Scholar]
- Azziz R.P.C.O.S. New insights into the genetics of polycystic ovary syndrome. Nat Rev Endocrinol. 2015, 2016.;12:183. doi: 10.1038/nrendo.2016.9. [DOI] [PubMed] [Google Scholar]
- Pinola P., Puukka K., Piltonen T.T., Puurunen J., Vanky E., Sundström-Poromaa I. Normo-and hyperandrogenic women with polycystic ovary syndrome exhibit an adverse metabolic profile through life. Fertil Steril. 2017;107:788–795. doi: 10.1016/j.fertnstert.2016.12.017. [DOI] [PubMed] [Google Scholar]
- Lemos A.J., Peixoto C.A., Teixeira Á.A., Luna R.L., Rocha S.W., Santos H.M. Effect of the combination of metformin hydrochloride and melatonin on oxidative stress before and during pregnancy, and biochemical and histopathological analysis of the livers of rats after treatment for polycystic ovary syndrome. Toxicol Appl Pharmacol. 2014;280:159–168. doi: 10.1016/j.taap.2014.05.015. [DOI] [PubMed] [Google Scholar]
- Ahmed H., Moawad A., Owis A., AbouZid S. Antioxidant capacity and HPLC determination of phenolic in different organs of Calligonum polygonoides subspecies comosum. J. Reports Pharmaceut. Sci. 2020;9(2):251–255. [Google Scholar]
- Shalabi M., Khilo K., Zakaria M.M., Elsebaei M.G., Abdo W., Awadin W. Anticancer activity of Aloe vera and Calligonum comosum extracts separetely on hepatocellular carcinoma cells. Asian Pacific J Trop Biomed. 2015;5(5):375–381. [Google Scholar]
- Badria F.A., Ameen M., Akl M.R. Evaluation of cytotoxic compounds from Calligonum comosum L. growing in Egypt. Zeitschrift für Naturforschung C. 2007;62:656–660. doi: 10.1515/znc-2007-9-1005. [DOI] [PubMed] [Google Scholar]
- Ashour O.M., Abdel-Naim A.B., Abdallah H.M., Nagy A.A., Mohamadin A.M., Abdel-Sattar E.A. Evaluation of the potential cardioprotective activity of some Saudi plants against doxorubicin toxicity. Z Naturforsch C. 2012;67:297–307. doi: 10.1515/znc-2012-5-609. [DOI] [PubMed] [Google Scholar]
- Abdel-Sattar E.A., Mouneir S.M., Asaad G.F., Abdallah H.M. Protective effect of Calligonum comosum on haloperidol-induced oxidative stress in rat. Toxicol Ind Health. 2014;30:147–153. doi: 10.1177/0748233712452601. [DOI] [PubMed] [Google Scholar]
- Askari Jahromi M., Movahedin M., Mazaheri Z., Amanlu M., Mowla S.J., Batooli H. Evaluating the effects of Escanbil (Calligonum) extract on the expression level of Catsper gene variants and sperm motility in aging male mice. Iran J Reprod Med. 2014;12:459–466. [PMC free article] [PubMed] [Google Scholar]
- El-Hallouty S.M., Soliman A.A.F., Nassrallah A., Salamatullah A., Alkaltham M.S., Kamal K.Y., Hanafy E.A., Gaballa H.S., Aboul-Soud M.A.M. Crude Methanol Extract of Rosin Gum Exhibits Specific Cytotoxicity Against Human Breast Cancer Cells via Apoptosis Induction. Anticancer Agents Med Chem. 2020;20(8):1028–1036. doi: 10.2174/1871520620666200423074826. [DOI] [PubMed] [Google Scholar]
- Aboul-Soud M.A.M., Ashour A.E., Challis J.K., Ahmed A.F., Kumar A., Nassrallah A., Alahmari T.A., Saquib Q., Siddiqui M.A., Al-Sheikh Y., El-Shemy H.A., Aboul-Enein A.M., Alghamdi K.M., Jones P.D., Giesy J.P. Biochemical and Molecular Investigation of In Vitro Antioxidant and Anticancer Activity Spectrum of Crude Extracts of Willow Leaves Salix safsaf. Plants. 2020;9:1295. doi: 10.3390/plants9101295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abo-Salem H.M., Nassrallah A., Soliman A.A.F., Ebied M.S., Elawady M.E., Abdelhamid S.A., El-Sawy E.R., Al-Sheikh Y.A., Aboul-Soud M.A.M. Synthesis and Bioactivity Assessment of Novel Spiro Pyrazole-Oxindole Congeners Exhibiting Potent and Selective in vitro Anticancer Effects. Molecules. 2020;3 doi: 10.3390/molecules25051124. 25(5):1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Yuan J.S., Reed A., Chen F., Stewart C.N. Statistical analysis of real–time PCR data. BMC Bioinformatics. 2006;7:85. doi: 10.1186/1471-2105-7-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan B.D. Multiple range test for correlated and heteroscedastic means. Biometrics. 1957;13:359–364. doi: 10.2307/2527799. [DOI] [Google Scholar]
- Jahan S., Abid A., Khalid S., Afsar T., Qurat-Ul-Ain S.G. Therapeutic potentials of Quercetin in management of polycystic ovarian syndrome using Letrozole induced rat model: a histological and a biochemical study. J Ovarian Res. 2018;11:26–35. doi: 10.1186/s13048-018-0400-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashour A.E., Ahmed A.F., Kumar A., Zoheir K.M., Aboul-Soud M.A., Ahmad S.F., Attia S.M., Abd-Allah A.R.A., Cheryan V.T., Rishi A.K. Thymoquinone inhibits growth of human medulloblastoma cells by inducing oxidative stress and caspase-dependent apoptosis while suppressing NF-κB signaling and IL-8 expression. Mol. Cell. Biochem. 2016;416:141–155. doi: 10.1007/s11010-016-2703-4. [DOI] [PubMed] [Google Scholar]
- Aboul-Soud M.A.M., El-Shemy H.A., Aboul-Enein K.M., Mahmoud A.M., Al-Abd A.M., Lightfoot D.A. Effects of plant-derived anti-leukemic drugs on individualized leukemic cell population profiles in Egyptian patients. Oncol. Lett. 2016;11:642–648. doi: 10.3892/ol.2015.3916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alehaideb Z, AlGhamdi S, Yahya WB, Al-Eidi H, Alharbi M, Alaujan M, Albaz A, Tukruni M, Nehdi A, Abdulla MH, Matou-Nasri S. Anti-Proliferative and Pro-Apoptotic Effects of Calligonum comosum (L'Her.) Methanolic Extract in Human Triple-Negative MDA-MB-231 Breast Cancer Cells. J Evid Based Integr Med. 2020 25:2515690X20978391. doi: 10.1177/2515690X20978391. [DOI] [PMC free article] [PubMed]
- Cheruth A.J., Al Naqbi K.M.A., El-Kaabi A.A.A.S. In vitro antioxidant activities and screening of phytochemicals from methanolic and ethyl acetate extracts of C. comosum L’Her. Orient Pharm Exp Med. 2016;16:209–215. [Google Scholar]
- Bailón-Moscoso N., Cevallos-Solorzano G., Romero-Benavides J.C., Orellana M.I.R. Natural Compounds as Modulators of Cell Cycle Arrest: Application for Anticancer Chemotherapies. Curr. Genom. 2017;18:106–131. doi: 10.2174/1389202917666160808125645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita K., Nomura Y., Sawajiri M., Mohapatra P.K., El-Shemy H.A., Nguyen N.T., Hosokawa M., Miyashita K., Maeda T., Saneoka H. The extracts of Japanese willow tree species are e_ective forapoptotic desperation or di_erentiation of acute myeloid leukemia cells. Pharmacogn. Mag. 2014;10:125–131. doi: 10.4103/0973-1296.131023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abaza M.S.I., Al-Attiyah R., Bhardwaj R., Abbadi G., Koyippally M., Afzal M. Syringic acid from Tamarix aucheriana possesses antimitogenic and chemo-sensitizing activities in human colorectal cancer cells. Pharm. Biol. 2013;51:1110–1124. doi: 10.3109/13880209.2013.781194. [DOI] [PubMed] [Google Scholar]
- Gheena S., Ezhilarasan D. Syringic acid triggers reactive oxygen species-mediated cytotoxicity in HepG2 cells. Hum. Exp. Toxicol. 2019;38:694–702. doi: 10.1177/0960327119839173. [DOI] [PubMed] [Google Scholar]